Summary
Organisms of all phyla express mechanosensitive ion channels with a wide range of physiological functions. In recent years, several classes of mechanically gated ion channels have been identified. Some of these ion channels are intrinsically mechanosensitive. Others depend on accessory proteins to regulate their response to mechanical force. The mechanotransduction machinery of cochlear hair cells provides a particularly striking example of a complex force-sensing machine. This molecular ensemble is embedded into a specialized cellular compartment that is crucial for its function. Notably, mechanotransduction channels of cochlear hair cells are not only critical for auditory perception. They also shape their cellular environment and regulate the development of auditory circuitry. Here we summarize recent discoveries that have shed light on the composition of the mechanotransduction machinery of cochlear hair cells and how this machinery contributes to the development and function of the auditory system.
In Brief:
Qiu and Müller review recent progress in the field of auditory mechanotransduction that has revealed that the mechanotransduction machinery of cochlear hair cells is a complex molecular machine. Notably, hair cell mechanotransduction is not only important for sensory perception but this process also shapes hair cell morphology and auditory circuits.
Introduction
Molecules designed to respond to mechanical stimuli are perhaps some of the earliest molecular sensors to emerge during evolution. These molecules are found throughout all phyla. Some of these sensors respond to osmotic pressure to prevent the rupture of the cell membrane during environmental fluctuations. Others are expressed in specialized sense organs and respond to defined stimuli such as touch and sound. In recent years, tremendous progress has been made towards the identification of the molecular components of a variety of force sensors. Two major concepts have emerged. First and perhaps not surprisingly, force-sensors are built from different molecular components reflecting the diversity of their functions to detect a wide range of mechanical stimuli. Second, at the heart of many (but not all) of these force-sensors are mechanically gated ion channels. Some of these ion channels, including the bacterial MscL/MscS proteins and the mammalian PIEZO proteins, are intrinsically mechanically sensitive when expressed in lipid membranes. Others such as the DEG/ENac proteins of C. elegans, the NOMPC protein of Drosophila and the mechanotransduction channel of cochlear hair cells are components of larger molecular assemblies that contain additional proteins critical to transmit force onto the pore-forming subunits of these ion channels (Jin et al., 2020; Kefauver et al., 2020).
Mechanotransduction by sensory hair cells of the inner ear has been an influential model to study mechanisms of mechanosensation. Recent progress in the field has been driven by the study of genes that are linked to deafness. New discoveries challenge some of the earlier concepts that had been developed based on biophysical studies alone. These new studies have revealed that the mechanotransduction machinery of cochlear hair cells is assembled from an astonishing number of diverse proteins. While the lack of a reconstituted system to study ion channel function outside hair cells has been a significant roadblock for the field, the study of genetically modified mice has begun to unravel the function of individual components of the mechanotransduction machinery of hair cells. Surprisingly, these studies have also revealed that mechanotransduction by hair cells is not only essential for auditory perception. Functional transduction channels are necessary for the normal development of hair cells and for the maturation of the afferent neurons that innervate them. We will review here the current status of the field of auditory mechanotransduction and will highlight remaining open questions.
The Organ of Corti: An Exquisitely Organized Cellular Ensemble
The perception of sound is an impressive task. Humans can distinguish sounds from near 0 dB up to 120 dB thus enabling us to hear the rustling of leaves in the wind or the roaring of an airplane engine. Humans perceive frequencies ranging from dozens of Hz up to 20 kHz. This frequency range is dramatically extended in other species. Mice hear sound frequencies of up to 70 kHz, while the frequency range of several bat species extends to an astonishing 200 kHz (Dale et al., 2001; Hopp et al., 1998). These variations in frequency range are adaptations optimized for species-specific tasks. Sound perception at the lower end of the frequency spectrum enables humans to communicate and mice to perceive predators. Mice use ultrasound communication for inter-species communication, while bats use ultrasound vocalization and detection to capture prey.
The end-organ for the perception of sound: a highly organized sensory epithelium
The basic building plan of the end-organ for the perception of sound is remarkably conserved across mammalian species and represents perhaps one of the most elegant examples of a mechanosensory structure dedicated to a specific task. The auditory sensory epithelium of mammals is embedded into the Organ of Corti, which is localized within the cochlear snail of the inner ear (Fig. 1A). The Organ of Corti contains one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs) that run along the length of the cochlear duct. These cells are the specialized mechanosensory cells of the inner ear. The function of hair cells depends on supporting cells that assume specific positions within the Organ of Corti (Fig. 1A). Transcriptomics studies have begun to reveal cell-type specific signatures for each cell type within the Organ of Corti, thus enabling new opportunities for a molecular investigation of their functions (https://umgear.org/). The cells of the Organ of Corti are sandwiched between two molecularly distinct extracellular matrix assemblies, the tectorial membrane that overlies the Organ of Corti, and the basilar membrane that runs below it (Fig. 1A) (Fettiplace, 2017; Schwander et al., 2010).
Figure 1. The auditory sense organ.
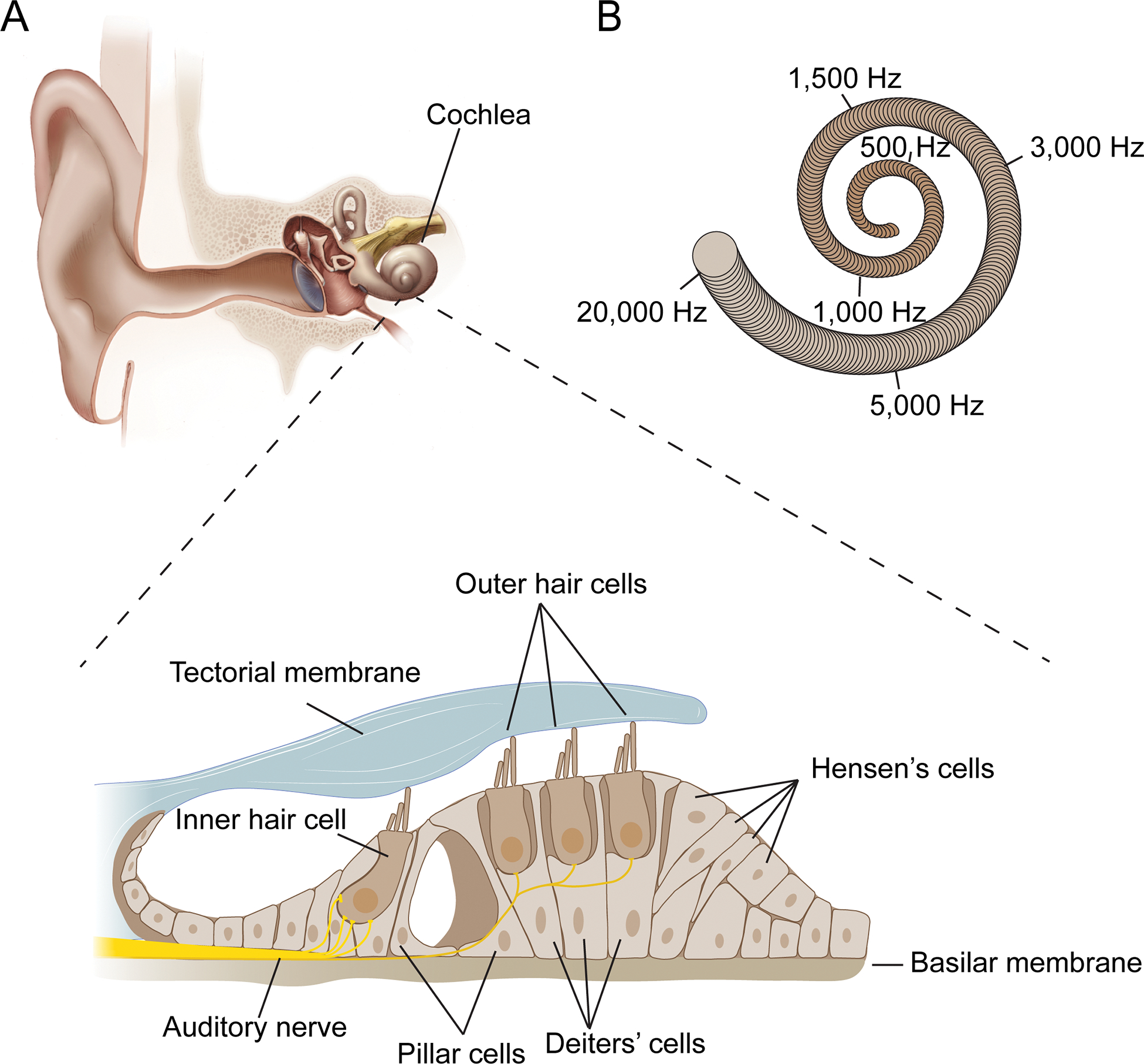
(A) Diagram of the outer, middle and inner ear. The bottom shows a cross section of the Organ of Corti that is situated within the snail-shaped cochlea of the inner ear. Sensory hair cells, including one row of inner hair cells (IHCs) and three rows of outer hair cells (OHCs), are indicated. Supporting cells such as Pillar cells, Deiters’ cells and Hensen’s cells separate and support hair cells. The cells of the Organ of Corti are sandwiched between two extracellular matrix assemblies, the tectorial membrane and the basilar membrane. (B) Schematic of the snail-shaped cochlea indicating that different sound frequencies are encoded by hair cells at different tonotopic positions along the cochlear duct. Frequency positions are for the human cochlea.
Hair cells derive their names from a bundle of specialized actin-rich microvilli, known as stereocilia, that crown their apical surface. The stereocilia are the mechanically sensitive organelle of a hair cell. Sound waves that reach the ear drum are converted into fluid motions that travel down the cochlear duct, leading to vibrations within the elastic basilar membrane. These vibrations cause deflections of the stereocilia of hair cells against the overlying tectorial membrane, thus leading to the activation of mechanotransduction channels within stereocilia (Fettiplace, 2017; Schwander et al., 2010). To achieve tight coupling of hair cells to the tectorial membrane, the longest stereocilia of OHCs form attachment crowns. Mutations in the genes that affect the formation of attachment crowns cause deafness, highlighting the importance of coupling between hair bundles and the tectorial membrane for sound perception (Avan et al., 2019; Verpy et al., 2011; Zeng et al., 2016). It was long thought that the stereocilia of IHCs are not in contact with the tectorial membrane, but this concept has recently been challenged. Using fluorescence confocal imaging in an acute organ preparation, it was shown that the stereocilia of both IHCs and OHCs appear to be in contact with the tectorial membrane, at least in guinea pig (Hakizimana and Fridberger, 2021). However, studies by others are consistent with the model that stereocilia of IHCs are not directly coupled to the tectorial membrane and instead are activated by fluid motion (Jia et al., 2007; Russell and Sellick, 1983).
Mechanosensation: distinct functions for IHCs and OHCs
The building plan of the cochlea provides first clues to the mechanisms that enable mammals to perceive sound of different frequencies and intensities. Anatomical features of the Organ of Corti vary systematically along the length of the cochlear duct. Because of such changes including a gradual increase in the stiffness and mass of the cochlear partition from the apex to the base, sounds of different frequencies induce maximal vibrations at different positions along the cochlea duct, thus stimulating sensory hair cells at specific so-called tonotopic positions. Highest sound frequencies are perceived by hair cells at the base of the cochlea and lowest frequencies at the apex, with a gradient in-between (Fig. 1B) (Fettiplace, 2017; Schwander et al., 2010).
Both IHCs and OHCs are mechanosensory cells, yet they have distinct functions. IHCs are the bona fide auditory receptors. In contrast, OHCs amplify input sound signals because these cells can change the length of their cell body in response to changes in membrane potential, a process called electromotility (Ashmore, 1987; Brownell et al., 1985; Kachar et al., 1986). The amplification process depends on the molecular motor protein prestin, which is embedded into the lateral membrane of the cell body of OHCs (Belyantseva et al., 2000; Zheng et al., 2000). Cryo-EM studies have determined the structure of prestin (Bavi et al., 2021; Butan et al., 2022; Ge et al., 2021). The structure of dolphin prestin in six conformations is consistent with a model where structural rearrangements in a voltage sensor domain are coupled to conformational changes at the protein-membrane interface that cause membrane expansion (Bavi et al., 2021). The shape changes in OHCs amplify the vibrations caused by input sound signals in a localized fashion within frequency-specific positions. The vibrations are then sensed by IHCs, leading ultimately to the initiation of action potentials in spiral ganglion neurons (SGNs) that innervate these sensory cells with tonotopic specificity to transmit sound information to the CNS.
Hair-cell stereocilia: form defines function
The stereocilia of a hair cell are an extraordinarily sensitive sensory compartment. Computationally derived estimates indicate that transducer currents in the mammalian cochlea saturate at ~2° angular rotation (He et al., 2004). The core of stereocilia consists of highly crosslinked parallel actin filaments. Near their base, stereocilia form a characteristic taper. During mechanical stimulation, stereocilia act as stiff rods that tilt around their base (Hudspeth, 1983). Within a hair bundle, stereocilia are organized into rows of increasing heights, and only deflection towards the longest stereocilia lead to the activation of mechanotransduction channels in stereocilia (Hudspeth and Corey, 1977; Shotwell et al., 1981). These ion channels are localized at the base of tip links, fine extracellular filaments that connect the stereocilia and are visible in the electron microscope (Fig. 2A) (Beurg et al., 2009; Pickles et al., 1984). Disruption of tip-links affects channel gating, indicating that tip links are critical to transmit force onto mechanotransduction channels (Assad et al., 1991; Zhao et al., 1996). This has perhaps been most directly demonstrated by elegant studies in bullfrog hair cells. Hudspeth and colleagues generated superparamagnetic beads that bind to the tip links of hair cells. Application of a magnetic-field gradient exerts mechanical force on the tip links that evokes mechanotransduction currents (Basu et al., 2016).
Figure 2. The mechanotransduction machinery of cochlear hair cells.
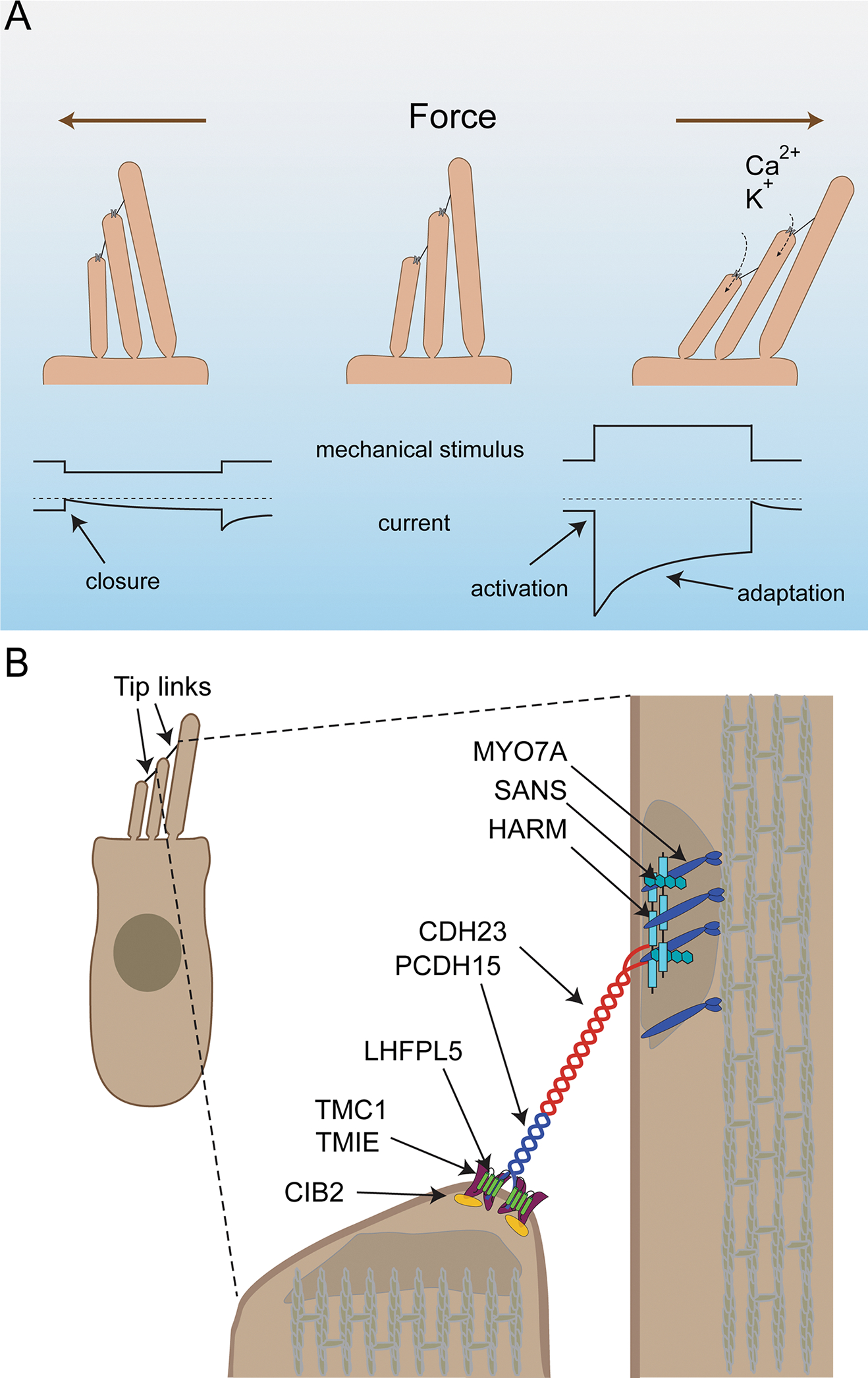
(A) Diagram of the mechanotransduction process. On top the direction of stereocilia deflection is shown, on the bottom idealized traces from recordings. Upper traces show mechanical deflections of the stereocilia: downward shift (left) indicates a deflection towards the shortest stereocilia, an upward shift (right) deflection towards the longest stereocilia. Deflection towards the shortest stereocilia (left) leads to a closing of mechanotransduction channels that are open at rest followed by slow channel re-opening. Deflection towards the longest stereocilia (right) leads to an increased influx of Ca2+ and K+ into stereocilia. This is reflected in the lower traces by a downward current, which then adapts due to channel closure even during a maintained stimulus. (B) Diagram of a hair cells indicating proteins important for mechanotransduction and their asymmetric localization at the upper and lower ends of the tip link.
