Abstract
Background
Data on response and safety of repeated vaccinations and hybrid immunity in patients with immune-mediated inflammatory diseases on immunosuppressive therapy is needed to further develop vaccination strategies in this vulnerable population. This study aimed to evaluate hybrid immunity and humoral immune response and safety of four SARS-CoV-2 vaccine doses in patients with immune-mediated inflammatory diseases on immunosuppressive therapy.
Methods
This prospective observational Norwegian study of vaccine response to COVID-19 (Nor-vaC) included adult patients aged 18 years and older with immune-mediated inflammatory diseases (rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, Crohn's disease, or ulcerative colitis) on immunosuppressive therapy, who had received four SARS-CoV-2 vaccine doses (vaccine group) or three vaccine doses followed by COVID-19 (hybrid group), and healthy controls receiving three vaccine doses (control group). Patients were recruited from the Division of Rheumatology at Diakonhjemmet Hospital, Oslo, and the Department of Gastroenterology at Akershus University Hospital, Lørenskog. Patients who had COVID-19 before the third vaccine dose, and patients with allergies or intolerances to elements of the vaccine were excluded. Antibodies to the receptor-binding domain of SARS-CoV-2 spike protein (anti-RBD antibodies) were assessed 2–4 weeks following vaccination or COVID-19. This study is registered at Clinialtrials.gov, NCT04798625.
Findings
Between Nov 12, 2021, and April 19, 2022, 1458 participants with immune-mediated inflammatory diseases provided post-vaccination samples at 2–4 weeks following a third vaccine dose. After 544 participants were excluded, 715 (78%) of the remaining 914 participants received the fourth dose of the vaccine, and of these, 536 (75%) provided post-vaccination samples 2–4 weeks after their fourth vaccination (vaccine group). 199 (22%) of the 914 had COVID-19 after their third dose of the vaccine and of these, 167 (84%) provided samples (hybrid group). 256 of the eligible 703 patients had rheumatoid arthritis, 107 had spondyloarthritis, 115 had psoriatic arthritis, 130 had Crohn's disease, and 95 had ulcerative colitis). Median age was 56 years [IQR 45–65], 398 (57%) were women, and 305 (43%) were men. Patients in the vaccine group had higher anti-RBD antibody concentrations following the fourth vaccine dose (median 6192 BAU/ml [IQR 2878–11 243]) than after the third dose (median 5087 BAU/ml [1250–9081]; p< 0·0001), but lower antibody concentrations than the control group following the third dose (median 7595 BAU/ml [5916–12 001]; p< 0·0001). Antibody concentrations were higher in the patients in the hybrid group (23 548 BAU/ml [IQR 11 440–35 935]) than in the vaccine group (p<0·0001). No difference was found in antibody concentrations between the fourth dose of BNT162b2 (full-dose) and mRNA-1273 (half-dose). Patients and controls had a comparable safety profile after both three and four vaccine doses.
Interpretation
Vaccine boosters improve humoral immune responses and are safe in patients with immune-mediated inflammatory diseases on immunosuppressive therapy, and administration should be considered regularly in this patient group. Hybrid immunity with omicron induces a strong humoral response suggesting longer intervals between booster doses in this patient group.
Funding
The South-Eastern Norway Regional Health Authority, The Coalition for Epidemic Preparedness Innovations, Akershus University Hospital
Introduction
Patients with immune-mediated inflammatory diseases on immunosuppressive therapy are known to have impaired humoral immune responses to standard SARS-CoV-2 vaccination regimens, and are at increased risk of severe COVID-19 and hospital admission.1, 2, 3, 4 Repeated vaccination might therefore be especially important in these patients and other vulnerable populations.5 Several countries now recommend three primary vaccine doses followed by a fourth booster dose for this patient group. Although studies regarding serological response and safety of a fourth dose of SARS-CoV-2 mRNA vaccines are available for healthy populations,6, 7, 8, 9, 10 data from patients with immune-mediated inflammatory diseases on immunosuppressive therapy are scarce.11, 12, 13
Research in context.
Evidence before this study
We searched PubMed and medRxiv for studies published in English between Jan 1, 2021, and Aug 15, 2022, using the following terms “immune-mediated inflammatory disorder”, “auto-immune”, “immunosuppressive therapy”, “immunocompromised”, “SARS-CoV-2”, “COVID-19”, “fourth vaccine dose”, “second booster dose”, “hybrid immunity”, “rheumatic disease”, and “inflammatory bowel disease” to identify studies on immune response to four vaccine doses in patients on immunosuppressive therapy or hybrid immunity. We identified two small studies and one case-series indicating an improved humoral immune response to a fourth vaccine dose in populations including mainly non-responders to previous vaccination. Currently, few data exist on a fourth vaccine dose in patients with immune-mediated inflammatory diseases receiving mRNA vaccines, and on hybrid immunity with the omicron variant in these patients. We have previously published data from the prospective, observational Norwegian study of vaccine response to COVID-19 on humoral and cellular immune responses among patients with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, and inflammatory bowel disease treated with immunosuppressive medication, demonstrating impaired humoral and cellular immune responses following standard vaccination in these patient groups.
Added value of this study
In this large cohort of patients with immune-mediated inflammatory diseases, we have shown that receiving a fourth mRNA vaccine dose was safe and associated with an increased humoral immune response compared with three vaccine doses. Rituximab and TNF inhibitor therapy was associated with lower humoral immune responses. There was a positive association between time from rituximab infusion and antibody concentration. Moreover, this study showed superiority of hybrid immunity (ie, humoral immune responses in patients who had COVID-19 following three vaccine doses) resulting in a stronger immune response than infection-naïve patients with four vaccine doses. Infections occurred during a time of omicron circulation, demonstrating that omicron induces a potent activation of humoral immune responses.
Implications of all the available evidence
Results from this study show that humoral immune responses can be improved by regular vaccine boosters and should be recommended to patients on immunosuppressive therapy. Importantly, humoral immune response following half-dose mRNA-1273 equals the response to full-dose BNT162b2 and might help economise global vaccine booster programmes. Hybrid immunity gives a very substantial humoral immune response in patients with immune-mediated inflammatory diseases on immunosuppressive therapy, implying that additional vaccine doses can be postponed in patients with hybrid immunity. Further studies are needed to address the clinical importance of hybrid immunity against the omicron variants and how infection with SARS-CoV-2 should be considered in an optimal regime for booster vaccinations in this vulnerable population.
Current mRNA-based vaccines against SARS-CoV-2 have induced neutralising immunity against the earlier SARS-CoV-2 variants,14, 15 and anti-spike antibody concentrations have been shown to correlate with protection against COVID-19.16 Two doses of the mRNA-1273 (Moderna) vaccine have been shown to induce a superior humoral response compared with the BNT162b2 (Pfizer–BioNtech) vaccine in patients with immune-mediated inflammatory diseases 17 and in the general population.18
Current vaccines have remained clinically effective in preventing severe disease, but the fast-spreading SARS-CoV-2 omicron variants (BA.1, BA.2, BA.3, BA.4 and BA.5) escape neutralisation by antibodies induced solely by vaccines19 and are associated with breakthrough infections.20 Thus, current research interest is focused on the effect of hybrid immunity with SARS-CoV-2 vaccination in combination with COVID-19. Hybrid immunity has been demonstrated to enhance neutralising antibodies in healthy individuals,21 and protection against severe disease by hybrid immunity in healthy people seems at least equal to repeated vaccination.22 There is a need to explore the humoral antibody response following hybrid immunisation in patients with immune-mediated inflammatory diseases on immunosuppressive therapy23 to develop optimal vaccination programmes regarding number of vaccinations, appropriate timing of booster doses, and choice of vaccine.
