Abstract
The bispectral index (BIS) is an attractive approach for monitoring level of consciousness in critically ill patients, particularly during paralysis, when commonly used sedation scales cannot be used. As a first step towards establishing the utility of BIS during paralysis, this review examines the strength of correlation between BIS and clinical sedation scales in a broad population of non-paralyzed, critically ill adults.
We included studies evaluating the strength of correlation between concurrent assessments of BIS and Richmond Agitation Sedation Scale (RASS), Ramsay Sedation Scale (RSS), or Sedation Agitation Scale (SAS) in critically ill adult patients. Studies involving assessment of depth of sedation during perioperative or procedural time periods, and those reporting BIS and sedation scale assessments conducted >5 minutes apart or while neuromuscular blocking agents (NMBA) was administered, were excluded. Data were abstracted on sedation scale, correlation coefficients, setting, patient characteristics, and BIS assessment characteristics that could impact the quality of the studies.
Twenty-four studies which enrolled 1,235 patients met inclusion criteria. The correlation between BIS and RASS, RSS, and SAS overall was 0.68 (95% confidence interval, 0.61–0.74, Ƭ2=0.06 I2=71.26%). Subgroup analysis by sedation scale indicated that the correlation between BIS and RASS, RSS, and SAS were 0.66 (95% confidence interval 0.58–0.73, Ƭ2=0.01 I2=30.20%), 0.76 (95% confidence interval 0.69–0.82, Ƭ2=0.04 I2=67.15%), and 0.53 (95% confidence interval 0.42–0.63, Ƭ2=0.01 I2=26.59%), respectively. Factors associated with significant heterogeneity included comparator clinical sedation scale, neurologic injury, and the type of intensive care unit (ICU) population.
BIS demonstrated moderate to strong correlation with clinical sedation scales in adult ICU patients, providing preliminary evidence for the validity of BIS as a measure of sedation intensity when clinical scales cannot be used. Futures studies should determine whether BIS monitoring is safe and effective in improving outcomes in patients receiving NMBA treatment.
Keywords: bispectral index, intensive care unit, Ramsay Sedation Scale, Richmond Agitation Sedation Scale, Sedation Agitation Scale
Introduction
Electroencephalographic monitoring with the bispectral index (BIS) is a method for assessing level of consciousness that can be used to titrate the dosage of sedative agents in a variety of settings. BIS monitoring was originally developed for the operating room setting, where it demonstrates a strong correlation with drug-induced loss of consciousness,1 and randomized trials have shown its use to significantly reduce the incidence of accidental awareness during anesthesia.2 The role of BIS in the critically ill population has yet to be clearly defined, given the lack of data showing a benefit of BIS compared to commonly used clinical sedation scales.3 However, clinical sedation scales cannot be used in patients treated with neuromuscular blocking agents (NMBA), because such scales require assessment of a patient’s movement in response to stimulus.
NMBA are a common adjuvant therapy for mechanically ventilated patients in the intensive care unit (ICU). An estimated one in five patients with acute respiratory distress syndrome (ARDS) are treated with a NMBA,4 and the prevalence of use has increased substantially since the advent of the coronavirus disease 2019 (COVID-19) pandemic.5,6 One of the most challenging aspects of NMBA treatment is the management of sedation. The inability to apply clinical sedation scales during NBMA treatment creates a substantial risk of harm from undersedation (i.e., awareness with paralysis) and oversedation (i.e., sedatives are infused beyond what is needed). Awareness during paralysis is a potentially devastating complication that can lead to post-traumatic stress disorder.7,8 Further, oversedation may be a mediator of increased mortality in patients treated with NMBA.9,10 Thus, establishing the utility of BIS as a monitoring tool in paralyzed, critically ill patients is of considerable interest.
An effective approach to establishing the validity of BIS would be to examine agreement between BIS and validated clinical sedation scales. However, doing so directly in NMBA-treated patients is not feasible. Thus, as a first step towards this goal, we conducted a systematic review and meta-analysis to provide a definitive evaluation of the literature supporting the validity of BIS in non-paralyzed, critically ill patients. Our primary aim was to determine the strength of correlation between BIS and the following validated clinical scales: Richmond Agitation Sedation Scale (RASS), Ramsay Sedation Scale (RSS), and Sedation Agitation Scale (SAS).11–13
MATERIALS AND METHODS
Protocol and Registration
This review and associated protocol were registered with the PROSPERO international prospective register of systematic reviews (Registration Number: CRD42020158314). This study did not require ethical approval.
Study Eligibility
We included studies evaluating the strength of correlation between concurrent assessments of BIS and RASS, RSS, or SAS in critically ill adult patients. We excluded studies involving assessment of depth of sedation during perioperative or procedural periods; however, patients admitted to the ICU for post-operative care were included. BIS and clinical sedation scale assessments had to be conducted concurrently and not during a period of neuromuscular blockade. The definition of “concurrent” was met if the methods stated broadly that assessments were done at the same time. If a specific time between assessments was mentioned, it must have been ≤5 minutes. Studies published in abstract form were included if there was no subsequent manuscript with the same dataset and only if outcomes of interest were reported.
Search Strategy, Sources, and Study Identification
We performed computerized searches of PubMed, Embase (Elsevier), Cochrane Library (includes Cochrane Database of Systematic Reviews and Cochrane Central Register of Controlled Trials), Scopus (Elsevier), and OpenGrey with the assistance of an experienced medical librarian (E.F.G.). Searches were conducted on August 18, 2018 and rerun on November 15, 2019, January 15, 2021, and April 14, 2022. Search strategies combined keywords and subject headings for the concepts of consciousness monitors, sedation scales, and ICUs (Supplemental Data File 1). No date or language restrictions were applied. Corresponding authors of studies with missing data were contacted one time to obtain data of interest.
Study Selection and Data Abstraction
Title and abstract screening for the initial search results was conducted in Excel. Full text screening for the initial search and all screening for search updates were completed in Covidence.14 Covidence was also used for data abstraction and quality assessment.
Article selection was independently conducted by two authors (D.D.L. and S.Y.A.Y.) and disagreements were reconciled by a third author (M.S.H.), who reviewed articles independently and determined relevance. If the third author agreed with either of the two authors, that determination was followed. If there was ambiguity identified by M.S.H., all three authors discussed disagreements for reconciliation. Data of interest were abstracted from full texts by three authors (D.D.L., S.Y.A.Y., and M.S.H.). Two authors independently collected and documented data of interest for each included study. Disagreements in data collected were reconciled by M.S.H., similar to the process defined above for article selection.
Author, publication year, sponsorship source, country of origin, ICU setting, corresponding author information, study design, number of patients, and number of assessments were collected. To gain an understanding of the study population and quality, we also collected data on whether neurologically injured patients were included, goal depth of sedation, if specified, use and timing of NMBA with respect to sedation assessments, monitoring of signal-quality index, monitoring of electromyogram input, number of BIS scores documented at each assessment, number of clinical sedation scores documented at each assessment, percentage of patients receiving mechanical ventilation, sedative and analgesic agents administered, severity of illness, and type of BIS monitor and electrodes used during the study. In addition, data on study quality or risk of bias, and outcomes of interest were collected for each full study included.
