Abstract
Babesia microti produces a self-limiting infection in mice, and recovered mice are resistant to reinfection. In the present study, the role of T cells in protective immunity against challenge infection was examined. BALB/c mice which recovered from primary infection showed strong protective immunity against challenge infection. In contrast, nude mice which failed to control the primary infection and were cured with an antibabesial drug did not show protection against challenge infection. Treatment of immune mice with anti-CD4 monoclonal antibody (MAb) diminished the protective immunity against challenge infection, but treatment with anti-CD8 MAb had no effect on the protection. Transfer of CD4+ T-cell-depleted spleen cells resulted in higher parasitemia than transfer of CD8+ T-cell-depleted spleen cells. A high level of gamma interferon (IFN-γ), which was produced by CD4+ T cells, was observed for the culture supernatant of spleen cells from immune mice, and treatment of immune mice with anti-IFN-γ MAb partially reduced the protection. Moreover, no protection against challenge infection was found in IFN-γ-deficient mice. On the other hand, treatment of immune mice with MAbs against interleukin-2 (IL-2), IL-4, or tumor necrosis factor alpha did not affect protective immunity. These results suggest essential requirements for CD4+ T cells and IFN-γ in protective immunity against challenge infection with B. microti.
Babesia species are hemoprotozoan parasites of animals which are transmitted by ticks. Babesia parasites infect a wide variety of wild and domestic animals, and enormous economic losses due to babesiosis are reported throughout the world (14). Some Babesia parasites also infect human beings. Human babesial infections are beginning to emerge as a public health concern in Europe and, especially, in the United States (9, 20, 29). In order to develop successful chemotherapy treatments, effective prevention methods, or an effective vaccine, it is critical to understand the immune mechanism of Babesia infections. However, the mechanisms of the mediating control of the primary infection or protective immunity against Babesia infections remain to be clarified.
Babesia microti, an intraerythrocytic parasite of rodents, is used as a useful experimental model to study immune mechanisms for animal infection and is also the etiologic agent for human babesiosis (9, 27). Mice infected with B. microti produce transient high parasitemias, but they subsequently recover from the acute infection (11, 23). The role of T cells in the resolution of primary infection in mice has been suggested. Congenitally athymic nude mice (4); lethally irradiated, thymectomized mice reconstituted with anti-theta serum-treated bone marrow cells (24); or hamsters administered antilymphocyte serum (39) failed to suppress B. microti parasitemia. Recently, it has been demonstrated that CD4+ T cells play an essential role in the resolution of primary infection with B. microti (11, 28) and that gamma interferon (IFN-γ) produced by CD4+ T cells is partially responsible for resolution of primary infection with B. microti (11).
After recovery from the primary infection, mice are protected against reinfection with B. microti. The spleen appears to play an important role in immunity to Babesia (12). Immunity to reinfection with B. microti was successfully transferred by immune spleen cells (5, 18, 25). The importance of T cells for the protection against reinfection was demonstrated with anti-theta serum-treated immune spleen cells (25), Sephadex G-10-adherent spleen cells (19), or T-cell clones (8). These results suggest that T-cell-mediated immunity plays a significant role in protective immunity against reinfection with B. microti in mice. However, the specific subset of T lymphocytes and the mechanism responsible for protective immunity against B. microti are not yet known. In the present study, the role of T cells in B. microti reinfection was examined with BALB/c mice and BALB/c nude mice. To identify T-cell subsets, immune mice were treated with anti-CD4 or anti-CD8 monoclonal antibodies (MAbs) during the course of challenge infection, and thereafter, the subpopulation of T cells responsible for adoptive transfer of immunity was determined. The role of cytokines in protective immunity was also studied by administration of MAb against cytokines or by using IFN-γ-deficient mice.
MATERIALS AND METHODS
Mice.
Female BALB/c mice and BALB/c nu/nu mice were purchased from CLEA Japan (Tokyo, Japan). IFN-γ-deficient mice were generated as previously described (33). Male and female IFN-γ-deficient mice were backcrossed to BALB/c for seven generations and maintained by interbreeding heterozygous animals. Homozygous (−/−) and wild-type (+/+) littermates were identified by isolation of genomic tail DNA by proteinase K digestion and one extraction with Tris-EDTA-saturated phenol. After precipitation with ethanol, the DNA was dissolved in distilled water. An aliquot of the genomic DNA was amplified in a PCR with one sequence within the neomycin cassette (antisense, 5′-ACG TGC ATG GAT CTG CAA CAT GTC-3′) and two adjacent sequences of the IFN-γ gene (sense, 5′-AAC AGA GGA TGG TTT GCA TCT GGG-3′; antisense, 5′-AAA GCC AAG ATG CAG TGT GTA GCG-3′). PCR conditions were as follows: one incubation at 94°C for 4 min and 40 cycles of 94°C for 1 min, 66°C for 2 min, and 72°C for 3 min. The final incubation was at 72°C for 7 min, followed by agarose gel separation and ethidium bromide staining of the products. All mice were between 5 and 7 weeks old at the time of the experiment. They were housed in filter-topped autoclaved cages and given autoclaved food and water.
