Abstract
During the summers of 2020 and 2021, the number of confirmed cases of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infections in Switzerland remained at relatively low levels, but grew steadily over time. It remains unclear to what extent epidemic growth during these periods was a result of the relaxation of local control measures or increased traveling and subsequent importation of cases. A better understanding of the role of cross-border-associated cases (imports) on the local epidemic dynamics will help to inform future surveillance strategies.
We analyzed routine surveillance data of confirmed cases of SARS-CoV-2 in Switzerland from 1 June to 30 September 2020 and 2021. We used a stochastic branching process model that accounts for superspreading of SARS-CoV-2 to simulate epidemic trajectories in absence and in presence of imports during summer 2020 and 2021.
The Swiss Federal Office of Public Health reported 22,919 and 145,840 confirmed cases of SARS-CoV-2 from 1 June to 30 September 2020 and 2021, respectively. Among cases with known place of exposure, 27% (3,276 of 12,088) and 25% (1,110 of 4,368) reported an exposure abroad in 2020 and 2021, respectively. Without considering the impact of imported cases, the steady growth of confirmed cases during summer periods would be consistent with a value of that is significantly above the critical threshold of 1. In contrast, we estimated at 0.84 (95% credible interval, CrI: 0.78–0.90) in 2020 and 0.82 (95% CrI: 0.74–0.90) in 2021 when imported cases were taken into account, indicating that the local was below the critical threshold of 1 during summer.
In Switzerland, cross-border-associated SARS-CoV-2 cases had a considerable impact on the local transmission dynamics and can explain the steady growth of the epidemic during the summers of 2020 and 2021.
Keywords: SARS-CoV-2, Import, Cross-border-associated cases, Travel, Summer
1. Introduction
In early 2020, the emergence and spread of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in Europe was facilitated by travelers (Worobey et al., 2020). A simulation study showed that targeting of air-travelers with symptom-based screening at exit or entry points of countries likely delayed the local spread of SARS-CoV-2 during the first months of the pandemic. If travel-related control measures were introduced with a delay, i.e. after local transmission had already been triggered by earlier arrivals of travelers, then control measures only delayed local spread by a few days (Clifford et al., 2020). The role of travelers in the spread of SARS-CoV-2 changed after local transmission had been established with varying levels of incidence across countries. Russel et al. showed that the contribution of imported cases on the local epidemic dynamics strongly depends on the local incidence of SARS-CoV-2, whether local epidemics are close to tipping points for exponential growth, and travel volumes (Russell et al., 2021).
A phylogenetic analysis reported on a novel SARS-CoV-2 variant, 20E (EU1), which was identified in Spain and subsequently spread to countries across Europe in summer 2020 (Hodcroft et al., 2021). The study estimated that 20E (EU1) was introduced multiple times into various European countries including Switzerland. This illustrated how the rising incidence in the country of origin, lack of screening and surveillance efforts, and resumption of travel can quickly establish local outbreaks and epidemics and undermine local efforts to control SARS-CoV-2 transmission. The rapid spread of 20E (EU1) revealed that travel guidelines and restrictions in Europe were mostly not sufficient to reduce cross-border transmission as holiday travel to countries with a higher incidence was not accompanied with adequate screening and surveillance efforts. These findings were supported by a phylogeographic study that showed how the impact of imported viral lineages on the local transmission dynamics was negatively associated with the local incidence (Lemey et al., 2021). Furthermore, they estimated that Belgium, the Netherlands, Norway, and Switzerland had more introductions than exports during summer 2020, highlighting that imported cases were impacting the SARS-CoV-2 epidemic in these countries.
Like most European countries, Switzerland experienced rapid spread of SARS-CoV-2 after reporting the first confirmed case on 25 February 2020 (Lemaitre et al., 2020). The federal government issued a number of major non-pharmaceutical interventions (NPIs) that included mandatory working from home, closing of schools and universities, non-essential shops, and restaurants, prohibition of gatherings of more than five people, and restricted movement across borders. Switzerland lifted major measures on 10 May 2020 and reopened its borders to countries of the Schengen Area on 15 June 2020 (Federal Office of Public Health, 2020). Switzerland lies at the heart of Europe, neighboring five countries, and has a significant fraction of foreign residents and cross-border commuters. In a survey conducted in Switzerland in May 2020, almost 80% of participants indicated that traveling is an important part of their holidays (Heim and Müller, 2020). In another survey conducted in June 2020, 15% planned to spend their summer holidays abroad compared to 49% before the pandemic (Bosshardt et al., 2020). Around 4% were planning to go to Germany, 3% to France, and 2% to Italy or Austria. More distant holiday destinations like Spain or Greece were planned to be visited by less than 1%. In summer 2020, travel-related control measures were limited. Countries with a higher incidence compared to Switzerland were typically added to a quarantine list, but often with considerable delays or not at all. The epidemic changed between the summers of 2020 and 2021 with high infection levels in winter and the start of the vaccination campaign. With the introduction of the COVID-19 certificate on 7 June 2021 (Federal Council, 2020), travelers had to test negative for entering Switzerland, be recovered from, or be vaccinated against SARS-CoV-2. On 26 June 2021, quarantine requirement for those who had recovered from SARS-CoV-2 or had been vaccinated was lifted (Federal Council, 2020). Hence, in both summers travel-associated cases were to be expected, but the impact of these cases on the local level of transmission of SARS-CoV-2 in Switzerland remained unknown.
The local level of transmission can be quantified by the effective reproduction number , which represents the average number of secondary cases per infected case at a given time in an epidemic (Gostic et al., 2020). Typically, estimates of are based on confirmed case counts from a certain country or region and do not consider imported cases, which were infected abroad. When the proportion of imported cases among all confirmed cases is sufficiently high, conclusions about the efficacy of local control measures can be hampered. In addition, it is important to consider stochastic effects of transmission and the potential for superspreading when incidence is low and even a small number of imports can have a large impact on the local epidemic dynamics. Stochastic branching process models can account for the observed overdispersion in the number of secondary SARS-CoV-2 cases (Laxminarayan et al., 2020, Bi et al., 2020, Kremer et al., 2021), and were used to estimate the basic reproduction number during the early transmission of SARS-CoV-2 in Wuhan, China (Riou and Althaus, 2020).
