Abstract
Background
Extracorporeal membrane oxygenation (ECMO) has been widely used in patients with COVID-19, but uncertainty remains about the determinants of in-hospital mortality and data on post-discharge outcomes are scarce. The aims of this study were to investigate the variables associated with in-hospital outcomes in patients who received ECMO during the first wave of COVID-19 and to describe the status of patients 6 months after ECMO initiation.
Methods
EuroECMO-COVID is a prospective, multicentre, observational study developed by the European Extracorporeal Life Support Organization. This study was based on data from patients aged 16 years or older who received ECMO support for refractory COVID-19 during the first wave of the pandemic—from March 1 to Sept 13, 2020—at 133 centres in 21 countries. In-hospital mortality and mortality 6 months after ECMO initiation were the primary outcomes. Mixed-Cox proportional hazards models were used to investigate associations between patient and management-related variables (eg, patient demographics, comorbidities, pre-ECMO status, and ECMO characteristics and complications) and in-hospital deaths. Survival status at 6 months was established through patient contact or institutional charts review. This study is registered with ClinicalTrials.gov, NCT04366921, and is ongoing.
Findings
Between March 1 and Sept 13, 2020, 1215 patients (942 [78%] men and 267 [22%] women; median age 53 years [IQR 46–60]) were included in the study. Median ECMO duration was 15 days (IQR 8–27). 602 (50%) of 1215 patients died in hospital, and 852 (74%) patients had at least one complication. Multiorgan failure was the leading cause of death (192 [36%] of 528 patients who died with available data). In mixed-Cox analyses, age of 60 years or older, use of inotropes and vasopressors before ECMO initiation, chronic renal failure, and time from intubation to ECMO initiation of 4 days or more were associated with higher in-hospital mortality. 613 patients did not die in hospital, and 547 (95%) of 577 patients for whom data were available were alive at 6 months. 102 (24%) of 431 patients had returned to full-time work at 6 months, and 57 (13%) of 428 patients had returned to part-time work. At 6 months, respiratory rehabilitation was required in 88 (17%) of 522 patients with available data, and the most common residual symptoms included dyspnoea (185 [35%] of 523 patients) and cardiac (52 [10%] of 514 patients) or neurocognitive (66 [13%] of 512 patients) symptoms.
Interpretation
Patient's age, timing of cannulation (<4 days vs ≥4 days from intubation), and use of inotropes and vasopressors are essential factors to consider when analysing the outcomes of patients receiving ECMO for COVID-19. Despite post-discharge survival being favourable, persisting long-term symptoms suggest that dedicated post-ECMO follow-up programmes are required.
Funding
None.
Introduction
Since the end of 2019, the SARS-CoV-2 pandemic has affected more than 600 million people worldwide, with more than 6·5 million deaths (as of Nov 4, 2022).1 COVID-19 represents an unprecedented cause of hospitalisations, requiring intensive care unit (ICU) admission in 5–32% of admitted patients.1, 2, 3 Almost 90% of patients admitted to an ICU in the first wave of the pandemic required mechanical ventilation,2, 3 with the most severe cases of acute respiratory distress syndrome (ARDS) requiring extracorporeal membrane oxygenation (ECMO).2, 3, 4, 5, 6 Despite the lessons learned from the 2009 H1N1 influenza pandemic,7 scepticism and uncertainty regarding appropriate selection of patients for ECMO were common during the initial phase of the first wave of COVID-19 from February to March, 2020. These doubts were exacerbated by scarcity of resources, differences in health-care systems, variability in institutional protocols, and cultural factors, such as heterogeneity concerning population characteristics (race and ethnicity, genetic background, and socioeconomic status).8 Once again, the patient selection process and ECMO management optimisation became crucial. Nevertheless, more than 2 years after the beginning of the COVID-19 pandemic, much remains unknown about the determinants of mortality, factors that could serve as a guide to prevent the futile initiation of extracorporeal support, or modifiable factors that could be targeted to improve outcomes. Even less evidence is available regarding outcomes after discharge;9, 10, 11 however, this information will be essential to understand the disease burden in patients with COVID-19 who survived ECMO. Furthermore, the post-COVID-19 condition, also known as long COVID, has been shown to have consequences for post-discharge quality of life and even delayed mortality after acute disease, which could play a part in the health trajectory after ECMO.
Research in context.
Evidence before this study
We searched PubMed for articles published in English or with an abstract written in English from database inception to April 15, 2021, with the medical subject heading terms “extracorporeal membrane oxygenation” and “COVID-19”. A second search was done from April 16, 2021, to Sept 15, 2022, with the supplementary concept “follow-up”. The search identified 252 manuscripts: 26 included data for extracorporeal membrane oxygenation (ECMO) in patients with COVID-19 with follow-up. 24 studies identified frames of follow-up after ECMO treatment. These reports had a mean follow-up time of 30 days and a maximum follow-up time of 90 days. We excluded one single-centre cohort study with a small number of patients (n=43) that reported 6-month follow-up; six different studies that focused on lung transplantation due to severe forms of respiratory failure with follow-up at 350 days because of their small population and the different ECMO pathophysiological approach; and the largest cohort study (n=1733) with 6-month follow up, in a population with mild to severe COVID-19, with only ten patients included who received ECMO support.
Added value of this study
The novelty of this EuroECMO-COVID study rests on the 6-month follow-up of a unique population: patients supported with ECMO for respiratory failure during the first wave of the COVID-19 pandemic (March 1 to Sept 13, 2020). The study explored the interactions between a new pathophysiological respiratory condition—COVID-19-associated acute respiratory distress syndrome (ARDS), which is fundamentally different from other forms of ARDS—and its clinical management at 133 centres across 21 different European and adjacent countries, with variability in patient selection (eg, age limitations) and previous experience with ECMO use for ARDS, absence of specific data to guide a consistent approach to managing ARDS, and strain on resources, including human resources. The study identified the pre-ECMO need for inotropes and vasopressors as a sign of evolving cardiorespiratory compromise that might influence survival and require a hybrid and dynamic ECMO approach or configuration change. Age (≥60 years) and time from intubation to ECMO initiation (≥4 days) were also associated with higher in-hospital mortality. In addition, although the study showed a low 6-month mortality after hospital discharge, a range of health impairments at 6 months—including respiratory, cardiac, and neurocognitive symptoms—were found in patients who underwent ECMO support for COVID-19 during the first pandemic wave and survived.
Implications of all the available evidence
As COVID-19 is still a new disease, clinicians require data to estimate long-term outcomes, including those specifically associated with ECMO use. The EuroECMO-COVID study provides evidence for excellent post-discharge survival and helps to identify early interventions that might improve in-hospital outcomes and reduce the pressure on health systems due to subsequent health complications. Of note, the study also supports the development of dedicated post-ECMO programmes to enhance patient monitoring and recovery in terms of physical and neurocognitive conditions and reintegration into pre-ECMO working activities. In our study, on the basis of their association with in-hospital mortality, patient's age, timing of cannulation (<4 days or ≥4 days from intubation), and use of inotropes or vasopressors represent essential factors to be considered in the care of patients, and the role of these factors needs to be confirmed in analyses of subsequent waves. Finally, the data shed light on the heart–lung interplay and the importance of choosing the right ECMO configuration, on the basis of the degree of cardiorespiratory compromise, in patients with COVID-19.
The EuroECMO-COVID study was developed by the European Extracorporeal Life Support Organization (ELSO) to provide weekly reports on patients undergoing ECMO for refractory COVID-19 in Europe and adjacent countries, including information on demographics, support type, and health status (deceased, alive, or still on ECMO).12, 13 The aim of this analysis of data from the EuroECMO-COVID study was to investigate in-hospital outcomes in addition to survival and health status at 6 months after ECMO initiation—including respiratory, cardiac, and neurocognitive symptoms, and employment status—of patients with COVID-19 who were supported by ECMO during the first wave of the COVID-19 pandemic.
Methods
Study design and participants
The EuroECMO-COVID study is a prospective, international, multicentre observational study that was started in March, 2020, to follow the status of patients undergoing ECMO for refractory COVID-19 in Europe and adjacent countries, including Belarus, Israel, Norway, Russia, and Switzerland.12, 13 Data collection is still ongoing. Currently, the study includes 204 participating centres and releases near real-time information on ECMO use in patients with COVID-19 every week throughout the European chapter of the ELSO (Euro-ELSO). Patients aged 16 years or older who received ECMO support for refractory COVID-19 from March 1, 2020, to Sept 13, 2020—the first wave—were included in this analysis. Diagnosis of COVID-19 was confirmed at each centre through a PCR test on a nasopharyngeal specimen. All patients receiving ECMO for other concomitant causes were excluded.
This study complied with the Declaration of Helsinki, and the use of data was approved by the Ethics Committee of the Maastricht University Medical Centre, Maastricht, Netherlands (METC 2020-1343), which issued a consent exemption for the current analysis of anonymised data due to the increased benchmark risks during the pandemic. All participating centres had the study approved by the local ethics committee.
Procedures
The EuroECMO-COVID study collects data prospectively on patients undergoing ECMO for refractory COVID-19. Participating hospitals regularly deliver a predefined dataset to the coordinating centre and data collection is done on the basis of a predefined protocol.
Variables collected as part of the study dataset reported here addressed pre-ECMO profile, ECMO management, and outcomes until 6 months after initiation or death (appendix pp 16–19). Data on in-hospital complications and causes of death were collected, including end-of-life decisions by the attending health-care personnel.
Follow-up continued until March 14, 2021, through direct patient contact or institutional charts review. Data on 6-month status included hospitalisation status, respiratory symptoms, neurocognitive complications (physical disability, muscle pain, attention disorders, and headache), and cardiovascular symptoms (chest pain, peripheral oedema, fatigue, and tachycardia). Finally, 6-month survivors were asked to report about their return to work in part-time or full-time employment.
