Abstract
The hypothalamus is a brain region that integrates signals from the periphery and the environment to maintain organismal homeostasis. To do so, specialized hypothalamic neuropeptidergic neurons control a range of processes, such as sleep, feeding, the stress response, and hormone release. These processes are altered with age, which can affect longevity and contribute to disease status. Technological advances such as single cell RNA sequencing, are upending assumptions about the transcriptional identity of cell types in the hypothalamus and revealing how distinct cell types change with age. In this review, we summarize current knowledge about the contribution of hypothalamic functions to aging. We highlight recent single cell studies interrogating distinct cell types of the mouse hypothalamus and suggest ways in which single cell ‘omics technologies can be used to further understand the aging hypothalamus and its role in longevity.
Keywords: Single Cell RNA-seq, homeostasis, longevity, metabolism
Single cell technologies provide new insights into the aging hypothalamus
Aging is the most significant risk factor for a host of diseases, many of particular interest to neuroscientists, such as stroke[1], brain cancer[2], and neurodegenerative diseases[3]. Aging is also accompanied by alterations in basic homeostatic processes (Figure 1), many of which have been linked to disease[4–6]. The hypothalamus is a brain region that receives homeostatic information from the periphery to coordinate behaviors such as circadian rhythms, food intake, the stress response, and hormone release[7]. This brain area harbors a diverse collection of specialized neuropeptidergic neurons that regulate these distinct physiological functions. Many hypothalamic-regulated processes are altered with age, and their disruption can affect longevity and contribute to age-related disease[8–13].
Figure 1. Schematic of hypothalamic nuclei and their functions.
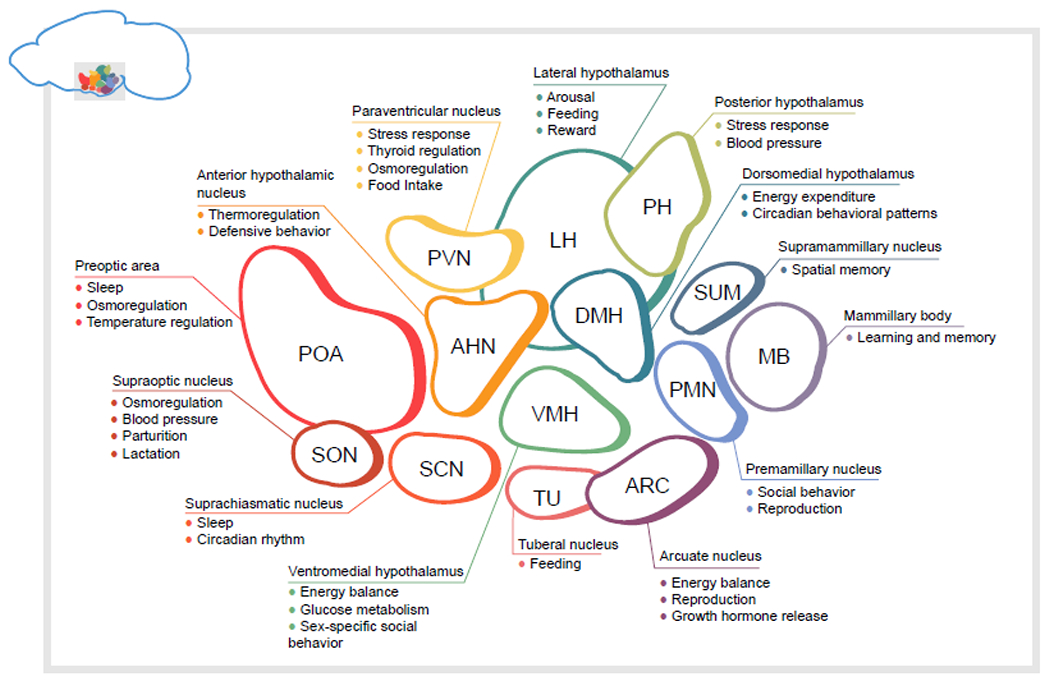
In the following, the figure’s nuclei are described counterclockwise, starting with the preoptic area. Many of the specific behaviors described relate to studies in rodents, although some of the functions are relevant to other species as well. POA. Preoptic area neurons are involved in sleep and temperature regulation[25]. SON. The supraoptic nucleus contains two major populations: vasopressin (encoded by Avp) expressing neurons control osmoregulation, while oxytocin neurons (encoded by Oxt) play an essential role in parturition, lactation, and other social behaviors[30,31]. ANH. The anterior hypothalamic nucleus is understudied, but has a known role in defensive attack behaviors[32]. SCN. The suprachiasmatic nucleus is the core of the internal circadian clock, and entrains body processes and behaviors to the light cycle. TU. TUSst+ (encoding somatostatin) neurons are involved in feeding[33]. ARC. The arcuate nucleus of the hypothalamus is a critical region for energy homeostasis and reproduction[34,35]. ARCKiss1 (kisspeptin neurons) are essential for proper pulsatile release of reproductive hormones [34]. DMH. Leptin sensitive neurons in the dorsomedial hypothalamus are essential for energy expenditure via brown adipose tissue thermogenesis, as well as the circadian timing of feeding and activity[36]. DMHBrs3 neurons are involved in heart rate and maintenance of body temperature[24]. VMH. Leptin sensitive neurons on the ventromedial hypothalamus protect against diet-induced obesity, and regulate the interaction between estrogen and body composition[37,38]. Glucose-excited and glucose-inhibited neurons in the VMH control whole-body glucose homeostasis[39]. VMHEsr1 neurons coordinate social behaviors such as attack and mounting[40]. PMH. The ventral premamillary nucleus links conspecific odorant cues and energy balance signals to reproductive function[41,42]. The dorsal premammillary nucleus controls escape behavior in response to threat[43]. MB. The mammillary bodies encode information about head direction, and projections from the mammillary bodies to anterior thalamic nuclei are necessary for spatial memory[44]. SUM. The supramamillary nucleus projects to the dentate gyrus and is involved in spatial memory tasks and can promote hippocampal neurogenesis[45,46]. PH. The posterior hypothalamus is activated under chronic unpredictable stress[47,48]. Together with the supramamillary nucleus, stimulation of the posterior hypothalamus can induce theta oscillations in the hippocampus[49]. LH. Lateral hypothalamus neurons are implicated in arousal and feeding, especially motivation to eat[26,50]. PVN. The paraventricular nucleus relays information from the hypothalamus back to the body. PVNTrh neurons release thyrotropin-releasing hormone to the pituitary to control the hypothalamic-pituitary-thyroid axis. PVNCrh expressing neurons are the central regulators of the hypothalamic-pituitary-adrenal (HPA) axis [51,52]. PVNMC4R neurons receive inputs from hypothalamic nuclei such as ARC and promote feeding[53,54].
Despite decades of interest in the hypothalamus and its role in aging, the transcriptional and functional complexity of the neurons in this area has remained a roadblock for the field. Recently developed technologies, such as single-cell RNA sequencing, single-cell ATAC sequencing, and spatial transcriptomics, offer new opportunities to investigate the cellular makeup and functions of the hypothalamus. In this review, we discuss findings, primarily from humans and mouse models, that implicate the hypothalamus in the aging process. We then focus on how single cell profiling technologies contribute to these discoveries, and highlight recent lines of inquiry toward the ultimate goal of promoting healthy aging and combating age-related diseases.
