Abstract
Host defenses against Streptococcus pneumoniae depend largely on opsonophagocytosis mediated by antibodies and complement. Since pneumococcus is a respiratory pathogen, mucosal immune responses may play a significant role in the defense against pneumococcal infections. Thus, mucosal vaccination may be an alternative approach to current immunization strategies, but effective adjuvants are required. Protein antigens induce significant mucosal immunoglobulin A (IgA) and systemic IgG responses when administered intranasally (i.n.) with the glyceride-polysorbate based adjuvant RhinoVax (RV) both in experimental animals and humans. The immunogenicity and efficacy of pneumococcal polysaccharide conjugate vaccines (PNC) of serotypes 1 and 3 was studied in mice after i.n. immunization with RV. Antibodies were measured in serum (IgM, IgG, and IgA) and saliva (IgA) and compared to antibody titers induced by parenteral immunization. The PNCs induced significant systemic IgG and IgA antibodies after i.n. immunization only when given with RV and, for serotype 1, serum IgG titers were comparable to titers induced by subcutaneous immunization. In addition, i.n. immunization with PNC-1 in RV elicited detectable mucosal IgA. These results demonstrate that RV is a potent mucosal adjuvant for polysaccharides conjugated to proteins. A majority of the PNC-1-immunized mice were protected against both bacteremia and pneumonia after i.n. challenge with a lethal dose of serotype 1 pneumococci, and protection correlated significantly with the serum IgG titers. Similarly, the survival of mice immunized i.n. with PNC-3 in RV was significantly prolonged. These results indicate that mucosal vaccination with PNC and adjuvants may be an alternative strategy for prevention against pneumococcal infections.
The mucosal surfaces of the respiratory, genitourinary, and gastrointestinal tracts are covered by a specialized epithelium which creates an efficient physical barrier against environmental pathogens (19). However, a majority of bacterial and viral infections directly affect or enter the body through mucosal surfaces, and colonization at these sites is often the first step in pathogenesis. Streptococcus pneumoniae is a major pathogen which enters the body through the respiratory mucosa (34) and may cause serious infections such as pneumonia, bacteremia, and meningitis, especially in elderly people with a variety of chronic diseases and in young children. It is also a common cause of mucosal infections such as otitis media and sinusitis (2, 9, 10).
The pneumococcus is surrounded by pneumococcal polysaccharides (PPS), which are the main virulence factors and protect the pneumococci from defense mechanisms of the host (1, 37), particularly phagocytosis of bacteria opsonized by type-specific immunoglobulin G (IgG) antibodies and complement (24, 35, 37). PPS can induce antibody production in the absence of T-cell help and are classified as thymus-independent type 2 (TI-2) antigens. It is thought that the TI-2 antigens only activate mature B cells, which may be one reason why infants respond poorly to polysaccharide antigens (23). However, the responses of children to PPS of different serotypes varies with age (7, 20). Conjugation of PPS to proteins makes them immunogenic in infants (18, 25, 28), and opsonic activity of antibodies has been demonstrated (30, 36). The immunogenicity of such pneumococcal polysaccharide conjugate vaccines (PNC) is assumed to be related to their thymus-dependent-like character (29), although the mechanism is not known in detail.
Systemic vaccination has led to a significant reduction in morbidity and mortality caused by a variety of pathogens, where protection has been shown to correlate with serum IgG antibody titers (26). Nevertheless, systemic immunization does not induce mucosal immune responses, which may be important against infection of the respiratory tract (4, 21). Protection at mucosal sites may be obtained by stimulation of the mucosal-associated lymphoid tissue (MALT), which elicits systemic IgG response in addition to secretory IgA (S-IgA), the major antibody isotype at mucosal surfaces (4, 32). S-IgA may inhibit the adherence and invasion of mucosal pathogens and neutralize the virulence of enzymes and toxins (22, 32, 38). However, the enormous potential of MALT has not been adequately exploited in the design of vaccines, partly due to lack of mucosal adjuvants acceptable for human use.
Two potent enterotoxins, cholera toxin and Escherichia coli heat-labile enterotoxin, are powerful mucosal adjuvants (4, 16). The wild-type forms are toxic, but mutants with reduced toxicity have been developed (5, 6, 8). RhinoVax (RV) is an adjuvant formulation based on caprylic-capric glycerides dissolved in polysorbate 20 and water, and various protein antigens administered with RV intranasally (i.n.) induce significant mucosal IgA, as well as systemic IgG responses, both in experimental animals (13, 15) and in humans (12). RV is nontoxic and is thus feasible for human use but at high concentrations (>46%) it may cause increased secretion and a slight initial stinging, which disappear 5 to 10 min after administration (11).
Susceptibility to different serotypes of S. pneumoniae varies with the host species, but both types 1 and 3 are virulent in mice (3, 33). Challenge of NMRI mice i.n. with these serotypes leads to severe lung infection and bacteremia, although bacteremia develops more rapidly with serotype 1 than with serotype 3 (33). Both serotypes are important causes of human diseases (2, 9).
To investigate the potential of mucosal immunization to protect against pneumococcal infections, mice were immunized i.n. with PNC of serotypes 1 and 3 mixed with RV and antibody responses measured. At 2 weeks after booster immunization, the mice were challenged with the respective pneumococcal serotype to determine the level of protection against bacteremia and pulmonary infection.
MATERIALS AND METHODS
Mice.
