Abstract
Noninvasive prenatal testing (NIPT) is widely used to screen for common fetal chromosomal aneuploidies. However, the ability of NIPT-Plus to detect copy number variation (CNV) is debatable. Accordingly, we assessed the efficiency of NIPT-Plus to detect clinically significant fetal CNV. We performed a prospective analysis of 31,260 singleton pregnancies, included from June 2017 to December 2020. Cell-free fetal DNA was directly sequenced using the semiconductor sequencing platform for women with high-risk CNV with clinically significant results. Fetal karyotyping and chromosomal microarray analysis (or next-generation sequencing) are recommended for invasive diagnostic procedures. Women at low risk with no other abnormal results continued their pregnancies. We analyzed the expanded NIPT results, diagnostic test results, and follow-up information to evaluate its performance in detecting fetal CNV. Of the 31,260 pregnant women who received NIPT-Plus, 31,256 cases were tested successfully, a high risk of clinically significant CNV was detected in 221 cases (0.71%); 18 women refused further diagnosis; 203 women underwent invasive prenatal diagnosis; and 78 true positive cases and 125 false positive cases, with an overall positive predictive value (PPV) of 38.42% and a false positive rate of 0.40%. For known microdeletion/microduplication syndromes (n = 27), the PPVs were 75% DiGeorge syndrome (DGS), 80% 22q11.22 microduplication, 50% Prader–Willi syndrome, and 50% cri-du-chat. For the remaining clinically significant fetal CNVs (n = 175), the combined PPVs were 46.5% (CNVs > 10 Mb) and 28.57% (CNVs ≤ 10 Mb). NIPT-Plus screening for CNV has certain clinical value. NIPT-Plus yielded relatively high PPVs for 22q11.2 microduplication syndrome and DGS, and low to moderate PPVs for other CNVs.
Subject terms: Molecular biology, Biomarkers, Health care, Medical research, Molecular medicine
Introduction
Chromosomal anomaly, including submicroscopic copy number variation (CNV), is a major cause of birth defects1. Studies have shown that the proportion of fetuses carrying pathogenic CNVs can reach 1.6–1.7%, which is much higher than the prevalence of common fetal trisomies2. Noninvasive prenatal testing (NIPT) is more popular than the traditional maternal serum screening because of its higher sensitivity and specificity in screening trisomy 21, 18, and 133,4, reducing unnecessary invasive diagnostic procedures and the associated risk of fetal loss5. With the rapid development of high-throughput sequencing and bioinformatics analysis, a growing number of studies have indicated that NIPT can be used to detect microdeletion/microduplication syndromes (MMSs)6–8.
A large proportion of CNVs can cause severe genomic diseases. Clinically relevant CNVs occur in 6% of fetuses with structural anomalies9. Unlike chromosomal aneuploidies, the risk of fetal CNVs is independent of maternal age10. Thus, it is beneficial to detect clinically significant fetal CNVs in all pregnant women, irrespective of maternal age, including younger pregnant women. Thus, the application of NIPT, expanded from common aneuploidies to MMS, will guide pregnancy management.
MMSs though relatively rare, collectively account for 1–2% of all congenital malformations in newborns11. Many commercially available NIPT cover the detection of specific MMS12,13. The NIPT-Plus showed varying performance in detecting specific MMS, with only low to moderate positive predictive value (PPV) of DiGeorge syndrome (DGS), 1p36 deletion syndrome, cri-du-chat (CDC), and Prader–Willi/Angelman syndromes (PWS/AS)14–16. In recent years, several studies have demonstrated that it is promising and feasible to utilize NIPT in detecting fetal MMS10,17–27. However, there have been few prospective large-scale population studies8,19, and its performance remains challenging and controversial.
At present, karyotyping and chromosomal microarray analysis (CMA) or CNV sequencing (CNV-seq), have been recommended to prenatally identify fetal clinically significant CNVs as a first-tier technique28. In this study, we prospectively investigated 31,256 singleton pregnancies using NIPT-Plus and investigated its efficacy in detecting fetal clinically significant CNVs.
Material and methods
Study subjects and expanded NIPT data sources
This prospective study enrolled 31,260 pregnant women with a singleton pregnancy who underwent NIPT-Plus, in Fujian Maternity and Child Health Hospital, of whom four failed to be detected. The study was approved by the Ethics Committee of the Hospital (2015KYLLD01051). All methods were performed in accordance with the relevant guidelines and regulations. The test method, screening-covered diseases, and limitations and risks were informed.
Blood samples were sent for the NIPT-Plus test, generating 36-bp genomic sequence reads. Reads were assigned to consecutive non-overlapping 100 Kb bins to further filter bins with low coverage and GC content < 30% or > 70%. Thus, data regarding data regarding clinically significant CNV cases were obtained from Ion Proton semiconductor sequencing platform (Da An Gene Co., Ltd., Shenzhen, China), the sequencing depth was 0.4 ×, and the data volume was 8 million reads. The ENET algorithm was applied to calculate the fetal fraction (FF)29. The redraw rate was 0.49%. The median FF of the samples passing quality control was 11.2% (4.0–48.3%).
Pregnant woman demographics
The demographic characteristics of maternal age, gestational week, gravidity, and prior screening are shown in Table 1. The pregnant women were 19–48 years old (mean age, 32.2 years). The range for gestational weeks at NIPT was 12–32 weeks. The mean values of gestational week at NIPT and invasive testing were 17.3 ± 2.0 and 22.7 ± 2.6 weeks, respectively. Prior maternal serological screening (MSS) tests before NIPT, including abnormal MSS results [high risk, critical risk and abnormal multiple of the median for single marker value (AFP, β-HCG, uE3)] and low risk. Invasive diagnositic procedures include amniocentesis during 16 and 24 gestational weeks, and fetal blood sampling beyond 24 gestational weeks. Autosomal and all chromosome aneuploidies were excluded. Totally, 221 women were suspected to have fetal CNVs, after genetic counseling, and 203 women voluntarily opted invasive testing by karyotype and CMA/CNV-seq, and 18 women refused invasive testing (Fig. 1).
Table 1.
Clinical characteristic of pregnant women undergoing NIPT-Plus.
| Variable | Value |
|---|---|
| Age(years) | No. rate (%) |
| 19–26 | 6628 (15.3) |
| 27–34 | 26,555 (61.3) |
| 35–42 | 9877 (22.8) |
| > 42 | 260 (0.6) |
| Gestational weeks | |
| 12–15+6 | 13,169 (30.4) |
| 16–19+6 | 21,487 (49.6) |
| 20–23+6 | 8231 (19.0) |
| 24–26+6 | 390 (0.9) |
| ≥ 27 | 43 (0.1) |
| Specimens, n(%) | |
| Amniotic fluid | 100 (81.3) |
| Cord blood | 23 (18.7) |
| Pregnancy types | |
| Singleton pregnancy | 41,804(96.5) |
| Twin pregnancy | 1516(3.5) |
| Clinical features | |
| AMA | 10,137 (23.4) |
| Abnormal serologic screening(MSS) | 12,953 (29.9) |
| High risk | 2513 (5.8) |
| Critical risk | 8794 (20.3) |
| Abnormal single marker MOM | 1646 (3.8) |
| Only NIPT | 17,371 (40.1) |
| Soft ultrasound markers | 1733 (4.0) |
| Adverse reproductive history | 390 (0.9) |
| Other* | 736 (1.7) |
AMA advanced maternal age, NIPT noninvasive prenatal testing, n number, MoM multiple of the median, MSS maternal serological screening.
*Pregnant women with contraindications for invasive diagnostic procedures.
Figure 1.
Flowchart of NIPT-Plus results and clinical outcome of pregnant women. NIPT noninvasive prenatal testing, TOP terminate of pregnancy, UA ultrasound anomaly. *CMA detection of induced labor tissue indicated true positive.
Invasive prenatal diagnostic testing by karyotyping and CNV analysis
For further validation, women with NIPT-positive clinically significant CNVs results were recommended to undergo invasive diagnostic procedures, and 30 mL of amniotic fluid or 4 mL of cord blood was obtained. In addition, in cases with NIPT-negative CNVs results, fetuses anomaly were examined by either ultrasound prenatally or physical examination postnatally, and were also advised chromosome testing.
Karyotypes were scanned on Leica GSL120. At least 20 metaphases were counted, and five metaphases were analyzed. The naming of abnormal karyotypes were based on ISCN 2020.
CMA was performed using Affymetrix CytoScan 750 K array (Affymetrix Inc., Santa Clara, CA), the experimental processes were performed as previously described30, and data analysis was carried out using Affymetrix Chromosome Analysis Suite Software (version 3.1.0.15). The reporting threshold was set at copy number gains/losses ≥ 500 Kb and loss of heterozygosity (LOH) ≥ 10 Mb.
In regard to CNV-seq, library construction and purification operation was conducted by Biosan chromosomal CNV assay kit (reversible terminal termination sequencing), the concentration of library was quantitatively determined by quantification through KAPA Library KTS. Post-quantitative library pooling was sequenced on Illumina NextSeq 500 sequencing platform at ~ 1 × depth, and software was used for chromosomal CNV above 0.1 Mb finally. The number of reads after sample quality control is more than 8 M Sequence depth is ~ 0.1 ×. Burrows-Wheeler algorithm for calculating CNV was performed according to the previous study29.
CNVs were classified through OMIM, UCSC, International Standard Cytogenomic Array, Database of Genome Variants, and Decipher databases into pathogenic, likely pathogenic, variants of unknown significance (VOUS), likely benign (LB), and benign. Data were analyzed using the human genome hg19 reference sequence. The pathogenicity significance of CNVs was evaluated following the ACMG guidelines31. For fetuses with confirmed abnormal CNVs, parental testing was performed to determine its origin.
NIPT result is true positive (TP) if confirmed by diagnostic testing of the fetus, mother, or placenta. When diagnostic testing of placenta, fetus, or mother do not confirm the NIPT results, the results are considered as false positive (FP), and when fetal chromosomal anomalies are detected which are not identified via NIPT, the NIPT results are considered false negative (FN). True negative (TN) refers to cases with negative NIPT results and the diagnostic test is normal.
