Abstract
A major limitation of organ allotransplantation is the insufficient supply of donor organs. Consequently, thousands of patients die every year while waiting for a transplant. Progress in xenotransplantation that has permitted pig organ graft survivals of years in non-human primates has led to renewed excitement about the potential of this approach to alleviate the organ shortage. In 2022, the first pig-to-human heart transplant was performed on a compassionate use basis, and xenotransplantation experiments using pig kidneys in deceased human recipients provided encouraging data. Many advances in xenotransplantation have resulted from improvements in the ability to genetically modify pigs using CRISPR–Cas9 and other methodologies. Gene editing has the capacity to generate pig organs that more closely resemble those of humans and are hence more physiologically compatible and less prone to rejection. Despite such modifications, immune responses to xenografts remain powerful and multi-faceted, involving innate immune components that do not attack allografts. Thus, the induction of innate and adaptive immune tolerance to prevent rejection while preserving the capacity of the immune system to protect the recipient and the graft from infection is desirable to enable clinical xenotransplantation.
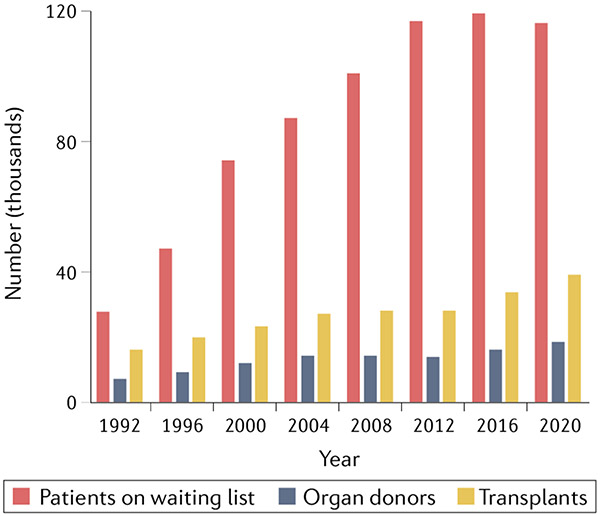
The heart transplant pioneer Norman Shumway famously stated that xenotransplantation is the future of transplantation, and always will be. However, advances in the field that belie this pessimistic statement have led to widespread hope that xenotransplantation will provide a near-term solution to the dire shortage of human organs for transplantation. In 2020, the Organ Procurement and Transplantation Network reported that 116,577 patients in the USA were on waiting lists for organ transplantation, including 97,541 who were waiting for kidney allografts1. On average, 20 patients on transplant waiting lists die every day because they do not obtain an organ. The disparity between the number of patients who are waiting for a transplant and the number of available organs has narrowed slightly in the past decade, but is still very large (FIG. 1). In addition, many patients whose lives could be saved by transplantation do not meet the criteria to be added to waiting lists.
Fig. 1 ∣. The growing organ shortage.
The disparity between the number of patients on waiting lists and the number of transplants performed in the USA has grown markedly over the past three decades. Data obtained from the OPTN database1.
Strategies that could potentially close the gap between organ supply and demand include increasing deceased donor donation, bioengineering organs for transplantation and xenotransplantation. As less than 1% of deaths (i.e. brain deaths and cardiac deaths) result in organs that can be used for transplantation, the potential to increase human organ donation is limited. Even countries with presumed consent for deceased donation have experienced an increasing disparity between organ need and availability2. Attempts to bioengineer transplantable organs using stem or progenitor cells to populate scaffolds produced by 3D printing3 or by organ decellularization4 face substantial challenges in replicating the complex structure–function relationships of multiple cell types that are required for normal organ function. Bioengineering approaches that are personalized to use cells of the individual recipient are expensive and cumbersome, whereas those that utilize an ‘off-the-shelf’ universal product will require immunosuppressive therapy or genetic modifications to prevent rejection.
The use of organs from animals could ensure a reliable supply of quality-controlled organs for transplantation. Such xenotransplantation would provide the opportunity to ensure that transplanted organs are of optimal size, structure and function and are free of potentially harmful infectious organisms. Transplantation could be performed on an elective basis and would be available to patients at early stages in the course of organ failure who are not currently eligible for inclusion on allograft waiting lists, likely improving outcomes and quality of life. Early attempts at xenotransplantation utilized chimpanzees and other non-human primates (NHPs)5-7. However, the pig is now widely accepted as an appropriate xenograft source animal owing to its size, availability, breeding characteristics and physiological similarities to humans. Given that 100 million pigs are used for food each year in the USA alone, ethical considerations are unlikely to preclude the use of pig organs to save the lives of humans.
In this Review, we summarize the current state of knowledge regarding immunological barriers to xenotransplantation. We also discuss the major approaches that are being used to overcome these barriers, including immunosuppression, genetic engineering of pigs and tolerance induction.
Immunological barriers
Xenografts are subject to powerful rejection responses in recipients that involve both innate and adaptive immune responses.
Innate immunity
Monocytes and macrophages.
Macrophages have an important physiological role in the clearance of senescent or damaged cells. They have also been implicated in cellular xenograft rejection, including that of islets8 and haematopoietic cells (HCs)9,10. T cell-independent macrophage infiltration was reported in a rodent xenograft model11, whereas T cell-dependent macrophage activation and recruitment were shown in other models12-15, including recruitment of human macrophages during porcine islet graft rejection in human immune system (HIS) mice. Human monocytes might contribute to solid organ xenograft rejection by producing inflammatory cytokines and activating porcine endothelial cells16. Healthy erythrocytes and leukocytes express CD47, which transmits ‘don’t eat me’ signals to macrophages via the inhibitory receptor SIRPα. Transgenic expression of human CD47 on pig cells might therefore be beneficial in xenotransplantation (discussed further below).
Natural killer cells.
Natural killer (NK) cells are a prominent component of cellular infiltrates in rejecting pig-to-primate solid organ xenografts17,18, suggesting a possible pathogenic role. NK cells express inhibitory receptors that recognize class I major histocompatibility molecules, preventing killing of normal autologous cells. Failure of many inhibitory receptors to bind to xenogeneic MHC molecules contributes to NK cell-mediated destruction of xenogeneic cells19,20, which is augmented by xenoantigen recognition by NK cell activating receptors21 and T cell-derived IL-2 (REF.22). Human NK cell-mediated destruction of porcine cells can be mitigated by transgenic expression of human leukocyte antigen (HLA) molecules23-25. For example, the endothelial cells of transgenic HLA-E-expressing swine are resistant to human NK cell-mediated cytotoxicity26. NK cells can also kill their targets via antibody-dependent cell-mediated cytotoxicity27, which involves binding of NK cell FcγRIII (also known as CD16) to the Fc portion of IgG xenoantibodies28.
Although a T cell-independent or antibody-independent role for NK cells has not been clearly demonstrated in solid organ xenotransplantation, these cells clearly represent a much greater barrier to xenogeneic than to allogeneic HC engraftment29. However, xenogeneic HCs that engraft and induce mixed chimerism can tolerize recipient NK cells (discussed further below).
Complement and coagulation.
Complement contributes to innate and adaptive immune responses via the classical (antibody triggered), alternative and lectin pathways30,31. Activated complement components mediate opsonization, which promotes macrophage-mediated destruction of cells. Several complement regulatory proteins (CRPs) that are expressed on cell surfaces, namely CD46, CD55 and CD59, mitigate constant activation of complement in the microcirculation. However, these complement regulatory interactions might not be fully functional across species barriers32.
Antibody-mediated complement activation on vascular endothelium also activates the coagulation pathway, which might be amplified in xenotransplantation owing to a failure of coagulation inhibitors expressed on the xenogeneic vascular endothelium to interact effectively with the recipient’s activated coagulation components33. Binding of NAbs, fixation of complement and activation of the coagulation cascade results in catastrophic hyperacute rejection (HAR), which is characterized by thrombosis resulting in graft ischaemia. Coagulation dysregulation resulting from complement activation also likely contributes to thrombotic microangiopathy in pig-to-baboon kidney and cardiac transplants17,34-36. Antibody-independent complement activation might also contribute to the destruction of xenogeneic (porcine) HCs in mice37. The coagulation and complement pathways are major targets of efforts to humanize pigs to make their organs less susceptible to antibody-mediated rejection (ABMR).
Natural antibodies.
NAbs are often classified as innate immune components because they arise without exposure to their known ligands. However, their presence is likely driven by exposure to microorganisms with antigens on their cell walls or capsids that are shared with known NAb ligands or cross-recognized by NAbs. The most important NAb specificity for xenotransplantation is the Gal terminal carbohydrate moiety. Gal is widely displayed on cell surface glycoproteins and glycolipids of pigs and most other animals, with the exception of humans and Old World monkeys, in which a frameshift mutation in the α-1,3-galactosyltransferase gene (GGTA1) prevents its synthesis38. Anti-Gal NAbs include IgM and IgG antibody classes and constitute about 1–4% of circulating human immunoglobulin39,40.
The development of nuclear transfer technology in the early 2000s enabled removal of the GGTA1 gene from pigs. Transplantation of organs from these genetically modified pigs prevented HAR in NHPs, thus overcoming a major hurdle in xenotransplantation41-43. However, NAbs against other pig antigens that are less prominent than Gal can initiate acute vascular rejection44, which is also known as delayed xenograft rejection45,46 because of its slower evolution (days to weeks) than HAR (minutes to hours). Anti-non-Gal NAbs bind to multiple pig cell surface antigens47. Some of these NAbs recognize the SDa blood group antigen B4Gal (a terminal carbohydrate produced by B4GALNT2)48 or the NeuGc ligand49,50 (produced by the enzyme CMAH)51. As NeuGc is expressed by glycoproteins and gangliosides on the endothelial cells of all mammals except humans49, anti-NeuGc Nabs are present in human sera but not in NHP sera. Removal of the NeuGc epitope from pigs results in the expression of a new antigenic target for baboon NAbs52, limiting the utility of NHPs as preclinical xenotransplantation models.
Antibody binding to vascular endothelium can also have complement-independent activating effects on endothelial cells that result in morphological changes, vascular leakiness and apoptosis, contributing to the rapid rejection of vascularized xenografts53,54. Conversely, antibody binding can have a beneficial effect by inducing accommodation, in which vascular endothelium becomes resistant to complement-mediated rejection55,56.
Adaptive immunity
B cells.
The role of Nabs may be thought of as innate immunity, despite the likely role of exposure to microbial carbohydrate moieties in driving their formation. IgG antibodies formed by prior exposure to human alloantigens clearly belong to the adaptive immune response. However, another class of anti-pig antibodies has been reported in highly sensitized individuals whose anti-HLA alloantibodies cross-react to swine leukocyte antigen (SLA, that is porcine MHC) class II57 and SLA class I58 antigens, suggesting that knocking out or mutating certain SLA alleles might improve xenotransplant outcomes59,60. In early xenotransplantation trials, exclusion of patients with high levels of IgG NAbs in crossmatch to potential donor pigs will be necessary; however, desensitization procedures might be evaluated in later studies.
T cells.
The development of IgG antibody responses to source pig antigens following xenotransplantation usually indicates the existence of a T cell response. Although T cells are generally assigned to the adaptive immune response, γδ T cells have features of both innate and adaptive immunity61,62. γδ T cells have an important role in rejecting rat bone marrow in mice63; however, their potential role in organ xenograft rejection has not been widely explored.
Conventional αβ T cells pose a powerful barrier to xenotransplantation, both by directly attacking the graft and by promoting antibody and NK cell responses. T cell responses that include cytotoxicity, cytokine production and the recruitment and activation of innate cytotoxic cells are difficult to overcome in pig to primate xenotransplantation, even with high levels of immunosuppression64,65. Early studies that reported weak mouse anti-pig direct T cell xenoresponses66 likely reflected the failure of several key receptor–ligand interactions between these very evolutionarily disparate species67 and did not necessarily indicate reduced T cell receptor (TCR)-ligand interactions in xenogeneic compared with allogeneic combinations.
MHC molecules from pigs can positively select a diverse repertoire of murine68-70 and human T cells71, suggesting that these xenogeneic TCR–MHC interactions are quite effective. In contrast to mouse anti-pig responses, most of the tested molecular interactions seem to be effective in the human anti-pig T cell response. Porcine SLA class I and class II molecules can directly activate human CD8+ and CD4+ T cells, respectively, and human direct T cell responses to pig and HLA-mismatched human antigens are similar in magnitude72. Interactions of human T cells with porcine ligands for human LFA1 (also known as CD11a–CD18), CD2 and CD28 are effective72-77. Human CD4+ T cells (through the Fas–FasL pathway) and, to a lesser degree, CD8+ T cells, are capable of directly killing porcine target cells78,79. However, a lack of signalling from human IFN-γ80 through porcine receptors might limit the ability of human T cells to promote the upregulation of MHC and costimulatory molecules on porcine antigen-presenting cells (APCs).
Powerful indirect xenorecognition, in which recipient APCs process and present donor antigens on recipient MHC molecules, occurs in the human anti-pig direction72,73. This indirect xenorecognition response seems to be stronger than the indirect response to alloantigens72, consistent with the much greater number of protein polymorphisms between different species than between individuals of the same species. Studies in rhesus macaques implicated indirect activation of IFN-γ-producing recipient T cells in the rejection of porcine islet xenografts, which also showed macrophage infiltration. A strong immunosuppressive regimen that prevented allograft rejection was insufficient to prevent this xenograft rejection, underscoring the strength of the T cell xenoresponse81. Indirect memory T cell responses to porcine antigens in NHPs are refractory to immunosuppression sufficient to suppress direct xenoresponses82. As induction of T cell-dependent antibodies involves presentation of donor antigens by antigen-specific B cells to peptide-reactive CD4 T cells, the early IgG responses seen in NHPs receiving porcine heart or kidney transplants44,83,84 may reflect the strength of the indirect T cell xenoresponse.
Porcine B7 molecules can costimulate human T cells through CD28 (REF.85), making this interaction a viable target for immunosuppression in xenotransplantation. Blockade of this costimulation pathway attenuated the anti-non-Gal antibody response in pig to baboon heart transplantation86. Although CD40–CD154 blockade did not suppress the human anti-pig proliferative T cell response in vitro85, such blockade has been used extensively to prevent organ87,89 and islet64,90 graft rejection in large animal xenotransplantation and seems to be essential to prevent rapid ABMR of cardiac xenografts in baboons91.
Human CD4+CD25+FOXP3+ regulatory T (Treg) cells can suppress anti-pig responses in vitro92. Treg cells can also suppress porcine islet xenograft rejection by human T cells in HIS mice93 and CD4 T cell-dependent human macrophage activation in vitro94. Prolonged porcine skin graft survival was achieved in baboons that received polyclonally expanded recipient Treg cells in combination with donor HCs95. However, autologous Treg cells failed to enhance porcine islet graft survival in immunosuppressed rhesus macacques96. In vitro xenogeneic studies suggest that modified porcine dendritic cells can reduce human anti-pig responses97,98. CD8+CD28− Treg cells have also been reported to suppress human anti-pig CD4 responses in vitro99.
