Abstract
Background
In hepatic damage, Hepatic stellate cells (HSCs) become active, proliferate, and change to myofibroblasts. Increasing the fibrogenic genes, such as Transforming growth factor-β (TGF-β), Alpha Smooth Muscle Actin (α-SMA), and Collagen1 α (COL 1α) show that the activation of HSCs can lead to hepatic fibrosis.
Purpose
These days people consume much cholesterol, palmitic acid, and glucose which can have adverse effects on an individuals’ health, but their influences on activating human HSCs and inducing liver fibrosis have not been assessed. Our purpose is to investigate the effects of these three main and abundant ingredients in the diet on the activation of human HSCs and inducing liver fibrosis.
Methods
To measure cholesterol, palmitic acid, and glucose cytotoxic effects on the viability of the cells, the MTT technique was used. Then the treated cells were incubated in media containing cholesterol, palmitic acid, and glucose with different concentrations for 24 h. At last, the α-SMA, COL 1α, and TGF-β, genes mRNA expression were measured by real-time PCR.
Results and Conclusions
Our results demonstrated that high concentrations of cholesterol and palmitic acid can activate human HSCs that lead to an increase in the mRNA expressions of fibrogenic genes. Thus, controlling fat intaking and knowing its mechanism is crucial to prevent and attenuate hepatic fibrosis.
Keywords: Hepatic fibrosis, Human HSCs, Cholesterol, Palmitic acid, Glucose
Introduction
Liver fibrosis disease is considered a significant unsolved problem in the world. Nowadays, higher liver fibrosis level is associated with increased liver disease and overall mortality. Until now, there is no definite cure for it. So preventing and knowing the factors that cause it, are very important [1]. Hepatic fibrogenesis is the effect of chronic liver injury after several chronic wound-healing responses. Hepatic fibrosis shows the reaction of the liver to different damages and is happened by increasing the production of extracellular matrix (ECM) when synthesis and degradation of ECM are unbalanced. Progressed Hepatic fibrosis can ultimately lead to cirrhosis and liver failure [2, 3]. Viral infections (hepatitis B and C), the consumption of alcohol, autoimmune and metabolic diseases are the routine and important reasons to induce hepatic fibrosis [4].
Injuries and damages to the liver can be managed by lifestyle and nutritional modifications [5]. Nutrition has not been the main focus of studying liver fibrosis, despite its importance in the progression and the severity of this disease. Certainly, improper nutrition can onset liver fibrosis. Therefore, we should know the elements in our food which can cause this disease [6].
The stellate cells are the main cells type involved in liver fibrosis, which become active in response to liver damage. In normal liver, stellate cells are in a quiescent state and the lipid droplets in them store vitamin A as retinol ester. The function of quiescent hepatic stellate cells is unknown. When the liver is damaged, stellate cells can change into an activated state [7]. Factors such as the toxicity of alcohol, viral hepatitis, or metabolic diseases can be considered as injuries to activate these cells The activated stellate cell is characterized by proliferation, contractility, migration, and changing to myofibroblasts. This state of the stellate cell is the main source of extracellular matrix production in liver injury, which in chronic injuries can lead to liver fibrosis [8, 9]. Transforming growth factor-β (TGF-β) is one of the key cytokines in this process [10]. Activated HSCs express so much TGF-β. TGF-β has important effects on hepatic fibrosis.it is a pre-fibro genic factor in chronic liver injury [11, 12]. TGF-β can activate HSCs by the autocrine effects. The expression of TGF-β maybe is one of the main signals that can activate other quiescent and inactivated HSCs and change them to myofibroblasts.To prevent hepatic fibrosis and preserve organs function, Inhibition of TGF-β is effective [13]. In hepatic fibrosis, activated HSCs express high levels of Collagen1 α (COL 1α) and Alpha Smooth Muscle Actin (α-SMA), which is a cytoskeleton factor in the cell cytoplasm. Increasing in COL 1α and α-SMA expression are known as markers that shows activation of HSCs [14].
The factors which can cause liver fibrosis are important because there is no definite remedy for this disease, so by knowing the factors which can cause liver fibrosis, we can manage and prevent this disease. Dietary factors are maybe important for hepatic fibrosis beginning and progression. In one laboratory research, after long-time use of a high-cholesterol diet, rodents or rabbits developed hepatic fibrosis [15, 16]. Diet with high Cholesterol aggravated hepatic fibrosis in mice because free cholesterol had accumulated in HSCs and sensitized HSC to TGF-β [17].
In persons which are suffering from non-alcoholic fatty liver disease (NAFLD), circulating free fatty acids in the blood, are commonly increased to the upper level [18]. When the capacity of the liver to keep surplus FFA (free fatty acids) in the form of TGs (triglycerides) is exceeded, Liver damages may happen. Mitochondrial dysfunction, Oxidative stress, and expression of many pro-inflammatory cytokines may be the result of cellular lipid excess [19, 20], and can contribute to inflammatory damage in the liver and fibrogenesis [21].