The hypothalamus regulates homeostatic processes that change with age
Changes in homeostatic processes such as sleep, circadian rhythms, and body composition are hallmarks of aging. The hypothalamus contains a diverse array of neuropeptidergic cell types that respond to cues from the periphery to regulate these processes and maintain homeostasis (Figure 1). Understanding how these cell types are affected during aging may be key to unlocking therapeutics to treat particular age-related conditions.
Energy homeostasis is a broad category of biological functions which include food intake, fat storage, as well as energy expenditure in the form of locomotion and body temperature maintenance. These processes are tightly regulated by a suite of neuronal subtypes across the hypothalamus (Figure 1). Aging is accompanied by alterations to body composition, including increased abdominal adiposity and decreased lean muscle mass[14]. Concomitantly, there is a decline in basal metabolic rate and total energy expenditure, and increased risk for metabolic disease such as type II diabetes[15,16]. These organismal-level changes are concurrent with changes in the hypothalamus; for example, there is reduced response to central leptin administration and reduced leptin receptor expression, increased inhibition by ARCAGRP onto ARCPOMC, and age-related proteostasis defects. All of these changes likely contribute to age-related obesity and metabolic alterations[17–19].
Maintenance of body temperature is a homeostatic process that intersects with energy homeostasis, as food intake is affected by external body temperature, and energy is expended through heat generation in brown adipose tissue[20]. Studies in humans have shown that older individuals have a decreased average internal body temperature compared to young adults, and reduced amplitude of body temperature changes with the circadian rhythm [21,22]. Additionally, aged individuals are less able to mount a fever in response to infection [23]. Internal body temperature is maintained by the warm- and cold- sensitive thermoregulatory neurons of the preoptic area, as well as DMHBrs3 neurons[24,25].
Changes in circadian rhythm and sleep/wake cycles are also common in aged individuals. Middle-aged adults have advanced sleep and wake times compared to younger adults, as well as increased daytime sleeping, increased latency to fall asleep, and less deep sleep overall[8]. Circadian rhythm is controlled by neuropeptidergic cell types in the suprachiasmatic nucleus, which receive retinal inputs to entrain body rhythms to the day/night cycle. Neurons expressing hypocretin/orexin in the lateral hypothalamus (LHHCRT/OX) are also essential for arousal[26]. With age, there is a reduction of expression of hypocretin/orexin, and reduced circadian rhythmicity of SCN neurons, which may contribute to age related changes in sleep and circadian rhythm[27,28]. Recent research has also shown that with age, LHHCRT/OX neurons become hyperexcitable, and pharmacological inhibition of this hyperexcitability in aged mice can restore proper sleep [29].
Endocrine changes with aging implicate the hypothalamus
The hypothalamic-pituitary axis controls release of hormones that have profound consequences for health, including thyroid hormone, growth hormone, cortisol, and gonadal hormones[55]. While measurement of pituitary hormones, such as thyroid stimulating hormone, luteinizing hormone, and follicle stimulating hormone, can be performed in humans via blood test, studying the hypothalamic neuronal populations controlling the timing and amplitude of pituitary hormone release is difficult. Consequently, much more is known about how levels of pituitary hormones and their downstream effects change with age, than the hypothalamic neuron alterations associated with these changes. One of the changes observed with age, for example, is attenuation of growth hormone release, with decreased amplitude of pulses but not frequency of pulses[56]. Release of growth hormone from the pituitary is controlled by neurons in the arcuate nucleus that release growth hormone-releasing hormone (GHRH). However, the extent to which ARCGHRH neurons are involved in age-related changes in growth hormone release not well understood. Neurons in the hypothalamus control release of other hormones known to change with age, including thyroid stimulating hormone, luteinizing hormone, follicle stimulating hormone, and adrenocorticotropic hormone. The role of hypothalamic neuronal populations in driving these age-related changes remains understudied and is an important goal for future work (see Outstanding Questions).
Outstanding Questions.
Many options exist to analyze single cell data and cluster cell types, however, there is high variability in number and composition of clusters across datasets generated in different laboratories. How can we improve cross-laboratory interoperability of these datasets? How well do transcriptionally defined clusters match with functionally distinct neuronal subtypes?
In worms and flies, the proper function of neuropeptidergic energy-sensing neurons is required for the lifespan-extending effects of dietary restriction. How are hypothalamic energy-homeostasis neurons affected by dietary restriction at different ages? Which (if any) energy-sensing hypothalamic neurons mediate the effect(s) of dietary restriction on lifespan?
The hypothalamus contains sex-specific neuronal populations and populations that function in a sex-specific manner. However, single-cell studies comparing sexes or across hormone states are rare. Do other, undiscovered, sex-specific neuronal populations or activities exist in the hypothalamus? Do these populations contribute to sex differences in lifespan or diseases susceptibility?
There is a growing appreciation for the link between neurodegenerative diseases, such as Alzheimer’s Disease, and changes in hypothalamic function. How are individual neuronal subtypes altered with age and in neurodegeneration in humans?
Hypothalamic inflammation has been proposed as a major driver of whole-body aging, and single nuclei RNA-seq of the hypothalamus has uncovered age-related changes in female hypothalamic microglia. How does microglia-neuron crosstalk at the single cell level influence aging? How are other glial cells, such as tanycytes or astrocytes, affected by inflammation and aging?
Age-related neurodegenerative diseases are associated with hypothalamic neuropathology
Neuropathological studies in humans implicate the hypothalamus in the development of a variety of neurodegenerative diseases. For example, neuroimaging and post-mortem analysis of human brains indicate hypothalamic atrophy may be a feature of Alzheimer’s disease[57]. Interestingly, sleep disturbance and circadian changes are key features of Alzheimer’s Disease and amyloid deposition is associated with poorer sleep quality[58]. Degradation of the suprachiasmatic nucleus has been reported in AD patients, and loss of orexin/hypocretin neurons occurs in advanced AD[59,60]. However, other hypothalamic regions, such as the supraoptic nucleus, seem to be spared from neurodegeneration[59].
Obesity is a major risk factor for Alzheimer’s disease and alterations in body weight may precede or predict the course of neurodegenerative disease. Loss of weight late in the lifespan is associated with the development of mild cognitive impairment or preclinical Alzheimer’s Disease[61,62]. In patients with Amyotrophic lateral sclerosis (ALS), hypothalamic TDP-43 inclusions were identified in one third of patient brains. In patients with inclusions in the lateral hypothalamus, body mass index (BMI) was significantly reduced[63]. Reductions in BMI are associated with accelerated disease progression in ALS, suggesting that the TDP-43 inclusions in the lateral hypothalamus may be clinically significant[64].
The involvement of the hypothalamus in neurodegenerative diseases remains critically understudied. Addressing the timing and trajectory of neuropathology in this brain region may uncover treatments for the non-cognitive symptoms of neurodegenerative disease, which would improve quality of life for the patient and caregivers (see Outstanding Questions).
The impact of energy sensing and the hypothalamus on lifespan and healthspan
A major goal in the aging field is to understand the mechanisms underlying the aging process to improve organismal healthspan, the period of an individual’s life spent free from disease. The study and manipulation of organismal energy states are a mainstay of aging research, with interventions such as caloric restriction being one of the most conserved and reproducible paradigms for lifespan extension[65]. For example, in C57BL/6J mice, 20% caloric restriction (CR) increases mean lifespan by 40.6% in females and 24.4% in males compared to ad libitum fed animals[66]. Studies performed in worms[67], flies[68], and mice[69–72] implicate peptidergic neurons as key regulators of lifespan changes in response to alterations to energy sensing pathways. However, the molecular nature of the cellular changes and neuronal subtypes involved in the response to caloric restriction in mammals remains unclear, in part due to the transcriptional and functional complexity of the hypothalamus.
