Abstract
Background
The relationship between liver enzymes and Metabolic Syndrome (MetS) in different populations, including Canadians, is not consistent and well understood. We used the Canadian Health Measures Survey data (Cycles 3 and 4) to examine the cross-sectional relationships between select liver biomarkers and MetS in the adult Canadian population. The biomarkers selected were gamma-glutamyl transferase (GGT), aspartate aminotransferase (AST), and alkaline phosphatase (ALKP).
Methods
Fasting blood samples (FBS) were collected from adults above the age of 20 years for Cycle 3 and Cycle 4 (n = 3003). MetS was diagnosed if the subjects had three or more risk determinants according to the Joint Interim Statement criteria. Primary risk factors included quartile cut-offs for each of the biomarkers ALKP, AST, GGT for males and females separately. A multivariable logistic regression technique based on a maximum likelihood approach was used to evaluate the association between quartiles of ALKP, AST, and GGT, other individual and contextual factors, and the prevalence of MetS.
Results
MetS was prevalent in 32.3% of subjects. BMI was an effect modifier in the relationship between GGT and MetS prevalence, while sex was an effect modifier in the relationship between ALKP and MetS prevalence; and age was an effect modifier in the relationship between AST and MetS prevalence.
Conclusions
Since the mechanisms to underpin the associations between the liver enzymes activity and MetS are unknown, further epidemiologic investigations using longitudinal designs are necessary to understand these associations.
Keywords: Metabolic syndrome, Adults, Risk factors, Liver enzymes
Introduction
Metabolic syndrome (MetS) has become an important global public health issue [1, 2]. In Canada, data from the nationally representative survey (the Canadian Health Measures Survey-CHMS) reported an increase in the MetS prevalence in adults aged 18 years and greater from 19.1% [3] during Cycle 1 (2007–2009) to 21% [4] during Cycle 3 (2012–2013). MetS is known to be associated with increased risk of cardiovascular diseases (CVD) and other chronic diseases such as diabetes, chronic kidney disease [7, 8]. Recent epidemiologic studies have reported associations between elevated serum levels of liver enzymes and increased cardiovascular risk [5, 6]. For example, in prospective studies, elevated alanine aminotransferase (ALT) and gamma-glutamyl transferase (GGT) have been shown to predict CVD.
Plasma levels of liver enzymes, including ALT and Aspartate aminotransferase (AST), GGT, and alkaline phosphatase (ALKP), have been widely explored as the indicators of MetS and its components among different populations [9–14]. Previous epidemiological studies [9, 11, 12, 14–19] have suggested that liver enzymes showed high sensitivity to metabolic disorders, suggesting their potential as novel biomarkers of MetS. Many large-scale prospective studies have reported that high levels of GGT is a strong and independent predictor of increased risk of MetS components [20–22]. Other liver enzymes, including ALT, AST, and ALKP were also reported to be positively related to an increased risk of MetS and related disorders [10, 17, 23–26]. However, inconsistent findings have been shown when examining that link between liver enzymes and MetS in different populations. Koskinen et al. [27] found no evidence that increased enzyme levels (ALT and GGT) would amplify the atherogenicity of MetS. In contrast, among Thai adults, elevated liver enzymes were significantly(?) related to MetS risk [11] and similar trends were found in Korean [13, 28] and Chinese [17] adults. To our best knowledge, no such information with regard to this association among Canadian adult population is available.
Literature also suggested that demographics (age, sex, family history of disease, etc.), socioeconomic status (household income and education), lifestyle risk factors (personal cigarette smoking and alcohol consumption), environmental exposure (household smoking) are associated with MetS [18, 19]. In Canada, the prevalence of metabolic syndrome was found to be higher among people in households with lower education and income levels [3]. Given the inconsistency in the literature and a scarcity of comparable research in Canadian population, this study aimed to use the CHMS data (Cycles 3 and 4) to investigate the association between elevated GGT, AST, and ALKP and MetS and to examine the cross-sectional relationships between these biomarkers and MetS in the adult Canadian population.
Methods and Materials
Study Population and Data Sources
We included data from for Cycle 3 (2012–2013) and Cycle 4 (2014–2015) of the Canadian Health Measures Survey. Procedures and methods for data collection for the survey have been described previously [29, 30]. In brief, the CHMS are a series of cross-sectional national surveys led by Statistics Canada, in partnership with Health Canada and the Public Health Agency of Canada, completed by a representative sample covering about 96.3% of the Canadian population aged 6–79 years. The survey covered Canadians living at home in the 10 provinces and 3 territories. People living on reserves or in institutions, full-time members of the Armed Forces and people living in remote areas were excluded.