Outcomes
In-hospital mortality and mortality 6 months after ECMO initiation were the primary outcomes. Secondary in-hospital outcomes were the median duration of ECMO support, median duration of ICU and hospital stay, in-hospital complications (eg, renal failure, major bleeding, neurological complications, gastrointestinal complications, and respiratory complications) from ECMO initiation to hospital discharge or death, cause of death, in-hospital time of death (on ECMO or after ECMO weaning), and heart or lung transplantation. Secondary 6-month outcomes were any kind of hospitalisation, work status, and respiratory, cardiac, and neurocognitive symptoms (appendix pp 16–19).
Statistical analysis
Demographic and clinical variables were expressed as n (%) for categorical variables and median (IQR) for continuous variables after evaluation for normality. Categorical data were compared with a χ2 test. Continuous variables were analysed with a t test or a Mann-Whitney U test, as appropriate.
First, we described the population characteristics, ECMO details, and in-hospital outcomes for the whole cohort and separately for patients who died in hospital (in-hospital non-survivors) and those who did not die in hospital (in-hospital survivors). In-hospital survival was investigated with the Kaplan-Meier method and log-rank test for comparison. A patient's discharge or transfer to another facility was treated as a censoring event. To estimate the association between variables and mortality, we fitted a frailty mixed-Cox proportional hazards model that included both fixed and random effects. The random effect was used to consider differences between centres.14 We considered different sets of variables that we deemed to be important in terms of an association with in-hospital mortality. Each variable set was developed on the basis of clinical practice and the authors' search of the literature. The mixed-Cox model estimated the hazard ratio (HR) for death in hospital as a function of the best discriminatory set of variables. Specifically, we included two models: one accounting only for pre-ECMO initiation variables along with initial ECMO mode, and another model with pre-ECMO and post-ECMO initiation variables.
The variables considered were as follows: age categories (<60 years, 60–69 years, or ≥70 years); sex; body-mass index (BMI) categories (≤29·9 kg/m2 or obesity class 1 [30·0–34·9 kg/m2], class 2 [35·0–39·9 kg/m2], or class 3 [≥40·0 kg/m2] according to the WHO classification); race; history of chronic obstructive pulmonary disease (COPD), chronic renal failure, arterial hypertension, diabetes, or pre-existing cardiovascular disease; use of antiviral drugs, vasopressors, or inotropes; ECMO indication; pre-ECMO prone positioning; duration of pre-ECMO endotracheal intubation (<4 days or ≥4 days); initial ECMO mode (venovenous vs venoarterial); ECMO duration; ECMO configuration change; renal failure with and without renal replacement therapy; lung complications (pneumothorax, embolism, and superinfection); ischaemic stroke; major bleeding (intracranial, gastrointestinal, and lung bleeding); and bowel ischaemia. The cutoff of 4 days for pre-ECMO endotracheal intubation was found using time receiver operating characteristic package to identify the best predicting cutoff at different timepoints (0, 30, 60, 90, 120, 150, and 180 days). The 4-day cutoff showed the best combination of predictive parameters: sensitivity 50·7, specificity 54·1, positive predictive value of 56·2, negative predictive value of 48·7 at 180 months (see appendix p 26). The final models were then assessed for goodness of fit using Akaike's information criterion (AIC) and Bayesian information criterion (BIC) as reported. Discrimination power was assessed with Harrell's C index. A calibration plot was drawn using bootstrap resampling in 500 new samples and mean absolute prediction error was reported (appendix pp 26–27).
We chose only variables with missing data of 20% or less to be included in the model; for selected variables that still had missing data, we applied a multiple imputation process. Briefly, we used fully specified chained equations in the R package (version 1.1.463).15 Mechanisms underlying missing data were investigated using the Little test. Because missing data were not missing completely at random (MCAR) and monotonic pattern was not found, chained equations or the Markov chain Monte Carlo (MCMC) method was used, checking for healthy convergence. Ten imputed datasets were created and pooled (appendix pp 20–21).15 We plotted marginal distribution of observed data and ten imputed datasets per variable to verify whether the imputations were reasonable; in all cases, we achieved a reasonable imputation.
The parameters of interest from a mixed-Cox model was the hazard ratio (HR; 95% CI; p value) for death in hospital. Survival curves were obtained with Kaplan-Meier and log-rank tests for comparison. A p value of less than 0·0001 was considered statistically significant.
A subgroup analysis focused on hospital survivors to describe their status 6 months after ECMO initiation. Each patient's follow-up period was defined as the time from the start of ECMO to death, 6-month follow-up, or last clinical contact. The 6-month outcomes are reported as counts and percentages of available data. Data completeness regarding the 6-month follow-up is reported in the appendix (p 22).
All data were merged from separate deidentified files into SPSS (version 26.0), Prism (version 8.0), and R studio, for data management and statistical analysis. The study was registered with ClinicalTrials.gov, NCT04366921.
Role of the funding source
There was no funding source for this study.
Results
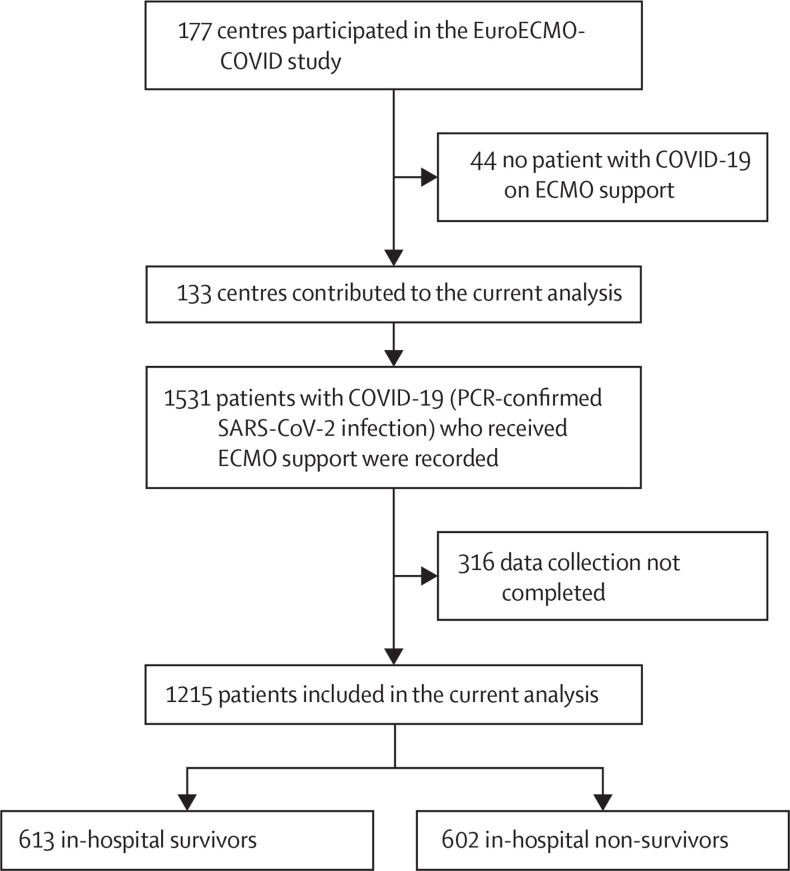
During the first COVID-19 pandemic wave, 133 centres from 21 countries (appendix pp 23–24) provided in-hospital and 6-month follow-up information for patients with COVID-19 who received ECMO. An additional 44 centres participated in the study but did not implant any ECMO in patients with COVID-19 during the first wave (figure 1 ). Data were available for 1215 patients who were supported with ECMO (942 [78%] men and 267 [22%] women of 1209 patients for whom sex data were available; median age 53 years [IQR 46–60]) and diagnosed with SARS-CoV-2 infection. Patients' geographical distribution is presented in the appendix (p 23). Baseline patient characteristics and comparisons between in-hospital survivors and non-survivors are reported in table 1 . Pre-ECMO use of inotropes was required in 361 (32%) of 1119 patients and vasopressors in 934 (79%) of 1178 patients (table 1). Time from hospital presentation to ICU admission was 7 days (IRQ 4–10), and the median time from intubation to ECMO initiation was 4 days (2–8). 1105 (91%) of 1215 patients received isolated venovenous ECMO; the remaining patients had combined cardiorespiratory or isolated cardiac support (table 2 ). The median ECMO support duration was 15 days (IRQ 8–27). Overall, 109 (11%) of 1039 patients required an ECMO reconfiguration: 23 (26%) of 87 due to refractory hypoxaemia, five (6%) of 87 due to left ventricular failure, seven (8%) of 87 due to right ventricular failure, and 19 (22%) of 87 due to biventricular failure. 590 (54%) of 1097 patients had tracheostomy; the median time from intubation to tracheostomy was 16 days (IQR 8–25; table 2).
Figure 1.
Study profile
Patients receiving ECMO support who were recruited in the EuroECMO-COVID study during the first pandemic wave (March 1 to Sept 13, 2020) were included in the current analysis. ECMO=extracorporeal membrane oxygenation.
Table 1.
