Abstract
Opacity-associated protein A (OapA), which is responsible for the transparent-colony phenotype of Haemophilus influenzae, has been implicated in the colonization of the nasopharynx in an infant rat model of carriage. In this report, we show that OapA mediates attachment to Chang epithelial cells examined by using genetically defined type b and nontypeable H. influenzae strains with or without OapA. We also showed that OapA was conserved among H. influenzae strains by comparing deduced amino acid sequences. Both recombinant OapA and polyclonal anti-OapA antiserum blocked the binding of H. influenzae to Chang epithelial cells, suggesting that the interaction of H. influenzae is specific to OapA. Moreover, the binding of recombinant OapA to epithelial cells further provided evidence that OapA can promote attachment of H. influenzae. Expression of oapA gene in a nonadherent Escherichia coli strain significantly increased the binding to Chang epithelial cells, and disruption of the oapA gene with kanamycin resistance cassette insertion resulted in a significant loss of binding. These findings demonstrate that OapA plays a role in H. influenzae binding to human conjunctival epithelial cells.
The initial event in the pathogenesis of Haemophilus influenzae infection is the colonization of the respiratory mucosal surfaces (9). Contiguous spread within the respiratory tract may lead to infections of the upper and lower respiratory tract in susceptible hosts. H. influenzae type b (Hib) is capable of disseminating in young children, which may result in sepsis and meningitis. The introduction of Hib conjugate vaccines has largely eliminated infections caused by Hib but has not affected respiratory tract and other infections by other types of H. influenzae, particularly nontypeable strains (11, 17).
The process of colonization requires special microbial factors that allow the binding of H. influenzae to host cells. The most common form of bacterial colonization factor is the pilus or fimbria, a hair-like surface appendage which mediates the adherence of H. influenzae to host cells. In vitro, fimbriated Hib strains have been shown to exhibit increased adherence to buccal and pharyngeal epithelial cells (12) and nasopharyngeal mucosa (5) compared to adherence by nonfimbriated strains. However, the majority of Hib strains isolated from the nasopharynges of children are nonfimbriated (8, 20). In addition, a fimbria-deficient strain was able to persist in the nasopharynx, although in reduced numbers compared with the fimbriated parent strain, in a simian model of H. influenzae carriage (21). The microbial structures responsible for the interactions with host cells in the absence of fimbriae are incompletely understood. In search of nonpilus adhesins, recent work has been focused on outer membrane proteins in nontypeable H. influenzae (6, 10). Several outer membrane proteins, including the high-molecular-weight (HMW) proteins HMW-1 and HMW-2, related to filamentous hemagglutinin and an exported protein with similarity to a family of immunoglobulin A (IgA) proteases, have been shown to contribute to the attachment of bacteria to cultured epithelial cells, although their role in colonization has not been determined (18, 19).
Spontaneous phase variation in colony morphology of H. influenzae has been shown to play a role in the pathogenesis of infection with Hib (22). Variants with the transparent-colony phenotype were able to colonize the nasopharynx efficiently in an infant rat model of H. influenzae colonization, whereas variants with intermediate or opaque-colony phenotype were relatively deficient at colonization. Expression of more- opaque colony phenotypes is linked to the phase variation of lipopolysaccharide structures and has been associated with differences in quantity of capsular polysaccharide in encapsulated strains (14, 22, 24). Weiser et al. have identified a gene encoding a cell envelope protein, termed opacity-associated protein A (OapA), which is responsible for transparent-colony phenotype of H. influenzae and is required for efficient colonization of the nasopharynx in an infant rat model of H. influenzae carriage (23). Inactivation of oapA was associated with rapid clearance of H. influenzae from the infant rat nasopharynx; however, OapA has not been shown to play an important role in pathogenesis once organisms have become invasive. In this report we show that OapA contributes to the binding of H. influenzae strains to Chang epithelial cells examined by using genetically defined Hib and nontypeable strains with or without OapA.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
H. influenzae strains used in this study are shown in Table 1 and were previously described (23). H. influenzae strains were grown on chocolate agar supplemented with 1% IsoVitale X or in brain heart infusion (BHI) broth supplemented with hemin and NAD. Escherichia coli strains, transformed with plasmid pE214 containing the oapA gene, were grown in Luria-Bertani broth with chloramphenicol (12.5 μg/ml). Kanamycin (20 μg/ml) was used in all culture media for strains having OapA mutations. All chemicals were purchased from Sigma Chemical Co. (St. Louis, Mo.) unless otherwise specified. Pasteur-Merieux-Connaught Co. (Toronto, Canada) provided the recombinant OapA protein (rOapA) and guinea pig antiserum to OapA.
TABLE 1.