Follow-up and pregnancy management
All pregnant women received pre- and post-test counseling from a senior genetic counselor. Pregnant couples confirmed to have fetuses with pathogenic/likely pathogenic (P/LP) CNVs go through multi-disciplinary treatment, and they take an informed decision on whether to continue pregnancy. Follow-up began three months after delivery, including ultrasound examination report, diagnostic testing results, final pregnancy outcomes, infant’s sex, and physical examination of newborn results. Any FN clinically significant CNVs results subsequently identified by either ultrasound prenatally or physical examination postnatally were subjected to chromosome analysis. The pregnancy outcomes of the FN samples were recorded via telephone or through a follow-up registry.
Statistics
SPSS software version 19.0 (SPSS, Inc., Chicago, IL) was used for statistical analysis. Measurement data were expressed as mean ± standard deviation, statistical comparisons were performed using χ2 test, and p < 0.05 was considered statistically significant.
Ethics approval and consent to participate
This study was approved by the ethics committee of Fujian Maternity and Child Health Hospital, affiliated to Fujian Medical University (No. 2018KYLLD01051), and informed consent was obtained from all the pregnant women.
Results
Locations of CNVs detected by NIPT-plus
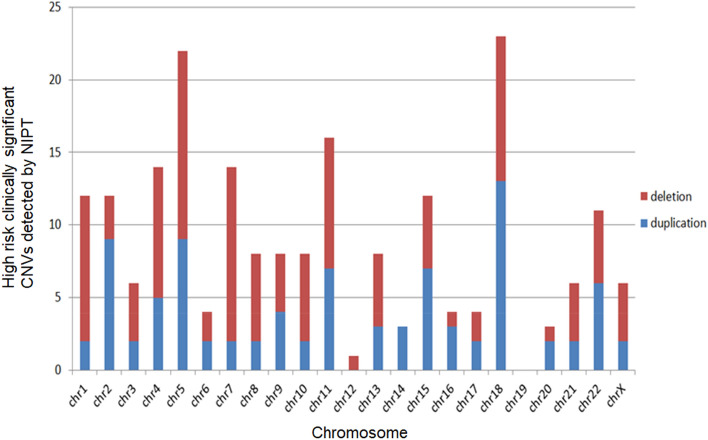
Totally, 31,256 pregnant women who received NIPT-Plus were enrolled finally in this study due to 4 cases failed by NIPT-Plus. The enrollment, and flowchart are presented in Fig. 1. A total of 221 women were suspected to have fetal clinically significant CNVs, thus, the screening positive rate of fetal clinically significant CNVs was 0.71% (221/31,256), including 128 CNVs with microduplications ranging in size from 2.0 to 46 Mb and 98 CNVs with microdeletions ranging in size from 2.2 to 75.29 Mb (and more than one abnormality were detected in 5 cases), CNVs detected by NIPT were distributed in chromosome X and each autosome except chromosome 19, of which, and CNVs on chromosomes 4, 5, 7, 11, 15 and 18 were the most common, as shown in Fig. 2.
Figure 2.
Autosomal and sexual CNVs detected by NIPT in 221 cases were distributed on each autosome and sexual except chromosome 19, and CNVs on chromosomes 4, 5, 7, 11, 15 and 18 were the most common. CNV copy number variation, NIPT noninvasive prenatal testing, chr chromosome.
Detection efficiency of NIPT-Plus in screening clinically significant fetal CNVs
Of the 221 cases with clinically significant CNVs, 203 (91.86%) cases underwent invasive diagnostic testing via amniocentesis or fetal blood sampling. The remaining 18 pregnant women decilined invasive testing. Among the 203 cases with clinically significant CNVs detected by invasive diagnostic testing, 78 cases were TP (of which, 33 were microduplications, 45 were microdeletions), and 125 cases were FP, with an overall PPV of 38.42% and an overall false positive rate (FPR) of 0.40%. Among 101 fetal positive confirmatory invasive diagnostic testing results, 59 were pathogenic, 11 were likely pathogenic, 25 were VOUS, 6 were LB (Table 2).
Table 2.
A 101 fetal positive confirmatory invasive diagnostic testing results with fetal CNVs indicated by NIPT-Plus.
| Case ID | MA | GW | NIPT-PLUS results | Fetal karyotype results | Fetal CMA/CNV-seq results/ pathogenicity classification | Fetal ultrasound finding | Pregnancy outcome | Chromosome disease syndrome indicated by NIPT-Plus |
|---|---|---|---|---|---|---|---|---|
| Copy number gain TP (≤ 16 GW) | ||||||||
| 18 | 24 | 13+6 |
Dup 9q13 (~ 30 Mb) |
46,XN,add(9)(?p13) pat |
arr[GRCh37]9p13.1p24.3 (208,454_38,772,005) × 3 LP |
FGR at 22 GW | TOP | Nonsyndromic |
| 17 | 39 | 14 |
Dup 9p24.3p11.2 (~ 46 Mb) |
46,XN,add(9)(?p24) |
arr[GRCh37]9p24.3q21.13 (208,454_77,662,508) × 3 P |
NT thickening | TOP | Nonsyndromic |
| 27 | 37 | 14+6 |
Dup 13q31.1 (~ 4.3 Mb) |
46,XN |
arr[GRCh37]13q31.1 (78,894,976–81,994,976)) × 3 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 7 | 23 | 15 |
Dup 17p12 (~ 3.34 Mb) |
46,XN |
arr[GRCh37]17p12 (14,060,293–15,416,912) × 3 P |
None | Born (normal phenotype except high arch) | Charcot-Marie-Tooth 1A type syndrome |
| 26 | 32 | 15+6 |
Dup 21q21.1q22 (~ 3.5 Mb) |
46,XN |
arr[GRCh37]21q21.3q22.11 (29,962,609_32,659,168) × 3 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 6 | 32 | 16 |
Dup 2q23.3q24.2 (~ 9.93 Mb) |
46,XN |
arr[GRCh37]2q23.3q24.2 (154,042,539–162,258,705) × 3 dn LP |
None | TOP | Non-syndromic |
| TP (> 16 GW) | ||||||||
| 9 | 31 | 16+3 |
Dup 15q21.2 (~ 5.8 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (22,770,421_28,704,050) × 3 dn P |
None | TOP | 15q11-q13 duplication syndrome |
| 25 | 26 | 17 |
Dup 4p13p12 (15 Mb) |
47, XN, + mar |
arr[hg19]4p13q12 (41,896,801–57,724,715) × 3 mat LP |
None | TOP | Nonsyndromic |
| 16 | 28 | 17+1 |
Dup 11q23.3q24 (~ 17 Mb) |
46,XN |
arr[GRCh37]11q23.3q25 (117,097,362_134,937,416) × 3 P |
Dandy–Walker malformation | TOP | Nonsyndromic |
| 5 | 29 | 17+2 |
Dup 22q11.21q12.1 (~ 2.0 Mb) |
46,XN |
arr[GRCh37]22q11.22q11.23 (22,997,928_25,043,045) × 3 mat P |
None | Born (normal phenotype) | 22q11 duplication syndrome |
| 22 | 28 | 17+3 |
Dup 11q23.3q24 (~ 17 Mb) |
46,XN |
arr[GRCh37]11q23.3q25 (117,097,362–134,937,416) × 3 P |
Dandy–Walker malformation at 23 GW | TOP | Nonsyndromic |
| 21 | 26 | 18 |
Dup 15q11.21q13.1 (~ 6.3 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (22,102,621–28,315,618) × 3 pat* P |
None | Born (normal phenotype) | 15q11q13 duplication syndrome |
| 19 | 38 | 18+1 |
Dup 3p26.3p22.3 (~ 35 Mb) |
46,XN,add(3)(?p22) |
arr[GRCh37]3p26.3p22.3 (1_43,682,691) × 3 pat P |
HPE | TOP | Nonsyndromic |
| 23 | 34 | 18+5 |
Dup 11q24.3q25 (~ 4.1 Mb) |
46,XN |
arr[GRCh37]11q24.3q25 (130,308,334–134,937,46) × 3 P |
None | TOP | Nonsyndromic |
| 10 | 36 | 19 |
Dup 5q14.2 (~ 10 Mb) |
46,XN,add(5)(?p14) |
arr[GRCh37]5q14.2 (90,230,935–91,382,020) × 3 LP |
None | TOP | Nonsyndromic |
| 4 | 35 | 19+2 |
Dup 22q11.2 (~ 2.7 Mb) |
46,XN |
arr[GRCh37]22q11.21 18,648,855–21,454,872) × 3 pat P |
None | TOP | 22q11 duplication syndrome |
| 1 | 25 | 19+2 |
Dup 15q11.2 q13.1 (~ 4.9 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (23,281,885–28,526,905) × 3 mat P |
None | TOP | 15q11q13 duplication syndrome |
| 28 | 29 | 19+4 |
Dup 17q21.3q31.32 (~ 3.0 Mb) |
46,XN |
arr[GRCh37]17q21.31 (44,187,491–44,784,639) × 3 mat VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 29 | 31 | 19+5 |
Dup 9q21.11q22.3 (~ 25 Mb) |
46,XN |
arr[GRCh37]9q21.11q22.31 (71,013,799–95,657,711) × 3 dn VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 15 | 25 | 19+6 |
Dup 4p16.315.2 (~ 25 Mb) |
46,XN |
arr[GRCh37]4p16.3p15.2 (68,345–25,296,039) × 3 P |
None | TOP | Nonsyndromic |
| 24 | 31 | 20 |
Dup 20q13.2q13.3 (12 Mb) |
46,XN |