Baseline patient characteristics
| Valid cases* | All patients (n=1215) | In-hospital survivors (n=613) | In-hospital non-survivors (n=602) | p value | ||
|---|---|---|---|---|---|---|
| Age, years | 1161 (95·6%) | 53 (46–60) | 50 (43–57) | 57 (49–62) | <0·0001 | |
| Age groups | .. | .. | .. | .. | <0·0001 | |
| <60 years old | .. | 893 (74%) | 536 (82%) | 357 (63%) | .. | |
| 60–69 years old | .. | 271 (22%) | 107 (16%) | 164 (29%) | .. | |
| ≥70 years old | .. | 51 (4%) | 9 (7%) | 42 (8%) | .. | |
| Sex | 1209 (99·5%) | .. | .. | .. | 0·15 | |
| Male | .. | 942 (78%) | 467 (76%) | 475 (80%) | .. | |
| Female | .. | 267 (22%) | 146 (24%) | 121 (20%) | .. | |
| Race | 1107 (91·1%) | .. | .. | .. | 0·05 | |
| White | .. | 745 (67%) | 355 (63%) | 390 (71%) | .. | |
| Asian | .. | 134 (12%) | 81 (14%) | 53 (10%) | .. | |
| Black | .. | 90 (8%) | 49 (9%) | 41 (8%) | .. | |
| Hispanic | .. | 77 (7%) | 44 (8%) | 33 (6%) | .. | |
| Other | .. | 61 (6%) | 32 (6%) | 29 (5%) | .. | |
| BMI | 1154 (95%) | 29·1 (26·0–33·0) | 29·3 (26·0–33·0) | 29·0 (26·0–32·9) | 0·71 | |
| Coexisting condition | ||||||
| Obesity (BMI >29·9 kg/m2) | 1155 (95·1%) | 511 (44%) | 259 (44%) | 252 (44%) | 1·00 | |
| BMI class | 1155 (95·1%) | .. | .. | .. | 0·35 | |
| ≤29·9 kg/m2 | .. | 644 (56%) | 326 (56%) | 318 (56%) | .. | |
| Obesity class 1 (30·0–34·9 kg/m2) | .. | 303 (26%) | 151 (26%) | 152 (27%) | .. | |
| Obesity class 2 (35·0–39·9 kg/m2) | .. | 118 (10%) | 55 (9%) | 63 (11%) | .. | |
| Obesity class 3 (≥40·0 kg/m2) | .. | 90 (8%) | 53 (9%) | 37 (7%) | .. | |
| Current smoker | 1076 (88·6%) | 137 (13%) | 58 (11%) | 79 (15%) | 0·02 | |
| Pre-existing comorbidities | ||||||
| Diabetes | 1187 (97·7%) | 279 (24%) | 125 (21%) | 154 (27%) | 0·01 | |
| Arterial hypertension | 1149 (94·6%) | 476 (41%) | 213 (36%) | 263 (47%) | <0·0001 | |
| Cardiovascular disease | 1183 (97·4%) | 139 (12%) | 50 (8%) | 89 (15%) | <0·0001 | |
| Renal failure | 1168 (96·1%) | 55 (5%) | 15 (3%) | 40 (7%) | <0·0001 | |
| Chronic obstructive pulmonary disease | 1172 (96·5%) | 79 (7%) | 27 (5%) | 52 (9%) | 0·0021 | |
| COVID-19 medical therapy | ||||||
| Antibiotics | 1129 (92·9%) | 1077 (95%) | 545 (95%) | 532 (96%) | 0·16 | |
| Antiviral drugs | 1131 (93·1%) | 584 (52%) | 275 (48%) | 309 (56%) | 0·0050 | |
| Steroids | 837 (68·9%) | 523 (63%) | 258 (64%) | 265 (61%) | 0·43 | |
| Other immunomodulators | 800 (65·8%) | 225 (28%) | 115 (29%) | 110 (27%) | 0·64 | |
| Convalescent plasma | 856 (70·5%) | 95 (11%) | 52 (12%) | 43 (10%) | 0·23 | |
| Pre-ECMO support | ||||||
| Prone positioning before ECMO | 1124 (92·5%) | 995 (83%) | 505 (83%) | 490 (93%) | <0·0001 | |
| Neuromuscular blockade | 710 (58·4%) | 561 (79%) | 259 (79%) | 302 (80%) | 0·78 | |
| Inhaled nitric oxide | 698 (57·4%) | 163 (23%) | 70 (22%) | 93 (25%) | 0·28 | |
| Inotropes | 1119 (92·1%) | 361 (32%) | 142 (25%) | 219 (40%) | <0·0001 | |
| Vasopressors | 1178 (97%) | 934 (77%) | 444 (74%) | 490 (85%) | <0·0001 | |
| Indication for ECMO | 1210 (99·6%) | 934 (79%) | .. | .. | <0·0001 | |
| ARDS | .. | 966 (80%) | 517 (85%) | 449 (75%) | .. | |
| Pneumonia | .. | 177 (15%) | 74 (12%) | 103 (17%) | .. | |
| Septic shock | .. | 15 (1%) | 5 (1%) | 10 (2%) | .. | |
| Myocarditis/cardiac dysfunction | .. | 28 (2%) | 10 (2%) | 18 (3%) | .. | |
| Isolated ventricular failure | .. | 6 (1%) | 1 (<1%) | 5 (1%) | .. | |
| Pulmonary embolism | .. | 10 (1%) | 4 (1%) | 6 (1%) | .. | |
| Cardiac arrest | .. | 7 (1%) | 0 | 7 (1%) | .. | |
| Postpartum | .. | 1 (<1%) | 1 (<1%) | 0 | .. | |
| Blood gases before ECMO | ||||||
| pH | 683 (56·2%) | 7·3 (7·2–7·4) | 7·3 (7·2–7·4) | 7·3 (7·2–7·4) | 0·38 | |
| PaO2, mm Hg | 799 (65·8%) | 64·5 (56–75·8) | 65·0 (56·2–77·7) | 64·0 (55·5–75·0) | 0·19 | |
| PaCO2, mm Hg | 691 (56·9%) | 59·0 (49–73) | 58·0 (49·5–73) | 60·0 (48·0–73·0) | 0·48 | |
| PaO2/FiO2 ratio | 779 (64·1%) | 67·0 (57·7–80·3) | 67·0 (58–81·7) | 66·7 (57·5–79·8) | 0·82 | |
| Time from hospital admission to ICU admission, days | 810 (66·7%) | 7·0 (4·0–10·0) | 8·0 (5–11) | 6·0 (3–10) | <0·0001 | |
| Time from ICU admission to intubation, days | 859 (70·7%) | 0·0 (0·0–2·0) | 0·0 (0–1) | 0·0 (0–1) | 0·03 | |
| Time from intubation to ECMO cannulation, days | 1202 (98·9%) | 4·0 (2·0–8·0) | 4·0 (1–7) | 4·0 (2–9) | 0·03 | |
Data are reported as n (%) or median (IQR). ARDS=acute respiratory distress syndrome. BMI=body-mass index. ECMO=extracorporeal membrane oxygenation. FiO2=fraction of inspired oxygen. ICU=intensive care unit. PaCO2=partial pressure of arterial carbon dioxide. PaO2=partial pressure of arterial oxygen.
For the characteristics listed, valid cases were the patients for whom data were available and considered to be accurate and consistent for statistical analysis.
Table 2.
Details of management with extracorporeal membrane oxygenation
| Valid cases* | All patients (n=1215) | In-hospital survivors (n=613) | In-hospital non-survivors (n=602) | p value | ||
|---|---|---|---|---|---|---|
| Time on ECMO support, days | 1200 (98·8%) | 15 (8–27) | 16 (9–27) | 14 (6–27) | <0·0001 | |
| Type of ECMO | 1215 (100%) | .. | .. | .. | <0·0001 | |
| VV-ECMO | .. | 1105 (91%) | 575 (94%) | 530 (88%) | .. | |
| VA-ECMO | .. | 89 (7%) | 36 (6%) | 53 (9%) | .. | |
| VAV-ECMO | .. | 10 (1%) | 0 | 10 (2%) | .. | |
| VVA-ECMO | · | 7 (1%) | 2 (0·3%) | 5 (1%) | .. | |
| OxyRVAD | .. | 0 | 0 | 0 | .. | |
| Other ECMO | .. | 4 (<1%) | 0 | 4 (1%) | .. | |
| Maximum ECMO blood flow, L/min | 1137 (93·6%) | 4·8 (4·2–5·3) | 4·8 (4·2–5·2) | 4·9 (4·2–5·5) | 0·0020 | |
| ECMO configuration change | 1039 (85·5%) | 109 (11%) | 35 (7%) | 74 (14%) | <0·0001 | |
| Median time to configuration change, days | 123 (10·1%) | 4 (1–10) | 4·0 (1–13) | 4·0 (1–10) | 0·58 | |
| Tracheostomy | 1097 (90·3%) | 590 (54%) | 357 (64%) | 233 (43%) | <0·0001 | |
| Time from intubation to tracheostomy, days | 581 (47·8%) | 16·0 (8–25) | 19·0 (10–28) | 12·0 (5–19) | <0·0001 | |
| Anticoagulation therapy | 1196 (98·4%) | 1115 (93%) | 566 (93%) | 549 (94%) | 0·42 | |
| Anticoagulation therapy type | 1118 (92%) | .. | .. | .. | 0·12 | |
| Heparin | .. | 947 (85%) | 477 (84%) | 470 (85%) | .. | |
| Bivalirudin | .. | 81 (7%) | 38 (7%) | 43 (8%) | .. | |
| Argatroban | .. | 81 (7%) | 42 (7%) | 39 (7%) | .. | |
| Other | .. | 9 (1%) | 8 (1%) | 1 (<1%) | .. | |
| Antiplatelet therapy | 699 (57·5%) | 94 (16%) | 38 (13%) | 56 (18%) | 0·04 | |
| Antiplatelet therapy type | 94 (7·7%) | .. | .. | .. | 0·64 | |
| Aspirin | .. | 84 (89%) | 33 (87%) | 51 (91%) | .. | |
| Clopidogrel | .. | 7 (7%) | 4 (11%) | 3 (5%) | .. | |
| Other | .. | 3 (3%) | 1 (1%) | 2 (2%) | .. | |
| Adjunctive therapy | 923 (76%) | .. | .. | .. | 0·02 | |
| None | .. | 245 (27%) | 145 (31%) | 100 (22%) | .. | |
| Renal replacement therapy | .. | 480 (52%) | 224 (48%) | 256 (56%) | .. | |
| Plasmapheresis | .. | 36 (4%) | 19 (4%) | 17 (4%) | .. | |
| Cytosorb | .. | 64 (7%) | 28 (6%) | 36 (8%) | .. | |
| Leukophoresis | .. | 0 | 0 | 0 | .. | |
| MARS | .. | 0 | 0 | 0 | .. | |
| IABP | .. | 0 | 0 | 0 | .. | |
| Combination | .. | 98 (11%) | 51 (11%) | 47 (10%) | .. | |
Data are reported as n (%) or median (IQR). ECMO=extracorporeal membrane oxygenation. IABP=intra-aortic balloon pump. MARS=molecular adsorbent recirculating system. OxyRVAD=oxygenator in right ventricular assist device. VA-ECMO=venoarterial ECMO. VAV-ECMO=venoarteriovenous ECMO. VV-ECMO=venovenous ECMO. VVA-ECMO=venovenoarterial ECMO.
