Abstract
Background
The worldwide pandemic SARS-CoV-2 infection is associated with clinical course including a very broad spectrum of clinical manifestations, including death. Several studies and meta-analyses have evaluated the role of hypertension on prognosis, but with important limitations and conflicting results. Therefore, we decided to perform a new meta-analysis of the observational studies that explored the relationship between pre-existing hypertension and mortality risk in patients with SARS-CoV-2 infection, using more stringent inclusion criteria to overcome the limitations inherent previous meta-analyses.
Methods
A systematic search of the on-line databases available up to 31 March 2022 was conducted, including peer-reviewed original articles, involving the adult population, where the role of hypertension on mortality due to SARS-CoV-2 infection was determined by Cox-proportional hazard models. Pooled hazard ratio (HR) was calculated by a random effect model. Sensitivity, heterogeneity, publication bias, subgroup and meta-regression analyses were performed.
Results
Twenty-six studies (222,083 participants) met the pre-defined inclusion criteria. In the pooled analysis, pre-existing hypertension was significantly associated with mortality due to SARS-CoV-2 infection, both in unadjusted and adjusted models (HR: 1.55; 95% CI: 1.22 to 1.97). However, in separate analyses including results adjusted for crucial and strong predictors of mortality during SARS-CoV-2 infection (e.g. body weight), the association disappeared.
Conclusions
The results of this meta-analysis indicate that pre-existing hypertension is not an independent predictor of mortality during SARS-CoV-2 infection. Further studies should nevertheless be carried out worldwide to evaluate this role, independent of, or in interaction with, other confounders that may affect the mortality risk.
Keywords: Hypertension, SARS-CoV-2, COVID-19, Mortality, Meta-analysis
1. Introduction
Infection by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) has been affecting millions of people around the world since December 2019 [1].
This infection has a very broad spectrum of clinical manifestations, from asymptomatic illness to the development of the critical illness – Corona Virus Disease 2019 (COVID-19) – and death [2]. Several studies have explored the potential risk factors leading to the development of severe COVID-19 and death. Among the different risk factors assessed, those associated with poor prognosis were cardiovascular conditions (such as hypertension, diabetes and obesity) [3,4]. The involvement of the renin-angiotensin-aldosterone system (RAAS) in the SARS-CoV-2 infection mechanism, especially the imbalance between angiotensin-converting-enzyme (ACE) and ACE2 activity, could explain the key role of cardiovascular risk factors, in particular hypertension [5]. However, the results of the observational studies on the association between hypertension and mortality risk in patients affected by COVID-19 are not univocal and, especially in some studies, the role of hypertension based on the adjusted effect estimates is significantly reduced or even disappears [6], [7], [8], [9].
Some of the studies carried out are flawed by low statistical power [see Table 1 ], a cross-sectional design [Supplemental Table 1, ref 1–28], or because they fail to assess possible confounders of the relationship [see Table 1]. Likewise, the meta-analyses performed also tried to provide definite evidence of the unfavorable role of hypertension on mortality risk in COVID-19 patients [10], [11], [12], but these also have major limitations, such as the inclusion of studies with cross-sectional design, or with large heterogeneity of the participants’ features, or without exploration of potential sources of heterogeneity.
Table 1.
Characteristics of the studies included in the meta-analysis.
| Author (ref) | Country | N. of participants/ Events | Gender (M/F%) |
Age (yrs) [Range] |
BMI (kg/m2) |
Hypertension (%)[RAAS%] | Other cardiovascular risk factors (%) | Adjusted variables |
|---|---|---|---|---|---|---|---|---|
| Abayomi [17] | Nigeria | 2075/73 | 66/34 | 40 [18–98] |
- | 17.8 | Diabetes 7.2 CVDs 0.6 CKD 0.5 |
Age, gender, CVDs, diabetes, CKD, HIV/HBV co-infection, asthma, cancer. |
| Alguwaihes [6] | Saudi Arabia | 439/77 | 68/32 | 55 [19- 101] |
29.7 | 42.6 [25.3] |
Diabetes 68.3 CVDs 18 CKD 5 Obesity 42.2 Smoke 2.6 |
Age, BMI, Obesity, Nationality, Hypertension, Diabetes, heart failure, CKD, CVDs, Stroke, Smoke, Vit D deficiency, Medications, Symptoms, Vital signs, Liver profile, Lipid profile, Inflammatory markers, Renal profile, Complete blood count, Thyroid profile, Glycaemic profile. |
| An [18] | South Korea | 10,237/228 | 40/60 | 45 | - | 18 [10] |
Diabetes 10.0 CVDs 5.3 Hyperlipidemia 18.0 |
Age, gender, income level, residence, household type, disability, symptom, infection route. |
| Bonnet [7] | France | 2878/360 | 58/42 | 66.6 | 27.8 | 51 [56] |
Diabetes 23.7% CVDs 36.4 CKD 14.3 Obesity 30.3 Hyperlipidemia 28 Smoke 13.5 |
Age, gender, BMI, Cardiovascular complications, Asthma, Chronic respiratory failure, Cancer, Atrial fibrillation, Previous heart surgery, Chronic medications, Laboratory values, Electrocardiogram, Chest CT, qSOFA score. |
| Chen [19] | China | 1303/108 | 50/50 | 56 [42-66] |
- | 27.3 | Diabetes15.3 CVDs 8.2 Hyperlipidemia 7.7 Smoking 8.1 |
Age, gender, smoking, Onset of symptoms, Wuhan exposure, Symptoms, Vital signs, WBC (Neutrophil count, Lymphocyte count), PLT, Hb, PT, APTT, D-dimer, Albumin, ALT, AST, Total bilirubin, Na, K, Ca, P, Creatinine, CK, Troponin I, Procalcitonin, CRP, HDL, LDL, Cholesterol, Uric acid, Homocysteine, serum glucose, Imaging features. |
| Cheng [20] | China |
220/29 |
48/52 |
59.5 [48.3-70] | - | 31.8 [10.4] | Diabetes 15.4 CVDs 13.6 |
Age, gender, CVDs, diabetes,Cancer, Chronic liver disease, Symptoms, Treatment. |
| Cummings [21] | USA | 257/101 | 67/33 | 62 [51–72] |
30.8 | 63 | Diabetes 36 CVDs 19 CKD 14 Obesity 46 Smoke 13 |
Age, CVDs, chronic pulmonary disease, higher concentrations of IL-6, higher concentrations of D-dimer. |
| Czapla [22] | Poland | 286/194 | 68/32 | 60.5 |
31 | 50.7 | Diabetes 32.2 CVDs 41.2 CKD 2.8 Hyperlipidemia 21.1 Obesity 46 |
- |
| De Sousa R., 2021 [23] | India | 689/156 | 49/51 | 46.5 | - | 9.6 | Diabetes 9.7 CVDs 2.5 CKD 2.9 |
