Abstract
Background
Between May and November, 2022, global outbreaks of human monkeypox virus infection have been reported in more than 78 000 people worldwide, predominantly in men who have sex with men. We describe the epidemiological and clinical characteristics of monkeypox virus infection in cisgender (cis) and transgender (trans) women and non-binary individuals assigned female sex at birth to improve identification and understanding of risk factors.
Methods
International collaborators in geographical locations with high numbers of diagnoses of monkeypox virus infection were approached and invited to contribute data on women and non-binary individuals with confirmed monkeypox virus infection. Contributing centres completed deidentified structured case-report spreadsheets, adapted and developed by participating clinicians, to include variables of interest relevant to women and non-binary individuals assigned female at birth. We describe the epidemiology and clinical course observed in the reported infections.
Findings
Collaborators reported data for a total of 136 individuals with monkeypox virus infection who presented between May 11 and Oct 4, 2022, across 15 countries. Overall median age was 34 years (IQR 28–40; range 19–84). The cohort comprised 62 trans women, 69 cis women, and five non-binary individuals (who were, because of small numbers, grouped with cis women to form a category of people assigned female at birth for the purpose of comparison). 121 (89%) of 136 individuals reported sex with men. 37 (27%) of all individuals were living with HIV, with a higher proportion among trans women (31 [50%] of 62) than among cis women and non-binary individuals (six [8%] of 74). Sexual transmission was suspected in 55 (89%) trans women (with the remainder having an unknown route of transmission) and 45 (61%) cis women and non-binary individuals; non-sexual routes of transmission (including household and occupational exposures) were reported only in cis women and non-binary individuals. 25 (34%) of 74 cis women and non-binary individuals submitted to the case series were initially misdiagnosed. Overall, among individuals with available data, rash was described in 124 (93%) of 134 individuals and described as anogenital in 95 (74%) of 129 and as vesiculopustular in 105 (87%) of 121. Median number of lesions was ten (IQR 5-24; range 1–200). Mucosal lesions involving the vagina, anus, or oropharynx or eye occurred in 65 (55%) of 119 individuals with available data. Vaginal and anal sex were associated with lesions at those sites. Monkeypox virus DNA was detected by PCR from vaginal swab samples in all 14 samples tested. 17 (13%) individuals were hospitalised, predominantly for bacterial superinfection of lesions and pain management. 33 (24%) individuals were treated with tecovirimat and six (4%) received post-exposure vaccinations. No deaths were reported.
Interpretation
The clinical features of monkeypox in women and non-binary individuals were similar to those described in men, including the presence of anal and genital lesions with prominent mucosal involvement. Anatomically, anogenital lesions were reflective of sexual practices: vulvovaginal lesions predominated in cis women and non-binary individuals and anorectal features predominated in trans women. The prevalence of HIV co-infection in the cohort was high.
Funding
None.
Introduction
Between May and November, 2022, more than 78 000 monkeypox virus infections have been described in 109 countries that have not historically reported monkeypox infections.1 WHO declared the outbreak a Public Health Emergency of International Concern (PHEIC) in July, 2022.2 Transmissions during the global outbreaks have been overwhelmingly associated with sexual contact and have almost exclusively affected sexually active gay, bisexual, and other men who have sex with men (GBMSM).1 28–47% of individuals diagnosed with monkeypox infection are living with HIV, and a majority of those without HIV are on HIV pre-exposure prophylaxis (PrEP).3, 4, 5, 6, 7, 8, 9 Consequently, international case definitions specify GBMSM as the at-risk group, and prevention efforts have focused on GBMSM on PrEP, individuals living with HIV, men with sexually transmitted infections (STIs), and individuals attending sex-on-site venues, including sexual activity at mass gatherings. So far, sustained spread outside of GBMSM networks has not occurred; however, the spread of monkeypox virus to women is a concern, especially because of the potential for serious consequences for fetuses if pregnant individuals become infected.10
Research in context.
Evidence before this study
Scientific literature on human monkeypox virus infection began in the 1970s, and two periods can be distinguished: before and since 2022. Before 2022, scientific reports focused on outbreaks in central and western Africa, where the disease is endemic, and on sporadic imported cases and very limited outbreaks in high-income countries. 30–50% of infections were reported in women. The 2022 global outbreaks began with monkeypox virus infections being reported almost exclusively among gay, bisexual, and other men who have sex with men and in countries not historically affected by this disease. We searched PubMed for the terms “monkeypox AND (women OR females)” from May 10 to Oct 16, 2022 with no language restrictions. Most publications were letters, perspectives, and case reports. One preprint described seven cases in heterosexual women in Nigeria occurring since May, 2022. We also reviewed epidemiological reports from WHO and the US Centers for Disease Control and Prevention in 2022. In these series, women represented 3·8% of infections, without distinction between cisgender (cis) women, transgender (trans) women, and non-binary individuals (ie, with gender identities outside the gender binary) who were assigned female at birth. There were no published series or cohorts of women diagnosed with monkeypox virus infection in 2022. Therefore, data on transmission routes and clinical features among women and non-binary individuals are scarce.
Added value of this study
In our international convenience case series of 62 trans women, 69 cis women, and five non-binary individuals diagnosed with monkeypox virus infection since May, 2022, we describe the epidemiological, clinical, and diagnostic features, and complications in these under-studied populations. HIV prevalence was very high in trans women (31 [50%] of 62) but lower in cis women and non-binary people (six [8%] of 74). Overall, sexual contact was the most common suspected route of transmission (55 [89%] of 62 trans women and 45 [61%] of 74 cis women and non-binary people). In trans women, commercial sex work was reported in 34 (55%) of 62 individuals and was the strongest occupational link to infection. 24 (34%) of 71 cis women and non-binary people had vaginal mucosal involvement and 42 (59%) of 71 had vulvar lesions. One trans woman had vulvar involvement, and no disease of the vagina was described, perhaps reflecting sexual activity, as few trans women had undergone gender-affirming surgery (vaginoplasty). Anal mucosal involvement (proctitis or ulceration) occurred in 25 (56%) of 45 trans women and eight (11%) of 70 cis women and non-binary people. Overall, oral presentations (both perioral and mucosal) occurred in around 31 (24%) of 129 individuals. Fewer individuals without sexual exposure had anogenital lesions, but otherwise the presentation did not differ from previously reported, predominantly male cohorts. The median incubation period was estimated at 7 days (IQR 4–11) on the basis of the individual's recall of presumed exposure date and date of first symptom. Complications requiring hospitalisation occurred for similar reasons (mainly pain management and bacterial superinfection) and at similar frequencies (13%) as in predominantly male case series. No deaths were reported.
Implications of all the available evidence
The evidence available to date indicates that women represent a small but important part of the overall population infected with monkeypox virus during the 2022 outbreaks. However, special attention must be paid to avoid delayed diagnosis and misdiagnosis in women. In our case series, characteristic clinical findings typically included a self-limiting genital and anal vesiculopustular rash, often involving the mucosa, with or without systemic symptoms before the onset of rash. In trans women and cis women and non-binary people, the site of the lesions largely corresponded to the type of sexual activity reported. Clinicians must be made aware of the differing clinical presentations according to gender identity and sexual practices.
Over the past 50 years, monkeypox virus infections have been reported in several central and western African countries, with sporadic cases or limited outbreaks in high-income countries, linked to the exotic pet trade and international travel.11 Before 2017, human monkeypox virus infections in western and central Africa had shown a similar incidence in males and females.12, 13, 14, 15, 16, 17, 18 However, during the 2017–18 outbreak in Nigeria, an increased proportion of infections occurred in males (65%).19
Similarly, large case series describing the 2022 outbreaks of monkeypox virus have included no or few women,3, 4, 5, 6, 7, 8, 9 and large epidemiological surveillance datasets have not differentiated between cisgender (cis) and transgender (trans) women.20, 21 The US Centers for Disease Control and Prevention (CDC) reported that, of more than 25 000 infections in the USA, 3·8% were in cis women and 0·8% in trans women.20 The total number of women infected with monkeypox virus is unknown and likely to be underestimated because of underdiagnosis, given international case definitions specifying GBMSM as the major at-risk group.
