Background.
Donor-derived cell-free DNA (dd-cfDNA) is a useful biomarker for the diagnosis of acute allograft injury within the first 1 to 2 y after lung transplant, but its utility for diagnosing chronic lung allograft dysfunction (CLAD) has not yet been studied. Understanding baseline dd-cfDNA kinetics beyond the initial 2 y posttransplant is a necessary first step in determining the utility of dd-cfDNA as a CLAD biomarker. We seek to establish baseline dd-cfDNA% levels in clinically stable lung allograft recipients who are >2 y posttransplant.
Methods.
We performed a prospective, single-center, observational study to identify plasma dd-cfDNA levels in clinically stable lung allograft recipients >2 y posttransplant.
Results.
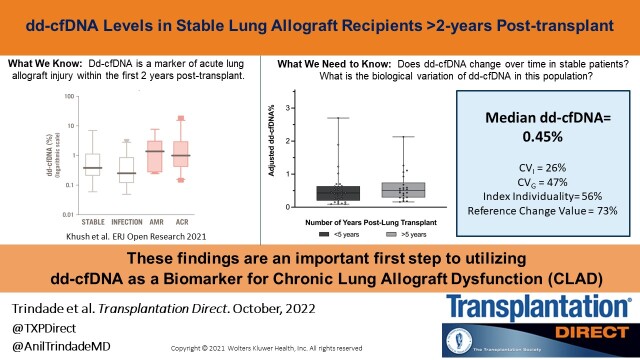
Fifty-one subjects were enrolled and ≥3 baseline dd-cfDNA measurements were acquired during a median of 252 d. The median baseline percent dd-cfDNA level in our cohort was 0.45% (interquartile range [IQR], 0.26–0.69). There were statistically significant differences in dd-cfDNA based on posttransplant duration (≤5 y posttransplant median 0.41% [IQR, 0.21–0.64] versus >5 y posttransplant median 0.50% [IQR, 0.33–0.76]; P < 0.02). However, the clinical significance of this small change in dd-cfDNA is uncertain because this magnitude of change is within the biologic test variation of 73%.
Conclusions.
This study is the first to define levels of dd-cfDNA in clinically stable patients who are >2 y post–lung transplant. These findings lay the groundwork for the study of dd-cfDNA as a possible biomarker for CLAD.
INTRODUCTION
Long-term survival after lung transplantation is limited, with a median survival of 6.7 y.1 The leading cause of mortality and allograft failure beyond the initial year posttransplant is chronic lung allograft dysfunction (CLAD).2 CLAD is progressive, irreversible fibrosis of the lung parenchyma with differences in prognosis based on the phenotype of bronchiolitis obliterans or restrictive allograft syndrome.3 CLAD develops because of an amalgam of acute insults over time; risk factors for CLAD include acute cellular rejection (ACR), antibody-mediated rejection, primary graft dysfunction, gastroesophageal reflux disease, and infections with Staphylococcus species, Pseudomonas, cytomegalovirus (CMV), or community-acquired respiratory viruses.4 By 5 y posttransplant, >50% of lung transplant recipients will develop CLAD.5 Although anti-inflammatory therapies can delay CLAD onset or stabilize lung function once CLAD is diagnosed, there are no known cures.6 As such, prevention and early diagnosis are paramount. Recent work has identified donor-derived‚ cell-free DNA (dd-cfDNA) as a novel biomarker with increased utility for CLAD prediction within the first 3 mo posttransplant.7
Circulating cell-free DNA are short fragments of double-stranded DNA 50 to 200 base pairs in length, released by apoptotic and necrotic cells. During times of allograft injury, dd-cfDNA levels increase, which upon cessation of injury levels return to baseline.8 Given differences in single-nucleotide polymorphisms across the genome between donor and recipient, the percent of DNA originating from the allograft can be determined without knowledge of either donor or recipient genotype.9 Levels of dd-cfDNA correlate with ACR in lung transplant recipients, with a sensitivity of 77%, specificity of 84%, positive predictive value of 60%, and negative predictive value of 90% at a threshold of 1%.10 dd-cfDNA is also a biomarker of antibody-mediated rejection.11
Given the diagnostic utility of dd-cfDNA for acute lung allograft dysfunction (ALAD), we postulated that dd-cfDNA might identify CLAD in patients who are >2 y posttransplant. However, little is known about dd-cfDNA kinetics in patients beyond the initial 2 y posttransplant. Therefore, an important initial step is to understand the relationship between time and baseline levels of dd-cfDNA in clinically stable patients at time points more distant from transplantation.12 Given that findings that altered senescence, telomere length, and other aging mechanisms may contribute to CLAD, it is possible that levels of dd-cfDNA in clinically stable lung allograft recipients might change with time.13-15 We performed a single-center, prospective, observational study in clinically stable lung allograft recipients >2 y posttransplant, measuring plasma dd-cfDNA% at routine ambulatory clinic appointments to define the trajectory of dd-cfDNA release beyond 2 y after lung transplantation. We included subjects with stable lung function, with an absence of symptoms of acute respiratory illness, and with ≥3 dd-cfDNA measurements separated by at least 1 mo. The primary outcome was median dd-cfDNA% for 2 distinct subcohorts based on time posttransplant (≤5 y, >5 y). Secondary outcomes included intra- and interindividual coefficients of variation (CVI and robust CVG, respectively), index of individuality (II), and reference change value (RCV). We hypothesized that baseline dd-cfDNA% in patients >2 y posttransplant would increase over time.
