Abstract
Monkeypox is a zoonotic illness caused by the monkeypox virus, an Orthopoxvirus in the same genus as the variola, vaccinia, and cowpox viruses. Since the detection of the first human case in the Democratic Republic of the Congo in 1970, the disease has caused sporadic infections and outbreaks, mainly restricted to some countries in west and central Africa. In July, 2022, WHO declared monkeypox a Public Health Emergency of International Concern, on account of the unprecedented global spread of the disease outside previously endemic countries in Africa and the need for global solidarity to address this previously neglected disease. The 2022 outbreak has been primarily associated with close intimate contact (including sexual activity) and most cases have been diagnosed among men who have sex with men, who often present with novel epidemiological and clinical characteristics. In the 2022 outbreak, the incubation period ranges from 7 days to 10 days and most patients present with a systemic illness that includes fever and myalgia and a characteristic rash, with papules that evolve to vesicles, pustules, and crusts in the genital, anal, or oral regions and often involve the mucosa. Complications that require medical treatment (eg, antiviral therapy, antibacterials, and pain control) occur in up to 40% of patients and include rectal pain, odynophagia, penile oedema, and skin and anorectal abscesses. Most patients have a self-limited illness; between 1% and 13% require hospital admission (for treatment or isolation), and the case-fatality rate is less than 0·1%. A diagnosis can be made through the presence of Orthopoxvirus DNA in PCRs from lesion swabs or body fluids. Patients with severe manifestations and people at risk of severe disease (eg, immunosuppressed people) could benefit from antiviral treatment (eg, tecovirimat). The current strategy for post-exposure prophylaxis or pre-exposure prophylaxis for people at high risk is vaccination with the non-replicating modified vaccinia Ankara. Antiviral treatment and vaccines are not yet available in endemic countries in Africa.
Introduction
Monkeypox is a zoonotic viral infection caused by the monkeypox virus that results in a rash similar to that of smallpox. However, person-to-person spread beyond immediate close contacts and case-fatality rates are substantially lower in monkeypox than in smallpox infection.
Monkeypox was first described in 1958 among monkeys shipped from Singapore to Denmark.1 During the following decade, additional outbreaks were reported in captive monkeys in the USA, the Netherlands, and France.2 The first case of monkeypox infection in humans was reported in 1970 in the Democratic Republic of the Congo in a boy aged 9 months who was the only member of his family without a smallpox vaccination. Receiving a previous vaccination against smallpox was estimated to be 85% effective in preventing monkeypox,3 although the long-term efficacy of smallpox vaccination is unclear.4
After the first human case, sporadic outbreaks were reported in some countries in west and central Africa, mainly among children in rural, rainforest areas. On the basis of clinical presentation and genomic sequencing results, monkeypox virus isolates were classified into two clades. Between 1981 and 2017, monkeypox virus clade 1 caused several outbreaks in the Democratic Republic of the Congo, with high fatality rates (1–12%).5, 6, 7, 8, 9, 10, 11, 12 Most of these cases were not laboratory confirmed because of a scarcity of local diagnostic infrastructure, most patients living in difficult-to-reach rural settings, and challenges associated with civil unrest and the existing health system. During this period, very few human monkeypox cases were reported in west Africa but, in 2017, Nigeria had a large outbreak with 122 PCR-confirmed cases of the monkeypox virus clade 2.13, 14 The progressive rise of cases in the Democratic Republic of the Congo and the re-emergence of monkeypox in Nigeria in 2017 were attributed to the discontinuation of smallpox vaccination in 1980, waning immunity, frequent hunting or butchering of bushmeat for subsistence, and the spreading urbanisation with encroachment into forest and swamp areas.15 Despite this concern, there was a global neglect of the African outbreaks and dearth of related research.
Monkeypox generated some international attention in 2003, when 71 human cases were reported in the USA.16, 17, 18, 19 Between 2003 and 2022, a few travel-related cases were reported outside endemic countries in Europe, North America, and Asia.20, 21, 22, 23, 24, 25, 26, 27, 28 However, from May 13, 2022, a global outbreak—consisting of community spread of a new monkeypox virus lineage, clade 2b, in newly affected countries worldwide—led to the declaration of a Public Health Emergency of International Concern. By November, 2022, more than 78 000 cases in more than 100 countries had been reported,29, 30, 31 and several clinical studies have suggested that the disease has novel epidemiological and clinical characteristics.32, 33, 34, 35
In this Seminar, we discuss the virology, epidemiology, clinical presentation, treatment, and prevention of monkeypox in light of the 2022 global outbreak.
Virology
Monkeypox is an orthopoxvirus (a double-stranded DNA virus) in the same genus as the variola virus (the causative agent of smallpox), vaccinia virus (the virus used in the smallpox vaccine), and cowpox virus. Electron microscopy of cells infected with monkeypox virus shows a brick-like virion ranging from 200 nm to 250 nm, indistinguishable from the virions of variola or vaccinia viruses.
The monkeypox genome is large, with about 200 kilobase pairs, and encodes approximately 190 proteins to build viral particles and modulate numerous host processes. Two distinct clades of monkeypox that show approximately 0·5% genomic sequence difference had been historically identified in different geographical regions of Africa.36 Clade 1 (which has case-fatality rates of 1–12%5, 6, 7, 8, 9, 10, 11, 12, 37) is usually responsible for disease in central Africa and the Congo Basin, whereas clade 2 (which is less virulent, with case-fatality rates less than 0·1%) is found in west Africa.36, 37, 38 The genomic differences between clade 1 and 2 viruses occur in regions that encode for important virulence genes39 and probably explain the differences in clinical severity. For example, the gene encoding a complement control protein that prevents initiation of the complement pathway is missing in clade 2 viral strains, and animal models of monkeypox using the clade 1 virus with a complement control protein deletion led to reduced morbidity and mortality in prairie dogs.40
A new lineage B.1, classified as clade 2b for its close relationship to clade 2, has been identified in the 2022 global outbreak.41, 42 Unlike RNA viruses, the double-stranded DNA of orthopoxviruses is very stable and their DNA polymerase has proofreading exonuclease activity, resulting in a low mutation rate (one to two nucleotide changes per year). The new B.1 lineage has been associated with strains circulating in Nigeria during the 2017 outbreak;13 however, there has been a divergence of up to 50 single nucleotide polymorphisms (especially APOBEC3-related mutations), which represents a mutation rate 6–12 times higher than previously estimated.42 Since the APOBEC3 human protein serves as a cellular defence mechanism by introducing errors into the viral genome, mutations of this type are indicative of a large amount of human-to-human transmission. One key question is how these variations affect the monkeypox virus transmissibility, virulence, and human adaptation.
Epidemiology
Within the decade after the first case in humans in 1970, 59 cases of human monkeypox were reported in west and central Africa,5, 6, 7, 12 with a mortality rate of 17% in children younger than 10 years.8, 43 After smallpox eradication and subsequent discontinuation of routine smallpox immunisation in 1980,44 WHO monitored human monkeypox cases with the concern that lower immunity rates to smallpox would increase population susceptibility to the monkeypox virus.45 From 2000 to 2015, there were three spatiotemporal clusters indicative of outbreaks of suspected monkeypox cases in the Democratic Republic of the Congo,9 including 760 laboratory-confirmed cases between 2005 and 2007.10 Between 1981–86 and 2006–07, the cases increased by 20 (from 0·72 per 10 000 to 14·42 per 10 000). Similarly, an increase in monkeypox cases in Nigeria has been reported since 2017,13 after nearly 40 years of no reported cases.
According to a 2022 WHO report,46 monkeypox was considered endemic in several African countries, including Benin, Cameroon, Central African Republic, the Democratic Republic of the Congo, Gabon, Ghana (identified in animals only), Côte d'Ivoire, Liberia, Nigeria, Sierra Leone, and South Sudan. Between January and May, 2022, the Democratic Republic of the Congo was the country that reported the greatest number of suspected monkeypox cases, with 1284 cases and 58 deaths.46 However, there is a considerable and regrettable gap between confirmed and suspected cases, which indicates an inadequate laboratory testing capacity.
Aside from the generally increasing rates in endemic countries, several sporadic cases and outbreaks of monkeypox have been reported in non-endemic countries (eg, the UK, the USA, Israel, and Singapore) after travel to, or animal importation from, endemic areas.18, 20, 21, 27, 28 In 2003, 71 cases (35 of which were laboratory confirmed) of human infection in the USA were related to prairie dogs that had been in contact with African rodents.16, 17, 19 Between 2018 and 2021, seven cases of monkeypox were diagnosed in the UK, four of them related to travel from endemic countries.23, 24 In July, 2021, two returning travellers from Nigeria were diagnosed with monkeypox in Texas and Maryland (USA), respectively.25, 26
In the 2022 global outbreak, the first cases of monkeypox were reported to WHO in May;47 since then, the number of cases has continued to increase worldwide. Many early cases occurred in people who had attended an international LGBT+ Pride event held on the Spanish island of Gran Canaria, which linked to transmission chains in several European countries.48, 49, 50, 51 However, by the end of May, locally acquired infections and community transmission became predominant in all affected countries.48 On July 23, 2022, WHO declared this outbreak of monkeypox a Public Health Emergency of International Concern.52
Pathogenesis
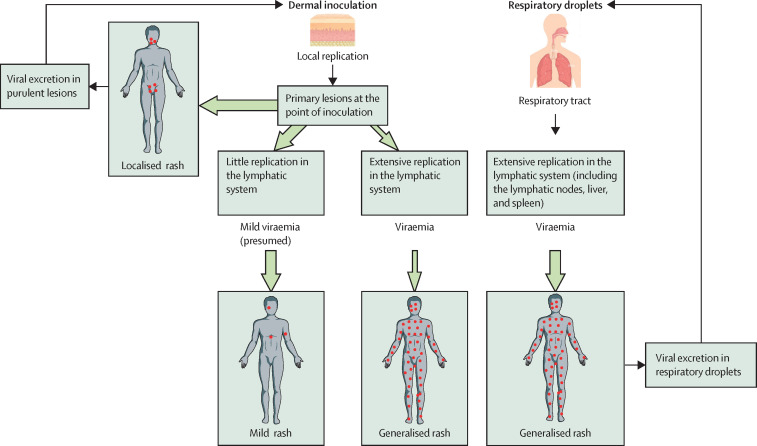
Monkeypox viruses can enter the host via a respiratory route or a dermal route (figure 1 ).53, 54 The route of entry and the clade of monkeypox virus might affect how the illness manifests.
Figure 1.
Proposed mechanism for the spread of the monkeypox virus throughout the body and its relation to the transmission route
The clinical presentation of monkeypox might be influenced by microorganism virulence factors, host immunity, and the transmission route. Unlike in previous outbreaks, the monkeypox virus in the 2022 outbreak is thought to spread through close contact or sexual contact, causing predominantly localised lesions instead of extensive disseminated lesions. It is possible that the localised nature of the disease results in lower concentrations of viraemia and, consequently, less virus in respiratory excretions. As the respiratory route becomes less important, transmission continues to occur through direct contact via dermal inoculation, perpetuating the cycle of clinical presentation and transmission. Nevertheless, the monkeypox virus in previous outbreaks, mainly from the Democratic Republic of the Congo, is thought to be transmitted primarily through the respiratory tract and was followed by disseminated disease presentation. Both the dermal inoculation and respiratory routes could contribute to animal-to-human transmission. The size of the green arrows indicates the frequency of manifestation; a large arrow indicates a frequent pathway and a small arrow indicates an infrequent pathway.