Parasite passage and infection of mice.
The Munich strain of B. microti was maintained by blood passage in mice as previously described (11). Immune animals were prepared by intraperitoneal (i.p.) infection of BALB/c mice with 107 parasitized erythrocytes. Mice which resolved infections by 30 days after the primary infection were used for challenge infection or as immune cell donors. Infected nude and IFN-γ−/− mice were treated intramuscularly with diazoaminodibenzamidine diaceturate (Ganaseg; Ciba-Geigy; 1 mg/mouse) for 6 consecutive days starting at 30 days after primary infection. These drug-cured mice were used for challenge experiments. Challenge infections in mice were initiated by the i.p. injection of 107 infected erythrocytes. Parasitemia was monitored by examining between 200 and 104 erythrocytes in Giemsa-stained thin blood films.
Treatment of mice with MAbs.
Immune mice were treated i.p. with anti-CD4 (GK1.5) or anti-CD8 (53-6.72) MAbs which were prepared as previously described by Igarashi et al. (11). Half a milligram of either anti-CD4 or anti-CD8 MAb was injected into each mouse for 3 successive days before challenge infection and twice weekly thereafter for the duration of the experiment. Greater than 96% depletion of CD4+ or CD8+ T cells in immune mice was indicated by flow cytometric analysis of spleen cells with a FACScan flow cytometer (Becton Dickinson, Mountain View, Calif.) as previously described (11). Anti-IFN-γ (XMG1.2), anti-interleukin 2 (IL-2) (S4B6), anti-IL-4 (11B11), and anti-tumor necrosis factor alpha (TNF-α) (MP6-XT22) MAbs were prepared for neutralization of lymphokines as previously described (11, 38). After parasite inoculation, mice were injected i.p. with 1 mg of anti-IFN-γ, anti-IL-2, or anti-IL-4 MAb or 2 mg of anti-TNF-α MAb for 3 consecutive days and thereafter every other day until day 10. Control mice were treated with 0.5, 1, or 2 mg of normal rat immunoglobulin G (IgG) (Caltag Laboratories, South San Francisco, Calif.).
Passive transfer of immune spleen cells and immune serum.
Immune spleen cells were taken from immune donors 2 to 3 weeks after resolution of the primary infection. CD4+- or CD8+-depleted spleen cells were prepared by treating immune mice with 1 mg of anti-CD4 or anti-CD8 MAb 1 day before cell transfer. Ninety-five percent of CD4+ and 98% of CD8+ cells were depleted by MAb treatment as confirmed by the flow cytometry described above. BALB/c naive mice were immunized by adoptive transfer of spleen cells (5 × 107 cells/mouse) and challenged i.p. with 107 infected erythrocytes.
Immune sera were collected from immune mice prepared as described above. The antibody titer in the pooled sera was 1:4,096, which was determined by the indirect immunofluorescent antibody test with a slight modification as previously described (37). Naive BALB/c mice were injected intravenously with 0.5 ml of immune serum or normal serum on days 8, 5, and 2 before infection and challenged with 107 infected erythrocytes. The control group of mice received an identical regimen of saline.
Detection of IFN-γ and TNF-α in immune mice.
Spleen cells were prepared from challenged mice in RPMI 1640 (ICN Biomedicals, Cleveland, Ohio) containing 5% fetal bovine serum and cultured in a six-well plate at 2.5 × 107 cells/5 ml/well with 200 μl (equivalent to 2.5 × 108 infected erythrocytes) of B. microti lysate antigen prepared as described by Igarashi et al. (11). Briefly, blood with 60 to 80% parasitemia was obtained from infected mice, and infected erythrocytes were frozen-thawed three times. The sample was centrifuged at 10,000 × g for 30 min at 4°C, and the obtained supernatant was used as B. microti lysate antigens. Culture supernatants were collected after 72 h and used for the determination of IFN-γ production. Serum samples for the determination of TNF-α levels were collected on indicated days. Concentrations of IFN-γ in culture supernatants and of TNF-α in serum were measured with enzyme-linked immunosorbent assay kits (Endogen, Cambridge, Mass.) according to the manufacturer’s instructions.
Statistics.
All statistical analyses were performed by using the unpaired Student t test.
RESULTS
The role of T cells in protection against challenge infection with B. microti.