The mechanisms underlying lifespan extension in response to dietary restriction have been studied extensively (note that dietary restriction is an umbrella term for a variety of manipulations, and includes caloric restriction, time-restricted feeding, and specific macronutrient manipulations (e.g. carbohydrates, fats, amino acids)). These mechanisms are in part cell non-autonomous and involve nutrient sensing neurons (reviewed in [73,74]). In two invertebrate models, the effects of dietary restriction depend on neuropeptidergic energy-sensing neurons. In C. elegans, dietary restriction is dependent on the gene skn-1, which plays a role in autophagy; loss-of-function mutations in skn-1 prevent the lifespan extending effects of dietary restriction. Skn-1 has several isoforms, one of which is expressed uniquely in the neuropeptergic ASI neurons. Ablation of the neuron-specific skn-1 isoform, as well as ablation of the ASI neurons themselves prevented dietary restriction from increasing lifespan[67]. Similarly, in Drosophila, ablation of median neurosecretory cells, which produce insulin-like peptides, extends lifespan in both dilute food conditions (dietary restriction) and in conditions of surplus food, suggesting a cell non-autonomous effect of these neurons on mediating lifespan in response to food intake in invertebrates[68].
In mice, specific manipulations to the hypothalamus are sufficient to extend lifespan. The NAD+ dependent deacetylase SIRT1 is expressed at higher levels during food restriction than during ad libitum feeding, and Sirt1 is required for the normal upregulation of the hypocretin (orexin) receptor 2 (Hcrtr2) in response to DR[69]. Transgenic Sirt1 overexpression in the lateral hypothalamus and dorsomedial hypothalamus of mice is itself sufficient to increase lifespan, with a ~16% increase in median lifespan for females and 9% for males[70]. NF-κB activity in the hypothalamus has also been shown to impact lifespan. With age, hypothalamic inflammation increases, and abrogation of inflammation by overexpressing a dominant-negative form of IκB-α which inhibits NF-κB significantly extended lifespan in male mice, although precise effect size was not reported[71]. In addition, ablation of hypothalamic progenitor cells in middle-age male mice results in a reduction in mean lifespan of 13.5%[72]. Manipulations to the hypothalamus can also improve organismal function. For example, overexpression of the nicotinamide mononucleotide transporter Slc12a8 in the lateral hypothalamus restored skeletal muscle function of aged mice[75].
The growth hormone signaling axis has long been implicated in longevity, with mice deficient in growth hormone or lacking the growth hormone receptor living longer than their wild-type counterparts (reviewed in [76]). Growth hormone-releasing hormone (GHRH) is released by neurons in the hypothalamus to promote growth hormone release in the pituitary. In GHRH knock-out animals, lifespan is markedly increased (43% increase in median lifespan in females and 51% increase in males)[77]. Interestingly, GHRH knock-out mice display an overall increase in survival in response to caloric restriction, indicating that the mechanisms by which GHRH neurons in the hypothalamus impact lifespan are at least partially distinct from those of caloric restriction.
Together, these data implicate the hypothalamus in the central regulation of lifespan, and suggest that it may regulate multiple longevity-associated processes with additive effects on lifespan. While published studies lay a strong foundation implicating hypothalamic nuclei in healthy aging, the impact of different hypothalamic functions on longevity is only beginning to be clarified. More work is required in several areas, including reproductive aging, the stress response, social behavior, sleep and circadian rhythms, and body temperature regulation.
Longevity interventions involve multiple hypothalamic functional networks.
Distinct regions of the hypothalamus interact in order to coordinate behaviors and maintain organismal homeostasis (Figure 1). Lifespan interventions can simultaneously affect several hypothalamic functions. Thus, determining the impact of any specific hypothalamic region or cell type in longevity presents a challenge. For example, caloric restriction affects metabolic state and causes a concomitant reduction in internal body temperature[78], raising the question of the extent to which body temperature influences longevity under restricted feeding conditions. Studies have shown lifespan-extending effects of reduced internal body temperature despite ad libitum food intake[79,80]. In contrast, mice overexpressing brain-specific Sirt1 live longer than wild-type controls despite their higher internal body temperature and increased food intake[70], suggesting that lowered internal body temperature is one mechanism among many for lifespan extension. Further, the timing of feeding, especially in regards to circadian time of day, can affect caloric restriction paradigms. In one study, animals were fed a calorically restricted diet at the start of the active period, at the end of the active period, or throughout the day[81]. While median lifespan for all CR conditions was longer than for ad libitum animals, animals a CR diet at the start of the active period had a 35% increase in median lifespan compared to ad libitum fed animals, highlighting the intersection of circadian rhythms, feeding patterns, and longevity. Together, these data exemplify the difficulty of untangling the role of the hypothalamus and homeostatic processes in lifespan.
Finally, many studies have been hampered by the lack of specific molecular markers for cell types in hypothalamic regions or cell types of interest. It has been noted, for instance, that the lack of Cre driver mice to target specific cell types in the lateral hypothalamus and dorsomedial hypothalamus presents a barrier to dissecting the specific roles of these subregions in the context of studies of metabolic regulation [70]. Identifying cell type-specific molecular markers for hypothalamic cell types is a critical step toward deeper understanding of the hypothalamus in the context of aging.
Single cell ‘omics complements existing tools
Many approaches have been used to understand the role of the hypothalamus in aging and longevity, but studies have been limited by available technologies. For investigators interested in a particular cellular subtype, cell sorting (FACS, MACS) followed by bulk RNA sequencing allows for the analysis of transcriptional changes in cell types for which a reporter mouse can be used or which has known surface markers with reliable antibodies available. However, bulk sequencing approaches cannot be used to discern transcriptional and functional heterogeneity within a population. Microdissection of hypothalamic subregions for bulk transcriptomic analysis is similarly hampered by the transcriptomic diversity within the region. Low cell numbers for many neuronal subtypes, as well as difficulties in obtaining intact neurons from dissociated adult brain tissue, contribute to the challenges of these studies. The low cell number limitation can be circumvented through the use of increased animal numbers[86], but in some cases this is not a practical solution, particularly for studies involving aged mice or disease models. Techniques in which the spatial identity of a cell is preserved, such as in situ hybridization (ISH), offer an approach in which expression patterns in individual cells can be discerned[87]. However, large screens using ISH or immunohistochemistry (IHC) require probes or antibodies for known targets, and targeting multiple transcripts or proteins in a single cell is arduous. Moreover, these methods are less quantitative than available sequencing methods.
Methods to observe or manipulate neuronal activity rely on the existence of known marker genes. Tools such as optogenetics and chemogenetics have been essential for causally linking the activity of specific neuronal populations to changes in organismal state or behavior. In optogenetics experiments, cell type-specific activation or inhibition of a neuronal population can be achieved for instance by combining transgenic mice expressing cell type-specific Cre drivers with Cre-dependent viral opsin expression[88]. DREADDs (designer receptors exclusively activated by designer drugs) are a class of chemogenetic tools that can be used to activate or suppress neuronal subtypes over extended timescales. Similar to optogenetic approaches, DREADDs can be expressed in a neuronal subtype-specific manner by combining viral Cre-dependent DREADD expression with cell type-specific Cre-driver mice[89]. Additionally, genetically encoded calcium sensors or voltage sensors allow researchers to record neuronal activity in awake and behaving animals (reviewed in [90]). While there are multiple strategies to target the sensors to specific cell types, including genetically encoded or viral methods, these methods require a known marker gene. Importantly, regions of the hypothalamus that impact lifespan such as the lateral hypothalamus or preoptic area are some of the least molecularly defined, and currently, specific Cre driver lines targeting these cells are lacking.