Six-week-old female outbred NMRI mice obtained from Gl. Bomholtgård Ltd. (Ry, Denmark) were housed under standard conditions with regulated daylength and kept in cages with free access to commercial pelleted food and water.
Vaccines and adjuvants.
Experimental tetanus-toxoid conjugated polysaccharide vaccines (i.e., PNC) were produced by Pasteur Merieux Connaught, Marcy l’Etoile, France. PPS were purchased from American Type Culture Collection (ATCC, Rockville, Md.). For i.n. immunization, the PNC or PPS were diluted in saline or mixed with 20% RV, a mucosal adjuvant based on caprylic-capric glycerides dissolved in polysorbate 20-water and produced at the Department of Pharmacy, University of Iceland, Reykjavík, Iceland (11). For parenteral immunization, PNC-1 was diluted in saline, but PNC-3, which is poorly immunogenic in mice when administered subcutaneously (s.c.) in saline (unpublished observation), was emulsified with 50% Freund adjuvant (FA; Sigma Chemical Co., St. Louis, Mo.), which was complete for primary (FCA) and incomplete for booster (FIA) treatments.
Immunization.
The mice, at 10 animals per group, were immunized with 0.5 or 2.0 μg of PNC or PPS. For i.n. immunization, a 10-μl vaccine solution in RV or saline was slowly delivered into the nares of mice sedated by s.c. injection with Hypnorm (Jansen Pharmaceutica, Beerse, Belgium). For parenteral immunization, PNC-1 in saline was injected s.c. in the scapular girdle region, and PNC-3 in FA was injected intraperitoneally (i.p.). All groups received a booster with the same dose and by the same route 4 weeks after the primary immunization. Nonimmunized mice were used as controls.
Blood and saliva sampling for antibody measurements.
The mice were bled from the retro-orbital sinus 15 days after the boosting; serum was then isolated and stored at −70°C. Saliva was collected from each mouse by the insertion of absorbent sticks (Polyfiltronics, Inc., Rockland, Maine) to the mouth. After 5 min, the sticks were transferred to phosphate-buffered saline, pH 7.4 (PBS), containing 10.0 μg of protease inhibitor (Aprotinin; Sigma) per ml to prevent the proteolysis of antibodies. The dissolved saliva was pooled for each group of mice and was stored at −70°C.
Antibodies to PPS.
Specific antibodies (IgM, IgG, and IgA) to PPS were determined by enzyme-linked immunosorbent assay (ELISA) designed according to the standardized ELISA protocol (Workshop at the Centers for Disease Control, Atlanta, Georgia, 1996) with few modifications. Microtiter plates (MaxiSorp; Nunc AS, Roskilde, Denmark) were coated with 10 μg of polysaccharide of serotypes 1 and 3 (ATCC) per ml of PBS and incubated for 5 h at 37°C. For neutralization of antibodies to cell wall polysaccharide (CWPS; Statens Serum Institute, Copenhagen, Denmark), serum samples and standards were diluted 1:50 in PBS with 0.05% Tween 20 (Sigma) and incubated in 500 μg of CWPS per ml for 30 min at room temperature. The neutralized sera were serially diluted and incubated in PPS-coated microtiter plates at room temperature for 2 h.
For detection, horseradish peroxidase-conjugated goat antibodies to mouse IgG (Caltac Laboratories, Burlingame, Calif.), IgM, or IgA (Sera-Lab, Sussex, United Kingdom) were used. The conjugates were diluted 1:5,000 in PBS-Tween and incubated for 2 h at room temperature. For development, 3,3′,5,5′-tetramethylbenzidine peroxidase (Kirkegaard & Perry Laboratories, Gaithersburg, Md.) was incubated for 10 min according to the manufacturer’s instructions, and the reaction was stopped by the addition of 0.18 M H2SO4. The absorbance was measured at 450 nm in an ELISA spectrophotometer (Titertek Multiscan Plus MK II; Flow Laboratories, Irvine, United Kingdom). Reference serum obtained from Pasteur Mérieux Connaught was included on each microtiter plate for the calculation of the titers expressed in ELISA units (EU) per ml. The titers of the reference sera (EU/milliliters) corresponded to the inverse of the serum dilution giving an optical density of 1.0.
The assays were performed at room temperature. All sera were tested in duplicate, and 100-μl volumes were used in all incubation steps with three washings with PBS-Tween after each step.
Pneumococci.
The bacteria were cultured as described by Saeland et al. (33). S. pneumoniae of serotypes 1 (ATCC 6301) and 3 (ATCC 6303), maintained in Tryptoset broth with 20% glycerol at −70°C, were plated on blood agar (Difco Laboratories, Detroit, Mich.) and incubated at 37°C in 5% CO2 overnight. Isolated colonies were transferred to a heart infusion broth (Difco) with 10% horse serum, cultured at 37°C to log phase for 3.5 h, and resuspended in 0.9% sterile saline. Serial 10-fold dilutions were plated on blood agar to determine the inoculum density.
Pneumococcal challenge.
The challenge experiments (33) were performed 2 days after the mice were bled. The animals were anesthetized with pentobarbitone sodium BP (50 mg/kg; Icelandic Pharmaceuticals, Reykjavík, Iceland) injected i.p. and challenged i.n. with 50 μl of bacterial suspension. To evaluate bacteremia, blood was collected from the tail vein at various time points after challenge and plated on blood agar for culturing at 37°C in 5% CO2 overnight. Bacteremia was determined as the number of CFU per milliliter of blood. When the mice were sacrificed, the lungs were removed and homogenized in sterile 0.9% saline, and serial dilutions were plated on blood agar, including Staph/Strep selective supplement containing nalidixic acid and colistin sulfate (Unipath Ltd., Hamshire, United Kingdom). Pneumococcal lung infection was determined as the number of CFU per milliliter of lung homogenate. Depending on the first dilution used, the detection limit was 2.2 CFU/ml of lung homogenate and 1.6 CFU/ml of blood.