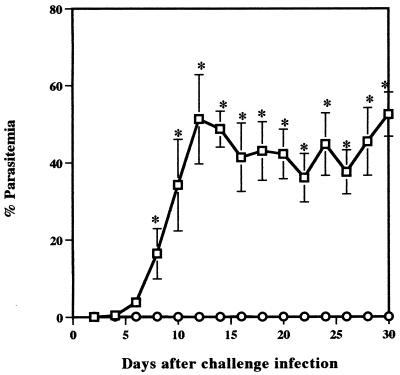
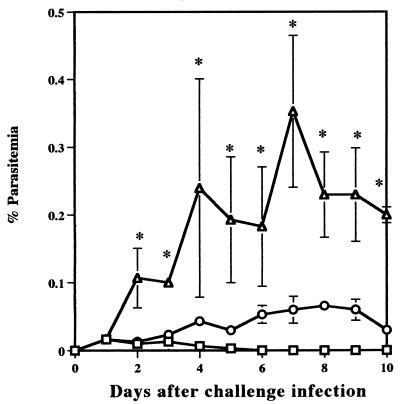
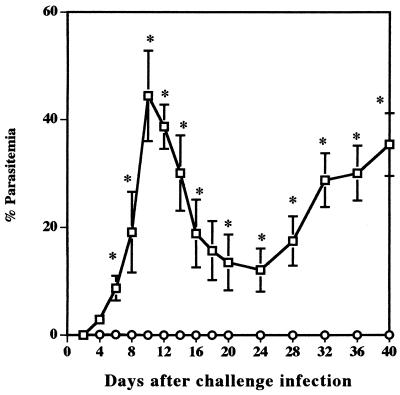
The effect of T cells on resistance to reinfection with B. microti was examined by comparing parasitemias in BALB/c and in nude mice. The infection with B. microti in BALB/c mice was self-limiting and was controlled by 30 days after primary infection. In contrast, nude mice failed to resolve acute infection, and persistent parasitemias of between 40 and 70% were observed until 30 days after primary infection. After treatment with Ganaseg for 6 days from day 30 after primary infection, all parasites had disappeared in infected nude mice. Fifty days after primary infection, both BALB/c and nude mice were challenged with 107 B. microti-infected erythrocytes and the percent parasitemia was determined (Fig. 1). BALB/c mice had strong protection against challenge infection. They showed only latent parasitemias, which reached a very low level with a maximum individual parasitemia of 0.15%. However, nude mice showed significantly higher parasitemia on day 8 after challenge infection and thereafter developed high parasitemias of between 30 and 60% by the 30th day. These results demonstrate that T cells are essential for protective immunity against reinfection with B. microti in mice.
FIG. 1.
Comparison of B. microti infection in BALB/c (○) and in nude (□) mice. BALB/c mice recovered from primary infection or nude mice cured with chemotherapy were challenged with 107 parasites. Each value represents the mean parasitemia ± standard error of the mean for six mice. Asterisks indicate a significant difference (P < 0.05) between two groups. Data are representative of two separate experiments.
Effects of anti-CD4 treatment in immune mice.
Immune BALB/c mice were treated with anti-CD4 or anti-CD8 MAb to determine the T-cell subset required for the protective immunity against B. microti challenge infection. On day 0 (50 days after primary infection), mice were challenged with i.p. injection of 107 B. microti-infected erythrocytes. CD4+ T-cell-depleted mice had an average peak parasitemia of 27.2% on day 16 after challenge infection and demonstrated a significantly higher parasitemia than control mice on days 6 to 24 after challenge infection (Fig. 2A). In contrast, CD8+ T-cell-depleted immune mice showed a level of parasitemia similar to that of control mice, with a maximum parasitemia of 0.15%. The levels of Babesia-specific IgG antibodies in serum were compared in CD4+ and CD8+ T-cell-depleted mice. All mice showed high antibody titers (1:4,096) before challenge infection. High IgG titers (1:4,096) were maintained in the control and CD8+-depleted mice after challenge infection. CD4+-depleted mice tended to show lower IgG titers than did control and CD8+-depleted mice from 12 to 24 days after challenge infection, but differences were not significant (Fig. 2B).
FIG. 2.
Effect of MAb treatment on the course of infection (A) and antibody titers (B) in mice challenged with B. microti. Mice recovered from primary infection were challenged with 107 parasites. □, untreated control; ○, treated with anti-CD4 MAb; ▵, treated with anti-CD8 MAb. Each value represents mean parasitemia ± standard error of the mean for four mice. Asterisks indicate a significant difference (P < 0.05) between anti-CD4 MAb-treated and control groups. Data are representative of three separate experiments.
Effect of transfer of spleen cells and immune serum on the challenge infection with B. microti.
