Abstract
We investigated the antimycobacterial role of myeloperoxidase (MPO), one of the most abundant granule proteins in human neutrophils. Our data indicate that purified MPO, in the presence of hydrogen peroxide, exerts a consistent killing activity against Mycobacterium tuberculosis H37Rv and against a clinical isolate. The activity is time and dose dependent and requires the presence of chloride ions in the assay medium.
Neutrophilic granulocytes are the first cells which, in response to microbial invasion, migrate from the blood into tissue sites where they participate in the early inflammatory response. Their involvement in the host response against Mycobacterium tuberculosis is controversial (6, 7, 9, 10, 24); mycobacteria, M. tuberculosis included, induce neutrophil influx in animal models (2–5, 26), and isolated neutrophils phagocytize M. tuberculosis and initiate a respiratory burst (17). In addition, neutropenia has been cited as a risk factor for mycobacterial infection (18). It has been shown that eosinophilic granulocytes may participate in the inflammatory response initiated by M. tuberculosis (8), but even in this case the role of these granulocytes remains to be established. In contrast, convincing evidence for the role of macrophages in both intracellular replication and resistance to M. tuberculosis infection has been presented (13). These findings have led researchers to concentrate their efforts on establishing the M. tuberculosis-inactivating mechanisms of macrophages rather than on those of granulocytes. In these latter, however, at least two kinds of antimycobacterial proteins have been described: peroxidases, which are able to strongly inactivate the infectivity of Mycobacterium leprae (15), and defensins, which have been shown to kill Mycobacterium avium (22). To the best of our knowledge, the effects of human neutrophil granule proteins on M. tuberculosis are largely unexplored. Brown et al. (7) have claimed that, employing a metabolic test based on the liberation of 14CO2 from 14C-palmitate oxidation, myeloperoxidase (MPO), a well-known antibacterial enzyme (14), does not exert on M. tuberculosis growth the same strong effect it exerts on M. leprae infectivity (15). They did not provide, however, a dose-response curve for MPO, nor did they consider times of incubation longer than 30 min or monitor the effect of MPO by a method that specifically evaluates the growth of M. tuberculosis. Considering that there is increasing evidence for a role of neutrophilic granulocytes in host defense against M. tuberculosis, that leukocyte peroxidases inhibit the growth of M. leprae, and that no conclusive evidence exists for or against an anti-M. tuberculosis role for MPO, we decided to investigate in depth the possibility that this peroxidase may exert a microbicidal effect against M. tuberculosis.
MATERIALS AND METHODS
Phosphate-buffered saline (PBS), horseradish peroxidase (HRP), and hydrogen peroxide were purchased from Sigma (Sigma Chemical Co. St. Louis, Mo.); the other chemicals were of reagent grade.
M. tuberculosis H37Rv (Pasteur Institute, Paris, France) and strain H19, a clinical isolate from human bronchial aspirate (our own strain collection), and alternatively, M. avium 485 (Istituto Superiore della Sanità, Rome, Italy) were used.
MPO was obtained from human blood granulocytes by combining cationic exchange and gel filtration chromatography as previously described (29). Purified peroxidase showed the characteristic absorption spectrum (23), and the Rz (the ratio between absorption at 428 and that at 280 nm, which is commonly used as a criterion of purity for heme peroxidases), was 0.75, which indicates a high degree of purification (19, 23). The activity of MPO (protein concentration, 1.6 mg/ml in 0.025 M phosphate buffer [pH 7.0]) was 700 guaiacol units/ml (1 guaiacol unit [GU] = 1 μmol of guaiacol oxidized in 1 min), corresponding to 1,320 ortodianisidine units/ml.
The bacteria were grown on Lowenstein Jensen medium (Difco Labs, Detroit, Mich.) for 2 to 4 weeks, resuspended, and brought to a concentration of 108/ml in Middlebrook 7H9 broth (Difco) containing 0.05% (wt/vol) Tween 80. A total of 1 × 106 to 5 × 106 M. tuberculosis cells/ml were then incubated for different lengths of time (0 to 90 min) in PBS, either in the presence or in the absence of hydrogen peroxide (0.5 mM final concentration), with increasing amounts of purified MPO. At each time interval, 0.1-ml volumes of serial 10-fold dilutions of the mixture were plated in triplicate on Middlebrook 7H11 agar (Difco) plates. The agar plates were then incubated at 37°C, and the numbers of CFU were determined after 3 to 4 weeks.