PATIENTS AND METHODS
Study Cohort
We performed a prospective, single-center, observational study at the Vanderbilt University Medical Center (VUMC) between January 1, 2021, and June 1, 2022 (VUMC Institutional Review Board #200233). Adult (aged >18 y) recipients of either single or bilateral transplants transplanted before January 1, 2019, and followed for routine care at VUMC who had stable lung function (absence of CLAD) compared with peak baseline average values (FEV1 >90%, forced vital capacity >90%, and forced expiratory flow between 25% and 75% of exhaled breath >75% peak baseline average, defined as the best 2 values measured >3 wk apart) were eligible for inclusion.16 After obtaining written informed consent, patients were enrolled in subgroups based on time posttransplant to ensure inclusion of patients over a wide range of times since transplant to allow for possible CLAD development; we planned to have an equal number of patients <5 and >5 y posttransplant.17 Patients were invited to participate via email at the start of the study and every 3 mo thereafter. We excluded subjects who were recipients of allogeneic stem cell transplants, had prior solid organ transplants (including prior lung transplant), could not participate in spirometry, had a diagnosis of a nondermatologic malignancy posttransplant, received organs from syngeneic donors, or were pregnant during the study period. At any point during the study, dd-cfDNA samples were excluded if patients had symptoms concerning for ALAD (such as acute onset of cough, fever, dyspnea), acute infiltrates on chest imaging, or a reduction in FEV1 >10% from baseline. Samples from patients with prior episodes of ALAD were included if there was resolution of symptoms and return of spirometry to pre-ALAD values and as long as >30 d had passed after the ALAD episode. Patients were only included in the analysis if there were ≥3 dd-cfDNA measurements, each >1 mo apart. We used Research Electronic Data Capture (REDCap) for data collection and management.18,19
Management of Patients Posttransplant
All patients received induction immunosuppression with basiliximab. Standard maintenance immunosuppression included a calcineurin inhibitor (tacrolimus preferred, goal trough 10–14 ng/mL within the first year posttransplant, followed by goal 8–12 ng/mL thereafter), an antiproliferative agent (mycophenolate mofetil 1000 mg twice daily preferred), and prednisone (tapered from 20 to 5 mg/d during the first 3 mo posttransplant).20 The antiproliferative was reduced or held if a patient had persistent or recurrent infection, malignancy, or cytopenias. Standard infection prophylaxis included trimethoprim–sulfamethoxazole, valganciclovir (for 6–12 mo posttransplant depending on donor/recipient CMV serostatus), and an antifungal (posaconazole preferred through 2 mo posttransplant). Patients were routinely followed in ambulatory clinic every 3 to 4 mo after the initial year posttransplant. Routine follow-up included laboratory analysis (complete metabolic panel, complete blood count with differential, CMV polymerase chain reaction, calcineurin inhibitor trough level, spirometry, and 2-view chest radiography).
