Abstract
Objective:
Adverse childhood experiences have been robustly associated with poor sexual health in later life. In low-income countries, there is growing evidence that children experience greater adversity than those in higher income countries. Research suggests this may contribute to later sexual risk taking and HIV infection, though most studies to date have been cross-sectional.
Design:
We use longitudinal data on adolescents to examine the temporal relationship between adversity and HIV-related behavioral and biological outcomes.
Methods:
We interviewed 1,878 adolescents living in Malawi in 2017–18 (age 10–16) and again in 2021 (age 13–20). Adolescents completed the Adverse Childhood Experience – International Questionnaire. HIV-risk was assessed through both behavioral (e.g., condom use) and biological (HIV and HSV2 infection) outcomes. OLS and logistic multivariate regression models are used to explore associations between adversity and HIV risk.
Results:
In longitudinal analyses, ACEs were significantly associated with intimate partner violence and girls’ behavioral risk scores only. HIV incidence was too low to model; there were no significant associations with HSV2. In cross-sectional analyses, ACEs were additionally associated with an early sexual debut, lack of condom use, a greater number of sexual partnerships, and STI symptoms.
Conclusions:
Our findings emphasize the importance of collecting prospective data: results from longitudinal and cross-sectional analyses drew qualitatively different conclusions. Cross-sectional analyses may not be accurate representations of longitudinal processes. However, they suggest that recent adversity and distress drives HIV-related behavior, perhaps more than early adversity. Interventions that combat emotional abuse or peer violence during adolescence could potentially reduce HIV risk.
Keywords: HIV/AIDS, adverse childhood experiences (ACEs), HSV2, intimate partner violence (IPV), sexual risk
Introduction
There is mounting evidence that adverse childhood experiences (ACEs) explain why some individuals engage in high-risk behaviors and are more likely to contract HIV. ACEs include events that are potentially traumatizing, such as neglect, abuse, family dysfunction, and community violence, experienced during childhood. A recent review pooled global data representing over a quarter million adults from middle and high-income countries. They found that 57% had experienced at least one ACE and 13% reported ≥4 [1]. Importantly, emerging evidence suggests ACEs are even more prevalent in low- and middle-income countries (LMIC) than high-income countries [2–7]. For example, studies in South Africa, Nigeria and Kenya found that 35%, 41% and 78% of adults respectively reported experiencing ≥ 4 ACEs [4–6].
Childhood adversity is robustly associated with poor later-life health – including sexual health [8–17]. A growing number of African studies show that adversity increases HIV infection. In Zimbabwe, childhood abuse was associated with a three-fold increase in HIV infection among adult, post-natal women [18]. A Tanzanian study similarly found that adults with HIV reported greater numbers of childhood traumas [19]. The lone longitudinal study found that emotional, physical, and sexual abuse were each associated with HIV incidence in young South African women (15–26 years), though not in young men [20]. While the evidence is growing, it is not entirely consistent: other studies have reported null results when testing links between ACEs and HIV [5, 21]. For example, a Kenyan study found no significant association between adversity and HIV for women [5].
Thus, it is useful to contextualize HIV studies within larger literatures focused on related sexual health outcomes. For instance, a 2017 meta-analysis of 37 studies found a sixfold increase in sexually transmitted infections (STIs) when comparing individuals with ≥4 ACEs to those with none; it also found a fourfold increase in the odds of multiple sexual partners, early sexual debut and teenage pregnancy [1]. Critically, ACEs emerge as independent risk factors even in challenging, HIV endemic contexts. For example, a South African study of youth (18–30 years) in poor informal settlements found that childhood adversity was associated with transactional sex, multiple partners, intimate partner violence (IPV), and not using condoms [22]. Likewise, a study of youth (19–24) in Malawi, one of the poorest countries grappling with HIV, found childhood adversity was positively associated with infrequent condom use [23].
In our own work, we found that adolescents (10–16 years) in rural Malawi reported high adversity that was positively associated with sexual debut [24]. We have now followed these same adolescents an additional 3–4 years, which allows us to expand on the original study in three critical ways: 1) extend the analyses into a new developmental period (late adolescence); 2) capture a greater range of both behavioral and biological outcomes; and 3) capitalize on longitudinal data to establish the temporality of associations, thus filling an important evidentiary gap on the relationship between adversity and HIV risk.
Methods
Setting and Sample:
Our study took place in Malawi, where 74,000 adolescents were living with HIV in 2020 [25]. The sample is derived from the Malawi Longitudinal Study of Families and Health (MLSFH). Established in 1998, the MLSFH is an adult cohort drawn from three rural districts (Mchinji, Balaka, Rumphi). For each prior MLSFH respondent (alive or deceased), we selected children from household rosters who were projected to be age 10–16 in 2017.
At baseline (2017–18), we interviewed 2,062 adolescents and their caregivers. At follow-up (2021), we located and re-interviewed 1,878 adolescents. Adolescents who had migrated were tracked to their new homes if they resided in the same district or a major city. Respondents lost to follow-up had similar sociodemographic characteristics, but slightly higher ACE counts on average (5.36 versus 5.08) than those retained.
All interviews were conducted face-to-face at the participants’ homes and in local languages. Adolescents below age 18 gave assent after caregiver consent; adolescents ≥18 gave their own consent. Stony Brook University and the Malawian National Health Science Research Committee gave IRB approval.
Measures:
The key exposure evaluated was childhood adversity, measured using the WHO Adverse Childhood Experience – International Questionnaire (ACE-IQ) [26]. The ACE-IQ covers exposure to 13 domains (e.g., neglect, parental death, bullying, community violence). The ACE-IQ begins with “When you were growing up, during the first 18 years of your life…” Because respondents were below the age of 18, we omitted the latter part of the phrase. Studies have shown a dose-response relationship between ACEs and poor health [8, 16], so ACEs were summed to create cumulative scores ranging from 0–13.
HIV-risk was assessed through both behavioral and biological outcomes. Questions focused on proximal risk factors that have been consistently linked to HIV infection [e.g., 27, 28]. Because engagement in any one sexual risk behavior during adolescence is relatively low, a composite score (as opposed to rarer dichotomous outcomes on individual indicators) can better capture risk-taking [29]. It has the added benefit of simplifying data presentation when examining multiple model specifications, and has been used to capture HIV risk in prior studies [e.g., 30, 31]. We created composite behavioral risk scores using four binary indicators: no condom use at last sex; early sexual debut (<16 years); ≥3 lifetime sexual partners; and age-disparate partnerships (>5 years difference). We tested alternative composite scores and results remained substantively similar.