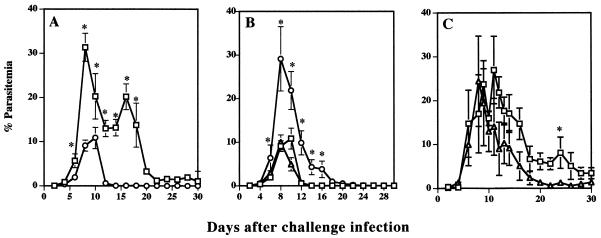
The role of CD4+ T cells was also examined by passive transfer of spleen cells from immune donors. BALB/c naive mice received 5 × 107 immune or normal spleen cells and were challenged with 107 infected erythrocytes on the same day as cell transfer. BALB/c mice which received immune spleen cells developed parasitemias slowly and showed significantly lower peak parasitemia than did the mice which received normal spleen cells on days 8 to 30 after challenge infection, and parasites were rapidly cleared (Fig. 3A). However, BALB/c mice which received normal spleen cells showed significantly higher peak parasitemia, and the clearance of parasites from the circulation was delayed. Mice which received CD8+-depleted spleen cells resolved their infections with kinetics similar to those of mice that received immune spleen cells. However, mice which received CD4+-depleted spleen cells showed significantly higher parasitemia on days 6 to 16 after challenge infection, and the clearance of parasites from the circulation was also delayed (Fig. 3B). These results strongly suggested that CD4+ T cells are required for protective immunity against reinfection with B. microti in mice.
FIG. 3.
Effect of spleen cell transfer and immune serum on the protective immunity against B. microti infection in mice. (A) Mice received spleen cells of mice recovered from primary infection (○) or of normal mice (□). (B) Mice received spleen cells from mice treated with anti-CD4 MAb (○), mice treated with anti-CD8 MAb (▵), or nontreated mice (□). (C) Mice received anti-B. microti immune serum (▵) or normal mouse serum (□). Each value represents mean parasitemia ± standard error of the mean. Asterisks indicate a significant difference (P < 0.05). Data are representative of three separate experiments.
To determine the protective effect of antibody on challenge infection with B. microti, mice were injected intravenously with 0.5 ml of serum from B. microti-immune mice before challenge infection. All of the four injected mice showed high antibody titers (1:4,096) after three injections of immune serum. Higher parasitemias were observed in normal serum recipients than in immune serum recipients after peak parasitemia, but the differences were not significant except on day 22 after challenge infection (Fig. 3C).
Production of IFN-γ and TNF-α.
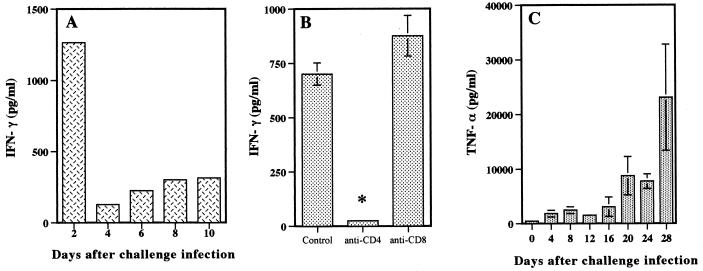
The production of IFN-γ by spleen cells and of TNF-α in serum was examined in mice challenged with B. microti. Spleen cells had to be stimulated with parasite antigen to release IFN-γ in culture. The cells obtained from mice 2 days after the challenge inoculation produced IFN-γ most efficiently (1,226 pg/ml) (Fig. 4A), and this high level of IFN-γ production was completely suppressed by the treatment of mice with anti-CD4 MAb but not by treatment with anti-CD8 MAb (Fig. 4B). IFN-γ production by spleen cells was then reduced drastically on day 4 and gradually recovered thereafter. On the other hand, a first minor peak of TNF-α was observed in serum 8 days after reinfection, and a second major peak of TNF-α was observed in serum between days 16 and 28 (Fig. 4C).
FIG. 4.
IFN-γ and TNF-α production during the course of challenge infection with B. microti. (A) Spleen cells from immune mice were cultured with B. microti lysate antigen, and IFN-γ concentrations were measured in supernatants at 72 h. (B) Spleen cells from mice treated with MAb against CD4+ or CD8+ T cells were cultured as described for panel A. (C) Serum samples were collected on the indicated days for determination of TNF-α production in the serum of mice. Error bars indicate standard errors of the means. An asterisk indicates a significant difference (P < 0.01) between anti-CD4 MAb-treated and control groups. Data are representative of two separate experiments.
Effect of anticytokine MAbs on the course of challenge infection with B. microti.
Immune mice were challenged with B. microti and given neutralizing MAbs to analyze the participation of cytokines in the protective immunity. Mice treated with anti-IFN-γ MAb showed higher parasitemias than did control mice treated with anti-TNF-α MAb or rat IgG (Fig. 5). Treatment of mice with MAbs against IL-2 or IL-4 did not cause an increase in parasitemia (data not shown), compared with treatment with normal rat IgG.