In addition, the modes of transmission and clinical presentation of monkeypox virus infection during the current global outbreak differ from previous descriptions from before 2017.19 In this outbreak, painful anal, genital, and oral mucosal lesions have commonly been described, often as presenting features and frequently without previous systemic symptoms.3, 4, 5, 6, 7, 8, 9 Transmission via skin and mucosal contact related to sexual activity and possibly via semen is likely to explain the location of these lesions, as suggested in other case series.8
We hypothesised that the transmission routes and clinical presentation of monkeypox virus in the current outbreaks might not be the same for women as for GBMSM, and that presentations might also differ between cis and trans women.
Building on the Share-Net international clinical network established at the beginning of the global monkeypox outbreak, we report a cohort from 15 countries of women and non-binary individuals with human monkeypox virus infection, with the aim of describing the epidemiological associations and clinical outcomes.
Methods
Overview
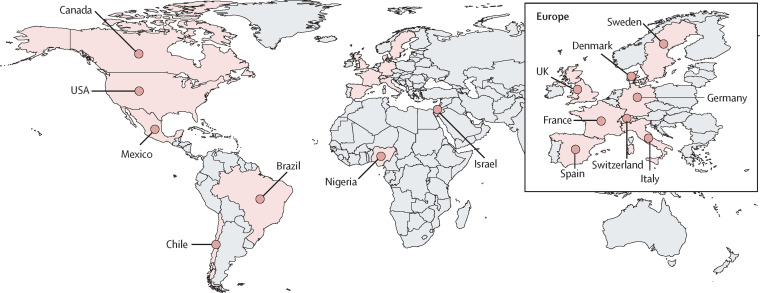
In response to the PHEIC, academic researchers within the London-based Sexual Health and HIV All East Research Collaborative re-energised the international collaboration Share-Net, as previously described.9 Researchers in geographical locations reporting high numbers of monkeypox virus infections were approached and invited to contribute to the case series. A convenience-sample case series was collated to describe epidemiological and clinical features in cis and trans women and non-binary individuals assigned female sex at birth (figure 1 ). Participating clinicians identified women and non-binary individuals with monkeypox virus infection at their site, and invited these individuals to participate in the case series. Informed consent for inclusion was obtained and maintained in accordance with local standards, along with local institutional review board approval per local requirements. Image-specific consent was obtained for use of images. Deidentified data were securely transferred to the coordinating site, stored, and analysed within the Queen Mary University of London Bart's Cancer Institute data safe haven.
Figure 1.
Global distribution of Share-Net contributing sites
Case definition and identification
We used the UK Health Security Agency definition of a confirmed case: a PCR-confirmed monkeypox virus infection in a specimen from any anatomical site. Diagnosis of monkeypox virus infection by PCR is based on detection of unique sequences of monkeypox virus DNA, which were published by public health agencies and adopted by clinical laboratories worldwide for the development of local PCR testing platforms. We included nine individuals without PCR confirmation but with typical clinical presentation from a site in France where, as of July 2022, local guidelines prohibited confirmation by PCR when at-risk individuals had classic symptoms of monkeypox virus infection and known contacts with people infected with monkeypox virus.
Data collection
Each contributing centre was provided with and completed a deidentified structured case-report spreadsheet, adapted and developed by participating clinicians on Sept 1, 2022 to include variables of interest relevant to women and non-binary individuals assigned female at birth (appendix p 10).3 The case-report spreadsheet used drop-down menus and free-text fields to allow contributing clinicians to capture data from the site's electronic or paper medical record. We described routine clinical care that was not part of a research protocol. The case-report spreadsheet particularly focused on potential exposures, demographic characteristics, occupation, early symptoms, clinical findings, diagnosis, HIV status, concomitant STIs, and complications. Clinicians were asked to designate a single suspected route of infection or to choose “unknown” if a route could not be designated. Infections diagnosed since May 1, 2022, were reported between Sept 10 and Oct 4, 2022.
Statistical analysis
Data were analysed using SPSS software (version 28) and descriptive statistics were reported. Aggregate and deidentified data are presented to avoid deductive disclosure of the identities of individuals with monkeypox virus infection. During the analysis, data from the five non-binary individuals assigned female at birth were grouped with data from cis women (forming a category of people assigned female at birth) as the numbers were too small for meaningful comparison.
Role of the funding source
There was no funding source for this study.
Results
136 women and non-binary individuals with human monkeypox virus infection who presented between May 11 and Oct 4, 2022, were included from 15 countries and three WHO regions. 68 (50%) were from the WHO European region, 65 (48%) were from the region of the Americas (USA, Canada, Mexico, Brazil, and Chile), and three (2%) were from the African region (appendix p 6). Demographic and epidemiological characteristics of the cohort are shown in table 1 . Median age was 34 years (IQR 28–40; range 19–84). Most individuals were either Latinx (61 [45%]), White (40 [29%]), or Black (28 [21%]). 69 (51%) were cis women, 62 (46%) were trans women, and five (4%) people were non-binary individuals assigned female at birth. Overall, 121 (89%) individuals reported having sex with men within the past month. 34 (55%) of 62 trans women and two (3%) of 74 cis women and non-binary people reported current sex work. 14 (10%) of all individuals reported injecting drugs (seven [11%] trans women and seven [9%] cis women and non-binary individuals, four (3%) were known to be migrants, and eight (6%) were experiencing homelessness. 19 (14%) individuals (18 cis women and one non-binary individual) had children at home, among whom two children subsequently contracted monkeypox.
Table 1.
Patient demographics
| Trans women (n=62) | Cis women and non-binary individuals (n=74) | Overall (n=136) | ||
|---|---|---|---|---|
| WHO region | ||||
| Region of the Americas | 29/62 (47%) | 36/74 (49%) | 65/136 (48%) | |
| European region | 33/62 (53%) | 35/74 (47%) | 68/136 (50%) | |
| African region | 0/62 | 3/74 (4%) | 3/136 (2%) | |
| Age, years | N=62 | N=74 | N=136 | |
| Median (IQR) | 34 (30–39) | 33 (26–45) | 34 (28–40) | |
| Range | 22–52 | 19–84 | 19–84 | |
| Gender identity | ||||
| Cis woman (assigned female sex at birth) | .. | 69/74 (93%) | 69/136 (51%) | |
| Non-binary (assigned female sex at birth) | .. | 5/74 (7%) | 5/136 (4%) | |
| Trans woman (assigned male sex at birth) | 62/62 (100%) | .. | 62/136 (46%) | |
| Ethnicity | ||||
| White | 8/62 (13%) | 32/74 (43%) | 40/136 (29%) | |
| Latinx | 38/62 (61%) | 23/74 (31%) | 61/136 (45%) | |
| Black | 13/62 (21%) | 15/74 (20%) | 28/136 (21%) | |
| Asian | 2/62 (3%) | 1/74 (1%) | 3/136 (2%) | |
| Mixed | 1/62 (2%) | 1/74 (1%) | 2/136 (1%) | |
| First Nation, Inuit, or Métis | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Other or unknown | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Sexual orientation | ||||
| Heterosexual | 43/62 (69%) | 65/74 (88%) | 108/136 (79%) | |
| Bisexual | 5/62 (8%) | 5/74 (7%) | 10/136 (7%) | |
| Lesbian | 0/62 | 2/74 (3%) | 2/136 (1%) | |
| Unknown | 14/62 (23%) | 2/74 (3%) | 16/136 (12%) | |
| Occupation | ||||
| Sex work | 34/62 (55%) | 1/74 (1%) | 35/136 (26%) | |
| Health care | 3/62 (5%) | 10/74 (14%) | 13/136 (10%) | |