The prospective, observational Norwegian study of vaccine response to COVID-19 (Nor-vaC) included patients with immune-mediated inflammatory diseases on immunosuppressive medication. The objectives of this study were to evaluate humoral immune response and safety of four vaccine doses and hybrid immunisation in patients with immune-mediated inflammatory diseases on immunosuppressive therapy, compared with three vaccine doses in the same patients and in healthy controls. Furthermore, we aimed to compare humoral immune responses induced by a fourth dose of BNT162b2 (full-dose) versus mRNA-1273 (half-dose), and humoral immunity across diseases and medication groups.
Methods
Study design and participants
Nor-vaC is an ongoing longitudinal observational study conducted at two Norwegian centres; the Division of Rheumatology at Diakonhjemmet Hospital, Oslo, and the Department of Gastroenterology at Akershus University Hospital, Lørenskog. Adult patients (aged ≥18 years) with rheumatoid arthritis, spondyloarthritis, psoriatic arthritis, Crohn's disease, or ulcerative colitis treated with immunosuppressive medication were identified by hospital records and included in the study (appendix p 2). Only patients with the intention of obtaining SARS-CoV-2 vaccination were included in the study, and any patients with allergies or intolerance to elements of the vaccine were excluded. Patients were consecutively recruited into the study before initiation of the national vaccination programme in February, 2021, and consenting health-care workers were recruited as controls.
All participants received primary vaccination recommended by the national vaccination programme administered by the Norwegian Institute of Public Health. Three different SARS-CoV-2 vaccines were initially available: BNT162b2, mRNA-1273, and ChAdOx1 nCoV-19. The ChAdOx1 nCoV-19 (AstraZeneca) vaccine was withdrawn from the Norwegian vaccination programme in March, 2021. Primary vaccination in patients with immune-mediated inflammatory diseases consisted of three doses followed by a fourth booster dose at least 12 weeks after the third dose (vaccine group). Healthy controls received two vaccine doses as primary vaccination and a third booster dose at least 12 weeks after the second dose (control group). The recommended interval between the first two doses of the mRNA vaccines was 3–6 weeks in patients with immune-mediated inflammatory diseases and the general population, with the third dose in patients with immune-mediated inflammatory diseases after a minimum of 4 weeks. In people receiving the ChAdOx1 nCoV-19 vaccine as the first dose, one of the mRNA vaccines were offered as a second dose with an interval of 9–12 weeks. Patients with COVID-19 after the initial vaccine series of three doses were not eligible for a booster dose (hybrid group). According to the national programme, boosters were administered as either a half-dose of mRNA-1273 or as a full dose of BNT162b2.
Patients and controls provided serum samples on a regular basis throughout the study: before vaccination, and at 2–4 weeks and 12 weeks following each vaccine dose or COVID-19. In the present analyses, we included patients who underwent serological testing 2–4 weeks after the third and fourth vaccine dose (vaccine group), patients who provided serum samples 2–4 weeks following COVID-19 after the third vaccine dose (hybrid group), and healthy controls who provided serum samples 2–4 weeks after the third vaccine dose (control group). Patients who had COVID-19 before the third vaccine dose were excluded from the analyses.
The study was approved by the Norwegian Regional Committees for Medical and Health Research Ethics South East (reference numbers 235424 and 135924), and by appropriate institutional review boards. All participants provided written informed consent.
Procedures
IgG antibodies to the receptor binding domain (RBD) of the SARS-CoV-2 spike protein were assessed and reported in standardised units (BAU/mL) by using an in-house bead-based method validated against a micro-neutralisation assay at the Department of Immunology at Oslo University Hospital.24 Data from Diakonhjemmet Hospital were collected using Nettskjema, an electronic questionnaire tool hosted by University of Oslo and stored in the research portal Services for Sensitive Data. Data from Akershus University Hospital were collected and managed using Viedoc (version 4; Sweden).
Demographic data were collected at baseline, and immunosuppressive medication data were collected at time of vaccination. Gender data were based on the national identity number indicating sex, with male and female as available options. Patients were regularly reminded to report any cases of COVID-19 verified by either a PCR test or a rapid antigen test. Adverse events were reported in patients and controls 14 days following each vaccine dose. Data on administered vaccine types were provided from the national database of COVID-19 vaccinations, The Norwegian Immunisation Registry, SYSVAK.
The primary outcomes were anti-RBD antibody concentrations assessed 2–4 weeks following the third and fourth vaccine dose in the vaccine group, 2–4 weeks after COVID-19 in the hybrid group, and 2–4 weeks following the third vaccine dose in the control group. Secondary outcomes were self-reported adverse events after each vaccine dose in patients and controls. All outcomes were prespecified.
Statistical analyses
Descriptive statistics were used to summarise the baseline characteristics of people in the vaccine, hybrid, and control groups. The following comparisons in anti-RBD antibody concentrations were carried out: (1) between the third and fourth dose of the vaccine group, (2) between the vaccine and control group, (3) between the vaccine and hybrid group, (4) between vaccine types, and finally across (5) medications and (6) diseases. Group-level antibody data were summarised in the form of medians (IQRs) and graphically presented using violin plots. Unadjusted group comparisons were done using non-parametric methods, (ie, Mann-Whitney U test for independent groups (comparisons 2–5) and Wilcoxon-test for the paired comparison (1). Adjusted comparisons of (2–5) were done using multivariable median regression. The selected adjustment variables were prespecified and based on clinical knowledge and previous evidence. For the comparison between vaccine and control groups, we adjusted for age, sex, vaccine type, vaccine sequence, time between vaccination and blood sampling, and time between past two vaccinations categorised according to elapsed months. For comparisons between the vaccine and hybrid group, and between vaccine types additional adjustments were made for medication and disease. For the comparisons across medications, additional adjustments were made for disease, and for the comparison across diseases, adjustments were made for medication. The comparison between third and fourth doses of the vaccine group was done separately for patients on rituximab, where Spearmans ρ was used to assess the association between antibody response and time since last infusion.
Although the analyses were primarily explorative and descriptive, several comparisons were carried out (about 34 unadjusted and adjusted comparisons in total), requiring correction of the p value for multiplicity. Therefore, we only considered comparisons with p values of 0·0017 or below (=0·05/30) as significant, which by the Bonferroni adjustment controls the overall type 1 error to a maximum of 5%. Data were analysed using Stata (version 17; StataCorp) and R (version 4.0.3). This study is registered at Clinialtrials.gov, NCT04798625.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report.
Results
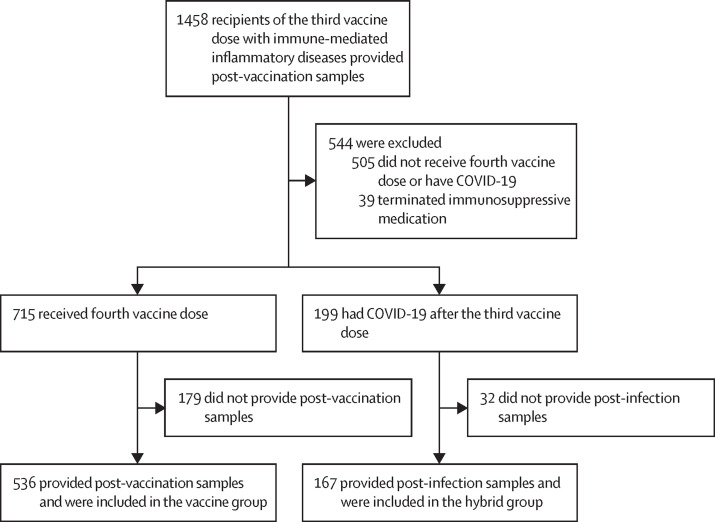
Between Nov 12, 2021, and April 19, 2022, 1458 participants with immune-mediated inflammatory diseases who had received the third vaccine dose provided post-vaccination samples at 2–4 weeks. 505 were excluded for not receiving the fourth vaccine dose or having COVID-19 before the third vaccine dose, and 39 were excluded for terminating their immunosuppressive medication. 715 (78%) of the remaining 914 participants received the fourth dose of the vaccine, and of these, 536 (75%) provided post-vaccination samples 2–4 weeks after their fourth vaccination (vaccine group). 199 (22%) of the 914 participants had COVID-19 after the third vaccine dose and of these, 167 (84%) provided post-infection samples after hybrid immunisation with a series of three vaccine doses followed by COVID-19 (hybrid group; figure 1 ).