In the respiratory tract, the monkeypox virus can infect airway epithelial cells, whereas in the skin, the virus infects keratinocytes, fibroblasts, and endothelial cells, establishing productive and cytopathic infection.55, 56 Moreover, antigen-presenting cells, such as macrophages, dendritic cells, and (in the skin) Langerhans cells, are infected abortively, allowing them to survive long enough to carry antigens to draining lymph nodes. Viral replication, gene expression, and virion assembly in the host's cell cytoplasm results in mature virions with a single lipid membrane, followed by the release of extracellular virions with an additional envelope.57 These are two antigenically distinct forms that contain 25 (mature virions) or six (extracellular virions) surface proteins.
The monkeypox virus spreads from the initial site of infection to draining lymph nodes by migration of antigen-presenting cells and by direct viral access to lymphatic vessels. After initial replication in the lymph nodes, resulting in a low-grade primary viraemia, the monkeypox virus can target other large organs, the spleen, and the liver, where it amplifies and results in a second major viraemia wave that could then allow the virus to further spread to distant organs such as the lung, kidneys, intestines, and skin.
In the non-human primate models of respiratory-acquired, clade 1 monkeypox virus,58, 59, 60 the virus replicates in the respiratory epithelium during the incubation period (up to post-challenge day 4),59 then it spreads to the regional lymph nodes and lymphoid organs, including the tonsils, spleen, liver, and colon, where it amplifies up to day 6. Finally, the virus is detected in the blood on day 8, with increasing concentration until day 10, along with widespread lesions in the skin and mucous membranes. Ulcerating lesions in the mouth and pharynx release large quantities of virus particles, disseminated through large respiratory droplets.
The primate models of subcutaneous inoculation show viral replication only in the skin and lymphatic system, displaying mild, localised disease after clade 2 monkeypox virus infection.60 The respiratory, gastrointestinal, and genitourinary tracts might be affected after skin inoculation of clade 1 monkeypox virus.60 Data on skin inoculation in humans are limited to those obtained from vaccination with the vaccinia virus or variola virus (ie, variolation), which resulted in locally restricted lesions around the point of entry.61 Similarly, during the 2022 outbreak, patients sexually acquiring the monkeypox virus present with local oral and anogenital lesions and some develop a small number of distant lesions (face, limbs, and trunk), but extensive disseminated skin lesions are rare.32, 33, 34, 35
Histopathological analysis of skin lesions in the vesicular stage can reveal ballooning degeneration of keratinocytes, prominent spongiosis, dermal oedema, and acute inflammation.62 In the pustule stage, apoptotic keratinocyte debris and inflammatory cells are predominant, along with few viable keratinocytes. Viable keratinocytes can be multinucleated or exhibit cytopathic damage, such as eosinophilic inclusion bodies, prominent nucleoli, and so-called ground glass chromatin. Immunochemistry shows the virus in the cytoplasm of all keratinocytes within the affected (but not the non-affected) epidermis. The lymphocytic infiltrate is predominantly T cells with CD4+ and CD8+ elements.63
Monkeypox virus infection stimulates humoral and cellular immune responses that restrict viral replication and induce long-term immunity in recovering patients.4, 64, 65, 66, 67, 68 Humoral immune response after natural infection with monkeypox or vaccinia virus vaccination consists of orthopoxvirus-specific IgM and IgG antibodies against multiple antigen targets with long-term persistence of residual IgG-memory B cells4, 65, 67 that protects from reinfection or from developing severe disease.4 After vaccinia virus vaccination, specific memory B cells can last for decades, although only 50% of individuals have protective concentrations of neutralising antibodies after 20 years. It is likely that cross-protective immunity against monkeypox might similarly wane over time.
Cellular immune response after natural infection with monkeypox or vaccinia virus vaccination is characterised by a rapid expansion of activated effector CD4+ and CD8+ T cells,64, 66, 68 followed by a decrease over time, which tends to normalise after 12–20 days from symptom onset.66 Most patients have specific T cells able to produce several Th1 inflammatory cytokines (IFN-γ, IL-1β, IL-6, IL-8, TNF, and MCP-1). After vaccinia virus vaccination, memory T cells were found to persist for up to 50 years (half-life of 8–15 years),69 but this does not necessarily provide robust protection against the monkeypox virus, as shown in numerous cases of monkeypox virus breakthrough infections in people vaccinated in childhood. Effector CD4+ T cells play a role in enhancing recall and differentiation of B cells into antibody-secreting cells, whereas CD8+ T cells kill infected macrophages to prevent viral spread. People with HIV with high CD4+ T-cell counts (>350 cells) also show a similar poxvirus-specific T-cell response,4 but there is no data in those with low CD4 counts (<350 cells). In previously immunised non-human primates, B-cell responses are essential for protection, whereas depletion of CD4+ or CD8+ T cells have a minor effect on disease protection.67 However, CD4 depletion before immunisation decreased the development of such protective B-cell responses and antibodies, and increased infection severity, which is concerning.70
Transmission
Animal-to-human and human-to-human transmission can occur. Although monkeypox virus has been isolated from several rodents and non-primate animals in Africa (eg, rope squirrels, tree squirrels, Gambian rats, dormice, and monkeys),71 the exact animal reservoir of the virus is still unknown. It has been suggested that monkeys and humans are incidental hosts of the infection.45
Animal-to-human transmissions could occur from non-invasive exposures to infected animals (eg, touching the animal, cleaning its cage, and hunting or processing its meat) or from a bite or scratch from an infected animal, with the former having a lower risk of transmission.72, 73 Genomic analyses have revealed a number of zoonotic spillovers into human populations, suggesting that monkeypox virus might persist in wildlife reservoirs and occasionally infect humans.74, 75
Human-to-human transmission of monkeypox virus can occur by respiratory secretions, direct contact, vertical transmission, percutaneous transmission, or indirect contact through fomites.
Respiratory transmission occurs when large respiratory droplets from the transmitter host deposit on to the mucous membranes of the mouth and nose of the recipient host. Extended face-to-face contact, such as household contact, might be required for transmission to occur via this route.26 Activities resulting in resuspension of dried material from lesions (eg, shaking contaminated linens) could also present a risk and should be avoided.
Direct contact with infectious sores or lesions on mucous membranes has been the primary mode of transmission during the 2022 outbreak.32, 33, 34, 35, 76 The monkeypox virus can spread during activities that include close, intimate contact with an infected individual, and transmission might be facilitated by a breach in the recipient's skin or mucosa, such as microscopic abrasions that occur during sexual activity.
During the 2022 outbreak, although monkeypox virus DNA is nearly always found in skin samples, it is detected less frequently and at lower viral loads in other body parts. For example, monkeypox virus DNA can be detected in only 60–70% of anus and throat samples, 50% of semen samples, and 20% of blood and urine samples.32, 34, 77, 78 Moreover, the viral load in skin samples is higher by about two orders of magnitude compared with that in other body locations (viral load 106 copies per mL vs 104 copies per mL; cycle threshold values approximately 22 vs approximately 28).77, 78, 79, 80 In addition, evidence of replication-competent virus isolation has been reported more frequently from skin samples than from throat swabs and semen samples.78, 80 Altogether these data support that intimate sexual contact is the main route of transmission, with the respiratory route playing a less important role.
The infectious period lasts from the onset of clinical manifestations until all skin lesions have scabbed over and re-epithelialisation has occurred. In immunocompetent patients with mild monkeypox disease, viral DNA is detectable by quantitative PCR for a median time of 25 days in the skin, 16 days in the pharynx, 16 days in the rectum, 13 days in semen, and 1 day in blood; and it would take until day 39 and day 41 for 90% of cases to have undetectable viral DNA concentrations in semen and in skin lesions, respectively.80 Semen is unlikely to be a major potential source of transmission during the course of disease or after complete recovery, as previously observed in other zoonotic viruses, because viral loads in semen are generally low and viral clearance is fast.80 A severe clinical course is associated with a longer period of viraemia and viral shedding than mild monkeypox disease.24
Vertical transmission to the fetus can occur, sometimes leading to congenital monkeypox, although the risk at different stages of pregnancy has not been determined.71 Between 2007 and 2011 there were four pregnant women with monkeypox in the Democratic Republic of the Congo; one had a healthy infant, two had a miscarriage, and one had a fetal death with diffuse maculopapular skin lesions, consistent with vertical transmission.81 At least 12 pregnant women have been infected during the 2022 outbreak, but vertical transmission was not observed in any case.82 This difference might be partly related to the greater invasiveness of clade 1 than clade 2.
Percutaneous transmission has been reported after needlestick injuries from supplies used to collect cutaneous lesion samples.83, 84 The monkeypox lesions appeared at the site of the needlestick. The recommendation is to avoid both using sharp instruments to open or aspirate monkeypox lesions and recapping used needles to reduce the risk of injuries.
Environmental contamination with monkeypox virus DNA in both the household and patient-care environment of people with monkeypox has been reported, including detection of replication-competent virus samples.85, 86, 87 Investigations in respiratory isolation rooms of a hospital found viral DNA in rooms, bathrooms, anterooms, health-care workers' personal protective equipment, and non-touch surfaces (eg, >1·5 m from the bed).88 It is unclear whether indirect contact with fomites is a frequent transmission pathway; however, the widespread surface contamination of the patient-care environment calls for a systematic approach to surface cleaning and appropriate use of personal protective equipment by health-care workers.
Risks of transmission can vary in different settings, including households, congregated settings, health-care facilities, and in the community. Before the 2022 outbreak, transmission occurred mainly within the household, and sustained human-to-human spread was rare. In one report from the Democratic Republic of the Congo, the secondary attack rate in households was as high as 9%.89 Additionally, monkeypox outbreaks had been described in congregate living situations, such as prisons.90 By contrast, household transmission has been rare during the 2022 outbreak, accounting for only 0·6–3·0% of cases,32, 34 including several paediatric cases and one neonatal infant who lived in a home with an infected adult.91, 92 Most infections in 2022 have been associated with community transmission, with an estimated reproductive number ranging from 1·40 to 1·80, which implies a potential for sustainable local transmission.93 Health-care-associated transmission of monkeypox had been reported in a dozen cases in Africa,94 and in one case outside endemic regions before May, 2022.23 The risk of transmission during the 2022 outbreak has been low, with only a small number of transmission events reported after exposure to fomites or needlestick injuries.83, 84, 95, 96
Subclinical or asymptomatic monkeypox infection is considered rare. However, seroepidemiological studies in Africa,3, 97 and retrospective PCR detection in male sexual health clinic attendees in France98 and Belgium,99 suggest that some patients could have an asymptomatic infection, and modelling studies suggest that in the 2022 outbreak approximately half of the transmissions occurred in the pre-symptomatic phase.100 Further research is needed to determine the potential for transmission from individuals with an asymptomatic infection.