For the complications and outcomes listed, valid cases were the patients for whom data were available and considered to be accurate and consistent for statistical analysis.
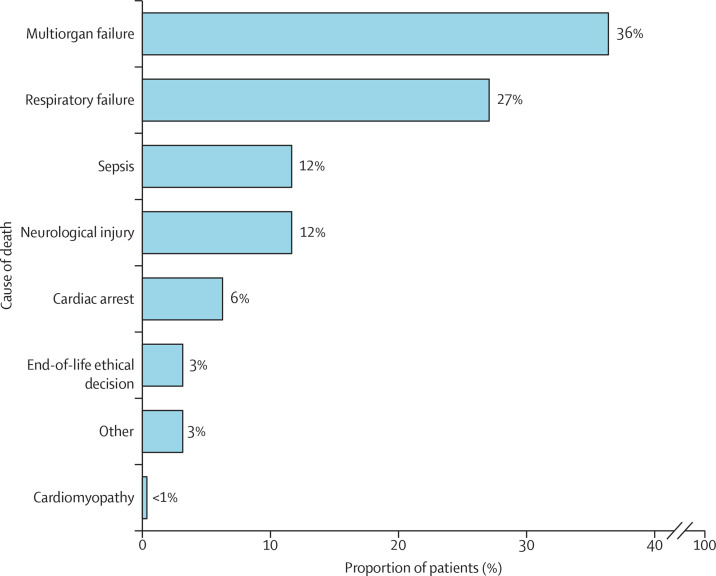
During the index hospitalisation, 852 (74%) of 1149 patients had major complications, with a different distribution between the 613 (50%) in-hospital survivors and the 602 (50%) in-hospital non-survivors (table 3 ). The most frequent cause of death was multiorgan failure, followed by irreversible respiratory compromise, sepsis, neurological complication, acute pulmonary embolism, and cardiac arrest (figure 2 ). Patients who died had a median ICU stay of 24 days (IQR 14–39). Patients who did not die in hospital had a median ICU stay of 37 days (23–58) and an overall hospital stay length of 52 days (34–81; table 3).
Table 3.
Complications and outcomes
| Valid cases* | All patients (n=1215) | In-hospital survivors (n=613) | In-hospital non-survivors (n=602) | p value | ||
|---|---|---|---|---|---|---|
| Any complication | 1149 (94·6%) | 852 (74%) | 380 (65%) | 472 (84%) | <0·0001 | |
| Renal failure | 1148 (94·5%) | .. | .. | .. | <0·0001 | |
| Renal failure without RRT | .. | 388 (34%) | 152 (26%) | 236 (42%) | .. | |
| Renal failure with RRT | .. | 259 (23%) | 111 (19%) | 148 (27%) | .. | |
| Major bleeding | 792 (65·2%) | 164 (21%) | 65 (16%) | 99 (25%) | 0·002 | |
| Neurological complication | ||||||
| Ischaemic stroke | 1142 (94%) | 61 (5%) | 21 (4%) | 40 (7%) | 0·012 | |
| Haemorrhagic stroke | 1007 (82·9%) | 54 (5%) | 10 (2%) | 44 (9%) | <0·0001 | |
| Intracranial bleeding | 988 (81·3%) | 95 (10%) | 27 (5%) | 68 (14%) | <0·0001 | |
| Seizures | 909 (74·8%) | 19 (2%) | 13 (3%) | 6 (14%) | 0·25 | |
| Delirium | 913 (75·1%) | 156 (17%) | 135 (28%) | 21 (5%) | <0·0001 | |
| Gastrointestinal complication | ||||||
| Bowel ischaemia | 1072 (88·2%) | 54 (5%) | 13 (2%) | 41 (8%) | <0·0001 | |
| Gastrointestinal bleeding | 1096 (90·2%) | 92 (8%) | 46 (8%) | 46 (9%) | 0·75 | |
| Ileus requiring medications | 887 (73%) | 123 (14%) | 75 (16%) | 48 (12%) | 0·064 | |
| Gastrointestinal perforation | 906 (74·6%) | 11 (1%) | 5 (1%) | 6 (1%) | 0·77 | |
| Respiratory complication | ||||||
| Pneumothorax or pneumomediastinum | 1143 (94·1%) | 207 (18%) | 85 (15%) | 122 (22%) | 0·0010 | |
| Lung bleeding | 1045 (86%) | 136 (13%) | 50 (9%) | 86 (17%) | <0·0001 | |
| Pulmonary embolism | 985 (81·1%) | 135 (14%) | 78 (15%) | 57 (12%) | 0·16 | |
| Haemothorax | 928 (76·4%) | 65 (7%) | 18 (4%) | 47 (10%) | <0·0001 | |
| Pulmonary superinfection | 927 (76·3%) | 377 (41%) | 187 (39%) | 190 (43%) | 0·29 | |
| Pulmonary abscess | 926 (76·2%) | 19 (2%) | 8 (2%) | 11 (3%) | 0·49 | |
| HAP or VAP | 897 (73·8%) | 338 (38%) | 169 (36%) | 169 (40%) | 0·27 | |
| Major bleeding | 1184 (97·5%) | 373 (31%) | 151 (25%) | 222 (37%) | <0·0001 | |
| Weaning from ECMO | 1215 (100%) | 728 (60%) | 613 (100%) | 115 (19%) | <0·0001 | |
| Cause of death | 528 (43·5%) | .. | .. | .. | NA | |
| Respiratory failure | .. | 143 (27%) | NA | 143 (27%) | .. | |
| Cardiac arrest | .. | 33 (6%) | NA | 33 (6%) | .. | |
| Neurological injury | .. | 62 (12%) | NA | 62 (12%) | .. | |
| Sepsis | .. | 62 (12%) | NA | 62 (12%) | .. | |
| Multiorgan failure | .. | 192 (36%) | NA | 192 (36%) | .. | |
| Other | .. | 17 (3%) | NA | 17 (3%) | .. | |
| Bleeding | .. | 0 | NA | 0 | .. | |
| End-of-life ethical decision | .. | 17 (3·2%) | NA | 17 (3%) | .. | |
| Cardiomyopathy | .. | 2 (<1%) | NA | 2 (<1%) | .. | |
| ICU length of stay, days | 1172 (96·5%) | 31 (18–49) | 37 (23–58) | 24 (14–39) | <0·0001 | |
| ICU stay off ECMO, days | 1200 (98·8%) | 11 (5–23) | 18 (8–32) | 7 (3–13) | <0·0001 | |
| Hospital length of stay, days | 1104 (90·9%) | 39 (25–63) | 52 (34–81) | 30 (19–45) | <0·0001 | |
| Lung transplant | 564 (46·4%) | 6 (1%) | 6 (2%) | 0 | NA | |
| Heart transplant | 559 (46%) | 3 (1%) | 3 (1%) | 0 | NA | |
Data are reported as n (%) or median (IQR). ECMO=extracorporeal membrane oxygenation. HAP=hospital-acquired pneumonia. ICU=intensive care unit. RRT=renal replacement therapy. VAP=ventilator-acquired pneumonia.
For the complications and outcomes listed, valid cases were the patients for whom data were available and considered to be accurate and consistent for statistical analysis.
Figure 2.
Reasons for in-hospital death
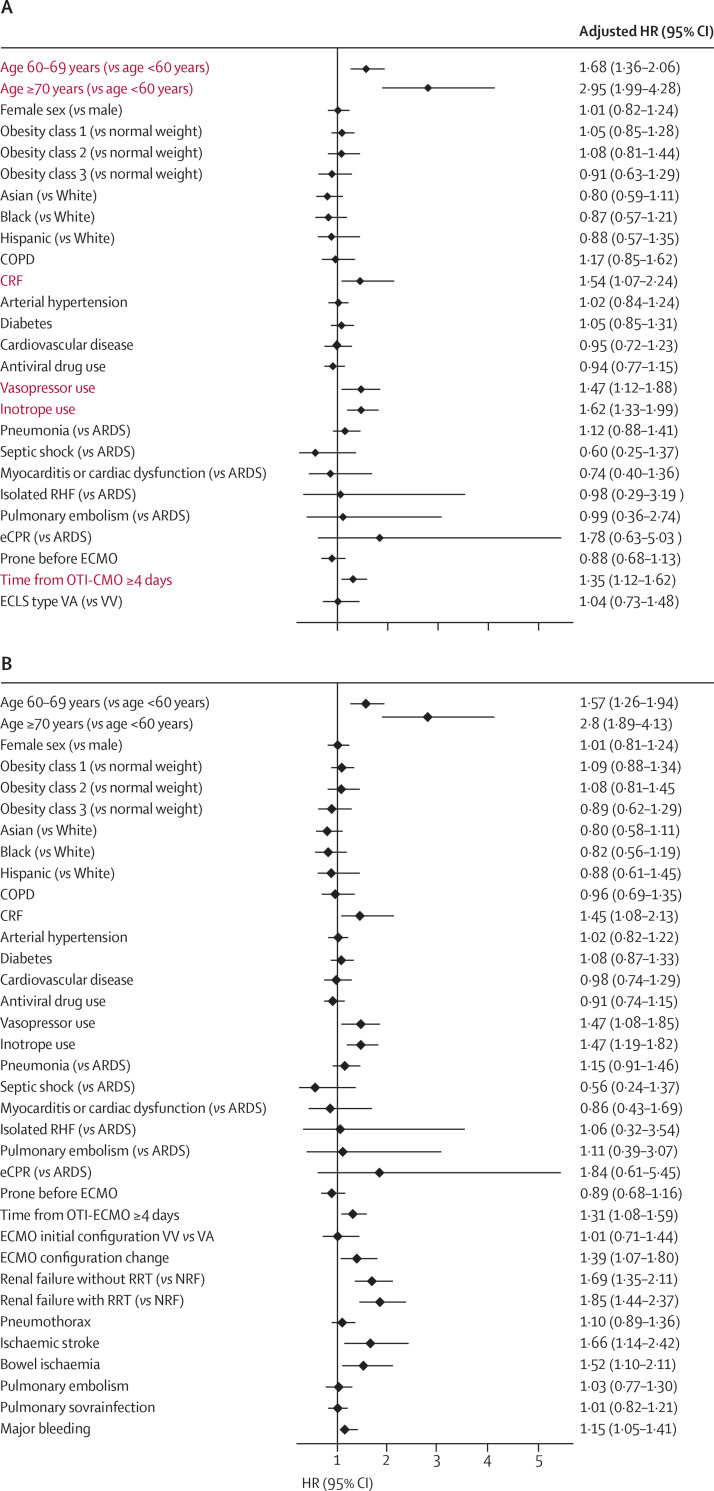
Two multivariable models (one based on pre-ECMO parameters and the other on pre-ECMO and post-ECMO initiation parameters) were created with the best possible performance. The first model reported the association between pre-ECMO initiation parameters and in-hospital mortality: age of 60 years or older, chronic renal failure, use of inotropes or vasopressors before ECMO initiation, time from intubation to ECMO initiation of 4 days or more were found to be independent predictors (figure 3A , appendix p 28). The model showed good performance (AIC 149; BIC 31; Harrell's C 0·67; and mean absolute error 0·12). In the model that used pre-ECMO and post-ECMO initiation parameters, in addition to the associations shown in the first model, the need for ECMO configuration change, renal failure with or without renal replacement therapy, major bleeding, ischaemic stroke, and bowel ischaemia were also associated with higher in-hospital mortality (figure 3B, appendix p 29). The second model also showed good performance (AIC 201; BIC 41; Harrell's C 0·69; and mean absolute error 0·020).