Age, gender, Symptoms at the time of presentation, Chronic pulmonary disease, Liver disease, Cancer, Oxygen by mask/cannula, NIV, Ventilator support, Number of comorbid conditions, Treatment (HCQ, Azithromycin, Azithromycin+HCQ, Azithromycin+Steroids, Azithromycin+Lopinavir-ritonavir, Azithromycin+ Oseltamivir SARI), Respiratory support. |
| Gao C., 2020 [24] | China | 2877/56 | 51/49 | 59.5 | - | 29.5 [6.4] | Diabetes 13.4 CVDs 10.8 CKD 1 Smoke 6.6 |
Age, gender, DM, insulin-treated, myocardial infarction, underwent PCI/CABG, CKD, stroke, heart failure. |
| Ge E., 2021 [25] | Canada | 167500/4747 | 48/52 |
42.7 | - | 24 | Diabetes 14.7 CVDs 4.9 CKD 3.4 |
Age, gender, Income, Rual, LTC resident, n of comorbidities, Asthma, Dementia, HIV, Cancer, Rheumatoid arthritis, Inflammatory bowel disease, Liver disease, Severe mental illness, Solid organ transplant. |
| Geng L., 2020 [26] | China | 123/57 | - | - | - | 61 | Diabetes 23.5 CVDs 30 CKD 13 |
- |
| Giorgi Rossi P., 2020 [27] | Italy | 2653/217 |
50/50 | 63.2 | - | 18.1 [30.9] |
Diabetes 12 CVDs 15.5 CKD 2.5 Hyperlipidemia 5 Obesity 2.7 |
Age, gender, Obesity, DM, Hypertension, CAD, heart failure, Arrhythmia, HLP, CVDs, Use of drugs in previous year RAAS inhibitors, CKD, Cancer, Calendar period, Time from symptoms to diagnosis, Place of birth Charlson Comorbidity Index, Dementia. |
| Gu H., 2021 [28] | China - UK | 380/93 |
58/42 | 58 | 26.7 | 47.9 | Diabetes 28.2 CVDs 13.9 CKD 8.2 Smoke 19.7 |
Age, gender, ethnicity, BMI, Heart rate, COPD, BNP, CRP, D-dimer, TnI, Echocardiographic parameters, SOFA score. |
| Haase N., 2020 [8] | Denmark | 323/118 | 74/26 | 68 [59-75] |
27 | 49.5 | Diabetes 21 CVDs 18 CKD 12 |
Age, gender, BMI, CAD, heart failure. Hypertension, Chronic pulmonary Disease, diabetes, CKD, Liver cirrhosis, Active cancer, Hematologic cancer, Immunocompromised. |
| Ioannou G. N., 2020 [9] | USA | 10,131/1094 |
91/9 | 63.6 | - | 62.1 | Diabetes 38.1 CVDs 21.7 Hyperlipidemia 55.6 Obesity 44.8 Smoke 11.2 |
Age, gender, BMI, Obesity, Ethnicity, coronary disease, heart failure, Cerebrovascular disease, diabetes, Cancer, Dialysis, CKD, Cirrhosis, Asthma, hypoventilation, Alcohol dependence, HLP, Smoking. |
| Kim E., 2021 [29] | Korea | 7590/224 | 41/59 | 46.6 | - | 25.8 [2.6] | Diabetes 21.5 CVDs 23.9 CKD 4.8 |
Age, gender, Socioeconomic status, Baseline conditions, Underlyng disease, Cancer, Mental disorders, Cardiac arrest, Pneumonia, Arrythmia, Hospitalization, HCQ, Lopinavir/Ritonavir, Ribavirin, Interferon, Steroid, |
| Kim S., 2020 [30] | Korea | 2254/179 | 36/64 | 58 [42-70] |
23.2 | 28.7 | Diabetes 16.6 CVDs 6.8 CKD 1.6 Obesity 28.5 Smoke 6.9 |
- |
| Marateb H. R., 2021 [31] | Iran | 630/45 | 61/39 | 57.1 | - | 34.9 | - | Age, gender, Hypertension, Oxygen Saturation, CCI. |
| Pezel T., 2021 [32] | France | 481/66 | 61/39 | 68.4 | 27.7 | 39.5 | Diabetes 23.3 CVDs 56.8 CKD 0.4 Hyperlipidemia 13.7 Obesity 10.2 Smoke 21 |
Indications for stress CMR, Cardiac rhythm, Medical history, LVEF, Early revascularization < 3 month after CMR, LV end diastolic volume index, RVEF. |
| Qin W., 2021 [33] | China | 262/23 | 47/53 | 63.5 [53-70] | - | 35.5 | Diabetes 16.4 CVDs 15.7 CKD 1.1 Hyperlipidemia 16.4 |
Age, gender, Symptoms, COPD, Biochemical parameters, Haematological parameters, Oxygen support, Treatmnet, Shortness of breath, AST, CK-MB, LDH, WBC, Dyspnea. |
| Tu Y., 2021 [34] | China | 74/60 | 72/28 | 68 [61-74] | - | 39.2 | Diabetes 18.9 CVDs 34.5 CKD 1.4 |
- |
| Wang F., 2020 [35] | China | 7283/649 | 51/49 | 64 [53-71] |
- | 4.1 | Diabetes 1.7 CVDs 2.2 |
Age, gender, Source, Location, Occupations, Symptoms, Initial oxygen Therapy, Highest oxygen therapy, Final oxygen therapy. |
| Wang L., 2020 [36] | China | 339/65 | 49/51 | 71 | - | 40.8 | Diabetes 16 CVDs 15.7 |
- |
| Wu C., 2020 [37] | China | 201/44 | 64/36 | 51 [43-60] |
- | 19.4 | Diabetes 10.9 CVDs 4 |
- |
| Xu K., 2020 [38] | China | 598/79 | 58/42 | 57 [42–66] |
- | 33.9 | Diabetes 13.2 CVDs 9.8 Smoke 7.5 |
- |
ACE-i : ACE inhibitors; CVDs: Cardiovascular diseases; HLP: hyperlipidemia; CKD: Chronic kidney disease; CCB: Calcium channel blockers; ARB: Angiotensin receptor blockers; MRA: Mineralcorticoid Receptor Antagonist; PAD; ARDS: Acute Respiratory Distress Syndrome.
Therefore, considering the worldwide diffusion of SARS-CoV-2 infection and the high correlated mortality after 2 years of pandemic, the huge prevalence and future incidence of hypertension, the important limitations of previous meta-analyses, and continuously emerging evidence on this issue, we decided to perform a new systematic review with a meta-analysis of the observational studies to explore the relationship between pre-existing hypertension and risk of mortality in patients with SARS-CoV-2 infection. To this end, we used more stringent inclusion criteria and tried to overcome the limitations inherent in the previous meta-analyses [4,[10], [11], [12]].
2. Methods
2.1. Data sources and search strategy
This meta-analysis was designed, conducted and reported according to the PRISMA statement [13] (Supplemental Table 2); the study protocol was preregistered (CRD42022335826). A systematic search of the available publications up to 31 March 2022 was performed using MEDLINE/PubMed, Web of Science, and Scopus. The search strategy, without restrictions, included the terms "Covid" OR "COVID-19″ OR "SARS-CoV-2″ AND "blood pressure" OR "hypertension", or a combination thereof, either in medical subject headings or in the title/abstract (Supplemental Table 3). Further information was retrieved through a manual search of references from recent reviews and relevant published original studies.
2.2. Study selection and data extraction
Data selection was conducted and reported in accordance with the PRISMA statement [13] by A.G. and A.F.Z., and was checked for accuracy by L.D. The titles and abstracts of the studies retrieved in the searches were screened to identify the studies that met the predefined inclusion criteria. The full texts of the potentially eligible studies were then retrieved and assessed for eligibility. Discrepancies over the inclusion of studies and the interpretation of data were resolved in conference. The data was then extracted from the studies selected for inclusion by A.G. and A.F.Z. in accordance with the PRISMA statement, and was checked for accuracy by L.D.
