Abstract
Between 2020 and 2021, 31,525 hematopoietic stem cell transplantations (HSCTs) were reported to the Chinese Blood and Marrow Transplantation Registry Group throughout mainland China. In this report, we describe the activity and current trends for HSCT in China during the SARS-CoV-2 pandemic. In 2020, a total of 13,415 cases of HSCT were reported from 166 transplantation teams, and 75% (10,042 cases) were allogeneic HSCTs. In 2021, a total of 18,110 cases of HSCT were reported from 174 transplantation teams, and 70% (12,744 cases) were allogeneic HSCTs. Haploidentical donor (HID) transplantation accounted for 63% (7977 cases) of allogeneic HSCTs in 2021. The most common indications for allogeneic HSCT for malignant disease were acute myeloid leukemia (37%) and acute lymphoblastic leukemia (23%), and the largest proportion of nonmalignant disease comprised aplastic anemia (13%). The peripheral blood stem cell source accounted for 41% of HIDs and 75% of matched sibling donors. The BuCy-based regimen (57%) was the most popular conditioning regimen for allogeneic HSCT, followed by the BuFlu-based regimen (28%) and total body irradiation–based regimen (11%). This survey provides comprehensive information about the current activities and might benefit clinical physicians’ decision planning for HSCT.
Keywords: Hematopoietic stem cell transplantations, SARS-CoV-2 pandemic, Survey
Hematopoietic stem cell transplantation (HSCT) is an established therapy for many malignant and nonmalignant hematologic diseases, as well as other life-threatening diseases 1, 2, 3, 4. The Chinese Blood and Marrow Transplantation Registry Group (CBMTRG) was established in 2007 and collects transplantation data in China every 6 months. The analysis using data from the CBMTRG illustrated a steady increase in the annual numbers of HSCTs since 2008 [5,6]. Coronavirus disease 2019 (COVID-19), a severe respiratory illness caused by the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), emerged at the beginning of 2020 and was declared a pandemic by the World Health Organization on March 11, 2020. This pandemic imposed unprecedented stress on the health care system, including programs performing allogeneic and autologous HSCT. Data analysis from the European Society for Blood and Marrow Transplantation in 2020 showed a decrease in transplant activity for the first time [7]. There was a similar descending trend for the annual numbers of transplants in the U.S. in 2020 [8]. During the prevalence of COVID-19, this retrospective analysis was performed using the CBMTRG survey data of 2020 to 2021 to deliver an overview of the current state of HSCT in China.
METHODS
Data collection
A nationwide registration of HSCT has been organized by the Stem Cell Application Section of the Hematology Branch of the Chinese Medical Association since 2008. For the recipients of HSCT in China, most of the costs are covered by public health insurance. Data including transplantation numbers, patient sex and age, disease, donor type, stem cell source, conditioning regimen, and protocols for graft-versus-host disease prophylaxis and so on are reported from participating teams to the CMBTRG using an Excel form every 6 months. Data were collected at the patient level and reported by transplantation centers. The first transplant or multiple transplants for a patient are not distinguished [5,6]. Based on our contacts with regulatory agencies and transplantation centers, the reporting rate for HSCT centers was estimated to be 90%. Data were validated by several quality control measures; through confirmation of the entered data by the reporting teams, selective comparison of the survey data in the CBMTRG database and crosschecking with source documents. This was a retrospective study based on data collected from HSCT centers in China in 2020 and 2021. The teams involved in this study are listed in the Supplementary Appendix by hospital names.
Results participating teams
A total of 166 and 174 transplantation teams participated in the 2020 and 2021 surveys, respectively, and these teams were located in 27 provinces, municipalities, or autonomous regions. In the last 2 years, the Beijing area contributed the largest number of transplantations (5176 [16%]), followed by Guangdong Province (4037 [13%]), and Jiangsu Province (2921 [9%]). A total of 135 teams (81%) in 2020 and 153 teams (88%) in 2021 performed both allogeneic and autologous transplantations. Transplantation activity was restricted to auto HSCT for 17 teams (10%) and 16 teams (9%) in 2020 and 2021, respectively. Haploidentical donor (HID) HSCT was reported by 145 teams (87%) in 2020 and 152 teams (87%) in 2021. A total of 118 teams reported both adult and pediatric transplantations, 35 teams reported only adult transplantations, and 13 teams reported only pediatric transplantations in 2020. In 2021, 119 teams reported both adult and pediatric transplantations, 38 teams reported only adult transplantations, and 17 teams reported only pediatric transplantations.