Statistical analysis.
A nonparametric t test (Mann-Whitney on ranks) was used to compare antibody titers and CFU numbers between groups. Correlation was calculated by using Pearson’s coefficients. The chi-square test and Kaplan-Meier survival test were used to compare survival rates. A P value of <0.05 was considered to be statistically significant.
RESULTS
Antibody responses to serotype 1.
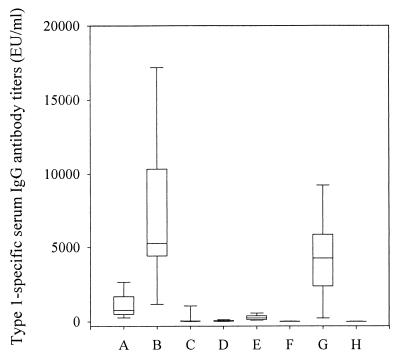
Mice were immunized i.n. with either 0.5 or 2.0 μg of PNC-1 or PPS-1, with or without the mucosal adjuvant RV. Immunization i.n. with either 0.5 and 2.0 μg of PNC-1 in saline elicited very low systemic responses, but the IgG levels were significantly higher than in unimmunized control mice (P < 0.001). There was a highly significant increase in systemic IgG response when PNC-1 was mixed with RV (P < 0.001; Fig. 1 and Table 1), and immunization with 2.0 μg of PNC-1 in RV elicited a significantly higher systemic IgG response than did 0.5 μg of PNC-1 (P = 0.003). In preliminary studies, we observed that s.c. immunization with 0.5 μg of PNC-1 elicited the highest antibody response, and thus this dose was used. A 0.5-μg dose of PNC-1 in saline administered s.c. elicited a higher IgG response than the same dose given i.n. with RV (P = 0.038), but 2.0 μg of PNC-1 in RV induced the highest IgG antibody response of all immunized groups. An i.n. immunization with PPS-1 in RV or saline elicited very low IgG antibody responses, although the levels were significantly higher than in unimmunized control mice (P < 0.001 and P = 0.004, respectively); they were significantly lower than after i.n. immunization with PNC-1 (P < 0.001). Only those mice immunized with PNC-1 given s.c. (P = 0.008) and PPS-1 in RV given i.n. (P = 0.001) showed significant IgM responses compared to unimmunized control mice (Table 1). Significant systemic IgA responses (Table 1) were only elicited in mice immunized i.n. with 2.0 μg of PNC-1 in RV (P < 0.001), i.n. with PPS-1 in RV (P = 0.030), and s.c. with PNC-1 in saline (P = 0.014). Moreover, a significantly higher systemic IgA response was observed for the RV group than for the group immunized s.c. (P < 0.001).
FIG. 1.
Type 1-specific serum IgG antibody titers after immunization with PNC-1 and PPS-1. The box plot shows the median value with the 25th through the 75th percentiles; the error bars indicate the 5th through the 95th percentiles. Groups: A, PNC-1 (0.5 μg) in RV given i.n.; B, PNC-1 (2.0 μg) in RV given i.n.; C, PPS-1 (2.0 μg) in RV given i.n.; D, PNC-1 (0.5 μg) in saline given i.n.; E, PNC-1 (2.0 μg) in saline given i.n.; F, PPS-1 (2.0 μg) in saline given i.n.; G, PNC-1 (0.5 μg) in saline given s.c.; H, nonimmunized control.
TABLE 1.
Type-specific antibodies in sera after immunization with PPS and PNC
| Type | Vaccine (dose [μg]) | Route | Adjuvant | Geometric mean titer (EU/ml)a of:
|
||
|---|---|---|---|---|---|---|
| IgG | IgM | IgA | ||||
| Type 1 | ||||||
| A | PNC-1 (0.5) | i.n. | RV | 771 (384–1550) | 40 (26–62) | 42 (29–62) |
| B | PNC-1 (2.0) | i.n. | RV | 5,365 (2,720–10,583) | 55 (33–91) | 341 (207–564) |
| C | PPS-1 (2.0) | i.n. | RV | 38 (11–125) | 71 (47–106) | 52 (37–72) |
| D | PNC-1 (0.5) | i.n. | Saline | 33 (15–74) | 11 (5–24) | 26 (21–33) |
| E | PNC-1 (2.0) | i.n. | Saline | 220 (132–371) | 23 (13–43) | 49 (28–85) |
| F | PPS-1 (2.0) | i.n. | Saline | 7 (5–11) | 33 (22–48) | 45 (28–72) |
| G | PNC-1 (0.5) | s.c. | Saline | 2,562 (882–7,441) | 99 (31–312) | 62 (40–95) |
| H | Control | 3 (3–4) | 17 (10–28) | 28 (20–42) | ||
| Type 3 | ||||||
| A | PNC-3 (2.0) | i.n. | RV | 48 (12–193) | 68 (50–92) | |
| B | PPS-3 (2.0) | i.n. | RV | 5 (4–6) | 49 (34–70) | |
| C | PNC-3 (2.0) | i.n. | Saline | 3 (1–6) | 37 (15–78) | |
| D | PPS-3 (2.0) | i.n. | Saline | 4 (3–8) | 44 (16–121) | |
| E | PNC-3 (2.0) | i.p. | FCA or FIA | 1,650 (1,131–2,407) | 147 (67–327) | |
| F | Control | 2 (2–3) | 38 (15–97) | |||
The 95% confidence interval is given in parentheses.