Palmitic acid (C16:0) forms a large proportion of total dietary SFA intake and can be found in palm oil, meat, and butter [22]. Palmitic acid is one of the most common long chain saturated free fatty acids in food and the human body, and they are most closely related to insulin resistance and type 2 diabetes. Human studies have revealed that circulating SFAs in blood, especially C16:0 is associated with higher metabolic disease risk like diabetes [23, 24]. In one study, the HSCs activation genes expression level, such as α-SMA, COL 1α, and TGFβ in Primary HSCs isolated from Sprague Dawley rats, significantly increased with palmitic Acid [25]. High hyperglycemia and uncontrolled diabetes can lead to fatty liver disease, so hyperglycemia is a factor to induce liver injury. In one study, the levels of COL 1α did not significantly increase in human HSCs exposed to Hyperglycemia [26]. Recent studies have looked at the effects of diet on liver injuries [27]. But, few studies have been focused on hepatic fibrosis to investigate the roles of cholesterol, palmitic acid, glucose, and their mechanisms of action. Also, because these three compounds, namely cholesterol, palmitic acid, and glucose, are mainly present in diets and the body, by comparing the effects of these three compounds on activation of human HSCs, the genes expression involved in hepatic fibrosis and its progression, helpful results can be obtained. The purpose of this study is to know the causing elements in our diets which lead to liver fibrosis. In this research, we investigated and compared the mRNA expression of major genes involved in hepatic fibrogenesis (TGFβ, COL1α α, and α-SMA,) in human HSCs treated by different concentrations of cholesterol, glucose, and Palmitic acid.
Materials and methods
Human hepatic stellate cells culture and preparation glucose, cholesterol, and palmitic acid solutions
Cholesterol, palmitate, and glucose specific for cell culture, The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-2 H-tetrazolium bromide (MTT) assay, FBS, DMEM Low Glucose, penicillin, and antibiotics all were got (Sigma-Aldrich, US). To do this study, the LX-2 human hepatic stellate cells line (gotten from Dr. Scott L. Friedman) was used. The cells were seeded in low DMEM with FBS (10%), penicillin and streptomycin (100 mg/ml each) in the incubator with 37 °C and 5% CO2.
To prepare cholesterol and glucose solutions, cholesterol powder was dissolved in acetic acid. Glucose was dissolved in bi-distilled water and filter-sterilized to BSA without fatty acids. For this purpose, the palmitate with different concentrations was dissolved in 50% ethanol, and then each concentration was added to DMEM containing 1% BSA [28]. Then, the solution was incubated on a shaker for 2 h at 37 °C. After 2 h, this medium was filtered and ready to give to the cells.
Human hepatic stellate cells viability after treatment with different concentrations of palmitic acid, cholesterol and glucose
To study the effects of various concentrations of glucose, cholesterol, and palmitic acid on the human HSCs survival, in all 96-well culture plates, about 4 × 103 cells of the desired cell line were cultured. After overnight incubation, the cells were treated with different concentrations of cholesterol (20,40,60,80,100 and120 µM) [29], glucose (10,15,22,32,and 44 mM) [28], and palmitic acid (25,50,75,100,125 and 150 µM) [30] which they were for 24 h. The supernatant was detached and then, the HSC cells were incubated with MTT (100 µl) for four hours. Then, we used DMSO to solve the formazan crystals.at 570 nm, the absorbance was read with an ELISA reader. For all different concentrations, the experiments were repeated 3 times.
RNA extraction from Human hepatic stellate cells and doing Real Time PCR after treatment of the cells with different cholesterol, glucose, and palmitic acid concentrations
First, the cells were seeded in six wells (2 × 105 cells/well) for 24 or 48 h to reach Confluences of about 70–80%. Before treatment, the cells were under starvation for 24 h. After starvation, the medium was renewed with DMEM containing 0.1% FBS. Different concentrations of glucose (10,15,22,32,and 44 mm), cholesterol (20,40,60,80, and 100 µM), and palmitic acid (25,50,75,100, and 125 µM) were used to treat Human HSCs for 24 h. Our control group had exactly the same situation as the treated groups in the laboratory. After starvation, it was treated with DMEM Low Glucose for 24 h.