While the basic building plan of hair bundles is similar across species, variations between different types of hair cells and species have been observed. In mice each cochlear hair cell contains three rows of stereocilia, but the stereocilia of OHCs form a bundle with a more eccentric V shape compared to the flatter V shape of IHC. Hair cells in other species such as bullfrogs, have more rows of stereocilia per bundle and form a tighter bundle with no obvious V shape (Barr-Gillespie, 2015; Schwander et al., 2010). The different organization of stereocilia likely has significant impact on the way a hair bundle moves in response to mechanical inputs (O’Maoileidigh and Ricci, 2019). Stereocilia across hair bundles in bullfrogs and turtles move as a unit during mechanical stimulation, thus allowing for exquisite sensitivity and coherent gating of mechanotransduction channels across the stereociliary bundle (Crawford and Fettiplace, 1985; Karavitaki and Corey, 2010; Kozlov et al., 2007). It is less clear if this mode of motion applies to the mammalian hair bundle, which has a more extreme shape with less coherence (Nam et al., 2015; Wang et al., 2021b). Variations in the way mechanical stimuli of different intensities deflect the whole bundle or only parts of it could have significant effects on downstream signaling mechanisms.
The Mechanotransduction Process: Channel Activation and Adaptation
At rest, mechanotransduction channels in cochlear hair cells are partially open. In rodents, the resting open probability of OHCs is ~50% and of IHCs ~20% when measured in vitro at the low Ca2+ concentration typical for endolymph (Beurg et al., 2010; Corns et al., 2014; Johnson et al., 2011; Peng et al., 2013). This defines the set point of the channel along the activation curve in the absence of a mechanical stimulus and keeps the channels at the position of greatest sensitivity to mechanical stimulation. Sound induced deflections of the stereocilia in the direction of the longest stereocilia activate the mechanotransduction channel followed by channel adaptation. Deflections in the opposite direction close the channels that are open at rest (Fig. 2A) (O’Maoileidigh and Ricci, 2019; Fettiplace, 2017).
Mechanotransduction channels of cochlear hair cells are non-selective for cations with a four times higher permeability for Ca2+ compared to K+ and Na+ (Beurg et al., 2006; Ohmori, 1985). Since the endolymph fluid that surrounds the stereocilia is high in K+ and low in Ca2+, most of the transduction current in vivo is carried by K+. However, Ca2+ profoundly regulates channel activity (Fettiplace, 2017). This was perhaps most quantitatively evaluated in turtle hair cells, where a reduction in the extracellular Ca2+ concentration leads to an increase in single channel conductance, a leftward shift of the activation curve and a reduction in the extent of adaptation (Ricci and Fettiplace, 1998).
Studies in turtle and rodent hair cells have determined a unitary channel conductance of 100–300 pS, depending on species, Ca2+ concentration and cochlear localization. In turtle hair cells and rodent OHCs, highest conductance values are observed towards the base of the cochlea and lowest values towards the apex indicative of molecular differences in the transduction complex along the tonotopic axis (Beurg et al., 2006; Ricci et al., 2003). There are no significant tonotopic differences in the conductance of IHCs (Beurg et al., 2006). Changes in conductance as well as differences in the kinetics of channel gating along the tonotopic axis could be critical for the detection and discrimination of a wide range of frequencies (Ricci, 2002).
Recent studies in mice suggest that the unitary conductance of the mechanotransduction channel may be closer to 50 pS. This conclusion is based on the observation that each MET channel complex exhibits multiple conductance states in ~50 pS increments (Beurg et al., 2018). However the lowest conductance states are observed extremely rarely in murine hair cells and have not been observed in turtle (Ricci et al., 2003). Further studies are necessary to define the single channel conductance of the mechanotransduction channel and possible variations between species.
Channel activation: ultrafast signal transmission
The activation of the hair cell mechanotransduction channel is much faster than for most ion channels and cannot be accurately determined with traditional stiff probe stimulation (Doll et al., 2012; Ricci et al., 2005). Early studies from bullfrog hair cells measured field potentials and concluded that channels open in response to mechanical stimulation within tens of μs (Corey and Hudspeth, 1983). Direct measurements of the activation time constant in turtle provided similar evidence for channel opening within μs (Crawford et al., 1989). This has led to the idea that the channel is directly gated by mechanical force (Corey and Hudspeth, 1983). Recent measurements in mouse cochlear hair cells combined with modeling estimate the value for activation at about 4 μs for basal and 10 μs for apical cochlear hair cells (Beurg et al., 2021a). A similar tonotopic gradient of activation kinetics had been reported in turtle (Ricci et al., 2005).
Studies in bullfrog hair cells have revealed a non-linear displacement of the hair bundle upon force application and a gating compliance, which has led to the influential gating spring model. This model proposes that mechanical force is transmitted to the mechanotransduction channel through an elastic element, or gating spring. The stiffness of the gating spring was determined to be at about 1 pN/nm (Howard and Hudspeth, 1988). More recently, the gating spring stiffness has been analyzed in the rat cochlea. This elegant study revealed that gating spring stiffness increases in hair cells with increasing characteristic frequency (Tobin et al., 2019). This tonotopic variation in gating spring stiffness could be important to optimize the sensitivity of the mechanotransduction channel to forces generated by different sound frequencies. In addition, cAMP affects mechanotransduction by hair cells (Ricci and Fettiplace, 1997), and an increase in cAMP levels leads to a decrease in gating spring stiffness (Mecca et al., 2022). While the mechanisms that regulate cAMP levels in hair cells are not known, this observation suggests that gating spring stiffness might be modulated by cellular signaling mechanisms.
How to adapt: old friends and new models
Following activation, mechanotransduction channels in hair cells adapt, which is reflected by a decrease in transducer currents during a sustained hair bundle displacement (Fig. 2A). Adaptation is thought to be important to extend the dynamic range of a hair cell and to contribute to frequency selectivity and signal amplification. Studies in non-mammalian vertebrates identified two components of adaptation, fast and slow, progressing with a time constant of several milliseconds and tens of milliseconds, respectively. Fast adaptation has been proposed to depend on the binding of Ca2+ to the mechanotransduction channel or a side near the channel leading to channel closure. Slow adaptation has been proposed to depend on a motor protein localized at the upper end of tip links (Fettiplace, 2017; Gillespie and Müller, 2009). This motor protein, originally proposed to be MYO1C (Gillespie and Cyr, 2004), climbs up the actin filaments toward the tips of the stereocilia to establish tension in the transduction machinery. The motor is released from F-actin when Ca2+ flows into stereocilia and tension on the transduction machinery is reduced thus leading to channel closure (Assad et al., 1991; Howard and Hudspeth, 1988). The motor model also proposes that adaptation is critical to set the resting tension of the mechanotransduction channel, which keeps the channel partially open at rest. Notably, adaptation rates vary tonotopically with higher adaptation rates in hair cells responding to high frequencies (Ricci and Fettiplace, 1997; Ricci et al., 2005; Waguespack et al., 2007), which could yet be another specialization critical for frequency discrimination.
As discussed below, recent observations in mammals suggest that this motor model of adaptation is not universally true and likely does not apply to cochlear hair cells. These studies also suggest that adaptation and resting tension in mammals are regulated by distinct mechanisms.
The Mechanotransduction Complex: A Dazzling Array of Molecules
The mammalian inner ear contains only a small number of hair cells, approximately 3,000 in mice and 15,000 in humans. Due to this sparsity of material, biochemical methods have been largely unsuccessful to provide insights into the molecular composition of the tip link and mechanotransduction channel. Models of mechanotransduction have largely been developed based on biophysical studies without a molecular framework. A breakthrough in the field came with the positional cloning of genes that are linked to deafness. The study of the affected genes has revealed that mechanotransduction by hair cells depends on a molecular machine consisting of many parts (Fig. 2B). Structural biology has provided first insights into the atomic structure of some of the transduction molecules.
Molecular composition of the tip-link: a striking molecular asymmetry
The first molecules that were definitively linked by genetic studies to the mechanotransduction machinery of cochlear hair cells are two members of the cadherin superfamily, PCDH15 and CDH23. Mutations in the genes encoding these proteins cause deafness and defects in mechanotransduction (Kazmierczak and Müller, 2012). CDH23 and PCDH15 have subsequently been shown to be components of tip links (Ahmed et al., 2006; Kazmierczak et al., 2007; Siemens et al., 2004; Sollner et al., 2004). These adhesion receptors form cis-homodimers that interact in trans via their N-termini to form the upper and lower parts of tip links (Fig. 2B) (Kazmierczak et al., 2007). Distinct from classic cadherins that contain 5 extracellular cadherin (EC) repeats, PCDH15 and CDH23 contain 11 and 27 EC repeats, respectively. A complete tip-link thus contains an astonishing 76 EC domains to make up an extracellular filament that is approximately 150 nm in length (Kazmierczak et al., 2007).
Crystal structures of fragments of PCDH15 and CDH23 have confirmed the classic cadherin fold for their EC domains (Araya-Secchi et al., 2016; Choudhary et al., 2020; De-la-Torre et al., 2018; Dionne et al., 2018; Elledge et al., 2010; Jaiganesh et al., 2018; Narui and Sotomayor, 2018; Powers et al., 2017; Sotomayor et al., 2010; 2012). Single particle reconstruction of the entire EC1-EC11 domain of PCDH15 as well as a high-resolution structures of smaller PCDH15 fragments have shown that dimerization is mediated by the EC3 domains of two PCDH15 molecules (Fig. 3) (Dionne et al., 2018). A second dimerization site is localized closer to the cell membrane (Fig. 3) (Ge et al., 2021). Mutations that perturb PCDH15 dimerization affect mechanotransduction, demonstrating the importance of the dimeric tip-link structure for tip-link function (Dionne et al., 2018). Mechanisms that drive dimerization of CDH23 are less clear.
Figure 3. Structure of the PCDH15-LHFPL5 complex and the PCDH15-CDH23 binding site.
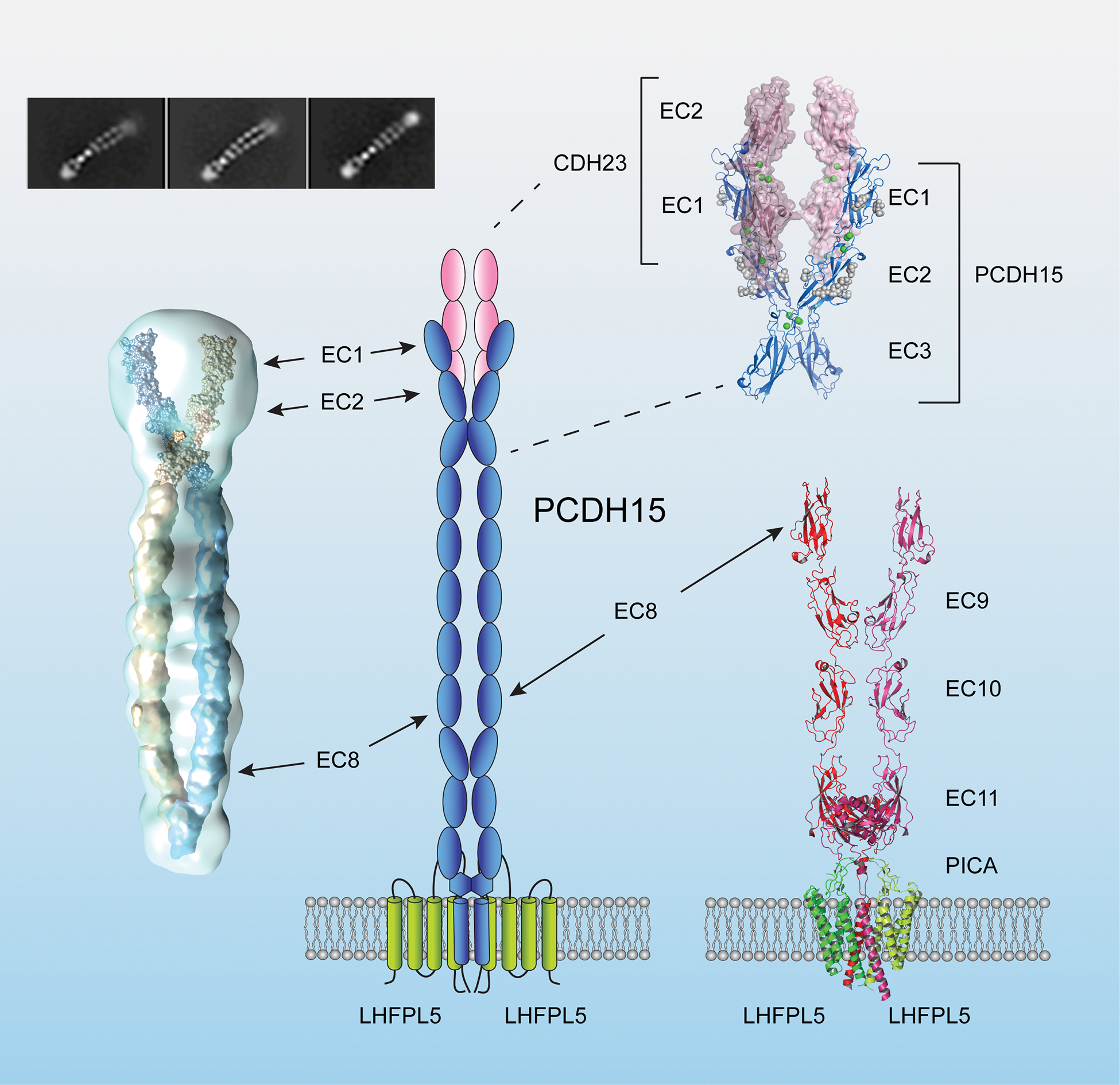
A diagram of the lower end of tip links. The interaction between PCDH15 and CDH23 is mediated by N-terminal EC1 and EC2 domains (upper right, PDB 4APX and 6CV7) (Sotomayor et al., 2012). Dimerization of PCDH15 is mediated by two sites. The first dimerization site is at EC3 and was observed by single particle reconstruction of the entire 11 EC domains (left) and a high-resolution X-ray crystallographic structure of an EC1–3 fragment (upper right: PDB:6CV7) (Dionne et al., 2018). The second dimerization sited is close to the membrane proximal domain observed in crystals of a C-terminal fragment of PCDH15 (Ge et al., 2018). A PCDH15 dimer interacts with an LHFPL5 dimer (bottom right). The PICA domain of PCDH15 forms a collar above the membrane that might have structural flexibility (PDB: 6C13 and 6C14) (Ge et al., 2018).
In classical cadherins, the basic adhesive unit consists of two cadherin monomers that interact with each other via their N-termini. Trp residues in the first N-terminal cadherin repeats are buried into complementary binding pockets in the first N-terminal cadherin repeat of the trans binding-partner (Boggon et al., 2002; Haussinger et al., 2004; Patel et al., 2006). Trp residues are not conserved in EC1 of PCDH15 and CDH23, and structures of the N-terminus of CDH23 reveal significant differences to classical cadherins (Elledge et al., 2010; Sotomayor et al., 2010). Instead, the adhesive units of tip links are dimers of PCDH15 and CDH23 that interact via an enlarged binding surface formed by EC1 and EC2 of the two molecules (Fig. 3) (Sotomayor et al., 2012).
The dimeric structure of the tip-link cadherins combined with a new and enlarged binding surface (compared to classical cadherins) are likely evolutionary adaptations to achieve the necessary binding strength that is required to maintain tip-link integrity during mechanical stimulation. This is consistent with single-molecule force spectroscopy experiments that compared the binding strength of monomeric and dimeric N-terminal fragments of tip-link cadherins (Mulhall et al., 2021). The remarkable molecular asymmetry in the tip link is suggestive of distinct functions for the divergent cytoplasmic domains of the two cadherins at the upper and lower ends of tip links.