Immunization i.n. with adjuvanted PNC-1 induced mucosal IgA antibodies, and a 10-fold increase was found for the group that received 2.0 μg of PNC-1 (3.8 EU/ml) compared to the control group (<0.2 EU/ml). IgA antibodies were undetectable in saliva after i.n. immunization with either dose of PNC-1 in saline (<0.2 EU/ml). Although parenteral immunization with 0.5 μg of PNC-1 in saline induced a strong serum antibody response, type 1-specific IgA in saliva was hardly detectable (0.7 EU/ml).
Antibody responses to serotype 3.
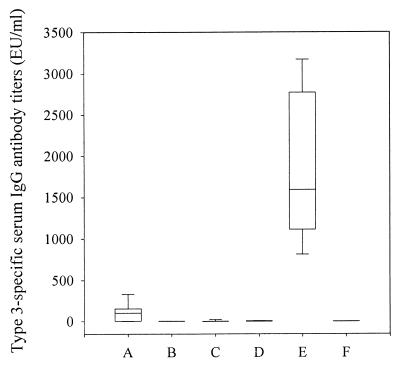
Mice were immunized with 2.0 μg of PNC-3, given i.n. in saline or RV or given i.p. in FCA or FIA, since PNC-3 is poorly immunogenic when given s.c. in saline (data not shown). Immunization i.p. with PNC-3 emulsified with FCA or FIA elicited by far the highest type 3-specific IgG antibody titer in serum (Fig. 2). When PNC-3 was administered i.n. with RV, a significant serum IgG antibody response was observed (P = 0.003). Immunization i.n. with PNC-3 alone or with PPS-3 with or without RV did not induce significant IgG responses. Neither i.n. nor i.p. immunization with PNC-3 elicited detectable type 3-specific IgA antibody levels in saliva.
FIG. 2.
Type 3-specific serum IgG antibody titers after immunization with PNC-3 and PPS-3. The box plot description is as described for Fig. 1. Groups: A, PNC-3 in RV given i.n.; B, PPS-3 in RV given i.n.; C, PNC-3 in saline given i.n.; D, PPS-3 in saline given i.n.; E, PNC-3 in FCA or FIA given i.p.; F, nonimmunized control.
Protection against pneumococcal infection caused by serotype 1.
To evaluate the efficacy of the PNC-1 against bacteremia and pulmonary infection, mice were challenged i.n. with 4 × 106 CFU of serotype 1 pneumococci in 50 μl of saline 2 weeks after booster immunization. Serotype 1 is very virulent in mice, causing heavy lung infection and bacteremia, with 100% deaths occurring between 24 and 30 h after challenge. In previous experiments, pneumococci were undetectable in lungs and blood at 24 h in mice vaccinated parenterally with PNC-1. Thus, we used this time point to evaluate vaccine-induced protection against invasive pneumococcal infections.
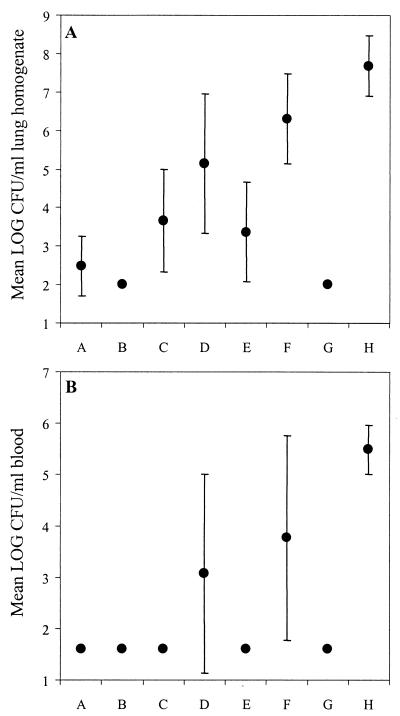
The results of a representative experiment are shown in Fig. 3. Serotype 1 was very virulent and caused severe lung infection (mean, 7.70 log CFU/ml of lung homogenate [Fig. 3A]) and bacteremia (mean, 5.50 log CFU/ml of blood [Fig. 3B]) in unimmunized control mice 24 h after i.n. challenge.
FIG. 3.
Pneumococcal density (mean log ± the standard deviation) in the lungs (A) and blood (B) in groups of mice at 24 h after i.n. challenge with serotype 1. Each group is represented by one dot (n = 10). Groups: A, PNC-1 (0.5 μg) in RV given i.n.; B, PNC-1 (2.0 μg) in RV given i.n.; C, PPS-1 (2.0 μg) in RV given i.n.; D, PNC-1 (0.5 μg) in saline given i.n.; E, PNC-1 (2.0 μg) in saline given i.n.; F, PPS-1 (2.0 μg) in saline given i.n.; G, PNC-1 (0.5 μg) in saline given s.c.; H, nonimmunized control.