In additional analyses, we examined each of the above indicators separately. We also measured both IPV victimization and self-reported STI symptoms. Adolescents who reported either sexual debut and/or current-or-past romantic partners were asked about IPV. Both girls and boys completed questions adapted from the WHO’s Violence Against Women instrument (VAWI) [32]. Adolescents were considered to have been victims if they reported ever experiencing physical, sexual or emotional IPV. Finally, STI symptoms were assessed by asking girls whether they had experienced bad smelling abnormal genital discharges, genital sores or ulcers in the past year.
HIV status was assessed at baseline and follow-up following Malawian Ministry of Health protocols. Trained HIV counselors first used the Determine® HIV rapid test; if the test was indeterminate, a second test was performed using the Unigold® rapid test. Both tests have very high sensitivity (>99%) and specificity (>99%) in African trials [33]. At follow-up, we also tested for the herpes simplex virus 2 (HSV-2), which is a biomarker often used to indicate risky sexual behavior [34, 35]. HSV-2 infection is potentially also an independent risk factor for HIV acquisition [36–38]. We collected dried-blood spots and assessed HSV-2 positivity using the Focus Technologies HerpeSelect 2 ELISA IgG test. This test has been used in African trials [39], including Malawian adolescent studies. Based on the results of previous African studies [40], we used a more conservative cutoff (2.1) shown to have better sensitivity (94%) and specificity (91%) than that recommended by the manufacturer. This test was conducted only on a subset of respondents due to supply-chain delays.
Analyses:
Our first analyses focused on the composite HIV risk score. We graphed average HIV-risk scores by ACEs score to examine bivariate relations. Next, we estimated OLS longitudinal regressions using three alternative operationalizations of ACEs: 1) cumulative ACEs, 2) tertiles of cumulative ACEs, and 3) binary indicators for each of the 13 individual ACEs. Adjusted multivariate models included age, gender, marital status, socioeconomic status (a continuous asset ownership measure, weighted by inverse of the population proportion owning each asset), and origin region. All models used standard errors clustered by early-childhood caregivers to account for the MLSFH-ACE sampling frame. We plotted HIV-risk scores by baseline cumulative ACEs, and assumptions of linearity appear to largely hold. We plotted predicted composite HIV risk scores by computing marginal effects of increasing cumulative ACEs by one, holding other covariates at their means. As prior studies documented gender relationships between ACEs and HIV behavior, we also presented associations stratified by gender.
Our second group of analyses examines a wider range of behavioral and biological HIV-risk factors. We considered three sets of models, with different timing assumptions: (1) longitudinal associations by regressing follow-up HIV risk factors on baseline ACE tertiles; (2) baseline cross-sectional associations; and (3) follow-up cross-sectional associations. All models used the same set of covariates and accounted for clustering as above, and are designed to contrast longitudinal and cross-sectional approaches. With the exception of the composite risk score, all outcomes were binary and modeled using logistic regressions.
Results
Study sample:
On average, adolescents were 13 at baseline and 16.5 at follow-up, with 12% married or cohabitating by that time (Table 1).
Table 1.
Sample means and proportions: Malawian adolescents (N=1,878)
| Baseline (2017–18) | Follow-up (2021) | |
|---|---|---|
| Demographics | ||
| Age (years) | 13.21 | 16.53* |
| Female | 0.49 | 0.49 |
| Married | 0.01 | 0.12* |
| SES (wealth score) | 2.37 | 2.47 |
| Central (Mchinji) | 0.33 | 0.33 |
| South (Balaka) | 0.35 | 0.35 |
| North (Rumphi) | 0.33 | 0.32 |
| Adversity | ||
| Lifetime ACEs (mean score) | 5.08 | 5.91* |
| Sexual Risk Factors | ||
| HIV Behavioral Risk Score1 | 0.42 | 0.74* |
| Early Sexual Debut | 0.22 | 0.29* |
| No Condom Use | 0.14 | 0.24* |
| 3+ Lifetime Partners | 0.07 | 0.20* |
| Age-Disparate Partnership | NA | 0.04 |
| IPV | 0.07 | 0.16* |
| STI Symptoms2 | NA | 0.09 |
| HSV-2 Infection3 | NA | 0.20 |
| N | 1,878 | 1,878 |
HIV-risk score baseline range is 0–3; follow-up range is 0–4.
Only female respondents
Only collected in Mchinji (N=562)
Denotes that the measure is significantly higher at follow-up (p<0.001)
Distribution of ACEs:
At baseline, adolescents reported ~5 lifetime adversities (Table 2). Most commonly reported was witnessing community violence (88%), emotional neglect (86%), emotional abuse (53%), witnessing domestic violence (59%), and physical abuse (53%).