Tip links were originally proposed as candidates for the gating spring of the mechanotransduction channel (Assad et al., 1991). However, initial structure-based predictions indicated otherwise. Modeling studies of the classical C-cadherin suggest that its extracellular domain is stiff in the presence of Ca2+ and shows limited elasticity in the absence of Ca2+ (Oroz et al., 2019). Three conserved amino acids within the linker domains between EC domains are required for Ca2+ binding and rigidification (Oroz et al., 2019; Sotomayor and Schulten, 2008). Modeling studies for the EC1-EC2 fragment of CDH23 predicted a stiffness of 570 nN/m. When these values were extrapolated to all EC domains of the tip link, a stiffness of 40–60 nN/m was predicted (Sotomayor et al., 2010). These values are too high compared to the measured stiffness of the gating spring (1 pN/nm)(Howard and Hudspeth, 1988). However, not all linkers between EC domains of PCDH15 and CDH23 contain the three conserved amino acids that bind Ca2+ and rigidify cadherins (Araya-Secchi et al., 2016; Jaiganesh et al., 2018; Powers et al., 2017). This could significantly affect the elasticity of tip links. In fact, single molecule measurements of the stiffness of a monomeric extracellular domain of PCDH15 using a high-precision optical trap revealed a stiffness lower than the gating spring stiffness. Considering that PCDH15 are dimeric and interact with CDH23, the predicted values for the stiffness of the entire tip link were estimated to be in line with the stiffness of the gating spring (Bartsch et al., 2021). While further studies are warranted, tip links are good candidates to contribute to the gating spring. Several molecules that are in series with tip links could also be part of the gating spring, which might include both proteins and membrane lipids. Recent data suggest that the membrane of stereocilia contributes to calcium-modulated viscoelastic element that can regulate hair cell mechanotransduction (George et al., 2020).
The tetraspan LHFPL5: tight coupling to the lower end of tip links
In the search for additional components of the mechanotransduction complex of hair cells, a large-scale forward genetic screen was carried out to identify mice that are deaf. This screen also analyzed mouse mutants generated by other researchers (Schwander et al., 2007; Xiong et al., 2012). One of the first mouse lines to be identified from the screen carries a mutation in Cdh23 that affects tip link function, thus providing evidence for a role of CDH23 in mechanotransduction (Schwander et al., 2009). A second affected mouse line carries a mutation in the gene encoding the tetraspan LHFPL5/TMHS (Longo-Guess et al., 2005; Xiong et al., 2012). Mutations in the LHFPL5 gene also cause deafness in humans (Shabbir et al., 2006). LHFPL5 binds to PCDH15 and co-localizes with PCDH15 to the lower end of tip links, thus reinforcing the concept of molecular asymmetry in the mechanotransduction machinery (Fig. 2B) (Li et al., 2019; Mahendrasingam et al., 2017; Xiong et al., 2012)
Structural studies have shown that a dimer of LHFPL5 binds to a dimer of PCDH15 where extensive contact between the two molecules is mediated by transmembrane helices. In addition, an extracellular loop of LHFPL5 is in contact with the membrane proximal extracellular domain of PCDH15 that follows EC11 and forms a collar above the membrane (Fig. 3) (Ge et al., 2018). This domain was dubbed as the PCDH15 interacting-channel associated (PICA) domain because it could be involved in mediating interactions with the mechanotransduction channel (Dionne et al., 2018). Cryo-EM studies suggest that the collar has structural flexibility (Ge et al., 2018), which could be important to regulate force-transfer from the tip link to the transduction channel.
In mice carrying an Lhfpl5 null mutation, localization of PCDH15 to stereocilia is affected, thus leading to reduced numbers of tip links and a concomitant reduction of transducer currents by 70–90% (Xiong et al., 2012). Some tip links remain in the mutant mice, allowing for the study of mechanotransduction currents carried by channels in the absence of LHFPL5. The properties of these currents are strikingly altered, including a delay in channel activation and lack of fast adaptation (Xiong et al., 2012). These findings provide evidence that LHFPL5 is an essential component of the MET channel complex that forms a tight unit with the tip link. Since mechanotransduction is not completely abolished in LHFPL5-deficient hair cells, LHFPL5 is unlikely to be an essential pore-forming subunit of the MET channels. Both in overall structure and function LHFPL5 resembles TARP subunits of glutamatergic AMPA receptors (Xiong et al., 2012). These ion channel accessory subunits regulate the localization of AMPA receptors to synaptic sites, and they allosterically affect the pore properties of these ion channels (Jackson and Nicoll, 2011). LHFPL5 is thus most likely a TARP-like molecule for the mechanotransduction channel of cochlear hair cells (Xiong et al., 2012).
The mechanism by which LHFPL5 contributes to the gating of the mechanotransduction channel in hair cells is unclear. Based on the fast activation time constant for the mechanotransduction channel, it seems reasonable to hypothesize that this ion channel binds to the tip link. Data summarized in the next section support this view. Alternatively, the tip-link complex might pull on the membrane to change membrane curvature, thus leading to channel activation. In support of the latter model, the membrane at the lower end of tip links pulls away from the underlying cytoskeleton, suggesting that the tip link applies tension to the membrane (Assad et al., 1991; Kachar et al., 2000). However, it cannot be excluded that this membrane tenting is caused by shrinkage of the stereocilia during fixation. Nevertheless, membrane components such as PIP2 are concentrated at stereociliary tips and have dramatic effects on channel gating and function (Effertz et al., 2017; Hirono et al., 2004). LHFPL5 is a distant member of the tetraspanin family, which form multimers and are components of lipid microdomains (van Deventer et al., 2017). It would be interesting to determine whether LHFPL5 can affect PIP2 distribution and membrane properties at the tips of stereocilia.
The mechanotransduction channel revealed: TMC1 and TMC2 as pore-forming subunits
Experiments by the Ricci and Fettiplace laboratories demonstrated that mechanotransduction channels in hair cells are only present at lower ends of tip links (Fig. 2B). Without knowledge of the molecular identity of the transduction channel, these laboratories took advantage of the observation that transduction channels are non-selective cation channels with a preference for Ca2+. Imaging of Ca2+ entry into stereocilia during mechanical stimulation revealed that mechanotransduction channels are only present at the tips of shorter stereocilia near PCDH15 (Beurg et al., 2009), thus reinforcing the concept of molecular asymmetry at tip links.
Genetic studies in mice were once again instrumental for the identification of subunits of the mechanotransduction channel of hair cells. Mutations in the gene encoding the multi-transmembrane protein TMC1 were linked to dominant and recessive forms of deafness (Kurima et al., 2002). TMC1 and its close relative TMC2 are expressed in mechanosensory hair cells of the cochlea, where TMC2 is the predominant isoform in the first few postnatal days before it becomes progressively replaced by TMC1 (Kawashima et al., 2011; Kurima et al., 2015). Mechanotransduction currents are abolished in hair cells from mice lacking both TMC1 and TMC2, while some transducer currents are maintained in Tmc1 mutant mice in the first few days after birth (Kawashima et al., 2011; Kim and Fettiplace, 2013). The reason for the developmentally regulated expression patterns of TMC1 and TMC2 is not known, but both molecules confer distinct properties to hair cells. Hair cells expressing only TMC1 have reduced Ca2+ permeability and larger single channel conductance compared to hair cells expressing only TMC2 (Corns et al., 2017; Kim and Fettiplace, 2013; Pan et al., 2013). In addition, Ca2+ permeability is affected in a mouse line expressing a dominant p.M412K mutation in Tmc1 that is linked to deafness in humans (Corns et al., 2017; Kim and Fettiplace, 2013; Pan et al., 2013). One study reported reduced single channel conductance caused by the p.M412K mutation (Pan et al., 2013), although a second study could not repeat these findings (Beurg et al., 2015a). These studies suggested that TMC1 and TMC2 affect pore-properties of the mechanotransduction channel of cochlear hair cells.
TMC1 and TMC2 are localized in hair cells near the lower end of tip links (Kurima et al., 2015), although the vast amount of TMC1 is found in the ER (Cunningham et al., 2020). In addition, TMC1 and TMC2 can bind to PCDH15 and to LHFPL5 (Beurg et al., 2015b; Maeda et al., 2014; Yu et al., 2020), consistent with the model that transduction channels might be coupled to the tip link. The localization of PCDH15 and TMC1 to stereocilia is perturbed in Lhfpl5 mutant mice (Beurg et al., 2015b; Xiong et al., 2012), reinforcing the concept that these proteins are part of a larger protein complex near tip links.
Sequence alignments suggest that TMC1 and TMC2 are evolutionarily related to anoctamins, a family of 10 genes that encode Ca2+ activated Cl− channels and lipid scramblases (Hahn et al., 2009). The structure of TMEM16A, one of the anoctamins, was solved and used as a template for homology modelling, predicting that TMC1 has a similar 10 membrane topography as TMEM16A (Fig. 4A,B). The model also revealed a putative ion conducting pathway lined by TM4-TM7 and predicted that TMC1 forms dimers indicative of two pores within a functional ion channel complex (Ballesteros et al., 2018; Pan et al., 2018). Two laboratories interrogated the model that TMCs are pore-forming subunits of the mechanotransduction channel.
Figure 4. Diagram of the ion channel complex.
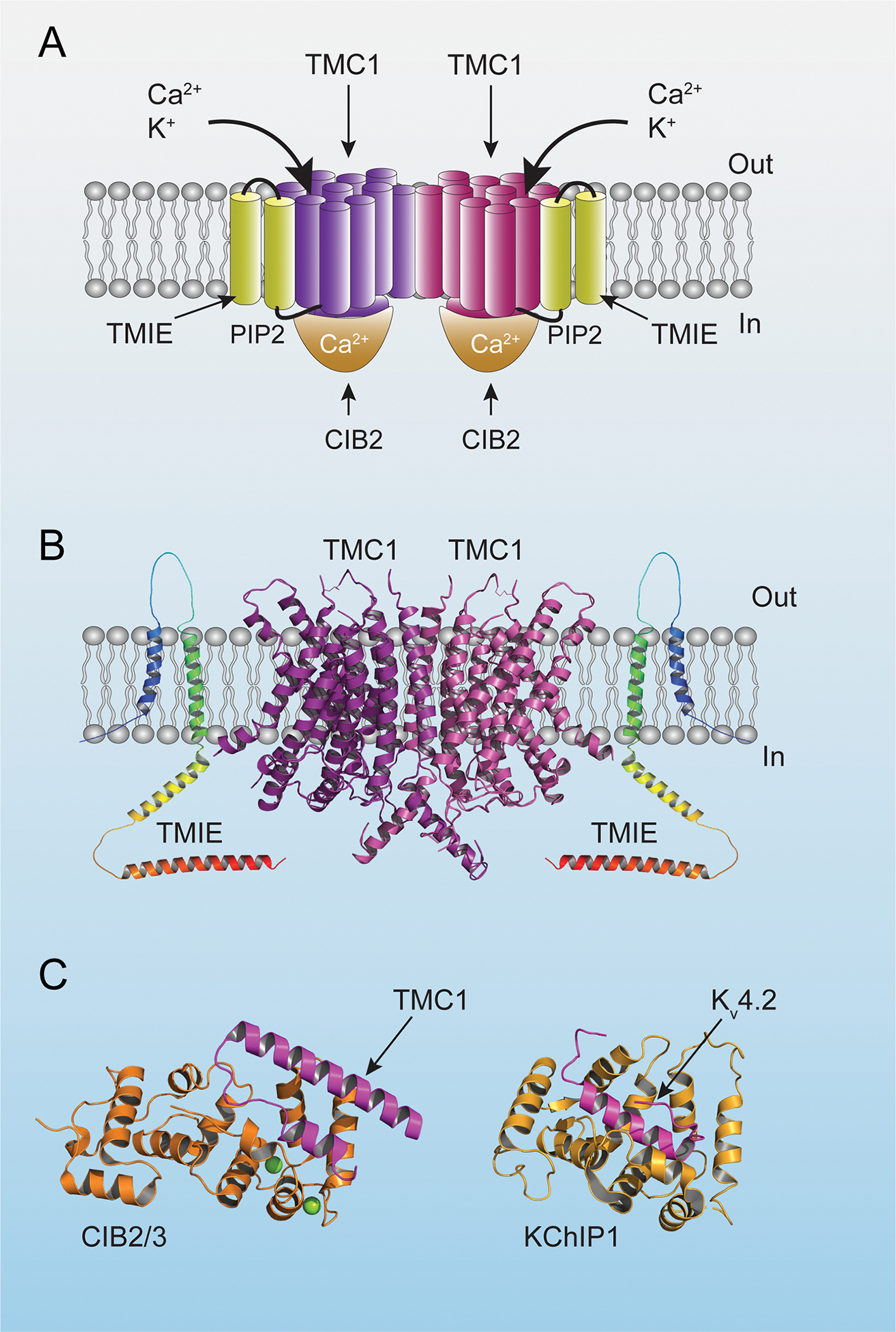
(A) Diagram of the mechanotransduction-channel complex based on structural data from C. elegans (Jeong et al., 2022). Transmembrane domains of TMC1 are in purple and burgundy to highlight the dimeric nature of the complex. (B) Structural model of TMC1 based on the structure of TMEM16A (Ballesteros et al., 2018). The structure of TMIE was predicted by alpha-fold (https://alphafold.ebi.ac.uk/). (C) Crystal structure of CIB2/3 and KChiP1 in complex with the first cytoplasmic loop of TMC1 and the N-terminal cytoplasmic domain of Kv4.2, respectively (PDB: 6WUD and 7E84). Note the similar fold for CIB2/3 and KChiP1 with a hydrophobic groove occupied by α-helices of the TMC1/Kv4.2 binding partners (Liang et al. 2021).
The Holt laboratory substituted single amino acids within the putative pore region of TMC1 with cysteines (Pan et al., 2018). These mutations might change the properties of the channel pore by themselves, or by reacting with bulky cysteine modification agents such as MTSET to form covalent disulfide bonds leading to obstruction of the channel pore. The mutant proteins were expressed in hair cells from Tmc1/Tmc2 double mutant animals using AAV vectors. Out of 17 cysteine substitutions tested, 11 showed reduced Ca2+ permeability (compared to wild-type) that was caused by the mutation itself and/or following the addition of MTSET. Three mutations also caused reduction of single channel conductance. For those three mutants, effects of MTSET were reduced by channel blockers that enter the open pore of the channel, consistent with the model that the amino acids are within the pore (Pan et al., 2018).
The Holt and Fettiplace laboratories investigated effects of Tmc1 mutations that are linked to deafness for their effects on TMC1-dependent MET currents (Beurg et al., 2019; Beurg et al., 2015a; Beurg et al., 2021b; Goldring et al., 2019; Pan et al., 2013). These findings similarly provide evidence that amino acid residues in the predicted TM4-TM7 region of TMC1 affect the Ca2+ permeability of the mechanotransduction channel. A p.D528N mutation had drastic effects on channel conductance, suggesting that this amino acid is in a particular narrow region of the TMC1 pore (Beurg et al., 2021b), which is consistent with data obtained by cysteine scanning mutagenesis (Pan et al., 2018).
Taken together, the studies provide strong evidence that TMC1 and TMC2 are pore-forming subunits of the mechanotransduction channel in hair cells.
The curious case of TMIE: an additional essential ion channel subunit affecting the pore
Surprisingly, parallel studies demonstrated that TMC1 and TMC2 cannot form functional ion channels in hair cells without a small transmembrane protein termed TMIE (Cunningham et al., 2020). TMIE has two predicted transmembrane domains (Fig. 4A,B) (Mitchem et al., 2002; Naz et al., 2002), although the first transmembrane domain might be a signal sequence that is cleaved off during transport of the protein to the cell surface (Gu et al., 2020; Naz et al., 2002). TMIE binds to LHFPL5, PCDH15 and TMC1, and it co-localizes with LHFPL5 and TMC1 at the lower end of tip links (Cunningham et al., 2020; Zhao et al., 2014). Mechanotransduction is abolished in hair cells from mice with a Tmie null mutation (Zhao et al., 2014). This is due at least in part because TMC1 transport or retention in stereocilia is affected by TMIE (Cunningham et al., 2020). TMIE similarly regulates the localization of TMC proteins in zebrafish (Pacentine and Nicolson, 2019). However, overexpression of TMC1/2 in the hair cells from Tmie mutant mice drives TMC1/2 back into stereocilia without rescuing mechanotransduction currents. Conversely, mechanotransduction is not observed in stereocilia containing TMIE but not TMC1/2. Finally, TMIE binds to TMC1 through its C-terminal cytoplasmic domain, and this interaction is crucial for mechanotransduction (Cunningham et al., 2020). A functional mechanotransduction channel in hair cells thus consists of TMC1 (or TMC2) and TMIE.
The model that TMIE is an essential ion channel subunit is further supported by studies motivated by human genetics. Point mutations in TMIE linked to deafness have been identified in the C-terminal cytoplasmic domain of TMIE (Naz et al., 2002). These mutations are within a polar region of TMIE that binds to TMC1 and to phospholipids, including PIP2 (Cunningham et al., 2020). The mutations affect PIP2 binding and pore properties of the mechanotransduction channel as revealed by a reduction in single channel conductance and a change in the Ca2+ selectivity (Cunningham et al., 2020).
It is currently unclear by which mechanism TMIE regulates the pore properties of the mechanotransduction channel of cochlear hair cells. The TMEM16A-based model structure of TMC1 reveals an unusually large cavity for the pore that is open to the lipid bilayer (Ballesteros et al., 2018; Pan et al., 2018). Perhaps, the transmembrane domains of TMIE shield the channel pore from the lipid environment, while the C-terminal cytoplasmic domain, which binds to TMC1 and PIP2, is close to the pore region and thus regulates pore properties.