The halide dependence of MPO activity was tested according to the method of Klebanoff and Shepard (15). Briefly, MPO (3 GU/106 M. tuberculosis) was added to a 0.2 × 106 M. tuberculosis H37Rv suspension in 0.02 M sodium phosphate buffer (pH 7.0) containing 0.067 M sodium sulfate and, where indicated, 0.1 M NaCl, 0.2 mM NaI, or 0.2 mM NaBr, either in the presence or in the absence of 0.5 mM H2O2. After 60 min of incubation at 37°C the suspensions were appropriately diluted, and the CFU were calculated as reported above.
[U-14C]palmitate (Amersham Pharmacia Biotech, Rainham, Essex, United Kingdom) oxidation was evaluated in 25-ml flasks equipped with a center well in which 0.5 ml of 20% KOH was added. A total of 0.02 × 106 to 0.2 × 106 M. tuberculosis H37Rv cells, in 1.5 ml of 7H9 medium containing 1 μCi of [U-14C]palmitate, was added to the flask after a 90-min preincubation in PBS with MPO and/or H2O2 as indicated. The spontaneous oxidation of 14C-palmitate, alone or in the presence of MPO, H2O2, or MPO and H2O2 (background values), was evaluated by omitting the bacteria in the assay mixture. The flasks were then hermetically closed and incubated for 6 days at 37°C. Subsequently, 1 ml of HCl N was added to the bacteria in order to free 14CO2 from the medium, and after 30 min, 0.2 ml of KOH from the center well was added to 10 ml of Ready Safe liquid scintillation cocktail (Beckman Instruments Inc., Fullerton, Calif.). The radioactivity was evaluated with an LS-6000 liquid scintillation counter (Beckman Instruments) and expressed as counts per minute.
RESULTS AND DISCUSSION
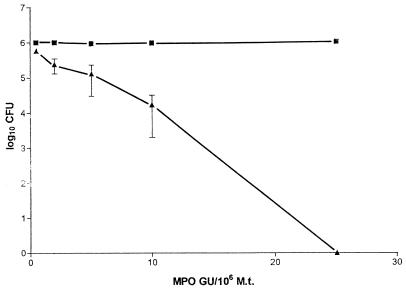
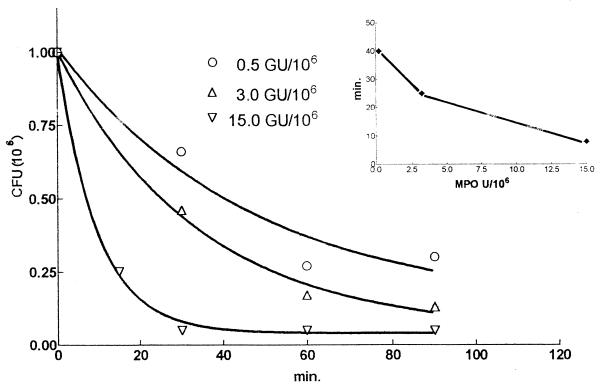
Figure 1 shows that MPO exerted a consistent microbicidal effect on the growth of M. tuberculosis H37Rv after an incubation of 60 min. This effect started to be evident at a concentration of 0.5 GU of MPO/106 bacteria, became clearly evident at a concentration of 2 GU of MPO/106 bacteria (the MPO activity corresponding to about 4 × 106 neutrophils), and reached a value of 1 log at 5 to 10 GU of MPO/106 bacteria. At an MPO concentration of 25 GU/ml, virtually no CFU were detectable. Comparable results were obtained when challenging M. tuberculosis H37Rv with pure eosinophil peroxidase (not shown). Figure 2 shows that the time course of the killing activity of MPO depended on the ratio of the number of MPO units to the number of bacteria, since the time required for 50% growth inhibition diminished progressively as this ratio increased (Fig. 2, inset). To see whether or not a metabolic assay could also reveal the killing activity of MPO, we monitored both the CFU and the extent of 14C-palmitate oxidation in M. tuberculosis H37Rv exposed to MPO. Table 1 shows that, as expected, the CFU were significantly reduced in the presence of MPO and H2O2 and that 14C-palmitate oxidation failed to reveal any significant killing activity of MPO either after 3 or after 6 days of incubation. The table also shows that the extent of palmitate oxidation in untreated M. tuberculosis was not proportional to the CFU values. However, when 0.2 × 104 CFU were added the 14C-palmitate oxidation became negligible and was not significantly different from the background value (not shown). These findings confirm the observations that a CFU assay is required to evaluate M. tuberculosis inactivation and that metabolic assays are not quantitative (20, 21) and therefore do not prove that MPO is unable to kill M. tuberculosis. Brown et al. (7) failed to detect an MPO anti-M. tuberculosis activity using the equivalent of 0.08 GU of MPO/106 M. tuberculosis cells and incubating the mixture for only 30 min. Under these conditions, the microbicidal activity of MPO against M. tuberculosis is hard to detect even with the CFU assay.
