Abstract
The liposome is the first nanomedicine transformed into the market and applied to human patients. Since then, such phospholipid bilayer vesicles have undergone technological advancements in delivering small molecular-weight compounds and biological drugs. Numerous investigations about liposome uses were conducted in different treatment fields, including anti-tumor, anti-fungal, anti-bacterial, and clinical analgesia, owing to liposome's ability to reduce drug cytotoxicity and improve the therapeutic efficacy and combinatorial delivery. In particular, two liposomal vaccines were approved in 2021 to combat COVID-19. Herein, the clinically used liposomes are reviewed by introducing various liposomal preparations in detail that are currently proceeding in the clinic or on the market. Finally, we discuss the challenges of developing liposomes and cutting-edge liposomal delivery for biological drugs and combination therapy.
Keywords: Liposomes, Drug delivery, Clinical application, COVID-19 pandemic
Graphical abstract
Liposomes have been extensively used in various clinical treatments, including anti-tumor, anti-fungal, anti-bacterial, and clinical analgesia. In particular, two liposomal vaccines were approved in 2021 to combat COVID-19. The challenges of developing liposomes and the cutting-edge liposome delivery for combination and RNA therapy will be discussed in this review.
1. Introduction
Since Alec Bangham first observed in 1961 that a spherical lipid bilayer structure could be spontaneously formed by phospholipids in aqueous systems [1], liposomes are the most promising drug carriers in the medical field. The spherical liposomes, composed of phospholipids and cholesterol, have a water core and a lipid bilayer with a diameter range of 50-1000 nm (Fig. 1) [2]. Due to the mutual repulsion between the hydrophobic tail and the surrounding medium, hydrophobic tails tend to avoid the water phase, and the hydrophilic heads are exposed to the water phase when amphiphilic phospholipid molecules are dispersed in the water phase [3]. The existence of lipid bilayers and water cores can theoretically encapsulate various small molecules and biomacromolecules. Liposomes can encapsulate drugs to enhance efficacy and sustained release [4]. Liposomes are able to reduce cytotoxicity, improve the therapeutic effects of the drugs and load multiple drugs [5], [6], [7], [8], [9]. Furthermore, liposomes demonstrate advantages over other drug carriers such as polymer micelles and dendrimers, including higher payload ability, enhanced stability, drug protection and biocompatibility, and more straightforward modification and industrialization [6].
Fig. 1.
The basic structure of a liposome.
According to the number of lipid bilayers contained, liposomes are classified into unilamellar vesicles (ULV), multilamellar vesicles (MLV), and multivesicular vesicles (MVV) (Fig. 2) [10]. ULV is segmented into small ULV (SUV), large ULV (LUV) and giant ULV (GUV) [11]. ULVs have only a single phospholipid bilayer structure, while MLVs have a structure similar to onions [12]. The liposome can be categorized into anionic liposomes, cationic liposomes, and neutral liposomes, according to the charge of the phospholipids [13]. Typically, positively charged liposomes have a more substantial capacity to deliver gene drugs, while negatively charged liposomes have lower cytotoxicity [14]. Understanding the factors that affect liposome size, drug loading, stability and drug release is critical to the rational design of liposomes with the desired pharmacokinetics. The formulation parameters, such as lipid chain length, lipid unsaturation, lipid phase transition temperature (Tc), and cholesterol content, could impact the liposome properties. E.g., incorporating lipids with long-acyl chains could increase the drug-loading capacity and encapsulation efficiency of liposomes. Formulating lipids with high unsaturation and low Tc demonstrates faster drug release from liposomes. In addition, increasing the cholesterol can improve the polydispersity index (PDI) and modulate the fluidity of the liposome membrane [15]. Meanwhile, functional ligand modification is contributed to prolonging the circulation time and target distribution, such as PEGylation [16,17].
Fig. 2.
Classification of liposomes, (a) MVV, (b) MLV, (c) ULV, (c1) GUV, (c2) LUV, (c3) SUV.
The liposome preparation includes four primary stages: the organic phase acquisition of dry lipids, redispersion of lipids in an aqueous environment, purification of the resulting liposomes, and detection of the final liposome [18]. The thin-film dispersion method is the most commonly used preparation method in the laboratory, and other methods include ethanol injection, reverse-phase evaporation, and high-pressure homogenization [19]. Even though only a few methods are applied in the industries, the technique extensively used in scale-up manufacturing was the ethanol injection method followed by extrusion [20]. Industrial manufacturing of liposomes is a complex and laborious process, including buffer solution preparation, phospholipid solution preparation, lipid membrane hydration, liposome extrusion, diafiltration, final product dilution, sterilization, and filtration between every step [20,21].
As an attractive nanocarrier in medicine, liposomes have been extensively used in various clinical treatments, i.e., cancer treatment, anti-fungal, anti-viral, and anti-bacterial (Fig. 3) [22]. This article reviewed the clinical applications of liposomes and liposome-like nanoparticles (LLPs) to combat various diseases. Finally, we summarized various new technology or pathophysiological phenomena utilized in liposome-related drug delivery and provided perspectives on their use in the delivery of biological drugs and combined treatment.
Fig. 3.
The development history of essential liposome products on the market.
2. Marketed liposome and LLP preparations
The first marked liposomes were Ambisome® used against anti-fungal infections, reducing the drug toxicity significantly [23]. Since then, liposomes have been gaining attention for disease therapy. In 1995, the first anti-tumor liposome Doxil® was approved for clinical use, and liposomes began to be extensively investigated to treat different diseases [24]. In recent years, flexible and versatile liposomal nanoparticles have been developed due to the finding of ionizable lipids, facilitating their application in biological drug delivery. Especially, mRNA-carrying liposome nanoparticle vaccines were approved for use against the COVID-19 pandemic in 2021 [25,26]. Various marketed liposome and LLP products are summarized as follows (Table 1).
Table 1.
Liposome products on the market.
| Product Name | Approval Year | Active Ingredient | Administration route | Formulation | Indication | Side Effects | |
|---|---|---|---|---|---|---|---|
| 1 | Ambisome® | 1990 | Amphotericin B | intravenous | HSPC, DSPG, Cholesterol | Fungal infection | Gastrointestinal reaction |
| 2 | Epaxal® | 1993 | Inactivated hepatitis A virus (strain RGSB) | intramuscular | DOPC, DOPE | Hepatitis A | Injection site reactions |
| 3 | Abelcet® | 1995 | Amphotericin B | intravenous | DMPC and DMPG | Invasive severe fungal infections | Transient chills and/or fever during infusion of the drug |
| 4 | Doxil®/ Caelyx® | 1995/1996 | Doxorubicin | intravenous | HSPC, Cholesterol, PEG 2000-DSPE | Ovarian cancer and KS | Skin toxicity, “Hand-foot syndrome” |
| 5 | Amphotec® | 1996 | Amphotericin B | intravenous | Cholesteryl sulphate | Severe fungal infections | Infusion-related reactions |
| 6 | DaunoXome® | 1996 | Daunorubicin | intravenous | DSPC and Cholesterol | KS infected with HIV | Bone marrow suppression |
| 7 | Inflexal® V | 1997 | Inactivated hemagglutinin of Influenza virus strains A and B | intramuscular | DOPC and DOPE | Influenza | Injection site reactions |
| 8 | Depocyt® | 1999 | Cytarabine | Spinal | DOPC, DPPG, Cholesterol and Triolein | NM | Chemical arachnoiditis and neurotoxicity |
| 9 | Visudyne® | 2000 | Verteporfin | intravenous | DMPC and EPG | Choroidal neovascularisation | Local adverse reactions after injection and vision decreased |
| 10 | Myocet® | 2001 | Doxorubicin | intravenous | EPC and Cholesterol | Combination therapy with cyclophosphamide in metastatic breast cancer | Neutropenic fever |
| 11 | Lipusu® | 2003 | Paclitaxel | intravenously guttae | Lecithin, Cholesterol, Threonine and Glucose | Ovarian cancer | Bone marrow suppression, neurotoxicity, cardiovascular toxicity and liver toxicity |
| 12 | DepoDur™ | 2004 | Morphine Sulfate | Epidural | DOPC, DPPG, Cholesterol and Triolein | Pain management | Typical of opiate medications |
| 13 | Mepact® | 2009 | Mifamurtide | intravenous | DOPS and POPC | Non-metastatic osteosarcoma | Anaemia, headache and dizziness |
| 14 | Exparel® | 2011 | Bupivacaine | intravenous | DEPC, DPPG, Cholesterol and Tricaprylin | Pain management | Chondrolysis and methemoglobinemia |
| 15 | Marqibo® | 2012 | Vincristine | intravenous | Sphingomyelin and Cholesterol | ALL | Peripheral neuropathy and neutropenia |
| 16 | Onivyde™ | 2015 | Irinotecan | intravenous | DSPC, mPEG-2000 and DSPE | Metastatic pancreatic cancer | Neutropenia, lung disease |
| 17 | Vyxeos® | 2017 | Daunorubicin and Cytarabine | intravenous | DSPC, DSPG and Cholesterol | AML-MRC and t-AML | Febrile neutropenia, heart-related side effects, allergic reactions |
| 18 | Shingrix® | 2017 | Recombinant VZV glycoprotein E |
intramuscular | DOPC, Chol | Against shingles and post-herpetic neuralgia | General disorders and administration site conditions |
| 19 | Onpattro™ | 2018 | siRNA | intravenous | DLin-MC3-DMA, DSPC, Cholesterol and PEG2000-C-DMG | Polyneuropathy caused by hATTR | Infusion-related reactions and Peripheral edema |
| 20 | Arikayce® Kit | 2018 | Amikacin | inhalation administration | DPPC and CHO-HP | NTM lung disease caused by MAC | Bronchospasm, ototoxicity, nephrotoxicity, neuromuscular blockade |
| 21 | Comirnaty® | 2021 | BNT162b2 | intramuscular | ALC-0315, ALC-0159, DSPC and Cholesterol | COVID-19 | Injection site reactions |
| 22 | Moderna | 2021 | mRNA-1273 | intramuscular | SM-102, PEG2000-DMG, Cholesterol, and DSPC | COVID-19 | Injection site reactions |
Table 2.
The structure of partially ionizable cationic lipids.
| Name | Structure | ||
|---|---|---|---|
| DLin-MC3-DMA | Lipid N | ||
| Lipid 319 | 2,2(8,8) 4C CH3 | 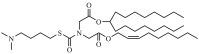 |
|
| 306Oi10 | CL1 | ||
| Lipid H (SM-102) | 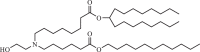 |
A9 | 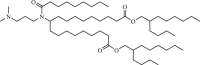 |
| Lipid M | ALC-0315 | 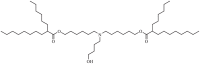 |
|
| Lipid P | 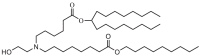 |
C12-200 | |
| Lipid Q | 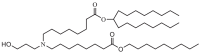 |
5A2-SC8 |  |
The two C18 linoleic acid tails and the dimethylamine group head of DLin-MC3-DMA, which is a well-defined feature, can appropriately adjust the pKa. Lipid H, M, P, Q, N retained the pKa requirement and pursued greater endosomal solubility properties by introducing more branches at the alkyl tail. Other lipids are developed by different companies [94,175]
2.1. Liposomes for small-molecule drug delivery
2.1.1. Anti-tumor therapy
Cancer is one of the most severe public health issues threatening the world, and many new patients are diagnosed with cancer every year. According to Global Cancer Statistics 2020, there are approximately 19.3 million new cases of cancer diagnosed worldwide, and the number of cancer deaths is expected to reach 10 million [27]. Cancer is still a significant health problem in China, although tremendous efforts have been made for its management [28,29]. Chemotherapy is the most widely used regimen against cancer in the clinic. However, traditional chemotherapeutics also kill normal cells, causing severe toxic side effects to patients. Liposomes are practical nanocarriers for anti-tumor and clinical applications, owing to their improved efficiency, low toxicity, and tumor-targeting ability [30]. So far, several anti-tumor liposomes have been approved for clinical trials and translated into the market.
Doxorubicin liposomes
Anthracycline doxorubicin is an orange-red loose lump or powder, easily soluble in water, dimethyl sulfoxide (DMSO), tetrahydrofuran, ethanol, insoluble in acetone, chloroform, benzene, and ether. Doxorubicin, with a strong cytotoxic effect, can inhibit the synthesis of nucleic acids by intercalating DNA, and generate free radicals to induce DNA and cell membrane damage [31], which is clinically used for the treatment of various cancers. Doxorubicin disappears rapidly in plasma after intravenous injection and is generally distributed in the liver, spleen, kidney, lung, and heart. Therefore, dose-related cardiotoxicity of doxorubicin limits the use of traditional doxorubicin formulations [32]. In 1995, the first doxorubicin liposome Doxil® was developed by Sequus and approved by the United States Food and Drug Administration (FDA). The approved indications were recurrent ovarian tumors and the Kaposi's Sarcoma (KS) infected with human immunodeficiency virus (HIV) [33,34]. Then, doxorubicin liposomes have gradually become the most studied anti-tumor drug liposomes. It can improve drug accumulation at the tumor sites and produce higher local doxorubicin concentrations than standard soluble preparations [35,36]. In addition, doxorubicin encapsulated in phospholipids avoids being absorbed by myocardial cells, reducing its cardiotoxicity. Doxil® encapsulates doxorubicin in polyethylene glycol (PEG)-modified stealth liposomes to prolong blood circulation, improve anti-tumor efficacy, and significantly increase patient compliance during application. In 1996, Doxil®, the PEGylated doxorubicin liposomes, was approved by the European Medicines Agency (EMA). And the trade name is Caelyx® on the European market. As the second-line recommended drugs for treating advanced ovarian cancer, doxorubicin liposomes have a good relieving effect on patients who are refractory to free platinum, paclitaxel, and other drugs [37]. Caelyx® was listed in China in 2003 and imported by Xi'an Janssen Pharmaceutical Co., Ltd.
Both Doxil® and Caelyx® use synthetic phospholipids as carriers and have high stability. PEG modification enhances the sustained release effect; meanwhile, it evades the interception of the immune system [38]. Although PEG modification brings the advantages of low cardiotoxicity, it can also cause carrier-related toxicity. After patients use high-dose PEGylated doxorubicin liposomes, a few of the drugs will leak out through the capillaries on the hands and feet, causing skin toxicity, causing numbness or pain in the hands and feet of the patient, and symptoms such as skin swelling, erythema, desquamation, chapped, and indurated blisters, namely “hand-foot syndrome” [39], [40], [41]. On the market in Europe in 2001, Myocet®, a non-PEG-modified doxorubicin citrate liposome developed by Elan Pharmaceuticals, does not have side effects on the skin and is used to treat metastatic breast cancer. Compared with PEG-modified doxorubicin liposomes, Myocet® is quickly swallowed by macrophages, has a short circulating half-life period, and has low targeting in tumor tissues [42].