Unlike bulk RNA-seq, ISH, or IHC, single cell sequencing technologies allow for the discovery and investigation of new cell types without a priori knowledge of specific markers. In the context of the hypothalamus, single cell RNA-seq has uncovered novel neuronal subtypes and revealed previously under-reported heterogeneity among known cell types. However, how to define clusters and cell types in the hypothalamus continues to be debated. Further, experimental variables such as sample type, preparation, cell number, and sequencing depth can greatly affect downstream outcomes and complicated interpretation across datasets. To date, there is no consensus method for defining hypothalamic cell types in single cell data, and so the overall number of clusters, as well as the markers or labels used for each cluster, vary greatly from study to study. For example, in two studies examining the makeup of the whole hypothalamus using DropSeq, the number of neuronal clusters reported varied from 34 to 62[91,92]. Interestingly, the paper reporting the higher number of clusters analyzed fewer cells (3,131), while the study with the lower number of clusters analyzed over four times as may cells (14,000). Relatedly, in a recent study by our group, we sequenced nuclei rather than whole cells, and found 34 transcriptionally distinct neuronal clusters using 40,064 nuclei from the whole hypothalamus as input[93].
Somewhat counterintuitively, studies focused on particular hypothalamic subregions often report more transcriptionally distinct subclusters than are found in some studies on the whole hypothalamus (summarized in Table 1). For example, one analysis of 31,299 cells from the preoptic area found 69 neuronal clusters[94]. While computationally these clusters may represent transcriptionally distinct entities, whether these distinctions or groupings have functional relevance remains an open question (see Outstanding Questions). Moreover, since there are currently no established firm criteria for what defines and delineates a cluster, comparison between studies for validation or discovery purposes remains a challenge.
Table 1.
Single cell studies of adult mouse hypothalamus
| Region/cell type | Approach | Total cells | Number of neuronal clusters | Ref. |
|---|---|---|---|---|
| Whole hypothalamus | Drop-Seq | 898 | 62 | [92] |
| Arcuate nucleus and median eminence | Drop-Seq | 20,921 | 34 | [97] |
| Whole hypothalamus | Drop-Seq | 14,000 | 34 | [91] |
| Sorted POMC-EGFP neurons | SMART-seq | 163 | 5 | [96] |
| Whole CNS, including hypothalamus | 10x | 509,876 | 15 peptidergic | [98] |
| Preoptic region | Drop-Seq | 31,299 | 69 | [94] |
| Lateral hypothalamic area | 10x | 7,218 | 30 | [99] |
| Lateral hypothalamic area | Drop-Seq | 20194 | 4 | [100] |
| Ventrolateral ventromedial hypothalamus | SMART-seq and 10x | 4574 (SMART-seq), 41,385 (10x) | 29 | [101] |
| Sorted GiprEYFP cells | 10x | 2420 | 1 | [102] |
| Suprachiasmatic nucleus | Drop-Seq | 62,083 | 16 | [103] |
| Sorted SF1-tdTomato neurons | SMART-seq | 530 | 6 | [104] |
| Whole hypothalamus | 10x | 51,199 | 45 | [105] |
| Arcuate nucleus | 10x | 21,017 | 14 | [106] |
| Whole hypothalamus | 10x | 125,224 | 50 | [107] |
| Whole hypothalamus | 10x | 15,291 | N/A | [108] |
| Ventral posterior hypothalamus | 10x | 16,991 | 20 | [109] |
| Median eminence and mediobasal hypothalamus | 10x | 54470 | 1 | [110] |
| Sorted CRH-tdTomato neurons | SMART-seq | 254 | 5 | [111] |
| Arcuate nucleus | 10x | 7136 | 4 | [112] |
| Sorted Hcrt-dsRed neuronal nuclei | 10x | N/A | 4 | [29] |
| Median eminence | 10x | 28,292 | 1 | [113] |
| Anterior ventral preoptic area | 10x | 16,430 | 23 | [114] |
| Whole hypothalamus | 10x | 40,064 | 35 | [93] |
| Arcuate nucleus | 10x | 19,995 | 1 | [115] |
| Ventral posterior hypothalamus | 10x | 16,991 | 20 | [109] |
Traditionally, hypothalamic neurons have been categorized by their neuropeptide expression, or by expression of specific receptor genes. However, across the hypothalamus, single cell approaches have revealed transcriptional heterogeneity within cell populations sharing neuropeptide identity. In some cases, functional heterogeneity of the population was known prior to transcriptional profiling, as is the case with POMC neurons of the arcuate nucleus[95]. This well-studied neuronal population is known to have subpopulations responding uniquely to serotonin or leptin[95]. In a study using SMART-Seq of sorted POMC-EGFP+ cells, four clusters of Pomc-positive, Agrp-negative cells emerged[96]. Importantly, these clusters differed based on expression of receptor genes and neuropeptide co-expression, indicating that neurons in these clusters may be sensitive to different stimuli and thus have differing functions. Single cell transcriptomic approaches present a timely opportunity to identify important subpopulations of neurons in other areas of the hypothalamus as well.
Recent single cell transcriptomic studies reveal hypothalamic neurons that may impact aging
Single cell approaches present an opportunity to identify and develop tools to target specific types of neurons that impact healthy aging, including neuronal subtypes involved in body temperature regulation, feeding and energy homeostasis, stress, and circadian rhythms. Studies using single cell transcriptomics to understand these specific functions in more depth are beginning to emerge, and here we highlight a few specific advances that uncover neuronal subtypes of interest.
Internal body temperature has been identified as a key mediator of longevity, and the search for molecular markers of hypothalamic warm-sensing neurons in the preoptic area is ongoing[79,80]. Previous research identified the genes Bdnf and Adcyap1 as potential markers for this population, and these genes were co-enriched in multiple clusters in a preoptic area single cell sequencing dataset[94]. By combining transcriptomic and functional cFOS data, the authors identified a temperature-sensitive cluster that could be distinguished from other Bdnf+ Adcyap1+ clusters by its co-expression of Sngc. Further research is needed to fully determine the role of this Bdnf+ Adcyap1+ Sngc+ neuronal population in regulating internal body temperature, but this study highlights the potential of single cell transcriptomics to identify candidate neuronal populations of interest to the aging field.
While dietary restriction paradigms have been studied for decades, the molecular identity of cell populations involved in feeding and energy expenditure are just now being described due to advances in single cell technology. Classically, the neuron populations involved in feeding and energy homeostasis have been defined by expression of a single neuropeptide, however, single cell analysis suggests that the same neuropeptide can be expressed in functionally distinct subpopulations. For example, single cell analysis of the lateral hypothalamus confirmed two transcriptionally distinct MCH subtypes[99] as well as transcriptional heterogeneity in canonical leptin sensitive populations of POMC and AgRP/NPY neurons[96,97]. Understanding the function of these discrete neuronal populations may pave the way for pharmacological interventions targeting them to mimic the lifespan-enhancing effects of dietary restriction[116].