In this study, we quantified the impact of cross-border-associated (imported) cases on the SARS-CoV-2 epidemic in Switzerland during the summer of 2020 and 2021. To this end, we first described routine surveillance data of confirmed cases by country of exposure to SARS-CoV-2. We then calibrated a stochastic branching process model that accounts for superspreading of SARS-CoV-2 to the observed cases in Switzerland using different scenarios for the number of imports. This allowed us to obtain estimates of the local in absence and presence of imports, highlighting the potential role of travelers on the local epidemic.
2. Methods
2.1. Data
We analyzed routine surveillance data of confirmed cases of SARS-CoV-2 in Switzerland from 1 June to 30 September 2020 and 2021 that were provided by the Swiss Federal Office of Public Health (FOPH). Data included age, sex, case date (earliest available date of sample, test or registration), and the country of exposure, i.e., the country where confirmed cases had been within the last 14 days before they were tested or began to have symptoms. We compared the age of cases from Switzerland and abroad using Student’s t-test. To compare the incidence of confirmed cases between Switzerland and other countries, we retrieved data from ‘Our World in Data’ (Ritchie et al., 2020, Hasell et al., 2020).
2.2. Stochastic simulations
We simulated epidemic trajectories of SARS-CoV-2 in Switzerland from 1 June to 30 September 2020 and 2021. To this end, we used a stochastic branching process model that accounts for superspreading in transmission of SARS-CoV-2 (Althaus, 2015, Riou and Althaus, 2020). The branching process was based on a negative binomial distribution to describe the number of secondary cases, with a mean of and overdispersion . The generation time for each transmission event was sampled from the gamma distribution with a mean of 5.2 days and a standard deviation (sd) of 1.72 days (Ganyani et al., 2020).
We considered different parameter combinations of , , and the seed, i.e., the number of infectious individuals during the week preceding the start of the simulation. For , we assumed a uniform prior distribution between 0.5 and 1.5. was normally distributed between 0.49 and 0.52 (Laxminarayan et al., 2020). In a sensitivity analysis, we also considered values of 0.1 and 1 (Taube et al., 2021). To obtain the seed, we fitted a negative binomial generalized linear model with a weekend effect to account for varying testing rates to the daily number of confirmed cases during the study period. We then sampled from a uniform distribution of the 95% prediction interval of confirmed cases for the week before the study period (16–30 cases per day in 2020, and 98–300 cases per day in 2021).
We derived the number of imported cases from the routine surveillance data. In November 2020, registration procedures changed and the country of exposure was only available for hospitalized cases, deceased cases, and cases in nursing homes. Information on the place of exposure was frequently missing (see Table 1 and Supplementary Table 1). To account for a potential reporting bias, we considered different scenarios for the total number of imported cases among all confirmed cases: (a) we assumed imported cases among reported cases were representative and extrapolated to the total number of cases (missing at random), (b) we assumed imported cases were less likely among cases with missing information (‘lower limit’ of (a)), (c) we assumed imported cases were more likely among cases with missing information (‘upper limit’ of (a)). The lower and upper limits correspond to 50% and 150% of the daily imports of the first scenario. We compared these three scenarios to a baseline scenario without any imported cases, i.e., a scenario where the number of imported cases was set to 0.
Table 1.
Confirmed cases of SARS-CoV-2 with exposure in Switzerland and abroad. Percentages for cases with reported exposure correspond to all cases with known exposure. Scenario (a) Missing at random. Scenario (b) Lower limit (50% of imports in scenario (a). Scenario (c) Upper limit (150% of imports in scenario (a).
| Summer 2020 (Jun–Sep) | Summer 2021 (Jun–Sep) | |
|---|---|---|
| Total confirmed cases | 22,919 | 145,840 |
| Confirmed cases with known exposure | 12,088 (53%) | 4,368 (3%) |
| Confirmed cases with exposure in Switzerland | 8,812 (73%) | 3,258 (75%) |
| Confirmed cases with exposure abroad | 3,276 (27%) | 1,110 (25%) |
| Total number of imports (scenario a) | 6,211 | 37,061 |
| Total number of imports (scenario b) | 3,106 | 18,530 |
| Total number of imports (scenario c) | 9,317 | 55,591 |
2.3. Inference
We simulated epidemic trajectories by randomly sampling parameter values. For the scenarios with imported cases, we added the imported cases to the simulations according to their case date. For the lower and upper limit scenarios, we decreased and increased the extrapolated number of imported cases by 50% and 150% for each day. We assumed that reported cases were representative of the overall infection dynamics, i.e., that the reporting rate was constant throughout each study period. Furthermore, we assumed that imported cases were equally likely to transmit and initiate new branches of transmission as did the local cases (see discussion). For computational reasons, we stopped and removed simulated trajectories that exceeded a cumulative incidence of cases before the end of the simulation. As this value is orders of magnitude higher than the target incidence for model calibration, this did not affect our results.
In order to infer for the different scenarios, we applied Approximate Bayesian Computation (ABC) and rejected the parameter combinations where the simulated cumulative and final incidence fell outside specific ranges. The ranges for each period were defined as the 95% prediction interval of the cumulative incidence and of the final incidence of confirmed cases, assuming a negative binomial distribution. For the final incidence, we considered the average incidence over the last week. We quantitatively compared the quality of the calibrated simulations in describing the observed number of confirmed cases in summer 2020 and 2021. We computed the sum of squared residuals (SSR) between each of 1,000 randomly selected trajectories and the daily incidence of confirmed cases (7-day moving average), and the root-mean-square error (RMSE) for each scenario. We then compared the RMSE and density distribution of the SSR between the different scenarios.
All analyses were performed using R version 4.0 with the following packages: MASS, MCMCglmm, doParallel, foreach, lubridate, reshape2, ggplot2, ggpubr, grid, gridExtra, RColorBrewer (R. Core Team, 2020). Code is available on GitHub (https://github.com/ISPMBern/covid_summer).
