PURPOSE
To examine COVID-19 mRNA vaccine–induced binding and neutralizing antibody responses in patients with non–small-cell lung cancer (NSCLC) to SARS-CoV-2 614D (wild type [WT]) strain and variants of concern after the primary 2-dose and booster vaccination.
METHODS
Eighty-two patients with NSCLC and 53 healthy volunteers who received SARS-CoV-2 mRNA vaccines were included in the study. Blood was collected longitudinally, and SARS-CoV-2–specific binding and neutralizing antibody responses were evaluated by Meso Scale Discovery assay and live virus Focus Reduction Neutralization Assay, respectively.
RESULTS
A majority of patients with NSCLC generated binding and neutralizing antibody titers comparable with the healthy vaccinees after mRNA vaccination, but a subset of patients with NSCLC (25%) made poor responses, resulting in overall lower (six- to seven-fold) titers compared with the healthy cohort (P = < .0001). Although patients age > 70 years had lower immunoglobulin G titers (P = < .01), patients receiving programmed death-1 monotherapy, chemotherapy, or a combination of both did not have a significant impact on the antibody response. Neutralizing antibody titers to the B.1.617.2 (Delta), B.1.351 (Beta), and in particular, B.1.1.529 (Omicron) variants were significantly lower (P = < .0001) compared with the 614D (WT) strain. Booster vaccination led to a significant increase (P = .0001) in the binding and neutralizing antibody titers to the WT and Omicron variant. However, 2-4 months after the booster, we observed a five- to seven-fold decrease in neutralizing titers to WT and Omicron viruses.
CONCLUSION
A subset of patients with NSCLC responded poorly to the SARS-CoV-2 mRNA vaccination and had low neutralizing antibodies to the B.1.1.529 Omicron variant. Booster vaccination increased binding and neutralizing antibody titers to Omicron, but antibody titers declined after 3 months. These data highlight the concern for patients with cancer given the rapid spread of SARS-CoV-2 Omicron variant.
INTRODUCTION
SARS-CoV-2 infection has caused severe respiratory illness all over the world, making it a global pandemic.1,2 More than 5.7 million people have succumbed to SARS-CoV-2 infection with the highest mortality rate among elderly people. mRNA vaccines BNT162b2 by Pfizer and mRNA-1273 by Moderna have more than 95% efficacy in controlling SARS-CoV-2 infection.3,4 However, variants of concern (VOCs) have emerged, resulting in breakthrough infections. Notably, the B.1.1.529 (Omicron) variant carries 37 mutations in its spike protein, with 15 of these mutations in the receptor-binding domain (RBD), which is an important target of neutralizing antibodies. These mutations enable the Omicron variant to escape both vaccine-induced and therapeutic antibodies.5-11 The administration of a third mRNA booster dose to healthy individuals increases SARS-CoV-2–specific antibody responses substantially, resulting in a detectable neutralizing response against the Omicron variant.5,8,12,13
CONTEXT
Key Objective
Patients with non–small-cell lung cancer (NSCLC) are at increased risk for SARS-CoV-2 infection especially with the emergence of new variants of concern. Here, we examined neutralizing antibody responses in patients with NSCLC against the SARS-CoV-2 wild type (WT) virus and the B.1.1.529 (Omicron) variant after two-dose primary and booster immunization with mRNA vaccines.
Knowledge Generated
Most patients with NSCLC had normal antibody responses to the mRNA vaccines, but a subset (25%) of patients responded very poorly. In general, neutralizing antibody titers to the Omicron variant were significantly lower (P = < .0001) than those to WT virus. After booster immunization, antibody responses to both WT virus and Omicron variants increased significantly (P = .0001), but declined rapidly within 3-6 months.
Relevance
It is important to understand why a subset of patients with NSCLC respond poorly to the COVID-19 mRNA vaccines. This knowledge will provide insight into optimizing vaccination strategies for patients with NSCLC.
Approximately two million patients are diagnosed with lung cancer every year globally; it is the leading cause of cancer-related deaths with nearly 1.76 million deaths per year. As the median age of lung cancer diagnosis is 70 years and immune dysregulation because of tumor malignancy and immunomodulatory therapies is seen during lung cancer, it is important to evaluate the immune response after SARS-CoV-2 vaccination in these patients.14,15 Recent studies in patients with thoracic cancer receiving the BNT162b2 vaccine (99.3%) have shown to be efficient in generating protective antibody responses against the SARS-CoV-2 wild-type strain.16,17 However, vaccine-induced immune response to emerging VOCs in patients with non–small-cell lung cancer (NSCLC) has not been studied in detail. Here, we examined the binding and neutralizing antibody responses to SARS-CoV-2 wild type (WT; 614D) strain and B.1.1.529 (Omicron) variant in patients with NSCLC after primary mRNA vaccination and after booster dose.
METHODS
Refer to Appendix 1 (online only).
RESULTS
Binding Antibody Response Induced by SARS-CoV-2 mRNA Vaccines in Patients With NSCLC
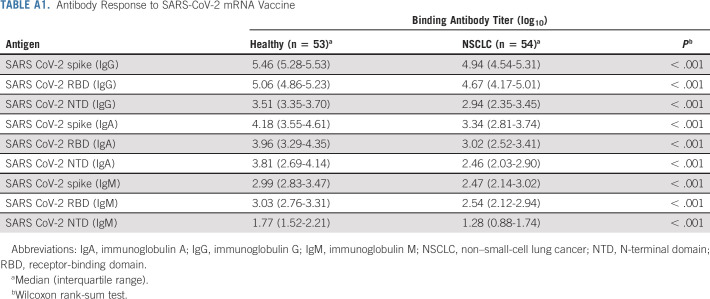
First, we evaluated the vaccine-specific antibody response in our NSCLC patient cohort (Table 1) compared with the healthy cohort (Table 1). Prepandemic plasma samples collected from healthy individuals were used to set the detection limit of the assay. A month after the second dose of primary vaccination, most patients with NSCLC had binding immunoglobulin G (IgG) titers comparable with healthy controls. A subset of patients with NSCLC had poor vaccine-specific binding antibody titers, resulting in an overall lower spike, RBD, and N-terminal domain (NTD)–specific IgG titers (six-fold for spike, seven-fold for RBD, and eight-fold for NTD) compared with the healthy controls (P = < .0001 for spike, .0002 for RBD, and < .0001 for NTD).
TABLE 1.
NSCLC Patient and Healthy Cohort Characteristics
Patients with a history of SARS-CoV-2 infection were identified by the presence of high antinucleocapsid antibody titer (N+) in their plasma as SARS-CoV-2 nucleocapsid is not a component of the mRNA vaccine. These N+ patients had 9- to 12-fold higher levels of spike-, RBD-, and NTD-specific antibodies compared with patients with NSCLC who did not have a history of SARS-CoV-2 infection (Figs 1A-1C and Appendix Table A1, online only). These patients were not included in the subsequent analysis.
FIG 1.
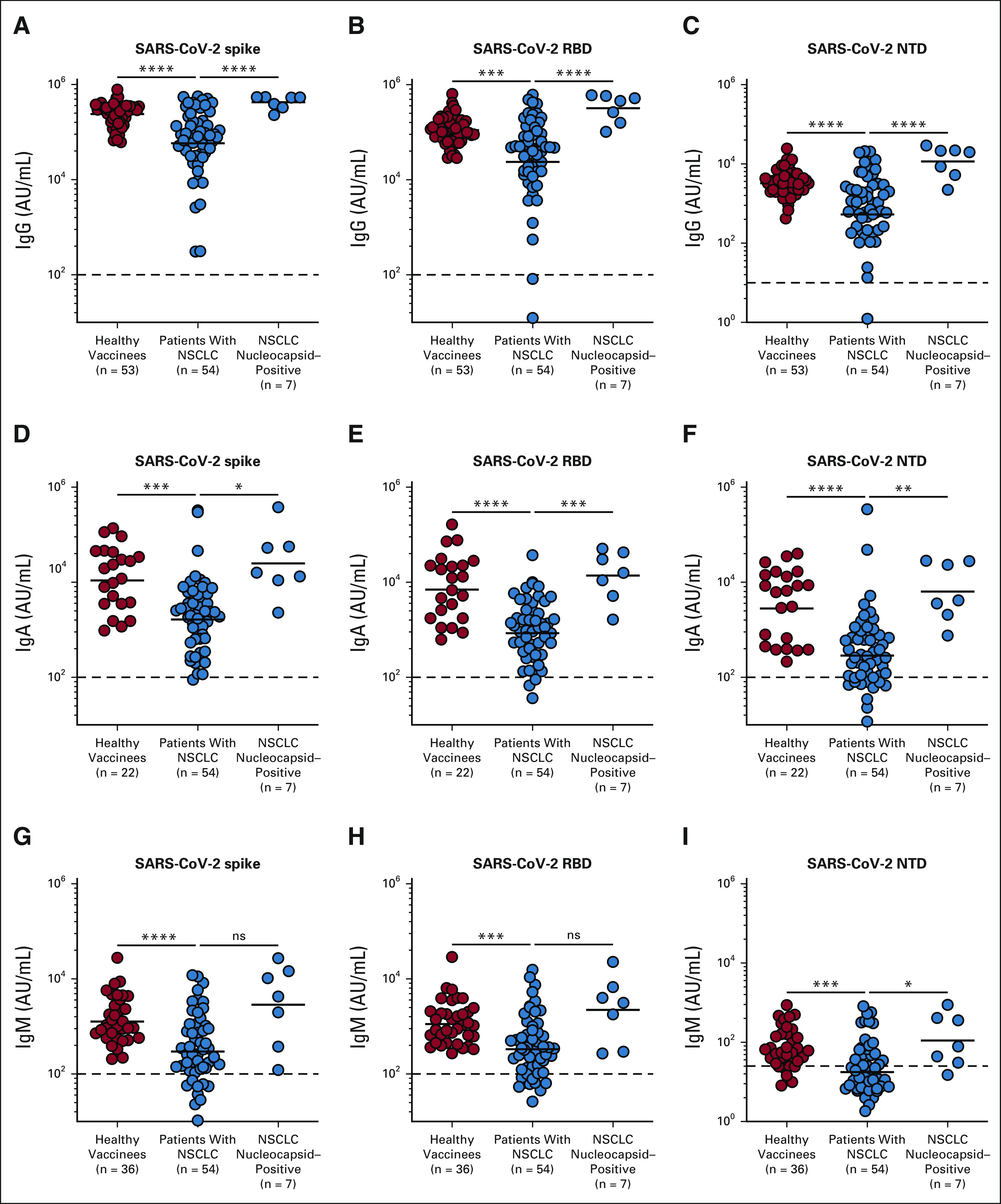
Antibody response to SARS-CoV-2 mRNA vaccine in patients with NSCLC. Binding antibody titers to spike, RBD, and NTD antigens were measured by MSD assay 1 month after the two-dose primary vaccination. Spike-, RBD-, and NTD-specific antibody titers in plasma from healthy vaccinees, patients with NSCLC, and patients with NSCLC with prior exposure to SARS-CoV-2 infection are shown for (A-C) IgG, (D-F) IgA, and (G-I) IgM, respectively. Prepandemic plasma samples from healthy individuals were used to set the detection limit for SARS-CoV-2–specific antibody titers. The figures show the mean and SEM. *P ≤ .05, **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001. IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; MSD, Meso Scale Discovery; ns, not significant; NSCLC, non–small-cell lung cancer; NTD, N-terminal domain; RBD, receptor-binding domain.