FIG. 1.
Dose-response curve of the inhibitory effect of MPO on the growth of 106 M. tuberculosis (H37Rv). Data are logs of the number of CFU (106) after 60 min of incubation at 37°C in the presence of MPO alone (squares) or in the presence of MPO and hydrogen peroxide (0.5 mM final concentration). The initial numbers of CFU (1 × 106 to 5 × 106/ml), which did not change significantly after 90 min of incubation at 37°C with MPO, were rationalized to 106/ml for the sake of homogeneity of the data, and the relative CFU values obtained in the presence of MPO and H2O2 were recalculated accordingly. Values represent the mean numbers of CFU obtained in at least three different experiments ± standard errors of the means, except for MPO at concentrations of 0.5 and 10 GU/ml (upper curve), for which values are the means of CFU obtained in two experiments.
FIG. 2.
Time course of the inhibitory effect of MPO on the growth of 106 M. tuberculosis (H37Rv) cells. Numbers of CFU (106) were plotted as described in the legend to Fig. 1, and the values are the means of data obtained in two different experiments. The dependence on the amount of MPO added to the assay medium is shown. In the inset, the graph shows the reduction of time required for 50% inhibition of bacterial growth as the amount of MPO in the assay medium increased; the data are calculated from those of Fig. 2.
TABLE 1.
Comparison between the extent of 14C-palmitate oxidation and the number of CFU of M. tuberculosis (H37Rv) in a suspension exposed to MPOa
| Exptl condition | CFU (106 [%]) |
14CO2 from 14C-palmitate (cpm [%]) at:
|
|
|---|---|---|---|
| 3 days | 6 days | ||
| H37Rv (0.2 × 105) | 372 | 543 ± 100 | |
| H37Rv (0.2 × 106) | 0.180 (100) | 480 (100) | 802 ± 138 (100) |
| H37Rv + MPO | 0.170 (94) | 405 (84) | 947 ± 210 (118) |
| H37Rv + H2O2 | 0.150 (83) | 307 (64) | 537 ± 188 (67) |
| H37Rv + MPO + H2O2 | 0.025 (13.8) | 386 (80) | 745 ± 228 (92.8) |
Values (CFU, 106) are the means of two separate experiments. The concentration of MPO was 10 GU/106 M. tuberculosis cells, and the concentration of H2O2 was 0.5 mM. The preincubation period was 90 min. CFU were counted after 3 weeks. The amount of 14CO2 released from 14C-palmitate was evaluated after 3 or 6 days, and the values are the means (± standard deviations) of either three experiments carried out with two center-well-equipped flasks per condition per experiment or of one experiment carried out with three separate center-well-equipped flasks per condition. The background value (already subtracted) in the absence of bacteria was 55 ± 15 cpm. See Materials and Methods for more details.
The susceptibility of mycobacteria to MPO is not specific to the H37Rv strain. Table 2 shows that the growth of strain H19, a clinical isolate, is similarly affected. The table also shows that the growth of M. avium, as well as that of M. microti (28), was affected by the presence of hydrogen peroxide alone and this effect was not enhanced by MPO. Notably, an ongoing peroxidase activity did not seem to be sufficient to inhibit M. tuberculosis growth. The table shows, in fact, that a high HRP concentration (18 GU/106 bacteria) in the assay did not affect the growth of M. tuberculosis H37Rv even when iodide (0.2 mM), which is used as a substrate by this peroxidase (1), was substituted for chloride in the assay medium, suggesting that the cationic property of MPO (14) might play a significant role in its anti-M. tuberculosis activity.
TABLE 2.