arr[hg19]20q13.2q13.33 (51,504,974–62,913,645) × 3 P |
None | TOP | Nonsyndromic |
| 32 | 22 | 20 |
Dup13q12.11q12.13 (~ 3.0 Mb) |
46,XN |
arr[GRCh37]13q12.11q12.12 (22,073,046–25,230,759) × 3 LB |
None | Born (normal phenotype) | Nonsyndromic |
| 33 | 25 | 20 |
Dup 5p15.2p15.1 (~ 2.5 Mb) |
46,XN |
arr[GRCh37]5p15.2p15.1 (14,860,000–16,860,000) × 3 LB |
None | Born (normal phenotype) | Nonsyndromic |
| 3 | 20 | 20 |
Dup 22q11.21q11.21 (~ 2.88 Mb) |
46,XN |
arr[GRCh37]22q11.21 (18,648,855–21,461,017) × 3 mat P |
None | TOP | 22q11 duplication syndrome |
| 2 | 24 | 20+2 |
Dup 22q11.2 (~ 4.0 Mb) |
46,XN | arr[GRCh37]22q11.21 (18,919,477–21,915,207) × 3 mat P | None | Born (normal phenotype) | 22q11 duplication syndrome |
| 14 | 33 | 21 |
Dup 5p15.33p15.2 (~ 31 Mb) |
46,XN,add(21)(p11.2) |
arr[GRCh37]5p15.33p13.3 (113,584–32,448,253) × 3 P |
None | TOP | Nonsyndromic |
| 30 | 29 | 21 |
Dup 2p22.3 (~ 3.1 Mb) |
46,XN |
arr[GRCh37]2p22.3 (34,049,512–35,045,602) × 3 LB |
None | Born (normal phenotype) | Nonsyndromic |
| 11 | 31 | 21 |
Dup 5p15.33p15.2 (~ 15 Mb) |
46,XN,add(21)(p11.2) |
arr[GRCh37]5p15.33p13.3 (113,576–32,448,169) × 3 P |
None | TOP | Nonsyndromic |
| 8 | 33 | 21 |
Dup 2q23.324.2 (~ 9.9 Mb) |
46,XN dup(2)(q23.3q24.2) |
arr[GRCh37]2q23.3q24.2 154,042,539–162,258,705) × 3 dn LP |
None | TOP | Nonsyndromic |
| 13 | 31 | 23 |
Dup 20q13.2q13.3 (~ 12 Mb) |
46,XN |
arr[GRCh37]20q13.2q13.33 (51,504,974–62,913,645) × 3 P |
Fetal lung cystic adenoma | TOP | Nonsyndromic |
| 20 | 40 | 23+1 |
Dup 2q34q37.2 (~ 27.08 Mb) |
46,XN,add(2)(?q34) |
arr[GRCh37]2q34q37.2 (215,025,029–236,132,136) × 3 mat LP |
Polyhydramnios | Lost follow-up | Nonsyndromic |
| 12 | 39 | 23+4 |
Dup 5p15.33p11 (~ 41 Mb) |
47,XN, + mar |
arr[GRCh37]5p15.33p11 (113,576_46,242,541) × 3 P |
None | TOP | Nonsyndromic |
| 31 | 32 | 24 |
Dup 4p16.1 (~ 7.1 Mb) |
46,XN |
arr[GRCh37]4p16.2p15.33 (5,431,644–12,413,075) × 3 LB |
None | Born (normal phenotype) | Nonsyndromic |
| Copy number gain FP (≤ 16 GW) | ||||||||
| 35 | 35 | 14+6 |
Dup 6p25.3p22.2 (~ 4.2 Mb) |
46,XN |
arr[GRCh37]4p16.3 (68,345–4,277,002) × 1 dn P |
None | TOP | Nonsyndromic |
| 37 | 37 | 15+6 |
Dup 8p12 (~ 2.5 Mb) |
46,XN |
arr[GRCh37]16p11.2 (29,351,826–30,190,029) × 1 dn VOUS |
Separation of renal pelvis | Born (normal phenotype) | Nonsyndromic |
| 38 | 39 | 15+6 |
Dup 4p16.3 (~ 3.4 Mb) |
46,XN |
arr[GRCh37]5p13.2 (37,044,025–37,233,386) × 3 pat VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| FP (> 16 GW) | ||||||||
| 39 | 43 | 16+6 |
Dup 7q21.11 (~ 5.1 Mb) |
46,XN |
arr[GRCh37]16p11.2 (29,428,531–30,176,508) × 1 pat VOUS |
Separation of renal pelvis | Born (normal phenotype) | Nonsyndromic |
| 34 | 27 | 17+2 |
Dup 2p12 (~ 2.6 Mb) |
46,XN |
arr[GRCh37]22q11.21 (18,301,185–21,184,093) × 3 P |
Intracardiac echogenic focus | TOP | Nonsyndromic |
| 36 | 34 | 17+6 |
Dup 2p12 (~ 2.1 Mb) |
46,XN |
arr[GRCh37]22q11.21 (78,345,501–79,629,001) × 3 dn P |
None | TOP | Nonsyndromic |
| 40 | 31 | 18+1 |
Dup 16p13.3p11.1 (~ 34.2 Mb) |
46,XN |
arr[GRCh37]16p13.12p11.2 (14628204–32924046) × 2 hmz VOUS |
None | TOP | Nonsyndromic |
| Copy number loss TP (≤ 16 GW) | ||||||||
| 78 | 36 | 14+4 |
Del 13q31.1 (~ 3.71 Mb) |
46,XN |
arr[GRCh37]13q31.1 (83, 494, 767–86, 543, 280) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 69 | 38 | 14+6 |
Del 18p11.3p11.21 (~ 13 Mb) |
47,XN, + mar |
arr[GRCh37]18p11.32p11.21 (136,227–15,099,116) × 1 P |
FGR, HPE | TOP | Nonsyndromic |
| 50 | 32 | 15 |
Del 15q11q13 (~ 5.0 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (23,290,787–28,560,664) × 1 P |
Intracardiac echogenic focus | TOP | PWS/AS |
| 67 | 23 | 15 |
Del Xq22.3 (~ 45 Mb) |
46,XN |
arr[GRCh37]Xq22.3q28 (107,912,179–155,233,098) × 1 P |
None | TOP | Nonsyndromic |
| 45 | 25 | 15 |
Del 22q11 (~ 3.0 Mb) |
46,XN |
arr[hg19]22q11.21 (18,648,855–21,800,471) × 1 P |
None | TOP | DGS |
| 74 | 34 | 15 |
Del 4q31.3q32.2 (11 Mb) |
46,XN |
seq[hg19]4q32.1-q32.2 (155,800,001–164,960,000) × 1 LP |
None | TOP | Nonsyndromic |
| 72 | 23 | 15+5 |
Del 6q25 (~ 17 Mb) |
46,XN |
arr[GRCh37]6q25.1q27 (152,176,966–170,914,297) × 1 P |
None | TOP | Nonsyndromic |
| TP (> 16 GW) | ||||||||
| 70 | 30 | 16 |
Del 7q32q36 (~ 2.3 Mb) |
46,XN |
arr[GRCh]7q36.1 (149,828,703–152,102,066) × 1 P |
None | TOP | Nonsyndromic |
| 53 | 23 | 16+2 |
Del Xq23q28 (~ 3.68 Mb) |
46,XX |
arr[GRCh37]Xq28 (147550751–155233098) × 1 (female) P |
Intestinal dilatation | Born (normal phenotype) | Nonsyndromic |
| 68 | 30 | 16+3 |
Del 7q34q36 (~ 17 Mb) |
46,XN |
arr[GRCh37]7q34q36.38 (142,044,268–159,119,707) × 1 P |
None | TOP | Nonsyndromic |
| 42 | 37 | 16+3 |
Del 22q11.21 (~ 2.99 Mb) |
46,XN |
arr[GRCh37]22q11.21 (18,066,280–21,630,621) × 1 P |
None | TOP | DGS |
| 58 | 27 | 16+4 |
Del 18p11.32 (~ 3.0 Mb) |
46,XN |
arr[GRCh37]18p11.32p11.31 (2,186,353–5,675,587) × 1 mat P |
None | TOP | Nonsyndromic |
| 84 | 25 | 17 |
Del 11q22.3 (~ 5.18 Mb) |
46,XN |
arr[GRCh37]11q22.3 (104,181,493–106,629,690) × 1 mat LB |
None | Born (normal phenotype) | Nonsyndromic |
| 85$ | 33 | 17 |
Del 21q22.12q22.3 (~ 11 Mb) |
46,XN |
arr[GRCh37]21q22.12q22.3 ((36,746,514–48,093,361) × 1 P 8q24.22q24.3 (134,400,222–146,295,771) × 3 LP |
None | TOP | Nonsyndromic |
| 44 | 27 | 17 |
Del 22q11.21 (~ 2.8 Mb) |
46,XN |
arr[hg19]22q11.21 (18,916,842–21,800,471) × 1 P |
VSD | TOP | DGS |
| 66 | 25 | 17 |
Del 6q25 (~ 17 Mb) |
46,XN |
arr[GRCh37]6q25.1q27 (152,176,966–170,914,297) × 1 P |
Intestinal dilatation | Lost follow-up | Nonsyndromic |
| 60 | 28 | 17 |
Del 8q12.1q21.2 (~ 6.69 Mb) |
46,XN |
arr[GRCh37]8q12.3q13.2 (63,249,055–69,695,857) × 1 dn LP |
None | TOP | Nonsyndromic |
| 63$ | 28 | 17 |
Del 18q21.31q23 (~ 35 Mb) |
46,XN,-18, + mar |
arr[GRCh37]18p11.32p11.31 (136,227–3,348,254) × 1, P 18p11.31p11.21 (3,350,736–13,083,388) × 3, P 18p11.21 (13,090,666–15,170,636) × 1, P 18p11.21q21.31 (15,181,207–54,008,143) × 3, P 18q21.31q23 (54,020,488–78,013,728) × 1 P |
Fetal aorta coarctation, pulmonary artery stenosis, VSD | TOP | Nonsyndromic |
| 75 | 31 | 17+2 |
Del 1p31 (~ 4.5 Mb) |
46,XN |
arr[GRCh37]1p31.1 (78,282,099–84,553,373) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 76 | 28 | 17+2 |
Del 18q22.3 (~ 2.2 Mb) |
46,XN |
arr[GRCh37]18q22.3 (69,288,001–71,535,501) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 71 | 28 | 17+3 |
Del 9p24.3p24.2 (~ 2.5 Mb) |
46,XN |
arr[GRCh37]9p24..3p24.2 (208,454–2,920,085) × 1 P (152,093,040–159,118,443) × 2 hmz VOUS |
Dandy–Walker malformation | TOP | Nonsyndromic |
| 81 | 20 | 17+6 |
Del 15q25.2q26.3 (~ 18 Mb) |
46,XN |
arr[GRCh37]15q25.2q26.3 (83,759,214–102,397,317) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 49 | 37 | 18 |
Del 15q11.2q13.1 (~ 5.0 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (23,290,787–28,659,911) × 1 P |
None | TOP | PWS/AS |
| 46 | 33 | 18+1 |
Del 22q11 (~ 2.5 Mb) |
46,XN |
arr[hg19]22q11.21 (18,636,749–21,136,749) × 1 P |
