Abstract
Nontypeable Haemophilus influenzae is an exclusive human pathogen which infects the respiratory epithelium. We have initiated studies to explore the interaction of the nontypeable H. influenzae strain 2019 with primary human airway epithelial cells by electron and confocal microscopy. Primary human airway cell cultures were established as monolayers on glass collagen-coated coverslips or on semipermeable membranes at an air-fluid interface. Scanning electron microscopy indicated that bacteria adhered to nonciliated cells in the population. The surface of infected cells showed evidence of cytoskeletal rearrangements manifested by microvilli and lamellipodia extending toward and engaging bacteria. Confocal microscopic analysis demonstrated that infection induced actin polymerization with an increase in cortical actin as well as evidence of actin strands around the bacteria. Transmission electron microscopic analysis showed lamellipodia and microvilli surrounding organisms, as well as organisms adherent to the cell surface. These studies also demonstrated the presence of bacteria within vacuoles inside of airway cells. Confocal microscopic studies with Texas red-labeled dextran (molecular weight, 70,000) indicated that H. influenzae cells were entering cells by the process of macropinocytosis. These studies indicate that nontypeable H. influenzae can initiate cytoskeletal rearrangement within human airway epithelium, resulting in internalization of the bacteria within nonciliated human airway epithelial cells by the process of macropinocytosis.
Nontypeable Haemophilus influenzae (NTHI) is a nonencapsulated, gram-negative pleomorphic rod-shaped bacterium which colonizes the upper airway of the majority of individuals (25). An opportunistic pathogen, it frequently infects airway surfaces that have been compromised by obstruction or loss of mucociliary clearance mechanisms. It is the pathogen most frequently isolated from sputa of patients with acute exacerbations of chronic bronchitis (25, 27) and is isolated from approximately 30% of children with purulent otitis media (32).
A number of studies have examined the pathogenesis of H. influenzae by experimental infection of human tissue. Studies by Farley and coworkers (11, 12) using infected adenoidal explants showed that H. influenzae type b strains did not enter the airway epithelial cells but appeared to pass between cells which were losing lateral contact with neighboring cells. St. Geme and Falkow showed that H. influenzae could invade non-airway-derived tissue culture cells (28). Recently, Holmes and Bakaletz demonstrated attachment of nontypeable H. influenzae using human oropharyngeal cells in suspension (18). These authors also demonstrated cytoskeletal changes in these cells following attachment.
We have utilized a system to culture primary human airway epithelial cells in order to study the interaction of NTHI and human airway epithelium. These studies have been performed on cells grown submerged on collagen-coated glass coverslips or at an air-fluid interface on polycarbonate membranes. For comparison, infection studies were also performed on a simian virus 40 (SV40)-transformed human bronchial epithelial line, designated 16HBE14. Both types of cells grown submerged or at the air-fluid interface were studied by scanning electron microscopy (SEM), confocal laser scanning microscopy (CLSM), and transmission electron microscopy (TEM). These studies demonstrated that NTHI adhered primarily to nonciliated airway epithelial cells and induced cytoskeletal changes manifested by directed extension of microvilli and formation of lamellipodia. Electron and confocal microscopic analysis indicate that macropinocytosis is a mechanism of NTHI entry into airway epithelial cells.
MATERIALS AND METHODS
Bacteria.
Experimental infections of airway cells were carried out by using NTHI strains 2019, 3198, 1479, and 7502. Bacteria were reconstituted from frozen stock cultures and plated on brain heart infusion supplemented with 2% Fildes (Difco, Detroit, Mich.). These strains were obtained from our own collection and were originally isolated from the sputa of adult males with chronic bronchitis (5). Electron microscopy studies confirmed the presence of circumferential pili and fibrils on all NTHI strains that were used in the studies described. PCR analysis indicated that the genomes of all strains contained hmw1 and that strain 2019 also contained hmw2. The gene for hemagglutination inhibition could not be demonstrated in the genomes of any of the NTHI strains used in these studies.
Primary cell cultures.
Primary human airway epithelial cells were obtained from nasal polyps (one patient), from normal bronchial tissue (one individual) from a surgical specimen, and from bronchial brushings taken from healthy human volunteers by bronchoscopy. Tissue samples from nasal polyps or bronchial tissue were treated at 4°C in Ca-free/Mg-free Hanks’ balanced salt solution freshly supplemented with pronase at 1.5 mg/ml and DNase at 0.1 mg/ml (both reagents were obtained from Sigma Chemical Co., St. Louis, Mo.). Digestion of the tissue was halted after 48 h by the addition of fetal bovine serum to 10% (vol/vol). The resulting cell suspension was washed twice with Airway Medium (AM). AM consists of high-glucose Dulbecco’s modified Eagle medium and Ham’s F-12 medium (Gibco BRL, Grand Island, N.Y.) combined in equal volumes and supplemented with 5% heat-inactivated fetal bovine serum, 1% nonessential amino acids, 1% penicillin-streptomycin mix, and human insulin to 5 μg/ml (Sigma Chemical Co.).
Samples obtained by bronchial brushing were collected into Ham’s F-12 medium containing penicillin-streptomycin and gentamicin and were stored cold until they could be distributed onto the desired growth surfaces, usually within 24 h of the time of collection. From this point, the brushings and the suspensions of airway epithelial cells were handled similarly. The cell suspensions were pelleted and resuspended in AM at an approximate concentration of 5 × 105 cells per ml. For submerged cultures, cell suspensions in AM were seeded at 50 to 60 μl onto sterile 12-mm-diameter glass coverslips previously coated with a solution of 0.5 mg of bovine collagen (Worthington Biochemical Corp., Freehold, N.J.) per ml in distilled water. These cells were used for SEM and CLSM studies. Airway cells were also grown submerged on tissue culture well insert units (BioCoat; Becton Dickinson, Bedford, Mass.). These cells were used for TEM studies. In both types of submerged cultures, the medium was replaced with defined Bronchial Epithelial Cell Growth Medium (BEGM; Clonetics Corp., San Diego, Calif.) following a 24-h initial incubation at 37°C and 5% CO2. The cells on the surface of the BioCoat membrane were also covered with BEGM. The cells were allowed to grow for 5 to 7 days before the initial medium was replaced with fresh BEGM, and thereafter, the BEGM was replaced twice weekly.