Genetic approaches to avoid rejection
As technological advances have greatly enhanced the capacity to genetically engineer pigs, this approach has demonstrated its potential to overcome immune barriers to xenotransplantation (BOX 1).
Box 1 |. Genetic engineering of source pigs.
Advances in technologies for editing the DNA of somatic cells, such as CRISPR, have permitted modification of the nuclei of fibroblasts in culture, followed by transfer of these nuclei into enucleated oocytes that are then implanted into surrogate sows to obtain genetically modified pigs. Many transgenes and knockouts of potential benefit for xenotransplantation strategies are currently under study.
Transgenes
Complement inhibition
Human DAF
Human CD46
Human CD59
Coagulation inhibition
Human CD39
Human thrombomodulin
Human endothelial protein C receptor
Anti-inflammatory genes
HO-1
A20
Immunosuppressive molecules
Anti-CD2
CTLA4Ig
Human CD47
PD-L1
FasL
NK cell inhibition
Class I MHC
Prevention of infection
Porcine endogenous retrovirus short interfering RNA
Knockouts
Prevention of natural antibody-mediated rejection
α1,3-galactosyl transferase
CMAH
B4GalNT2
Prevention of infection
Porcine endogenous retroviruses
Limitation of organ size
GHR
Knock-ins
Prevention of graft thrombosis
Human vWF
Antibody-mediated rejection
NAbs that bind to porcine cell surface antigens and fix complement are responsible for HAR in pig-to-primate transplantation. Recognizing the potential incompatibility of porcine CRPs with primate activated complement, early attempts to produce genetically modified pigs for xenotransplantation involved introducing human CRPs, including CD55, CD46 and CD59, into porcine fertilized ova via pro-nuclear injection100. This approach generated pigs that expressed human CRPs on endothelial cells and could be bred. However, human CRP expression mitigated, but did not eliminate, HAR. Removal of NAbs by absorption procedures enabled organs from CRP-transgenic pigs to survive for days to weeks in NHPs101 but rapid recovery of NAbs, often at increased levels, was associated with delayed ABMR102. Complete knockout of Gal using nuclear transfer was required to prevent HAR and markedly extend survival of pig-to-baboon xenotransplants in recipients with high titres of anti-Gal NAbs83,103.
Further advances in genome editing, including the use of zinc finger nucleases, transcription activator-like effector nucleases (TALENS) and Clustered Regularly Interspaced Short Palindromic Repeats (CRISPR)–Cas9 (REF.104) have sped up and enhanced the feasibility of humanizing the pig genome to improve the success of xenotransplantation. All of these techniques require transfer of nuclei from gene-edited somatic cells into enucleated oocytes and implantation into receptive sows to generate cloned pigs.
CRISPR has been used to generate pigs with knockout of the genes responsible for production of the B4Gal and NeuGc carbohydrate epitopes in addition to Gal. Human sera show markedly reduced levels of NAbs binding to pig cells with knockout of GGTA1, B4GALNT2 and CMAH (known as triple knockout (TKO) cells) compared with pig cells that lack Gal and NeuGc, whereas pig cells with double knockout of CMAH and GGTA1 show increased binding of baboon antibodies compared with pig cells with knockout of GGTA1 alone or TKO pig cells105. The binding of antibodies in sera from transplant-waitlisted patients to TKO pig target cells is reportedly similar to their binding to allogeneic targets58. As highly sensitized patients with alloantibodies against multiple HLA alleles have difficulty finding suitable allograft donors, they might be appropriate candidates for initial trials of pig TKO kidney or heart xenotransplantation. Two studies have shown a lack of correlation between panel-reactive antibody levels and reactivity to GGTA1 knockout pig PBMCs106,107, suggesting that highly allosensitized patients might be appropriate candidates for porcine organ transplants108.
The analysis of sera from prospective transplant recipients to detect antibodies against potential source pigs does not account for the possibility that xenoantibodies can be rapidly induced after transplantation, causing delayed xenograft reaction a few days after placement of a xenograft, as was observed in early hamster to rat transplants109,110. Given the potential for the generation of new carbohydrate epitopes when enzymes producing NAb targets are deleted from pigs, the induction of tolerance among B cells that produce all NAb specificities that recognize donor antigens (discussed below), may be the optimal approach to avoiding non-Gal NAb-mediated rejection.
Additional genetic modifications have been introduced into pigs with the goal of overcoming early ABMR. Transgenic expression of human haem oxygenase-1 (HO-1), which converts haem to bilirubin, carbon monoxide and free iron, protects cells from oxidative injury through various mechanisms including anti-inflammatory, cytoprotective and anti-apoptotic effects111. Although several NHP transplant studies have used pigs with transgenic human HO-1 expression112, the effect of human HO1 on xenograft survival has not been systematically assessed. In pig hearts, expression of human A20 (also known as TNFAIP3), a TNF-induced zinc finger protein enzyme that inhibits NF-κB activation and TNF-mediated apoptosis, protected porcine endothelial cells from CD95-mediated cell death and complement-mediated cytotoxicity in vitro and, in combination with human HO1, delayed ABMR in pig kidneys perfused ex vivo with human blood113.
Given the prominent activation of coagulation processes during ABMR and the species incompatibility of some regulators of coagulation, source pigs that produce human inhibitors of clotting and coagulation have also been generated. Transgenic expression of human thrombomodulin (TM, also known as CD141), an endothelial cell protein that inhibits coagulation by converting thrombin from a procoagulant to an anticoagulant enzyme, is thought to be instrumental in enabling long-term survival of heart xenografts in baboons91,114. Humanization of porcine von Willebrand Factor (vWF), a glycoprotein that interacts with Factor VIII to promote platelet adhesion at sites of vascular damage, has been found to be protective ex vivo and in organ perfusion studies115, but its effect on xenograft survival is unknown. A transgene for human CD39 (also known as ENTPD1), an enzyme that hydrolyses ATP and ADP to AMP, which is subsequently hydrolysed to adenosine (which has anti-thrombotic and cardiovascular protective effects), has been included in several pig-to-primate xenograft models116. Like many of the human transgenes introduced into pigs, the effect of human CD39 has not been systematically examined separately from other transgenes, making its possible benefit difficult to ascertain.
Serial nuclear transfer has been used to produce pigs that have GGTA1 and CMAH knocked out and express multiple human CRPs and transgenes encoding the anti-inflammatory and anti-apoptotic molecules HO1 and A20 (REF.117). Although no studies have included experiments to pinpoint the activity of individual genetic modifications added to double knockout pigs or to GGTA1, B4GALNT2 and CMAH TKO pigs, one study reported that longer rejection-free survival (up to 217 days) could be achieved using kidneys from animals that expressed higher rather than lower levels of human CRPs118. All grafts were eventually lost owing to rejection and/or thrombotic microangiopathy; infectious complications of the immunosuppressive therapies were also an important limitation. Whether TKO source pigs have any advantage over GGTA1 knockout pigs for xenotransplantation is unclear from existing data, as they have not been compared directly. Comparable survival of kidney xenografts has been obtained in NHPs using GGTA1 knockout pigs transgenically expressing human CD55 and using TKO pigs expressing human CRPs118,119. Limitations of the Old World primate model, in which a new epitope is revealed by the CMAH knockout, make it difficult to draw conclusions about the best genetic modifications to use for human xenotransplantation. However, in some cases kidneys from GGTA1 and B4GALNT2 double knockout pigs underwent rapid ABMR within a week after transplantation into Rhesus monkeys, indicating that additional Nab targets exist in this species combination, although survival of up to 435 days was achieved in one animal120.
T cell-mediated rejection
Genetic engineering could potentially be used to avoid T cell responses and thereby enable reduction of immunosuppressive therapy. Several groups have explored this approach by introducing FasL121, CTLA4Ig122, PD-L1 (REF.118) or anti-CD2 monoclonal antibodies123 into pigs with the aim of achieving local immunosuppression in the xenograft following transplantation. Corneal transplants from pigs expressing transgenic CTLA4Ig showed improved survival compared with wild type corneal transplants in NHPs124 and transgenic CTLA4Ig expression in beta cells improved porcine islet graft survival in HIS mice125. Localized expression of CTLA4Ig is advantageous compared with generalized expression, which may compromise the immunocompetence of the source animals122.
Another potential approach to enabling xenografts to evade host T cell responses is to knock out class I SLA from TKO source pigs126. The results of transplants from these pigs into NHPs have not yet been reported. However, loss of class I SLA might increase the susceptibility of source pigs and xenografts to infection as well as increase the risk of NK cell-mediated rejection. The latter risk might be mitigated by transgenic expression of HLA25,26,127. However, lack of class I SLA would not prevent indirect T cell recognition of xenoantigens that can promote antibody-mediated and cytokine-mediated graft injury and therefore would not provide complete immune evasion.
Islet and organ xenotransplantation
Use of genetic modification approaches, combined with advances in immunosuppressive therapies, has permitted long-term pig islet, kidney and heart graft survival in NHPs and revived interest in clinical xenotransplantation (TABLE 1). Blockade of the B7-CD28 (REF.86) and CD40–CD154 costimulation pathways has had a substantial role in these successes. Liver xenotransplantation has proven to be much more challenging and such grafts have not yet survived for longer than around a month in NHPs128.
Table 1 ∣.
Chronology of progress in xenotransplantation from pigs into non-human primates
| Date | Innovation | Organ xenograft survival |
Key refs. |
|---|---|---|---|
| 1980s | Natural antibody absorption | Minutes to hours | 263 |
| 1990s | Human CRP transgenic donor pigs | Days to weeks | 264 |
| 2000s | GGTA1-knockout donor pigs | Months | 83,103 |
| 2010s | New transgenic (CRPs, hCD47, coagulation inhibitors, anti-inflammatory proteins) and knockout (B4GalNT2, CMAH, porcine endogenous retrovirus) donor pigs using CRISPR | Months to years | 91,102,105, 112-120 |
| 2020s | First human xenotransplants, potential clinical trials, development of tolerance induction approaches | NA | 145,146,150,176,237 |
CRP, complement regulatory protein; NA, not available.
Islet xenotransplantation
Islet xenotransplantation is relatively non-invasive and the graft is not life supporting, potentially simplifying its clinical application. Long-term islet xenograft survival has been achieved in NHPs but grafts were eventually rejected despite heavy immunosuppression including costimulatory blockade64,90,129. Persistent innate inflammatory responses seem to limit pig islet survival in NHPs130. Genetic modifications such as human CRPs and CTLA4Ig have been included in some studies, but their effects are uncertain131. As adult pig islets do not express Gal132, the GGTA1 knockout is of less relevance than other modifications in this setting. However, human CRPs and anti-coagulant protein transgenes could be important in controlling the immediate innate immune response that destroys islets injected into the portal circulation133.
As diabetes is usually manageable with insulin treatment and islet transplantation does not typically cure the disease134, islet xenotransplantation could only be justified on a large scale if the need for immunosuppression could be avoided, for example, by islet encapsulation or tolerance induction. To date, clinical trials of xenogeneic islet encapsulation have not demonstrated prolonged porcine insulin production135-138. Tolerance to partially class II MHC-matched allogeneic islets in NHPs has been achieved using donor apoptotic cell administration with costimulatory blockade, rapamycin and anti-inflammatory treatments139. This approach has been extended to xenograft models in rodents140 but has not yet succeeded in NHP xenograft models.
Kidney xenotransplantation
Survival of GGTA1 knockout pig kidney grafts for >400 days has been reported in a few NHPs with low levels of non-Gal Nabs89,120,141,142. However, grafts were eventually rejected despite immunosuppression that included T and B cell depletion, steroids and costimulatory blockade together with rapamycin or mycophenolate mofetil. One group reported that long-term depletion of CD4+ cells was required to achieve long-term xenograft survival119 but others found that this approach was not necessary118. Similar results have been achieved with TKO kidneys expressing various human transgenes in NHP recipients with differing levels of anti-donor antibodies in their sera118. Given these data, some researchers have suggested that kidney xenotransplantation would be appropriate for patients who are unlikely to receive an allograft for a number of reasons, including high levels of presensitization to alloantigens, primary kidney disease that is likely to recur rapidly in an allograft or a lack of vascular access for dialysis143. The poor translatability of GGTA1, B4GALNT2 and CMAH TKO kidney transplants from NHPs to humans justifies additional human decedent studies and limited clinical trials of TKO porcine kidney transplantation144.
In 2022, several human xenotransplantation experiments were carried out using GGTA1 knockout and TKO porcine kidneys in brain-dead recipients. GGTA1 knockout pig kidneys were connected to the circulations of two deceased individuals and ex vivo perfused for 54 h at New York University Medical Center, USA145. The kidneys were functional and did not undergo HAR. The TKO experiment involved implantation of two pig kidneys containing human transgenes for CD47, HO-1, several CRPs, TM and EPCR and with knockout of growth hormone receptor (GHR), into a deceased individual. The kidneys produced urine but did not clear creatinine and the grafts underwent thrombotic microangiopathy during the 3-day period of the experiment146. The disrupted physiology due to prolonged brain death and multi-organ failure in the recipient at the time of transplantation makes this outcome difficult to interpret.
Although xenotransplantation experiments using deceased humans are challenging from an ethical standpoint, much could be learned from additional similar studies, for example, with regard to the threshold levels of anti-donor IgM and IgG antibodies that would result in early ABMR with different genetically modified pigs. Establishment of such standards would greatly facilitate future clinical trials of xenotransplantation.
Heart transplantation
NHP models of orthotopic, life-sustaining heart xenotransplantation have been published in the last 5 years. Previous studies involved heterotopic transplants, in which the graft serves as an accessory rather than as a functioning heart. Long-term (several years) survival of heterotopic pig GGTA1 knockout heart grafts that expressed human CRP and TM was achieved in baboons with an immunosuppressive regimen that required CD40 blockade91. Subsequently, 6–9 months’ survival of life-sustaining orthotopic GGTA1 knockout pig hearts that expressed human CD46 and TM was achieved in baboons treated with rituximab, anti-thymocyte globulin, anti-CD40/CD40L, mycophenolate mofetil and steroids147,148. Success was dependent on non-ischaemic preservation of the heart prior to transplantation. Myocardial hypertrophy was an important initial limitation to long-term survival that could be controlled by maintaining low blood pressure in the recipient and using rapamycin. Control of graft growth achieved by knocking out GHR in source pigs with knockout of B4GALNT2 and GGTA1 that were transgenic for human CRPs, CD47, HO-1, TBM and EPCR resulted in further improvements in survival148. However, GHR-knockout pigs may not have normal health and metabolic function149; therefore, use of miniature pigs might be advantageous for cardiac xenotransplantation.