Table 2.
Prevalence and association (coefficient and 95% confidence interval) with Follow-up HIV-related behavioral risks by reported baseline lifetime adverse childhood experience among Malawian adolescents (N=1, 878)
| Baseline Adverse Childhood Experiences | Estimated Associations with Follow-up HIV Behavioral Risks | |||||||
|---|---|---|---|---|---|---|---|---|
| All | Girls | Boys | ||||||
| bivariate | adjusted | bivariate | adjusted | bivariate | adjusted | |||
| Cumulative ACEs (0–13) | 5.08 | 2.18 | 0.05*** | 0.01 | 0.06*** | 0.04** | 0.03 | 0 |
| [0.03, 0.07] | [−0.00, 0.03] | [0.04, 0.09] | [0.01, 0.06] | [−0.00, 0.06] | [−0.02, 0.03] | |||
| ACE Tertiles | n | Prop. | ||||||
| 1st Tertile (0–4 ACEs) | 804 | 0.43 | - | - | - | - | - | - |
| - | - | - | - | - | - | |||
| 2nd Tertile (5–6 ACEs) | 563 | 0.3 | 0.06 | −0.04 | 0.09 | 0.01 | 0 | −0.08 |
| [−0.05, 0.17] | [−0.14, 0.05] | [−0.05, 0.24] | [−0.11, 0.14] | [−0.16, 0.17] | [−0.22, 0.07] | |||
| 3rd Tertile (7–13 ACEs) | 508 | 0.27 | 0.20*** | 0.06 | 0.32*** | 0.21** | 0.07 | −0.02 |
| [0.08, 0.32] | [−0.04, 0.16] | [0.16, 0.47] | [0.07, 0.34] | [−0.09, 0.24] | [−0.17, 0.13] | |||
| Individual ACEs | n | Prop. | ||||||
| Abuse & Neglect | ||||||||
| Emotional neglect | 1,606 | 0.86 | 0.22*** | 0.05 | 0.20* | 0.05 | 0.21* | 0.08 |
| [0.09, 0.34] | [−0.06, 0.15] | [0.05, 0.36] | [−0.07, 0.17] | [0.02, 0.39] | [−0.09, 0.24] | |||
| Emotional abuse | 991 | 0.53 | 0.23*** | 0.02 | 0.26*** | 0.15* | 0.18* | −0.06 |
| [0.13, 0.32] | [−0.07, 0.11] | [0.14, 0.38] | [0.03, 0.26] | [0.04, 0.32] | [−0.19, 0.08] | |||
| Physical neglect | 612 | 0.33 | 0.02 | 0.09* | 0.03 | 0.07 | 0 | 0.11 |
| [−0.08, 0.11] | [0.00, 0.17] | [−0.11, 0.16] | [−0.04, 0.18] | [−0.14, 0.14] | [−0.02, 0.24] | |||
| Physical abuse | 997 | 0.53 | −0.02 | −0.01 | −0.05 | 0.04 | −0.01 | −0.05 |
| [−0.12, 0.07] | [−0.10, 0.07] | [−0.18, 0.07] | [−0.06, 0.15] | [−0.15, 0.13] | [−0.18, 0.07] | |||
| Sexual abuse | 121 | 0.06 | 0.30** | 0.11 | 0.40** | 0.17 | 0.2 | 0.09 |
| [0.09, 0.51] | [−0.07, 0.29] | [0.14, 0.66] | [−0.05, 0.39] | [−0.14, 0.53] | [−0.19, 0.38] | |||
| Family Dysfunction | ||||||||
| Substance abuser in household | 414 | 0.22 | 0.1 | 0.03 | 0.06 | −0.01 | 0.12 | 0.08 |
| [−0.01, 0.21] | [−0.07, 0.13] | [−0.09, 0.22] | [−0.13, 0.11] | [−0.04, 0.29] | [−0.07, 0.23] | |||
| Someone with mental health issues | 114 | 0.06 | 0.11 | 0.08 | 0.33** | 0.21 | −0.14 | −0.08 |
| [−0.08, 0.30] | [−0.09, 0.24] | [0.08, 0.57] | [−0.00, 0.42] | [−0.43, 0.15] | [−0.32, 0.16] | |||
| Incarcerated household member | 233 | 0.12 | 0.19** | 0.09 | 0.39*** | 0.25** | 0 | −0.03 |
| [0.05, 0.34] | [−0.03, 0.22] | [0.19, 0.60] | [0.07, 0.43] | [−0.20, 0.20] | [−0.21, 0.15] | |||
| Domestic violence | 1,096 | 0.59 | 0.05 | 0 | 0.1 | 0.04 | 0.02 | −0.02 |
| [−0.04, 0.14] | [−0.08, 0.08] | [−0.03, 0.23] | [−0.06, 0.14] | [−0.12, 0.15] | [−0.15, 0.10] | |||
| Parents dead or divorced | 931 | 0.5 | 0.14** | 0.03 | 0.28*** | 0.1 | 0.02 | −0.02 |
| [0.05, 0.24] | [−0.05, 0.11] | [0.15, 0.40] | [−0.00, 0.20] | [−0.12, 0.15] | [−0.14, 0.10] | |||
| Peer | ||||||||
| Bullied | 588 | 0.31 | 0.11* | 0.05 | 0.22** | 0.20*** | −0.01 | −0.03 |
| [0.01, 0.21] | [−0.03, 0.14] | [0.07, 0.37] | [0.08, 0.32] | [−0.15, 0.13] | [−0.16, 0.10] | |||
| Community | ||||||||
| Community violence | 1,654 | 0.88 | 0.25*** | −0.05 | 0.12 | −0.09 | 0.39*** | 0 |
| [0.12, 0.39] | [−0.17, 0.06] | [−0.05, 0.29] | [−0.22, 0.05] | [0.19, 0.59] | [−0.18, 0.18] | |||
| Collective violence | 165 | 0.09 | 0.20* | 0.06 | 0.16 | 0.02 | 0.25 | 0.12 |
| [0.03, 0.37] | [−0.08, 0.21] | [−0.06, 0.38] | [−0.16, 0.20] | [−0.00, 0.50] | [−0.10, 0.35] | |||
p<0.05,
p<0.01,
p<0.001
Control variables in the adjusted models are age, gender, follow-up SES, follow-up marital status, and region
Distribution of HIV risk:
At follow-up, more than half of the sample had sexually debuted, with about a third reporting early (<16) sexual debut, 24% reporting not using a condom, 20% reporting having at ≥3 sexual partners, and 16% reporting IPV. One in five adolescents tested positive for HSV-2 infection. There were 6 new HIV infections at follow-up, too few to power further analyses.
Longitudinal relationships between ACEs and HIV risk:
Figure 1 illustrates the positive relationship between the baseline ACE cumulative counts and follow-up HIV-risk scores. This relationship is similarly evident in Table 2: there is a significant bivariate association between baseline ACE scores and follow-up composite HIV-risk scores, though it loses significance when adjusted for sociodemographics. Figure 2 illustrates the HIV-risk scores predicted for the total sample.
Figure 1.