Airway cells were grown at an air-fluid interface by the method of Smith and coworkers (26). Briefly, the cell suspensions were seeded at 50 to 60 μl onto the surface of the microporous membrane in tissue culture well insert units and incubated as described above. Following the initial 24-h incubation, the medium in the tissue culture wells below the insert units was replaced with Widdicombe’s Medium (WM) (33) containing 2% Ultroser-G (BioSepra S.A., France) and 1% penicillin-streptomycin. The residual AM was aspirated from the upper surface of the insert units, and the incubation was continued. Medium was aspirated daily from the apical surface of the insert units until cell growth prevented leakage of medium from the well. Cell growth was maintained by replacing the WM in the well below the microporous membrane as needed. To confirm the integrity of the epithelial cell monolayer, transepithelial resistance was measured utilizing an EVOM epithelial voltohmmeter (World Precision Instruments, Sarasota, Fla.) according to the directions supplied by the manufacturer.
SV40-transformed bronchial epithelial cells.
The SV40-transformed bronchial epithelial cell line, 16HBE14, was kindly provided by D. C. Gruenert of the University of California, San Francisco (16).
Bacterial infections.
Bacteria used in the infection studies were collected from fresh overnight plate cultures. The concentration of bacterial cells in phosphate-buffered saline (PBS) was adjusted to 50 Klett units (∼108 CFU/ml), and the mixture was subsequently diluted 10-fold in BEGM without antibiotics. Submerged cultures were infected by washing the cells once in BEGM without antibiotics and replacing the cell culture medium with 500 μl of this bacterial cell suspension in BEGM. Epithelial cells grown at an air-fluid interface were infected with 10 μl of a mixture containing ∼4 × 106 CFU of NTHI 2019 in WM, delivered to the apical surface of the insert unit after the medium in the wells below the insert units had been replaced with antibiotic-free WM.
Incubation of the airway epithelial cells with bacteria was carried out at 37°C in a 5% CO2 environment. The airway epithelial cells were washed at the termination of the infection period and immediately fixed for 30 min at room temperature with 2% paraformaldehyde in PBS. The fixative was aspirated and replaced with PBS for storage at 4°C until the cells were processed for microscopic examination.
In order to investigate the role of actin polymerization on bacterial infection of primary airway epithelial cells, confluent monolayers were incubated for 30 min in antibiotic-free BEGM supplemented with cytochalasin D (Sigma) at a final concentration of 1 μg/ml. This level of cytochalasin D was maintained throughout the subsequent 2- or 4-h infection with NTHI 2019. Protein synthesis by the airway epithelial cells was inhibited in experiments by preincubating the cells for 30 min with cycloheximide (Sigma) at a final concentration of 100 μg/ml in antibiotic-free BEGM (21). The level of cycloheximide was maintained during the NTHI 2019 infection, as with the cytochalasin D. Previous studies in our laboratory had shown that NTHI cells were resistant to the effects of 100 μg of cycloheximide/ml (data not shown).
Processing for microscopy.
Fixed cell cultures were processed for CLSM, SEM, or TEM according to standard techniques. Primary human airway epithelial cells grown submerged on collagen-coated coverslips were processed for CLSM or for SEM. Cells grown on the microporous membranes, either submerged or at an air-fluid interface, could be processed for SEM and/or TEM by partitioning the membranes.
CLSM analysis.
CLSM was used to examine the role of actin polymerization in the airway epithelial cells due to infection by NTHI 2019 (17). Fixed cells on coverslips could be processed and stained directly in the wells of the original tissue culture plate.
To study the effect of infection on actin polymerization, the cells were first permeabilized by a 15-min incubation at room temperature with 0.2% Triton X-100. Following washes with PBS, the cultures were exposed to rhodamine-phalloidin, a fluorophore that specifically labels F-actin, for 30 min according to the protocol recommended by the manufacturer, Molecular Probes, Inc. (Eugene, Oreg.). The cells were washed again, blocked with 5% normal goat serum, and incubated with either monoclonal antibody (MAb) 3B9 or a rabbit antiserum to whole NTHI 2019. MAb 3B9 is specific for a configurational epitope on the P6 membrane protein of H. influenzae (3). The cells were then incubated with a fluorescein isothiocyanate-conjugated goat antiserum to murine immunoglobulin G (IgG) or goat anti-rabbit immunoglobulin-fluorescein isothiocyanate conjugate (Molecular Probes). The treated coverslips were mounted with Vectashield mounting medium (Vector Labs, Burlingame, Calif.) on microscope slides, covered with square glass coverslips, and examined by dual-wavelength laser in the Bio-Rad 1024 confocal laser scanning microscope.
Uptake of bacteria by the process of macropinocytosis was studied by using dextran 70,000 (molecular weight) labeled with Texas red (Molecular Probes, Inc). This marker of endocytosis was introduced into the media at the onset of infection. At the termination of the period of infection, the residual marker was removed by washing the cells once with PBS followed by fixation with 2% paraformaldehyde. Prior to viewing, the bacteria were labeled with the nucleic acid stain YOYO-1 (Molecular Probes, Inc.) at 0.5 μM in PBS for 8 min.
SEM.
SEM processing included treatment with 1% osmium tetroxide prior to dehydration through a graded ethanol series, with a final clearance in hexamethyl-disilazane (HMDS; Polysciences, Inc., Warrington, Pa.). After a light coating with gold-palladium, the specimens were viewed with an S-4000 Hitachi scanning electron microscope at 5-kV accelerating voltage.
TEM.
Samples for TEM were processed to allow for labeling with immunospecific reagents. The airway epithelial cell monolayers could be dehydrated through a graded ethanol series for embedment in LR White resin (Ted Pella, Inc., Redding, Calif.) and sectioned to approximately 85-nm thickness by using an ultramicrotome. NTHI 2019 was detected with either MAb 3B9 or an affinity-purified polyclonal rabbit antibody made against strain 2019. These labels were tagged with the appropriate secondary antibodies conjugated to 10- or 30-nm-diameter gold beads (AuroProbe; Amersham Life Science, Arlington Heights, Ill.), and the cells were counterstained with 5% uranyl acetate for viewing with an H-7000 Hitachi transmission electron microscope at 75-kV accelerating voltage.