Assessment of Methodologic Quality
QUADAS-2 was used to evaluate the risk of bias in included studies. Four key domains of patient selection, index test, reference standard, and flow and timing were evaluated for each study. Three authors (D.D.L., S.Y.A.Y., and M.S.H.) evaluated studies for quality and risk of bias. Two authors independently evaluated and documented assessment for quality and risk of bias for each domain of QUADAS-2. Disagreements were reconciled by M.S.H. using the previously defined approach.
Data Analysis
Data analysis was conducted by T.A.M. Pooled analyses were based on the Pearson correlation coefficient (r). Values from studies reporting other correlation measures (Spearman’s Rank correlation, Kendall rank correlation) were converted to r values using published equations.15 The primary analysis pooled results from all studies. If a study examined the correlation between BIS and more than one clinical scale, the results for only one scale were included in the primary analysis according to the following hierarchy, based on how widely the scales have been reportedly used in practice:16 RASS is primary, if RASS is not reported, RSS is primary, if RSS not reported, SAS is primary. Estimation of 95% confidence intervals and pooled estimates were obtained after applying the Fischer Z transformation to approximate a normal sampling distribution, with transformation back to the correlation scale for presentation. Pooled estimates were obtained from a random-effects meta-analysis using the method of DerSimonian and Laird.17 Heterogeneity of correlation estimates between studies was examined by calculating the Q statistic, derived from the chi-square test, and the inconsistency index (I2). We considered an I² > 50% to indicate important heterogeneity between studies and a p-value ≤ 0.10 as indicating statistically significant heterogeneity.18
We specified several subgroup analyses a priori to examine potential sources of heterogeneity: sedation scale (RASS vs. RSS vs. SAS), depth of sedation targeted (deep sedation [RASS < −3, RSS < 4, SAS < 2] vs. higher levels), signal quality index assessed (yes vs. no), exclusion of patients with prior NMBA use (yes vs. no), ICU population type (mixed vs. medical vs. surgical), inclusion of patients with neurological injury (yes vs. no), and whether the correlation analysis was the study’s primary outcome (yes vs. no). We also performed several post-hoc subgroup analyses, including: electromyographic assessment (yes vs. no), approach to BIS measurement (single measure vs. average of multiple measurements), BIS monitor type (XP vs. non-XP), APACHE II score (0–10, 11–20, >20),19 and risk of bias (based on bias and applicability ratings).
For the depth of sedation subgroup analysis, we classified scales as follows (from deepest to lightest and excluding the agitated states for clinical scales): BIS 0–39 (ultra-deep), 40–59 (deep), 60–79 (moderate), 80–100 (light); RASS −5 to −4 (deep), −3 (moderate), −2 to 0 (light); RSS 6 to 5 (deep), 4 (moderate), 3 to 2 (light); and SAS 1 to 2 (deep), 3 (moderate), 4 (light).20
We considered a correlation coefficient between 0.0–0.09 to be negligible, 0.10–0.39 to be weak, 0.40–0.69 to be moderate, 0.70–0.89 to be strong, and 0.90–1.0 to be a very strong correlation.21 We examined the risk of publication bias by visual inspection of funnel plots. All analyses were performed with Stata version 17.1 (College Station, TX).
RESULTS
Study Identification and Selection
The comprehensive electronic search yielded 2,973 citations. Removal of duplicates and screening for inclusion criteria yielded 59 studies. After elimination of 35 studies for exclusion criteria, 24 studies enrolling 1,235 patients were included in the final analysis.22–45 Figure 1 depicts the Preferred Reporting Items for Systematic Review and Meta-Analyses (PRISMA) flow diagram.
Figure 1.
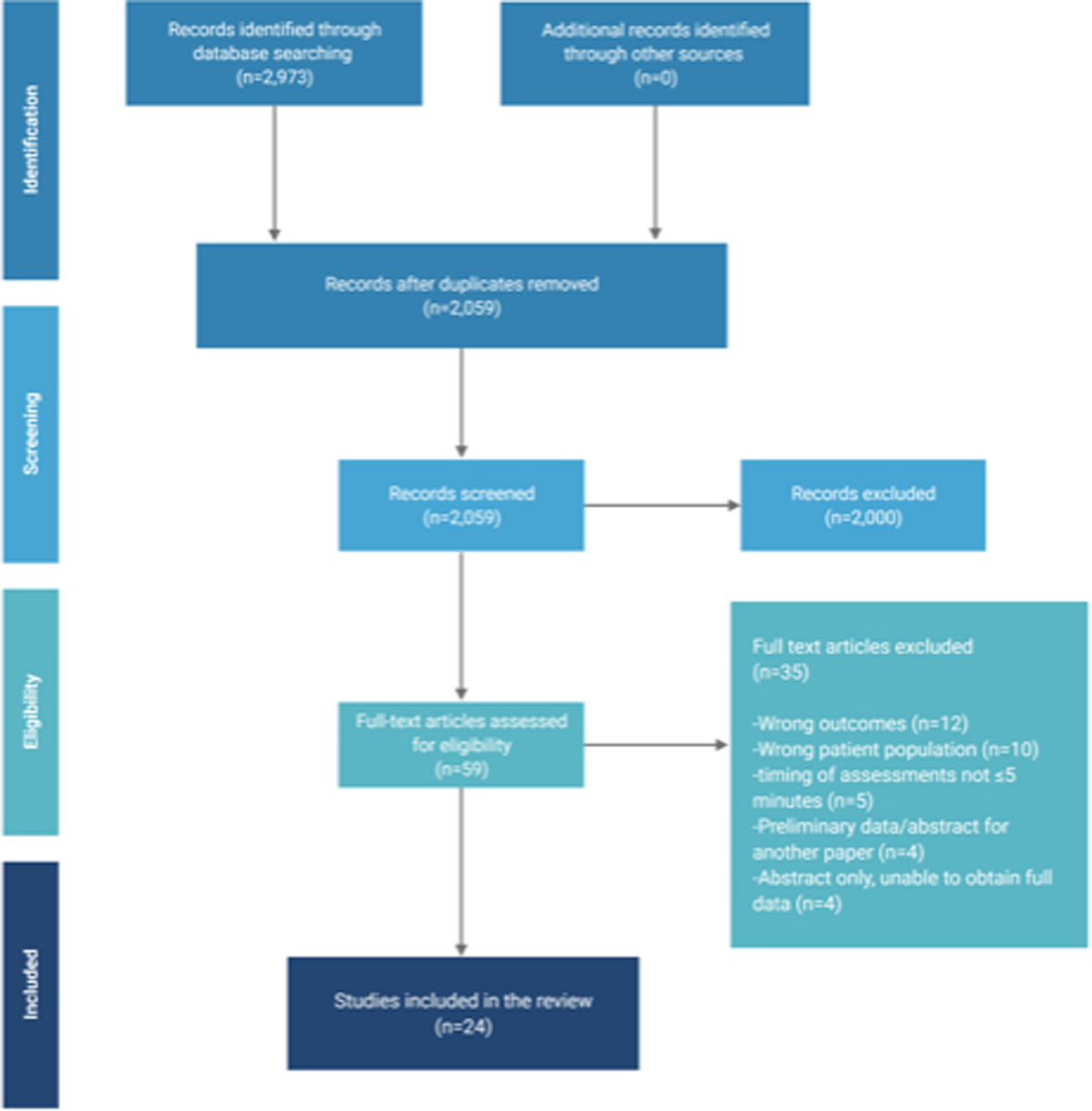
Preferred Reporting Items for Systematic Review and Meta-Analyses flow diagram for systematic review phases for correlation between concurrent measurements of bispectral index (BIS) and clinical sedation scale assessments.