| Business or office work | 1/62 (2%) | 10/74 (14%) | 11/136 (8%) | |
| Student | 1/62 (2%) | 6/74 (8%) | 7/136 (5%) | |
| Cleaning | 2/62 (3%) | 1/74 (1%) | 3/136 (2%) | |
| Food service | 2/62 (3%) | 2/74 (3%) | 4/136 (3%) | |
| Beauty | 1/62 (2%) | 1/74 (1%) | 2/136 (1%) | |
| Sales or marketing | 0/62 | 5/74 (7%) | 5/136 (4%) | |
| Arts | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Teaching or childcare | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Other | 2/62 (3%) | 4/74 (5%) | 6/136 (4%) | |
| Unemployed | 6/62 (10%) | 13/74 (18%) | 19/136 (14%) | |
| Unknown | 10/62 (16%) | 19/74 (26%) | 29/136 (21%) | |
| Current sex work | ||||
| No | 20/62 (32%) | 69/74 (93%) | 89/136 (65%) | |
| Yes | 34/62 (55%) | 2/74 (3%) | 36/136 (26%) | |
| Not known | 8/62 (13%) | 3/74 (4%) | 11/136 (8%) | |
| Types of sexual partners within past month | ||||
| Casual female partner | 1/62 (2%) | 0/74 | 1/136 (1%) | |
| Casual male partner | 2/62 (3%) | 6/74 (8%) | 8/136 (6%) | |
| Male and female partners | 0/62 | 7/74 (9%) | 7/136 (5%) | |
| Multiple female partners | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Multiple male partners | 45/62 (73%) | 9/74 (12%) | 54/136 (40%) | |
| Regular female partner | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Regular male partner | 8/62 (13%) | 44/74 (59%) | 52/136 (38%) | |
| No sexual partners | 0/62 | 5/74 (7%) | 5/136 (4%) | |
| Not known | 6/62 (10%) | 1/74 (1%) | 7/136 (5%) | |
| Number of sexual partners in past 3 months | N=46 | N=68 | N=114 | |
| Median (IQR) | 10 (2–50) | 1 (1–2) | 2 (1–10) | |
| Range | 1–360 | 0–22 | 1–360 | |
| Type of sex | ||||
| Anal only | 3/62 (5%) | 0/74 | 3/136 (2%) | |
| Oral and anal | 47/62 (76%) | 1/74 (1%) | 48/136 (35%) | |
| Oral only | 1/62 (2%) | 1/74 (1%) | 2/136 (1%) | |
| Vaginal and anal | 0/62 | 1/74 (1%) | 1/136 (1%) | |
| Vaginal and oral | 0/62 | 25/74 (34%) | 25/136 (18%) | |
| Vaginal only | 0/62 | 17/74 (23%) | 17/136 (13%) | |
| Vaginal, oral, and anal | 0/62 | 9/74 (12%) | 9/136 (7%) | |
| Not known | 11/62 (18%) | 20/74 (27%) | 31/136 (23%) | |
| Suspected route of transmission* | ||||
| Occupational exposure (health-care workers) | 0/62 | 4/74 (5%) | 4/136 (3%) | |
| Household | 0/62 | 7/74 (9%) | 7/136 (5%) | |
| Non-sexual close contact | 0/62 | 7/74 (9%) | 7/136 (5%) | |
| Sexual contact | 55/62 (89%) | 45/74 (61%) | 100/136 (74%) | |
| Unknown | 7/62 (11%) | 11/74 (15%) | 18/136 (13%) | |
| Living with HIV | ||||
| No or not known | 31/62 (50%) | 68/74 (92%) | 99/136 (73%) | |
| Yes | 31/62 (50%) | 6/74 (8%) | 37/136 (27%) | |
| Other immune suppression | ||||
| No or not known | 59/62 (95%) | 73/74 (99%) | 132/136 (97%) | |
| Yes, corticosteroids | 1/62 (2%) | 1/74 (1%) | 2/136 (1%) | |
| Yes, primary immunodeficiency | 2/62 (3%) | 0/74 | 2/136 (1%) | |
| Tested for HIV at monkeypox diagnosis | ||||
| Yes | 20/31 (65%) | 36/68 (53%) | 56/99 (57%) | |
| No or not known | 11/31 (35%) | 32/68 (47%) | 43/99 (43%) | |
| Not applicable (known HIV) | 31 | 6 | 37 | |
| PrEP use in those without HIV | ||||
| No or not known | 13/31 (42%) | 67/68 (99%) | 80/99 (81%) | |
| Yes | 18/31 (58%) | 1/68 (1%) | 19/99 (19%) | |
| Concurrent sexually transmitted infections at presentation | ||||
| 0 | 46/62 (74%) | 68/74 (92%) | 114/136 (84%) | |
| 1 | 8/62 (13%) | 4/74 (5%) | 12/136 (9%) | |
| ≥2 | 4/62 (6%) | 1/74 (1%) | 5/136 (4%) | |
| Not known | 4/62 (6%) | 1/74 (1%) | 5/136 (4%) | |
| Children in household | ||||
| No | 50/62 (81%) | 45/74 (61%) | 95/136 (70%) | |
| Yes | 0/62 | 19/74 (26%) | 19/136 (14%) | |
| Not known | 12/62 (19%) | 10/74 (14%) | 22/136 (16%) | |
| Children infected with monkeypox | ||||
| No or not known | 62/62 (100%) | 72/74 (97%) | 134/136 (99%) | |
| Yes | 0/62 | 2/74 (3%) | 2/136 (1%) | |
| Pregnant at time of monkeypox infection | ||||
| No | 62/62 (100%) | 69/74 (93%) | 131/136 (96%) | |
| Yes | 0/62 | 2/74 (3%) | 2/136 (1%) | |
| Not known | 0/62 | 3/74 (4%) | 3/136 (2%) | |
| Known contact with monkeypox virus | ||||
| No or not known | 56/62 (90%) | 42/74 (57%) | 98/136 (72%) | |
| Yes | 6/62 (10%) | 32/74 (43%) | 38/136 (28%) | |
| Attended Pride events or similar within month preceding symptom onset | ||||
| No or not known | 56/62 (90%) | 71/74 (96%) | 127/136 (93%) | |
| Yes | 6/62 (10%) | 3/74 (4%) | 9/136 (7%) | |
| Attended large event or festival within month preceding symptom onset | ||||
| No | 58/62 (94%) | 68/74 (92%) | 126/136 (93%) | |
| Yes | 4/62 (6%) | 6/74 (8%) | 10/136 (7%) | |
| Current intravenous drug use | ||||
| No or not known | 55/62 (89%) | 67/74 (91%) | 122/136 (90%) | |
| Yes | 7/62 (11%) | 7/74 (9%) | 14/136 (10%) | |
| Migrant or asylum seeker | ||||
| No or not known | 59/62 (95%) | 73/74 (99%) | 132/136 (97%) | |
| Yes | 3/62 (5%) | 1/74 (1%) | 4/136 (3%) | |
| Experiencing homelessness | ||||
| No or not known | 59/62 (95%) | 69/74 (93%) | 128/136 (94%) | |
| Yes | 3/62 (5%) | 5/74 (7%) | 8/136 (6%) | |
Data are n/N (%), median (IQR), or range; N signifies the number of individuals with available data. PrEP=pre-exposure prophylaxis.
Only one suspected route of transmission was allowed.
37 (27%) individuals were living with HIV, with a higher proportion among trans women (31 [50%]) than cis women or non-binary individuals (six [8%]). Of those with HIV, 36 (97%) of 37 were on antiretroviral therapy, 29 (81%) of 36 with available data had an undetectable viral load (<50 copies per mL), and the median CD4 cell count was 600 cells per μL (IQR 487–805). Among individuals without HIV, 18 (58%) of 31 trans women were reported to be taking PrEP for HIV, compared with one (2%) of 68 cis women and non-binary individuals.
Trans women had a higher median number of sexual partners in the past 3 months (10 [IQR 2–50]) than cis women and non-binary individuals did (1 [1–2]). Five (7%) of 74 cis women and non-binary individuals reported no sexual partner in the past month. The proportion of individuals who reported having a single regular sexual partner was higher among cis women and non-binary individuals (45 [61%]) than among trans women (eight [13%]).
Sexual contact was suspected as the most likely route of transmission in 100 (74%) of 136 individuals (45 [61%] cis women and non-binary individuals and 55 [89%] trans women). Non-sexual suspected routes of transmission in cis women and non-binary individuals included occupational exposure in health-care workers (four [5%]), household contact (seven [10%]), and non-sexual close contact (seven [10%]; table 1). The probable route of transmission was unknown in 18 (13%) individuals from the overall cohort (11 [15%] women and non-binary people and seven [11%] trans women). 32 (43%) cis women and non-binary individuals and six (10%) trans women were known contacts of people with monkeypox infection. In the overall cohort, attendance at large gatherings (ten [7%]) and LGBTQ+ Pride events (nine [7%]) within the month preceding the onset of the monkeypox symptoms was uncommon. Of the 131 individuals tested for STIs, a concurrent STI at presentation was diagnosed in 17 (13%) people, most commonly syphilis (seven [5%]), chlamydia (six [5%]), and gonorrhoea (six [5%]). More STIs were reported among trans women (12 [21%] of 58) than cis women or non-binary individuals (five [7%] of 73). HIV testing among individuals without known HIV was documented in 20 (65%) of 31 trans women and 36 (53%) of 68 cis women and non-binary individuals.