Daunorubicin liposomes
Like doxorubicin, daunorubicin is an anthracycline antineoplastic drug. Daunorubicin is easily soluble in water, and its aqueous solution is relatively stable. The effect is the same as that of doxorubicin, which can inhibit RNA and DNA synthesis by intercalating DNA, and has a pronounced effect on RNA, selectively acting on purine nucleosides. The daunorubicin liposome, DaunoXome®, was developed by NeXstar in the United States and was approved by the FDA for HIV-related KS treatment in 1996 [43]. DaunoXome® can modulate the drug release in the circulation by phagocytosis to overcome the side effects of high doses of the free drug [44]. A single daunorubicin liposome has a narrow indication range, and the main toxicity is bone marrow suppression.
The FDA approved a new drug manufactured by Jazz Pharmaceuticals in August 2017, named Vyxeos® (CPX-351), that was employed in the treatment of therapy-related acute myeloid leukemia (t-AML) and AML with myelodysplasia-related changes (AML-MRC) [45,46]. Vyxeos® is different from conventional daunorubicin liposomes and simultaneously encapsulates daunorubicin and cytarabine at a specific molar ratio (cytarabine: daunorubicin = 5:1) [45,47]. Vyxeos® is the first liposome formulation that loads two active pharmaceutical ingredients (API). The successful development of Vyxeos® represents a significant advancement in liposome technology and provides novel ideas for various treatment programs involving combination drugs.
Cytarabine liposomes
Depocyt® was a cytarabine liposome developed by SkyPharm Inc. As a pyrogen-free parenteral suspension, the release rate of cytarabine can be controlled. Depocyt® was approved by the FDA for neoplastic meningitis (NM) therapy in 1999. Cytarabine can interfere with DNA synthesis and act on DNA/RNA polymerases, reducing the replication ability of cells [48]. However, cytarabine has a short plasma half-life, poor stability, and bioavailability, and its continuous infusion always causes severe side effects [49]. Depocyt® is a sustained-release preparation using DepoFoam technology to load the drug. The delivery of cytarabine through liposome technology can reduce the frequency of administrations from once every two days to once every two weeks, significantly improving patient compliance [50].
Paclitaxel liposomes
Paclitaxel is an anti-microtubule drug. By reversibly binding to microtubules, the drug destroys the dynamic balance between tubulin and tubulin dimers to stabilize microtubules and inhibit tumor cell mitosis, exerting an anti-tumor effect [51], [52], [53]. Paclitaxel is an insoluble drug. Polyoxyethylene castor oil and absolute ethanol are usually used as mixed solvents in intravenous injection preparations, frequently inducing unwanted effects such as muscle pain and allergic reactions. Liposomal encapsulation effectively enhances paclitaxel solubility without using mixed solvents, improves safety, and reduces adverse reactions. The only liposomal paclitaxel formulation on the market is Lipusu®, developed by Luye Pharma, which was launched in China in January 2003 [54].
Vincristine liposomes
Vincristine is a common drug used for acute lymphoblastic leukemia (ALL) treatment. Vincristine is an M-phase cell cycle-specific anti-cancer drug that can be combined with other drugs. Concentration and exposure time determine the activity of vincristine [55], [56], [57]. The pharmacokinetic profile of vincristine exhibits a bi-exponential elimination pattern with a very short initial distribution half-life followed by a longer elimination half-life, and vincristine also has a large volume of distribution [57]. However, due to the neurotoxicity, vincristine's dosage is often limited in clinical application, and it is always manifested as insufficient dosage for patients with large body surface areas. The application of liposome technology can achieve passive accumulation at the site where the permeability of the vasculature is increased and reduce adverse reactions relative to free drugs [55]. A vincristine sulfate liposome injection, Marqibo®, was developed by Talon Therapeutics in the United States, and the FDA accelerated its approval for ALL therapy in 2012 [58].
Irinotecan liposomes
Irinotecan is a semi-synthetic water-soluble camptothecin derivative and an inhibitor of DNA topoisomerase I [59]. The complex formed by irinotecan, topoisomerase I, and DNA can cause DNA single-strand breaks, prevent DNA replication, and inhibits RNA synthesis. Irinotecan has a highly complex metabolic process; its active metabolite is 100 to 1000 times more active than irinotecan. However, during its treatment, there is dose-limiting toxicity and large interindividual pharmacokinetic variability, and its metabolism is susceptible to environmental and genetic influences [60]. Onivyde™ was developed by Merrimack Pharmaceuticals and was approved by the FDA in 2015. It is a liposome that encapsulates irinotecan and is especially suitable for combination use with fluorouracil and leucovorin for gemcitabine chemotherapy, affecting poorly metastatic pancreatic cancer therapy [61]. Onivyde™ was prepared by encapsulating drugs in long-circulating liposomal nanovesicles [62]. Compared with conventional preparations, Onivyde™ was developed to maximize its anti-tumor efficacy while minimizing drug-related toxicity. When Onivyde™ is combined with fluorouracil and leucovorin, it has controllable safety and prolongs the patients’ survival. Compared with fluorouracil and leucovorin alone, it has significant clinical benefits in adjusting the survival rate [63].
2.1.2. Anti-fungal therapy
Amphotericin B liposomes
Amphotericin B is extensively used for various systemic fungal infections treatment. When the body's immune function is deficient or suppressed, fungal infections are prone to occur, and amphotericin B is commonly used. Amphotericin B is a lipophilic macrolide antibiotic. Because of the asymmetric distribution of hydrophilic and hydrophobic groups in the molecular structure has low solubility in water and poor absorption in gastrointestinal mucosa and skin. The most concerning adverse effect is nephrotoxicity [64]. The application of liposome technology can reduce the toxicity of amphotericin B, mainly through two mechanisms. One is to adjust the rate of amphotericin B transfer from the carrier to the cell membrane, which usually leads to the decreased uptake of fungal target cells; however, it can be solved by increasing the amount of the drug. The second is to adjust the clearance rate of the complex from the blood [64]. Liposomes will be quickly absorbed by the mononuclear phagocyte system (MPS), and lowering its plasma concentration can achieve low toxicity. Amphotericin B liposomes can be internalized by macrophages, carried to the site of infection by macrophages, and locally released slowly. Its high affinity for ergosterol can cause it to accumulate in fungi and play a role in anti-fungal infections.
The FDA has approved three amphotericin B liposome preparations for marketing, including Ambisome®, Abelcet®, and Amphotec®. Ambisome® was a liposome formulation of amphotericin B successfully developed by NexStar Pharmaceuticals in 1997. It was the world's first liposome formulation for fungal severe infections therapy, first approved by EMA in 1990. Ambisome® quickly penetrates the fungal cell wall and enters the cell. After releasing amphotericin B, it can combine with the membrane sterol components (mainly ergosterol) to achieve a potent bactericidal activity in vitro and inhibit fungi replication in immunocompetent animals and animals with impaired immune function [23]. At the same time, the liposome can maintain its integrity in the presence of mammalian cells and achieve low toxicity to mammalian cells [23].
Abelcet® was a sterile, preservative-free phospholipid complex preparation for intravenous injection developed by the United State Sigma-Tau Pharmaceutical Company. It is used to treat invasive fungal infections that are difficult to respond to traditional amphotericin B deoxycholate treatment or when the use of traditional amphotericin B is forbidden in the case of kidney injury. When Abelcet® is observed under a cryo-electron microscope, it shows a ribbon-like structure [65], and there is no imitation product on the market.
Amphotec® was a parenteral liposome formulation of amphotericin B for injection. It was manufactured by Ben Venue Laboratories and was approved by the FDA in 1996 for severe fungal infections and leishmaniasis treatment [66]. Amphotec® is a uniform small spherical ULV liposome with a particle size of approximately 100 nm. When reconstituted in an aqueous medium, it will form a colloidal solution composed of tiny disc-shaped particles. Compared with the traditional amphotericin B preparation, the unique colloidal preparation properties reduce adverse reactions such as hemolysis and acute toxicity [19]. This product has been withdrawn from the market in the United States.
2.1.3. Anti-bacterial therapy
Amikacin liposomes
Arikayce® Kit was a liposomal inhalation formulation developed by Insmed and approved by the FDA in 2018. Administration using the eFlow nebulization system manufactured by PARI Pharma GmbH for non-tuberculous mycobacteria (NTM) pulmonary disease therapy, especially NTM lung disease caused by Mycobacterium avium complex (MAC) [67], [68], [69]. This is the first and only treatment in the United States specifically designed to treat that unique pulmonary disease. Amikacin is an aminoglycoside antibiotic. The therapeutic mechanism of amikacin is to act on ribosomes in bacteria, inhibit bacterial protein synthesis, and destroy the integrity of bacterial cell walls, resulting in bacterial cell death. It is administered by intramuscular injection or intravenous infusion, and the half-life of intravenous infusion is about 2 hours. Systemic toxic effects and drug efficacy are of paramount concern when treating with amikacin [68]. The Arikayce® Kit delivers amikacin directly to the lungs by inhalation using charge-neutral liposomes. The drug is taken up by NTM-infected pulmonary macrophages, prolonging the amikacin release time in the lungs, and decreasing entry into the systemic circulation, thereby reducing the systemic toxicity of amikacin. The study demonstrated that the endpoint of sputum culture transformation could be reached at 6 months when NTM is treated with the Arikayce® Kit [70]. The addition of Arikayce® Kit to clinical therapy found that 29% of patients achieved a negative NTM sputum culture result at 6 months compared to 8.9% of patients receiving conventional therapy alone [68]. However, limited data on the safety of the Arikayce® Kit is currently only useful in special populations, especially in patients with lung disease caused by NTM. The clinical safety of the Arikayce® Kit requires further study [69].
2.1.4. Clinic analgesic
Morphine liposomes
DepoDur™, a sustained-release liposome-encapsulated morphine sulfate, was produced by SkyePharma and approved by the FDA in 2004 for preoperative anesthesia or postpartum analgesia. Epidural injection of morphine is usually used to relieve postoperative pain through a single injection, intermittent additional doses, continuous infusion, and other methods. A single epidural injection of conventional morphine typically provides only 24 h of analgesia and is often insufficient for postoperative pain relief in patients who suffered significant surgery. Although intermittent supplemental doses or continuous epidural infusion can provide long-term pain relief, the need for indwelling catheters will hinder the patient's activities and bring the risk of epidural hematoma to patients receiving preventive anticoagulation therapy, so the application is limited [71].
DepoDur™ is a sustained-release morphine liposome for better postoperative analgesia. A single epidural injection of DepoDur™ can provide pain relief for up to 48 h [72]. It consists of multivesicular morphine liposomes with a diameter of 17-23 µm and then dispersed in a preservative-free 0.9% sodium chloride solution. Multivesicular morphine liposomes constructed by “DepoFoam" technology, each vesicle can be ruptured time-dependent, slowly and continuously releasing the encapsulated morphine sulfate into the epidural space to maintain more pain relief time [73,74]. In addition, DepoDur™ also avoids excessive peak concentration and its possible side effects [75].
Bupivacaine liposomes
Exparel® was a bupivacaine liposome injection produced by Pacira BioSciences and approved by the FDA in 2011, used for local infiltration anesthesia [76]. Bupivacaine has a short analgesic duration (approximately 9 hours) and requires delivery through a perineural catheter to maintain a continuous drug infusion. Nevertheless, this technique is limited by various infection risks associated with perineural catheters [77]. Exparel® is a multivesicular liposome encapsulated with bupivacaine based on DepoFoam technology, achieving 72 h of analgesic effect in the body [78]. And Exparel® is used for postoperative analgesia through peripheral nerve block (PNB) [79], and the FDA approved it for intermuscular sulcus brachial plexus block in 2018. The application of Exparel® can reduce the consumption of opioids without the need for catheters or other equipment and is easy to dilute with saline [78]. However, due to the instability of liposomes, when Exparel® is used in combination with other free local anesthetics, a large amount of bupivacaine will be released owing to liposome reorganization. Therefore, Exparel® cannot be used simultaneously with other local anesthetics for at least 20 min [80].
2.1.5. Photodynamic therapy
Verteporfin liposomes
Visudyne® was a liposome of the photosensitizing drug verteporfin developed by Novartis AG. The FDA approved it for age-related macular degeneration (AMD) and choroidal neovascularization (CNV) treatment in 2000. AMD is one of the significant causes of blindness, and it is divided into non-exudative (dry type) and exudative (neovascular type) [81]. In exudative AMD, abnormal blood vessels grow from under the choroidal capillaries into the retina, called choroidal neovascularization. These new blood vessels will bleed and leak, accompanied by fibrous tissue, causing severe vision deterioration [81]. After Visudyne® is administered by intravenous injection, it is activated by a laser via photodynamic therapy (PDT). The activated verteporfin can produce reactive oxygen free radicals to damage local neovascular endothelial cells, resulting in vascular closure and preventing vision loss or blindness caused by vascular abnormalities and leakage.
PDT is used not only in treating AMD but is also considered a promising tumor treatment method. Using photosensitizers and lasers can result in reactive oxygen species generation, which can kills tumor cells [82]. Due to the strong fluidity of the liposome membrane of Visudyne®, it is believed that after injection into the blood, the internal API can be easily transferred to lipoproteins, thereby being transported into tumor tissue through the bloodstream [82], which is beneficial for the cancer treatment.
2.2. Liposomes and LLPs for biological drug delivery
The biological drug, such as active proteins, peptides, and gene drugs, have attracted increasing attention [83], along with numerous biological drugs approved for clinical use [84], [85], [86]. However, the high molecular weight and complex structure of biological drugs always result in the sensitivity to denaturation and deactivation and poor ability to enter the cell, restricting the administration of dosage forms and routes. The liposome technology demonstrates huge advantages in improving the delivery of biologics via elevating stability, facilitating cellular entry, and enhancing bioavailability [87]. Some liposomes and LLPs loading biological drugs have been marketed.
2.2.1. siRNA LLPs
The FDA approved Onpattro™ (Patisiran) in 2018 to treat hereditary transthyretin amyloidosis (hATTR) polyneuropathy. Onpattro™ is the first marketed small interference RNA (siRNA) drug, and its approval demonstrates a milestone significance in RNA therapy [88]. hATTR is a rare and fatal autosomal dominant gene mutation by which misfolded mutant transthyretin (TTR) fibrils deposit in tissues and organs of the body, forming abnormal sediment called amyloid substances that breaks the normal function of tissues and organs [89]. This protein deposition often occurs in the peripheral nervous system and causes loss of sensation, pain, or inability to move in the limbs [90]. Onpattro™ is a TTR-targeted siRNA therapy that silencing the RNA. Onpattro™ encapsulates siRNA in LLPs and delivers the drug directly to the liver by intravenous infusion. Onpattro™ works by reducing the production of abnormal TTR RNA and its accumulation in body tissues [91]. The study results showed the LLPs had an average serum TTR clearance of 86.8% following a single injection [92].