Immunization with PNC-1 conferred protection against lung infection caused by serotype 1 (Fig. 3A). Bacteria were cultured from lung homogenate of all unimmunized control mice 24 h after challenge, whereas all mice immunized parenterally with PNC-1 were completely protected. In addition, 100% protection in lungs was observed in the group that received 2.0 μg of PNC-1 with RV given i.n. Mice that received 0.5 μg of PNC-1 mixed with RV had significantly reduced numbers of pneumococci in the lungs compared to unimmunized control mice (P < 0.001), and 7 of 10 were fully protected. The three mice in this group, which had detectable pneumococci (3 to 4 log CFU) in the lungs, all had low serum antibody titers (100 to 1,000 EU/ml). Both groups of mice immunized i.n. with PNC-1 in saline had reduced pneumococcal density in the lungs compared to the unimmunized control mice (P < 0.001), and of those receiving 2.0 μg of PNC-1, 3 of 10 were completely protected. In contrast, pneumococci were cultured from the lungs of all mice immunized i.n. with 0.5 μg of PNC-1 in saline. All mice immunized i.n. with PPS-1 in saline were heavily infected in the lungs, but mice immunized i.n. with PPS-1 mixed with RV had a reduced pneumococcal density (P < 0.001), and 4 of 10 mice were protected.
Similarly, immunization with PNC-1 protected against pneumococcal bacteremia (Fig. 3B), and the numbers of CFU in the blood correlated with the numbers of CFU in the lung homogenate (r = 0.852; P < 0.001). Whereas all control mice had severe bacteremia, 100% protection was observed for the two groups that received PNC-1 mixed with RV given i.n.: the parenterally immunized group and the group immunized i.n. with 2.0 μg of PNC-1 in saline (Fig. 3B). Of the mice immunized i.n. with 0.5 μg of PNC-1 in saline, 6 of 10 were protected against bacteremia. Administration i.n. of PPS-1 in RV gave 100% protection from bacteremia, but 5 of 10 mice immunized i.n. with PPS-1 in saline had detectable pneumococci in the blood.
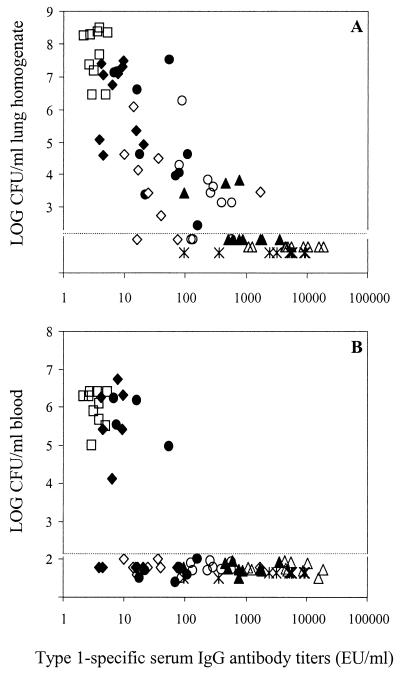
The relationship between type 1-specific serum IgG antibodies and pneumococcal density in the lungs and blood is shown in Fig. 4. Unimmunized mice had hardly detectable levels of IgG antibodies and were heavily infected. Protection against lung infection was significantly correlated with type 1-specific IgG and IgA antibody levels in serum (r = −0.44 and P < 0.001 for IgG; r = −0.350 and P = 0.002 for IgA), and in all mice with >1,000 EU/ml of IgG pneumococci were not detectable in the lungs (Fig. 4A). However, ∼100 EU/ml of IgG in serum was sufficient to provide protection against bacteremia (Fig. 4B).
FIG. 4.
Relationship between pneumococcal lung infection (A) and bacteremia (B) (as log CFU) and type 1-specific IgG antibody titers in serum. Each symbol represents one mouse. Groups: PNC-1 (0.5 μg) in RV given i.n. (▴), PNC-1 (2.0 μg) in RV given i.n. (▵), PPS-1 in RV given i.n. (◊), PNC-1 (0.5 μg) in saline given i.n. (●), PNC-1 (2.0 μg) in saline given i.n. (○), PPS-1 in saline given i.n. (⧫), PNC-1 (0.5 μg) in saline given s.c. (✽), nonimmunized control (□). The dotted lines represent the detection limits for CFU.
Protection against pneumococcal infection caused by serotype 3.
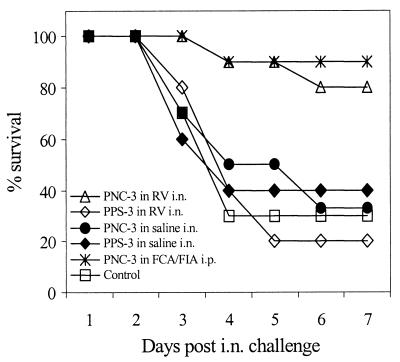
We have already demonstrated that serotype 3 is virulent and causes severe lung infection in mice after i.n. challenge (33). Challenge with >107 CFU of this serotype may also cause bacteremia, which kills the mice in 1 to 2 days. However, by reducing the challenge dose, survival may be prolonged. Figure 5 shows one representative experiment, in which mice were challenged i.n. with ∼104 CFU of type 3 pneumococci in 50 μl of saline and the survival was recorded over a period of 7 days, when the experiment was terminated. At day 7 only 30% of unimmunized control mice had survived, and there was no difference in survival between the control mice and mice immunized i.n. with PNC-3 in saline (30%) and with PPS-3 in saline (40%) or RV (20%). However, 8 of 10 mice immunized i.n. with PNC-3 in RV (P = 0.006) and 9 of 10 mice immunized i.p. with PNC-3 in FCA or FIA (P = 0.0003) had survived and looked healthy by day 7. When survival was compared for the entire duration of the experiment (Kaplan-Meier survival test), significant protection was observed in groups immunized with PNC-3 in FCA or FIA given i.p. (P = 0.0062) and given i.n. in RV (P = 0.0164). Nevertheless, when sacrificed at this time point, low levels of pneumococci (2 to 4 log CFU/ml of lung homogenate) were detectable in lungs of all mice.