Measurement of dd-cfDNA
Patients enrolled in the study underwent quantification of plasma dd-cfDNA at each routine ambulatory visit using AlloSure Lung kits (CareDx, Inc, Brisbane, CA). Briefly, this test uses next-generation sequencing to distinguish between dd-cfDNA and recipient-derived circulating cell-free DNA fragments in peripheral blood based on differences in single-nucleotide polymorphisms across 405 single-nucleotide polymorphisms. Peripheral venous blood was collected in 2 to 10 mL Streck containers, sealed according to package directions, and delivered to CareDx for processing, next-generation sequencing performance, and data analysis per the manufacturer’s proprietary methodology.9 Numeric results were reported to VUMC for each patient expressed as the percent of cell-free DNA that is donor-derived (dd-cfDNA%). For recipients of single lung transplants, dd-cfDNA% levels were adjusted by doubling the values.21 In studies of patients within 1 y of lung transplant, a value of >1% is interpreted as indicative of allograft injury.7,8
Assessment of Biologic Variation
We assessed intraindividual CVI, CVG (robust)), II, and RCVs for the entire cohort. CVI was calculated by dividing the standard deviation by the mean of the samples for each subject. Robust CVG was calculated by obtaining the median value of the median absolute deviation (|median-X1|) and dividing it by the median.22 II was the ratio of CVI/CVG. RCV was calculated using the formula RCV = 21/2 × 1.96 × (CVA2 + CVI2)½. Analytic coefficient of variation (CVA) for the AlloSure test has been previously established as 2.7%.23
Statistics
Categorical values were compared using the Pearson chi-square test or Fisher exact test, where appropriate. Continuous values were compared using the Mann-Whitney U test. Correlations between continuous variables were performed using Spearman’s coefficient. Statistical calculations were performed on Stata/BE‚ version 17.0 (College Station, TX).
RESULTS
Demographics of Study Cohort
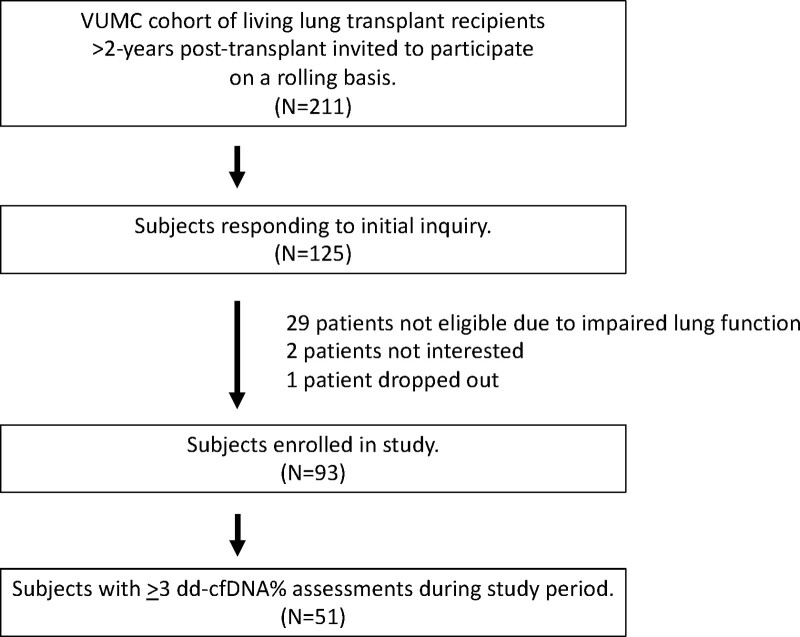
Among the cohort of 310 living lung transplant recipients followed at VUMC, 211 were >2 y posttransplant at the time of study enrollment. Patients in this eligible pool were invited to participate via email, of which 125 patients expressed interest. Ultimately, 93 patients were enrolled in the study (Figure 1). Twenty-nine patients did not meet enrollment criteria because of impaired lung function, 2 patients did not provide consent, and 1 patient dropped out before blood sample collection. Patients included in the analysis were a median of 1762 d posttransplant (interquartile range [IQR], 1104–2563); the subcohort of patients transplanted ≤5 y were a median of 1149 d posttransplant (IQR, 969–1492), whereas those transplanted >5 y were a median 2796 d posttransplant (IQR, 2191–3405). Fifty-one patients had ≥3 viable samples of dd-cfDNA within the study period and were included in the final analysis. Baseline demographics of subjects included in the analysis are listed in Table 1 and are divided into groups based on time posttransplant (≤5 y [N = 29], >5 y [N = 22]). The cohort that was ≤5 y posttransplant had statistically fewer episodes of ACR (50% versus 82%; P = 0.04).
FIGURE 1.
Study recruitment and enrollment. Flowchart describing participant screening and enrollment for this study. dd-cfDNA, donor-derived, cell-free DNA; VUMC, Vanderbilt University Medical Center.
TABLE 1.