Population at risk
Historical risk factors for acquiring the infection in African countries include living in forested areas (specifically near sites habitable to squirrels), living in a home with monkeypox, being male, and being younger than 15 years.10, 11 During 2022, most patients diagnosed with monkeypox have been identified among men who have sex with men (98% of the patients in a report of 528 cases from 16 countries)32 and many reported high-risk sexual behaviour as a potential risk factor. Some of the patients have reported having multiple or anonymous sexual partners in the previous 2 weeks, attending sex-on-premises venues (eg, saunas or bathhouses) or group-sex sessions, and using recreational drugs during sex. Concomitant sexually transmitted infections have been reported in 16–29% of individuals tested in the published cohorts,32, 33, 34, 35 with gonorrhoea, chlamydia, and syphilis being the most common infections. A substantial proportion (ie, 33–42%) of patients infected with monkeypox virus are on pre-exposure prophylaxis to prevent acquiring HIV (ie, sexually active HIV-negative adults),32, 101 and a high percentage are people living with HIV (36–42%).32, 33, 34 However, it is not yet known whether HIV infection affects a person's risk of acquiring monkeypox, and future studies shall determine the relative contribution of sexual behaviour, access to sexual health care, and biological risk. Women infected with the monkeypox virus during the 2022 outbreak represent a very small part of the overall infected population. One report102 found that 61% of 74 cis women and non-binary individuals with monkeypox and 89% of 62 trans women with monkeypox acquired it through sexual contact, with the trans women sampled having a higher rate of HIV infection and sex work than the cis women.
Groups at higher risk for progressing to severe disease in African countries were children,19 pregnant women,81 and immunocompromised individuals, including people with uncontrolled HIV infection.13, 14 However, among the few children, adolescents, and pregnant women infected during the 2022 outbreak, there have been no severe cases or adverse neonatal outcomes.82, 91 The risk of severe disease in people with HIV appears to vary depending on their immunological status. Reports from individuals admitted to hospital during the 2017–18 outbreak in Nigeria indicate that people with advanced and uncontrolled HIV infection might be at higher risk for severe, extensive, or prolonged monkeypox disease after infection.13 During the 2022 outbreak, several people with HIV and CD4 counts of less than 200 cell per mL have presented with severe manifestations of monkeypox and some have died.103 In contrast, studies in which most patients were receiving effective antiretroviral therapy (and had undetectable HIV RNA) have noted no evident excess in complications, hospital admissions, or deaths among people with HIV infection and monkeypox to date.32, 34, 104 Two studies32, 34 that, respectively, had 241 and 72 people living with HIV found no differences in clinical features or clinical outcomes between people with or without HIV.34 Another study found that, although patients with HIV were more likely to have a higher rash burden, there was no association between HIV status and severe illness.104
Clinical presentation
The clinical presentation of monkeypox cases associated with the 2022 outbreak32, 33, 34, 35 differs from previous reports (table ).12, 13, 14, 19 Before the 2022 outbreak, the mean incubation period of monkeypox virus infection was 5–13 days (range 4–21).19, 105 People with a history of an animal bite or scratch might have a shorter incubation period than those with only tactile exposures (9 days vs 13 days, respectively).73 During the 2022 outbreak, the mean incubation period generally spans 7–10 days after exposure.32, 34, 106, 107 The shorter incubation period could be due to the direct viral inoculation through sexual transmission.
Table.
Comparison of the clinical presentation in the 2022 outbreak with previous outbreaks
| 2022 outbreak | Previous outbreaks | |
|---|---|---|
| Population features | ||
| Mean age | 37–41 years | 26–32 years |
| Smallpox vaccination in childhood | 11–18% | 20% |
| Incubation period | 6–7 days | 12 days |
| Sex | ||
| Male | 97–100% | 53–78% |
| Female | 0–3% | 22–47% |
| Systemic features | ||
| Systemic symptoms | Fever (54–72%), fatigue or myalgia (24–81%), and headache (25–53%) | Fever (45–90%), fatigue or myalgia (73–85%), and headache (48–79%) |
| Lymphadenopathy | 55–87%, localised in the lymph catchment area of lesions | 57–87%, localised or generalised |
| Systemic symptoms start after rash | 38–52% | 15–66% |
| Clinical features of the rash | ||
| More than 10 lesions | 22–36% | 100% |
| More than 20 lesions | 12% | 46% |
| More than 100 lesions | 0–4% | 20–42% |
| Progression | Lesions present at different stages simultaneously; not all lesions progressed from one phase to another in order | Progression from one phase to another occurs in order |
| Distribution | Commonly localised to 1–3 body regions | Commonly disseminated to >3 body regions |
| Localisation | Genitalia (55–61%), perianal (34–44%), oropharyngeal (14–43%), trunk (25–57%), arms and legs (50–60%), face (20–39%), and palms or soles (0–10%) | Genitalia (67–68%), perianal (not reported), oropharyngeal (38%), trunk (80–93%), arms and legs (81–91%), face (96–98%), palms (28–55%), and soles (10–64%) |
| Outcome | ||
| Complications | Rectal pain (14–36%), sore throat (17–36%), difficulty swallowing related to tonsillar or pharyngeal ulcer (5–14%), penile oedema (8–16%), proctitis (11–25%), secondary bacterial infection (3–4%), and conjunctivitis (1%) | Secondary bacterial infection of skin lesions (19%), bronchopneumonia (12%), sepsis (1%), encephalitis (0·4%), keratitis (0·4%), and retropharyngeal abscess (0·4%) |
| Hospital admission | 1–13% | 26% |
| Risk factors for severe disease | Unknown | Age (younger ages are more at risk), living with HIV and not being on antiretroviral therapy |
| Fatality rate | <0·1% | Clade 1 had 1–12%, clade 2 had <0·1% |
| Sexual health | ||
| Living with HIV | 36–67% | ND |
| Concomitant STI | 16–76% | ND |
| History of STI in past 12 months | 54–55% | ND |
Monkeypox has historically caused systemic symptoms attributable to a viraemic phase of illness that typically occurs before the skin rash, lasts 1–5 days, and includes fever, myalgias, sore throat, and generalised lymphadenopathy.14 In the 2022 outbreak, systemic symptoms are common and can occur before the rash (prodromal stage) or shortly after the rash appears (early clinical stage), although rashes without systemic illness have been reported.34, 105 In this outbreak, generalised swelling of the lymph nodes has not been commonly seen, although regional lymphadenopathy is often associated with the lymph catchment area of skin lesions.34
The skin eruption of monkeypox usually lasts for 2–3 weeks and progresses through several stages in the following order: macules of 2–5 mm that evolve into papules, vesicles, and then pseudopustules (ie, papules that resemble pustules but contain solid debris instead of fluid or pus).35 Lesions are well circumscribed and often develop umbilication (a central depression on the top of the lesion). Between 7 days and 14 days after the rash begins, the lesions crust over, dry up, and fall off. The lesions typically appear and evolve simultaneously on any given part of the body.32, 33, 34, 35 Some cases diagnosed in the 2022 outbreak present lesions at different stages simultaneously,104 and not all lesions progress from one phase to another in order.33, 34
The number of skin lesions in monkeypox virus infection can vary from a few to approximately 1000. In endemic regions, between 20% and 42% of patients present with more than 100 lesions,13, 14, 19 and immunocompromised individuals can present with more than 1000 lesions. In the 2022 outbreak, most patients present with 1–20 skin lesions and cases with more than 100 lesions have been extremely rare (0–4%).32, 33, 34, 35 Single lesions are reported at a rate of 10–12% and represent a significant risk of misdiagnosis, particularly with syphilis chancres.32, 33, 34
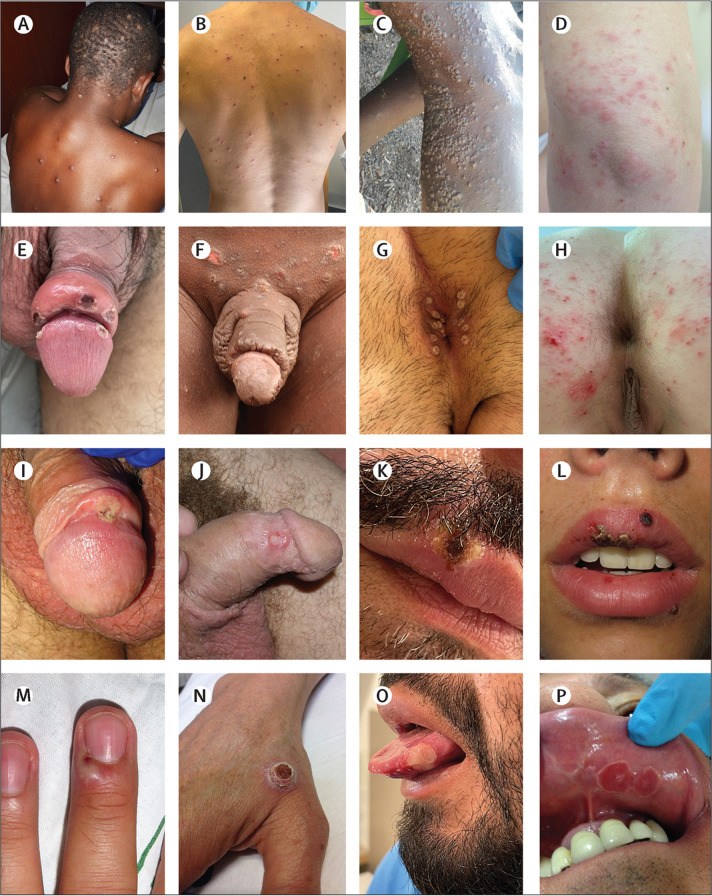
The rash location during previous outbreaks usually involved all parts of the body, with the face, trunk, and limbs being the most affected (figure 2A, C ).12, 13, 14, 19 Conversely, during the 2022 outbreak, lesions were mainly located on the anogenital and perioral areas (figure 2E, G, I, K, O).32, 33, 34, 35 Genital lesions in male patients might present as one or two solitary lesions (figure 2I) or multiple lesions that affect the penis, scrotum, and pubis. Genital lesions are commonly accompanied by surrounding oedema, which can progress to severe swelling of the penile glans or foreskin so that the retracted foreskin cannot be returned to its normal position (ie, paraphimosis; figure 2E). In the perianal region, lesions can involve the buttocks, the anal margin (figure 2G), or the anorectal mucosa resulting in proctitis (ie, rectal pain, pain on defecation, tenesmus, serosanguineous discharge, or bleeding). In the perioral region, lesions of the oral mucosa or lips are ulcers or crusts (figure 2K); lesions on the tongue are usually circular, white, and centrally depressed (figure 2O); and tonsillar lesions are painful and cause difficulty swallowing. Among individuals who started with a rash presenting in the genital and oral areas (possibly the site of inoculation), some presented with subsequent spread to the face and trunk; for others, the face or extremities have not been involved at all; and a few individuals presented with solitary primary lesions in the face or fingers alone (sometimes around the nail, causing paronychia; figure 2M). Occasionally, secondary bacterial infections can cause abscesses, and coalescing lesions in any region can lead to large plaques or ulcerations. In a report of 74 cis women and non-binary individuals with monkeypox and 62 trans women with monkeypox, cis women had vulvar lesions more frequently than perianal lesions (59% vs 24%), whereas trans women had more anal lesions (2% vs 74%), perhaps reflecting sexual activity as few of the trans women had undergone gender affirming surgery.102
Figure 2.