Changes in circadian rhythm and sleep quality are common in aging[8]. The SCN is the master regulator of the circadian rhythm, and consists of neuropeptidergic neurons which receive inputs from the retina and other areas to synchronize behavior to the light/dark cycle. Canonically, SCN VIP neurons and GRP neurons are considered to be two separate populations. However, two single cell studies have identified two distinct Vip+ populations, one of which also expresses Grp[103,117]. Whether any (or all) of these populations are responsible for age-associated changes in circadian rhythms has not been determined, but single cell technologies have been useful to identify new neuronal populations that may be involved.
Machine learning applications in single cell analysis and aging research
Many studies have leveraged advances in machine learning (ML) to uncover processes underlying brain aging. Trajectory analysis is one method used to discover genes that underlie the transition between cell states[118]. While trajectory analysis methods were initially developed with the goal of identifying changes in cell state during development or reprogramming, two recent papers have used trajectory analysis to understand brain aging[119]. In one study on brain tissue from patients with Alzheimer’s Disease, Monocle3 was used to uncover transcriptional changes across the trajectory from healthy to disease state in glial cell types[120]. In our study comparing young and aging hypothalamus, we found that a clear inflammatory signal emerged across age in microglia and macrophages[93].
One of the most well-established applications of ML in the aging field is the DNA methylation clock, in which sets of CpGs can predict chronological age with high accuracy using supervised ML methods[121,122]. Similarly, transcriptomic profiles have been used to build aging clocks to predict the age of a given cell in a single cell dataset and define the transcriptional signatures of aging[123]. For example, a study of the mouse subventricular zone neurogenic region generated transcriptomic clocks to predict a cell’s chronological age. The study reported that methylation clock genes were cell type-specific but interferon and lipid metabolism signatures were shared across cell-types. Our analysis of the aging female mouse hypothalamus using single nuclei RNA-seq revealed that the X chromosome gene, Xist, was the most important feature to predict neuronal age using the XGBoost model[93]. Thus, ML models have been useful for identifying new markers of cellular age from single cell transcriptome data across species. In future work, integration of methylation clocks with single cell expression datasets will provide a more complete understanding of how regulatory networks impact the aging process in a cell type-specific manner.
Moving forward, with increased quantities of data, sophisticated deep learning and artificial intelligence methods can be applied to geroscience to optimize age prediction, biomarker development, and drug design[124]. However, the interpretability of deep learning models can be challenging, so in applications such as medical diagnosis, models should be carefully selected and applied.
Concluding remarks and future perspectives.
Single cell ‘omics methods have ignited interest in the diverse cell types of the hypothalamus. Despite decades of interest in this brain region, many outstanding questions regarding the molecular identity of cell types controlling basic processes remain. As interest grows in defining a functional role for molecularly defined subtypes, the field will need to develop consensus definitions for specific cell types. Ideally, such consensus markers would be stable between studies, and across sexes, strains, and age of the animals within a species. While some cell-type markers may not be conserved across taxa, markers that are expressed similarly in different species will be highly useful, particularly for investigation of the human hypothalamus.
Several methods of lifespan extension in animal models, including specific diets and manipulations to internal body temperate, involve the hypothalamus. Thus, identifying interventions that target specific hypothalamic neuronal subtypes may be a valid path toward extending healthspan without requiring arduous fasting regimens. However, as recent studies have revealed, we are only beginning to understand which cell types should be targeted, and how neurons regulating distinct processes interact. Deep sequencing of the hypothalamus across aging, under different conditions, and in disease states will provide important insight into the development of future interventions.
Figure 2. Analysis pipeline for single cell RNA-seq experiments.
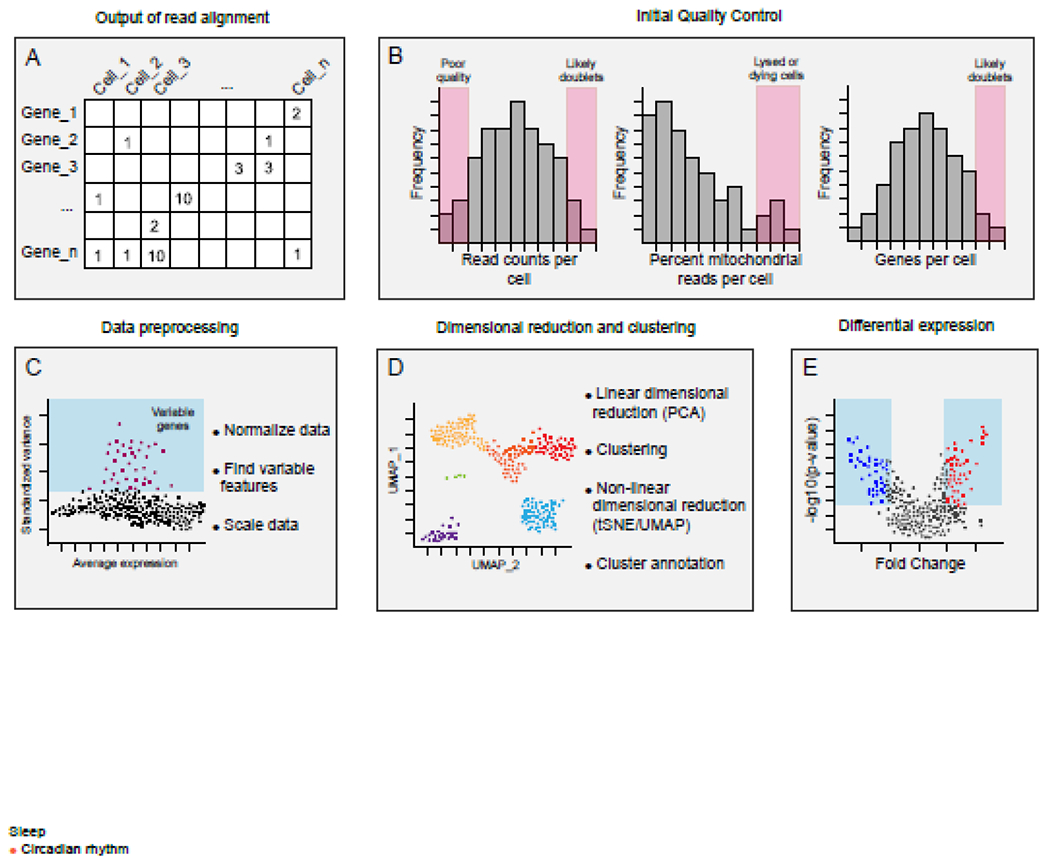
A) The output of most alignment software is a barcode by gene matrix. Due to variability among cells, and the technical limitations of single cell RNA-seq sampling, datasets are sparse[82]. B) Quality control and filtering steps are essential and impact downstream analysis. Threshold values should be carefully considered and can vary depending on the tissue analyzed and the sample preparation[83]. The number of counts and features per cell can indicate whether a cell is low quality (few counts or features), or a doublet (very high features compared to other cells). However, some cell types may have more features than others, so strict cut-offs may remove sources of legitimate biological variability. Similarly, the percentage of mitochondrial reads can indicate whether a cell is dead or dying, but some cells do have naturally occurring higher mitochondrial counts. C) Data preprocessing steps include data normalization, the identification of highly variable genes, and data scaling. The subset of genes which are highly variable can be used for downstream analysis. D) Single-cell datasets have high dimensionality, therefore dimensional reduction is used for clustering and data visualization. The results of clustering can vary based on upstream quality control[83]. E) Testing for differential gene expression between groups is a major goal of many studies. Because of the sparse nature of single cell data distributions, single cell RNA-seq can require different statistical approaches from traditional bulk approaches. Pseudoreplication, in which cells from the same animal are treated as statistically independent replicates, can be avoided through the use of mixed models[84,85].