Evaluation of gene expression by RNA extraction kit and real-time reverse transcription-polymerase chain reaction technic (RT-PCR)
We used the Real-time PCR technique for mRNA quantitation of hepatic fibrogenesis main genes. (TGFβ, αSMA, and COL 1α). After the treatments of the cells with different concentrations of Glucose, palmitic acid, and Cholesterol for 24 h, total RNA was isolated from the cells utilizing an RNeasy mini kit (Qiagen Company, Germany) according to the protocols. The quality and quantity of extracted RNA were assessed utilizing a spectrophotometer (NanoDrop 2000, Thermo Fisher Scientific, USA), and 1.0% agarose gel electrophoresis. After that, cDNA synthesis was done with a cDNA kit (the company of Yekta Tajhiz Azma, Iran), oligo-dT primers, and a random hexamer. To evaluate the mRNA quantitation level of TGF-β, COL1α, and αSMA, Real-Time PCR technic was performed (Applied Biosystems, US) by using Amplicon SYBR green Master Mix low ROX kit(US). In order to detect the expression of the genes, the primers we used for RT-PCR are in the below table:
| TGF-β | Sequence of forward | 5’-AGCCGTGGAGGGGAAATTG-3’ |
| Sequence of reverse | 5’-CGGTAGTGAACCCGTTGATG- 3’ | |
| αSMA | Sequence of Forward | 5’-TATCCCCGGGACTAAGACGG- 3’, |
| Sequence of reverse | 5’-CACCATCACCCCCTGATGTC-3’ | |
| COL 1α | Sequence of Forward | 5’- TGAAGGACACAGAGGTTCAG- 3’ |
| Sequence of reverse | 5’- GTAGCACATCATTTCCACGA- 3’ | |
| GAPDH | Sequence of Forward | 5’- GTCTCCTCTGACTTCAACAGCG − 3’ |
| Sequence of reverse | 5’- ACCACCCTGTTGCTGTAGCCAA − 3’ |
Analysis statistical
All steps were repeated three times. The information is presented in means ± standard error of the mean (SEM). The difference significance between the group’s means was determined by ANOVA and GraphPad Prism 9 software. For determining the statistical significance of changes in the groups, Tukey’s tests and ANOVA were used. P values (less than 0.05) were as significant.
Results
Effect of different concentrations of cholesterol, glucose, and palmitic acid on the viability of human HSC cells
MTT test technic was done after 24 h to find proper concentrations of glucose, cholesterol, and palmitic acid for treatment. First, Human HSCs were treated with different concentrations of glucose (10,15,22,32,and 44 mM), palmitic acid (25,50,75,100,125 and 150 µM), and cholesterol (20,40,60,80,100 and120 µM) for 24 h. The percentage of cell survival at all concentrations of glucose was not changed compared to the control group (Fig. 1A). At a concentration of 150 µM palmitic acid, the cell’s survival percentage was remarkably reduced in comparison to the control (****p < 0.0001, Fig. 1B). Therefore, concentrations under IC50 were chosen to treat. The cell viability percentage at 120 µM cholesterol concentration was significantly reduced in comparison to the control (***p < 0.001, Fig. 1C), Therefore, other concentrations which were under IC50 were chosen to treat.