The CHMS consists of an in-home interview and a physical assessment conducted at a mobile examination center. Information about demographic, socioeconomic, family history and general health information was collected from the interview survey. The physical assessment includes measures of anthropometry, spirometry, blood pressure, fitness and oral health and involves collecting biological specimens.
For the purpose of this current study, we included data only for participants from whom fasting blood samples were available. The reason for this selection was that fasting glucose and plasma triglycerides were required to meet the definition of metabolic syndrome. Therefore, adults 20+ years of age who provided fasting blood samples (herein sub-fasting sample) for Cycle 3 or Cycle 4 were included as respondents (n = 3003). Inclusion criteria included: not pregnant, not having liver diseases, not having hepatitis, having information of valid waist circumference, triglycerides, HDL, blood pressure, glucose, and liver enzymes. Sample weights specific to the fasting subgroup were provided by Statistics Canada to ensure prevalence estimates to be representative of Canadian population.
Measures
Outcome Variable
MetS was diagnosed if the subjects had three or more risk determinants according to the Joint Interim Statement criteria [8]. These included: (1) abdominal obesity, defined as waist circumference ≥ 90 cm for men and ≥ 85 cm for women; (2) fasting blood glucose levels ≥110 mg/dL; (3) triglycerides ≥150 mg/dL; (4) high-density lipoprotein (HDL) cholesterol <40 mg/dL for men, and < 50 mg/dL for women; and (5) hypertension, defined as systolic blood pressure ≥ 130 mmHg or diastolic blood pressure ≥ 85 mmHg, and taking antihypertensive medication.
Primary risk factors
Biomarkers, primary risk factors used in this report, were available from laboratory test values. Biomarkers of interest were ALKP, ALT, AST, and GGT in units per liter (U/L). Since ALT was not available for both Cycle 3 and 4 of the CHMS cycles, it was excluded from our analysis. Quartile cut-offs were obtained for males and females separately for each of the biomarkers ALKP, AST, GGT to be used in the analysis (Table 1).
Table 1.
Quartiles of ALKP Alkaline phosphatase (U/L), AST Asparate aminotransferase (AST) (U/L), and Gamma-glutamyltransferase (GGT) (U/L) stratified by sex (N = 3003)
| Males | Females | |||||
|---|---|---|---|---|---|---|
| ALKP | AST | GGT | ALKP | AST | GGT | |
| Lower quartile, Q1 | 61.00 | 23.00 | 19.00 | 60.00 | 20.00 | 15.00 |
| Middle quartile, Q2 | 73.00 | 27.00 | 26.00 | 72.00 | 23.00 | 19.00 |
| Upper quartile, Q3 | 86.00 | 33.00 | 37.50 | 86.00 | 28.00 | 26.00 |
Covariates
The prevalence of MetS involves an interplay among several factors, such as demographic, traditional risk factors (socioeconomic and environmental), and recently researched biomarkers, and the interaction between them.
Covariates of interest used in this report were obtained from household questionnaires: demographics (age, sex, etc.), socioeconomic status (household income and education), lifestyle factors (personal cigarette smoking and alcohol consumption, total number of hours per week spent in sedentary activities), environmental exposure (household smoking), and ethnicity (white, Aboriginal, and other). Specifically, each variable was defined as: Age (20–39 years, 40–59 years, and ≥ 60 years), marital status (single/not single), sedentarity (sedentary for <= 14 hours, 15–29 hours, or ≥ 30 hours), Body Mass Index (BMI) measured by kg/m2 (neither overweight nor obese, overweight, and obese), personal smoking status (never a smoker, ex-smoker, and current smoker), drinking status (never, ex-, and current), education (post-secondary and less or equal secondary), income (low income, middle income, and high income), environmental smoking (Yes/No), ethnicity (White, Aboriginal, and others), and immigrant status (Yes/No).
Statistical analyses
Statistical analysis was conducted using SPSS version 24 and STATA version 15 (Stata Corp, USA). For descriptive analyses, chi-square tests were conducted to compare GGT, AST, and ALKP levels, individual (e.g. Body Mass Index [BMI]) and contextual (e.g. socioeconomic) factors, and other population characteristics between the two groups (presence or absence of MetS). Results were presented in terms of weighted percentages by using the clinical weight for sub-fasting samples (the combination of weights for Cycle 3 and 4). Then, a series of univariable dichotomous logistic regression was conducted to obtain the unadjusted odds ratios (ORunadj), and 95% confidence intervals (95% CI) to examine the associations of MetS prevalence with each risk factors (liver enzymes, individual, and contextual factors). In order to select variables to include in the multivariable model, we utilized standard model building techniques. All statistically significant variables (with p < 0.20) and clinically significant variables based on univariable logistic regression were selected. A multivariable logistic regression technique based on a maximum likelihood approach [31] was used to evaluate the association between quartiles of ALKP, AST, and GGT, other individual and contextual factors, and the prevalence of MetS. At this stage, all statistically significant (p < 0.05), biological and clinical variables were retained. All potential scientifically important two-way interactions were examined for statistical significance (p < 0.05).