Baseline demographics
| Variable | Time posttransplant at study enrolment | P | ||
|---|---|---|---|---|
| All patients(N = 51) | ≤5 ypost-TXP(N = 29) | >5 ypost-TXP(N = 22) | ||
| Time posttransplant at enrolment, d | 1762(1104–2563) | 1149(969–1492) | 2796(2191–3405) | <0.01 |
| Age at transplant, y | 58 (50–62) | 59 (51–63) | 58 (47–61) | 0.55 |
| Female sex | 20 (39) | 13 (45) | 7 (32) | 0.40 |
| Bilateral transplant | 43 (84) | 23 (79) | 20 (91) | 0.44 |
| White race | 43 (84) | 24 (83) | 19 (86) | 1.0 |
| Body mass index at transplant | 25.4 (20.6–27.8) | 23.2 (20.4–27.5) | 26.4 (23.1–28.3) | 0.25 |
| Lung allocation score at transplant | 39.5 (36.0–52.4) | 39.0 (35.8–52.0) | 40.0 (37.7–52.4) | 0.47 |
| CMV D+/R− | 12 (24) | 4 (14) | 8 (36) | 0.10 |
| Diagnosis group | 0.47 | |||
| Obstructive | 13 (25) | 8 (28) | 5 (23) | |
| Pulmonary vascular | 3 (6) | 2 (10) | 1 (5) | |
| Cystic fibrosis | 2 (4) | 2 (10) | 0 | |
| Interstitial | 33 (65) | 17 (59) | 16 (73) | |
| Total ischemic time, h | 5.46 (4.43–6.15) | 5.8 (4.22–6.33) | 5.28 (4.96–5.82) | 0.71 |
| Gastroesophageal reflux diagnosis posttransplant | 27 (53) | 16 (55) | 11 (50) | 0.78 |
| Esophageal dysmotility | 11 (22) | 5 (20) | 6 (27) | 0.50 |
| Primary graft dysfunction grade 3 at 72 h posttransplant | 5 (10) | 4 (15) | 1 (11) | 0.38 |
| Acute cellular rejection history | 33 (65) | 15 (50) | 18 (82) | 0.04 |
| De novo donor-specific antibody (MFI >3000) | 8 (16) | 5 (17) | 3 (16) | 1.0 |
Patients who underwent ≥3 assessments of donor-derived, cell-free DNA, each separated by >1 mo, who were asymptomatic with stable forced expiratory volume in 1 s were included (N = 51). Categorical values are expressed as number (%) and compared using the Pearson chi-square test vs the Fisher exact test, where appropriate. Continuous values are expressed as median (interquartile range) and compared using the Mann-Whitney U test or the Kruskal-Wallis test, where appropriate.
CMV, cytomegalovirus; D, donor; MFI, mean fluorescent intensity; R, recipient; TXP, transplant.
dd-cfDNA Levels in Patients >2 y Posttransplant
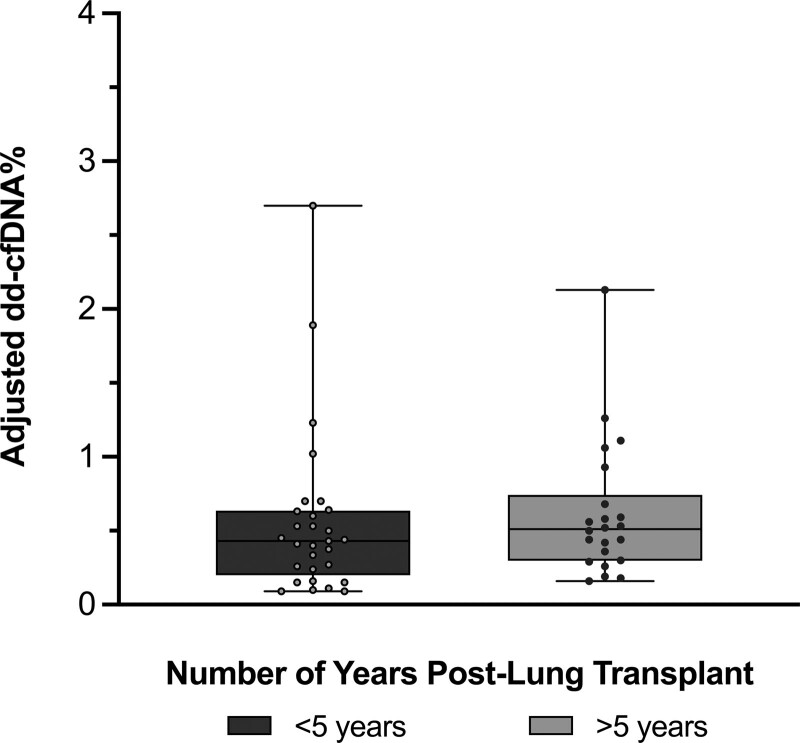
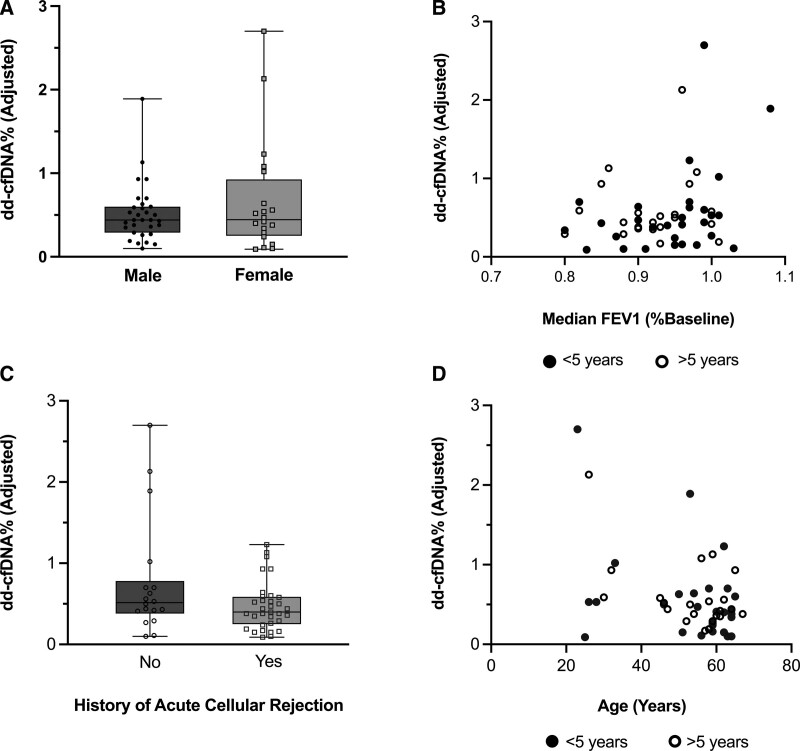