For HSCTs, the median number per team was 44 (1-838) and 51.5 (1-1205) for 2020 and 2021, respectively. For allogeneic transplantations, the median number per team was 28 (1-775) in 2020, which increased to 40 (1-1101) in 2021. For autologous transplantations, the median number per team was 15 (1-182) in 2020 and 19 (1-265) in 2021. Four teams reported >500 HSCTs in 2021, including Peking University People's Hospital, Peking University Institute of Hematology (1205 cases); The First Affiliated Hospital of Soochow University (1004 cases); Hebei Yanda Lu Daopei Hospital (731 cases); and Blood Diseases Hospital, Chinese Academy of Medical Science (662 cases). Data are shown in Table 1 . Compared with 2019, the total number of autologous HSCTs increased by 24%, and allogeneic HSCTs increased by 5% in 2020. Compared with 2020, the total number of autologous and allogeneic HSCTs increased by 59% and 27% in 2021, respectively. As shown in Table 1, <25, 25 to 100, and >100 HSCTs per team were defined as small, medium, and large transplantation groups. Between 2020 and 2021, the number of medium and large groups continued to increase, with a significant increase in the median number of HSCTs per team of large groups.
Table 1.
Numbers of Teams and Transplants by Size Category
| Team size | No. of teams | Percentage of total teams | No. of HSCT | Percentage of total transplants | HSCT per team, median (range) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | 2020 | 2021 | |
| HSCT | ||||||||||
| <25 HSCTs per team | 49 | 39 | 30% | 22% | 629 | 452 | 5% | 2% | 14 (1-24) | 11 (1-24) |
| 25-100 HSCTs per team | 79 | 84 | 48% | 48% | 4054 | 4476 | 30% | 25% | 48 (25-100) | 48 (25-99) |
| >100 HSCTs per team | 38 | 51 | 23% | 29% | 8732 | 13,182 | 65% | 73% | 171.5 (101-838) | 18 7 (102-1205) |
| Total | 166 | 174 | 13,415 | 18,110 | 44 (1-838) | 51.5 (1- 1205) | ||||
| Allogeneic | ||||||||||
| <25 HSCTs per team | 71 | 63 | 48% | 40% | 866 | 701 | 9% | 6% | 11 (1-24) | 10 (1-24) |
| 25-100 HSCTs per team | 49 | 63 | 33% | 40% | 2600 | 3474 | 26% | 27% | 51 (25-89) | 51 (25-96) |
| >100 HSCTs per team | 29 | 32 | 19% | 20% | 6576 | 8569 | 65% | 67% | 183 (102-775) | 208 (101-1101) |
| Total | 149 | 158 | 10,042 | 12,744 | 28 (1-775) | 40 (1-1101) | ||||
| Autologous | ||||||||||
| <25 HSCTs per team | 109 | 100 | 72% | 59% | 1186 | 1082 | 35% | 20% | 10 (1-24) | 10 (1-24) |
| 25-100 HSCTs per team | 41 | 60 | 27% | 36% | 1889 | 2915 | 56% | 54% | 38 (25-100) | 43.5 (25-99) |
| >100 HSCTs per team | 2 | 9 | 1% | 5% | 296 | 1357 | 9% | 25% | 148 (114-182) | 140 (104-265) |
| Total | 152 | 169 | 3371 | 5354 | 15 (1-182) | 19 (1-265) | ||||
No. indicates number.
Number of transplantations
During the last 2 years, a total of 31,525 HSCTs were reported, with 13,415 cases in 2020 and 18,110 cases in 2021. In 2020, 25% (3371 cases) of the annual transplants were autologous, and the remaining 75% (10042 cases) were allogeneic HSCT. In 2021, the number of autologous HSCTs was more than 5000 for the first time, accounting for 30% (5354 cases) of the total transplants, with the other 12,744 patients (70%) receiving allogeneic HSCT. In 2020 and 2021, the number of annual transplantations was also increased after removing the effect of the new additional HSCT centers.
Among the allogeneic HSCTs, the proportion of HIDs increased to 63% (7977 cases) in 2021, followed by matched sibling donor (MSD), unrelated donor (URD), and cord blood (CB) HCSTs accounting for 20%, 12%, and 5%, respectively. The trend of total HSCT and transplant by donor type over the past 14 years is illustrated in Figure 1 a and 1b. For the last 2 years, 42% (13,250 cases) of the HSCT patients were female, and 58% (18,275 cases) were male. The median age of the HSCT patients was 35 (0.2-87) years. Specifically, the median ages of auto- and allo-HSCT patients were 51 (0.5-87) and 29 (0.2-79) years, respectively. A total of 8499 pediatric patients (≤18 years old) underwent HSCT, 94% of whom received allogeneic HSCT. A total of 7564 elderly patients (>50 years old) received HSCT, 39% of whom received allogeneic HSCT.