Figure 3.
Mixed-Cox regression analysis of determinants of in-hospital mortality
Red text indicates discriminatory set of variables. (A) Pre-ECMO parameters. (B) Pre-ECMO initiation and post-ECMO initiation parameters. HRs were adjusted for differecnes between centre. ARDS=acute respiratory distress syndrome. COPD=chronic obstructive pulmonary disease. CRF=chronic renal failure. ECLS=extracorporeal life support. ECMO=extracorporeal membrane oxygenation. eCPR=extracorporeal cardiopulmonary resuscitation. HR=hazard ratio. OTI=oro-tracheal intubation. NRF=normal renal function. RRT=renal replacement therapy. VA=venoarterial. VV=venovenous.
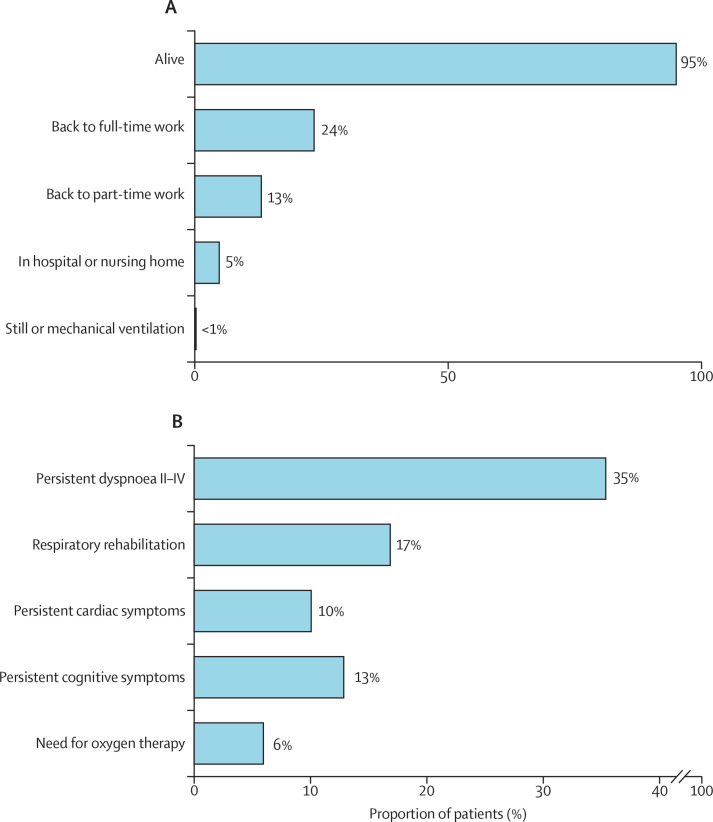
At 6-month follow-up, for the 613 in-hospital survivors, data collection completeness ranged from 70% (n=428) for data on returning to full-time work, to 94% (n=577) for mortality (appendix p 22). 30 (5%) of the 577 patients with data on 6-month survival died after hospital discharge. Lung transplantation was required for six (2%) of 309 hospital survivors and heart transplantation was required for three (1%) of 305 hospital survivors with reported data. One patient died soon after lung transplantation. 26 (5%) of 520 patients were still hospitalised or admitted to a nursing home facility at 6-month follow-up. Post-discharge respiratory rehabilitation was still required in 88 (17%) of 522 patients. Two patients (<1%) were still on mechanical ventilation, and 31 (6%) of 521 patients needed oxygen therapy. The most common residual symptoms at follow-up were dyspnoea (185 [35%] of 523 patients), cardiac symptoms (52 [10%] of 514), and neurocognitive symptoms (66 [13%] of 512) symptoms. 102 (24%) of 431 discharged patients had returned to full-time work; 57 (13%) of 428 were employed in part-time work (figure 4 ).
Figure 4.
Status (A) and functional symptoms (B) of survivors 6 months after extracorporeal membrane oxygenation initiation
Discussion
We report patient characteristics, in-hospital outcomes, and the associations between variables, such as patient demographics, comorbid status, pre-ECMO settings, ECMO characteristics and complications, and in-hospital mortality in 1215 patients with COVID-19 who were undergoing ECMO support, and we describe the largest 6-month follow-up during the first wave of the pandemic in this patient setting. Our study had four main findings. First, in patients who required ECMO for SARS-CoV-2 infection during the first pandemic wave, in-hospital mortality was high (50%), with death occurring after a median ICU stay of 24 days. Second, age of 60 years or more, use of inotropes and vasopressors before ECMO initiation, chronic renal failure, and a time from intubation to ECMO initiation of 4 days or more were associated with higher risk of death in hospital. Third, of the patients who were discharged, 5% died within 6 months of ECMO initiation. Finally, a substantial number of patients still had respiratory, cardiac, or neurocognitive symptoms and required hospitalisation or rehabilitation at 6-month follow-up. Only 24% of in-hospital survivors returned to full-time employment at 6 months.
Scepticism initially characterised the use of ECMO for patients with COVID-19 with respiratory failure. However, it soon became clear that selected patients might benefit from extracorporeal support.12, 13, 16, 17, 18, 19, 20, 21 A first analysis of the ELSO Registry, reporting on 1035 patients with COVID-19 undergoing ECMO during the first pandemic wave, showed a 90-day mortality of 38%, with 68 patients still in hospital at the time of data analysis.17 Despite these early promising results, the first pandemic wave was characterised by uncertainty regarding appropriate COVID-19 therapies and optimal ECMO selection criteria. Moreover, the ethical circumstances surrounding the allocation of scarce resources during the first COVID-19 wave tended to favour a utilitarian approach, minimising the use of ECMO.22, 23 In addition, significant differences were noticed in local and regional mortality rates, duration of ECMO support, and resource constraints.8, 18 Our study highlights the substantial amount of resources required to manage a patient receiving ECMO during the first wave of the pandemic and the complex in-hospital outcomes of these patients. Almost half of the patients in this study died after a median of 14 days on ECMO support, an ICU stay of 24 days, and an overall hospital stay of 30 days. Patients who did not die were supported on ECMO for a median of 16 days; they remained in the ICU for 37 days and in hospital for 52 days.
In times of restricted resources and challenges in the ethical allocation of treatments, the early recognition of determinants of mortality and the identification of safe and effective treatments became fundamental to patient care. Initially, ELSO provided well defined indications and patient selection criteria for the use of ECMO, based on previous experience.4 However, COVID-19 is a new disease, and although several lessons from the past can be adapted to its management, many other aspects of care need special consideration.24
Time from intubation to ECMO start has previously been shown to be a determinant of patient outcome, leading to the ELSO guideline of 7 days as the upper time limit for ECMO consideration.7 Although a single-centre experience25 and a multi-institutional analysis26 questioned such a time threshold, our findings confirm that an even shorter time from intubation to ECMO initiation (<4 days) might be a determinant of death in patients with COVID-19 receiving ECMO. Similar results have been described by Hall and colleagues,27 who estimated that an ECMO patient intubated on day 14 after the diagnosis of COVID-19 versus day 4 had a relative hazard of survival of 0·65 (95% CI 0·44–0·96).