The structure of the mammalian mechanotransduction channel has not been reported yet, but a recent study in bioRxiv reported the structure of a TMC1 protein complex purified from C. elegans (Jeong et al., 2022). The native complex contains both TMC1 and TMIE, confirming that the two proteins form a complex. The TMC1 structure is in good agreement with the model structure for mammalian TMC1 revealing a dimeric architecture and 10 transmembrane domains. Unlike the prediction from the mammalian channel, the structural data indicate that the pore of the channel is formed by TM6–8. TMIE is directly adjacent to the pore region. Interestingly, the TM domain of TMIE does not directly interact with the TM domains of TMC1 but instead forms a striking intramembranous cavity that is occupied by lipid molecules within the putative pore-forming TMC-1 helices TM6 and TM8. A cysteine at the cytosolic boundary of the TM domain in TMIE is palmitoylated and close to the TMC1 pore region, suggesting a possible role of TMIE and lipids in regulating MET channel gating (Jeong et al., 2022). The structure of the C. elegans TMC1 complex appears to reveal the closed conformation of the ion channel, which could undergo substantial structural rearrangements that alter the relative position of TMC1 and TMIE.
There are several unresolved issues. The mammalian MET channel contains a large permeation pathway permeable for cations and large organic molecules. The charge of the predicted pore of the nematode TMC1 channel suggests that it might not be selective for cations (Jeong et al., 2022). Nematode TMC1 might have similar permeation properties as the OSCA channel, a mechanically gated ion channel that displays non-selective cation currents with 17–21% Cl− permeability (Murthy et al., 2018). In addition, TMC1 in nematodes is required for mechanosensation by OLQ neurons, acts as a pH sensor in ASH neurons, and as a leak channel in HSN neurons and vulval muscles (Tang et al., 2020; Wang et al., 2016; Yue et al., 2018). Interestingly, in hair cells TMC1 is also required to establish a background Na+ leak current (Liu et al., 2019). It is not clear which channel complex is represented by the available nematode structure. Finally, nematodes do not have orthologs of CDH23 and PCDH15 and thus lack tip links. The structural relationship of the nematode channel complex to the mammalian mechanotransduction channel complex at tip links remains to be established.
CIB2 and CIB3: resemblance to accessory subunits of voltage gated K+ channels
The study of families afflicted with a recessive form of deafness led to the identification of causative mutations in CIB2, a member of the calcium and integrin binding protein family, which consists in mammals of four members (CIB1–4) (Gentry et al., 2005). Studies in mouse models have demonstrated that null mutations in Cib2 or a point mutation linked to deafness impair mechanotransduction and lead to degenerative changes in the hair bundle of cochlear hair cells (Giese et al., 2017). CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction by cochlear hair cells (Giese et al., 2017; Liang et al., 2021; Michel et al., 2017). Vestibular function is not affected by Cib2 mutations, which is likely explained by the fact that vestibular hair cells co-express CIB2 and its close homologue CIB3 (Giese et al., 2017; Michel et al., 2017). In support of redundancy between the two proteins, mechanotransduction defects are rescued in cochlear hair cells from Cib2 mutant mice by overexpression of CIB3 (Liang et al., 2021).
CIB2 and its relatives contain four EF-hand motifs, which are helix-loop-helix structural elements commonly found in Ca2+ binding proteins. CIB2 family proteins share this domain organization with KChIP proteins, which are members of the large family of neuronal Ca2+ sensor (NCS) proteins. Member of the NCS family have important functions in the nervous system including in phototransduction, neurotransmitter release, and in the regulation of ion channel trafficking and function (Burgoyne et al., 2019). KChIP proteins bind to the N-terminal cytoplasmic tail of Kv4 and regulate channel assembly, cell surface expression, and function (An et al., 2000; Wang, 2008). The structure of KChIP1 has been determined and identified a hydrophobic groove that mediates interactions with an alpha-helix formed by a protein domain within the N-terminal cytoplasmic domain of Kv4 (Fig. 4C) (Kise et al., 2021; Pioletti et al., 2006). CIB2 and its relative CIB3 bind to TMC1 and TMC2 through the N-terminal cytoplasmic domain and through a second stronger binding site in the first cytoplasmic loop between TM domains 2 and 3 (Giese et al., 2017; Liang et al., 2021). The co-crystal structure of a fragment of TMC1 encompassing the first cytoplasmic loop in complex with CIB3 revealed that the cytoplasmic loop of TMC1 folds into a long alpha helix connected by a linker to a shorter alpha helix that binds into a groove in CIB3 resembling the groove recognized by Kv4 in KChIP1 (Fig. 4C) (Liang et al., 2021). Molecular modelling studies predict that TMC1 and TMC2 bind to CIB2 in similar ways (Liang et al., 2021). When TMC1 was purified from C. elegans, it was bound not only by TMIE but also by CALM1, the C. elegans ortholog of CIB2/3 (Jeong et al., 2022). CALM1 forms a cap on the cytoplasmic surface of the ion channel complex and the structure revealed a similar longer and shorter alpha helix within the first cytoplasmic loop of TMC1 that is buried into a groove of CALM1 (Jeong et al., 2022).
In mouse hair cells, CIB2 is required for TMC1 transport to or retention within stereocilia (Liang et al., 2021). CIB2 resemble in this regard KChIP proteins, which also regulate the localization of K+ channels (Burgoyne et al., 2019). In addition, CIB2 and KChIP regulate ion channel function directly, although in distinct ways. While KChIP proteins regulate channel inactivation kinetics, CIB2 affects the open probability of the mechanotransduction channels at rest prior to mechanical stimulation (Liang et al., 2021). The latter conclusion was reached by the study of a point mutation in Cib2 (p.R186W) that causes deafness. The p.R186W mutation reduces the binding affinity of CIB2 for TMC1, indicative of structural changes that affect the protein-protein interaction surface between the two proteins (Liang et al., 2021).
The mechanism by which CIB2 affects ion channel function needs further study. The activity of mechanotransduction channels in cochlear hair cells is regulated by the extracellular and intracellular Ca2+ concentration (Fettiplace, 2017). NMR studies suggest that the structure of CIB2 is affected by Ca2+ (Vallone et al., 2018). Ca2+ ions that enter hair cells through transduction channels could affect the conformation of CIB2 and its interaction with TMC1. The p.R186W mutation in Cib2 slightly affects the unitary conductance of the mechanotransduction channel in OHCs (Liang et al., 2021). Perhaps this can be explained by allosteric effects of CIB2 on the conformation of the TMC1 protein. The p.R186W mutation is located in the extreme C-terminus of CIB2 that is not well structured in crystals, indicative of flexibility (Liang et al., 2021). The point mutation converts a charged amino acid to one that is hydrophobic. Perhaps the N-terminus of CIB2 is close enough to the ion conductance pathway to affect ion permeability. The analysis of additional mutations in Cib2 that affect Ca2+ binding might be informative to define the extent to which CIB2 mediates effects of Ca2+ on the mechanotransduction channel. However, the affinity of CIB2 for Ca2+ is low and much higher for Mg2+, suggesting that CIB2 may not work as Ca2+ sensor under physiological conditions (Vallone et al., 2018).
Recent studies have proposed a different function for CIB2 that might not be related to its function as a Ca2+ binding protein. These studies suggest that CIB2 might serve as a linker to anchor the mechanotransduction channel to the cytoskeleton. CIB2 binds to the adapter protein whirlin (WHRN) and to the motor protein MYO7A (Riazuddin et al., 2012). WHRN and its binding partner MYO15A are localized to tips of stereocilia and are required for the development of stereocilia of normal heights (Belyantseva et al., 2005). Interactions of CIB2 with the WHRN/MYO15A could mediate interactions between the mechanotransduction channel complex and the actin cytoskeleton.
Studies in nematodes have provided evidence that CALM1, the C. elegans ortholog of CIB2, binds to the ankyrin domain protein UNC-44, which could be yet another way to connect the mechanotransduction channel to the cytoskeleton (Tang et al., 2020). Earlier measurements indicated spring-like properties of ankyrin repeats (Lee et al., 2006; Sotomayor et al., 2005). Recent cryo-EM data and functional studies indicate that ankyrin repeats function as gating springs for the mechanically activated NOMPC ion channel (Jin et al., 2017; Wang et al., 2021a; Zhang et al., 2015). UNC-44 might fulfill a similar role for the mechanotransduction channel in hair cells, but UNC-44 was not present in the purified mechanotransduction complex from C. elegans used for structural studies (Jeong et al., 2022). The role of mammalian ankyrins in cochlear hair cells has not been analyzed. Thus, further studies will thus be necessary to define the extent to which UNC-44 is required to anchor the mechanotransduction channel to the cytoskeleton or whether the channel is in fact anchored to the cytoskeleton at all.
Model of the mechanotransduction-channel complex of hair cells: two-fold symmetry and multisubunit composition
The findings summarized above suggest a striking architecture for the mechanotransduction channel of cochlear hair cells. In this model, the basic channel unit is a symmetrical structure that contains two pores and consists of dimers of TMC1, and two TMIE and CIB2 molecules (Fig. 4A). Symmetry and dimeric structure are also defining features of the tip-link complex including PCDH15 and LHFPL5 (Fig. 3). It is tempting to speculate that the dimeric ion channel complex is tethered to the dimeric tip link via LHFPL5, although no structural data are available to support this model. CIB2 might provide a connection to the cytoskeleton by binding to WHRN, UNC44 or perhaps LOXHD1, which has recently been linked to mechanotransduction by hair cells (Trouillet et al., 2021).
Alternatively, the mechanotransduction channel might be gated by changes in membrane curvature. In support of the latter model, truncated TMC1 and TMC2 proteins from turtle and budgerigar have been reconstituted into liposomes and appear to be activated by negative pressure on the membrane (Jia et al., 2020). A recent report also suggests a tonotopic gradient in the number of TMC1 proteins with up to 20 copies per tip link (Beurg et al., 2018). This observation would suggest that direct coupling of the mechanotransduction channel to the tip link is not essential for channel gating.
Perhaps, the activation mechanism of the mechanotransduction channel of hair cells is not solely explained by either the tethered channel or membrane curvature model. Both mechanisms might cooperate to establish the exquisite force-sensitivity of this ion channel.
Adaptation: Quo Vadis?
Much attention has recently focused on the identification of the molecular components of the mechanotransduction channel at the lower end of tip links. In addition, proteins have been identified that are localized to the upper end of tip links. These proteins are thought to regulate resting tension in the mechanotransduction channel and perhaps adaptation.
The upper end of tip links: adapters and molecular motor proteins
The classical motor model of adaptation predicts that the upper end of tip links is connected to a protein complex that includes myosin motors that establish resting tension in the mechanotransduction complex and regulate slow adaptation (Markin and Hudspeth, 1995). MYO1C was proposed to be the adaptation motor (Holt et al., 2002). The motor model also predicts that Ca2+ entering through the mechanotransduction channel regulates myosin motor function. In its original form, the model cannot apply to cochlear hair cells since mechanotransduction channels are only localized at the lower end of tip links (Beurg et al., 2009). In addition, MYO1C is not concentrated at the upper end of tip links of mammalian hair cells (Schneider et al., 2006).
Motivated by the study of genes linked to deafness, a different protein complex has been identified at the upper end of tip links that consists of the adapter protein HARM-B, the ankyrin-domain containing protein SANS and the molecular motor protein MYO7A (Grati and Kachar, 2011; Grillet et al., 2009). These proteins bind to each other and to the cytoplasmic domain of CDH23, and the crystal structures highlighting some of these interactions have been determined (Fig. 5) (Kazmierczak and Müller, 2012). SANS, MYO7A and HARM-B also bind to F-actin thus establishing a potential link between the tip link and the cytoskeleton of hair cells (Kazmierczak and Muller, 2012). It is tempting to speculate that each strand of CDH23 assembles a similar protein complex thus extending the concept of a symmetric dimeric structure throughout the entire tip-link structure.
Figure 5. Diagram of the protein complex at the upper end of the tip link.
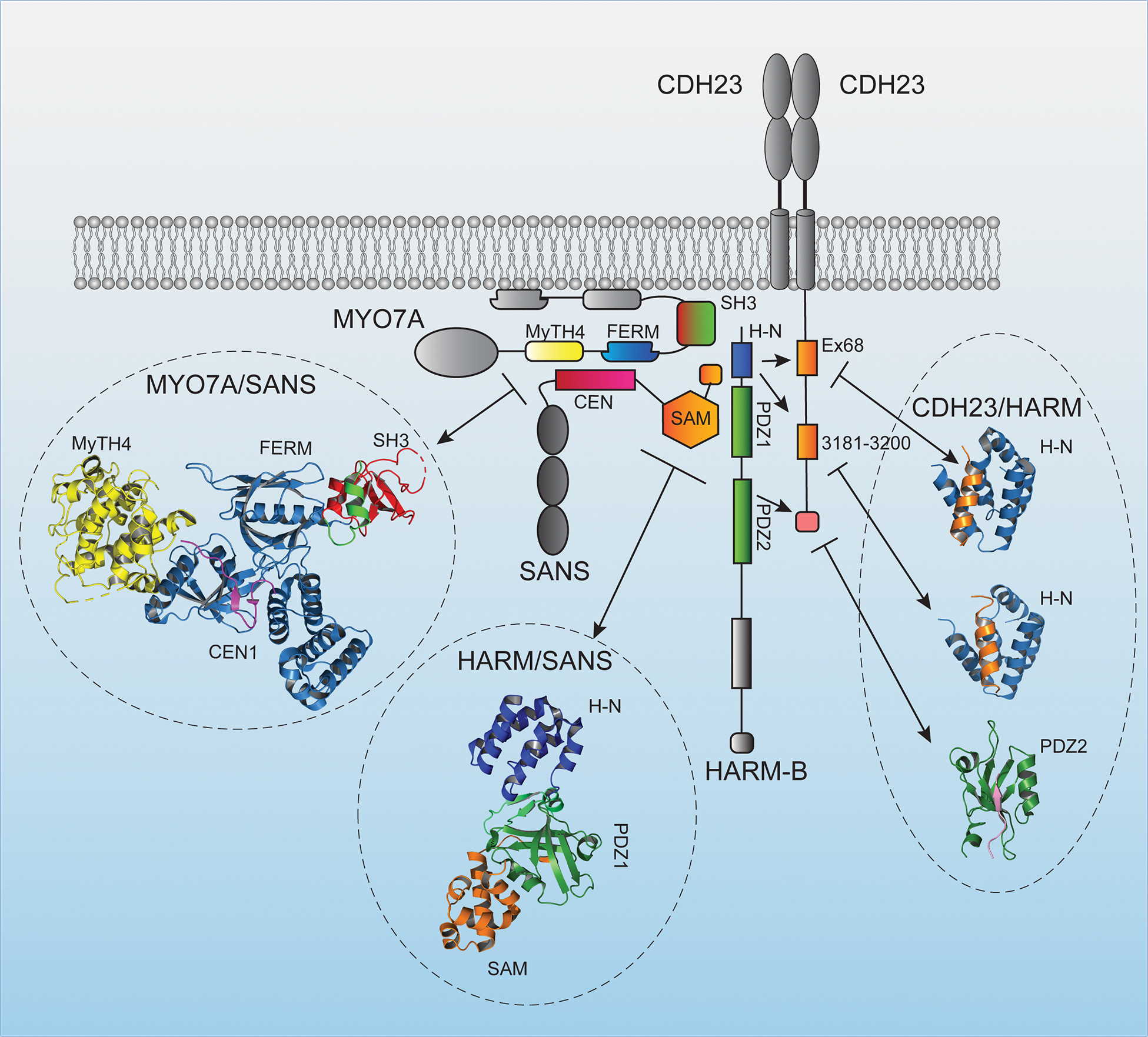
Diagram of proteins that are localized to the upper end of the tip link consisting of the adapter protein HARM-B, the ankyrin-domain containing protein SANS, and the motor protein MYO7A. Known protein-protein interaction domains are indicated. Protein domains in the diagram for which structures have been determined are color coded and the structures are shown (PDB:3PVL, 3K1R, 2LSR, 2KBR, 2KBS) (Pan et al., 2009; Wu et al., 2011; Wu et al., 2012; Yan et al., 2010). The structure of the interaction site between the N-terminus of CDH23 and HARM-B was omitted.
The importance of SANS, MYO7A and HARM-B for mechanotransduction has been investigated with the help of genetically modified mice. Deaf circler (dfcr) mice carry a mutation that prevents interaction of HARM-B with F-actin (Grillet et al., 2009; Johnson et al., 2003). These mice preserve their tip links but HARM-B is displaced from the upper end of tip links. Gating of transduction channels throughout the hair bundle is less well coordinated in the mutants, and transducer current activation and adaptation are slowed (Grillet et al., 2009). Similar observations have been made for dfcr2J mice, which entirely lack HARM-B (Michalski et al., 2009). The analysis of the function of SANS and MYO7A in mechanotransduction has been complex because null mutations in the two genes lead to defects in hair bundle development (Caberlotto et al., 2011; Self et al., 1998). However, MYO7A is expressed in several isoforms. The longest isoform MYO7A-C contains an extension of 11 amino acids at the N-terminus of a shorter MYO7A-S isoform (Li et al., 2020). Hair bundles maintain their normal shape in genetically modified mice expressing only the shorter MYO7A-S isoform, but MYO7A levels in IHC are diminished. Resting tension is reduced and there is a significant delay in current onset following mechanical stimulation (Li et al., 2020). These findings are consistent with a model where the protein complex at the upper end of tip links might provide a stable anchor point that keeps the tip link and transduction complex under tension.