Similar to IgG titers, about 75% of the patients with NSCLC had vaccine-specific IgA and IgM titers comparable with the healthy volunteers, whereas about 25% of the patients had markedly lower titers. The overall IgA and IgM responses were about 7- to 10-fold lower in patients with NSCLC compared with the healthy controls. N+ patients had 10- to 12-fold higher IgA and IgM titers compared with SARS-CoV-2–naïve NSCLC patients (Figs 1D-1I and Appendix Table A1). Next, we examined if spike-specific antibody titers correlate with RBD- and NTD-specific antibody levels. As shown in Appendix Figures A1A-A1C (online only), the binding spike–specific IgG, IgA, and IgM titers strongly correlate with the RBD- and NTD-specific titers, respectively (Appendix Figs A1D-A1F).
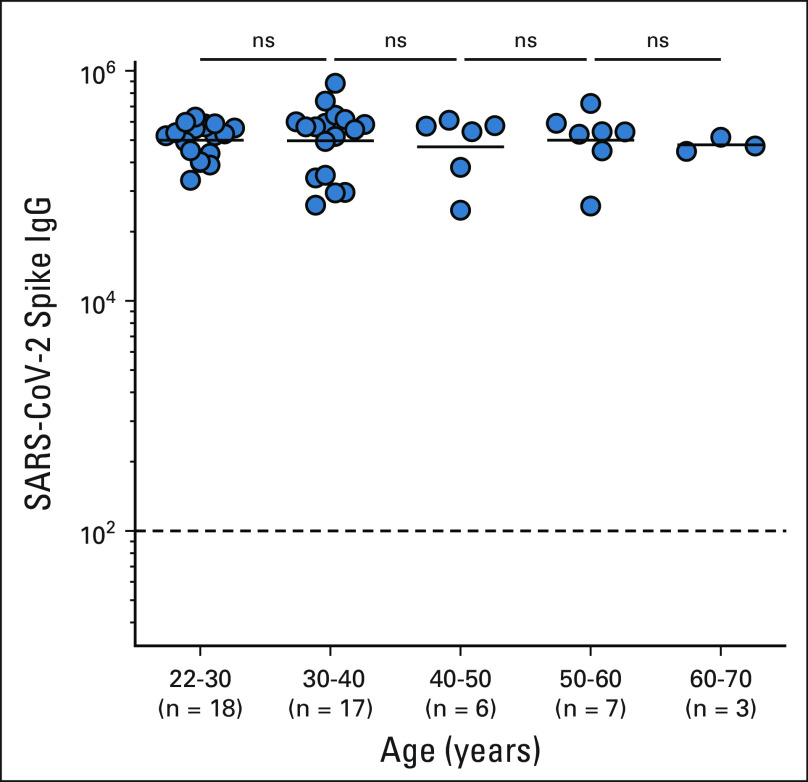
The age of our healthy cohort is skewed toward younger people and is not ideally matched with the age of our NSCLC cohort. However, we have some older people in our healthy cohort with age ranging from 20 to 70 years. We did not see any significant differences in the antibody response to COVID-19 mRNA vaccine for the healthy cohort across these age groups (Appendix Fig A2, online only). These data are in line with the previously published findings where healthy adults age between 56 and 70 years did not show decreased antibody response to mRNA vaccines.18
Neutralizing Antibody Response after SARS-CoV-2 mRNA Vaccination of Patients With NSCLC
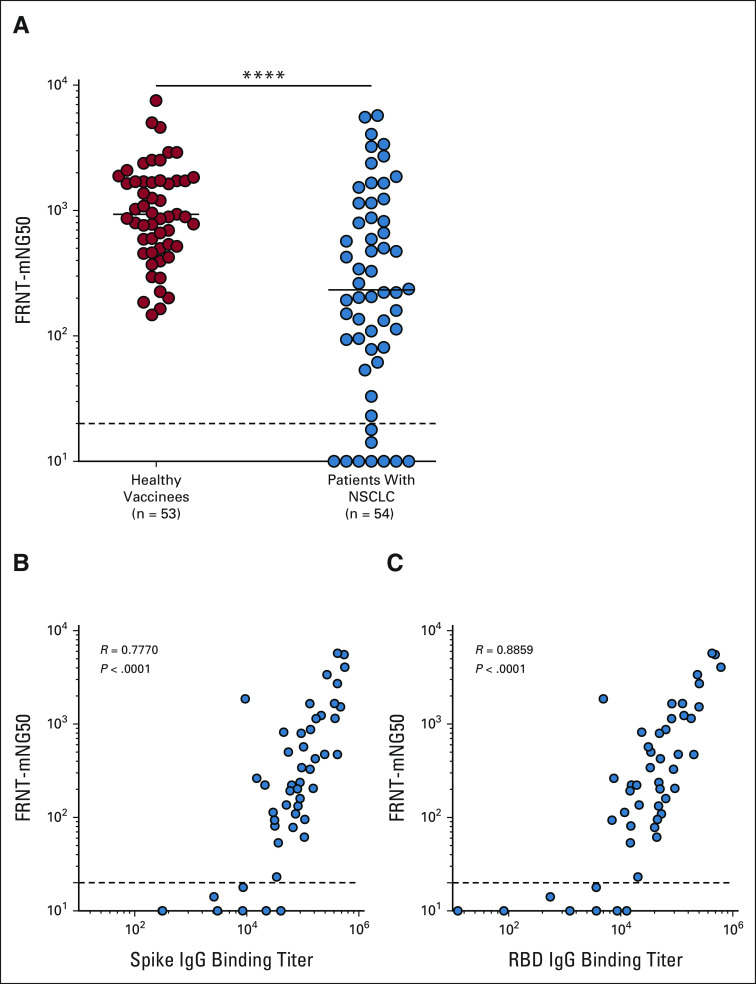
Next, we performed a live virus neutralization assay to determine the presence of neutralizing antibodies against the WT (614D) virus in healthy vaccinees and patients with NSCLC in response to mRNA vaccination. Although 65% of the patients with NSCLC generated neutralizing antibody titers comparable with the healthy cohort, 25% of the patients had lower neutralizing antibody titers. About 18% of the patients failed to generate any detectable neutralizing antibody titers. Overall, the neutralizing antibody titers in patients with NSCLC were seven-fold lower compared with those in the healthy vaccinees (P = < .0001; Fig 2A). Of note, the focus reduction neutralization test (FRNT)50 titers for live virus closely correlated with the binding spike and RBD antibody titers (P = < .0001; Figs 2B and 2C). Taken together, these data demonstrate that in response to vaccination, most patients with NSCLC generate detectable neutralizing antibody titers; however, a significant fraction of patients with NSCLC (9 of 54) failed to mount a detectable neutralizing antibody response.
FIG 2.
Live virus–neutralizing antibody response to SARS-CoV-2 mRNA vaccine in patients with NSCLC. Neutralizing antibody titers to WT (614D) virus were evaluated 1 month after the two-dose primary vaccination by FRNT assay. (A) Live virus neutralization of WT (614D) virus by sera from patients with NSCLC compared with the samples from healthy vaccinees. (B) Correlation of spike-specific IgG titer and FRNT50 titer (neutralization titer). (C) Correlation between RBD-specific IgG titer and FRNT50 titer (neutralization titer). Simple linear regression analysis was performed to do correlation analysis, and P values were obtained from the Pearson r correlation method. The figure shows the mean and SEM. ****P ≤ .0001. FRNT, Focus Reduction Neutralization Test; IgG, immunoglobulin G; NSCLC, non–small-cell lung cancer; RBD, receptor-binding domain; WT, wild type.
Longitudinal Analysis of Spike and RBD-Specific Antibody Response in Vaccinated Patients With NSCLC
To evaluate the persistence of the vaccine-specific antibody response, we longitudinally measured the binding antibody response to mRNA vaccines in our patients with NSCLC over 6 months. Antispike and anti-RBD IgG titers increased by 30- and 25-fold, respectively, after a week following the second vaccine dose (P = < .0001). Both antispike and anti-RBD IgG titers tended to decrease approximately 3 months after the second dose although this did not reach statistical significance. However, there was a significant decrease in both spike- (six-fold; P = < .0003) and RBD-specific (eight-fold; P = < .0009) antibodies at 6 months after the second dose of vaccination compared with the respective peak IgG titers (Appendix Figs A3A-A3D, online only).