Alternatively, specimens for TEM could be labeled prior to embedment in resin, following the protocol recommended by Aurion Co. (Wagenungen, The Netherlands). The fixed cells were washed in buffer, incubated in a solution containing 5% normal goat serum to block nonspecific labeling in the samples, and then incubated overnight with rabbit polyclonal antiserum against NTHI 2019. After being thoroughly washed, the specimens were incubated overnight with a goat secondary antibody to rabbit IgG, conjugated to ultrasmall gold beads (beads smaller than 1 nm in diameter; available from Aurion Co. [GAR/GP-US]). The labeled samples were again washed thoroughly, additionally fixed in 2% glutaraldehyde and treated with 1% osmium tetroxide, and then dehydrated through a graded ethanol series. Final embedment was in Eponate 12 (Ted Pella, Inc.). The ultrathin sections cut from these samples required silver enhancement of the gold beads, performed according to the method of Danscher (8). Counterstaining and viewing by TEM could then proceed as described above (1).
RESULTS
Analysis of primary human airway epithelial cell cultures.
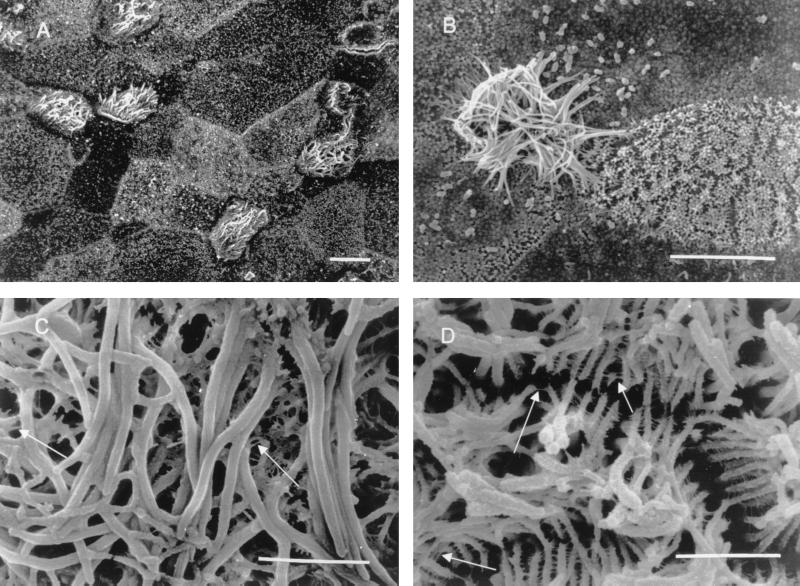
Figure 1A shows the typical SEM appearance of an air-fluid interface bronchial epithelial cell culture at 14 days after implantation. Transepithelial resistance was 2,760 Ω/cm2, indicating that the cells had polarized. Based on surface morphology, three types of cells could be distinguished. Ciliated cells comprised about 10 to 15% of the epithelium. The remaining two cell types had differences in the density of microvilli on their surfaces. One cell type had a small number of small microvillus-like structures, while the second was covered by these structures. The surface of human bronchial epithelial cells grown on bovine collagen-covered glass coverslips in submerged cultures appeared morphologically similar to that of cells grown at the air-fluid interface, with the exception of the absence of ciliated cells in the former.
FIG. 1.
A series of SEM studies of uninfected (A and C) and infected (B and D) airway epithelial cells grown at an air-fluid interface. Panel A demonstrates a culture that was approximately 14 days old. The cilia were actively beating at the time of fixation. Ciliated and nonciliated cells can be seen within the population. Panel B shows NTHI binding to nonciliated cells in the population after 4 h of infection. The binding of bacteria to specific nonciliated cells within the population was characteristic of infected air interface and submerged cultures. The scale bars in panels A and B represent 10 μm. Panel C shows cilia from an uninfected air interface culture. The ciliary surface is smooth, and there are a limited number of strands between the cilia (arrows). Panel D shows cilia from an air interface culture infected for 30 min. The surfaces of individual cilia appear to be roughened. The cilia are organized in clumps, with multiple proteinaceous strands cross-linking individual cilia (arrows). The scale bars in panels C and D represent 2 μm.
Because of the ease of use of an immortalized cell line, we elected to study an SV40-transformed bronchial epithelial cell line in parallel with the primary cells. The morphology of the SV40-transformed bronchial epithelial cell line, 16HBE14, was similar to that of the primary cells in air-fluid interface cultures. Ciliated cells were seen when 16HBE14 cells were grown at an air-fluid interface with WM, but the number was markedly reduced compared to the primary cells. The gross morphology of the surface of the 16HBE14 cells was indistinguishable from that of primary airway cells grown in a submerged culture.
Examination of NTHI-infected airway epithelial cells.
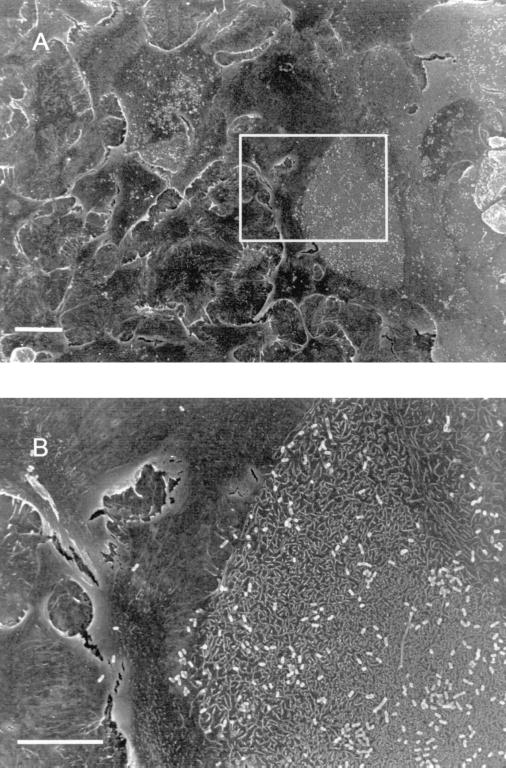
The majority of our studies were carried out with the NTHI strain 2019, since this is the strain in which we have made our lipo-oligosaccharide mutations. SEM and CLSM studies were performed with NTHI strains 1479, 7502, and 3198. Similar results were obtained with all four NTHI strains studied. Figure 1B shows the result of 4-h NTHI 2019 cell infection of primary human cells grown at an air-fluid interface. NTHI 2019 cells did not adhere to the cilia or ciliated cells. However, the cilia in the infected sample appeared to be clumped together, and proteinaceous strands could be seen connecting individual cilia (Fig. 1D). This was not observed on cilia from uninfected cells (Fig. 1C). NTHI 2019 cells attached only to a limited repertoire of nonciliated cells within the airway epithelial cell populations in primary cells grown at an air-fluid interface or in submerged cultures. A similar pattern of organism attachment was also seen when 16HBE14 cells were infected with NTHI 2019. Figures 2A and B show a typical example of a heavily infected epithelial cell surrounded by cells with no adherent bacteria. Attachment could be observed in as little as 15 min after initiation of infection, although the actual number of bacterial cells was much smaller in shorter exposure times.