Fig. 1.
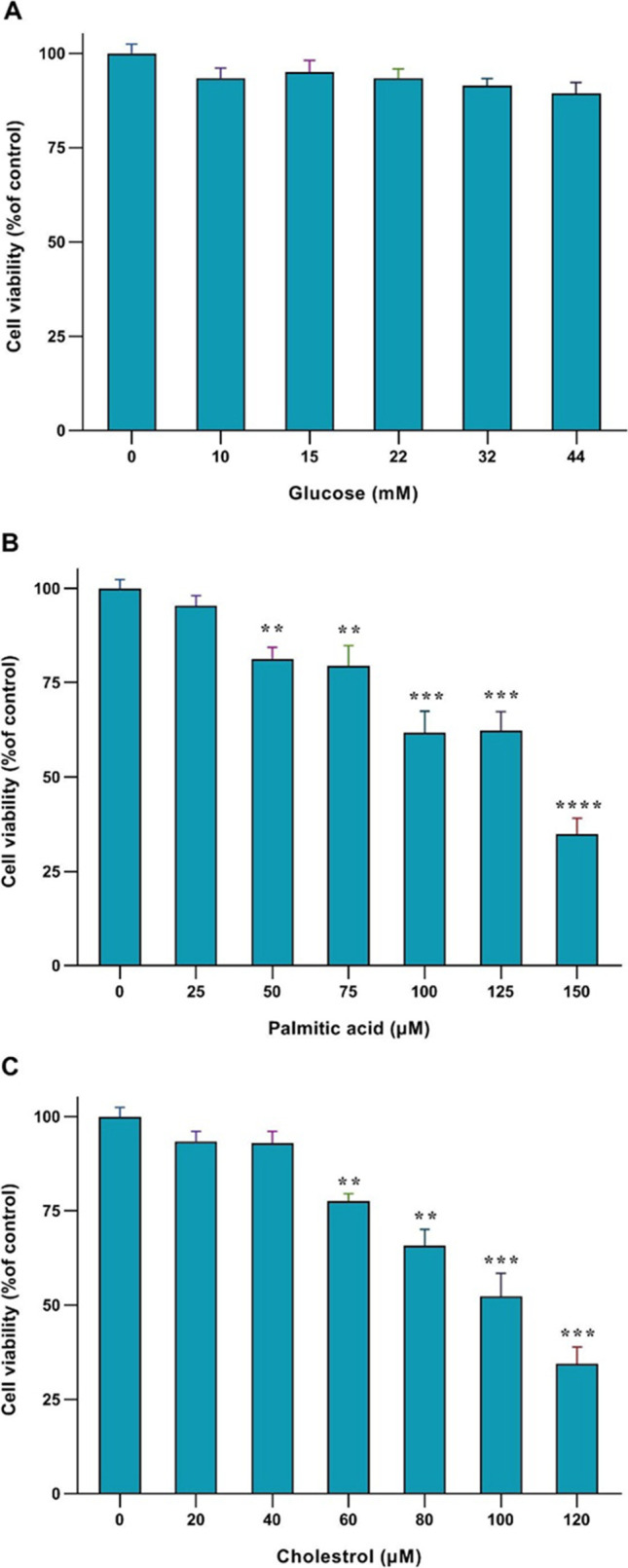
Effects of, glucose, palmitic acid, and cholesterol in various concentrations on the human HSCs survival. The MTT assay results show the viability of the cells treated to different cholesterol, glucose, and palmitic acid concentrations after 24 h. obtained results are shown with mean ± SEM. Analysis was done by Tukey test, one-way ANOVA, and GraphPad Prism 8 program. (**p < 0.01, ***p < 0.001, ****p < 0.0001)
Comparison of the effects of different concentrations of cholesterol, glucose, and palmitic acid on the liver fibrosis genes expression
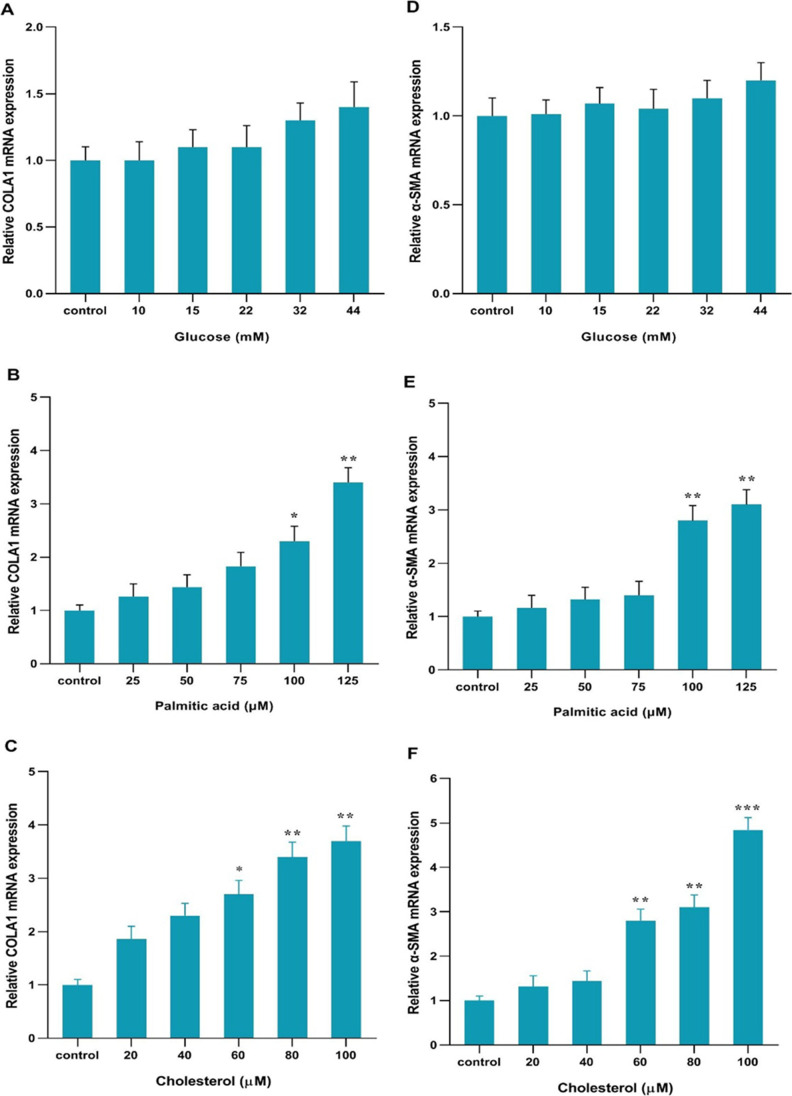
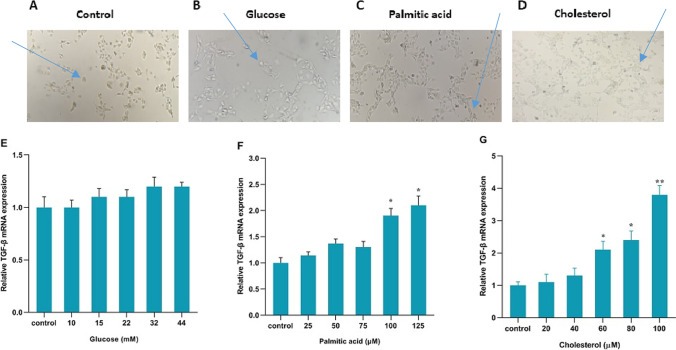
According to phenotype and appearance, changing quiescent HSC cells into fibrogenic Myofibroblasts are a major sign of the activation of these cells [26]. The shape of the cells is shown in the control group and in the presence of glucose, palmitic acid, and cholesterol treatments. (Fig. 2A, B, C, and D) .Activated cells are spindle-shaped and more elongated and changed to Myofibroblasts. Inactive cells are almost round. The results got from Real-time PCR indicated that the TGF-β, COL 1α, and α-SMA genes mRNA expressions were not significantly changed and not increased in response to 10, 15, 22, 32, and 44 mM glucose compared to the control group. (Figs. 2E, 3A and D)The TGF-β, COL 1α, and α-SMA genes mRNA expressions in response to palmitic acid were not changed at 25,50 and 75 µM concentrations but palmitic acid at 100 and 125 µM concentrations significantly increased the mRNA expression of the genes. Palmitic acid at the concentrations of 100 and 125 µM significantly increased the mRNA expression of genes by Fold Change 1.9and 2.1 for TGF-β, 2.3, and 3.3 for COL 1α, 2.8and 3 for α-SMA relative to the control respectively. (* p < 0.05, ** p < 0.01, Figs. 2F, 3B and E). Cholesterol at 20 and 40 µM concentrations did not change the TGF-β, COL 1α, and α-SMA genes mRNA expressions but significantly increased the expression of these genes at each 60, 80, and 100 µM concentration. Cholesterol at the concentrations of 60, 80, and 100 mM significantly increased the mRNA expression of genes by Fold Change 2, 2.2, and 3.8 for TGF-β, 2.7, 3.4, and 3.7 for COL 1α, 2.9,3.1and 4.9 for α-SMA relative to the control respectively. (* p < 0.05, ** p < 0.01, *** p < 0.001 Figs. 2G, 3C and F).
