Under normal conditions, the immune system protects the brain from outside invaders, including viruses, bacteria, toxins, and fungi. However, an abnormal immune response within the brain affects its function and contributes to the progression of neurological diseases. For example, the immune system attacks the myelin sheath surrounding the nerve fibers in multiple sclerosis, causing nerve signal transmission problems [1], and excessive glial activation exacerbates Alzheimer’s disease and contributes to its progression [2]. The discovery of the gut-brain axis indicates that the immune system in the central nervous system is also regulated by peripheral organ systems [3]. Numerous studies have demonstrated that the microbiome in the gastrointestinal tract is one of the critical regulators of the brain’s immune response [4]. However, the roles of the microbiomes in other peripheral organs in regulating the brain’s immune systems remain unclear.
In a recent study, Hosang et al. elucidated the role of the lung microbiome in regulating the brain’s immune reactivity [5]. Hosang et al. first analyzed whether the lung microbiota affects the lung autoimmune process in a rat model of experimental autoimmune encephalomyelitis (EAE). The EAE model is a well-established animal model of T-cell-mediated autoimmune disease of the central nervous system (CNS) [6]. This animal model has been established in various animals and is induced by administering CNS-derived antigens. Myelin basic protein (MBP) peptide fragment-induced EAE is a widely used model of multiple sclerosis. In the study by Hosang et al., lung EAE was established by intravenous administration of MBP-specific T cells (TMBP cells) followed by intratracheal administration of MBP. They found that daily application of intratracheal neomycin completely blocks the lung EAE and regulates the diversity and abundance of the lung microbiome. To exclude the possibility that the improvement of lung EAE was due to the changes in gut microbiota, Hosang et al. analyzed the gut microbiota after intratracheal neomycin treatment. They did not detect any significant changes in abundance or diversity of the gut bacterial strains. Moreover, direct neomycin application in the gastrointestinal tract at the dose used in the intratracheal treatment or 10-fold-higher doses does not ameliorate clinical EAE, suggesting the improvement of lung EAE has no relationship with the microbiome diversity of the gut. Furthermore, they found that subcutaneous neomycin injection that lacks a microbial environment did not affect the lung EAE, indicating that the lung microbiota is necessary for the effect of neomycin in lung EAE. Interestingly, the number of T cells within the CNS was significantly reduced following local neomycin treatment. However, no significant changes were detected in the proliferation and migration of T cells into the blood following intratracheal neomycin treatment. This finding suggests the disease-suppressing effects of the intratracheal neomycin treatment is not due to the changes in T-cell activation within the lung tissue and further confirms that local microbiota dysregulation plays a vital role in the disease-suppressing effects of intratracheal neomycin.
Hosang et al. next asked whether peripheral EAE induced by subcutaneous immunization or transfer EAE by the transfer of effector T cells could also be affected by changes in the lung microbiome [5]. Interestingly, the authors found that the peripheral EAE and transfer EAE induced outside the lung were significantly impaired after treatment with intratracheal neomycin. Next, lung microbiota transfer experiments were conducted to further confirm the role of the lung microbiota in the peripheral EAE model. Intriguingly, animals that received lung microbiota from the neomycin-treated rats showed significant improvement in the clinical symptoms of transfer EAE but not in the animals that received microbiota isolated from control animals without neomycin treatment. Similarly, in both peripheral and transfer EAE, neomycin treatment did not influence T cell numbers and migration in the periphery but rather it reduced T cell numbers within the CNS.
The authors also analyzed changes in the autoimmune process within the CNS following neomycin treatment [5]. They found that CNS inflammation was significantly decreased following the neomycin treatment. However, the reduced CNS inflammation could not be explained by the changes in TMBP cells. Therefore, the authors speculated that the changes in CNS inflammation might be due to changes in the microglia, the brain’s primary resident immune cells. To confirm the role of microglia in EAE pathogenesis following neomycin treatment, the authors treated transfer EAE animals with minocycline, a microglial activation inhibitor. Interestingly, the transfer EAE was significantly inhibited, and pretreatment with neomycin did not show any additional disease-dampening effect. Similar results were found when the microglial depletion strategy was applied, suggesting that microglia mediate the altered autoimmune response after neomycin-induced lung microbiome changes. However, morphological changes of microglia were seen after intratracheal neomycin treatment. In addition, global transcriptome analyses of total tissue and microglia found that the gene changes in microglia and whole tissue had considerable overlap, suggesting that microglial changes play a crucial role in the lung-microbiota-induced changes in CNS tissue.