In our analysis, only 54% of patients had a tracheostomy. More patients who did not die in hospital underwent a tracheostomy, suggesting that it was a practice reserved for patients with signs of positive outcomes.28 According to the recommendations by several major societies, tracheostomy was done 2–3 weeks after intubation only in selected patients because of the initial alarming high death rate in ICUs and the procedural risks for the attending staff.29
Among other more obvious dependent variables for mortality, pre-ECMO need for inotropes and vasopressors were associated with unfavourable outcome. Their use might indicate a certain degree of cardiac injury and a possible need for circulatory support in addition to respiratory support.21, 22 Despite this, cardiac support was the first-choice treatment in 9% of patients in our analysis. However, 11% of patients required an ECMO reconfiguration, with several patients characterised by univentricular or biventricular cardiac dysfunction, and ECMO configuration change was a determinant of increased in-hospital mortality. Cardiac injury, particularly related to right ventricular failure, has been repeatedly described in patients with COVID-19.5, 6, 21, 22 Whether early recognition of cardiac dysfunction or direct cardiocirculatory support could improve outcomes is still a matter of debate.22 However, case series with prophylactic right ventricular support while extracorporeal respiratory assistance is provided have shown encouraging results.30
Finally, our findings confirmed that renal failure represents risk factors for in-hospital mortality;17 bowel ischaemia, neurological complications, and major bleeding also characterised a less favourable prognosis for these patients. By contrast, our data do not provide confirmation for chronic respiratory disease and cardiac arrest before ECMO as a risk factor for in-hospital mortality, as shown by data in the ELSO Registry.17
The post-discharge outcomes of patients treated for COVID-19 are not yet recorded in sufficient detail, particularly in patients who have received ECMO.31, 32 Overall, survivors of ECMO are reported to have adverse changes in quality of life, with 12% of patients losing their jobs and almost 30% having significant disabilities affecting their survival.33, 34 In patients with COVID-19, the effects on quality of life might be worsened by long COVID,31, 35, 36 which is characterised by severe fatigue, muscle pain, breathlessness, heart palpitations, and problems with attention, memory, and cognition.23, 24, 25, 26, 27, 28 Our study confirms the hypothesis of long-lasting symptoms in patients who receive ECMO, despite a very low post-discharge mortality rate at 6 months for hospital survivors. In line with the results of previous smaller reports,9 only 24% of patients in our study returned to full-time work and 13% of patients returned to part-time work at 6 months. Furthermore, a substantial number of patients still required oxygen therapy or had shortness of breath and cardiac or neurocognitive symptoms. Whether these symptoms can be attributed to ECMO, COVID-19 itself, or a combination of both still needs to be clarified with larger specific studies. Nevertheless, our findings highlight the importance of a multidisciplinary post-ECMO follow-up programme tailored to address physical and psychological rehabilitation in patients who have received ECMO, especially in the COVID-19 era.9, 34, 37
The structured and systematic prospective data collection, regular weekly reports, and participation of large-volume centres in the EuroECMO-COVID study guarantee data robustness and granularity. Nevertheless, our study is observational by nature, limiting causal inferences. Moreover, under-reporting of ECMO implants or complications is plausible. Furthermore, specific data on ECMO details, such as selection criteria or local ECMO protocols, are not captured by the database and could not be included in this analysis. Indeed, each country might have followed different guidelines according to the local health-care system, resources, and ethical guidance.8 Perception of futility or the legal possibility of withdrawing support might have varied between centres. Nevertheless, this issue was addressed in our mixed-Cox analysis by using random effect to consider the differences between centres. Patients who did not die were either discharged home or transferred to another hospital, but this specification is missing, and this information might have been additionally informative. Furthermore, data on baseline functional status, previous physical limitations or working status, and an in-depth analysis of the patient's functional status at 6 months—including the 6 min walk test or spirometry, and an extended evaluation of psychological status, cognitive impairment, and quality of life through the use of specific tools—were either not available or not possible. Due to the particularity of this cohort in pandemic conditions, findings could differ in analyses of subsequent waves of COVID-19, although the EuroELSO study has reported a provisional comparison of in-hospital survival between the first and second waves (lower survival rate during the second wave).12 A partial overlapping of the cohort reported here with previously reported series cannot be excluded.17, 18 In particular, we estimate a 15–20% overlap between this study and the ELSO Registry analysis of the first COVID-19 wave.17 Finally, a comparison between patients with COVID-19 and those with other causes of hypoxaemic respiratory failure—with potential differences in intrinsic pathophysiological characteristics, clinical presentation, and management—is not provided here due to the well known differences that distinguish them.
In our study, in-hospital mortality after initiation of ECMO for COVID-19 during the first wave of the pandemic reached almost 50%. On the basis of their association with in-hospital mortality, patient's age, timing of cannulation (<4 days or ≥4 days from intubation), and use of inotropes or vasopressors represent essential factors to be considered in the care of patients, and the role of these factors needs to be confirmed in analyses of subsequent waves. Furthermore, identifying evolving cardiorespiratory compromise, guided by appropriate monitoring, represents an essential step in targeting a patient's physiological needs and a consequently adopting a more efficient ECMO configuration that could improve in-hospital results. The post-discharge prognosis in our study was favourable in terms of survival, but dedicated post-ECMO follow-up programmes with an emphasis on the psychological care of patients are warranted to support patients' recovery in view of the expected effects of persistent, disabling symptoms on health, employment status, and quality of life.
Data sharing
The data dictionary and EuroELSO policies are available online. All submitting institutions have unrestricted access to their own data and routine reports generated using these data. Reports are produced by EuroELSO, which include standard outcome reports, data analyses as a result of specific requests to EuroELSO and scientific publications using submitted data. Any scientific study, abstract, presentation, or manuscript resulting from use of data requested from EuroELSO must be presented to the EuroELSO Scientific Committee or its delegate for review before presentation or submission for publication and must comply with the EuroELSO Publication Policy and Data Access. Data and reports will be provided through a secure transfer mechanism and comply with the GDPR EU regulation.
Declaration of interests
RL and JBe are past chairmen of the European Extracorporeal Life Support Organization (ELSO) Steering Committee. NB is the current chairman of the European ELSO Steering Committee. MVA, NB, JBe, MB, MDM, TM, JR, and JS are European ELSO Steering Committee members. RL reports personal fees from Abiomed and Xenios and honoraria payed institution from Medtronic, LivaNova, Getinge and Eurosets, unrelated to the submitted works. FST is advisory member for Eurosets unrelated to the submitted work. DW is proctor for Abbott and scientific advisor for Fresenius. NB is advisory board for ALung. JR reports lecture and advisory fees from Medtronic and Werfen outside the submitted work. PS reports advisory fees from Getinge but not related to the submitted work. AV received consultant fees from Inspira Technologies. MB is an advisory member for Eurosets, congress speaker for Hamilton Medical, and course speaker for Estor, all unrelated to the submitted work. LMB is advisory board for Eurosets and Xenios and is board member of EuroELSO Enterprise, unrelated to the present work. JBe reports consultancy and lecture fees from Abiomed and Getinge and is advisory board member of AstraZeneca and Boehringer Ingelheim. SL is advisory member of Inspira Technologies, unrelated to the present work. All other authors declare no competing interests.
Acknowledgments
Acknowledgments
We thank all contributors for their continuous commitment to sharing information and supporting each other during all phases of the COVID-19 pandemic. Additional contributors and collaborators are listed in the appendix (pp 4–12). There were no funding sources for this study.
Contributors
RL, MEDP, JBe, FST, LMB, TM, JS, JR, and NB conceived the study. SM and MDM conceived the analysis plan. RL, MEDP, SM, MDM, VLC, LMB, TM, and JBe designed the study. RL, MEDP, SM, and MDM drafted the initial manuscript. RL, MEDP, SM, MDM, TF, ST, LC, JS, DM, MB, LMB, AV, YK, AMS, VF, PG, SL, JBa, UB, SM, AK, BM, MP, FMP, PS, KK, NB, JR, TM, JBe, and all members of the EuroECMO-COVID Study Group analysed and interpreted the results, critically edited the manuscript, approved the final work, and agreed to be accountable for the accuracy and integrity of the work. RL, MEDP, SM, and MDM accessed and verified the data. The Chief Investigator and the Coordinating Scientific Committee had complete access to data. Other study members had access to the data upon approval of the Coordinating Scientific Committee and all participating centres, in compliance with the EuroELSO Publication Policy and Data Access and verified the data, RL, NB, TM, and JBe were responsible for the decision to submit the manuscript. The corresponding author confirms that all authors and contributors have seen and approved the final work.
EuroECMO-COVID Study Group
Members of the EuroECMO-COVID Study Group (authors and additional contributors to this study) were Roberto Lorusso, Maria Elena De Piero, Silvia Mariani, Michele Di Mauro, Valeria Lo Coco, Thierry Folliguet, Fabio Silvio Taccone, Luigi Camporota, Iwan C C van der Horst, Bas C T van Bussel, Ronny M Schnabel, Thijs Delnoij, Justyna Swol, Dominik Wiedemann, Mirko Belliato, Lars Mikael Broman, Alain Vuylsteke, Gil Bolotin, Yigal Kassif, Anna Mara Scandroglio, Vito Fanelli, Philippe Gaudard, Luca Lorini, Stephane Ledot, Julian Barker, Martin O Schmiady, Udo Boeken, David Schibilsky, Sven Maier, Alexander Kersten, Bart Meyns, Matteo Pozzi, Mariusz Kowalewski, Finn M Pedersen, Peter Schellongowski, Luis F Pinto, Pedro E Silva, Igor Kornilov, Kaan Kirali, Aaron Blandino Ortiz, Leen Vercaemst, Simon Finney, Peter Paul Roeleveld, Matteo Di Nardo, Felix Hennig, Marta Velia Antonini, Mark Davidson, Tim J Jones, Nicholas Barrett, Jordi Riera, Thomas Mueller, Jan Belohlavek. Affiliations for group members are provided in the appendix (pp 4–5).