Figure 1.
Study profile
Of the 703 eligible patients in the vaccine group and the hybrid group, 256 [36%] had rheumatoid arthritis, 107 [15%] had spondyloarthritis, 115 [16%] had psoriatic arthritis, 130 [18%] had Crohn's disease, and 95 [14%] had ulcerative colitis. The median age for these two groups was 56 years [IQR 45–65], and 398 (57%) were women and 305 (43%) were men. The median age of the control group was 45 years (IQR 35–56), 131 (88%) of 149 were women and 18 (12%) were men.
Sera for antibody assessment were obtained at a median of 25 days (IQR 21–29) after the third vaccine dose and a median of 23 days (20–30) after the fourth vaccine dose in the vaccine group, and a median of 27 days (21–34) following COVID-19 in the hybrid group. In the control group, sera were assessed at a median of 29 days [IQR 22–47] after the third vaccine dose. Baseline characteristics for patients and controls including vaccine distribution are presented in table 1 .
Table 1.
Baseline characteristics
| All patients (n=703) | Vaccine group (n=536) | Hybrid group (n=167) | Controls (n=149) | |
|---|---|---|---|---|
| Demographics | ||||
| Age, years | 56 (45–65) | 59 (49–67) | 44 (34–55) | 45 (35–56) |
| Female | 398 (57%) | 307 (57%) | 91 (54%) | 131 (88%) |
| Male | 305 (43%) | 229 (43%) | 76 (46%) | 18 (12%) |
| Body-mass index, kg/m2 | 26 (23–29) | 26 (23–29) | 26 (23–29) | .. |
| Current smoker | 62 (9%) | 50 (9%) | 12 (7%) | .. |
| Immune-mediated inflammatory disease | ||||
| Rheumatoid arthritis | 256 (36%) | 225 (42%) | 31 (19%) | .. |
| Spondyloarthritis | 107 (15%) | 85 (16%) | 22 (13%) | .. |
| Psoriatic arthritis | 115 (16%) | 89 (17%) | 26 (16%) | .. |
| Crohn's disease | 130 (18%) | 85 (16%) | 45 (27%) | .. |
| Ulcerative colitis | 95 (14%) | 52 (10%) | 43 (26%) | .. |
| Medication | ||||
| TNF inhibitor, monotherapy* | 275 (39%) | 189 (35%) | 86 (51%) | .. |
| TNF inhibitor, combination therapy† | 152 (22%) | 111 (21%) | 41 (25%) | .. |
| Methotrexate | 126 (18%) | 111 (21%) | 15 (9%) | .. |
| Vedolizumab | 28 (4%) | 21 (4%) | 7 (4%) | .. |
| Janus kinase inhibitor | 21 (3%) | 18 (3%) | 3 (2%) | .. |
| Rituximab | 47 (7%) | 44 (8%) | 3 (2%) | .. |
| Interleukin inhibitor‡ | 39 (6%) | 30 (6%) | 9 (5%) | .. |
| Abatacept | 6 (1%) | 6 (1%) | 0 | .. |
| Other§ | 9 (1%) | 6 (1%) | 3 (2%) | .. |
| First vaccine | ||||
| BNT162b2 | 527 (75%) | 407 (76%) | 120 (72%) | 66 (44%) |
| mRNA-1273 | 159 (23%) | 119 (22%) | 40 (24%) | 38 (26%) |
| ChAdOx1 nCoV-19 | 17 (2%) | 10 (2%) | 7 (4%) | 45 (30%) |
| Second vaccine | ||||
| BNT162b2 | 542 (77%) | 415 (77%) | 127 (76%) | 110 (74%) |
| mRNA-1273 | 161 (23%) | 121 (23%) | 40 (24%) | 39 (26%) |
| Third vaccine | ||||
| BNT162b2 | 428 (61%) | 319 (60%) | 109 (65%) | 140 (94%) |
| mRNA-1273 | 275 (39%) | 217 (40%) | 58 (35%) | 9 (6%) |
| Fourth vaccine or COVID-19 | ||||
| BNT162b2 | 241 (34%) | 241 (45%) | .. | .. |
| mRNA-1273 | 295 (42%) | 295 (55%) | .. | .. |
| COVID-19¶ | 167 (24%) | .. | 167 (100%) | .. |
| Vaccine series | ||||
| BNT162b2, homogenous series | .. | 172 (32%) | 90 (54%) | 60 (40%) |
| mRNA-1273, homogenous series | .. | 72 (13%) | 27 (16%) | 5 (3%) |
| Combination of vaccines‖ | .. | 292 (54%) | 50 (30%) | 84 (56%) |
Data are median (IQR) or n (%).
TNF inhibitors: infliximab, etanercept, adalimumab, golimumab, certolizumab pegol.
Combination therapy: TNF inhibitor in combination with methotrexate, sulfasalazine, leflunomide, or azathioprine.
Ustekinumab, secukinumab, and tocilizumab.
Leflunomide, sulfasalazine, and azathioprine.
COVID-19 after three vaccine doses (hybrid immunity).
Combination of the following vaccines: ChAdOx1 nCoV-19, BNT162b2, mRNA-1273.
In the hybrid immunity group, COVID-19 cases following a series of three vaccine doses were registered between Oct 23, 2021, and March 18, 2022. Most people in this group (143 [86%] of 167) had COVID-19 after January, 2022, when the omicron variant was dominant in Norway (representing 94% of all cases detected in the general population according to the Norwegian Surveillance System for Communicable Diseases). Of the remaining 24 (14%) who had COVID-19 before January, 2022, sequenced PCR tests showed that eight had the omicron variant (B.1.1.529), 12 had the delta variant (B.1.617.2), and four people did not have their variant identified. Patients had COVID-19 a median of 92 days (IQR 62–118) after the third vaccine dose. Sex disaggregated data are presented in the appendix (pp 6–11).