The most important lipid moiety in Onpattro™ is an ionizable positive charged lipid, (6Z,9Z,28Z,31Z)-heptatriacont-6,9,28,31-tetraene-19-yl 4-(dimethylamino) butanoate (DLin-MC3-DMA). When Onpattro™ enters the systemic circulation, its PEGylated lipid moiety is replaced by proteins in the blood, primarily apolipoprotein E (ApoE), and binds to the cholesterol moiety to transport the drug to the liver. The liver cells then take up the lipid nanoparticles and transfer them to the endosome. After reaching the endosome, the ionizable DLin-MC3-DMA becomes cationic in the low pH environment, causing penetration into the endosome until the endosome ruptures [93]. The most critical characteristic of ionizable lipids is that their pKa should be 6-7 [94]. When released into the cytoplasm, siRNA works to reduce serum TTR levels and protein tissue deposition to improve the symptoms of polyneuropathy in patients with hATTR. After the launch of Onpattro™, ionizable lipids gradually became the focus of study. Since siRNA products require repeated administration to treat chronic diseases, the slow degradation of lipid moieties in vivo often leads to lipid accumulation and potential toxicity. Various novel ionizable lipids have been synthesized (Table 2).
2.2.2. Peptide liposomes
Mepact®, an orphan drug approved by the EMA in 2004 and marketed in Europe in 2009, is a mifamurtide (MFT) liposome developed by IDM Pharma and is mainly used to treat resectable non-metastatic osteosarcoma. The liposome product is the first medicine to treat osteosarcoma and effectively prolongs the survival period of patients.
Mepact® is a multilamellar liposome with a diameter of less than 100 nm and is formulated with cell wall tripeptide phosphatidylethanolamine (L-MTP-PE) [95]. The liposomes release MFT after being taken up by macrophages, leading to cytokine release and cancer-cell killing [96].
2.2.3. Liposome and LLP vaccine
Liposome vaccine for hepatitis and influenza
Virosome is a lipid nanoparticle prepared by removing the viral nucleocapsid of the enveloped virus after being treated with a detergent and is mainly used as a vaccine carrier. Compared with traditional liposomes, the phospholipid bilayer is embedded with viral membrane proteins. These proteins can ensure the fusion of virosome and immune cells and present the encapsulated specific antigens to immune cells. Virosome does not contain immune adjuvants because of its immunogenicity and tolerability, whereas traditional vaccines require immune adjuvants [97]. The virosome preparation can simulate natural antigens' presentation and induce humoral and cellular immunity, enhancing vaccinization efficacy.
Epaxal® was the first liposome vaccine for hepatitis A using virosome technology. The vaccine was developed by Swiss Crucell Berna Biotech and launched in Europe in 1993. Epaxal® is prepared by mixing a fixed molar ratio of dioleoly-sn-glycerol-phosphoethanolamine (DOPE) and dioleoylphosphatidylcholine (DOPC) (DOPE: DOPC=25: 75). The viral glycoprotein is inserted in the phospholipid bilayer, and the vaccine strain RG-SB is anchored on a 150 nm liposome [98]. Epaxal® does not encompass aluminum salts and thimerosal as immune adjuvants compared with traditional aluminum-containing vaccines. Therefore, it relieves the injection site's local pain and side effects [97]. As a result, Epaxal® is often utilized to vaccinate infants and young children over 12-month age [99].
In addition to Epaxal®, Inflexal® V was another representative liposome vaccine developed by Swiss company Crucell Berna Biotech using the virosome technology. Inflexal® V launched in Europe in 1997 was an inactivated and virosome-assisted influenza vaccine. Inflexal® V is a 150 nm ULV liposome loading the surface molecules of hemagglutinin in the lipid bilayer. Due to excellent immunogenicity and tolerability, the vaccine is extensively utilized in various age groups, including children, young adults, and the elderly [100].
Vaccines are usually classified into gene-based and protein-based vaccines. Protein-based vaccines are safe; however, they are weak in immunogenicity and generally cannot activate pattern recognition receptors (PRRs). Therefore, adjuvants that can initiate and amplify adaptive immune responses are always formulated into such vaccines [101]. Shingrix® is a state-of-the-art liposome vaccine adjuvant delivery system (VADS) based on a new subunit vaccine produced by pharmaceutical giant GSK (GlaxoSmithKline). Shingrix® was approved by the FDA in October 2017 for shingles (or herpes zoster, HZ) prophylaxis in the elderly aged 50 years and over. The product is a liposome-based anti-HZ subunit vaccine consisting of AS01b as the VADS and freeze-dried powder of recombinant varicella-zoster virus (VZV) glycoprotein E (gE) as the antigen. AS01b is a VADS consisting of an aqueous suspension of 50–100 nm DOPC/CHO/MPLA liposomes bound by QS21 to liposome CHO [102,103]. According to the completed clinical studies, the vaccine's efficacy in preventing shingles development could last up to 4 years. The efficacy in people older than 50 was 97%, of which approximately 90% of participants were older than 70. More notably, the anti-antigen response appears to be preserved with age, demonstrating that the protecting effectiveness dropped a little over four years and was effectively maintained in 70-year older people [104,105].
LLP vaccine against COVID-19
The emergence of messenger RNA (mRNA) vaccines breaks through the immune activation model of traditional vaccines. Using the body's cells to produce antigens activates dual-specific immunity and provides a longer-lasting immune response. The delivery of mRNA vaccines via lipid technology is still a promising solution. In December 2019, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbroke, which subsequently triggered a global pandemic of coronavirus disease 19 (COVID-19) [106], [107], [108]. Since then, global health and the economy have been facing a massive crisis. Based on this public health security crisis, innovative solutions are urgently needed to curb the spread of COVID-19. Coronavirus is mainly composed of four main structural proteins: spike (S) protein, nucleocapsid (N) protein, membrane (M) protein, and envelope (E) protein [106,109]. The four proteins work together to complete the activities of the virus. S protein is responsible for mediating the adhesion and fusion between the virus and the host cell receptor. N protein can bind viral RNA, support nucleocapsid formation, and participate in the virus's replication cycle. M protein is an essential part of the virus membrane. E protein is the minor complete membrane structural protein in the virus envelope [109]. The mechanism of E protein is not precise, but it seems to play an essential role in the virus's production, maturation, release, and pathogenesis [106]. The S protein of SARS-CoV-2 consists of two subunits: the S1 subunit contains a receptor-binding domain (RBD), which binds to angiotensin-converting enzyme 2 (ACE2) on the surface of host cells, and the S2 subunit mediates the fusion between virus membrane and host cells [109], [110], [111].
The main obstacles to COVID-19 vaccine expansion are the development of an appropriate vaccine technology platform and the inability of long-term immunization due to the high variability of the virus. In addition to conventional vaccine forms such as inactivated or attenuated viruses and virus vectors, nanotechnology has strong adaptability and accelerates vaccine development. Countries' efforts worldwide have marketed two mRNA vaccines using lipid technology. They are the COVID-19 vaccine Moderna (mRNA-1273), industrialized by the United State vaccine company Moderna, and the COVID-19 vaccine Pfizer-BioNTech (product name is Comirnaty®) jointly developed by Pfizer and German BioNTech. The two LLP vaccines contain ionizable lipids, cholesterol, phospholipids, PEG2000, and non-replicating in vitro transcribed (IVT) mRNAs, demonstrating a similar structure with which liposomes (Fig. 4) [94,112].
Fig. 4.
Schematic illustration of liposome and LLP.
IVT mRNA used for treatment has many advantages for clinical applications: 1) The steps to produce mRNA are fast and straightforward, which can ensure rapid and straightforward production under pharmaceutical conditions; 2) since IVT mRNA is essentially a transient molecule in the body, it will be completely degraded by the abundant RNase inside and outside the cell, which has a safe and clear therapeutic window; 3) single antigenicity can avoid triggering and enhancing the immune response [112]. Comirnaty® is the first COVID-19 vaccine officially approved by the FDA in 2021. The vaccine is a nucleoside-modified RNA vaccine formulated with lipid nanoparticles, encoding the entire length of the spike protein of SARS-CoV-2 that is stable and membrane-anchored before fusion. According to published clinical data, Comirnaty® has high protection and safety, which two-dose regimen provides 95% COVID-19 protection for people 16 years of age and older [113]. Moderna vaccine is also an mRNA vaccine encapsulated in lipid nanoparticles and can afford 94% efficacy for symptomatic COVID-19 patients [114]. This vaccine has received emergency use authorization from multiple global regulatory agencies, including the FDA.
Moderna vaccine is based on mRNA-1273 molecules that can translate S2-protein antigens (composed of SARS-CoV-2 glycoprotein and complete S1-S2 cleavage sites), delivered by LLPs [115,116]. Comirnaty® is similar to the Moderna vaccine as both use LLPs as a carrier to deliver the mRNA molecule BNT162b2 that encodes the full-length S protein of SARS-CoV-2 [115]. After such mRNA molecules are delivered into the cytoplasm, they are translated to produce S protein capable of stimulating an immune response, leading to the body producing antibodies. After the mRNA vaccine is injected into the host, the internalized mRNA molecules are usually trapped in endosomal vesicles [117]. Therefore, the mRNA must escape from the endosome for translation. Therefore, effectively achieving the intracellular delivery of large-molecule drugs such as mRNA is a crucial issue that must be concerned in the application process.
The lipid part of both mRNA vaccines contains an ionizable cationic lipid component (ALC-0315 and SM-102, respectively), which can effectively enhance the vaccine's ability to endosomal escape during the biological delivery process. In the formation process of LLPs, positively charged lipids and negatively charged mRNA molecules can form stable complexes through electrostatic interactions to achieve drug loading [118], and this ionizable lipid, which is sensitive to pH, will be positively charged in the low pH condition of the endosome, causing the instability of the endosomal membrane. Cationic lipids preferentially form complexes with anionic lipids in the membrane of the endosome, forming a “cone”-shaped ion pair, which promotes the generation of a hexagonal HII phase structure on the lipid bilayer phase and the formation of local pores [87,118,119], destroying the membrane structure of the endosome and facilitating the mRNA molecule to escape from the endosome and achieve intracellular delivery. In addition to the two mRNA vaccines that have been approved for marketing, several mRNA vaccines based on lipid formulations are in clinical trials (Table 3), and other non mRNA COVID-19 vaccines have also been marketed or are in different stages of clinical trials in response to the spread of COVID-19 (Table 4).
Table 3.
COVID-19 mRNA liposomal vaccine in the clinical trial.
| Name | Company | Location | Phase | |
|---|---|---|---|---|
| 1 | mRNA-1273 | Moderna | America | Phase Ⅲ NCT04470427 |
| 2 | BNT 162b2 | BioNTech | Germany | Phase Ⅲ NCT04368728 |
| 3 | CVnCoV | CureVac AG | Germany | Phase Ⅲ NCT04674189 |
| 4 | ARCoV | Abogen | China | Phase Ⅱ ChiCTR2000039212 |
| 5 | ARCT-021 | Arcturus | America | Phase Ⅱ NCT04668339 |
| 6 | LNP-nCoVsaRNA | Imperial College London | England | Phase Ⅰ SRCTN17072692 |
| 7 | ChulaCoV19 mRNA vaccine | Chulalongkom University | Thailand | Phase Ⅱ NCT05231369 |
Administration route: all intramuscular
Details may be found at the website in the supplementary material
Table 4.
Other COVID-19 vaccines in the clinical trial.
| Name | Company | Location | Vaccine type | Phase | |
|---|---|---|---|---|---|
| 1 | CoronaVac | Sinovac | China | Inactivated virus | Phase Ⅳ ChiCTR2100052697 |
| 2 | COVID-19 Vaccine (Vero Cell), Inactivated | Wuhan Institute of Biological Products | China | Inactivated virus | Phase Ⅲ ChiCTR2000034780 |
| 3 | Sinopharm COVID-19 vaccine | Beijing Institute of Biological Products | China | Inactivated virus | Phase Ⅱ ChiCTR2000032459 |
| 4 | Inactivated ASRS-CoV-2 vaccine | Chinese Academy of Medical Science | China | Inactivated virus | Phase Ⅱ NCT04412538 |
| 5 | BBV152 | Bharat Biotech International Limited | India | Inactivated virus | Phase Ⅱ NCT04471519 |
| 6 | AG0301-COVID19 | AnGes, Inc. | Japan | DNA | Phase Ⅱ NCT04463472 |
| 7 | ZyCoV-D | Zydus Cadila | India | DNA | Phase Ⅱ CTRI/2020/07/026352 |
| 8 | GX-19 | Genexine, Inc. | Korea | DNA | Phase Ⅱ NCT04445389 |
| 9 | INO-4800 | International Vaccine Institute | Korea | DNA | Phase Ⅱ NCT04447781 |
| 10 | AZD1222 | AstraZeneca | Russian | Viral vector | Phase Ⅲ NCT04540393 |
| 11 | Ad26.COV2.S | Janssen Vaccines & Prevention B.V. | America | Viral vector | Phase Ⅱ NCT04436276 |
| 12 | Gam-COVID-Vac Lyo | Gamaleya Research Institute of Epidemiology and Microbiology | Russian | Viral vector | Phase Ⅱ NCT04437875 |
| 13 | Gam-COVID-Vac | Gamaleya Research Institute of Epidemiology and Microbiology | Russian | Viral vector | Phase Ⅱ NCT04436471 |
| 14 | Ad5-nCoV | CanSino Biological Inc. | China | Viral vector | Phase Ⅲ ChiCTR2100044249 |
| 15 | GRAd-COV2 | ReiThera Srl | Italy | Viral vector | Phase Ⅲ NCT04791423 |
| 16 | TMV-083 | Institut Pasteur | Belgium | Viral vector | Phase Ⅰ NCT04497298 |
| 17 | SCB-2019 | Clover Biopharmaceuticals AUS Pty Ltd | Australia | Recombinant protein | Phase Ⅰ NCT04405908 |
| 18 | Covax-19 | Vaxine Pty Ltd | Australia | Recombinant protein | Phase Ⅰ NCT04453852 |
| 19 | UQ-1-SARS-CoV-2-Sclamp | Queensland/CSL/Seqirus | Australia | Recombinant protein | Phase Ⅰ NCT04495933 |
| 20 | Recombinant new CoV vaccine (CHO cell) | Anhui Zhifei Longcom Biopharmaceutical Inc | China | Recombinant protein | Phase Ⅱ NCT04466085 |
| 21 | MVC-COV1901 | Medigen Vaccine Biologics Corp. | Taiwan, China | Recombinant protein | Phase Ⅰ NCT04487210 |
| 22 | SARA-CoV-2 rS | Novavax | America | Recombinant protein | Phase Ⅱ NCT04368988 |
| 23 | Covac 1 | University Hospital Tübinge | German | Peptide | Phase Ⅰ NCT04546841 |
| 24 | CoVLP | Medicago | Canada | Virus-like particles (VLP) | Phase Ⅰ NCT04450004 |
Administration route: all intramuscular
Details may be found at the website in the supplementary material
3. Liposomes and LLPs under clinical trials
The liposomes continue to evolve rapidly, driven by several factors such as new delivery strategies, preparation technologies, and the emergence of new drugs entering clinical trials. In this section, we summarized liposome and LLP formulations that entered clinical trials or failed in clinical trials recently (Table 5) and discussed the translation limitation.