None | TOP | DGS |
| 52 | 33 | 18+3 |
Del 5p15.32p15.2 (~ 5.3 Mb) |
46,XN |
arr[GRCh37]5p15.33p15.2 (113,576–10,477,490) × 1 P |
Single umbilical artery | TOP | Cri-Du-Chat |
| 77 | 30 | 18+6 |
Del 15p13.1p14 (~ 2.7 Mb) |
46,XN |
arr[GRCh37]15q13.2q13.3 (30,241,910–32,991,173) × 1 VOUS |
Subependymal cyst | Born (normal phenotype) | Nonsyndromic |
| 73 | 28 | 19 |
Del 17p13.3–13.2 (4.2 Mb) |
46,XN |
arr[GRCh37]17p13.3p13.2 (525–4,669,796) × 1 P |
None | TOP | Miller–Dieker syndrome |
| 79 | 31 | 19 |
Del 11p15.1p13 (~ 11.18 Mb) |
46,XN,del(11)(p13p15) |
arr[GRCh37]11p15.1p13 (19,973,767–31,001,449) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 59 | 26 | 19+1 |
Del 10q26.13q26.3 (~ 7.0 Mb) |
46,XN,del(10)(q26.1) |
arr[GRCh37]10q26.13q26.3 (125,262,198–135,426,386) × 1 P |
None | TOP | Nonsyndromic |
| 57 | 28 | 19+2 |
Del 17p13.213.3 (~ 4.2 Mb) |
46,XN |
arr[GRCh37]17p13.3p13.2 (525–4,669,796) × 1 P |
None | TOP | Nonsyndromic |
| 80 | 31 | 19+2 |
Del 4q26 (~ 10 Mb) |
46,XN |
seq[GRCh37]4q26 (114,340,000–119,800,000) × 1 VOUS |
Enhanced liver echo | Born (normal phenotype) | Nonsyndromic |
| 43 | 25 | 19+2 |
Del 22q11.21 (~ 3.72 Mb) |
46,XN |
arr[GRCh37]22q11.21 (18631365–21800471) × 1 P |
None | TOP | DGS |
| 65 | 27 | 19+4 |
Del 5q23.1 (~ 12 Mb) |
46,XN |
arr[GRCh37]5q21.3q23.1 (107,915,007–120,847,610) × 1 P |
None | TOP | Nonsyndromic |
| 47 | 32 | 19+4 |
Del 22q11.21 (~ 3.0 Mb) |
46,XN |
arr[hg19]22q11.21 (18,916,842–21,800,471) × 1 P |
None | TOP | DGS |
| 82 | 31 | 19+5 |
Del 9p23p13.1 (~ 24 Mb) Del 9q21.11q22.3 (~ 25 Mb) |
46,XN |
arr[GRCh37]9p23p13.1 (13,107,600–38,771,831) × 1 dn VOUS arr[GRCh37]9q21.11q22.31 (13,107,600–38,771,831) × 1 dn VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 83 | 31 | 19+5 |
Del 9p23p13.1 (~ 25.6 Mb) |
46,XN |
arr[GRCh37]9p23p13.1 (13,107,600–38,771,831)x1dn VOUS |
Subependymal cyst | Born (normal phenotype) | Nonsyndromic |
| 55*$ | 35 | 19+5 |
Del Xp22.31 (~ 2.6 Mb) Del 10q21.1 (~ 3.8 Mb) |
46,XY |
arr[GRCh37]Xp22.31 (6,455,152–8,141,076) × 0,mat (male) P 10q21.1 (55,657,551–57,504,582) × 1 VOUS |
Fetal mild tricuspid regurgitation | Born (mild ichthyosis phenotype) | XLR ichthyosis |
| 54 | 37 | 20 |
Del Xp22.31 (~ 21 Mb) |
46,XY |
arr[GRCh37]Xp22.31 (6455152_8135568) × 0 mat P |
None | Born (mild ichthyosis phenotype) | XLR ichthyosis |
| 62 | 33 | 21 |
Del 18q21.33q23 (~ 18 Mb) |
46,XN,del(18)(?q21) |
arr[GRCh37]18q21.33q23 (59,280,654–78,013,728) × 1 P |
None | TOP | Nonsyndromic |
| 48 | 30 | 22+1 |
Del 15q11.1q13.1 (~ 8.75 Mb) |
46,XN |
arr[GRCh37]15q11.2q13.1 (22,770,422–28,928,730) × 1 P |
None | TOP | PWS/AS |
| 61 | 34 | 22+4 |
Del 18q11.2q12.1 (~ 6.0 Mb) |
46,XN |
arr[GRCh37]18q11.2q12.1 (19,886,814–27,306,978) × 1 P |
None | TOP | Nonsyndromic |
| 51 | 31 | 22+5 |
Del 15q15.33p14.3 (~ 21 Mb) |
46,XN,del(5)(p14.3) |
arr[GRCh37]5p15.33p14.3 (113,577–21,810,739) × 1 P |
None | TOP | Cri-Du-Chat |
| 56 | 34 | 26 |
Del 4q31.3q32.2 (11 Mb) |
46,XN |
seq[GRCh37]4q32.1q32.3 (155,800,001–164,960,000) × 1 LP |
Abnormal posture of right foot | TOP | Nonsyndromic |
| 64 | 39 | 26+2 |
Del 13q32.1q34 (~ 17.8 Mb) |
46,XN,r(13) (?p11q32) [61]/45,XX,-1324 |
arr[GRCh37]13q31.3q34 (94,929,201–115,107,733) × 1 P |
FGR, absence of a-wave of ductus venosus, HPE, corpus callosum dysplasia | TOP | Nonsyndromic |
| 41$ | 30 | 26 |
Del 18p11.3q22.3 (~ 5 Mb) |
46,XN,r(18)(p11q22)[97]/46,XN,\idic r(18)(p11q22)13 /45,XN,-183/47,XN,idic r(18) (p11q22) × 22 |
arr[GRCh37]18p11.32p11.31 (136,227–3,334,683) × 1, LP 18p11.31q22.3 (3,342,699–72,722,952) × 3, P 18q22.3q23 (72,723,195–78,013,728) × 1 LP |
None | TOP | Nonsyndromic |
| Copy number loss FP (≤ 16 GW) | ||||||||
| 94 | 39 | 15 |
Del 18p23.3p23.1 (~ 5.1 Mb) |
46,XN,15ph |
arr[GRCh37]8p23.3p23.1 (168,483–6,999,220) × 2 hmz VOUS 8p23.1p12 (8,117,564–32,069,805) × 2 hmz VOUS |
Atrial septal defect | Born (normal phenotype) | Nonsyndromic |
| 90 | 34 | 15 |
Del 22q11.21 (~ 3.0 Mb) |
46,XN |
arr[hg19]4q13.2 (69,344,443–69,565,861) × 1 VOUS |
None | Born (normal phenotype) | DGS |
| 98 | 25 | 15 |
Del 1p36.32p36.31 (5.7 Mb) |
46,XN |
arr[GRCh37]4q24q25 (107033067–109404131) × 1 LP |
None | TOP | 1p36 deletion syndrome |
| 95 | 29 | 15+2 |
Del Xp21.1q28 (~ 75.29 Mb) |
46,XN |
arr[GRCh37]8p21.2 (23,725,923–24, 936,161) × 3 pat VOUS |
ARSA | Born (normal phenotype) | TS |
| 88 | 25 | 15+5 |
Del 22q11.21 (~ 3.5 Mb) |
46,XN |
arr[GRCh37]4q13.2 (69,344,443–69,565,861) × 1 VOUS |
None | Born (normal phenotype) | DGS |
| 99 | 27 | 16 |
Del 7q22.1q31.1 (10 Mb) |
46,XN |
arr[GRCh37]11p14.1p12 (30,211,776–36,615,043) × 1 P |
Bilateral pleural effusion | Born | Nonsyndromic |
| FP (> 16 GW) | ||||||||
| 101 | 39 | 17 |
Del 15q11.2q13.1 (~ 4.2 Mb) |
46,XN |
arr[GRCh37]17p11.2 (16,727,490–20,433,723) × 1 P |
VSD | Born | AS/PWS |
| 89 | 26 | 17 |
Del 4q31-qter (~ 10 Mb) |
46,XN |
arr[GRCh37]4q32.3q35.2 (167230247–190921709) × 2 hmz VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 91 | 34 | 17+2 |
Del 2p13.3p11.2 (~ 4.9 Mb) |
46,XN |
arr[GRCh37]8q11.1q11.2 (46,919,156–51,932,566) × 1, VOUS 9p23(9,216,123–12,914,396) × 1 VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 93 | 34 | 17+4 |
Del 1q31.1q32.2 (~ 4.8 Mb) |
46,XN |
arr[GRCh37]6p22.3 (17,867,202–18,765,914) × 1 pat VOUS |
None | Born (normal phenotype) | Nonsyndromic |
| 86 | 28 | 18 |
Del 7q21.11q31.2 (~ 31 Mb) |
46,XN |
arr[GRCh37]22q11.21q11.22 (21,464,764–22,962,962) × 1 dn P |
None | TOP | Nonsyndromic |
| 87 | 25 | 18 |
Del 18p21.3q23 (~ 22.13 Mb) |
46,XN |
seq[hg19] dup (17)( p13.3p13.3) (1–712,489) × 3 LP |
None | Born (normal phenotype except high arch) | Nonsyndromic |
| 92 | 29 | 18+6 |
Del 21q22.3 (~ 3.4 Mb) |
46,XN |
arr[GRCh37]13q21.2 (59608821–60,709,021) × 1 pat VOUS |
VSD | Born (normal phenotype) | Nonsyndromic |
| 96 | 29 | 20 |
Del 13q12 (~ 3.2 Mb) |
46,XN |
arr[GRCh37]5q14.1 (76,983,283–77,512,158) × 3 mat LB |
VSD | Born | Nonsyndromic |
| 97 | 38 | 22 |
Dup Xq28 (~ 7 Mb) |
46,XN |
arr[GRCh37]4q31.3q32.2 (155,463,038–162,158,990) × 1 dn P |
Complete endocardial cushion defect (unbalanced), coarctation of the aorta | Born | Nonsyndromic |
| 100 | 35 | 23 |
Del 4p16.3p15.33 (12 Mb) |
46,XN |
arr[GRCh37]7q36.2q36.3 (152,747,657–159,119,707) × 1 P |
FGR | Born (SGA) | WHS |
MA maternal age, GW gestional weeks, LFU, TOP terminate of pregnancy, MS-MLPA methylation-specific multiplex ligation-dependent probe amplification, mat maternal, pat paternal, P pathogenic, LP likely pathogenic, VOUS variants of uncertain significance, CMA chromosomal microarray, CNV copy number variation, NIPT noninvasive prenatal testing, TS turner syndrome, UA ultrasound anomalies, WHS wolf-hirschhorn syndrome, XLR X-linked recessive, SGA small for gestational age, ARSA aberrant right abuclavian artery, VSD ventricular septal defect, FGR fetal growth restriction, HPE holoprosencephaly.
