PURPOSE
Photon involved-field radiotherapy (IFRT) is the standard-of-care radiotherapy for patients with leptomeningeal metastasis (LM) from solid tumors. We tested whether proton craniospinal irradiation (pCSI) encompassing the entire CNS would result in superior CNS progression-free survival (PFS) compared with IFRT.
PATIENTS AND METHODS
We conducted a randomized, phase II trial of pCSI versus IFRT in patients with non–small-cell lung cancer and breast cancers with LM. We enrolled patients with other solid tumors to an exploratory pCSI group. For the randomized groups, patients were assigned (2:1), stratified by histology and systemic disease status, to pCSI or IFRT. The primary end point was CNS PFS. Secondary end points included overall survival (OS) and treatment-related adverse events (TAEs).
RESULTS
Between April 16, 2020, and October 11, 2021, 42 and 21 patients were randomly assigned to pCSI and IFRT, respectively. At planned interim analysis, a significant benefit in CNS PFS was observed with pCSI (median 7.5 months; 95% CI, 6.6 months to not reached) compared with IFRT (2.3 months; 95% CI, 1.2 to 5.8 months; P < .001). We also observed OS benefit with pCSI (9.9 months; 95% CI, 7.5 months to not reached) versus IFRT (6.0 months; 95% CI, 3.9 months to not reached; P = .029). There was no difference in the rate of grade 3 and 4 TAEs (P = .19). In the exploratory pCSI group, 35 patients enrolled, the median CNS PFS was 5.8 months (95% CI, 4.4 to 9.1 months) and OS was 6.6 months (95% CI, 5.4 to 11 months).
CONCLUSION
Compared with photon IFRT, we found pCSI improved CNS PFS and OS for patients with non–small-cell lung cancer and breast cancer with LM with no increase in serious TAEs.
INTRODUCTION
Leptomeningeal metastasis (LM), the spread of a malignancy into the CSF-filled leptomeningeal space surrounding the brain and spinal cord, is associated with marked morbidity and mortality. LM is clinically detected in 5%-10% of patients with solid tumors1 but is frequently underdiagnosed; approximately 30% of all patients with malignancy and neurologic symptoms harbor LM at autopsy.2-4 The incidence of LM is rising, likely because of improved imaging techniques5 and systemic disease control.6-8 LM incidence varies widely depending on histology, with lung cancer and breast cancer being most associated with LM (5%-25%), followed by melanoma (6%-18%) and gastrointestinal malignancies (4%-14%).3,5,9,10
CONTEXT
Key Objective
Solid tumor leptomeningeal metastasis (LM) is associated with significant morbidity and mortality. Standard-of-care photon involved-field radiotherapy (IFRT) is effective in relieving local symptoms because of LM; however, it does not stop progression of LM along the neuroaxis. We tested whether proton craniospinal irradiation (pCSI) would improve progression-free survival compared with IFRT. This phase II trial is the first randomized evaluation of the optimal radiotherapy approach for LM.
Knowledge Generated
pCSI resulted in significantly improved CNS progression-free survival compared with IFRT in patients with metastatic non–small-cell lung cancer and breast cancer with LM, meeting the primary end point of the study, which led to early discontinuing of the trial at planned interim analysis. pCSI also showed overall survival advantage compared with IFRT with no increase in high-grade adverse events.
Relevance
Our results support the superior efficacy in CNS disease control of pCSI compared with standard-of-care IFRT in patients with non–small-cell lung cancer and breast cancer LM. Additional investigation is warranted to evaluate the observed overall survival benefit of pCSI.
Once within the leptomeningeal space, tumor cells disseminate throughout the CNS. As a result, patients with LM may develop multiple debilitating neurologic dysfunctions that can be life-threatening. Treatments for LM are palliative with the goals of stabilizing or improving neurologic symptoms. The prognosis for patients with LM is poor. Untreated LM can lead to death within 4-6 weeks. With treatments, the overall survival (OS) remains poor at between 4-6 months.5,11 Responses to therapies and outcomes vary widely and are affected by performance status, tumor histology, and disease outside the CNS.10,12-16
Radiotherapy (RT) effectively relieves local symptoms because of LM, and, in the form of photon involved-field RT (IFRT), is commonly used to treat symptomatic or bulky disease sites.17-19 Because LM disseminates throughout the entire CNS compartment, standard-of-care IFRT, such as whole-brain radiotherapy (WBRT) or focal spine RT, cannot halt the progression of LM along the entire neuroaxis. Craniospinal irradiation (CSI), conversely, treats the whole leptomeningeal compartment and may therefore achieve superior symptom and disease control. In fact, photon CSI has demonstrated efficacy with LM in retrospective studies, but toxicities limit the widespread applicability of this technique.20 When delivering photon radiation to the entire neuroaxis, photons exit the body anteriorly, exposing the entire spinal column and anterior organs to radiation. This can lead to unacceptable toxicities in patients who already have significant morbidities. By contrast, when using protons for CSI, most commonly, protons are planned to deposit the bulk of their energy at the last few millimeters of their range, resulting in diminished delivery of radiation beyond the neuroaxis and significantly less toxicity.21
We had previously identified in a phase Ib clinical trial that hypofractionated proton CSI (pCSI) at a dose of 3 Gy × 10 fractions is a safe treatment for patients with solid tumor LM with promising long-term CNS control observed.22 Here, we report the results of a randomized phase II trial of pCSI versus standard-of-care photon IFRT in patients with LM.
PATIENTS AND METHODS
Study Design and Participants
We conducted an open-label, randomized, phase II trial to assess the efficacy of pCSI in patients with pathologically proven solid tumor malignancies with LM established radiographically and/or through CSF cytology. Additional eligibility criteria included Karnofsky performance score ≥ 60; ability in developing a treatment plan respective of normal tissue tolerance; and adequate bone marrow function. Patients with treated and untreated parenchymal brain metastases were eligible. Systemic therapy was held during protocol therapy. Patients resumed or began new systemic therapy per physician's choice after protocol therapy. The Protocol (online only) was approved by the Memorial Sloan Kettering Cancer Center institutional review board, registered with ClinicalTrials.gov (identifier: NCT04343573), and completed in accordance with the protocol and Good Clinical Practice guidelines. All patients provided informed consent.
Random Assignment and Masking
Patients with metastatic non–small-cell lung cancer (NSCLC) or breast cancer were randomly assigned in a 2:1 ratio to pCSI versus standard-of-care photon IFRT, stratified by histology (NSCLC v breast) and systemic disease status (progressive v stable/none). Patients with other solid tumor histologies were enrolled to an exploratory pCSI group without random assignment.