Binding of H. influenzae strains to Chang epithelial cells
| Strain | Phenotype | Binding (CFU/well)a (% of inoculum) |
|---|---|---|
| Eagan | Type b, clinical isolate | 3.1 × 106 ± 0.4 × 106 (11)b |
| H229 | Eagan, OapA− | 1.2 × 105 ± 0.2 × 105 (1.1) |
| Rd | Unencapsulated type d clinical isolate | 4.1 × 106 ± 0.6 × 106 (13)b |
| H209 | Rd, OapA−, Kanr | 1.4 × 105 ± 0.2 × 105 (1.5) |
| H217 | H209, OapA+, Kans | 3.4 × 106 ± 0.4 × 106 (11.3)b |
| H233 | Nontypeable, clinical isolate | 6.0 × 106 ± 1.0 × 106 (20)b |
| H487 | H233, Oap−, Kanr | 7.9 × 105 ± 1.3 × 105 (6.5) |
Mean ± standard deviation of at least five separate experiments done in triplicate.
P < 0.01 by two-tailed unpaired t test compared to the corresponding OapA mutant.
Generation of OapA mutants.
OapA mutants of Eagan and Rd strains were obtained as described previously (23). Briefly, a 3.0-kb BamHI-to-XhoI fragment containing complete open reading frames of oapA and oapB was cloned in plasmid pE214. Then, the kanamycin resistance cassette derived from Tn903 was inserted into a unique EcoRI site in the oapA gene and the resulting plasmid, pE219, was linearized and used to transform strains Rd and Eagan to generate H209 and H229 strains, respectively. The mutation in H209 was then corrected by allelic exchange with pE214, which contains wild-type oapA gene, to generate H217. Loss of the kanamycin resistance marker in generating the corrected mutant, H217, correlated with acquisition of oapA expression. Similarly, the nontypeable H. influenzae strain H233 was used to generate the OapA-negative mutant H487. In addition, the oapA sequence was amplified along with pE214 vector sequence with two primers to exclude the oapB sequence, and a kanamycin resistance cassette from pUC4K was inserted in the place of the deleted oapB to obtain the plasmid pEL1. E. coli strain DH5α was transformed with either pE214, pE219, or pEL1 by electroporation according to the manufacturer’s instructions (Bio-Rad Co., Richmond, Calif.).
Cloning and sequencing of oapA.
The oapA gene was obtained from chromosomal DNA by PCR by using primers 5′-GCACGAGAAATTGCGGG-3′ and 5′-GAGACAGATTGCGTTGC-3′, which are based on the nucleotide sequence previously determined (23). The PCR products were cloned into the pCR vector (Invitrogen Inc., Carlsbad, Calif.), and the nucleotide sequence was obtained from both strands of a single clone by using the dideoxy chain termination method (16). Sequence analysis was carried out with the Genetics Computer Group software package from the University of Wisconsin (4).
Binding assays.
Binding assays were performed by using Chang epithelial cells (Wong-Kilbourne derivative, clone 1-5c-4 [human conjunctiva]; ATCC CCL 20.2) that were seeded into 24-well tissue culture plates. Bacteria were inoculated into BHI broth and allowed to grow overnight at 37°C. The bacteria were washed three times with saline and adjusted to optical density at 620 nm of 0.3 to 0.35 (approximately 108 CFU/ml) in experimental medium (M199–Ham’s F-12 [1:1, vol/vol] containing 5% heat-inactivated fetal calf serum, 2 mM l-glutamine, and 1 mM sodium pyruvate). After reaching confluence, the monolayers were infected with approximately 107 CFU of H. influenzae in 500 μl of experimental medium and incubated for 2 h at 37°C in 5% CO2 without shaking. The monolayers were rinsed four times with RPMI 1640 medium and treated with 0.3% Triton X-100 in water to dissolve the monolayers along with the adherent bacteria. The well contents were agitated, and the dilutions were plated on chocolate agar to determine the number of adherent bacteria per monolayer. The concentration of Triton X-100 used in these experiments did not show any effect on the viability of H. influenzae. The actual number of bacteria added to the monolayers was determined for each experiment by colony plate count. Percent adherence was calculated by dividing the number of adherent CFU per monolayer by the number of inoculated CFU and multiplying by 100. For inhibition studies, the rOapA was incubated with epithelial cells for 1 h at 37°C in experimental medium followed by the addition of bacteria. The anti-OapA antibody was incubated with bacteria for 1 h on ice before it was added to the wells. The anti-OapA antibody did not cause any agglutination of bacteria under the experimental conditions employed. Bovine serum albumin (BSA) and preimmune serum were used as negative controls.
Western blotting.