FIG. 2.
SEM of submerged primary airway epithelial cell culture that had been infected for 4 h with NTHI 2019. (A) An example of the distribution of binding of NTHI to the surfaces of cells within the population. Bacteria are bound to a limited repertoire of cells in the population. The scale bar in panel A represents 20 μm. (B) A higher-magnification view of a region on the same sample. The sharp demarcation in NTHI 2019 binding to the surface of one cell with minimal binding of bacteria to an adjacent cell can be seen. This was a typical observation. The scale bar in panel B represents 10 μm.
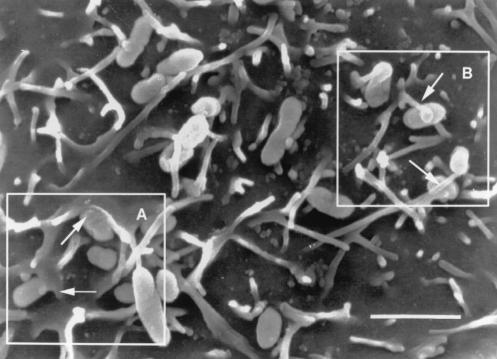
The airway epithelial cell apical surface morphology changed after infection. High-magnification SEM analysis revealed that cytoskeletal changes occurred within the infected cell as demonstrated by lamellipodia enfolding bacteria (Fig. 3A) and elongated microvilli (Fig. 3B) extending toward and wrapping around individual bacterial cells.
FIG. 3.
This SEM study demonstrates a view of the surface of a primary airway epithelial cell from a submerged culture, infected for 4 h with NTHI 2019. The field shows typical cytoskeletal changes induced by infection. Boxed area A shows lamellipodia emerging from the airway cell surface and surrounding a bacterium (arrows). Boxed area B shows microvilli engaging the NTHI on the surface of the cell. The scale bar represents 2 μm.
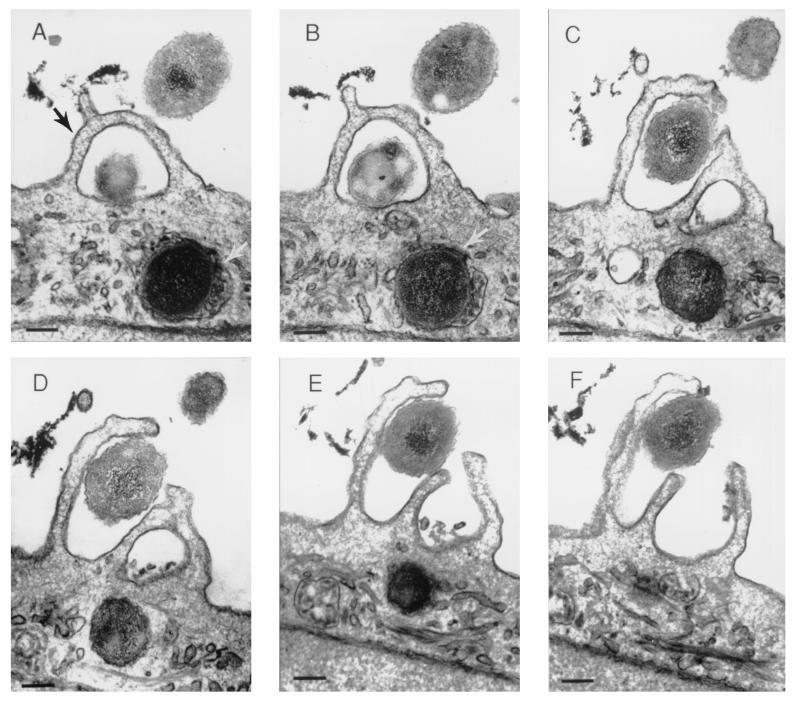
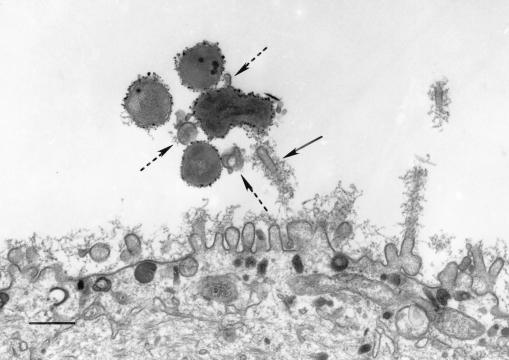
The observations made by SEM were confirmed by TEM and by CLSM. Figure 4 shows six serial sections through an infected airway epithelial cell, grown in a submerged culture, that shows lamellipodia surrounding NTHI 2019 cells after 4 h of infection. A bacterium within a vacuole can also be seen within the airway cell immediately below the lamellipodia in the same figure.
FIG. 4.
Airway cells on a sample of the BioCoat membrane from the same specimen as shown in Fig. 3, embedded in Epon resin and serially sectioned for TEM. The series (A through F) demonstrates lamellipodia surrounding NTHI 2019 (black arrow) at the surface of a submerged airway cell culture after 4 h of infection. Immediately below the lamellipodia can be seen an intracellular organism that is surrounded by a vacuolar membrane (white arrows, panels A and B). The scale bar represents 200 nm.