2.3. Inclusion criteria
To be included in the meta-analysis, the studies published had to meet the following criteria:
(a) peer-reviewed original articles, (b) studies involving adult populations, (c) studies involving the assessment of SARS-CoV-2 infection as the baseline exposure, (d) studies in which the role of pre-existing hypertension on mortality by SARS-CoV-2 infection was determined by Cox-proportional hazard models.
2.4. Risk of bias
The risk of bias of the studies included in the meta-analyses was assessed according to criteria established by the Newcastle-Ottawa Scale [14].
2.5. Grading of evidence
The quality of the entire body of evidence was evaluated using the GRADE (grading of recommendations assessment, development, and evaluation) methodology [15]. Evidence was graded as high, moderate or low. Observational studies were initially graded as low by default and were downgraded or upgraded based on specified criteria. Criteria to downgrade included study limitations (risk of bias), inconsistency (substantial unexplained heterogeneity), indirectness (factors that limit generalizability), imprecision (95% CI cross a minimally important difference of 5%, and publication bias (significant evidence of small-study effects). Criteria to upgrade the certainty of evidence included a large magnitude of effect, a dose-response gradient, and attenuation by plausible confounding factors.
2.6. Statistical analysis
The statistical analyses were performed using the Stata Corp. software (version 11.2; College Station, Texas, USA) and MIX software (version 1.7, Kitasato Clinical Research Center, Kanagawa, Japan). Unadjusted and adjusted hazard ratios (HRs) were extracted from the publications selected, and their standard errors (SEs) were calculated from the respective 95% confidence intervals (CIs). The value from each study and the corresponding SE were transformed into their natural logarithms to stabilize the variances and normalize their distribution. The pooled HR (and 95% CI) was estimated using a random-effect model by DerSimonian and Laird (DL) and the likelihood-based method (Profile Likelihood -PL). The influence of the individual cohorts or of a particular study was estimated by sensitivity analysis, omitting one cohort at a time to verify to which extent the inferences depend on a particular study or group of studies. The Cochrane Q test and the I2 statistic were used to evaluate statistical heterogeneity across the studies. Funnel plots were constructed and visually assessed for possible publication bias [16]. Egger's, Begg's and Macaskill's tests were also used to explore potential publication bias. Subgroup and meta-regression analyses were used to identify associations between outcome risk and relevant study or patient characteristics, as possible sources of heterogeneity. The meta-regression analysis was performed by STATA syntax “metareg”. First, univariate meta-regression was performed including a single covariate; then, a final adjusted model by multivariable meta-regression was performed, including the factors with p < 0.1 in the univariate analysis. In order to reduce the risk of identifying false associations, only the models including a minimum of 10 studies were considered in the multivariable meta-regression. Adjusted-R2 was considered to quantify the proportion of variance in the model predicted by the independent variables.
It was a priori estimated that 15 studies were required to provide 90% power at 5% probability level (two-sided) (expected effect size: 1.2, expected study size 200, I2= 90%) (“metapower” package R, version 4.2.1, R Foundation for Statistical Computing).
3. Results
3.1. Characteristics of the studies included in the meta-analysis
Of a total of 25,447 publications retrieved, 377 studies were identified to undergo a qualitative evaluation (Supplemental Figure 1). However, 351 of them were excluded because the data reported were unsuitable (Supplemental Figure 1). Thus, eventually 26 studies were used for the analysis [[6], [7], [8], [9],[17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]]. The main characteristics of the studies identified and of the respective study populations are recorded and reported in Table 1 (Supplemental Tables 4–5). Overall, the meta-analysis involved 222,083 participants from 12 countries (Asia-Far East and Middle East-, Europe, America and Africa). All studies recruited both male and female patients (from 36% to 91% of prevalence of men) and with a mean/median age range from 40 to 71 years.
Sixteen of the total studies included reported both unadjusted and adjusted data, 6 only unadjusted and another 4 only adjusted data. All multivariate models included age, 19 of them also gender, 13 cardiovascular diseases, 7 body weight, 4 dyslipidemia, and only 3 smoking habit. All but three [9,18,27] provided cohorts only including in-hospital mortality. Almost all the studies retrospectively evaluated the data, while four studies had a prospective design [7,21,23,29]. Only 5 studies assessed the proportional hazard assumption [17,20,21,24,32].
All but one analyzed data collected in first half of 2020, and one until June 2021 [22].
The evaluation of the “risk of bias” indicated that all studies were low-risk (Supplemental Table 6).
3.2. Hypertension and mortality
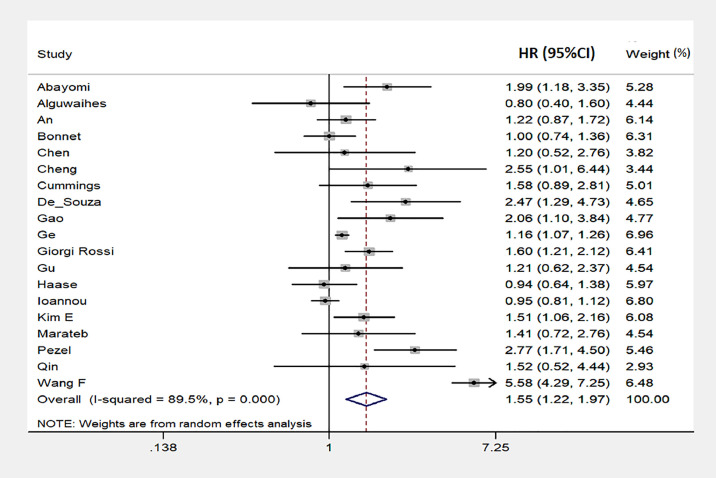
Pooling data of 22 studies reporting unadjusted results (50,504 total participants, 4013 total deaths) (Table 1) [[6], [7], [8], [9],17,18,[20], [21], [22], [23], [24],[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]] showed that hypertensive patients had significantly higher mortality risk compared with non-hypertensive patients (DL/PL, HR= 2.58; 95% CI: 1.66 to 4.02; p<0.001), with significant between-study heterogeneity (p<0.01; I2=97%) (Supplemental Figure 2). These results were confirmed when data from multivariate models were included (19 studies; 218,208 total participants; 8441 total deaths) (Table 1). Indeed, pre-existing hypertension status at baseline was associated with significantly higher mortality risk (DL/PL, HR: 1.55; 95% CI: 1.22 to 1.97; p<0.001), with significant heterogeneity among studies (p<0.001, I2=89%) (Fig. 1 ). Visual analysis of the funnel plot indicated some asymmetry (Supplemental Figure 3), whereas Egger's,Begg's and Macaskill's tests failed to detect significant evidence of publication bias (Egger: p = 0.2, Begg: p = 0.6, Macaskill: 0.2). The evaluation of individual studies showed a trend toward a direct association between hypertensive status at baseline and risk of mortality in 15 studies, with significantly association in 9 of them, whereas a non-significant opposite trend was observed in 3 studies, and a neutral association in one (Fig. 1). Sensitivity analysis showed that the risk of mortality did not vary substantially when excluding any individual study.
Fig. 1.
Forest plot of the predicting role of hypertension on the risk of mortality in SARS-CoV2 infection (results from adjusted data). Results are expressed as Hazard Ratio (HR) and 95% confidence intervals (95% CI). Squares indicate study-specific risk estimates (size of the square reflects the study-specific statistical weight); horizontal lines indicate 95% CI; diamond indicates the overall risk with its 95% CI.