Sensitivity of two M. tuberculosis strains and one M. avium strain to peroxidase activitiesa
| Exptl condition | % Growth of:
|
||
|---|---|---|---|
| M. tuberculosis H37Rv | M. tuberculosis H19b | M. avium 485 | |
| Control | 100 | 100 A | 100 |
| MPO + H2O2 | 15 | 29 ± 14 B | 45 |
| MPO | 92 | 80 ± 34 C | 100 |
| HRP + H2O2c | 89 | NDd | ND |
| H2O2 | 100 | 80 ± 15 D | 52 |
Values are expressed as percentages (± standard deviations) of growth, taking the number of CFU of untreated bacteria as 100%. The concentration of MPO was 3 GU/106 M. tuberculosis and M. avium cells, and the concentration of HRP was 18 GU/106 M. tuberculosis cells. Two experiments each were conducted for M. tuberculosis H37Rv and M. avium 485; three experiments were conducted for M. tuberculosis H19. See Materials and Methods for more details.
B versus A, P < 0.015 (significant); C versus A and A versus D, not significant; B versus C, P < 0.002 (very significant). All results by two-tailed Student’s t test.
These experiments were carried out in 0.02 M sodium phosphate buffer (pH 7.0) containing 0.067 M sodium sulfate and 0.2 mM NaI.
ND, not done.
All the results reported above concerning the M. tuberculosis-inhibiting activity of MPO were obtained with an assay medium containing chloride. The bactericidal activity of peroxidases may, however, change in the presence of different halides. We therefore tested the halide requirement of the M. tuberculosis microbicidal activity of MPO. Table 3 shows that a reduction of CFU could only be obtained in the presence of chloride, while in the absence of chloride or with the presence of bromide or iodide in the assay medium, no growth inhibition was detected. This finding was expected for iodide, since it has been reported that for MPO antimycobacterial activity the requirement of halide is Cl− = Br− > I− (15). However, in the case of M. tuberculosis, the MPO killing activity appears to be completely chloride dependent, at least under these assay conditions, since no activity was detected even in the presence of bromide. We cannot exclude the possibility, however, that at a different pH and/or halide concentration in the assay medium, iodide and bromide could be active as well.
TABLE 3.
Effects of different halides on the microbicidal activity of MPO on M. tuberculosis H37Rva
| Halide added | % Growth of M. tuberculosis H37Rv in the presence of:
|
||
|---|---|---|---|
| MPO | H2O2 | MPO + H2O2 | |
| None | 100 | 95 | 101 |
| Cl− | 92 | 99 | 40 |
| I− | 90 | 92 | 122 |
| Br− | 100 | 101 | 100 |
Values (means of two separate experiments) are expressed as percentages of growth taking the number of CFU exposed to MPO alone, in the absence of halide, as 100%. The concentrations used were as follows: MPO, 5 GU/106 M. tuberculosis cells; H2O2, 0.5 mM; Cl−, 100 mM; I−, 0.2 mM; Br−, 0.2 mM. There was a 60-min period of incubation. See Materials and Methods for more details.
In this study we did not investigate the mechanisms by which MPO inactivates M. tuberculosis growth. It is known that peroxidase can affect bacterial viability, by reducing H2O2 to H2O and oxidizing substrates (such as halide ions), in various ways (14). As far as the M. tuberculosis-killing activity of MPO is concerned, however, the dependence on chloride ions suggests that chlorination or hypochlorous acid (HClO) production (11) may be principally involved both in our in vitro model and in intact neutrophils.
In conclusion, our findings indicate that human MPO exerts a microbicidal activity against M. tuberculosis. The killing effect was shown to be about 1 log (90%) when MPO reached a concentration of 5 to 10 GU/106 bacteria. However, when MPO reached a concentration of 25 GU/106 M. tuberculosis bacteria, the peroxidase completely abolished M. tuberculosis growth. The effect was not as strong as that previously described for M. leprae, since for M. leprae a strong inactivating effect by MPO was reported at 0.16 GU/106 bacteria (15). We do not know the reason why the MPO concentration required for M. tuberculosis killing activity is higher than that reported for M. leprae. It is possible that MPO does not adhere to M. tuberculosis with the same avidity and that a threshold of bound MPO is required for bacterial killing.
We are presently investigating whether MPO can fight mycobacterial infections in animal models. We believe that if an adequate pathway (27) is provided to target MPO to the infection site, together with recruited macrophages (perhaps even in their endosomes if MPO is endocytosed), MPO could exert its antimycobacterial activity with the endogenously produced hydrogen peroxide, independently of lysosome-phagosome fusion. It has indeed been shown that exogenously added eosinophil peroxidase can improve the candidacidal activity of macrophages (16). Furthermore, data reported many years ago showed that MPO injected into the peritoneal cavity of the rat was able to reach the lung (25) and that intravenously injected MPO alone did not induce glomerular injury (12). Our findings suggest that it is worth reconsidering these studies on cytotoxicity, organ distribution, and clearance of injected MPO in animal models in view of using MPO as an antimycobacterial agent.