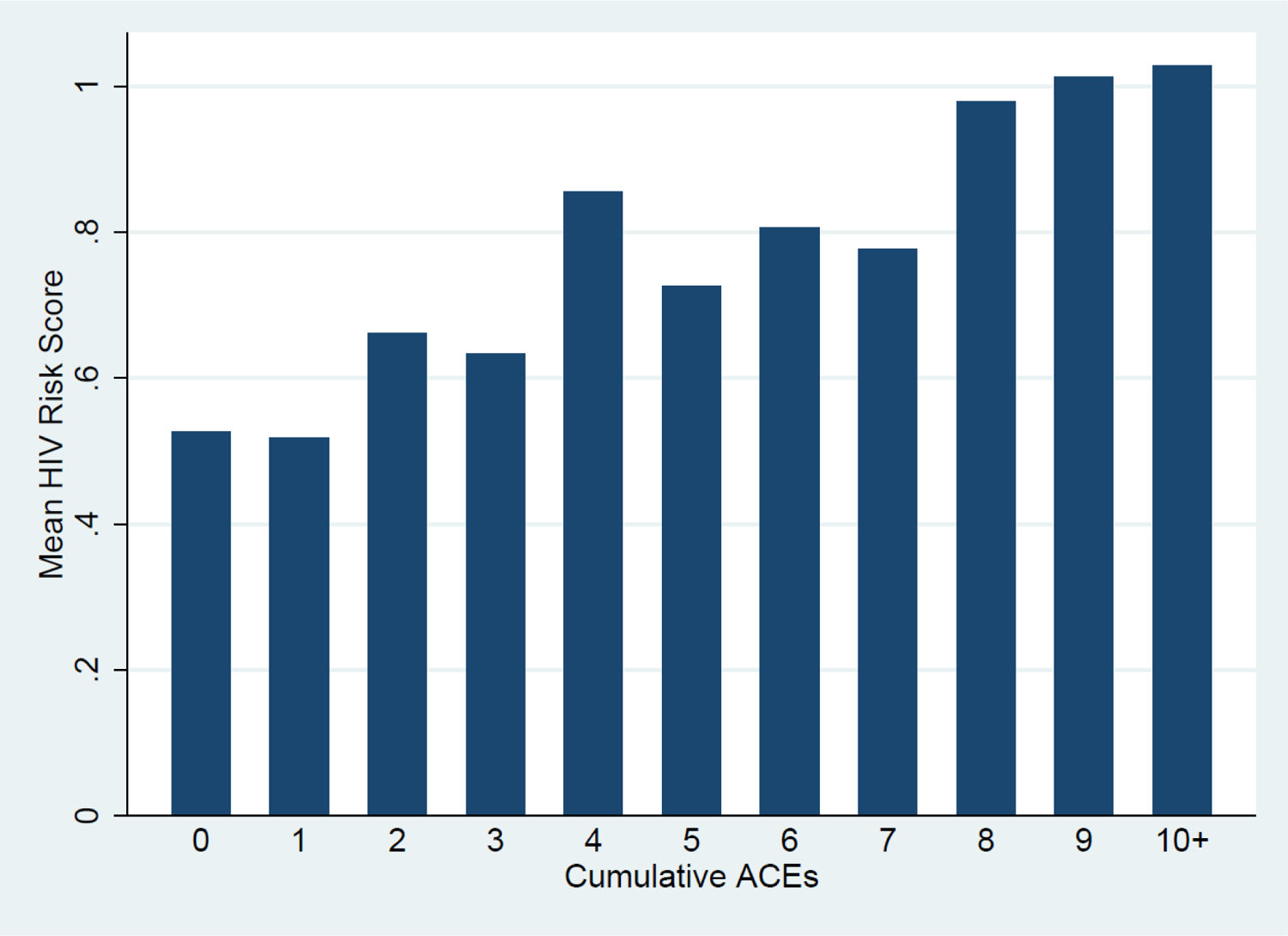
Average follow-up HIV-risk scores by baseline cumulative ACEs
Figure 2.
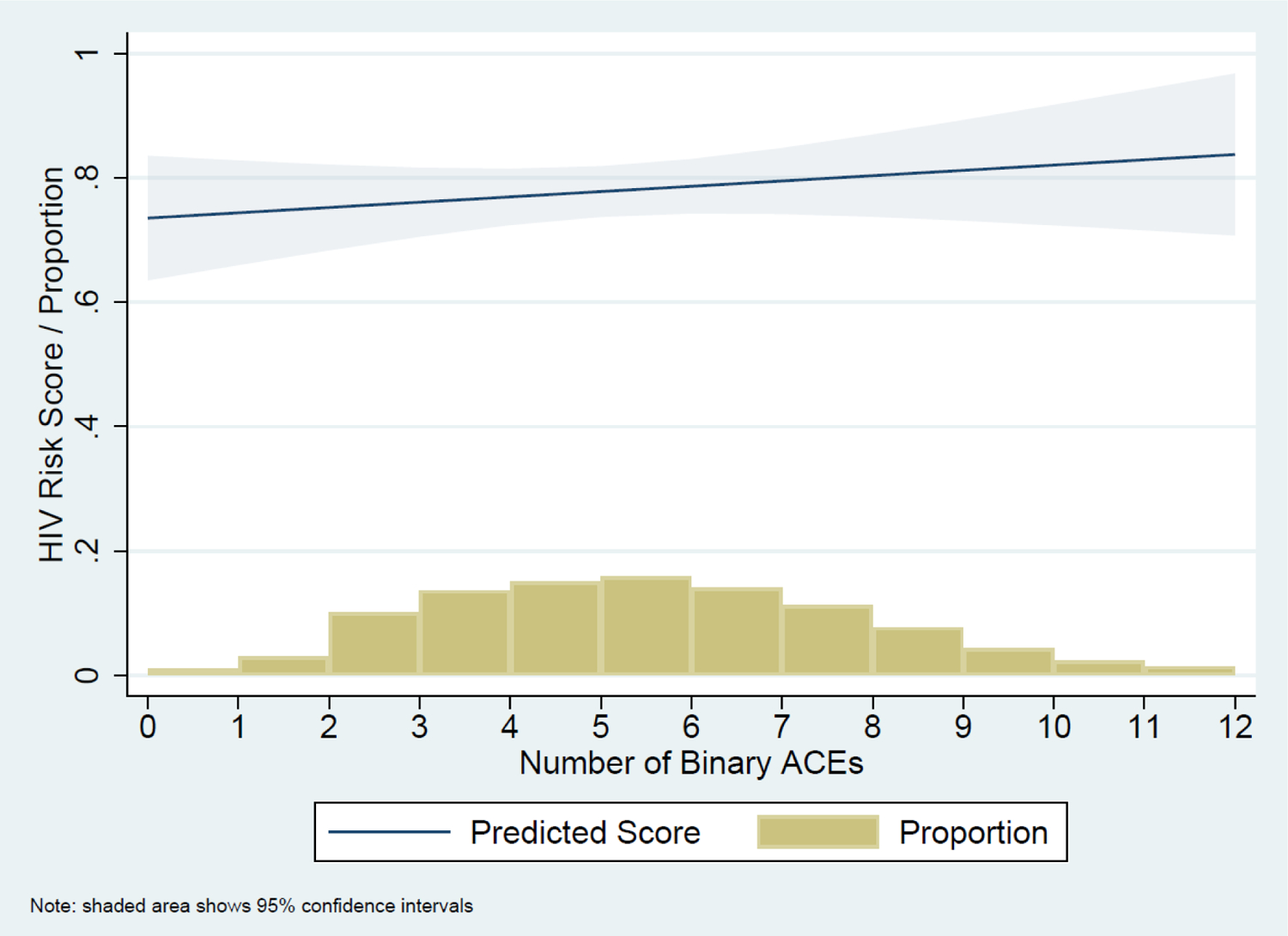
The proportion of respondents reporting each count of binary ACEs and associated the predicted follow-up HIV-risk scores
When we stratified results by gender, we saw a significant association between ACEs and HIV risk for girls but not boys. This held whether we modelled a cumulative score (Risk Difference (RD)=0.04), ACEs tertiles (RD=0.20 for the highest tertile), or individual ACEs (emotional abuse RD=0.15; having a household member who was incarcerated RD=0.26; being bullied by peers RD=0.20).