Study Characteristics
The 24 studies included in the analyses were published between 2001 and 2015. Eighteen studies enrolled patients in mixed or general ICUs. All studies were prospectively conducted and included only mechanically ventilated patients except one study that did not report this information. Nineteen studies evaluated BIS and clinical sedation scale correlation as the primary outcome. Nine studies correlated BIS with RASS, 11 with RSS, and 9 with SAS.
Meta-Analysis Results
When data from all studies were aggregated, the correlation between BIS and clinical sedation scales was 0.68 (95% confidence interval, 0.61–0.74, I2=71.26%), demonstrating substantial heterogeneity across studies (Figure 2). The correlation with BIS varied significantly by sedation scale (Figure 3), showing the strongest correlation with the RSS scale. The correlation between BIS and clinical scales varied significantly depending on whether patients with neurological injury were included; the correlation was significantly lower in the three studies including patients with neurological injury. (Figure 4). When the studies were stratified by depth of sedation, the correlation between BIS and clinical sedation scales was stronger with studies including patients undergoing deep sedation (correlation coefficient 0.76 for deep sedation versus 0.68 for light to moderate sedation). However, heterogeneity was lower across studies with light to moderate sedation (Figure 5). Significant heterogeneity was also observed when analysis was stratified by ICU type (Supplemental Data File 2). No significant heterogeneity was observed in the remaining subgroup analyses (Supplemental Data File 2).
Figure 2.
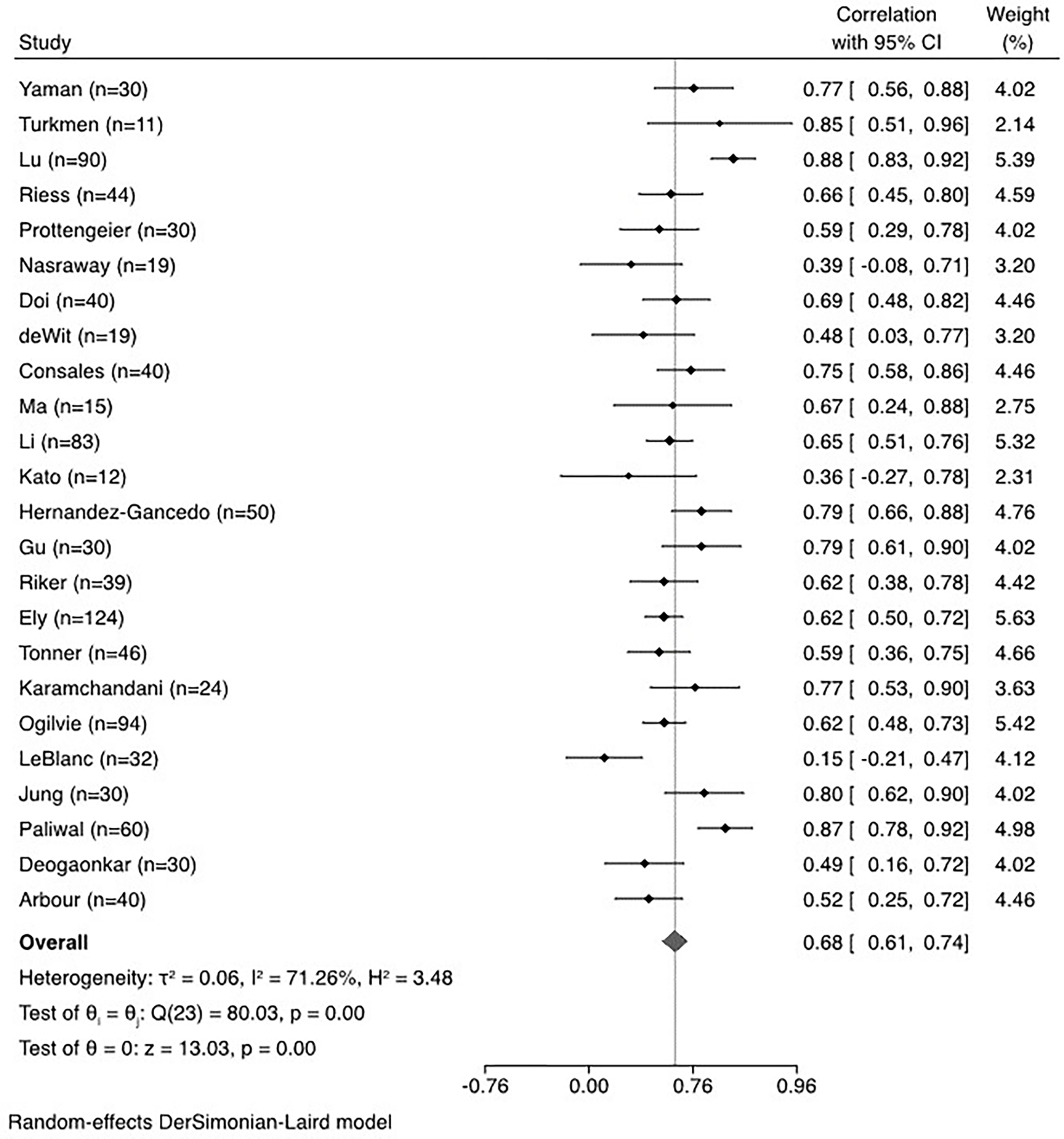
Meta-analysis and forest plot of overall correlation between bispectral index (BIS) and clinical sedation scales.
Figure 3.