Procedures
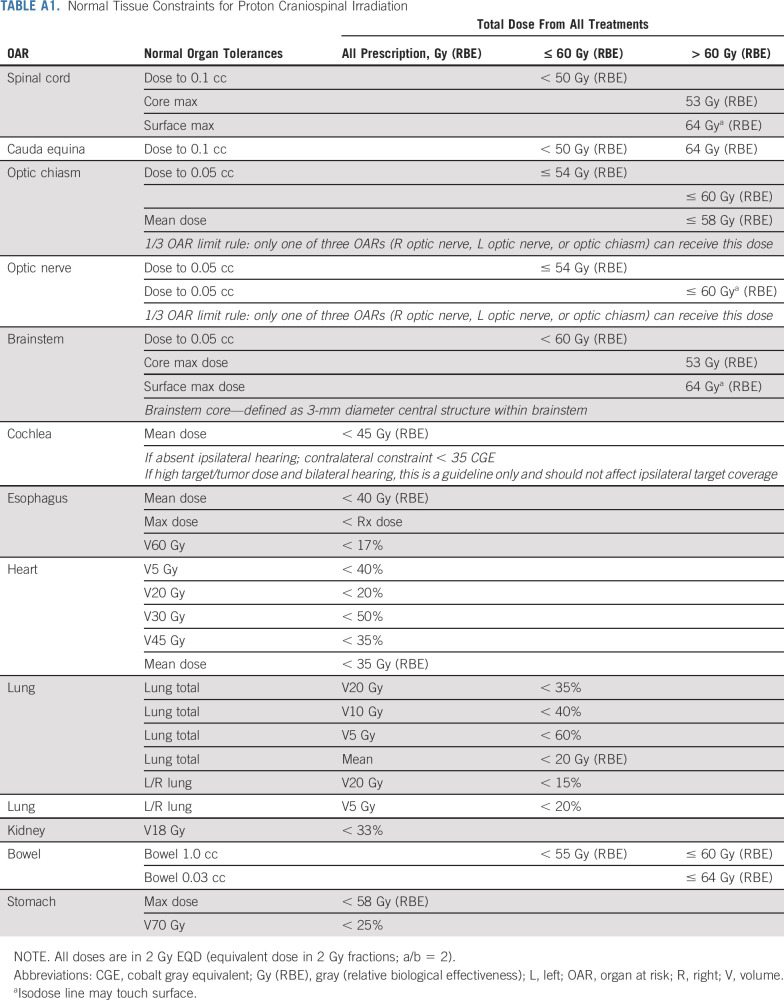
Pretreatment evaluations included history and physical examination; complete blood count, electrolytes, and renal and liver function tests; lumbar puncture for CSF assessment, including cytology and circulating tumor cell (CTC) using CellSearch platform23; and contrast-enhanced magnetic resonance imaging of the neuroaxis. Protocol RT was delivered at a dose of 3 Gy × 10 daily fractions, with RT planning time ≤ 2 weeks per protocol. Cumulative dose-volume constraint guidelines for organs at risk were followed (Appendix Table A1, online only). Patients who received pCSI were treated with pencil beam scanning proton therapy to the entire CNS compartment.24 Patients who received photon IFRT, including WBRT and/or focal spine RT, underwent three-dimensional planning to symptomatic sites. All patients received memantine prophylaxis.25 During protocol therapy, patients underwent weekly clinical assessment and complete blood count. Patients were followed every 3 months with repeat neuroaxis magnetic resonance imaging and lumbar puncture for up to 1 year or until CNS progression. Treatment-related adverse events (TAEs, Common Terminology Criteria for Adverse Events version 5.0) were assessed weekly during protocol therapy and at each follow-up.
Outcomes
The primary end point was CNS progression-free survival (CNS PFS), defined as the time from random assignment to CNS disease progression or death among patients with NSCLC and breast cancer randomly assigned to pCSI versus IFRT. Patients were censored at the last follow-up (April 11, 2022) or the date of withdrawal from the study per protocol. Patients with new neurologic deficits not related to therapeutic intervention, progressive radiographic change using Leptomeningeal Assessment in Neuro-Oncology scale,26 and/or new positive CSF cytology after previously negative CSF cytology were considered to have progression. Enrollment of 81 patients with NSCLC and breast cancer LM would provide a one-sided α of .025 and power of 0.8 on the basis of stratified log-rank test for an estimated CNS PFS of 3 months for IFRT and 6 months for pCSI. An interim analysis was planned after half of the expected events were observed (25 events). Lan-DeMets spending function was used. At the interim analysis, if P < .0015 favoring pCSI, the trial would stop for efficacy. If P > .288, the trial would stop for futility. At the final analysis, we would declare the pCSI superior if P < .024 favoring pCSI.
Secondary end points included OS, defined as the time from random assignment to death or last on study follow-up, and TAEs. Protocol-defined exploratory end points included outcomes for patients with other solid tumor histologies enrolled on the exploratory pCSI group, and evaluation of the utility of CSF CTC as a potential biomarker.
Statistical Analysis
The Kaplan-Meier method was used to estimate CNS PFS and OS. The cumulative incidence was estimated for time to CNS progression (CNS TTP) with death treated as a competing event. For patients in the randomized groups, stratified log-rank test and stratified Gray's test were used to compare CNS TTP, CNS PFS, and OS. Multivariable Cox proportional hazard regressions were performed to examine associations of CNS PFS and OS with treatment, age, Karnofsky performance score, histology, and systematic disease status. Backward variable selection on the basis of z test was applied. TAEs were reported for all patients. Incidences of high-grade TAEs (grade ≥ 3 nonhematologic toxicities and grade ≥ 4 hematologic toxicities) were compared between the randomized groups using the chi-squared test with Rao and Scott's27 second-order correction to take into account multiple adverse events per patient.
As an exploratory assessment, CSF CTC count and its correlation with outcomes were analyzed. Baseline CTC count was analyzed as a categorical predictor dichotomized by the median count. We tested whether there was a significant interaction between baseline CTC count and treatment groups, and reported association of CTC count with CNS TTP, CNS PFS, and OS. We then analyzed CTC counts over time (baseline and after protocol therapy) as described previously with joint modeling of longitudinal and survival outcomes with shared random effects and an unspecified baseline risk function,23 allowing modeling of a linear mixed-effect model that is then used as a predictor in a Cox proportional hazard model. The interpretation of the joint model coefficients reveals whether trends in CTC count over time are associated with outcomes.28 We modeled the relationship between CTC counts over time and CNS TTP (censored at death or no CNS progression), CNS PFS, and OS, comparing the randomized pCSI and IFRT groups, adding fixed-effect terms to the model for treatment arm, histology, and their interaction. The results from models treating CTC count as a continuous covariate were reported per 10-cell increment. The association between treatment group and histology was determined using analysis of variance of nested JM models. All statistical analyses were performed in R version 4.1 (The R Foundation for Statistical Computing, Vienna, Austria).
RESULTS
Between April 16, 2020, and October 11, 2021, 63 NSCLC and breast cancer patients with LM were randomly assigned to either pCSI (n = 42) or photon IFRT (n = 21), and 35 patients with other solid tumor histologies with LM were enrolled to an exploratory pCSI group (Fig 1). Baseline demographic and relevant clinical variables were not different between the randomized groups (Table 1).
FIG 1.