In addition, we carried out an analysis also including the results reported by Geng et al. [26] on the predictive role of stage-3 hypertension. The pooled HR only changed from 1.55 to 1.53 (95% CI: 1.23 to 1.91; p<0.001). There was again a significant heterogeneity among studies (p<0.001, I2=89%) and a little asymmetry of the funnel plot. However, there was no evidence of publication bias (Egger: p = 0.2, Begg: p = 0.4, Macaskill: 0.2).
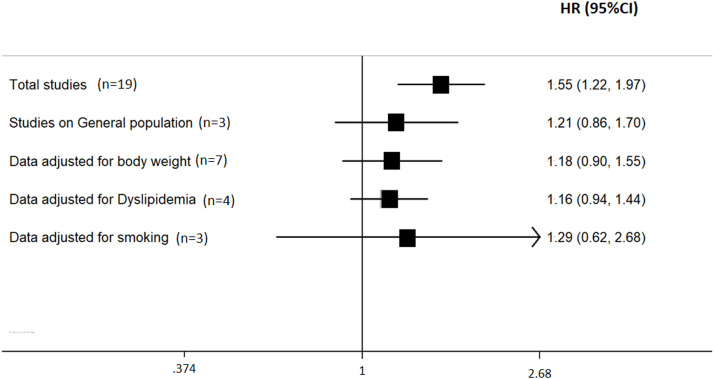
Additional Analyses (Fig. 2 , Table 2, Table 3 ). A further analysis that included studies with only in-hospital mortality [[6], [7], [8],17,[19], [20], [21], [22], [23], [24], [25], [26],[28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38]] detected both a similar association (HR= 1.64; 95% CI: 1.19 to 2.25; p = 0.002), and between-study heterogeneity (p<0.01; I2=90%). Conversely, a pooled analysis of studies with in-hospital and not in-hospital mortality [9,18,27] indicated no significant association (HR= 1.21; 95% CI: 0.86 to 1.70; p = 0.27; heterogeneity: p<0.01; I2=81%).
Fig. 2.
Sub-group analysis of the predicting role of hypertension on the risk of mortality in SARS-CoV2 infection (results from adjusted data). Results are expressed as Hazard Ratio (HR) and 95% confidence intervals (95% CI).
Table 2.
Subgroup analysis of the relationship between pre-existing hypertension and mortality.
| Results from adjusted data | |||||
|---|---|---|---|---|---|
| Variables (n. of cohorts) | HR | 95% CI | I2 | P for interaction | |
| Country of origin | Asia [East Asia] (8) | 2.02 | 1.18 to 3.48 | 89% | 0.13 |
| Europe (4) | 1.40 | 0.92 to 2.12 | 82% | ||
| America (3) | 1.10 | 0.91 to 1.33 | 67% | ||
| Asia [Middle East] (2) | 1.07 | 0.61 to 1.86 | 24% | ||
| Africa (1) | 1.99 | 1.18 to 3.35 | – | ||
| Mix [Europe +East Asia] (1) | 1.21 | 0.62 to 2.37 | – | ||
|
Number of participants [Median: 1000] |
< Median (9) | 1.53 | 1.10 to 2.12 | 58% | 0.93 |
| > Median (10) | 1.56 | 1.12 to 2.18 | 94% | ||
|
Age [Median: 60 years] |
< Median (11) | 1.41 | 1.17 to 1.70 | 43% | 0.60 |
| > Median (8) | 1.64 | 0.97 to 2.79 | 95% | ||
|
Gender [Median Prevalence of men: 50%] |
< Median (6) | 1.40 | 1.12 to 1.76 | 49% | 0.67 |
| > Median (13) | 1.54 | 1.05 to 2.26 | 92% | ||
|
Hypertension [Median Prevalence: 35%] |
< Median (11) | 1.84 | 1.26 to 2.70 | 93% | 0.06 |
| > Median (8) | 1.19 | 0.92 to 1.55 | 66% | ||
|
Diabetes [Median Prevalence: 15%] |
< Median (7) | 1.99 | 1.18 to 3.33 | 95% | 0.12 |
| > Median (10) | 1.27 | 1.00 to 1.61 | 66% | ||
|
Cardiovascular Disease [Median Prevalence: 15%] |
< Median (9) | 1.89 | 1.14 to 3.14 | 94% | 0.17 |
| > Median (9) | 1.28 | 1.01 to 1.64 | 73% | ||
| Study design | Retrospective (15) | 1.56 | 1.17 to 2.09 | 91% | 0.75 |
| Prospective (4) | 1.45 | 1.02 to 2.07 | 61% | ||
| Proportional hazard assumption | Yes (5) | 2.14 | 1.65 to 2.78 | 0% | 0.15 |
| No (14) | 1.40 | 1.06 to 1.85 | 92% | ||
Table 3.
Results of meta-regression analysis.
| Variable included in univariate analysis (number of studies) |
Coefficient (95%Confidence interval) |
p-value | I2-residual (%) | R2 | |
|---|---|---|---|---|---|
| Age (year) (19) | .0037202 (−0.0249393 0.0323796) |
0.787 | 89.51 | −6.29 | |
| Gender (male-%) (19) | −0.0125623 (−0.0315228 0.0063982) |
0.180 | 89.36 | 5.54 | |
| Total participants (n) (19) | −1.88e-06 (−8.02e-06 4.27e-06) |
0.528 | 89.13 | −4.67 | |
| BMI (kg/m2) (6) | .0123467 (−0.3903656 0.415059) |
0.936 | 75.39 | −32.18 | |
| Obesity (%) (5) | −0.0140209 (−0.0502218 0.0221799) |
0.306 | 70.51 | 13.30 | |
| CVD (%) (18) | −0.004839 (−0.0247176 0.0150395) |
0.613 | 90.51 | −4.45 | |
| Hypertension (%) (19) | −0.0174232 (−0.0296499 −0.0051965) |
0.008 | 85.85 | 39.06 | |
| Diabetes (%) (17) | −0.0206864 (−0.037172 −0.0042008) |
0.017 | 87.64 | 33.14 | |
| CKD (%) (13) | −0.042925 (−0.0809309 −0.0049191) |
0.030 | 61.20 | 46.72 | |
| Hyperlipidemia (%) (7) | −0.0120645 (−0.0284748 0.0043457) |
0.117 | 49.07 | 48.57 | |
| Smoke (%) (7) | .0322809 (−0.0385474 0.1031091) |
0.294 | 61.35 | 11.80 | |
| NOS (score) (19) | −0.1787508 (−0.7542938 0.3967923) |
0.521 | 89.09 | −1.04 | |
| RAAS – inhibitors use (%) (7) | −0.0078018 (−0.0220101 0.0064065) |
0.217 | 39.08 | 21.55 | |
| Stringency index (unit) (19) | .0014277 (−0.0123503 0.0152056) |
0.830 | 88.81 | −5.37 | |
| Fatality rate (%) (19) | .0121743 (−0.0674449 0.0917934) |
0.751 | 89.96 | −6.17 | |
| Reproduction rate (unit) (19) | −0.0856447 (−0.4453866 0.2740973) |
0.622 | 89.99 | −4.31 | |
| New cases per 1 M (n) (19) | −0.0050174 (−0.0117136 0.0016789) |
0.132 | 87.06 | 10.58 | |
| Hospitalized patients per 1 M (n) (6) | −0.0001298 (−0.0053418 0.0050822) |
0.948 | 84.14 | −42.08 | |
| Mortality (day) (7) | −0.115428 (−0.2602414 0.0293854) |
0.096 | 64.92 | 42.28 | |
| Multivariate analysis (12) | |||||
| Hypertension | .0161822 (−0.0126633 0.0450277) |
0.232 |
0.105* |
66.59 |
47.32 |
| Diabetes | −0.0131651 (−0.034144 0.0078138) |
0.186 | |||
| CKD | −0.0716798 (−0.1465653 0.0032057) |
0.060 | |||
With Knapp-Hartung modification; BMI: body mass index; CKD: chronic kidney disease; CVD: cardiovascular damage; NOS: Newcastle Ottawa score; RAAS: renin-angiotensin-aldosterone system.