The classical motor model of adaptation was developed based on studies in hair cells tuned to low frequencies (i.e bullfrogs and turtles). Although the activity of mechanotransduction channels in both mammalian and non-mammalian species is dependent on the intracellular and extracellular Ca2+ concentration, cochlear hair cells respond to much higher frequencies and recent findings suggest that fast adaptation predominates in cochlear hair cells and may be independent of Ca2+ (Caprara et al., 2019; Peng et al., 2013). While data by others do not necessarily support this conclusion (Beurg et al., 2015a; Corns et al., 2014), a recent study also has provided evidence that there is no strict correlation between Ca2+ entry into stereocilia and adaptation rate (Goldring et al., 2019).
Slow adaptation in cochlear hair cells is still observed and requires Ca2+ entry and myosin motor function, although the specific myosin appears not to be MYO1C (Caprara et al., 2020). In addition, channel open probability at rest and adaptation are regulated in cochlear hair cells by distinct mechanisms (Caprara et al., 2020; Liang et al., 2021; Peng et al., 2016). Peng and colleagues therefore proposed a revised motor model, which suggests that different myosin motors located at upper and lower tip-link insertions are required for tension generation and slow adaptation, respectively (Caprara et al., 2020). In this model, slow adaptation is not related to the modulation of tip-link tension, requires activity of myosin motors other than MYO1C and other modulators, such as PIP2. It is still unclear whether Ca2+ entry through the mechanotransduction channel at the lower end of the tip link can influence the proteins at the upper end of the tip link. Further studies will be necessary to test this model and to identify the molecules that regulate adaptation in cochlear hair cells.
Mechanotransduction: Beyond Processing Input Sound Stimuli
Exciting new evidence has revealed important functions for mechanotransduction channels in hair cells that go beyond roles in sensory transduction. New evidence suggests that active mechanotransduction channels in hair cells affect the fine structure of the hair bundle and the assembly of auditory circuits.
Mechanotransduction and hair bundle morphogenesis
The morphogenesis of the hair bundles is a complex process. Studies in chickens have defined four phases of bundle development. In stage I, postmitotic hair cells start to develop morphological specializations at their apical surface. In stage II, stereocilia start to form, elongate and develop a rudimentary staircase. In stage III, stereocilia widen, while in stage IV, stereocilia elongate to reach their mature length (Tilney et al., 1992). The development of hair bundles in rodents progresses through four stages that are very similar to those in chickens (Krey et al., 2020). Studies in mice have provided insights into the molecular machinery that shape the hair bundle. In rodents, mature hair bundles of cochlear hair cells contain three rows of stereocilia. A protein complex consisting of the short isoform of the motor protein MYO15A-S, the adapter protein WHRN, the actin regulatory proteins EPS8 and the planar cell polarity proteins GPSM2 and GNAI3 becomes stabilized at the tips of row 1 stereocilia, the longest stereocilia (Fig. 6A). Mutations in the murine genes encoding these proteins lead to abnormally short stereocilia indicative of a function for this protein complex in regulating elongation of the actin core of stereocilia (Belyantseva et al., 2005; Fang et al., 2015; Manor et al., 2011; Mauriac et al., 2017; Tadenev et al., 2019; Zampini et al., 2011). A different set of proteins including the long isoform of the molecular motor MYO15A-L, as well as the actin capping proteins CAPZB, TWF2 and EPS8L2 are concentrated in row 2 stereocilia (Fig. 6A) (Avenarius et al., 2017; Fang et al., 2015; Furness et al., 2013).
Figure 6. Mechanotransduction and hair cell development.
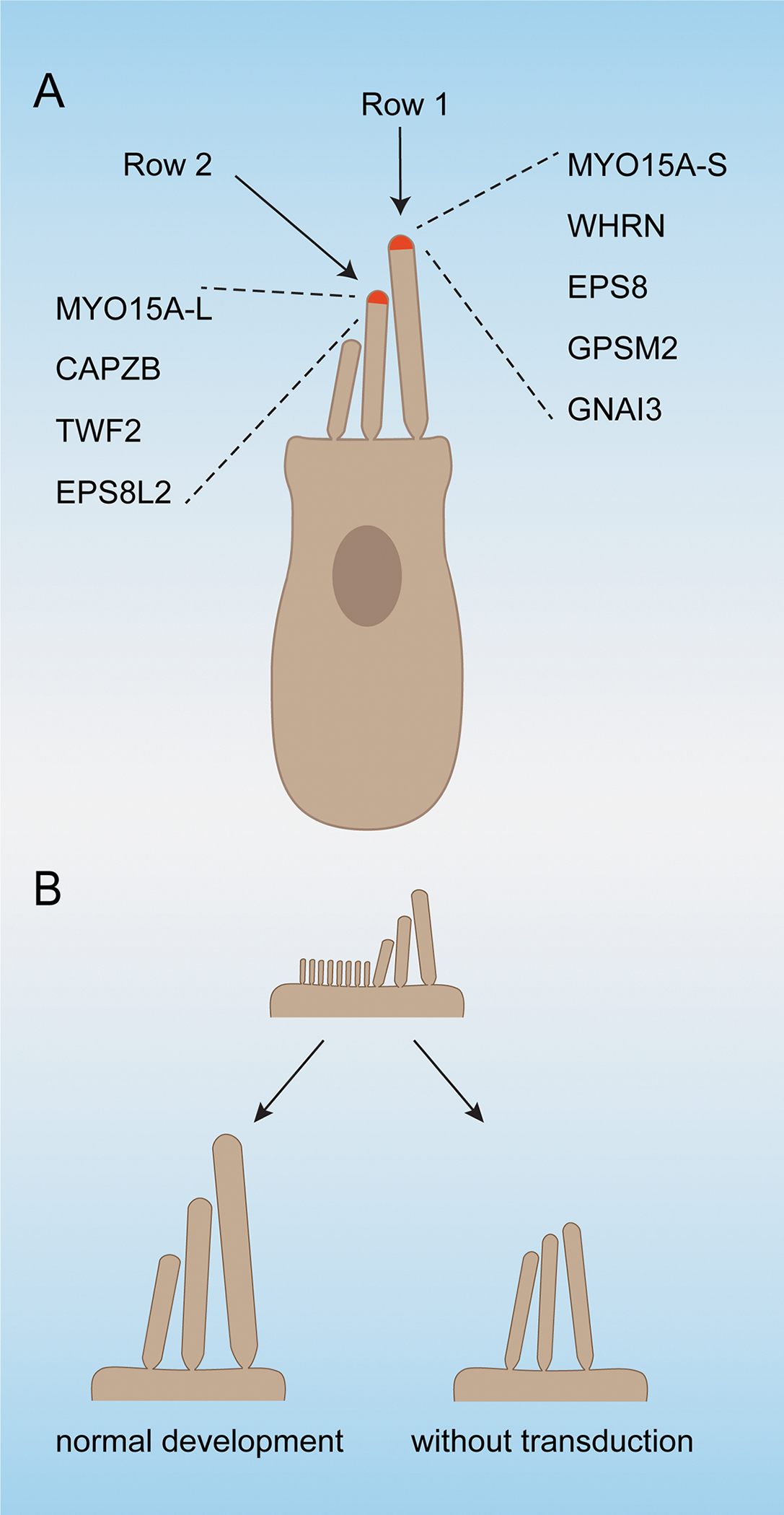
(A) Diagram of a hair cell indicating the protein complexes that are stabilized in row 1 and row 2 stereocilia and that are required for normal hair bundle development and/or maintenance. (B) Diagram of the shape of an immature hair bundle and how it matures in wild-type mice, and in mice with defects in mechanotransduction. In the absence of mechanotransduction, a hair bundles containing stereocilia of graded heights still develop, but the dimension of stereocilia is changes and the heights-gradient between stereocilia is reduced.
The mechanotransduction machinery of cochlear hair cells in rodents matures in the early postnatal phase coincident with the development of the stereociliary bundle (Lelli et al., 2009; Waguespack et al., 2007). These findings suggested a potential role for mechanotransduction in determining bundle shape, which could explain why hair bundles in mutant mice that affect the mechanotransduction machinery lose their typical V shape (Beurg et al., 2018; Giese et al., 2017). Tilney had already suggested that Ca2+ might be a driving force in regulating the morphogenesis of stereocilia (Tilney et al., 1992). This is a compelling concept, especially because the mechanotransduction channel of cochlear hair cells is permeant for Ca2+, which could regulate actin polymerization at stereocilia tips. Consistent with a role for mechanotransduction in hair bundle morphogenesis, mutations in the gene encoding SANS affect tip-link maintenance and mechanotransduction, as well as the length of stereocilia in rows 2 and 3, the transducing stereocilia (Caberlotto et al., 2011). Similarly, within 5 hours of treatment of inner ear organ cultures with mechanotransduction channel blockers, the tips of transducing stereocilia start to thin and they shorten over the next 24 hours. Shrinkage is reversible when the blockers are removed (Velez-Ortega et al., 2017).
To gain insights into the mechanisms by which mechanotransduction affects the shape of the hair bundle, a recent study has investigated how mechanotransduction affects distinct stages of stereocilia morphogenesis across the hair bundle. The findings demonstrated that mutations that affect mechanotransduction channels lead to changes in the widening of stereocilia, especially in row 2. In addition, while a stereocilia staircase still develops, the difference in stereocilia length across rows is less pronounced (Fig. 6B). These morphological changes are accompanied by molecular changes where stabilization of protein complexes typical for row 1 and row 2 no longer occurs. MYO15A-S, MYO15A-L as well as EPS8 and EPS8L2 are found at all stereociliary tips, rather than segregated to either row 1 or row 2 tips. By the third postnatal week, GNAI3, GPSM2, CABZB2 and TWF2 are no longer concentrated at stereociliary tips (Krey et al., 2020).
The mechanisms that link mechanotransduction defects to altered hair bundle morphology need further study. Hair bundle morphogenesis in mice progresses in the first week after birth, but mice only hear sound by about P12-P14 when the ear canal opens (Mikaelian and Ruben, 1965). Thus, mechanotransduction channels are not activated by incoming sound signals while hair bundles develop. However, mechanotransduction channels are not closed at rest leading to an influx of Ca2+ into the cells even in the absence of a sound stimulus (Beurg et al., 2010; Corns et al., 2014; Johnson et al., 2011; Peng et al., 2013). Influx of Ca2+ may locally regulates the function of proteins that are important for stereocilia growth. To explore the mechanisms by which mechanotransduction affects stereocilia morphology, it would be interesting to analyze in real time how acute block of mechanotransduction channels affects the distribution and movement of proteins within stereocilia. Some proteins of the mechanotransduction complex, such as the Ca2+ binding proteins CIB2, might also have direct roles in morphogenesis. This is consistent with the findings that hair bundles in Cib2 mutant mice develop significant morphological defects (Giese et al., 2017).
Mechanotransduction and auditory circuit development
SGNs are the sensory afferent neurons of the auditory systems. Type I SGNs constitute 95% of the total pool of SGNs and mono-synaptically innervate IHCs, where each IHC is contacted by 5–30 type I SGNs. Type II SGNs constitute the remaining 5% of the total pool of SGNs and innervate OHCs, where each type II SGNs branches out onto dozens of OHCs (Fig. 7A). Type I SGNs transmit sound information to the brain, while type II SGNs are thought to have a role in damage perception or pain signaling. Similar to hair cells, type I SGNs are tonotopically organized, where each SGN is most sensitive to a particular frequency that coincides with its innervation specificity on IHCs along the tonotopic axis of the cochlea (Appler and Goodrich, 2011; Sun et al., 2021).
Figure 7. Development of spiral ganglion neurons.
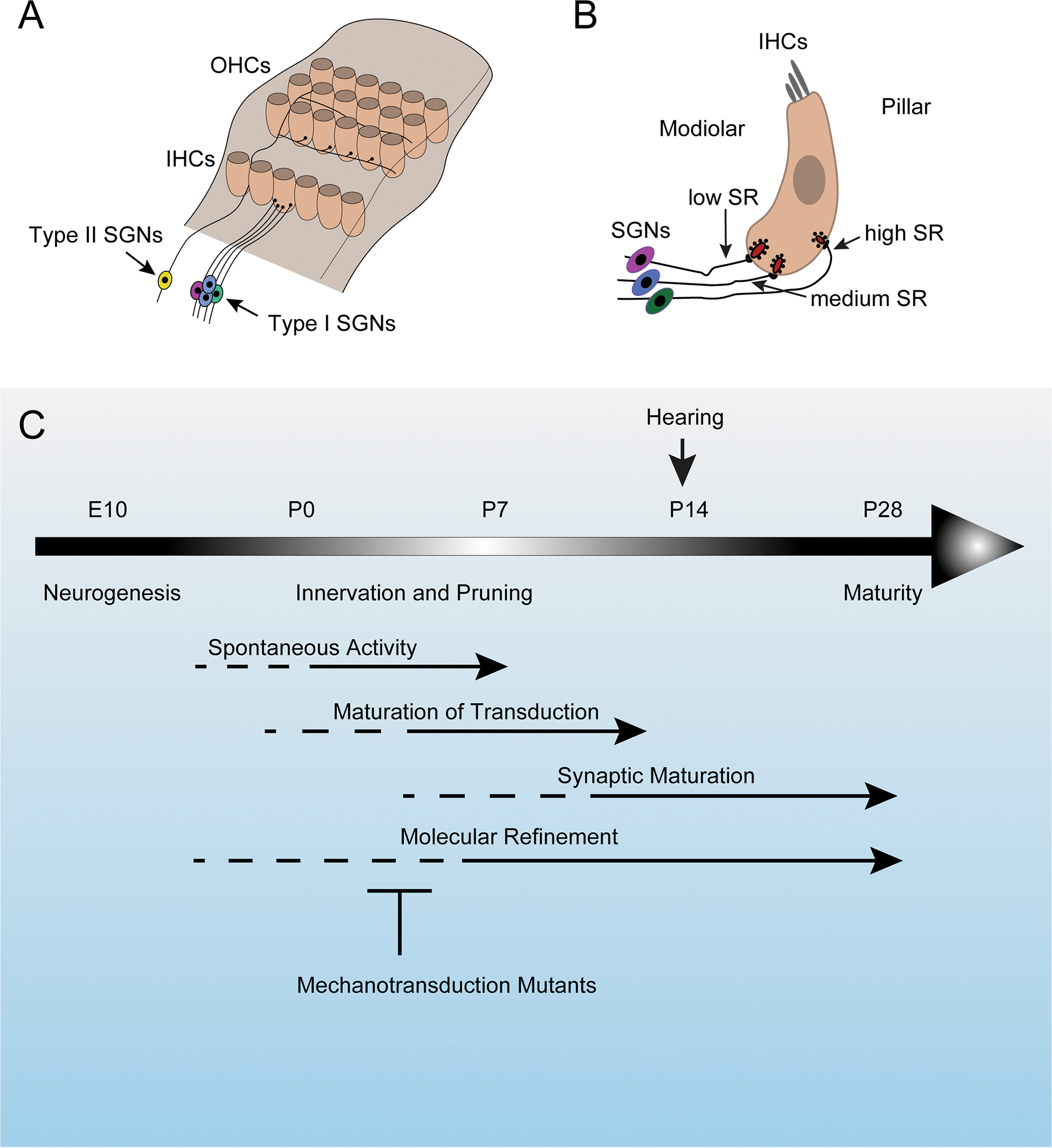
(A) Diagram of the auditory sensory epithelium highlighting type I SGNs and type II SGNs and their innervation specificity onto IHC and OHCs, respectively. (B) Diagram of an IHC showing the innervation specificity of type I SGNs with different spontaneous rates (SRs) along the modiolar-pillar axis of the hair cell. (C) Timeline of the development of SGNs highlighting key events. Mechanotransduction defects affect synaptic maturation and molecular refinement.
Type I SGNs innervating a single IHCs vary in their properties and innervation specificity onto IHCs. Type I SGNs fire spontaneous action potentials at varying rates. Based on differences in spontaneous rates (SRs) and sensitivity to sound, these neurons have been divided into high-SR, medium-SR and low-SR fibers, although in many species SR rates seem to show a more gradual increase from low to high that cannot so easily be fit into three groups (Heil and Peterson, 2015). In cats, low and high-SR fibers preferentially contact the modiolar and pillar side of IHCs, respectively (Liberman, 1982). Single rodent IHCs also appear to be innervated by SGNs with different SRs (Fig. 7B) (Liberman et al., 2011; Wu et al., 2016).
Single cell RNA sequencing studies have shown that type I SGNs are at birth molecularly diverse and that they further refine their molecular phenotype over the next few postnatal weeks to define three subclasses of type I SGNs (Petitpre et al., 2018; Shrestha et al., 2018; Sun et al., 2018). This refinement process is severely disrupted in mice carrying mutations in genes that encode components of the mechanotransduction machinery including Tmie, Lhfpl5 and Pcdh15 (Sun et al., 2018). The molecular refinement process coincides with the time frame when mechanotransduction currents can first be recorded from hair cells and when type I SGNs establish their innervation specificity by a process that involves the pruning of excessive nerve sprouts (Fig. 7C) (Sun et al., 2018). The findings suggest that signals from hair cells that depend on active mechanotransduction channels are critical to direct hair cell innervation by type I SGNs. The formation or maintenance of ribbon synapses is affected in Tmc1/2 mutant mice (Lee et al., 2021), further demonstrating the importance of mechanotransduction channels for patterning auditory afferents.