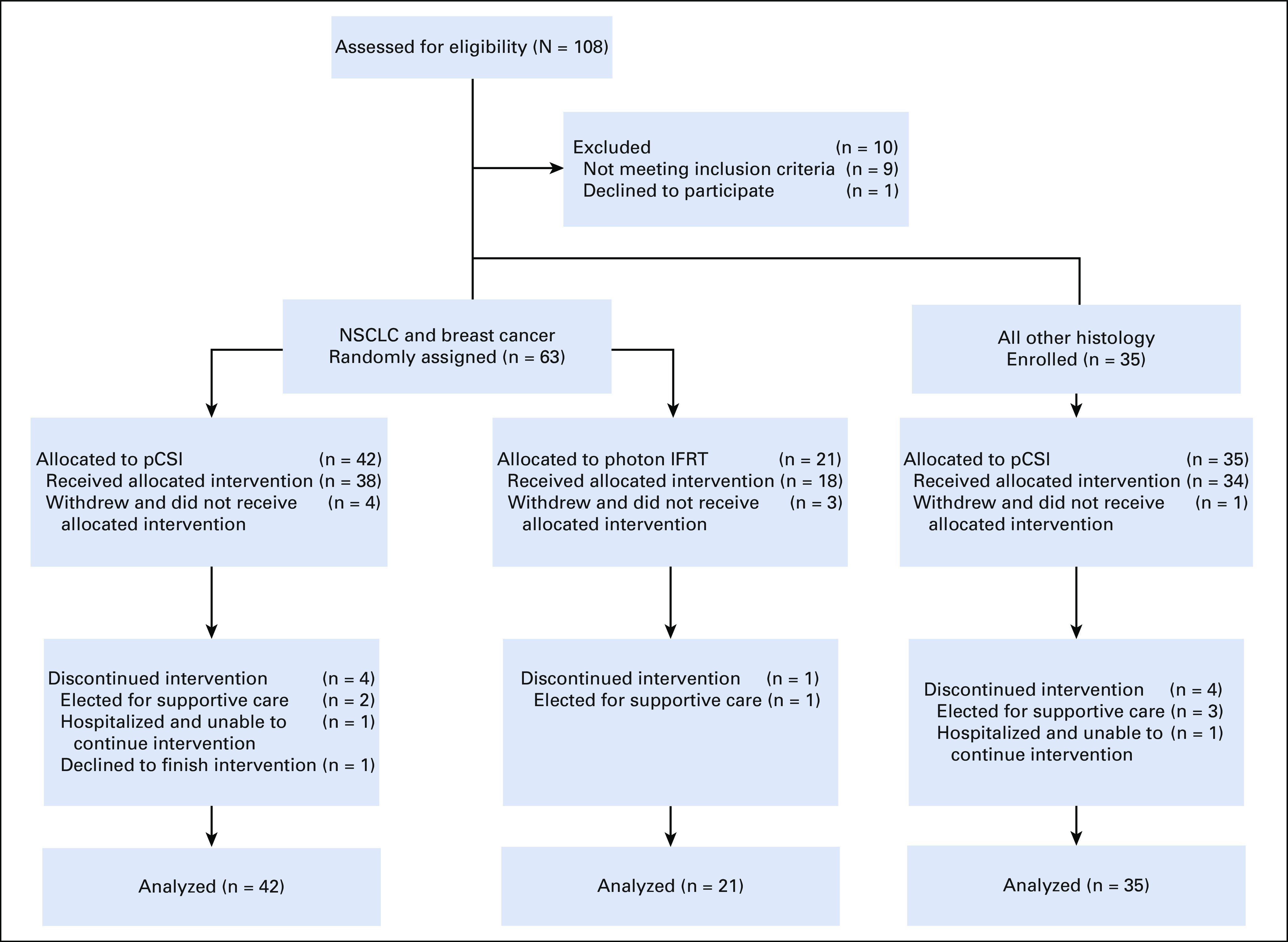
CONSORT diagram. IFRT, involved-field radiotherapy; NSCLC, non–small-cell lung cancer; pCSI, proton craniospinal irradiation.
TABLE 1.
Clinical Characteristics of All Evaluable Patients (N = 98)
Randomized pCSI Versus Photon IFRT for Patients With NSCLC and Breast Cancer LM
At the time of analysis, 12/42 patients in the pCSI group experienced CNS progression (five with radiographic and clinical progression, two with clinical progression, two with CSF cytologic progression, two with radiographic progression, and one with radiographic and CSF cytologic progression), and 16/21 patients in the IFRT group experienced CNS progression (seven with radiographic and clinical progression, four with clinical progression, three with radiographic progression, and two with CSF cytologic and clinical progression). Of the 16/42 pCSI patients who died, eight died with systemic disease progression, four with CNS and systemic disease progression, and four with CNS progression. Of the 14/21 IFRT patients who died, nine died with CNS progression, and five died with CNS and systemic disease progression. The median duration of follow-up was 9.3 months (95% CI, 7.8 to 17.6 months; range, 0-18.9 months).
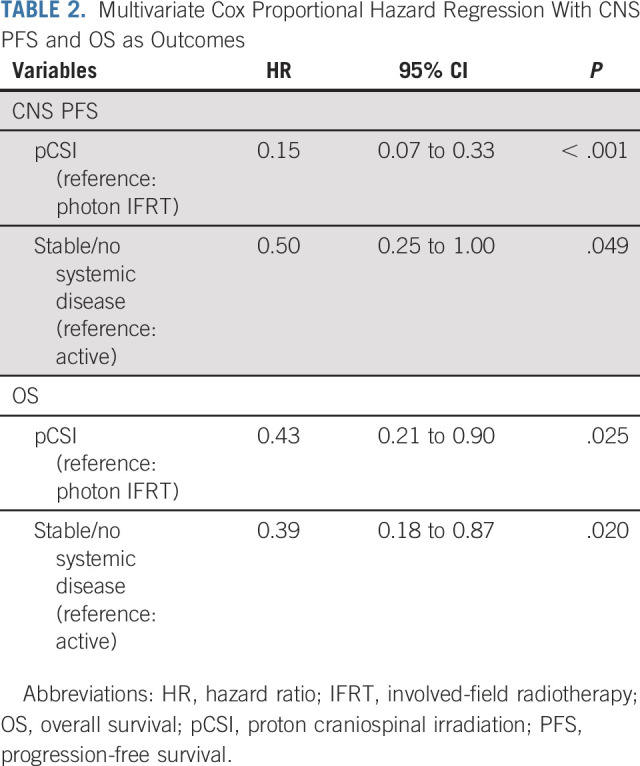
pCSI patients had significantly longer estimated CNS TTP compared with those who received photon IFRT (P < .001), with 6.3% (95% CI, 1.1 to 18) and 22% (95% CI, 9.5 to 38) of pCSI patients and 70% (95% CI, 40 to 87) and 92% (95% CI, 37 to 99) of IFRT patients experiencing CNS progression at 3 months and 6 months, respectively (Fig 2A). Significant improvement in CNS PFS was observed with pCSI, with a median CNS PFS of 7.5 months (95% CI, 6.6 months to not reached) compared with 2.3 months (95% CI, 1.2 to 5.8 months; P < .001; Fig 2B) with IFRT. As a result, the Memorial Sloan Kettering Cancer Center Data and Safety Monitoring Committee recommended early discontinuation of the trial. In addition, an OS benefit with pCSI was observed with median OS of 9.9 months (95% CI, 7.5 months to not reached) compared with 6.0 months (95% CI, 3.9 months to not reached; P = .029; Fig 2C) with IFRT. In a multivariable analysis, pCSI remained significantly associated with improved CNS PFS (hazard ratio [HR], 0.15; 95% CI, 0.07 to 0.33; P < .001) and OS (HR, 0.43; 95% CI, 0.21 to 0.90; P = .025; Table 2).
FIG 2.