FIG. 5.
Effect of MAb against IFN-γ (▵), TNF-α (○), or normal rat IgG (□) on the course of challenge infection with B. microti. Mice recovered from primary infection were challenged with 107 parasites on day 0, and in vivo treatment was started on the same day. Each value represents the mean parasitemia ± standard error of the mean for five mice. Asterisks indicate a significant difference (P < 0.05) between anti-IFN-γ MAb-treated and control groups. Data are representative of two separate experiments.
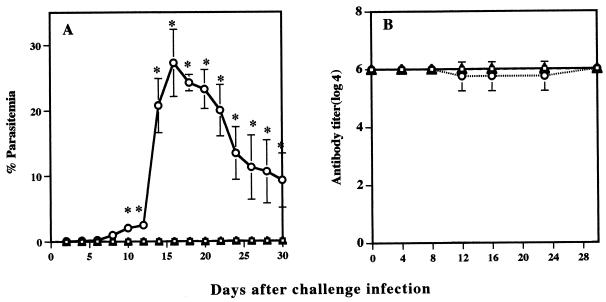
Course of infection of B. microti in IFN-γ-deficient mice.
Since the treatment of immune mice with anti-IFN-γ suggested the possible role of IFN-γ in protective immunity, IFN-γ-deficient mice were used to further examine the role of IFN-γ in protective immunity against B. microti infection. IFN-γ−/− mice failed to resolve the primary infection (data not shown). IFN-γ−/− mice were then treated with a babesiacidal drug and challenged with B. microti. Wild-type mice showed strong protection against challenge infection. They showed only latent parasitemias, which reached a very low level with a maximum individual parasitemia of 0.28%. However, IFN-γ−/− mice showed significantly higher peak parasitemia on day 10 after challenge infection (Fig. 6). Although parasitemia dropped once to a low of 15% on day 24, it increased again thereafter until the end of the experiment. These results strongly suggested that IFN-γ is an essential mediator of protective immunity against challenge infection with B. microti.
FIG. 6.
Time course of B. microti infection in IFN-γ-deficient mice (□) and wild-type control mice (○). Wild-type mice recovered from primary infection or IFN-γ-deficient mice cured with chemotherapy were challenged with 107 parasites. Each value represents the mean parasitemia ± standard error of the mean for five mice. Asterisks indicate a significant difference (P < 0.05) between the two groups. Data are representative of three separate experiments.
DISCUSSION
In the present study, the importance of T cells for protection against reinfection with B. microti was clearly demonstrated by using nude mice. BALB/c mice recovered from primary infection showed strong immunity to challenge infection, with maximum parasitemia of <0.15%, while nude mice which were infected and treated with an antibabesial drug did not develop such immunity and showed very high parasitemia. The importance of T cells for protective immunity against reinfection with B. microti has been suggested in earlier reports. For example, Ruebush et al. (26) reported that a delayed-type hypersensitivity response occurs in parallel with resistance against B. microti. Transfer of immune spleen cells to recipient mice resulted in lower peak parasitemia after challenge infection than did transfer of normal spleen cells, and treatment with anti-theta serum abrogated the protective immunity of immune spleen cells (25). Meeusen et al. (19) demonstrated adoptive transfer of immunity against reinfection with T-cell-enriched spleen cells. Our findings agree with those previous findings and give clearer evidence of the absolute requirement for T cells in protective immunity against B. microti infection.
In rodent malarial infection, the requirement for CD4+ T cells in protective immunity has been demonstrated for infection with Plasmodium berghei (40), Plasmodium chabaudi (1, 2, 15, 32), and Plasmodium yoelii (13, 36). In B. microti infection, protection was achieved by the passive transfer of in vitro-generated CD4+ T-cell clones. However, the protection was partial and short-lived (8). In the present study, immune BALB/c mice were treated with either anti-CD4 or anti-CD8 MAb in order to identify the T-cell subpopulation responsible for protective immunity against reinfection with B. microti. Depletion of CD4+ T cells decreased the protective immunity in immune mice, while depletion of CD8+ T cells did not affect protective immunity. The requirement for CD4+ T cells was also demonstrated by the adoptive transfer of CD4-enriched spleen cells to naive mice. These results suggested that CD4+ T-cell-mediated immunity plays a major role in protection against reinfection with B. microti.
To examine the mechanism by which CD4+ T cells provide protective immunity, the effect of antibody was studied because the Th2 cells among the CD4+ T cells act as helper cells for antibody production (21). Antibody-mediated immunity was suggested elsewhere to be important in protection against B. microti in hamsters (10, 39) and mice (16). In the present study, high antibody titers were observed both for mice treated with anti-CD4 MAb and for those treated with anti-CD8 MAb during challenge infection, although a decrease in protective immunity was observed only for mice treated with anti-CD4 MAb. Protective immunity could not be conferred on naive mice with passive transfer of immune serum. In addition, treatment of immune mice with anti-IL-4 MAb did not affect protective immunity. Cavacini et al. (3) demonstrated that B-cell-deficient mice could control primary infection with B. microti. Taken together, the results of our experiments suggest that antibody does not play a major role in protective immunity against reinfection in mice.