Whole-bacterial-cell lysates corresponding to 107 cells per lane or cell fractions were separated on sodium dodecyl sulfate (SDS)–10% polyacrylamide gels. The proteins were transferred to an Immobilon-P membrane (Millipore Co.) by using a Millipore semidry blotter. Blots were incubated for 2 h with 5% milk followed by antiserum raised against purified OapA in guinea pig (diluted 1:5,000). Binding of the antibody to OapA was detected by using antiserum to guinea pig immunoglobulins conjugated to alkaline phosphatase (diluted 1:10,000). Similarly, 3D6 monoclonal antibody, which recognizes a common epitope on HMW-1 and HMW-2 proteins of nontypeable H. influenzae, was used to detect the presence of HMW proteins. The nontypeable H. influenzae strains 12 and 5 and their mutants lacking HMW proteins were used as positive and negative controls, respectively.
Immunocytochemical staining.
Confluent Chang epithelial cell monolayers were grown in eight-well chamber slides (Lab-Tek) and fixed with 2% paraformaldehyde in phosphate-buffered saline (PBS) for 15 min at room temperature. Then the monolayers were washed with PBS five times and preincubated with 1% normal goat serum for 1 h at room temperature. The monolayers were then incubated with biotinylated rOapA or BSA (10 μg/200 μl) in 1% normal goat serum for 1 h at 37°C. After being washed with RPMI 1640 four times, the monolayers were incubated with streptavidin peroxidase for 1 h at room temperature. The color was developed with diaminobenizidine and hydrogen peroxide as a substrate.
Light microscopy.
The binding assays were carried out as described above, and the epithelial cell monolayers with adherent bacteria in eight-well chamber slides were stained with Giemsa stain and examined by light microscopy (18).
RESULTS
Binding of H. influenzae strains and their OapA mutant strains to Chang epithelial cells.
OapA has been shown to contribute to the colonization of mucosal surfaces in an animal model by H. influenzae (23). Thus, to verify whether the OapA protein has any role in the binding of H. influenzae, we examined the binding to Chang epithelial cells of both wild-type and OapA-negative mutants of Eagan, Rd, and H233 strains. Optimal binding of H. influenzae to Chang epithelial cells under the experimental conditions employed was observed with an inoculum of 107 bacteria for 2-h incubation at 37°C. As shown in Table 1, all the parental strains, Eagan, Rd, and H233, bound to epithelial cells in significantly greater numbers than their corresponding OapA mutants. Eagan and Rd bound ≈10 to 15% of inoculum, whereas H233 binding was ≈20%. The binding of H229 (OapA mutant of Eagan) and H209 (OapA mutant of Rd), however, was reduced to the level of ≈1%, whereas the OapA mutant of H233 (H487) showed 6.5% binding. The reversal of OapA mutation by allelic exchange with the oapA gene in the OapA-negative strain H209 (strain H217) restored the binding capacity to the level for the parent strain (binding was 11.3% ± 1.2% for H217 and 13.0% ± 1.9% for Rd). The binding of these H. influenzae strains was also verified by light microscopic examination after staining with Giemsa stain. The strains Eagan and Rd (Fig. 1A and C) bound to the epithelial cell surface in clusters, particularly on a subpopulation of epithelial cells, whereas the binding of OapA mutants (Fig. 1B and D) was significantly reduced. The binding of strain H217 was similar to that of Rd (data not shown). Interestingly, the binding of the nontypeable H. influenzae strain H233 was diffusely distributed all over the cells although some clustering was observed (Fig. 1E). The binding was significantly reduced for OapA mutant H487 (Fig. 1F). The number of H. influenzae cells bound to Chang epithelial cells as observed by light microscope appeared to be fewer than the number of bound bacteria obtained by binding assays; this discrepancy could be due to uneven binding of bacteria to epithelial cells. The growth curves of the OapA mutant strains were indistinguishable from those of the parent H. influenzae strains (data not shown), thus eliminating the possibility that OapA mutants lost their binding capacity due to impaired growth. These results suggest that OapA of H. influenzae contributes to the binding of the bacteria to Chang epithelial cells, an observation that correlates with its contribution to colonization observed in animal studies (23).
FIG. 1.
Light micrographs demonstrating the binding of H. influenzae strains having or lacking OapA to Chang epithelial cells. The epithelial cell monolayers were infected with the bacterial strains Eagan (A), H229 (B), Rd (C), H209 (D), H233 (E), and H487 (F) as described in the Materials and Methods section, rinsed with RPMI, and stained with Giemsa stain. Prior to inoculation of bacteria, in some experiments epithelial cell monolayers were incubated with 10 μg of rOapA/ml (G). For antibody inhibition, the anti-OapA antibody generated in guinea pig was incubated with the bacteria on ice for 1 h before infection of the epithelial cell monolayers (H). Original magnification; ×400.
Conservation and expression of OapA in H. influenzae.