3. Results
The number of daily confirmed cases of SARS-CoV-2 increased continuously in Switzerland during early summer 2020 and 2021 before it declined in September of both years (Fig. 1A). Between 1 June to 30 September 2020, FOPH reported 22,919 confirmed cases of SARS-CoV-2 (Table 1 and Supplementary Table 1). For 12,088 (53%) cases, the reported country of exposure was available. Of these, 3,276 (27%) cases reported an exposure abroad. The highest number of imports was reported on 18 August 2020. The number of confirmed cases was considerably higher during summer 2021. Between 1 June to 30 September 2021, FOPH received 145,840 notifications of SARS-CoV-2 cases (Table 1 and Supplementary Table 1). Due to changes in the registration of cases, the country of exposure in 2021 was only available for hospitalized cases, deceased cases and cases in nursing homes (4,368 or 3%). Of these, 1,110 (25%) cases reported an exposure abroad. Extrapolation of reported imports resulted in 3,106 to 9,317 and 18,530 to 55,591 imports for summer 2020 and 2021, respectively (Fig. 1B and Table 1). The highest number of imports was reported on 23 August 2021. In both years, the highest number of imports coincided with the end of school holidays/resumption of school in most cantons in Switzerland (Supplementary Figure 1A). Among imported cases, the countries of the reported most likely exposure were France and Croatia in 2020, and North Macedonia and Kosovo in 2021. During both years, the mean age of all imported cases was lower compared to local cases (Supplementary Table 1 and Supplementary Figure 2). Note that the mean age of all cases for which the country of exposure was available was substantially higher in 2021 compared to 2020 due to differences in reporting guidelines.
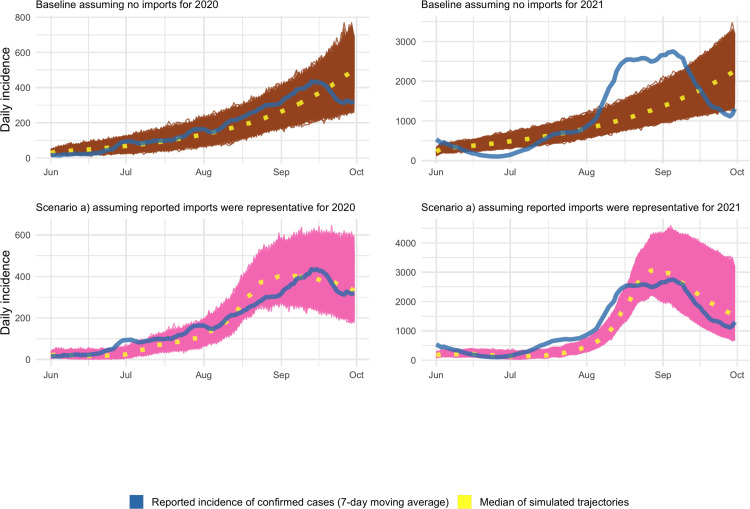
Fig. 1.
SARS-CoV-2 epidemic in Switzerland during summer 2020 and 2021. (A) Daily number of all confirmed cases (blue) and reported imported cases (red). (B) Imported cases as reported (red), and three different scenarios extrapolating the total number of imports from reported imports. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
The incidence of SARS-CoV-2 cases in countries that were reported as a common place of exposure among Swiss cases varied widely (Supplementary Figure 3 and Supplementary Table 1). In 2020, the incidence per million in the 19 countries with at least 10 reported imports was higher compared to Switzerland during 52 of 122 days (IQR: 40–107 days) during the study period on average. At the beginning of June 2020, testing was not mandatory to enter Switzerland and none of these 19 countries were listed on ’Ordinance on Measures to Combat the Coronavirus (COVID-19) in International Passenger Transport’ (Federal Council, 2021). This list has been regularly updated by the Swiss authorities based on the global epidemiological situation and their strategy to combat SARS-CoV-2. It is important to note that quarantine for travelers in Switzerland was not enforced, but was based on trust. Several countries were subsequently added to the quarantine list until the end of September 2020 (Supplementary Table 1). In 2021, the incidence in the 14 countries with at least 10 reported imports was higher compared to Switzerland during 52 of 122 days (IQR: 26–86 days) during the study period on average. During the study period of 2021, quarantine was only mandatory for travelers returning from specific countries, i.e., countries with unreliable surveillance data, a high number of imports, and countries where the incidence per million cases during the last two weeks was 60 times higher compared to Switzerland (Federal Council, 2021).
To understand the role of imported cases on the local dynamics of SARS-CoV-2 in Switzerland, we simulated epidemic trajectories for different import scenarios using a stochastic branching process model (Fig. 2 and Supplementary Figure 4). Calibrating the different scenarios for the total number of imports to the confirmed number of cases allowed us to estimate the values of the effective reproduction number that are most compatible with the observed epidemic dynamics. In our baseline scenario (without imports), only an above the critical threshold of 1 is compatible with the observed overall increase in the daily number of cases. Hence, we estimated a local (95% credible interval, CrI: 1.08–1.16) in summer 2020 and (95% CrI: 1.06–1.12) in summer 2021, suggesting that the local in Switzerland was above the critical threshold of 1 during those time periods (Fig. 3). In contrast, taking imports into account does not require a continuous exponential growth of the local epidemic to be compatible with the data. In scenario (a), assuming reported imports were representative, i.e., unreported imports were missing at random, we estimated a considerably lower local (95% CrI: 0.78–0.90) and (95% CrI: 0.74–0.90) in summer 2020 and 2021, respectively (Fig. 2, Fig. 3). Despite being below the critical threshold of 1, the simulated epidemics were still growing due to imported cases and subsequently declined when imports decreased after mid-August. The additional scenarios (b) (lower limit that assumed imports were overreported) and (c) (upper limit that assumed imports were underreported) also resulted in estimates of that were below the critical threshold of 1 (Fig. 3 and Supplementary Table 2).
Fig. 2.
Simulated epidemic trajectories of the SARS-CoV-2 epidemic in Switzerland during summer 2020 and 2021. Top row: Baseline scenario without imports, which leads to an estimated local Re above on. Bottom row: Scenario a) (missing at random) assuming 27% and 25% of confirmed cases were imports in 2020 and 2021, respectively. Colored areas represent all calibrated epidemic trajectories. The median of the epidemic trajectories and the reported incidence of confirmed cases (7-day moving average) are given as yellow and blue lines, respectively. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Fig. 3.
Estimates of the effective reproduction number of SARS-CoV-2 during summer 2020 and 2021. Three different import scenarios are compared to the baseline scenario that does not consider imports. (For interpretation of the references to color in this figure legend,the reader is referred to the web version of this article.).