Impact of Patient Therapy and Baseline Characteristics on Vaccine Response
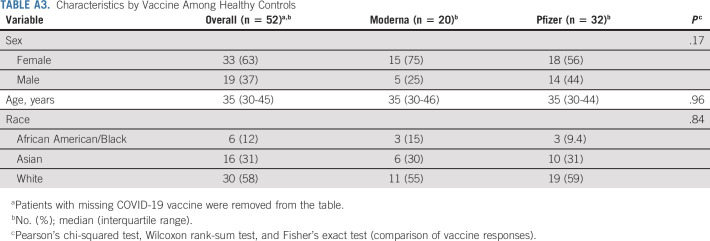
We next examined if patient therapy and demographic characteristics of our NSCLC cohort influenced the antibody response to vaccination. Patients with NSCLC who were older than 70 years had nine-fold lower spike-specific IgG titers compared with the patients who were age < 60 years (P = < .0034; Fig 3A and Appendix Table A2, online only). Race and sex did not have an impact on the spike-specific IgG titers in both patients with NSCLC and healthy volunteers (Appendix Tables A2 and A3, online only).
FIG 3.
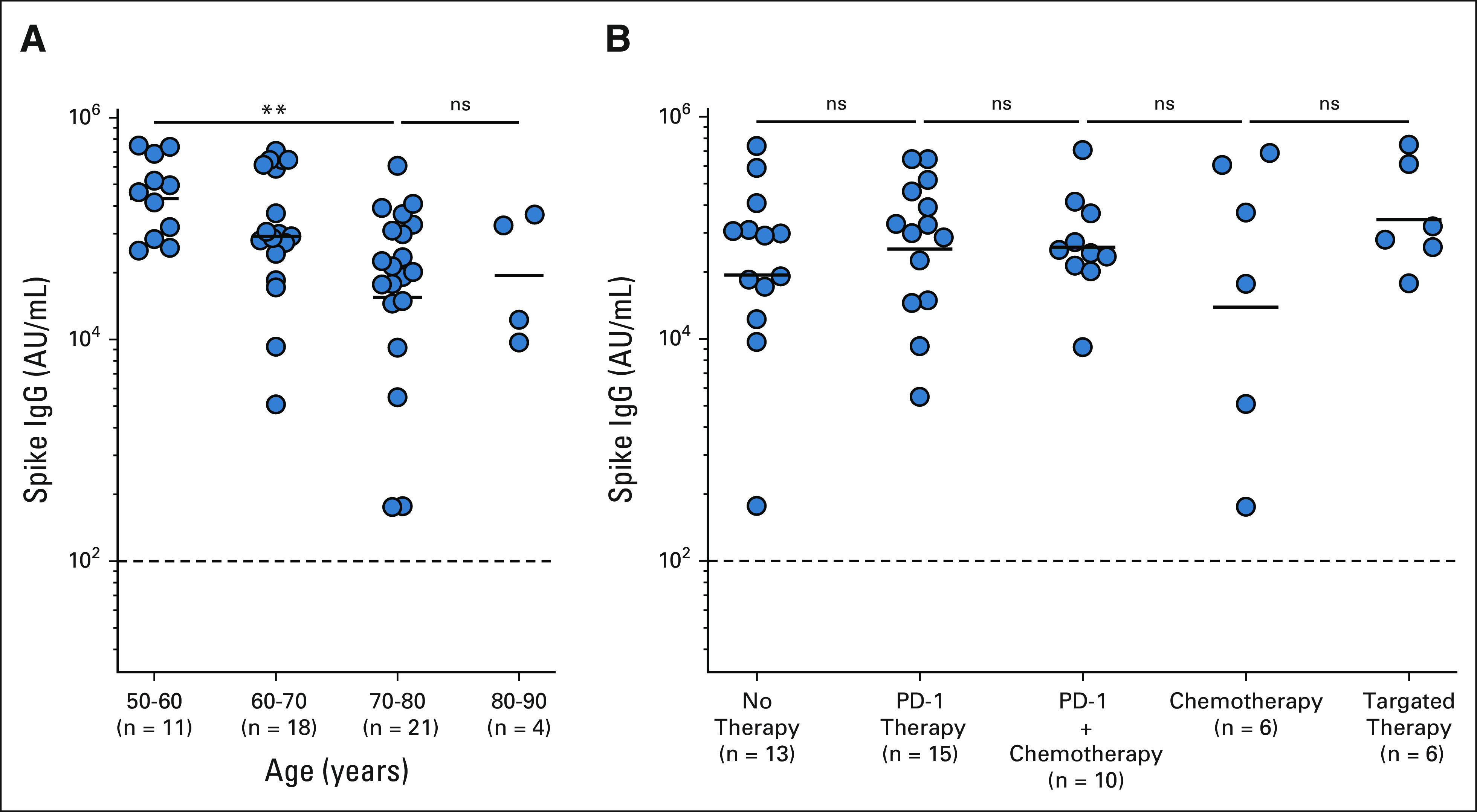
Influence of age and treatment regimens on the antibody response to mRNA vaccination in patients with NSCLC. (A) Patients with NSCLC were divided into four age groups (50-60, 60-70, 70-80, and 80-90 years) and their binding antispike IgG titers were determined. (B) Effect of different cancer therapies (no therapy, PD-1 therapy, and PD-1 with chemotherapy, chemotherapy, and targeted therapy) on the spike-specific IgG titers in response to the mRNA vaccines. The figure shows the mean and SEM. **P ≤ .01. IgG, immunoglobulin G; ns, not significant; NSCLC, non–small-cell lung cancer; PD-1, programmed death-1.
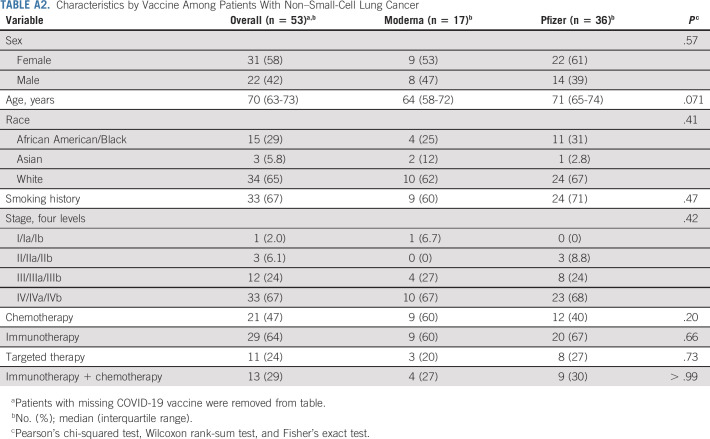
As many of the patients with NSCLC in our cohort are actively receiving cancer therapy, we evaluated if different cancer therapies had an influence on the antibody response to vaccination. On the basis of the type of therapy, the NSCLC cohort was divided into five subsets (patients receiving programmed death-1 (PD-1)–targeted therapy (n = 14), PD-1 and chemotherapy (n = 10), chemotherapy (n = 6), and other targeted therapy (n = 6)). At the time of SARS-CoV-2 vaccination, 13 patients received no therapy. We did not observe notable differences in the binding spike–specific IgG titers among patients receiving different cancer therapies compared with patients receiving no therapy (Fig 3B and Appendix Table A2).
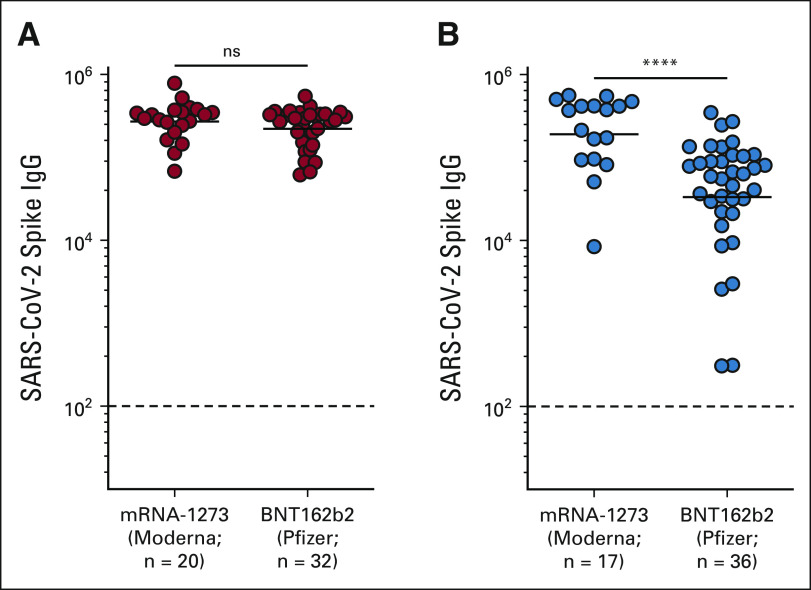
Vaccine type did not have a measurable impact on the antibody response in our healthy cohort; however, we observed lower spike-specific binding IgG titers in patients receiving the BNT162b2 compared with mRNA-1273 recipients (P = < .0001; Appendix Fig A4, online only and Appendix Tables A2 and A3). The three-fold higher mRNA dose in mRNA-1273 (100 μg) compared with BNT162b2 (30 μg) could have contributed to the higher antibody titer in patients with NSCLC. Taken together, these data suggest that although vaccine type and age of the patient influenced the antibody response, cancer therapy did not have any observable effect on the antibody response to SARS-CoV-2 vaccination in our NSCLC cohort.