The mechanisms by which mechanotransduction channels affect the differentiation of SGNs is unclear. Hair cells do not receive sound-driven sensory input prior to P12-P14 when the ear canal opens (Mikaelian and Ruben, 1965). However, prior to the onset of hearing, waves of spontaneous activity that are initiated by supporting cells in the auditory sensory epithelium are propagated to SGNs and from there along the auditory pathway to the CNS (Fig. 7C). These waves of spontaneous activity depend on glutamate release from IHCs (Wang and Bergles, 2015). This release of glutamate depends on the vesicular glutamate transporter VGLUT3 that is expressed in IHCs (Seal et al., 2008). In mice with mutations in Vglut3, spontaneous activity can still be observed, but its pattern is changed (Babola et al., 2018). The spontaneous activity patterns of SGNs and the molecular diversification of type I SGN are also perturbed in Vglut3 and Tmie mutant mice (Sun et al., 2018), revealing an interesting interplay between mechanotransduction, spontaneous activity, and neuronal subtype specification. How mechanotransduction participate in this regulatory circuit remains to be established, but some hints have emerged. Prior to the onset of hearing, spontaneous activity is initiated by release of ATP from inner supporting cells. ATP release induces crenations in supporting cells (Wang and Bergles, 2015). Perhaps, these crenations activate mechanotransduction channels in hair cells, thus triggering glutamate release from hair cells to contribute to the propagation of spontaneous activity patterns to SGNs.
Challenges Ahead
The field of auditory mechanotransduction has made a leap forward. Core components of the mechanotransduction machinery have been identified, but we still have a limited understanding of their role within this protein complex. A significant stumbling block for the field has been the lack of a reconstituted system that allows to study ion channel function. Properties of the mechanotransduction channel have been inferred from the study of hair cells where single channel features are evaluated in the whole-cell patch configuration and not by directly patching on membrane segments containing the ion channel. These studies have led to the model that TMC1 and TMIE affect pore properties of the mechanotransduction channel. How Ca2+ regulates channel function is unclear, but CIB2 is a good candidate to mediate some effect of Ca2+ on this ion channel. The atomic structure of a TMC1-containing ion channel complex has been revealed in C. elegans, but the structure of the mammalian channel complex and how it couples to the tip link are not known. While candidate gating spring molecules have been identified, further studies are necessary to study the function of these molecules. Mechanisms of adaptation appear to differ between hair cells in different species and the molecular mechanisms of adaptation remain to be clarified. Finally, we lack an understanding of the mechanisms by which mechanical force leads to structural changes in the mechanotransduction channel that allow ion flux through the channel pore. The identification of core components of the mechanotransduction machinery of hair cells opens the door to resolve these questions. The demonstration that active mechanotransduction channels are required for the normal development of hair cells and SGNs opens up entirely new areas for investigation.
Acknowledgements
We thank members of the laboratory and Christopher L. Cunningham for comments. This work was supported by the NIH (RO1DC005965, RO1DC019514, RODC16960) and the David M. Rubenstein Fund for Hearing Research. U.M. is a Bloomberg Distinguished Professor for Neuroscience and Biology.
Footnotes
Declaration of Interests
Dr. Mueller is a co-founder of Decibel Therapeutics.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmed ZM, Goodyear R, Riazuddin S, Lagziel A, Legan PK, Behra M, Burgess SM, Lilley KS, Wilcox ER, Riazuddin S, et al. (2006). The tip-link antigen, a protein associated with the transduction complex of sensory hair cells, is protocadherin-15. J Neurosci 26, 7022–7034. 10.1523/JNEUROSCI.1163-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An WF, Bowlby MR, Betty M, Cao J, Ling HP, Mendoza G, Hinson JW, Mattsson KI, Strassle BW, Trimmer JS, and Rhodes KJ (2000). Modulation of A-type potassium channels by a family of calcium sensors. Nature 403, 553–556. 10.1038/35000592. [DOI] [PubMed] [Google Scholar]
- Appler JM, and Goodrich LV (2011). Connecting the ear to the brain: Molecular mechanisms of auditory circuit assembly. Prog Neurobiol 93, 488–508. 10.1016/j.pneurobio.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araya-Secchi R, Neel BL, and Sotomayor M (2016). An elastic element in the protocadherin-15 tip link of the inner ear. Nat Commun 7, 13458. 10.1038/ncomms13458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashmore JF (1987). A fast motile response in guinea-pig outer hair cells: the cellular basis of the cochlear amplifier. J Physiol 388, 323–347. 10.1113/jphysiol.1987.sp016617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assad JA, Shepherd GM, and Corey DP (1991). Tip-link integrity and mechanical transduction in vertebrate hair cells. Neuron 7, 985–994. 10.1016/0896-6273(91)90343-x. [DOI] [PubMed] [Google Scholar]
- Avan P, Le Gal S, Michel V, Dupont T, Hardelin JP, Petit C, and Verpy E (2019). Otogelin, otogelin-like, and stereocilin form links connecting outer hair cell stereocilia to each other and the tectorial membrane. Proc Natl Acad Sci U S A 116, 25948–25957. 10.1073/pnas.1902781116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avenarius MR, Krey JF, Dumont RA, Morgan CP, Benson CB, Vijayakumar S, Cunningham CL, Scheffer DI, Corey DP, Muller U, et al. (2017). Heterodimeric capping protein is required for stereocilia length and width regulation. J Cell Biol 216, 3861–3881. 10.1083/jcb.201704171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babola TA, Li S, Gribizis A, Lee BJ, Issa JB, Wang HC, Crair MC, and Bergles DE (2018). Homeostatic Control of Spontaneous Activity in the Developing Auditory System. Neuron 99, 511–524 e515. 10.1016/j.neuron.2018.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballesteros A, Fenollar-Ferrer C, and Swartz KJ (2018). Structural relationship between the putative hair cell mechanotransduction channel TMC1 and TMEM16 proteins. Elife 7. 10.7554/eLife.38433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barr-Gillespie PG (2015). Assembly of hair bundles, an amazing problem for cell biology. Mol Biol Cell 26, 2727–2732. 10.1091/mbc.E14-04-0940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartsch TF, Villasante CM, Hengel FE, Toure A, Firester DM, Oswald A, and Hudspeth AJ (2021). Measurement of hindered diffusion in complex geometries for high-speed studies of single-molecule forces. Sci Rep 11, 2196. 10.1038/s41598-021-81593-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu A, Lagier S, Vologodskaia M, Fabella BA, and Hudspeth AJ (2016). Direct mechanical stimulation of tip links in hair cells through DNA tethers. Elife 5. 10.7554/eLife.16041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavi N, Clark MD, Contreras GF, SHen R, Reddy BG, Milewski W, and Perozo E (2021). The conformational cycle of prestin underlies outer-hair cell electromotility. Nature 600, 553–558. [DOI] [PubMed] [Google Scholar]
- Belyantseva IA, Adler HJ, Curi R, Frolenkov GI, and Kachar B (2000). Expression and localization of prestin and the sugar transporter GLUT-5 during development of electromotility in cochlear outer hair cells. J Neurosci 20, RC116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belyantseva IA, Boger ET, Naz S, Frolenkov GI, Sellers JR, Ahmed ZM, Griffith AJ, and Friedman TB (2005). Myosin-XVa is required for tip localization of whirlin and differential elongation of hair-cell stereocilia. Nat Cell Biol 7, 148–156. 10.1038/ncb1219. [DOI] [PubMed] [Google Scholar]
- Beurg M, Barlow A, Furness DN, and Fettiplace R (2019). A Tmc1 mutation reduces calcium permeability and expression of mechanoelectrical transduction channels in cochlear hair cells. Proc Natl Acad Sci U S A 116, 20743–20749. 10.1073/pnas.1908058116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Cui R, Goldring AC, Ebrahim S, Fettiplace R, and Kachar B (2018). Variable number of TMC1-dependent mechanotransducer channels underlie tonotopic conductance gradients in the cochlea. Nat Commun 9, 2185. 10.1038/s41467-018-04589-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Evans MG, Hackney CM, and Fettiplace R (2006). A large-conductance calcium-selective mechanotransducer channel in mammalian cochlear hair cells. J Neurosci 26, 10992–11000. 10.1523/JNEUROSCI.2188-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Fettiplace R, Nam JH, and Ricci AJ (2009). Localization of inner hair cell mechanotransducer channels using high-speed calcium imaging. Nat Neurosci 12, 553–558. 10.1038/nn.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Goldring AC, and Fettiplace R (2015a). The effects of Tmc1 Beethoven mutation on mechanotransducer channel function in cochlear hair cells. J Gen Physiol 146, 233–243. 10.1085/jgp.201511458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, Chen Q, and Fettiplace R (2010). Calcium balance and mechanotransduction in rat cochlear hair cells. J Neurophysiol 104, 18–34. 10.1152/jn.00019.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Nam JH, and Fettiplace R (2021a). The speed of the hair cell mechanotransducer channel revealed by fluctuation analysis. J Gen Physiol 153. 10.1085/jgp.202112959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Schimmenti LA, Koleilat A, Amr SS, Oza A, Barlow AJ, Ballesteros A, and Fettiplace R (2021b). New Tmc1 Deafness Mutations Impact Mechanotransduction in Auditory Hair Cells. J Neurosci 41, 4378–4391. 10.1523/JNEUROSCI.2537-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beurg M, Xiong W, Zhao B, Muller U, and Fettiplace R (2015b). Subunit determination of the conductance of hair-cell mechanotransducer channels. Proc Natl Acad Sci U S A 112, 1589–1594. 10.1073/pnas.1420906112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, and Shapiro L (2002). C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science 296, 1308–1313. 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- Brownell WE, Bader CR, Bertrand D, and de Ribaupierre Y (1985). Evoked mechanical responses of isolated cochlear outer hair cells. Science 227, 194–196. 10.1126/science.3966153. [DOI] [PubMed] [Google Scholar]
- Burgoyne RD, Helassa N, McCue HV, and Haynes LP (2019). Calcium Sensors in Neuronal Function and Dysfunction. Cold Spring Harb Perspect Biol 11. 10.1101/cshperspect.a035154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butan C, Song Q, Bai JP, Tan WJT, Navaratnam D, and Santos-Sacchi J (2022). Single particle cryo-EM structure of the outer hair cell motor protein prestin. Nat Commun 13, 290. 10.1038/s41467-021-27915-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caberlotto E, Michel V, Foucher I, Bahloul A, Goodyear RJ, Pepermans E, Michalski N, Perfettini I, Alegria-Prevot O, Chardenoux S, et al. (2011). Usher type 1G protein sans is a critical component of the tip-link complex, a structure controlling actin polymerization in stereocilia. Proc Natl Acad Sci U S A 108, 5825–5830. 10.1073/pnas.1017114108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara GA, Mecca AA, and Peng AW (2020). Decades-old model of slow adaptation in sensory hair cells is not supported in mammals. Sci Adv 6, eabb4922. 10.1126/sciadv.abb4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caprara GA, Mecca AA, Wang Y, Ricci AJ, and Peng AW (2019). Hair Bundle Stimulation Mode Modifies Manifestations of Mechanotransduction Adaptation. J Neurosci 39, 9098–9106. 10.1523/JNEUROSCI.1408-19.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choudhary D, Narui Y, Neel BL, Wimalasena LN, Klanseck CF, De-la-Torre P, Chen C, Araya-Secchi R, Tamilselvan E, and Sotomayor M (2020). Structural determinants of protocadherin-15 mechanics and function in hearing and balance perception. Proc Natl Acad Sci U S A 117, 24837–24848. 10.1073/pnas.1920444117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corey DP, and Hudspeth AJ (1983). Kinetics of the receptor current in bullfrog saccular hair cells. J Neurosci 3, 962–976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns LF, Jeng JY, Richardson GP, Kros CJ, and Marcotti W (2017). TMC2 Modifies Permeation Properties of the Mechanoelectrical Transducer Channel in Early Postnatal Mouse Cochlear Outer Hair Cells. Front Mol Neurosci 10, 326. 10.3389/fnmol.2017.00326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corns LF, Johnson SL, Kros CJ, and Marcotti W (2014). Calcium entry into stereocilia drives adaptation of the mechanoelectrical transducer current of mammalian cochlear hair cells. Proc Natl Acad Sci U S A 111, 14918–14923. 10.1073/pnas.1409920111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, Evans MG, and Fettiplace R (1989). Activation and adaptation of transducer currents in turtle hair cells. J Physiol 419, 405–434. 10.1113/jphysiol.1989.sp017878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford AC, and Fettiplace R (1985). The mechanical properties of ciliary bundles of turtle cochlear hair cells. J Physiol 364, 359–379. 10.1113/jphysiol.1985.sp015750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham CL, Qiu X, Wu Z, Zhao B, Peng G, Kim YH, Lauer A, and Muller U (2020). TMIE Defines Pore and Gating Properties of the Mechanotransduction Channel of Mammalian Cochlear Hair Cells. Neuron 107, 126–143 e128. 10.1016/j.neuron.2020.03.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D, O.M., and Ricci AJ (2019). A Bundle of Mechanisms: Inner-Ear Hair-Cell Mechanotransduction. Trends Neurosci 42, 221–236. 10.1016/j.tins.2018.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale P, Augustine GJ, Fitzpatrick D, Katz LC, La Mantia A-S, McNamara JO, and Williams SM (2001). The Audible Spectrum. Neuroscience (Oxfore University Press, Sinauer Associates). [Google Scholar]
- De-la-Torre P, Choudhary D, Araya-Secchi R, Narui Y, and Sotomayor M (2018). A Mechanically Weak Extracellular Membrane-Adjacent Domain Induces Dimerization of Protocadherin-15. Biophys J 115, 2368–2385. 10.1016/j.bpj.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dionne G, Qiu X, Rapp M, Liang X, Zhao B, Peng G, Katsamba PS, Ahlsen G, Rubinstein R, Potter CS, et al. (2018). Mechanotransduction by PCDH15 Relies on a Novel cis-Dimeric Architecture. Neuron 99, 480–492 e485. 10.1016/j.neuron.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doll JC, Peng AW, Ricci AJ, and Pruitt BL (2012). Faster than the speed of hearing: nanomechanical force probes enable the electromechanical observation of cochlear hair cells. Nano Lett 12, 6107–6111. 10.1021/nl3036349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Effertz T, Becker L, Peng AW, and Ricci AJ (2017). Phosphoinositol-4,5-Bisphosphate Regulates Auditory Hair-Cell Mechanotransduction-Channel Pore Properties and Fast Adaptation. J Neurosci 37, 11632–11646. 10.1523/JNEUROSCI.1351-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elledge HM, Kazmierczak P, Clark P, Joseph JS, Kolatkar A, Kuhn P, and Muller U (2010). Structure of the N terminus of cadherin 23 reveals a new adhesion mechanism for a subset of cadherin superfamily members. Proc Natl Acad Sci U S A 107, 10708–10712. 10.1073/pnas.1006284107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Q, Indzhykulian AA, Mustapha M, Riordan GP, Dolan DF, Friedman TB, Belyantseva IA, Frolenkov GI, Camper SA, and Bird JE (2015). The 133-kDa N-terminal domain enables myosin 15 to maintain mechanotransducing stereocilia and is essential for hearing. Elife 4. 10.7554/eLife.08627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fettiplace R (2017). Hair Cell Transduction, Tuning, and Synaptic Transmission in the Mammalian Cochlea. Compr Physiol 7, 1197–1227. 10.1002/cphy.c160049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness DN, Johnson SL, Manor U, Ruttiger L, Tocchetti A, Offenhauser N, Olt J, Goodyear RJ, Vijayakumar S, Dai Y, et al. (2013). Progressive hearing loss and gradual deterioration of sensory hair bundles in the ears of mice lacking the actin-binding protein Eps8L2. Proc Natl Acad Sci U S A 110, 13898–13903. 10.1073/pnas.1304644110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Elferich J, Dehghani-Ghahnaviyeh S, Zhao Z, Meadows M, von Gersdorff H, Tajkhorshid E, and Gouaux E (2021). Molecular mechanism of prestin electromotive signal amplification. Cell 184, 4669–4679 e4613. 10.1016/j.cell.2021.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge J, Elferich J, Goehring A, Zhao H, Schuck P, and Gouaux E (2018). Structure of mouse protocadherin 15 of the stereocilia tip link in complex with LHFPL5. Elife 7. 10.7554/eLife.38770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentry HR, Singer AU, Betts L, Yang C, Ferrara JD, Sondek J, and Parise LV (2005). Structural and biochemical characterization of CIB1 delineates a new family of EF-hand-containing proteins. J Biol Chem 280, 8407–8415. 10.1074/jbc.M411515200. [DOI] [PubMed] [Google Scholar]