Another analysis, including the studies that reported both unadjusted and adjusted data [6,-9,17,18,20,21,23,24,28,29,[31], [32], [33],35], confirmed the significant and direct association between pre-existing hypertension and mortality (HR=1.61, 95% CI: 1.15 to 2.26, p = 0.006), and the between-study heterogeneity (p<0.01, I2: 90%).
A separate analysis including studies that considered markers of body weight in the assessment showed a non-significant association between pre-existing hypertension and mortality (HR: 1.18, 95%CI: 0.90 – 1.56) [[6], [7], [8], [9],21,28,32]. On the other hand, a pooled analysis of studies not adjusted for this covariate indicated a significant association between hypertension and mortality (HR=1.75, 95% CI: 1.23 to 2.51).
Likewise, an additional analysis including studies that adjusted for dyslipidemia showed a non-significant role of hypertension on mortality (HR: 1.16, 95%CI: 0.89 – 1.50) [7,9,18,19]. Similar results were detected when a further analysis included studies that adjusted for smoking habit (HR: 1.29, 95%CI: 0.62–2.67) [6,9,32].
On the other hand, the meta-regression analysis indicated that markers of body weight (BMI: coeff.= 0.01, p = 0.9; obesity: coeff.= 0.01, p = 0.3), and prevalence of dyslipidemia (coeff.=−0.01, p = 0.12) and smoking habit (coeff.=0.03, p = 0.3) of the single cohorts were not significant sources of heterogeneity (Table 3, Supplemental Figure 4).
Moreover, the meta-regression analysis found that the percentage of pre-existing cardiovascular risk factors was a significant source of heterogeneity. In particular, there was an inverse relationship between the percentage of pre-existing hypertension (coeff:−0.02, p = 0.008), diabetes (coeff: −0.02, p = 0.02) and chronic kidney disease (coeff:−0.04, p = 0.03), and risk of mortality. The multivariate meta-regression result did not confirm these significant trends, with a residual-I2 of 66.59% and R2 of 47.3% (Table 3).
Similar trends were also detected by subgroup analysis, but without significant difference (Table 2).
Moreover, the univariate meta-regression analysis did not detect age (coeff.= 0.004; p = 0.8), gender (coeff.=−0.01; p = 0.2), total number of participants (coeff.= −1.88e-06; p = 0.5), pre-existing cardiovascular damage (coeff.=−0.005; p = 0.6), RAAS inhibitors use (coeff.= −0.008; p = 0.2), “risk of bias” score (coeff.= −0.18; p = 0.5) and time interval between diagnosis of SARS-CoV-2 infection and death (−0.11, p = 0.1) as significant source of heterogeneity (Table 3, Supplemental Figure 4). Among other potential sources of heterogeneity, relevant country data at the time of the study was evaluated by meta-regression analysis. The analysis did not find any significant result: stringency index (coeff.: 0.001, p = 0.8), fatality rate (coeff.: 0.01, p = 0.7), reproduction rate (coeff.: −0.09, p = 0.6), new cases per 1 M (coeff.:−0.005, p = 0.13), hospitalized patients per 1 M (coeff.: −0.0001, p = 0.9). (Table 3, Supplemental Figure 4).
Subgroup analyses confirmed the trends for age, gender, total number of participants, prevalence of hypertension, diabetes and cardiovascular damage (Table 2). In addition, also country of origin, study design and evaluation of proportional hazard assumption were not significant sources of heterogeneity (Table 2).
3.3. Quality of body of evidence
According to the GRADE criteria, the evidence for the association between pre-existing hypertension and mortality risk was of moderate quality for both unadjusted and adjusted data. Despite the GRADE methodology defines observational evidence from cohort studies as low quality, there was an upgrade of the score due to large magnitude of effect (for unadjusted data analysis) and attenuation by plausible confounding factors (for adjusted data analysis).
4. Discussion
The results of this meta-analysis seem to suggest a not independent predictive role of pre-existing hypertension on mortality for SARS-CoV-2 infection. Despite a direct effect was found when unadjusted data or general adjusted data were combined, the association was not confirmed when the data were adjusted for crucial and strong predictors of SARS-CoV-2 mortality, or in the general population.
Indeed, a separate analysis that also included the markers of body weight as covariate–a well-documented feature involved in the prognosis of SARS-CoV-2 infection [39], [40], [41], [42], [43] and commonly associated with hypertension–showed a non-significant predictive role of hypertension on mortality. By contrast, a pooled analysis of studies unadjusted for these markers indicated a significant association between hypertension and mortality. In particular, most of the studies including body weight as covariate suggested a non-significant association between pre-hypertension and mortality [[6], [7], [8], [9],21,28], and only one study found a significant direct association [32]. By contrast, all studies without body weight as covariate showed a direct association between hypertension and mortality, which was statistically significant in 7 of 12 studies.
Similar results were detected taking into account the influence of the smoking habit or dyslipidemia–other features involved in the prognosis of SARS-CoV-2 infection [40,44,45] and in general associated with hypertension. These results as well emphasize the interaction between these risk factors and pre-existing hypertension on mortality during SARS-CoV-2 infection.
Nevertheless, data on body weight, dyslipidemia and smoking habit of the single cohorts did not affect the relationship between pre-existing hypertension and mortality by meta-regression analysis.
The predictive role of pre-existing hypertension seems more pronounced in studies from East Asia than in other countries. However, this non-significant difference is likely due to the large number of studies included in this subgroup and to the first impact of the pandemic with respect to other regions.
Our analyses also suggest a more consistent role of hypertension on mortality in studies involving relatively younger patients (average age in the studies included was less than 60 years), despite previous studies suggested a worse prognosis in older patients [46], [47], [48], [49]. This result might be explained by the low comorbidities in these cohorts, which may lead to more pronounced event rate according to comorbidities. Moreover, of course, the result should be contextualized to the studies that analyze the role of hypertension, not comparable to those that carried out a general exploration of several predictive risk factors. Likewise, gender was also an important cause of heterogeneity. Although several studies reported a worse prognosis in men [49], [50], [51], our analysis on gender highlighted a worse prognosis in cohorts with lower number of men. Also in this case, the result should be contextualized to the studies that evaluate potential influence on the role of hypertension.
On the other hand, a lower prevalence of hypertension seems be associated with greater risk of mortality in relation to a higher prevalence, both in unadjusted and adjusted data. A greater risk of mortality was also detected in the cohorts with a lower prevalence of diabetes. Probably, the interaction among other risk factors in cohorts with higher prevalence of hypertension and diabetes (and other comorbidities) could conceal the effect of hypertension.