Next, we looked at individual risk factors. There were no significant findings for girls’ or boys’ biological outcomes (HSV2) (Table 3, Model Set 1). For behavioral outcomes, being in the highest baseline ACE tertile compared to the lowest tertile was positively associated with follow-up IPV for both genders (OR=1.97), and with overall risk scores (RD=0.20) and early sexual debuts (OR=1.88) for girls.
Table 3.
Synthesis of linear and logistic regression models showing associations (coefficient and 95% confidence interval) between ACEs and individual HIV risk factors among adolescents in Malawi (N=1, 878), specific to the timing of measurement
| Model Set 1 | Model Set 2 | Model Set 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline ACEs -> Follow-up Outcomes | Baseline ACEs -> Baseline Outcomes | Follow-up ACEs -> Follow-up Outcomes | |||||||
| HIV-related outcome | All | Girls | Boys | All | Girls | Boys | All | Girls | Boys |
| HIV-behavioral-risk score | RD=0.06 | RD=0.21** | RD=−0.02 | RD=0.12* | RD=0.11* | RD=0.13 | RD=0.18*** | RD=0.21** | RD=0.18* |
| [−0.04, 0.16] | [0.07, 0.34] | [−0.17, 0.13] | [0.03, 0.21] | [0.01, 0.22] | [−0.01, 0.28] | [0.08, 0.28] | [0.08, 0.34] | [0.03, 0.33] | |
| Early sexual debut | OR=1.11 | OR=1.86** | OR=0.81 | OR=1.89*** | OR=1.93** | OR=1.82** | OR=1.51** | OR=1.64* | OR=1.45* |
| [0.84, 1.45] | [1.21, 2.87] | [0.57, 1.15] | [1.40, 2.57] | [1.19, 3.14] | [1.22, 2.71] | [1.14, 1.99] | [1.06, 2.54] | [1.01, 2.08] | |
| No condom use | OR=1.16 | OR=1.45 | OR=1.00 | OR=1.28 | OR=1.23 | OR=1.26 | OR=1.80*** | OR=1.99** | OR=1.68* |
| [0.83, 1.61] | [0.87, 2.39] | [0.64, 1.57] | [0.91, 1.80] | [0.70, 2.16] | [0.82, 1.94] | [1.29, 2.51] | [1.21, 3.27] | [1.06, 2.68] | |
| 3+ lifetime partners | OR=1.31 | OR=1.87* | OR=1.12 | OR=1.44 | OR=5.67* | OR=1.17 | OR=1.56** | OR=2.54** | OR=1.27 |
| [0.96, 1.78] | [1.05, 3.33] | [0.77, 1.63] | [0.90, 2.32] | [1.28, 25.18] | [0.69, 1.98] | [1.12, 2.17] | [1.39, 4.62] | [0.85, 1.88] | |
| Age-disparate partner | OR=1.05 | OR=0.95 | OR=3.76 | NA | NA | NA | OR=0.88 | OR=0.84 | OR=1.73 |
| [0.55, 1.99] | [0.49, 1.85] | [0.32, 44.05] | NA | NA | NA | [0.44, 1.77] | [0.40, 1.74] | [0.10, 29.14] | |
| IPV | OR=1.98*** | OR=2.02** | OR=1.91** | OR=2.83*** | OR=5.09*** | OR=1.84 | OR=4.97*** | OR=5.20*** | OR=4.89*** |
| [1.43, 2.74] | [1.25, 3.24] | [1.20, 3.06] | [1.70, 4.71] | [2.23, 11.64] | [0.92, 3.70] | [3.44, 7.18] | [3.15, 8.57] | [2.83, 8.46] | |
| STI symptoms# | NA | OR=1.24 | NA | NA | NA | NA | NA | OR=2.22** | NA |
| NA | [0.67, 2.27] | NA | NA | NA | NA | NA | [1.24, 3.99] | NA | |
| HSV2 infection | OR=0.95 | OR=1.17 | OR=0.83 | NA | NA | NA | OR=0.87 | OR=0.91 | OR=0.85 |
| [0.56, 1.61] | [0.51, 2.69] | [0.42, 1.65] | NA | NA | NA | [0.52, 1.46] | [0.45, 1.87] | [0.40, 1.77] | |
p<0.05,
p<0.01,
p<0.001
Girls only
Risk differences (RD) and odds ratios (OR) shown only for significant associations (at p<0.05) comparing the 3rd tertile of ACEs (7–13 ACEs) with the 1st tertile of ACEs (0–4 ACEs)
NA = not applicable
All models control for respondent’s age, gender, follow-up SES, follow-up marital status and region.
Cross-sectional relationships between ACEs and HIV risk:
In cross-sectional analyses (Table 3, Model Sets 2 & 3), we found ACE tertiles were positively associated with composite scores of HIV behavioral risks, sexual debut and IPV for both genders at both time points (e.g., RD=0.18; OR=1.51; and OR=4.93 respectively at follow-up). ACEs were also associated with a lack of condom use (OR=1.79), greater number of sexual partnerships (OR=1.53), and STI symptoms (OR=2.20 for girls only) at follow-up, when the cohort was in late adolescence. The follow-up cross-sectional associations were much stronger and more consistently present across risk categories and genders than the longitudinal associations. For example, there was no association between ACEs and condom use in the longitudinal analyses; however being in the highest ACE tertile was significantly associated with such in the cross-sectional analyses at follow-up (OR=1.79).
Discussion
Main findings:
We hypothesized that childhood adversities would drive adolescent sexual risk taking and STIs. We tested such with prospective data, and found inconsistent support: there was a significant longitudinal associations for girls’ behavioral risk; there were no significant findings for girls’ biological outcomes (HSV2) or for boys generally. This closely mirrors the pattern found in one of the only other longitudinal studies of childhood adversities and HIV. In South Africa, Jewkes et al. found that emotional, physical, sexual abuse were each individually associated with HIV incidence in young women (15–26 years) [20], yet abuse showed no association with HSV2 incidence over the same two-year follow-up. Moreover, abuse did not predict HIV or HSV2 incidence for young men in that study, consistent with our findings.
Our findings emphasize the importance of collecting prospective data on adolescents in an HIV endemic context. Much of the work on childhood adversities has focused on adults, with retrospective recall of events in childhood. We are able to overcome this limitation by collecting data on adversities prior to measurement of HIV-risk behaviors. Moreover, by focusing on adolescents, there is no confusion about whether the events reported occurred before age 18. The use of longitudinal data as compared to cross-sectional data led to qualitatively different conclusions. In contrast to our longitudinal findings, our repeated cross-sectional estimates were generally in line with previous research showing that higher levels of adversities are associated with increased sexual risk-taking during adolescence, both in high- [e.g., 13, 17] and lower-income countries [e.g., 22]. Thus, cross-sectional associations may not be accurate representations of longitudinal processes, but may be an indicator of current distress.