The role of IFN-γ in protective immunity against reinfection was examined in the present study. The importance of IFN-γ has been shown in P. chabaudi infection with neutralizing MAb treatment (17, 30), and a more profound effect of IFN-γ was observed with IFN-γ knockout mice (35). In B. microti infection, the administration of natural human IFN-α to mice inhibited development of parasitemia (22). Our previous study showed that IFN-γ was detected in culture 4 to 5 days prior to the peak parasitemia and that IFN-γ produced by CD4+ T cells is responsible for the resolution of the primary infection (11). In the present study, IFN-γ was detected in cultures of immune spleen cells as early as day 2 after challenge infection, and the amount of IFN-γ (1,226 pg/ml) detected during challenge infection was much higher than that (100.8 pg/ml) detected during primary infection (11). This higher IFN-γ level after the challenge infection was produced by CD4+ T cells and may contribute to the strong protective immunity. Mice treated with anti-IFN-γ MAb showed higher peak parasitemia than did untreated mice or the anti-TNF-α MAb-treated group. However, the decrease in protective immunity caused by anti-IFN-γ MAb treatment was not as great as that caused by anti-CD4 MAb. We suspected that the effect might have been partial because treatment with MAb was not sufficient to completely deplete the IFN-γ activity in mice. Therefore, we infected IFN-γ-deficient mice with B. microti after chemotherapy and observed a more profound effect of IFN-γ on the protective immunity. IFN-γ-deficient mice had higher parasitemia than did wild-type controls and could not develop protective immunity. Our present results indicate that IFN-γ plays an important role in the protective immunity against reinfection with B. microti and suggest that Th1 cells are apparently involved in protective immunity against B. microti.
Our results are not in agreement with those of the report of Hanafusa et al. (8), in which there was no direct correlation between IFN-γ production in vitro and protective activity in vivo. The difference in the role of IFN-γ in protective immunity may simply be due to the different strains of parasites used in the experiments (16). Alternatively, IFN-γ alone may not mediate all the effects of CD4+ cells in protective immunity. As IFN-γ can activate macrophages or the production of other cytokines, additional factors such as TNF, reactive oxygen intermediates, or IL-12 may be involved in the control and elimination of parasites, as has been suggested for malaria (6, 7, 31, 34, 40). In the present study, TNF-α levels were low during the early phase of reinfection and were gradually increased during later stages of reinfection, at which time a decrease of parasitemia was observed in IFN-γ-deficient mice. Therefore, TNF-α may be responsible for the decrease in parasitemia to some extent. Further studies are necessary to clarify the roles of CD4+ T cells and cytokines in protective immunity against B. microti.
ACKNOWLEDGMENTS
We thank Toshikazu Shirahata for supplying the MAb against TNF-α.
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Science, Culture and Sports of Japan.
REFERENCES
- 1.Brake D A, Long C A, Weidanz W P. Adoptive protection against Plasmodium chabaudi adami malaria in athymic nude mice by a cloned cell line. J Immunol. 1988;140:1989–1993. [PubMed] [Google Scholar]
- 2.Cavacini L A, Long C A, Weidanz W P. T-cell immunity in murine malaria: adoptive transfer of resistance to Plasmodium chabaudi adami in nude mice with splenic T cells. Infect Immun. 1986;52:637–643. doi: 10.1128/iai.52.3.637-643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cavacini L A, Parke L A, Weidanz W P. Resolution of acute malarial infections by T-cell-dependent non-antibody-mediated mechanisms of immunity. Infect Immun. 1990;58:2946–2950. doi: 10.1128/iai.58.9.2946-2950.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Clark I A, Allison A C. Babesia microti and Plasmodium berghei yoelii infection in nude mice. Nature. 1974;252:328–329. doi: 10.1038/252328a0. [DOI] [PubMed] [Google Scholar]