Antibody Titer in Patients With NSCLC Against SARS-CoV-2 Variants
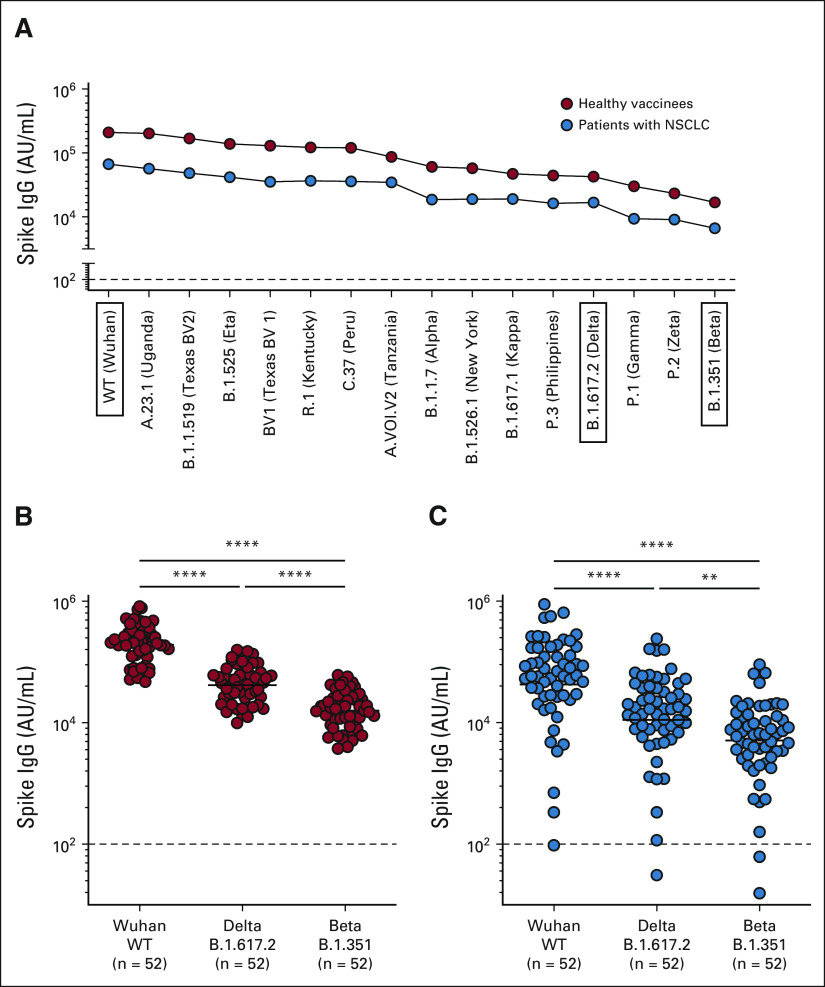
Next, we determined the vaccine-induced binding antibody response to SARS-CoV-2 VOCs in our healthy and NSCLC patient cohorts. Appendix Figure A5A (online only) shows the median spike-specific IgG titers in the plasma of both healthy vaccinees (n = 52) and the patients with NSCLC (n = 54) to 15 different SARS-CoV-2 variants. Spike-specific IgG titers to the variants are shown in descending order with the highest IgG titer to the WT (Wuhan) spike and the lowest to the SARS-CoV-2 B.1.351 (Beta) variant (Appendix Fig A5A). In both healthy vaccinees and patients with NSCLC, spike-specific IgG titers to the B.1.617.2 (Delta) and the B.1.351 (Beta) variants were significantly lower compared with the WT (614D) titer (Appendix Figs A5B and A5C).
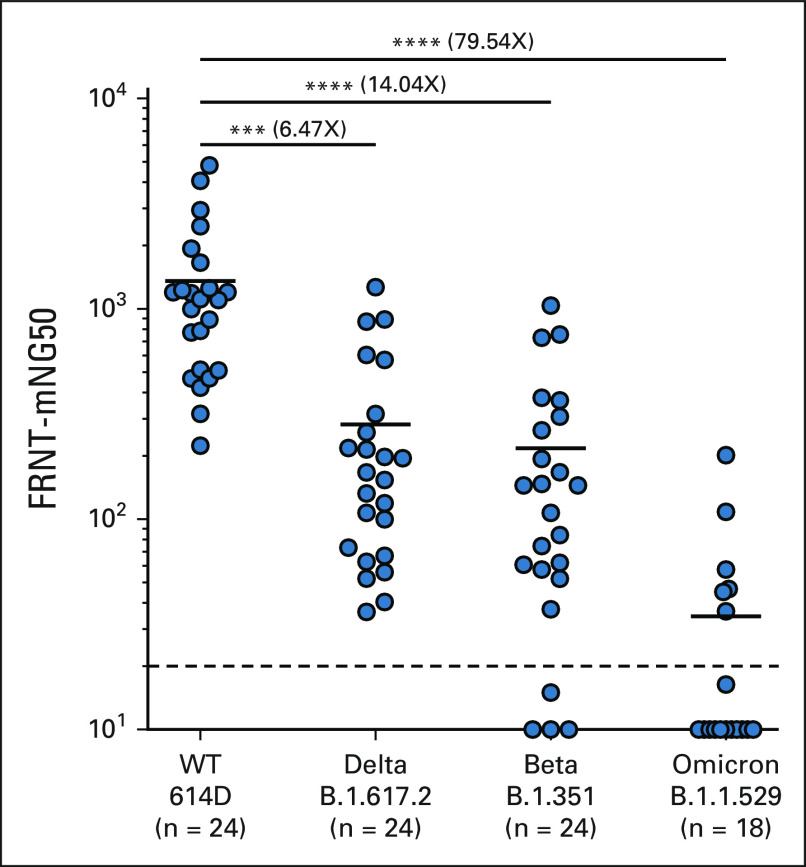
We next performed a live virus neutralization assay to determine if SARS-CoV-2 mRNA vaccination in patients with NSCLC induced neutralizing antibodies VOCs. Patient samples 1 month after the primary vaccination were used for the VOC neutralization assay. As shown in Figure 4, patients with NSCLC had 6.5- and 14-fold lower neutralizing antibody titers against the Delta and Beta variants, respectively, and importantly, > 50-fold lower neutralizing antibody titer to the Omicron variant compared with the 614D strain (Fig 4). These data show that after a two-dose SARS-CoV-2 mRNA vaccination, there is considerable neutralizing activity against the WT virus. However, the capacity of this antibody response to neutralize the Omicron variant is severely limited.
FIG 4.
Neutralizing antibody response to Delta, Beta, and Omicron SARS-CoV-2 variants in patients with NSCLC after mRNA vaccination. Live virus neutralization of WT (614D), B.1.617.2 (Delta), B.1.351 (Beta), and B.1.1.529 (Omicron) virus by patients with NSCLC 30 days after two-dose primary mRNA vaccination. The figure shows the mean and SEM. ***P ≤ .001, ****P ≤ .0001. FRNT, Focus Reduction Neutralization Test; NSCLC, non–small-cell lung cancer; WT, wild type.
Binding and Neutralizing Antibody Response Against the SARS-CoV-2 WT Virus and Omicron (B.1.1.529) Variant in Patients With NSCLC After Booster Vaccination
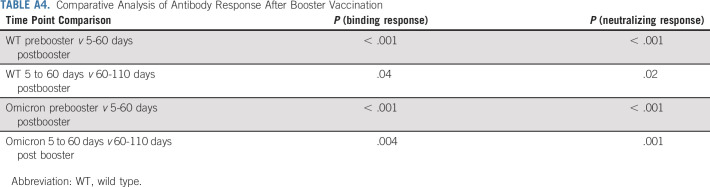
The Omicron variant now accounts for more than 95% of the current SARS-CoV-2 cases in the United States. We thus assessed the binding antibody responses in patients with NSCLC to the Omicron variant before and after booster vaccination. As shown in Figure 5A, patients with NSCLC received a booster dose 8 months after the primary vaccination. Before receiving a booster, patients with NSCLC had significantly lower spike-specific IgG titer to the Omicron variant compared with the WT strain (P = < .0001). After booster, we observed a 12- and 15-fold increase in the binding IgG titer to the WT and the Omicron variant compared with the prebooster samples, respectively. It is important to note that Omicron-specific IgG titers were still about five-fold lower compared with the WT spike titers (P = .0001). There was also a five- and eight-fold decrease in binding IgG titer to wild-type and Omicron variants after 60-110 days of booster vaccination compared with the peak binding titers observed at days 5-30 (Fig 5A and Appendix Table A4, online only).
FIG 5.