In addition, in line with these latest results, a lower chronic kidney disease prevalence seems be associated with higher mortality risk; instead, pre-existing cardiovascular diseases, study design, score of the “risk of bias and total number of participants did not affect the role of hypertension on mortality during SARS-CoV-2 infection.
Previous meta-analyses have assessed the role of hypertension on mortality in patients with SARS-CoV-2 infection [40,[52], [53], [54]], the main results indicating a direct effect of hypertension on mortality. However, these meta-analyses were limited by the inclusion of not updated evidence, combination of heterogeneous results (e.g. different design – cross-sectional, retrospective, prospective, case-control and outcome expression, HR, relative risk, odds ratio), and by limited evaluation of potential sources of heterogeneity.
4.1. Study strengths and limitations
This study has several strengths: a) the inclusion of studies reporting time-dependent outcomes; b) the stringent inclusion criteria; c) the “low-risk” of bias of the studies; d) a relatively large number of participants for mortality evaluation from different countries; e) the robustness of the findings by sensitivity and sub-group analysis; f) the comprehensive exploration of possible sources of heterogeneity; g) the substantial lack of evidence of publication bias; h) the gradual association detected from unadjusted and adjusted data analysis; i) the assessment of the overall quality of evidence using the GRADE assessment approach.
Nevertheless, our study also has limitations. The observational nature of the studies does not allow conclusions to be drawn on a possible cause-effect relationships. The experimental data showed an involvement of RAAS, in particular of the imbalance between ACE and ACE2 activity [55], [56], an involvement of the innate and adaptive immunity [57], and a contribution of the chronic inflammatory status by hypertension and of acute inflammation by SARS-CoV-2 infection [58]. Despite this evidence, our results did not completely confirm this association. At the beginning of the pandemic, there were contrasting results on RAAS inhibitors use, because some studies suggested that ACE inhibitors or AT1 receptor blockers cause increased ACE2expression, which could allow the virus to spread more easily, leading to a massive and ineffective inflammatory response [59,60]. By contrast, subsequent studies and pooled analyses [61,62] suggested a lack of harmful effects of RAAS inhibitors use. Indeed, there is evidence that their use along with controlled blood pressure at baseline is associated with better prognosis [63,64]. Unfortunately, none of the studies included in our meta-analysis adjusted for RAAS inhibitors use (also because it may be an over-adjustment since the diagnosis of hypertension included antihypertensive treatment), and only seven studies reported data on this treatment. On the other hand, the meta-regression analysis including these cohorts indicated that RAAS inhibitors did not affect the role of pre-existing hypertension on mortality during SARS-CoV-2 infection.
Another limitation is the difficulty to draw definitive conclusions regarding the interaction between the main features of the participants and the role of hypertension, given the peculiar composition of the study cohorts available. Likewise, the heterogeneity among study characteristics may be a limitation, such as the proportional hazard assumption assessed in few studies only. However, this limitation was explored by sub-group and meta-regression analysis, which found evidence of subgroup differences. In addition, in some subgroup analyses or meta-regression analyses, the tests were performed including relatively few studies; hence, in those cases no definitive conclusions could be reached. Finally, all but one study included data collected in first half of 2020, limiting the results to first phase of the pandemic.
5. Conclusions and perspectives
The results of this meta-analysis allow to hypothesize a non-independent predictive role of pre-existing hypertension on mortality in SARS-CoV-2 infection. Noteworthy, our systematic review highlights the limitation of most of the studies screened, which is an incomplete assessment of the independent role of pre-existing hypertension or its interaction with other risk factors on mortality risk. Therefore, to further extend current knowledge in this field, future studies should be carried out to prospectively evaluate, worldwide, the role of pre-existing hypertension, and to better assess this effect independently of or in interaction with other potential confounders (e.g. body weight, diabetes, smoking, cancer) that may affect the risk of mortality.
Supplementary data
Supplemental Figure 1. Stepwise procedure for selection of the studies. Flowchart indicating the results of the systematic review with inclusions and exclusions.
Supplemental Figure 2. Forest plot of the predicting role of hypertension on the risk of mortality in SARS-CoV2 infection (results from unadjusted data)
Supplemental Figure 3. Funnel plot of the predicting role of hypertension on the risk of mortality in SARS-CoV2 infection (adjusted data). HR: hazard ratio; SE: standard error.
Supplemental Figure 4. Bubble plot for random-effects meta-regression of hazard ratio (HR) against characteristics of the studies for the longitudinal association of pre-existing hypertension with mortality.
Bubbles each represent one study and are plotted according to the study's HR(ln) and a single characteristic of the study; bubble sizes reflect the relative weight apportioned to studies in the random-effects meta-regression; the solid line indicates the line of best fit.
Declaration of Competing Interest
The authors have not conflict of interest to disclose.
Acknowledgments
Acknowledgments
We thank Rosanna Scala for the language editing.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ejim.2022.11.018.
Appendix. Supplementary materials
References
- 1.WHO Coronavirus (COVID-19) dashboard 2022. https://covid19.who.int/ [Accessed to July 20th, 2022].
- 2.Huang C., Wang Y., Li X., et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China [published correction appears in Lancet. 2020 Jan 30] Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Richardson S., Hirsch J.S., Narasimhan M., et al. Presenting characteristics, comorbidities, and outcomes among 5700 patients hospitalized with COVID-19 in the New York City Area [published correction appears in JAMA. 2020 May 26;323(20):2098] JAMA. 2020;323(20):2052–2059. doi: 10.1001/jama.2020.6775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Meng M., Zhao Q., Kumar R., Bai C., Deng Y., Wan B. Impact of cardiovascular and metabolic diseases on the severity of COVID-19: a systematic review and meta-analysis. Aging. 2020;12(22):23409–23421. doi: 10.18632/aging.103991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sarzani R., Giulietti F., Di Pentima C., Giordano P., Spannella F. Disequilibrium between the classic renin-angiotensin system and its opposing arm in SARS-CoV-2-related lung injury. Am J Physiol Lung Cell Mol Physiol. 2020;319(2):L325–L336. doi: 10.1152/ajplung.00189.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Alguwaihes A.M., Al-Sofiani M.E., Megdad M., et al. Diabetes and Covid-19 among hospitalized patients in Saudi Arabia: a single-centre retrospective study. Cardiovasc Diabetol. 2020;19(1):205. doi: 10.1186/s12933-020-01184-4. Published 2020 Dec 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bonnet G., Weizman O., Trimaille A., et al. Characteristics and outcomes of patients hospitalized for COVID-19 in France: the Critical COVID-19 France (CCF) study. Arch Cardiovasc Dis. 2021;114(5):352–363. doi: 10.1016/j.acvd.2021.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Haase N., Plovsing R., Christensen S., et al. Characteristics, interventions, and longer term outcomes of COVID-19 ICU patients in Denmark-A nationwide, observational study. Acta Anaesthesiol Scand. 2021;65(1):68–75. doi: 10.1111/aas.13701. [DOI] [PubMed] [Google Scholar]