Most trans women (41 [66%]) presented to sexual health or HIV clinics, and 13 (21%) presented to emergency departments. Cis women and non-binary individuals most commonly presented to emergency departments (26 [35%]), with 21 (24%) presenting to sexual health or HIV clinics and the remainder presenting to other hospital departments (including dermatology, gynaecology, and obstetrics) or primary care (table 2 ). Misdiagnosis before a diagnosis of monkeypox virus infection was more common among cis women and non-binary individuals (25 [34%]) than among trans women (six [10%]). Additionally, delayed diagnosis was more common among cis women and non-binary individuals, with 48 (77%) trans women diagnosed on their first visit compared with 43 (58%) cis women and non-binary individuals (table 2).
Table 2.
Clinical characteristics
| Trans women (n=62) | Cis women and non-binary people (n=74) | Overall (n=136) | |
|---|---|---|---|
| Confirmed monkeypox virus infection | |||
| High clinical suspicion | 9/62 (15%) | 1/74 (1%) | 10/136 (7%) |
| Yes, by PCR | 53/62 (85%) | 73/74 (99%) | 126/136 (93%) |
| Department presented to | |||
| Sexual health clinic | 35/62 (56%) | 18/74 (24%) | 53/136 (39%) |
| Emergency department | 13/62 (21%) | 26/74 (35%) | 39/136 (29%) |
| Dermatology | 0/62 | 7/74 (9%) | 7/136 (5%) |
| HIV clinic | 6/62 (10%) | 3/74 (4%) | 9/136 (7%) |
| Gynaecology or obstetrics | 0/62 | 2/74 (3%) | 2/136 (1%) |
| Other hospital clinic | 6/62 (10%) | 12/74 (16%) | 18/136 (13%) |
| Primary care or general practice | 2/62 (3%) | 5/74 (7%) | 7/136 (5%) |
| Not known | 0/62 | 1/74 (1%) | 1/136 (1%) |
| Misdiagnosed before monkeypox diagnosis | |||
| No or not known | 56/62 (90%) | 49/74 (66%) | 105/136 (77%) |
| Yes | 6/62 (10%) | 25/74 (34%) | 31/136 (23%) |
| Number of health-care visits before diagnosis | |||
| 1, diagnosed at first visit | 48/62 (77%) | 43/74 (58%) | 91/136 (67%) |
| 2 | 11/62 (18%) | 19/74 (26%) | 30/136 (22%) |
| 3 | 1/62 (2%) | 4/74 (5%) | 5/136 (4%) |
| 4 | 0/62 | 0/74 | 0/136 |
| ≥5 | 0/62 | 1/74 (1%) | 1/136 (1%) |
| Not known or missing data | 2/62 (3%) | 7/74 (5%) | 9/136 (7%) |
| Incubation period, days | N=9 | N=42 | N=51 |
| Median (IQR) | 7 (5–11) | 7 (4–11) | 7 (4–11) |
| Range | 1–20 | 1–24 | 1–24 |
| Systemic symptoms before rash | |||
| No | 23/54 (43%) | 23/68 (34%) | 46/122 (38%) |
| Yes | 31/54 (57%) | 45/68 (66%) | 76/122 (62%) |
| Not known or missing data | 8 | 6 | 14 |
| Fever | |||
| No | 22/58 (38%) | 26/74 (35%) | 48/132 (36%) |
| Yes | 36/58 (62%) | 48/74 (65%) | 84/132 (64%) |
| Not known or missing data | 4 | 0 | 4 |
| Lymphadenopathy | |||
| No | 28/59 (47%) | 36/73 (49%) | 64/132 (48%) |
| Yes | 31/59 (53%) | 37/73 (51%) | 68/132 (52%) |
| Not known or missing data | 3 | 1 | 4 |
| Lethargy or tiredness | |||
| No | 34/58 (59%) | 28/69 (41%) | 62/127 (49%) |
| Yes | 24/58 (41%) | 41/69 (59%) | 65/127 (51%) |
| Not known or missing data | 4 | 5 | 9 |
| Headache | |||
| No | 47/59 (80%) | 46/71 (65%) | 93/130 (72%) |
| Yes | 12/59 (20%) | 25/71 (35%) | 37/130 (28%) |
| Not known or missing data | 3 | 3 | 6 |
| Sore throat or pharyngitis | |||
| No | 46/60 (77%) | 47/73 (64%) | 93/133 (70%) |
| Yes | 14/60 (23%) | 26/73 (36%) | 40/133 (30%) |
| Not known or missing data | 2 | 1 | 3 |
| Myalgia | |||
| No | 49/58 (84%) | 41/70 (59%) | 90/128 (70%) |
| Yes | 9/58 (16%) | 29/70 (41%) | 38/128 (30%) |
| Not known or missing data | 4 | 4 | 8 |
| Meningism, encephalitis, or seizure | |||
| No | 59/60 (98%) | 74/74 (100%) | 133/134 (99%) |
| Yes | 1/60 (2%) | 0/74 | 1/134 (1%) |
| Not known or missing data | 2 | 0 | 2 |
| Low mood or anxiety | |||
| No | 44/56 (79%) | 43/61 (70%) | 87/117 (74%) |
| Yes | 12/56 (21%) | 18/61 (30%) | 30/117 (26%) |
| Not known or missing data | 6 | 13 | 19 |
| Arthralgia | |||
| No | 54/57 (95%) | 58/70 (83%) | 112/127 (88%) |
| Yes | 3/57 (5%) | 12/70 (17%) | 15/127 (12%) |
| Not known or missing data | 5 | 4 | 9 |
| Rash, including viral exanthem | |||
| No | 4/61 (7%) | 6/73 (8%) | 10/134 (7%) |
| Yes | 57/61 (93%) | 67/73 (92%) | 124/134 (93%) |
| Not known or missing data | 1 | 1 | 2 |
| Duration of rash*, days | N=11 | N=40 | N=51 |
| Median (IQR) | 10 (10–22) | 18 (13–21) | 15 (10–21) |
| Range | 7–56 | 2–30 | 2–56 |
| Viral exanthem (rash all over body) | |||
| No | 53/60 (88%) | 61/72 (85%) | 114/132 (86%) |
| Yes | 7/60 (12%) | 11/72 (15%) | 18/132 (14%) |
| Not known or missing data | 2 | 2 | 4 |
| Type of rash | |||
| Vesiculopustular rash | 45/52 (87%) | 60/69 (87%) | 105/121 (87%) |
| Multiple ulcers | 1/52 (2%) | 5/69 (7%) | 6/121 (5%) |
| Single ulcer | 3/52 (6%) | 2/69 (3%) | 5/121 (4%) |
| Macular | 0/52 | 1/69 (1%) | 1/121 (1%) |
| Other | 3/52 (6%) | 1/69 (1%) | 4/121 (3%) |
| Not known | 10 | 5 | 15 |
| Number of lesions | N=56 | N=70 | N=126 |
| Median (IQR) | 10 (3–20) | 10 (5–33) | 10 (5–24) |
| Range | 1–100 | 1–200 | 1–200 |
| Any anogenital lesions† | |||
| No | 12/59 (20%) | 22/70 (31%) | 34/129 (26%) |
| Yes | 47/59 (80%) | 48/70 (69%) | 95/129 (74%) |
| Not known | 3 | 4 | 7 |
| Any oral lesions‡ | |||
| No | 42/58 (72%) | 56/71 (79%) | 98/129 (76%) |
| Yes | 16/58 (28%) | 15/71 (21%) | 31/129 (24%) |
| Not known | 4 | 3 | 7 |
| Any mucosal lesions§ | |||
| No | 16/48 (33%) | 38/71 (54%) | 54/119 (45%) |
| Yes | 32/48 (67%) | 33/71 (46%) | 65/119 (55%) |
| Not known | 14 | 3 | 17 |
| Vulvar skin lesions (non-mucosal) | |||
| No | 59/60 (98%) | 29/71 (41%) | 88/131 (67%) |