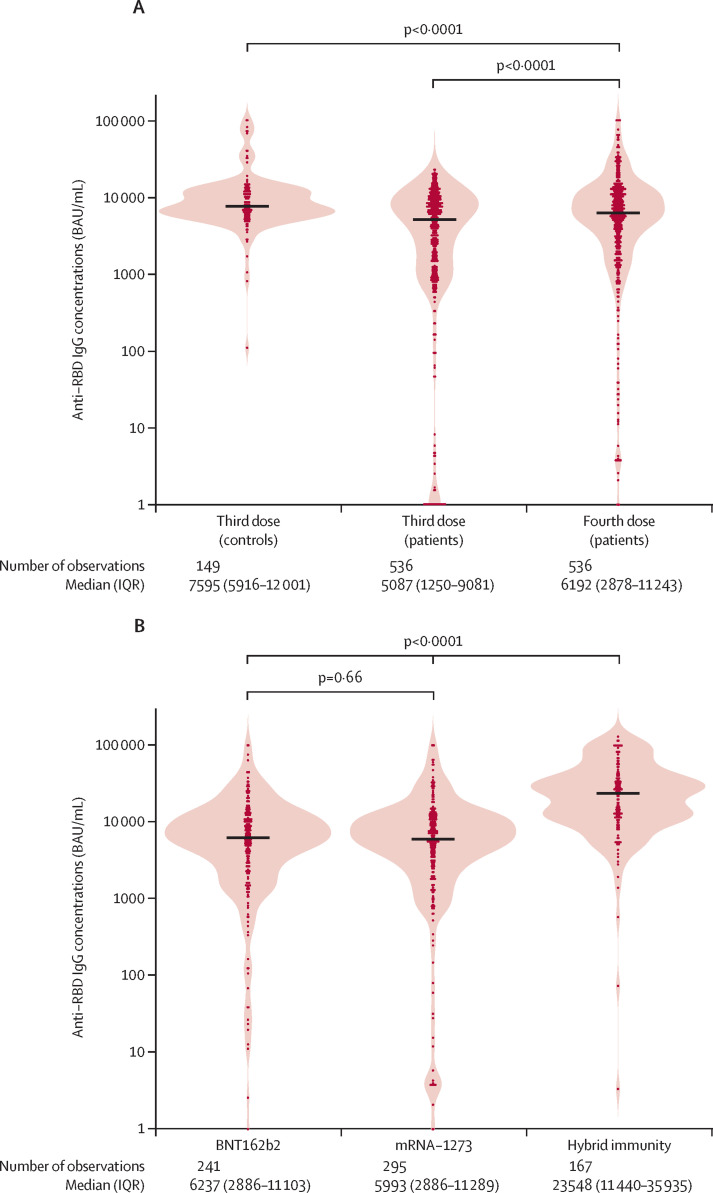
In the vaccine group, anti-RBD antibody concentrations were higher following the fourth vaccine dose (median 6192 BAU/ml [IQR 2878–11 243]) than the third dose (median 5087 BAU/ml [1250–9081]; p<0·0001), but were lower than in the control group vaccinated with three doses (median 7595 BAU/ml [5916–12 001]; p<0·0001; figure 2A ). The comparison between patients and controls remained robust after adjusting for possible confounders in multivariable median regression analysis (p<0·0001; appendix p 3). In the vaccine group, there was no significant difference in anti-RBD antibody concentrations between fourth vaccine dose with full-dose BNT162b2 versus half-dose mRNA-1273 (median 6237 BAU/ml [IQR 2886–11 103] vs 5993 BAU/ml [2886–11 289]; p=0·66; figure 2B).
Figure 2.
Anti-RBD IgG antibody concentrations
(A) Anti-RBD IgG antibody concentrations following the third and fourth vaccine doses in the vaccine group and third vaccine dose in the control group. P values show unadjusted comparisons between the vaccine group and control group, and between third and fourth vaccine dose in the vaccine group. (B) Anti-RBD IgG antibody concentrations following a fourth vaccine dose in the vaccine group and COVID-19 after three vaccine doses in the hybrid group. P values show unadjusted comparisons between the vaccine types (BNT162b2 or mRNA-1273) in the vaccine group, and between the hybrid group and the vaccine group. Violin plot of probability densities, smoothed by a kernel density estimator. Points denote participants, and solid black lines show group medians. RBD=receptor binding domain.
In the vaccine group, patients received the fourth vaccine dose a median of 106 (IQR 97–125) days after the third dose. In unadjusted analyses, patients who received the fourth dose after more than 150 days after the third dose (n=43) had significantly lower antibody concentrations than did patients who received the fourth dose 90–120 days following the third dose (n=357; median 1366 BAU/ml [IQR 147–5652]) vs 6918 BAU/ml [3807–12 100]); p<0·0001). This association was not robust in a multivariable regression analysis adjusting for age, sex, diagnosis, medication, vaccine type, vaccine sequence, time from vaccine dose to sampling, and time between third and fourth vaccine dose (adjusted median 4994 BAU/ml [IQR 2966–7022] vs 6435 BAU/ml [5774–7095]; p=0·19).
In the hybrid group, anti-RBD antibody concentrations were higher than in the vaccine group (23 548 BAU/ml [IQR 11 440–35 935] vs 6192 BAU/ml [2878–11 243]; p<0·0001). Significantly higher antibody concentrations in the hybrid group were seen only compared with those in the vaccine group who received a fourth dose with the BNT162b2 vaccine (figure 2B, table 2 ). The comparison between the hybrid group and participants in the vaccine group who received a fourth dose with BNT162b2 remained robust after adjusting for possible confounders in multivariable median regression analysis (p<0·0001; table 2). Antibody concentrations in the hybrid group were also higher than in the control group (7595 BAU/ml [IQR 5916–12 001]; p<0·0001).
Table 2.
Effect of a fourth SARS-CoV-2 vaccine dose and hybrid immunity on anti-RBD antibody concentrations, with adjustment for possible confounding factors
| Univariable analysis (unadjusted) |
Multivariable analyses (adjusted) |
|||
|---|---|---|---|---|
| Median (BAU/mL) | Median (BAU/mL) | 95% CI | p value | |
| BNT162b2 | 6237 | 6647 | 5036–8258 | 1 (ref) |
| mRNA-1273 | 5993 | 5811 | 4342–7281 | 0·44 |
| COVID-19 | 23 548 | 23513 | 21 226–25 801 | <0·0001 |
Multivariable median regression model for the vaccine group versus the hybrid group with adjustments for possible confounding factors. All variables were prespecified. Covariates included in this model were age, sex, diagnosis, medication group, vaccine type, vaccine series, time from fourth vaccine dose or COVID-19 to sampling, time between third and fourth vaccine dose, and time from third vaccine dose to COVID-19. The dependent variable was anti-RBD antibody concentrations (BAU/mL). BAU=binding antibody units. RBD=receptor binding domain.
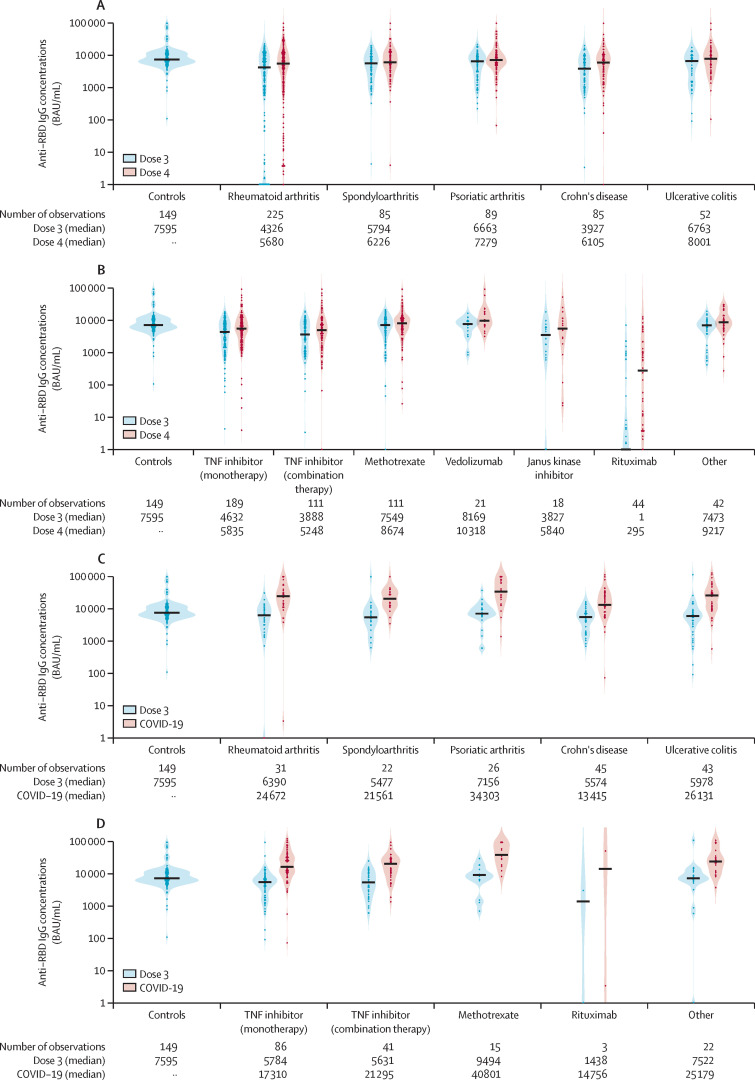
In the vaccine group, we found higher anti-RBD antibody concentrations following the fourth vaccine dose compared with the third dose, a finding that was consistent across diseases (figure 3A ).