FIG. 5.
Survival of mice (n = 10 per group) after i.n. challenge with pneumococci of serotype 3.
Although there was a highly significant difference in serum IgG antibody titers between mice immunized i.n. with PNC-3 in RV and those immunized i.p. in FA (P < 0.001), there was no difference in survival between the immunization routes (P = 0.290), indicating that serum IgG antibodies elicited by i.n. immunization (Fig. 2 and Table 1) were sufficient to protect the mice from severe pneumococcal lung infection by type 3.
DISCUSSION
Since the mucosal epithelium of the nasopharynx is the primary site of pneumococcal colonization (34, 37), i.n. vaccination may offer an alternative approach to current strategies since it induces mucosal as well as systemic immune responses. In addition, such vaccination is painless and easy to perform, which may favor these strategies for the vaccination of infants and children.
Immunization i.n. has been investigated by the administration of antigens together with adjuvants, such as cholera toxin B (CTB) (4) and mutants of Escherichia coli heat-labile enterotoxin with reduced toxicity (5). These enterotoxins change the permeability of the mucosa and enhance the transepithelial flux of antigens from the lumen to the lamina propria (14). In this study we used the mucosal adjuvant RV, which is based on caprylic-capric glycerides dissolved in polysorbate 20 and water. RV enhances mucosal as well as systemic antibody responses to various bacterial and viral protein antigens, both in experimental animals (13, 15) and in humans (12). The biological mechanisms of this adjuvant are still under investigation.
The immunogenicity of two PNC (PNC-1 and PNC-3) was studied when administered i.n. with RV, and the extent of protection against infection with the corresponding pneumococcal serotypes was evaluated. For PNC-1, i.n. immunization with RV was as efficient as immunization by the s.c. route, both in terms of immunogenicity and protection against pneumococcal pneumonia and bacteremia. Even though i.n. immunization with the corresponding polysaccharide PPS-1 in RV elicited a significant systemic IgG response, this was not sufficient to protect against pulmonary infection. Thus, among the doses tested, PPS was less effective than PNC for mucosal immunization against pneumococcal infections.
We have previously shown a prolonged survival of up to 11 days in mice immunized i.p. with PNC-3 in FCA or FIA and that most of the deaths occur between days 2 and 4 in unimmunized mice, with very few deaths occurring after day 5 (unpublished data). We now compared the survival of mice immunized i.n. with PNC-3 in RV and PNC-3 in FCA or FIA given i.p. Although use of the i.n. route induced a significantly lower systemic IgG response compared to use of the i.p. route, the i.n. immunization reduced the severity of infection caused by type 3 pneumococci and prolonged survival to a similar degree.
Mucosal IgA antibody response to PNC-1 in RV but not to PNC-3 was observed. Thus, salivary IgA does not seem to be necessary for protection against infection following i.n. challenge with a large inoculum in this mouse model. Although measurements of IgA to various protein antigens were found to be comparable in saliva and mucosal tissues (10a), low levels of mucosal IgA antibodies may not be detected in saliva. Immunization of mice i.n. with pneumococcal surface protein A with CTB as a adjuvant has been shown to induce salivary IgA antibodies and to provide protection against carriage of S. pneumoniae, indicating a role of IgA antibodies in protection of mucosal surfaces. Immunization with 6B conjugate and CTB induced marginal salivary IgA response but detectable serum IgG and reduced nasopharyngeal colonization (38).
Systemic IgG antibodies to PPS are known to correlate with protection against pneumococcal infections, and passive immunization with IgG preparations containing high levels of antibodies to bacterial polysaccharides protects infants and young children at high risk (27, 31). We have demonstrated the immunogenicity of the octavalent PNC in infants, and the infant sera had both opsonic activity in vitro (17) and were protective against serotypes 6A and 6B by passive immunization in this mouse model (unpublished data). It has also been postulated that inflammatory responses to infections at mucosal surfaces induce transudation of serum IgG and phagocytes, which may clear the infections at the mucosal surfaces (26).
RV is well tolerated, and its adjuvant activity has been demonstrated in humans (12). In this study we demonstrated that i.n. immunization with PNC-1 and PNC-3 in RV protected mice against infection after i.n. challenge with the respective pneumococcal serotypes and that the protection was related to the levels of type-specific serum IgG antibodies. These results indicate that mucosal vaccination with PNC may offer an alternative approach to current strategies for preventing pneumococcal diseases.
ACKNOWLEDGMENTS
We acknowledge the excellent technical assistance of Gunnhildur Ingólfsdóttir and Vera Gudmundsdóttir and the valuable scientific advice of Bernard Danve at Pasteur Mérieux Connaught, Marcy l’Etoile, France. We also thank Örn Ólafsson for his help with the statistical analysis. We are grateful for the facilities provided by the Department of Microbiology, National University Hospital, and the Department of Physiology, University of Iceland.
This work was supported by the Research Fund and the Student Innovation Fund of the University of Iceland and by Pasteur Mérieux Connaught, Marcy l’Etoile, France.