Figure 1.
(a). Annual number of HSCT recipients in China by transplant. (b) Allogeneic HSCT in China by donor type. (c) Allogeneic HSCT in China by disease indications.
There were 1874 patients >60 years old, and 27% of them underwent allogeneic HSCT. We show the proportion of allogeneic HSCT donor types by recipient age in Figure 2 .
Figure 2.
Proportion of allogeneic HSCT donor types by recipient age in China 2020 to 2021.
Disease indication
The distribution of disease indications for allogeneic and autologous HSCT is shown as pie charts in Figure 3 a and 3b. The main indications for HSCT were acute leukemia (acute myeloid leukemia [AML], acute lymphoblastic leukemia [ALL], and mixed phenotype acute leukemia), with a total of 14,399 cases. In terms of allogeneic HSCT, the predominant indications were AML (8419 cases, 37%) and ALL (5164 cases, 23%). In addition, aplastic anemia (AA) (2858 cases, 13%), myelodysplastic syndrome (MDS) (2233 cases, 10%), thalassemia (1316 cases, 6%), and lymphoma (693 cases, 3%) were also identified. The trend for the numbers of main indications for allogeneic HSCT is shown in Figure 1c.
Figure 3.
Disease indications for HSCT recipients. (a) Allogeneic HSCT. (b) Autologous HSCT. (c) Allogeneic HSCT for pediatric patients. (d) Autologous HSCT for elderly patients. (e) Allogeneic HSCT for elderly patients.
The indications and donor type are shown in Table 2 . In 2020, the total number of transplantations for AML (3889 cases) and ALL patients (2397 cases) increased by 5% and 2%, respectively, compared to that in 2019. In 2021, the number of HSCTs for AML (4964 cases) and ALL patients (2903 cases) was 28% higher than that in 2020. Among AML patients (8853 cases), 5511 patients (62%) received HID-HSCT, 1721 patients (19%) received MSDs, 793 patients (9%) received URDs, 394 patients (4%) received CB, and 430 patients (5%) received autologous transplants. Among ALL patients (5300), HID transplantation was the most frequent, accounting for 65% (3461 cases) of the total transplants, and the proportions of MSD, URD, CB, and autologous HSCT were 17% (903 cases), 10% (548 cases), 5% (252 cases), and 3% (133 cases), respectively. Additionally, 62% (1385 cases) of MDS patients received HID HSCT, and the remaining cases were MSD (548 cases, 24%), URD (228 cases, 10%), CB (72 cases, 3%), and autologous HSCT (3 cases). For 352 CML patients in the last two years, 226 (65%) received HID HSCT. The number of allogeneic transplantations in CML patients decreased by 34 in 2020 and increased by 50 in 2021 compared with the previous year.
Table 2.
Numbers of HSCT in China 2020-2021 Indication, Donor Type, and Stem Cell Source
| MSD |
HID |
URD | CB | Syngeneic | Auto | Total | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PB | BM | BM + PB | Unknown | Total | PB | BM | BM + PB | Unknown | Total | |||||||||||||||||||
| PB only | PB + Third-Party CB | BM only | BM + Third-Party CB | BM + PB only | BM + PB + Third-Party CB | PB only | PB + Third-Party CB | BM Only | BM+ Third-Party CB | BM + PB Only | BM + PB + Third-Party CB | |||||||||||||||||