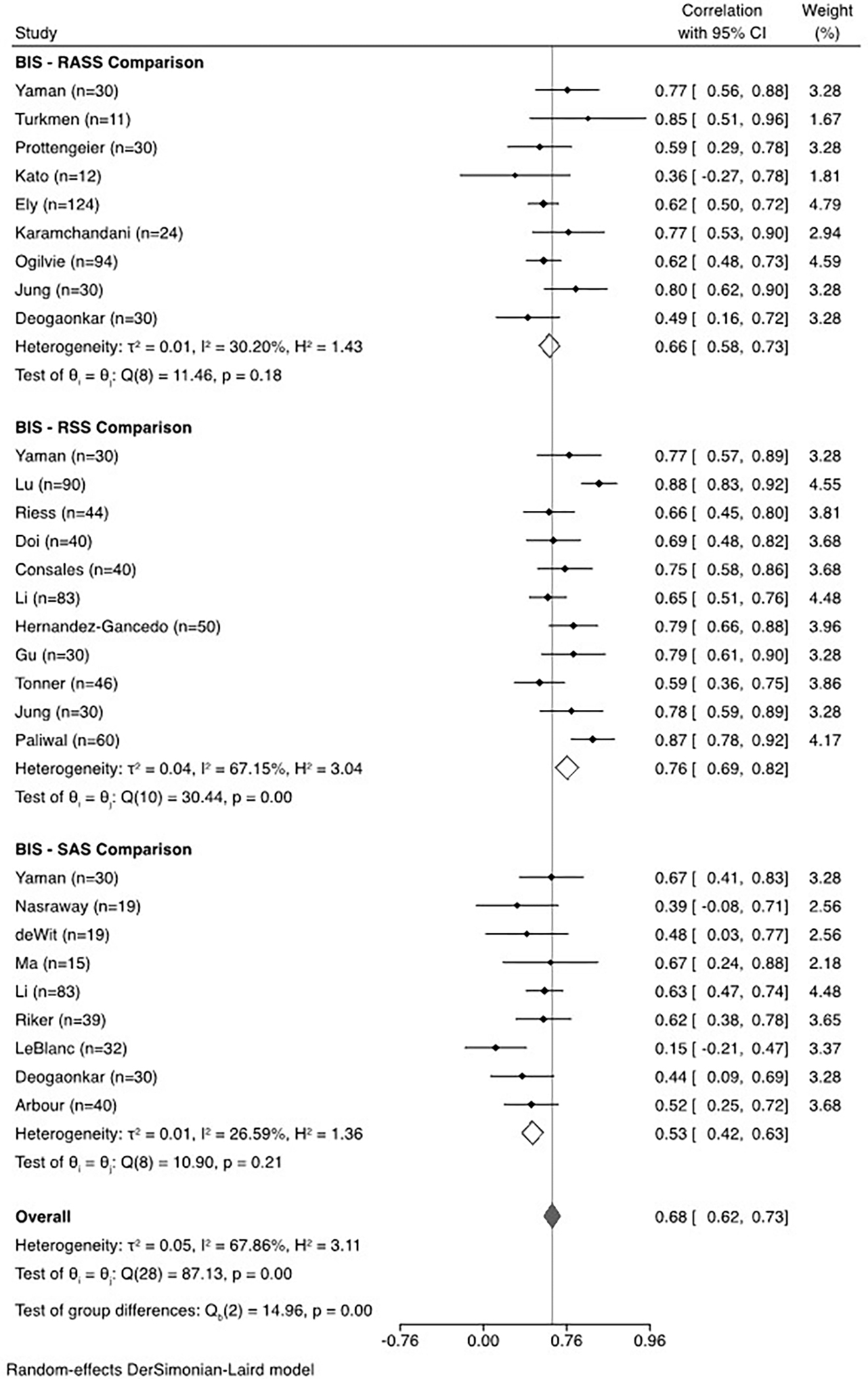
Sub-group meta-analysis and forest plot of correlation between bispectral index (BIS) and Richmond Agitation Sedation Scale (RASS), Ramsay Sedation Scale (RSS), or Sedation Agitation Scale (SAS).
Figure 4.
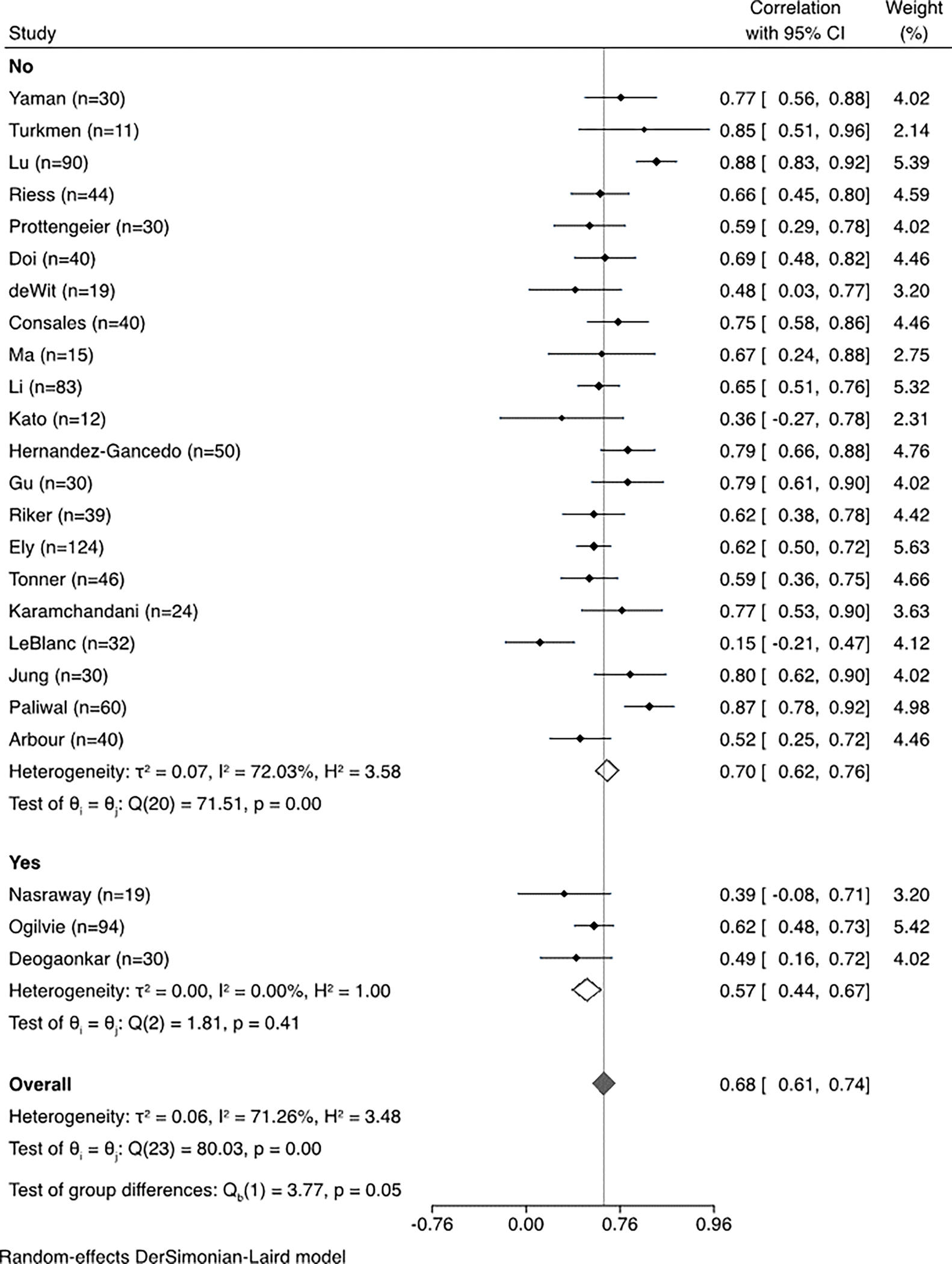
Sub-group meta-analysis and forest plot of correlation between bispectral index (BIS) and clinical sedation scales stratified by inclusion of patients with neurologic injury.
Figure 5.