In contrast to the baseline scenario, the scenarios with imported cases can qualitatively account for the initial increase in incidence during summer, and the subsequent decrease in the second half of September during both years. We further conducted a quantitative comparison of the quality of the calibrated scenarios in describing the observed number of cases (Supplementary Figure 5). For 2020, scenario (a) resulted in the lowest RMSE (664) followed by scenario (b) (697), scenario (c) (732), and the baseline scenario (746) For 2021, the RMSE was lowest for scenario (a) (6,328) and scenario (c) (6,327), followed by scenario (b) (6,525), and the baseline scenario (8,252). The quantitative comparison of the different scenarios further illustrates that taking into account imported cases results in a better description of the initial increase in confirmed cases during summer and the subsequent decline in the second half of September during both summers.
In a sensitivity analysis, we evaluated the effect of different levels for the overdispersion (superspreading) on the estimated values of . We hypothesized that overdispersion can strongly affect the role of imports on the local dynamics. However, we found that higher and lower values of , representing more and less superspreading, did not result in notable differences estimates of (Supplementary Table 2).
4. Discussion
We analyzed routine surveillance data and used a stochastic branching process model to quantify the impact of imported cases on the SARS-CoV-2 epidemic in Switzerland during summer 2020 and 2021. We found that 27% and 25% of cases with a known place of exposure reported an exposure abroad in 2020 and 2021, respectively. Stochastic simulations suggested that the local was likely below the critical threshold of 1, highlighting that transmission within Switzerland was under sufficient control during the summers of both years. Our results indicate that imported cases caused the local epidemic to cross the tipping point of sustained growth. Imports led to a steady rise in cases from June to August and a drop in September in both years. Together, our results highlight that the high number of imported cases had a considerable impact on the dynamics of the Swiss SARS-CoV-2 epidemic during periods when local transmission was low.
Our study relied on detailed surveillance data from FOPH that provide information about the place of exposure for a subset of all confirmed SARS-CoV-2 cases. This allowed us to track imported cases by country of exposure and over time in detail. The stochastic modeling approach was particularly suitable to describe the impact of single importation events on the epidemic dynamics in low incidence settings. Another strength of our study was that our main findings and conclusions are supported by both data sets from summer 2020 and 2021.
There are a number of limitations to our study. First, information on the place of exposure was missing for a significant fraction of confirmed cases. This was because the place of exposure was only reported via the clinical report form, which could have been filled in for all cases up to 2 November 2020 and thereafter only for hospitalized cases, deceased cases, and cases in nursing homes. Since hospitalized cases, deceased cases, and patients in nursing homes tend to be older, cross-border-associated cases were older in 2021 than in 2020. To account for uncertainty of missing data, we compared the results of our baseline scenario (missing at random) with alternative scenarios that assumed imported cases were less or more likely to have unknown place of exposure than local cases. Second, the data did not allow to distinguish between different types of traveling, like daily commuting across borders to Germany, France, Italy, Austria, and the Principality of Liechtenstein, Swiss residents returning from trips abroad, or foreign tourists. There was also no information about the modes of transport like plane, car or public transport. Hence, the results of our study do not allow deriving specific interventions that would reduce the number of imported cases. Third, we assumed that the ascertainment of confirmed cases was constant and that they reflected the underlying dynamics of the SARS-CoV-2 epidemic. During the study periods, the degree of under-ascertainment was relatively low, suggesting that the confirmed cases represented the summer epidemics well (Nadeau et al., 2020, Stringhini et al., 2021). Fourth, we restricted the study periods to the summer of 2020 and 2021 and assumed a constant in our model simulations. We specifically chose these time periods because earlier research has shown the importance of holiday travel for the spread of SARS-CoV-2 during summer (Hodcroft et al., 2021). Furthermore, the locally implemented NPIs varied little throughout the study periods (the KOF Stringency Index remained largely constant from July to October of both years (Pleninger et al., 2021) and Supplementary Figure 1B) and the impact of seasonal effects on virus transmission is unlikely to change substantially during summer months (Neher et al., 2020). In contrast, varied considerably from autumn to spring and our stochastic modeling framework would not accurately capture the transmission dynamics during this time period. It is worth noting that the emergence of the variant Delta resulted in a higher intrinsic transmissibility of SARS-CoV-2 in summer 2021 compared to 2020 (Campbell et al., 2021). Fifth, we assumed that imported cases were as likely to generate secondary cases as local cases. In summer 2020, incoming travelers were not routinely tested but should have followed quarantine when they came from certain countries. Since quarantine was not enforced as in other countries (e.g., Australia or New Zealand) but based on trust, the adherence to this measure and its impact on further transmission of SARS-CoV-2 from imported cases – compared to local cases – remains unclear. Furthermore, mandatory quarantine for travelers from countries with many imported cases (e.g., France, Croatia, Italy, Germany) were only introduced in September 2020 or not at all. In the future, more detailed models that are informed by behavioral data and include different transmission rates could further improve our understanding of transmission from imported and local cases (Ashcroft et al., 2021). Finally, we did not consider different age groups and age-specific contact patterns in our model (Coletti et al., 2020, Jarvis et al., 2020). Our finding that the median age of imported cases was lower compared to local cases indicates that future studies should also consider social contact patterns of travelers to better understand their impact on SARS-CoV-2 epidemics.
Other estimates of the for Switzerland during the same time period were similar to our baseline scenario (https://github.com/covid-19-Re) (Huisman et al., 2020). However, these values likely represent overestimates. The results from our main scenario accounting for imports suggest that the local was below 1 and that imported cases led to a steady growth in the number of confirmed cases. Our study therefore supports the notion that travelers play an important role in the spread of SARS-CoV-2 (Worobey et al., 2020, Hodcroft et al., 2021, Shearer et al., 2022), and provides further evidence that the contribution of imported cases on the local epidemic dynamics depends on the local incidence (Russell et al., 2021, Lemey et al., 2021). Furthermore, the risk of getting infected with SARS-CoV-2 while abroad can differ from the risk at home due to differences in incidence and behavior. Many infected travelers returned to Switzerland from countries with a similar incidence to Switzerland, but incidence levels varied widely within countries and were arguably higher in tourist hotspots. An earlier study indeed showed that the number of imported cases is typically higher than what one would expect based on the incidence and travel volume (Hodcroft et al., 2021). In addition, it is likely that traveling is associated with leisure activities that result in a higher risk of infection (Coletti et al., 2021).