| Myeloid malignancies | 1929 | 113 | 3 | 297 | 20 | 63 | 2425 | 3314 | 1045 | 33 | 22 | 1680 | 1121 | 139 | 7354 | 1099 | 502 | 6 | 433 | 11819 | ||||||||
| AML | 1388 | 72 | 3 | 201 | 13 | 44 | 1721 | 2447 | 788 | 19 | 18 | 1272 | 887 | 80 | 5511 | 793 | 394 | 3 | 430 | 8852 | ||||||||
| CML | 59 | 1 | 11 | 2 | 2 | 75 | 110 | 29 | 2 | 1 | 45 | 33 | 6 | 226 | 38 | 13 | 352 | |||||||||||
| MDS | 424 | 36 | 71 | 3 | 14 | 548 | 667 | 172 | 10 | 3 | 321 | 166 | 46 | 1385 | 228 | 72 | 2 | 3 | 2238 | |||||||||
| MDS/MPN | 40 | 4 | 9 | 1 | 3 | 57 | 71 | 49 | 2 | 33 | 25 | 7 | 187 | 33 | 23 | 300 | ||||||||||||
| MPN | 18 | 5 | 1 | 24 | 19 | 7 | 9 | 10 | 45 | 7 | 1 | 77 | ||||||||||||||||
| Lymphoid malignancies | 877 | 43 | 1 | 0 | 135 | 3 | 29 | 1088 | 1739 | 555 | 11 | 7 | 847 | 723 | 44 | 3926 | 616 | 291 | 4 | 8048 | 13973 | |||||||
| ALL | 730 | 36 | 119 | 2 | 16 | 903 | 1514 | 477 | 9 | 6 | 761 | 665 | 29 | 3461 | 548 | 252 | 3 | 133 | 5300 | |||||||||
| MM | 22 | 1 | 23 | 18 | 3 | 2 | 23 | 2 | 1 | 4072 | 4121 | |||||||||||||||||
| other plasma cell disorders | 3 | 1 | 4 | 5 | 2 | 2 | 2 | 11 | 2 | 1 | 201 | 219 | ||||||||||||||||
| HL | 1 | 1 | 4 | 1 | 5 | 1 | 264 | 271 | ||||||||||||||||||||
| NHL | 121 | 7 | 1 | 14 | 1 | 13 | 157 | 198 | 73 | 2 | 1 | 81 | 56 | 15 | 426 | 63 | 38 | 3378 | 4062 | |||||||||
| Mixed phenotype acute leukemia | 28 | 3 | 2 | 3 | 36 | 69 | 19 | 2 | 1 | 47 | 37 | 175 | 27 | 8 | 1 | 247 | ||||||||||||
| Solid tumors | 5 | 0 | 0 | 0 | 1 | 1 | 0 | 7 | 10 | 1 | 0 | 0 | 5 | 3 | 1 | 20 | 3 | 10 | 223 | 264 | ||||||||
| Neuroblastoma | 1 | 1 | 1 | 1 | 1 | 3 | 1 | 9 | 178 | 192 | ||||||||||||||||||
| other solid tumors | 4 | 1 | 1 | 6 | 9 | 4 | 3 | 1 | 17 | 2 | 2 | 45 | 72 | |||||||||||||||
| Nonmalignant disorders | 576 | 49 | 10 | 28 | 382 | 26 | 7 | 1078 | 631 | 516 | 21 | 9 | 963 | 425 | 20 | 2585 | 951 | 411 | 3 | 8 | 5036 | |||||||
| SAA | 348 | 20 | 3 | 261 | 10 | 6 | 648 | 357 | 185 | 12 | 2 | 793 | 207 | 18 | 1574 | 430 | 214 | 3 | 2869 | |||||||||
| Other bone marrow failure | 18 | 1 | 7 | 26 | 23 | 5 | 30 | 11 | 2 | 71 | 62 | 20 | 179 | |||||||||||||||
| Thalassemia | 143 | 26 | 7 | 27 | 100 | 14 | 1 | 318 | 147 | 276 | 8 | 6 | 76 | 147 | 660 | 325 | 13 | 6 | 1322 | |||||||||
| Inherited disorders of metabolism | 14 | 2 | 16 | 26 | 10 | 1 | 9 | 3 | 49 | 36 | 63 | 1 | 165 | |||||||||||||||
| Primary immune deficiencies | 17 | 1 | 1 | 5 | 2 | 26 | 12 | 23 | 1 | 11 | 26 | 73 | 64 | 90 | 253 | |||||||||||||
| Hemophagocytic lymphohistiocytosis | 36 | 1 | 7 | 44 | 66 | 17 | 44 | 31 | 158 | 34 | 11 | 1 | 248 | |||||||||||||||
| others | 29 | 2 | 1 | 3 | 35 | 26 | 19 | 32 | 25 | 2 | 104 | 32 | 3 | 13 | 187 | |||||||||||||
| Total | 3444 | 210 | 15 | 28 | 820 | 50 | 102 | 4669 | 5789 | 2155 | 67 | 39 | 3574 | 2334 | 206 | 14164 | 2728 | 1225 | 14 | 8725 | 31525 | |||||||
Not all centers reported stem cell source data, and that is classified as unknown; 98% of URD and 99% of autologous HSCT used PB as stem cell source, so total number of URD and autologous HSCT are shown in the table.
CML indicates chronic myelogenous leukemia; MPN, myeloproliferative neoplasm; HL, Hodgkin lymphoma.