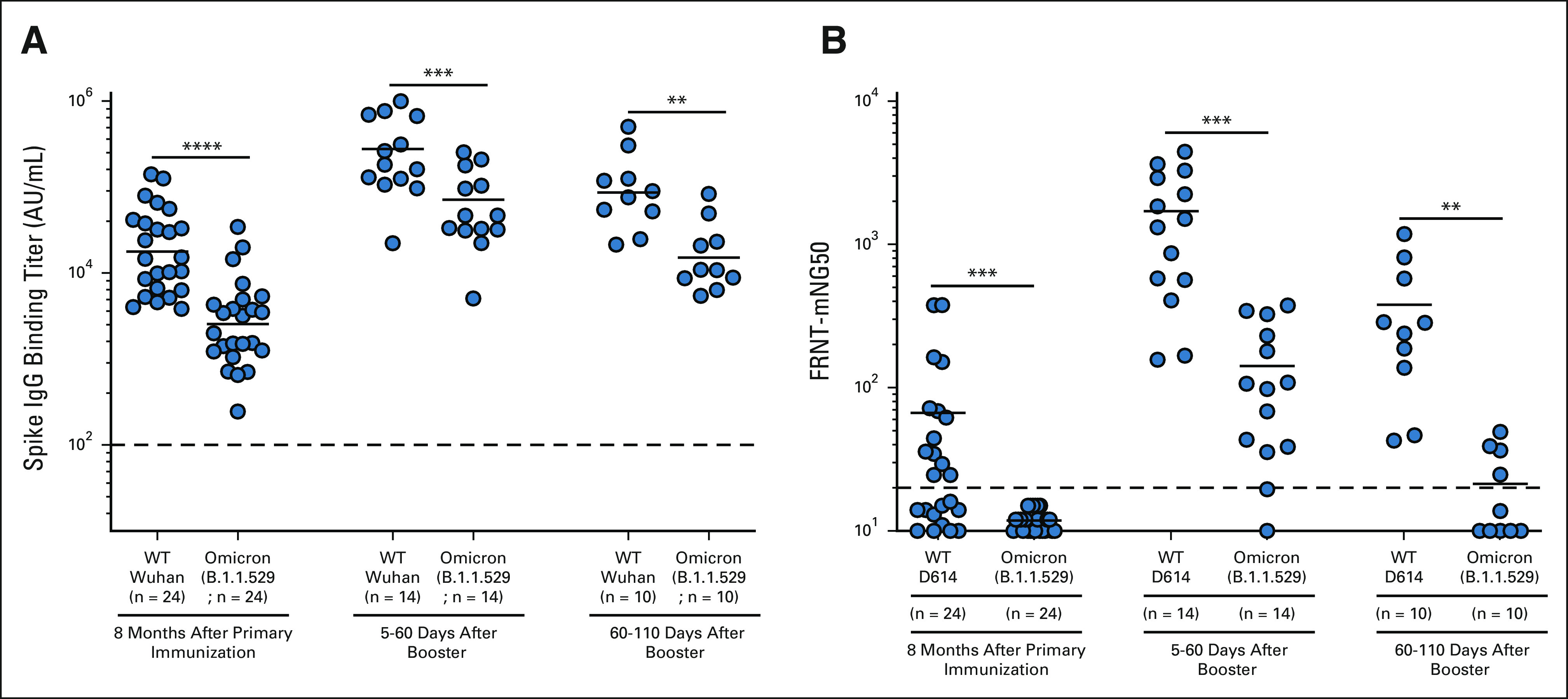
(A) Binding and (B) neutralizing antibody titer responses to SARS-CoV-2 wild-type and Omicron variants in patients with NSCLC before booster vaccination and 5-60 days and 60-110 days after booster vaccination. The figure shows the mean and SEM. **P ≤ .01, ***P ≤ .001, and ****P ≤ .0001. FRNT, Focus Reduction Neutralization Test; IgG, immunoglobulin G; NSCLC, non–small-cell lung cancer; WT, wild type.
We also examined the effect of booster vaccination of patients with NSCLC on the neutralizing antibody titer to the WT virus and Omicron variant (Fig 5B). Before booster, 13 of the 24 analyzed patients with NSCLC had detectable neutralizing antibody titers to the wild-type (D614) virus, but none of the patients had neutralizing antibodies to the Omicron variant (P = .0006). After booster vaccination, we observed a significant increase (P = .0001) in neutralizing antibody titers to the WT and Omicron variant, compared with the prebooster titers. Neutralizing antibody titers to the Omicron variant were 12-fold lower compared with the WT virus (P = .0001). After 60-110 days of booster vaccination, we observed lower neutralizing antibody titers to both WT and Omicron viruses compared with peak titers observed at days 5-30 (P = .0048 for WT and P = .0012 for Omicron). Of the 10 patients with NSCLC tested for neutralizing antibodies on 60-110 days after booster, only four patients still had detectable neutralizing antibody titers to the Omicron variant, whereas all patients had neutralizing antibody titer to the WT virus (P = .002). These data show that, although booster vaccination significantly increased the neutralizing antibody to the Omicron variant, the antibody titers to Omicron declined significantly (P = .0012) within 3-4 months after booster (Fig 5B and Appendix Table A4).
DISCUSSION
Immunosuppression in patients with cancer increases susceptibility to infections and reduces response to vaccination.19,20 Recent studies show that a subset of patients with cancer with solid and hematologic malignancies fail to respond to SARS-CoV-2 mRNA vaccination.21-23 Numerous SARS-CoV-2 VOCs are currently evolving, which evade vaccine-induced antibody response even in vaccinated healthy individuals.24 This leaves patients with cancer with immune dysfunction at a higher risk to contract severe SARS-CoV-2 infection.
We studied the vaccine-induced reactogenicity to SARS-CoV-2 mRNA vaccination in patients with NSCLC with emphasis on the antibody response to SARS-CoV-2 VOCs including the Omicron variant. Although most patients with NSCLC generated a vaccine-specific antibody response comparable with the healthy volunteers, a subset of patients responded poorly to vaccination. Antibody titers in patients with NSCLC correlated with age, as patients older than 70 years had lower vaccine-induced antibody titers compared with younger patients. This is in line with a recent study that showed healthy elderly individuals with a median age of 72 years having lower SARS-CoV-2 vaccine–specific antibody response compared with healthy young adults.14
PD-1–targeted therapies are effective in the treatment of patients with NSCLC.25 At the time of vaccination, more than 50% of the patients in our NSCLC cohort received either PD-1 monotherapy or PD-1 therapy in combination with chemotherapy. Cancer therapies that result in lymphocyte depletion are known to influence immune responses to infection and vaccination, but the effect of PD-1 therapy on vaccine response is not well known.19,20 We did not see a significant difference in the antibody response to SARS-CoV-2 vaccination in patients receiving either PD-1 monotherapy or a combination of chemotherapy and PD-1–targeted therapy compared with patients receiving no cancer therapy at the time of vaccination. Further studies with larger cohorts may be required to address if cancer therapy influences antibody response to SARS-CoV-2 vaccination.
Booster vaccination in heathy individuals increases neutralizing antibody response to the variants including the Omicron variant.5,26 Before booster vaccination, patients with NSCLC had very low to undetectable levels of neutralizing antibody response to the Omicron variant. Similar to the findings in healthy individuals, booster vaccination in patients with NSCLC induced an increase in binding and neutralizing antibody titers to the Omicron variant, albeit at lower levels compared with the WT (614D) virus. However, the booster dose failed to establish a durable antibody response in patients with NSCLC as both binding and neutralizing antibody titers declined significantly within 3-4 months of booster. Similar to our findings in patients with NSCLC, recent studies show a trend in waning antibody response to VOCs after booster vaccination in healthy individuals notably in elderly vaccinees.27 Given the spread of evolving VOCs, these findings are of concern especially for elderly patients with NSCLC.
In conclusion, this study highlights an important issue that needs to be addressed in more detail in future studies. The key question is why a subset of patients with lung cancer responded poorly to the COVID-19 mRNA vaccines. Although age and type of vaccine (Pfizer v Moderna) had an influence on the immune response, the very suboptimal vaccine responses generated by these patients with NSCLC cannot be explained solely by these factors. In our study, we also did not find a correlation with the type of cancer treatment and the magnitude of the antibody response. Thus, it is important to perform additional studies to identify the underlying mechanisms for the poor antibody responses in these individuals. These studies should also examine vaccine-induced T-cell responses in the patients to determine if both cellular and humoral immune responses are compromised. A better understanding of these critical issues will provide insight into optimizing vaccination strategies for patients with lung cancer, not only for COVID-19 disease but also more broadly for vaccines in general.
ACKNOWLEDGMENT
We acknowledge the support of Cancer Tissue and Pathology, Data and Technology Applications, and Biostatistics-shared resource of the Winship Cancer Institute of Emory University and NIH/NCI under award No. P30CA138292. M.V.D. was supported in part by R35CA197603.
APPENDIX 1. METHODS
NSCLC Patient Cohort
Peripheral blood samples from patients with non–small-cell lung cancer (NSCLC) were collected at the Winship Cancer Institute after written informed consent approval by the Institutional Review Board at Emory University. Blood samples were processed to isolate plasma and mononuclear cells. The patient demographics for all 82 patients with NSCLC enrolled in the study are given in Appendix Table A1. Peripheral blood samples were collected for analyzing antibody response longitudinally from all enrolled patients with NSCLC. Of the enrolled 82 patients, samples were collected from 61 patients one month after two-dose primary vaccination for the peak titer analysis.
Healthy Control Cohort
A total of 53 healthy volunteers vaccinated with COVID mRNA vaccines were recruited, and samples were collected at the Hope Clinic, Atlanta, after written informed consent approval by the Institutional Review Board at Emory University. Of these, 22 volunteers received mRNA-1273 and 31 received BNT162b2. Healthy volunteer demographics and characteristics are tabulated in Appendix Table A2.
Meso Scale Discovery Assay
A V-PLEX COVID-19 Respiratory Panel 2 Kit (K15372U panel 2) was used to measure the immunoglobulin G (IgG), IgM, and IgA antibodies against the antigens SARS-CoV-2 spike, receptor-binding domain, N-terminal domain, and nucleocapsid, following the manufacturer's recommendations. To measure spike-specific IgG against SARS-CoV-2 variants, V-PLEX COVID-19 Respiratory Panel 13, Panel 14, and Panel 23 Kits (K15463U, K15468U, and K15567U) were used. To assess IgG binding, plasma samples were diluted at 1:5,000, 1:25,000 or 1:40,000, and MSD Reference Standard-1 was diluted per MSD instructions in MSD Diluent 100. Fifty microliter of each sample and Reference Standard-1 dilution was added to the plates and incubated for 2 hours at room temperature, shaking at a speed of 700 rpm. After this, 50 µL per well of 1× MSD SULFO-TAG Anti-Human IgG Antibody was added and incubated for 1 hour at room temperature, shaking at a speed of 700 rpm. After the detection reagent step, 150 µL per well of MSD Gold Read Buffer B was added to each plate immediately before reading on an MSD plate reader. Plates were washed three times with 150 µL of wash solution B provided in the kit between each step. The limit of detection was defined as 1,000 Relative Luminescence Unit for each assay.