Potential candidates for cardiac xenotransplantation would likely include patients experiencing failure of left ventricular assist devices (LVADs), alloantibody formation while on LVADs, other contraindications to LVADs, failure of primary cardiac allografts, and complications of or contraindications to total artificial hearts143. In 2022, the field was galvanized by a report of cardiac xenotransplantation from a pig with 10 genetic modifications, including knockout of GGTA1, B4GALNT2, CMAH and GHR and transgenic expression of human CRPs, CD47, HO-1, TM and EPCR, to a patient at the University of Maryland, USA150. The pig heart did not undergo rapid rejection and was life sustaining for 7 weeks, providing a milestone of clinical xenotransplantation. Although the heart failed, the finding that a pig heart is capable of sustaining human life is very encouraging. Studies are in progress to determine the precise cause of the failure of the heart. Pig-specific cytomegalovirus was detected in the patient but the role, if any, of this infection in causing graft loss is currently unclear. Such infections could potentially be avoided by more rigorous screening and elimination of viruses from source pigs.
Advances in xenograft tolerance
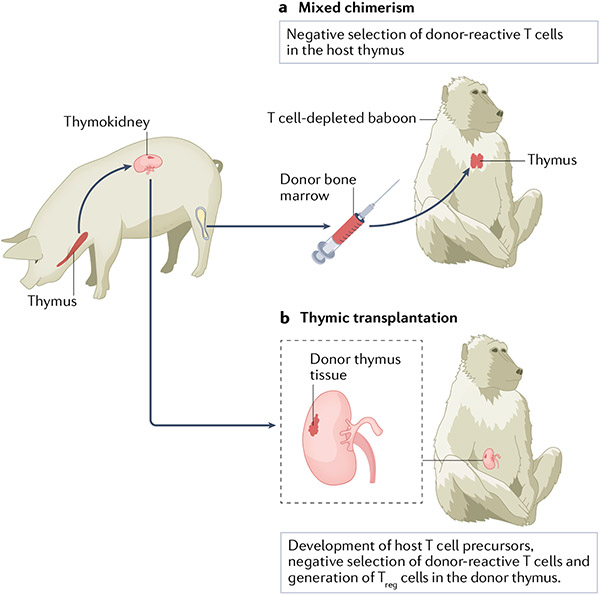
Although advances in immunosuppression and genetic engineering have markedly improved xenograft survival in NHPs, whether these approaches will reliably ensure rejection-free survival with the use of clinically tolerable levels of immunosuppression is unclear. Achievement of tolerance — the absence of a destructive response to the graft with an otherwise normal, functioning immune system — is therefore a desirable goal. Two tolerance induction strategies, mixed chimerism and thymic transplantation, have shown evidence of benefit in NHPs (FIG. 2).
Fig. 2 ∣. Tolerance induction strategies.
Two strategies are currently being developed as a means of inducing tolerance across the pig-to-primate barrier. a ∣ In mixed haematopoietic chimerism, bone marrow from the donor pig is injected into a T cell-depleted baboon. Donor bone marrow-derived dendritic cells migrate to the host thymus, where they negatively select developing donor-reactive host T cells, resulting in tolerance to donor cells. b ∣ In thymus transplantation, the donor pig thymus is transplanted into a T cell-depleted, thymectomized baboon, either as a vascularized thymic lobe (not shown) or as part of a composite thymokidney in which autologous pig thymic tissue is grafted under the renal capsule. T cells develop from host T cell precursors in the donor thymus in which tolerance to the donor may be induced both by negative selection and by the generation of regulatory T (Treg) cells. Thus, both methods of tolerance induction are dependent on the thymus.
Mixed haematopoietic chimerism
Mixed allogeneic chimerism, in which donor and recipient haematopoietic elements coexist, has been shown to induce robust allograft tolerance in rodent, large animal and clinical studies (reviewed in151). Similarly, mixed xenogeneic chimerism has achieved xenograft tolerance in rodent, humanized mouse and, to some extent, NHP models152. In rodents, mixed chimerism has been demonstrated to induce xenograft tolerance not only in the T cell compartment, but also among NAb-producing B cells153 and NK cells154. However, the initial innate and adaptive immune barriers to xenogeneic HC engraftment are much stronger than those to allogeneic HC engraftment. A major challenge for the use of HC transplantation as a tolerance induction strategy is the need for conditioning regimens that do not ablate recipient haematopoiesis, have fairly low toxicity, and are not associated with a risk of graft-vs-host disease. In rat to mouse transplantation, these criteria were met when mice were conditioned with monoclonal antibodies to deplete T cells and NK cells, low-dose total body irradiation and local thymic irradiation prior to administration of T cell-depleted rat bone marrow155. In addition to exhaustive depletion of conventional αβ T cells in the recipient, depletion of recipient NK cells and γδ T cells63 was essential to achieve rat chimerism. By contrast, in allogeneic HC transplantation, depletion of γδ T cells156 and/or NK cells157 is not required. Multilineage donor chimerism was stable in the mouse allogeneic model156, but slowly declined over time in the rat–mouse xenogeneic model, despite evidence of persistent T cell, B cell and NK cell tolerance155,158-161. This phenomenon reflects a competitive advantage of recipient HCs over xenogeneic HCs, suggesting a role for species-specific preferential interactions with haematopoietic cytokines and adhesion molecules. These physiological barriers increase as the donor species disparity increases, making it almost impossible to achieve porcine haematopoiesis in non-transgenic immunodeficient mice162. However, substantial porcine haematopoiesis was achieved in immunodeficient mouse recipients that expressed porcine haematopoietic cytokine transgenes163. Thus, genetic modifications of pigs that promote the ability of their HCs to respond to human haematopoietic cytokines could potentially advance the use of mixed chimerism to promote xenograft tolerance.
NAbs pose an additional barrier to xenogeneic HC engraftment164. Importantly, once engraftment of these cells is achieved, pre-existing natural IgM antibodies against the rat donor disappear in rat–mouse mixed xenogeneic chimeras160,165-167, even as chimerism declines to low levels159. Studies using GGTA1-knockout mice, which produce NAb against αGal168, demonstrated that mixed chimerism rapidly tolerizes anti-donor NAb-forming B cells169-171. In GGTA1-knockout mouse recipients that were presensitized to Gal and had high levels of anti-Gal IgG antibodies, the barrier to engraftment of Gal-positive HCs could be overcome by using increased doses of bone marrow and tolerance of anti-αGal antibody-forming cells was achieved172. Mixed chimerism prevented cellular and humoral rejection of primarily vascularized rat heart xenografts171, demonstrating that this approach can robustly tolerize donor-reactive T cells, prevent induced antibody responses and tolerize T cell-independent Nab-producing B cells.
T cell tolerance.
The mechanisms of T cell tolerance induction in the rat-to-mouse mixed xenogeneic chimaera model are similar to those in the allogeneic model, with central deletion of donor-reactive thymocytes158 correlating with the presence of rat MHC class II + APCs in the recipient thymus173. Achievement of tolerance was evident in in vitro assays and in vivo, with marked and specific prolongation of the survival of donor rat skin grafts155. In a robust HIS mouse model in which human HC and thymus were transplanted174 into transgenic mice that expressed porcine haematopoietic cytokines, mixed pig-human xenogeneic chimerism led to specific tolerance of human T cells to porcine donor antigens and porcine APCs were detected in the human thymus grafts175.
B cell tolerance.
Specific tolerance of human Nab-producing B cells to porcine xenoantigens has been achieved in HIS mice with mixed pig–human chimerism153. The demonstration that both B cells and T cells of HIS are tolerized by mixed porcine chimerism provides a powerful impetus to develop this approach for clinical use. Parallel results in the pig to baboon model176 suggest that mixed chimerism can tolerize primate anti-pig xenoantibodies.
The use of GGTA1-knockout mice enabled investigation of the mechanism of tolerance of Nab-producing recipient B cells induced by mixed chimerism. In these mice, anti-Gal-producing B cells have a B1b-like phenotype but do not express CD11b. These CD11b− cells are derived from CD11b + B1b cells in the peritoneal cavity that become antibody secreting and migrate to the spleen upon TLR stimulation in vitro and antigenic exposure in vivo177,178. Whether a human B1 cell subset exists is uncertain179-183; however, anti-αGal IgM-producing cells in humans and baboons have similar features to those in mice, including a largely splenic location and expression of CD11b184.
Initial B cell tolerization by induction of rat to GGTA1 KO mouse mixed chimerism was shown to reflect the anergy of Gal-binding B cells, whereas long-term tolerance reflected clonal deletion and/or receptor editing170,171,185. Early, anergy-dependent tolerance required persistence of Gal+ chimeric cells, whereas deletional tolerance, once established, did not185. The requirement for complement and complement receptor (CR1/2) expression on non-HCs for B cell tolerance186, together with the lack of requirement for soluble IgM (H.W Li, P. Bardwell and M. Sykes, unpublished work) and the observation that complement and complement receptor expression were also needed to maximize the anti-Gal response in non-tolerant mice, suggest a novel model in which complement fixation on the surface of anti-Gal surface Ig-bearing cells creates an immune complex that interacts with complement receptors on follicular dendritic cells187. The involvement of a Gal+ cell in the interaction tolerizes the Gal-binding B cells, which would otherwise be activated.
In contrast to genetic modification of pigs to eliminate targets of Nabs, mixed chimerism has the potential to tolerize all specificities of Nab-forming B cells such that identification of these specificities is not required. Progressively knocking out porcine carbohydrate-producing genes might ultimately compromise the health of the pigs and risks revealing new antigenic targets as known carbohydrate targets are removed. Thus, mixed chimerism may provide the optimal way to overcome the Nab barrier to xenografts.
Macrophage tolerance.
Macrophages impose an additional innate immune barrier to xenogeneic HC engraftment10,188,189 that at least partly results from failure of functional interactions between CD47 and SIRPα from different species. Binding of pig CD47 to human SIRPα fails to result in SIRPα phosphorylation, which is needed to transmit a signal that prevents rapid destruction of the porcine HCs190-194. This barrier can be overcome by introducing a human CD47 gene into porcine HCs195. Interestingly, NOD mice harbour a SIRPα allele that interacts effectively with human CD47 (REF.196) and transgenic expression of the human CD47 gene by porcine HCs greatly enhances their engraftment in NOD-Scid-common gamma chain knockout mice197,198. However, macrophage tolerance is not induced by haematopoietic chimerism199. Importantly, transgenic expression of human CD47 by pig HC donors increased the level of pig chimerism in conditioned recipient baboons and markedly prolonged the survival of donor pig skin grafted without any immunosuppression200. Chimerism and tolerance induction in baboons have been further enhanced by injecting human CD47 transgenic pig HCs directly into bone176. Intrabone injection enhances porcine HC survival201, possibly by evading recipient macrophages in the lungs, liver, and spleen. Infusion of polyclonally expanded recipient Treg cells with human CD47 transgenic pig HCs further prolonged donor pig skin graft survival on baboons95.
NK cell tolerance.
Mixed porcine xenogeneic chimerism in HIS mice results in human NK cell tolerance154 as well as T cell and B cell tolerance. In contrast to allogeneic mixed chimerism models in which NK cell unresponsiveness is specific to the donor and NK function is otherwise preserved202, NK cell tolerance in the rat–mouse xenogeneic mixed chimerism model is associated with global unresponsiveness of NK cells154,161. A plausible explanation for these disparate results is that the presence of a cell population lacking inhibitory ligands for any subset of recipient NK cells, as may be the case for xenogeneic but not allogeneic cells, results in chronic activation and hence global anergy of all NK cells. Inhibitory NK cell receptors have generally been shown to be non-functional between species, whereas activating receptors are functional24,26,203-213 and continuous engagement of these receptors renders NK cells hyporesponsive214,215. The ‘dominant’ tolerance imposed on recipient NK cells achieved by low levels of xenogeneic chimerism suggests that all donor and recipient cells encountered by an NK cell must express an inhibitory ligand in order for that NK cell to function normally. We have extended these observations to HIS mice, in which the presence of mixed porcine chimerism led to a marked reduction of the cytolytic activity of human NK cells against porcine lymphoblasts, suggesting that human NK cell tolerance was achieved. In some of these mice, this tolerance was specific for pig cells, whereas in others, global NK cell hyporesponsiveness was observed154. These results are consistent with the inability of some, but not all, human killer inhibitory receptors (KIRs) to recognize porcine MHC molecules216. In mixed xenogeneic chimeras, introduction of a human inhibitory ligand such as HLA-E, which binds to NKG2A–CD94 expressed on most human NK cells, might be advantageous in preserving normal function of human NK cells to provide protection against viruses and tumours161. Transgenic HLA-E expression on porcine HCs could also diminish the initial NK cell barrier to engraftment, thereby enhancing mixed xenogeneic chimerism in humans.
Thymic transplantation
Rodent studies.
Xenogeneic thymus transplantation can tolerize the T cell arm of the immune response. Normal self-tolerance induction in the thymus involves deletion, anergy or Treg cell differentiation of potentially autoreactive T cells by exposure to the appropriate self-antigens presented by either bone marrow-derived cells or thymic stromal cells. The tolerance induced by mixed chimerism depends on negative selection of developing T cells in the thymus by both donor and host bone marrow-derived cells that migrate to the host thymus, specifically deleting reactivity to both host and donor. Conversely, transplantation of a donor thymus into a recipient that is depleted of mature T cells results in intrathymic generation of new recipient T cells that are subjected to negative selection by host bone marrow-derived APCs entering the thymic graft and by donor APCs and thymic epithelial cells, resulting in loss of reactivity to both host and donor217,218. Intrathymic self-tolerance induction also involves exposure to tissue-restricted antigens (TRAs) expressed by medullary thymic epithelial cells, which results in deletion or Treg cell differentiation of thymocytes that recognize these antigens.
Small animal studies.
A pig-to-immunocompetent mouse model was used to demonstrate the capacity of xenogeneic thymic tissue to reconstitute functional host T cells in thymectomized T cell-depleted mice. These mice accepted fetal porcine thymus tissue grafted under the kidney capsule. Normal mouse thymopoiesis occurred in the thymus grafts, generating thymocyte populations that were phenotypically indistinguishable from normal mouse thymocytes. Mature CD4+ mouse T cells repopulated the periphery and demonstrated tolerance to the donor and the recipient. Most remarkably, the recipient mice accepted donor pig skin grafts with no immunosuppression217,218. Intrathymic clonal deletion was shown to be a major mechanism of both xenogeneic donor and recipient-specific tolerance69,219. The development of Treg cells in the porcine thymus graft also contributed to tolerance220. Studies that used T cell receptor (TCR) transgenic recipient mice with known MHC alleles demonstrated that positive selection in porcine thymus grafts was mediated solely by porcine thymic MHC, whereas negative selection was mediated by both pig and mouse MHC, in keeping with the detection of class II MHC + APCs from both species in the thymus grafts68-70. The peripheral CD4 T cells responded to protein antigens presented by murine class II MHC and protected the mice from infection221. Thus, the pig thymus generates a sufficiently diverse TCR repertoire to permit recognition of foreign antigens on recipient MHC despite positive selection by porcine MHC.
Studies in HIS mice provided important proof-of-principle that porcine thymic transplantation can achieve human T cell tolerance. Human T cells developed normally from haematopoietic progenitors and were centrally tolerized to porcine xenoantigens in pig thymic grafts222,223. A diverse T cell repertoire was generated71 and specific tolerance towards donor pig and human haematopoietic stem cell (HSC) antigens was shown in vitro, with intact responses to third-party pig and allogeneic human antigens222,223. Lack of response to the human HSC donor and the murine recipient reflects the presence of both human donor and murine recipient APCs in thymic xenografts222,224. Most strikingly, human T cells developing in porcine thymus grafts demonstrated pig donor-specific skin graft tolerance223.