HIGHLIGHTS.
Homeostatic processes such as food intake/energy balance, sleep and circadian rhythms, and stress responses are controlled in part by specific neurons of the hypothalamus. These processes are altered with age, and can contribute to increased age-associated disease.
Despite decades of interest in the hypothalamus, molecular markers for many of the specific neuronal subtypes in the hypothalamus remain elusive.
Recently, single cell RNA-sequencing technologies have uncovered new and functionally distinct neuronal subtypes in the hypothalamus. Studies have also revealed significant transcriptional and functional heterogeneity among cell types previously thought to be homogeneous.
Comprehensive transcriptomic mapping across hypothalamic cell types will provide important insights into the aging process and may pave the way for new healthspan-enhancing interventions.
Acknowledgements
This work was supported by: a Carney Institute for Neuroscience Zimmerman Innovation Award to A.E.W., Carney Graduate Awards to K.H.H. and D.Y., and NIH/NIA R21 AG070527 to A.E.W.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interests
The authors declare no conflicts of interest in relation to this work.
References
- 1.Yousufuddin M and Young N (2019) Aging and ischemic stroke. Aging 11, 2542–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deorah S et al. (2006) Trends in brain cancer incidence and survival in the United States: Surveillance, Epidemiology, and End Results Program, 1973 to 2001. Neurosurg. Focus 20, E1. [DOI] [PubMed] [Google Scholar]
- 3.Hou Y et al. (2019) Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol 15, 565–581 [DOI] [PubMed] [Google Scholar]
- 4.Ma Y et al. (2020) Higher risk of dementia in English older individuals who are overweight or obese. Int. J. Epidemiol 49, 1353–1365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sabia S et al. (2021) Association of sleep duration in middle and old age with incidence of dementia. Nat. Commun 12, 2289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Corriere M et al. (2013) Epidemiology of Diabetes and Diabetes Complications in the Elderly: An Emerging Public Health Burden. Curr. Diab. Rep 13, 10.1007/s11892-013-0425-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sternson SM (2013) Hypothalamic Survival Circuits: Blueprints for Purposive Behaviors. Neuron 77, 810–824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mander BA et al. (2017) Sleep and Human Aging. Neuron 94, 19–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kuk JL et al. (2009) Age-related changes in total and regional fat distribution. Ageing Res. Rev 8, 339–348 [DOI] [PubMed] [Google Scholar]
- 10.Morrison JH et al. (2006) Estrogen, Menopause, and the Aging Brain: How Basic Neuroscience Can Inform Hormone Therapy in Women. J. Neurosci 26, 10332–10348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rance NE (2009) Menopause and the human hypothalamus: Evidence for the role of kisspeptin/neurokinin B neurons in the regulation of estrogen negative feedback. Peptides 30, 111–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Matsumoto AM (2002) Andropause: Clinical Implications of the Decline in Serum Testosterone Levels With Aging in Men. J. Gerontol. Ser. A 57, M76–M99 [DOI] [PubMed] [Google Scholar]
- 13.Veldhuis JD (2008) Aging and Hormones of the Hypothalamo-Pituitary Axis: gonadotropic axis in men and somatotropic axes in men and women. Ageing Res. Rev 7, 189–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Barzilai N et al. (2012) The Critical Role of Metabolic Pathways in Aging. Diabetes 61, 1315–1322 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Roberts DE et al. (2012) Neuron numbers in the hypothalamus of the normal aging rhesus monkey: stability across the adult lifespan and between the sexes. J. Comp. Neurol 520, 1181–1197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kalyani RR and Egan JM (2013) Diabetes and Altered Glucose Metabolism with Aging. Endocrinol. Metab. Clin. North Am 42, 333–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scarpace PJ et al. (2001) Hypothalamic leptin resistance is associated with impaired leptin signal transduction in aged obese rats. Neuroscience 104, 1111–1117 [DOI] [PubMed] [Google Scholar]
- 18.Newton AJ et al. (2013) AgRP Innervation onto POMC Neurons Increases with Age and Is Accelerated with Chronic High-Fat Feeding in Male Mice. Endocrinology 154, 172–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kaushik S et al. (2012) Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Rep. 13, 258–265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu S et al. (2018) The Hypothalamic Preoptic Area and Body Weight Control. Neuroendocrinology 106, 187–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Geneva II et al. (2019) Normal Body Temperature: A Systematic Review. Open Forum Infect. Dis 6, ofz032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vitiello MV et al. (1986) Circadian temperature rhythms in young adult and aged men. Neurobiol. Aging 7, 97–100 [DOI] [PubMed] [Google Scholar]
- 23.Norman DC (2000) Fever in the Elderly. Clin. Infect. Dis 31, 148–151 [DOI] [PubMed] [Google Scholar]
- 24.Piñol RA et al. (2018) Brs3 neurons in the mouse dorsomedial hypothalamus regulate body temperature, energy expenditure, and heart rate, but not food intake. Nat. Neurosci 21, 1530–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rothhaas R and Chung S (2021) Role of the Preoptic Area in Sleep and Thermoregulation. Front. Neurosci 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown JA et al. (2015) To ingest or rest? Specialized roles of lateral hypothalamic area neurons in coordinating energy balance. Front. Syst. Neurosci 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hunt NJ et al. (2015) Changes in orexin (hypocretin) neuronal expression with normal aging in the human hypothalamus. Neurobiol. Aging 36, 292–300 [DOI] [PubMed] [Google Scholar]
- 28.Mattis J and Sehgal A (2016) Circadian Rhythms, Sleep, and Disorders of Aging. Trends Endocrinol. Metab 27, 192–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li S-B et al. (2022) Hyperexcitable arousal circuits drive sleep instability during aging. Science 375, eabh3021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown CH et al. (2013) Physiological regulation of magnocellular neurosecretory cell activity: Integration of intrinsic, local and afferent mechanisms. J. Neuroendocrinol 25, 10.1111/jne.12051 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Benarroch EE (2013) Oxytocin and vasopressin: Social neuropeptides with complex neuromodulatory functions. Neurology 80, 1521–1528 [DOI] [PubMed] [Google Scholar]
- 32.Xie Z et al. (2022) Mechanically evoked defensive attack is controlled by GABAergic neurons in the anterior hypothalamic nucleus. Nat. Neurosci 25, 72–85 [DOI] [PubMed] [Google Scholar]
- 33.Luo SX et al. (2018) Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361, 76–81 [DOI] [PubMed] [Google Scholar]
- 34.Beale KE et al. (2014) The Physiological Role of Arcuate Kisspeptin Neurons in the Control of Reproductive Function in Female Rats. Endocrinology 155, 1091–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jais A and Brüning JC (2022) Arcuate Nucleus-Dependent Regulation of Metabolism-Pathways to Obesity and Diabetes Mellitus. Endocr. Rev 43, 314–328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Faber CL et al. (2021) Leptin receptor neurons in the dorsomedial hypothalamus regulate diurnal patterns of feeding, locomotion, and metabolism. eLife 10, e63671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dhillon H et al. (2006) Leptin Directly Activates SF1 Neurons in the VMH, and This Action by Leptin Is Required for Normal Body-Weight Homeostasis. Neuron 49, 191–203 [DOI] [PubMed] [Google Scholar]