*Fetal MS-PLPA: methylation-specific multiplex ligation-dependent probe amplification, paternal duplication.
$When there are multiple CNVs, only the highest pathogenicity classification is calculated.
Among the 203 cases with validation, P/LP CNVs were identified in 70 cases, including 19 cases with susceptibility loci (SL) for neurodevelopmental disorders (NDD) and 18 cases with abnormal karyotypes. Of the 18 fetuses, 14 had confirmed CNVs ≥ 10 Mb and 4 had CNVs < 10 Mb. In addition, CMA also detected 5 LOH (Tables 2, 3).
Table 3.
The overall PPV and the rate of TP in each of these two cohorts (at ≤ 16 weeks and > 16 weeks).
| GW at NIPT | n | NIPT positive | TP | FP$ | FN* | Refused invasive testing | PPV(%) |
|---|---|---|---|---|---|---|---|
| ≤ 16 | 13,172 | 34 | 13 | 14 | 3 | 7 | 48.1 |
| > 16 | 18,084 | 187 | 65 | 111 | 4 | 11 | 36.9 |
*,$13 of 18 pregnancies who declined invasive testing with no confirmed test and 23 pregnancies that have been lost follow-up with low-risk results were excluded when making data statistics.
There were 27 cases of CNVs associated with classical MMS. This comprised 8 cases at high risk of DGS, 5 cases at high risk of 22q11.2 microduplication syndrome, 6 cases of PWS/AS, 4 cases of CDC, and 4 cases of 1p36 deletion syndromes. Of the eight cases of suspected DGS, there were 6 TPs and 2 FPs yielding a PPV of 75%. Of the five cases of suspected 22q microduplication syndrome, there were four TPs and one FP, yielding a PPV of 80%. For the six suspected PWS/AS cases, there were three TPs and three FPs; for the four suspected CDC cases, there were two TPs and two FPs. Finally, four cases indicated as a 1p36 deletion proved to be FP (Table 4).
Table 4.
Performance of NIPT-Plus for detection of clinically significant CNVs in 31,256 pregnancies.
| Clinically significant CNVs | TP | FP/FPR | PPV | TN | FN/FNR | NPV | Specificity |
|---|---|---|---|---|---|---|---|
| Classical MMS | 15 | 12/0.038% | 55.56% | 31,226 | 4/21.1% | 99.99% | 99.96% |
| 22q11.2 deletion syndrome | 6 | 2/0.006% | 75% | 31,247 | 1/14.29% | 100% | 99.99% |
| 22q11.2 duplication syndrome | 4 | 1/0.003% | 80% | 31,249 | 2/33.3% | 99.99% | 100% |
| Cri-du-Chat syndrome | 2 | 2/0.006% | 50% | 31,252 | 0/0% | 100% | 99.99% |
| Prader–Willi syndrome/Angelman syndrome | 3 | 3/0.01% | 50% | 31,250 | 0/0% | 100% | 99.99% |
| 1p36 deletion syndrome | 0 | 4/0.01% | 0% | 31,251 | 1/100% | 100% | 99.99% |
| Other genome-wide CNVs | 63 | 113/0.36% | 35.80% | 31,077 | 3/4.55% | 99.99% | 99.64% |
| ˃ 10 Mb | 33 | 38/0.12% | 46.5% | 31,183 | 2/5.71% | 99.99% | 99.88% |
| ≤ 10 Mb | 30 | 75/0.24% | 28.57% | 31,150 | 1/3.23% | 100% | 99.76% |
| Total | 78 | 125/0.40% | 38.42% | 31,046 | 7/8.24% | 99.98% | 99.60% |
CNV copy number variation, TP true positive, FP false positive, FPR false positive rate, PPV positive predictive value, TN true negative, FN false negative, FNR false negative rate, NPV negative predictive value.
The remaining 175 cases of fetal CNVs were segmental CNVs that were classified as other genome-wide CNVs. Of these, there were 34 TPs and 40 FPs for CNVs ≥ 10 Mb (PPV, 45.95%) and 28 TPs and 73 FPs for CNVs < 10 Mb (PPV, 27.72%) (Tables 4, 5).
Table 5.
The PPVs for all fetal CNVs indicated by NIPT-Plus according to different CNV sizes.
| CNV size detected by NIPT | NIPT positive | TP | FP | Refused invasive testing | PPV(%) |
|---|---|---|---|---|---|
| Within 2–4 Mb | 97 | 24 | 70 | 3 | 25.5 |
| Within 4–7 Mb | 29 | 14 | 13 | 2 | 51.9 |
| Within 7–10 Mb | 16 | 6 | 3 | 7 | 66.7 |
| > 10 Mb | 79 | 34 | 39 | 6 | 46.6 |
| Total | 221 | 78 | 125 | 18 | 38.4 |
TP true positive, FP false positive, PPV positive predictive value.
Pregnancy outcomes
All pregnant women with TP NIPT results underwent genetic counseling to discuss pregnancy intention. While the majority of women diagnosed with fetal clinically significant CNVs elected termination of pregnancy (TOP), a relatively small proportion of pregnant women chose to continue their pregnancies. The TOP rates were much higher in pregnancies diagnosed with known MMS, including DGS (100%), PWS/AS (100%), and CDC (100%). In contrast, elective TOP rates were much lower in women carrying a fetus with 22q11.2 microduplication syndrome (50%) (Table 2).
Seven pregnancies with pathogenic CNVs were missed by NIPT-Plus (negative predictive value 99.98%) (Table 7). The FN cases with clinically significant CNVs included one of eight cases with confirmed DGS, one of five cases with confirmed 22q11.2 microduplication syndrome, one of four cases with confirmed 1p36 deletion syndrome, as well as a Wolf-Hirschhorn syndrome (WHS) case, a 8q24.22q24.3 duplication case, a 16p11.2 deletion case, and a 15q11.2 deletion case. In one of the seven (14.29%) FN cases, prenatal ultrasound detected fetal abnormalities, and pregnancies were terminated upon confirmation via invasive diagnostic testing. The remaining six FN cases were identified only at birth and subsequently confirmed by postnatal chromosome analysis. In the 3–12 months follow-up period after birth, no other FN cases were identified.
Table 7.
Seven cases with false negative NIPT results missed by NIPT-Plus with validation.
| Case no. | Clinically significant CNVs by CMA | Z-score of CNV | Fetal fraction | Prenatal/postnatal findings |
|---|---|---|---|---|
| FN-1 |
arr[GRCh37] 22q11.21(18631365_21800471) × 1 DGS |
− 2.51 | 9.1% | NOT identified by ultrasound prenatally, detected due to ventricular septal defect postnatally |
| FN-2 |
arr[GRCh37]1p36.33p36.32(849,466–4,894,800) × 1 1p36 deletion syndrome |
1.34 | 11.58% | NOT identified by ultrasound prenatally, detected due to language retardation postnatally |
| FN-3 |
arr[GRCh37] 16p11.2(29,428,531–30,177,916) × 1 Nonsyndromic |
2.78 | 5% | Fetal ultrasound anomalies: single umbilical artery, left renal parenchyma echogenicity enhancement, upper ureter dilatation on ultrasound |
| FN-4 |
arr[GRCh37] 4p16.3p15.2(68,345–22,489,538) × 1 WHS |
− 2.97 | 13.8% | NOT identified by ultrasound prenatally, detected due to VSD postnatally |
| FN-5 |
arr[GRCh37]22q11.21 (18,919,477_21,915,207) × 3 22q11.2 duplication syndrome |
2.56 | 10.8% | NOT identified by ultrasound prenatally, detected due to developmental delay and cleft palate postnatally |
| FN-6 |
arr[GRCh37] 8q24.11q24.3(117,830,985_146,295,771) × 3 Nonsyndromic |
2.83 | 13.2% | NOT identified by ultrasound prenatally, detected due to seizure postnatally |
| FN-7 |
arr[GRCh37] 15q11.2(22,770,421–23,625,785) × 1 Nonsyndromic |
2.71 | 6.8% | NOT identified by ultrasound prenatally, detected due to mental retardation postnatally |
DGS DiGeorge syndrome, WHS Wolf-Hirschhorn syndrome, FN false negative, VSD ventricular septal defect.
The underlying causes of these seven FNs were further investigated. In all seven pregnancies, low FF was unlikely because FF values ranged from 5 to 13.8%. The other five women refused further placenta studies; thus, each one case of WHS and 1p36 deletion syndrome was further investigated via placental tissue chromosomal analysis. From four placental biopsy samples, no evidence of 4p16.3 deletion and 1p36 deletion was identifiable, suggesting possible confined placental mosaicism (CPM) as a cause of the two FN results.
The follow-up of 18 pregnant women with high-risk CNVs detected by NIPT-Plus is shown in Table 6. Interestingly, there were three FP cases with normal fetal and placental anatomies but complicated with multiple 5–10.5 cm uterine leiomyomas detected via ultrasound, though their prior obstetrical and gynecologic history was negative. In one case, NIPT indicated a 38 Mb deletion at 7q21.11q31.31 (FF: 6.4%); in another case, NIPT indicated multiple CNVs involving chromosomes 3, 4, 7, 10, and 12 (a 40 Mb deletion at 3q25.2q29, a 20 Mb deletion at 4q24q28.1, a 70 Mb deletion at 7q11.23q34, a 24 Mb deletion at 10q22.3q24.31, and a 25 Mb deletion at 12q12q14.2 (FF: 10.8%). In the third case (case 86 in Table 2), NIPT indicated a 31 Mb deletion at 7q21.11q31.2; further CMA on amniocytes identified a 1.5 Mb microdeletion at 22q11.21q11.22, which is associated with DGS, and the pregnancy was terminated. Confirmatory CMA on amniocytes did not show any pathogenic CNV in the other two cases. CMA studies of the three placentas after induction or postpartum did not show the existence of abnormal CNVs.