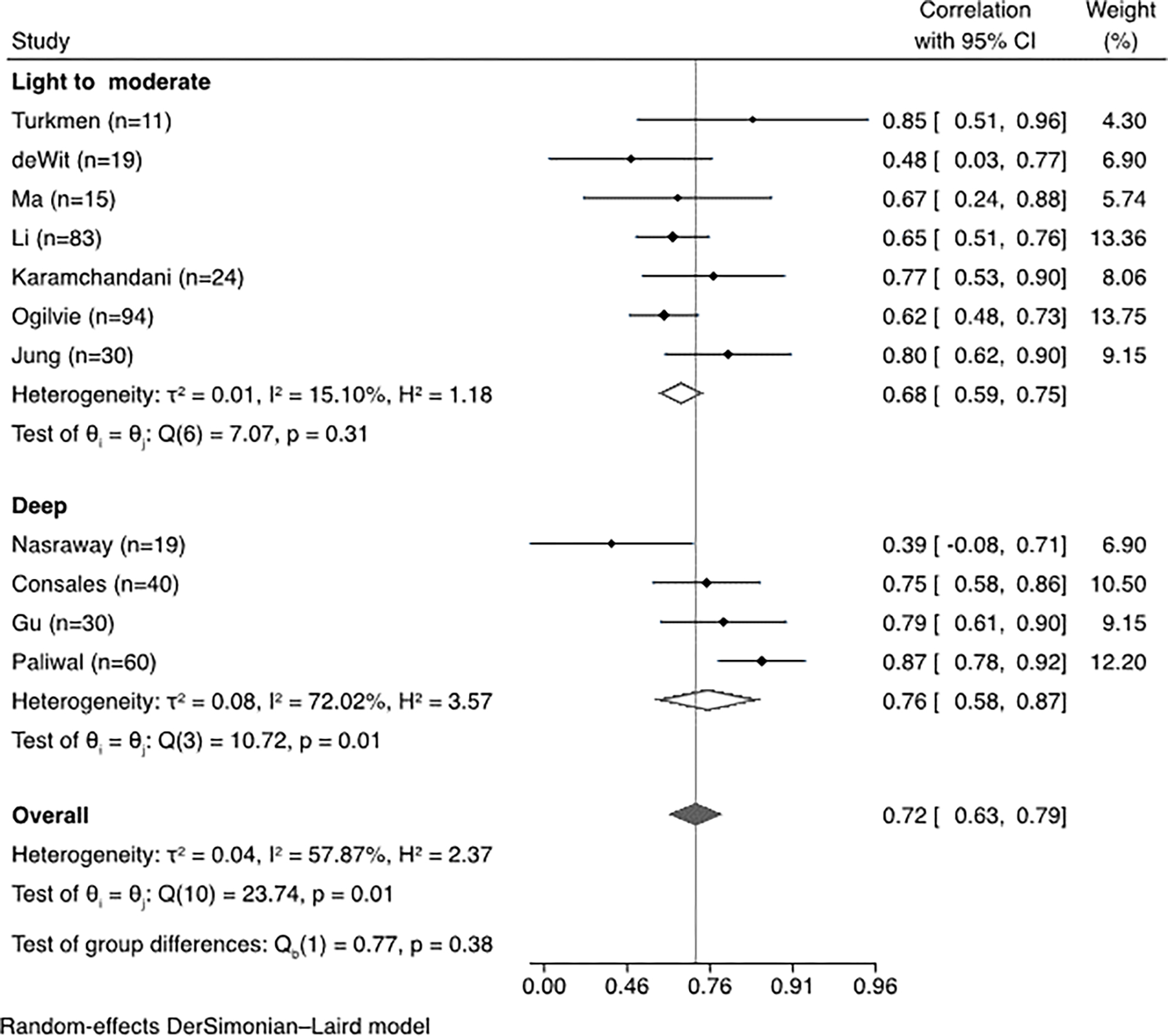
Sub-group meta-analysis and forest plot of correlation between bispectral index (BIS) and clinical sedation scales stratified by depth of sedation.
Assessment of Methodologic Quality
Table 1 summarizes assessments for risk of bias for each study, in each of domain of the QUADAS-2 tool. Ten studies were found to have low risk of bias, four studies with 1 domain considered high or unclear risk of bias, and 10 studies with 2 or more domains considered high or unclear risk of bias. Seventeen studies had low risk in the applicability rating and seven studies with 1 or more domains with high or unclear risk in the applicability rating.
Table 1.
Risk of Bias Assessment
| Study | Risk of Bias | Applicability Concerns | |||||
|---|---|---|---|---|---|---|---|
| Patient Selection | Index Tests | Reference Standard | Flow and Timing | Patient Selection | Index Tests | Reference Standard | |
| Riker, 2001 | high | high | low | low | low | low | low |
| Nasraway, 2002 | high | low | low | low | low | low | low |
| Riess, 2002 | low | low | low | low | low | low | low |
| deWit, 2003 | low | low | low | low | low | low | low |
| Deogaonkar, 2004 | low | low | low | low | high | low | low |
| Ely, 2004 | low | low | low | low | low | low | low |
| Doi, 2005 | low | high | unclear | low | high | low | low |
| Tonner, 2005 | unclear | high | low | low | low | low | low |
| Consales, 2006 | low | low | unclear | low | low | low | low |
| Ma, 2006 | low | unclear | unclear | low | low | low | low |
| Turkmen, 2006 | high | unclear | unclear | unclear | low | high | low |
| Gu, 2007 | low | low | low | low | low | low | low |
| Hernandez-Gancedo, 2007 | low | low | low | low | low | low | low |
| Lu, 2008 | low | high | low | low | low | high | low |
| Arbour, 2009 | unclear | high | low | low | low | low | low |
| Li, 2009 | low | high | unclear | low | low | low | low |
| Karamchandani, 2010 | low | low | low | low | low | low | low |
| Ogilvie, 2011 | low | high | low | low | high | high | low |
| Jung, 2012 | low | low | low | low | low | low | low |
| Kato, 2012 | low | high | high | low | low | high | low |
| LeBlanc, 2012 | low | low | low | low | low | low | low |
| Yaman, 2012 | low | high | low | unclear | low | low | low |
| Prottengeier, 2014 | unclear | low | low | unclear | low | low | low |
| Paliwal, 2015 | low | low | low | low | low | high | low |
DISCUSSION
In this systematic review and meta-analysis, we observed a moderate to strong correlation between BIS and validated clinical sedation scales in a population of critically ill patients who were predominantly receiving light sedation. This finding suggests that BIS monitoring potentially provides clinically relevant information on level of consciousness in critically ill patients receiving sedation. Application of this finding is limited, however, by substantial heterogeneity of the correlation across included studies. We included 24 studies in varied patient populations, using three different clinical sedation scales, and employing numerous differences in methodology. Methodological inconsistencies such as type of BIS monitor or electrodes used, timing of clinical and BIS assessments, monitoring of electromyogram input or signal quality index to ensure the appropriateness of the BIS measurements, etc., could explain some of the heterogeneity observed across studies. However, subgroup analyses based on these methodological factors did not explain substantial heterogeneity. Factors that were associated with significant heterogeneity included comparator clinical sedation scale, neurologic injury, and the type of ICU population.
Subgroup analysis across clinical scales showed the highest correlation between RSS and BIS (0.76), with lower values between BIS and SAS or BIS and RASS (0.53 and 0.66, respectively). This finding may be due in part to “ceiling effects” at higher levels of consciousness. BIS reaches a maximum value in patients who are awake.10 Similarly, the RSS scale assigns the same score to all levels of consciousness above “alert and calm.” Thus, both BIS and RSS have a ceiling at higher levels of consciousness (e.g., agitation), whereas RASS and SAS continue to differentiate increasing levels of agitation. Similarly, “floor effects” would be expected on the other end of the spectrum; in patients who are deeply sedated, clinical scales reach a minimum value at “unarousable.” In contrast, BIS values can, at least theoretically, continue to differentiate lower levels of consciousness.10 Although this potential non-linear association between BIS and clinical scales is plausible based on mechanistic grounds, subgroup analysis across “targeted depth of sedation” categories did not explain significant heterogeneity. This analysis is limited by the fact that most studies did not report on targeted level of sedation, and further, the targeted level might not reflect the achieved level of sedation at the time of BIS measurement. Although our analysis is not designed to show this, we postulate that a lack of correlation between BIS and clinical sedation scales may represent a potential advantage of BIS in the setting of lower levels of consciousness, which is particularly relevant for patients receiving NMBA. We propose this as an important area for future prospective evaluation, in a critically ill patient population receiving NMBA.