Public health measures to control the spread of SARS-CoV-2 from travelers will continue to play an important role in the global response against the SARS-CoV-2 pandemic. During the beginning of the pandemic, response strategies have varied enormously ranging from containment (e.g., Australia, New Zealand or Taiwan) to mitigation (e.g., European countries and the United States). Compared to 2020 and 2021, the situation had changed considerably due to the increasing levels of naturally acquired and vaccine-elicited immunity that substantially reduced COVID-19-related morbidity and mortality. Hence, the continuation of travel measures such as screening, quarantine, isolation, contact tracing, vaccination passports, and travel restrictions have to be carefully balanced against the high societal and economic costs that accompany these measures (Ashcroft et al., 2021, Clifford et al., 2021, McCrone et al., 2022). The choice of an objective for (re-)implementing travel measures will likely depend on the local context and the global situation with respect to SARS-CoV-2 transmission and the prevalence of variants of concern (VoCs). The expected seasonal variation in the level of SARS-CoV-2 transmission, the rise of the Omicron variant (Viana et al., 2022), and the expected emergence of future VoCs with altered transmission underlines the importance of internationally coordinated and evidence-based strategies to travel measures (Kucharski et al., 2022, McCrone et al., 2022). Though the outlook for SARS-CoV-2 may suggest that travel-restrictions may play a less prominent role in the future, they are particularly critical before other methods of treatment or prevention, like vaccination, are available, or when they are limited. Thus, developing frameworks to better understand the role of imported transmissions is critical for being prepared for future pandemics, so that effective measures can be successfully implemented while minimizing disruption.
The results of this study advance our understanding of the role of imported cases on the local epidemic dynamics of SARS-CoV-2. We found that imported cases can explain the steady growth of the epidemic in Switzerland during summer 2020 and 2021, a period without strong surveillance and control measures for travelers. Improved screening and surveillance efforts targeting travelers can continue to be valuable tools for controlling transmission of SARS-CoV-2, especially when local incidence is low.
CRediT authorship contribution statement
Martina L. Reichmuth: Conceived and designed the study, Performed the analysis, Interpretation of the results, Wrote the manuscript, Commented on the manuscript, Approved the final version. Emma B. Hodcroft: Interpretation of the results, Commented on the manuscript, Approved the final version. Julien Riou: Conceived and designed the study, Interpretation of the results, Commented on the manuscript, Approved the final version. Richard A. Neher: Interpretation of the results, Commented on the manuscript, Approved the final version. Niel Hens: Interpretation of the results, Commented on the manuscript, Approved the final version. Christian L. Althaus: Conceived and designed the study, Interpretation of the results, Wrote the manuscript, Commented on the manuscript, Approved the final version.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgment
We would like to thank the Swiss Federal Office of Public Health (FOPH) for providing surveillance data. Model simulations were performed on UBELIX (http://www.id.unibe.ch/hpc), the HPC cluster at the University of Bern.
Funding
MR, NH, and CA received funding from the European Union’s Horizon 2020 research and innovation program - project EpiPose (grant numbers 101003688). EH, RH, and CA were supported by the Swiss National Science Foundation (grant numbers 196046). JR was funded by the Swiss National Science Foundation (grant numbers 174281).
Footnotes
Supplementary material related to this article can be found online at https://doi.org/10.1016/j.epidem.2022.100654.
Appendix A. Supplementary data
The following is the Supplementary material related to this article.
.
Data availability
The authors do not have permission to share data.
References
- Althaus C.L. Ebola superspreading. Lancet Infect. Dis. 2015;15(5):507–508. doi: 10.1016/S1473-3099(15)70135-0. URL https://linkinghub.elsevier.com/retrieve/pii/S1473309915701350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashcroft P., Lehtinen S., Angst D.C., Low N., Bonhoeffer S. Quantifying the impact of quarantine duration on COVID-19 transmission. eLife. 2021;10 doi: 10.7554/eLife.63704. URL https://elifesciences.org/articles/63704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bi Q., Wu Y., Mei S., Ye C., Zou X., Zhang Z., Liu X., Wei L., Truelove S.A., Zhang T., Gao W., Cheng C., Tang X., Wu X., Wu Y., Sun B., Huang S., Sun Y., Zhang J., Ma T., Lessler J., Feng T. Epidemiology and transmission of COVID-19 in 391 cases and 1286 of their close contacts in Shenzhen, China: a retrospective cohort study. Lancet Infect. Dis. 2020;20(8):911–919. doi: 10.1016/S1473-3099(20)30287-5. URL https://linkinghub.elsevier.com/retrieve/pii/S1473309920302875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosshardt L., Bühler G., Bütikofer S., Craviolini J., Hermann M., David K., Müller E., Bruno W. Forschungsstelle sotomo; 2020. Die Schweiz und die Corona-Krise. URL https://sotomo.ch/site/wp-content/uploads/2020/06/SRG_sotomo_Monitoring_Coronakrise_W4.pdf. [Google Scholar]