In terms of nonmalignant indications for allogeneic HSCT, AA and thalassemia were the most frequent. Among the 2869 AA patients, HID HSCT accounted for 55% (1574 cases), and MSD, URD, and CB accounted for 23% (648 cases), 15% (430 cases), and 7% (214 cases), respectively, with syngeneic donors (3 cases). An increase of 4% and 20% in the allo-HSCT activity for AA patients was seen in 2020 and 2021, respectively, compared with the previous year. For thalassemia, HID HSCT accounted for 50% (660 cases), and the proportions of URD and MSD HSCT were close at 25% (325 cases) and 24% (318 cases), respectively.
Regarding autologous HSCT, multiple myeloma (MM) and non-Hodgkin's lymphoma (NHL) were the most common indications, with proportions of 47% and 39%, respectively. For NHL, the number of patients receiving HSCT increased by 11% and 53% in 2020 and 2021, respectively, compared with the previous year. Approximately 83% of the 4062 NHL patients (3378 cases) received autologous HSCT, more often than other donor types, including HID (426 cases, 10%), MSD (157 cases, 4%), URD (63 cases, 2%), and cord blood (38 cases, 1%) HSCT. For MM, the number of transplants increased by 39% and 61% in 2020 and 2021, respectively, compared with the year before. Almost all of the MM patients received autologous HSCT (4072 of 4121 cases).
Ninety-four percent (8015 cases) of pediatric patients (≤18 years of age) received allogeneic HSCTs, with AML (1915 cases, 24%) and ALL (1822 cases, 23%) being the most common indications, followed by AA (1481 cases, 18%) and thalassemia (1312 cases, 16%). Patients with congenital diseases, including primary immunodeficiency diseases, inherited metabolic disorders, and inherited bone marrow failure, with a proportion of 7% altogether, received stem cells from CB (33%), HID (31%), URD (26%), and MSD (10%). The main indications of allogeneic HSCT for pediatric patients are shown in Fig. 3c. For elderly patients, 4628 cases (61%) underwent autologous HSCTs, main indications including MM (2964 cases, 64%) and NHL (1406 cases, 31%) (Fig. 3d). Another 2936 elderly patients (39%) followed allogeneic HSCTs, and the most prevalent indications were AML (1468 cases, 50%), MDS (704 cases, 24%) and ALL (400 cases, 14%) (Figure 3e).
Stem cell source
In accordance with our previous study, peripheral blood (PB) stem cell source was the main source for URD (98%) and autologous (99%) HSCT in China; thus, we focused on the stem cell sources of HID and MSD (Table 2). A single stem cell source of PB was the main graft source for HIDs and MSDs, with proportions of 41% and 75%, respectively. On the other hand, 26% of HIDs and 18% of MSDs had multiple stem cell sources (BM + PB), which is typical in China. Thirty-two percent of HID and 6% of MSD HSCTs used CB as a supplemental component to PB, BM, or PB + BM. Allogeneic transplantations in this study were all performed with fresh stem cells.
Conditioning regimen
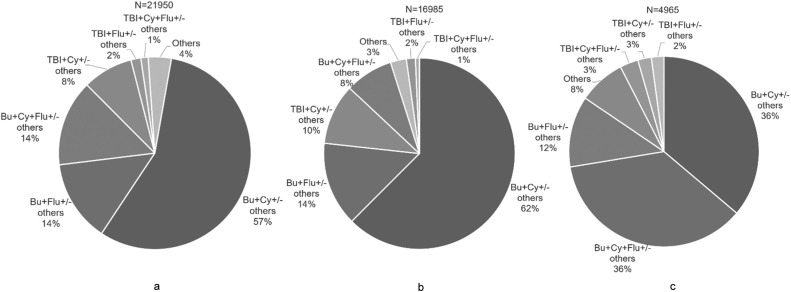
Because reduced-intensity or myeloablative conditioning data were not included, we focused on the drug composition of the conditioning regimen, which is described in Figure 4 a–c. For allogeneic HSCT, the BuCy-based regimen was most popular, with a proportion of 57%. The BuFlu-based regimen was the second most common regimen, accounting for 28% of allogeneic HSCT. For the TBI-based regimen, the proportion was 11%, including TBICy-based (73%) and TBIFlu-based (27%) regimens. For patients with malignant diseases undergoing allogeneic HSCT, the proportions of BuCy-based and BuFlu-based regimens were 62% and 22%, respectively. Thirteen percent of patients with malignant diseases received a TBI-based regimen (ALL 66%, AML 17%, and NHL 9%). However, for nonmalignant disease, the proportions of BuCy-based, BuFlu-based and TBI-based regimens were 36%, 48%, and 8%, respectively.