Table 5.
Liposome and LLP formulations in clinical trials.
| Name | Company | Active Ingredient | Phase | Reference | |
|---|---|---|---|---|---|
| 1 | ThermoDox | Celsion Corporation | Doxorubicin | Phase Ⅲ (Failure) NCT00617981 | [131] |
| 2 | L-CsA-i | Breath Therapeutics | Cyclosporine A | Phase Ⅲ (Failure) NCT01334892 | [176] |
| 3 | SPI-77 | Alza Corporation | Cisplatin | Phase Ⅱ NCT00004083 | [177] |
| 4 | LE-SN38 | Neopharm | SN-38 | Phase Ⅰ NCT00046540 | [178] |
| 5 | LEP-ETU | Neopharm | Paclitaxel | Phase Ⅱ NCT01190982 | [54,179] |
| 6 | Lipo-MERIT | BioNTech SE | BNT111 | Phase Ⅰ NCT02410733 | [128] |
| 7 | Tecemotide | Merk Serono | BLP-25 | Phase Ⅲ (Failure) NCT01015443 | [180] |
| 8 | mRNA-4157 | Moderna | mRNA-4157 | Phase Ⅰ NCT03313778 | [122] |
| 9 | EndoTAG-1 | MediGene | Paclitaxel | Phase Ⅲ NCT03126435 | [181] |
| 10 | MM-310 | Merrimack Pharmaceuticals | Docetaxel | Phase Ⅰ NCT03076372 | [121] |
| 11 | MM-302 | Merrimack Pharmaceuticals | Doxorubicin | Phase Ⅲ (Failure) NCT02213744 | [121] |
| 12 | C225-ILS-Dox | University Hospital, Basel | Doxorubicin | Phase Ⅰ NCT03603379 | [121] |
| 13 | Alprostadil liposome | Guangzhou Yipinhong Pharmaceutical CO., LTD | Alprostadil | Phase Ⅰ NCT02889822 | [182] |
| 14 | Liposomal Annamycin | Moleculin Biotech, Inc. | Annamycin | Phase Ⅱ NCT03315039 | [183] |
| 15 | Anti-EGFR-immunoliposomes | Swiss Group for Clinical Cancer Research | Doxorubicin | Phase Ⅱ (Failure) NCT02833766 | [184] |
| 16 | MRT5201 | Translate Bio, Inc. | MRT5201 | Phase Ⅱ (Failure) NCT03767270 |
[185] |
| 17 | MRT5005 | Translate Bio, Inc. | MRT5005 | Phase Ⅱ NCT03375047 | [185] |
| 18 | IVAC_W_bre1_uID | BioNTech SE | IVAC_W_bre1_uID | Phase Ⅰ NCT02316457 | [186] |
| 19 | MTL-CEBPA | Mina Alpha Limited | MTL-CEBPA | Phase Ⅰ NCT02716012 | [187] |
| 20 | TLD-1 | Swiss Group for Clinical Cancer Research | Doxorubicin | Phase Ⅰ NCT03387917 | [188] |
| 21 | mRNA-1944 | Moderna | mRNA-1944 | Phase Ⅰ NCT03829384 | [189] |
3.1. Liposomes entering clinical trials
3.1.1. Active-targeted liposomes
Active-targeted liposomes under clinical trials are mainly immunoliposomes prepared by covalently binding monoclonal antibodies or genetic antibodies to liposomes. Target cells are killed explicitly with the help of antibody binding to target cell surface antigens or receptors [120]. The immunoliposomes under clinical trial include MM-302 formulation, C225-ILS-Dox (the doxorubicin liposome decorated with an antigen-binding fragment of cetuximab [121]) and MM-310 (the docetaxel liposome decorated with an antibody targeted to the ephrin A2 receptor [122]). The first immunoliposomes approved for clinical trials is the MM-302 formulation developed by Merrimack Pharmaceuticals, which consists of a doxorubicin liposome and a single-chain portion decorated with an anti-HER-2 monoclonal antibody. The modification of HER-2 targeting ligands allows the liposomes to be especially internalized by tumor cells overexpressing HER-2 receptors [123]. One study found a 35-fold increase of Cu-64 labeled MM-302 in tumor accumulation 24 to 48 hours after dosing [124]. Nonetheless, a phase II clinical trial (NCT02213744) demonstrated no clinical benefit when trastuzumab was combined with MM-302 compared to trastuzumab plus conventional anti-tumor agents, leading to the trial termination [125].
3.1.2. mRNA-liposome tumor vaccine
mRNA tumor vaccines can be divided into two categories: mRNA vaccines based on dendritic cell (DC) delivery and directly injected mRNA vaccines. DC vaccines are mRNA-transfected DCs able to activate the immune system and kill tumor cells [126]. Using granulocyte-macrophage colony-stimulating factor (GM-CSF) as an adjuvant, directly injected mRNA vaccines stimulate the body to produce antibodies and inhibit the growth of tumor cells [126,127].
Antigen selection is critical to developing effective cancer vaccines. Tumor vaccines can be designed to target tumor-associated antigens (TAAs) that are preferentially expressed in malignant tumor cells. A well-known example is Lipo-MERIT. Lipo-MERIT is prepared by complexing mRNA with cationic lipids such as DOTMA or DOTAP. Lipo-MERIT encapsulates BNT111, an mRNA capable of encoding four TAAs, including NY-ESO-1, Tyrosinase, MAGE-A3, and TPTE [128]. Activation of the immune system in patients was demonstrated shortly after vaccination and was strengthened by increasing the dose from 7.2 to 100 µg. T cells in the patient acquired the ability to specifically recognize at least one of the TAAs after vaccinization [128].
Vaccines against TTAs may trigger central and peripheral immune tolerance responses, resulting in reduced vaccine efficiency. Several barriers, such as the TAA variability and TAAs in normal tissue, limit the vaccine application. E.g., the TAA variability leads to immune escape and the development of drug resistance [126]. Consequently, tumor-specific antigens, neoantigens, are emerging as core targets for mRNA vaccines. Neoantigens are derived from random somatic mutations in tumor cells and are not present in normal cells [129]. Neoantigens contribute to the development of personalized tumor vaccines. Some clinical trials are underway to investigate the safety and efficacy of mRNA vaccines encoding neoantigens. mRNA-4157, developed by Moderna, is a personalized vaccine encapsulated in LLPs and used as monotherapy or in combination with pembrolizumab (NCT03313778) against resected solid tumors, displaying an acceptable safety profile and significant neoantigen-specific T-cell responses [126].
3.2. Failed clinical trials
Despite clinical trials of liposome formulations being conducted every year, most products did not reach the primary endpoints. ThermoDox is a doxorubicin thermosensitive liposome containing a lipid DPPC with a phase transition temperature of 42 °C. When the temperature is above 42 °C, the drug in ThermoDox is released. ThermoDox was combined with radiofrequency ablation (RFA) to treat liver cancer in a phase III trial (NCT00617981). RFA is a minimally invasive tumor in situ treatment technique. It can position and guide the electrode needle to insert directly into the tumor under the assistance of ultrasound or CT and other imaging technologies, generating high temperature in situ through radiofrequency energy, drying and ultimately coagulating and inactivating tumor cells [130]. Hence, the researchers proposed that RFA-induced local heating could stimulate ThermoDox drug release and serve as a combined treatment for liver cancer. Yet, this phase III trial failed to meet the critical endpoint [131]. The failure of ThermoDox is likely due to its poor targeting and short liver presence, partly resulting from the high liver blow speed. So, the response of RFA + ThermoDox was shown to be consistent with RFA alone in the trial results. Moreover, ThermoDox has limited clinical experience and the preliminary data failed to display a clear dose-response. Nonetheless, this trial still could inspire the design of liposomes.
Another failed clinical trial (NCT01015443) was the lung cancer trial vaccine Tecemotide. Tecemotide targeting the tumor-associated antigen MUC1 to combat unresectable stage IIIA/IIIB non-small cell lung cancer (NSCLC) was utilized as maintenance therapy after chemoradiotherapy [132]. The study discovered no significant difference in overall survival for unresectable stage III NSCLC patients dosed with Tecemotide after radiotherapy compared to the placebo. Of note, the use of immunotherapy for maintenance treatment is still in the experimental stage. Tecemotide may also benefit patients treated with initial concurrent radiotherapy; further studies are still needed.
4. Revolutionary changes in the preparation
The liposome preparation can be accomplished by traditional methods such as thin film hydration, ethanol injection, reverse-phase evaporation, and freeze-drying. With an increasing understanding of the lipid-assembly mechanisms from colloidal and intermolecular forces, several new techniques of liposome preparation are emerging, including the microfluidic method and intelligent manufacturing. Has and Sunthar reviewed various preparation methods recently [133].
4.1. Microfluidic method
Microfluidics refers to the current situation in which fluid handling procedures are performed in a finite volume, typically defined by sub-millimeter length scales and low Reynolds numbers [134]. Microfluidics provides excellent control of liposome size homogeneity and has a significant advantage. For instance, liposome size and encapsulation volume can be precisely regulated by changing the flow parameters of the fluid phase [135]. The microfluidic liposome preparation techniques are divided into droplet-based and continuous flow-based systems [136].
Droplet-based microfluidic systems formulate liposomes by manipulating two immiscible phases to form micron- or submicron-sized droplets (typically O/W emulsions) within a microfluidic device, followed by liposome formation in the droplets. Davies et al. fabricated phospholipid liposomes from a double-emulsion template generated in a microfluidic 3D flow-focused PDMS droplet generator [137]. The microfluidic droplet method could improve encapsulation efficacy and polydispersity. Continuous flow technology is another simple microfluidic strategy for liposome production, first developed by Jahn et al. [138]. This method uses a hydrodynamic focusing approach to control the formation of liposomes in the particle size range of 50 to 500 nm by modifying the classical ethanol injection method. In this method, lipids are dissolved in isopropanol and then passed through the center of a dual channel containing an aqueous medium. The lipid stream in isopropanol is subsequently mixed to form liposomes [139]. The liposome size is controlled by the lipid concentration in the laminar flow and microfluidic channels. Compared to continuous flow fluid focusing, microfluidic droplets have a larger specific surface area, better control of the size and homogeneity of the formed liposomes, and superior encapsulation efficacy. However, the main drawback of droplet-based microfluidic system methods is the involvement of organic solvents, leading to the degradation of the active ingredients and potential toxicity to the body.
4.2. Smart engineering for liposome preparation
Biomembranes that alter their morphology in response to environmental stimuli is nature's ultimate intelligent molecular systems. The design effort of drug delivery is shifting to innovative drug carriers that release cargo reacting to microenvironment changes caused by internal or external triggers. The marketed liposomes release their drugs at the target without control manner. In contrast, well-designed smart or stimulus-responsive liposomes enable drug release by answering to endogenous or exogenous motivations. Endogenous triggers, such as pH changes, hormone levels, enzyme expression, glucose and redox gradients, etc., are generally associated with pathological features of the disease [140]. While exogenous triggers, including temperature, magnetic field, ultrasound, light and electrical pulses/high energy radiation, can also be used to trigger or enhance drug release in disease areas.
Various stimuli can be utilized for controlled liposomal drug release, but temperature stimulation is often selected as a trigger for its safety [141]. ThermoDox, a temperature-sensitive Doxorubicin liposome developed by Celsion, is a typical example. Its phase III clinical trial failed; however, it offers an experience translating intelligent drug delivery systems [142]. Other smart liposomes, such as pH-triggering [143], enzyme-sensitive [144] and ultrasound-responsive liposomes [145], are attracting increasing attention. Although applying these liposomes remains a challenge, it is believed that more smart liposome platforms will be available soon along with collaboration improvement of multidisciplinary.
5. Discussion and perspectives
Liposomes with high biocompatibility have excellent drug adaptability and protection. As a result, they improve some drugs' druggability and overcome their clinical applications' limitations [10]. Liposomes have undergone long-term exploration and development from concept to clinical application to industrial production. The appearance of various new technology such as PEGylation and ionizable lipids or pathophysiological phenomena of diseases such as enhanced permeability and retention (EPR) effect significantly promotes the development of liposome technology.
The appearance of PEGylation is the first milestone in the development of liposome technology. PEGylation prolongs the blood-circulation time and reduces RES clearance, hindering cellular uptake and endosomal escape [10,146,147]. Since the FDA first approved Doxil® with a PEGylated liposome formulation in 1995, numerous liposome formulations have investigated the PEGylation technique. Whether the siRNA liposome Onpattro® approved for marketing in 2018 or the mRNA liposome vaccine Comiranty® approved for COVID-19 in 2021, the formulation still contains PEGylated lipid materials. However, as PEGylated preparations are used repeatedly, the body can produce antibodies against PEG, which leads to faster blood clearance, termed the accelerated blood clearance (ABC) phenomenon [148,149]. Therefore, if PEGylated preparations are dosed recurrently, the ABC phenomenon is a problem worthy of attention. Cleavable PEGylation represents a potential approach to compromise the ABC effect. E.g., incorporating cysteine (Cys)-cleavable PEG5000 into the 80-100 nm liposomes allowed less liver accumulation than conventional PEGylated liposomes [150]. Modifying other polymers such as hyaluronic acid (HA) and hydroxyethyl L-asparagine could alleviate the ABC effect [151,152]. Zhang et al. reported that HA (5.6kDa)-modified 137 nm liposomes did not demonstrate the ABC effect and increased the liver uptake after repeated injection compared with 120 nm PEGylated liposomes [152]. Nonetheless, PEGylation is still the most effective strategy to combat the “protein corona”. PEG chains grafted onto the surface of liposomes can create steric hindrance and, as a result, significantly inhibits protein adsorption and reduces recognition by macrophages. In addition, PEGylation affects the properties of liposomes, including effects on particle size and zeta potential [153], promoting liposome stability by reducing particle aggregation [154]. PEGylation remains a promising approach to improving the translation of liposome-based carriers.