Fig. 3.
COLA1 and, α-SMA gene expression with cholesterol, glucose, and palmitic acid in cells line. A COLA1 expression in glucose-treated cells B COLA1 expression in palmitic acid-treated cells. C COLA1 expression in cholesterol-treated cells. D α-SMA expression in glucose-treated cells (E) α-SMA expression in palmitic acid-treated cells for. F α-SMA expression in cholesterol-treated cells. All cells were treated for 24 h. The data are shown with the mean ± SEM and 3 replicates. They are shown as fold changes in expressions in comparison to the control. For the reference gene, GAPDH was taken to use. (* p < 0.05 vs. treated control, ** p < 0.01 vs. treated)
Fig. 2.
Effect of the action of palmitic acid, cholesterol and, glucose on hepatic stellate cells. Human HSCs before treating (control group) (A). Human HSCs after treatment with glucose, palmitic acid, and cholesterol respectively (B, C, and D) (10 magnification). TGFβ1 gene expression with glucose, palmitic acid, and cholesterol in the cells. E TGF-β expression in glucose-treatedcells. F TGF-β expression in palmitic acid-treated cells. G TGF-β expression in cells treated with cholesterol. All cells were treated for 24 h. The data are shown with the mean ± SEM and 3 replicates. They are shown as fold changes in expressions in comparison to the control. For the reference gene, GAPDH was taken to use. (* P < 0.05 vs. treated control, **p < 0.01 vs. treated)
Discussion
Nowadays, liver fibrosis disease is known as one of the most common reasons for death in the world, and its incidence is growing every year. Recognizing the factors which can cause and advance liver fibrosis is a serious matter in the medical field today [29]. If chronic liver damages continue, because of any reason like the toxicity of alcohol, viral hepatitis, or metabolic diseases, Hepatic fibrosis will happen [28, 30], but few attention and studies have been done on the effect of nutrition and food in causing liver fibrosis. At present, it is better to know the factors in the diet that cause hepatic fibrosis to choose better food. The absence of efficient drugs to treat hepatic fibrosis is a major global subject, thus, prevention of hepatic fibrosis is important. Hepatic stellate cells (HSCs) which stand in the liver, are the source of activated myofibroblasts cells that yield ECM (extracellular matrix) in the liver [31]. When HSCs become activated and change to myofibroblasts, they have important roles in developing hepatic fibrosis because they can produce much ECM. Several inflammatory and fibrogenic pathways have a role in the activation of HSCs [32].