Viruses and Cells
VeroE6 cells were obtained from ATCC (clone E6, ATCC, #CRL-1586) and cultured in complete DMEM medium consisting of 1× DMEM (VWR, #45000-304), 10% Fetal Bovine Serum, 25 mM HEPES buffer (Corning Cellgro, Corning, NY) 2 mM L-glutamine, 1 mM sodium pyruvate, 1× nonessential amino acids, and 1× antibiotics. VeroE6-TMPRSS2 cells were generated and cultured as previously described.18 nCoV/USA_WA1/2020 (WA/1), closely resembling the original Wuhan strain and resembling the spike used in the mRNA-1273 and Pfizer-BioNTech vaccine, was propagated from an infectious SARS-CoV-2 clone as previously described.19 icSARS-CoV-2 was passaged once to generate a working stock. The B.1.351 variant isolate (hCoV-19/USA/MD-HP01542/2021), kindly provided by Dr Andy Pekosz (John Hopkins University, Baltimore, MD), was propagated once in VeroE6-TMPRSS2 cells to generate a working stock. hCoV19/EHC_C19_2811C (referred to as the B.1.1.529 variant) was derived from a mid-turbinate nasal swab collected in December 2021.20 This SARS-CoV-2 genome is available under GISAID accession number EPI_ISL_7171744. Using VeroE6-TMPRSS cells, the B.1.1.529 variant was plaque purified directly from the nasal swab, propagated once in a 12-well plate, and expanded in a confluent T175 flask to generate a working stock. All viruses used in this study were deep sequenced and confirmed as previously described.18
Focus Reduction Neutralization Assay
FRNT-mNG assays were performed on VeroE6 cells, and Focus Reduction Neutralization Test assays were performed on Vero-TMPRSS2 cells as previously described.18,21,22 Samples were diluted at three-fold in eight serial dilutions using DMEM (VWR, #45000-304) in duplicates with an initial dilution of 1:10 in a total volume of 60 μL. Serially diluted samples were incubated with an equal volume of icSARS-CoV-2-mNG, WA1/2020, or B.1.1.529 (100-200 foci per well on the basis of the target cell) at 37°C for 45 minutes in a round-bottom 96-well culture plate. The antibody-virus mixture was then added to cells and incubated at 37°C for 1 hour. Postincubation, the antibody-virus mixture was removed and 100 μL of prewarmed 0.85% methylcellulose (Sigma-Aldrich, St Louis, MO, #M0512-250G) overlay was added to each well. Plates were incubated at 37°C for 18 hours, and the methylcellulose overlay was removed and washed six times with phosphate-buffered saline (PBS). For Omicron infections, cells were incubated for 40-48 hours. Cells were fixed with 2% paraformaldehyde in PBS for 30 minutes. After fixation, plates were washed twice with PBS and permeabilization buffer (0.1% Bovine Serum Albumin [VWR, #0332], saponin [Sigma, 47036-250G-F] in PBS) was added to permeabilized cells for at least 20 minutes. Cells were incubated with either an anti–SARS-CoV spike primary antibody directly conjugated to Alexaflour-647 (CR3022-AF647) or an anti–SARS-CoV spike primary antibody directly conjugated to biotin (CR3022-biotin) for at least 4 hours at room temperature. For CR3022-AF647, cells were washed three times in PBS and foci were visualized on an ELISPOT reader (CTL). For CR3022-biotin, cells were washed three times in PBS and avidin-HRP was added for 1 hour at room temperature followed by three washes in PBS. Foci were visualized using TrueBlue HRP substrate (KPL, # 5510-0050) and imaged on an ELISPOT reader (CTL). Antibody neutralization was quantified by counting the number of foci for each sample using the Virodot program.23 The neutralization titers were calculated as follows: 1 – (ratio of the mean number of foci in the presence of sera and foci at the highest dilution of the respective sera sample). Each specimen was tested in duplicates. Samples that did not neutralize at the limit of detection at 50% were plotted at 12 and were used for geometric mean calculations.
Statistical Analysis
Statistical analysis was conducted using Graphpad Prism V9 and R 4.1.2. For all analyses, the significance level was set at P < .05, two-tailed. Descriptive statistics were performed to tabulate patients' demographic and clinical characteristics by COVID vaccine type. Frequency, percentage, mean, and standard deviation or median with the interquartile range were reported on the basis of the data structure of variable. Statistical differences were assessed by group using one-way analysis of variance, Kruskal-Wallis test, student's t-test, or Mann-Whitney test for continuous variables and chi-square or Fischer's exact test for categorical variables. The strength of association between laboratory biomarkers was tested with the Pearson or Spearman test. Further univariate analysis was conducted with simple linear regression on patients' assays collected in the study. Appropriate statistical tests were selected by validity of assumptions of the variables in analyses. The plots of the residuals (Q-Q plots) from each variable were used to examine whether the parametric or nonparametric statistical test would be used. Multiple comparison is accounted for by using Dunn's or Tukey test on the basis of the selected statistical test and validity of assumptions. Antibody responses after booster were analyzed using linear models with log of antibody as the dependent variable and time as a categorical independent variable. Models were fit using packages censReg, lme4, and lmec and the R programming language. Repeated measures were handled by including individual-level intercepts as random effects, and neutralizing titers < 20 were treated as left censored.
TABLE A1.
Antibody Response to SARS-CoV-2 mRNA Vaccine
TABLE A2.
Characteristics by Vaccine Among Patients With Non–Small-Cell Lung Cancer
TABLE A3.
Characteristics by Vaccine Among Healthy Controls
TABLE A4.
Comparative Analysis of Antibody Response After Booster Vaccination
FIG A1.
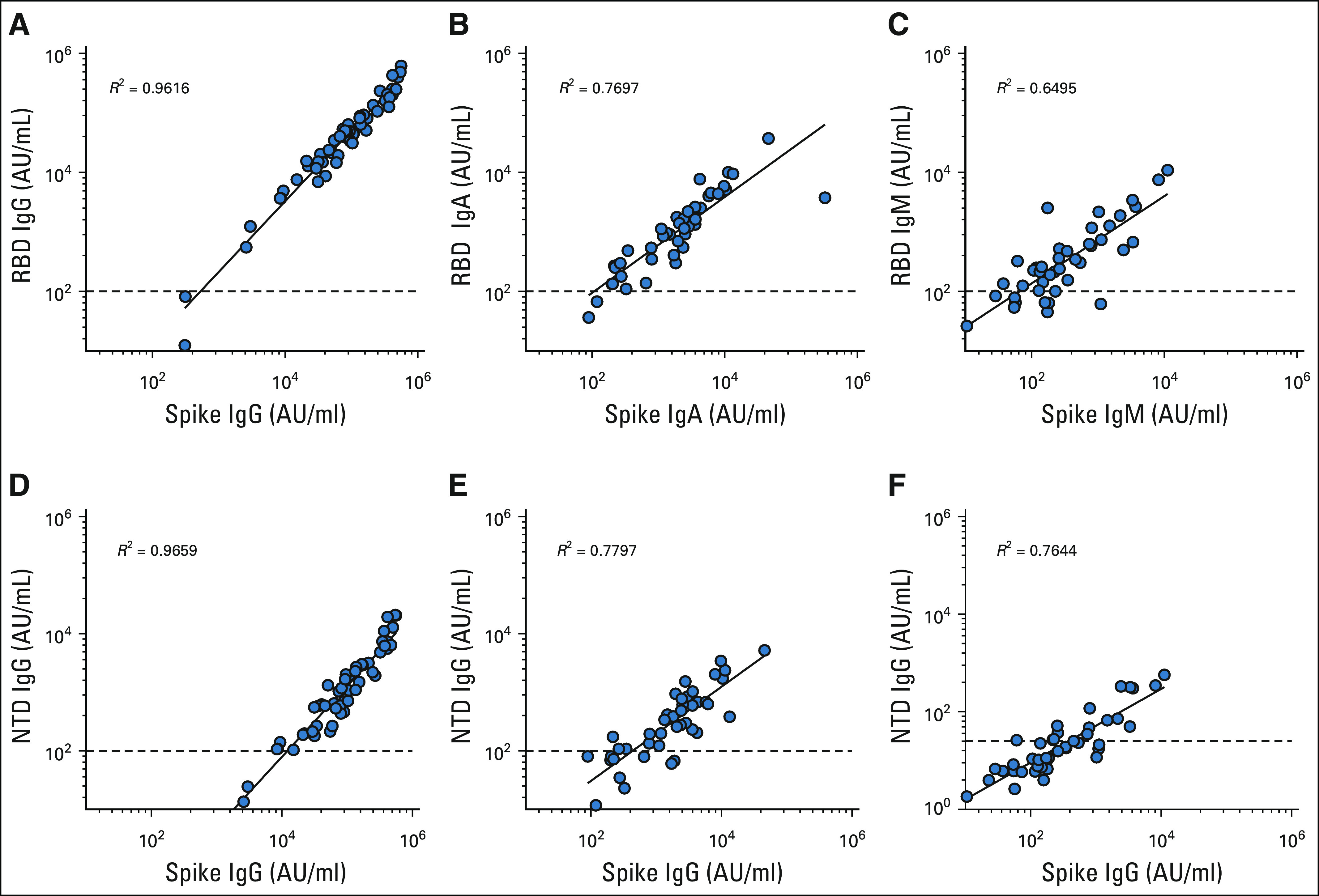
(A-C) Spike versus RBD and (D-F) spike versus NTD. IgG, immunoglobulin G; NTD, N-terminal domain; RBD, receptor-binding domain.
FIG A2.
Age range of the healthy cohort. IgG, immunoglobulin G; ns, not significant.
FIG A3.