Monkeypox clinical presentations and differential diagnosis
Discrete rash on the thorax caused by monkeypox (Nigeria; A) and varicella (Spain; B); a generalised monkeypox rash (Democratic Republic of the Congo; C) and a blistering rash caused by dermatitis herpetiformis (Spain; D); localised monkeypox lesions causing penile oedema (Spain; E) and impetigo associated with scabies (Malawi; F); localised perianal rash caused by monkeypox (Spain; G) and molluscum contagiosum (Spain, H); a solitary monkeypox genital ulcer (Spain; I) and a primary syphilis chancre (Spain; J); lip lesion caused by monkeypox (Spain; K) and herpes simplex (Spain; L); hand lesions caused by monkeypox (Spain; M) and Orf virus infection (Spain; N); monkeypox lesions on the tongue (Spain; O) and aphthous ulcer on the labial mucosa (Spain; P). Photo credits: Dimie Ogoina (A), Fernando Gruber (B), Cristina Galván (C, D, F, H, N, P), Adrià Mendoza (E), José Miguel Cabrera (G, K, O), Irene Fuertes (I, M), Martí Vall-Mayans (J), and Rosa Taberner (L).
Data gathered during the 2022 outbreak suggest a relationship between the location of the lesions and the site of inoculation. For instance, men who have sex with men and who engage in anal-receptive sex present with proctitis more frequently than men who have sex with men and who do not engage in anal-receptive sex.34 Similarly, individuals reporting oral-receptive sex are more likely to present with tonsillitis.
The differential diagnosis should consider several skin infections, poxviruses infections, and sexually transmitted infections. Varicella (chickenpox; figure 2B) is the most likely diagnostic consideration in a patient presenting with a vesicular rash. Lesions appear in successive crops, normally coexist with lesions in different stages of development, and have a fluid content. Herpes simplex virus is a differential diagnosis of monkeypox in cases of perioral vesicles and anal ulcers (figure 2L). Additionally, impetigo (figure 2F) caused by infection with group A streptococcus should be considered because of the presentation of vesicles and pustules, although the characteristic golden crust suggests impetigo. Other non-infectious skin conditions might show a similar clinical picture including erythema multiforme, pompholyx, blistering diseases (eg, dermatitis herpetiformis [figure 2D] that is associated with gluten-sensitive enteropathy), and aphthous ulcers that are very common and are associated with many diseases (figure 2P).
The differential diagnosis should consider other poxviruses, including Molluscum contagiosum (figure 2H), which presents as single or multiple small papules with central umbilication (the anogenital location in adults could be a confounding sign in the diagnosis); the replication-competent smallpox vaccine (ACAM2000; Emergent, Gaithersburg, MD, USA), which can cause local skin lesions; and Tanapox virus, another African poxvirus that causes a febrile prodrome and skin lesions lasting several weeks without sequelae.108 Orf and bovine stomatitis (caused by parapoxviruses) can produce localised skin lesions similar to those of monkeypox that are difficult to distinguish clinically (figure 2N), but a previous contact with sheep, goats, or dairy cows facilitates the differential diagnosis of these conditions.
Several sexually transmitted infections can present with signs and symptoms that overlap with those of monkeypox, including penile, vaginal, and ulcerated lesions in primary syphilis (figure 2J), or lymphogranuloma venereum. In patients with proctitis associated with monkeypox, it could be confused with lymphogranuloma venereum, chlamydia, gonorrhoea, and syphilis. Additionally, throat features of monkeypox could be mistaken for bacterial tonsillitis or primary syphilis.
Complications
Historically, severe complications of monkeypox infections include bronchopneumonia, sepsis, ocular infection, and neurological manifestations. Ocular involvement can consist of conjunctivitis and lesions on the eyelids,109 as well as keratitis that can lead to corneal scarring and blindness.110, 111 A systematic review of studies from 2003 to 2021 reported neurological clinical features such as encephalitis, seizures, and confusion in about 2% of monkeypox cases.112 Radiographic imaging of some cases of encephalitis are consistent with acute demyelinating encephalomyelitis but PCR tests of cerebrospinal fluid were negative for poxvirus DNA.113 Low mood and other mental health issues, including a case of suicide, were reported in Nigeria,114 although it is unclear whether these conditions are caused by the neurological tropism of monkeypox virus or if they are rather the result of stigma and isolation. In the 2022 outbreak, some novel (albeit rare) severe complications have been identified, including a few sporadic cases of myocarditis,32 epiglottitis,32 peritonsillar abscess,110 rectal wall perforation with associated abscess in patients with proctitis,33 and haemophagocytic lymphohistiocytosis. Other less severe but more commonly reported complications during 2022 include rectal pain or pain on defecation (14–36% of cases); difficulty swallowing (5–14%); inflammation of the penis (8–16%); and secondary bacterial infection (3–4%).32, 33, 34, 35 Finally, some patients might present with a morbilliform rash of pink-to-red spots on the trunk, arms, and legs after the administration of specific antibiotics (eg, ampicillin or amoxicillin).34
During the 2022 outbreak, the hospital admission rate was low (1–13%) and primarily aimed at isolating the patient32 or providing adequate pain management and treating secondary infections.32, 115 Some nasal, conjunctival, corneal, and perianal lesions have also led to hospital admission. Most individuals have a self-limited disease with symptoms lasting from 2 to 4 weeks.
The historically reported case-fatality rate associated with monkeypox infection is heterogeneous and varies from 1% to 12% in cases infected with clade 1 in central Africa (deaths occurred primarily within the second week of illness)5, 6, 7, 8, 9, 10, 11, 12 to less than 0·1% in most outbreaks caused by clade 2a.19, 37 One exception was an outbreak of clade 2a in Nigeria (2017–18) that resulted in a fatality rate of 3·6%, with several deaths among HIV-positive immunosuppressed individuals.13, 14 During the 2022 outbreak, the case-fatality rate has been less than 0·1% (41 deaths have been reported of the 78 229 cases up to Nov 4, 2022);30 a few of them were related to encephalitis31 or in immunocompromised people.103
Diagnostic investigations
Monkeypox is diagnosed on the basis of suspected epidemiological and clinical findings and confirmed by nucleic acid amplification testing (NAAT), such as real-time or conventional PCR tests.
The diagnosis should be suspected in patients who present with an unexplained acute rash, including mucosal lesions in the conjunctiva, mouth, penis, vagina, or anorectal area; in patients who present with proctitis or lymphadenopathy; and in patients with influenza-like symptoms after high-risk exposure.116 A probable case is defined as someone with a clinical suspicion and epidemiological risk factors for infection (eg, close or intimate contact with a case of monkeypox, part of a social network or community experiencing monkeypox activity, or who have travelled to areas where large outbreaks of monkeypox have been reported).
Patients with suspected or probable monkeypox should be offered NAAT testing, either generic to orthopoxviruses or specific to the monkeypox virus (preferable). The recommended specimen type for laboratory confirmation of monkeypox is skin lesion material, including swabs of lesion surface or exudate and lesion crusts. Swabbing of the lesion should be done vigorously to ensure adequate viral DNA is collected. Unlike herpes simplex lesions, which are usually filled with fluid, monkeypox virus lesions can be filled with solid material (as shown on the histopathology), making it difficult to unroof the lesions.62 If there are multiple lesions, a few of them can be sampled. DNA testing of a throat swab for the monkeypox virus might serve for research or epidemiological purposes but is generally not used in the clinical setting. A positive NAAT result has been found in some blood specimens, but the clinical significance of viraemia is not well established.24, 80
West and central Africa face diagnostic challenges associated with scarce access to NAAT platforms and cold chain requirements for sample preservation, which hamper case confirmation in remote areas and, therefore, an understanding of monkeypox epidemiology. In rural health clinics or regional hospitals in low-income countries without access to high-precision PCR instruments, loop-mediated isothermal amplification diagnostic assays could be a viable alternative.117 This approach has been proposed as a point-of-care diagnostic tool for several neglected tropical diseases,118 and showed promising results in other emerging viruses, such as chikungunya virus119 or hantaviruses causing haemorrhagic fevers.120 Another approach for determining the cause of an outbreak of rash illness involves using a field analytical facility to conduct PCR assays from blotting papers soaked in pustular exudate collected in remote areas.121
Serological testing for the monkeypox virus can be used to support a diagnosis of monkeypox, particularly if NAAT testing cannot be performed. IgM detection from acutely ill patients (4–56 days after rash onset) or IgG in paired serum samples (collected at least 21 days apart, with the first being collected during the first week of illness) can aid diagnosis. Patients with monkeypox have detectable amounts of antiorthopoxvirus.122
Skin tissue biopsies are additional clinical samples that can be considered for diagnostic testing, only if clinically indicated. The histological features of monkeypox are very similar to those of smallpox, vaccinia, and cowpox, but are useful to differentiate it from other infections, such as herpes simplex virus and varicella.
In patients with proctitis, proctoscopy could show evidence of mucosal inflammation or friability. However, distinguishing monkeypox from other sexually transmitted infections is difficult with only a visual inspection. In many cases, routine proctoscopy is not possible due to the severe pain. If rectal wall perforation is suspected, rectal MRI should be done as part of the evaluation. Some patients with a sore throat and impaired swallowing present with ulcerative pharyngitis or tonsillitis. The presence of these symptoms with a negative result in the group A streptococcus rapid test suggests monkeypox as a possible cause.
Treatment
The approach to the clinical management of monkeypox includes both general supportive care and use of antivirals with activity against the monkeypox virus. Approximately half of patients during the 2022 outbreak have required pain relief medications (eg, for oral or anogenital lesions). Additionally, for the treatment of proctitis, stool softeners and topical lidocaine have been used, and for the treatment of pruritus, warm baths and oral antihistamines could prove beneficial. Supportive care requiring catheterisation could be warranted for people who are dehydrated or are at risk for dehydration, people who require more intensive pain management, and people with severe disease or complications. In patients with extensive anogenital ulcers or abscesses, drainage, debridement, and wound management are required; antibiotics are prescribed for secondary bacterial infections.
The efficacy of any antiviral agent against monkeypox infection has not been evaluated in randomised or non-randomised trials. Three antivirals, tecovirimat (intravenous and oral),123, 124, 125 cidofovir (intravenous and topical),126 and brincidofovir (oral), are potential options for treating monkeypox. These antivirals, approved for the treatment of smallpox on the basis of animal models and safety data in healthy individuals, are expected to be effective against monkeypox as well.127, 128
The preferred agent for monkeypox treatment is tecovirimat, which is recommended in selected patients with severe illness (eg, infection of the eye, encephalitis, severe proctitis, or pharyngitis) or those at risk of developing severe illness (immunocompromised patients, children younger than 8 years, patients with atopic dermatitis, pregnant people, and nursing mothers).129, 130 Early commencement and extended duration of therapy should be considered in highly immunocompromised patients, including people living with HIV with a CD4 count of less than 200, patients who had a solid organ transplantation, or patients with haematological malignancy.103 Tecovirimat inhibits an orthopoxvirus protein that is essential for dissemination within an infected host. Human randomised controlled studies on the efficacy of tecovirimat are currently missing. In non-human primate models of monkeypox disease, tecovirimat improves survival,123 and case studies in humans show anecdotal improvement of symptoms and viral clearance.24, 130, 131 The medication is well tolerated with the most commonly reported side-effects being headache, nausea, and abdominal pain, although the adverse effects profile was similar to placebo in a trial involving 360 healthy volunteers.123 Tecovirimat appears effective in vitro against the monkeypox virus lineage responsible for the 2022 outbreak;132, 133 however, cases of genotypic and phenotypic resistance have been reported and alternative drugs should be considered in patients who do not respond to tecovirimat (ie, continue to develop lesions or complications after a 14-day course).134 Currently, tecovirimat is available only under emergency authorisation; clinical trials evaluating its efficacy in the treatment of human monkeypox are in progress.135, 136, 137
Cidofovir competitively inhibits the incorporation of deoxycytidine triphosphate into viral DNA by the viral DNA polymerase, disrupting chain elongation. Cidofovir has in vitro activity against monkeypox138 and has been shown to be effective against lethal monkeypox challenge in animal models.126 The most important safety concern of cidofovir is the dose-dependent nephrotoxicity, which can be reduced by coadministration with probenecid.128, 139 Cidofovir is contraindicated in people with proteinuria (≥2+ or ≥100 mg/dL) or baseline serum creatinine greater than 1·5 mg/dL, and it is not recommended during pregnancy due to embryotoxicity found in rats and rabbits and the absence of adequate studies in pregnant people.140 The oral analogue of cidofovir, brincidofovi, might offer a better renal safety profile than cidofovir; however, three patients in the UK had a derangement in liver function requiring discontinuation of the treatment.24 In the EU, the use of cidofovir for monkeypox is not authorised, whereas in the USA, the Centers for Disease Control and Prevention has an expanded access protocol. In the UK, only tecovirimat is recommended because of insufficient evidence on cidofovir.