Large animal studies.
Although thymic tissue transplantation induced long-term tolerance to pig in normal and HIS mice, we achieved more transient effects in the pig-to-primate large animal model when we transplanted minced fetal pig thymus into various sites (muscle, omentum, subrenal capsule) in thymectomized, T cell-depleted NHPs. Conditioning included T cell depletion with anti-CD3-CRM9, an effective T cell-depleting conjugate of an anti-monkey CD3 mAb and a diphtheria toxin binding site mutant. The grafted animals demonstrated donor-specific hyporesponsiveness in vitro and prolongation of porcine skin grafts, but only a small amount of thymic epithelium remained at the implantation site by day 60 (REF.225). Control animals receiving similar treatment without a pig thymus graft rejected pig skin grafts rapidly and showed no evidence of tolerance in vitro.
A possible reason for the difficulty in achieving tolerance with thymic transplantation in large animals is incomplete T cell depletion. In mice, CD4 T cell depletion must be exhaustive to avoid rejection of the thymus graft226 and the T cell depletion achieved in baboons was much less complete than that achieved in mice225. Consistent with this hypothesis, a thymic tissue graft was reported to achieve cardiac allograft tolerance in a patient who had complete DiGeorge syndrome and therefore lacked a thymus and pre-existing T cells227.
Thymic tissue grafts must establish a new vascular supply to survive and are susceptible to ischaemic damage during the process of revascularization. In addition, vascularized grafts are relatively tolerogenic, whereas skin and tissue grafts are relatively immunogenic owing to their ability to sensitize the host by releasing cells or antigens into the lymphatic system228. During the sensitive revascularization period, complete T cell depletion is likely essential to protect implanted thymic tissue grafts. We therefore examined the potential of vascularized thymic tissue to induce tolerance in our miniature swine model. For this purpose, we prepared a composite thymus-plus-kidney graft (thymokidney) using juvenile thymic tissue. Pigs aged 6–8 weeks underwent partial thymectomy and received autologous thymic tissue grafts under the renal capsule. The autologous thymic tissues successfully engrafted without immunosuppression229. In thymectomized class I disparate pigs receiving a short course of ciclosporin A, which induced tolerance to renal allografts in non-thymectomized recipients but not in thymectomized recipients, non-vascularized allogeneic thymic grafts were rejected within 4 weeks and kidney grafts were rejected in the second post-operative month. By contrast, all thymectomized recipients accepted vascularized composite thymokidney grafts indefinitely with stable kidney function and donor-specific unresponsiveness230. The same strategy permitted tolerance induction across a full MHC mismatch231. The thymokidney recipients demonstrated donor-specific unresponsiveness in vitro. These promising data demonstrate the functional capacity of vascularized thymic grafts and indicate the necessity of a vascularized thymic graft for the success of this tolerance strategy in large animal models.
In addition to the thymokidney approach, a technique was developed for simultaneous transplantation of a vascularized thymic lobe (VTL) graft and a donor kidney in pigs232. The researchers reasoned that this technique would be broadly applicable for induction of tolerance to any organ simultaneously transplanted from the same donor. Subsequent studies demonstrated the validity of this hypothesis, showing that simultaneous transplantation of a donor VTL led to tolerance of allogeneic cardiac transplants in pigs233.
Given the success of thymokidney transplantation and VTL transplantation for induction of tolerance across fully allogeneic barriers, these techniques were applied in efforts to induce tolerance across the xenogeneic pig-to-primate barrier. Early studies utilized human CD55 transgenic kidney donors, immunoabsorption of natural anti-Gal antibodies, thymectomy, cobra venom factor to deplete complement and T cell depletion along with immunosuppressive drugs234. The vascularized thymic grafts supported initial reconstitution of naïve-type CD45RAhigh baboon CD4+ T cells. In addition, donor-specific unresponsiveness in mixed lymphocyte reactions was achieved for approximately 2 months. However, all grafts were subsequently lost owing to humoral rejection, following the inexorable return of anti-Gal antibodies.
Further exploration of the thymokidney and VTL approach made use of GGTA1 knockout swine donors to avoid rejection owing to anti-Gal antibodies. Predictably, this approach remarkably improved kidney xenograft survival to >80 days, with most animals dying from causes other than rejection83,235. These results suggest that the thymokidney and VTL tolerance-inducing regimens could prevent T cell-mediated responses as well as new T cell-dependent antibody responses, thereby permitting much longer survival of the xenografted organs.
Further modification of the induction regimen with thymokidney or VTL transplantation has permitted survival of life-supporting porcine kidneys for over 6 months in baboons, with eventual loss due to cortical necrosis without evidence of rejection236,237. As even miniature swine kidneys become too large for a 10-kg baboon, we hypothesize that cortical necrosis might be caused by excessive growth of the transplanted organ. In animals euthanized at >6 months owing to an intolerable increase in graft size, there was no evidence of the development of anti-pig antibodies and the thymokidney graft showed well-preserved glomeruli and vessels with no glomerulitis, endothelialitis, or vasculopathy and minimal cellular infiltrate. Ischaemic tubular injury with dilatation of some renal tubules and interstitial oedema were consistent with the pathological diagnosis of cortical ischaemia. Importantly, the immunosuppression administered to these animals was tapered after the first 1–2 months, such that by 6 months only minimal immunosuppression was given. Baboons that survived with life-supporting porcine thymokidneys for >3 months developed T cells with the phenotype of new thymic emigrants (CD4+CD31+CD45RA+) that showed donor-specific unresponsiveness in vitro83,237. As the recipients were completely thymectomized before transplantation, these thymic emigrants must have developed in the vascularized thymic grafts of the composite thymokidneys, suggesting that these transplants had achieved tolerance and immunocompetence.
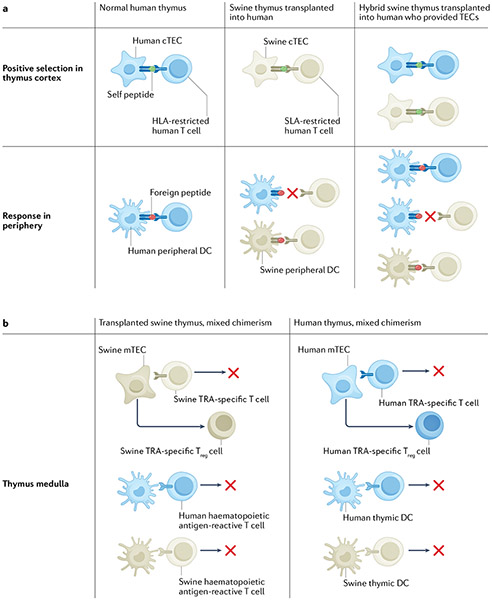
Limitations that might be associated with the generation of a human T cell repertoire in a xenogeneic porcine thymus must be considered. Positive selection by porcine MHC would result in a T cell repertoire that preferentially recognizes peptide antigens presented by SLA. This preference would result in excellent protection of the xenograft from infection, but protection of host cells against pathogens might be less effective. Indeed, human T cells that developed in a pig thymus in HIS mice showed weaker responses to peptides presented by human APCs than those developing in human thymus grafts223. In addition, negative selection of TRA-specific conventional T cells and positive selection of TRA-specific Treg cells by porcine medullary epithelium might result in gaps in tolerance for human TRAs that are not conserved in pigs or re-presented by human APCs. We have therefore developed an alternative approach involving construction of a ‘hybrid thymus’, in which human (“recipient”) thymic epithelial cells are injected into porcine thymic tissue in HIS mice238 (FIG. 3a). This approach overcame incomplete tolerance to recipient antigens in the pig-to-immunocompetent mouse model described above239. In HIS mice, we demonstrated that human thymic epithelial progenitors (TEPs) generated from pluripotent stem cells could positively influence human T cell development in a porcine thymus graft and improve human immune reconstitution when the TEPs and HSCs shared an HLA allele240. With further development of this approach, patient-specific induced pluripotent stem cell-derived TEPs might prove to be ideal for construction of swine–human hybrid thymuses. An alternative approach to achieving a T cell repertoire with optimal recognition of microbial antigens presented by both pig and human MHC molecules, while achieving a combination of deletion of conventional T cells and selection of Treg cells recognizing pig and human antigens, could involve a combination of porcine thymic transplantation and mixed xenogeneic chimerism induction in recipients with an intact native thymus (FIG. 3b).
Fig. 3 ∣. Tolerance and immune function with transplantation of swine (or hybrid) thymus and mixed xenogeneic chimerism.
a ∣ Human thymocyte selection in the normal human thymus, swine thymus transplanted into a human and hybrid swine thymus containing human cortical thymic epithelial cells (cTECs) transplanted into the human who provided the TECs. The hybrid thymus is generated by injecting TECs obtained from the recipient’s thymus at the time of thymectomy or generated de novo from induced pluripotent stem cells of the recipient into the swine thymus prior to transplantation. In the human thymus, human cTECs mediate positive selection so that T cell responses in the periphery are largely restricted by the human leukocyte antigen (HLA) of the recipient, resulting in immune protection against infection. In the transplanted swine thymus, swine cTECs mediate positive selection, so that T cell responses in the periphery are restricted by swine leukocyte antigen (SLA), resulting in immune protection against infection of a swine xenograft but not of the recipient. In the hybrid thymus, both human and swine cTECs participate in positive selection. The peripheral immune system is therefore able to recognize foreign peptides presented by recipient HLA and by donor SLA, resulting in good immune protection of the recipient and of a swine xenograft. In the transplanted swine thymus and the hybrid swine thymus, positive selection on swine cTECs will enable selection of swine donor-specific regulatory T (Treg) cells and the presence of swine antigen-presenting cells (APCs) in the graft will result in negative selection of swine-reactive T cells, resulting in tolerance to the donor (not shown). b ∣ Advantages of combined mixed chimerism and swine thymus transplantation. In the transplanted swine thymus, swine haematopoietic antigen-reactive T cells would be deleted, as mixed chimerism ensures a constant supply of swine APCs. In addition, swine tissue-restricted antigen (TRA)-specific T cells would be deleted and swine TRA-specific Treg cells positively selected by swine medullary thymic epithelial cells (mTECs), resulting in robust tolerance to the swine donor. However, human TRA-specific T cells would be released to the periphery owing to the absence of human mTECs, potentially predisposing the recipient to autoimmunity. The presence of a human thymus (in addition to the swine thymus) may be permissible in the setting of mixed chimerism, as many swine haematopoietic antigen-reactive T cells would be deleted by the presence of swine APCs in the human thymus. Swine TRA-specific T cells that escape the human thymus (owing to the absence of swine mTECs) would be suppressed by Treg cells emigrating from the swine thymus graft, where swine mTECs are abundant. The presence of a human thymus would enhance human TRA-specific tolerance and HLA-restricted protective immunity owing to the participation of human mTECs in selection.
Considerations for source pigs
The favourable breeding characteristics of pigs as well as their availability and physiological similarities to humans make them an attractive source animal for clinical xenotransplanation241. With the heightened capacity for genetic engineering, source pigs could be tailored to meet the needs of specific organs for transplantation.
Inbreeding
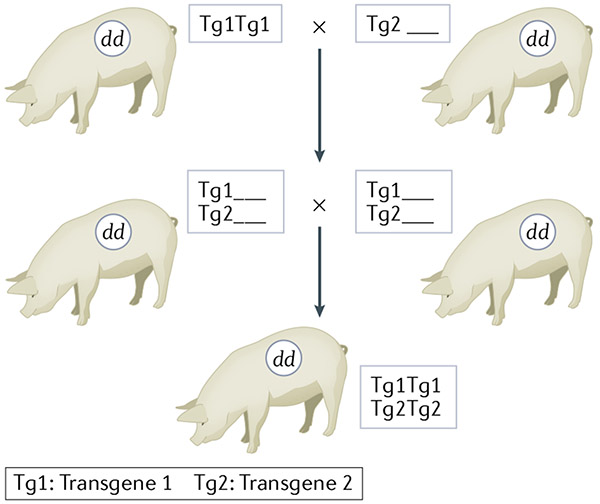
The large litter sizes (5–10 offspring), early sexual maturity (5 months), short gestation time (114 days), and frequent oestrus cycles (every 3 weeks) of pigs enabled rapid selective breeding programs that produced miniature swine homozygous for MHC in a fairly short time242. In addition, an inbred line of miniature swine that has reached a >94% coefficient of inbreeding enables transplants within the line to be accepted without immunosuppression243. Inbreeding provides major advantages for genetic engineering of pigs and tolerance induction. Using inbred animals, any number of independently segregating transgenes can be combined into the same genetic background by simple breeding and selection, likely maintaining the expression patterns of the original pig lines (FIG. 4). This fixation of the genetic background bypasses issues related to epigenetic differences and mitochondrial DNA differences in addition to practical limitations of cloning pigs for xenotransplantation. Use of inbred animals also avoids the variability of multiple genetic backgrounds when breeding genetically modified pigs. Use of inbred pigs facilitates xenograft tolerance because genetically modified cells optimized for tolerance induction from one animal can be used to tolerize to an organ from another animal carrying different genetic modifications tailored to optimize the function of the organ. If for any reason the transplanted organ needed to be replaced, the recipient would remain tolerant to the replacement organ. Inbreeding of animals might, however, be associated with substantial challenges, including potential reductions in fecundity or compromised health due to fixation of recessive alleles.
Fig. 4 ∣. Breeding for multiple transgenes in inbred miniature swine.
Breeding of genetically modified inbred miniature swine permits combination of multiple different modifications into pigs with the same inbred genetic background. Breeding of cloned outbred animals leads to random assortment of background genes (not shown), whereas a cross and intercross of edits made on the same inbred background can produce offspring with both edits still on the same background. dd denotes the homozygous SLA genotype of the pig.
Pathogens
During the late 1990s, the observation that a porcine cell line could transmit porcine endogenous retroviruses (PERVs) to human cell lines in vitro led to concerns about the risk of infectious transmission from pigs to humans244. If PERVs were able to infect human xenograft recipients, such infections could theoretically result in new infectious diseases, perhaps owing to recombination with human endogenous viruses in the genome. However, extensive research demonstrated that it is very difficult to infect primary human cells with PERVs owing to the presence of restriction factors and that the PERVs that infected human cell lines were primarily PERC A-C recombinants (reviewed in Fishman245). Although sensitive assays have been developed for PERV detection246, no evidence of infection has been found in >200 human recipients of porcine cells or xenografts or in hundreds of NHP recipients of porcine xenografts (reviewed in Fishman245). The lack of infection in NHPs might be partly explained by the incompatibility of the receptors needed for PERV infection. In addition, primary human and NHP cells limit PERV infectivity because of the expression of intracellular restriction factors that prevent PERV replication247. Thus, with appropriate safety measures in place, the risk of PERV infection in xenotransplantation is now generally considered to be both acceptable and manageable245,248. Genetic engineering methodologies have been developed to further reduce this risk249, including use of CRISPR–Cas9 technology to knock out PERVs250,251. However, this approach could potentially lead to off-target genetic modifications with deleterious effects252, particularly as so many loci are targeted simultaneously.