Contributor Information
EuroECMO-COVID Study Group:
Roberto Lorusso, Maria Elena De Piero, Silvia Mariani, Michele Di Mauro, Thierry Folliguet, Fabio Silvio Taccone, Luigi Camporota, Justyna Swol, Dominik Wiedemann, Mirko Belliato, Lars Mikael Broman, Alain Vuylsteke, Yigal Kassif, Anna Mara Scandroglio, Vito Fanelli, Philippe Gaudard, Stephane Ledot, Julian Barker, Udo Boeken, Sven Maier, Alexander Kersten, Bart Meyns, Matteo Pozzi, Finn M Pedersen, Peter Schellongowski, Kaan Kirali, Nicholas Barrett, Jordi Riera, Thomas Mueller, Jan Belohlavek, Valeria Lo Coco, Iwan C C Van der Horst, Bas C T Van Bussel, Ronny M Schnabel, Thijs Delnoij, Gil Bolotin, Luca Lorini, Martin O Schmiady, David Schibilsky, Mariusz Kowalewski, Luis F Pinto, Pedro E Silva, Igor Kornilov, Aaron Blandino Ortiz, Leen Vercaemst, Simon Finney, Peter P Roeleveld, Matteo Di Nardo, Felix Hennig, Marta Velia Antonini, Mark Davidson, Tim J Jones, Thomas Staudinger, Peter Mair, Juliane Kilo, Christoph Krapf, Kathrin Erbert, Andreas Peer, Nikolaos Bonaros, Florian Kotheletner, Niklas Krenner Mag, Liana Shestakova, Greet Hermans, Dieter Dauwe, Philippe Meersseman, Bernard Stockman, Leda Nobile, Olivier Lhereux, Alexandre Nrasseurs, Jacques Creuter, Daniel De Backer, Simone Giglioli, Gregoire Michiels, Pierre Foulon, Matthias Raes, Inez Rodrigus, Matthias Allegaert, Philippe Jorens, Gerd Debeucklare, Michael Piagnerelli, Patrick Biston, Harlinde Peperstraete, Komeel Vandewiele, Olivier Germay, Dimitri Vandeweghe, Sven Havrin, Marc Bourgeois, Marc-Gilbert Lagny, Genette Alois, Nathalie Lavios, Benoit Misset, Romain Courcelle, Philippe J Timmermans, Alaaddin Yilmaz, Michiel Vantomout, Jerone Lehaen, Ame Jassen, Herbert Guterman, Maarten Strauven, Piet Lormans, Bruno Verhamme, catherine Vandewaeter, Frederik Bonte, Dominique Vionne, Martin Balik, Jan Blàha, Michal Lips, Michal Othal, Filip Bursa, Radim Spacek, Steffen Christensen, Vibeke Jorgensen, Marc Sorensen, Soren A Madsen, Severin Puss, Aleksandr Beljantsev, gabriel Saiydoun, Antonio Fiore, Pascal Colson, Florian Bazalgette, Xavier Capdevila, Sebastien Kollen, Laurent Muller, Jean-Francois Obadia, Pierre-Yves Dubien, Lucrezia Ajrhourh, Pierre G Guinot, Jonathan Zarka, Patricia Besserve, Maximilian V Malfertheiner, Esther Dreier, Birgit Heinze, Payam Akhyari, Artur Lichtenberg, Hug Aubin, Alexander Assman, Diyar Saeed, Holger Thiele, Matthias Baumgaertel, Jan D Schmitto, Natanov Ruslan, Axel Haverich, Matthias Thielmann, Thorsten Brenner, Arjang Ruhpawar, Christoph Benk, Martin Czerny, Dawid L Staudacher, Fridhelm Beyersdorf, Johannes Kalbhenn, Philipp Henn, Aron-Frederik Popov, Torje Iuliu, Ralf Muellenbach, Christian Reyher, Caroline Rolfes, Gosta Lotz, Michael Sonntagbauer, Helen Winkels, Julia Fichte, Robert Stohr, Sebastian Kalverkamp, Christian Karagiannidis, Simone Schafer, Alexei Svetlitchny, Julia Fichte, Hans-Bernd Hopf, Dominik Jarczak, Heinirich Groesdonk, Magdalena Rommer, Jan Hirsch, Christian Kaehny, Dimitros Soufleris, Georgios Gavriilidis, Kostantinos Pontikis, Magdalini Kyriakopoulou, Anna Kyriakoudi, Serena O'Brien, Ian Conrick-Martin, Edmund Carton, Maged Makhoul, Josef Ben-Ari, Amir Hadash, Alexander Kogan, Reut Kassif Lerner, Anas Abu-Shakra, Moshe Matan, Ahmad Balawona, Erez Kachel, Roman Altshuler, Ori Galante, Lior Fuchs, Yaniv Almog, Yaron S Ishay, Yael Lichter, Amir Gal-oz, Uri Carmi, Asaph Nini, Arie Soroksky, Hagi Dekel, Ziv Rozman, Emad Tayem, Eduard Ilgiyaev, Yuval Hochman, daniel Miltau, Avigal Rapoport, Arieh Eden, Dmitry Kompanietz, Michael Yousif, Miri Golos, Lorenzo Grazioli, Davide Ghitti, Antonio Loforte, Daniela Di Luca, Massimo Baiocchi, Davide Pacini, Antioco Cappai, Paolo Meani, Michele Mondino, Claudio F Russo, Marco Ranucci, Dario Fina, Marco Cotza, Andrea Ballotta, Giovanni Landoni, Pasquale Nardelli, Eygeny V Fominski, Luca Brazzi, Giorgia Montrucchio, Gabriele Sales, Umberto Simonetti, Sergio Livigni, Daniela Silengo, Giulia Arena, Stefania S Sovatzis, Antonella Degani, Mariachiara Riccardi, Elisa Milanesi, Giuseppe Raffa, Gennaro Martucci, Antonio Arcadipane, Giovanna Panarello, Giovanni Chiarini, Sergio Cattaneo, Carmine Puglia, Stefano Benussi, Giuseppe Foti, Marco Giani, Michela Bombino, Maria Cristina Costa, Roberto Rona, Leonello Avalli, Abele Donati, Roberto Carozza, Francesco Gasparri, Andrea Carsetti, Marco Picichè, Anna Marinello, Vinicio Danzi, Anita Zanin, Ignazio Condello, Flavio Fiore, Marco Moscarelli, Giuseppe Nasso, Giuseppe Speziale, Luca Sandrelli, Andrea Montalto, Francesco Musumeci, Alessandro Circelli, Emanuele Russo, Vanni Agnoletti, Ruggero Rociola, Aldo D Milano, Emanuele Pilato, Giuseppe Comentale, Andrea Montisci, Francesco Alessandri, Antonella Tosi, Francesco Pugliese, Giovanni Giordano, Simone Carelli, Domenico L Grieco, Antonio M Dell'Anna, Massimo Antonelli, Enrico Ramoni, Josè Zulueta, Mauro Del Giglio, Sebastiano Petracca, Pietro Bertini, Fabio Guarracino, Luigi De Simone, Paolo M Angeletti, Francesco Forfori, Francesco Taraschi, Veronica N Quintiliani, Robertas Samalavicius, Agne Jankuviene, Nadezda Scupakova, Karolis Urbonas, Juozas Kapturauskas, Gro Soerensen, Piotr Suwalski, Luis Linhares Santos, Ana Marques, Marisa Miranda, Sonia Teixeira, Andrea Salgueiro, Filipe Pereira, Michail Ketskalo, Sergey Tsarenko, Alexandra Shilova, Ivan Afukov, Konstantin Popugaev, Sergei Minin, Daniil Shelukhin, Olga Malceva, Moroz Gleb, Alexander Skopets, Roman Kornelyuk, Alexandr Kulikov, Vadim Okhrimchuk, Alexandr Turchaninov, Daniil Shelukhin, Maxim Petrushin, Anastasia Sheck, Akhmed Mekulov, Svetlana Ciryateva, Dmitry Urusov, Vojka Gorjup, Alenka Golicnik, Tomaz Goslar, Ricard Ferrer, Maria Martinez-Martinez, Eduard Argudo, Neiser Palmer, Raul De Pablo Sanchez, Lucas Juan Higuera, Lucas Arnau Blasco, Josè A Marquez, Fabrizio Sbraga, Mari Paz Fuset, Pablo Ruiz De Gopegui, Luis M Claraco, Josè A De Ayala, Maranta Peiro, Pilar Ricart, Sergio Martinez, Fernando Chavez, Marc Fabra, elena Sandoval, David Toapanta, Albert Carraminana, Adrian Tellez, Jeysson Ososio, Pablo Milan, Jorge Rodriguez, Garcia Andoni, Carola Gutierrez, Enrique Perez de la Sota, Andrea Eixeres-Esteve, Maria Teresa Garcia-Maellas, Judit Gutierrez-Gutierrez, Rafael Arboleda-Salazar, Patricia Santa Teresa, Alexis Jaspe, Alberto Garrido, Galo Castaneda, Sara Alcantara, Nuria Martinez, Marina Perez, Hector Villanueva, Anxela Vidal Gonzalez, Juan Paez, Arnoldo Santon, Cesar Perez, Marta Lopez, Maria Isabel Rubio Lopez, Antonio Gordillo, Jose Naranjo-Izurieta, Javier Munoz, Immaculada Alcalde, Fernando Onieva, Ricardo Gimeno Costa, Francisco Perez, Isabel Madrid, Monica Gordon, Carlos L Albacete Moreno, Daniel Perez, Nayara Lopez, Domingo Martinenz, Pablo Blanco-Schweizer, Cristina Diez, David Perez, Ana Prieto, Gloria Renedo, Elena Bustamante, Ramon Cicuendez, Rafael Citores, Victoria Boado, Katherine Garcia, Roberto Voces, Monica Domezain, Jose Maria Nunez Martinez, Raimundo Vicente, David Martin, Antonio Andreu, Vanesa Gomez Casal, Ignacio Chico, Eva Maria Menor, Sabela Vara, Jose Gamacho, Helen Perez-Chomon, Francisco Javier Gonzales, Irene Barrero, Luis Martin-Villen, Esperanza Fernandez, Maria Mendoza, Joaquin Navarro, Joaquin Colomina Climent, Alfredo Gonzales-Perez, Guillermo Muniz-Albaceita, Laura Amado, Raquel Rodriguez, Emilio Ruiz, Maria Eiras, Edgars Grins, Rosen Magnus, Mikael Kanetoft, Marcus Eidevald, Pia Watson, Paul R Vogt, Peter Steiger, Tobias Aigner, Alberto Weber, Jurg Grunefelder, Martin Kunz, Martin Grapow, Thierry Aymard, Diana Reser, Gianluca Agus, Jolanda Consiglio, Matthias Haenggi, Jenni Hansjoerg, Manuela Iten, Thomas Doeble, Urs Zenklusen, Xavier Bechtold, Giovanni Faedda, Manuel Iafrate, Amanda Rohjer, Layla Bergamaschi, Jos Maessen, Dinis Reis Miranda, H Endeman, D Gommers, C Meuwese, Jacinta Maas, MJ Van Gijlswijk, RN Van Berg, Dario Candura, Marcel Van der Linden, Merijin Kant, JJ Van der Heijden, Eric Scholten, Nicole Van Belle-van Haren, WK Lagrand, Alexander P Vlaar, Syste De Jong, Basar Cander, Murat Sargin, Murat Ugur, Mehmet A Kaygin, Kathleen Daly, Nicola Agnew, Laura Head, Laura Kelly, Gunawardena Anoma, Clare Russell, Verna Aquino, Ian Scott, Lucy Flemming, Stuart Gillon, Olivia Moore, Elton Gelandt, George Auzinger, Sameer Patel, and Robert Loveridge
Supplementary Material
References
- 1.WHO COVID-19 Dashboard. 2021. https://covid19.who.int/ (accessed Nov 4, 2022).