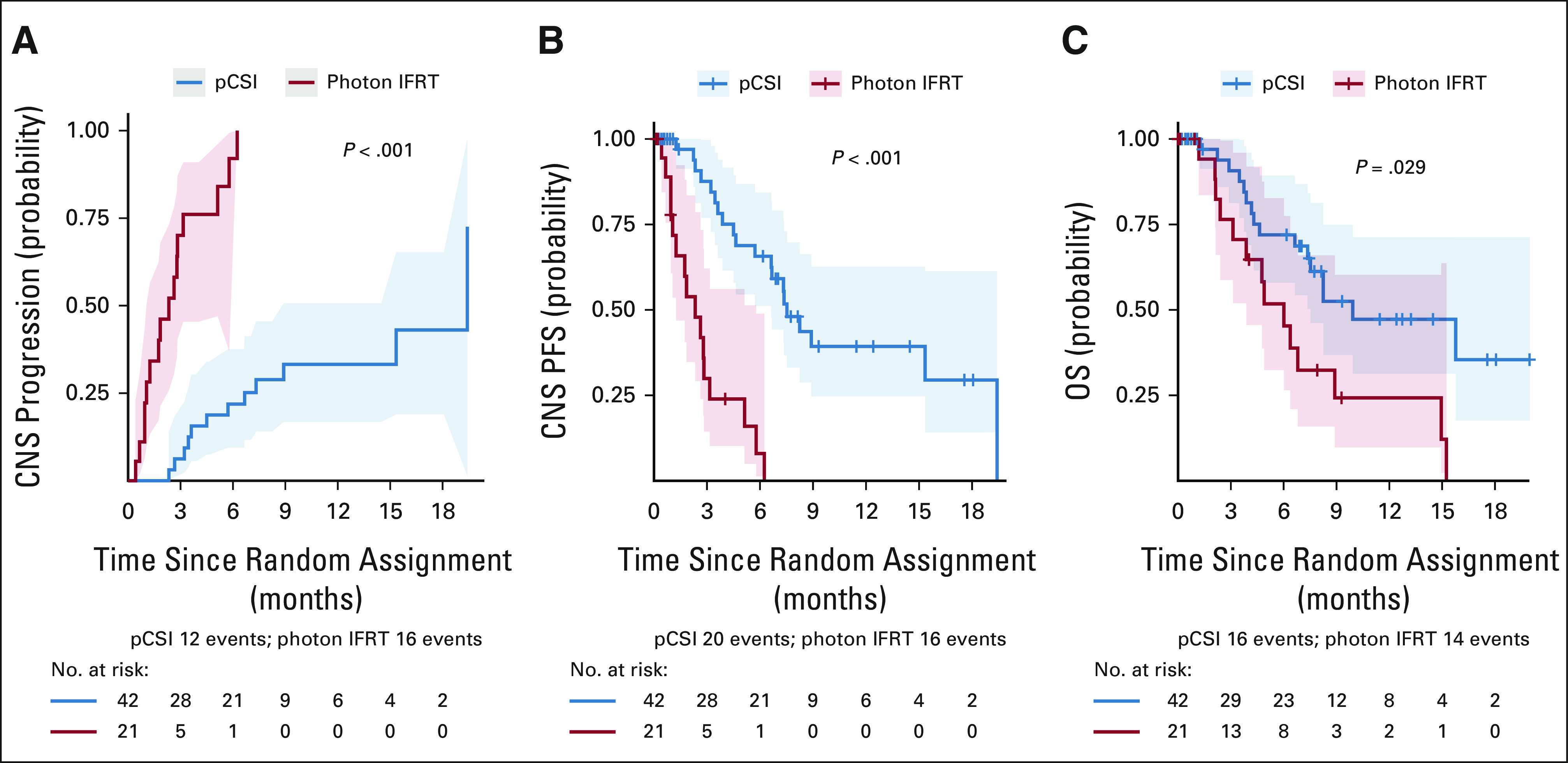
Patients who were randomly assigned to pCSI had significantly improved (A) CNS time to progression, (B) CNS PFS, and (C) OS. IFRT, involved-field radiotherapy; OS, overall survival; PFS, progression-free survival; pCSI, proton craniospinal irradiation.
TABLE 2.
Multivariate Cox Proportional Hazard Regression With CNS PFS and OS as Outcomes
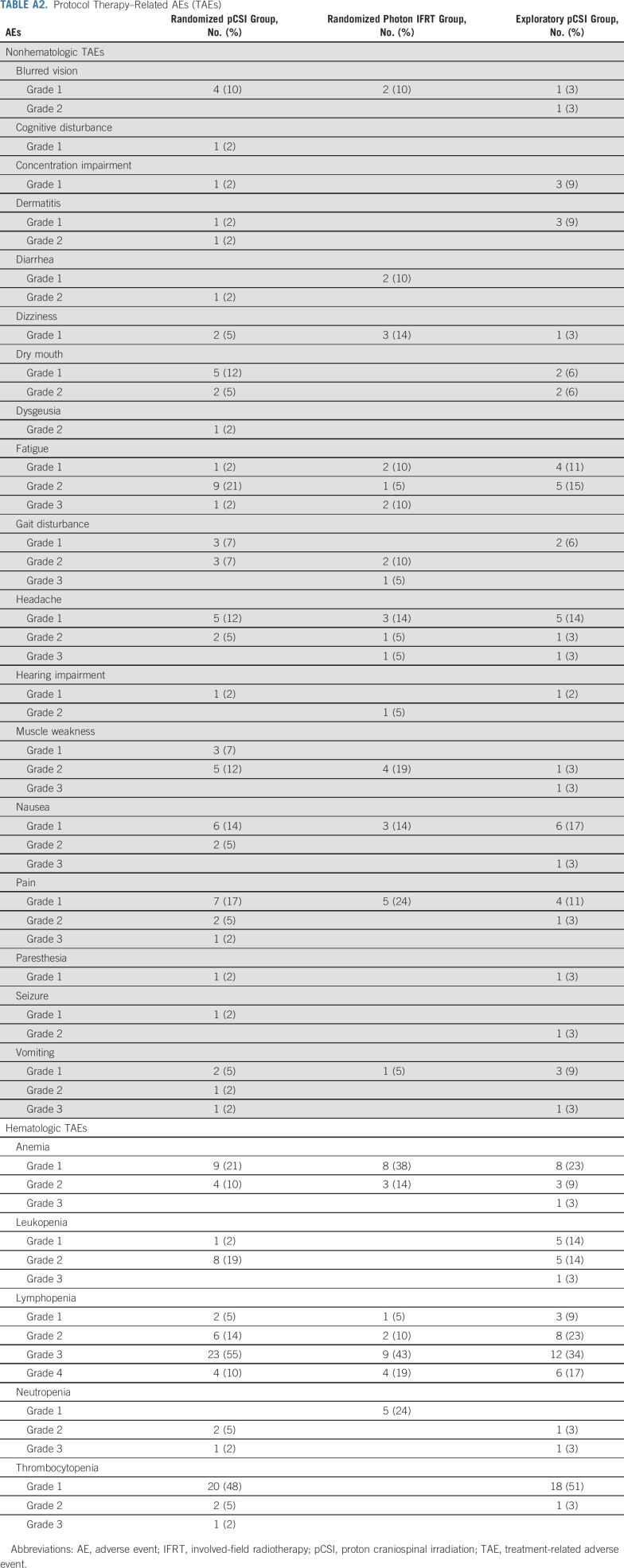
TAEs were similar between groups (Appendix Table A2, online only). No patient in either group experienced grade 5 toxicity. In the pCSI group, lymphopenia was the only grade 4 TAE reported (n = 4; 10%). Grade 3 nonhematologic toxicities included fatigue (n = 1; 2%), pain (n = 1; 2%), and vomiting (n = 1; 2%). In the IFRT group, lymphopenia was the only grade 4 TAE reported (n = 4; 19%). Grade 3 nonhematologic toxicities included fatigue (n = 2; 10%), gait disturbance (n = 1; 5%), and headache (n = 1; 5%). There was no difference in the rate of high-grade TAE between the two cohorts (P = .19).
Exploratory pCSI for Patients With Other Solid Tumor Histologies
The most common histology in the exploratory pCSI group was ovarian cancer (n = 7; 20%), followed by esophageal cancer (n = 6; 17%) and melanoma (n = 6; 17%). At the time of analysis, 9/35 patients experienced CNS progression (four with radiographic and clinical progression, two with clinical progression, two with radiographic progression, one with CSF cytologic progression), and 23/35 patients died (11 with systemic disease progression, five with CNS progression, four with CNS and systemic disease progression). The median duration of follow-up was 12 months (95% CI, 6.3 months to not reached; range, 0.2-18.9 months). The estimated CNS progression rates at 3 months and 6 months were 7% (95% CI, 1.2 to 20) and 21% (95% CI, 8.4 to 38), respectively. The median CNS PFS in this cohort was 5.8 months (95% CI, 4.4 to 9.1 months). Median OS was 6.6 months (95% CI, 5.4 to 11 months).