Table 6.
The follow-up of 18 pregnant women with high‐risk CNVs detected by NIPT-Plus refused invasive testing prenatally due to fetal ultrasound structural anomalies or contraindications to prenatal diagnosis.
| Case ID | Prenatal ultrasound finding | Postnatal cord blood CMA results/pathogenicity classification) | Cord blood karyotyping | Associated disease with validation | Pregnancy outcome | NIPT-plus result |
|---|---|---|---|---|---|---|
| 1 | Complete endocardial cushion defect, hydramnios | Not done | Not done | No result | TOP | Del 20q11.23q13.31 (18 Mb) |
| 2 | FGR | arr[GRCh37]46, XY | 46, XY | No result | Preterm birth at 35+4w, normal phenotype | Del 1p36.32p36.31 (5.5 Mb) |
| 3 | Bilateral pleural effusion | arr[GRCh37]3p26.3 (61,891–2,441,042) × 1 VOUS | Normal | No result | TOP |
Del 7q22.1-q31.1 (8 Mb) |
| 4 | FGR,fetal BPD was less than the mean value 3.7SD, HC was less than the mean value 4.7SD | Not done | Not done | No result | TOP |
Del 4p16.3p15.33 (13 Mb) |
| 5 | Fetal ventricular septal defect | Not done | Not done | No result | Born (normal phenotype) |
Del Xp22.31 (~ 5.8 Mb) |
| 6 | Partial absence of corpus callosum | arr[GRCh37]46,XX | Normal | No result | Born (normal phenotype) |
Del 1p36.32-p36.23 (~ 5.1 Mb) |
| 7* | Fetal pelvic ectopic kidney with multiple cystic changes? | Not done | Not done | No result | Born (normal phenotype) |
DupXq28 (~ 7 Mb) |
| 8 | Fetal FL and HL were less than the mean value 2SD | Not done | Not done | No result | Born (normal phenotype) |
Del 10q25.310q26.3 (~ 16.8 Mb) |
| 9 | Right hydrocephalus | arr[GRCh37]46, XX | 46, XX | No result | Born (normal phenotype) | Del 16p12.1-p11.2 (4.66 Mb) |
| 10 | Fetal double kidney echo enhanced | arr[GRCh37]46, XY | 46, XY | No result | Born (normal phenotype) |
Del 15q11.2–13 (5 Mb) |
| 11 | cerebellar dysplasia, smoon brain? strephenopodia | Not done | Not done | No result | TOP |
Del 22q11.2 (5.2 Mb) |
| 12* | Normal | Not done | Not done | No result | Born (normal phenotype) |
Del 15q11.2–13 (4.5 Mb) |
| 13 | Normal | Not done | Not done | No result | Born (normal phenotype) |
Del 4p16.3-p15.33 (12 Mb) |
| 14* | Single umbilical artery | Not done | Not done | No result | Born (normal phenotype) |
Del 7q22.1-q31.1 (10 Mb) |
| 15 | Intestinal echo enhancement | Not done | Not done | No result | Born (normal phenotype) |
Del 10q25.3q26.3 (16.8 Mb) |
| 16 | Minimal pulmonary regurgitation, skin thickening of head and neck back | Not done | Not done | No result | Born (normal phenotype) |
Dup 14q31.1q31.32 (22 Mb) |
| 17* | Persistent left superior vena cava | Not done | Not done | No result | Born (normal phenotype) |
Dup 2p25.3p24.3 (11.5 Mb) |
| 18* | Fetal nasal bone dysplasia | Not done | Not done | No result | Born (normal phenotype) |
Dup 4p16.17.1 (7.1 Mb) |
VOUS variants of uncertain significance.
*Contraindications to prenatal diagnosis.
Low‐risk NIPT CNV results
Of the 31,035 cases with fetal low-risk P/LP CNVs, 23 were lost to follow-up; thus, 99.93% (31,012/31,035) of thees cases were successfully followed. Twenty-five fetuses underwent diagnostic tests because of abnormal ultrasound findings. Of which, 24 cases showed normal karyotype as well as CMA results, and had normal live births. One fetus harbored pathogenic CNVs (FN-3 in Table 7), and the pregnant couple terminated the pregnancy given the test results. Among the 24 cases with normal karyotype and CMA results, one fetus died in utero owing to preeclampsia and multiple malformations, one fetus died in utero owing to oligohydramnios, and three fetuses were born preterm because of fetal growth restriction (FGR), premature rupture of membranes, intrauterine cytomegalovirus infection, respectively, and 15 women terminated the pregnancies due to fetal multiple ultrasound anomalies. No abnormalities were found in the remaining low-risk pregnant women during the 3–12 months postnatal follow-up.
Discussion
Chromosomal abnormality is one of the most important causes of birth defects, and there is no effective method to deal with it. The aim of prenatal screening is to identify fetal chromosomal abnormalities. Currently, in comparison to traditional MSS for Down syndrome, NIPT screening for common trisomy and sex chromosome aneuploidy is more popular among pregnant women; however, it is still controversial whether NIPT should screen for MMS2,32. In the present study, we investigated the performance of NIPT-Plus for fetal P/LP CNVs in 31,256 pregnant women and assessed its clinical value.
Opponents have argued that the relatively low PPV, high FPR, and uncertain pathogenesis of CNVs cause a dilemma in interpreting reports on high-risk results, putting significant psychological stress on pregnant women, and even increasing unnecessary invasive diagnostic procedures and their associated risks. However, proponents have debated that the purpose of prenatal screening and prenatal diagnosis is to prevent the birth of infants with the burden of fetal chromosomal anomalies, even for MMS with low PPVs. Most MMSs occur randomly, because the risk of fetal CNVs is not related to the age of pregnant women, which is beneficial for pregnant women of all ages on NIPT screening for CNVs, which are observed in 1.0–2.0% of birth defects without ultrasound anomalies33. Individually, although the incidence of MMSs is low, they are more frequent than that of Down syndrome. Previous studies have shown that NIPT has a certain sensitivity in identifying some classic MMSs. In our study, 19 (9.36%, 19/203) fetuses harbored CNVs associated with SL for NDD; thus, NIPT would also provide the possibility of screening certain fetal chromosomal anomalies such as SL for NDD, which do not show significant abnormalities on ultrasound, such as neurodevelopmental abnormalities, mental retardation, developmental delays, autism, and so on, in addition, the size of CNVs associated with SL is usually below 5–10 Mb, which cannot usually be detected by karyotyping. Despite this, ACMG does not currently recommend NIPT screening for P/LP CNVs31. Recently, routine screening for MMS has been recommended for younger women because microdeletions are more common than aneuploidies2.
In this study, among 203 confirmed cases with fetal suscepted CNVs screened via NIPT, 78 were TP and 125 were FP, with an overall PPV of 38.42%, suggesting that NIPT demonstrates some efficiency in screening P/LP CNV, although the chance of FP cases was relatively high. In our study, P/LP CNVs through diagnostic testing were observed in 70 cases; Eighteen cases had abnormal karyotypes, of which fourteen fetuses with CNVs > 10 Mb and four fetuses with CNVs ≤ 10 Mb were confirmed (Table 2). Although NIPT has a relatively high FPR for CNVs, it is difficult to detect CNVs less than 10 Mb by conventional karyotype analysis. From this point of view, NIPT can compensate for the deficiency of karyotype analysis to some extent. Our data suggest that NIPT screening performance for CNV is not relatively good, which may be related to the small sample size, refusal of further diagnostic testing of a small proportion of pregnant women, CPM, and maternal CNV34. Overall, our data indicate that NIPT has clinical significance for the detection of fetal MMSs, which can provide an important basis for interventional prenatal diagnosis.
The study reported by Liang et al.19 showed that NIPT exhibited high sensitivity and specificity for the detection of clinically significant CNVs. In our study, for classic MMSs (n = 27), the PPV were 75% (DGS), 80% (22q11.22 microduplication), 50% (PWS/AS), and 50% (CDC). For the remaining clinically significant fetal CNVs (n = 175), combined PPVs were 45.95% (CNVs ≥ 10 Mb) and 27.18% (CNVs < 10 Mb), which is slightly higher than that reported by Liang et al.19 The slight difference may be related to the different sample sizes and NIPT sequencing depth.
For the classic MMSs in our study, the PPV for 22q11.2 microduplication syndrome in this study was very high (80%). The overall PPV for the detection of other MMSs varied. For DGS, PWS/AS, CDC, and 1p36 deletion syndrome, the PPVs were 75%, 50%, 50%, and 0%, respectively. The PPVs for PWS and CDC were slightly lower than those reported, with reported PPVs of 75% and 40%, respectively19. Petersen et al. reported that the PPV for CDC, PWS, 1p36 deletion syndrome, and DGS was 0%, 0%, 14%, and 21%, respectively35. Low-level CPM resulted in one FP case of DGS36; Thus, we speculate that CPM may also be the potential etiology in two FP DGS cases, three FP PWS/AS cases, two FP CDC cases, and one four 1p36 deletion cases.
The combined frequency of FN in MMS was 0.022% (7/31,256). These included one fetus identified via ultrasound prenatally and six detected only at birth. Thus, the frequency of FNs can be reduced to 0.019% by prenatal ultrasound examination. Thus, prenatal ultrasound results should be combined to consider the need for further invasive testing, consequently improving the detection rate of fetal MMSs37. We speculate that the FNs may be caused by biological factors other than a low FF. In two FN cases of WHS and 1p36 deletion syndrome, placental chromosomal analysis revealed no 4p16.3 and 1p36 deletion, which could explain the two FN NIPT results, suggesting possible CPM is considered as a cause of the two FN results. Although placental chromosome studies of the other four FNs cases are lacking, we speculated that low-level CPM might be the underlying cause of FN.