The EPR effect refers to the phenomenon that nanoparticles with a specific size (a few nanometers to 100 nm) accumulate in tumor tissue [155]. This is because the growth rate of tumors is significantly higher than normal tissues, and there are defects among tumor vascular endothelial cells, contributing to their loose arrangement and high permeability [156] The transendothelial pathway is an effective mechanism of nanoparticle extravasation in tumors [157]. Vascular spaces in tumors have 100-780 nm pores, facilitating nanoparticle accumulation in the tumor [155]. In addition to anatomical features such as vascular structural defects and significant gaps between vascular endothelial cells, the EPR effect is also associated with abundant vascular mediators such as bradykinin [158], nitric oxide [159], carbon monoxide [160], and vascular endothelial growth factor [161]. Due to insufficient lymphatic drainage and low blood flow inside the tumor, nanoparticles will selectively penetrate and remain in the tumor site once they enter. The EPR effect has played a guiding role in developing anti-tumor nanomedicines for a long time. The EPR effect is related to various physicochemical properties of nanoparticles, including size, shape, hardness, and surface properties [155]. However, long-term clinical practical studies found that the EPR effect in animal models has not been well demonstrated in humans [162]. Therefore, EPR effects exhibited in animal models should not be overly relied upon to assess the efficacy of nanomedicines. The EPR effect should be used as a determination index to improve the translation of nanomedicine. Additional methods should be established to enhance the EPR effect, such as boosting blood flow in solid tumors and arterial infusion through tumor-feeding arteries and preparing large animal tumor models [155,162].
The liposome can also load multiple drugs to increase the therapeutic efficacy. Vyxeos®, approved by the FDA in 2017, allows enhanced therapeutic efficacy and decreased risks of employing two drugs alone by co-loading cytarabine and daunorubicin in the liposomes via CombiPlex® technology. Vyxeos® simultaneously encapsulates cytarabine and daunorubicin in a 5:1 molar ratio and is composed of distearoylphosphatidylcholine (DSPC), distearoylphosphatidylglycerol (DSPG), and cholesterol at a 7:2:1 molar ratio [45,47]. The strength of Vyxeos® is 5 units/mL, where 1 unit equals 1.0 mg cytarabine plus 0.44 mg daunorubicin [163]. Combination therapy usually assumes that combining drugs at the maximum tolerated dose could achieve a better therapeutic effect. Nonetheless, using two free drugs directly cannot obtain a sustained synergistic therapeutic effect in practical clinical applications due to the kinetic difference between the drugs. CombiPlex® technology is a unique technology platform that boosts anti-tumor activity by determining the ratio of synergistic drug therapy in vitro, selecting appropriate nanocarriers to maintain this ratio, and enhancing targeted delivery to tumor cells [164]. The launch of Vyxeos®, the first liposomal formulation containing two APIs, potentially satisfies the expectation of multiple drugs in liposomes. Vyxeos® is not only the first clinically validated example of the CombiPlex® platform but also a representative event in the development of liposome technology.
Biomacromolecule drugs have potential merits over small-molecule chemical drugs in disease treatment [165], but their applications are greatly limited due to their significant drawbacks, such as poor stability and modest delivery into cells [87]. For example, due to their powerfully negatively charged backbone [166], gene drugs are difficult to interact with cells to enter cells, while liposomes are expected to be carriers for intracellular gene-drug delivery, especially positive charged liposomes. Traditional liposomes easily enter endosomes through the endosome pathway after being phagocytosed by cells, resulting in the degradation of drugs in the acidic environment of endosomes, dramatically reducing the therapeutic effect of drugs [167]. Achieving endosomal escape is another important direction for the development of liposome technology. Onpattro™, the first siRNA liposomes, used ionizable cationic lipids to prepare liposomes for the first time. Through its unique ionizable properties, it is positively charged in the endosome environment resulting in the appearance of local holes in the endosomal membrane to achieve endosomal escape of siRNA [91]. The launch of Onpattro™ is a breakthrough for genetic medicine from concept to clinical, representing a considerable leap in liposome technology. Ionizable cationic lipids have set off a research boom in new excipients, including two mRNA vaccines approved for marketing in 2021, using ionizable cationic lipids. The liposomal delivery of biological drugs has additional advantages. These advantages include improving the accumulation and long-term retention of biological drugs in target organs or target cells and being able to control drug release to ensure prolonged therapeutic effects, reducing the side effects and toxicity of biological drugs, strengthening the protection of biological drugs and preventing them from being inactivated by the complex internal environment in the body [168]. Generally, liposome and LLP technology has become an indispensable and vital carrier for biological drug delivery.
Liposomes always possess two sites for drug loading. Hydrophilic drugs are encapsulated in the aqueous core, while insoluble drugs are often loaded in the phospholipid bilayer. Previous studies demonstrated that the liposome surface also had the potential to be a drug-loading site. The authors argued that the conjugate of an HA-active drug, such as HA-PTX and HA-oridonin, could self-assemble with drug-encapsulated temperature-sensitive liposomes or other liposomes such as traditional and acid-sensitive liposomes [29,[169], [170], [171], [172]]. The loaded active compounds released after mild high-temperature stimulation targeted tumor cells and microenvironment and inhibited tumor growth. The surface anchoring potentially enriched the drug-carrying sites of liposomes, which is beneficial to combination therapy and can improve the liposome's ability to target the diseased lesion. In addition, liposome preparation has been reformed due to the emergence of microfluidics and intelligent manufacturing. The liposomes prepared by microfluidic technology can precisely control the boundary conditions and regulate various physical parameters according to the demand compared to the former experimental methods. The resulting liposomes are size-uniform and -controllable and demonstrate high encapsulation efficacy. At the same time, smart manufacturing enables liposomes to release their cargo precisely at the target site.
The key to addressing the clinical translation of liposome formulations is technical issues such as scaled synthesis, performance optimization, and performance prediction [143]. Clinical translation relies on consistent, reproducible products. Most liposome formulations used in preclinical or clinical studies are almost always synthesized in small batches, and their scaled-up synthesis always is challenging. Obtaining a consistent and highly reproducible formulation before the clinical trial phase is critical because of the complexity of human disease. In addition, formulations that provide the best performance in animal models are generally candidates for clinical trials in human disease. However, preclinical studies to identify clinical candidates demand systematic design optimization. In conjunction with devices that simulate physiological tissues and conditions, the computational or theoretical modeling could predict liposome performance [173]. Besides, personalized medicine is attracting increasing attention along with nanomedicine development. Liposomal formulations failed in clinical trials due to not reaching the endpoints. Several failures stem from the initial clinical study design, ignoring trends in outliers. Therefore, individualized clinical studies are needed to create a system demonstrating individual variability [174].
Overall, liposomes appear to be the most promising drug delivery system. In the future, we look forward to seeing more biological drugs encapsulated by liposomes, which will be manufactured with a simple technology, successfully marketed, and applied in clinical practice. We also expected that clinic and commercial application of liposomes would stimulate and drive the clinic transformation of other nanomedicine.
Conflicts of interest
The authors declare that there is no conflicts of interest.
Acknowledgements
This study was supported by the National Natural Science Foundation of China (Nos. 81872823 and 82073782), the Shanghai Science and Technology Committee (No. 19430741500), and the Key Laboratory of Modern Chinese Medicine Preparation of Ministry of Education of Jiangxi University of Traditional Chinese Medicine (zdsys-202103)
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajps.2022.11.002.
Contributor Information
Wei Chen, Email: w.chen@cpu.edu.cn.
Wei He, Email: weihe@cpu.edu.cn.
Appendix. Supplementary materials
References
- 1.Bangham AD, Horne RW. Negative staining of phospholipids and their structural modification by surface-active agents as observed in the electron microscope. J Mol Biol. 1964;8(5):660–668. doi: 10.1016/s0022-2836(64)80115-7. [DOI] [PubMed] [Google Scholar]
- 2.Langner M, Kral TE. Liposome-based drug delivery systems. Pol J Pharmacol. 1999;51(3):211–222. [PubMed] [Google Scholar]
- 3.Lasic D. Novel applications of liposomes. Trends Biotechnol. 1998;16(7):307–321. doi: 10.1016/s0167-7799(98)01220-7. [DOI] [PubMed] [Google Scholar]
- 4.Pattni BS, Chupin VV, Torchilin VP. New developments in liposomal drugdelivery. Chem Rev. 2015;115(19):10938–10966. doi: 10.1021/acs.chemrev.5b00046. [DOI] [PubMed] [Google Scholar]
- 5.Abu Lila AS, Ishida T. Liposomal delivery systems: design optimization and current applications. Biol Pharm Bull. 2017;40(1):1–10. doi: 10.1248/bpb.b16-00624. [DOI] [PubMed] [Google Scholar]
- 6.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 7.Goyal P, Goyal K, Kumar SV, Singh A, Katare O, Mishra D. Liposomal drug delivery systems-clinical applications. Acta Pharm. 2005;55(1):1–25. [PubMed] [Google Scholar]
- 8.Al-Jamal WT, Kostarelos K. Liposomes: from a clinically established drug delivery system to a nanoparticle platform for theranostic nanomedicine. Acc Chem Res. 2011;44(10):1094–1104. doi: 10.1021/ar200105p. [DOI] [PubMed] [Google Scholar]
- 9.Lyu Y, Xiao Q, Yin L, Yang L, He W. Potent delivery of an MMP inhibitor to the tumor microenvironment with thermosensitive liposomes for the suppression of metastasis and angiogenesis. Signal Transduct Target Ther. 2019;4:26. doi: 10.1038/s41392-019-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Magar KT, Boafo GF, Li XT, Chen ZJ, He W. Liposome-based delivery of biological drugs. Chin Chem Lett. 2022;33(2):587–596. [Google Scholar]
- 11.Amarnath Sharma. Liposomes in drug delivery: progress and limitations. Int J Pharmaceut. 1997;154(2):123–140. [Google Scholar]
- 12.Kindt JT, Szostak JW, Wang A. Bulk self-assembly of giant, unilamellar vesicles. ACS Nano. 2020;14(11):14627–14634. doi: 10.1021/acsnano.0c03125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Patil Y P, Jadhav S. Novel methods for liposome preparation. Chem Phys Lipids. 2014;177:8–18. doi: 10.1016/j.chemphyslip.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 14.Li M, Du C, Guo N, Teng Y, Meng X, Sun H, et al. Composition design and medical application of liposomes. Eur J Med Chem. 2019;164:640–653. doi: 10.1016/j.ejmech.2019.01.007. [DOI] [PubMed] [Google Scholar]
- 15.Maritim S, Boulas P, Lin Y. Comprehensive analysis of liposome formulation parameters and their influence on encapsulation, stability and drug release in glibenclamide liposomes. Int J Pharm. 2021;592 doi: 10.1016/j.ijpharm.2020.120051. [DOI] [PubMed] [Google Scholar]
- 16.Yuba E. Development of functional liposomes by modification of stimuli-responsive materials and their biomedical applications. J Mater Chem B. 2020;8(6):1093–1107. doi: 10.1039/c9tb02470k. [DOI] [PubMed] [Google Scholar]
- 17.Martinez-Lopez AL, Gonzalez-Navarro CJ, Aranaz P, Vizmanos JL, Irache JM. In vivo testing of mucus-permeating nanoparticles for oral insulin delivery using Caenorhabditis elegans as a model under hyperglycemic conditions. Acta Pharm Sin B. 2021;11(4):989–1002. doi: 10.1016/j.apsb.2021.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Akbarzadeh A, Rezaei-Sadabady R, Davaran S, Joo SW, Zarghami N, Hanifehpour Y, et al. Liposome: classification, preparation, and applications. Nanoscale Res Lett. 2013;8(1):102. doi: 10.1186/1556-276X-8-102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bulbake U, Doppalapudi S, Kommineni N, Khan W. Liposomal formulations in clinical use: an updated review. Pharmaceutics. 2017;9(2):12. doi: 10.3390/pharmaceutics9020012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shah S, Dhawan V, Holm R, Nagarsenker MS, Perrie Y. Liposomes: advancements and innovation in the manufacturing process. Adv Drug Del Rev. 2020;154-155:102–122. doi: 10.1016/j.addr.2020.07.002. [DOI] [PubMed] [Google Scholar]
- 21.Abraham SA, Waterhouse DN, Mayer LD, Cullis PR, Madden TD, Bally MB. The liposomal formulation of doxorubicin. Methods Enzymol. 2005;391:71–97. doi: 10.1016/S0076-6879(05)91004-5. [DOI] [PubMed] [Google Scholar]