Vaccination
The smallpox vaccine has gone through three genera_tions of medical technology but only the second and third generation vaccines are currently licensed: ACAM2000, a replication-competent smallpox vaccine, and IMVANEX (also known as JYNNEOS or IMVAMUNE; Bavarian Nordic, Hellerup, Denmark), a live, nonreplicating vaccine.141 These can be used in two situations: pre-exposure to prevent infection and disease among those at high risk, or post exposure (ideally within 4 days of exposure) to improve infection and disease outcomes.142
First-generation vaccines (eg, Dryvax; Wyeth Laboratories [now merged with Pfizer], Madison, NJ, USA) consist of the live unattenuated vaccinia virus. Its effectiveness in preventing monkeypox was shown in a surveillance study of human-to-human transmission of monkeypox in Africa.3 Vaccination was associated with a remarkable reduction in the secondary attack rate (7·5% vs 1·3%) among 2278 household contacts enrolled in the study. First-generation vaccines were withdrawn since they were manufactured using crude methods that would make them ineligible for licensure today.
ACAM2000 is a second-generation, replication-competent vaccine derived from a single clonal viral isolate from Dryvax that exhibited reduced neurovirulence in animal models.143 Immunogenicity testing showed non-inferiority to Dryvax and clinical trials showed a similar safety profile.144 Because ACAM2000 is infectious, it can cause serious side-effects (ie, progressive vaccinia, encephalitis, and eczema vaccinatum) and it is contraindicated in immunocompromised patients, people with skin disorders, people with underlying heart disease, and pregnant people.142
IMVANEX is a third-generation vaccine based on the replication-deficient modified vaccinia Ankara (MVA). In the non-human primate model, the vaccine elicits robust humoral and cellular immune responses,145 and clinical protection against severe monkeypox diseases and death,146 but clinical efficacy data against monkeypox in humans are currently pending. In human volunteers, overall peak neutralising antibody titres after two doses of an MVA vaccine are similar to those seen after single-dose ACAM2000, but with no serious adverse events noted in clinical trials.147 However, lower levels of monkeypox-specific neutralising antibodies have been found in one study.65
Because of their attenuated phenotype, these vaccines have an improved safety profile and can be administered to immunocompromised individuals. Third-generation MVA vaccines are administered subcutaneously in two doses, 4 weeks apart. However, owing to shortages in vaccine supplies, several countries have authorised intradermal administration, which requires one-fifth of the volume of the subcutaneous route, in individuals older than 18 years.148, 149 Intradermal administration is supported by extrapolations from other infections, in which the intradermal route enhances immunogenicity, and phase 2 studies showing equivalent antibody responses with both MVA administration routes.150 To reach larger populations, some countries have adopted single-dose intradermal administration.
Vaccination programmes in newly affected countries have focused primarily on men who have sex with men, who are at the highest risk of acquiring monkeypox. Globally, vaccines are being administered primarily in North America and Europe, whereas African countries remain without access to vaccines. Although global alliances, such as GAVI, the Vaccine Alliance or The Global Fund to Fight AIDS, Tuberculosis and Malaria, emphasise the importance of increasing vaccine coverage in African countries, so far supplies have been cornered by high-income countries.151
Disease control
In endemic countries, such as the Democratic Republic of the Congo and Nigeria, the national health authorities developed comprehensive disease control plans.152, 153 This included targeted epidemiological investigations in high-risk areas, improved laboratory-based surveillance capacity, laboratory diagnostics, the development of regional capacities to implement effective responses at the local level, and enhanced research activities.152 However, the absence of access to vaccines154 precludes these countries from stopping the transmission or developing a clear strategy for immunisation once these vaccines become available. To be effective, vaccination might need to target risk groups in forested areas of west and central Africa where zoonotic spillover traditionally causes outbreaks. Additionally, understanding how emerging sexual transmission affects local epidemics is crucial.
Current guidance for the 2022 outbreak control does not support mass vaccination of the entire population and relies on surveillance, contact tracing, and vaccination of high-risk groups to control the monkeypox outbreak.155 Therefore, newly affected countries will require intervention prioritisation in key populations. Although early associations with men who have sex with men might have been influenced by social, environmental, or biological factors, the epidemiology of this emerging infection could change over time.156 Of particular interest are congregated settings, such as prisons, dormitories, and schools, as well as sexual networks of heterosexual individuals. On the basis of the above recommendations, the USA and Europe have started mobilising their stockpiles of smallpox vaccines. Conversely, the corresponding strategic supplies of WHO for smallpox outbreaks in Africa are not yet being distributed.154 Despite the enormous difference in access to vaccines, WHO has explicitly committed to a unified response to the 2022 monkeypox emergency, including the discontinuation of distinguishing between endemic and non-endemic countries.46
At this time, it is uncertain whether the outbreak can be controlled within specific countries. However, even if some countries in Europe and North America can eliminate the monkeypox virus, other countries in Africa will remain affected, which will not only be inequitable but also a threat to future outbreaks worldwide. Therefore, high-income nations must do more to control the spread of the virus in human populations in Africa. Moreover, the monkeypox virus is unlikely to be eradicated due to its wide host range and elusive animal reservoir, in contrast to its close relative Variola, which primarily causes infections in humans.157 The animal reservoir probably lies in Africa; therefore, a clear OneHealth framework must be directed towards that region. The monkeypox virus could also expand its endemic range through reverse zoonosis within newly infected countries. The establishment of a reservoir of the monkeypox virus in a wild animal population (eg, rodents) in a previously non-endemic region would make control and eradication increasingly challenging.
Controversies and uncertainties
Many aspects of the epidemiology of human monkeypox, especially relating to the natural reservoirs, modes of transmission, and predictors of clinical disease course remain largely unknown (panel ). The knowledge gaps must be addressed through further research and intervention, including topics relevant to African nations such as behavioural changes regarding animal exposure, and addressing misconceptions and stigma associated with monkeypox.
Panel. Unanswered research questions.
Virology
-
•
Has the monkeypox virus genetically changed, with mutations conferring an advantage over other forms of the virus?
-
•
Does the B.1 lineage result in milder clinical presentation than previous lineages?
-
•
Are different complications associated with specific strains and what is the role of genomic variation?
Pathogenesis
-
•
Is the paucisymptomatic or localised presentation (seen in the 2022 outbreak) associated with a lower degree of immune protection after infection than the disseminated presentation of typical monkeypox disease?
Transmission
-
•
How much does the respiratory route vs the dermal route contribute to the spread of monkeypox infection in the 2022 outbreak?
-
•
What is the relative transmissibility by type of sexual exposure?
-
•
To what extent and for what duration do viable viruses remain present in different body fluids? Could this duration lead to asymptomatic carriage of the virus and the possibility of onward transmission?
-
•
Can the monkeypox virus be lodged in privileged sites (the eyeball, synovial fluid, spinal cord, or the testicles) and pass the virus to others a long time after the host recovers?
-
•
What is the extent to which viable replication-competent viruses persist in the environment?
-
•
What is the role and how much does asymptomatic infection contribute to spread of the infection?
Population at risk
-
•
Does HIV infection affect a person's risk of acquiring monkeypox and why are people with HIV overly represented in published cohorts?
-
•
What are the risk factors for severe disease? How important is immune suppression in the context of uncontrolled HIV infection, solid organ transplantation, haematological disorders, etc?
Clinical presentation and complications
-
•
What are the best methods for identifying complications early and managing complications effectively?
-
•
What is the precise cause of death among patients who die of a monkeypox virus infection?
Diagnostics
-
•
Can adapted point-of-care diagnostics, such as antigen-detection-based rapid tests, circumvent the shortage of nucleic acid amplification tests in west and central Africa?
Treatment
-
•
What is the efficacy and optimal timing and duration of antiviral therapy?
-
•
Should early initiation of treatment and an extended duration of treatment be recommended for individuals who are highly immunocompromised (eg, HIV with a CD4 count <200)?
-
•
What are the risks of resistance to tecovirimat and how should it be detected and managed?
Vaccines
-
•
What is the effectiveness, optimal number of doses, optimal administration route, and duration of protection against the monkeypox virus from third-generation vaccines?
-
•
Does previous vaccination against smallpox decades ago protect against monkeypox today?
Disease control
-
•
Will the outbreak remain limited to specific risk groups or will it bridge to new target populations? What are the populations at highest risk of the monkeypox virus transmission other than men who have sex with men?
-
•
Is there community transmission that is undetected?
-
•
Is it feasible for some countries to stop transmission and eliminate the monkeypox virus?
-
•
Upon elimination in a given country, how likely is it that new outbreaks will occur due to imported cases from countries without the ability to eliminate the disease?
Search strategy and selection criteria
Information for this Seminar was obtained from peer-reviewed articles (retrieved from PubMed by searching with the terms “monkeypox”,”smallpox”, “vaccinia”, “orthopoxvirus”, “tecovirimat”, “cidofovir”, and “modified vaccinia Ankara”, in combination with the words “pathophysiology”, “animal model”, “immunology”, “sexually-transmitted infections“, “HIV”, “immunosuppression”, “global outbreak”, “risk factors”, “treatment”), releases from reference institutions (eg, the US Food and Drug Administration, WHO, the US Centers for Disease Control and Prevention, and the European Centre for Disease Prevention and Control). We mainly selected publications from the 2022 global monkeypox outbreak, but we also included relevant publications from previous years that related to basic science or clinical reports, commonly from Africa. Highly regarded older publications from the twentieth century are cited to provide readers with more details regarding the extensive knowledge learnt from smallpox infections before mass vaccination, and monkeypox in Africa. No language or time restrictions were applied.
Declaration of interests
Acknowledgments
Acknowledgments
The authors would like to thank Gerard Carot-Sans for providing medical writing support during the preparation of the manuscript. There was no funding source for writing this Seminar. All patients whose photographs were taken provided written consent.
Contributors
All authors planned, wrote, and revised the manuscript. OM wrote the first draft and led the work.
CMO reports research grants and honoraria for travel, lectures, and advisory boards from Gilead Science, ViiV Healthcare, Janssen, MSD, and AstraZeneca, outside of the submitted work. All other authors report no competing interests.