The mean number of samples per patient was 3.5. The median time between the first and last sample collected was 252 d (IQR, 212–328). There was no significant difference in median collection time between patients ≤5 y and those >5 y posttransplant (238 d [IQR, 205–307] versus 270 d [IQR, 233–324]; P = 0.08). The median dd-cfDNA% value overall was 0.45% (IQR, 0.26%–0.68%; Figure 2). The 95th percentile value was 1.54%‚ and the 97.5th percentile was 1.90%. There was a statistically significant difference in median dd-cfDNA% between patients ≤5 y posttransplant versus those >5 y (≤5 y group median 0.41% [IQR, 0.21%–0.64%], >5 y group median 0.50% [IQR, 0.33%–0.76%]; P = 0.02). There was no significant relationship between dd-cfDNA% and recipient sex, FEV1, previous history of ACR, or recipient age (Figure 3).
FIGURE 2.
Adjusted dd-cfDNA% in stable subjects >2 y post–lung transplant. Median dd-cfDNA% levels in clinically stable subjects are shown, categorized by time posttransplant. Values displayed are adjusted for single vs bilateral lung transplant status. Patients >5 y post–lung transplant have numerically greater dd-cfDNA% than patients ≤5 y post–lung transplant (P = 0.02). n = 29 in ≤5 y posttransplant group, n = 22 in >5 y posttransplant group. In the box and whisker plots, the following are depicted: median value = line, interquartile range = box, error bars = minimum and maximum values. Comparisons were performed using the Mann-Whitney U test. dd-cfDNA, donor-derived, cell-free DNA.
FIGURE 3.
Median dd-cfDNA% levels in clinically stable lung allograft recipients >2 y posttransplant do not vary by recipient sex, relative FEV1 at measurement, history of acute cellular rejection, or age. We assessed whether dd-cfDNA% levels were associated with demographics or relevant clinical history. A, Subject sex vs median dd-cfDNA% (P = 0.26). B, Median dd-cfDNA% vs median FEV1 (relative to the average peak baseline FEV1 posttransplant) at each measurement (r = 0.100, P = 0.48). C, Acute cellular rejection history vs dd-cfDNA% (P = 0.16). D, Subject age vs median dd-cfDNA% (r = −0.264, P = 0.06). Mann-Whitney U test was used to compare baseline dd-cfDNA% by sex and history of acute cellular rejection. Spearman’s correlation was used to assess the relationships between FEV1 or age and dd-cfDNA%. In the box and whisker plots, the following are depicted: median value = line, interquartile range = box, error bars = minimum and maximum values. dd-cfDNA, donor-derived, cell-free DNA; FEV1, forced expiratory volume in 1 s.