Concerns regarding the theoretical risk of PERV infection might have obscured the fact that quality control of source pigs results in reduced infectious risk compared with human allografts245,253. Organs from deceased donors can only be screened for limited pathogens, resulting in substantial rates of unexpected infectious transmission from allografts254. Concerns regarding infections can be minimized by careful control and monitoring of source pigs and their environment according to regulatory guidelines255,256 and recommendations245,257,258. In addition to pathogens that could cause zoonoses in humans, porcine pathogens that may pose a risk to the graft, such as porcine CMV, should also be excluded, particularly as human recipients will typically not have T cell immunity to porcine viral antigens presented by SLA.
Organ size
Most groups that are developing genetically modified pigs for xenotransplantation use domestic swine, which attain adult weights of >450 kg. After the age of about 1 year, the organs of these animals would be too large for human transplantation. Use of GHR knockout pigs could potentially overcome this problem148. Alternatively, we have developed miniature swine that achieve maximum adult weights of 90–140 kg, similar to those of humans. The organs of these animals could be of potential use for transplantation at any age. How much of the growth potential of a transplanted organ is intrinsic rather than controlled by the size of the recipient is unclear. Transplantation of kidneys from conventional swine to miniature swine with a tolerance induction regimen was associated with persistent growth of the grafts in the recipients. In a 3-month period following transplantation into miniature swine, the size of conventional swine grafts increased to 3.7 times their initial volume, whereas miniature swine grafts increased to 1.2 times their initial volume237. Lung allografts showed similarly increased growth ratios, which were associated with impaired function of the organs237. Excessive growth could outstrip the blood supply of the organ, perhaps explaining the cortical necrosis seen in porcine kidneys transplanted to baboons237.
Biological incompatibilities
Differences in organ-specific biological products, for example, coagulation factors produced by the liver, are expected between pigs and humans. Selective genetic modifications or biological replacement therapies may be able to overcome many of these incompatibilities. For example, pig liver xenograft survival in baboons was prolonged to 28 days by replacing liver-derived porcine coagulation factors that did not function with baboon components to prevent coagulopathy and thrombocytopenia259,260. More subtle incompatibilities might become apparent as prolonged pig xenograft survival is achieved in NHPs and ultimately in humans. Differing metabolic requirements between pigs and humans have become apparent in pig-to-primate islet xenotransplantation261 and differences in the intrinsic blood pressures of pigs versus baboons might promote myocardial hypertrophy following orthotopic pig heart transplantation147. Suboptimal xenogeneic HC engraftment partly reflects physiological incompatibilities in the haematopoietic microenvironment in recipients and may be partially corrected by introducing human cytokine receptors into the porcine source animals.
Conclusions
Improvements in methodologies for genetic engineering of pigs and immunosuppression have led to exciting advances in xenotransplantation, which are reflected in long-term organ xenograft survival in NHPs and early forays into clinical xenotransplantation. Criteria for kidney xenotransplantation in living humans and for recipient selection have been previously discussed262 and such studies are likely imminent. Enormous potential exists for further modifications to make pig organs more compatible with human immune systems and physiology. Nevertheless, immune barriers to xenotransplantation are likely to remain formidable and tolerance may ultimately be required to enable xenotransplantation to become the standard of care. Stepwise advances towards this goal are anticipated in the near future, and it is hoped that the aim of organ transplantation for all who need it will ultimately be achieved.
Key points.
As the demand for human organs for transplantation far exceeds the supply, many patients die while waiting for a transplant; xenotransplantation provides a potential near-term solution to the organ shortage.
Advances in genetic engineering, immunosuppression and tolerance approaches have contributed to improved pig organ survival in non-human primates (NHPs).
Functioning pig islet and heart grafts have survived in NHPs for months and functioning pig kidney grafts have survived in NHPs for years.
In 2022, a pig heart was transplanted into a patient with heart failure and functioned for 7 weeks and xenotransplantation experiments using pig kidneys in deceased human recipients provided encouraging data.
Mixed haematopoietic chimerism and thymic transplantation approaches have been successfully used to tolerize human T cells, B cells and natural killer cells in human immune system mice, providing proof of their potential to induce immune tolerance.
Advances in applying tolerance approaches in NHPs, including genetic engineering of source pigs, support their potential to provide a long-term solution to the powerful immune barriers to xenotransplantation.
Footnotes
Competing interests
The authors receive sponsored research support from Choironex, a fully-owned subsidiary of Nephro Health.
References
- 1.OPTN. http://optn.transplant.hrsa.gov(2022).
- 2.Levitt M Could the organ shortage ever be met? Life Sci. Soc. Policy 11, 6 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kang HW, Yoo JJ & Atala A Bioprinted scaffolds for cartilage tissue engineering. Methods Mol. Biol 1340, 161–169 (2015). [DOI] [PubMed] [Google Scholar]
- 4.Gilpin SE & Ott HC Using nature’s platform to engineer bio-artificial lungs. Ann. Am. Thorac. Soc 12, S45–S49 (2015). [DOI] [PubMed] [Google Scholar]
- 5.Reemtsma K, McCracken BH & Schlegel JU Renal heterotransplantation in man. Ann. Surg 160, 384 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hardy JD et al. Heart transplantation. J. Miss. State Med. Assoc 9, 105–110 (1968). [PubMed] [Google Scholar]
- 7.Starzl TE, Marchioro TL & Peters GN Renal heterotransplantation from baboon to man: experience with six cases. Transplantation 2, 752 (1964). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fu Y et al. Selective rejection of porcine islet xenografts by macrophages. Xenotransplantation 15, 307–312 (2008). [DOI] [PubMed] [Google Scholar]
- 9.Abe M et al. Elimination of porcine hematopoietic cells by macrophages in mice. J. Immunol 168, 621–628 (2002). [DOI] [PubMed] [Google Scholar]
- 10.Basker M et al. Clearance of mobilized porcine peripheral blood progenitor cells is delayed by depletion of the phagocytic reticuloendothelial system in baboons. Transplantation 72, 1278–1285 (2001). [DOI] [PubMed] [Google Scholar]
- 11.Candinas D et al. T cell independence of macrophage and natural killer cell infiltration, cytokine production, and endothelial activation during delayed xenograft rejection. Transplantation 62, 1920–1927 (1996). [DOI] [PubMed] [Google Scholar]
- 12.Yi S et al. CD4+ T cells initiate pancreatic islet xenograft rejection via an interferon-gamma-dependent recruitment of macrophages and natural killer cells. Transplantation 73, 437–446 (2002). [DOI] [PubMed] [Google Scholar]
- 13.Benda B, Karlsson-Parra A, Ridderstad A & Korsgren O Xenograft rejection of porcine islet-like cell clusters in immunoglobulin- or Fc-receptor γ-deficient mice. Transplantation 62, 1207–1211 (1996). [DOI] [PubMed] [Google Scholar]
- 14.Gill RG, Wolf L, Daniel D & Coulombe M CD4+ T cells are both necessary and sufficient for islet xenograft rejection. Transplant. Proc 26, 1203 (1994). [PubMed] [Google Scholar]
- 15.Yi S et al. T cell-activated macrophages are capable of both recognition and rejection of pancreatic islet xenografts. J. Immunol 170, 2750–2758 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Millan MT et al. Human monocytes activate porcine endothelial cells, resulting in increased E-selectin, interleukin-8, monocyte chemotactic protein-1, and plasminogen activator inhibitor-type-1 expression. Transplantation 63, 421–429 (1997). [DOI] [PubMed] [Google Scholar]
- 17.Shimizu A, Yamada K, Robson SC, Sachs DH & Colvin RB Pathologic characteristics of transplanted kidney xenografts. J. Am. Soc. Nephrol 23, 225–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hisashi Y et al. Rejection of cardiac xenografts transplanted from α1,3-galactosyltransferase gene-knockout (GalT-KO) pigs to baboons. Am. J. Transplant 8, 2516–2526 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Seebach JD & Waneck GL Natural killer cells in xenotransplantation. Xenotransplantation 4, 201–211 (1997). [Google Scholar]
- 20.Middleton D, Curran M & Maxwell L Natural killer cells and their receptors. Transpl. Immunol 10, 147–164 (2002). [DOI] [PubMed] [Google Scholar]
- 21.Yang YG & Sykes M Xenotransplantation: current status and a perspective on the future. Nat. Rev. Immunol 7, 519–531 (2007). [DOI] [PubMed] [Google Scholar]
- 22.Donnelly CE, Yatko C, Johnson EW & Edge AS Human natural killer cells account for non-MHC class I-restricted cytolysis of porcine cells. Cell. Immunol 175, 171–178 (1997). [DOI] [PubMed] [Google Scholar]
- 23.Seebach JD et al. HLA-Cw3 expression on porcine endothelial cells protects against xenogeneic cytotoxicity mediated by a subset of human NK cells. J. Immunol 159, 3655–3661 (1997). [PubMed] [Google Scholar]
- 24.Forte P, Baumann BC, Schneider MK & Seebach JD HLA-Cw4 expression on porcine endothelial cells reduces cytotoxicity and adhesion mediated by CD158a+ human NK cells. Xenotransplantation 16, 19–26 (2009). [DOI] [PubMed] [Google Scholar]
- 25.Lilienfeld BG, Crew MD, Forte P, Baumann BC & Seebach JD Transgenic expression of HLA-E single chain trimer protects porcine endothelial cells against human natural killer cell-mediated cytotoxicity. Xenotransplantation 14, 126–134 (2007). [DOI] [PubMed] [Google Scholar]
- 26.Weiss EH et al. HLA-E/human beta2-microglobulin transgenic pigs: protection against xenogeneic human anti-pig natural killer cell cytotoxicity. Transplantation 87, 35–43 (2009). [DOI] [PubMed] [Google Scholar]
- 27.Seebach JD, Yamada K, McMorrow I, Sachs DH & DerSimonian HD Xenogeneic human anti-pig cytotoxicity mediated by activated natural killer cells. Xenotransplantation 3, 188–197 (1996). [Google Scholar]
- 28.Watier H et al. Human NK cell-mediated direct and IgG-dependent cytotoxicity against xenogeneic porcine endothelial cells. Transpl. Immunol 4, 293–299 (1996). [DOI] [PubMed] [Google Scholar]
- 29.Nikolic B, Cooke DT, Zhao G & Sykes M Both γδ T cells and NK cells inhibit the engraftment of xenogeneic rat bone marrow cells and the induction of xenograft tolerance in mice. J. Immunol 166, 1398–1404 (2001). [DOI] [PubMed] [Google Scholar]
- 30.Sacks SH, Chowdhury P & Zhou W Role of the complement system in rejection. Curr. Opin. Immunol 15, 487–492 (2003). [DOI] [PubMed] [Google Scholar]
- 31.Java A, Atkinson J & Salmon J Defective complement inhibitory function predisposes to renal disease. Annu. Rev. Med 64, 307–324 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cozzi E & White DJG The generation of transgenic pigs as potential organ donors for humans. Nat. Med 1, 964–967 (1995). [DOI] [PubMed] [Google Scholar]
- 33.Robson SC, Cooper DK & d’Apice AJ Disordered regulation of coagulation and platelet activation in xenotransplantation. Xenotransplantation 7, 166–176 (2000). [DOI] [PubMed] [Google Scholar]
- 34.Shimizu A et al. Thrombotic microangiopathy associated with humoral rejection of cardiac xenografts from α1,3-galactosyltransferase gene-knockout pigs in baboons. Am. J. Pathol 172, 1471–1481 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shimizu A et al. Thrombotic microangiopathic glomerulopathy in human decay accelerating factor-transgenic swine-to-baboon kidney xenografts. J. Am. Soc. Nephrol 16, 2732–2745 (2005). [DOI] [PubMed] [Google Scholar]
- 36.Knosalla C et al. Renal and cardiac endothelial heterogeneity impact acute vascular rejection in pig-to-baboon xenotransplantation. Am. J. Transplant 9, 1006–1016 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yang Y-G, Chen AM, Sergio JJ, Zhou Y & Sykes M Role of antibody-independent complement activation in rejection of porcine bone marrow cells in mice. Transplantation 69, 163–190 (1999). [DOI] [PubMed] [Google Scholar]
- 38.Galili U, Shohet SB, Kobrin E, Stults CL & Macher BA Man, apes, and old world monkeys differ from other mammals in the expression of alpha-galactosyl epitopes on nucleated cells. J. Biol. Chem 263, 17755–17762 (1988). [PubMed] [Google Scholar]
- 39.Parker W, Bruno D, Holzknecht ZE & Platt JL Characterization and affinity isolation of xenoreactive human natural antibodies. J. Immunol 153, 3791–3803 (1994). [PubMed] [Google Scholar]
- 40.McMorrow IM, Comrack CA, Sachs DH & DerSimonian H Heterogeneity of human anti-pig natural antibodies cross-reactive with the Gal(α1,3) Galactose epitope. Transplantation 64, 501–510 (1997). [DOI] [PubMed] [Google Scholar]
- 41.Lai L et al. Production of {α}−1,3-Galactosyltransferase knockout pigs by nuclear transfer cloning. Science 295, 1089–1092 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Phelps CJ et al. Production of α1,3-galactosyltransferase-deficient pigs. Science 299, 411–414 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kolber-Simonds D et al. Production of {α}−1,3-galactosyltransferase null pigs by means of nuclear transfer with fibroblasts bearing loss of heterozygosity mutations. Proc. Natl Acad. Sci. USA 101, 7335–7340 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen G et al. Acute rejection is associated with antibodies to non-Gal antigens in baboons using Gal-knockout pig kidneys. Nat. Med 11, 1295–1298 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Le Bas-Bernardet S et al. Bortezomib, C1-inhibitor and plasma exchange do not prolong the survival of multi-transgenic GalT-KO pig kidney xenografts in baboons. Am. J. Transplant 15, 358–370 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Le Bas-Bernardet S et al. Xenotransplantation of galactosyl-transferase knockout, CD55, CD59, CD39, and fucosyl-transferase transgenic pig kidneys into baboons. Transplant. Proc 43, 3426–3430 (2011). [DOI] [PubMed] [Google Scholar]