ACKNOWLEDGMENTS
This work was supported by the Ministero dell’Università e della Ricerca Scientifica (MURST) 60, 40, and ex-40% cofinanziamento grants, by Primo Progetto di Recerche sulla Tubercolosi, Minister della Sanita (to M. Brai, Instituto di Patologia Generale, Universita di Palermo, Palermo, Italy), and by the Fondazione Carlo e Dirce Callerio.
We thank Alessandra Knowles for revising the text.
REFERENCES
- 1.Anderson R. Levamisol stimulation of neutrophil chemotaxis and chemokinesis by protection of the leukoattractant and the cellular chemotactic response from inactivation by the peroxidase/hydrogen peroxide/halide system “in vitro”. Int Arch Appl Immunol. 1981;65:257–265. doi: 10.1159/000232765. [DOI] [PubMed] [Google Scholar]
- 2.Appelberg R. Macrophage inflammatory proteins MIP-1 and MIP-2 are involved in T cell mediated neutrophil recruitment. J Leukoc Biol. 1992;52:303–306. doi: 10.1002/jlb.52.3.303. [DOI] [PubMed] [Google Scholar]
- 3.Appelberg R. Mycobacterial infection primes T cells and macrophages for enhanced recruitment of neutrophils. J Leukoc Biol. 1992;51:472–477. doi: 10.1002/jlb.51.5.472. [DOI] [PubMed] [Google Scholar]
- 4.Appelberg R. Interferon-gamma (IFN-γ) and macrophage inflammatory proteins (MIP-1 and MIP-2) are involved in the regulation of the T cell-dependent chronic peritoneal neutrophilia of mice infected with mycobacteria. Clin Exp Immunol. 1992;89:269–273. doi: 10.1111/j.1365-2249.1992.tb06943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Appelberg R, Silva M T. T cell dependent chronic neutrophilia during mycobacterial infections. Clin Exp Immunol. 1989;78:478–483. [PMC free article] [PubMed] [Google Scholar]
- 6.Bartold P M, Hay S, Vernon-Roberts B. Effect of cyclosporine A on connective tissue deposition in experimental inflammatory lesions. Matrix. 1989;9:293–300. doi: 10.1016/s0934-8832(89)80005-8. [DOI] [PubMed] [Google Scholar]
- 7.Brown A E, Holzer T J, Andersen B R. Capacity of human neutrophils to kill Mycobacterium tuberculosis. J Infect Dis. 1987;156:985–991. doi: 10.1093/infdis/156.6.985. [DOI] [PubMed] [Google Scholar]
- 8.Castro A G, Esaguy N, Macedo P M, Aguas A P, Silva M T. Live but not heat-killed mycobacteria cause rapid chemotaxis of large numbers of eosinophils in vivo and are ingested by the attracted granulocytes. Infect Immun. 1991;59:3009–3014. doi: 10.1128/iai.59.9.3009-3014.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denis M. Human neutrophils, activated with cytokines or not, do not kill virulent Mycobacterium tuberculosis. J Infect Dis. 1991;163:919–925. doi: 10.1093/infdis/163.4.919. [DOI] [PubMed] [Google Scholar]
- 10.Filley E A, Rook G A W. Effect of mycobacteria on sensitivity to the cytotoxic effect of tumor necrosis factor. Infect Immun. 1991;59:2567–2572. doi: 10.1128/iai.59.8.2567-2572.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hampton M B, Kettle A J, Winterbourn C C. Inside the neutrophil phagosome: oxidants, myeloperoxidase, and bacterial killing. Blood. 1998;92:3007–3017. [PubMed] [Google Scholar]
- 12.Johnson R J, Couser W G, Chi E Y, Adler S, Klebanoff S J. New mechanism of glomerular injury: myeloperoxidase-hydrogen peroxide-halide system. J Clin Investig. 1987;79:1379–1387. doi: 10.1172/JCI112965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kaufmann S H E. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- 14.Klebanoff S J. Oxygen metabolites from phagocytes. In: Gallin J I, Goldstein I M, Snyderman R, editors. Inflammation: basic principles and clinical correlates—1992. 2nd ed. New York, N.Y: Raven Press, Ltd.; 1992. pp. 541–588. [Google Scholar]