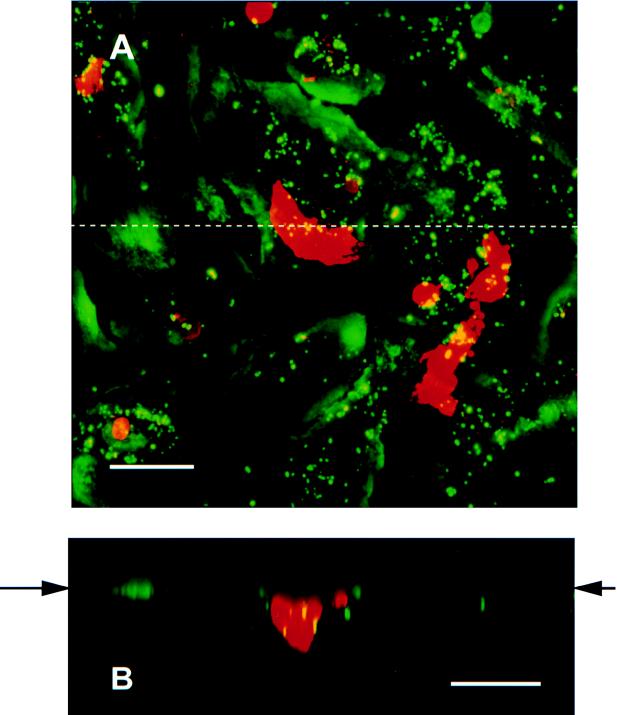
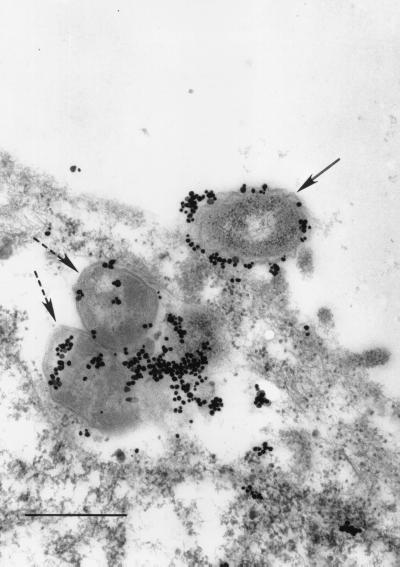
Figure 5 shows a section through an airway epithelial cell that shows a microvillus extending from the airway cell plasma membrane and engaging the bacterium. In addition, sectioned microvilli can be seen surrounding the bacterium. Similar results were obtained with primary airway cells and 16HBE14 cells whether they were grown at an air-fluid interface or in submerged cultures. This indicates that bacteria can enter human airway epithelial cells by the process of macropinocytosis. To further confirm that this process was involved in internalization of the bacteria by the airway cell, we performed studies using impermeant dextran 70,000 labeled with Texas red. Figure 6A shows a compiled Z series of NTHI within Texas red-dextran vacuoles within primary airway epithelial cells. Figure 6B is a vertical section (view in the x-z plane) showing the localization of the organisms within the vacuoles in this projection. As can be seen, large vacuoles containing bacteria are present in the infected cell. The colocalization of the bacteria within these vacuoles is confirmed by the color shift of the macropinocytosed bacteria from green to yellow. Smaller vacuoles containing the marker are present in the uninfected cell as a consequence of normal pinocytosis. In addition to the bacterial uptake by macropinocytosis, intracellular organisms that are not associated with Texas red-dextran 70,000 can be seen. This suggests that bacteria gain entry by another process as well as macropinocytosis.
FIG. 5.
TEM demonstrates microvillus engagement (solid arrow) of NTHI 2019 at the surface of a primary airway epithelial cell in an air interface culture after 4 h of infection. Portions of multiple microvilli, surrounding the bacteria (dashed arrows), can be seen in cross section. This specimen was labeled prior to Epon embedment with a rabbit polyclonal antiserum to NTHI 2019, followed by a secondary antiserum conjugated to ultrasmall gold beads. The label was enhanced with silver after embedment and sectioning. The scale bar represents 500 nm.
FIG. 6.
Images collected by dual-wavelength CLSM of primary airway cells infected for 3 h with NTHI 2019. Fluid entry into the cells is identified by using Texas red-labeled dextran (molecular weight, 70,000). NTHI and airway cell nuclei are labeled with the green fluorescing dye YOYO-1. A series of 1-μm optical sections, collected in the x-y axis, was compiled to produce the image shown in panel A. Colocalization of the green NTHI and red-labeled vacuoles can be seen as yellow-stained areas within the vacuoles, suggesting that some of the bacteria have been taken into the cells by macropinocytosis. This possibility is further explored in panel B. The image is a compilation of optical sections collected in the x-z axis, along the dotted line shown in panel A. The apical surface of the cell culture is indicated by the arrows in panel B. The cross section of the large red-stained vacuole shown in panel A shows several NTHI cells (yellow) within the host cell. The scale bars on both panels represent 50 μm.
Figure 7 shows an immunoelectron micrograph demonstrating bacteria adherent to the airway cell surface. In some instances, pedestal formation could also be seen beneath adherent bacteria. Bacteria were also seen within airway epithelial cells (Fig. 4 and 7). In Fig. 4A, 4B, and 7, a vacuolar membrane can be seen surrounding the bacterium.
FIG. 7.
16HBE14 cells infected for 24 h with NTHI 2019. The specimen was embedded in LR White resin, and after sectioning, the bacteria were labeled with a rabbit polyclonal antiserum to NTHI 2019 lipooligosaccharide. The secondary antibody was conjugated to ultrasmall gold beads, which were subsequently enhanced with silver. The figure demonstrates NTHI 2019 cells bound to the surface of the airway epithelial cell (arrow) and within the cell (dashed arrow). A vacuolar membrane can be seen surrounding the bacteria within the airway cell. The scale bar represents 500 nm.
Analysis of the cytoskeletal changes in infected airway epithelial cells.