Biological Variation of dd-cfDNA in Lung Allograft Recipients >2 y Posttransplant
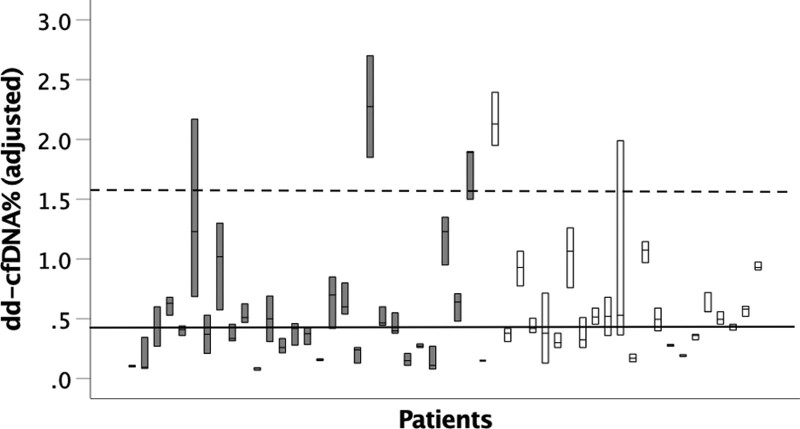
We determined the biological variability of plasma dd-cfDNA in this population (Figure 4). The coefficient of intraindividual variability (CVI) for the entire cohort was 26% (IQR, 17%–43%). Interindividual coefficient of variability (CVG) was estimated using the robust coefficient of variability and was 47%. II was 56%. RCV was 73%. These data demonstrate that the detected changes in dd-cfDNA% in patients ≤5 y posttransplant and those >5 y posttransplant fall within the biological variation of repeated testing.
FIGURE 4.
Variation in dd-cfDNA% measurements in stable patients >2 y post–lung transplant. dd-cfDNA% levels (≥3 samples) in clinically stable subjects are shown, categorized by time posttransplant. Values displayed are adjusted for single vs bilateral lung transplant status. n = 29 in ≤5 y posttransplant group (shown in gray boxes), n = 22 in >5 y posttransplant group (shown in white boxes). Intraindividual variation highlighted as median values (bars) and interquartile range (boxes). The solid line delineates median dd-cfDNA for the entire cohort, whereas the dashed line shows the 95th percentile. dd-cfDNA, donor-derived, cell-free DNA.
DISCUSSION
In a prospective, single-center, observational cohort study, we demonstrate that the median dd-cfDNA% in clinically stable lung allograft recipients >2 y posttransplant is 0.45%. Although there is a statistically significant change in median dd-cfDNA% based on time posttransplant, these values are within the normal biologic variation for this test.
Our study defines baseline levels of dd-cfDNA% in patients >2 y post–lung transplant. Levels in this time frame are similar to the baseline values of dd-cfDNA in patients within the first 2 y after lung transplantation (<0.5%).7,10,24 We initially hypothesized that dd-cfDNA may increase over time because of allograft aging processes related to cellular senescence, telomere biology, and other mechanisms. Although there is a statistically significant increase in dd-cfDNA% in the subcohort of patients >5 y posttransplant, the clinical significance of this small change is unclear because these levels are still within the variability of the test in this population. One possibility is that these patients have a greater propensity for subclinical CLAD, independent of normal aging processes, because of higher rates of previously resolved ACR. Assessing levels in a much larger cohort over time may elucidate whether this incremental increase in dd-cfDNA% reflects longitudinal allograft cellular turnover.