References
- 1.von Magnus P, Andersen EK, Petersen KB, Birch-Andersen A. A pox-like disease in cynomolgus monkeys. Acta Pathol Microbiol Scand. 1959;46:156–176. [Google Scholar]
- 2.Parker S, Buller RM. A review of experimental and natural infections of animals with monkeypox virus between 1958 and 2012. Future Virol. 2013;8:129–157. doi: 10.2217/fvl.12.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jezek Z, Marennikova SS, Mutumbo M, Nakano JH, Paluku KM, Szczeniowski M. Human monkeypox: a study of 2,510 contacts of 214 patients. J Infect Dis. 1986;154:551–555. doi: 10.1093/infdis/154.4.551. [DOI] [PubMed] [Google Scholar]
- 4.Karem KL, Reynolds M, Hughes C, et al. Monkeypox-induced immunity and failure of childhood smallpox vaccination to provide complete protection. Clin Vaccine Immunol. 2007;14:1318–1327. doi: 10.1128/CVI.00148-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.US Centers for Disease Control and Prevention Human monkeypox—Kasai Oriental, Democratic Republic of Congo, February 1996–October 1997. MMWR Morb Mortal Wkly Rep. 1997;46:1168–1171. [PubMed] [Google Scholar]
- 6.Heymann DL, Szczeniowski M, Esteves K. Re-emergence of monkeypox in Africa: a review of the past six years. Br Med Bull. 1998;54:693–702. doi: 10.1093/oxfordjournals.bmb.a011720. [DOI] [PubMed] [Google Scholar]
- 7.WHO . Technical advisory group on human monkeypox: report of a WHO meeting. World Health Organization; Geneva: 1999. [Google Scholar]
- 8.Foster SO, Brink EW, Hutchins DL, et al. Human monkeypox. Bull World Health Organ. 1972;46:569–576. [PMC free article] [PubMed] [Google Scholar]
- 9.Mandja BM, Brembilla A, Handschumacher P, et al. Temporal and spatial dynamics of monkeypox in Democratic Republic of Congo, 2000-2015. EcoHealth. 2019;16:476–487. doi: 10.1007/s10393-019-01435-1. [DOI] [PubMed] [Google Scholar]
- 10.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fuller T, Thomassen HA, Mulembakani PM, et al. Using remote sensing to map the risk of human monkeypox virus in the Congo Basin. EcoHealth. 2011;8:14–25. doi: 10.1007/s10393-010-0355-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jezek Z, Szczeniowski M, Paluku KM, Mutombo M. Human monkeypox: clinical features of 282 patients. J Infect Dis. 1987;156:293–298. doi: 10.1093/infdis/156.2.293. [DOI] [PubMed] [Google Scholar]
- 13.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017-18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 15.Nguyen P-Y, Ajisegiri WS, Costantino V, Chughtai AA, MacIntyre CR. Reemergence of human monkeypox and declining population immunity in the context of urbanization, Nigeria, 2017–2020. Emerg Infect Dis. 2021;27:1007. doi: 10.3201/eid2704.203569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.US Centers for Disease Control and Prevention Multistate outbreak of monkeypox—Illinois, Indiana, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:537–540. [PubMed] [Google Scholar]
- 17.US Centers for Disease Control and Prevention Update: multistate outbreak of monkeypox—Illinois, Indiana, Kansas, Missouri, Ohio, and Wisconsin, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:561–564. [PubMed] [Google Scholar]
- 18.US Centers for Disease Control and Prevention Monkeypox: past U.S. cases and outbreaks. 2022. https://www.cdc.gov/poxvirus/monkeypox/outbreak/us-outbreaks.html
- 19.Huhn GD, Bauer AM, Yorita K, et al. Clinical characteristics of human monkeypox, and risk factors for severe disease. Clin Infect Dis. 2005;41:1742–1751. doi: 10.1086/498115. [DOI] [PubMed] [Google Scholar]
- 20.Erez N, Achdout H, Milrot E, et al. Diagnosis of imported monkeypox, Israel, 2018. Emerg Infect Dis. 2019;25:980–983. doi: 10.3201/eid2505.190076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yong SEF, Ng OT, Ho ZJM, et al. Imported monkeypox, Singapore. Emerg Infect Dis. 2020;26:1826–1830. doi: 10.3201/eid2608.191387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Reed KD, Melski JW, Graham MB, et al. The detection of monkeypox in humans in the western hemisphere. N Engl J Med. 2004;350:342–350. doi: 10.1056/NEJMoa032299. [DOI] [PubMed] [Google Scholar]
- 23.Zachary KC, Shenoy ES. Monkeypox transmission following exposure in healthcare facilities in nonendemic settings: low risk but limited literature. Infect Control Hosp Epidemiol. 2022;43:920–924. doi: 10.1017/ice.2022.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Adler H, Gould S, Hine P, et al. Clinical features and management of human monkeypox: a retrospective observational study in the UK. Lancet Infect Dis. 2022;22:1153–1162. doi: 10.1016/S1473-3099(22)00228-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.US Centers for Disease Control and Prevention CDC and Texas confirm monkeypox in U.S. traveler. July 16, 2021. https://www.cdc.gov/media/releases/2021/s0716-confirm-monkeypox.html
- 26.US Centers for Disease Control and Prevention Emergency Preparedness and Response: potential exposure to person with confirmed human monkeypox infection—United States, 2021. July 17, 2021. https://emergency.cdc.gov/han/2021/han00446.asp
- 27.Vaughan A, Aarons E, Astbury J, et al. Two cases of monkeypox imported to the United Kingdom, September 2018. Euro Surveill. 2018;23:1800509. doi: 10.2807/1560-7917.ES.2018.23.38.1800509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Vaughan A, Aarons E, Astbury J, et al. Human-to-human transmission of monkeypox virus, United Kingdom, October 2018. Emerg Infect Dis. 2020;26:782–785. doi: 10.3201/eid2604.191164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.European Centre for Disease Prevention and Control, WHO Joint ECDC-WHO Regional Office for Europe monkeypox surveillance bulletin. Oct 26, 2022. https://monkeypoxreport.ecdc.europa.eu/ (accessed Nov 6, 2022)
- 30.US Centers for Disease Control and Prevention 2022 monkeypox outbreak global map. 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 31.WHO Multi-country outbreak of monkeypox, external situation report #8: 19 October 2022. Oct 19, 2022. https://www.who.int/publications/m/item/multi-country-outbreak-of-monkeypox--external-situation-report--8---19-october-2022
- 32.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 33.Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Català A, Clavo-Escribano P, Riera-Monroig J, et al. Monkeypox outbreak in Spain: clinical and epidemiological findings in a prospective cross-sectional study of 185 cases. Br J Dermatol. 2022 doi: 10.1111/bjd.21790. published online Aug 2. [DOI] [PubMed] [Google Scholar]
- 36.Chen N, Li G, Liszewski MK, et al. Virulence differences between monkeypox virus isolates from west Africa and the Congo Basin. Virology. 2005;340:46–63. doi: 10.1016/j.virol.2005.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Beer EM, Rao VB. A systematic review of the epidemiology of human monkeypox outbreaks and implications for outbreak strategy. PLoS Negl Trop Dis. 2019;13:e0007791. doi: 10.1371/journal.pntd.0007791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Likos AM, Sammons SA, Olson VA, et al. A tale of two clades: monkeypox viruses. J Gen Virol. 2005;86:2661–2672. doi: 10.1099/vir.0.81215-0. [DOI] [PubMed] [Google Scholar]
- 39.Weaver JR, Isaacs SN. Monkeypox virus and insights into its immunomodulatory proteins. Immunol Rev. 2008;225:96–113. doi: 10.1111/j.1600-065X.2008.00691.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hudson PN, Self J, Weiss S, et al. Elucidating the role of the complement control protein in monkeypox pathogenicity. PLoS One. 2012;7:e35086. doi: 10.1371/journal.pone.0035086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Happi C, Adetifa I, Mbala P, et al. Urgent need for a non-discriminatory and non-stigmatizing nomenclature for monkeypox virus. PLoS Biol. 2022;20:e3001769. doi: 10.1371/journal.pbio.3001769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Isidro J, Borges V, Pinto M, et al. Phylogenomic characterization and signs of microevolution in the 2022 multi-country outbreak of monkeypox virus. Nat Med. 2022;28:1569–1572. doi: 10.1038/s41591-022-01907-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breman JG, Henderson DA. Poxvirus dilemmas—monkeypox, smallpox, and biologic terrorism. N Engl J Med. 1998;339:556–559. doi: 10.1056/NEJM199808203390811. [DOI] [PubMed] [Google Scholar]
- 44.Arita I, Breman JG. Evaluation of smallpox vaccination policy. Bull World Health Organ. 1979;57:1–9. [PMC free article] [PubMed] [Google Scholar]
- 45.Jezek Z, Fenner F. Human monkeypox. Karger Publishers; Geneva: 1988. [Google Scholar]
- 46.WHO Multi-country monkeypox outbreak in non-endemic countries: update. May 29, 2022. https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON388
- 47.European Centre for Disease Prevention and Control Monkeypox cases reported in UK and Portugal. May 19, 2022. https://www.ecdc.europa.eu/en/news-events/monkeypox-cases-reported-uk-and-portugal
- 48.Selb R, Werber D, Falkenhorst G, et al. A shift from travel-associated cases to autochthonous transmission with Berlin as epicentre of the monkeypox outbreak in Germany, May to June 2022. Euro Surveill. 2022;27:2200499. doi: 10.2807/1560-7917.ES.2022.27.27.2200499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Perez Duque M, Ribeiro S, Martins JV, et al. Ongoing monkeypox virus outbreak, Portugal, 29 April to 23 May 2022. Euro Surveill. 2022;27:2200424. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Iñigo Martínez J, Gil Montalbán E, Jiménez Bueno S, et al. Monkeypox outbreak predominantly affecting men who have sex with men, Madrid, Spain, 26 April to 16 June 2022. Euro Surveill. 2022;27:2200471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Antinori A, Mazzotta V, Vita S, et al. Epidemiological, clinical and virological characteristics of four cases of monkeypox support transmission through sexual contact, Italy, May 2022. Euro Surveill. 2022;27:2200421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.WHO Second meeting of the International Health Regulations (2005) (IHR) Emergency Committee regarding the multi-country outbreak of monkeypox. July 23, 2022. https://www.who.int/news/item/23-07-2022-second-meeting-of-the-international-health-regulations-(2005)-(ihr)-emergency-committee-regarding-the-multi-country-outbreak-of-monkeypox
- 53.Dixon CW. Smallpox. J & A Churchill; London: 1962. pp. 5–56. [Google Scholar]
- 54.Elwood JM. Smallpox and its eradication. J Epidemiol Community Health. 1989;43:92. [Google Scholar]
- 55.Liu L, Xu Z, Fuhlbrigge RC, Peña-Cruz V, Lieberman J, Kupper TS. Vaccinia virus induces strong immunoregulatory cytokine production in healthy human epidermal keratinocytes: a novel strategy for immune evasion. J Virol. 2005;79:7363–7370. doi: 10.1128/JVI.79.12.7363-7370.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.MacLeod DT, Nakatsuji T, Wang Z, di Nardo A, Gallo RL. Vaccinia virus binds to the scavenger receptor MARCO on the surface of keratinocytes. J Invest Dermatol. 2015;135:142–150. doi: 10.1038/jid.2014.330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Realegeno S, Puschnik AS, Kumar A, et al. Monkeypox virus host factor screen using haploid cells identifies essential role of GARP complex in extracellular virus formation. J Virol. 2017;91:e00011–e00017. doi: 10.1128/JVI.00011-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zaucha GM, Jahrling PB, Geisbert TW, Swearengen JR, Hensley L. The pathology of experimental aerosolized monkeypox virus infection in cynomolgus monkeys (Macaca fascicularis) Lab Invest. 2001;81:1581–1600. doi: 10.1038/labinvest.3780373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Tree JA, Hall G, Pearson G, et al. Sequence of pathogenic events in cynomolgus macaques infected with aerosolized monkeypox virus. J Virol. 2015;89:4335–4344. doi: 10.1128/JVI.03029-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saijo M, Ami Y, Suzaki Y, et al. Virulence and pathophysiology of the Congo Basin and west African strains of monkeypox virus in non-human primates. J Gen Virol. 2009;90:2266–2271. doi: 10.1099/vir.0.010207-0. [DOI] [PubMed] [Google Scholar]