- 38.Xu Y et al. (2011) Distinct Hypothalamic Neurons Mediate Estrogenic Effects on Energy Homeostasis and Reproduction. Cell Metab. 14, 453–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yoon NA and Diano S (2021) Hypothalamic glucose-sensing mechanisms. Diabetologia 64, 985–993 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lee H et al. (2014) Scalable control of mounting and attack by Esr1+ neurons in the ventromedial hypothalamus. Nature 509, 627–632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Leshan RL et al. (2009) Direct Innervation of GnRH Neurons by Metabolic- and Sexual Odorant-Sensing Leptin Receptor Neurons in the Hypothalamic Ventral Premammillary Nucleus. J. Neurosci 29, 3138–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ross RA et al. (2018) PACAP neurons in the ventral premammillary nucleus regulate reproductive function in the female mouse. eLife 7, e35960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang W et al. (2021) Coordination of escape and spatial navigation circuits orchestrates versatile flight from threats. Neuron 109, 1848–1860.e8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Nelson AJD and Vann SD (2014) Mammilliothalamic Tract Lesions Disrupt Tests of Visuo-Spatial Memory. Behav. Neurosci 128, 494–503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li Y et al. (2020) Supramammillary nucleus synchronizes with dentate gyrus to regulate spatial memory retrieval through glutamate release. eLife 9, e53129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Li Y-D et al. (2022) Hypothalamic modulation of adult hippocampal neurogenesis in mice confers activity-dependent regulation of memory and anxiety-like behavior. Nat. Neurosci 25, 630–645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Flak JN et al. (2012) Identification of chronic stress-activated regions reveals a potential recruited circuit in rat brain. Eur. J. Neurosci 36, 2547–2555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nyhuis TJ et al. (2016) Evidence for the Integration of Stress-Related Signals by the Rostral Posterior Hypothalamic Nucleus in the Regulation of Acute and Repeated Stress-Evoked Hypothalamo-Pituitary-Adrenal Response in Rat. J. Neurosci 36, 795–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kowalczyk T et al. (2021) The Role of the Posterior Hypothalamus in the Modulation and Production of Rhythmic Theta Oscillations. Neuroscience 470, 100–115 [DOI] [PubMed] [Google Scholar]
- 50.Barbano MF et al. (2016) Feeding and Reward Are Differentially Induced by Activating GABAergic Lateral Hypothalamic Projections to VTA. J. Neurosci 36, 2975–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.McEwen BS (2007) Physiology and Neurobiology of Stress and Adaptation: Central Role of the Brain. Physiol. Rev 87, 873–904 [DOI] [PubMed] [Google Scholar]
- 52.Qin C et al. (2018) The Paraventricular Nucleus of the Hypothalamus: Development, Function, and Human Diseases. Endocrinology 159, 3458–3472 [DOI] [PubMed] [Google Scholar]
- 53.Shah BP et al. (2014) MC4R-expressing glutamatergic neurons in the paraventricular hypothalamus regulate feeding and are synaptically connected to the parabrachial nucleus. Proc. Natl. Acad. Sci 111, 13193–13198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Balthasar N et al. (2005) Divergence of Melanocortin Pathways in the Control of Food Intake and Energy Expenditure. Cell 123, 493–505 [DOI] [PubMed] [Google Scholar]
- 55.van den Beld AW et al. (2018) The physiology of endocrine systems with ageing. Lancet Diabetes Endocrinol. 6, 647–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hersch EC and Merriam GR (2008) Growth hormone (GH)–releasing hormone and GH secretagogues in normal aging: Fountain of Youth or Pool of Tantalus? Clin. Interv. Aging 3, 121–129 [PMC free article] [PubMed] [Google Scholar]
- 57.Ishii M and Iadecola C (2015) Metabolic and Non-Cognitive Manifestations of Alzheimer’s Disease: The Hypothalamus as Both Culprit and Target of Pathology. Cell Metab. 22, 761–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ju Y-ES et al. (2013) Sleep quality and preclinical Alzheimer Disease. JAMA Neurol. 70, 587–593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goudsmit E et al. (1990) The supraoptic and paraventricular nuclei of the human hypothalamus in relation to sex, age and Alzheimer’s disease. Neurobiol. Aging 11, 529–536 [DOI] [PubMed] [Google Scholar]
- 60.Fronczek R et al. (2012) Hypocretin (orexin) loss in Alzheimer’s disease. Neurobiol. Aging 33, 1642–1650 [DOI] [PubMed] [Google Scholar]
- 61.Jimenez A et al. (2017) Weight loss in the healthy elderly might be a non-cognitive sign of preclinical Alzheimer’s disease. Oncotarget 8, 104706–104716 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hiller AJ and Ishii M (2018) Disorders of Body Weight, Sleep and Circadian Rhythm as Manifestations of Hypothalamic Dysfunction in Alzheimer’s Disease. Front. Cell. Neurosci 12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cykowski MD et al. (2014) TDP-43 pathology in the basal forebrain and hypothalamus of patients with amyotrophic lateral sclerosis. Acta Neuropathol. Commun 2, 171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jawaid A et al. (2010) A decrease in body mass index is associated with faster progression of motor symptoms and shorter survival in ALS. Amyotroph. Lateral Scler 11, 542–548 [DOI] [PubMed] [Google Scholar]
- 65.Green CL et al. (2022) Molecular mechanisms of dietary restriction promoting health and longevity. Nat. Rev. Mol. Cell Biol 23, 56–73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mitchell SJ et al. (2016) Effects of Sex, Strain, and Energy Intake on Hallmarks of Aging in Mice. Cell Metab. 23, 1093–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bishop NA and Guarente L (2007) Two neurons mediate diet-restriction-induced longevity in C. elegans. Nature 447, 545–549 [DOI] [PubMed] [Google Scholar]
- 68.Broughton SJ et al. (2010) DILP-producing Median Neurosecretory Cells in the Drosophila Brain Mediate the Response of Lifespan to Dietary Restriction. Aging Cell 9, 336–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Satoh A et al. (2010) SIRT1 Promotes the Central Adaptive Response to Diet Restriction through Activation of the Dorsomedial and Lateral Nuclei of the Hypothalamus. J. Neurosci 30, 10220–10232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Satoh A et al. (2013) Sirt1 extends life span and delays aging in mice through the regulation of Nk2 homeobox 1 in the DMH and LH. Cell Metab. 18, 416–430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Zhang G et al. (2013) Hypothalamic programming of systemic ageing involving IKK-β, NF-κB and GnRH. Nature 497, 211–216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Zhang Y et al. (2017) Hypothalamic stem cells control ageing speed partly through exosomal miRNAs. Nature 548, 52–57 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Dacks PA et al. (2013) Role of the hypothalamus in mediating protective effects of dietary restriction during aging. Front. Neuroendocrinol 34, 95–106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Miller HA et al. (2020) Cell non-autonomous regulation of health and longevity. eLife 9, e62659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ito N et al. (2022) Slc12a8 in the lateral hypothalamus maintains energy metabolism and skeletal muscle functions during aging. Cell Rep. 40, 111131. [DOI] [PubMed] [Google Scholar]
- 76.Bartke A (2019) Growth Hormone and Aging: Updated Review. World J. Mens Health 37, 19–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sun LY et al. (2013) Growth hormone-releasing hormone disruption extends lifespan and regulates response to caloric restriction in mice. eLife 2, e01098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Guijas C et al. (2020) Metabolic adaptation to calorie restriction. Sci. Signal 13, eabb2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Conti B et al. (2006) Transgenic Mice with a Reduced Core Body Temperature Have an Increased Life Span. Science 314, 825–828 [DOI] [PubMed] [Google Scholar]
- 80.Zhao Z et al. (2022) Body temperature is a more important modulator of lifespan than metabolic rate in two small mammals. Nat. Metab DOI: 10.1038/s42255-022-00545-5 [DOI] [PubMed] [Google Scholar]