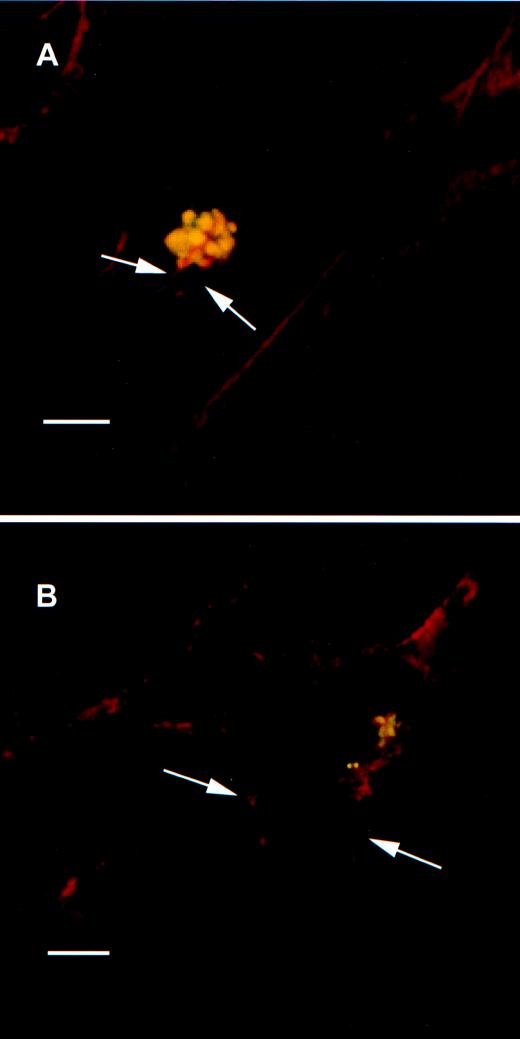
Confocal microscopy with rhodamine-phalloidin showed polymerized actin either in microvilli or lamellipodia covering NTHI 2019 at the time that the bacteria attached to the cell surface (Fig. 8A). There also appeared to be a marked increased in the amount of cortical actin present in the infected cells. Treatment of the airway cells with cytochalasin D resulted in disruption of the actin polymerization and an absence of microvillus formation or lamellipodia after infection for 2 and 4 h (Fig. 8B). Bacteria could still be seen adhering to the cell surfaces in both SEM and CLSM analyses. Studies with cycloheximide showed microvilli and lamellipodia extending toward adherent bacteria after treatment. This indicates that de novo protein synthesis was not required for the cytoskeletal rearrangements caused by NTHI infection of airway cells.
FIG. 8.
(A) Dual-wavelength CLSM of primary airway epithelial cells infected for 30 min with NTHI 2019. Actin filaments were stained with rhodamine-phalloidin; bacteria were labeled with the anti-NTHI P6 MAb 3B9 and a secondary antiserum to murine IgG conjugated to fluorescein. The image is a single optical section through a plane near the top of the specimen, showing actin strands in red (arrows) surrounding NTHI cells (yellow spheres) on the surface of an airway epithelial cell. Dense bands of cortical actin that form during the infection process can also be seen. The scale bar represents 5 μm. (B) The effect of cytochalasin D on the infection process. Primary airway epithelial cells were infected with NTHI 2019 for 2 h in the presence of 1 μg of cytochalasin D/ml. The specimen was stained for actin filaments and bacteria and examined by CLSM as described for panel A. The adherent bacteria in this single optical section stain yellow, as noted above; the red label shows the fragmentation of actin filaments due to the effect of cytochalasin D (arrows). The scale bar represents 10 μm.
DISCUSSION
The mechanisms used by nontypeable H. influenzae to damage human airway epithelial cells have not been clearly established. The organism produces at least two potential toxins, lipooligosaccharide and peptidoglycan, either of which may be implicated in the process. The question of whether the organism actually invades airway epithelial cells or resides in the mucus surface layer has also been a matter of speculation. Until recently, tissue samples from infected individuals have been unavailable, and hence the course of natural infection has not been studied in detail. In order to study the pathogenesis of nontypeable H. influenzae on human airway epithelium, we established primary airway epithelial cell cultures, studied them under different growth conditions (submerged and at an air-fluid interface), and compared these results with studies performed in an SV40-transformed bronchial epithelial cell line.
These studies indicate that nontypeable H. influenzae cells adhere to and invade a subset of human airway epithelial cells. The studies performed in primary airway epithelial cells and SV40-transformed bronchial epithelial cells grown at an air-fluid interface and in submerged cultures gave identical results. Adherence of the NTHI to the airway epithelial cell is accompanied by microvillus elongation that appears to be directed toward the adhering organisms. The microvilli appear to be engaging the bacterium with adhesion to the bacterial surface. Formation of lamellipodia with enfolding of the NTHI is also frequently seen in TEM and SEM. Invasion of the airway epithelial cell occurs with entry into vacuoles. Cytochalasin D treatment of airway cells ablates the response of the microvilli and lamellipodia, while cycloheximide treatment does not alter these cytoskeletal changes.
Previous studies have shown that NTHI cells can adhere to and invade a variety of tissue culture cells (28). A number of factors in bacterial adherence to epithelial cells and mucin associated with the outer membrane of H. influenzae have been identified (2, 9, 10, 15, 28–31). Holmes and Bakaletz have shown that NTHI cells adhere to and induce cytoskeletal changes in oropharyngeal cells suspended in cold PBS. These studies showed that cytochalasin D could inhibit these changes (18). Studies by St. Geme and Falkow showed that Chang conjunctival epithelial cells were invaded by unencapsulated H. influenzae type b variants (28). Farley and coworkers studied H. influenzae type b infection of human adenoidal explants (11). These studies demonstrated that the organism adhered selectively to nonciliated cells on this surface. The ciliary cells, while not directly adhered to by H. influenzae type b, lost motility and eventually sloughed from the surface. Invasion of the epithelial surface occurred by disruption of the epithelial tight junctions with passage of organisms between epithelial cells.
Moller and coworkers studied patients with a variety of chronic pulmonary diseases using culture, immunoperoxidase staining, and PCR (23). Immunoperoxidase staining and PCR were positive for 24 patients. From only two of these patients could H. influenzae be cultured. These authors concluded that H. influenzae was present in the respiratory epithelium and in the subepithelial layers in these patients. Forsgren and coworkers used in situ hybridization to demonstrate that H. influenzae could be found within macrophage-like cells in the adenoidal crypts of normal children undergoing adenoidectomy (13). Using immunofluorescence, we have studied frozen sections from 39 biopsy specimens from patients with chronic bronchitis. Four of these specimens demonstrated the presence of intracellular NTHI (data not shown).
NTHI cells adhere to a specific but unidentified nonciliated cell type within the primary human airway population. Others have observed that H. influenzae binds to nonciliated cells in human adenoidal explant studies (11). In contrast, SEM studies performed after infection of primary human airway epithelial cells with Moraxella catarrhalis show that this bacterium adheres to all of the cells in the population (data not shown). The epithelium lining the human bronchial surface consists of many morphologically distinct cell types with different but sometimes overlapping functions. At least eight different epithelial cell types have been delineated, with the number identified depending on the mammalian species (19). In the bronchi, ciliated cells predominate, and these are interspersed with mucus-secreting cells, serous and dense core granulated cells of the surface epithelium, and possibly Clara cells. The type of nonciliated cell to which NTHI binds remains to be determined.