- 47.Byrne GW et al. Proteomic identification of non-Gal antibody targets after pig-to-primate cardiac xenotransplantation. Xenotransplantation 15, 268–276 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Byrne GW, Stalboerger PG, Du Z, Davis TR & McGregor CG Identification of new carbohydrate and membrane protein antigens in cardiac xenotransplantation. Transplantation 91, 287–292 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miwa Y et al. Are N-glycolylneuraminic acid (Hanganutziu-Deicher) antigens important in pig-to-human xenotransplantation? Xenotransplantation 11, 247–253 (2004). [DOI] [PubMed] [Google Scholar]
- 50.Zhu A & Hurst R Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation 9, 376–381 (2002). [DOI] [PubMed] [Google Scholar]
- 51.Irie A, Koyama S, Kozutsumi Y, Kawasaki T & Suzuki A The molecular basis for the absence of N-glycolylneuraminic acid in humans. J. Biol. Chem 273, 15866–15871 (1998). [DOI] [PubMed] [Google Scholar]
- 52.Yamamoto T et al. Old World Monkeys are less than ideal transplantation models for testing pig organs lacking three carbohydrate antigens (Triple-Knockout). Sci. Rep 10, 9771 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Platt JL et al. The role of natural antibodies in the activation of xenogeneic endothelial cells. Transplantation 52, 1037–1043 (1991). [DOI] [PubMed] [Google Scholar]
- 54.Grehan JF, Levay-Young BK, Benson BA, Abrahamsen MS & Dalmasso AP aGal ligation of pig endothelial cells induces protection from complement and apoptosis independently of NF-κB and inflammatory changes. Am. J. Transplant 5, 712–719 (2005). [DOI] [PubMed] [Google Scholar]
- 55.Magee JC et al. Prevention of hyperacute xenograft rejection by intravenous immunoglobulin. Transplant. Proc 27, 271–271 (1995). [PubMed] [Google Scholar]
- 56.Dalmasso AP, He T & Benson BA Human IgM xenoreactive natural antibodies can induce resistance of porcine endothelial cells to complement-mediated injury. Xenotransplantation 3, 54–62 (1996). [Google Scholar]
- 57.Ladowski JM et al. Swine leukocyte antigen (SLA) class II is a xenoantigen. Transplantation 102, 249–254 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Martens GR et al. Humoral reactivity of renal transplant-waitlisted patients to cells from GGTA1/CMAH/B4GalNT2, and SLA class I knockout pigs. Transplantation 101, e86–e92 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Martens GR et al. HLA class I-sensitized renal transplant patients have antibody binding to SLA class I epitopes. Transplantation 103, 1620–1629 (2019). [DOI] [PubMed] [Google Scholar]
- 60.Ladowski JM et al. Examining epitope mutagenesis as a strategy to reduce and eliminate human antibody binding to class II swine leukocyte antigens. Immunogenetics 71, 479–487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Barcy S et al. γδ+ T cells involvement in viral immune control of chronic human herpesvirus 8 infection. J. Immunol 180, 3417–3425 (2008). [DOI] [PubMed] [Google Scholar]
- 62.Wu Y et al. Human γδ T cells: a lymphoid lineage cell capable of professional phagocytosis. J. Immunol 183, 5622–5629 (2009). [DOI] [PubMed] [Google Scholar]
- 63.Nikolic B, Cooke DT, Zhao G & Sykes M Both γ/δ T cells and NK cells inhibit the engraftment of xenogeneic rat bone marrow cells in mice. J. Immunol 166, 1398–1404 (2001). [DOI] [PubMed] [Google Scholar]
- 64.Hering BJ et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat. Med 12, 301–303 (2006). [DOI] [PubMed] [Google Scholar]
- 65.Cardona K et al. Long-term survival of neonatal porcine islets in nonhuman primates by targeting costimulation pathways. Nat. Med 12, 304–306 (2006). [DOI] [PubMed] [Google Scholar]
- 66.Moses RD, Pierson RN III, Winn HJ & Auchincloss HJ Xenogeneic proliferation and lymphokine production are dependent on CD4+helper T cells and self antigen-presenting cells in the mouse. J. Exp. Med 172, 567–575 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Moses RD, Winn HJ & Auchincloss H Jr. Evidence that multiple defects in cell-surface molecule interactions across species differences are responsible for diminished xenogeneic T cell responses. Transplantation 53, 203–209 (1992). [DOI] [PubMed] [Google Scholar]
- 68.Zhao Y et al. Maturation and function of mouse T cells with a transgeneic TCR positively selected by highly disparate xenogeneic porcine MHC. Cell. Mol. Biol 47, 217–228 (2000). [PubMed] [Google Scholar]
- 69.Zhao Y et al. Positive and negative selection of functional mouse CD4 cells by porcine MHC in pig thymus grafts. J. Immunol 159, 2100–2107 (1997). [PubMed] [Google Scholar]
- 70.Zhao Y, Swenson K, Sergio JJ & Sykes M Pig MHC mediates positive selection of mouse CD4+ T cells with a mouse MHC-restricted TCR in pig thymus grafts. J. Immunol 161, 1320–1326 (1998). [PubMed] [Google Scholar]
- 71.Shimizu I, Fudaba Y, Shimizu A, Yang YG & Sykes M Comparison of human T cell repertoire generated in xenogeneic porcine and human thymus grafts. Transplantation 86, 601–610 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Dorling A, Lombardi G, Binns R & Lechler RI Detection of primary direct and indirect human anti-porcine T cell responses using a porcine dendritic cell population. Eur. J. Immunol 26, 1378–1387 (1996). [DOI] [PubMed] [Google Scholar]
- 73.Yamada K, Sachs DH & DerSimonian H Human anti-porcine xenogeneic T cell response. Evidence for allelic specificity of mixed leukocyte reaction and for both direct and indirect pathways of recognition. J. Immunol 155, 5249–5256 (1995). [PubMed] [Google Scholar]
- 74.Rollins SA et al. Evidence that activation of human T cells by porcine endothelium involves direct recognition of porcine SLA and costimulation by porcine ligands for LFA-1 and CD2. Transplantation 57, 1709–1716 (1994). [PubMed] [Google Scholar]
- 75.Yamada K, Sachs DH & DerSimonian H Direct and indirect recognition of pig class II antigens by human T cells. Transplant. Proc 27, 258–259 (1995). [PubMed] [Google Scholar]
- 76.Godwin JW, D’Apice AJ & Cowan PJ Characterization of pig intercellular adhesion molecule-2 and its interaction with human LFA-1. Am. J. Transplant 4, 515–525 (2004). [DOI] [PubMed] [Google Scholar]
- 77.Murray AG, Khodadoust MM, Pober JS & Bothwell AL Porcine aortic endothelial cells activate human T cells: direct presentation of MHC antigens and costimulation by ligands for human CD2 and CD28. Immunity 1, 57–63 (1994). [DOI] [PubMed] [Google Scholar]
- 78.Zheng L, Ben LH, Pober JS & Bothwell AL Porcine endothelial cells, unlike human endothelial cells, can be killed by human CTL via Fas ligand and cannot be protected by Bcl-2. J. Immunol 169, 6850–6855 (2002). [DOI] [PubMed] [Google Scholar]
- 79.Yi S, Feng X, Wang Y, Kay TW & O’Connell PJ CD4+ cells play a major role in xenogeneic human anti-pig cytotoxicity through the Fas/Fas ligand lytic pathway. Transplantation 67, 435–443 (1999). [DOI] [PubMed] [Google Scholar]
- 80.Sultan P et al. Pig but not human interferon-γ initiates human cell-mediated rejection of pig tissue in vivo. Proc. Natl Acad. Sci. Usa 94, 8767–8772 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hering BJ & Walawalkar N Pig-to-nonhuman primate islet xenotransplantation. Transpl. Immunol 21, 81–86 (2009). [DOI] [PubMed] [Google Scholar]
- 82.Buhler L et al. Persistence of indirect but not direct T cell xenoresponses in baboon recipients of pig cell and organ transplants. Am. J. Transplant 16, 1917–1922 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yamada K et al. Marked prolongation of porcine renal xenograft survival in baboons through the use of α1,3-galactosyltransferase gene-knockout donors and the cotransplantation of vascularized thymic tissue. Nat. Med 11, 32–34 (2005). [DOI] [PubMed] [Google Scholar]
- 84.Davila E et al. T-cell responses during pig-to-primate xenotransplantation. Xenotransplantation 13, 31–40 (2006). [DOI] [PubMed] [Google Scholar]
- 85.Lee RS et al. Blockade of CD28-B7, but not CD40-CD154, prevents costimulation of allogeneic porcine and xenogeneic human anti-porcine T cell responses. J. Immunol 164, 3434–3444 (2000). [DOI] [PubMed] [Google Scholar]
- 86.Wu G et al. Co-stimulation blockade targeting CD154 and CD28/B7 modulates the induced antibody response after a pig-to-baboon cardiac xenograft. Xenotransplantation 12, 197–208 (2005). [DOI] [PubMed] [Google Scholar]
- 87.Brenner PM et al. Orthotopic cardiac xenotransplantation of GalKO/hCD46/hTM-transgenic pig hearts into baboons (40 days survival) using a CD40mAb or CD40L-Ab costimulation blockade. Xenotransplantation 24, 29 (2017). [Google Scholar]
- 88.Mohiuddin MM et al. One-year heterotopic cardiac xenograft survival in a pig to baboon model. Am. J. Transplant 14, 488–489 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Iwase H et al. Pig kidney graft survival in a baboon for 136 days: longest life-supporting organ graft survival to date. Xenotransplantation 22, 302–309 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Cardona K et al. Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am. J. Transplant 7, 2260–2268 (2007). [DOI] [PubMed] [Google Scholar]
- 91.Mohiuddin MM et al. Chimeric 2C10R4 anti-CD40 antibody therapy is critical for long-term survival of GTKO.hCD46.hTBM pig-to-primate cardiac xenograft. Nat. Commun 7, 11138 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Jin X et al. Enhanced suppression of the xenogeneic T-cell response in vitro by xenoantigen stimulated and expanded regulatory T cells. Transplantation 97, 30–38 (2014). [DOI] [PubMed] [Google Scholar]
- 93.Yi S et al. Adoptive transfer with in vitro expanded human regulatory T cells protects against porcine islet xenograft rejection via interleukin-10 in humanized mice. Diabetes 61, 1180–1191 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Fu Y et al. In vitro suppression of xenoimmune-mediated macrophage activation by human CD4+CD25+regulatory T cells. Transplantation 86, 865–874 (2008). [DOI] [PubMed] [Google Scholar]
- 95.Stern JK et al. Extended pig skin graft survival in baboons receiving Hcd47 Tg/Galt-KO pig PBSC plus host Treg infusion. Xenotransplantation 24, 9 (2017). [Google Scholar]
- 96.Shin JS et al. Failure of transplantation tolerance induction by autologous regulatory T cells in the pig-to-non-human primate islet xenotransplantation model. Xenotransplantation 23, 300–309 (2016). [DOI] [PubMed] [Google Scholar]
- 97.Layton DS et al. Differential cytokine expression and regulation of human anti-pig xenogeneic responses by modified porcine dendritic cells. Xenotransplantation 15, 257–267 (2008). [DOI] [PubMed] [Google Scholar]
- 98.Madelon N, Puga Yung GL & Seebach JD Human anti-pig NK cell and CD8+ T-cell responses in the presence of regulatory dendritic cells. Xenotransplantation 23, 479–489 (2016). [DOI] [PubMed] [Google Scholar]
- 99.Ciubotariu R et al. Specific suppression of human CD4+ Th cell responses to pig MHC antigens by CD8+CD28− regulatory T cells. J. Immunol 161, 5193–5202 (1998). [PubMed] [Google Scholar]
- 100.Niemann H & Rath D Progress in reproductive biotechnology in swine. Theriogenology 56, 1291–1304 (2001). [DOI] [PubMed] [Google Scholar]
- 101.Buhler L, Friedman T, Iacomini J & Cooper DK Xenotransplantation — state of the art — update 1999. Front. Biosci 4, D416–D432 (1999). [DOI] [PubMed] [Google Scholar]
- 102.Kozlowski T et al. Apheresis and column absorption for specific removal of Gal-α-1,3 Gal natural antibodies in a pig-to-baboon model. Transplant. Proc 29, 961 (1997). [DOI] [PubMed] [Google Scholar]
- 103.Kuwaki K et al. Heart transplantation in baboons using α1,3-galactosyltransferase gene-knockout pigs as donors: initial experience. Nat. Med 11, 29–31 (2005). [DOI] [PubMed] [Google Scholar]
- 104.Hsu PD, Lander ES & Zhang F Development and applications of CRISPR-Cas9 for genome engineering. Cell 157, 1262–1278 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Estrada JL et al. Evaluation of human and non-human primate antibody binding to pig cells lacking GGTA1/CMAH/beta4GalNT2 genes. Xenotransplantation 22, 194–202 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Wong BS et al. Allosensitization does not increase the risk of xenoreactivity to α1,3-galactosyltransferase gene-knockout miniature swine in patients on transplantation waiting lists. Transplantation 82, 314–319 (2006). [DOI] [PubMed] [Google Scholar]
- 107.Hara H et al. Allosensitized humans are at no greater risk of humoral rejection of GT-KO pig organs than other humans. Xenotransplantation 13, 357–365 (2006). [DOI] [PubMed] [Google Scholar]
- 108.Cooper DK, Tseng YL & Saidman SL Alloantibody and xenoantibody cross-reactivity in transplantation. Transplantation 77, 1–5 (2004). [DOI] [PubMed] [Google Scholar]
- 109.Chong AS-F et al. Delayed xenograft rejection in the concordant hamster heart into Lewis rat model. Transplantation 62, 90–96 (1996). [DOI] [PubMed] [Google Scholar]
- 110.Lin Y, Sobis H, Vandeputte M & Waer M Mechanism of leflunomide-induced prevention of xenoantibody formation and xenograft rejection in the hamster to rat heart transplantation model. Transplant. Proc 27, 305–306 (1995). [PubMed] [Google Scholar]
- 111.Lundvig DM, Immenschuh S & Wagener FA Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly? Front. Pharmacol 3, 81 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Mohiuddin MM et al. Progressive genetic modifications of porcine cardiac xenografts extend survival to 9 months. Xenotransplantation 29, e12744 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Niemann H & Petersen B The production of multi-transgenic pigs: update and perspectives for xenotransplantation. Transgenic Res. 25, 361–374 (2016). [DOI] [PubMed] [Google Scholar]
- 114.Singh AK et al. Cardiac xenografts show reduced survival in the absence of transgenic human thrombomodulin expression in donor pigs. Xenotransplantation 26, e12465 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Connolly MR et al. Humanized von Willebrand factor reduces platelet sequestration in ex vivo and in vivo xenotransplant models. Xenotransplantation 28, e12712 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Lee SJ et al. Seven years of experiences of preclinical experiments of Xeno-Heart transplantation of pig to non-human primate (Cynomolgus Monkey). Transplant. Proc 50, 1167–1171 (2018). [DOI] [PubMed] [Google Scholar]
- 117.Fischer K et al. Efficient production of multi-modified pigs for xenotransplantation by ‘combineering’, gene stacking and gene editing. Sci. Rep 6, 29081 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Ma D et al. Kidney transplantation from triple-knockout pigs expressing multiple human proteins in cynomolgus macaques. Am. J. Transplant 22, 46–57 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kim SC et al. Long-term survival of pig-to-rhesus macaque renal xenografts is dependent on CD4 T cell depletion. Am. J. Transplant 19, 2174–2185 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Adams AB et al. Xenoantigen deletion and chemical immunosuppression can prolong renal xenograft survival. Ann. Surg 268, 564–573 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tsuyuki S, Kono M & Bloom ET Cloning and potential utility of porcine Fas ligand: overexpression in porcine endothelial cells protects them from attack by human cytolytic cells. Xenotransplantation 9, 410–421 (2002). [DOI] [PubMed] [Google Scholar]