Understanding how adversity influences HIV risks:
It is thought that both biologic and social processes may mediate the relationship between childhood adversities and adolescent HIV risks [41]. First, chronic stress-response activation can disrupt cognitive, emotional and physiological processes [16, 42, 43]. This could impair impulse control, increase depression, and diminish abilities to recognize potentially risky situations – all of which could impact HIV risks [16, 42, 44–48]. Second, adversities may lead to a cascade of negative events that culminate in high-risk situations. For example, early marriage and school dropout are strong predictors of HIV [49–51].
While our study found some indication that earlier adversities were linked to later risks, we found that HIV-related risks were more strongly and consistently related to adolescent adversities reported concurrently. Thus, the mechanism linking adversities and sexual risks may have less to do with neurodevelopmental pathways, and more to do with recent distress or disrupted social transitions. This suggests the importance of recency, an element not adequately considered in life-course models of childhood adversities and adult health. There is too little rigorous evidence to reach any conclusions, but more research on the timing and mechanisms linking adversities to HIV risks is certainly called for.
One of the most consistent – though also likely the most researched – mediator between childhood adversities and later HIV risks is mental health. Brown et. al. found that psychopathology mediated associations between childhood adversities and HIV/STIs in American adults [52]. Pence et. al. similarly found that current mental-health symptoms, specifically PTSD and depression, seemed to mediate relationships between childhood adversities and HIV in Tanzanian adults [19]. A study of South African girls (12 to 17 years) found no direct pathways between ACEs and HIV risks, but did observe an indirect pathway through mental-health distress [31]. Notably, they found that interventions (in this case schooling) could interrupt this pathway and reduce HIV-risk behavior. This is consistent with theoretical models that take timing of exposure into account. Specifically, Dunn et. al. found that psychopathology in childhood is better explained by recency of exposure, and only to a lesser extent by life-course accumulation of adversities [53]. In our study, current reports of lifetime adversities were a much stronger predictor of HIV-risk behaviors; prior reports were significant only for girls. Though our study does not probe specific mediators, the findings do suggest current perceptions of adversities may be critical.
We note an important caveat: mental distress could reflect a causal pathway, or alternatively reflect confounding. The latter could occur if distress influences both reports of past adversities and engagement in sexual risk behavior. In our cohort, we found inconsistencies where some adolescents seemed to “lose” reported ACEs over time; that is, they failed to report ACEs at follow-up that they had previously reported at baseline [54]. Depression was a significant predictor of these inconsistencies. If current distress is correlated with reporting ACEs, this may confound the cross-sectional association between adversities and HIV risk and explain the significant cross-sectional findings.
Strengths and limitations:
One strength was the incorporation of biological outcomes. We could not model the direct influence of adversities on HIV incidence because the latter was very low. HSV2 was more prevalent, and was used as a proxy since it shares the same mode of sexual transmission [38]. The HSV2 prevalence in our study (20%) falls within the range reported by other recent studies (e.g., 26% among girls 13–26 in South Africa [55]; 17% among girls in secondary school in Kenya [56]). We found no association between childhood adversities and HSV2 infections. This is surprising given the observed association between adversities and sexual-risk behaviors. The above-mentioned studies among African adolescents, however, similarly reported no associations between HSV2 infections and sexual behaviors [55, 56]. We note that HSV2 was only measured at follow-up in our study, and thus cannot be defined as incident cases. It is possible that HSV2 preceded adversities for some, though the young ages of the cohort at baseline makes it unlikely to have been common enough to explain the lack of associations.
A limitation is that while our longitudinal study can establish temporal order between adversity and HIV risk factors, we do not know the specific ages at which adversities occurred. Thus, we do not investigate whether there are sensitive or critical developmental periods, and questions around recency remain unanswered. Even with prospective data collection, moreover, we cannot measure all potentially important covariates. In addition, the follow-up survey wave was conducted a year into the covid-19 pandemic. It is possible that the external threat of the pandemic altered risk-behavior patterns. For example, we observed a drop in sexual debut among younger adolescents (aged 13–16) surveyed in 2021 as compared to 2017–18 [57]. However, we would not expect this drop to change the relative relationship between adversities and HIV risks. Of greater importance is that the new external threat may have changed how adolescents interpret or respond to past adversities, and thus how they influence current risk taking.
Implications for practice:
We cannot end the HIV pandemic without a focus on adolescents [58]. In 2020, 150,000 adolescents (10–19 years) were newly infected, bringing the number of adolescents living with HIV to almost 1.8 million [25]. Risk continues to mount as adolescents transition into adulthood [59]. While there is still much to learn, the high prevalence of adversities and strong correlations with HIV risks across multiple studies justifies more immediate intervention. The UNAIDS Global AIDS Strategy 2021–2026 highlights the disproportionate HIV incidence among adolescent girls and young women [59]. The Strategy also asserts that “a central reason why inequalities in the HIV response persist is that we have not successfully addressed the social and structural determinants that increase HIV vulnerability.” To bring these to the fore, the plan sets ambitious “societal enabler targets.” They estimate that failure to meet these targets will lead to 2.5 million new HIV infections by 2030. Through this lens, the Strategy calls for addressing the unique determinants that make young people vulnerable to HIV, and suggests targeted programming around schooling, social protection, employment and gender-based violence. It may be time to put childhood adversities on that list. Our study adds to the mounting evidence that that reducing violence and other traumas children face in the home could attenuate HIV risk – particularly for girls.
We hypothesized that adversities encountered earlier in childhood would have a sustained impact on HIV risk during later adolescence. Instead, what we observe is that recent appraisal of adversities was more strongly correlated with multiple HIV risk factors for both genders. This may indicate that current events and distress are more important targets for intervention. If so, interventions that focus on reducing abuse and adversities at home [e.g., 60, 61], or reducing peer violence among adolescents [e.g., 62], may have outsized impacts. Adversities reported during earlier periods had weaker impacts, but were none-the-less important predictors for girls. More research is needed to characterize sensitive developmental periods and understand the pathways that may be linking childhood adversities to HIV risks.
Acknowledgements:
Kidman and Kohler participated in the concept and design of the paper. Breton conducted the analysis. Kidman drafted the paper. Kidman, Breton, Behrman and Kohler collaborated on the interpretation of data and refinement of the manuscript. All authors have read and approved the text as submitted to AIDS. The authors thank the respondents for their time and willingness to share their experiences. We also thank the staff (field supervisors, interviewers, counselors, and many others) at Invest in Knowledge International for their time, effort, and support of this project. Research reported in this publication was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under Award Numbers R01HD090988. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
Conflicts of Interest: The authors declare no conflicts of interest.