In this research, we studied the effects of different glucose, cholesterol, and palmitic acid concentrations on the expression of fibrosis genes in human HSCs. Our results showed that Both high concentrations of cholesterol and palmitic acid could increase the mRNA expression of the main fibrosis genes (α-SMA, COL 1α, and TGF-β genes)while different glucose concentrations didn’t affect the expression of these genes. Compared with palmitic, Cholesterol increases the α-SMA, COL 1α, and TGF-β genes mRNA expression more significantly. “Tomita and his colleagues in a study after giving a high-fat to mice, found that the liver becomes fibrosis and the higher cholesterol causes fibrosis to become more severe and faster. It was reported that free cholesterol could activate HSCs, and adding excess cholesterol to a diet could lead to more accumulation of cholesterol in mice cells. They further observed that fibrosis was caused by the accumulation of free cholesterol in mice HSCs, which increased the expression of genes involved in the progression of fibrosis. Also, they found that the cause of cholesterol accumulation in HSCs was impaired regulation of cholesterol homeostasis” [33]. “A study conducted by Meissen JK showed that high glucose concentration increases the accumulation of triglyceride in the hepatocyte cells” [34] but its effect on hepatic fibrosis in humans has not been studied yet. Experiments of Katalin Kiss and his colleagues in vitro indicate that chronic exposure to high glucose concentration initiates profound alteration of cells. We guess maybe chronic exposure to glucose can activate the cells and increase the expression of genes, but it needs to test [26]. “Hella Wobser and his colleagues in a study found that palmitic acid treatment induces cellular lipid accumulation in humans, but its effect on hepatic fibrosis has remained unclear” [35]. “Z Dong in his study investigated the role of palmitic acid in the activation of rat HSCs. After treating the cells with palmitic acid, they evaluated the TLR4-NF-κb signaling pathway as well as the expression of genes such as TGFβ and concluded that palmitic acid could activate HSCs and cause hepatic fibrosis by increasing the TLR4-NF-κB signaling pathway” [25]. The activated HSCs express more TGF-β which leads to hepatic fibrosis by increasing ECM deposition and inhibits collagenase activity in HSCs cells [36]. Increasing the expression of COL 1α and α-SMA genes are the markers of activated HSCs [32]. According to these researches, we decided to study the effect of cholesterol, palmitic acid, and glucose on mRNA expression of fibrogenic genes in human HSCs. As shown in this study, the human HSCs were activated by cholesterol and palmitic acid treatment (dose-dependent), and the TGFβ1, COL 1α, and α-SMA genes mRNA expressions (hepatic fibrogenic genes) were significantly increased, but mRNA expressions of these genes were not increased by glucose treatment. Moreover, our results showed that cholesterol more than palmitic acid activates HSCs and increases the expression of hepatic fibrogenic genes.
Our study has some limitations. In this study, all experiments were done on cell line and in the laboratory (in vitro), and the results may be different from those referring to living organisms. So, we recommend doing all these experiments on living organisms, mice, or humans. Our study shows that high cholesterol and palmitic acid can activate human HSCs and increase hepatic fibrogenic genes expression, but glucose does not have this effect. According to these data, it is recommended to reduce cholesterol and free fatty acids in the diet to prevent liver fibrosis. Once again, we realize the role of proper nutrition in preventing diseases.
Acknowledgements
The authors thank the University of Medical Sciences of the Ahvaz Jundishapur for the financial support of the study.
Abbreviations
- TGF-β
1-Transforming growth factor-β
- α-SMA
2-Alpha Smooth Muscle Actin
- COL 1α
3-Collagen1 α
- HSCS
Human hepatic stellate cells
- ECM
Extracellular matrix
- NAFLD
Non-alcoholic fatty liver disease
- MTT assay
3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyl-2 H-tetrazolium bromide
- FFA
free fatty acids
- TGs
triglycerides
Authors’ contributions
GHM planned the research. ESH did assay. RA analyses the obtained results. SSB and ESH wrote the manuscript and revised it. FA and SAZ interpreted the data. All authors confirmed the final article.
Funding
Medical Sciences Ahvaz Jundishapur University has supported this study (grant number. HLRC- CMRC-0009). The funding has not affected any steps of the research.
Data availability
The data in this study are present from the corresponding author if requested.