- 22.He W., Turkeshi A., Li X., Zhang H. Progress in systemic co-delivery of microRNAs and chemotherapeutics for cancer treatment by using lipid-based nanoparticles. Therapeutic Delivery. 2020;11(9):591–603. doi: 10.4155/tde-2020-0052. [DOI] [PubMed] [Google Scholar]
- 23.Adler-Moore J, Proffitt RT. AmBisome: liposomal formulation, structure, mechanism of action and pre-clinical experience. J Antimicrob Chemother. 2002;49(Suppl 1):21–30. doi: 10.1093/jac/49.suppl_1.21. [DOI] [PubMed] [Google Scholar]
- 24.Anselmo C, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou X, Zaks T, Langer R, Dong Y. Lipid nanoparticles for mRNA delivery. Nat Rev Mater. 2021;6(12):1078–1094. doi: 10.1038/s41578-021-00358-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zhang Y, Almazi G, Ong X, Johansen D, Ledger S, Traini D, et al. Nanoparticle delivery platforms for RNAi therapeutics targeting COVID-19 disease in the respiratory tract. Int J Mol Sci. 2022;23(5):2408. doi: 10.3390/ijms23052408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2021;71(3):209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 28.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer statistics, 2022. CA Cancer J Clin. 2022;72(1):7–33. doi: 10.3322/caac.21708. [DOI] [PubMed] [Google Scholar]
- 29.Xiao Q, Li X, Liu C, Jiang Y, He Y, Zhang W, et al. Improving cancer immunotherapy via co-delivering checkpoint blockade and thrombospondin-1 downregulator. Acta Pharm Sin B. 2022 doi: 10.1016/j.apsb.2022.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Y, Wang J, Zhu D, Wang Y, Qing G, Zhang Y, et al. Effect of physicochemical properties on in vivo fate of nanoparticle-based cancer immunotherapies. Acta Pharm Sin B. 2021;11(4):886–902. doi: 10.1016/j.apsb.2021.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rivankar S. An overview of doxorubicin formulations in cancer therapy. J Cancer Res Ther. 2014;10(4):853–858. doi: 10.4103/0973-1482.139267. [DOI] [PubMed] [Google Scholar]
- 32.Tacar O, Sriamornsak P, Dass CR. Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systems. J Pharm Pharmacol. 2013;65(2):157–170. doi: 10.1111/j.2042-7158.2012.01567.x. [DOI] [PubMed] [Google Scholar]
- 33.Rizzardini G, Pastecchia C, Vigevani GM, Milella AM, Cremoni L, Milazzo F. Stealth liposomal doxorubicin or bleomycin/vincristine for the treatment of AIDS-related Kaposi's sarcoma. J Acquir Immune Defic Syndr Hum Retroviro. 1997;14(4):17. [Google Scholar]
- 34.Neale MH, Lamont A, Hindley A, Kurbacher CM, Cree IA. The ex vivo effect of high concentrations of doxorubicin on recurrent ovarian carcinoma. Anticancer Drugs. 2000;11(10):865–871. doi: 10.1097/00001813-200011000-00011. [DOI] [PubMed] [Google Scholar]
- 35.Gabizon A, Goren D, Cohen R, Barenholz Y. Development of liposomal anthracyclines: from basics to clinical applications. J Control Release. 1998;53(1-3):275–279. doi: 10.1016/s0168-3659(97)00261-7. [DOI] [PubMed] [Google Scholar]
- 36.Yang Y, Zuo S, Li L, Kuang X, Li J, Sun B, et al. Iron-doxorubicin prodrug loaded liposome nanogenerator programs multimodal ferroptosis for efficient cancer therapy. Asian J Pharm Sci. 2021;16(6):784–793. doi: 10.1016/j.ajps.2021.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Bauknecht T. Caelyx (R) in the treatment of advanced ovarian cancer. Onkologie. 2000;23:40–45. [Google Scholar]
- 38.Cattel L, Ceruti M, Dosio F. From conventional to stealth liposomes: a new frontier in cancer chemotherapy. J Chemotherapy. 2004;16(Supplement-1):94–97. doi: 10.1179/joc.2004.16.Supplement-1.94. [DOI] [PubMed] [Google Scholar]
- 39.Park JW. Liposome-based drug delivery in breast cancer treatment. Breast Cancer Res. 2002;4(3):93–97. doi: 10.1186/bcr432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gabizon A, Isacson R, Libson E, Kaufman B, Uziely B, Catane R, et al. Clinical studies of liposome-encapsulated doxorubicin. Acta Oncol. 1994;33(7):779–786. doi: 10.3109/02841869409083948. [DOI] [PubMed] [Google Scholar]
- 41.Zhang R, Zhang Y, Zhang Y, Wang X, Gao X, Liu Y, et al. Ratiometric delivery of doxorubicin and berberine by liposome enables superior therapeutic index than Doxil(R) Asian J Pharm Sci. 2020;15(3):385–396. doi: 10.1016/j.ajps.2019.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leonard RCF, Williams S, Tulpule A, Levine AM, Oliveros S. Improving the therapeutic index of anthracycline chemotherapy: focus on liposomal doxorubicin (Myocet (TM)) Breast. 2009;18(4):218–224. doi: 10.1016/j.breast.2009.05.004. [DOI] [PubMed] [Google Scholar]
- 43.Kaposi's sarcoma: DaunoXome approved. AIDS Treat News. 1996;(no 246):3–4. [PubMed] [Google Scholar]
- 44.Dawidczyk CM, Kim C, Park JH, Russell LM, Lee KH, Pomper MG, et al. State-of-the-art in design rules for drug delivery platforms: lessons learned from FDA-approved nanomedicines. J Control Release. 2014;187:133–144. doi: 10.1016/j.jconrel.2014.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Krauss AC, Gao X, Li L, Manning ML, Patel P, Fu W, et al. FDA approval summary: (daunorubicin and cytarabine) liposome for injection for the treatment of adults with high-risk acute myeloid leukemia. Clin Cancer Res. 2019;25(9):2685–2690. doi: 10.1158/1078-0432.CCR-18-2990. [DOI] [PubMed] [Google Scholar]
- 46.Alfayez M, Kantarjian H, Kadia T, Ravandi-Kashani F, Daver N. CPX-351 (vyxeos) in AML. Leuk Lymphoma. 2020;61(2):288–297. doi: 10.1080/10428194.2019.1660970. [DOI] [PubMed] [Google Scholar]
- 47.Crommelin DJA, Van Hoogevest P, Storm G. The role of liposomes in clinical nanomedicine development. What now? Now what? J Control Release. 2020;318:256–263. doi: 10.1016/j.jconrel.2019.12.023. [DOI] [PubMed] [Google Scholar]
- 48.Salehi B, Selamoglu Z, Ksenija SM, Pezzani R, Redaelli M, Cho WC, et al. Liposomal cytarabine as cancer therapy: from chemistry to medicine. Biomolecules. 2019;9(12):773. doi: 10.3390/biom9120773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chhikara B S, Parang K. Development of cytarabine prodrugs and delivery systems for leukemia treatment. Expert Opin Drug Deliv. 2010;7(12):1399–1414. doi: 10.1517/17425247.2010.527330. [DOI] [PubMed] [Google Scholar]
- 50.Murry DJ, Blaney SM. Clinical pharmacology of encapsulated sustained-release cytarabine. Ann Pharmacother. 2000;34(10):1173–1178. doi: 10.1345/aph.19347. [DOI] [PubMed] [Google Scholar]
- 51.Dong S, Ma S, Chen H, Tang Z, Song W, Deng M. Nucleobase-crosslinked poly(2-oxazoline) nanoparticles as paclitaxel carriers with enhanced stability and ultra-high drug loading capacity for breast cancer therapy. Asian J Pharm Sci. 2022;17(4):571–582. doi: 10.1016/j.ajps.2022.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang TH, Wang HS, Soong YK. Paclitaxel-induced cell death - where the cell cycle and apoptosis come together. Cancer. 2000;88(11):2619–2628. doi: 10.1002/1097-0142(20000601)88:11<2619::aid-cncr26>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 53.Weaver BA. How taxol/paclitaxel kills cancer cells. Mol Biol Cell. 2014;25(18):2677–2681. doi: 10.1091/mbc.E14-04-0916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Koudelka S, Turanek J. Liposomal paclitaxel formulations. J Control Release. 2012;163(3):322–334. doi: 10.1016/j.jconrel.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 55.Batta B, Trechot F, Cloche V, George J L, Angioi K. Vincristine-induced unilateral ptosis: case report and review of the literature. J Fr Ophtalmol. 2013;36(8):683–686. doi: 10.1016/j.jfo.2013.03.007. [DOI] [PubMed] [Google Scholar]
- 56.Larson RA, Dodge RK, Burns CP, Lee EJ, Stone RM, Schulman P, et al. A five-drug remission induction regimen with intensive consolidation for adults with acute lymphoblastic leukemia: cancer and leukemia group B study 8811. Blood. 1995;85(8):2025–2037. [PubMed] [Google Scholar]
- 57.Silverman JA, Deitcher SR. Marqibo(R) (vincristine sulfate liposome injection) improves the pharmacokinetics and pharmacodynamics of vincristine. Cancer Chemoth Pharm. 2013;71(3):555–564. doi: 10.1007/s00280-012-2042-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Schiller GJ, Damon LE, Stock W, Coutre SE, Hsu P, Prasad L, et al. Marqibo (R), vincristine sulfate liposome injection, for the treatment of advanced, relapsed or refractory philadelphia chromosome-negative (Ph-) acute lymphoblastic leukemia (ALL) in an adolescent young adult (AYA) population. Blood. 2015;126(23):1291. [Google Scholar]
- 59.Shao RG, Cao CX, Zhang H, Kohn KW, Wold MS, Pommier Y. Replication-mediated DNA damage by camptothecin induces phosphorylation of RPA by DNA-dependent protein kinase and dissociates RPA:DNA-PK complexes. EMBO J. 1999;18(5):1397–1406. doi: 10.1093/emboj/18.5.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.De Man FM, Goey AKL, Van Schaik RHN, Mathijssen RHJ, Bins S. Individualization of irinotecan treatment: a review of pharmacokinetics, pharmacodynamics, and pharmacogenetics. Clin Pharmacokinet. 2018;57(10):1229–1254. doi: 10.1007/s40262-018-0644-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhang H. Onivyde for the therapy of multiple solid tumors. Onco Targets Ther. 2016;9:3001–3007. doi: 10.2147/OTT.S105587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Drummond DC, Noble CO, Guo Z, Hong K, Park JW, Kirpotin DB. Development of a highly active nanoliposomal irinotecan using a novel intraliposomal stabilization strategy. Cancer Res. 2006;66(6):3271–3277. doi: 10.1158/0008-5472.CAN-05-4007. [DOI] [PubMed] [Google Scholar]
- 63.Frampton JE. Liposomal irinotecan: a review in metastatic pancreatic adenocarcinoma. Drugs. 2020;80(10):1007–1018. doi: 10.1007/s40265-020-01336-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Plotnick AN. Lipid-based formulations of amphotericin B. J Am Vet Med Assoc. 2000;216(6):838–841. doi: 10.2460/javma.2000.216.838. [DOI] [PubMed] [Google Scholar]
- 65.Lister J. Amphotericin B lipid complex (Abelcet) in the treatment of invasive mycoses: the North American experience. Eur J Haematol Suppl. 1996;57:18–23. doi: 10.1111/j.1600-0609.1996.tb01348.x. [DOI] [PubMed] [Google Scholar]
- 66.Minodier P, Retornaz K, Horelt A, Garnier JM. Liposomal amphotericin B in the treatment of visceral leishmaniasis in immunocompetent patients. Fundam Clin Pharmacol. 2003;17(2):183–188. doi: 10.1046/j.1472-8206.2003.00168.x. [DOI] [PubMed] [Google Scholar]
- 67.Li Z, Perkins W, Cipolla D. Robustness of aerosol delivery of amikacin liposome inhalation suspension using the eFlow(R) technology. Eur J Pharm Biopharm. 2021;166(9):10–18. doi: 10.1016/j.ejpb.2021.05.021. [DOI] [PubMed] [Google Scholar]
- 68.Khan O, Chaudary N. The use of amikacin liposome inhalation suspension (Arikayce) in the treatment of refractory nontuberculous mycobacterial lung disease in adults. Drug Des Devel Ther. 2020;14:2287–2294. doi: 10.2147/DDDT.S146111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hoy SM. Amikacin liposome inhalation suspension in refractory mycobacterium avium complex lung disease: a profile of its use. Clin Drug Investig. 2021;41(4):405–412. doi: 10.1007/s40261-021-01010-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Zhang J, Leifer F, Rose S, Chun DY, Thaisz J, Herr T, et al. Amikacin liposome inhalation suspension (ALIS) penetrates non-tuberculous mycobacterial biofilms and enhances amikacin uptake into macrophages. Front Microbiol. 2018;9:915. doi: 10.3389/fmicb.2018.00915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Horlocker TT, Wedel DJ, Benzon H, Brown DL, Enneking FK, Heit JA, et al. Regional anesthesia in the anticoagulated patient: defining the risks (the second ASRA Consensus Conference on Neuraxial Anesthesia and Anticoagulation) Reg Anesth Pain Med. 2003;28(3):172–197. doi: 10.1053/rapm.2003.50046. [DOI] [PubMed] [Google Scholar]
- 72.Pasero C, Mccaffery M. Extended-release epidural morphine (DepoDur) J Perianesth Nurs. 2005;20(5):345–350. doi: 10.1016/j.jopan.2005.07.004. [DOI] [PubMed] [Google Scholar]
- 73.Alam M, Hartrick CT. Extended-release epidural morphine (DepoDur): an old drug with a new profile. Pain Pract. 2005;5(4):349–353. doi: 10.1111/j.1533-2500.2005.00048.x. [DOI] [PubMed] [Google Scholar]
- 74.Vaughan C. Development and implementation of a process to ensure safe use of morphine sulfate extended-release liposome injection. Am J Health Syst Pharm. 2008;65(5):458–461. doi: 10.2146/ajhp070241. [DOI] [PubMed] [Google Scholar]
- 75.Carvalho B, Riley E, Cohen S E, Gambling D, Palmer C, Huffnagle HJ, et al. Single-dose, sustained-release epidural morphine in the management of postoperative pain after elective cesarean delivery: results of a multicenter randomized controlled study. Anesth Analg. 2005;100(4):1150–1158. doi: 10.1213/01.ANE.0000149544.58230.FF. [DOI] [PubMed] [Google Scholar]
- 76.Kaye AD, Armstead-Williams C, Hyatali F, Cox KS, Kaye RJ, Eng LK, et al. Exparel for postoperative pain management: a comprehensive review. Curr Pain Headache Rep. 2020;24(11):73. doi: 10.1007/s11916-020-00905-4. [DOI] [PubMed] [Google Scholar]
- 77.Tong YC, Kaye AD, Urman RD. Liposomal bupivacaine and clinical outcomes. Best Pract Res Clin Anaesthesiol. 2014;28(1):15–27. doi: 10.1016/j.bpa.2014.02.001. [DOI] [PubMed] [Google Scholar]