- 2.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA. 2020;323:1574–1581. doi: 10.1001/jama.2020.5394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395:497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shekar K, Badulak J, Peek G, et al. Extracorporeal life support organization coronavirus disease 2019 interim guidelines: a consensus document from an international group of interdisciplinary extracorporeal membrane oxygenation providers. ASAIO J. 2020;66:707–721. doi: 10.1097/MAT.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jaffe AS, Cleland JGF, Katus HA. Myocardial injury in severe COVID-19 infection. Eur Heart J. 2020;41:2080–2082. doi: 10.1093/eurheartj/ehaa447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Madjid M, Safavi-Naeini P, Solomon SD, Vardeny O. Potential effects of coronaviruses on the cardiovascular system: a review. JAMA Cardiol. 2020;5:831–840. doi: 10.1001/jamacardio.2020.1286. [DOI] [PubMed] [Google Scholar]
- 7.Peek GJ, Mugford M, Tiruvoipati R, et al. Efficacy and economic assessment of conventional ventilatory support versus extracorporeal membrane oxygenation for severe adult respiratory failure (CESAR): a multicentre randomised controlled trial. Lancet. 2009;374:1351–1363. doi: 10.1016/S0140-6736(09)61069-2. [DOI] [PubMed] [Google Scholar]
- 8.Mesotten D, Meijs DAM, van Bussel BCT, et al. Differences and similarities among COVID-19 patients treated in seven ICUs in three countries within one region: an observational cohort study. Crit Care Med. 2022;50:595–606. doi: 10.1097/CCM.0000000000005314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Taylor LJ, Jolley SE, Ramani C, et al. Early posthospitalization recovery after extracorporeal membrane oxygenation in survivors of COVID-19. J Thorac Cardiovasc Surg. 2022 doi: 10.1016/j.jtcvs.2021.11.099. published online March 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Smith DE, Chang SH, Geraci TC, et al. One-year outcomes with venovenous extracorporeal membrane oxygenation support for severe COVID-19. Ann Thorac Surg. 2022;114:70–75. doi: 10.1016/j.athoracsur.2022.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Taboada M, Moreno E, Cariñena A, et al. Quality of life, functional status, and persistent symptoms after intensive care of COVID-19 patients. Br J Anaesth. 2021;126:e110–e113. doi: 10.1016/j.bja.2020.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Broman LM, Eksborg S, Lo Coco V, De Piero ME, Belohlavek J, Lorusso R. Extracorporeal membrane oxygenation for COVID-19 during first and second waves. Lancet Respir Med. 2021;9:e80–e81. doi: 10.1016/S2213-2600(21)00262-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lorusso R, Combes A, Lo Coco V, et al. ECMO for COVID-19 patients in Europe and Israel. Intensive Care Med. 2021;47:344–348. doi: 10.1007/s00134-020-06272-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balan TA, Putter H. A tutorial on frailty models. Stat Methods Med Res. 2020;29:3424–3454. doi: 10.1177/0962280220921889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Buuren S, Groothuis-Oudshoorn K. mice: multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 16.Melhuish TM, Vlok R, Thang C, Askew J, White L. Outcomes of extracorporeal membrane oxygenation support for patients with COVID-19: a pooled analysis of 331 cases. Am J Emerg Med. 2021;39:245–246. doi: 10.1016/j.ajem.2020.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation support in COVID-19: an international cohort study of the Extracorporeal Life Support Organization registry. Lancet. 2020;396:1071–1078. doi: 10.1016/S0140-6736(20)32008-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbaro RP, MacLaren G, Boonstra PS, et al. Extracorporeal membrane oxygenation for COVID-19: evolving outcomes from the international Extracorporeal Life Support Organization Registry. Lancet. 2021;398:1230–1238. doi: 10.1016/S0140-6736(21)01960-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lebreton G, Schmidt M, Ponnaiah M, et al. Extracorporeal membrane oxygenation network organisation and clinical outcomes during the COVID-19 pandemic in Greater Paris, France: a multicentre cohort study. Lancet Respir Med. 2021;9:851–862. doi: 10.1016/S2213-2600(21)00096-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li X, Guo Z, Li B, et al. Extracorporeal membrane oxygenation for Coronavirus Disease 2019 in Shanghai, China. ASAIO J. 2020;66:475–481. doi: 10.1097/MAT.0000000000001172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suwalski P, Staromłyński J, Brączkowski J, et al. Transition from Simple V-V to V-A and hybrid ECMO configurations in COVID-19 ARDS. Membranes. 2021;11:434. doi: 10.3390/membranes11060434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mariani S, De Piero ME, Ravaux JM, et al. Temporary mechanical circulatory support for COVID-19 patients: a systematic review of literature. Artif Organs. 2022;46:1249–1267. doi: 10.1111/aor.14261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Emanuel EJ, Persad G, Upshur R, et al. Fair allocation of scarce medical resources in the time of Covid-19. N Engl J Med. 2020;382:2049–2055. doi: 10.1056/NEJMsb2005114. [DOI] [PubMed] [Google Scholar]
- 24.Supady A, Combes A, Barbaro RP, et al. Respiratory indications for ECMO: focus on COVID-19. Intensive Care Med. 2022;48:1326–1337. doi: 10.1007/s00134-022-06815-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hermann M, Laxar D, Krall C, et al. Duration of invasive mechanical ventilation prior to extracorporeal membrane oxygenation is not associated with survival in acute respiratory distress syndrome caused by coronavirus disease 2019. Ann Intensive Care. 2022;12:6. doi: 10.1186/s13613-022-00980-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Urner M, Barnett AG, Bassi GL, et al. Venovenous extracorporeal membrane oxygenation in patients with acute covid-19 associated respiratory failure: comparative effectiveness study. BMJ. 2022;377:e068723. doi: 10.1136/bmj-2021-068723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hall CA, Jacobs JP, Stammers AH, et al. Multi-institutional analysis of 505 patients with coronavirus disease-2019 supported with extracorporeal membrane oxygenation: predictors of survival. Ann Thorac Surg. 2022;114:61–68. doi: 10.1016/j.athoracsur.2022.01.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Al Omari A, Al-Ashqar R, Alabd Alrhman R, Nuseir A, Allan H, Alzoubi F. Assessment of the harms and potential benefits of tracheostomy in COVID-19 patients: narrative review of outcomes and recommendations. Am J Otolaryngol. 2021;42:102972. doi: 10.1016/j.amjoto.2021.102972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shah R, Priyadarshini G, Parsana M. A systematic review on guidelines and recommendations for tracheostomy during COVID-19 pandemic. Indian J Otolaryngol Head Neck Surg. 2021 doi: 10.1007/s12070-021-02517-9. published online April 29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mustafa AK, Joshi DJ, Alexander PJ, et al. Comparative propensity matched outcomes in severe COVID-19 respiratory failure-extracorporeal membrane oxygenation or maximum ventilation alone. Ann Surg. 2021;274:e388–e394. doi: 10.1097/SLA.0000000000005187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Huang C, Huang L, Wang Y, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. 2021;397:220–232. doi: 10.1016/S0140-6736(20)32656-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tariq M, Acharekar MV, Guerrero Saldivia SE, et al. Just when we thought that COVID was over: a systematic review. Cureus. 2022;14:e27441. doi: 10.7759/cureus.27441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cho HW, Song IA, Oh TK. Quality of life and long-term mortality among survivors of extracorporeal membrane oxygenation: a nationwide cohort study in South Korea. Crit Care Med. 2021;49:e771–e780. doi: 10.1097/CCM.0000000000005015. [DOI] [PubMed] [Google Scholar]
- 34.Kanji HD, Chouldechova A, Harris-Fox S, et al. Quality of life and functional status of patients treated with venovenous extracorporeal membrane oxygenation at 6 months. J Crit Care. 2021;66:26–30. doi: 10.1016/j.jcrc.2021.07.010. [DOI] [PubMed] [Google Scholar]
- 35.Taboada M, Cariñena A, Moreno E, et al. Post-COVID-19 functional status six-months after hospitalization. J Infect. 2021;82:e31–e33. doi: 10.1016/j.jinf.2020.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Carfì A, Bernabei R, Landi F. Persistent symptoms in patients after acute COVID-19. JAMA. 2020;324:603–605. doi: 10.1001/jama.2020.12603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Higgins V, Sohaei D, Diamandis EP, Prassas I. COVID-19: from an acute to chronic disease? Potential long-term health consequences. Crit Rev Clin Lab Sci. 2021;58:297–310. doi: 10.1080/10408363.2020.1860895. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data dictionary and EuroELSO policies are available online. All submitting institutions have unrestricted access to their own data and routine reports generated using these data. Reports are produced by EuroELSO, which include standard outcome reports, data analyses as a result of specific requests to EuroELSO and scientific publications using submitted data. Any scientific study, abstract, presentation, or manuscript resulting from use of data requested from EuroELSO must be presented to the EuroELSO Scientific Committee or its delegate for review before presentation or submission for publication and must comply with the EuroELSO Publication Policy and Data Access. Data and reports will be provided through a secure transfer mechanism and comply with the GDPR EU regulation.