FP CNVs detected by NIPT can also be attributed to CPM38 and the death of a twin in utero. In this study, 125 of the 203 cases were confirmed to be FP. Unfortunately, placental biopsies were obtained after delivery or pregnancy termination for 15 of the 125 fetuses with normal genetic results, and 2 of them ultimately turned out to be CPM with CNVs, which presented with FGR. This supports the fact that CPM involving some P/LP CNVs may be associated with adverse pregnancy outcomes39. In our cohort, there were three FP pregnancies with normal fetal and placental anatomies but complicated with multiple 5–10.5 cm uterine leiomyomas detected via ultrasound. Further diagnostic results revealed that fetal and placental lesions were normal except in case 86. Thus, we speculated that uterine leiomyoma may confound the results of NIPT screening for CNV and lead to FP40. Therefore, when the medical history of the pregnant woman should be further understood when NIPT screening for CNV is positive.
Given the performance of NIPT for detecting MMSs in the present and other reported studies, compared to traditional serological screening, we propose that NIPT could be a candidate for first-line screening of pathigenic CNVs for all pregnancies, irrespective of maternal age9. Currently, there are no other methods available to screen for MMSs, although NIPT has a high FPR.
Factors that influence the performance of CNVs detection include CNV size, sequencing depth, FF, and GC content41. In our study, CNVs detected by NIPT were distributed in chromosome X and each autosome, except for chromosome 19. CNVs were frequently found on chromosomes 2, 4, 15, and 18. We speculated that chromosome 19 is very rarely involved, primarily because of its high GC content.
NIPT has a better detection performance for fetal CNVs ≥ 10 Mb at conventional sequencing depths42. However, their ability to detect smaller CNVs is reduced. In our study, the PPV of nonsyndromic CNVs greater than 10 Mb was slightly higher than that of CNVs less than 10 Mb detected by NIPT (45.95% vs 27.18%, p > 0.05), and our data showed that NIPT demonstrates good performance in detecting fetal CNVs, especially for CNVs ≥ 10 Mb, similar to the results of the study by Yu et al.43.
This study had some limitations. First, studies on placentas and maternal CNVs were not routinely conducted to explore the cause of discordance between NIPT results and normal invasive diagnostic results. Second, the sample size was not large enough, and further research is required to accumulate more data. Third, the data are based on a cohort from a single tertiary referral center, and there exists regional bias.
Fourth, the genetic information is incomplete due to 23 pregnancies with low-risk results that were lost follow-up and 18 pregnancies who declined invasive testing.
Our data indicate the potential significance of NIPT in screening clinically significant CNVs. NIPT exhibited high performance for the detection of 22q11.2 duplication syndrome and DGS, low to moderate detection performance for other clinically significant CNVs. We believe that NIPT-Plus combined with ultrasound examination and maternal history examination screening for MMS may be more effective in further multicenter studies with a larger population, increased sequencing depth, and improved bioinformatics analysis algorithms.
Acknowledgements
We thank the family members who participated in our study. We also appreciate the obstetricians, radiographers, sonographer and pediatricians who offered assistance to our study.
Abbreviations
- NIPT
Noninvasive prenatal testing
- CNVs
Copy number variations
- CMA
Chromosomal microarray analysis
- LOH
Loss of heterozygosity
- VOUS
Variants of unknown significance
- AMA
Advanced maternal age
- MMS
Microduplication/microdeletion syndrome
- FPR
False positive rate
- CPM
Confined placental mosaicism
- NDD
Neurodevelopmental disorders
- SL
Susceptibility loci
- PPV
Positive predictive value
- TP
True positive
- FP
False positive
- TN
True negative
- FN
False negative
- PWS/AS
Prader–Willi/Angelman syndromes
- CDC
Cri-du-chat
- DGS
DiGeorge syndrome
- TOP
Termination of pregnancy
- WHS
Wolf-Hirschhorn syndrome
Author contributions
H.X., M.L. and A.Y. prepared the main manuscript; X.C., L.X., H.H. and Q.G. prepared the figures 1-3 and all the Tables. All authors reviewed the manuscript.
Funding
This study was sponsored by the Joint Funds for the Innovation of Science and Technology, Fujian Province (No. 2020Y9149), 2021 Fujian provincial health technology project (No. 2021GGA051), and Natural Science Foundation of Fujian Province (No. 2022J01421).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
These authors contributed equally: Huili Xue and Aili Yu.
Contributor Information
Huili Xue, Email: xhuili345@163.com.
Hailong Huang, Email: hl-hai@163.com.
References
- 1.Webber DM, MacLeod SL, Bamshad MJ, Shaw GM, Finnell RH, Shete SS, Witte JS, Erickson SW, Murphy LD, Hobbs C. Developments in our understanding of the genetic basis of birth defects, birth defects research, Part A. Clin. Mol. Teratol. 2015;103:680–691. doi: 10.1002/bdra.23385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Evans MI, Wapner RJ, Berkowitz RL. Noninvasive prenatal screening or advanced diagnostic testing: Caveat emptor. Am. J. Obstet. Gynecol. 2016;215:298–305. doi: 10.1016/j.ajog.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 3.Gil MM, Accurti V, Santacruz B, Plana MN, Nicolaides KH. Analysis of cell-free DNA in maternal blood in screening for aneuploidies: Updated meta-analysis. Ultrasound Obstet. Gynecol. 2017;50:302–314. doi: 10.1002/uog.17484. [DOI] [PubMed] [Google Scholar]
- 4.Norton ME, Jacobsson B, Swamy GK, Laurent LC, Ranzini AC, Brar H, Tomlinson MW, Pereira L, Spitz JL, Hollemon D, Cuckle H, Musci TJ, Wapner RJ. Cell-free DNA analysis for noninvasive examination of trisomy. N. Engl. J. Med. 2015;372:1589–1597. doi: 10.1056/NEJMoa1407349. [DOI] [PubMed] [Google Scholar]
- 5.Wong FC, Lo YM. Prenatal diagnosis innovation: Genome sequencing of maternal plasma. Annu. Rev. Med. 2016;67:419–432. doi: 10.1146/annurev-med-091014-115715. [DOI] [PubMed] [Google Scholar]
- 6.Zhao C, Tynan J, Ehrich M, Hannum G, McCullough R, Saldivar JS, Oeth P, van den Boom D, Deciu C. Detection of fetal subchromosomal abnormalities by sequencing circulating cell-free DNA from maternal plasma. Clin. Chem. 2015;61:608–616. doi: 10.1373/clinchem.2014.233312. [DOI] [PubMed] [Google Scholar]
- 7.Snyder MW, Simmons LE, Kitzman JO, Coe BP, Henson JM, Daza RM, Eichler EE, Shendure J, Gammill HS. Copy-number variation and false positive prenatal aneuploidy screening results. N. Engl. J. Med. 2015;372:1639–1645. doi: 10.1056/NEJMoa1408408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dar P, Jacobsson B, Clifton R, Egbert M, Malone F, Wapner RJ, Roman AS, Khalil A, Faro R, Madankumar R, Edwards L, Strong N, Haeri S, Silver R, Vohra N, Hyett J, Demko Z, Martin K, Rabinowitz M, Flood K, Carlsson Y, Doulaveris G, Daly S, Hallingström M, MacPherson C, Kao C, Hakonarson H, Norton ME. Cell-free DNA screening for prenatal detection of 22q112 deletion syndrome. Am. J. Obstet. Gynecol. 2022;222:79. doi: 10.1016/j.ajog.2022.01.002. [DOI] [PubMed] [Google Scholar]
- 9.Wapner RJ, Martin CL, Levy B, Ballif BC, Eng CM, Zachary JM, Savage M, Platt LD, Saltzman D, Grobman WA, Klugman S, Scholl T, Simpson JL, McCall K, Aggarwal VS, Bunke B, Nahum O, Patel A, Lamb AN, Thom EA, Beaudet AL, Ledbetter DH, Shaffer LG, Jackson L. Chromosomal microarray versus karyotyping for prenatal diagnosis. N. Engl. J. Med. 2012;367:2175–2184. doi: 10.1056/NEJMoa1203382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wapner RJ, Babiarz JE, Levy B, Stosic M, Zimmermann B, Sigurjonsson S, Wayham N, Ryan A, Banjevic M, Lacroute P, Hu J, Hall MP, Demko Z, Siddiqui A, Rabinowitz M, Gross SJ, Hill M, Benn P. Expanding the scope of noninvasive prenatal testing: Detection of fetal microdeletion syndromes. Am. J. Obstet. Gynecol. 2015;212(332):e331–339. doi: 10.1016/j.ajog.2014.11.041. [DOI] [PubMed] [Google Scholar]
- 11.Watson CT, Marques-Bonet T, Sharp AJ, Mefford HC. The genetics of microdeletion and microduplication syndromes: An update. Annu. Rev. Genomics Hum. Genet. 2014;15:215–244. doi: 10.1146/annurev-genom-091212-153408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dan S, Wang W, Ren J, Li Y, Hu H, Xu Z, Lau TK, Xie J, Zhao W, Huang H, Xie J, Sun L, Zhang X, Wang W, Liao S, Qiang R, Cao J, Zhang Q, Zhou Y, Zhu H, Zhong M, Guo Y, Lin L, Gao Z, Yao H, Zhang H, Zhao L, Jiang F, Chen F, Jiang H, Li S, Li Y, Wang J, Wang J, Duan T, Su Y, Zhang X. Clinical application of massively parallel sequencing-based prenatal noninvasive fetal trisomy test for trisomies 21 and 18 in 11,105 pregnancies with mixed risk factors. Prenat. Diagn. 2012;32:1225–1232. doi: 10.1002/pd.4002. [DOI] [PubMed] [Google Scholar]