- 5.Clark I A, Allison A C, Cox F E. Protection of mice against Babesia and Plasmodium with BCG. Nature. 1976;259:309–311. doi: 10.1038/259309a0. [DOI] [PubMed] [Google Scholar]
- 6.Clark I A, Hunt N H, Butcher G A, Cowden W B. Inhibition of murine malaria (Plasmodium chabaudi) in vivo by recombinant interferon-γ or tumor necrosis factor, and its enhancement by butylated hydroxyaniole. J Immunol. 1987;139:3493–3496. [PubMed] [Google Scholar]
- 7.Dockrell H M, Playfair J H L. Killing of blood-stage murine malaria parasites by hydrogen peroxide. Infect Immun. 1983;39:456–459. doi: 10.1128/iai.39.1.456-459.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hanafusa Y, Onuma M, Kamiyama T. Partial protection of severe combined immunodeficient mice against infection with Babesia microti by in vitro-generated CD4+ T cell clones. J Vet Med Sci. 1998;60:401–404. doi: 10.1292/jvms.60.401. [DOI] [PubMed] [Google Scholar]
- 9.Healy G, Ristic M. Human babesiosis. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press, Inc.; 1985. pp. 209–225. [Google Scholar]
- 10.Hu R, Yeh M-T, Hyland K E, Mather T N. Experimental Babesia microti infection in golden hamsters: immunoglobulin G response and recovery from severe hemolytic anemia. J Parasitol. 1996;82:728–732. [PubMed] [Google Scholar]
- 11.Igarashi I, Waki S, Ito M, Omata Y, Saito A, Suzuki N. Role of CD4+ T cells in the control of primary infection with Babesia microti in mice. J Protozool Res. 1994;4:164–171. [Google Scholar]
- 12.Irvin A D, Young E R, Osborne G D, Francis L M A. A comparison of Babesia infection in intact, surgically splenectomized, and congenitally asplenic (Dh/+) mice. Int J Parasitol. 1981;11:251–255. doi: 10.1016/0020-7519(81)90057-6. [DOI] [PubMed] [Google Scholar]
- 13.Jayawardena A N, Murphy D B, Janeway C A, Gershon R K. T cell-mediated immunity in malaria. I. The Ly phenotype of T cells mediating resistance to Plasmodium yoelii. J Immunol. 1982;129:377–381. [PubMed] [Google Scholar]
- 14.Kuttler K L. World-wide impact of babesiosis. In: Ristic M, editor. Babesiosis of domestic animals and man. Boca Raton, Fla: CRC Press, Inc.; 1985. pp. 2–22. [Google Scholar]
- 15.Langhorne J, Simon-Haarhaus B, Meding S J. The role of CD4+ T cells in the protective immune response to Plasmodium chabaudi in vivo. Immunol Lett. 1990;25:101–107. doi: 10.1016/0165-2478(90)90099-c. [DOI] [PubMed] [Google Scholar]
- 16.Matsubara J, Koura M, Kamiyama T. Infection of immunodeficient mice with a mouse-adapted substrain of the gray strain of Babesia microti. J Parasitol. 1993;79:783–786. [PubMed] [Google Scholar]
- 17.Meding S J, Cheng S C, Simon-Haarhaus B, Langhorne J. Role of gamma interferon during infection with Plasmodium chabaudi chabaudi. Infect Immun. 1990;58:3671–3678. doi: 10.1128/iai.58.11.3671-3678.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Meeusen E, Lloyd S, Soulsby E J. Babesia microti in mice. Adoptive transfer of immunity with serum and cells. Aust J Exp Biol Sci. 1984;62:551–566. doi: 10.1038/icb.1984.53. [DOI] [PubMed] [Google Scholar]
- 19.Meeusen E, Lloyd S, Soulsby E J. Babesia microti in mice. Subpopulations of cells involved in the adoptive transfer of immunity with immune spleen cells. Aust J Exp Biol Med Sci. 1984;62:567–575. doi: 10.1038/icb.1984.54. [DOI] [PubMed] [Google Scholar]
- 20.Moro M H, David C S, Magera J M, Wettstein P J, Barthold S W, Persing D H. Differential effects of infection with a Babesia-like piroplasm, WA1, in inbred mice. Infect Immun. 1998;66:492–498. doi: 10.1128/iai.66.2.492-498.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mosmann T R, Coffman R L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 22.Orinda G O, Waltisbuhl D J, Young A S, Wright I G. Low doses of natural human interferon alpha inhibit the development of Babesia microti infection in BALB/c mice. Vet Parasitol. 1994;53:53–58. doi: 10.1016/0304-4017(94)90016-7. [DOI] [PubMed] [Google Scholar]
- 23.Ruebush M J, Hanson W L. Susceptibility of five strains of mice to Babesia microti of human origin. J Parasitol. 1979;65:430–433. [PubMed] [Google Scholar]