Declarations
Ethics approval to participate
Ethical clearance was not needed and not sought from the Review Board of Ahwaz Jundishapur University of Medical Sciences, because the study was done on cell lines in vitro, and did not use human samples.
Publication consent
No applicable.
Competing interests
The authors report no conflicts of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Roehlen N, Crouchet E, Baumert TF. Liver fibrosis: mechanistic concepts and therapeutic perspectives. Cells. 2020;9(4):875. doi: 10.3390/cells9040875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Friedman SL, Maher JJ, Bissell DM. Mechanisms and therapy of hepatic fibrosis: report of the AASLD Single Topic Basic Research Conference. 2000, Wiley Online Library. [DOI] [PubMed]
- 3.Wells RG. The role of matrix stiffness in hepatic stellate cell activation and liver fibrosis. J Clin Gastroenterol. 2005;39(4):S158–61. doi: 10.1097/01.mcg.0000155516.02468.0f. [DOI] [PubMed] [Google Scholar]
- 4.Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Ann Hepatol. 2017;16(1):48–56. doi: 10.5604/16652681.1226814. [DOI] [PubMed] [Google Scholar]
- 5.Smith A, Baumgartner K, Bositis C. Cirrhosis: diagnosis and management. Am Family Phys. 2019;100(12):759–70. [PubMed] [Google Scholar]
- 6.Juakiem W, Torres DM, Harrison SA. Nutrition in cirrhosis and chronic liver disease. Clin Liver Dis. 2014;18(1):179–90. doi: 10.1016/j.cld.2013.09.004. [DOI] [PubMed] [Google Scholar]
- 7.Winau F, et al. Ito cells are liver-resident antigen-presenting cells for activating T cell responses. Immunity. 2007;26(1):117–29. doi: 10.1016/j.immuni.2006.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Eng FJ, Friedman SL. Fibrogenesis I. New insights into hepatic stellate cell activation: the simple becomes complex. Am J Physiology-Gastrointestinal Liver Physiol. 2000;279(1):G7–11. doi: 10.1152/ajpgi.2000.279.1.G7. [DOI] [PubMed] [Google Scholar]
- 9.Krizhanovsky V, et al. Senescence of activated stellate cells limits liver fibrosis. Cell. 2008;134(4):657–67. doi: 10.1016/j.cell.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu X, Hu H, Yin JQ. Therapeutic strategies against TGF-β signaling pathway in hepatic fibrosis. Liver Int. 2006;26(1):8–22. doi: 10.1111/j.1478-3231.2005.01192.x. [DOI] [PubMed] [Google Scholar]
- 11.Bissell D, et al. Cell-specific expression of transforming growth factor-beta in rat liver. Evidence for autocrine regulation of hepatocyte proliferation. J Clin Investig. 1995;96(1):447–55. doi: 10.1172/JCI118055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bissell DM, Roulot D, George J. Transforming growth factor β and the liver. Hepatology. 2001;34(5):859–67. doi: 10.1053/jhep.2001.28457. [DOI] [PubMed] [Google Scholar]
- 13.Martin M, Lefaix J-L, Delanian S. TGF-β1 and radiation fibrosis: a master switch and a specific therapeutic target? Int J Radiation Oncology* Biology* Phys. 2000;47(2):277–90. doi: 10.1016/S0360-3016(00)00435-1. [DOI] [PubMed] [Google Scholar]
- 14.Kweon Y-O, et al. Gliotoxin-mediated apoptosis of activated human hepatic stellate cells. J Hepatol. 2003;39(1):38–46. doi: 10.1016/S0168-8278(03)00178-8. [DOI] [PubMed] [Google Scholar]
- 15.Sumiyoshi M, Sakanaka M, Kimura Y. Chronic intake of a high-cholesterol diet resulted in hepatic steatosis, focal nodular hyperplasia and fibrosis in non-obese mice. Br J Nutr. 2010;103(3):378–85. doi: 10.1017/S0007114509991772. [DOI] [PubMed] [Google Scholar]
- 16.Kainuma M, et al. Cholesterol-fed rabbit as a unique model of nonalcoholic, nonobese, non-insulin-resistant fatty liver disease with characteristic fibrosis. J Gastroenterol. 2006;41(10):971–80. doi: 10.1007/s00535-006-1883-1. [DOI] [PubMed] [Google Scholar]
- 17.Teratani T, et al. A high-cholesterol diet exacerbates liver fibrosis in mice via accumulation of free cholesterol in hepatic stellate cells. Gastroenterology. 2012;142(1):152–64. doi: 10.1053/j.gastro.2011.09.049. [DOI] [PubMed] [Google Scholar]
- 18.Nehra V, et al. Nutritional and metabolic considerations in the etiology of nonalcoholic steatohepatitis. Dig Dis Sci. 2001;46(11):2347–52. doi: 10.1023/A:1012338828418. [DOI] [PubMed] [Google Scholar]