- 122.Bahr A et al. Ubiquitous LEA29Y expression blocks T cell co-stimulation but permits sexual reproduction in genetically modified pigs. PLoS One 11, e0155676 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nottle MB et al. Targeted insertion of an anti-CD2 monoclonal antibody transgene into the GGTA1 locus in pigs using FokI-dCas9. Sci. Rep 7, 8383 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Vabres B et al. hCTLA4-Ig transgene expression in keratocytes modulates rejection of corneal xenografts in a pig to non-human primate anterior lamellar keratoplasty model. Xenotransplantation 21, 431–443 (2014). [DOI] [PubMed] [Google Scholar]
- 125.Klymiuk N et al. Xenografted islet cell clusters from INSLEA29Y transgenic pigs rescue diabetes and prevent immune rejection in humanized mice. Diabetes 61, 1527–1532 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Fischer K et al. Viable pigs after simultaneous inactivation of porcine MHC class I and three xenoreactive antigen genes GGTA1, CMAH and B4GALNT2. Xenotransplantation 27, e12560 (2019). [DOI] [PubMed] [Google Scholar]
- 127.Puga Yung G et al. Release of pig leukocytes and reduced human NK cell recruitment during ex vivo perfusion of HLA-E/human CD46 double-transgenic pig limbs with human blood. Xenotransplantation 25, 12357 (2018). [DOI] [PubMed] [Google Scholar]
- 128.Shah JA et al. A bridge to somewhere: 25-day survival after pig-to-baboon liver xenotransplantation. Ann. Surg 263, 1069–1071 (2016). [DOI] [PubMed] [Google Scholar]
- 129.Markmann JF et al. Executive summary of IPITA-TTS opinion leaders report on the future of beta-cell replacement. Transplantation 100, e25–e31 (2016). [DOI] [PubMed] [Google Scholar]
- 130.Graham ML et al. Clinically available immunosuppression averts rejection but not systemic inflammation after porcine islet xenotransplant in cynomolgus macaques. Am. J. Transplant 22, 745–760 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Bottino R et al. Pig-to-monkey islet xenotransplantation using multi-transgenic pigs. Am. J. Transplant 14, 2275–2287 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Dor FJ, Cheng J, Alt A, Cooper DK & Schuurman HJ Galα1,3 Gal expression on porcine pancreatic islets, testis, spleen, and thymus. Xenotransplantation 11, 101–106 (2004). [DOI] [PubMed] [Google Scholar]
- 133.Ozmen L et al. Inhibition of thrombin abrogates the instant blood-mediated inflammatory reaction triggered by isolated human islets: possible application of the thrombin inhibitor melagatran in clinical islet transplantation. Diabetes 51, 1779–1784 (2002). [DOI] [PubMed] [Google Scholar]
- 134.Barton FB et al. Improvement in outcomes of clinical islet transplantation: 1999–2010. Diabetes Care 35, 1436–1445 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cooper DK et al. Progress in clinical encapsulated islet xenotransplantation. Transplantation 100, 2301–2308 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Morozov VA et al. No PERV transmission during a clinical trial of pig islet cell transplantation. Virus Res. 227, 34–40 (2017). [DOI] [PubMed] [Google Scholar]
- 137.Wynyard S, Nathu D, Garkavenko O, Denner J & Elliott R Microbiological safety of the first clinical pig islet xenotransplantation trial in New Zealand. Xenotransplantation 21, 309–323 (2014). [DOI] [PubMed] [Google Scholar]
- 138.Garkavenko O et al. The first clinical xenotransplantation trial in New Zealand: efficacy and safety. Xenotransplantation 19, 6 (2012). [Google Scholar]
- 139.Singh A et al. Long-term tolerance of islet allografts in nonhuman primates induced by apoptotic donor leukocytes. Nat. Commun 10, 3495 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang S et al. Transient B-cell depletion combined with apoptotic donor splenocytes induces xeno-specific T- and B-cell tolerance to islet xenografts. Diabetes 62, 3143–3150 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Higginbotham L et al. Pre-transplant antibody screening and anti-CD154 costimulation blockade promote long-term xenograft survival in a pig-to-primate kidney transplant model. Xenotransplantation 22, 221–230 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kim SC et al. Fc-Silent anti-CD154 domain antibody effectively prevents nonhuman primate renal allograft rejection. Am. J. Transplant 17, 1182–1192 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Cooper DKC et al. Selection of patients for initial clinical trials of solid organ xenotransplantation. Transplantation 101, 1551–1558 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Cooper DKC et al. Xenotransplantation — the current status and prospects. Br. Med. Bull 125, 5–14 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Montgomery RA et al. Results of two cases of pig-to-human kidney xenotransplantation. N. Engl. J. Med 386, 1889–1898 (2022). [DOI] [PubMed] [Google Scholar]
- 146.Porrett PM et al. First clinical-grade porcine kidney xenotransplant using a human decedent model. Am. J. Transplant 22, 1037–1053 (2022). [DOI] [PubMed] [Google Scholar]
- 147.Langin M et al. Consistent success in life-supporting porcine cardiac xenotransplantation. Nature 564, 430–433 (2018). [DOI] [PubMed] [Google Scholar]
- 148.Goerlich CE et al. The growth of xenotransplanted hearts can be reduced with growth hormone receptor knockout pig donors. J. Thorac. Cardiovasc. Surg 10.1016/j.jtcvs.2021.07.051 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Riedel EO et al. Functional changes of the liver in the absence of growth hormone (GH) action — proteomic and metabolomic insights from a GH receptor deficient pig model. Mol. Metab 36, 100978 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Griffith BP et al. Genetically modified porcine-to-human cardiac xenotransplantation. N. Eng. J. Med 387, 35–44 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Sykes M Hematopoietic cell transplantation for tolerance induction: animal models to clinical trials. Transplantation 87, 309–316 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sykes M IXA honorary member lecture, 2017: the long and winding road to tolerance. Xenotransplantation 25, e12419 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Waffarn EE et al. Mixed xenogeneic porcine chimerism tolerizes human anti-pig natural antibody-producing cells in a humanized mouse model. Xenotransplantation 28, e12691 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Li HW et al. Impact of mixed xenogeneic porcine hematopoietic chimerism on human NK cell recognition in a humanized mouse model. Am. J. Transplant 17, 353–364 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sharabi Y, Aksentijevich I, Sundt TM III, Sachs DH & Sykes M Specific tolerance induction across a xenogeneic barrier: production of mixed rat/mouse lymphohematopoietic chimeras using a nonlethal preparative regimen. J. Exp. Med 172, 195–202 (1990). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Sharabi Y & Sachs DH Mixed chimerism and permanent specific transplantation tolerance induced by a non-lethal preparative regimen. J. Exp. Med 169, 493–502 (1989). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee LA, Sergio JJ & Sykes M Natural killer cells weakly resist engraftment of allogeneic long-term multilineage-repopulating hematopoietic stem cells. Transplantation 61, 125–132 (1996). [DOI] [PubMed] [Google Scholar]
- 158.Tomita Y, Lee LA & Sykes M Engraftment of rat bone marrow and its role in negative selection of murine T cells in mice conditioned with a modified non-myeloablative regimen. Xenotransplantation 1, 109–117 (1994). [Google Scholar]
- 159.Lee LA, Sergio JJ & Sykes M Evidence for non-immune mechanisms in the loss of hematopoietic chimerism in rat-mouse mixed xenogeneic chimeras. Xenotransplantation 2, 57–66 (1995). [Google Scholar]
- 160.Aksentijevich I, Sachs DH, Sharabi Y, Sundt TMI & Sykes M Humoral tolerance in mixed xenogeneic chimeras prepared by a non-myeloablative conditioning regimen. Transplant. Proc 23, 880–882 (1991). [PubMed] [Google Scholar]
- 161.Kawahara T, Rodriguez-Barbosa JI, Zhao Y, Zhao G & Sykes M Global unresponsiveness as a mechanism of natural killer cell tolerance in mixed xenogeneic chimeras. Am. J. Transplant 7, 2090–2097 (2007). [DOI] [PubMed] [Google Scholar]
- 162.Gritsch HA et al. The importance of non-immune factors in reconstitution by discordant xenogeneic hematopoietic cells. Transplantation 57, 906–917 (1994). [DOI] [PubMed] [Google Scholar]
- 163.Chen AM et al. Porcine stem cell engraftment and seeding of murine thymus with class II+ cells in mice expressing porcine cytokines: toward tolerance induction across discordant xenogeneic barriers. Transplantation 69, 2484–2490 (2000). [DOI] [PubMed] [Google Scholar]
- 164.Aksentijevich I, Sachs DH & Sykes M Natural antibodies can inhibit bone marrow engraftment in the rat-mouse species combination. J. Immunol 147, 4140–4146 (1991). [PubMed] [Google Scholar]
- 165.Aksentijevich I, Sachs DH & Sykes M Humoral tolerance in xenogeneic BMT recipients conditioned with a non-myeloablative regimen. Transplantation 53, 1108–1114 (1992). [DOI] [PubMed] [Google Scholar]
- 166.Aksentijevich I, Sachs DH & Sykes M Normal mouse serum contains natural antibody against bone marrow cells of a concordant xenogeneic species. J. Immunol 147, 79–85 (1991). [PubMed] [Google Scholar]
- 167.Lee LA, Sergio JJ, Sachs DH & Sykes M Mechanism of tolerance in mixed xenogeneic chimeras prepared with a non-myeloablative conditioning regimen. Transplant. Proc 26, 1197–1198 (1994). [PubMed] [Google Scholar]
- 168.Thall AD Generation of α1,3Galactosyltransferase deficient mice. Subcell. Biochem 32, 259–279 (1999). [DOI] [PubMed] [Google Scholar]
- 169.Yang Y-G et al. Tolerization of anti-galα1-3 gal natural antibody-forming B cells by induction of mixed chimerism. J. Exp. Med 187, 1335–1342 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ohdan H, Yang Y-G, Shimizu A, Swenson KG & Sykes M Mixed bone marrow chimerism induced without lethal conditioning prevents T cell and anti-Galα1,3Gal-mediated graft rejection. J. Clin. Invest 104, 281–290 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Ohdan H, Yang Y-G, Swenson KG, Kitamura H & Sykes M T cell and B cell tolerance to galα1,3gal-expressing heart xenografts is achieved in α1,3-galactosyltransferase-deficient mice by non-myeloablative induction of mixed chimerism. Transplantation 71, 1532–1542 (2001). [DOI] [PubMed] [Google Scholar]
- 172.Ohdan H, Swenson KG, Kitamura H, Yang Y-G & Sykes M Tolerization of Galα1,3Gal-reactive B cells in presensitized α1,3-galactosyltransferase-deficient mice by nonmyeloablative induction of mixed chimerism. Xenotransplantation 8, 227–238 (2002). [DOI] [PubMed] [Google Scholar]
- 173.Nikolic B et al. Role of intrathymic rat class II cells in maintaining deletional tolerance in xenogeneic rat-mouse bone marrow chimeras. Transplantation 65, 1216–1224 (1998). [DOI] [PubMed] [Google Scholar]
- 174.Lan P, Tonomura N, Shimizu A, Wang S & Yang YG Reconstitution of a functional human immune system in immunodeficient mice through combined human fetal thymus/liver and CD34+ cell transplantation. Blood 108, 487–492 (2006). [DOI] [PubMed] [Google Scholar]
- 175.Lan P et al. Induction of human T cell tolerance to porcine xenoantigens through mixed hematopoietic chimerism. Blood 103, 3964–3969 (2004). [DOI] [PubMed] [Google Scholar]
- 176.Watanabe H et al. Intra-bone bone marrow transplantation from hCD47 transgenic pigs to baboons prolongs chimerism to >60 days and promotes increased porcine lung transplant survival. Xenotransplantation 27, e12552 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kawahara T, Ohdan H, Zhao G, Yang Y-G & Sykes M Peritoneal cavity B cells are precursors of splenic IgM natural antibody-producing cells. J. Immunol 171, 5406–5414 (2003). [DOI] [PubMed] [Google Scholar]
- 178.Ohdan H et al. Mac-1-negative B-1b phenotype of natural antibody-producing cells, including those responding to Galα1,3 Gal epitopes in α1,3-galactosyltransferase deficient mice. J. Immunol 165, 5518–5529 (2000). [DOI] [PubMed] [Google Scholar]
- 179.Griffin DO, Holodick NE & Rothstein TL Human B1 cells in umbilical cord and adult peripheral blood express the novel phenotype CD20+CD27+CD43+CD70−. J. Exp. Med 208, 67–80 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Griffin DO, Holodick NE & Rothstein TL Human B1 cells are CD3−: a reply to “A human equivalent of mouse B-1 cells?” and “The nature of circulating CD27+CD43+B cells”. J. Exp. Med 208, 2566–2569 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Griffin DO & Rothstein TL A small CD11b+ human B1 cell subpopulation stimulates T cells and is expanded in lupus. J. Exp. Med 208, 2591–2598 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Griffin DO & Rothstein TL Human “orchestrator” CD11b+ B1 cells spontaneously secrete IL-10 and regulate T cell activity. Mol. Med 18, 1003–1008 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Reynaud CA & Weill JC Gene profiling of CD11b+ and CD11b− B1 cell subsets reveals potential cell sorting artifacts. J. Exp. Med 209, 433–434 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Xu Y et al. Characterization of anti-Gal antibody-producing cells of baboons and humans. Transplantation 81, 940–948 (2006). [DOI] [PubMed] [Google Scholar]
- 185.Kawahara T, Shimizu I, Ohdan H, Zhao G & Sykes M Differing mechanisms of early and late B cell tolerance induced by mixed chimerism. Am. J. Transplant 5, 2821–2829 (2005). [DOI] [PubMed] [Google Scholar]
- 186.Shimizu I et al. B-cell extrinsic CR1/CR2 promotes natural antibody production and tolerance induction of anti-α-Gal-producing B-1 cells. Blood 109, 1773–1781 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Bardwell PD, Ohdan H & Sykes M B cell tolerance and xenotransplantation. Curr. Op. Organ. Transplant 10, 252–258 (2005). [Google Scholar]
- 188.Abe M et al. Elimination of porcine hemopoietic cells by macrophages in mice. J. Immunol 168, 621–628 (2002). [DOI] [PubMed] [Google Scholar]