Figure 4.
Conditioning regimen of allogeneic HSCT in China 2020 to 2021. (a) Total allogeneic HSCT. (b) Malignant disease. (c) Non-malignant disease.
DISCUSSION
On behalf of the CBMTRG, we conducted this survey using data from 2020 and 2021 to investigate the effects of the SARS-CoV-2 pandemic on Chinese transplantation activity. Between 2020 and 2021, the total number of HSCTs in China was 31,525; with 72% being allogeneic. Compared with the previous year, the number of HSCTs increased by 9% and 35% in 2020 and 2021, respectively. Several reasons might explain this increase. First, with progress in the transplant procedure and relative supportive care, probably resulted in an increase for number of medium and large groups, also for the median HSCT number. Next, the COVID-19 pandemic was relatively moderate in China. Thus transplantation-related activity was carried out smoothly under the careful practice of social distancing, masking, and education for patients and donors, which are recommended worldwide as major pillars of prevention 9, 10, 11.
According to the latest report of CIBMTR and European Society for Blood and Marrow Transplantation, for the first time, a decline in transplantation activity was observed in 2020. The annual number of allogeneic transplantations in the United States was 8326 in 2020, slightly decreasing from 8740 in 2019, with autologous HSCT accounting for 58% of the total transplantations [8]. In 2020, 18,796 (41%) allogeneic and 26,568 (59%) autologous transplantations were reported in Europe, indicating a drop in allogeneic and autologous HSCT by −5.1% and −7.5%, respectively [7]. A slight decrease in allogeneic transplantation for hematopoietic malignancies was seen in Europe in 2020, yet a decrease of −9.7% was reported for severe aplastic anemia (SAA) (n = 676) [7]. This trend was probably associated with the deferred strategy in transplantations for nonmalignant disorders [10,12]. However, SAA accounted for 13% of allogeneic transplantation in China in 2020 to 2021, the same as that in 2019. A large report on transplantation for SAA was published recently [13].
There was an increase in the use of haploidentical donors worldwide during this pandemic. In the United States, a steady increase was shown in the number of HID transplantations, which surpassed MSD transplantations for the first time in 2020, accounting for 24% of allogeneic HSCTs [8]. Similarly, an increase of 6.2% compared with the previous year was observed in HID HSCTs in Europe in 2020 [7]. In China, the proportion of HIDs increased to 63% of allogeneic HSCTs in 2021, with the number approaching 8000. This increase in the use of HID might be ascribed to its encouraging outcomes for many indications and more flexible availability, especially during this COVID-19 pandemic 13, 14, 15, 16, 17, 18, 19.
Before the pandemic, BM+PB was the most frequently used stem cell source for HID HSCT in China because higher acute and chronic graft-versus-host disease rates have been reported in PB HID HSCT 20, 21, 22, 23. Nevertheless, during the pandemic, BM harvesting has been made problematic by increased difficulties, including more complex procedures in donor hospital admission and less surgery room availability. Consequently, more HID HSCTs (41%) have been performed using PBSCs as a graft in China in 2020–2021, especially in malignant disease. A shift from marrow to peripheral blood as a stem cell source was also seen for allo-HSCT in the US and Europe in 2020, especially for HID HSCT [7,8]. This phenomenon was consistent with the DKMS report, which showed that the total number of stem cell products provided declined by 15% during the crisis, particularly a steep decrease in BM products [24]. Cryopreservation has been recommended as graft source in Europe, which made the graft collection more flexible during the SARS-CoV-2 pandemic [25,12]. In our study, HSCTs was still performed with fresh stem cells during this pandemic and several measures were adopted to ensure the success of HSCT. Under the organization of China Marrow Donor Program, stem cells from URDs were shipped by commercial express instead of personnel delivery. For patients following URD HSCT, an alternative donor was prepared as a backup before initiating the condition regimen.
In our database, the proportions of ≥40- and ≥65-year-old patients undergoing allogeneic transplantation were 30% and 1%, respectively, in the last two years. From the latest report from the United States, the relevant proportion was 66% and 20% during 2015 to 2020 [8]. The reason for this difference could be attributed to (1) the time for diagnosing hematopoietic diseases and from diagnosis to HSCT being probably longer in developing countries; thus some elderly patients might miss the chance of transplantation because of advanced disease, poor performance status, and more infection; (2) limited socioeconomic conditions preventing further therapy; and (3) less availability from sibling donors leading to fewer HSCT opportunities. In addition, an increase in the number of allogeneic transplantations was shown in elderly patients in China, with HID being the most popular donor type.