- 9.Ioannou G.N., Locke E., Green P., et al. Risk factors for hospitalization, mechanical ventilation, or death among 10 131 US veterans with SARS-CoV-2 infection. JAMA Netw Open. 2020;3(9) doi: 10.1001/jamanetworkopen.2020.22310. Published 2020 Sep 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang J., Zheng Y., Gou X., et al. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: a systematic review and meta-analysis. Int J Infect Dis. 2020;94:91–95. doi: 10.1016/j.ijid.2020.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ng W.H., Tipih T., Makoah N.A., et al. Comorbidities in SARS-CoV-2 patients: a systematic review and meta-analysis. MBio. 2021;12(1) doi: 10.1128/mBio.03647-20. e03647-20. Published 2021 Feb 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Huang D.Q., Zou B., et al. Epidemiology of COVID-19: a systematic review and meta-analysis of clinical characteristics, risk factors, and outcomes. J Med Virol. 2021;93(3):1449–1458. doi: 10.1002/jmv.26424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liberati A., Altman D.G., Tetzlaff J., et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate health care interventions: explanation and elaboration. PLoS Med. 2009;6(7) doi: 10.1371/journal.pmed.1000100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Higgins J.P.T., Altman D.G. In: Cochrane handbook for systematic reviews of interventions, version 5.0.1 [updated september 2008] Higgins JPT, Green S, editors. The Cochrane Collaboration; 2008. Chapter 8: assessing risk of bias in included studies.https://handbook-5-1.cochrane.org/chapter_13/13_5_2_3_toolsfor_assessing_methodological_quality_or_risk_of.htm Accessed 28 March 2022. [Google Scholar]
- 15.Guyatt G., Oxman A.D., Akl E.A., et al. GRADE guidelines: 1. Introduction-GRADE evidence profiles and summary of findings tables. J Clin Epidemiol. 2011;64(4):383–394. doi: 10.1016/j.jclinepi.2010.04.026. [DOI] [PubMed] [Google Scholar]
- 16.Sterne J.A., Sutton A.J., Ioannidis J.P., et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ. 2011;343:d4002. doi: 10.1136/bmj.d4002. Published 2011 Jul 22. [DOI] [PubMed] [Google Scholar]
- 17.Abayomi A., Osibogun A., Kanma-Okafor O., et al. Correction to: morbidity and mortality outcomes of COVID-19 patients with and without hypertension in Lagos, Nigeria: a retrospective cohort study. Glob Health Res Policy. 2021;6(1):28. doi: 10.1186/s41256-021-00215-1. Published 2021 Aug 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.An C., Lim H., Kim D.W., Chang J.H., Choi Y.J., Kim S.W. Machine learning prediction for mortality of patients diagnosed with COVID-19: a nationwide Korean cohort study. Sci Rep. 2020;10(1):18716. doi: 10.1038/s41598-020-75767-2. Published 2020 Oct 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q., Wang L., Li C., et al. Chronic cardio-metabolic disease increases the risk of worse outcomes among hospitalized patients with COVID-19: a multicenter, retrospective, and real-world study. J Am Heart Assoc. 2021;10(12) doi: 10.1161/JAHA.120.018451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheng X., Cai G., Wen X., et al. Clinical characteristics and fatal outcomes of hypertension in patients with severe COVID-19. Aging. 2020;12(23):23436–23449. doi: 10.18632/aging.104019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cummings M.J., Baldwin M.R., Abrams D., et al. Epidemiology, clinical course, and outcomes of critically ill adults with COVID-19 in New York City: a prospective cohort study. Lancet. 2020;395(10239):1763–1770. doi: 10.1016/S0140-6736(20)31189-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Czapla M., Juárez-Vela R., Gea-Caballero V., Zieliński S., Zielińska M. The Association between nutritional status and in-hospital mortality of COVID-19 in critically-ill patients in the ICU. Nutrients. 2021;13(10):3302. doi: 10.3390/nu13103302. Published 2021 Sep 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Souza R., Mhatre S., Qayyumi B., et al. Clinical course and outcome of patients with COVID-19 in Mumbai City: an observational study. BMJ Open. 2021;11(5) doi: 10.1136/bmjopen-2020-042943. Published 2021 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gao C., Cai Y., Zhang K., et al. Association of hypertension and antihypertensive treatment with COVID-19 mortality: a retrospective observational study. Eur Heart J. 2020;41(22):2058–2066. doi: 10.1093/eurheartj/ehaa433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ge E., Li Y., Wu S., Candido E., Wei X. Association of pre-existing comorbidities with mortality and disease severity among 167,500 individuals with COVID-19 in Canada: a population-based cohort study. PLoS ONE. 2021;16(10) doi: 10.1371/journal.pone.0258154. Published 2021 Oct 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Geng L., He C., Kan H., et al. The association between blood pressure levels and mortality in critically ill patients with COVID-19 in Wuhan, China: a case-series report. Hypertens Res. 2021;44(3):368–370. doi: 10.1038/s41440-020-00594-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Giorgi Rossi P., Marino M., Formisano D., et al. Characteristics and outcomes of a cohort of COVID-19 patients in the Province of Reggio Emilia, Italy. PLoS ONE. 2020;15(8) doi: 10.1371/journal.pone.0238281. Published 2020 Aug 27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gu H., Cirillo C., Nabeebaccus A.A., et al. First-phase ejection fraction, a measure of preclinical heart failure, is strongly associated with increased mortality in patients with COVID-19. Hypertension. 2021;77(6):2014–2022. doi: 10.1161/HYPERTENSIONAHA.121.17099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim E., Kim Y.C., Park J.Y., Jung J., Lee J.P., Kim H. Evaluation of the prognosis of COVID-19 patients according to the presence of underlying diseases and drug treatment. Int J Environ Res Public Health. 2021;18(10):5342. doi: 10.3390/ijerph18105342. Published 2021 May 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim S.W., Kim S.M., Kim Y.K., et al. Clinical characteristics and outcomes of COVID-19 cohort patients in daegu metropolitan city outbreak in 2020. J Korean Med Sci. 2021;36(1):e12. doi: 10.3346/jkms.2021.36.e12. Published 2021 Jan 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Marateb H.R., von Cube M., Sami R., et al. Absolute mortality risk assessment of COVID-19 patients: the Khorshid COVID Cohort (KCC) study. BMC Med Res Methodol. 2021;21(1):146. doi: 10.1186/s12874-021-01340-8. Published 2021 Jul 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pezel T., Garot P., Hovasse T., et al. Prognostic value of pre-hospitalization stress perfusion cardiovascular magnetic resonance to predict death in patients hospitalized for COVID-19. Arch Cardiovasc Dis. 2021;114(12):781–792. doi: 10.1016/j.acvd.2021.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Qin W., Bai W., Liu K., et al. Clinical course and risk factors of disease deterioration in critically ill patients with COVID-19. Hum Gene Ther. 2021;32(5–6):310–315. doi: 10.1089/hum.2020.255. [DOI] [PubMed] [Google Scholar]