- 78.Malik O, Kaye AD, Kaye A, Belani K, Urman RD. Emerging roles of liposomal bupivacaine in anesthesia practice. J Anaesthesiol Clin Pharmacol. 2017;33(2):151–156. doi: 10.4103/joacp.JOACP_375_15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Richard BM, Newton P, Ott LR, Haan D, Brubaker AN, Cole PI, et al. The safety of EXPAREL (R) (bupivacaine liposome injectable suspension) administered by peripheral nerve block in rabbits and dogs. J Drug Deliv. 2012;2012 doi: 10.1155/2012/962101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Richard BM, Rickert DE, Doolittle D, Mize A, Liu J, Lawson CF. Pharmacokinetic compatibility study of lidocaine with EXPAREL in yucatan miniature pigs. ISRN Pharm. 2011;2011 doi: 10.5402/2011/582351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Bakri SJ, Kaiser PK. Verteporfin ocular photodynamic therapy. Expert Opin Pharmaco. 2004;5(1):195–203. doi: 10.1517/14656566.5.1.195. [DOI] [PubMed] [Google Scholar]
- 82.Ichikawa K, Takeuchi Y, Yonezawa S, Hikita T, Kurohane K, Namba Y, et al. Antiangiogenic photodynamic therapy (PDT) using Visudyne causes effective suppression of tumor growth. Cancer Lett. 2004;205(1):39–48. doi: 10.1016/j.canlet.2003.10.001. [DOI] [PubMed] [Google Scholar]
- 83.Zhang Y, Sun T, Jiang C. Biomacromolecules as carriers in drug delivery and tissue engineering. Acta Pharm Sin B. 2018;8(1):34–50. doi: 10.1016/j.apsb.2017.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Teng C, Li B, Lin C, Xing X, Huang F, Yang Y, et al. Targeted delivery of baicalein-p53 complex to smooth muscle cells reverses pulmonary hypertension. J Control Release. 2022;341:591–604. doi: 10.1016/j.jconrel.2021.12.006. [DOI] [PubMed] [Google Scholar]
- 85.Du X, Hou Y, Huang J, Pang Y, Ruan C, Wu W, et al. Cytosolic delivery of the immunological adjuvant Poly I:C and cytotoxic drug crystals via a carrier-free strategy significantly amplifies immune response. Acta Pharm Sin B. 2021;11(10):3272–3285. doi: 10.1016/j.apsb.2021.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Simos YV, Spyrou K, Patila M, Karouta N, Stamatis H, Gournis D, et al. Trends of nanotechnology in type 2 diabetes mellitus treatment. Asian J Pharm Sci. 2021;16:62–76. doi: 10.1016/j.ajps.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.He W, Xing XY, Wang XL, Wu D, Wu W, Guo JL, et al. Nanocarrier-mediated cytosolic delivery of biopharmaceuticals. Adv Funct Mater. 2020;30(37) [Google Scholar]
- 88.Viana IMO, Roussel S, Defrene J, Lima EM, Barabe F, Bertrand N. Innate and adaptive immune responses toward nanomedicines. Acta Pharm Sin B. 2021;11(4):852–870. doi: 10.1016/j.apsb.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lamb YN, Deeks ED. Tafamidis: a review in transthyretin amyloidosis with polyneuropathy. Drugs. 2019;79(8):863–874. doi: 10.1007/s40265-019-01129-6. [DOI] [PubMed] [Google Scholar]
- 90.Hawkins PN, Ando Y, Dispenzeri A, Gonzalez-Duarte A, Adams D, Suhr OB. Evolving landscape in the management of transthyretin amyloidosis. Ann Med. 2015;47(8):625–638. doi: 10.3109/07853890.2015.1068949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Urits I, Swanson D, Swett MC, Patel A, Berardino K, Amgalan A, et al. Correction to: a review of Patisiran (ONPATTRO(R)) for the treatment of polyneuropathy in people with hereditary transthyretin amyloidosis. Neurol Ther. 2021;10(1):407. doi: 10.1007/s40120-020-00228-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kristen AV, Ajroud-Driss S, Conceicao I, Gorevic P, Kyriakides T, Patisiran Obici L. an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener Dis Manag. 2019;9(1):5–23. doi: 10.2217/nmt-2018-0033. [DOI] [PubMed] [Google Scholar]
- 93.Setten RL, Rossi JJ, Han SP. The current state and future directions of RNAi-based therapeutics. Nat Rev Drug Discov. 2019;18(6):421–446. doi: 10.1038/s41573-019-0017-4. [DOI] [PubMed] [Google Scholar]
- 94.Buschmann MD, Carrasco MJ, Alishetty S, Paige M, Alameh MG, Weissman D. Nanomaterial delivery systems for mRNA vaccines. Vaccines (Basel) 2021;9(1):65. doi: 10.3390/vaccines9010065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Alphandery E, Grand-Dewyse P, Lefevre R, Mandawala C, Durand-Dubief M. Cancer therapy using nanoformulated substances: scientific, regulatory and financial aspects. Expert Rev Anticancer Ther. 2015;15(10):1233–1255. doi: 10.1586/14737140.2015.1086647. [DOI] [PubMed] [Google Scholar]
- 96.Zarkovic M, Qin X, Nakatsuru Y, Zhang S, Yamazaki Y, Oda H, et al. Inhibitory effect of probucol on benzo[a]pyrene induced lung tumorigenesis. Carcinogenesis. 1995;16(10):2599–2601. doi: 10.1093/carcin/16.10.2599. [DOI] [PubMed] [Google Scholar]
- 97.Bovier PA. Epaxal: a virosomal vaccine to prevent hepatitis A infection. Expert Rev Vaccines. 2008;7(8):1141–1150. doi: 10.1586/14760584.7.8.1141. [DOI] [PubMed] [Google Scholar]
- 98.Zylberberg C, Matosevic S. Pharmaceutical liposomal drug delivery: a review of new delivery systems and a look at the regulatory landscape. Drug Deliv. 2016;23(9):3319–3329. doi: 10.1080/10717544.2016.1177136. [DOI] [PubMed] [Google Scholar]
- 99.Dagan R, Amir J, Livni G, Greenberg D, Abu-Abed J, Guy L, et al. Concomitant administration of a virosome-adjuvanted hepatitis a vaccine with routine childhood vaccines at age twelve to fifteen months: a randomized controlled trial. Pediatr Infect Dis J. 2007;26(9):787–793. doi: 10.1097/INF.0b013e318060acbd. [DOI] [PubMed] [Google Scholar]
- 100.Kunzi V, Dornseiff M, Horwath J, Hartmann K. Safe vaccination of children with a virosomal adjuvanted influenza vaccine. Vaccine. 2009;27(8):1261–1265. doi: 10.1016/j.vaccine.2008.12.008. [DOI] [PubMed] [Google Scholar]
- 101.Xu S, Yang K, Li R, Zhang L. mRNA Vaccine era-mechanisms, drug platform and clinical prospection. Int J Mol Sci. 2020;21(18):6582. doi: 10.3390/ijms21186582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Alving CR, Beck Z, Matyas GR, Rao M. Liposomal adjuvants for human vaccines. Expert Opin Drug Deliv. 2016;13(6):807–816. doi: 10.1517/17425247.2016.1151871. [DOI] [PubMed] [Google Scholar]
- 103.Beck Z, Matyas GR, Alving CR. Detection of liposomal cholesterol and monophosphoryl lipid A by QS-21 saponin and Limulus polyphemus amebocyte lysate. Biochim Biophys Acta. 2015;1848(3):775–780. doi: 10.1016/j.bbamem.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 104.Syed YY. Recombinant zoster vaccine (Shingrix (R)): a review in herpes zoster. Drugs Aging. 2018;35(12):1031–1040. doi: 10.1007/s40266-018-0603-x. [DOI] [PubMed] [Google Scholar]
- 105.Cunningham AL, Lal H, Kovac M, Chlibek R, Hwang SJ, Diez-Domingo J, et al. Efficacy of the herpes zoster subunit vaccine in adults 70 years of age or older. N Engl J Med. 2016;375(11):1019–1032. doi: 10.1056/NEJMoa1603800. [DOI] [PubMed] [Google Scholar]
- 106.Lopez-Cantu DO, Wang X, Carrasco-Magallanes H, Afewerki S, Zhang X, Bonventre JV, et al. From bench to the clinic: the path to translation of nanotechnology-enabled mRNA SARS-CoV-2 vaccines. Nanomicro Lett. 2022;14(1):41. doi: 10.1007/s40820-021-00771-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Zhu Y, Li J, Pang Z. Recent insights for the emerging COVID-19: drug discovery, therapeutic options and vaccine development. Asian J Pharm Sci. 2021;16(1):4–23. doi: 10.1016/j.ajps.2020.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Li JJ, Zhang K, Wu D, Ren LJ, Chu XY, Qin C, et al. Liposomal remdesivir inhalation solution for targeted lung delivery as a novel therapeutic approach for COVID-19. Asian J Pharm Sci. 2021;16(6):772–783. doi: 10.1016/j.ajps.2021.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chung JY, Thone MN, Kwon YJ. COVID-19 vaccines: the status and perspectives in delivery points of view. Adv Drug Deliv Rev. 2021;170:1–25. doi: 10.1016/j.addr.2020.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Park KS, Sun X, Aikins ME, Moon JJ. Non-viral COVID-19 vaccine delivery systems. Adv Drug Deliv Rev. 2021;169:137–151. doi: 10.1016/j.addr.2020.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Shin MD, Shukla S, Chung YH, Beiss V, Chan SK, Ortega-Rivera OA, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nat Nanotechnol. 2020;15(8):646–655. doi: 10.1038/s41565-020-0737-y. [DOI] [PubMed] [Google Scholar]
- 112.Pascolo S. Synthetic messenger RNA-based vaccines: from scorn to hype. Viruses. 2021;13(2):270. doi: 10.3390/v13020270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Polack FP, Thomas SJ, Kitchin N, Absalon J, Gurtman A, Lockhart S, et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med. 2020;383(27):2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Baden LR, El Sahly HM, Essink B, Kotloff K, Frey S, Novak R, et al. Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med. 2021;384(5):403–416. doi: 10.1056/NEJMoa2035389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Fathizadeh H, Afshar S, Masoudi M R, Gholizadeh P, Asgharzadeh M, Ganbarov K, et al. SARS-CoV-2 (Covid-19) vaccines structure, mechanisms and effectiveness: a review. Int J Biol Macromol. 2021;188:740–750. doi: 10.1016/j.ijbiomac.2021.08.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Huang L, Rong Y, Pan Q, Yi K, Tang X, Zhang Q, et al. SARS-CoV-2 vaccine research and development: conventional vaccines and biomimetic nanotechnology strategies. Asian J Pharm Sci. 2021;16(2):136–146. doi: 10.1016/j.ajps.2020.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tan L, Sun X. Recent advances in mRNA vaccine delivery. Nano Res. 2018;11(10):5338–5354. [Google Scholar]
- 118.Huang J, Yuen D, Mintern JD, Johnston APR. Opportunities for innovation: building on the success of lipid nanoparticle vaccines. Curr Opin Colloid Interface Sci. 2021;55 doi: 10.1016/j.cocis.2021.101468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hafez IM, Maurer N, Cullis PR. On the mechanism whereby cationic lipids promote intracellular delivery of polynucleic acids. Gene Ther. 2001;8(15):1188–1196. doi: 10.1038/sj.gt.3301506. [DOI] [PubMed] [Google Scholar]
- 120.Sawant RR, Torchilin VP. Challenges in development of targeted liposomal therapeutics. AAPS J. 2012;14(2):303–315. doi: 10.1208/s12248-012-9330-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Fraguas-Sanchez AI, Lozza I, Torres-Suarez AI. Actively targeted nanomedicines in breast cancer: from pre-clinal investigation to clinic. Cancers. 2022;14(5):1198. doi: 10.3390/cancers14051198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Anselmo AC, Mitragotri S. Nanoparticles in the clinic: an update. Bioeng Transl Med. 2019;4(3):e10143. doi: 10.1002/btm2.10143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Dumont N, Merrigan S, Turpin J, Lavoie C, Papavasiliou V, Geretti E, et al. Nanoliposome targeting in breast cancer is influenced by the tumor microenvironment. Nanomed-nanotechnol. 2019;17:71–81. doi: 10.1016/j.nano.2018.12.010. [DOI] [PubMed] [Google Scholar]
- 124.Lee H, Shields AF, Siegel BA, Miller KD, Krop I, Ma CX, et al. (64)Cu-MM-302 Positron emission tomography quantifies variability of enhanced permeability and retention of nanoparticles in relation to treatment response in patients with metastatic breast cancer. Clin Cancer Res. 2017;23(15):4190–4202. doi: 10.1158/1078-0432.CCR-16-3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Makwana V, Karanjia J, Haselhorst T, Anoopkumar-Dukie S, Rudrawar S. Liposomal doxorubicin as targeted delivery platform: current trends in surface functionalization. Int J Pharm. 2021;593:25. doi: 10.1016/j.ijpharm.2020.120117. [DOI] [PubMed] [Google Scholar]
- 126.Miao L, Zhang Y, Huang L. mRNA vaccine for cancer immunotherapy. Mol Cancer. 2021;20(1):41. doi: 10.1186/s12943-021-01335-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi: 10.1038/nrd.2017.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sahin U, Oehm P, Derhovanessian E, Jabulowsky RA, Vormehr M, Gold M, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi: 10.1038/s41586-020-2537-9. [DOI] [PubMed] [Google Scholar]
- 129.Guo YG, Lei KW, Tang L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front Immunol. 2018;9 doi: 10.3389/fimmu.2018.01499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lau WY, Lai ECH. The current role of radiofrequency ablation in the management of hepatocellular carcinoma a systematic review. Ann Surg. 2009;249(1):20–25. doi: 10.1097/SLA.0b013e31818eec29. [DOI] [PubMed] [Google Scholar]
- 131.Dou Y, Hynynen K, Allen C. To heat or not to heat: challenges with clinical translation of thermosensitive liposomes. J Control Release. 2017;249:63–73. doi: 10.1016/j.jconrel.2017.01.025. [DOI] [PubMed] [Google Scholar]
- 132.Wurz GT, Kao CJ, Wolf M, Degregorio MW. Tecemotide: an antigen-specific cancer immunotherapy. Hum Vaccin Immunother. 2014;10(11):3383–3393. doi: 10.4161/hv.29836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Has C, Sunthar P. A comprehensive review on recent preparation techniques of liposomes. J Liposome Res. 2020;30(4):336–365. doi: 10.1080/08982104.2019.1668010. [DOI] [PubMed] [Google Scholar]
- 134.van Swaay D, demello A. Microfluidic methods for forming liposomes. Lab Chip. 2013;13(5):752–767. doi: 10.1039/c2lc41121k. [DOI] [PubMed] [Google Scholar]
- 135.Andra VVSNL, Pammi SVN, Bhatraju LVKP, Ruddaraju LK. A comprehensive review on novel liposomal methodologies, commercial formulations, clinical trials and patents. Bionanoscience. 2022;12(1):274–291. doi: 10.1007/s12668-022-00941-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Balbino TA, Aoki NT, Gasperini AAM, Oliveira CLP, Azzoni AR, Cavalcanti LP, et al. Continuous flow production of cationic liposomes at high lipid concentration in microfluidic devices for gene delivery applications. Chem Eng J. 2013;226:423–433. [Google Scholar]
- 137.Davies RT, Kim D, Park J. Formation of liposomes using a 3D flow focusing microfluidic device with spatially patterned wettability by corona discharge. J Micromech Microeng. 2012;22(5) [Google Scholar]