In terms of autologous HSCT, differences exist because some countries adapted their transplantation procedures to the local COVID-19 situation and the emerging therapy. In China, the number of autologous HSCTs reached more than 5000 for the first time in 2021, with a rapid increase for MM and NHL. However, the number of lymphoma patients undergoing autologous transplant was slightly lower in the last 2 to 3 years in the United States, which might represent the increased availability of newer, approved therapeutic options or an impact in the first year of the pandemic [8]. When compared to 2019, a notable decrease in autologous HSCT activity for PCD (−6.8%) and NHL (−8.9%) was seen in Europe, which might not be clarified by the shift from autologous HSCT to CAR-T therapies [7].
This study has some limitations. We retrospectively analyzed transplantation activity during this pandemic, but there was a lack of the outcome data for HSCT recipients. Some significant factors, such as the disease status before transplantation, the number of transplantations, and the intensity of conditioning regimens, were not included in this survey. In general, this study did reflect current activity and trends in the field of transplantation. The data in our survey indicated an increase in HSCT, both in allogeneic and autologous transplants, especially for HID HSCT during this COVID-19 pandemic, reflecting that the impact of this crisis was relatively mild in China.
ACKNOWLEDGMENTS
The authors sincerely thank all participating centers and their staff. The authors sincerely thank JW Wang for the secretarial work. All participating teams are listed in the Appendix.
Financial disclosure: Supported by the National Key Research and Development Program of China (2017YFA0104500) and the National Natural Science Foundation of China (Grant Nos. 81930004).
Conflict of interest statement: There are no conflicts of interest to report.
Authorship statement: X.-J.H. and L.-P.X. designed the study and revised the manuscript, L.-P.X. and Y.-Y. collected and analyzed the data and drafted the manuscript. All authors contributed to data collection and interpretation.
Footnotes
Financial disclosure: See Acknowledgments on page 136.e7.
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.jtct.2022.11.011.
Appendix. Supplementary materials
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Duarte RF, Labopin M, Bader P, et al. Indications for haematopoietic stem cell transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe, 2019. Bone Marrow Transplant. 2019;54:1525–1552. doi: 10.1038/s41409-019-0516-2. [DOI] [PubMed] [Google Scholar]
- 3.Gratwohl A, Baldomero H, Aljurf M, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303:1617–1624. doi: 10.1001/jama.2010.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Xu L, Chen H, Chen J, et al. The consensus on indications, conditioning regimen, and donor selection of allogeneic hematopoietic cell transplantation for hematological diseases in China-recommendations from the Chinese Society of Hematology. J Hematol Oncol. 2018;11(1):33. doi: 10.1186/s13045-018-0564-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu LP, Wu DP, Han MZ, et al. A review of hematopoietic cell transplantation in China: data and trends during 2008-2016. Bone Marrow Transplant. 2017;52:1512–1518. doi: 10.1038/bmt.2017.59. [DOI] [PubMed] [Google Scholar]
- 6.Xu LP, Lu PH, Wu DP, et al. Hematopoietic stem cell transplantation activity in China 2019: a report from the Chinese Blood and Marrow Transplantation Registry Group. Bone Marrow Transplant. 2021;56:2940–2947. doi: 10.1038/s41409-021-01431-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Passweg JR, Baldomero H, Chabannon C, et al. Impact of the SARS-CoV-2 pandemic on hematopoietic cell transplantation and cellular therapies in Europe 2020: a report from the EBMT activity survey. Bone Marrow Transplant. 2022;57:742–752. doi: 10.1038/s41409-022-01604-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auletta JJ, KJ Chen M, Shaw BE. 2021. Current use and outcome of hematopoietic stem cell transplantation: CIBMTR US summary slides. [Google Scholar]