No patient experienced grade 5 toxicity. Lymphopenia was the only grade 4 TAE reported (n = 6, 17%). Grade 3 nonhematologic toxicities included headache (n = 1; 3%), muscle weakness (n = 1; 3%), nausea (n = 1; 3%), and vomiting (n = 1; 3%).
CSF CTCs
We measured baseline CSF CTC count in 36 (86%) patients in the randomized pCSI group, 17 (81%) patients in the randomized photon IFRT group, and 24 (69%) patients in the exploratory pCSI group. There was no statistically significant difference in baseline CTC count between treatment groups. For pCSI patients, baseline CTC > 136 cells/3 mL (median) was associated with worse CNS TTP (HR, 4.26; 95% CI, 1.36 to 13.4; P = .007), CNS PFS (HR, 2.05; 95% CI, 1.21 to 5.04; P = .011), and OS (HR, 2.94; 95% CI, 1.36 to 6.34; P = .004). For IFRT patients, baseline CTC > 199 cells/3 mL (median) was associated with worse CNS TTP (HR, 4.07; 95% CI, 1.02 to 16.27; P = .036) and CNS PFS (HR, 4.07; 95% CI, 1.02 to 16.27; P = .036), but not OS.
Numerical trends in CTC count following protocol therapy (Fig 3A) and the association with patient outcomes were investigated using joint modeling between the randomized pCSI and IFRT groups. After completion of RT, mean CTC count declined among patients treated with pCSI and increased among patients treated with IFRT (Fig 3B). For IFRT patients, the increase in CTC trend was significantly associated with worse CNS TTP (HR, 1.23 per 10-cell increment; 95% CI, 1.14 to 1.32; P < .001), CNS PFS (HR, 1.24 per 10 cells; 95% CI, 1.16 to 1.32; P < .001), and OS (HR, 1.18 per 10 cells; 95% CI, 1.10 to 1.26; P < .001). Histology was not significantly associated with CTC count change over time or outcomes.
FIG 3.
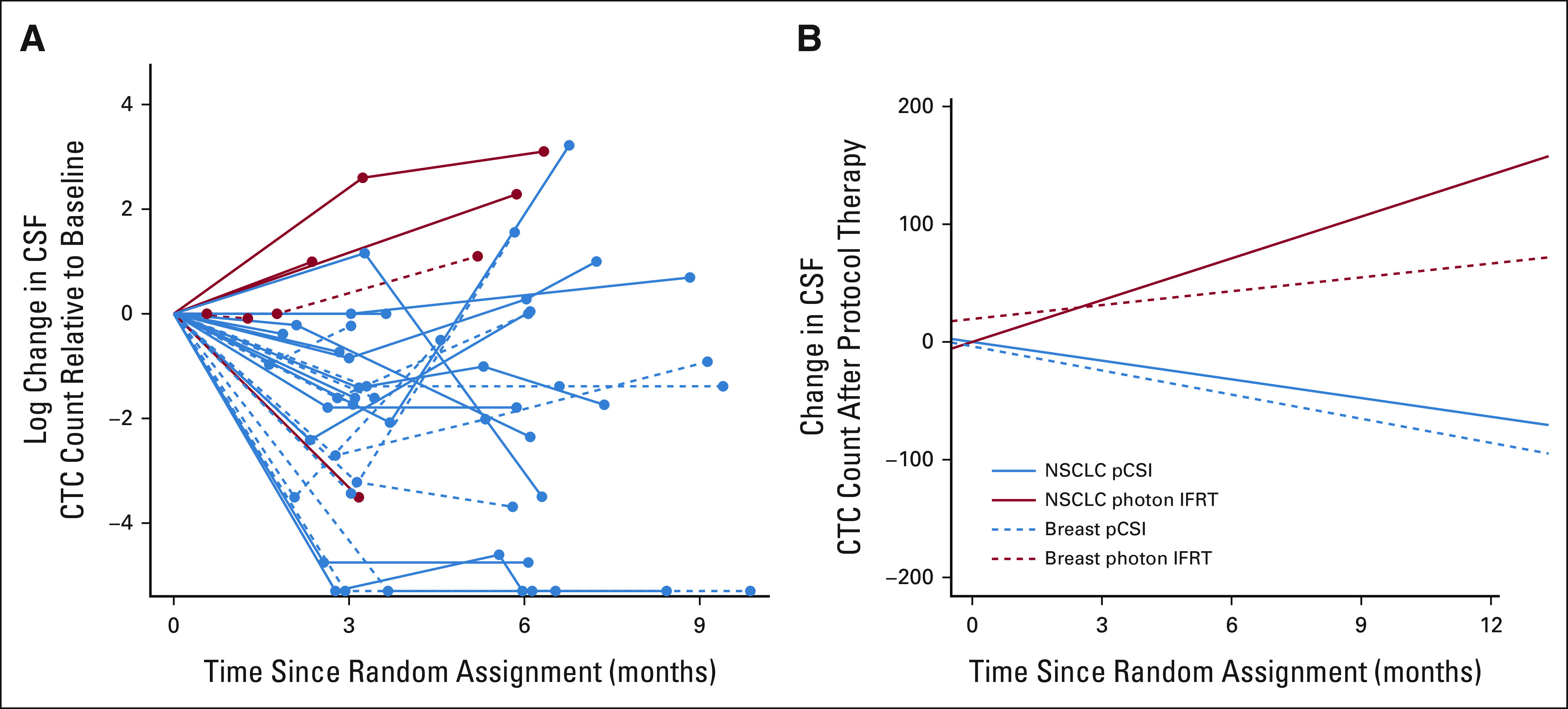
(A) The log CSF CTC count relative to baseline for each subject is shown over time for IFRT (red) and pCSI (blue). Patients who only had baseline CSF CTC counts are not shown. (B) Estimated effect of treatment on CSF CTC count assessed through joint modeling of longitudinal and survival outcomes with shared random effects and an unspecified baseline risk function.23 From the linear mixed-model component of the joint model of overall survival, we reported the slope from a nonsignificant interaction between histology and treatment arm. Although pCSI was associated with a decreasing CTC count after treatment, increasing CTC count was observed after IFRT. CTC, circulating tumor cell; IFRT, involved-field radiotherapy; NSCLC, non–small-cell lung cancer; pCSI, proton craniospinal irradiation.
DISCUSSION
This is the first randomized trial evaluating the optimal RT for patients with solid tumor LM. CNS PFS was significantly longer for patients with NSCLC and breast cancer LM who underwent pCSI compared with standard-of-care photon IFRT, resulting in early study closure recommended by the Data and Safety Monitoring Committee. We observed prolonged OS with pCSI compared with IFRT, and that pCSI was not associated with an increase in high-grade adverse events.
RT is essential in the treatment of solid tumor LM. Photon IFRT, in the form of WBRT and/or focal spine RT, is supported by guidelines for symptomatic and bulky disease.29,30 Several studies have supported a potential role for CSI for solid tumor LM with prolonged CNS disease control,20,31-33 including our institutional phase I trial, which demonstrated that hypofractionated pCSI is a safe and potentially effective treatment for LM.22 In this study, we conclude that pCSI is more efficacious than IFRT in CNS disease control, with an estimated 22% of pCSI patients and 97% of IFRT patients experiencing CNS progression at 6 months after therapy. The advantage in disease control translated into a significant improvement in CNS PFS with pCSI in patients with NSCLC and breast cancer LM (median 7.5 v 2.3 months with IFRT, P < .001). This result is comparable with our prior publications of pCSI for solid tumor LM that predominantly included patients with NSCLC or breast cancer.22,23 For the exploratory pCSI group including patients of other solid tumor histologies, a notably shorter CNS PFS was found. Although our study was not designed to compare the exploratory group to the randomized groups, the shorter CNS PFS in the exploratory group is likely because of the higher rate of systemic disease progression-related death (31%) compared with the randomized pCSI group (19%), as the CNS control was similar at 6 months for both groups (22% v 21%). Whether pCSI is a potentially efficacious approach for LM of other solid tumor histologies warrants further dedicated investigation.