References
- 1.Hughes K, et al. , The effect of multiple adverse childhood experiences on health: a systematic review and meta-analysis. The Lancet Public Health, 2017. 2(8): p. e356–e366. [DOI] [PubMed] [Google Scholar]
- 2.Tran QA, et al. , Adverse childhood experiences and the health of university students in eight provinces of Vietnam. Asia Pacific Journal of Public Health, 2015. 27(8_suppl): p. 26S–32S. [DOI] [PubMed] [Google Scholar]
- 3.Soares ALG, et al. , Adverse childhood experiences: Prevalence and related factors in adolescents of a Brazilian birth cohort. Child abuse & neglect, 2016. 51: p. 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Manyema M and Richter LM, Adverse childhood experiences: prevalence and associated factors among South African young adults. Heliyon, 2019. 5(12): p. e03003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Goodman ML, et al. , Testing and testing positive: childhood adversities and later life HIV status among Kenyan women and their partners. Journal of Public Health, 2017. 39(4): p. 720–729. [DOI] [PubMed] [Google Scholar]
- 6.Agbaje OS, et al. , Adverse childhood experiences and psychological distress among higher education students in Southeast Nigeria: an institutional-based cross-sectional study. Archives of Public Health, 2021. 79(1): p. 62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kappel RH, et al. , Prevalence of Adverse Childhood Experiences (ACEs) and associated health risks and risk behaviors among young women and men in Honduras. Child Abuse & Neglect, 2021. 115: p. 104993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Felitti VJ, et al. , Relationship of Childhood Abuse and Household Dysfunction to Many of the Leading Causes of Death in Adults. American Journal of Preventive Medicine, 1998. 14(4): p. 245–258. [DOI] [PubMed] [Google Scholar]
- 9.Chapman DP, et al. , Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord, 2004. 82(2): p. 217–25. [DOI] [PubMed] [Google Scholar]
- 10.Benjet C, Childhood adversities of populations living in low-income countries: prevalence, characteristics, and mental health consequences. Curr Opin Psychiatry, 2010. 23(4): p. 356–62. [DOI] [PubMed] [Google Scholar]
- 11.Jones DJ, et al. , Trajectories of childhood sexual abuse and early adolescent HIV/AIDS risk behaviors: the role of other maltreatment, witnessed violence, and child gender. J Clin Child Adolesc Psychol, 2010. 39(5): p. 667–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hillis SD, et al. , The association between adverse childhood experiences and adolescent pregnancy, long-term psychosocial consequences, and fetal death. Pediatrics, 2004. 113(2): p. 320–327. [DOI] [PubMed] [Google Scholar]
- 13.Hillis SD, et al. , Adverse childhood experiences and sexual risk behaviors in women: a retrospective cohort study. Fam Plann Perspect, 2001. 33(5): p. 206–11. [PubMed] [Google Scholar]
- 14.Dube SR, et al. , Adverse childhood experiences and the association with ever using alcohol and initiating alcohol use during adolescence. J Adolesc Health, 2006. 38(4): p. 444 e1–10. [DOI] [PubMed] [Google Scholar]
- 15.Dube SR, et al. , The impact of adverse childhood experiences on health problems: evidence from four birth cohorts dating back to 1900. Prev Med, 2003. 37(3): p. 268–77. [DOI] [PubMed] [Google Scholar]
- 16.Anda RF, et al. , The enduring effects of abuse and related adverse experiences in childhood. A convergence of evidence from neurobiology and epidemiology. Eur Arch Psychiatry Clin Neurosci, 2006. 256(3): p. 174–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fang L, Chuang D-M, and Lee Y, Adverse childhood experiences, gender, and HIV risk behaviors: Results from a population-based sample. Preventive medicine reports, 2016. 4: p. 113–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shamu S, et al. , Does a history of sexual and physical childhood abuse contribute to HIV infection risk in adulthood? A study among post-natal women in Harare, Zimbabwe. PLOS ONE, 2019. 14(1): p. e0198866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pence BW, et al. , Prevalence of Psychological Trauma and Association with Current Health and Functioning in a Sample of HIV-infected and HIV-uninfected Tanzanian Adults. PloS one, 2012. 7(5): p. e36304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jewkes RK, et al. , Associations between childhood adversity and depression, substance abuse and HIV and HSV2 incident infections in rural South African youth. Child abuse & neglect, 2010. 34(11): p. 833–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dunkle KL, et al. , Gender-based violence, relationship power, and risk of HIV infection in women attending antenatal clinics in South Africa. The lancet, 2004. 363(9419): p. 1415–1421. [DOI] [PubMed] [Google Scholar]
- 22.Gibbs A, et al. , Childhood traumas as a risk factor for HIV-risk behaviours amongst young women and men living in urban informal settlements in South Africa: A cross-sectional study. PloS one, 2018. 13(4): p. e0195369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanderEnde K, et al. , Adverse childhood experiences and HIV sexual risk-taking behaviors among young adults in malawi. Journal of interpersonal violence, 2018. 33(11): p. 1710–1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kidman R and Kohler H-PJA, Adverse childhood experiences, sexual debut and HIV testing among adolescents in a low-income high HIV-prevalence context. 2019. 33(14): p. 2245–2250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.UNICEF. HIV and AIDS in adolescents. 2021. [cited 2022 February 2]; https://data.unicef.org/topic/adolescents/hiv-aids/].