| Yes | 1/60 (2%) | 42/71 (59%) | 43/131 (33%) |
| Not known | 2 | 3 | 5 |
| Perianal skin lesions (non-mucosal) | |||
| No | 14/59 (24%) | 55/72 (76%) | 69/131 (53%) |
| Yes | 45/59 (76%) | 17/72 (24%) | 62/131 (47%) |
| Not known | 3 | 2 | 5 |
| Perioral skin lesions (non-mucosal) | |||
| No | 50/58 (86%) | 62/71 (87%) | 112/129 (87%) |
| Yes | 8/58 (14%) | 9/71 (13%) | 17/129 (13%) |
| Not known | 4 | 3 | 7 |
| Non-genital skin lesions—face | |||
| No | 44/59 (75%) | 48/72 (67%) | 92/131 (70%) |
| Yes | 15/59 (25%) | 24/72 (33%) | 39/131 (30%) |
| Not known | 3 | 2 | 5 |
| Non-genital skin lesions—trunk | |||
| No | 32/58 (55%) | 38/72 (53%) | 70/130 (54%) |
| Yes | 26/58 (45%) | 34/72 (47%) | 60/130 (46%) |
| Not known | 4 | 2 | 6 |
| Non-genital skin lesions—palms or soles | |||
| No | 44/57 (77%) | 49/72 (68%) | 93/129 (72%) |
| Yes | 13/57 (23%) | 23/72 (32%) | 36/129 (28%) |
| Not known | 5 | 2 | 7 |
| Mucosal lesions—lips or oral | |||
| No | 51/56 (91%) | 60/74 (81%) | 111/130 (85%) |
| Yes | 5/56 (9%) | 14/74 (19%) | 19/130 (15%) |
| Not known | 6 | 0 | 6 |
| Mucosal lesions—pharyngeal | |||
| No | 48/56 (86%) | 65/71 (92%) | 113/127 (89%) |
| Yes | 8/56 (14%) | 6/71 (8%) | 14/127 (11%) |
| Not known | 6 | 3 | 9 |
| Mucosal lesions—anorectal | |||
| No | 20/45 (44%) | 62/70 (89%) | 82/115 (71%) |
| Yes | 25/45 (56%) | 8/70 (11%) | 33/115 (29%) |
| Not known | 17 | 4 | 21 |
| Mucosal lesions—vaginal | |||
| No | 55/55 (100%) | 47/71 (66%) | 102/126 (81%) |
| Yes | 0/55 | 24/71 (34%) | 24/126 (19%) |
| Not known | 7 | 3 | 10 |
| Mucosal lesions—ocular | |||
| No | 58/58 (100%) | 72/73 (99%) | 130/131 (99%) |
| Yes | 0/58 | 1/73 (1%) | 1/131 (1%) |
| Not known | 4 | 1 | 5 |
| Mucosal lesions—urethral | |||
| No | 55/55 (100%) | 64/65 (98%) | 119/120 (99%) |
| Yes | 0/55 | 1/65 (2%) | 1/120 (1%) |
| Not known | 7 | 9 | 16 |
| Admitted to hospital for monkeypox virus infection | |||
| No or not known | 60/62 (97%) | 59/74 (80%) | 119/136 (88%) |
| Yes | 2/62 (3%) | 15/74 (20%) | 17/136 (13%) |
| Reason for admission | |||
| Abscess | 0/2 | 2/15 (13%) | 2/17 (12%) |
| Cellulitis or bacterial superinfection | 0/2 | 2/15 (13%) | 2/17 (12%) |
| Ocular lesion | 0/2 | 1/15 (7%) | 1/17 (6%) |
| Infection control | 0/2 | 2/15 (13%) | 2/17 (12%) |
| Altered mental status and worsening left-sided weakness | 0/2 | 1/15 (7%) | 1/17 (6%) |
| Odynophagia | 0/2 | 3/15 (20%) | 3/17 (18%) |
| Severe anorectal pain with or without tenesmus | 1/2 (50%) | 2/15 (13%) | 3/17 (18%) |
| Other | 1/2 (50%) | 2/15 (13%) | 3/17 (18%) |
| Antibiotic use during monkeypox virus infection | |||
| No or not known | 55/62 (89%) | 50/74 (68%) | 105/136 (77%) |
| Yes | 7/62 (11%) | 24/74 (32%) | 31/136 (23%) |
| Monkeypox-specific treatment | |||
| No or not known | 38/62 (61%) | 59/74 (80%) | 97/136 (71%) |
| Yes | 24/62 (39%) | 15/74 (20%) | 39/136 (29%) |
| Tecovirimat | 21/62 (34%) | 12/74 (16%) | 33/136 (24%) |
| Post-exposure vaccine | 3/62 (5%) | 3/74 (4%) | 6/136 (4%) |
| Vaccinated with smallpox vaccine since May, 2022 | |||
| No or not known | 54/62 (87%) | 72/74 (97%) | 126/136 (93%) |
| Yes | 8/62 (13%) | 2/74 (3%) | 10/136 (7%) |
| History of smallpox vaccination | |||
| No or not known | 59/62 (95%) | 70/74 (95%) | 129/136 (95%) |
| Yes | 3/62 (5%) | 4/74 (5%) | 7/136 (5%) |
Data are n/N (%), median (IQR), or range; N signifies the number of individuals with available data.
Time from first lesion to scab detaching.
Refers to at least one of the following: a vulvar or perianal skin lesion or an anorectal or vaginal mucosal lesion.
Refers to at least one of the following: a perioral skin lesion or an oral or lip or pharyngeal mucosal lesion.
Refers to any mucosal lesion in at least one of the following mucosal sites: pharyngeal, anorectal, vaginal, urethral, eye, or conjunctival.
Based on presumed dates of exposure and symptom onset (available for 51 individuals), the median incubation period reported was 7 days (IQR 4–11; range 1–24). Among those for whom data were available, 76 (62%) of 122 individuals presented with systemic features, the descriptions and frequency of which are shown in table 2. 124 (93%) of 134 individuals had skin lesions at presentation, which were described as vesiculopustular in 105 (87%) of 121 individuals. 95 (74%) of 129 individuals had at least one anogenital lesion. Mucosal lesions involving the vagina, anus, or oropharynx or eye occurred in 65 (55%) of 119 individuals. With regard to oral involvement, 17 (13%) of 129 individuals had perioral lesions and 19 (15%) of 130 had oral mucosal involvement. Among 71 cis women and non-binary individuals assigned female at birth, 42 (59%) had vulvar lesions and 24 (34%) had vaginal mucosal lesions (table 2; figure 2 ). One (2%) of 60 trans women had vulvar lesions, and none had vaginal lesions. Perianal skin lesions occurred in 45 (76%) of 59 trans women and 17 (24%) of 72 cis women and non-binary individuals (appendix p 7). Anorectal mucosal lesions were observed in 25 (56%) of 45 trans women and eight (11%) of 70 cis women and non-binary individuals. Anal and vaginal sex were commonly reported in association with lesions in those anatomical sites (ie, anal sex was reported in 49 of 64 individuals with anal lesions and vaginal sex was reported in 35 of 46 individuals with vaginal lesions; appendix p 7). The majority of those reporting oral sex did not report oral lesions (53 of 73). Overall, 34 (26%) of 129 individuals had no anogenital lesions.