- 61.Fenner F, Henderson DA, Arita I, et al. Smallpox and its eradication. World Health Organization; Geneva: 1988. [Google Scholar]
- 62.Bayer-Garner IB. Monkeypox virus: histologic, immunohistochemical and electron-microscopic findings. J Cutan Pathol. 2005;32:28–34. doi: 10.1111/j.0303-6987.2005.00254.x. [DOI] [PubMed] [Google Scholar]
- 63.Maronese CA, Beretta A, Avallone G, et al. Clinical, dermoscopic and histopathological findings in localized human monkeypox: a case from northern Italy. Br J Dermatol. 2022 doi: 10.1111/bjd.21773. published online July 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shao L, Huang D, Wei H, et al. Expansion, reexpansion, and recall-like expansion of Vgamma2Vdelta2 T cells in smallpox vaccination and monkeypox virus infection. J Virol. 2009;83:11959–11965. doi: 10.1128/JVI.00689-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zaeck LM, Lamers MM, Verstrepen BE, et al. Low levels of monkeypox virus neutralizing antibodies after MVA-BN vaccination in healthy individuals. Nat Med. 2022 doi: 10.1038/s41591-022-02090-w. published online Oct 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Agrati C, Cossarizza A, Mazzotta V, et al. Immunological signature in human cases of monkeypox infection in 2022 outbreak: an observational study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00662-4. published online Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Edghill-Smith Y, Golding H, Manischewitz J, et al. Smallpox vaccine-induced antibodies are necessary and sufficient for protection against monkeypox virus. Nat Med. 2005;11:740–747. doi: 10.1038/nm1261. [DOI] [PubMed] [Google Scholar]
- 68.Hammarlund E, Dasgupta A, Pinilla C, Norori P, Früh K, Slifka MK. Monkeypox virus evades antiviral CD4+ and CD8+ T cell responses by suppressing cognate T cell activation. Proc Natl Acad Sci USA. 2008;105:14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hammarlund E. Lewis MW, Hansen SG, et al. Duration of antiviral immunity after smallpox vaccination. Nat Med. 2003;9:1131–1137. doi: 10.1038/nm917. [DOI] [PubMed] [Google Scholar]
- 70.Edghill-Smith Y, Bray M, Whitehouse CA, et al. Smallpox vaccine does not protect macaques with AIDS from a lethal monkeypox virus challenge. J Infect Dis. 2005;191:372–381. doi: 10.1086/427265. [DOI] [PubMed] [Google Scholar]
- 71.WHO Fact sheet: monkeypox. May 19, 2022. https://www.who.int/news-room/fact-sheets/detail/monkeypox
- 72.Reynolds MG, Davidson WB, Curns AT, et al. Spectrum of infection and risk factors for human monkeypox, United States, 2003. Emerg Infect Dis. 2007;13:1332–1339. doi: 10.3201/eid1309.070175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Reynolds MG, Yorita KL, Kuehnert MJ, et al. Clinical manifestations of human monkeypox influenced by route of infection. J Infect Dis. 2006;194:773–780. doi: 10.1086/505880. [DOI] [PubMed] [Google Scholar]
- 74.Kugelman JR, Johnston SC, Mulembakani PM, et al. Genomic variability of monkeypox virus among humans, Democratic Republic of the Congo. Emerg Infect Dis. 2014;20:232–239. doi: 10.3201/eid2002.130118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Berthet N, Descorps-Declère S, Besombes C, et al. Genomic history of human monkey pox infections in the Central African Republic between 2001 and 2018. Sci Rep. 2021;11:13085. doi: 10.1038/s41598-021-92315-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.US Centers for Disease Control and Prevention Monkeypox: how it spreads. Oct 18, 2022. https://www.cdc.gov/poxvirus/monkeypox/if-sick/transmission.html
- 77.Peiró-Mestres A, Fuertes I, Camprubí-Ferrer D, et al. Frequent detection of monkeypox virus DNA in saliva, semen, and other clinical samples from 12 patients, Barcelona, Spain, May to June 2022. Euro Surveill. 2022;27:2200503. doi: 10.2807/1560-7917.ES.2022.27.28.2200503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lapa D, Carletti F, Mazzotta V, et al. Monkeypox virus isolation from a semen sample collected in the early phase of infection in a patient with prolonged seminal viral shedding. Lancet Infect Dis. 2022;22:1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Palich R, Burrel S, Monsel G, et al. Viral loads in clinical samples of men with monkeypox virus infection: a French case series. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00586-2. published online Sept 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Suner C, Ubals M, Tarín-Vicente EJ, et al. Viral dynamics in patients with monkeypox infection: a prospective cohort study in Spain. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00794-0. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 82.Khalil A, Samara A, O'Brien P, et al. Monkeypox in pregnancy: update on current outbreak. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00612-0. published online Sept 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Carvalho LB, Casadio LVB, Polly M, et al. Monkeypox virus transmission to healthcare worker through needlestick injury, Brazil. Emerg Infect Dis. 2022;28:2334–2336. doi: 10.3201/eid2811.221323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mendoza R, Petras JK, Jenkins P, et al. Monkeypox virus infection resulting from an occupational needlestick—Florida, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1348–1349. doi: 10.15585/mmwr.mm7142e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pfeiffer JA, Collingwood A, Rider LE, et al. High-contact object and surface contamination in a household of persons with monkeypox virus infection—Utah, June 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1092–1094. doi: 10.15585/mmwr.mm7134e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Atkinson B, Burton C, Pottage T, et al. Infection-competent monkeypox virus contamination identified in domestic settings following an imported case of monkeypox into the UK. Environ Microbiol. 2022;24:4561–4569. doi: 10.1111/1462-2920.16129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Nörz D, Brehm TT, Tang HT, et al. Clinical characteristics and comparison of longitudinal qPCR results from different specimen types in a cohort of ambulatory and hospitalized patients infected with monkeypox virus. J Clin Virol. 2022;155:105254. doi: 10.1016/j.jcv.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Gould S, Atkinson B, Onianwa O, et al. Air and surface sampling for monkeypox virus in a UK hospital: an observational study. Lancet Microbe. 2022 doi: 10.1016/S2666-5247(22)00257-9. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Hutin YJ, Williams RJ, Malfait P, et al. Outbreak of human monkeypox, Democratic Republic of Congo, 1996 to 1997. Emerg Infect Dis. 2001;7:434–438. doi: 10.3201/eid0703.010311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Pembi E, Omoleke S, Paul H, Augustine T, Cuevas LE. Monkeypox outbreak in a correctional center in North Eastern Nigeria. J Infect. 2022 doi: 10.1016/j.jinf.2022.09.010. published online Sept 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Aguilera-Alonso D, Alonso-Cadenas JA, Roguera-Sopena M, Lorusso N, Miguel LGS, Calvo C. Monkeypox virus infections in children in Spain during the first months of the 2022 outbreak. Lancet Child Adolesc Health. 2022;6:e22–e23. doi: 10.1016/S2352-4642(22)00250-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ramnarayan P, Mitting R, Whittaker E, et al. Neonatal monkeypox virus infection. N Engl J Med. 2022;387:1618–1620. doi: 10.1056/NEJMc2210828. [DOI] [PubMed] [Google Scholar]
- 93.Kwok KO, Wei WI, Tang A, Wong SYS, Tang JW. Estimation of local transmissibility in the early phase of monkeypox epidemic in 2022. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.06.025. published online July 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Ogoina D, Ogunsola FT. Monkeypox and the health-care environment. Lancet Microbe. 2022 doi: 10.1016/S2666-5247(22)00286-5. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Salvato RS, Rodrigues Ikeda ML, Barcellos RB, et al. Possible occupational infection of healthcare workers with monkeypox virus, Brazil. Emerg Infect Dis. 2022 doi: 10.3201/eid2812.221343. published online Sept 30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Marshall KE, Barton M, Nichols J, et al. Health care personnel exposures to subsequently laboratory-confirmed monkeypox patients—Colorado, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1216–1219. doi: 10.15585/mmwr.mm7138e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Fleischauer AT, Kile JC, Davidson M, et al. Evaluation of human-to-human transmission of monkeypox from infected patients to health care workers. Clin Infect Dis. 2005;40:689–694. doi: 10.1086/427805. [DOI] [PubMed] [Google Scholar]
- 98.Ferré VM, Bachelard A, Zaidi M, et al. Detection of monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann Intern Med. 2022;175:1491–1492. doi: 10.7326/M22-2183. [DOI] [PubMed] [Google Scholar]
- 99.De Baetselier I, Van Dijck C, Kenyon C, et al. Retrospective detection of asymptomatic monkeypox virus infections among male sexual health clinic attendees in Belgium. Nat Med. 2022 doi: 10.1038/s41591-022-02004-w. published online Aug 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Ward T, Christie R, Paton RS, Cumming F, Overton CE. Transmission dynamics of monkeypox in the United Kingdom: contact tracing study. BMJ. 2022;379:e073153. doi: 10.1136/bmj-2022-073153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Hoffmann C, Jessen H, Wyen C, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med. 2022 doi: 10.1111/hiv.13378. published online Sept 4. [DOI] [PubMed] [Google Scholar]
- 102.Thornill J, Palich R, Ghosn J, et al. Human monkeypox infection in women—a global case series. Lancet (in press). [DOI] [PMC free article] [PubMed]
- 103.Miller MJ, Cash-Goldwasser S, Marx GE, et al. Severe monkeypox in hospitalized patients—United States, August 10–October 10, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1412–1417. doi: 10.15585/mmwr.mm7144e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Angelo KM, Smith T, Camprubí-Ferrer D, et al. Epidemiological and clinical characteristics of patients with monkeypox in the GeoSentinel Network: a cross-sectional study. Lancet Infect Dis. 2022 doi: 10.1016/S1473-3099(22)00651-X. published online Oct 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Minhaj FS, Ogale YP, Whitehill F, et al. Monkeypox outbreak—nine states, May 2022. MMWR Morb Mortal Wkly Rep. 2022;71:764–769. doi: 10.15585/mmwr.mm7123e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Rodríguez BS, Guzmán Herrador BR, Díaz Franco A, et al. Epidemiologic features and control measures during monkeypox outbreak, Spain, June 2022. Emerg Infect Dis. 2022;28:1847–1851. doi: 10.3201/eid2809.221051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Miura F, van Ewijk CE, Backer JA, et al. Estimated incubation period for monkeypox cases confirmed in the Netherlands, May 2022. Euro Surveill. 2022;27:2200448. doi: 10.2807/1560-7917.ES.2022.27.24.2200448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Dhar AD, Werchniak AE, Li Y, et al. Tanapox infection in a college student. N Engl J Med. 2004;350:361–366. doi: 10.1056/NEJMoa031467. [DOI] [PubMed] [Google Scholar]