We also observed that the correlation between BIS and sedation scores was significantly lower in brain injury studies. Although the mechanism of this finding is unknown, it might suggest that the relationship between BIS and level of consciousness is altered by brain injury. Alternatively, lower correlation may reflect the difficulty of clinical assessment in patients with significant brain injury. Regardless of the mechanism, our data suggest caution with using BIS in the brain injured population.
We undertook this evaluation to determine if BIS could be an appropriate sedation assessment tool in critically ill adult patients treated with NMBA, when clinical sedation scales are impractical. The current standard in this group is to target deep sedation prior to initiation of NMBA.46 However, given the inability to continually assess depth of sedation over time, this strategy creates an important risk of oversedation, which has been associated with worse outcomes. An evaluation of sedation strategies during neuromuscular blockade in ARDS found that a higher proportion of deep sedation mediated the harmful effects of NMBA infusions on mortality and ventilator- and ICU-free days.9 A subsequent analysis of sedation strategies in mechanically ventilated patients with COVID-19 demonstrated that patients with COVID-19 have been more deeply sedated, with higher sedative doses, and for longer durations of time as compared with non-COVID-19 mechanically ventilated patients with ARDS. Mediation analysis in this study also showed a strong relationship between deep sedation and increased in-hospital mortality.10 The inability to assess sedation depth also creates an important risk of undersedation. Although awareness with chemical paralysis has been reported with a rate of 0.1% in the operating room, this incidence may be as high as 3.4% in the emergency department or ICU.47 Taken together, these data suggest that strategies for more accurate sedation titration could improve outcomes during paralysis. Based on the moderate correlation observed between BIS and clinical sedation scales in non-paralyzed patients, we hypothesize that BIS monitoring may provide meaningful information about level of consciousness that could improve sedation titration in patients receiving continuous NMBA.
Although our results provide preliminary evidence for the validity of BIS in the broad ICU population, important questions remain that limit routine use of this technology in paralyzed patients. In particular, the optimal target BIS range during NMBA treatment is unknown. A study in fully awake, healthy volunteers found that BIS values dropped significantly in some awake subjects after initiation of NMBA. This suggests that NMBA may directly lower BIS measurements, potentially by reducing electromyographic activity.48 Consequently, target BIS ranges must be developed that account for this direct lowering effect in order to avoid inappropriate down-titration of sedation.49 Alternatively, sedation algorithms that incorporate BIS monitoring might specify minimum sedative infusion rates below which patients are not titrated during chemical paralysis, even if BIS values are numerically below goal range for deep sedation.
Our analysis was restricted to studies of non-paralyzed patients. Such a restriction was unavoidable, as application of the clinical scales requires assessment of patient movement. Consequently, extrapolation of these results to paralyzed patients should be done with caution, and we consider our findings to be hypothesis generating. Future studies that directly examine BIS validity during NMBA administration are needed. A possible approach to this would be longitudinal concurrent assessments of BIS and clinical sedation scales during transition periods around NMBA administration. Additionally, studies that directly examine the association between BIS monitoring and clinical outcomes are needed. One study comparing BIS to clinical sedation found no difference in median daily sedation or analgesia exposure in patients receiving NMBA in the ICU; a more robust, prospective evaluation is needed.50 A systematic review and meta-analysis of BIS monitoring for sedation in critically ill mechanically ventilated adults on clinical outcomes or resource utilization found insufficient evidence on the effects of BIS due to uncertainty of the findings from low- and very low-quality evidence.51 Lastly, additional research is needed to determine if a strategy of NMBA holidays and clinical sedation assessment for titration of sedatives versus continuous titration using BIS would have better outcomes, given the considerations we have discussed.
CONCLUSIONS
Our results suggest that BIS has moderate to strong correlations with clinical sedation scales in adult ICU patients, providing preliminary evidence for the validity of BIS as a measure of sedation intensity when clinical scales cannot be used. However, mapping specific BIS values to validated clinical sedation scales is hindered by heterogeneity across studies, and potential ceiling effects at the extremes of consciousness. This makes implementation of BIS at the bedside challenging. Although our findings represent an important step toward defining a role for BIS monitoring during paralysis, additional research is required to use BIS safely during NMBA treatment. Prospective studies that directly examine the association between BIS scores and clinical outcomes are needed to identify optimal BIS ranges that could be applied in routine practice in patients receiving NMBA. Additionally, future research should evaluate the utility of BIS for titration of sedatives versus paralytic holidays and intermittent clinical assessment in patients undergoing neuromuscular blockade.
Supplementary Material
Acknowledgments and Credit:
All authors had full access to study data and contributed substantially to study design, data collection and interpretation, and writing of the manuscript. Author access to study data is on-going.
Funding:
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
T.A.M. is supported by a K08 career development award from NIDDK (K08DK124658)
Footnotes
Declaration of Conflicting Interests: D.D.L. is employed by Philips North America but was employed at Manchester University College of Pharmacy while this work was being conducted. M.S.H., E.F.G., S.Y.A.Y., and T.A.M. have no relevant relationships and activities to disclose.
Contributor Information
Mojdeh S. Heavner, Associate Professor and Vice Chair for Clinical Services, Department of Pharmacy Practice and Science, University of Maryland School of Pharmacy, Baltimore, Maryland.
Emily F. Gorman, Research and Education Librarian, Health Sciences and Human Services Library, University of Maryland, Baltimore, Maryland
Dustin D. Linn, Medical Science Liaison, Philips North America, Cambridge, Massachusetts
Siu Yan Amy Yeung, Clinical Pharmacy Specialist, Medical Intensive Care Unit, Department of Pharmacy Services, University of Maryland Medical Center, Baltimore, Maryland.