- 15.Klebanoff S J, Shepard C C. Toxic effect of the peroxidase-hydrogen peroxide-halide antimicrobial system on Mycobacterium leprae. Infect Immun. 1984;44:534–536. doi: 10.1128/iai.44.2.534-536.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lefkowitz D L, Lincoln J A, Howard K R, Stuart R, Lefkowitz S S, Allen R C. Macrophage-mediated candidacidal activity is augmented by exposure to eosinophil-peroxidase. Inflammation. 1997;21:159–172. doi: 10.1023/a:1027366119901. [DOI] [PubMed] [Google Scholar]
- 17.May M E, Spagnuolo P J. Evidence for activation of the respiratory burst in the interaction of human neutrophils with Mycobacterium tuberculosis. Infect Immun. 1987;55:2304–2307. doi: 10.1128/iai.55.9.2304-2307.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McWhinney P H, Yates M, Prentice H G, Thrussell M, Gillespie S H, Kibbler C C. Infection caused by Mycobacterium chelonae: a diagnostic and therapeutic problem in the neutropenic patient. Clin Infect Dis. 1992;14:1208–1212. doi: 10.1093/clinids/14.6.1208. [DOI] [PubMed] [Google Scholar]
- 19.Menegazzi R, Zabucchi G, Patriarca P. A simple procedure for the purification of eosinophil peroxidase from normal human blood. J Immunol Methods. 1986;91:283–288. doi: 10.1016/0022-1759(86)90491-6. [DOI] [PubMed] [Google Scholar]
- 20.Mitchison D A. Modern methods for assessing the drugs used in the chemotherapy of mycobacterial disease. J Appl Bacteriol. 1996;81:72S–80S. [PubMed] [Google Scholar]
- 21.O’Brien L, Roberts B, Andrew P W. “In vitro” interaction of Mycobacterium tuberculosis and macrophages: activation of anti-mycobacterial activity of macrophages and mechanism of anti-mycobacterial activity. In: Shinnic K, Thomas M, editors. Tuberculosis—1996. Berlin, Germany: Springer Verlag; 1996. pp. 97–130. [DOI] [PubMed] [Google Scholar]
- 22.Ogata K, Linzer B A, Zuberi R I, Ganz T, Lehrer R J, Catanzaro A. Activity of defensin from human neutrophilic granulocytes against Mycobacterium avium-Mycobacterium intracellulare. Infect Immun. 1992;60:4720–4725. doi: 10.1128/iai.60.11.4720-4725.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsson I, Olofsson T, Odeberg H. Myeloperoxidase-mediated iodination in granulocytes. Scand J Haematol. 1972;9:483–491. doi: 10.1111/j.1600-0609.1972.tb00974.x. [DOI] [PubMed] [Google Scholar]
- 24.Riedel D D, Kaufmann S H E. Chemokine secretion by human polymorphonuclear granulocytes after stimulation with Mycobacterium tuberculosis and lipoarabinomannan. Infect Immun. 1997;65:4620–4623. doi: 10.1128/iai.65.11.4620-4623.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schultz J, Baker A, Tucker B. Myeloperoxidase-enzyme therapy on rat mammary tumors. In: Shultz J, Ahmed F, editors. Cancer enzymology—1976. New York, N.Y: Academic Press; 1976. pp. 319–334. [Google Scholar]
- 26.Silva M T, Silva M N T, Appelberg R. Neutrophil-macrophage cooperation in the host defense against mycobacterial infections. Microb Pathog. 1989;6:369–380. doi: 10.1016/0882-4010(89)90079-x. [DOI] [PubMed] [Google Scholar]
- 27.Tournay C, Courtoy P J, Marodi L, Tottè P, Werenne J, Jacquet A, Garcia-Quintana L, Bollen A, Moguilevsky N. Uptake of recombinant myeloperoxidase, free or fused to Fcγ, by macrophages enhances killing activity toward micro-organisms. DNA Cell Biol. 1996;15:617–624. doi: 10.1089/dna.1996.15.617. [DOI] [PubMed] [Google Scholar]
- 28.Walker I, Lowrie D B. Killing of Mycobacterium microti by immunological activated macrophages. Nature. 1981;293:69–70. doi: 10.1038/293069a0. [DOI] [PubMed] [Google Scholar]
- 29.Zabucchi G, Soranzo M R, Menegazzi R, Bertoncin P, Nardon E, Patriarca P. Uptake of human eosinophil peroxidase and myeloperoxidase by cells involved in the inflammatory process. J Histochem Cytochem. 1988;37:499–508. doi: 10.1177/37.4.2538504. [DOI] [PubMed] [Google Scholar]