Figure 3.
Distribution of anti-RBD antibody concentrations across diagnoses and medication groups in the vaccine and hybrid groups
(A) The distribution of anti-RBD antibody concentrations across diagnoses in patients following a series of three and four vaccine doses (vaccine group), and in controls receiving three vaccine doses. (B) The distribution of anti-RBD antibody concentrations across medication groups in patients following a series of three and four vaccine doses (vaccine group), and in controls receiving three doses. (C) The distribution of anti-RBD antibody concentrations across diagnoses in patients with hybrid immunity with COVID-19 following a series of three vaccine doses (hybrid group). (D) The distribution of anti-RBD antibody concentrations across medication groups in patients with hybrid immunity with COVID-19 following a series of three vaccine doses (hybrid group). Violin plot of probability densities, smoothed by a kernel density estimator. Points denote participants, and solid black lines show group medians. Anti-RBD antibody concentrations after the third vaccine dose are marked in light blue, and after the fourth dose (vaccine group) or COVID-19 infection (hybrid group) in red. Anti-RBD antibody concentrations after the third vaccine dose in the control group are shown to the left. RBD=receptor binding domain.
Across medication groups, tumour necrosis factor (TNF) inhibitor, both in monotherapy and combination therapy, and rituximab treatment, were associated with lower humoral immune responses following the fourth vaccine dose than the reference methotrexate (appendix p 3). This pattern was robust in the adjusted analyses (appendix p 3). Janus kinase inhibitor was also associated with lower humoral immune responses, but this subcohort was limited (n=18) and was not significant in adjusted analyses (p=0·22; figure 3B; appendix p 3).
Patients with Crohn's disease and hybrid immunity had a lower serological response following COVID-19 than did patients with other diagnoses (figure 3C); however, the comparisons across diagnosis groups were not significant in multivariable analyses (Crohns disease compared with the reference rheumatoid arthritis; adjusted median 17 941 BAU/ml [IQR 6834–29048] vs 18 435 BAU/ml [3383–33488]; p=0·96). Anti-RBD antibody concentrations were comparable in patients with hybrid immunity on different medications (figure 3D). No conclusions could be drawn regarding ritixumab treatment in patients with hybrid immunity due to the small number in this group (n=3).
In the 44 patients on rituximab, anti-RBD antibody concentrations showed a median of 295 BAU/mL (IQR 4–1830) following the fourth vaccine dose compared with a median of 1 BAU/mL (IQR 1–77) following the third dose (p<0·0001; figure 3B). Time since last rituximab infusion was median 210 days (IQR 60–330) following the third dose and median 270 days (150–345) following the fourth dose. There was a positive association between time since last rituximab infusion and antibody concentration following the fourth vaccine dose (ρ=0·44, p=0·003; appendix p 12).
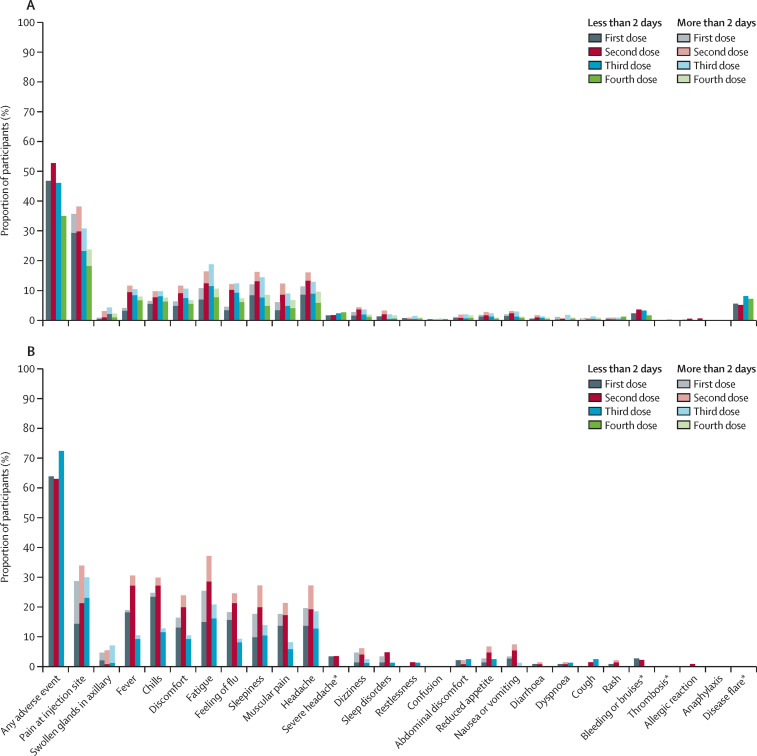
Adverse events after the third and fourth vaccine doses were reported by 283 (46%) of 616 patients after the third dose, and 171 (35%) of 491 patients after fourth dose, and by 63 (72%) of 87 healthy controls after the third dose (appendix pp 4–5). The safety profile was comparable between vaccine doses, and between patients and controls (figure 4 , appendix pp 4–5). No serious adverse events were reported, and no new safety signals emerged. Disease flare was reported by 35 (7%) of 491 patients following the fourth vaccine dose.
Figure 4.
Type and duration of adverse events reported after vaccination among patients (A) and controls (B)
Grey, red, blue, and green bars indicate adverse events reported after the first, second, third, and fourth vaccine doses. *Duration not measured.
Discussion
This large observational study encompassing patients with immune-mediated inflammatory diseases on a range of immunosuppressive therapies showed higher anti-RBD antibody concentrations following four vaccine doses than three vaccine doses, indicating that repeated vaccination gradually improves humoral immunity in this patient population. Additionally, we demonstrated a stronger humoral immune response in patients with hybrid immunity with three vaccine doses followed by COVID-19, mainly with the omicron variant, than in infection-naïve patients in the vaccine group receiving four vaccine doses. The serological response was comparable between full-dose BNT162b2 and half-dose mRNA-1273 given as the fourth dose, and patients (four doses) and controls (three doses) had a comparable safety profile.