Todd A. Miano, Critical Care Pharmacist, Instructor of Epidemiology, Department of Biostatistics, Epidemiology, and Informatics, Perelman School of Medicine at the University of Pennsylvania, Philadelphia, Pennsylvania
REFERENCES
- 1.Glass PS, Bloom M, Kearse L, Rosow C, Sebel P, Manberg P. Bispectral analysis measures sedation and memory effects of propofol, midazolam, isoflurane, and alfentanil in healthy volunteers. Anesthesiology. 1997;86(4):836–847. doi: 10.1097/00000542-199704000-00014 [DOI] [PubMed] [Google Scholar]
- 2.Lewis SR, Pritchard MW, Fawcett LJ, Punjasawadwong Y. Bispectral index for improving intraoperative awareness and early postoperative recovery in adults. Cochrane Database Syst Rev. 2019;9:CD003843. doi: 10.1002/14651858.CD003843.pub4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Devlin JW, Skrobik Y, Gélinas C, et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU: Crit Care Med. 2018;46(9):e825–e873. doi: 10.1097/CCM.0000000000003299 [DOI] [PubMed] [Google Scholar]
- 4.Bellani G, Laffey JG, Pham T, et al. Epidemiology, Patterns of Care, and Mortality for Patients With Acute Respiratory Distress Syndrome in Intensive Care Units in 50 Countries. JAMA. 2016;315(8):788–800. doi: 10.1001/jama.2016.0291 [DOI] [PubMed] [Google Scholar]
- 5.Coppock Kristen. Hospitals Reporting Short Supplies of Neuromuscular Blockers. Pharm Times. Published online May 8, 2020. Accessed March 14, 2022. https://www.pharmacytimes.com/view/short-supply-of-neuromuscular-blockers-reported-in-some-hospitals [Google Scholar]
- 6.American Society of Health-System Pharmacists. Status of Pharmacy Resources in U.S. Hospitals and Health Systems. ASHP COVID-19 Resour Cent. Published online July 9, 2020. Accessed March 15, 2022. https://www.ashp.org/covid-19/bi-weekly-ppe-survey-results-covid-19?loginreturnUrl=SSOCheckOnly
- 7.Leslie K, Chan MTV, Myles PS, Forbes A, McCulloch TJ. Posttraumatic stress disorder in aware patients from the B-aware trial. Anesth Analg. 2010;110(3):823–828. doi: 10.1213/ANE.0b013e3181b8b6ca [DOI] [PubMed] [Google Scholar]
- 8.Cook TM, Andrade J, Bogod DG, et al. The 5th National Audit Project (NAP5) on accidental awareness during general anaesthesia: patient experiences, human factors, sedation, consent and medicolegal issues. Anaesthesia. 2014;69(10):1102–1116. doi: 10.1111/anae.12827 [DOI] [PubMed] [Google Scholar]
- 9.Wongtangman K, Grabitz SD, Hammer M, et al. Optimal Sedation in Patients Who Receive Neuromuscular Blocking Agent Infusions for Treatment of Acute Respiratory Distress Syndrome—A Retrospective Cohort Study From a New England Health Care Network. Crit Care Med. 2021;Publish Ahead of Print. doi: 10.1097/CCM.0000000000004951 [DOI] [PubMed] [Google Scholar]
- 10.Wongtangman K, Santer P, Wachtendorf LJ, et al. Association of Sedation, Coma, and In-Hospital Mortality in Mechanically Ventilated Patients With Coronavirus Disease 2019–Related Acute Respiratory Distress Syndrome: A Retrospective Cohort Study*. Crit Care Med. 2021;49(9):1524–1534. doi: 10.1097/CCM.0000000000005053 [DOI] [PubMed] [Google Scholar]
- 11.Ramsay MAE, Savege TM, Simpson BRJ, Goodwin R. Controlled Sedation with Alphaxalone-Alphadolone. BMJ. 1974;2(5920):656–659. doi: 10.1136/bmj.2.5920.656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riker RR, Picard JT, Fraser GL. Prospective evaluation of the Sedation-Agitation Scale for adult critically ill patients: Crit Care Med. 1999;27(7):1325–1329. doi: 10.1097/00003246-199907000-00022 [DOI] [PubMed] [Google Scholar]
- 13.Sessler CN, Gosnell MS, Grap MJ, et al. The Richmond Agitation–Sedation Scale: Validity and Reliability in Adult Intensive Care Unit Patients. Am J Respir Crit Care Med. 2002;166(10):1338–1344. doi: 10.1164/rccm.2107138 [DOI] [PubMed] [Google Scholar]
- 14.Covidence Systematic Review Software, Veritas Health Innovation, Melbourne, Australia. Available at Www.Covidence.Org.
- 15.Rupinski MT, Dunlap WP. Approximating Pearson Product-Moment Correlations from Kendall’s Tau and Spearman’s Rho. Educ Psychol Meas. 1996;56(3):419–429. doi: 10.1177/0013164496056003004 [DOI] [Google Scholar]
- 16.Hughes CG, McGrane S, Pandharipande PP. Sedation in the intensive care setting. Clin Pharmacol Adv Appl. 2012;4:53–63. doi: 10.2147/CPAA.S26582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2 [DOI] [PubMed] [Google Scholar]
- 18.Higgins JPT, Thompson SG. Quantifying heterogeneity in a meta-analysis. Stat Med. 2002;21(11):1539–1558. doi: 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- 19.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: a severity of disease classification system. Crit Care Med. 1985;13(10):818–829. [PubMed] [Google Scholar]
- 20.Fraser GL, Riker RR. Bispectral Index Monitoring in the Intensive Care Unit Provides More Signal Than Noise. Pharmacotherapy. 2005;25(5 Part 2):19S–27S. doi: 10.1592/phco.2005.25.5_Part_2.19S [DOI] [PubMed] [Google Scholar]
- 21.Schober P, Boer C, Schwarte LA. Correlation Coefficients: Appropriate Use and Interpretation. Anesth Analg. 2018;126(5):1763–1768. doi: 10.1213/ANE.0000000000002864 [DOI] [PubMed] [Google Scholar]
- 22.Gu Q, Liu N, Ge M, Gao W. [Application of the bispectral index monitor in mechanical ventilation in patients under sedation in the intensive care unit]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue Chin Crit Care Med Zhongguo Weizhongbing Jijiuyixue. 2007;19(2):101–103. [PubMed] [Google Scholar]
- 23.Deogaonkar A, Gupta R, DeGeorgia M, et al. Bispectral Index monitoring correlates with sedation scales in brain-injured patients. Crit Care Med. 2004;32(12):2403–2406. doi: 10.1097/01.ccm.0000147442.14921.a5 [DOI] [PubMed] [Google Scholar]
- 24.Jung YJ, Chung WY, Lee M, et al. The significance of sedation control in patients receiving mechanical ventilation. Tuberc Respir Dis. 2012;73(3):151–161. doi: 10.4046/trd.2012.73.3.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hernández-Gancedo C, Pestaña D, Pérez-Chrzanowska H, Martinez-Casanova E, Criado A. Comparing Entropy and the Bispectral index with the Ramsay score in sedated ICU patients. J Clin Monit Comput. 2007;21(5):295–302. doi: 10.1007/s10877-007-9087-7 [DOI] [PubMed] [Google Scholar]
- 26.de Wit M, Epstein SK. Administration of sedatives and level of sedation: comparative evaluation via the Sedation-Agitation Scale and the Bispectral Index. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2003;12(4):343–348. [PubMed] [Google Scholar]
- 27.Doi M, Morita K, Mantzaridis H, Sato S, Kenny GNC. Prediction of responses to various stimuli during sedation: a comparison of three EEG variables. Intensive Care Med. 2005;31(1):41–47. doi: 10.1007/s00134-004-2516-x [DOI] [PubMed] [Google Scholar]