Keller et al23 demonstrated that normal biological variation within 2 y of transplant is 70%, up to the 95th percentile of 1.0. Our data show a similar amount of intraindividual variation in patients beyond 2 y posttransplant. We found that interindividual variation is numerically greater in our cohort of patients >2 y posttransplant than reported values.23
Interestingly, several patients had dd-cfDNA >1.50%, despite having normal baseline spirometry and being asymptomatic. Although these values may be falsely positive, an alternative explanation is that dd-cfDNA may detect subclinical allograft injury in this population. We did assess dd-cfDNA versus relative FEV1 (Figure 3D) to determine whether there was a direct correlation between dd-cfDNA and subclinical chronic injury states (“pre-CLAD”); there did not seem to be any relationship, although such an analysis is limited by our inclusion criteria that selected for patients without CLAD. Moreover, this trial was designed as a noninterventional study, so we did not direct management based on results of dd-cfDNA. Future studies should test the feasibility of using dd-cfDNA as a tool to detect subclinical injury by incorporating a diagnostic schema that includes bronchoscopy with transbronchial biopsies, screening for de novo donor-specific antibodies, and assessments for gastroesophageal reflux disease or tobacco use. Notably, it is possible that variability in recipient-derived cfDNA could also impact the proportion of total cfDNA that is donor-derived. For example, nonpulmonary infection or extreme exercise could reduce dd-cfDNA% by increased recipient-derived cfDNA release. In kidney transplant patients, there are some data suggesting that recipient cfDNA increases over time; this dilution results in a decrease of dd-cfDNA percentage.25
Our study has several strengths. We focused specifically on defining the characteristics of plasma dd-cfDNA over time after transplant in seemingly healthy lung transplant recipients. Our study cohort is relatively large compared with other studies of dd-cfDNA in lung transplant recipients. Analysis of serial samples in each patient increases the likelihood that dd-cfDNA in our cohort truly represents clinical stability. The main limitations of our study are its single-center design and the lack of additional objective measurements of potential subclinical graft injury. We included samples from 3 patients with previous ALAD with a documented resolution of symptoms and recovery of changes in lung function. These patients may not only contribute to the variability of dd-cfDNA beyond 2 y after transplant but also reflect a real-world patient population suggesting that dd-cfDNA levels at distant times after transplant may have clinical utility. Finally, our findings are based on the use of the AlloSure test kit and may not apply to other platforms for assaying dd-cfDNA, especially regarding biologic variation. However, dd-cfDNA% thresholds that define acute lung allograft injury have been comparable across different propriety platforms.8,24
In conclusion, this study defines baseline levels of dd-cfDNA in clinically stable patients who are >2 y post–lung transplant. We show that median values of 0.45% with biologic variation of 73% up to 1.54 are the baseline in this population. These findings lay the groundwork for dd-cfDNA for future studies to test if dd-cfDNA is a powerful tool for early, noninvasive detection of CLAD.
ACKNOWLEDGMENTS
The authors thank Dr Anna Hemnes, Brijesh Patel, and the Pulmonary Research Infrastructure for Shared Resources and Mentorship group for their help with study logistics. Study data were collected and managed using REDCap hosted at VUMC. REDCap is a secure, web-based software platform designed to support data capture for research studies and is funded through grant support from National Center for Advancing Translational Sciences/National Institutes of Health (UL1 TR000445). They thank their VUMC Lung Transplant Program patients and loved ones for their support of our research efforts.
Footnotes
This work was supported by an investigator-initiated trial grant by CareDx, Inc, (Brisbane, CA) to A.J.T. and by NIH K08 HL136888 to C.M.S..
A.J.T. is a member of the Lung Scientific Advisory Board for CareDx, Inc. The authors had full oversight over study design, data collection and analysis, and article preparation.
A.J.T. designed the study, performed analysis, and prepared the article. A.J.T., K.C., and A.M. collected the data. C.T.D., E.S.L., K.A.M., S.G.N., H.H., I.M.R., M.B., D.B.E., and C.M.S. provided critical discussion of the data and article. All authors approved the final article.
Supplemental digital content (SDC) is available for this article. Direct URL citations appear in the printed text, and links to the digital files are provided in the HTML text of this article on the journal’s Web site (www.transplantationdirect.com).