- Campbell F., Archer B., Laurenson-Schafer H., Jinnai Y., Konings F., Batra N., Pavlin B., Vandemaele K., Van Kerkhove M.D., Jombart T., Morgan O., le Polain de Waroux O. Increased transmissibility and global spread of SARS-CoV-2 variants of concern as at June 2021. Eurosurveillance. 2021;26(24) doi: 10.2807/1560-7917.ES.2021.26.24.2100509. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.24.2100509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S., Pearson C.A.B., Klepac P., Van Zandvoort K., Quilty B.J., Eggo R.M., Flasche S., CMMID COVID-19 working group Effectiveness of interventions targeting air travellers for delaying local outbreaks of SARS-CoV-2. J. Travel Med. 2020;27(5):taaa068. doi: 10.1093/jtm/taaa068. URL https://academic.oup.com/jtm/article/doi/10.1093/jtm/taaa068/5834629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifford S., Quilty B.J., Russell T.W., Liu Y., Chan Y.-W.D., Pearson C.A.B., Eggo R.M., Endo A., Flasche S., Edmunds W.J., CMMID COVID-19 Working Group Strategies to reduce the risk of SARS-CoV-2 importation from international travellers: modelling estimations for the United Kingdom, July 2020. Eurosurveillance. 2021;26(39) doi: 10.2807/1560-7917.ES.2021.26.39.2001440. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.39.2001440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti P., Libin P., Petrof O., Willem L., Abrams S., Herzog S.A., Faes C., Kuylen E., Wambua J., Beutels P., Hens N. A data-driven metapopulation model for the Belgian COVID-19 epidemic: assessing the impact of lockdown and exit strategies. BMC Infect. Dis. 2021;21(1):503. doi: 10.1186/s12879-021-06092-w. URL https://bmcinfectdis.biomedcentral.com/articles/10.1186/s12879-021-06092-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coletti P., Wambua J., Gimma A., Willem L., Vercruysse S., Vanhoutte B., Jarvis C.I., Van Zandvoort K., Edmunds J., Beutels P., Hens N. CoMix: comparing mixing patterns in the Belgian population during and after lockdown. Sci. Rep. 2020;10(1):21885. doi: 10.1038/s41598-020-78540-7. URL http://www.nature.com/articles/s41598-020-78540-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Federal Council . 2020. Press release by federal council: Coronavirus. URL https://www.admin.ch/gov/en/start/documentation/media-releases/media-releases-federal-council.html?dyn_startDate=01.05.2020, Bern, Switzerland. [Google Scholar]
- Federal Council . 2021. Ordinance on measures to combat the coronavirus (COVID-19) in international passenger transport. URL https://www.fedlex.admin.ch/eli/cc/2021/61/en. [Google Scholar]
- Federal Office of Public Health . 2020. New Coronavirus 2019-nCoV: first confirmed case in Switzerland. URL https://www.bag.admin.ch/bag/en/home/das-bag/aktuell/medienmitteilungen.msg-id-78233.html. [Google Scholar]
- Ganyani T., Kremer C., Chen D., Torneri A., Faes C., Wallinga J., Hens N. Estimating the generation interval for coronavirus disease (COVID-19) based on symptom onset data, March 2020. Eurosurveillance. 2020;25(17) doi: 10.2807/1560-7917.ES.2020.25.17.2000257. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.17.2000257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gostic K.M., McGough L., Baskerville E.B., Abbott S., Joshi K., Tedijanto C., Kahn R., Niehus R., Hay J.A., De Salazar P.M., Hellewell J., Meakin S., Munday J.D., Bosse N.I., Sherrat K., Thompson R.N., White L.F., Huisman J.S., Scire J., Bonhoeffer S., Stadler T., Wallinga J., Funk S., Lipsitch M., Cobey S. Practical considerations for measuring the effective reproductive number, Rt. Pitzer V.E., editor. PLoS Comput. Biol. 2020;16(12) doi: 10.1371/journal.pcbi.1008409. URL https://dx.plos.org/10.1371/journal.pcbi.1008409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasell J., Mathieu E., Beltekian D., Macdonald B., Giattino C., Ortiz-Ospina E., Roser M., Ritchie H. A cross-country database of COVID-19 testing. Sci. Data. 2020;7(1):345. doi: 10.1038/s41597-020-00688-8. URL http://www.nature.com/articles/s41597-020-00688-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heim N., Müller S. 2020. Corona und Reiseverhalten Ergebnisse einer Studie zur Untersuchung der Konsumentenpräferenzen. URL https://www.zhaw.ch/de/sml/institute-zentren/imm/newsdetail/event-news/drei-von-vier-reisen-werden-storniert/ [Google Scholar]
- Hodcroft E.B., Zuber M., Nadeau S., Vaughan T.G., Crawford K.H.D., Althaus C.L., Reichmuth M.L., Bowen J.E., Walls A.C., Corti D., Bloom J.D., Veesler D., Mateo D., Hernando A., Comas I., Candelas F.G., SeqCOVID-SPAIN consortium L.F., Comas I., González-Candelas F., Goig G.A., Chiner-Oms Á., Cancino-Muñoz I., López M.G., Torres-Puente M., Gomez-Navarro I., Jiménez-Serrano S., Ruiz-Roldán L., Bracho M.A., García-González N., Martínez-Priego L., Galán-Vendrell I., Ruiz-Hueso P., Marco G.D., Ferrús M.L., Carbó-Ramírez S., D’Auria G., Coscollá M., Ruiz-Rodríguez P., Roig-Sena F.J., Sanmartín I., Garcia-Souto D., Pequeno-Valtierra A., Tubio J.M.C., Rodríguez-Castro J., Rabella N., Navarro F., Miró E., Rodríguez-Iglesias M., Galán-Sanchez F., Rodriguez-Pallares S., de Toro M., Escudero M.B., Azcona-Gutiérrez J.M., Alberdi M.B., Mayor A., García-Basteiro A.L., Moncunill G., Dobaño C., Cisteró P., García-de Viedma D., Pérez-Lago L., Herranz M., Sicilia J., Catalán-Alonso P., Muñoz P., Muñoz-Cuevas C., Rodríguez-Rodríguez G., Alberola-Enguidanos J., Nogueira J.M., Camarena J.J., Rezusta A., Tristancho-Baró A., Milagro A., Martínez-Cameo N.F., Gracia-Grataloup Y., Martró E., Bordoy A.E., Not A., Antuori-Torres A., Benito R., Algarate S., Bueno J., del Pozo J.L., Boga J.A., Castelló-Abietar C., Rojo-Alba S., Alvarez-Argüelles M.E., Melon S., Aranzamendi-Zaldumbide M., Vergara-Gómez A., Fernández-Pinero J., Martínez M.J., Vila J., Rubio E., Peiró-Mestres A., Navero-Castillejos J., Posada D., Valverde D., Estévez-Gómez N., Fernandez-Silva I., de Chiara L., Gallego-García P., Varela N., Moreno R., Tirado M.D., Gomez-Pinedo U., Gozalo-Margüello M., Eliecer-Cano M., Méndez-Legaza J.M., Rodríguez-Lozano J., Siller M., Pablo-Marcos D., Oliver A., Reina J., López-Causapé C., Canut-Blasco A., Hernáez-Crespo S., Cordón M.L.A., Lecároz-Agara M.