To our knowledge, this is the largest study to investigate humoral immunity following repeated vaccination with a series of four mRNA or adenoviral vector vaccine doses in a population of patients with immune-mediated inflammatory diseases. Previous studies are few and with small sample sizes.12, 13 One previous study reported a positive serological response to a fourth mRNA vaccine dose in patients previously not responding to three inactivated vaccine doses and is thus not directly comparable to our study.11
Germinal centre formation is a key event following vaccination or infection. Through somatic hypermutation and cellular division, B cells expressing high affinity B-cell receptors are selected for differentiation into plasma cells that efficiently secrete antibodies that could neutralise infection. Importantly, the threshold for selection into the memory B-cell compartment is different, with memory B cells correspondingly displaying B-cell receptors with a wider selection of specificities for immunogenic epitopes.25 The implication is that with the establishment of a functional B-cell memory response, a wider range of antigenic variants might be recognised during later exposures. Thus, efficient memory B-cell formation also dictates a potential for recall responses to SARS-CoV-2 variants, and probably also the ability to prevent potential future severe disease following infection with circulating variants.
As efficient antibody activation is a marker of memory formation, it is important that the reduced antibody formation observed following the first vaccinations in patients with immune-mediated inflammatory diseases on immunosuppressive therapy can be augmented by repeated vaccinations.3, 5 Our results support the importance of administering repeated vaccination with additional SARS-CoV-2 vaccine doses to patients with immune-mediated inflammatory diseases, a patient population that is still considered to be at higher risk of serious outcomes from COVID-19.26, 27 Furthermore, this study showed that patients on TNF inhibitor therapy, either as monotherapy or in combination with other immunosuppressants, have a weaker humoral immune response than do patients on other medications. This finding is in line with previous data showing that TNF inhibitors negatively affect long-term humoral immunity,4 with a rapid decline in neutralising capacity being enhanced by a third vaccine dose. Previous studies suggest a third dose improved cross-variant neutralisation for non-omicron variants.28, 29 The long-term durability of humoral immunity after four vaccine doses should be investigated in further studies.
Several studies have shown that patients with immune-mediated inflammatory diseases treated with anti-CD20 therapy such as rituximab have an increased risk of hospital admission and death due to COVID-19.30, 31 Our findings indicate a benefit of administrating a fourth vaccine dose to patients on rituximab therapy in terms of increased anti-RBD antibody concentrations. This increased humoral immune response from the third to the fourth dose might in part be related to pausing of rituximab in some patients during the pandemic, resulting in longer intervals between the last rituximab infusion and the fourth dose versus the third dose. In line with previous data,3 we show an association between the concentration of antibodies and time since the last rituximab infusion. Only three patients on rituximab had COVID-19 after the third vaccine dose. Thus, we cannot draw any conclusions regarding hybrid immunity in rituximab-treated patients.
This study demonstrated a superior antibody response following hybrid immunity with three vaccine doses and COVID-19 compared with four vaccine doses in patients on immunosuppressive therapy. This finding remained robust when adjusting for confounders including age, time between vaccine doses, and time from COVID-19 to sampling. Importantly, patients in this study were mainly infected with omicron, the dominant variant in Norway from January, 2022, which suggests that undergoing SARS-CoV-2 infection induces a robust immunisation boost in patients on immunosuppressive therapy. Hybrid immunity has been shown to induce considerable anti-RBD antibody responses in healthy individuals;21, 32, 33 however, this is the first study to demonstrate superior humoral immune responses of hybrid immunisation with recent COVID-19 variants for patients on immunosuppressive therapy. Recent studies emphasise that antibodies from individuals undergoing omicron variant BA.1 infection lack neutralising capacity against other variants such as BA.4 and BA.5.34 However, several studies have shown superior protection by hybrid immunity against breakthrough omicron infections, even if infection was with pre-omicron variants.30, 35 Whether hybrid immunity increases clinical protection against these newer strains of the omicron variant is still unknown.
Three vaccinations followed by viral infection could more potently raise antibody responses than four vaccinations, in part due to the increased number of antigens able to activate immunity following a viral infection and possibly also due to increased germinal centre formation following exposure to a new antigenic variant. Repeated exposure to a similar antigen is typically not associated with increased affinity maturation,36 which from an evolutionary standpoint, might be important for maintaining protection to a variable environment. Viral infections potentially might support the maintenance of germinal centres more long-term, and as such also potentially increase the breadth of antigens that can efficiently be recognised.37
To our knowledge, a comparison of humoral immune responses following fourth dose vaccination with mRNA-1273 (half-dose) and BNT162b2 (full-dose) has not been described in patients on immunosuppressive therapy. There was no significant difference in anti-RBD antibody concentrations between patients with immune-mediated inflammatory diseases who received half-dose mRNA-1273 and full-dose BNT162b2 as a fourth vaccine dose, although antibody concentrations following mRNA-1273 were numerically higher. This result is in line with a smaller study of healthy individuals, which indicated that half-dose mRNA-1273 induces higher immunogenicity than does full-dose BNT162b2 as a fourth vaccine dose.8 Our findings suggest that patients on immunosuppressive therapy have similar immune responses to half-dose mRNA-1273 as they do to full-dose BNT162b2, and this might help inform and economise global vaccine booster programmes.
The optimal timing for booster doses in patients on immunosuppressive therapy is a current knowledge gap. In this study we found that receiving the fourth vaccine dose more than 150 days after the third dose was associated with a lower humoral immune response than was receiving the fourth dose between 90–120 days following the third dose. This finding was not robust when adjusting for possible confounders and there was little variation in the interval between vaccine doses in this study; therefore, this finding should be interpreted with caution.
Vaccine hesitancy is a widespread problem, partially due to concerns regarding vaccine side-effects.38 We found no serious adverse events after administration of a fourth vaccine dose, and the occurrence of reported adverse events was lower than after the third dose. Only 7% of patients reported a disease flare following vaccination. Our findings might help inform vaccine programmes and have clear clinical significance in that vaccine hesitant patients with immune-mediated inflammatory diseases can be reassured that there is a good benefit to risk ratio when receiving a fourth dose. Considering the superiority of hybrid immunity,33 patients who have had COVID-19 following primary vaccination and who have not yet been boosted, should be encouraged to get a booster vaccine.
Major strengths of this study include the prospective study design with regular anti-RBD antibody assessments, broad inclusion criteria, and a large sample size including well characterised patients with a wide range of diagnoses and therapies. We report concentrations of antibodies to the RBD from ancestral SARS-CoV-2, which have been shown to correlate with neutralising antibodies to multiple variants, including omicron BA.1.24
Limitations of this study include that COVID-19 cases relied on self-reported data. Consequently, we cannot exclude that some participants might have had subclinical COVID-19 or might not have reported a SARS-CoV-2 infection. However, throughout the study, patients received regular reminders regarding the importance of reporting COVID-19 and presumably had a strong self-interest in reporting infections and obtaining post-infection serology results. The main outcome of this study was serological response, which might not directly translate to clinical protection. Furthermore, in our study we report concentrations of antibodies to the RBD from ancestral SARS-CoV-2 and not against BA.5, which is currently the dominant variant.24 Hence, uncertainty remains regarding whether patients with higher antibody responses in the hybrid group are better protected against severe COVID-19 than are those who have not had COVID-19. However, earlier studies in healthy individuals have demonstrated a strong correlation between antibody concentrations and disease susceptibility, making such increased protection probable.15, 39 Another limitation is that T-cell responses were not evaluated in this study. Furthermore, the current study has not investigated the severity of omicron infection in patients, a factor that might influence the immune response.