In addition to CNS PFS, we observed a significant OS benefit with pCSI (median 9.9 months) compared with IFRT (median 6.0 months, P = .029) in patients with NSCLC and breast LM. The OS advantage of pCSI is likely a direct consequence of improved CNS disease control, as 19% of pCSI patients compared with 67% of IFRT patients died with CNS progression. It is undeniable that significant strides have been made in the management of NSCLC and breast cancer CNS metastases that harbor targetable mutations, including epidermal growth factor receptor (EGFR) exon 19 or L858R mutations,34,35 anaplastic lymphoma kinase and c-ros oncogene 1 (ALK/ROS1) rearrangements,36,37 and human epidermal growth factor receptor 2 (HER2) overexpression.12,38,39 Nevertheless, most patients in the randomized groups did not express these sensitizing mutations (52% in each group), and all patients with such mutations had received targeted therapies before trial enrollment (Table 1). As the groups were balanced and confounding factors were included in our multivariate analysis that confirmed pCSI as a prognostic factor for favorable OS, we are encouraged by this finding. Compared with patients of the randomized pCSI group, patients of the exploratory pCSI group demonstrated a shorter OS. Again, this is most likely related to the higher rate of systemic disease progression-related deaths in this group. It is difficult to conclude the effect of pCSI on OS in this heterogeneous group of patients; nevertheless, OS in this group compared favorably to recent reports of LM from gynecologic malignancies,40 melanoma,16 and those that include multiple histologies.5,33,41-44
CSF CTC count has been shown to be a valuable tool to predict survival in patients with LM.45 In an exploratory analysis, we determined that baseline CTC count is a predictive biomarker of CNS control and survival in patients treated with pCSI, confirming our prior results.23 Furthermore, although pCSI is associated with overall decrease in CTC count after treatment, increase in CTC count was observed after IFRT and was associated with poorer outcomes for these patients. Our results demonstrated that comprehensive treatment of the entire leptomeningeal compartment, such as with pCSI, is needed to reduce CSF LM burden.
To our knowledge, our study represents the first prospective trial of RT for solid tumor LM, and the first randomized trial that demonstrates survival benefit in solid tumor LM using RT. However, we had a relatively small sample size resulting from early discontinuation of the trial, and the study was not powered to evaluate differences in OS. Therefore, additional investigation is required to assess the impact of pCSI on OS in this population. We also recognize that the landscape of systemic therapy is continuously changing. However, our balanced random assignment of patients would in part account for this limitation.
In summary, our randomized phase II trial provides evidence supporting pCSI treatment over standard-of-care photon IFRT in patients with NSCLC and breast cancer LM in the form of improved CNS PFS with no increase in serious toxicity. We also observed an OS improvement after pCSI, which we aim to validate through a phase III randomized trial. CSF CTC count was associated with survival outcomes.
ACKNOWLEDGMENT
The authors thank all patients and their family members in participating in this trial. The authors also thank the physicians and staff at ProCure Proton Therapy Center, New Jersey, and New York Proton Center, New York.
APPENDIX
TABLE A1.
Normal Tissue Constraints for Proton Craniospinal Irradiation
TABLE A2.
Protocol Therapy–Related AEs (TAEs)
Jonathan T. Yang
Stock and Other Ownership Interests: Nanocan Therapeutics
Consulting or Advisory Role: Debiopharm Group, Galera Therapeutics, AstraZeneca, Nanocan Therapeutics
Research Funding: AstraZeneca, Kazia Therapeutics, X-RAD Therapeutics
Robert J. Young
Stock and Other Ownership Interests: Agios
Consulting or Advisory Role: Agios, Puma Biotechnology, NordicNeuroLab, ICON Clinical Research, Olema Pharmaceuticals
Research Funding: Agios (Inst)
Alexa Steckler
Employment: Memorial Sloan-Kettering Cancer Center
Allison Betof Warner
Consulting or Advisory Role: Nanobiotix, Iovance Biotherapeutics, Novartis, Shanghai Jo'Ann Medical Technology, BluePath Solutions, Pfizer, Instil Bio
Helena Yu
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Lilly (Inst), Novartis (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), Cullinan Oncology (Inst), Janssen Oncology (Inst), Erasca Inc (Inst), Blueprint Medicines
Travel, Accommodations, Expenses: Lilly
Other Relationship: Astellas Pharma
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Pfizer, Janssen, Sanofi, Daiichi-Sankyo, Novartis
Travel, Accommodations, Expenses: Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/234841
Andrew D. Seidman
Honoraria: Genomic Health, Lilly, Genentech/Roche, Novartis, AstraZeneca, Gilead Sciences, Pfizer, BeyondSpring Pharmaceuticals
Consulting or Advisory Role: Genentech/Roche, Novartis, Lilly, BeyondSpring Pharmaceuticals, Pfizer, Gilead/Forty Seven, Cancer Expert Now, G1 Therapeutics, Incyte, Affinia Therapeutics
Speakers' Bureau: Genomic Health, Genentech/Roche, Lilly, Novartis/Pfizer, Gilead Sciences
Research Funding: Bayer, Odonate Therapeutics (Inst), Nektar (Inst)
Andrew Lin
Stock and Other Ownership Interests: Novo Nordisk, Dexcom, Tandem Diabetes Care, Amedisys, Abbott Laboratories, Atea
Research Funding: Bristol Myers Squibb
Lisa M. DeAngelis
Consulting or Advisory Role: Sapience Therapeutics
Nancy Y. Lee
Consulting or Advisory Role: Merck, Pfizer, Merck Serono, Sanofi, Mirati Therapeutics, Roche/Genentech
Research Funding: AstraZeneca, Pfizer (Inst)
Simon N. Powell
Consulting or Advisory Role: Varian Medical Systems, Philips Healthcare, Rain Therapeutics
Research Funding: Philips Healthcare, Varian Medical Systems
Adrienne Boire
Patents, Royalties, Other Intellectual Property: Boire A and J Massagué, inventors. Sloan Kettering Institute, assignee. Modulating Permeability Of The Blood Cerebrospinal Fluid Barrier. United States Provisional Application No.: 62/258,044. November 20, 2015 (Inst), Boire A, Chen Q and J Massagué, inventors. Sloan Kettering Institute, assignee. Methods for Treating Brain Metastasis. United States 10413522, awarded September 17, 2019 (Inst), Boire A, inventor. Sloan Kettering Institute, assignee. Methods of Treating Leptomeningeal Metastasis. United States Provisional Application No.: 63/052,139. July 15, 2020 (Inst)
Uncompensated Relationships: Evren Technologies
No other potential conflicts of interest were reported.
PRIOR PRESENTATION
Presented at the 2022 ASCO Annual Meeting, June 6, 2022, Chicago, IL.
SUPPORT
Supported by grants from Cycle for Survival Equinox Innovation Initiative Award and the National Institutes of Health/National Cancer Institute (P30-CA008748).