- 19.Seki S, et al. In situ detection of lipid peroxidation and oxidative DNA damage in non-alcoholic fatty liver diseases. J Hepatol. 2002;37(1):56–62. doi: 10.1016/S0168-8278(02)00073-9. [DOI] [PubMed] [Google Scholar]
- 20.Pérez-Carreras M, et al. Defective hepatic mitochondrial respiratory chain in patients with nonalcoholic steatohepatitis. Hepatology. 2003;38(4):999–1007. doi: 10.1002/hep.1840380426. [DOI] [PubMed] [Google Scholar]
- 21.Reeves HL, et al. Hepatic stellate cell activation occurs in the absence of hepatitis in alcoholic liver disease and correlates with the severity of steatosis. J Hepatol. 1996;25(5):677–83. doi: 10.1016/S0168-8278(96)80238-8. [DOI] [PubMed] [Google Scholar]
- 22.Vazquez-Jimenez JG, et al. Palmitic acid but not palmitoleic acid induces insulin resistance in a human endothelial cell line by decreasing SERCA pump expression. Cell Signal. 2016;28(1):53–9. doi: 10.1016/j.cellsig.2015.10.001. [DOI] [PubMed] [Google Scholar]
- 23.Kleinfeld AM, et al. Increases in serum unbound free fatty acid levels following coronary angioplasty. Am J Cardiol. 1996;78(12):1350–4. doi: 10.1016/S0002-9149(96)00651-0. [DOI] [PubMed] [Google Scholar]
- 24.Takkunen MJ, et al. Longitudinal associations of serum fatty acid composition with type 2 diabetes risk and markers of insulin secretion and sensitivity in the Finnish Diabetes Prevention Study. Eur J Nutr. 2016;55(3):967–79. doi: 10.1007/s00394-015-0911-4. [DOI] [PubMed] [Google Scholar]
- 25.Dong Z, et al. Palmitic acid stimulates NLRP3 inflammasome activation through TLR4-NF-κB signal pathway in hepatic stellate cells. Ann Trans Med. 2020;8(5). [DOI] [PMC free article] [PubMed]
- 26.Kiss K, et al. Chronic hyperglycaemia induced alterations of hepatic stellate cells differ from the effect of TGFB1, and point toward metabolic stress. Pathol Oncol Res. 2020;26(1):291–9. [DOI] [PubMed]
- 27.Perdomo CM, Frühbeck G, Escalada J. Impact of nutritional changes on nonalcoholic fatty liver disease. Nutrients. 2019;11(3):677. doi: 10.3390/nu11030677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhou L, et al. miR-185 inhibits fibrogenic activation of hepatic stellate cells and prevents liver fibrosis. Mol Therapy-Nucleic Acids. 2018;10:91–102. doi: 10.1016/j.omtn.2017.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Iredale J. Defining therapeutic targets for liver fibrosis: exploiting the biology of inflammation and repair. Pharmacol Res. 2008;58(2):129–36. doi: 10.1016/j.phrs.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 30.Liu Z, et al. Transforming growth factor β (TGFβ) cross-talk with the unfolded protein response is critical for hepatic stellate cell activation. J Biol Chem. 2019;294(9):3137–51. doi: 10.1074/jbc.RA118.005761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Seki E, Brenner DA. Recent advancement of molecular mechanisms of liver fibrosis. J Hepato-Biliary‐Pancreatic Sci. 2015;22(7):512–8. doi: 10.1002/jhbp.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Carpino G, et al. Alpha-SMA expression in hepatic stellate cells and quantitative analysis of hepatic fibrosis in cirrhosis and in recurrent chronic hepatitis after liver transplantation. Dig Liver Dis. 2005;37(5):349–56. doi: 10.1016/j.dld.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 33.Tomita K, et al. Free cholesterol accumulation in hepatic stellate cells: mechanism of liver fibrosis aggravation in nonalcoholic steatohepatitis in mice. Hepatology. 2014;59(1):154–69. doi: 10.1002/hep.26604. [DOI] [PubMed] [Google Scholar]
- 34.Meissen JK, et al. Temporal metabolomic responses of cultured HepG2 liver cells to high fructose and high glucose exposures. Metabolomics. 2015;11(3):707–21. doi: 10.1007/s11306-014-0729-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wobser H, et al. Lipid accumulation in hepatocytes induces fibrogenic activation of hepatic stellate cells. Cell Res. 2009;19(8):996–1005. doi: 10.1038/cr.2009.73. [DOI] [PubMed] [Google Scholar]
- 36.Fabregat I, et al. TGF-β signalling and liver disease. FEBS J. 2016;283(12):2219–32. doi: 10.1111/febs.13665. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data in this study are present from the corresponding author if requested.