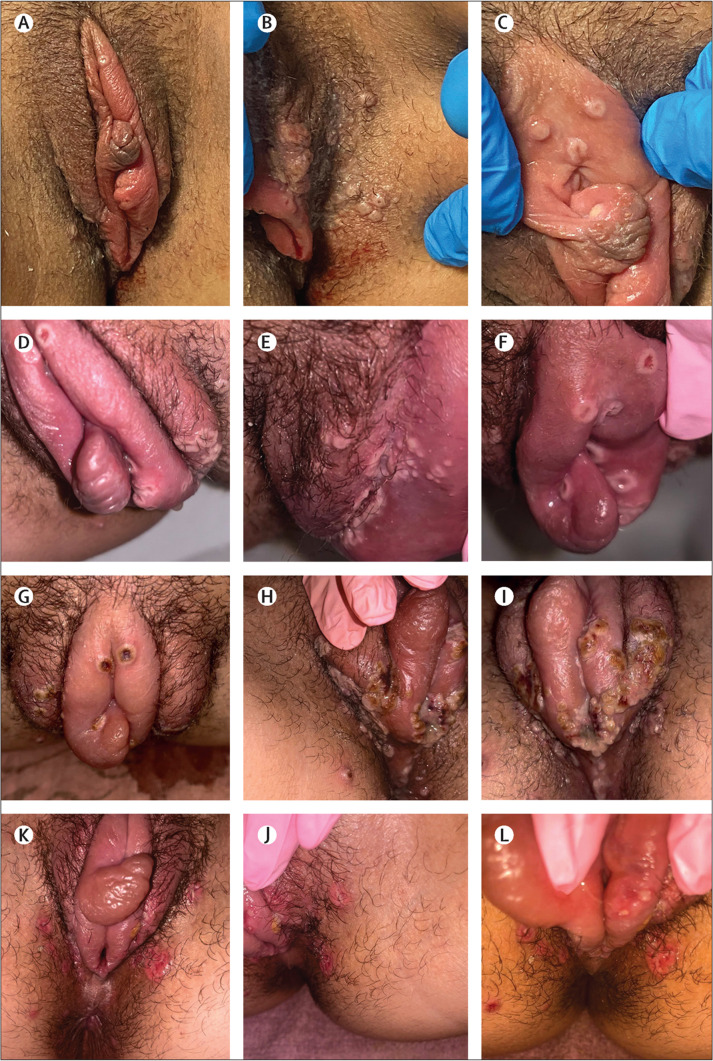
Figure 2.
Evolution of vulvovaginal manifestations of monkeypox in one individual
(A–C) Day 3 from symptom onset: vesicular or pustular lesions. (D–F) Day 5 from symptom onset: pustular lesions and erosion of the lesions, with initial swelling of the labia. (G–I) Day 8 from symptom onset: scab formation on most lesions with labial hypertrophy and severe oedema. (J–L) Day 14 from symptom onset: healing of most lesions and improvement of labial oedema.
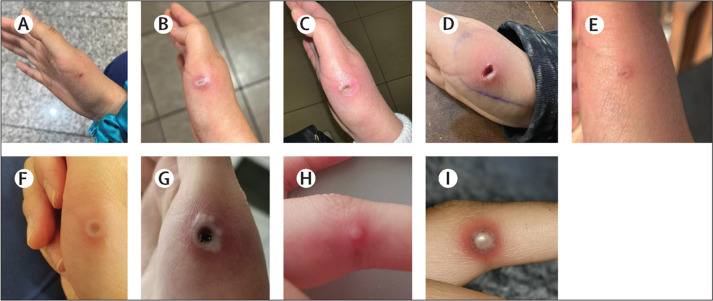
The median number of lesions reported was ten (IQR 5–24; range 1–200; N=126), and these numbers were similar for cis and trans women. In people in whom non-sexual routes of transmission were suspected (N=46), the median number of lesions (11 [IQR 5–40; range 1–100]) was very similar to that in people with suspected sexual routes of transmission (10 [IQR 4–20; range 1–200]), and no substantial differences were noted in the median incubation period (7 days [IQR 4–17] vs 7 days [4–10], respectively), the proportion of individuals with systemic symptoms before rash (23 [61%] of 38 vs 53 [63%] of 84, respectively), or the median duration of rash (18 days [13–21] vs 15 days [10–20], respectively). However, people with suspected non-sexual routes of transmission less commonly had anogenital lesions (21 [53%] of 40) or mucosal lesions (28 [65%] of 43) than people with suspected sexual transmission did (anogenital lesions 13 [15%] of 89; mucosal lesions 26 [34%] of 76; appendix p 9). Extragenital lesions are described in detail in two nurses infected through occupational exposure, who presented with lesions at the site of inoculation or skin contact (figure 3 ).
Figure 3.
Manifestations of monkeypox virus infection following occupational exposure in two nurses caring for individuals with monkeypox virus infection
(A–E) A scalpel wound on the right hand of a nurse (nurse 1), which occurred during the sampling of a monkeypox virus lesion in a person living with HIV. Monkeypox post-exposure prophylaxis was not prescribed because of a lack of availability. Nurse 1 received local wound care and HIV post-exposure prophylaxis. (F–I) Lesions on the hand of another nurse (nurse 2) exposed because of inadequate availability of personal protective equipment during handling of monkeypox virus samples. Images show thumb lesions on day 5 (F) and day 14 (G) after exposure and finger lesions day 14 (H) and day 15 (I) after exposure. Monkeypox virus post-exposure vaccination was not prescribed in the case of nurse 2 because of a lack of availability.
The proportion of samples from each anatomical site tested that were positive for monkeypox virus on PCR are shown in the appendix (p 8). Skin swabs were positive in 123 (100%) of 123 and vaginal swabs in 14 (100%) of 14 individuals from whom they were taken. Nasopharyngeal swabs were positive in 27 (73%) of 37, anorectal swabs in 19 (79%) of 24, and blood samples in three (75%) of four individuals from whom they were taken.
17 (13%) of 136 women (including 15 [20%] cis women and non-binary individuals and two [3%] trans women) were hospitalised. Reasons for admission included cellulitis, abscess, or bacterial superinfection; severe anorectal pain; odynophagia; infection control purposes; ocular lesion; and altered mental status and worsening left-sided weakness (table 2).
Monkeypox virus infection was treated with the antiviral tecovirimat in a higher proportion of trans women (21 [34%] of 62) than cis women and non-binary individuals (12 [16%] of 74). Six (4%) of 136 individuals overall received post-exposure vaccination. Pre-exposure vaccination (since May, 2022) was reported in eight (13%) of 62 trans women and two (3%) of 74 cis women and non-binary individuals. Tecovirimat treatment was more commonly given to individuals living with HIV (13 [35%] of 37) than to individuals not living with HIV (20 [20%] of 99), but the proportions of individuals hospitalised were similar between individuals living with HIV (four [11%] of 37) and individuals not living with HIV (13 [13%] of 99).
Of the two individuals who were pregnant at the time of at the time of writing this Article, both have ongoing pregnancies.
No deaths occurred in the cohort.
Discussion
Ours is the first case series to focus on and describe the risk factors and clinical presentations of monkeypox virus infection in cis and trans women and non-binary individuals assigned female at birth during the 2022 global outbreak. Previously published series or cohorts included almost exclusively men, primarily sexually active GBMSM, with the proportion of women ranging from 0% to 3·8%.3, 4, 5, 6, 7, 8, 9 Furthermore, most epidemiological surveillance datasets have not distinguished between cis and trans women, thereby prohibiting a detailed description and characterisation of any differences in these two subpopulations,21, 22 which are generally under-represented and under-reported in HIV and sexual health research.23 Although women account for a minority of infections reported in the current monkeypox outbreak (<5%),20 we anticipate that this might change as the outbreak evolves. It is important to collect and report on these infections to investigate sex and gender specificities in disease presentation. We observed many similarities in transmission and clinical characteristics in trans women to those that we previously reported for men,9 but noted several differences for cis women and non-binary individuals.
Inequalities and social determinants of health have been reported as a significant underlying problem, especially for Black and Latinx people in the USA, who have been disproportionately affected by monkeypox during the current outbreak .22 In our case series, White women and non-binary individuals represented only around a third of cases. Almost half of our cohort were trans women, a group more likely to be negatively affected by social determinants of health. Trans women have higher rates of HIV and STIs than cis women and non-binary individuals, which might influence the acquisition and clinical course of monkeypox virus infection, and also face barriers to accessing health care and social support.24, 25 Our data showed that a higher proportion of trans women engaged in sex work (55%) compared with the proportion of cis women and non-binary people (3%), suggesting higher levels of precarity and vulnerability, which might include factors like homelessness, injection drug use, and migrant status.