CLINICAL TRIAL INFORMATION
AUTHOR CONTRIBUTIONS
Conception and design: Jonathan T. Yang, Elena Pentsova, Suzanne Wolden, Robert J. Young, Zhigang Zhang, Allison Betof Warner, Helena Yu, Mark G. Kris, Andrew D. Seidman, Andrew Lin, Lisa M. DeAngelis, Nancy Y. Lee, Simon N. Powell, Adrienne Boire
Administrative support: Alexa Steckler, Weronika Bucwinska, Ashley Bernstein
Provision of study materials or patients: Jonathan T. Yang, Elena Pentsova, Suzanne Wolden, Allison Betof Warner, Helena Yu, Mark G. Kris, Andrew D. Seidman, Jessica A. Wilcox, Rachna Malani, Andrew Lin, Lisa M. DeAngelis, Nancy Y. Lee, Simon N. Powell, Adrienne Boire
Collection and assembly of data: Jonathan T. Yang, N. Ari Wijetunga, Elena Pentsova, Suzanne Wolden, Robert J. Young, Denise Correa, Alexa Steckler, Weronika Bucwinska, Ashley Bernstein, Helena Yu, Mark G. Kris, Andrew D. Seidman, Jessica A. Wilcox, Rachna Malani, Andrew Lin, Lisa M. DeAngelis, Nancy Y. Lee, Simon N. Powell, Adrienne Boire
Data analysis and interpretation: Jonathan T. Yang, N. Ari Wijetunga, Suzanne Wolden, Robert J. Young, Denise Correa, Zhigang Zhang, Junting Zheng, Allison Betof Warner, Helena Yu, Mark G. Kris, Andrew D. Seidman, Jessica A. Wilcox, Rachna Malani, Andrew Lin, Lisa M. DeAngelis, Nancy Y. Lee, Simon N. Powell, Adrienne Boire
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Randomized Phase II Trial of Proton Craniospinal Irradiation Versus Photon Involved-Field Radiotherapy for Patients With Solid Tumor Leptomeningeal Metastasis
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jonathan T. Yang
Stock and Other Ownership Interests: Nanocan Therapeutics
Consulting or Advisory Role: Debiopharm Group, Galera Therapeutics, AstraZeneca, Nanocan Therapeutics
Research Funding: AstraZeneca, Kazia Therapeutics, X-RAD Therapeutics
Robert J. Young
Stock and Other Ownership Interests: Agios
Consulting or Advisory Role: Agios, Puma Biotechnology, NordicNeuroLab, ICON Clinical Research, Olema Pharmaceuticals
Research Funding: Agios (Inst)
Alexa Steckler
Employment: Memorial Sloan-Kettering Cancer Center
Allison Betof Warner
Consulting or Advisory Role: Nanobiotix, Iovance Biotherapeutics, Novartis, Shanghai Jo'Ann Medical Technology, BluePath Solutions, Pfizer, Instil Bio
Helena Yu
Consulting or Advisory Role: AstraZeneca, Daiichi Sankyo, Blueprint Medicines, Janssen, C4 Therapeutics, Cullinan Oncology, Black Diamond Therapeutics
Research Funding: AstraZeneca (Inst), Astellas Pharma (Inst), Lilly (Inst), Novartis (Inst), Pfizer (Inst), Daiichi Sankyo (Inst), Cullinan Oncology (Inst), Janssen Oncology (Inst), Erasca Inc (Inst), Blueprint Medicines
Travel, Accommodations, Expenses: Lilly
Other Relationship: Astellas Pharma
Mark G. Kris
Consulting or Advisory Role: AstraZeneca, Pfizer, Janssen, Sanofi, Daiichi-Sankyo, Novartis
Travel, Accommodations, Expenses: Genentech
Open Payments Link: https://openpaymentsdata.cms.gov/physician/234841
Andrew D. Seidman
Honoraria: Genomic Health, Lilly, Genentech/Roche, Novartis, AstraZeneca, Gilead Sciences, Pfizer, BeyondSpring Pharmaceuticals
Consulting or Advisory Role: Genentech/Roche, Novartis, Lilly, BeyondSpring Pharmaceuticals, Pfizer, Gilead/Forty Seven, Cancer Expert Now, G1 Therapeutics, Incyte, Affinia Therapeutics
Speakers' Bureau: Genomic Health, Genentech/Roche, Lilly, Novartis/Pfizer, Gilead Sciences
Research Funding: Bayer, Odonate Therapeutics (Inst), Nektar (Inst)
Andrew Lin
Stock and Other Ownership Interests: Novo Nordisk, Dexcom, Tandem Diabetes Care, Amedisys, Abbott Laboratories, Atea
Research Funding: Bristol Myers Squibb
Lisa M. DeAngelis
Consulting or Advisory Role: Sapience Therapeutics
Nancy Y. Lee
Consulting or Advisory Role: Merck, Pfizer, Merck Serono, Sanofi, Mirati Therapeutics, Roche/Genentech
Research Funding: AstraZeneca, Pfizer (Inst)
Simon N. Powell
Consulting or Advisory Role: Varian Medical Systems, Philips Healthcare, Rain Therapeutics
Research Funding: Philips Healthcare, Varian Medical Systems
Adrienne Boire
Patents, Royalties, Other Intellectual Property: Boire A and J Massagué, inventors. Sloan Kettering Institute, assignee. Modulating Permeability Of The Blood Cerebrospinal Fluid Barrier. United States Provisional Application No.: 62/258,044. November 20, 2015 (Inst), Boire A, Chen Q and J Massagué, inventors. Sloan Kettering Institute, assignee. Methods for Treating Brain Metastasis. United States 10413522, awarded September 17, 2019 (Inst), Boire A, inventor. Sloan Kettering Institute, assignee. Methods of Treating Leptomeningeal Metastasis. United States Provisional Application No.: 63/052,139. July 15, 2020 (Inst)
Uncompensated Relationships: Evren Technologies
No other potential conflicts of interest were reported.
REFERENCES
- 1.Beauchesne P: Intrathecal chemotherapy for treatment of leptomeningeal dissemination of metastatic tumours. Lancet Oncol 11:871-879, 2010 [DOI] [PubMed] [Google Scholar]
- 2.Wasserstrom WR, Glass JP, Posner JB: Diagnosis and treatment of leptomeningeal metastases from solid tumors: Experience with 90 patients. Cancer 49:759-772, 1982 [DOI] [PubMed] [Google Scholar]
- 3.Kaplan JG, DeSouza TG, Farkash A, et al. : Leptomeningeal metastases: Comparison of clinical features and laboratory data of solid tumors, lymphomas and leukemias. J Neurooncol 9:225-229, 1990 [DOI] [PubMed] [Google Scholar]
- 4.Chamberlain MC: Leptomeningeal metastasis. Curr Opin Oncol 22:627-635, 2010 [DOI] [PubMed] [Google Scholar]
- 5.Clarke JL, Perez HR, Jacks LM, et al. : Leptomeningeal metastases in the MRI era. Neurology 74:1449-1454, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lai R, Dang CT, Malkin MG, et al. : The risk of central nervous system metastases after trastuzumab therapy in patients with breast carcinoma. Cancer 101:810-816, 2004 [DOI] [PubMed] [Google Scholar]
- 7.Omuro AMP, Kris MG, Miller VA, et al. : High incidence of disease recurrence in the brain and leptomeninges in patients with nonsmall cell lung carcinoma after response to gefitinib. Cancer 103:2344-2348, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Emoto S, Ishigami H, Yamaguchi H, et al. : Frequent development of leptomeningeal carcinomatosis in patients with peritoneal dissemination of gastric cancer. Gastric Cancer 14:390-395, 2011 [DOI] [PubMed] [Google Scholar]
- 9.Kesari S, Batchelor TT: Leptomeningeal metastases. Neurol Clin 21:25-66, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Mack F, Baumert BG, Schafer N, et al. : Therapy of leptomeningeal metastasis in solid tumors. Cancer Treat Rev 43:83-91, 2016 [DOI] [PubMed] [Google Scholar]
- 11.Le Rhun E, Devos P, Weller J, et al. : Prognostic validation and clinical implications of the EANO ESMO classification of leptomeningeal metastasis from solid tumors. Neuro Oncol 23:1100-1112, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morikawa A, Jordan L, Rozner R, et al. : Characteristics and outcomes of patients with breast cancer with leptomeningeal metastasis. Clin Breast Cancer 17:23-28, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Remon J, Le Rhun E, Besse B: Leptomeningeal carcinomatosis in non-small cell lung cancer patients: A continuing challenge in the personalized treatment era. Cancer Treat Rev 53:128-137, 2017 [DOI] [PubMed] [Google Scholar]