-C., Gómez-González C., Aguirre-Quiñonero A., López-Mirones J.I., Fernández-Torres M., Almela-Ferrer M.R., Gonzalo-Jiménez N., Ruiz-García M.M., Galiana A., Sanchez-Almendro J., Cilla G., Montes M., Piñeiro L., Sorarrain A., Marimón J.M., Gomez-Ruiz M.D., López-Hontangas J.L., González Barberá E.M., Navarro-Marí J.M., Pedrosa-Corral I., Sanbonmatsu-Gámez S., Pérez-González C., Chamizo-López F., Bordes-Benítez A., Navarro D., Albert E., Torres I., Gascón I., Torregrosa-Hetland C.J., Pastor-Boix E., Cascales-Ramos P., Fuster-Escrivá B., Gimeno-Cardona C., Ocete M.D., Medina-Gonzalez R., González-Cantó J., Martínez-Macias O., Palop-Borrás B., de Toro I., Mediavilla-Gradolph M.C., Pérez-Ruiz M., González-Recio Ó., Gutiérrez-Rivas M., Simarro-Córdoba E., Lozano-Serra J., Robles-Fonseca L., de Salazar A., Viñuela-González L., Chueca N., García F., Gómez-Camarasa C., Carvajal A., de la Puente R., Martín-Sánchez V., Fregeneda-Grandes J.-M., Molina A.J., Argüello H., Fernández-Villa T., Farga-Martí M.A., Domínguez-Márquez V., Costa-Alcalde J.J., Trastoy R., Barbeito-Castiñeiras G., Coira A., Pérez-del Molino M.L., Aguilera A., Planas A.M., Soriano A., Fernandez-Cádenas I., Pérez-Tur J., Marcos M.Á., Moreno-Docón A., Viedma E., Mingorance J., Galán-Montemayor J.C., Parra-Grande M., Stadler T., Neher R.A. Spread of a SARS-CoV-2 variant through Europe in the summer of 2020. Nature. 2021 doi: 10.1038/s41586-021-03677-y. URL http://www.nature.com/articles/s41586-021-03677-y. [DOI] [PubMed] [Google Scholar]
- Huisman J.S., Scire J., Angst D.C., Li J., Neher R.A., Maathuis M.H., Bonhoeffer S., Stadler T. Estimation and worldwide monitoring of the effective reproduc-tive number of SARS-CoV-2. eLife. 2020 doi: 10.1101/2020.11.26.20239368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis C.I., Van Zandvoort K., Gimma A., Prem K., Klepac P., Rubin G.J., Edmunds W.J., CMMID COVID-19 working group Quantifying the impact of physical distance measures on the transmission of COVID-19 in the UK. BMC Med. 2020;18(1):124. doi: 10.1186/s12916-020-01597-8. URL https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01597-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kremer C., Torneri A., Boesmans S., Meuwissen H., Verdonschot S., Vanden Driessche K., Althaus C.L., Faes C., Hens N. Quantifying superspreading for COVID-19 using Poisson mixture distributions. Sci. Rep. 2021;11(1):14107. doi: 10.1038/s41598-021-93578-x. URL http://www.nature.com/articles/s41598-021-93578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucharski A.J., Jit M., Logan J.G., Cotten M., Clifford S., Quilty B.J., Russell T.W., Peeling R.W., Antonio M., Heymann D.L. Travel measures in the SARS-CoV-2 variant era need clear objectives. Lancet. 2022;399(10333):1367–1369. doi: 10.1016/S0140-6736(22)00366-X. URL https://linkinghub.elsevier.com/retrieve/pii/S014067362200366X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laxminarayan R., Wahl B., Dudala S.R., Gopal K., Mohan B C., Neelima S., Jawahar Reddy K.S., Radhakrishnan J., Lewnard J.A. Epidemiology and transmission dynamics of COVID-19 in two Indian states. Science. 2020;370(6517):691–697. doi: 10.1126/science.abd7672. URL https://www.sciencemag.org/lookup/doi/10.1126/science.abd7672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemaitre J.C., Perez-Saez J., Azman A.S., Rinaldo A., Fellay J. Assessing the impact of non-pharmaceutical interventions on SARS-CoV-2 transmission in Switzerland. Swiss Med Wkly. 2020 doi: 10.4414/smw.2020.20295. URL https://doi.emh.ch/smw.2020.20295. [DOI] [PubMed] [Google Scholar]
- Lemey P., Ruktanonchai N., Hong S.L., Colizza V., Poletto C., Van den Broeck F., Gill M.S., Ji X., Levasseur A., Oude Munnink B.B., Koopmans M., Sadilek A., Lai S., Tatem A.J., Baele G., Suchard M.A., Dellicour S. Untangling introductions and persistence in COVID-19 resurgence in Europe. Nature. 2021 doi: 10.1038/s41586-021-03754-2. URL http://www.nature.com/articles/s41586-021-03754-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCrone J.T., Hill V., Bajaj S., Pena R.E., Lambert B.C., Inward R., Bhatt S., Volz E., Ruis C., Dellicour S., Baele G., Zarebski A.E., Sadilek A., Wu N., Schneider A., Ji X., Raghwani J., Jackson B., Colquhoun R., O’Toole Á., Peacock T.P., Twohig K., Thelwall S., Dabrera G., Myers R., Faria N.R., Huber C., Bogoch I.I., Khan K., du Plessis L., Barrett J.C., Aanensen D.M., Barclay W.S., Chand M., Connor T., Loman N.J., Suchard M.A., Pybus O.G., Rambaut A., Kraemer M.U.G., The COVID-19 genomics UK (COG-UK) consortium Context-specific emergence and growth of the SARS-CoV-2 Delta variant. Nature. 2022 doi: 10.1038/s41586-022-05200-3. URL https://www.nature.com/articles/s41586-022-05200-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadeau S., Beckmann C., Topolsky I., Vaughan T., Hodcroft E., Schär T., Nissen I., Santacroce N., Burcklen E., Ferreira P., Jablonski K.P., Posada-Céspedes S., Capece V., Seidel S., de Souza N.S., Martinez-Gomez J.M., Cheng P., Bosshard P.P., Levesque M.P., Kufner V., Schmutz S., Zaheri M., Huber M., Trkola A., Cordey S., Laubscher F., Gonçalves A.R., Leuzinger K., Stange M., Mari A., Roloff T., Seth-Smith H., Hirsch H.H., Egli A., Redondo M., Kobel O., Noppen C., Beerenwinkel N., Neher R.A., Beisel C., Stadler T. Quantifying SARS-CoV-2 spread in Switzerland based on genomic sequencing data. Epidemiology. 2020 doi: 10.1101/2020.10.14.20212621. URL http://medrxiv.org/lookup/doi/10.1101/2020.10.14.20212621. [DOI] [Google Scholar]