REFERENCES
- 1.Rana A, Gruessner A, Agopian VG, et al. Survival benefit of solid-organ transplant in the United States. JAMA Surg. 2015;150:252–259. [DOI] [PubMed] [Google Scholar]
- 2.Bos S, Vos R, Van Raemdonck DE, et al. Survival in adult lung transplantation: where are we in 2020? Curr Opin Organ Transplant. 2020;25:268–273. [DOI] [PubMed] [Google Scholar]
- 3.Verleden SE, Vos R, Vanaudenaerde BM, et al. Chronic lung allograft dysfunction phenotypes and treatment. J Thorac Dis. 2017;9:2650–2659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thabut G, Mal H. Outcomes after lung transplantation. J Thorac Dis. 2017;9:2684–2691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levy L, Huszti E, Renaud-Picard B, et al. Risk assessment of chronic lung allograft dysfunction phenotypes: validation and proposed refinement of the 2019 International Society for Heart and Lung Transplantation classification system. J Heart Lung Transplant. 2020;39:761–770. [DOI] [PubMed] [Google Scholar]
- 6.Benden C, Haughton M, Leonard S, et al. Therapy options for chronic lung allograft dysfunction-bronchiolitis obliterans syndrome following first-line immunosuppressive strategies: a systematic review. J Heart Lung Transplant. 2017;36:921–933. [DOI] [PubMed] [Google Scholar]
- 7.Agbor-Enoh S, Wang Y, Tunc I, et al. Donor-derived cell-free DNA predicts allograft failure and mortality after lung transplantation. EBioMedicine. 2019;40:541–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khush KK, De Vlaminck I, Luikart H, et al. Donor-derived, cell-free DNA levels by next-generation targeted sequencing are elevated in allograft rejection after lung transplantation. ERJ Open Res. 2021;7:00462–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Seeto RK, Fleming JN, Dholakia S, et al. Understanding and using AlloSure donor derived cell-free DNA. Biophys Rev. 2020;12:917–924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jang MK, Tunc I, Berry GJ, et al. Donor-derived cell-free DNA accurately detects acute rejection in lung transplant patients, a multicenter cohort study. J Heart Lung Transplant. 2021;40:822–830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Agbor-Enoh S, Jackson AM, Tunc I, et al. Late manifestation of alloantibody-associated injury and clinical pulmonary antibody-mediated rejection: evidence from cell-free DNA analysis. J Heart Lung Transplant. 2018;37:925–932. [DOI] [PubMed] [Google Scholar]
- 12.Wu AC, Kiley JP, Noel PJ, et al. Current status and future opportunities in lung precision medicine research with a focus on biomarkers. An American Thoracic Society/National Heart, Lung, and Blood Institute research statement. Am J Respir Crit Care Med. 2018;198:e116–e136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faust HE, Golden JA, Rajalingam R, et al. Short lung transplant donor telomere length is associated with decreased CLAD-free survival. Thorax. 2017;72:1052–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Courtwright AM, Lamattina AM, Takahashi M, et al. Shorter telomere length following lung transplantation is associated with clinically significant leukopenia and decreased chronic lung allograft dysfunction-free survival. ERJ Open Res. 2020;6:00003–02020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Parker SM, Goriwiec MR, Borthwick LA, et al. Airway epithelial cell senescence in the lung allograft. Am J Transplant. 2008;8:1544–1549. [DOI] [PubMed] [Google Scholar]
- 16.Verleden GM, Raghu G, Meyer KC, et al. A new classification system for chronic lung allograft dysfunction. J Heart Lung Transplant. 2014;33:127–133. [DOI] [PubMed] [Google Scholar]
- 17.Tissot A, Danger R, Claustre J, et al. Early identification of chronic lung allograft dysfunction: the need of biomarkers. Front Immunol. 2019;10:1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Harris PA, Taylor R, Thielke R, et al. Research electronic data capture (REDCap)—a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42:377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harris PA, Taylor R, Minor BL, et al. ; REDCap Consortium. The REDCap consortium: building an international community of software platform partners. J Biomed Inform. 2019;95:103208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheffert JL, Raza K. Immunosuppression in lung transplantation. J Thorac Dis. 2014;6:1039–1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keller MB, Meda R, Fu S, et al. Comparison of donor-derived cell-free DNA between single versus double lung transplant recipients. Am J Transplant. 2022;22:2451–2457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bromberg JS, Brennan DC, Poggio E, et al. Biological variation of donor-derived cell-free DNA in renal transplant recipients: clinical implications. J Appl Lab Med. 2017;2:309–321. [DOI] [PubMed] [Google Scholar]
- 23.Keller M, Mutebi C, Shah P, et al. Biological variation of donor-derived cell-free DNA in stable lung transplant recipients. J Appl Lab Med. 2022;7:901–909. [DOI] [PubMed] [Google Scholar]
- 24.Rosenheck JP, Ross DJ, Botros M, et al. Clinical validation of a plasma donor-derived cell-free DNA assay to detect allograft rejection and injury in lung transplant. Transplant Direct. 2022;8:e1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schütz E, Asendorf T, Beck J, et al. Time-dependent apparent increase in dd-cfDNA percentage in clinically stable patients between one and five years following kidney transplantation. Clin Chem. 2020;66:1290–1299. [DOI] [PubMed] [Google Scholar]