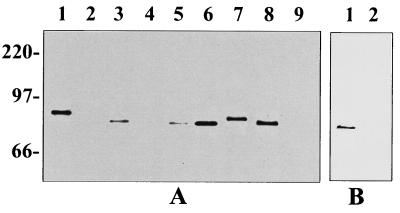
OapA-expressing H. influenzae strains bound in significantly greater numbers to Chang epithelial cells, as shown above, and more efficiently colonized the nasopharynx, than H. influenzae strains lacking OapA in an animal model (23). Thus, it is relevant to examine whether the presence of OapA on H. influenzae is universal or confined to specific strains in order to speculate as to the role of OapA in H. influenzae-related pathogenesis. The oapA region was sequenced from four unrelated H. influenzae strains (Fig. 2). Comparison of the deduced amino acid sequences of OapA from these strains showed that the sequence was highly conserved. Other than single amino acid substitutions, the only significant differences were an insertion of 12 amino acids in strain Eagan (type b) and a tandem repeat of a 16-amino-acid sequence in strain H142 (nontypeable). Western blot analysis confirmed the presence of OapA in encapsulated (Eagan), nonencapsulated (Rd), and nontypeable (H135, H142, and H233) strains (Fig. 3). Differences in the migration of OapA within the range between 78 and 84 kDa corresponded to variations in the lengths of the deduced amino acid sequences. Although equal numbers of cells were loaded in each lane, differences in the intensities of anti-OapA antibody-reactive bands between Eagan and Rd could represent differences in the levels of expression and/or strain-to-strain variation in epitopes. The OapA mutants H209, H229, and H487 had no detectable expression of OapA, as predicted (23). None of these strains was found to contain detectable amounts of HMW proteins by Western blot analysis performed by using 3D6 monoclonal antibody, which recognizes a common epitope present on all four HMW proteins (data not shown). The cellular location of OapA was examined by dividing cell membranes into sarcosyl-soluble and -insoluble fractions (3). As was previously noted for an OapA-PhoA fusion protein, the native protein detected by Western analysis was present in equal amounts in both membrane fractions (23). The presence of OapA on the cell surface was also examined by immunofluorescence by using the polyclonal anti-OapA antibody, which binds to intact cells. In comparison to the parent strains, the OapA-deficient mutants had very minimal reactivity with the OapA antiserum that was similar to the reactivity of control antibodies (data not shown). These results suggest that at least a portion of OapA is expressed on the surface of H. influenzae and conserved among several strains.
FIG. 2.
Sequence conservation of OapA. The deduced amino acid sequence of OapA from strain Rd is given with differences in the type b strain Eagan and two nontypeable isolates (H135 and H142) indicated below. Strain H142 contains a tandem repeat of 16 amino acids followed by a repeat of the sequence AKPV (underlined). Strain Eagan contains an insertion of 12 amino acids ending in a repeat of the sequence QAEQP (underlined).
FIG. 3.
Western blot showing expression of OapA in whole-cell lysates. (A) H. influenzae strains. Lanes: 1, Eagan (type b); 2, H229 (Eagan oapA mutant); 3, Rd; 4, H209 (Rd oapA mutant); 5, H217 (H209 corrected oapA mutant); 6, H135 (nontypeable); 7, H142 (nontypeable); 8, H233 (nontypeable); and 9, H487 (H233 oapA mutant). (B) E. coli strains. Lane: 1, OapA-positive DH5α; 2, OapA-negative DH5α. Molecular size markers are indicated at the left and expressed in kilodaltons.
rOapA and anti-OapA antibody block the binding of H. influenzae to Chang epithelial cells.