- 138.Jahn A, Vreeland WN, Devoe DL, Locascio LE, Gaitan M. Microfluidic directed formation of liposomes of controlled size. Langmuir. 2007;23(11):6289–6293. doi: 10.1021/la070051a. [DOI] [PubMed] [Google Scholar]
- 139.Jahn A, Stavis SM, Hong JS, Vreeland WN, Devoe DL, Gaitan M. Microfluidic mixing and the formation of nanoscale lipid vesicles. ACS Nano. 2010;4(4):2077–2087. doi: 10.1021/nn901676x. [DOI] [PubMed] [Google Scholar]
- 140.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 141.Kono K, Ozawa T, Yoshida T, Ozaki F, Ishizaka Y, Maruyama K, et al. Highly temperature-sensitive liposomes based on a thermosensitive block copolymer for tumor-specific chemotherapy. Biomaterials. 2010;31(27):7096–7105. doi: 10.1016/j.biomaterials.2010.05.045. [DOI] [PubMed] [Google Scholar]
- 142.Liu D, Yang F, Xiong F, Gu N. The smart drug delivery system and its clinical potential. Theranostics. 2016;6(9):1306–1323. doi: 10.7150/thno.14858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Simoes S, Moreira JN, Fonseca C, Duzgunes N, De Lima MC. On the formulation of pH-sensitive liposomes with long circulation times. Adv Drug Deliv Rev. 2004;56(7):947–965. doi: 10.1016/j.addr.2003.10.038. [DOI] [PubMed] [Google Scholar]
- 144.Wan Y, Han JF, Fan GR, Zhang ZR, Gong T, Sun X. Enzyme-responsive liposomes modified adenoviral vectors for enhanced tumor cell transduction and reduced immunogenicity. Biomaterials. 2013;34(12):3020–3030. doi: 10.1016/j.biomaterials.2012.12.051. [DOI] [PubMed] [Google Scholar]
- 145.Yudina A, De Smet M, Lepetit-Coiffe M, Langereis S, Van Ruijssevelt L, Smirnov P, et al. Ultrasound-mediated intracellular drug delivery using microbubbles and temperature-sensitive liposomes. J Control Release. 2011;155(3):442–448. doi: 10.1016/j.jconrel.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 146.Yaghmur A, Mu H. Recent advances in drug delivery applications of cubosomes, hexosomes, and solid lipid nanoparticles. Acta Pharm Sin B. 2021;11(4):871–885. doi: 10.1016/j.apsb.2021.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mazumdar S, Chitkara D, Mittal A. Exploration and insights into the cellular internalization and intracellular fate of amphiphilic polymeric nanocarriers. Acta Pharm Sin B. 2021;11(4):903–924. doi: 10.1016/j.apsb.2021.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Park K. Impact of anti-PEG antibodies on PEGylated nanoparticles fate in vivo. J Control Release. 2018;287:257. doi: 10.1016/j.jconrel.2018.09.014. [DOI] [PubMed] [Google Scholar]
- 149.Park H, Otte A, Park K. Evolution of drug delivery systems: from 1950 to 2020 and beyond. J Control Release. 2021;342:53–65. doi: 10.1016/j.jconrel.2021.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Kuai R, Yuan WM, Li WY, Qin Y, Tang J, Yuan MQ, et al. Targeted delivery of cargoes into a murine solid tumor by a cell-penetrating peptide and cleavable poly(ethylene glycol) comodified liposomal delivery system via systemic administration. Mol Pharmaceut. 2011;8(6):2151–2161. doi: 10.1021/mp200100f. [DOI] [PubMed] [Google Scholar]
- 151.Romberg B, Oussoren C, Snel CJ, Carstens MG, Hennink WE, Storm G. Pharmacokinetics of poly(hydroxyethyl-l-asparagine)-coated liposomes is superior over that of PEG-coated liposomes at low lipid dose and upon repeated administration. Biochim Biophys Acta. 2007;1768(3):737–743. doi: 10.1016/j.bbamem.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 152.Zhang Q, Deng C, Fu Y, Sun X, Gong T, Zhang Z. Repeated administration of hyaluronic acid coated liposomes with improved pharmacokinetics and reduced immune response. Mol Pharm. 2016;13(6):1800–1808. doi: 10.1021/acs.molpharmaceut.5b00952. [DOI] [PubMed] [Google Scholar]
- 153.Ryals RC, Patel S, Acosta C, Mckinney M, Pennesi ME, Sahay G. The effects of PEGylation on LNP based mRNA delivery to the eye. PLoS One. 2020;15(10) doi: 10.1371/journal.pone.0241006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Knop K, Hoogenboom R, Fischer D, Schubert US. Poly(ethylene glycol) in drug delivery: pros and cons as well as potential alternatives. Angew Chem Int Ed Engl. 2010;49(36):6288–6308. doi: 10.1002/anie.200902672. [DOI] [PubMed] [Google Scholar]
- 155.Ikeda-Imafuku M, Wang LL, Rodrigues D, Shaha S, Zhao Z, Mitragotri S. Strategies to improve the EPR effect: a mechanistic perspective and clinical translation. J Control Release. 2022;345:512–536. doi: 10.1016/j.jconrel.2022.03.043. [DOI] [PubMed] [Google Scholar]
- 156.Maeda H, Wu J, Sawa T, Matsumura Y, Hori K. Tumor vascular permeability and the EPR effect in macromolecular therapeutics: a review. J Control Release. 2000;65(1-2):271–284. doi: 10.1016/s0168-3659(99)00248-5. [DOI] [PubMed] [Google Scholar]
- 157.Sindhwani S, Syed AM, Ngai J, Kingston BR, Maiorino L, Rothschild J, et al. The entry of nanoparticles into solid tumours. Nat Mater. 2020;19(5):566–575. doi: 10.1038/s41563-019-0566-2. [DOI] [PubMed] [Google Scholar]
- 158.Fang J, Islam W, Maeda H. Exploiting the dynamics of the EPR effect and strategies to improve the therapeutic effects of nanomedicines by using EPR effect enhancers. Adv Drug Deliv Rev. 2020;157:142–160. doi: 10.1016/j.addr.2020.06.005. [DOI] [PubMed] [Google Scholar]
- 159.Maeda H. The link between infection and cancer: tumor vasculature, free radicals, and drug delivery to tumors via the EPR effect. Cancer Sci. 2013;104(7):779–789. doi: 10.1111/cas.12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Fang J, Islam R, Islam W, Yin H, Subr V, Etrych T, et al. Augmentation of EPR effect and efficacy of anticancer nanomedicine by carbon monoxide generating agents. Pharmaceutics. 2019;11(7):343. doi: 10.3390/pharmaceutics11070343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Fang J, Nakamura H, Maeda H. The EPR effect: unique features of tumor blood vessels for drug delivery, factors involved, and limitations and augmentation of the effect. Adv Drug Deliv Rev. 2011;63(3):136–151. doi: 10.1016/j.addr.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 162.Wu J. The enhanced permeability and retention (EPR) effect: the significance of the concept and methods to enhance its application. J Pers Med. 2021;11(8):771. doi: 10.3390/jpm11080771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Tzogani K, Penttila K, Lapvetelainen T, Hemmings R, Koenig J, Freire J, et al. EMA review of daunorubicin and cytarabine encapsulated in liposomes (Vyxeos, CPX-351) for the treatment of adults with newly diagnosed, therapy-related acute myeloid leukemia or acute myeloid leukemia with myelodysplasia-related changes. Oncologist. 2020;25(9):e1414–e1420. doi: 10.1634/theoncologist.2019-0785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tolcher AW, Mayer LD. Improving combination cancer therapy: the CombiPlex((R)) development platform. Future Oncol. 2018;14(13):1317–1332. doi: 10.2217/fon-2017-0607. [DOI] [PubMed] [Google Scholar]
- 165.Xiao Q, Li X, Li Y, Wu Z, Xu C, Chen Z, et al. Biological drug and drug delivery-mediated immunotherapy. Acta Pharm Sin B. 2021;11(4):941–960. doi: 10.1016/j.apsb.2020.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Shim MS, Kwon YJ. Stimuli-responsive polymers and nanomaterials for gene delivery and imaging applications. Adv Drug Deliv Rev. 2012;64(11):1046–1059. doi: 10.1016/j.addr.2012.01.018. [DOI] [PubMed] [Google Scholar]
- 167.Rayamajhi S, Marchitto J, Nguyen TDT, Marasini R, Celia C, Aryal S. pH-responsive cationic liposome for endosomal escape mediated drug delivery. Colloids Surf B Biointerfaces. 2020;188 doi: 10.1016/j.colsurfb.2020.110804. [DOI] [PubMed] [Google Scholar]
- 168.Antimisiaris SG, Marazioti A, Kannavou M, Natsaridis E, Gkartziou F, Kogkos G, et al. Overcoming barriers by local drug delivery with liposomes. Adv Drug Deliv Rev. 2021;174:53–86. doi: 10.1016/j.addr.2021.01.019. [DOI] [PubMed] [Google Scholar]
- 169.Lv Y, Xu C, Zhao X, Lin C, Yang X, Xin X, et al. Nanoplatform assembled from a CD44-targeted prodrug and smart liposomes for dual targeting of tumor microenvironment and cancer cells. ACS Nano. 2018;12(2):1519–1536. doi: 10.1021/acsnano.7b08051. [DOI] [PubMed] [Google Scholar]
- 170.Boafo GF, Shi Y, Xiao Q, Magar KT, Zoulikha M, Xing X, et al. Targeted co-delivery of daunorubicin and cytarabine based on the hyaluronic acid prodrug modified liposomes. Chin Chem Lett. 2022;33(10):4600–4604. [Google Scholar]
- 171.Li XT, Gu JY, Xiao QQ, Liu Y, Zhou P, Fan LF, et al. Liposomal codelivery of inflammation inhibitor and collagen protector to the plaque for effective anti-atherosclerosis. Chin Chem Lett. 2023;34(1) [Google Scholar]
- 172.Xiao QQ, Li XT, Liu C, Yang Y, Hou YQ, Wang Y, et al. Liposome-based anchoring and core-encapsulation for combinatorial cancer therapy. Chin Chem Lett. 2022;33(9):4191–4196. [Google Scholar]
- 173.Anselmo AC, Mitragotri S. A chemical engineering perspective of nanoparticle-based targeted drug delivery: a unit process approach. Aiche J. 2016;62(4):966–974. [Google Scholar]
- 174.Li M, KT Al-Jamal, Kostarelos K, Reineke J. Physiologically based pharmacokinetic modeling of nanoparticles. ACS Nano. 2010;4(11):6303–6317. doi: 10.1021/nn1018818. [DOI] [PubMed] [Google Scholar]
- 175.Hassett K J, Benenato K E, Jacquinet E, Lee A, Woods A, Yuzhakov O, et al. Optimization of lipid nanoparticles for intramuscular administration of mRNA vaccines. Mol Ther Nucleic Acids. 2019;15:1–11. doi: 10.1016/j.omtn.2019.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Neurohr C, Kneidinger N, Ghiani A, Monforte V, Knoop C, Jaksch P, et al. A randomized controlled trial of liposomal cyclosporine A for inhalation in the prevention of bronchiolitis obliterans syndrome following lung transplantation. Am J Transplant. 2022;22(1):222–229. doi: 10.1111/ajt.16858. [DOI] [PubMed] [Google Scholar]
- 177.Seetharamu N, Kim E, Hochster H, Martin F, Muggia F. Phase II study of liposomal cisplatin (SPI-77) in platinum-sensitive recurrences of ovarian cancer. Anticancer Res. 2010;30(2):541–545. [PubMed] [Google Scholar]
- 178.Pal A, Khan S, Wang YF, Kamath N, Sarkar AK, Ahmad A, et al. Preclinical safety, pharmacokinetics and antitumor efficacy profile of liposome-entrapped SN-38 formulation. Anticancer Res. 2005;25(1a):331–341. [PubMed] [Google Scholar]
- 179.Fetterly GJ, Grasela TH, Sherman JW, Dul JL, Grahn A, Lecomte D, et al. Pharmacokinetic/pharmacodynamic modeling and simulation of neutropenia during phase I development of liposome-entrapped paclitaxel. Clin Cancer Res. 2008;14(18):5856–5863. doi: 10.1158/1078-0432.CCR-08-1046. [DOI] [PubMed] [Google Scholar]
- 180.Mitchell P, Thatcher N, Socinski MA, Wasilewska-Tesluk E, Horwood K, Szczesna A, et al. Tecemotide in unresectable stage III non-small-cell lung cancer in the phase III START study: updated overall survival and biomarker analyses. Ann Oncol. 2015;26(6):1134–1142. doi: 10.1093/annonc/mdv104. [DOI] [PubMed] [Google Scholar]
- 181.Eichhorn ME, Ischenko I, Luedemann S, Strieth S, Papyan A, Werner A, et al. Vascular targeting by EndoTAG (TM)-1 enhances therapeutic efficacy of conventional chemotherapy in lung and pancreatic cancer. Int J Cancer. 2010;126(5):1235–1245. doi: 10.1002/ijc.24846. [DOI] [PubMed] [Google Scholar]
- 182.Chen L, Cheng WL, Wang Y, Ke YN, Fan SY, Pan L, et al. Alprostadil liposome microsphere preparation stabilizes vascular plaques and inhibits intra-plaque inflammation. Chinese Med J-peking. 2012;125(24):4380–4385. [PubMed] [Google Scholar]
- 183.Wetzler M, Thomas DA, Wang ES, Shepard R, Ford LA, Heffner TL, et al. Phase I/II trial of nanomolecular liposomal annamycin in adult patients with relapsed/refractory acute lymphoblastic leukemia. Cl Lymph Myelom Leu. 2013;13(4):430–434. doi: 10.1016/j.clml.2013.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mamot C, Ritschard R, Wicki A, Stehle G, Dieterle T, Bubendorf L, et al. Tolerability, safety, pharmacokinetics, and efficacy of doxorubicin-loaded anti-EGFR immunoliposomes in advanced solid tumours: a phase 1 dose-escalation study. Lancet Oncol. 2012;13(12):1234–1241. doi: 10.1016/S1470-2045(12)70476-X. [DOI] [PubMed] [Google Scholar]
- 185.Barbier AJ, Jiang AYJ, Zhang P, Wooster R, Anderson DG. The clinical progress of mRNA vaccines and immunotherapies. Nat Biotechnol. 2022;40(6):840–854. doi: 10.1038/s41587-022-01294-2. [DOI] [PubMed] [Google Scholar]
- 186.Zhang Z, Yao S, Hu Y, Zhao X, Lee RJ. Application of lipid-based nanoparticles in cancer immunotherapy. Front Immunol. 2022;13 doi: 10.3389/fimmu.2022.967505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Sarker D, Plummer R, Meyer T, Sodergren MH, Basu B, Chee CE, et al. MTL-CEBPA, a small activating RNA therapeutic upregulating C/EBP-a, in patients with advanced liver cancer: a first-in-human, multicenter, open-label, phase I trial. Clin Cancer Res. 2020;26(15):3936–3946. doi: 10.1158/1078-0432.CCR-20-0414. [DOI] [PubMed] [Google Scholar]
- 188.Hess D, Colombo I, Haefliger S, Bastian S, Rabaglio M, Metaxas Y, et al. 575P TLD-1, a novel liposomal doxorubicin, in patients (pts) with advanced solid tumours: dose escalation part of a multicenter open-label phase I trial (SAKK 65/16) Ann Oncol. 2020;31(4):S490. [Google Scholar]
- 189.August A, Attarwala HZ, Himansu S, Kalidindi S, Lu S, Pajon R, et al. A phase 1 trial of lipid-encapsulated mRNA encoding a monoclonal antibody with neutralizing activity against Chikungunya virus. Nat Med. 2021;27(12):2224–2233. doi: 10.1038/s41591-021-01573-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.