- 24.Ruebush M J, Hanson W L. Thymus dependence of resistance to infection with Babesia microti of human origin in mice. Am J Trop Med Hyg. 1980;29:507–515. doi: 10.4269/ajtmh.1980.29.507. [DOI] [PubMed] [Google Scholar]
- 25.Ruebush M J, Hanson W L. Transfer of immunity to Babesia microti of human origin using T lymphocytes in mice. Cell Immunol. 1980;52:255–265. doi: 10.1016/0008-8749(80)90347-0. [DOI] [PubMed] [Google Scholar]
- 26.Ruebush M J, Troutman E H, Kennedy D A. Delayed-type hypersensitivity to Babesia microti-infected erythrocytes in mice. Cell Immunol. 1986;98:289–299. doi: 10.1016/0008-8749(86)90289-3. [DOI] [PubMed] [Google Scholar]
- 27.Shih C M, Liu L P, Chung W C, Ong S J, Wang C C. Human babesiosis in Taiwan: asymptomatic infection with a Babesia microti-like organism in a Taiwanese woman. J Clin Microbiol. 1997;35:450–454. doi: 10.1128/jcm.35.2.450-454.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shimada T, Shikano S, Hashiguchi R, Matsuki N, Ono K. Effects of depletion of T cell subpopulations on the course of infection and anti-parasite delayed type hypersensitivity response in mice infected with Babesia microti and Babesia rodhaini. J Vet Med Sci. 1996;58:343–347. doi: 10.1292/jvms.58.343. [DOI] [PubMed] [Google Scholar]
- 29.Spielman A, Etkind P, Piesman J, Ruebush T K H, Juraneck D D, Jacobs M S. Reservoir host of human babesiosis on Nantucket Island. Am J Trop Med Hyg. 1981;30:560–566. doi: 10.4269/ajtmh.1981.30.560. [DOI] [PubMed] [Google Scholar]
- 30.Stevenson M M, Tam M F, Belosevic M, van der Meide P H, Podoba J E. Role of endogenous gamma interferon in host response to infection with blood-stage Plasmodium chabaudi AS. Infect Immun. 1990;58:3225–3232. doi: 10.1128/iai.58.10.3225-3232.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stevenson M M, Tam M F, Wolf S F, Sher A. IL-12-induced protection against blood-stage Plasmodium chabaudi AS requires IFN-γ and TNF-α and occurs via a nitric oxide-dependent mechanism. J Immunol. 1995;155:2545–2556. [PubMed] [Google Scholar]
- 32.Süss G, Eichmann K, Kury E, Linke A, Langhorne J. Roles of CD4- and CD8-bearing T lymphocytes in the immune response to the erythrocytic stages of Plasmodium chabaudi. Infect Immun. 1988;56:3081–3088. doi: 10.1128/iai.56.12.3081-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tagawa Y, Sekikawa K, Iwakura Y. Suppression of concanavalin A-induced hepatitis in IFN-γ−/− mice, but not in TNF-α−/− mice: role for IFN-γ in activating apoptosis of hepatocytes. J Immunol. 1997;59:1418–1428. [PubMed] [Google Scholar]
- 34.Traverne J, Tavernier J, Fiers W, Playfair J H L. Recombinant tumor necrosis factor inhibits malaria parasites in vivo but not in vitro. Clin Exp Immunol. 1987;67:1–4. [PMC free article] [PubMed] [Google Scholar]
- 35.van der Heyde H C, Pepper B, Batchelder J, Cigel F, Weidanz W P. The time course of selected malarial infections in cytokine-deficient mice. Exp Parasitol. 1997;85:206–213. doi: 10.1006/expr.1996.4132. [DOI] [PubMed] [Google Scholar]
- 36.Vinetz J M, Kumar S, Good M F, Fowlkes B J, Berzofsky J A, Miller L H. Adoptive transfer of CD8+ T cells from immune animals does not transfer immunity to blood stage Plasmodium yoelii malaria. J Immunol. 1990;44:1069–1074. [PubMed] [Google Scholar]
- 37.Waki S, Suzuki M. Development and decline of antiplasmodial indirect fluorescent antibodies in mice infected with Plasmodium berghei (NK65) and treated with drugs. Bull W H O. 1974;50:521–526. [PMC free article] [PubMed] [Google Scholar]
- 38.Waki S, Uehara K, Kanbe K, Suzuki M, Nariuchi H. The role of T cells in pathogenesis and protective immunity to murine malaria. Immunology. 1992;75:646–651. [PMC free article] [PubMed] [Google Scholar]
- 39.Wolf R E. Effect of anti-lymphocyte serum and splenectomy on the resistance to Babesia microti infection in hamsters. Clin Immunol Immunopathol. 1974;2:381–394. doi: 10.1016/0090-1229(74)90056-7. [DOI] [PubMed] [Google Scholar]
- 40.Yoshimoto T, Yoneto T, Waki S, Nariuchi H. Interleukin-12-dependent mechanisms in the clearance of blood-stage murine malaria parasite Plasmodium berghei XAT, an attenuated variant of P. berghei NK65. J Infect Dis. 1998;177:1674–1681. doi: 10.1086/515301. [DOI] [PubMed] [Google Scholar]