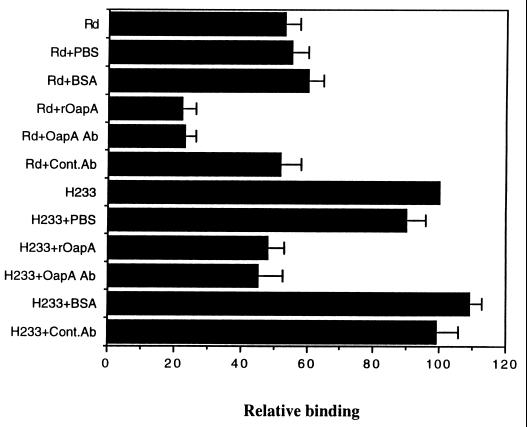
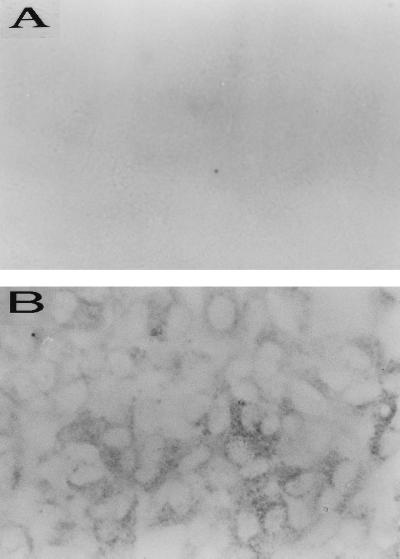
The results obtained by using OapA mutants presented thus far indicated that OapA contributes to the binding of H. influenzae to Chang epithelial cells. We further studied whether OapA has a direct role in H. influenzae binding to epithelial cells by examining the effect of rOapA and anti-OapA antibody on the binding. As shown in Fig. 4, in which the binding of each strain is expressed relative to the binding of H233, rOapA (10 μg of protein/ml) blocked the binding of Rd and H233 by more than 50%. Further increases in the concentration of rOapA (20 μg of protein/ml) produced only a slight increase in the inhibition of binding of H. influenzae. In contrast, controls containing equal amounts of BSA did not show any inhibitory effect on the binding. The inability of rOapA to block completely the binding of H. influenzae strains to epithelial cells could be due to a change in the conformation of rOapA in solution that is less favorable for binding compared to the conformation of OapA on the bacterial cell surface. Similar results were also obtained with anti-OapA antibody, which reduced the binding by 50% compared to that by control antibody. The effects of both the rOapA protein and anti-OapA antibody on the binding of Rd were also verified by light microscopy. As shown in Fig. 1G (rOapA) and Fig. 1H (anti-OapA antiserum), the binding was significantly reduced, providing further evidence that OapA of H. influenzae contributes to the binding to Chang epithelial cells. The inhibition of binding to Chang epithelial cells by rOapA and anti-OapA antibody for Eagan was similar to that for Rd (data not shown). To further substantiate the role of OapA in H. influenzae binding to Chang epithelial cells, we examined the binding of rOapA to epithelial cells by immunocytochemistry. Biotinylated BSA was used as a negative control. The binding of rOapA to epithelial cells showed punctate staining, interestingly, around certain population of cells, whereas BSA did not show any binding (Fig. 5). The pattern of rOapA binding to Chang epithelial cells was more or less similar to the binding of Eagan or Rd, i.e., clusters were present on certain epithelial cell populations. These results suggest that OapA may be responsible for the binding of H. influenzae by directly interacting with Chang epithelial cells.
FIG. 4.
Effects of rOapA and anti-OapA antibody on the binding of H. influenzae strains to Chang epithelial cells. The epithelial cell monolayers were incubated with 10 μg of either rOapA or BSA per ml for 1 h at 37°C before the addition of bacteria. In some experiments the H. influenzae strains were incubated with either anti-OapA antiserum (diluted 1:100) (OapA Ab), control antibody (Cont.Ab), or PBS before they were added to the epithelial cell monolayers. The results were expressed as relative binding, with the H233 binding taken as 100%, and the bars indicate standard deviations from the means for triplicate wells and from at least three separate experiments. The binding of H. influenzae was significantly reduced after incubation with either rOapA or anti-OapA antibody compared to the control protein or antibody (P < 0.01) by two-tailed unpaired t test.
FIG. 5.
Immunocytochemistry of the binding of rOapA to Chang epithelial cells. Confluent epithelial cell layers were incubated with 20 μg of biotinylated BSA (A) or rOapA (B) per ml for 1 h at 37°C. The bound proteins were identified with streptavidin peroxidase as described in the Materials and Methods section. Original magnification, ×200.
Introduction of the oapA gene into E. coli enhances the binding to Chang epithelial cells.