- 34.Tu Y., Yang P., Zhou Y., et al. Risk factors for mortality of critically ill patients with COVID-19 receiving invasive ventilation. Int J Med Sci. 2021;18(5):1198–1206. doi: 10.7150/ijms.50039. Published 2021 Jan 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang F., Cao J., Yu Y., et al. Epidemiological characteristics of patients with severe COVID-19 infection in Wuhan, China: evidence from a retrospective observational study [published correction appears in Int J Epidemiol. 2021 May 17;50(2):700] Int J Epidemiol. 2021;49(6):1940–1950. doi: 10.1093/ije/dyaa180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang L., He W., Yu X., et al. Coronavirus disease 2019 in elderly patients: characteristics and prognostic factors based on 4-week follow-up. J Infect. 2020;80(6):639–645. doi: 10.1016/j.jinf.2020.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wu C., Chen X., Cai Y., et al. Risk factors associated with acute respiratory distress syndrome and death in patients with coronavirus disease 2019 Pneumonia in Wuhan, China [published correction appears in JAMA Intern Med. 2020 Jul 1;180(7):1031] JAMA Intern Med. 2020;180(7):934–943. doi: 10.1001/jamainternmed.2020.0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu K., Zhou M., Yang D., et al. Application of ordinal logistic regression analysis to identify the determinants of illness severity of COVID-19 in China. Epidemiol Infect. 2020;148:e146. doi: 10.1017/S0950268820001533. Published 2020 Jul 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Manolis A.S., Manolis A.A., Manolis T.A., Apostolaki N.E., Melita H. COVID-19 infection and body weight: a deleterious liaison in a J-curve relationship. Obes Res Clin Pract. 2021;15(6):523–535. doi: 10.1016/j.orcp.2021.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mahamat-Saleh Y., Fiolet T., Rebeaud M.E., et al. Diabetes, hypertension, body mass index, smoking and COVID-19-related mortality: a systematic review and meta-analysis of observational studies. BMJ Open. 2021;11(10) doi: 10.1136/bmjopen-2021-052777. Published 2021 Oct 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Földi M., Farkas N., Kiss S., et al. Obesity is a risk factor for developing critical condition in COVID-19 patients: a systematic review and meta-analysis. Obes Rev. 2020;21(10):e13095. doi: 10.1111/obr.13095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Huang Y., Lu Y., Huang Y.M., et al. Obesity in patients with COVID-19: a systematic review and meta-analysis. Metabolism. 2020;113 doi: 10.1016/j.metabol.2020.154378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J., Hu J., Zhu C. Obesity aggravates COVID-19: a systematic review and meta-analysis. J Med Virol. 2021;93(1):257–261. doi: 10.1002/jmv.26237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hariyanto T.I., Kurniawan A. Dyslipidemia is associated with severe coronavirus disease 2019 (COVID-19) infection. Diabetes Metab Syndr. 2020;14(5):1463–1465. doi: 10.1016/j.dsx.2020.07.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Patanavanich R., Glantz S.A. Smoking is associated with COVID-19 progression: a meta-analysis. Nicotine Tob Res. 2020;22(9):1653–1656. doi: 10.1093/ntr/ntaa082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Peng M., He J., Xue Y., Yang X., Liu S., Gong Z. Role of hypertension on the severity of COVID-19: a review. J Cardiovasc Pharmacol. 2021;78(5):e648–e655. doi: 10.1097/FJC.0000000000001116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Leiva Sisnieguez C.E., Espeche W.G., Salazar M.R. Arterial hypertension and the risk of severity and mortality of COVID-19. Eur Respir J. 2020;55(6) doi: 10.1183/13993003.01148-2020. Published 2020 Jun 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kulkarni S., Jenner B.L., Wilkinson I. COVID-19 and hypertension. J Renin Angiotensin Aldosterone Syst. 2020;21(2) doi: 10.1177/1470320320927851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gao Y.D., Ding M., Dong X., et al. Risk factors for severe and critically ill COVID-19 patients: a review. Allergy. 2021;76(2):428–455. doi: 10.1111/all.14657. [DOI] [PubMed] [Google Scholar]
- 50.Zhou F., Yu T., Du R., et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [published correction appears in Lancet. 2020 Mar 28;395(10229):1038] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang J., Wang X., Jia X., et al. Risk factors for disease severity, unimprovement, and mortality in COVID-19 patients in Wuhan, China. Clin Microbiol Infect. 2020;26(6):767–772. doi: 10.1016/j.cmi.2020.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Du Y., Zhou N., Zha W., Lv Y. Hypertension is a clinically important risk factor for critical illness and mortality in COVID-19: a meta-analysis. Nutr Metab Cardiovasc Dis. 2021;31(3):745–755. doi: 10.1016/j.numecd.2020.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Xiang G., Xie L., Chen Z., et al. Clinical risk factors for mortality of hospitalized patients with COVID-19: systematic review and meta-analysis. Ann Palliat Med. 2021;10(3):2723–2735. doi: 10.21037/apm-20-1278. [DOI] [PubMed] [Google Scholar]
- 54.Bae S., Kim S.R., Kim M.N., Shim W.J., Park S.M. Impact of cardiovascular disease and risk factors on fatal outcomes in patients with COVID-19 according to age: a systematic review and meta-analysis. Heart. 2021;107(5):373–380. doi: 10.1136/heartjnl-2020-317901. [DOI] [PubMed] [Google Scholar]
- 55.Ferrario C.M. The renin-angiotensin system: importance in physiology and pathology. J Cardiovasc Pharmacol. 1990;15(Suppl 3):S1–S5. [PubMed] [Google Scholar]
- 56.Nicholls M.G., Richards A.M., Agarwal M. The importance of the renin-angiotensin system in cardiovascular disease. J Hum Hypertens. 1998;12(5):295–299. doi: 10.1038/sj.jhh.1000638. [DOI] [PubMed] [Google Scholar]
- 57.Rodríguez-Iturbe B., Pons H., Quiroz Y., Lanaspa M.A., Johnson R.J. Autoimmunity in the pathogenesis of hypertension. Nat Rev Nephrol. 2014;10(1):56–62. doi: 10.1038/nrneph.2013.248. [DOI] [PubMed] [Google Scholar]
- 58.Norlander A.E., Madhur M.S., Harrison D.G. The immunology of hypertension [published correction appears in J Exp Med. 2018 Jan 5;:] J Exp Med. 2018;215(1):21–33. doi: 10.1084/jem.20171773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hippisley-Cox J., Young D., Coupland C., et al. Risk of severe COVID-19 disease with ACE inhibitors and angiotensin receptor blockers: cohort study including 8.3 million people. Heart. 2020;106(19):1503–1511. doi: 10.1136/heartjnl-2020-317393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Yang G., Tan Z., Zhou L., et al. Effects of angiotensin ii receptor blockers and ACE (Angiotensin-Converting Enzyme) inhibitors on virus infection, inflammatory status, and clinical outcomes in patients with COVID-19 and hypertension: a single-center retrospective study. Hypertension. 2020;76(1):51–58. doi: 10.1161/HYPERTENSIONAHA.120.15143. [DOI] [PubMed] [Google Scholar]
- 61.Lee M.M.Y., Docherty K.F., Sattar N., et al. Renin-angiotensin system blockers, risk of SARS-CoV-2 infection and outcomes from CoViD-19: systematic review and meta-analysis. Eur Heart J Cardiovasc Pharmacother. 2022;8(2):165–178. doi: 10.1093/ehjcvp/pvaa138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Bavishi C., Whelton P.K., Mancia G., Corrao G., Messerli F.H. Renin-angiotensin-system inhibitors and all-cause mortality in patients with COVID-19: a systematic review and meta-analysis of observational studies. J Hypertens. 2021;39(4):784–794. doi: 10.1097/HJH.0000000000002784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lam K.W., Chow K.W., Vo J., et al. Continued in-hospital angiotensin-converting enzyme inhibitor and angiotensin ii receptor blocker use in hypertensive COVID-19 patients is associated with positive clinical outcome. J Infect Dis. 2020;222(8):1256–1264. doi: 10.1093/infdis/jiaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Meng J., Xiao G., Zhang J., et al. Renin-angiotensin system inhibitors improve the clinical outcomes of COVID-19 patients with hypertension. Emerg Microbes Infect. 2020;9(1):757–760. doi: 10.1080/22221751.2020.1746200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.