- Neher R.A., Dyrdak R., Druelle V., Hodcroft E.B., Albert J. Potential impact of seasonal forcing on a SARS-CoV-2 pandemic. Swiss Med Wkly. 2020 doi: 10.4414/smw.2020.20224. URL https://doi.emh.ch/smw.2020.20224. [DOI] [PubMed] [Google Scholar]
- Pleninger R., Streicher S., Sturm J.-E. 2021. Do COVID-19 Containment Measures Work? Evidence from Switzerland; p. 53. URL http://hdl.handle.net/20.500.11850/493408, Artwork Size: 53 p. Medium: application/pdf Publisher: ETH Zurich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- R. Core Team R. R Foundation for Statistical Computing; Vienna, Austria: 2020. R: A Language and Environment for Statistical Computing. URL https://www.R-project.org/ [Google Scholar]
- Riou J., Althaus C.L. Pattern of early human-to-human transmission of Wuhan 2019 novel coronavirus (2019-nCoV), December 2019 to January 2020. Eurosurveillance. 2020;25(4) doi: 10.2807/1560-7917.ES.2020.25.4.2000058. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2020.25.4.2000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritchie H., Edouard M., Lucas R.-G., Appel C., Giattino C., Ortiz-Ospina E., Hasell J., Macdonald B., Beltekian D., Roser M. Coronavirus Pandemic (COVID-19) Our World Data. 2020 URL https://ourworldindata.org/coronavirus. [Google Scholar]
- Russell T.W., Wu J.T., Clifford S., Edmunds W.J., Kucharski A.J., Jit M. Effect of internationally imported cases on internal spread of COVID-19: a mathematical modelling study. Lancet Public Health. 2021;6(1):e12–e20. doi: 10.1016/S2468-2667(20)30263-2. URL https://linkinghub.elsevier.com/retrieve/pii/S2468266720302632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shearer F.M., Walker J., Tellioglu N., McCaw J.M., McVernon J., Black A., Geard N. Rapid assessment of the risk of SARS-CoV-2 importation: case study and lessons learned. Epidemics. 2022;38 doi: 10.1016/j.epidem.2022.100549. URL https://linkinghub.elsevier.com/retrieve/pii/S175543652200010X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringhini S., Zaballa M.-E., Pullen N., Perez-Saez J., de Mestral C., Loizeau A.J., Lamour J., Pennacchio F., Wisniak A., Dumont R., Baysson H., Richard V., Lorthe E., Semaani C., Balavoine J.-F., Pittet D., Vuilleumier N., Chappuis F., Kherad O., Azman A.S., Posfay-Barbe K., Kaiser L., Guessous I., on behalf of the Specchio-COVID19 study group Seroprevalence of anti-SARS-CoV-2 antibodies 6 months into the vaccination campaign in Geneva, Switzerland, 1 June to 7 July 2021. Eurosurveillance. 2021;26(43) doi: 10.2807/1560-7917.ES.2021.26.43.2100830. URL https://www.eurosurveillance.org/content/10.2807/1560-7917.ES.2021.26.43.2100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taube J.C., Miller P.B., Drake J.M. An open-access database of infectious dis-ease transmission trees to explore superspreader epidemiology. Infectious Diseases(except HIV/AIDS) PLOS Bio. 2021 doi: 10.1101/2021.01.11.21249622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viana R., Moyo S., Amoako D.G., Tegally H., Scheepers C., Althaus C.L., Anyaneji U.J., Bester P.A., Boni M.F., Chand M., Choga W.T., Colquhoun R., Davids M., Deforche K., Doolabh D., du Plessis L., Engelbrecht S., Everatt J., Giandhari J., Giovanetti M., Hardie D., Hill V., Hsiao N.-Y., Iranzadeh A., Ismail A., Joseph C., Joseph R., Koopile L., Kosakovsky Pond S.L., Kraemer M.U.G., Kuate-Lere L., Laguda-Akingba O., Lesetedi-Mafoko O., Lessells R.J., Lockman S., Lucaci A.G., Maharaj A., Mahlangu B., Maponga T., Mahlakwane K., Makatini Z., Marais G., Maruapula D., Masupu K., Matshaba M., Mayaphi S., Mbhele N., Mbulawa M.B., Mendes A., Mlisana K., Mnguni A., Mohale T., Moir M., Moruisi K., Mosepele M., Motsatsi G., Motswaledi M.S., Mphoyakgosi T., Msomi N., Mwangi P.N., Naidoo Y., Ntuli N., Nyaga M., Olubayo L., Pillay S., Radibe B., Ramphal Y., Ramphal U., San J.E., Scott L., Shapiro R., Singh L., Smith-Lawrence P., Stevens W., Strydom A., Subramoney K., Tebeila N., Tshiabuila D., Tsui J., van Wyk S., Weaver S., Wibmer C.K., Wilkinson E., Wolter N., Zarebski A.E., Zuze B., Goedhals D., Preiser W., Treurnicht F., Venter M., Williamson C., Pybus O.G., Bhiman J., Glass A., Martin D.P., Rambaut A., Gaseitsiwe S., von Gottberg A., de Oliveira T. Rapid epidemic expansion of the SARS-CoV-2 Omicron variant in southern Africa. Nature. 2022;603(7902):679–686. doi: 10.1038/s41586-022-04411-y. URL https://www.nature.com/articles/s41586-022-04411-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worobey M., Pekar J., Larsen B.B., Nelson M.I., Hill V., Joy J.B., Rambaut A., Suchard M.A., Wertheim J.O., Lemey P. The emergence of SARS-CoV-2 in Europe and North America. Science. 2020;370(6516):564–570. doi: 10.1126/science.abc8169. URL https://www.sciencemag.org/lookup/doi/10.1126/science.abc8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
.
Data Availability Statement
The authors do not have permission to share data.