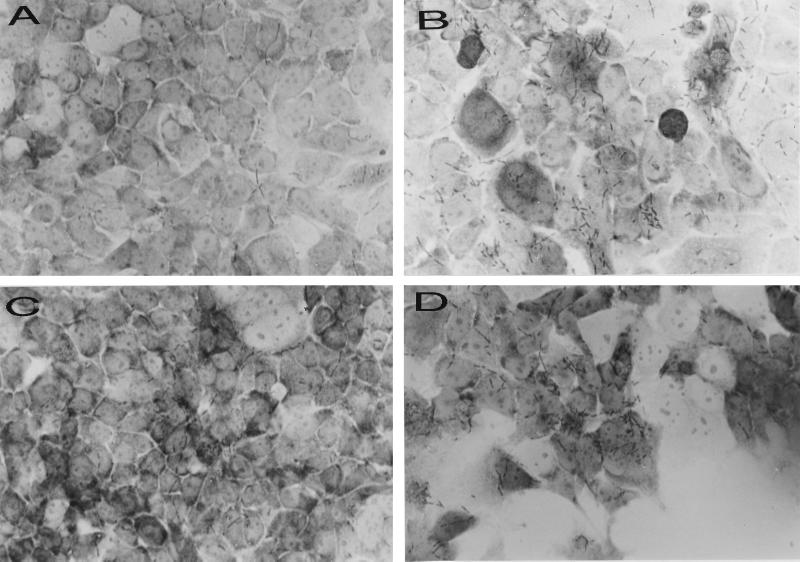
To confirm that OapA contributes to the binding of H. influenzae to Chang epithelial cells, a plasmid (pE214) carrying the 3.0-kb BamHI-to-XhoI fragment, containing both oapA and oapB genes, was introduced into the E. coli strain DH5α. In addition, plasmid pE219, which was obtained from plasmid pE214 after disruption of the oapA gene by kanamycin resistance cassette insertion, was transformed into DH5α. Western blot analysis demonstrated that a 70-kDa protein reacting with anti-OapA antibody is expressed only in DH5α containing pE214 and not in E. coli containing plasmid pE219 (Fig. 3B, lanes 1 and 2). As shown in Table 2, OapA-expressing E. coli bound at a level three times higher than that of E. coli either expressing no OapA or containing only vector. DH5α containing pEL1, from which the oapB sequence was deleted, showed binding to Chang epithelial cells similar to the level for DH5α containing pE214, indicating that OapB may not play a significant role in the binding of H. influenzae to epithelial cells. The binding of these strains to epithelial cells was further confirmed by light microscopic examination after staining with Giemsa stain (Fig. 6). The OapA-expressing E. coli bound in clusters on certain cell populations similarly to the binding of H. influenzae strains, whereas the binding was significantly reduced with strains having either the disrupted oapA gene or vector plasmid only. These results indicate that OapA protein can directly promote the attachment of E. coli strains to Chang epithelial cells.
TABLE 2.
Binding of E. coli strains expressing or not expressing OapA to Chang epithelial cells
| E. coli strains and phenotype | No. of bacteria bounda
|
|
|---|---|---|
| CFU/well | % of inoculum | |
| DH5α(pUC4) | 1.0 × 106 ± 0.2 × 106 | 3.9 ± 0.76 |
| DH5α(pE214) OapA+ | 4.65 × 106 ± 0.2 × 106 | 12.2 ± 0.53b |
| DH5α(pE219) OapA− | 1.23 × 106 ± 0.5 × 106 | 4.1 ± 1.74 |
| DH5α(pEL1) OapA+ | 5.35 × 106 ± 0.7 × 106 | 13.5 ± 1.76b |
Mean ± standard deviation of at least three separate experiments done in triplicate.
P < 0.01 by two-tailed t test compared to OapA− strains.
FIG. 6.
Binding of E. coli strains expressing OapA to Chang epithelial cells. E. coli strains were incubated with epithelial cell monolayers for 2 h, washed, and stained with Giemsa stain as described in the Materials and Methods section. (A) DH5α containing vector only (control); (B) DH5α containing pE214 expressing OapA; (C) DH5α containing pE219 expressing no OapA; and (D) DH5α containing pEL1 with deleted oapB sequence. Original magnification, ×600.
DISCUSSION
H. influenzae undergoes spontaneous phase variation in colony morphology. The transparent-colony phenotype of H. influenzae is associated with greater capacity for colonization in the nasopharynx of an infant rat model of H. influenzae carriage than the opaque-colony phenotype (23). OapA, a novel protein having no homologue in current sequence databases, is necessary for expression of the transparent-colony phenotype and is required for nasopharyngeal colonization. In this report, we provide evidence that OapA contributes to the binding of H. influenzae to Chang epithelial cells. Disruption of the oapA gene in both Hib and nontypeable H. influenzae strains significantly decreased their capacities for binding to human epithelial cells compared to those of wild-type OapA-positive strains.