Although 121 (89%) of the 136 individuals in this global case series reported having sex with men, 59% of cis women and non-binary individuals had a regular male partner, whereas 73% of trans women had multiple male partners. Having multiple sexual partners was a common risk factor for monkeypox virus infection in previous series in men.1, 2, 3, 4, 5, 6, 7, 8, 9 Sexual contact was thought to be the most likely route of transmission in 74% of our cohort overall. This value is lower than the 95–100% reported in series of men.7, 8, 9 However, we found differences in trans women compared with cis women and non-binary individuals. Although clinicians were required to select a single choice for the suspected route of transmission, no trans women (of those with a reported suspected route of transmission) were thought to have acquired monkeypox virus infection outside of sexual contact. Attendance at LGBTQ+ Pride events and large gatherings has been a prominent association in men during the global outbreak (32–36% attendance); by contrast, only 7% of all individuals in our series attended LGBTQ+ Pride or other large gatherings within the month preceding symptom onset.8, 9
18 (24%) of 74 cis women and non-binary individuals were thought to have contracted monkeypox without sexual contact, including via occupational contact and close non-sexual contact within and outside the household. Notably, those who acquired monkeypox through non-sexual routes were less likely to have the anogenital lesions that have been characteristic of the global outbreak. More cis women and non-binary individuals were reported as having known contact with people with confirmed monkeypox virus infection (43%) than did trans women (10%).
HIV has emerged as a highly significant association during the multicountry 2022 outbreak, with the CDC reporting HIV co-infection in 46% of individuals with monkeypox in the USA,3 and other cohorts reporting an HIV prevalence of 28–47% among individuals diagnosed with monkeypox virus infection.3, 4, 5, 6, 7, 8, 9 In the current case series, the HIV prevalence among trans women was very high at 50% (31 of 62 individuals), and much lower (six [8%] of 74), but still substantial, in cis women and non-binary individuals. Similar to global case series in men, the median CD4 cell count in the 37 individuals living with HIV in the current case series was high at 600 cells per μL (IQR 487–805), and 36 (97%) were on antiretroviral therapy.
In reports of the current monkeypox virus outbreak, 57–95% of individuals with monkeypox virus infection and without HIV were reported to be using PrEP.3, 5, 6, 7, 8, 9 In the current series, PrEP use was reported in 19 (19%) of 99 all individuals without known HIV infection, but was markedly more common in trans women (18 [58%] of 31) than cis women and non-binary individuals (one [2%] of 68) without HIV.26 65% of trans women and 53% of cis women and non-binary individuals without known HIV were tested for HIV at presentation for monkeypox, suggesting that the strong association between a monkeypox virus diagnosis and HIV infection has, regrettably, still not translated into systematic HIV screening for all individuals with monkeypox virus infection.23 More concurrent STIs were documented in trans women (21%) than in cis women and in non-binary individuals (7%). However, it is notable that in some countries, screening for Chlamydia trachomatis and Neisseria gonorrhoeae infections were not routinely done because of laboratory restrictions relating to biosafety.
In the historic literature, children are known to be at a higher risk of severe disease if infected with monkeypox virus. Transmission from women (and men) to children has been a great concern during the current outbreak.27 In our case series, despite children being in the home of 19 (26%) of 74 cis women and non-binary people, only two transmissions to a child were reported, suggesting very limited chains of transmission, similar to what has been previously reported.11 Severe consequences of monkeypox for pregnancy outcomes have also previously been reported, with high risks of severe congenital infection, pregnancy loss, and maternal morbidity and mortality.28 We reported only two cases in pregnant individuals who have not yet delivered, and hence the outcomes are unknown.
Although 66% of trans women in the current series presented to sexual health or HIV clinics, this proportion was much lower for cis women and non-binary individuals (28%), who presented to a wide variety of medical specialties, reinforcing the importance of awareness of the clinical features of monkeypox beyond sexual health, infectious diseases, and HIV specialists. Given that case definitions have emphasised the increased risk of monkeypox virus infection among GBMSM, women might be less commonly diagnosed. This observation is supported by the higher rates of misdiagnosis and multiple appointments before confirmation of diagnosis in cis women and non-binary individuals compared with trans women. This finding also highlights the overlap between the clinical manifestations of monkeypox virus and other clinical syndromes (such as varicella; hand, foot, and mouth disease; herpes simplex; and syphilis) and the need for ongoing clinician education to better recognise and test for monkeypox virus infection.3 Trans women, as in the male case series, often had more localised infections with mucocutaneous involvement, not always accompanied by systemic symptoms.
The type of sexual activity individuals reported was varied: among cis women and non-binary individuals, 69% reported vaginal sex and 14% reported anal sex, whereas no trans women reported vaginal sex and all reported anal sex, correlating with the clinical presentation.29 This finding is not surprising given the very low prevalence of vaginoplasty in the USA (around 12%) and the likelihood of lower prevalence in low-income and middle-income countries.29 Vulvovaginal lesions were more common in individuals who engaged in vaginal sex, and anal lesions or proctitis were more common when anal sex was reported. This is consistent with findings from a Spanish cohort, which showed that GBMSM who reported anal-receptive sex were significantly more likely to have proctitis than those who did not were.8
Hospital admission rates within different cohorts have ranged from 2% to 13%.3, 4, 5, 6, 7, 8, 9 In our series, 13% of individuals overall were managed as inpatients. It is not clear why more trans women received tecovirimat in comparison with cis women and non-binary people, but it could relate to the higher prevalence of HIV infection or decreased access to antivirals in low-income and middle-income countries.
Several limitations of our study should be highlighted. Our data are derived from an observational retrospective convenience case series from countries with high numbers of monkeypox virus infections. It is neither a population-based sample nor a prospective cohort. We are, therefore, unable to assess how well our sample represents the entire population of women and non-binary individuals with monkeypox virus infection. Our series includes individuals in whom monkeypox virus infection was confirmed with various (locally approved) PCR platforms, except for nine individuals in France (where PCR testing was restricted in individuals in whom classic symptoms of and known contacts with monkeypox virus were reported). Individuals in this case series had symptoms that led them to seek medical care, thus individuals who were asymptomatic, had milder symptoms, were pauci-symptomatic, or had little or no access to medical care could have been missed.30 Women and non-binary individuals might also be less likely to be investigated for monkeypox virus given the inclusion of GBMSM as the main at-risk group in case definitions.
Due to data collection from multiple sites with different health systems across many countries, some characteristics might have been collected in a heterogeneous manner. In our series, based on the recorded medical history, clinicians were asked to designate a single route of likely transmission (or “unknown”), and might not have captured all the potential transmission routes for some individuals. Furthermore, the relatively few individuals included, especially when we distinguish cis women and non-binary people from trans women, limits the generalisability of our findings. Data on paediatric infections were reported by individuals affected and were not verified, and are therefore subject to the potential biases of patient-reported outcomes.
Finally, the incubation period was estimated on the basis of reported date of presumed exposure and the reported date of first symptom. As in other case series, the accuracy of an individual's recollection of their potential exposure and symptom dates cannot be confirmed, and early and subtle symptoms might have been unrecognised and under-reported, limiting the accuracy of the estimated incubation period.
In summary, this series provides new insights on the epidemiology and clinical characteristics of monkeypox virus infection in cis women, non-binary individuals assigned female at birth, and trans women worldwide, who previously have been a small, undifferentiated percentage in international surveillance reports. It also reinforces emerging data correlating sexual practices with the clinical presenting lesions. Indeed, the prominent genital and mucosal features, which have been a defining feature of the global outbreak in men, have been replicated in cis women and non-binary individuals and trans women, as has the pattern of fewer lesions than previously described in the historical literature. We hope these findings will help clinicians consider the diagnosis and avoid misdiagnosis of monkeypox in women and non-binary individuals wherever they present, and emphasise the importance of a detailed sexual history and testing for other STIs, including HIV.
Data sharing
Deidentified participant data collected, including individual participant data and will be made available from the corresponding author on reasonable request.
Declaration of interests
We declare no competing interests.
Acknowledgments
Acknowledgments
We thank all the women and non-binary individuals with monkeypox infection who agreed to allow their medical team to share their data with their permission; the medical staff who looked after the participants and all of the listed contributors for their efforts in collecting the data; Sebastian Noe for creating figure 1 the world map; Sophie Strachan from the Sophia Forum, a community organisation serving women concerned about HIV and sexual health, for providing a community perspective on the case series; the clinicians who did not have cases to submit who nonetheless enthusiastically supported the collaboration and signposted us to colleagues caring for women and non-binary individuals with monkeypox virus, most notably Cristina Mussini (Italy), Alexandra Calmy (Switzerland), Jean-Michel Molina (France), Itsik Levy (Israel), Juergen Rockstroh, Christoph Boesecke and Christoph Spinner (Germany), Omar Sued and Laila Woc-Colburn (for Latin America), Jan Gerstoft (Denmark), Magnus Gisslen (Sweden), Sanjay Bhagani (UK), Monica Gandhi, Tara Palmore, and Daniel Kuritzkes (USA); and Sanjay Bhagani for suggesting the case series. The views expressed herein are those of the authors and do not reflect the official policy or position of the Uniformed Services University of the Health Sciences, Henry M Jackson Foundation for the Advancement of Military Medicine; National Institutes of Health; Department of Health and Human Services; Defense Health Agency; Departments of the Air Force, Navy, or Army; Department of Defense; or US Government.