- George SS, Steele CR, and Ricci AJ (2020). Rat Auditory Inner Hair Cell Mechanotransduction and Stereociliary Membrane Diffusivity Are Similarly Modulated by Calcium. iScience 23, 101773. 10.1016/j.isci.2020.101773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giese APJ, Tang YQ, Sinha GP, Bowl MR, Goldring AC, Parker A, Freeman MJ, Brown SDM, Riazuddin S, Fettiplace R, et al. (2017). CIB2 interacts with TMC1 and TMC2 and is essential for mechanotransduction in auditory hair cells. Nat Commun 8, 43. 10.1038/s41467-017-00061-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie PG, and Cyr JL (2004). Myosin-1c, the hair cell’s adaptation motor. Annu Rev Physiol 66, 521–545. 10.1146/annurev.physiol.66.032102.112842. [DOI] [PubMed] [Google Scholar]
- Gillespie PG, and Muller U (2009). Mechanotransduction by hair cells: models, molecules, and mechanisms. Cell 139, 33–44. 10.1016/j.cell.2009.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldring AC, Beurg M, and Fettiplace R (2019). The contribution of TMC1 to adaptation of mechanoelectrical transduction channels in cochlear outer hair cells. J Physiol 597, 5949–5961. 10.1113/JP278799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grati M, and Kachar B (2011). Myosin VIIa and sans localization at stereocilia upper tip-link density implicates these Usher syndrome proteins in mechanotransduction. Proc Natl Acad Sci U S A 108, 11476–11481. 10.1073/pnas.1104161108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillet N, Xiong W, Reynolds A, Kazmierczak P, Sato T, Lillo C, Dumont RA, Hintermann E, Sczaniecka A, Schwander M, et al. (2009). Harmonin mutations cause mechanotransduction defects in cochlear hair cells. Neuron 62, 375–387. 10.1016/j.neuron.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S, Knowland D, Matta JA, O’Carroll ML, Davini WB, Dhara M, Kweon HJ, and Bredt DS (2020). Hair cell alpha9alpha10 nicotinic acetylcholine receptor functional expression regulated by ligand binding and deafness gene products. Proc Natl Acad Sci U S A 117, 24534–24544. 10.1073/pnas.2013762117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn Y, Kim DS, Pastan IH, and Lee B (2009). Anoctamin and transmembrane channel-like proteins are evolutionarily related. Int J Mol Med 24, 51–55. 10.3892/ijmm_00000205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hakizimana P, and Fridberger A (2021). Inner hair cell stereocilia are embedded in the tectorial membrane. Nat Commun 12, 2604. 10.1038/s41467-021-22870-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussinger D, Ahrens T, Aberle T, Engel J, Stetefeld J, and Grzesiek S (2004). Proteolytic E-cadherin activation followed by solution NMR and X-ray crystallography. EMBO J 23, 1699–1708. 10.1038/sj.emboj.7600192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He DZ, Jia S, and Dallos P (2004). Mechanoelectrical transduction of adult outer hair cells studied in a gerbil hemicochlea. Nature 429, 766–770. 10.1038/nature02591. [DOI] [PubMed] [Google Scholar]
- Heil P, and Peterson AJ (2015). Basic response properties of auditory nerve fibers: a review. Cell Tissue Res 361, 129–158. 10.1007/s00441-015-2177-9. [DOI] [PubMed] [Google Scholar]
- Hirono M, Denis CS, Richardson GP, and Gillespie PG (2004). Hair cells require phosphatidylinositol 4,5-bisphosphate for mechanical transduction and adaptation. Neuron 44, 309–320. 10.1016/j.neuron.2004.09.020. [DOI] [PubMed] [Google Scholar]
- Holt JR, Gillespie SK, Provance DW, Shah K, Shokat KM, Corey DP, Mercer JA, and Gillespie PG (2002). A chemical-genetic strategy implicates myosin-1c in adaptation by hair cells. Cell 108, 371–381. 10.1016/s0092-8674(02)00629-3. [DOI] [PubMed] [Google Scholar]
- Hopp SL, Owren MJ, and Evans CS (1998). Acoustic Communication. (Berlin, Heidelberg: Springer; ). [Google Scholar]
- Howard J, and Hudspeth AJ (1988). Compliance of the hair bundle associated with gating of mechanoelectrical transduction channels in the bullfrog’s saccular hair cell. Neuron 1, 189–199. 10.1016/0896-6273(88)90139-0. [DOI] [PubMed] [Google Scholar]
- Hudspeth AJ (1983). The hair cells of the inner ear. They are exquisitely sensitive transducers that in human beings mediate the senses of hearing and balance. A tiny force applied to the top of the cell produces an electrical signal at the bottom. Sci Am 248, 54–64. [PubMed] [Google Scholar]
- Hudspeth AJ, and Corey DP (1977). Sensitivity, polarity, and conductance change in the response of vertebrate hair cells to controlled mechanical stimuli. Proc Natl Acad Sci U S A 74, 2407–2411. 10.1073/pnas.74.6.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson AC, and Nicoll RA (2011). The expanding social network of ionotropic glutamate receptors: TARPs and other transmembrane auxiliary subunits. Neuron 70, 178–199. 10.1016/j.neuron.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaiganesh A, De-la-Torre P, Patel AA, Termine DJ, Velez-Cortes F, Chen C, and Sotomayor M (2018). Zooming in on Cadherin-23: Structural Diversity and Potential Mechanisms of Inherited Deafness. Structure 26, 1210–1225 e1214. 10.1016/j.str.2018.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong H, Clark S, Goehring A, Dehghani-Ghahnaviyeh S, Rasouli A, Tajkhorshid E, and Gouaux E (2022). Structure of C. elegans TMC-1 complex illuminates auditory mechanosensory transduction. BioRxiv. 10.1101/2022.05.06.490478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S, Dallos P, and He DZ (2007). Mechanoelectric transduction of adult inner hair cells. J Neurosci 27, 1006–1014. 10.1523/JNEUROSCI.5452-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia Y, Zhao Y, Kusakizako T, Wang Y, Pan C, Zhang Y, Nureki O, Hattori M, and Yan Z (2020). TMC1 and TMC2 Proteins Are Pore-Forming Subunits of Mechanosensitive Ion Channels. Neuron 105, 310–321 e313. 10.1016/j.neuron.2019.10.017. [DOI] [PubMed] [Google Scholar]
- Jin P, Bulkley D, Guo Y, Zhang W, Guo Z, Huynh W, Wu S, Meltzer S, Cheng T, Jan LY, et al. (2017). Electron cryo-microscopy structure of the mechanotransduction channel NOMPC. Nature 547, 118–122. 10.1038/nature22981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin P, Jan LY, and Jan YN (2020). Mechanosensitive Ion Channels: Structural Features Relevant to Mechanotransduction Mechanisms. Annu Rev Neurosci 43, 207–229. 10.1146/annurev-neuro-070918-050509. [DOI] [PubMed] [Google Scholar]
- Johnson KR, Gagnon LH, Webb LS, Peters LL, Hawes NL, Chang B, and Zheng QY (2003). Mouse models of USH1C and DFNB18: phenotypic and molecular analyses of two new spontaneous mutations of the Ush1c gene. Hum Mol Genet 12, 3075–3086. 10.1093/hmg/ddg332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SL, Beurg M, Marcotti W, and Fettiplace R (2011). Prestin-driven cochlear amplification is not limited by the outer hair cell membrane time constant. Neuron 70, 1143–1154. 10.1016/j.neuron.2011.04.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kachar B, Brownell WE, Altschuler R, and Fex J (1986). Electrokinetic shape changes of cochlear outer hair cells. Nature 322, 365–368. 10.1038/322365a0. [DOI] [PubMed] [Google Scholar]
- Kachar B, Parakkal M, Kurc M, Zhao Y, and Gillespie PG (2000). High-resolution structure of hair-cell tip links. Proc Natl Acad Sci U S A 97, 13336–13341. 10.1073/pnas.97.24.13336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karavitaki KD, and Corey DP (2010). Sliding adhesion confers coherent motion to hair cell stereocilia and parallel gating to transduction channels. J Neurosci 30, 9051–9063. 10.1523/JNEUROSCI.4864-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawashima Y, Geleoc GS, Kurima K, Labay V, Lelli A, Asai Y, Makishima T, Wu DK, Della Santina CC, Holt JR, and Griffith AJ (2011). Mechanotransduction in mouse inner ear hair cells requires transmembrane channel-like genes. J Clin Invest 121, 4796–4809. 10.1172/JCI60405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, and Muller U (2012). Sensing sound: molecules that orchestrate mechanotransduction by hair cells. Trends Neurosci 35, 220–229. 10.1016/j.tins.2011.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazmierczak P, Sakaguchi H, Tokita J, Wilson-Kubalek EM, Milligan RA, Muller U, and Kachar B (2007). Cadherin 23 and protocadherin 15 interact to form tip-link filaments in sensory hair cells. Nature 449, 87–91. 10.1038/nature06091. [DOI] [PubMed] [Google Scholar]
- Kefauver JM, Ward AB, and Patapoutian A (2020). Discoveries in structure and physiology of mechanically activated ion channels. Nature 587, 567–576. 10.1038/s41586-020-2933-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KX, and Fettiplace R (2013). Developmental changes in the cochlear hair cell mechanotransducer channel and their regulation by transmembrane channel-like proteins. J Gen Physiol 141, 141–148. 10.1085/jgp.201210913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kise Y, Kasuya G, Okamoto HH, Yamanouchi D, Kobayashi K, Kusakizako T, Nishizawa T, Nakajo K, and Nureki O (2021). Structural basis of gating modulation of Kv4 channel complexes. Nature 599, 158–164. 10.1038/s41586-021-03935-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozlov AS, Risler T, and Hudspeth AJ (2007). Coherent motion of stereocilia assures the concerted gating of hair-cell transduction channels. Nat Neurosci 10, 87–92. 10.1038/nn1818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krey JF, Chatterjee P, Dumont RA, O’Sullivan M, Choi D, Bird JE, and Barr-Gillespie PG (2020). Mechanotransduction-Dependent Control of Stereocilia Dimensions and Row Identity in Inner Hair Cells. Curr Biol 30, 442–454 e447. 10.1016/j.cub.2019.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima K, Ebrahim S, Pan B, Sedlacek M, Sengupta P, Millis BA, Cui R, Nakanishi H, Fujikawa T, Kawashima Y, et al. (2015). TMC1 and TMC2 Localize at the Site of Mechanotransduction in Mammalian Inner Ear Hair Cell Stereocilia. Cell Rep 12, 1606–1617. 10.1016/j.celrep.2015.07.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurima K, Peters LM, Yang Y, Riazuddin S, Ahmed ZM, Naz S, Arnaud D, Drury S, Mo J, Makishima T, et al. (2002). Dominant and recessive deafness caused by mutations of a novel gene, TMC1, required for cochlear hair-cell function. Nat Genet 30, 277–284. 10.1038/ng842. [DOI] [PubMed] [Google Scholar]
- Lee G, Abdi K, Jiang Y, Michaely P, Bennett V, and Marszalek PE (2006). Nanospring behaviour of ankyrin repeats. Nature 440, 246–249. 10.1038/nature04437. [DOI] [PubMed] [Google Scholar]
- Lee J, Kawai K, Holt JR, and Geleoc GS (2021). Sensory transduction is required for normal development and maturation of cochlear inner hair cell synapses. Elife 10. 10.7554/eLife.69433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lelli A, Asai Y, Forge A, Holt JR, and Geleoc GS (2009). Tonotopic gradient in the developmental acquisition of sensory transduction in outer hair cells of the mouse cochlea. J Neurophysiol 101, 2961–2973. 10.1152/jn.00136.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Mecca A, Kim J, Caprara GA, Wagner EL, Du TT, Petrov L, Xu W, Cui R, Rebustini IT, et al. (2020). Myosin-VIIa is expressed in multiple isoforms and essential for tensioning the hair cell mechanotransduction complex. Nat Commun 11, 2066. 10.1038/s41467-020-15936-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Yu X, Chen X, Liu Z, Wang G, Li C, Wong EYM, Sham MH, Tang J, He J, et al. (2019). Localization of TMC1 and LHFPL5 in auditory hair cells in neonatal and adult mice. FASEB J 33, 6838–6851. 10.1096/fj.201802155RR. [DOI] [PubMed] [Google Scholar]
- Liang X, Qiu X, Dionne G, Cunningham CL, Pucak ML, Peng G, Kim YH, Lauer A, Shapiro L, and Muller U (2021). CIB2 and CIB3 are auxiliary subunits of the mechanotransduction channel of hair cells. Neuron 109, 2131–2149 e2115. 10.1016/j.neuron.2021.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman LD, Wang H, and Liberman MC (2011). Opposing gradients of ribbon size and AMPA receptor expression underlie sensitivity differences among cochlear-nerve/hair-cell synapses. J Neurosci 31, 801–808. 10.1523/JNEUROSCI.3389-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liberman MC (1982). Single-neuron labeling in the cat auditory nerve. Science 216, 1239–1241. 10.1126/science.7079757. [DOI] [PubMed] [Google Scholar]
- Liu S, Wang S, Zou L, Li J, Song C, Chen J, Hu Q, Liu L, Huang P, and Xiong W (2019). TMC1 is an essential component of a leak channel that modulates tonotopy and excitability of auditory hair cells in mice. Elife 8. 10.7554/eLife.47441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longo-Guess CM, Gagnon LH, Cook SA, Wu J, Zheng QY, and Johnson KR (2005). A missense mutation in the previously undescribed gene Tmhs underlies deafness in hurry-scurry (hscy) mice. Proc Natl Acad Sci U S A 102, 7894–7899. 10.1073/pnas.0500760102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda R, Kindt KS, Mo W, Morgan CP, Erickson T, Zhao H, Clemens-Grisham R, Barr-Gillespie PG, and Nicolson T (2014). Tip-link protein protocadherin 15 interacts with transmembrane channel-like proteins TMC1 and TMC2. Proc Natl Acad Sci U S A 111, 12907–12912. 10.1073/pnas.1402152111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahendrasingam S, Fettiplace R, Alagramam KN, Cross E, and Furness DN (2017). Spatiotemporal changes in the distribution of LHFPL5 in mice cochlear hair bundles during development and in the absence of PCDH15. PLoS One 12, e0185285. 10.1371/journal.pone.0185285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manor U, Disanza A, Grati M, Andrade L, Lin H, Di Fiore PP, Scita G, and Kachar B (2011). Regulation of stereocilia length by myosin XVa and whirlin depends on the actin-regulatory protein Eps8. Curr Biol 21, 167–172. 10.1016/j.cub.2010.12.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markin VS, and Hudspeth AJ (1995). Gating-spring models of mechanoelectrical transduction by hair cells of the internal ear. Annu Rev Biophys Biomol Struct 24, 59–83. 10.1146/annurev.bb.24.060195.000423. [DOI] [PubMed] [Google Scholar]
- Mauriac SA, Hien YE, Bird JE, Carvalho SD, Peyroutou R, Lee SC, Moreau MM, Blanc JM, Geyser A, Medina C, et al. (2017). Defective Gpsm2/Galphai3 signalling disrupts stereocilia development and growth cone actin dynamics in Chudley-McCullough syndrome. Nat Commun 8, 14907. 10.1038/ncomms14907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecca AA, Caprara GA, and Peng AW (2022). cAMP and voltage modulate rat auditory mechanotransduction by decreasing the stiffness of gating springs. Proc Natl Acad Sci U S A 119, e2107567119. 10.1073/pnas.2107567119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalski N, Michel V, Caberlotto E, Lefevre GM, van Aken AF, Tinevez JY, Bizard E, Houbron C, Weil D, Hardelin JP, et al. (2009). Harmonin-b, an actin-binding scaffold protein, is involved in the adaptation of mechanoelectrical transduction by sensory hair cells. Pflugers Arch 459, 115–130. 10.1007/s00424-009-0711-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel V, Booth KT, Patni P, Cortese M, Azaiez H, Bahloul A, Kahrizi K, Labbe M, Emptoz A, Lelli A, et al. (2017). CIB2, defective in isolated deafness, is key for auditory hair cell mechanotransduction and survival. EMBO Mol Med 9, 1711–1731. 10.15252/emmm.201708087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikaelian D, and Ruben RJ (1965). Development of hearing in the normal Cba-J mouse: correlation of physiological observations with behavioral responses and with cochlear anatomy. Acta Otolaryngol 59, 451–461. [DOI] [PubMed] [Google Scholar]
- Mitchem KL, Hibbard E, Beyer LA, Bosom K, Dootz GA, Dolan DF, Johnson KR, Raphael Y, and Kohrman DC (2002). Mutation of the novel gene Tmie results in sensory cell defects in the inner ear of spinner, a mouse model of human hearing loss DFNB6. Hum Mol Genet 11, 1887–1898. 10.1093/hmg/11.16.1887. [DOI] [PubMed] [Google Scholar]