- 9.Xiao H, Luo Y, Shi J, et al. How do we manage hematopoietic cell transplant during the SARS-CoV-2 pandemic? Acta Haematologica. 2021;144:500–507. doi: 10.1159/000513036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ljungman P, Mikulska M, de la Camara R, et al. The challenge of COVID-19 and hematopoietic cell transplantation; EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2020;55:2071–2076. doi: 10.1038/s41409-020-0919-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El Fakih R, Haroon A, Alfraih F, et al. Clinical course and outcomes of COVID-19 in hematopoietic cell transplant patients, a regional report from the Middle East. Bone Marrow Transplant. 2021;56:2144–2151. doi: 10.1038/s41409-021-01312-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Algwaiz G, Aljurf M, Koh M, et al. Real-world issues and potential solutions in hematopoietic cell transplantation during the COVID-19 pandemic: perspectives from the Worldwide Network for Blood and Marrow Transplantation and Center for International Blood and Marrow Transplant Research Health Services and International Studies Committee. Biol Blood Marrow Transplant. 2020;26:2181–2189. doi: 10.1016/j.bbmt.2020.07.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Xu L-P, Xu Z-L, Wang S-Q, et al. Long-term follow-up of haploidentical transplantation in relapsed/refractory severe aplastic anemia: a multicenter prospective study. Sci Bull. 2022;67:963–970. doi: 10.1016/j.scib.2022.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Wieduwilt MJ, Metheny L, Zhang MJ, et al. Haploidentical vs sibling, unrelated, or cord blood hematopoietic cell transplantation for acute lymphoblastic leukemia. Blood Adv. 2022;6:339–357. doi: 10.1182/bloodadvances.2021004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sanz J, Galimard JE, Labopin M, et al. Post-transplant cyclophosphamide containing regimens after matched sibling, matched unrelated and haploidentical donor transplants in patients with acute lymphoblastic leukemia in first complete remission, a comparative study of the ALWP of the EBMT. J Hematol Oncol. 2021;14:84. doi: 10.1186/s13045-021-01094-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zubicaray J, Pagliara D, Sevilla J, et al. Haplo-identical or mismatched unrelated donor hematopoietic cell transplantation for Fanconi anemia: results from the Severe Aplastic Anemia Working Party of the EBMT. Am J Hematol. 2021;96:571–579. doi: 10.1002/ajh.26135. [DOI] [PubMed] [Google Scholar]
- 17.Xu LP, Jin S, Wang SQ, et al. Upfront haploidentical transplant for acquired severe aplastic anemia: registry-based comparison with matched related transplant. J Hematol Oncol. 2017;10:25. doi: 10.1186/s13045-017-0398-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sanz J, Galimard JE, Labopin M, et al. Post-transplant cyclophosphamide after matched sibling, unrelated and haploidentical donor transplants in patients with acute myeloid leukemia: a comparative study of the ALWP EBMT. J Hematol Oncol. 2020;13:46. doi: 10.1186/s13045-020-00882-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhang XH, Chen J, Han MZ, et al. The consensus from The Chinese Society of Hematology on indications, conditioning regimens and donor selection for allogeneic hematopoietic stem cell transplantation: 2021 update. J Hematol Oncol. 2021;14:145. doi: 10.1186/s13045-021-01159-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holtick U, Albrecht M, Chemnitz JM, Theurich S, Skoetz N, Scheid C, et al. Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults. Cochrane Database Sys Rev. 2014;(4) doi: 10.1002/14651858.CD010189.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu X, Liu L, Xie Z, et al. Bone marrow versus peripheral blood as a graft source for haploidentical donor transplantation in adults using post-transplant cyclophosphamide-A systematic review and meta-analysis. Crit Rev Oncol/Hematol. 2019;133:120–128. doi: 10.1016/j.critrevonc.2018.05.017. [DOI] [PubMed] [Google Scholar]
- 22.Nagler A, Dholaria B, Labopin M, et al. Bone marrow versus mobilized peripheral blood stem cell graft in T-cell-replete haploidentical transplantation in acute lymphoblastic leukemia. Leukemia. 2020;34:2766–2775. doi: 10.1038/s41375-020-0850-9. [DOI] [PubMed] [Google Scholar]
- 23.Ma YR, Zhang X, Xu L, et al. G-CSF-primed peripheral blood stem cell haploidentical transplantation could achieve satisfactory clinical outcomes for acute leukemia patients in the first complete remission: a registered study. Front Oncol. 2021;11 doi: 10.3389/fonc.2021.631625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mengling T, Rall G, Bernas SN, et al. Stem cell donor registry activities during the COVID-19 pandemic: a field report by DKMS. Bone Marrow Transplant. 2021;56:798–806. doi: 10.1038/s41409-020-01138-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungman P, Mikulska M, de la Camara R, et al. Correction: The challenge of COVID-19 and hematopoietic cell transplantation: EBMT recommendations for management of hematopoietic cell transplant recipients, their donors, and patients undergoing CAR T-cell therapy. Bone Marrow Transplant. 2021;56:755. doi: 10.1038/s41409-020-0965-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.