At the current stage of the COVID-19 pandemic, a key question is how to maintain immunity to SARS-CoV-2 in vulnerable patient groups as well as in the general population. Knowledge on the benefit, side-effects, timing, and choice of vaccine booster types are key elements in developing optimal vaccine programmes. These questions are urgent in regions where vaccines are scarce, or vaccine hesitancy is prevalent. The current study adds important new knowledge pertaining to the utility of booster doses in the patient population on immunosuppressive therapy. We show a clear benefit regarding humoral immunity of a fourth dose with reassuring safety signals. This study adds to the current debate on how immunisation and omicron infection should be considered in an optimal regime for booster vaccinations in this vulnerable population. Although we cannot recommend that patients deliberately contract COVID-19, it seems clear that the people with immune-mediated inflammatory diseases obtain a significant immunological boost if they do get infected with SARS-CoV-2, even the omicron variant. Hence, immunisation in patients with immune-mediated inflammatory diseases who have had COVID-19 after three vaccine doses might be considered superior to a fourth vaccine dose, implying that additional vaccine doses might be postponed in patients with hybrid immunity.
Data sharing
A de-identified patient data set can be made available to researchers upon reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses and will have to be approved by the Norwegian study of vaccine response to COVID-19 steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.
Declaration of interests
KHB reports funding from Akershus University Hospital and speaker bureaus for Janssen-Cilag. TKK reports grants from AbbVie, Amgen, Bristol Myers Squibb, Merck Sharp & Dohme, Novartis, Pfizer, Union Chimique Belge; consulting fees from AbbVie, Biogen, Celltrion, Eli Lilly, Gilead, Mylan, Novartis, Pfizer, Sandoz, Sanofi; and speakers bureaus from Amgen, Celltrion, Egis, Evapharma, Ewopharma, Hikma, Oktal, Sandoz, and Sanofi. JJ reports grants from Boehringer-Ingelheim; speakers bureaus from AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, and Takeda; and participation on advisory board for AbbVie, Bristol-Myers Squibb, Galapagos, Gilead, Janssen, Pfizer, Roche, Sandoz, and Takeda. LAM reports funding from KG Jebsen foundation, support for infrastructure and biobanking from the university of Oslo and Oslo University Hospital, grants from the Coalition of Epidemic Preparedness Innovations, and speakers bureaus from Novartis, and Cellgene. EAH reports consultant fees from AbbVie, Boehringer-Ingelheim, Eli Lilly, and Gilead; and speakers bureaus from Pfizer and UCB. GG reports speaker bureaus from Bayer, Sanofi, ThermoFisher, and participation on advisory board for AstraZeneca. JTV reports grants from the Coalition of Epidemic Preparedness Innovations. GLG reports funding from the South-Eastern Norway Regional Health Authority, The Coalition for Epidemic Preparedness Innovations, RCN Covid (312693), a KG Jebsen Foundation (grant 19), Dr Trygve Gythfeldt og frues forskningsfond, Karin Fossum Foundation, the Research Foundation at Diakonhjemmet Hospital, and Oslo University Hospital; speakers bureaus from AbbVie, Galapagos, Pfizer, and Union Chimique Belge; and participation on advisory board of AbbVie, Galapagos, Pfizer, and Union Chimique Belge. KKJ reports speakers bureaus from Bristol-Myers Squibb and Roche. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank the patients and health-care workers who have participated in the Norwegian study of vaccine response to COVID-19. We are grateful for the time and effort they have invested in the project. We thank the patient representatives in the study group, Kristin Isabella Kirkengen Espe and Roger Thoresen. Many people have contributed to the study design and implementation of the study at Diakonhjemmet Hospital, Akershus University Hospital, and Oslo University Hospital. We thank all study personnel, laboratory personnel, and other staff involved at the clinical departments involved, particularly Synnøve Aure, Margareth Sveinsson, May Britt Solem, Elisabeth Røssum-Haaland, and Kjetil Bergsmark.
Contributors
KHB, HSØ, ATT, and JS verified all the data in the study. KHB, HSØ, ATT, SWS, GLG, and KKJ made the final decision to submit the manuscript for publication. KHB, HSØ, ATT, IJ, IEC, GBK, TKK, JJ, LAM, DJW, EAH, SAP, JTV, SWS, GLG, KKJ, and SM conceived and designed the study. KHB, HSØ, GG, SWS, GLG, and KKJ drafted the manuscript. TKK, JJ, LAM, EAH, GG, JTV, SWS, GLG, and KKJ supervised the study. KHB, ATT, IJ, TTT, AC, SWS, GLG, and KKJ contributed to acquisition of data. KHB and JS contributed to statistical analysis. ATT, JS, GBK, LAM, JTV, EAH, GLG, and KKJ contributed with administrative, technical, or material support. LAM, JTV, GLG, and KKJ obtained funding for this study. All authors contributed to interpretation and analysis of the data. All authors critically revised the manuscript and approved the final submitted version. All authors confirm they had full access to data in the study and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Parker EPK, Desai S, Marti M, et al. Response to additional COVID-19 vaccine doses in people who are immunocompromised: a rapid review. Lancet Glob Health. 2022;10:e326–e3e8. doi: 10.1016/S2214-109X(21)00593-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wieske L, van Dam KPJ, Steenhuis M, et al. Humoral responses after second and third SARS-CoV-2 vaccination in patients with immune-mediated inflammatory disorders on immunosuppressants: a cohort study. Lancet Rheumatol. 2022;4:e338–ee50. doi: 10.1016/S2665-9913(22)00034-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jyssum I, Kared H, Tran TT, et al. Humoral and cellular immune responses to two and three doses of SARS-CoV-2 vaccines in rituximab-treated patients with rheumatoid arthritis: a prospective, cohort study. Lancet Rheumatol. 2022;4:e177–ee87. doi: 10.1016/S2665-9913(21)00394-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Haberman RH, Um S, Axelrad JE, et al. Methotrexate and TNF inhibitors affect long-term immunogenicity to COVID-19 vaccination in patients with immune-mediated inflammatory disease. Lancet Rheumatol. 2022;4:e384–e3e7. doi: 10.1016/S2665-9913(22)00069-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Syversen SW, Jyssum I, Tveter AT, et al. Immunogenicity and safety of standard and third-dose SARS-CoV-2 vaccination in patients receiving immunosuppressive therapy. arthritis Rheumatol. 2022;74:1321–1332. doi: 10.1002/art.42153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bar-On YM, Goldberg Y, Mandel M, et al. Protection by a fourth dose of BNT162b2 against omicron in Israel. N Engl J Med. 2022;386:1712–1720. doi: 10.1056/NEJMoa2201570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Magen O, Waxman JG, Makov-Assif M, et al. Fourth dose of BNT162b2 mRNA Covid-19 vaccine in a nationwide setting. N Engl J Med. 2022;386:1603–1614. doi: 10.1056/NEJMoa2201688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munro APS, Feng S, Janani L, et al. Safety, immunogenicity, and reactogenicity of BNT162b2 and mRNA-1273 COVID-19 vaccines given as fourth-dose boosters following two doses of ChAdOx1 nCoV-19 or BNT162b2 and a third dose of BNT162b2 (COV-BOOST): a multicentre, blinded, phase 2, randomised trial. Lancet Infect Dis. 2022;22:1131–1141. doi: 10.1016/S1473-3099(22)00271-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Regev-Yochay G, Gonen T, Gilboa M, et al. Efficacy of a fourth dose of Covid-19 mRNA vaccine against omicron. N Engl J Med. 2022;386:1377–1380. doi: 10.1056/NEJMc2202542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Arbel R, Sergienko R, Friger M, et al. Effectiveness of a second BNT162b2 booster vaccine against hospitalization and death from COVID-19 in adults aged over 60 years. Nat Med. 2022;28:1486–1490. doi: 10.1038/s41591-022-01832-0. [DOI] [PubMed] [Google Scholar]