- Mulhall EM, Ward A, Yang D, Koussa MA, Corey DP, and Wong WP (2021). Single-molecule force spectroscopy reveals the dynamic strength of the hair-cell tip-link connection. Nat Commun 12, 849. 10.1038/s41467-021-21033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murthy SE, Dubin AE, Whitwam T, Jojoa-Cruz S, Cahalan SM, Mousavi SAR, Ward AB, and Patapoutian A (2018). OSCA/TMEM63 are an Evolutionarily Conserved Family of Mechanically Activated Ion Channels. Elife 7. 10.7554/eLife.41844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nam JH, Peng AW, and Ricci AJ (2015). Underestimated sensitivity of mammalian cochlear hair cells due to splay between stereociliary columns. Biophys J 108, 2633–2647. 10.1016/j.bpj.2015.04.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narui Y, and Sotomayor M (2018). Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15. Biochemistry 57, 1702–1710. 10.1021/acs.biochem.7b01075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naz S, Giguere CM, Kohrman DC, Mitchem KL, Riazuddin S, Morell RJ, Ramesh A, Srisailpathy S, Deshmukh D, Riazuddin S, et al. (2002). Mutations in a novel gene, TMIE, are associated with hearing loss linked to the DFNB6 locus. Am J Hum Genet 71, 632–636. 10.1086/342193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohmori H (1985). Mechano-electrical transduction currents in isolated vestibular hair cells of the chick. J Physiol 359, 189–217. 10.1113/jphysiol.1985.sp015581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oroz J, Galera-Prat A, Hervas R, Valbuena A, Fernandez-Bravo D, and Carrion-Vazquez M (2019). Nanomechanics of tip-link cadherins. Sci Rep 9, 13306. 10.1038/s41598-019-49518-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pacentine IV, and Nicolson T (2019). Subunits of the mechano-electrical transduction channel, Tmc1/2b, require Tmie to localize in zebrafish sensory hair cells. PLoS Genet 15, e1007635. 10.1371/journal.pgen.1007635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Akyuz N, Liu XP, Asai Y, Nist-Lund C, Kurima K, Derfler BH, Gyorgy B, Limapichat W, Walujkar S, et al. (2018). TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells. Neuron 99, 736–753 e736. 10.1016/j.neuron.2018.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, Geleoc GS, Asai Y, Horwitz GC, Kurima K, Ishikawa K, Kawashima Y, Griffith AJ, and Holt JR (2013). TMC1 and TMC2 are components of the mechanotransduction channel in hair cells of the mammalian inner ear. Neuron 79, 504–515. 10.1016/j.neuron.2013.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Yan J, Wu L, and Zhang M (2009). Assembling stable hair cell tip link complex via multidentate interactions between harmonin and cadherin 23. Proc Natl Acad Sci U S A 106, 5575–5580. 10.1073/pnas.0901819106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SD, Ciatto C, Chen CP, Bahna F, Rajebhosale M, Arkus N, Schieren I, Jessell TM, Honig B, Price SR, and Shapiro L (2006). Type II cadherin ectodomain structures: implications for classical cadherin specificity. Cell 124, 1255–1268. 10.1016/j.cell.2005.12.046. [DOI] [PubMed] [Google Scholar]
- Peng AW, Effertz T, and Ricci AJ (2013). Adaptation of mammalian auditory hair cell mechanotransduction is independent of calcium entry. Neuron 80, 960–972. 10.1016/j.neuron.2013.08.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng AW, Gnanasambandam R, Sachs F, and Ricci AJ (2016). Adaptation Independent Modulation of Auditory Hair Cell Mechanotransduction Channel Open Probability Implicates a Role for the Lipid Bilayer. J Neurosci 36, 2945–2956. 10.1523/JNEUROSCI.3011-15.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petitpre C, Wu H, Sharma A, Tokarska A, Fontanet P, Wang Y, Helmbacher F, Yackle K, Silberberg G, Hadjab S, and Lallemend F (2018). Neuronal heterogeneity and stereotyped connectivity in the auditory afferent system. Nat Commun 9, 3691. 10.1038/s41467-018-06033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pickles JO, Comis SD, and Osborne MP (1984). Cross-links between stereocilia in the guinea pig organ of Corti, and their possible relation to sensory transduction. Hear Res 15, 103–112. 10.1016/0378-5955(84)90041-8. [DOI] [PubMed] [Google Scholar]
- Pioletti M, Findeisen F, Hura GL, and Minor DL Jr. (2006). Three-dimensional structure of the KChIP1-Kv4.3 T1 complex reveals a cross-shaped octamer. Nat Struct Mol Biol 13, 987–995. 10.1038/nsmb1164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powers RE, Gaudet R, and Sotomayor M (2017). A Partial Calcium-Free Linker Confers Flexibility to Inner-Ear Protocadherin-15. Structure 25, 482–495. 10.1016/j.str.2017.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riazuddin S, Belyantseva IA, Giese AP, Lee K, Indzhykulian AA, Nandamuri SP, Yousaf R, Sinha GP, Lee S, Terrell D, et al. (2012). Alterations of the CIB2 calcium- and integrin-binding protein cause Usher syndrome type 1J and nonsyndromic deafness DFNB48. Nat Genet 44, 1265–1271. 10.1038/ng.2426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci A (2002). Differences in mechano-transducer channel kinetics underlie tonotopic distribution of fast adaptation in auditory hair cells. J Neurophysiol 87, 1738–1748. 10.1152/jn.00574.2001. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, Crawford AC, and Fettiplace R (2003). Tonotopic variation in the conductance of the hair cell mechanotransducer channel. Neuron 40, 983–990. 10.1016/s0896-6273(03)00721-9. [DOI] [PubMed] [Google Scholar]
- Ricci AJ, and Fettiplace R (1997). The effects of calcium buffering and cyclic AMP on mechano-electrical transduction in turtle auditory hair cells. J Physiol 501 (Pt 1), 111–124. 10.1111/j.1469-7793.1997.111bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, and Fettiplace R (1998). Calcium permeation of the turtle hair cell mechanotransducer channel and its relation to the composition of endolymph. J Physiol 506 (Pt 1), 159–173. 10.1111/j.1469-7793.1998.159bx.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci AJ, Kennedy HJ, Crawford AC, and Fettiplace R (2005). The transduction channel filter in auditory hair cells. J Neurosci 25, 7831–7839. 10.1523/JNEUROSCI.1127-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell IJ, and Sellick PM (1983). Low-frequency characteristics of intracellularly recorded receptor potentials in guinea-pig cochlear hair cells. J Physiol 338, 179–206. 10.1113/jphysiol.1983.sp014668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider ME, Dose AC, Salles FT, Chang W, Erickson FL, Burnside B, and Kachar B (2006). A new compartment at stereocilia tips defined by spatial and temporal patterns of myosin IIIa expression. J Neurosci 26, 10243–10252. 10.1523/JNEUROSCI.2812-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Kachar B, and Muller U (2010). Review series: The cell biology of hearing. J Cell Biol 190, 9–20. 10.1083/jcb.201001138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Sczaniecka A, Grillet N, Bailey JS, Avenarius M, Najmabadi H, Steffy BM, Federe GC, Lagler EA, Banan R, et al. (2007). A forward genetics screen in mice identifies recessive deafness traits and reveals that pejvakin is essential for outer hair cell function. J Neurosci 27, 2163–2175. 10.1523/JNEUROSCI.4975-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwander M, Xiong W, Tokita J, Lelli A, Elledge HM, Kazmierczak P, Sczaniecka A, Kolatkar A, Wiltshire T, Kuhn P, et al. (2009). A mouse model for nonsyndromic deafness (DFNB12) links hearing loss to defects in tip links of mechanosensory hair cells. Proc Natl Acad Sci U S A 106, 5252–5257. 10.1073/pnas.0900691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seal RP, Akil O, Yi E, Weber CM, Grant L, Yoo J, Clause A, Kandler K, Noebels JL, Glowatzki E, et al. (2008). Sensorineural deafness and seizures in mice lacking vesicular glutamate transporter 3. Neuron 57, 263–275. 10.1016/j.neuron.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Self T, Mahony M, Fleming J, Walsh J, Brown SD, and Steel KP (1998). Shaker-1 mutations reveal roles for myosin VIIA in both development and function of cochlear hair cells. Development 125, 557–566. 10.1242/dev.125.4.557. [DOI] [PubMed] [Google Scholar]
- Shabbir MI, Ahmed ZM, Khan SY, Riazuddin S, Waryah AM, Khan SN, Camps RD, Ghosh M, Kabra M, Belyantseva IA, et al. (2006). Mutations of human TMHS cause recessively inherited non-syndromic hearing loss. J Med Genet 43, 634–640. 10.1136/jmg.2005.039834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shotwell SL, Jacobs R, and Hudspeth AJ (1981). Directional sensitivity of individual vertebrate hair cells to controlled deflection of their hair bundles. Ann N Y Acad Sci 374, 1–10. 10.1111/j.1749-6632.1981.tb30854.x. [DOI] [PubMed] [Google Scholar]
- Shrestha BR, Chia C, Wu L, Kujawa SG, Liberman MC, and Goodrich LV (2018). Sensory Neuron Diversity in the Inner Ear Is Shaped by Activity. Cell 174, 1229–1246 e1217. 10.1016/j.cell.2018.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemens J, Lillo C, Dumont RA, Reynolds A, Williams DS, Gillespie PG, and Muller U (2004). Cadherin 23 is a component of the tip link in hair-cell stereocilia. Nature 428, 950–955. 10.1038/nature02483. [DOI] [PubMed] [Google Scholar]
- Sollner C, Rauch GJ, Siemens J, Geisler R, Schuster SC, Muller U, Nicolson T, and Tubingen Screen C (2004). Mutations in cadherin 23 affect tip links in zebrafish sensory hair cells. Nature 428, 955–959. 10.1038/nature02484. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, Corey DP, and Schulten K (2005). In search of the hair-cell gating spring elastic properties of ankyrin and cadherin repeats. Structure 13, 669–682. 10.1016/j.str.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Sotomayor M, and Schulten K (2008). The allosteric role of the Ca2+ switch in adhesion and elasticity of C-cadherin. Biophys J 94, 4621–4633. 10.1529/biophysj.107.125591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Weihofen WA, Gaudet R, and Corey DP (2010). Structural determinants of cadherin-23 function in hearing and deafness. Neuron 66, 85–100. 10.1016/j.neuron.2010.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sotomayor M, Weihofen WA, Gaudet R, and Corey DP (2012). Structure of a force-conveying cadherin bond essential for inner-ear mechanotransduction. Nature 492, 128–132. 10.1038/nature11590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Babola T, Pregernig G, So KS, Nguyen M, Su SM, Palermo AT, Bergles DE, Burns JC, and Muller U (2018). Hair Cell Mechanotransduction Regulates Spontaneous Activity and Spiral Ganglion Subtype Specification in the Auditory System. Cell 174, 1247–1263 e1215. 10.1016/j.cell.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun S, Siebald C, and Muller U (2021). Subtype maturation of spiral ganglion neurons. Curr Opin Otolaryngol Head Neck Surg 29, 391–399. 10.1097/MOO.0000000000000748. [DOI] [PubMed] [Google Scholar]
- Tadenev ALD, Akturk A, Devanney N, Mathur PD, Clark AM, Yang J, and Tarchini B (2019). GPSM2-GNAI Specifies the Tallest Stereocilia and Defines Hair Bundle Row Identity. Curr Biol 29, 921–934 e924. 10.1016/j.cub.2019.01.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang YQ, Lee SA, Rahman M, Vanapalli SA, Lu H, and Schafer WR (2020). Ankyrin Is An Intracellular Tether for TMC Mechanotransduction Channels. Neuron 107, 112–125 e110. 10.1016/j.neuron.2020.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilney LG, Tilney MS, and DeRosier DJ (1992). Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu Rev Cell Biol 8, 257–274. 10.1146/annurev.cb.08.110192.001353. [DOI] [PubMed] [Google Scholar]
- Tobin M, Chaiyasitdhi A, Michel V, Michalski N, and Martin P (2019). Stiffness and tension gradients of the hair cell’s tip-link complex in the mammalian cochlea. Elife 8. 10.7554/eLife.43473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trouillet A, Miller KK, George SS, Wang P, Ali NE, Ricci A, and Grillet N (2021). Loxhd1 Mutations Cause Mechanotransduction Defects in Cochlear Hair Cells. J Neurosci 41, 3331–3343. 10.1523/JNEUROSCI.0975-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallone R, Dal Cortivo G, D’Onofrio M, and Dell’Orco D (2018). Preferential Binding of Mg(2+) Over Ca(2+) to CIB2 Triggers an Allosteric Switch Impaired in Usher Syndrome Type 1J. Front Mol Neurosci 11, 274. 10.3389/fnmol.2018.00274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Deventer SJ, Dunlock VE, and van Spriel AB (2017). Molecular interactions shaping the tetraspanin web. Biochem Soc Trans 45, 741–750. 10.1042/BST20160284. [DOI] [PubMed] [Google Scholar]
- Velez-Ortega AC, Freeman MJ, Indzhykulian AA, Grossheim JM, and Frolenkov GI (2017). Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells. Elife 6. 10.7554/eLife.24661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verpy E, Leibovici M, Michalski N, Goodyear RJ, Houdon C, Weil D, Richardson GP, and Petit C (2011). Stereocilin connects outer hair cell stereocilia to one another and to the tectorial membrane. J Comp Neurol 519, 194–210. 10.1002/cne.22509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waguespack J, Salles FT, Kachar B, and Ricci AJ (2007). Stepwise morphological and functional maturation of mechanotransduction in rat outer hair cells. J Neurosci 27, 13890–13902. 10.1523/JNEUROSCI.2159-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HC, and Bergles DE (2015). Spontaneous activity in the developing auditory system. Cell Tissue Res 361, 65–75. 10.1007/s00441-014-2007-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K (2008). Modulation by clamping: Kv4 and KChIP interactions. Neurochem Res 33, 1964–1969. 10.1007/s11064-008-9705-x. [DOI] [PubMed] [Google Scholar]
- Wang X, Li G, Liu J, Liu J, and Xu XZ (2016). TMC-1 Mediates Alkaline Sensation in C. elegans through Nociceptive Neurons. Neuron 91, 146–154. 10.1016/j.neuron.2016.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Guo Y, Li G, Liu C, Wang L, Zhang A, Yan Z, and Song C (2021a). The push-to-open mechanism of the tethered mechanosensitive ion channel NompC. Elife 10. 10.7554/eLife.58388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Steele CR, Puria S, and Ricci AJ (2021b). In situ motion of individual inner-hair-cell stereocilia from stapes stimulation in adult mice. Communications Biology 4, 958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JS, Young ED, and Glowatzki E (2016). Maturation of Spontaneous Firing Properties after Hearing Onset in Rat Auditory Nerve Fibers: Spontaneous Rates, Refractoriness, and Interfiber Correlations. J Neurosci 36, 10584–10597. 10.1523/JNEUROSCI.1187-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L, Pan L, Wei Z, and Zhang M (2011). Structure of MyTH4-FERM domains in myosin VIIa tail bound to cargo. Science 331, 757–760. 10.1126/science.1198848. [DOI] [PubMed] [Google Scholar]
- Wu L, Pan L, Zhang C, and Zhang M (2012). Large protein assemblies formed by multivalent interactions between cadherin23 and harmonin suggest a stable anchorage structure at the tip link of stereocilia. J Biol Chem 287, 33460–33471. 10.1074/jbc.M112.378505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong W, Grillet N, Elledge HM, Wagner TF, Zhao B, Johnson KR, Kazmierczak P, and Muller U (2012). TMHS is an integral component of the mechanotransduction machinery of cochlear hair cells. Cell 151, 1283–1295. 10.1016/j.cell.2012.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan J, Pan L, Chen X, Wu L, and Zhang M (2010). The structure of the harmonin/sans complex reveals an unexpected interaction mode of the two Usher syndrome proteins. Proc Natl Acad Sci U S A 107, 4040–4045. 10.1073/pnas.0911385107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X, Zhao Q, Li X, Chen Y, Tian Y, Liu S, Xiong W, and Huang P (2020). Deafness mutation D572N of TMC1 destabilizes TMC1 expression by disrupting LHFPL5 binding. Proc Natl Acad Sci U S A 117, 29894–29903. 10.1073/pnas.2011147117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yue X, Zhao J, Li X, Fan Y, Duan D, Zhang X, Zou W, Sheng Y, Zhang T, Yang Q, et al. (2018). TMC Proteins Modulate Egg Laying and Membrane Excitability through a Background Leak Conductance in C. elegans. Neuron 97, 571–585 e575. 10.1016/j.neuron.2017.12.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zampini V, Ruttiger L, Johnson SL, Franz C, Furness DN, Waldhaus J, Xiong H, Hackney CM, Holley MC, Offenhauser N, et al. (2011). Eps8 regulates hair bundle length and functional maturation of mammalian auditory hair cells. PLoS Biol 9, e1001048. 10.1371/journal.pbio.1001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng WZ, Grillet N, Dewey JB, Trouillet A, Krey JF, Barr-Gillespie PG, Oghalai JS, and Muller U (2016). Neuroplastin Isoform Np55 Is Expressed in the Stereocilia of Outer Hair Cells and Required for Normal Outer Hair Cell Function. J Neurosci 36, 9201–9216. 10.1523/JNEUROSCI.0093-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Cheng LE, Kittelmann M, Li J, Petkovic M, Cheng T, Jin P, Guo Z, Gopfert MC, Jan LY, and Jan YN (2015). Ankyrin Repeats Convey Force to Gate the NOMPC Mechanotransduction Channel. Cell 162, 1391–1403. 10.1016/j.cell.2015.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao B, Wu Z, Grillet N, Yan L, Xiong W, Harkins-Perry S, and Muller U (2014). TMIE is an essential component of the mechanotransduction machinery of cochlear hair cells. Neuron 84, 954–967. 10.1016/j.neuron.2014.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y, Yamoah EN, and Gillespie PG (1996). Regeneration of broken tip links and restoration of mechanical transduction in hair cells. Proc Natl Acad Sci U S A 93, 15469–15474. 10.1073/pnas.93.26.15469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng J, Shen W, He DZ, Long KB, Madison LD, and Dallos P (2000). Prestin is the motor protein of cochlear outer hair cells. Nature 405, 149–155. 10.1038/35012009. [DOI] [PubMed] [Google Scholar]