These results suggest that the increased adherence of OapA-positive H. influenzae may explain the more efficient colonization associated with this phenotype. Although surface pili mediate adherence under certain circumstances, three well-defined nonpilus adhesins that mediate attachment have been observed in many different in vitro systems, suggesting that there is more than one mechanism of H. influenzae adherence to eukaryotic cells. Two distinct HMW proteins, HMW-1 and HMW-2, were shown to contribute to adherence in more than 75% of nontypeable H. influenzae strains (1). The absence of these HMW proteins conferred an 80% reduction in the adherence of H. influenzae strains to Chang epithelial cells (18). The nontypeable strain H233 used in this study showed significant reduction in the binding to Chang epithelial cells when the oapA gene was inactivated. Undetectable amounts of HMW proteins in this strain suggest that OapA may play a role in the binding of H. influenzae strains at least in the absence of other adhesins. In addition, the Hia/Hsf (2) family of adhesins and Hap protein (7) adhesins have been suggested to be involved in the adherence in the absence of HMW-1 and/or HMW-2 proteins. Despite the presence or absence of these families of adhesins (HMW proteins, Hia/Hsf, and Hap), the E. coli strains expressing OapA bound at levels three times higher than that for bacteria that usually show low or no adherence to epithelial cells, suggesting that OapA by itself can promote binding to epithelial cells. The binding of rOapA to epithelial cells further supported this activity of OapA. Inability of OapA mutants of H. influenzae or E. coli to bind to Chang epithelial cells could be due to polar effects on downstream genes. Although OapA mutation in H. influenzae has no discernible effect on LOS or outer membrane profiles (23), we cannot rule out the possibility of other pleiotropic effects. Knock-out mutation in oapB, the gene following oapA, had no effect either on binding of E. coli to Chang epithelial cells, as shown in this study, or on the colonization in an animal model of carriage (unpublished results). The presence of different adhesins on H. influenzae may be necessary to contribute to a certain extent to binding of H. influenzae to eukaryotic cells depending on the microenvironment the bacteria encounter in vivo. The relative contribution of OapA in the binding of H. influenzae in the presence of other adhesins remains to be established. Instead, it could be that either pili or Hia/Hsf proteins and HMW proteins or Hap protein contribute to the initial adherence to the eukaryotic cells and that OapA further enhances the intimate contact of H. influenzae with the cell surface. Loss of any one of these adhesins could result in a significant decrease in the binding of H. influenzae strains to epithelial cells.
Unlike other putative adhesins, OapA is present in all H. influenzae strains, both encapsulated and nontypeable, examined to date. We also showed that the OapA region from four H. influenzae strains was highly conserved by comparing nucleotide and deduced amino acid sequences. In addition to some single amino acid substitutions, a 12-amino-acid insertion was present in strain Eagan, whereas a 16-amino-acid tandem repeat was present in H142. Western analysis showed that the protein reactive to OapA antiserum has an apparent molecular mass of 68 kDa, in contrast to the expected molecular mass of OapA, 47 kDa. This difference in the molecular weights derived from the deduced amino acid sequences and from SDS-polyacrylamide gel electrophoresis could be due to the presence of high content of proline (15). Moreover, the demonstration of the cell surface localization of OapA by Western blotting and immunofluorescence suggests that OapA has a potential to interact with nasopharyngeal cell surface molecules. It is interesting that the binding of H. influenzae strains was clustered on a subset of Chang epithelial cells. The binding of rOapA and E. coli expressing OapA to Chang epithelial cells in a manner similar to H. influenzae binding (as clusters) further supports the role of OapA in the binding. This could be due to the interaction of OapA with specific receptors expressed by a subpopulation of epithelial cells. Similar distribution of receptor molecules on a particular fraction of cells has been shown previously for the E. coli invasin Ibe10 (13). Additional studies are necessary to determine whether expression of OapA binding molecules on a particular eukaryotic cell population increases the incidence of infection under specific conditions.
In summary, here we demonstrated that the expression of OapA enhances the binding of H. influenzae strains to Chang epithelial cells. We further showed that the OapA protein was conserved in different H. influenzae strains. Direct binding of rOapA to Chang epithelial cells suggests that OapA plays a role in the binding of H. influenzae strains to eukaryotic cells. Demonstration of increased attachment of E. coli strains expressing OapA to epithelial cells further confirms the role of OapA in H. influenzae binding. An understanding of the interrelationship among different adhesins for the colonization by H. influenzae may provide strategies to prevent the diseases caused by these strains.
ACKNOWLEDGMENTS
We thank S. J. Barenkamp for providing H. influenzae strains expressing HMW proteins and monoclonal antibody 3D6 and for critical review of the manuscript. We also thank Howard Faden, Children’s Hospital of Buffalo, for his comments on the manuscript. We thank Pasteur-Merieux-Connaught for providing purified rOapA and antiserum to OapA.
This work was supported by Public Health Service grants R29 AI40567 (N.V.P.) and AI38436 (J.N.W.).