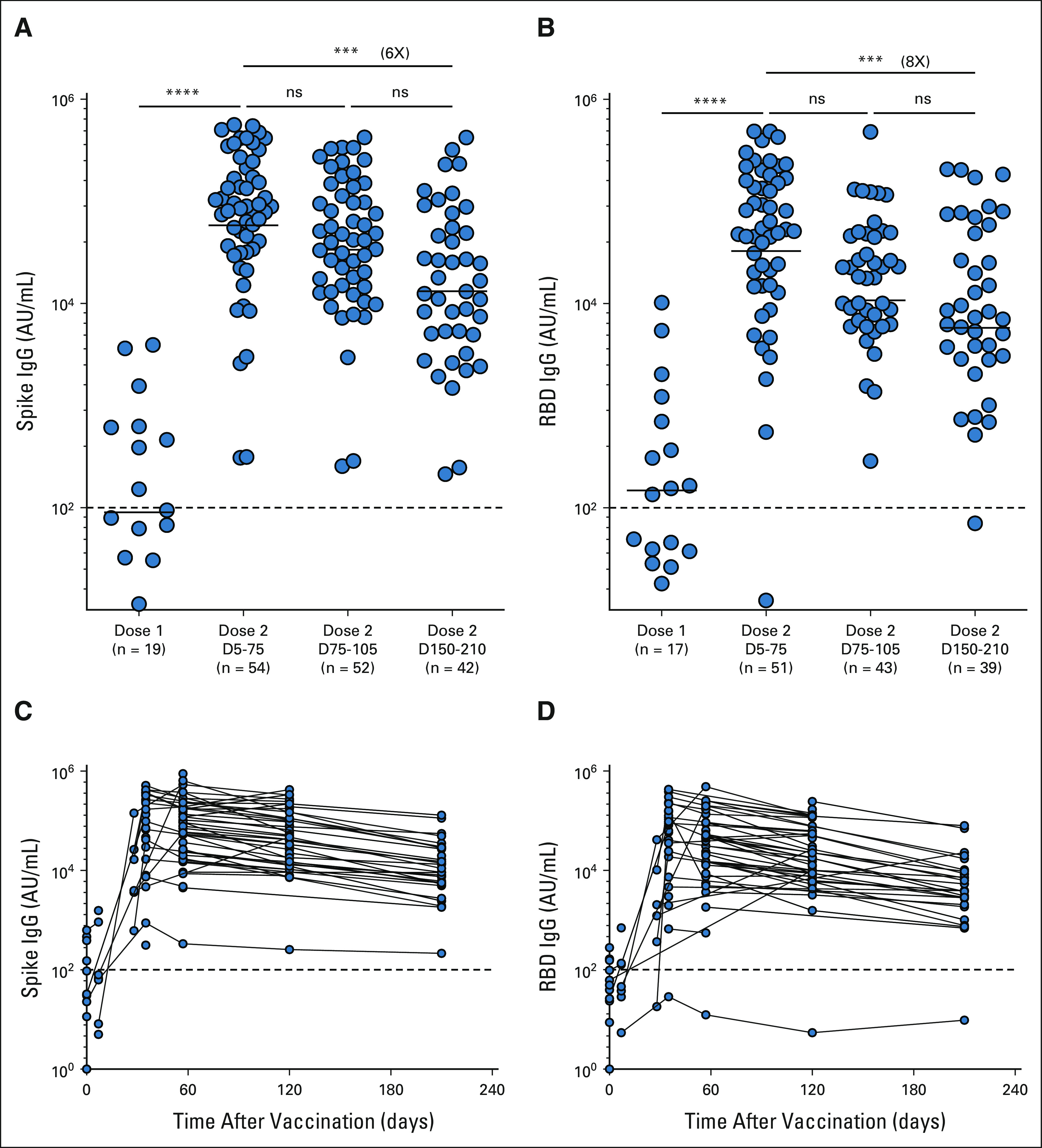
(A and C) Spike and (B and D) RBD. The figures show the mean and SEM. ***P < .001, ****P < .0001. IgG, immunoglobulin G; ns, not significant; RBD, receptor-binding domain.
FIG A4.
(A) Healthy vaccinees and (B) patients with NSCLC. The figures show the mean and SEM. ****P < .0001. IgG, immunoglobulin G; ns, not significant; NSCLC, non–small-cell lung cancer.
FIG A5.
(A) Spike specific IgG response to SARS-CoV-2 variants, (B) healthy vaccinees, and (C) patients with NSCLC. The figures show the mean and SEM. **P < .01, ****P < .0001. IgG, immunoglobulin G; NSCLC, non–small-cell lung cancer; WT, wild type.
Jennifer Carlisle
Research Funding: AstraZeneca (Inst)
Ralph Linwood Millett
Stock and Other Ownership Interests: Merck
Andreas Wieland
Patents, Royalties, Other Intellectual Property: Inventor on a patent (US patent application No. 16/971,627) filed by Emory University related to HPV-specific monoclonal antibodies and HPV E2 as a potential immunological target in HPV-positive cancers and inventor on a patent application filed by Emory University related to the use of HPV-specific TCR sequences
Conor Steur
Honoraria: Merck
Consulting or Advisory Role: AbbVie, BerGenBio, ARMO BioSciences, Lilly, Mirati Therapeutics, Sanofi/Regeneron, Caris Life Sciences
Research Funding: Vaccinex, Seattle Genetics, Daiichi Sankyo, Infinity Pharmaceuticals
Ajay K. Nooka
Honoraria: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst), Bristol Myers Squibb/Celgene (Inst), Arch Oncology (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Margaret A. Johns
Employment: Atlanta Center for Medicine
Veronika I. Zarnitsyna
Patents, Royalties, Other Intellectual Property: My husband Vladimir Zarnitsyn is receiving royalties from his contributions to the Clearside Biomedical family of patents (ocular drug delivery)
Meredith E. Davis-Gardner
Patents, Royalties, Other Intellectual Property: My husband holds two patents through Emmune, Inc related to HIV inhibitors and their delivery by AAV vectors
Stephanie L. Foster
Employment: DaVita Dialysis
Patents, Royalties, Other Intellectual Property: I have a patent application with the US Patent Office for a vaccine to prevent Nipah virus disease
Srilatha Edupuganti
Research Funding: Sanofi (Inst)
Mehul S. Suthar
Consulting or Advisory Role: Moderna Therapeutics, Ocugen
Research Funding: Moderna Therapeutics (Inst), Ocugen (Inst)
Madhav V. Dhodapkar
Consulting or Advisory Role: Roche/Genentech, Lava Therapeutics, Janssen Oncology
Suresh Ramalingam
Consulting or Advisory Role: Amgen, Genentech/Roche, Lilly/ImClone, Bristol Myers Squibb, AstraZeneca, Merck, Takeda, GlaxoSmithKline, Eisai, Mirati Therapeutics
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
Rafi Ahmed
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patents on PD-1–directed immunotherapy, I am listed as a coinventor on an Emory University–held patent for SARS-CoV-2 serology assays
No other potential conflicts of interest were reported.
See accompanying editorial on page 3787
DISCLAIMER
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
SUPPORT
Supported in part by grants (NIH P51 OD011132, 3U19AI057266-17S1, and 1U54CA260563 and NIH/NIAID CEIRR CEIRS contracts HHSN272201400004C and 75N93021C00017) from the National Institute of Allergy and Infectious Diseases (NIAID), by The Oliver S. and Jennie R. Donaldson Charitable Trust, Emory Executive Vice President for Health Affairs Synergy Fund award, the Pediatric Research Alliance Center for Childhood Infections and Vaccines and Children's Healthcare of Atlanta, COVID-Catalyst-I3 Funds from the Woodruff Health Sciences Center and Emory School of Medicine, and Woodruff Health Sciences Center 2020 COVID-19 CURE Award. The research reported in this publication was supported in part by the National Cancer Institute of the National Institutes of Health under award No. P50CA217691. The research reported in this publication was supported in part by the Biostatistics Shared Resource of Winship Cancer Institute of Emory University and NIH/NCI under award No. P30CA138292.
R.M.V. and J.C. contributed equally to this work. S.R. and R.A. contributed equally to this work.
AUTHOR CONTRIBUTIONS
Conception and design: Rajesh M. Valanparambil, Jennifer Carlisle, Suresh Ramalingam, Rafi Ahmed
Financial support: Rustom Antia, Mehul S. Suthar, Madhav V. Dhodapkar, Suresh Ramalingam, Rafi Ahmed
Administrative support: Suresh Ramalingam
Provision of study materials or patients: Lilin Lai, Conor Steur, Felicia Green, Margaret A. Johns, Uma Shanmugasundaram, Lindsay E. Nyhoff, Srilatha Edupuganti, Mehul S. Suthar, Madhav V. Dhodapkar, Suresh Ramalingam
Collection and assembly of data: Rajesh M. Valanparambil, Susanne L. Linderman, Akil Akthar, Ralph Linwood Millett, Lilin Lai, Andres Chang, Tahseen H. Nasti, Manpreet Saini, Kelly E. Manning, Madison Ellis, Kathryn M. Moore, Stephanie L. Foster, Katharine Floyd, Meredith E. Davis-Gardner, Venkata-Viswanadh Edara, Mit Patel, Conor Steur, Ajay K. Nooka, Felicia Green, Margaret A. Johns, Fiona O'Brein, Uma Shanmugasundaram, Lindsay E. Nyhoff, Grace Mantus, Michael Garett, Srilatha Edupuganti, Madhusmita Behra, Jens Wrammert, Mehul S. Suthar, Suresh Ramalingam, Rafi Ahmed
Data analysis and interpretation: Rajesh M. Valanparambil, Susanne L. Linderman, Akil Akthar, Andres Chang, Ashley A. McCook-Veal, Jeffrey Switchenko, Andreas Wieland, Conor Steur, Ajay K. Nooka, Veronika I. Zarnitsyna, Hasan Ahmed, Rustom Antia, Jens Wrammert, Mehul S. Suthar, Madhav V. Dhodapkar, Suresh Ramalingam, Rafi Ahmed
Manuscript writing: All authors
Final approval of manuscript: All authors
Accountable for all aspects of the work: All authors
AUTHORS' DISCLOSURES OF POTENTIAL CONFLICTS OF INTEREST
Antibody Response to COVID-19 mRNA Vaccine in Patients With Lung Cancer After Primary Immunization and Booster: Reactivity to SARS-CoV-2 WT Virus and Omicron Variant
The following represents disclosure information provided by authors of this manuscript. All relationships are considered compensated unless otherwise noted. Relationships are self-held unless noted. I = Immediate Family Member, Inst = My Institution. Relationships may not relate to the subject matter of this manuscript. For more information about ASCO's conflict of interest policy, please refer to www.asco.org/rwc or ascopubs.org/jco/authors/author-center.