- 11.Aikawa NE, Kupa LVK, Silva CA, et al. Strong response after 4th dose of mRNA COVID-19 vaccine in autoimmune rheumatic diseases patients with poor response to inactivated vaccine. Rheumatology (Oxford) 2022 doi: 10.1093/rheumatology/keac301. published online May 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mrak D, Simader E, Sieghart D, et al. Immunogenicity and safety of a fourth COVID-19 vaccination in rituximab-treated patients: an open-label extension study. Ann Rheum Dis. 2022 doi: 10.1136/ard-2022-222579. published 2022. [DOI] [PubMed] [Google Scholar]
- 13.Teles M, Connolly CM, Frey S, et al. Attenuated response to fourth dose SARS-CoV-2 vaccination in patients with autoimmune disease: a case series. Ann Rheum Dis. 2022;81:738–740. doi: 10.1136/annrheumdis-2021-221641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garcia-Beltran WF, St Denis KJ, Hoelzemer A, et al. mRNA-based COVID-19 vaccine boosters induce neutralizing immunity against SARS-CoV-2 omicron variant. Cell. 2022;185:457. doi: 10.1016/j.cell.2021.12.033. 66.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Khoury DS, Cromer D, Reynaldi A, et al. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat Med. 2021;27:1205–1211. doi: 10.1038/s41591-021-01377-8. [DOI] [PubMed] [Google Scholar]
- 16.Feng S, Phillips DJ, White T, et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat Med. 2021;27:2032–2040. doi: 10.1038/s41591-021-01540-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mitchell J, Connolly CM, Chiang TP, et al. Comparison of SARS-CoV-2 antibody response after 2-dose mRNA-1273 vs BNT162b2 vaccines in incrementally immunosuppressed patients. JAMA Netw Open. 2022;5:e2211897. doi: 10.1001/jamanetworkopen.2022.11897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaplonek P, Cizmeci D, Fischinger S, et al. mRNA-1273 and BNT162b2 COVID-19 vaccines elicit antibodies with differences in Fc-mediated effector functions. Sci Transl Med. 2022;14:eabm2311. doi: 10.1126/scitranslmed.abm2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cao Y, Wang J, Jian F, et al. Omicron escapes the majority of existing SARS-CoV-2 neutralizing antibodies. Nature. 2022;602:657–663. doi: 10.1038/s41586-021-04385-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andrews N, Stowe J, Kirsebom F, et al. Covid-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Khan K, Karim F, Cele S, et al. Omicron infection enhances Delta antibody immunity in vaccinated persons. Nature. 2022;607:356–359. doi: 10.1038/s41586-022-04830-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altarawneh HN, Chemaitelly H, Ayoub HH, et al. Effects of previous infection and vaccination on symptomatic omicron infections. N Engl J Med. 2022;387:21–34. doi: 10.1056/NEJMoa2203965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shenoy P, Ahmed S, Paul A, et al. Hybrid immunity versus vaccine-induced immunity against SARS-CoV-2 in patients with autoimmune rheumatic diseases. Lancet Rheumatol. 2022;4:e80–ee2. doi: 10.1016/S2665-9913(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tran TT, Vaage EB, Mehta A, et al. Multiplexed measurement of binding- and neutralizing antibodies to SARS-CoV-2 variants in 12·000 post-vaccine sera. bioRxiv. 2022 doi: 10.1101/2022.03.26.484261. published 2022. (preprint) [DOI] [Google Scholar]
- 25.Victora GD, Nussenzweig MC. Germinal Centers. Annu Rev Immunol. 2022;40:413–442. doi: 10.1146/annurev-immunol-120419-022408. [DOI] [PubMed] [Google Scholar]
- 26.Cordtz R, Lindhardsen J, Soussi BG, et al. Incidence and severeness of COVID-19 hospitalization in patients with inflammatory rheumatic disease: a nationwide cohort study from Denmark. Rheumatology (Oxford) 2021;60:Si59–si67. doi: 10.1093/rheumatology/keaa897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Akiyama S, Hamdeh S, Micic D, Sakuraba A. Prevalence and clinical outcomes of COVID-19 in patients with autoimmune diseases: a systematic review and meta-analysis. Ann Rheum Dis. 2021;80:384–391. doi: 10.1136/annrheumdis-2020-218946. [DOI] [PubMed] [Google Scholar]
- 28.Chen RE, Gorman MJ, Zhu DY, et al. Reduced antibody activity against SARS-CoV-2 B.1·617·2 delta virus in serum of mRNA-vaccinated individuals receiving tumor necrosis factor-α inhibitors. Med (N Y) 2021;2:1327. doi: 10.1016/j.medj.2021.11.004. 41.e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Geisen UM, Rose R, Neumann F, et al. The long term vaccine-induced anti-SARS-CoV-2 immune response is impaired in quantity and quality under TNFα blockade. J Med Virol. 2022;94:5780–5789. doi: 10.1002/jmv.28063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Boekel L, Besten YR, Hooijberg F, et al. SARS-CoV-2 breakthrough infections in patients with immune-mediated inflammatory diseases during the omicron dominant period. Lancet Rheumatol. 2022;4:e747–e750. doi: 10.1016/S2665-9913(22)00221-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Felten R, Duret PM, Bauer E, et al. B-cell targeted therapy is associated with severe COVID-19 among patients with inflammatory arthritides: a 1-year multicentre study in 1116 successive patients receiving intravenous biologics. Ann Rheum Dis. 2022;81:143–145. doi: 10.1136/annrheumdis-2021-220549. [DOI] [PubMed] [Google Scholar]
- 32.Hall V, Foulkes S, Insalata F, et al. Protection against SARS-CoV-2 after Covid-19 vaccination and previous infection. N Engl J Med. 2022;386:1207–1220. doi: 10.1056/NEJMoa2118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Epsi NJ, Richard SA, Lindholm DA, et al. Understanding ‘hybrid immunity': comparison and predictors of humoral immune responses to SARS-CoV-2 infection and COVID-19 vaccines. Clin Infect Dis. 2022 doi: 10.1093/cid/ciac392. published 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuekprakhon A, Nutalai R, Dijokaite-Guraliuc A, et al. Antibody escape of SARS-CoV-2 omicron BA.4 and BA.5 from vaccine and BA.1 serum. Cell. 2022;185:2422. doi: 10.1016/j.cell.2022.06.005. 33.e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Malato J, Ribeiro RM, Leite PP, et al. Risk of BA.5 infection among persons exposed to previous SARS-CoV-2 variants. N Engl J Med. 2022;387:953–954. doi: 10.1056/NEJMc2209479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tang J, Grubbs G, Lee Y, et al. Antibody affinity maturation and cross-variant activity following SARS-CoV-2 mRNA vaccination: Impact of prior exposure and sex. EBioMedicine. 2021;74:103748. doi: 10.1016/j.ebiom.2021.103748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang Z, Muecksch F, Schaefer-Babajew D, et al. Naturally enhanced neutralizing breadth against SARS-CoV-2 one year after infection. Nature. 2021;595:426–431. doi: 10.1038/s41586-021-03696-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Larson HJ, Gakidou E, Murray CJL. The Vaccine-hesitant moment. N Engl J Med. 2022;387:58–65. doi: 10.1056/NEJMra2106441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cromer D, Steain M, Reynaldi A, et al. Neutralising antibody titres as predictors of protection against SARS-CoV-2 variants and the impact of boosting: a meta-analysis. Lancet Microbe. 2022;3:e52–e61. doi: 10.1016/S2666-5247(21)00267-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
A de-identified patient data set can be made available to researchers upon reasonable request. The data will only be made available after submission of a project plan outlining the reason for the request and any proposed analyses and will have to be approved by the Norwegian study of vaccine response to COVID-19 steering group. Project proposals can be submitted to the corresponding author. Data sharing will have to follow appropriate regulations.