The authors next investigated the lung microbiome changes after intratracheal neomycin treatment to determine the relationship between microglial reactivity and lung microbiome changes. First, they found that Bacteroidetes were the most affected species of bacteria. Next, they transferred Prevotella melaninogenica, an inactivated strain of the Bacteroidetes phylum, to examine whether Bacteroidetes contributes to the clinical effects following neomycin treatment. Intriguingly, the intratracheal transfer of P. melaninogenica significantly improved clinical EAE rather than transfer by gastrointestinal gavage. This finding further supports the hypothesis that the lung microbiome regulates the immune reactivity of the CNS. Because lipopolysaccharide (LPS) is the main component of the bacterial cell wall and 80% of the LPS production in the gut is attributed to Bacteroidetes, the authors then measured its levels in bronchoalveolar lavage fluid. Interestingly, they found a significant increase of LPS in neomycin-treated rats, and antibiotic treatment that did not affect LPS did not affect microglial and had no effect in regulating EAE. In contrast, an antibiotic peptide that neutralizes LPS in the bronchoalveolar lavage fluid significantly exacerbates the severity of EAE. These results confirmed that LPS is a regulator of CNS immune functions and plays a vital role in regulation of brain autoimmunity by the lung microbiome
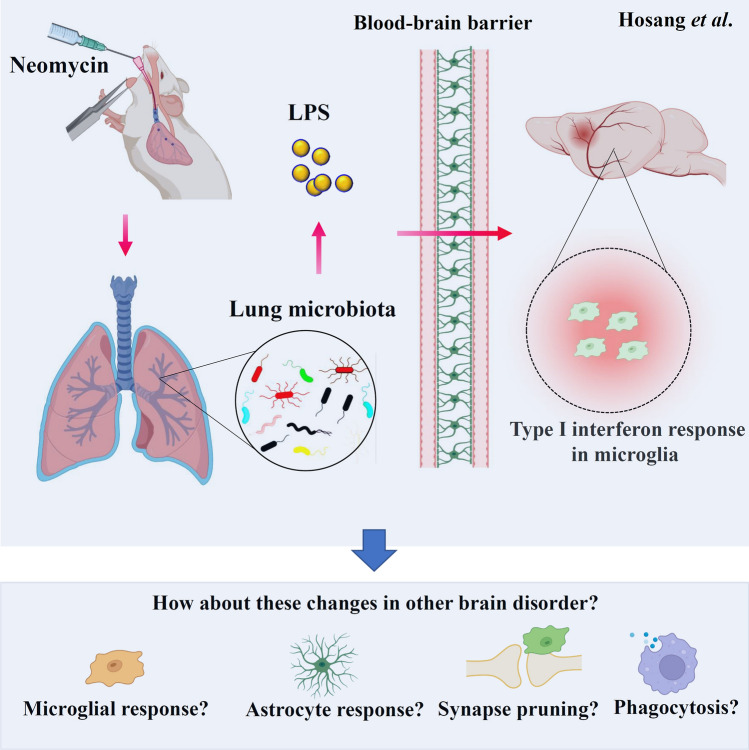
In summary, Hosang et al. confirmed that the lung microbiome regulates the autoimmune responses in the CNS. Moreover, they found that LPS-producing bacterial taxa play a central role in this process (Fig. 1). Although they could not rule out the effects of microbial metabolites and the changes of peripheral or recruited immune cells on the CNS autoimmunity, their study provides a new concept for the peripheral organ system controlling the CNS. It offers novel mechanisms underlying lung and CNS connections. Several intriguing questions arise based on these critical findings and are worth further investigation. First, although the astrocyte may not be involved in the lung-microbiota-induced CNS tissue changes [5], astrocyte changes may also be detected at a later stage or other brain disease models because of the crosstalk between astrocytes and microglia. Therefore, the role of astrocytes in the lung microbiome-regulated autoimmune inflammation of the brain deserves further investigation. Second, because microglia play an essential role in many processes in the brain, including microglia-mediated synaptic pruning, microglial phagocytosis, and neuroinflammation [7, 8], treatments influencing the lung microbiome may be able to regulate these processes. Further studies may examine the effect of the lung microbiome on microglia-mediated synaptic pruning during early brain development and microglial phagocytosis in neurological disorders. Third, more studies are needed to explore new avenues and possible therapeutic approaches targeting the lung microbiome. For example, is it possible to use inhaled probiotics to treat brain disorders? Finally, it would be interesting to explore whether other lung microbe-derived products influence CNS autoimmunity and whether chronic lung diseases are risk factors for neurodegenerative diseases. Looking deeper into the effects and the underlying mechanisms of the regulation of the lung microbiome in regulating brain autoimmunity in various brain diseases may provide a possible way to prevent or slow the progression of these brain disorders.