REFERENCES
- 1.Barenkamp S J, Leininger E. Cloning, expression, and DNA sequence analysis of genes encoding nontypeable Haemophilus influenzae high-molecular-weight surface-exposed proteins related to filamentous hemagglutinin of Bordetella pertussis. Infect Immun. 1992;60:1302–1313. doi: 10.1128/iai.60.4.1302-1313.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barenkamp S J, St. Geme J W., III Identification of a second family of high-molecular weight adhesion proteins expressed by nontypeable Haemophilus influenzae. Mol Microbiol. 1996;19:1215–1223. doi: 10.1111/j.1365-2958.1996.tb02467.x. [DOI] [PubMed] [Google Scholar]
- 3.Carlone G M, Thomas M L, Rumschlag H S, Sottnek F O. Rapid microprocedure for isolating detergent-insoluble outer membrane proteins from Haemophilus species. J Clin Microbiol. 1986;24:330–332. doi: 10.1128/jcm.24.3.330-332.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus and non-pilus mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 6.Foxwell A R, Kyd J M, Cripps A W. Nontypeable Haemophilus influenzae: pathogenesis and prevention. Microbiol Mol Biol Rev. 1998;62:294–308. doi: 10.1128/mmbr.62.2.294-308.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hendrixson D R, St. Geme J W., III The Haemophilus influenzae Hap serine protease promotes adherence and aggregation, potentiated by a soluble host protein. Mol Cell. 1998;2:841–850. doi: 10.1016/s1097-2765(00)80298-1. [DOI] [PubMed] [Google Scholar]
- 8.Mason E O, Kaplan S L, Wiedermann B L, Pinna Norrod E, Stenback W A. Frequency and properties of naturally occurring adherent piliated strains of Haemophilus influenzae type b. Infect Immun. 1985;49:98–103. doi: 10.1128/iai.49.1.98-103.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moxon E R, Wilson R. The role of Haemophilus influenzae in the pathogenesis of pneumonia. Rev Infect Dis. 1991;13:S518–S527. doi: 10.1093/clinids/13.supplement_6.s518. [DOI] [PubMed] [Google Scholar]
- 10.Murphy T F, Apicella M A. Non-typeable Haemophilus influenzae: a review of clinical aspects, surface antigens, and human immune response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 11.Murphy T F, White K E, Pastor P, Gabriel L, Medley F, Granoff D M, Osterholm M T. Declining incidence of Haemophilus influenzae type b disease since introduction of vaccination. JAMA. 1993;269:246–248. [PubMed] [Google Scholar]
- 12.Pichichero M E. Adherence of Haemophilus influenzae to human buccal and pharyngeal epithelial cells: relationship to piliation. J Med Microbiol. 1984;18:108–116. doi: 10.1099/00222615-18-1-107. [DOI] [PubMed] [Google Scholar]
- 13.Prasadarao N V, Wass C A, Huang S H, Kim K S. Identification and characterization of a novel Ibe10 binding protein that contributes to Escherichia coli invasion of brain microvascular endothelial cells. Infect Immun. 1999;67:1131–1138. doi: 10.1128/iai.67.3.1131-1138.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roche J R, Moxon E R. Phenotypic variation in Haemophilus influenzae: the interrelationship of colony opacity, capsule and lipopolysaccharide. Microb Pathog. 1995;18:129–140. doi: 10.1016/s0882-4010(95)90117-5. [DOI] [PubMed] [Google Scholar]
- 15.Sadler I, Crawford A W, Michelson J W, Berkele M C. Zyxin and cCRP: two interactive LIM domain proteins associated with cytoskeleton. J Cell Biol. 1992;119:1573–1587. doi: 10.1083/jcb.119.6.1573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shapiro E D. Infections caused by Haemophilus influenzae type b: the beginning of the end? JAMA. 1993;269:264–265. [PubMed] [Google Scholar]
- 18.St. Geme J W, III, Falkow S, Barenkamp S J. High-molecular weight proteins of non-typeable Haemophilus influenzae mediate attachment to human epithelial cells. Proc Natl Acad Sci USA. 1993;90:2875–2879. doi: 10.1073/pnas.90.7.2875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.St. Geme J W, III, de la Morena M, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 20.van Ham S M, Mooi F R, Sindhunata M G, Maris W R, van Alphen L. Cloning and expression in Escherichia coli of Haemophilus influenzae fimbrial genes establishes adherence to oropharyngeal epithelial cells. EMBO J. 1989;8:3535–3540. doi: 10.1002/j.1460-2075.1989.tb08519.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Weber A, Harris K, Lohrke S, Forney L, Smith A L. Inability to express fimbriae results in impaired ability of Haemophilus influenzae to colonize the nasopharynx. Infect Immun. 1991;59:4724–4728. doi: 10.1128/iai.59.12.4724-4728.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weiser J N. Relationship between colony morphology and the life cycle of Haemophilus influenzae: the contribution of lipopolysaccharide phase variation to pathogenesis. J Infect Dis. 1993;168:672–680. doi: 10.1093/infdis/168.3.672. [DOI] [PubMed] [Google Scholar]
- 23.Weiser J N, Chong S T H, Greenberg D, Fong W. Identification and characterization of a cell envelope protein of Haemophilus influenzae contributing to phase variation in colony opacity and nasopharyngeal colonization. Mol Microbiol. 1995;17:555–564. doi: 10.1111/j.1365-2958.1995.mmi_17030555.x. [DOI] [PubMed] [Google Scholar]
- 24.Weiser J N, Pan N. Adaptation of Haemophilus influenzae to acquired and innate humoral immunity based on phase variation of lipopolysaccharide. Mol Microbiol. 1998;30:767–775. doi: 10.1046/j.1365-2958.1998.01108.x. [DOI] [PubMed] [Google Scholar]