- 13.Yu SC, Jiang P, Choy KW, Chan KC, Won HS, Leung WC, Lau ET, Tang MH, Leung TY, Lo YM, Chiu RW. Noninvasive prenatal molecular karyotyping from maternal plasma. PLoS ONE. 2013;8:e60968. doi: 10.1371/journal.pone.0060968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Martin K, Iyengar S, Kalyan A, Lan C, Simon AL, Stosic M, Kobara K, Ravi H, Truong T, Ryan A, Demko ZP, Benn P. Clinical experience with a single-nucleotide polymorphism-based non-invasive prenatal test for five clinically significant microdeletions. Clin Genet. 2018;93:293–300. doi: 10.1111/cge.13098. [DOI] [PubMed] [Google Scholar]
- 15.Schwartz S, Kohan M, Pasion R, Papenhausen PR, Platt LD. Clinical experience of laboratory follow-up with noninvasive prenatal testing using cell-free DNA and positive microdeletion results in 349 cases. Prenat. Diagn. 2018;38:210–218. doi: 10.1002/pd.5217. [DOI] [PubMed] [Google Scholar]
- 16.Gross SJ, Stosic M, McDonald-McGinn DM, Bassett AS, Norvez A, Dhamankar R, Kobara K, Kirkizlar E, Zimmermann B, Wayham N, Babiarz JE, Ryan A, Jinnett KN, Demko Z, Benn P. Clinical experience with single-nucleotide polymorphism-based non-invasive prenatal screening for 22q11.2 deletion syndrome. Ultrasound Obstet. Gynecol. 2016;47:177–183. doi: 10.1002/uog.15754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yin AH, Peng CF, Zhao X, Caughey BA, Yang JX, Liu J, Huang WW, Liu C, Luo DH, Liu HL, Chen YY, Wu J, Hou R, Zhang M, Ai M, Zheng L, Xue RQ, Mai MQ, Guo FF, Qi YM, Wang DM, Krawczyk M, Zhang D, Wang YN, Huang QF, Karin M, Zhang K. Noninvasive detection of fetal subchromosomal abnormalities by semiconductor sequencing of maternal plasma DNA. Proc. Natl. Acad. Sci. USA. 2015;112:14670–14675. doi: 10.1073/pnas.1518151112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhao X, Fu L. Efficacy of copy-number variation sequencing technology in prenatal diagnosis. J. Perinat. Med. 2019;47:651–655. doi: 10.1515/jpm-2019-0005. [DOI] [PubMed] [Google Scholar]
- 19.Liang D, Cram DS, Tan H, Linpeng S, Liu Y, Sun H, Zhang Y, Tian F, Zhu H, Xu M, Wang H, Yu F, Wu L. Clinical utility of noninvasive prenatal screening for expanded chromosome disease syndromes. Genet. Med. 2019;21:1998–2006. doi: 10.1038/s41436-019-0467-4. [DOI] [PubMed] [Google Scholar]
- 20.Helgeson J, Wardrop J, Boomer T, Almasri E, Paxton WB, Saldivar JS, Dharajiya N, Monroe TJ, Farkas DH, Grosu DS, McCullough RM. Clinical outcome of subchromosomal events detected by whole-genome noninvasive prenatal testing. Prenat. Diagn. 2015;35:999–1004. doi: 10.1002/pd.4640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu H, Wang L, Wu J, Zhou P, Fu J, Sun J, Cai W, Liu H, Yang Y. Noninvasive prenatal testing for chromosome aneuploidies and subchromosomal microdeletions/microduplications in a cohort of 8141 single pregnancies. Hum. Genomics. 2019;13:14. doi: 10.1186/s40246-019-0198-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Peters D, Chu T, Yatsenko SA, Hendrix N, Hogge WA, Surti U, Bunce K, Dunkel M, Shaw P, Rajkovic A. Noninvasive prenatal diagnosis of a fetal microdeletion syndrome. N. Engl. J. Med. 2011;365:1847–1848. doi: 10.1056/NEJMc1106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jensen TJ, Dzakula Z, Deciu C, van den Boom D, Ehrich M. Detection of microdeletion 22q11.2 in a fetus by next-generation sequencing of maternal plasma. Clin. Chem. 2012;58:1148–1151. doi: 10.1373/clinchem.2011.180794. [DOI] [PubMed] [Google Scholar]
- 24.Lau TK, Jiang FM, Stevenson RJ, Lo TK, Chan LW, Chan MK, Lo PS, Wang W, Zhang HY, Chen F, Choy KW. Secondary findings from non-invasive prenatal testing for common fetal aneuploidies by whole genome sequencing as a clinical service. Prenat. Diagn. 2013;33:602–608. doi: 10.1002/pd.4076. [DOI] [PubMed] [Google Scholar]
- 25.Chen S, Lau TK, Zhang C, Xu C, Xu Z, Hu P, Xu J, Huang H, Pan L, Jiang F, Chen F, Pan X, Xie W, Liu P, Li X, Zhang L, Li S, Li Y, Xu X, Wang W, Wang J, Jiang H, Zhang X. A method for noninvasive detection of fetal large deletions/duplications by low coverage massively parallel sequencing. Prenat. Diagn. 2013;33:584–590. doi: 10.1002/pd.4110. [DOI] [PubMed] [Google Scholar]
- 26.Srinivasan A, Bianchi DW, Huang H, Sehnert AJ, Rava RP. Noninvasive detection of fetal subchromosome abnormalities via deep sequencing of maternal plasma. Am. J. Hum. Genet. 2013;92:167–176. doi: 10.1016/j.ajhg.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yin L, Tang Y, Lu Q, Pan A, Shi M. Application value of NIPT for uncommon fetal chromosomal abnormalities. Mol. Cytogenet. 2020;13:39. doi: 10.1186/s13039-020-00508-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Levy B, Wapner R. Prenatal diagnosis by chromosomal microarray analysis. Fertil. Steril. 2018;109:201–212. doi: 10.1016/j.fertnstert.2018.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang S, Huang S, Ma L, Liang L, Zhang J, Zhang J, Cram DS. Maternal X chromosome copy number variations are associated with discordant fetal sex chromosome aneuploidies detected by noninvasive prenatal testing. Clin. Chim. Acta Int. J. Clin. Chem. 2015;444:113–116. doi: 10.1016/j.cca.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 30.Xue H, Yu A, Lin N, Chen X, Lin M, Wang Y, Huang H, Xu L. Detection of copy number variation associated with ventriculomegaly in fetuses using single nucleotide polymorphism arrays. Sci. Rep. 2021;11:5291. doi: 10.1038/s41598-021-83147-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gregg AR, Skotko BG, Benkendorf JL, Monaghan KG, Bajaj K, Best RG, Klugman S, Watson MS. Noninvasive prenatal screening for fetal aneuploidy, 2016 update: A position statement of the American College of Medical Genetics and Genomics. Genet. Med. 2016;18:1056–1065. doi: 10.1038/gim.2016.97. [DOI] [PubMed] [Google Scholar]
- 32.Rose NC, Benn P, Milunsky A. Current controversies in prenatal diagnosis 1: Should NIPT routinely include microdeletions/microduplications? Prenat. Diagn. 2016;36:10–14. doi: 10.1002/pd.4710. [DOI] [PubMed] [Google Scholar]
- 33.Callaway JL, Shaffer LG, Chitty LS, Rosenfeld JA, Crolla JA. The clinical utility of microarray technologies applied to prenatal cytogenetics in the presence of a normal conventional karyotype: A review of the literature. Prenat. Diagn. 2013;33:1119–1123. doi: 10.1002/pd.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hartwig TS, Ambye L, Sørensen S, Jørgensen FS. Discordant non-invasive prenatal testing (NIPT): A systematic review. Prenat. Diagn. 2017;37:527–539. doi: 10.1002/pd.5049. [DOI] [PubMed] [Google Scholar]
- 35.Petersen AK, Cheung SW, Smith JL, Bi W, Ward PA, Peacock S, Braxton A, Van Den Veyver IB, Breman AM. Positive predictive value estimates for cell-free noninvasive prenatal screening from data of a large referral genetic diagnostic laboratory. Am. J. Obst. Gynecol. 2017;217:e691–e696. doi: 10.1016/j.ajog.2017.10.005. [DOI] [PubMed] [Google Scholar]
- 36.Bunnell M, Zhang C, Lee C, Bianchi DW, Wilkins-Haug L. Confined placental mosaicism for 22q11.2 deletion as the etiology for discordant positive NIPT results. Prenat. Diagn. 2017;37:416–419. doi: 10.1002/pd.5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xiang J, Ding Y, Song X, Mao J, Liu M, Liu Y, Huang C, Zhang Q, Wang T. Clinical utility of SNP array analysis in prenatal diagnosis: A cohort study of 5000 pregnancies. Front. Genet. 2020;11:571219. doi: 10.3389/fgene.2020.571219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu Y, Chen L, Liu Y, Hao Y, Xu Z, Deng L, Xie J. Screening, prenatal diagnosis, and prenatal decision for sex chromosome aneuploidy. Expert Rev. Mol. Diagn. 2019;19:537–542. doi: 10.1080/14737159.2019.1613154. [DOI] [PubMed] [Google Scholar]
- 39.Nagamatsu T, Kamei Y, Yamashita T, Fujii T, Kozuma S. Placental abnormalities detected by ultrasonography in a case of confined placental mosaicism for trisomy 2 with severe fetal growth restriction. J. Obstet. Gynaecol. Res. 2014;40:279–283. doi: 10.1111/jog.12145. [DOI] [PubMed] [Google Scholar]
- 40.Dharajiya NG, Namba A, Horiuchi I, Miyai S, Farkas DH, Almasri E, Saldivar JS, Takagi K, Kamei Y. Uterine leiomyoma confounding a noninvasive prenatal test result. Prenat. Diagn. 2015;35:990–993. doi: 10.1002/pd.4629. [DOI] [PubMed] [Google Scholar]
- 41.Li R, Wan J, Zhang Y, Fu F, Ou Y, Jing X, Li J, Li D, Liao C. Detection of fetal copy number variants by non-invasive prenatal testing for common aneuploidies. Ultrasound Obstet. Gynecol. 2016;47:53–57. doi: 10.1002/uog.14911. [DOI] [PubMed] [Google Scholar]
- 42.Qian YQ, Wang XQ, Chen M, Luo YQ, Yan K, Yang YM, Liu B, Wang LY, Huang YZ, Li HG, Pan HY, Jin F, Dong MY. Detection of fetal subchromosomal aberration with cell-free DNA screening led to diagnosis of parental translocation: Review of 11344 consecutive cases in a university hospital. Eur. J. Med. Genet. 2019;62:115–123. doi: 10.1016/j.ejmg.2018.06.012. [DOI] [PubMed] [Google Scholar]
- 43.Yu D, Zhang K, Han M, Pan W, Chen Y, Wang Y, Jiao H, Duan L, Zhu Q, Song X, Hong Y, Chen C, Wang J, Hui F, Huang L, Chen C, Du Y. Noninvasive prenatal testing for fetal subchromosomal copy number variations and chromosomal aneuploidy by low-pass whole-genome sequencing. Mol. Genet. Genomic Med. 2019;7:e674. doi: 10.1002/mgg3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.