- 189.Wang H & Yang YG Innate cellular immunity and xenotransplantation. Curr. Opin. Organ. Transplant 17, 162–167 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Wang H et al. Attenuation of phagocytosis of xenogeneic cells by manipulating CD47. Blood 109, 836–842 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Ide K et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc. Natl Acad. Sci. USA 104, 5062–5066 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Wang H, Wu X, Wang Y, Oldenborg P A. & Yang, Y. G. CD47 is required for suppression of allograft rejection by donor-specific transfusion. J. Immunol 184, 3401–3407 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Navarro-Alvarez N & Yang YG Lack of CD47 on donor hepatocytes promotes innate immune cell activation and graft rejection: a potential barrier to hepatocyte xenotransplantation. Am. J. Transplant 11, 107–108 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Wang Y, Wang H, Bronson R, Fu Y & Yang YG Rapid dendritic cell activation and resistance to allotolerance induction in anti-CD154-treated mice receiving CD47-deficient donor-specific transfusion. Cell Transpl. 23, 355–363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Ide K et al. Role for CD47-SIRPα signaling in xenograft rejection by macrophages. Proc. Natl Acad. Sci. USA 104, 5062–5066 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Wong AS et al. Polymorphism in the innate immune receptor SIRPα controls CD47 binding and autoimmunity in the nonobese diabetic mouse. J. Immunol 193, 4833–4844 (2014). [DOI] [PubMed] [Google Scholar]
- 197.Tena A et al. Transgenic expression of human CD47 markedly increases engraftment in a murine model of pig-to-human hematopoietic cell transplantation. Am. J. Transplant 14, 2713–2722 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Wang C et al. Human CD47 expression permits survival of porcine cells in immunodeficient mice that express SIRPα capable of binding to human CD47. Cell Transpl. 20, 1915–1920 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang H et al. Lack of CD47 on nonhematopoietic cells induces split macrophage tolerance to CD47null cells. Proc. Natl Acad. Sci. USA 104, 13744–13749 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tena AA et al. Prolonged survival of pig skin on baboons after administration of pig cells expressing human CD47. Transplantation 101, 316–321 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Tasaki M et al. High incidence of xenogenic bone marrow engraftment in pig-to-baboon intra-bone bone marrow transplantation. Am. J. Transplant 15, 974–983 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Zhao Y, Ohdan H, Manilay JO & Sykes M NK cell tolerance in mixed allogeneic chimeras. J. Immunol 170, 5398–5405 (2003). [DOI] [PubMed] [Google Scholar]
- 203.Sasaki H, Xu XC, Smith DM, Howard T & Mohanakumar T HLA-G expression protects porcine endothelial cells against natural killer cell-mediated xenogeneic cytotoxicity. Transplantation 67, 31–37 (1999). [DOI] [PubMed] [Google Scholar]
- 204.Torgersen KM et al. Major histocompatibility complex class I-independent killing of xenogenic targets by rat allospecific natural killer cells. Transplantation 63, 119–123 (1997). [DOI] [PubMed] [Google Scholar]
- 205.Forte P et al. HLA-G inhibits rolling adhesion of activated human NK cells on porcine endothelial cells. J. Immunol 167, 6002–6008 (2001). [DOI] [PubMed] [Google Scholar]
- 206.Silver ET, Lavender KJ, Gong DE, Hazes B & Kane KP Allelic variation in the ectodomain of the inhibitory Ly-49G2 receptor alters its specificity for allogeneic and xenogeneic ligands. J. Immunol 169, 4752–4760 (2002). [DOI] [PubMed] [Google Scholar]
- 207.Forte P, Baumann BC, Weiss EH & Seebach JD HLA-E expression on porcine cells: protection from human NK cytotoxicity depends on peptide loading. Am. J. Transplant 5, 2085–2093 (2005). [DOI] [PubMed] [Google Scholar]
- 208.Benoit LA, Shannon J, Chamberlain JW & Miller RG Influence of xenogeneic β2-microglobulin on functional recognition of H-2Kb by the NK cell inhibitory receptor Ly49C. J. Immunol 175, 3542–3553 (2005). [DOI] [PubMed] [Google Scholar]
- 209.Nakamura MC et al. Natural killing of xenogeneic cells mediated by the mouse Ly-49D receptor. J. Immunol 163, 4694–4700 (1999). [PubMed] [Google Scholar]
- 210.Tran PD et al. Porcine cells express more than one functional ligand for the human lymphocyte activating receptor NKG2D. Xenotransplantation 15, 321–332 (2008). [DOI] [PubMed] [Google Scholar]
- 211.Lilienfeld BG, Garcia-Borges C, Crew MD & Seebach JD Porcine UL16-binding protein 1 expressed on the surface of endothelial cells triggers human NK cytotoxicity through NKG2D. J. Immunol 177, 2146–2152 (2006). [DOI] [PubMed] [Google Scholar]
- 212.Forte P, Lilienfeld BG, Baumann BC & Seebach JD Human NK cytotoxicity against porcine cells is triggered by NKp44 and NKG2D. J. Immunol 175, 5463–5470 (2005). [DOI] [PubMed] [Google Scholar]
- 213.Clark EA & Sturge JC Phylogeny of NK cell reactivity against human and nonhuman primate lymphoblastoid cell lines: evolving and conserved target antigens. J. Immunol 126, 969–974 (1981). [PubMed] [Google Scholar]
- 214.Coudert JD, Scarpellino L, Gros F, Vivier E & Held W Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood 111, 3571–3578 (2008). [DOI] [PubMed] [Google Scholar]
- 215.Tripathy SK et al. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med 205, 1829–1841 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.del Rio ML, Seebach JD, Fernandez-Renedo C & Rodriguez-Barbosa JI ITIM-dependent negative signaling pathways for the control of cell-mediated xenogeneic immune responses. Xenotransplantation 20, 397–406 (2013). [DOI] [PubMed] [Google Scholar]
- 217.Lee LA et al. Specific tolerance across a discordant xenogeneic transplantation barrier. Proc. Natl Acad. Sci. USA 91, 10864–10867 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Zhao Y et al. Skin graft tolerance across a discordant xenogeneic barrier. Nat. Med 2, 1211–1216 (1996). [DOI] [PubMed] [Google Scholar]
- 219.Zhao Y et al. Despite efficient intrathymic negative selection of host-reactive T cells, autoimmune disease may develop in porcine thymus-grafted athymic mice: evidence for failure of regulatory mechanisms suppressing autoimmunity. Transplantation 75, 1832–1840 (2002). [DOI] [PubMed] [Google Scholar]
- 220.Rodriguez-Barbosa JI et al. Enhanced CD4 reconstitution by grafting neonatal porcine tissue in alternative locations is associated with donor-specific tolerance and suppression of pre-existing xenoreactive T cells. Transplantation 72, 1223–1231 (2001). [DOI] [PubMed] [Google Scholar]
- 221.Zhao Y et al. Immune restoration by fetal pig thymus grafts in T cell-depleted, thymectomized mice. J. Immunol 158, 1641–1649 (1997). [PubMed] [Google Scholar]
- 222.Nikolic B et al. Normal development in porcine thymus grafts and specific tolerance of human T cells to porcine donor MHC. J. Immunol 162, 3402–3407 (1999). [PubMed] [Google Scholar]
- 223.Kalscheuer HO et al. Xenograft tolerance and immune function of human T cell developing in pig thymus xenografts. J. Immunol 192, 3442–3450 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kalscheuer H et al. A model for personalized in vivo analysis of human immune responsiveness. Sci. Transl. Med 4, 125ra130 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Wu A et al. Xenogeneic thymus transplantation in a pig-to-baboon model. Transplantation 75, 282–291 (2003). [DOI] [PubMed] [Google Scholar]
- 226.Zhao Y et al. The critical role of mouse CD4+ cells in the rejection of highly disparate xenogeneic pig thymus grafts. Xenotransplant 7, 129–137 (2000). [DOI] [PubMed] [Google Scholar]
- 227.deBlecourt M World’s first heart transplant, cultured thymus implantation performed at Duke. Milestone could eliminate need for anti-rejection medications., https://www.dukehealth.org/blog/worlds-first-heart-transplant-cultured-thymus-implantation-performed-duke (2022). [Google Scholar]
- 228.Auchincloss H Jr, Mayer T, Ghobrial R & Winn HJ T-cell subsets, bm mutants, and the mechanisms of allogeneic skin graft rejection. Immunol. Res 8, 149–164 (1989). [DOI] [PubMed] [Google Scholar]
- 229.Yamada K et al. Thymic transplantation in miniature swine. I. Development and function of the “thymokidney”. Transplantation 68, 1684–1692 (1999). [DOI] [PubMed] [Google Scholar]
- 230.Yamada K et al. Thymic transplantation in miniature swine. II. Induction of tolerance by transplantation of composite thymokidneys to thymectomized recipients. J. Immunol 164, 3079–3086 (2000). [DOI] [PubMed] [Google Scholar]
- 231.Yamada K et al. Thymic transplantation in miniature swine: III. Induction of tolerance by transplantation of composite thymokidneys across fully major histocompatibility complex-mismatched barriers. Transplantation 76, 530–536 (2003). [DOI] [PubMed] [Google Scholar]
- 232.Kamano C et al. Vascularized thymic lobe transplantation in miniature swine: thymopoiesis and tolerance induction across fully MHC-mismatched barriers. Proc. Natl Acad. Sci. USA 101, 3827–3832 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Nobori S et al. Long-term acceptance of fully allogeneic cardiac grafts by cotransplantation of vascularized thymus in miniature swine. Transplantation 81, 26–35 (2006). [DOI] [PubMed] [Google Scholar]
- 234.Barth RN et al. Xenogeneic thymokidney and thymic tissue transplantation in a pig-to-baboon model: I. Evidence for pig-specific T-cell unresponsiveness. Transplantation 75, 1615–1624 (2003). [DOI] [PubMed] [Google Scholar]
- 235.Griesemer AD et al. Results of gal-knockout porcine thymokidney xenografts. Am. J. Transplant 9, 2669–2678 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Takeuchi K et al. Expression of human CD47 in pig glomeruli prevents proteinuria and prolongs graft survival following pig-to-baboon xenotransplantation. Xenotransplantation 28, e12708 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Tanabe T et al. Role of intrinsic (Graft) versus extrinsic (Host) factors in the growth of transplanted organs following allogeneic and xenogeneic transplantation. Am. J. Transplant 17, 1778–1790 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Khosravi Maharlooei MH et al. Generation of human/pig hybrid thymus to achieve immune tolerance to pig antigens with optimal immune function. Xenotransplantation 24, 15 (2017). [Google Scholar]
- 239.Fudaba Y et al. Abnormal regulatory and effector T cell function predispose to autoimmunity following xenogeneic thymic transplantation. J. Immunol 181, 7649–7659 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Gras-Pena R et al. Human stem cell-derived thymic epithelial cells enhance human T cell development in a xenogeneic thymus. J. Allergy Clin. Immunol 149, 1755–1771 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Sachs DH The pig as a potential xenograft donor. Vet. Immunol. Immunopathol 43, 185–191 (1994). [DOI] [PubMed] [Google Scholar]
- 242.Sachs DH in Swine as Models in Biomedical Research (eds Swindle MM, Moody DC & Phillips LD) Ch. 1, 3–15 (Iowa State University Press, 1992). [Google Scholar]
- 243.Mezrich JD et al. Histocompatible miniature swine: an inbred large-animal model. Transplantation 75, 904–907 (2003). [DOI] [PubMed] [Google Scholar]
- 244.Patience C, Takeuchi Y & Weiss RA Infection of human cells by an endogenous retrovirus of pigs. Nat. Med 3, 282–286 (1997). [DOI] [PubMed] [Google Scholar]
- 245.Fishman JA Infectious disease risks in xenotransplantation. Am. J. Transplant 18, 1857–1864 (2018). [DOI] [PubMed] [Google Scholar]
- 246.Wynyard S, Garkavenko O & Elliot R Multiplex high resolution melting assay for estimation of Porcine Endogenous Retrovirus (PERv) relative gene dosage in pigs and detection of PERV infection in xenograft recipients. J. Virol. Methods 175, 95–100 (2011). [DOI] [PubMed] [Google Scholar]
- 247.Denner J Why was PERV not transmitted during preclinical and clinical xenotransplantation trials and after inoculation of animals? Retrovirology 15, 28 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Cooper DKC et al. Regulation of clinical xenotransplantation-time for a reappraisal. Transplantation 101, 1766–1769 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dieckhoff B et al. Knockdown of porcine endogenous retrovirus (PERV) expression by PERV-specific shRNA in transgenic pigs. Xenotransplantation 15, 36–45 (2008). [DOI] [PubMed] [Google Scholar]
- 250.Yang L et al. Genome-wide inactivation of porcine endogenous retroviruses (PERVs). Science 350, 1101–1104 (2015). [DOI] [PubMed] [Google Scholar]
- 251.Niu D et al. Inactivation of porcine endogenous retrovirus in pigs using CRISPR-Cas9. Science 357, 1303–1307 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Schaefer KA et al. Unexpected mutations after CRISPR-Cas9 editing in vivo. Nat. Methods 14, 547–548 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Nellore A & Fishman JA Donor-derived infections and infectious risk in xenotransplantation and allotransplantation. Xenotransplantation 25, e12423 (2018). [DOI] [PubMed] [Google Scholar]
- 254.Ison MG The epidemiology and prevention of donor-derived infections. Adv. Chronic Kidney Dis 16, 234–241 (2009). [DOI] [PubMed] [Google Scholar]
- 255.Food and Drug Administration. Source animal, product, preclinical, and clinical issues regarding the use of xenotransplantation products in humans: guidance for industry. (2016).
- 256.Bloom ET National policies for xenotransplantation in the USA. Xenotransplantation 14, 345–346 (2007). [DOI] [PubMed] [Google Scholar]
- 257.World Health Organization. Second WHO global consultation on regulatory requirements for xenotransplantation clinical trials, October 17–19 2011, WHO, Geneva, Switzerland. https://apps.who.int/iris/handle/10665/341817 (2011).
- 258.Hering BJ et al. First update of the International Xenotransplantation Association consensus statement on conditions for undertaking clinical trials of porcine islet products in type 1 diabetes — executive summary. Xenotransplantation 23, 3–13 (2016). [DOI] [PubMed] [Google Scholar]
- 259.Navarro-Alvarez N et al. The effects of exogenous administration of human coagulation factors following pig-to-baboon liver xenotransplantation. Am. J. Transplant 16, 1715–1725 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Shah JA et al. Prolonged survival following pig-to-primate liver xenotransplantation utilizing exogenous coagulation factors and costimulation blockade. Am. J. Transplant 17, 2178–2185 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Graham ML, Bellin MD, Papas KK, Hering BJ & Schuurman HJ Species incompatibilities in the pig-to-macaque islet xenotransplant model affect transplant outcome: a comparison with allotransplantation. Xenotransplantation 18, 328–342 (2011). [DOI] [PubMed] [Google Scholar]
- 262.Hansen-Estruch C, Porrett PM, Kumar V & Locke JE The science of xenotransplantation for nephrologists. Curr. Opin. Nephrol. Hypertens 31, 387–393 (2022). [DOI] [PubMed] [Google Scholar]
- 263.Cooper DKC et al. Effects of cyclosporine and antibody adsorption on pig cardiac xenograft survival in the baboon. J. Heart Transplant 7, 238–246 (1988). [PubMed] [Google Scholar]
- 264.Cozzi E et al. in Xenotransplantation: The Transplantation of Organs and Tissues Between Species (eds Cooper DKC, et al) Ch. 49, 665–682 (Springer-Verlag, 1997). [Google Scholar]