- 81.Acosta-Rodríguez V et al. (2022) Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science 0, e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Jiang R et al. (2022) Statistics or biology: the zero-inflation controversy about scRNA-seq data. Genome Biol. 23, 31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Luecken MD and Theis FJ (2019) Current best practices in single-cell RNA-seq analysis: a tutorial. Mol. Syst. Biol 15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Finak G et al. (2015) MAST: a flexible statistical framework for assessing transcriptional changes and characterizing heterogeneity in single-cell RNA sequencing data. Genome Biol. 16, 278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Zimmerman KD et al. (2021) A practical solution to pseudoreplication bias in single-cell studies. Nat. Commun 12, 738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ren H et al. (2012) FoxO1 Target Gpr17 Activates AgRP Neurons to Regulate Food Intake. Cell 149, 1314–1326 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Shimogori T et al. (2010) A genomic atlas of mouse hypothalamic development. Nat. Neurosci 13, 767–775 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Allen BD et al. (2015) Principles of designing interpretable optogenetic behavior experiments. Learn. Mem 22, 232–238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Smith KS et al. (2016) DREADDS: Use and application in behavioral neuroscience. Behav. Neurosci 130, 137–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Campos P et al. (2020) Diving into the brain: deep-brain imaging techniques in conscious animals. J. Endocrinol 246, R33–R50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Chen R et al. (2017) Single-Cell RNA-Seq Reveals Hypothalamic Cell Diversity. Cell Rep. 18, 3227–3241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Romanov RA et al. (2017) Molecular interrogation of hypothalamic organization reveals distinct dopamine neuronal subtypes. Nat. Neurosci 20, 176–188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hajdarovic KH et al. (2022) Single-cell analysis of the aging female mouse hypothalamus. Nat. Aging DOI: 10.1038/s43587-022-00246-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Moffitt JR et al. (2018) Molecular, spatial, and functional single-cell profiling of the hypothalamic preoptic region. Science 362, eaau5324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Toda C et al. (2017) POMC Neurons: From Birth to Death. Annu. Rev. Physiol 79, 209–236 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lam BYH et al. (2017) Heterogeneity of hypothalamic pro-opiomelanocortin-expressing neurons revealed by single-cell RNA sequencing. Mol. Metab 6, 383–392 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Campbell JN et al. (2017) A Molecular Census of Arcuate Hypothalamus and Median Eminence Cell Types. Nat. Neurosci 20, 484–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zeisel A et al. (2018) Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014.e22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Mickelsen LE et al. (2019) Single-cell transcriptomic analysis of the lateral hypothalamic area reveals molecularly distinct populations of inhibitory and excitatory neurons. Nat. Neurosci 22, 642–656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Rossi MA et al. (2019) Obesity remodels activity and transcriptional state of a lateral hypothalamic brake on feeding. Science 364, 1271–1274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Kim D-W et al. (2019) Multimodal Analysis of Cell Types in a Hypothalamic Node Controlling Social Behavior. Cell 179, 713–728.e17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Adriaenssens AE et al. (2019) Glucose-Dependent Insulinotropic Polypeptide Receptor-Expressing Cells in the Hypothalamus Regulate Food Intake. Cell Metab. 30, 987–996.e6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Wen S et al. (2020) Spatiotemporal single-cell analysis of gene expression in the mouse suprachiasmatic nucleus. Nat. Neurosci 23, 456–467 [DOI] [PubMed] [Google Scholar]
- 104.van Veen JE et al. (2020) Hypothalamic oestrogen receptor alpha establishes a sexually dimorphic regulatory node of energy expenditure. Nat. Metab 2, 351–363 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Romanov RA et al. (2020) Molecular design of hypothalamus development. Nature 582, 246–252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Deng G et al. (2020) Single-Nucleus RNA Sequencing of the Hypothalamic Arcuate Nucleus of C57BL/6J Mice After Prolonged Diet-Induced Obesity. Hypertension 76, 589–597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kim DW et al. (2020) The cellular and molecular landscape of hypothalamic patterning and differentiation from embryonic to late postnatal development. Nat. Commun 11, 4360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Bentsen MA et al. (2020) Transcriptomic analysis links diverse hypothalamic cell types to fibroblast growth factor 1-induced sustained diabetes remission. Nat. Commun 11, 4458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mickelsen LE et al. (2020) Cellular taxonomy and spatial organization of the murine ventral posterior hypothalamus. eLife 9, e58901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yoo S et al. (2021) Control of neurogenic competence in mammalian hypothalamic tanycytes. Sci. Adv 7, eabg3777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Short AK et al. (2021) Single-Cell Transcriptional Changes in Hypothalamic Corticotropin-Releasing Factor–Expressing Neurons After Early-Life Adversity Inform Enduring Alterations in Vulnerabilities to Stress. Biol. Psychiatry Glob. Open Sci DOI: 10.1016/j.bpsgos.2021.12.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Huisman C et al. (2022) Critical changes in hypothalamic gene networks in response to pancreatic cancer as found by single-cell RNA sequencing. Mol. Metab 58, 101441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Chen Z-H et al. (2022) Single-cell transcriptomic profiling of the hypothalamic median eminence during aging. J. Genet. Genomics DOI: 10.1016/j.jgg.2022.01.001 [DOI] [PubMed] [Google Scholar]
- 114.Osterhout JA et al. (2022) A preoptic neuronal population controls fever and appetite during sickness. Nature 606, 937–944 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lutomska LM et al. Diet triggers specific responses of hypothalamic astrocytes in time and region dependent manner. Glia n/a [DOI] [PubMed] [Google Scholar]
- 116.de Cabo R et al. (2014) The Search for Antiaging Interventions: From Elixirs to Fasting Regimens. Cell 157, 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Todd WD et al. (2020) Suprachiasmatic VIP neurons are required for normal circadian rhythmicity and comprised of molecularly distinct subpopulations. Nat. Commun 11, 4410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Trapnell C et al. (2014) The dynamics and regulators of cell fate decisions are revealed by pseudotemporal ordering of single cells. Nat. Biotechnol 32, 381–386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Saelens W et al. (2019) A comparison of single-cell trajectory inference methods. Nat. Biotechnol 37, 547–554 [DOI] [PubMed] [Google Scholar]
- 120.Morabito S et al. (2021) Single-nucleus chromatin accessibility and transcriptomic characterization of Alzheimer’s disease. Nat. Genet 53, 1143–1155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seale K et al. (2022) Making sense of the ageing methylome. Nat. Rev. Genet DOI: 10.1038/s41576-022-00477-6 [DOI] [PubMed] [Google Scholar]
- 122.Horvath S and Raj K (2018) DNA methylation-based biomarkers and the epigenetic clock theory of ageing. Nat. Rev. Genet 19, 371–384 [DOI] [PubMed] [Google Scholar]
- 123.Buckley MT et al. (2022) Cell type-specific aging clocks to quantify aging and rejuvenation in regenerative regions of the brainbioRxiv, 2022.01.10.475747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Zhavoronkov A et al. (2021) Artificial intelligence in longevity medicine. Nat. Aging 1, 5–7 [DOI] [PubMed] [Google Scholar]