Cytochalasin D studies confirm the role of actin polymerization in the process of adherence and invasion. Exposure of the airway cells to cycloheximide over 4 h does not inhibit the cytoskeletal changes, indicating that de novo protein synthesis is not necessary for initiation of these changes. Killed organisms do not initiate the cytoskeletal response. This result combined with the directed nature of the cytoskeletal response seen with live bacteria suggests that NTHI produces a factor which results in signaling to the airway cell to activate the cytoskeleton. In addition, the directed nature of the cytoskeletal response suggests that a factor similar to those that promote phagocytosis may be involved in the process. The surfaces of cells of many types have a variety of protrusions or extensions that are involved in cell movement, phagocytosis, or specialized functions, such as absorption of nutrients. Most of these cell surface extensions are based on actin filaments, which are organized into either relatively permanent or rapidly emerging bundles or networks (24). Microvilli are the best characterized of these actin-based cell surface extensions and contain parallel bundles each formed of between 20 and 30 actin filaments. The filaments in these bundles are cross-linked by fimbrin and/or villin, both actin-bundling proteins. During macropinocytosis membrane ruffling (resulting in lamellipodia) occurs: the rims of the membrane folds extending from the surface fuse back with the plasma membrane (22). Studies by Galan have demonstrated that this is the mechanism of entry of Salmonella into enterocytes (14).
Lamellipodia are sheet-like extensions of the plasma membrane. They are supported by a flattened web of actin filaments rather than discrete bundles of actin (7). In the chemotactic response of phagocytic cells, G proteins have been implicated in the signaling processes that activate the actin cortex. There is evidence that two Ras-related proteins, known as Rac and Rho, act downstream (4, 20). These proteins have been shown to have a distinct effect on the actin cytoskeleton in fibroblasts. Microinjection of Rac protein into cultured cells causes a dramatic increase in the formation of lamellipodia within 5 min (6). The nature of the cytoskeletal changes we have observed indicates that a Rho-dependent pathway is activated in the airway cells upon exposure to H. influenzae. Vesicles or vacuoles formed during macropinocytosis can be quite large, i.e., 1 to 5 μm in diameter, and can transport extracellular fluid and macromolecules specifically or nonspecifically bound to the plasma cell membrane.
These studies indicate that NTHI cells bind to specific nonciliated airway epithelial cells and initiate cytoskeletal rearrangement. The enfolding of bacteria by lamellipodia and the engagement by microvilli appear to be a step in the process of internalization of the bacteria. Our future experiments will be directed at the NTHI factors involved in signaling the airway epithelial cells.
ACKNOWLEDGMENTS
We express our appreciation to Michael Welsh and the members of his laboratory at The University of Iowa for their help with the development of the primary human cell cultures, the staff of the Central Microscopy Research Facility at The University of Iowa, and Barbara Baxter of Baylor College of Medicine for help with the collection of bronchial brushing samples.
The work described in this paper was supported by NIAID grant R37AI24616 and NIAID contract NO1AI65298.
REFERENCES
- 1.Apicella M A, Ketterer M, Lee F K, Zhou D, Rice P A, Blake M S. The pathogenesis of gonococcal urethritis in men: confocal and immunoelectron microscopic analysis of urethral exudates from men infected with Neisseria gonorrhoeae. J Infect Dis. 1996;173:636–646. doi: 10.1093/infdis/173.3.636. [DOI] [PubMed] [Google Scholar]
- 2.Barenkamp S J, St. Geme J W., III Genes encoding high-molecular-weight adhesion proteins of nontypeable Haemophilus influenzae are part of gene clusters. Infect Immun. 1994;62:3320–3328. doi: 10.1128/iai.62.8.3320-3328.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bogdan J A, Jr, Apicella M A. Mapping of a surface-exposed, conformational epitope of the P6 protein of Haemophilus influenzae. Infect Immun. 1995;63:4395–4401. doi: 10.1128/iai.63.11.4395-4401.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Braga V M, Machesky L M, Hall A, Hotchin N A. The small GTPases Rho and Rac are required for the establishment of cadherin-dependent cell-cell contacts. J Cell Biol. 1997;137:1421–1431. doi: 10.1083/jcb.137.6.1421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campagnari A A, Gupta M R, Dudas K C, Murphy T F, Apicella M A. Antigenic diversity of lipooligosaccharides of nontypeable Haemophilus influenzae. Infect Immun. 1987;55:882–887. doi: 10.1128/iai.55.4.882-887.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cassimeris L, Zigmond S H. Chemoattractant stimulation of polymorphonuclear leukocyte locomotion. Semin Cell Biol. 1990;1:125–134. [PubMed] [Google Scholar]
- 7.Condeelis J. Life at the leading edge: the formation of cell protrusions. Annu Rev Cell Biol. 1993;9:411–444. doi: 10.1146/annurev.cb.09.110193.002211. [DOI] [PubMed] [Google Scholar]
- 8.Danscher G. Localization of gold in biological tissue. A photochemical method for light and electron microscopy. Histochemistry. 1981;71:81–88. doi: 10.1007/BF00592572. [DOI] [PubMed] [Google Scholar]
- 9.Davies J, Carlstedt I, Nilsson A-K, Håkansson A, Sabharwal H, van Alphen L, van Ham M, Svanborg C. Binding of Haemophilus influenzae to purified mucins from the human respiratory tract. Infect Immun. 1995;63:2485–2492. doi: 10.1128/iai.63.7.2485-2492.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farley M M, Stephens D S, Kaplan S L, Mason E O., Jr Pilus- and non-pilus-mediated interactions of Haemophilus influenzae type b with human erythrocytes and human nasopharyngeal mucosa. J Infect Dis. 1990;161:274–280. doi: 10.1093/infdis/161.2.274. [DOI] [PubMed] [Google Scholar]