REFERENCES
- 1.Alonsode-Velasco E, Verheul A F M, Verhoef J, Snippe H. Streptococcus pneumoniae: virulence factors, pathogenesis, and vaccines. Microbiol Rev. 1995;59:591–603. doi: 10.1128/mr.59.4.591-603.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Austrian R, Gold J. Some observations on the pneumococcus and on the current status of pneumococcal disease and its prevention. Rev Infect Dis. 1981;3:1–17. doi: 10.1093/clinids/3.supplement_1.s1. [DOI] [PubMed] [Google Scholar]
- 3.Briles D E, Crain M J, Gray B M, Forman C, Yother J. Strong association between capsular type and virulence for mice among human isolates of S. pneumoniae. Infect Immun. 1992;60:111–116. doi: 10.1128/iai.60.1.111-116.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Czerkinsky C, Holmgren J. The mucosal immune system and prospects for anti-infectious and anti-inflammatory vaccines. Immunologist. 1995;3:97–103. [Google Scholar]
- 5.Douce G, Turcotte C, Cropley I, Roberts M, Pizza M, Domenighini M, Rappuoli R, Dougan G. Mutants of Escherichia coli heat-labile toxin lacking ADP-ribosyltransferase activity act as nontoxic, mucosal adjuvants. Proc Natl Acad Sci USA. 1995;92:1644–1648. doi: 10.1073/pnas.92.5.1644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Douce G, Fontana M R, Pizza M, Rappuoli R, Dougan G. Intranasal immunogenicity and adjuvanticity of site-directed mutant derivatives of cholera toxin. Infect Immun. 1997;65:2821–2828. doi: 10.1128/iai.65.7.2821-2828.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Douglas R M, Paton J C, Duncan S J, Hansman D J. Antibody response to pneumococcal vaccination in children younger than five years of age. J Infect Dis. 1983;148:131–137. doi: 10.1093/infdis/148.1.131. [DOI] [PubMed] [Google Scholar]
- 8.Fontana M R, Manetti R, Gianelli V, Magagnoli C, Marchini A, Olivieri R, Domenighini M, Rappuoli R, Pizza M. Construction of nontoxic derivatives of cholera toxin and characterization of the immunological response against the A subunit. Infect Immun. 1995;63:2356–2360. doi: 10.1128/iai.63.6.2356-2360.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Giebink G S. Preventing pneumococcal diseases in children: recommendations for using pneumococcal vaccine. Pediatr Infect Dis J. 1985;4:343–348. doi: 10.1097/00006454-198507000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Giebink G S. The microbiology of otitis media. Pediatr Infect Dis J. 1989;8:18–20. [PubMed] [Google Scholar]
- 10a.Gizurarson, S. Unpublished data.
- 11.Gizurarson S, Georgsson G, Aggerbeck H, Thorarinsdóttir H, Heron I. Evaluation of the local toxicity inside the nasal cavity after intranasal vaccination. Toxicology. 1996;107:61–68. doi: 10.1016/0300-483x(95)03201-p. [DOI] [PubMed] [Google Scholar]
- 12.Gizurarson S, Aggerbeck H, Gudbrandsson F K, Valdimarsson H, Heron I. Intranasal vaccination against diptheria and tetanus in human subjects. Vaccine Res. 1998;6:41–47. [Google Scholar]
- 13.Gizurarson S, Aggerbeck H, Arnadottir S G, Mordhorst C H, Heron I. Intranasal vacciantion against influenza using pharmaceutical excipients as immunological adjuvants. Vaccine Res. 1996;5:69–75. [Google Scholar]
- 14.Gizurarson S, Tamura S, Aizawa C, Kurata T. Stimulation of the transepithelial flux of influenza vaccine by cholera toxin b subunit. Vaccine. 1992;10:101–106. doi: 10.1016/0264-410x(92)90025-f. [DOI] [PubMed] [Google Scholar]
- 15.Gizurarson S, Jonsdottir V M, Heron I. Intranasal administration of diptheria toxoid. Selecting antibody isotypes using formulations having various lipophilic characteristics. Vaccine. 1995;13:617–621. doi: 10.1016/0264-410x(94)00066-v. [DOI] [PubMed] [Google Scholar]
- 16.Holmgren J, Lycke N, Czerkinsky C. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine. 1993;11:1179–1184. doi: 10.1016/0264-410x(93)90039-z. [DOI] [PubMed] [Google Scholar]
- 17.Jonsdottir I, Sigurdardottir S T, Vidarsson G, Ingolfsdottir G, Gudnason T, Davidsdottir K, Kjartansson S, Kristinsson K G, Leroy O. Program and abstracts of the 37th Interscience Conference on Antimicrobial Agents and Chemotherapy. Washington, D.C: American Society for Microbiology; 1997. Functional activity of antibodies elicited by octavalent pneumococcal polysaccharide conjugate vaccines, PncT and PncD, abstr. G-90. [Google Scholar]
- 18.Käyhty H, Ahman H, Ronnberg P R, Tillikainen R, Eskola J. Pneumococcal polysaccharide-meningococcal outer membrane protein complex conjugate vaccine is immunogenic in infants and children. J Infect Dis. 1995;172:1273–1278. doi: 10.1093/infdis/172.5.1273. [DOI] [PubMed] [Google Scholar]
- 19.Kraehenbuhl J P, Hopkins S A, Kernéis E, Pringault E. Antigen sampling by epithelial tissues: Implication of vaccine design. Behring Inst Mitt. 1997;98:24–32. [PubMed] [Google Scholar]