- 26.World Health Organization. Adverse Childhood Experiences International Questionnaire (ACE-IQ)
- 27.Chen L, et al. , Sexual Risk Factors for HIV Infection in Early and Advanced HIV Epidemics in Sub-Saharan Africa: Systematic Overview of 68 Epidemiological Studies. PLoS ONE, 2007. 2(10): p. e1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberg NE, et al. , Identifying Adolescent Girls and Young Women at High Risk for HIV Acquisition: A Risk Assessment Tool From the Girl Power-Malawi Study. Sexually Transmitted Diseases, 2020. 47(11): p. 760–766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barker DH, et al. , Using Composite Scores to Summarize Adolescent Sexual Risk Behavior: Current State of the Science and Recommendations. Archives of Sexual Behavior, 2019. 48(8): p. 2305–2320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fergus S, Zimmerman MA, and Caldwell C.H.J.A.j.o.p.h., Growth trajectories of sexual risk behavior in adolescence and young adulthood. 2007. 97(6): p. 1096–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meinck F, Orkin F, and Cluver L, Does free schooling affect pathways from adverse childhood experiences via mental health distress to HIV risk among adolescent girls in South Africa: a longitudinal moderated pathway model. Journal of the International AIDS Society, 2019. 22(3): p. e25262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.García-Moreno C, et al. , WHO multi-country study on women’s health and domestic violence against women. 2005, World Health Organization: Geneva. p. 1–18. [Google Scholar]
- 33.Plate DK, Evaluation and implementation of rapid HIV tests: the experience in 11 African countries. AIDS research and human retroviruses, 2007. 23(12): p. 1491–1498. [DOI] [PubMed] [Google Scholar]
- 34.Cowan FM, et al. , Antibody to herpes simplex virus type 2 as serological marker of sexual lifestyle in populations. BMJ, 1994. 309(6965): p. 1325–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Van de Laar M, et al. , Prevalence and correlates of herpes simplex virus type 2 infection: evaluation of behavioural risk factors. International journal of epidemiology, 1998. 27(1): p. 127–134. [DOI] [PubMed] [Google Scholar]
- 36.Freeman EE, et al. , Herpes simplex virus 2 infection increases HIV acquisition in men and women: systematic review and meta-analysis of longitudinal studies. Aids, 2006. 20(1): p. 73–83. [DOI] [PubMed] [Google Scholar]
- 37.Looker KJ, et al. , Effect of HSV-2 infection on subsequent HIV acquisition: an updated systematic review and meta-analysis. The Lancet infectious diseases, 2017. 17(12): p. 1303–1316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kouyoumjian SP, et al. , Global population-level association between herpes simplex virus 2 prevalence and HIV prevalence. AIDS (London, England), 2018. 32(10): p. 1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Biraro S, et al. , Performance of commercial herpes simplex virus type-2 antibody tests using serum samples from Sub-Saharan Africa: a systematic review and meta-analysis. Sexually transmitted diseases, 2011. 38(2): p. 140–147. [DOI] [PubMed] [Google Scholar]
- 40.Mujugira A, et al. , Performance of the Focus HerpeSelect-2 enzyme immunoassay for the detection of herpes simplex virus type 2 antibodies in seven African countries. Sexually transmitted infections, 2011. 87(3): p. 238–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kuh D, et al. , Life course epidemiology. J Epidemiol Community Health, 2003. 57(10): p. 778–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shonkoff JP, Boyce WT, and McEwen BS, Neuroscience, molecular biology, and the childhood roots of health disparities: building a new framework for health promotion and disease prevention. JAMA, 2009. 301(21): p. 2252–9. [DOI] [PubMed] [Google Scholar]
- 43.Felitti VJ, Adverse childhood experiences and adult health. Academic Pediatrics, 2009. 9(3): p. 131–132. [DOI] [PubMed] [Google Scholar]
- 44.Brady KT and Sinha R, Co-occurring mental and substance use disorders: the neurobiological effects of chronic stress. American Journal of Psychiatry, 2005. 162(8): p. 1483–1493. [DOI] [PubMed] [Google Scholar]
- 45.Shrier LA, et al. , Associations of Depression, Self-Esteem, and Substance Use with Sexual Risk among Adolescents. Preventive Medicine, 2001. 33(3): p. 179–189. [DOI] [PubMed] [Google Scholar]
- 46.Lehrer JA, et al. , Depressive symptoms as a longitudinal predictor of sexual risk behaviors among US middle and high school students. Pediatrics, 2006. 118(1): p. 189–200. [DOI] [PubMed] [Google Scholar]
- 47.Mazzaferro KE, et al. , Depression, stress and social support as predictors of high-risk sexual behaviors and STIs in young women. Journal of Adolescent Health, 2006. 39: p. 601–603. [DOI] [PubMed] [Google Scholar]
- 48.Seth P, et al. , Psychological distress as a correlate of a biologically confirmed STI, risky sexual practices, self-efficacy and communication with male sex partners in African-American female adolescents. Psychology, Health & Medicine, 2009. 14: p. 291–300. [DOI] [PubMed] [Google Scholar]
- 49.Clark S, Early Marriage and HIV Risks in Sub-Saharan Africa. Studies in Family Planning, 2004. 35(3): p. 149–160. [DOI] [PubMed] [Google Scholar]
- 50.Jukes M, Simmons S, and Bundy D, Education and vulnerability: the role of schools in protecting young women and girls from HIV in southern Africa. AIDS, 2008. 22: p. S41–S56. [DOI] [PubMed] [Google Scholar]
- 51.Boileau C, et al. , Sexual and marital trajectories and {HIV} infection among ever-married women in rural {Malawi}. Sexually Transmitted Infections, 2009. 85: p. i27--i33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Brown MJ, et al. , Sex disparities in adverse childhood experiences and HIV/STIs: mediation of psychopathology and sexual behaviors. AIDS and behavior, 2017. 21(6): p. 1550–1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dunn EC, et al. , What life course theoretical models best explain the relationship between exposure to childhood adversity and psychopathology symptoms: recency, accumulation, or sensitive periods? Psychological medicine, 2018. 48(15): p. 2562–2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breton E, et al. , Consistency of Self-Reports of Adverse Childhood Experiences Among Adolescents in a Low-Income Setting. Manuscript in preparation. [DOI] [PMC free article] [PubMed]
- 55.Fong W, et al. , P320 Sexual behaviours and Herpes Simplex Virus Type-2 Incidence and Prevalence among adolescent girls and young women in KwaZulu-Natal, South Africa. 2021, BMJ Publishing Group Ltd. [Google Scholar]
- 56.Zulaika G, et al. , Factors associated with the prevalence of HIV, HSV-2, pregnancy, and reported sexual activity among adolescent girls in rural western Kenya: A cross-sectional analysis of baseline data in a cluster randomized controlled trial. PLoS medicine, 2021. 18(9): p. e1003756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kidman R, et al. , Returning to school after COVID-19 closures: who is missing in Malawi?. Manuscript under review. [DOI] [PMC free article] [PubMed]
- 58.Lake A and Sidibé M, To end the AIDS epidemic, start focusing on adolescents. 2015, UNAIDS. [Google Scholar]
- 59.UNAIDS, End inequalities. End AIDS. Global AIDS strategy 2021–2026, UNAIDS, Editor. 2021: Geneva. [Google Scholar]
- 60.World Health Organization, INSPIRE: seven strategies for ending violence against children. 2016, World Health Organization: Geneva. [Google Scholar]
- 61.Control, C.f.D. and Prevention, Preventing Adverse Childhood Experiences (ACEs): leveraging the best available evidence. 2020. 2020.
- 62.Organization, W.H., Preventing youth violence: an overview of the evidence. 2015: World Health Organization. [Google Scholar]