- 11.Farley M M, Stephens D S, Mulks M H, Cooper M D, Bricker J V, Mirra S S, Wright A. Pathogenesis of IgA1 protease-producing and -nonproducing Haemophilus influenzae in human nasopharyngeal organ cultures. J Infect Dis. 1986;154:752–759. doi: 10.1093/infdis/154.5.752. [DOI] [PubMed] [Google Scholar]
- 12.Farley M M, Whitney A M, Spellman P, Quinn F D, Weyant R S, Mayer L, Stephens D S. Analysis of the attachment and invasion of human epithelial cells by Haemophilus influenzae biogroup aegyptius. J Infect Dis. 1992;165(Suppl. 1):S111–S114. doi: 10.1093/infdis/165-supplement_1-s111. [DOI] [PubMed] [Google Scholar]
- 13.Forsgren J, Samuelson A, Ahlin A, Jonasson J, Rynnel-Dagoo B, Lindberg A. Haemophilus influenzae resides and multiplies intracellularly in human adenoid tissue as demonstrated by in situ hybridization and bacterial viability assay. Infect Immun. 1994;62:673–679. doi: 10.1128/iai.62.2.673-679.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galan J E. Interactions of bacteria with non-phagocytic cells. Curr Opin Immunol. 1994;6:639–642. doi: 10.1016/0952-7915(94)90146-5. [DOI] [PubMed] [Google Scholar]
- 15.Gilsdorf J R, McCrea K W, Marrs C F. Role of pili in Haemophilus influenzae adherence and colonization. Infect Immun. 1997;65:2997–3002. doi: 10.1128/iai.65.8.2997-3002.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gruenert D C, Finkbeiner W E, Widdicombe J H. Culture and transformation of human airway epithelial cells. Am J Physiol. 1995;268:L347–L360. doi: 10.1152/ajplung.1995.268.3.L347. [DOI] [PubMed] [Google Scholar]
- 17.Harvey H A, Ketterer M R, Preston A, Lubaroff D, Williams R, Apicella M A. Ultrastructural analysis of primary human urethral epithelial cell cultures infected with Neisseria gonorrhoeae. Infect Immun. 1997;65:2420–2427. doi: 10.1128/iai.65.6.2420-2427.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Holmes K A, Bakaletz L O. Adherence of non-typeable Haemophilus influenzae promotes reorganization of the actin cytoskeleton in human or chinchilla epithelial cells in vitro. Microb Pathog. 1997;23:157–166. doi: 10.1006/mpat.1997.0145. [DOI] [PubMed] [Google Scholar]
- 19.Jeffery P K. Airway mucosa: secretory cell mucus and mucin genes. Eur Respir J. 1997;10:1655–1662. doi: 10.1183/09031936.97.10071655. [DOI] [PubMed] [Google Scholar]
- 20.Mackay D J, Esch F, Furthmayr H, Hall A. Rho- and Rac-dependent assembly of focal adhesion complexes and actin filaments in permeabilized fibroblasts: an essential role for ezrin/radixin/moesin proteins. J Cell Biol. 1997;138:927–938. doi: 10.1083/jcb.138.4.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Maier R, Ganu V, Lotz M. Interleukin-11, an inducible cytokine in human articular chondrocytes and synoviocytes, stimulates the production of the tissue inhibitor of metalloproteinases. J Biol Chem. 1993;268:21527–21532. [PubMed] [Google Scholar]
- 22.Mellman I. Endocytosis and molecular sorting. Annu Rev Cell Dev Biol. 1996;12:575–625. doi: 10.1146/annurev.cellbio.12.1.575. [DOI] [PubMed] [Google Scholar]
- 23.Moller L V, Timens W, van der Bij W, Kooi K, de Wever B, Dankert J, van Alphen L. Haemophilus influenzae in lung explants of patients with end-stage pulmonary disease. Am J Respir Crit Care Med. 1998;157:950–956. doi: 10.1164/ajrccm.157.3.9707010. [DOI] [PubMed] [Google Scholar]
- 24.Mooseker M S. Organization, chemistry, and assembly of the cytoskeletal apparatus of the intestinal brush border. Annu Rev Cell Biol. 1985;1:209–241. doi: 10.1146/annurev.cb.01.110185.001233. [DOI] [PubMed] [Google Scholar]
- 25.Murphy T F, Apicella M A. Nontypeable Haemophilus influenzae: a review of clinical aspects, surface antigens and the human response to infection. Rev Infect Dis. 1987;9:1–15. doi: 10.1093/clinids/9.1.1. [DOI] [PubMed] [Google Scholar]
- 26.Smith J J, Travis S M, Greenberg E P, Welsh M J. Cystic fibrosis airway epithelia fail to kill bacteria because of abnormal airway surface fluid. Cell. 1996;85:229–236. doi: 10.1016/s0092-8674(00)81099-5. [DOI] [PubMed] [Google Scholar]
- 27.St. Geme J W. Nontypeable Haemophilus influenzae disease: epidemiology, pathogenesis and prospects for prevention. Infect Agents Dis. 1993;2:1–16. [PubMed] [Google Scholar]
- 28.St. Geme J W, III, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St. Geme J W, Cutter D. Evidence that surface fibrils expressed by Haemophilus influenzae type b promote attachment to human epithelial cells. Mol Microbiol. 1994;15:77–85. doi: 10.1111/j.1365-2958.1995.tb02222.x. [DOI] [PubMed] [Google Scholar]
- 30.St. Geme J W, III, Cutter D, Barenkamp S J. Characterization of the genetic locus encoding Haemophilus influenzae type b surface fibrils. J Bacteriol. 1996;178:6281–6287. doi: 10.1128/jb.178.21.6281-6287.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.St. Geme J W, III, Morena M, Falkow S. A Haemophilus influenzae IgA protease-like protein promotes intimate interaction with human epithelial cells. Mol Microbiol. 1994;14:217–233. doi: 10.1111/j.1365-2958.1994.tb01283.x. [DOI] [PubMed] [Google Scholar]
- 32.Teele D W, Klein J O, Rosner B Greater Boston Otitis Media Group. Epidemiology of otitis media during the first seven years of life in children in greater Boston: a prospective, cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 33.Yamaya M, Finkbeiner W E, Chun S Y, Widdicombe J H. Differentiated structure and function of cultures from human tracheal epithelium. Am J Physiol. 1992;262:L713–L724. doi: 10.1152/ajplung.1992.262.6.L713. [DOI] [PubMed] [Google Scholar]