- 14.Lassman AB, Abrey LE, Shah GD, et al. : Systemic high-dose intravenous methotrexate for central nervous system metastases. J Neurooncol 78:255-260, 2006 [DOI] [PubMed] [Google Scholar]
- 15.Morris PG, Reiner AS, Szenberg OR, et al. : Leptomeningeal metastasis from non-small cell lung cancer: Survival and the impact of whole brain radiotherapy. J Thorac Oncol 7:382-385, 2012 [DOI] [PubMed] [Google Scholar]
- 16.Glitza IC, Smalley KSM, Brastianos PK, et al. : Leptomeningeal disease in melanoma patients: An update to treatment, challenges, and future directions. Pigment Cell Melanoma Res 33:527-541, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brem SS, Bierman PJ, Black P, et al. : Central nervous system cancers: Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 3:644-690, 2005 [DOI] [PubMed] [Google Scholar]
- 18.Buszek SM, Chung C: Radiotherapy in leptomeningeal disease: A systematic review of randomized and non-randomized trials. Front Oncol 9:1224, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El Shafie RA, Bohm K, Weber D, et al. : Palliative radiotherapy for leptomeningeal carcinomatosis-analysis of outcome, prognostic factors, and symptom response. Front Oncol 8:641, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Devecka M, Duma MN, Wilkens JJ, et al. : Craniospinal irradiation (CSI) in patients with leptomeningeal metastases: Risk-benefit-profile and development of a prognostic score for decision making in the palliative setting. BMC Cancer 20:501, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown AP, Barney CL, Grosshans DR, et al. : Proton beam craniospinal irradiation reduces acute toxicity for adults with medulloblastoma. Int J Radiat Oncol Biol Phys 86:277-284, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yang TJ, Wijetunga NA, Yamada J, et al. : Clinical trial of proton craniospinal irradiation for leptomeningeal metastases. Neuro Oncol 23:134-143, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wijetunga NA, Boire A, Young RJ, et al. : Quantitative cerebrospinal fluid circulating tumor cells are a potential biomarker of response for proton craniospinal irradiation for leptomeningeal metastasis. Neurooncol Adv 3:vdab181, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lin H, Ding X, Kirk M, et al. : Supine craniospinal irradiation using a proton pencil beam scanning technique without match line changes for field junctions. Int J Radiat Oncol Biol Phys 90:71-78, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Brown PD, Pugh S, Laack NN, et al. : Memantine for the prevention of cognitive dysfunction in patients receiving whole-brain radiotherapy: A randomized, double-blind, placebo-controlled trial. Neuro Oncol 15:1429-1437, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le Rhun E, Devos P, Boulanger T, et al. : The RANO Leptomeningeal Metastasis Group proposal to assess response to treatment: Lack of feasibility and clinical utility and a revised proposal. Neuro Oncol 21:648-658, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rao JNK, Scott AJ: On chi-squared tests for multiway contingency tables with cell proportions estimated from survey data. Ann Stat 12:46-60, 1984 [Google Scholar]
- 28.Choi YH, Briollais L, He W, et al. : FamEvent: An R package for generating and modeling time-to-event data in family designs. J Stat Softw 97, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Rhun E, Weller M, Brandsma D, et al. : EANO-ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up of patients with leptomeningeal metastasis from solid tumours. Ann Oncol 28:iv84-iv99, 2017 [DOI] [PubMed] [Google Scholar]
- 30.National Comprehensive Cancer Network : Central nervous system cancers (version 2.2021). https://www.nccn.org/professionals/physician_gls/pdf/cns.pdf [Google Scholar]
- 31.Hermann B, Hultenschmidt B, Sautter-Bihl ML: Radiotherapy of the neuroaxis for palliative treatment of leptomeningeal carcinomatosis. Strahlenther Onkol 177:195-199, 2001 [DOI] [PubMed] [Google Scholar]
- 32.Harada HM, Mitsuya K, Asakura H, et al. : Cranio-spinal irradiation for leptomeningeal carcinomatosis: A pilot study. Int J Radiat Oncol Biol Phys 90:S310, 2014 [Google Scholar]
- 33.El Shafie RA, Bohm K, Weber D, et al. : Outcome and prognostic factors following palliative craniospinal irradiation for leptomeningeal carcinomatosis. Cancer Manag Res 11:789-801, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang JCH, Kim SW, Kim DW, et al. : Osimertinib in patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer and leptomeningeal metastases: The BLOOM study. J Clin Oncol 38:538-547, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ahn MJ, Chiu CH, Cheng Y, et al. : Osimertinib for patients with leptomeningeal metastases associated with EGFR T790M-positive advanced NSCLC: The AURA leptomeningeal metastases analysis. J Thorac Oncol 15:637-648, 2020 [DOI] [PubMed] [Google Scholar]
- 36.Solomon BJ, Besse B, Bauer TM, et al. : Lorlatinib in patients with ALK-positive non-small-cell lung cancer: Results from a global phase 2 study. Lancet Oncol 19:1654-1667, 2018 [DOI] [PubMed] [Google Scholar]
- 37.Shaw AT, Solomon BJ, Chiari R, et al. : Lorlatinib in advanced ROS1-positive non-small-cell lung cancer: A multicentre, open-label, single-arm, phase 1-2 trial. Lancet Oncol 20:1691-1701, 2019 [DOI] [PubMed] [Google Scholar]
- 38.Lin NU, Borges V, Anders C, et al. : Intracranial efficacy and survival with tucatinib plus trastuzumab and capecitabine for previously treated HER2-positive breast cancer with brain metastases in the HER2CLIMB trial. J Clin Oncol 38:2610-2619, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morikawa A, Wang R, Patil S, et al. : Characteristics and prognostic factors for patients with HER2-overexpressing breast cancer and brain metastases in the era of HER2-targeted therapy: An argument for earlier detection. Clin Breast Cancer 18:353-361, 2018 [DOI] [PubMed] [Google Scholar]
- 40.Yano H, Nagao S, Yamaguchi S: Leptomeningeal metastases arising from gynecological cancers. Int J Clin Oncol 25:391-395, 2020 [DOI] [PubMed] [Google Scholar]
- 41.Brastianos PK, Strickland MR, Lee EQ, et al. : Phase II study of ipilimumab and nivolumab in leptomeningeal carcinomatosis. Nat Commun 12:5954, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Srinivasalu VK, Subramaniam N, Philip A, et al. : Triple intrathecal chemotherapy for leptomeningeal carcinomatosis in solid tumors: Treatment outcomes, response and their determinants. Indian J Cancer 58:84-90, 2021 [DOI] [PubMed] [Google Scholar]
- 43.Deepak S, Lima A, Rajat S, et al. : Classification of leptomeningeal metastases from solid organ malignancies and clinical outcomes: Series from a Cancer Research Centre. Indian J Surg Oncol 11:308-312, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Meng Z, Zhang Q, Hong K, et al. : Clinical outcome and prognostic analysis of meningeal carcinomatosis treated by intrathecal chemotherapy. Expert Rev Pharmacoecon Outcomes Res 18:455-460, 2018 [DOI] [PubMed] [Google Scholar]
- 45.Diaz M, Singh P, Kotchetkov IS, et al. : Quantitative assessment of circulating tumor cells in cerebrospinal fluid as a clinical tool to predict survival in leptomeningeal metastases. J Neurooncol 157:81-90, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]