- 109.Hughes C, McCollum A, Pukuta E, et al. Ocular complications associated with acute monkeypox virus infection, DRC. Int J Infect Dis. 2014;21:276–277. [Google Scholar]
- 110.Mailhe M, Beaumont A-L, Thy M, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.08.012. published online Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Ježek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 112.Badenoch JB, Conti I, Rengasamy ER, et al. Neurological and psychiatric presentations associated with human monkeypox virus infection: a systematic review and meta-analysis. EClinicalMedicine. 2022;52:101644. doi: 10.1016/j.eclinm.2022.101644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Pastula DM, Copeland MJ, Hannan MC, et al. Two cases of monkeypox-associated encephalomyelitis—Colorado and the District of Columbia, July–August 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1212–1215. doi: 10.15585/mmwr.mm7138e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ogoina D, Mohammed A, Yinka-Ogunleye A, Ihekweazu C. A case of suicide during the 2017 monkeypox outbreak in Nigeria. IJID Reg. 2022;3:226–227. doi: 10.1016/j.ijregi.2022.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Basgoz N, Brown CM, Smole SC, et al. Case 24–2022: a 31-year-old man with perianal and penile ulcers, rectal pain, and rash. N Engl J Med. 2022;387:547–556. doi: 10.1056/NEJMcpc2201244. [DOI] [PubMed] [Google Scholar]
- 116.WHO Surveillance, case investigation and contact tracing for monkeypox: interim guidance, 22 May 2022. 2022. https://apps.who.int/iris/handle/10665/354486
- 117.Iizuka I, Saijo M, Shiota T, et al. Loop-mediated isothermal amplification-based diagnostic assay for monkeypox virus infections. J Med Virol. 2009;81:1102–1108. doi: 10.1002/jmv.21494. [DOI] [PubMed] [Google Scholar]
- 118.Bharadwaj M, Bengtson M, Golverdingen M, Waling L, Dekker C. Diagnosing point-of-care diagnostics for neglected tropical diseases. PLoS Negl Trop Dis. 2021;15:e0009405. doi: 10.1371/journal.pntd.0009405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Hayashida K, Orba Y, Sequeira PC, et al. Field diagnosis and genotyping of chikungunya virus using a dried reverse transcription loop-mediated isothermal amplification (LAMP) assay and MinION sequencing. PLoS Negl Trop Dis. 2019;13:e0007480. doi: 10.1371/journal.pntd.0007480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zeng Y, Feng Y, Zhao Y, et al. An HFman probe-based multiplex reverse transcription loop-mediated isothermal amplification assay for simultaneous detection of Hantaan and Seoul viruses. Diagnostics. 2022;12:1925. doi: 10.3390/diagnostics12081925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Dumont C, Irenge LM, Magazani EK, et al. Simple technique for in field samples collection in the cases of skin rash illness and subsequent PCR detection of orthopoxviruses and varicella zoster virus. PLoS One. 2014;9:e96930. doi: 10.1371/journal.pone.0096930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Karem KL, Reynolds M, Braden Z, et al. Characterization of acute-phase humoral immunity to monkeypox: use of immunoglobulin M enzyme-linked immunosorbent assay for detection of monkeypox infection during the 2003 North American outbreak. Clin Diagn Lab Immunol. 2005;12:867–872. doi: 10.1128/CDLI.12.7.867-872.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Grosenbach DW, Honeychurch K, Rose EA, et al. Oral tecovirimat for the treatment of smallpox. N Engl J Med. 2018;379:44–53. doi: 10.1056/NEJMoa1705688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Huggins J, Goff A, Hensley L, et al. Nonhuman primates are protected from smallpox virus or monkeypox virus challenges by the antiviral drug ST-246. Antimicrob Agents Chemother. 2009;53:2620–2625. doi: 10.1128/AAC.00021-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Matias WR, Koshy JM, Nagami EH, et al. Tecovirimat for the treatment of human monkeypox: an initial series from Massachusetts, United States. Open Forum Infect Dis. 2022;9:ofac377. doi: 10.1093/ofid/ofac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Stittelaar KJ, Neyts J, Naesens L, et al. Antiviral treatment is more effective than smallpox vaccination upon lethal monkeypox virus infection. Nature. 2006;439:745–748. doi: 10.1038/nature04295. [DOI] [PubMed] [Google Scholar]
- 127.US Food & Drug Administration FDA approves the first drug with an indication for treatment of smallpox. 2018. https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-indication-treatment-smallpox
- 128.US Food & Drug Administration FDA approves drug to treat smallpox. June 4, 2021. https://www.fda.gov/drugs/news-events-human-drugs/fda-approves-drug-treat-smallpox
- 129.US Centers for Disease Control and Prevention Monkeypox: treatment information for healthcare professionals. Oct 31, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/treatment.html
- 130.Sherwat A, Brooks JT, Birnkrant D, Kim P. Tecovirimat and the treatment of monkeypox—past, present, and future considerations. N Engl J Med. 2022;387:579–581. doi: 10.1056/NEJMp2210125. [DOI] [PubMed] [Google Scholar]
- 131.Hernandez LE, Jadoo A, Kirsner RS. Human monkeypox virus infection in an immunocompromised man: trial with tecovirimat. Lancet. 2022;400:e8. doi: 10.1016/S0140-6736(22)01528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Warner BM, Klassen L, Sloan A, et al. In vitro and in vivo efficacy of tecovirimat against a recently emerged 2022 monkeypox virus isolate. Sci Transl Med. 2022 doi: 10.1126/scitranslmed.ade7646. published online Nov 1. [DOI] [PubMed] [Google Scholar]
- 133.Frenois-Veyrat G, Gallardo F, Gorgé O, et al. Tecovirimat is effective against human monkeypox virus in vitro at nanomolar concentrations. Nat Microbiol. 2022 doi: 10.1038/s41564-022-01269-8. published online Nov 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.US Food & Drug Administration FDA monkeypox response: therapeutics. 2022. https://www.fda.gov/emergency-preparedness-and-response/mcm-issues/fda-monkeypox-response#therapeutics
- 135.ISRCTN Registry Antiviral treatment with tecovirimat for patients managed at home with monkeypox: ISRCTN17461766. 2022. https://www.isrctn.com/ISRCTN17461766
- 136.ISRCTN Registry Expanded access protocol for the use of tecovirimat for the treatment of monkeypox infection: ISRCTN43307947. 2022. https://www.isrctn.com/ISRCTN43307947
- 137.ClinicalTrials.gov. Study of tecovirimat for human monkeypox virus (STOMP). 2022. https://clinicaltrials.gov/ct2/show/NCT05534984
- 138.Baker RO, Bray M, Huggins JW. Potential antiviral therapeutics for smallpox, monkeypox and other orthopoxvirus infections. Antiviral Res. 2003;57:13–23. doi: 10.1016/S0166-3542(02)00196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.US Food & Drug Administration TEMBEXA: highlights of prescribing information. 1996. https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/214460s000,214461s000lbl.pdf
- 140.European Medicines Agency Vistide—product information. https://www.ema.europa.eu/en/documents/product-information/vistide-epar-product-information_en.pdf
- 141.See KC. Vaccination for monkeypox virus infection in humans: a review of key considerations. Vaccines. 2022;10:1342. doi: 10.3390/vaccines10081342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.US Centers for Disease Control and Prevention Monkeypox vaccine guidance. June 2, 2022. https://www.cdc.gov/poxvirus/monkeypox/clinicians/smallpox-vaccine.html
- 143.Weltzin R, Liu J, Pugachev KV, et al. Clonal vaccinia virus grown in cell culture as a new smallpox vaccine. Nat Med. 2003;9:1125–1130. doi: 10.1038/nm916. [DOI] [PubMed] [Google Scholar]
- 144.Frey SE, Newman FK, Kennedy JS, et al. Comparison of the safety and immunogenicity of ACAM1000, ACAM2000 and Dryvax in healthy vaccinia-naive adults. Vaccine. 2009;27:1637–1644. doi: 10.1016/j.vaccine.2008.11.079. [DOI] [PubMed] [Google Scholar]
- 145.US Food & Drug Administration JYNNEOS: highlights of prescribing information. 2019. https://www.fda.gov/media/131078/download
- 146.Hatch GJ, Graham VA, Bewley KR, et al. Assessment of the protective effect of Imvamune and Acam2000 vaccines against aerosolized monkeypox virus in cynomolgus macaques. J Virol. 2013;87:7805–7815. doi: 10.1128/JVI.03481-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Pittman PR, Hahn M, Lee HS, et al. Phase 3 efficacy trial of modified vaccinia Ankara as a vaccine against smallpox. N Engl J Med. 2019;381:1897–1908. doi: 10.1056/NEJMoa1817307. [DOI] [PubMed] [Google Scholar]
- 148.US Food & Drug Administration Monkeypox update: FDA authorizes emergency use of JYNNEOS vaccine to increase vaccine supply. Aug 9, 2022. https://www.fda.gov/news-events/press-announcements/monkeypox-update-fda-authorizes-emergency-use-jynneos-vaccine-increase-vaccine-supply
- 149.European Medicines Agency EMA's Emergency Task Force advises on intradermal use of Imvanex/Jynneos against monkeypox. Aug 19, 2022. https://www.ema.europa.eu/en/news/emas-emergency-task-force-advises-intradermal-use-imvanex-jynneos-against-monkeypox
- 150.Frey SE, Wald A, Edupuganti S, et al. Comparison of lyophilized versus liquid modified vaccinia Ankara (MVA) formulations and subcutaneous versus intradermal routes of administration in healthy vaccinia-naïve subjects. Vaccine. 2015;33:5225–5234. doi: 10.1016/j.vaccine.2015.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.GAVI, the Vaccine Alliance Monkeypox cases waning, but global threat remains. Oct 6, 2022. https://www.gavi.org/vaccineswork/monkeypox-cases-waning-global-threat-remains
- 152.Nigeria Centre for Disease Control Monkeypox outbreak response—interim national guidelines. October, 2017. https://ncdc.gov.ng/themes/common/docs/protocols/50_1508912430.pdf
- 153.Shiferaw ML, Doty JB, Maghlakelidze G, et al. Frameworks for preventing, detecting, and controlling zoonotic diseases. Emerg Infect Dis. 2017;23:S71–S76. doi: 10.3201/eid2313.170601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Francis E. Nations eye vaccines for monkeypox, but WHO says mass immunization not urgent. May 24, 2022. https://www.washingtonpost.com/world/2022/05/24/monkeypox-vaccine-smallpox-cdc-who/
- 155.WHO Vaccines and immunization for monkeypox: interim guidance. June 14, 2022. https://apps.who.int/iris/bitstream/handle/10665/356120/WHO-MPX-Immunization-2022.1-eng.pdf
- 156.Petersen E, Abubakar I, Ihekweazu C, et al. Monkeypox—enhancing public health preparedness for an emerging lethal human zoonotic epidemic threat in the wake of the smallpox post-eradication era. Int J Infect Dis. 2019;78:78–84. doi: 10.1016/j.ijid.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Kozlov M. Monkeypox in Africa: the science the world ignored. June 23, 2022. https://www.nature.com/articles/d41586-022-01686-z [DOI] [PubMed]