Contributors
CMO conceived and designed the study. CMO, MT, BKT, and EAM coordinated and engaged the global collaboration. CMO, JPT, DO, RP, MT, CPC, CMO, BKT, BC-R, RMG, and VA developed the case report form. JPT and CMO analysed and interpreted and vouch for the data. All authors except CMO, MT, VA, and JPT submitted cases. JPT did the statistical analysis. CMO, RP, JPT, SW, and JG contributed to the first draft of the manuscript. DM, CPC, and JG prepared the image library. All authors reviewed and edited the manuscript and vouch for the accuracy and completeness of the data. All authors were responsible for the final decision to submit for publication and have seen and approved the manuscript. CMO and JPT had full access to all data.
Supplementary Material
References
- 1.Centers for Disease Control and Prevention 2022 monkeypox outbreak global map. Oct 14, 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html
- 2.Wenham C, Eccleston-Turner M. Monkeypox as a PHEIC: implications for global health governance. Lancet. 2022 doi: 10.1016/S0140-6736(22)01437-4. published online Aug 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Curran KG, Eberly K, Russell OO, et al. HIV and sexually transmitted infections among persons with monkeypox—eight U.S. jurisdictions, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1141–1147. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Girometti N, Byrne R, Bracchi M, et al. Demographic and clinical characteristics of confirmed human monkeypox virus cases in individuals attending a sexual health centre in London, UK: an observational analysis. Lancet Infect Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoffmann C, Jessen H, Wyen C, et al. Clinical characteristics of monkeypox virus infections among men with and without HIV: a large outbreak cohort in Germany. HIV Med. 2022 doi: 10.1111/hiv.13378. published online Sept 4. [DOI] [PubMed] [Google Scholar]
- 6.Mailhe M, Beaumont AL, Thy M, et al. Clinical characteristics of ambulatory and hospitalized patients with monkeypox virus infection: an observational cohort study. Clin Microbiol Infect. 2022 doi: 10.1016/j.cmi.2022.08.012. published online Aug 23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Patel A, Bilinska J, Tam JCH, et al. Clinical features and novel presentations of human monkeypox in a central London centre during the 2022 outbreak: descriptive case series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tarín-Vicente EJ, Alemany A, Agud-Dios M, et al. Clinical presentation and virological assessment of confirmed human monkeypox virus cases in Spain: a prospective observational cohort study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thornhill JP, Barkati S, Walmsley S, et al. Monkeypox virus infection in humans across 16 countries—April–June 2022. N Engl J Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 10.Dashraath P, Nielsen-Saines K, Mattar C, Musso D, Tambyah P, Baud D. Guidelines for pregnant individuals with monkeypox virus exposure. Lancet. 2022;400:21–22. doi: 10.1016/S0140-6736(22)01063-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCollum AM, Damon IK. Human monkeypox. Clin Infect Dis. 2014;58:260–267. doi: 10.1093/cid/cit703. [DOI] [PubMed] [Google Scholar]
- 12.Doshi RH, Guagliardo SAJ, Doty JB, et al. Epidemiologic and ecologic investigations of monkeypox, Likouala department, Republic of the Congo, 2017. Emerg Infect Dis. 2019;25:281–289. doi: 10.3201/eid2502.181222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoff NA, Morier DS, Kisalu NK, et al. Varicella coinfection in patients with active monkeypox in the Democratic Republic of the Congo. EcoHealth. 2017;14:564–574. doi: 10.1007/s10393-017-1266-5. [DOI] [PubMed] [Google Scholar]
- 14.Jezek Z, Grab B, Szczeniowski M, Paluku KM, Mutombo M. Clinico-epidemiological features of monkeypox patients with an animal or human source of infection. Bull World Health Organ. 1988;66:459–464. [PMC free article] [PubMed] [Google Scholar]
- 15.Mwanbal PT, Tshioko KF, Moudi A, et al. Human monkeypox in Kasai Oriental, Zaire (1996–1997) Euro Surveill. 1997;2:33–35. doi: 10.2807/esm.02.05.00161-en. [DOI] [PubMed] [Google Scholar]
- 16.Rimoin AW, Kisalu N, Kebela-Ilunga B, et al. Endemic human monkeypox, Democratic Republic of Congo, 2001–2004. Emerg Infect Dis. 2007;13:934–937. doi: 10.3201/eid1306.061540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rimoin AW, Mulembakani PM, Johnston SC, et al. Major increase in human monkeypox incidence 30 years after smallpox vaccination campaigns cease in the Democratic Republic of Congo. Proc Natl Acad Sci USA. 2010;107:16262–16267. doi: 10.1073/pnas.1005769107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yinka-Ogunleye A, Aruna O, Dalhat M, et al. Outbreak of human monkeypox in Nigeria in 2017–18: a clinical and epidemiological report. Lancet Infect Dis. 2019;19:872–879. doi: 10.1016/S1473-3099(19)30294-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ogoina D, Iroezindu M, James HI, et al. Clinical course and outcome of human monkeypox in Nigeria. Clin Infect Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 20.Centers for Disease Control and Prevention Monkeypox cases by age and gender, race/ethnicity, and symptoms. Oct 12, 2022. https://www.cdc.gov/poxvirus/monkeypox/response/2022/demographics.html
- 21.Vaughan AM, Cenciarelli O, Colombe S, et al. A large multi-country outbreak of monkeypox across 41 countries in the WHO European Region, 7 March to 23 August 2022. Euro Surveill. 2022;27:2200620. doi: 10.2807/1560-7917.ES.2022.27.36.2200620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Philpott D, Hughes CM, Alroy KA, et al. Epidemiologic and clinical characteristics of monkeypox cases—United States, May 17–July 22, 2022. MMWR Morb Mortal Wkly Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Orkin C, Apea V. International Women's Day—how can I help? Lancet HIV. 2022;9:e228–e229. doi: 10.1016/S2352-3018(22)00060-1. [DOI] [PubMed] [Google Scholar]
- 24.Silva MST, Jalil EM, Torres TS, et al. Monkeypox and transgender women: the need for global initiative. Travel Med Infect Dis. 2022;50:102479. doi: 10.1016/j.tmaid.2022.102479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Gerwen OT, Jani A, Long DM, Austin EL, Musgrove K, Muzny CA. Prevalence of sexually transmitted infections and human immunodeficiency virus in transgender persons: a systematic review. Transgend Health. 2020;5:90–103. doi: 10.1089/trgh.2019.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Maharaj B, Karim QA. Engaging young women in Africa for PrEP use and adherence. Lancet HIV. 2021;8:e122–e123. doi: 10.1016/S2352-3018(20)30335-0. [DOI] [PubMed] [Google Scholar]
- 27.Khanna U, Bishnoi A, Singh K, Vinay K. Clinical considerations in pediatric cases of monkeypox. J Am Acad Dermatol. 2022 doi: 10.1016/j.jaad.2022.09.009. published online Sept 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbala PK, Huggins JW, Riu-Rovira T, et al. Maternal and fetal outcomes among pregnant women with human monkeypox infection in the Democratic Republic of Congo. J Infect Dis. 2017;216:824–828. doi: 10.1093/infdis/jix260. [DOI] [PubMed] [Google Scholar]
- 29.Sandy JJH, Susan R, Mara K, Lisa M, Ma'ayan A. The report of the 2015 U.S. Transgender Survey. December, 2016. https://transequality.org/sites/default/files/docs/usts/USTS-Full-Report-Dec17.pdf
- 30.Ferré VM, Bachelard A, Zaidi M, et al. Detection of monkeypox virus in anorectal swabs from asymptomatic men who have sex with men in a sexually transmitted infection screening program in Paris, France. Ann Intern Med. 2022;175:1491–1492. doi: 10.7326/M22-2183. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Deidentified participant data collected, including individual participant data and will be made available from the corresponding author on reasonable request.