- 28.Tonner PH, Wei C, Bein B, Weiler N, Paris A, Scholz J. Comparison of two bispectral index algorithms in monitoring sedation in postoperative intensive care patients. Crit Care Med. 2005;33(3):580–584. doi: 10.1097/01.ccm.0000156291.04287.7f [DOI] [PubMed] [Google Scholar]
- 29.Prottengeier J, Moritz A, Heinrich S, Gall C, Schmidt J. Sedation assessment in a mobile intensive care unit: a prospective pilot-study on the relation of clinical sedation scales and the bispectral index. Crit Care Lond Engl. 2014;18(6):615. doi: 10.1186/s13054-014-0615-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ely EW, Truman B, Manzi DJ, Sigl JC, Shintani A, Bernard GR. Consciousness monitoring in ventilated patients: bispectral EEG monitors arousal not delirium. Intensive Care Med. 2004;30(8):1537–1543. doi: 10.1007/s00134-004-2298-1 [DOI] [PubMed] [Google Scholar]
- 31.Riker RR, Fraser GL, Simmons LE, Wilkins ML. Validating the Sedation-Agitation Scale with the Bispectral Index and Visual Analog Scale in adult ICU patients after cardiac surgery. Intensive Care Med. 2001;27(5):853–858. doi: 10.1007/s001340100912 [DOI] [PubMed] [Google Scholar]
- 32.Arbour R, Waterhouse J, Seckel MA, Bucher L. Correlation between the Sedation-Agitation Scale and the Bispectral Index in ventilated patients in the intensive care unit. Heart Lung J Crit Care. 2009;38(4):336–345. doi: 10.1016/j.hrtlng.2008.10.010 [DOI] [PubMed] [Google Scholar]
- 33.Riess ML, Graefe UA, Goeters C, Van Aken H, Bone HG. Sedation assessment in critically ill patients with bispectral index. Eur J Anaesthesiol. 2002;19(1):18–22. doi: 10.1017/s0265021502000030 [DOI] [PubMed] [Google Scholar]
- 34.LeBlanc JM, Dasta JF, Pruchnicki MC, Gerlach A, Cook C. Bispectral index values, sedation-agitation scores, and plasma Lorazepam concentrations in critically ill surgical patients. Am J Crit Care Off Publ Am Assoc Crit-Care Nurses. 2012;21(2):99–105. doi: 10.4037/ajcc2012777 [DOI] [PubMed] [Google Scholar]
- 35.Consales G, Chelazzi C, Rinaldi S, De Gaudio AR. Bispectral Index compared to Ramsay score for sedation monitoring in intensive care units. Minerva Anestesiol. 2006;72(5):329–336. [PubMed] [Google Scholar]
- 36.Yaman F, Ozcan N, Ozcan A, Kaymak C, Basar H. Assesment of correlation between bispectral index and four common sedation scales used in mechanically ventilated patients in ICU. Eur Rev Med Pharmacol Sci. 2012;16(5):660–666. [PubMed] [Google Scholar]
- 37.Paliwal B, Rai P, Kamal M, et al. Comparison Between Dexmedetomidine and Propofol with Validation of Bispectral Index For Sedation in Mechanically Ventilated Intensive Care Patients. J Clin Diagn Res JCDR. 2015;9(7):UC01–05. doi: 10.7860/JCDR/2015/14474.6258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kato T, Koitabashi T, Ouchi T, Serita R. The utility of bispectral index monitoring for sedated patients treated with low-dose remifentanil. J Clin Monit Comput. 2012;26(6):459–463. doi: 10.1007/s10877-012-9379-4 [DOI] [PubMed] [Google Scholar]
- 39.Karamchandani K, Rewari V, Trikha A, Batra RK. Bispectral index correlates well with Richmond agitation sedation scale in mechanically ventilated critically ill patients. J Anesth. 2010;24(3):394–398. doi: 10.1007/s00540-010-0915-4 [DOI] [PubMed] [Google Scholar]
- 40.Ogilvie MP, Pereira BMT, Ryan ML, et al. Bispectral index to monitor propofol sedation in trauma patients. J Trauma. 2011;71(5):1415–1421. doi: 10.1097/TA.0b013e3182178b8b [DOI] [PubMed] [Google Scholar]
- 41.lin Ma P, zhu Zhao J, wen Su J, Li Q, Wang Y [Comparison of reliability of bispectral index and sedation-agitation scale in assessing the depth of sedation in patients treated with mechanical ventilation]. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue Chin Crit Care Med Zhongguo Weizhongbing Jijiuyixue. 2006;18(6):323–326. [PubMed] [Google Scholar]
- 42.Turkmen A, Altan A, Turgut N, Vatansever S, Gokkaya S. The correlation between the Richmond agitation-sedation scale and bispectral index during dexmedetomidine sedation. Eur J Anaesthesiol. 2006;23(4):300–304. doi: 10.1017/S0265021506000081 [DOI] [PubMed] [Google Scholar]
- 43.Lu CH, Ou-Yang HY, Man KM, et al. Relative reliability of the auditory evoked potential and Bispectral Index for monitoring sedation level in surgical intensive care patients. Anaesth Intensive Care. 2008;36(4):553–559. doi: 10.1177/0310057X0803600409 [DOI] [PubMed] [Google Scholar]
- 44.Nasraway SA SA, Wu EC, Kelleher RM, Yasuda CM, Donnelly AM. How reliable is the Bispectral Index in critically ill patients? A prospective, comparative, single-blinded observer study. Crit Care Med. 2002;30(7):1483–1487. doi: 10.1097/00003246-200207000-00014 [DOI] [PubMed] [Google Scholar]
- 45.Li W, Abraham K, Yost R, et al. 1148: IMPACT OF BIS MONITORING ON SEDATION AND ANALGESIA IN PATIENTS WITH ARDS ON NMBA. Crit Care Med. 2019;47:551. doi: 10.1097/01.ccm.0000551893.12476.e5 [DOI] [Google Scholar]
- 46.Murray MJ, DeBlock H, Erstad B, et al. Clinical Practice Guidelines for Sustained Neuromuscular Blockade in the Adult Critically Ill Patient: Crit Care Med. 2016;44(11):2079–2103. doi: 10.1097/CCM.0000000000002027 [DOI] [PubMed] [Google Scholar]
- 47.Pappal RD, Roberts BW, Mohr NM, et al. The ED-AWARENESS Study: A Prospective, Observational Cohort Study of Awareness With Paralysis in Mechanically Ventilated Patients Admitted From the Emergency Department. Ann Emerg Med. 2021;77(5):532–544. doi: 10.1016/j.annemergmed.2020.10.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schuller PJ, Newell S, Strickland PA, Barry JJ. Response of bispectral index to neuromuscular block in awake volunteers. Br J Anaesth. 2015;115:i95–i103. doi: 10.1093/bja/aev072 [DOI] [PubMed] [Google Scholar]
- 49.Poole BR, Reese ZA, Dechen T, et al. Patient and Care Delivery Characteristics Associated With Harm From Neuromuscular Blockade. Crit Care Explor. 2020;2(6):e0147. doi: 10.1097/CCE.0000000000000147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Bass S, Vance ML, Reddy A, et al. Bispectral Index for Titrating Sedation in ARDS Patients During Neuromuscular Blockade. Am J Crit Care. 2019;28(5):377–384. doi: 10.4037/ajcc2019917 [DOI] [PubMed] [Google Scholar]
- 51.Shetty RM, Bellini A, Wijayatilake DS, et al. BIS monitoring versus clinical assessment for sedation in mechanically ventilated adults in the intensive care unit and its impact on clinical outcomes and resource utilization. Cochrane Emergency and Critical Care Group, ed. Cochrane Database Syst Rev. 2018;2019(1). doi: 10.1002/14651858.CD011240.pub2 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