Fig. 1.
The lung microbiome regulates brain microglia. Intratracheal neomycin administration shifts the lung microbiota towards LPS-enriched types. The released LPS can cross the blood-brain barrier and target the brain, where the increased LPS in neomycin-treated rats induces a microglial type I interferon response within the CNS, resulting in the alleviation of EAE. Several intriguing questions arise and are worth further investigation, including the role of the lung microbiome in regulating the microglial response, the long-term astrocyte response, microglial pruning, and microglial phagocytosis in other brain disorders.
Acknowledgements
This highlight was supported by the National Natural Science Foundation of China (32100918), and the project was funded by the China Postdoctoral Science Foundation (2021M690060), and the Sigma Xi Grants in Aid of Research program (G03152021115804390).
Conflict of interest
The authors declare no competing financial interests.
Footnotes
Luoman Yang and Shu Feng contributed equally to this work.
Contributor Information
Chongyun Wu, Email: 2017010064@m.scnu.edu.cn.
Luodan Yang, Email: luodan.yang@lsuhs.edu.
References
- 1.Shaharabani R, Ram-On M, Talmon Y, Beck R. Pathological transitions in myelin membranes driven by environmental and multiple sclerosis conditions. Proc Natl Acad Sci USA. 2018;115:11156–11161. doi: 10.1073/pnas.1804275115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Yang LD, Wu CY, Parker E, Li Y, Dong Y, Tucker L, et al. Non-invasive photobiomodulation treatment in an Alzheimer Disease-like transgenic rat model. Theranostics. 2022;12:2205–2231. doi: 10.7150/thno.70756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rutsch A, Kantsjö JB, Ronchi F. The gut-brain axis: How microbiota and host inflammasome influence brain physiology and pathology. Front Immunol. 2020;11:604179. doi: 10.3389/fimmu.2020.604179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Carabotti M, Scirocco A, Maselli MA, Severi C. The gut-brain axis: Interactions between enteric microbiota, central and enteric nervous systems. Ann Gastroenterol. 2015;28:203–209. [PMC free article] [PubMed] [Google Scholar]
- 5.Hosang L, Canals RC, van der Flier FJ, Hollensteiner J, Daniel R, Flügel A, et al. The lung microbiome regulates brain autoimmunity. Nature. 2022;603:138–144. doi: 10.1038/s41586-022-04427-4. [DOI] [PubMed] [Google Scholar]
- 6.Baxter AG. The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol. 2007;7:904–912. doi: 10.1038/nri2190. [DOI] [PubMed] [Google Scholar]
- 7.Wu CY, Yang LD, Youngblood H, Liu TCY, Duan R. Microglial SIRPα deletion facilitates synapse loss in preclinical models of neurodegeneration. Neurosci Bull. 2022;38:232–234. doi: 10.1007/s12264-021-00795-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yang LD, Tucker D, Dong Y, Wu CY, Lu YJ, Li Y, et al. Photobiomodulation therapy promotes neurogenesis by improving post-stroke local microenvironment and stimulating neuroprogenitor cells. Exp Neurol. 2018;299:86–96. doi: 10.1016/j.expneurol.2017.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]