- 20.Mäkela P H, Leinonen M, Ukander J, Karma P A. A study of the pneumococcal vaccine in prevention of clinical acute attacks of recurrent otitis media. Rev Infect Dis. 1981;S3:124–132. doi: 10.1093/clinids/3.supplement_1.s124. [DOI] [PubMed] [Google Scholar]
- 21.McGhee J R, Mestecky J, Dertzbaugh M T, Eldridge J H, Hirasawa M, Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10:75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- 22.Mestecky J, Michalek S M, Moldoveanu Z, Russel M W. Routes of immunization and antigen delivery systems for optimal mucosal immune responses in humans. Behring Inst Mitt. 1997;98:33–43. [PubMed] [Google Scholar]
- 23.Mond J J, Lees A, Snapper C M. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 24.Musher D M, Chapman A J, Goree A, Jonsson S, Briles D E, Baughn E. Natural and vaccine-related immunity to Streptococcus pneumoniae. J Infect Dis. 1986;154:245–256. doi: 10.1093/infdis/154.2.245. [DOI] [PubMed] [Google Scholar]
- 25.Robbins J B, Schneerson R. Polysaccharide-protein conjugates: a new generation of vaccines. J Infect Dis. 1990;161:821–832. doi: 10.1093/infdis/161.5.821. [DOI] [PubMed] [Google Scholar]
- 26.Robbins J B, Schneerson R, Szu S C. Perspective: hypothesis: serum IgG antibody is sufficient to confer protection against infectious diseases by inactivating the inoculum. J Infect Dis. 1995;171:1387–1398. doi: 10.1093/infdis/171.6.1387. [DOI] [PubMed] [Google Scholar]
- 27.Santosham M, Reid R, Ambrosino D M. Prevention of Haemophilus influenzae type b infections in high-risk infants treated with bacterial polysaccharide immune globuline. N Engl J Med. 1987;317:923–929. doi: 10.1056/NEJM198710083171503. [DOI] [PubMed] [Google Scholar]
- 28.Sarnaik S, Kaplan J, Schiffman G, Bryla D, Robbins J B, Schneerson R. Studies on Pneumococcus vaccine alone or mixed with DTP and on Pneumococcus type 6B and Haemophilus influenzae type b capsular polysaccharide-tetanus toxoid conjugates in two- to five-year-old children with sickle cell anemia. Pediatr Infect Dis J. 1990;9:181–186. doi: 10.1097/00006454-199003000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Siber G R. Pneumococcal disease: prospects for a new generation of vaccines. Science. 1994;265:1385–1387. doi: 10.1126/science.8073278. [DOI] [PubMed] [Google Scholar]
- 30.Sigurdardottir S T, Vidarsson G, Gudnason T, Kjartansson S, Kristinsson K G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Immune responses of infants vaccinated with serotype 6B pneumococcal polysaccharide conjugated with tetanus toxoid. Pediatr Infect Dis J. 1997;16:667–674. doi: 10.1097/00006454-199707000-00009. [DOI] [PubMed] [Google Scholar]
- 31.Singelton R J, Davidson N M, Desmet I J, Berner J E, Wainwright R B, Bulkow L R, Lilly C M, Siber G R. Decline of Haemophilus influenzae type b disease in a region of high risk: impact of passive and active immunization. Pediatr Infect Dis J. 1994;13:362–367. doi: 10.1097/00006454-199405000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Steinmetz I. Comparative in vivo analysis of IgA- and IgG-mediated mucosal defense against bacterial pathogens. Behring Inst Mitt. 1997;98:53–55. [PubMed] [Google Scholar]
- 33.Saeland, E., G. Vidarsson, and I. Jonsdottir. Pneumococcal infection model in mice for analysis of protective human antibodies. Submitted for publication. [DOI] [PubMed]
- 34.Toumanen E I, Austrian R, Masure H R. Pathogenesis of pneumococcal infection. N Engl J Med. 1995;11:1280–1284. doi: 10.1056/NEJM199505113321907. [DOI] [PubMed] [Google Scholar]
- 35.Vidarsson G, Jonsdottir I, Jonsson S, Valdimarsson H. Opsonization and antibodies to capsular and cell wall polysaccharides of Streptococcus pneumoniae. J Infect Dis. 1994;170:592–599. doi: 10.1093/infdis/170.3.592. [DOI] [PubMed] [Google Scholar]
- 36.Vidarsson G, Sigurdardottir S T, Gudnason T, Kjartansson S, Kristinsson K G, Ingolfsdottir G, Jonsson S, Valdimarsson H, Schiffman G, Schneerson R, Jonsdottir I. Isotypes and opsonophagocytosis of pneumococcus type 6B antibodies elicited in infants and adults by experimental pneumococcus type 6B-tetanus toxoid vaccine. Infect Immun. 1998;66:2866–2870. doi: 10.1128/iai.66.6.2866-2870.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Watson D A, Musher D M, Verhoef J. Pneumococcal virulence factors and host immune responses to them. Eur J Clin Microbiol Infect Dis. 1995;14:479–490. doi: 10.1007/BF02113425. [DOI] [PubMed] [Google Scholar]
- 38.Wu H-Y, Nahm M H, Guo Y, Russell M W, Briles D E. Intranasal immunization of mice with PspA (pneumococcal surface protein A) can prevent intranasal carriage, pulmonary infection, and sepsis with Streptococcus pneumoniae. J Infect Dis. 1997;175:839–846. doi: 10.1086/513980. [DOI] [PubMed] [Google Scholar]