Open Payments is a public database containing information reported by companies about payments made to US-licensed physicians (Open Payments).
Jennifer Carlisle
Research Funding: AstraZeneca (Inst)
Ralph Linwood Millett
Stock and Other Ownership Interests: Merck
Andreas Wieland
Patents, Royalties, Other Intellectual Property: Inventor on a patent (US patent application No. 16/971,627) filed by Emory University related to HPV-specific monoclonal antibodies and HPV E2 as a potential immunological target in HPV-positive cancers and inventor on a patent application filed by Emory University related to the use of HPV-specific TCR sequences
Conor Steur
Honoraria: Merck
Consulting or Advisory Role: AbbVie, BerGenBio, ARMO BioSciences, Lilly, Mirati Therapeutics, Sanofi/Regeneron, Caris Life Sciences
Research Funding: Vaccinex, Seattle Genetics, Daiichi Sankyo, Infinity Pharmaceuticals
Ajay K. Nooka
Honoraria: Amgen, Janssen Oncology, Bristol Myers Squibb/Celgene, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Consulting or Advisory Role: Amgen, Janssen Oncology, Bristol Myers Squibb, GlaxoSmithKline, Takeda, Oncopeptides, Karyopharm Therapeutics, Adaptive Biotechnologies, Genzyme, BeyondSpring Pharmaceuticals, Secura Bio
Research Funding: Amgen (Inst), Janssen Oncology (Inst), Takeda (Inst), Bristol Myers Squibb/Celgene (Inst), Arch Oncology (Inst), GlaxoSmithKline (Inst)
Travel, Accommodations, Expenses: GlaxoSmithKline
Margaret A. Johns
Employment: Atlanta Center for Medicine
Veronika I. Zarnitsyna
Patents, Royalties, Other Intellectual Property: My husband Vladimir Zarnitsyn is receiving royalties from his contributions to the Clearside Biomedical family of patents (ocular drug delivery)
Meredith E. Davis-Gardner
Patents, Royalties, Other Intellectual Property: My husband holds two patents through Emmune, Inc related to HIV inhibitors and their delivery by AAV vectors
Stephanie L. Foster
Employment: DaVita Dialysis
Patents, Royalties, Other Intellectual Property: I have a patent application with the US Patent Office for a vaccine to prevent Nipah virus disease
Srilatha Edupuganti
Research Funding: Sanofi (Inst)
Mehul S. Suthar
Consulting or Advisory Role: Moderna Therapeutics, Ocugen
Research Funding: Moderna Therapeutics (Inst), Ocugen (Inst)
Madhav V. Dhodapkar
Consulting or Advisory Role: Roche/Genentech, Lava Therapeutics, Janssen Oncology
Suresh Ramalingam
Consulting or Advisory Role: Amgen, Genentech/Roche, Lilly/ImClone, Bristol Myers Squibb, AstraZeneca, Merck, Takeda, GlaxoSmithKline, Eisai, Mirati Therapeutics
Research Funding: AbbVie (Inst), Bristol Myers Squibb (Inst), Pfizer (Inst), Merck (Inst), AstraZeneca/MedImmune (Inst), Vertex (Inst), Takeda (Inst), EMD Serono (Inst), Genmab (Inst), Advaxis (Inst), Amgen (Inst)
Travel, Accommodations, Expenses: AstraZeneca
Other Relationship: American Cancer Society
Rafi Ahmed
Research Funding: Merck
Patents, Royalties, Other Intellectual Property: Patents on PD-1–directed immunotherapy, I am listed as a coinventor on an Emory University–held patent for SARS-CoV-2 serology assays
No other potential conflicts of interest were reported.
REFERENCES
- 1.Carvalho T, Krammer F, Iwasaki A: The first 12 months of COVID-19: A timeline of immunological insights. Nat Rev Immunol 21:245-256, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hu B, Guo H, Zhou P, et al. : Characteristics of SARS-CoV-2 and COVID-19. Nat Rev Microbiol 19:141-154, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Polack FP, Thomas SJ, Kitchin N, et al. : Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine. N Engl J Med 383:2603-2615, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baden LR, El Sahly HM, Essink B, et al. : Efficacy and safety of the mRNA-1273 SARS-CoV-2 vaccine. N Engl J Med 384:403-416, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Flemming A: Omicron, the great escape artist. Nat Rev Immunol 22:75-75, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cameroni E, Bowen JE, Rosen LE, et al. : Broadly neutralizing antibodies overcome SARS-CoV-2 Omicron antigenic shift. Nature 602:664-670, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harvey WT, Carabelli AM, Jackson B, et al. : SARS-CoV-2 variants, spike mutations and immune escape. Nat Rev Microbiol 19:409-424, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edara V-V, Manning KE, Ellis M, et al. : mRNA-1273 and BNT162b2 mRNA vaccines have reduced neutralizing activity against the SARS-CoV-2 Omicron variant. bioRxiv, 2021. 2021.12.20.473557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kupferschmidt K: New mutations raise specter of “immune escape.” Science 371:329-330, 2021 [DOI] [PubMed] [Google Scholar]
- 10.Hu J, Peng P, Cao X, et al. : Increased immune escape of the new SARS-CoV-2 variant of concern Omicron. Cell Mol Immunol 19:293-295, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lopez Bernal J, Andrews N, Gower C, et al. : Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med 385:585-594, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doria-Rose NA, Shen X, Schmidt SD, et al. : Booster of mRNA-1273 strengthens SARS-CoV-2 Omicron neutralization. medRxiv, 2021. 2021.12.15.21267805 [Google Scholar]
- 13.Planas D: Considerable escape of SARS-CoV-2 Omicron to antibody neutralization. Nature 602:671-675, 2021 [DOI] [PubMed] [Google Scholar]
- 14.Collier DA, Ferreira IATM, Kotagiri P, et al. : Age-related immune response heterogeneity to SARS-CoV-2 vaccine BNT162b2. Nature 596:417-422, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Passaro A, Bestvina C, Velez Velez M, et al. : Severity of COVID-19 in patients with lung cancer: Evidence and challenges. J Immunother Cancer 9:e002266, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Naranbhai V, Pernat CA, Gavralidis A, et al. : Immunogenicity and reactogenicity of SARS-CoV-2 vaccines in patients with cancer: The CANVAX cohort study. J Clin Oncol 40:12-23, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gounant V, Ferré VM, Soussi G, et al. : Efficacy of severe acute respiratory syndrome Coronavirus-2 vaccine in patients with thoracic cancer: A prospective study supporting a third dose in patients with minimal serologic response after two vaccine doses. J Thorac Oncol 17:239-251, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson EJ, Rouphael NG, Widge AT, et al. : Safety and immunogenicity of SARS-CoV-2 mRNA-1273 vaccine in older adults. N Engl J Med 383:2427-2438, 2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Verma R, Foster RE, Horgan K, et al. : Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res 18:10, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shroff RT, Chalasani P, Wei R, et al. : Immune responses to two and three doses of the BNT162b2 mRNA vaccine in adults with solid tumors. Nat Med 27:2002-2011, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greenberger LM, Saltzman LA, Senefeld JW, et al. : Antibody response to SARS-CoV-2 vaccines in patients with hematologic malignancies. Cancer cell 39:1031-1033, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Oosting SF, van der Veldt AAM, GeurtsvanKessel CH, et al. : mRNA-1273 COVID-19 vaccination in patients receiving chemotherapy, immunotherapy, or chemoimmunotherapy for solid tumours: a prospective, multicentre, non-inferiority trial. Lancet Oncol 22:1681-1691, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Massarweh A, Eliakim-Raz N, Stemmer A, et al. : Evaluation of seropositivity following BNT162b2 messenger RNA vaccination for SARS-CoV-2 in patients undergoing treatment for cancer. JAMA Oncol 7:1133-1140, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tao K, Tzou PL, Nouhin J, et al. : The biological and clinical significance of emerging SARS-CoV-2 variants. Nat Rev Genet 22:757-773, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Garon EB, Rizvi NA, Hui R, et al. : Pembrolizumab for the treatment of non–small-cell lung cancer. N Engl J Med 372:2018-2028, 2015 [DOI] [PubMed] [Google Scholar]
- 26.Bar-On YM, Goldberg Y, Mandel M, et al. : Protection of BNT162b2 vaccine booster against Covid-19 in Israel. N Engl J Med 385:1393-1400, 2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferdinands JM, Rao S, Dixon BE, et al. : Waning 2-dose and 3-dose effectiveness of mRNA vaccines against COVID-19–Associated Emergency Department and Urgent Care Encounters and Hospitalizations among adults during periods of Delta and Omicron variant predominance—VISION Network, 10 states, August 2021–January 2022. MMWR Morb Mortal Wkly Rep 71:255-263, 2022 [DOI] [PMC free article] [PubMed] [Google Scholar]