The strength of associations was presented as adjusted odds ratios (ORadj) and 95% CIs. Appropriate weight variables computed by Statistics Canada methodologists were used to account for unequal probability of selection. In our analysis, we used the sub-fasting sample weight variable combining Cycle 3 and 4. Bootstrap weights were used for the robust variance estimation to account for designs effects, such as stratification and clustering, inherent in the study design of the cross-sectional complex survey. To compute the standard errors of regression coefficients, we used balanced repeated replication method [32]. Two-sided P values < 0.05 were considered statistically significant.
Results
MetS was prevalent in 32.3% of subjects (Table 2). The prevalence of MetS components were: 44.4% for abdominal obesity, 25.9% for high blood pressure, 43.6% for high triglycerides, 44.9% for low HDL-cholesterol, and 21.5% for high blood glucose. Women tend to have lower MetS prevalence compared to men (31.5% v. 33.1%; p < 0.001).
Table 2.
Prevalence of MetS and its components stratified by sex (N = 3003)
| Males | Females | Total | p value* | |
|---|---|---|---|---|
| % | % | % | <0.001 | |
| Metabolic syndrome | 33.1 | 31.5 | 32.3 | <0.001 |
| Abdominal obesity | 36.6 | 52.4 | 44.4 | <0.001 |
| High triglyceride | 25.6 | 18.0 | 43.6 | <0.001 |
| Low HDL-cholesterol | 44.1 | 45.7 | 44.9 | <0.001 |
| High blood pressure | 27.5 | 24.4 | 25.9 | <0.001 |
| High fasting blood glucose levels | 28.1 | 14.9 | 21.5 | <0.001 |
*P values were calculated using weighted χ2 test to compare the proportions between males and females
The characteristics (in terms of weighted proportions) of adult Canadian population were stratified according to presence/absence of MetS (Table 3). Study participants with MetS tended to have higher level of liver enzyme compared to those without MetS. Also, MetS prevalence was greater in older age, male gender, and higher income, greater sedentary activities, overweight/obese, ex-smokers, and ex-drinkers.
Table 3.
Characteristics (in terms of weighted proportions) of adult Canadian population stratified according to presence/absence of MetS (N = 3003)
| Metabolic syndrome | p value | |||
|---|---|---|---|---|
| No | Yes | |||
| Weighted % | Weighted % | |||
| AST | <Q1 | 27.0 | 15.7 | <0.001 |
| Q1-Q2 | 24.0 | 21.7 | ||
| Q2-Q3 | 24.8 | 32.7 | ||
| >Q3 | 24.2 | 29.9 | ||
| GGT | <Q1 | 30.2 | 11.6 | <0.001 |
| Q1-Q2 | 28.5 | 22.0 | ||
| Q2-Q3 | 23.2 | 30.6 | ||
| >Q3 | 18.0 | 35.8 | ||
| ALKP | <Q1 | 26.7 | 13.6 | <0.001 |
| Q1-Q2 | 28.5 | 25.1 | ||
| Q2-Q3 | 27.2 | 30.4 | ||
| >Q3 | 17.7 | 30.9 | ||
| Age groups | 20–39 years | 48.3 | 14.1 | <0.001 |
| 40–59 years | 36.4 | 43.9 | ||
| 60+ years | 15.3 | 42.1 | ||
| Sex | Male | 49.8 | 51.6 | <0.001 |
| Female | 50.2 | 48.4 | ||
| Household income | Low income | 5.2 | 4.5 | <0.001 |
| Middle income | 39.7 | 39.5 | ||
| High income | 55.2 | 56 | ||
| Total number of hours per week spent in sedentary activities | <=14 hours | 20.8 | 15.7 | <0.001 |
| 15–29 hours | 43.8 | 36.6 | ||
| > = 30 hours | 35.4 | 47.7 | ||
| Body Mass Index (BMI)-kg/m2 | Neither overweight nor obese | 47.4 | 11.1 | <0.001 |
| Overweight | 35.8 | 37.9 | ||
| Obese | 16.8 | 51.1 | ||
| Environmental tobacco smoking (ETS) exposures | No | 67.3 | 69.6 | <0.001 |
| Yes | 32.7 | 30.4 | ||
| Educational attainment | Less than secondary | 5.9 | 18.6 | <0.001 |
| Secondary grad/other post-secondary | 23.7 | 23.4 | ||
| post-secondary graduate | 70.4 | 58.1 | ||
| Immigrant | Yes | 22.1 | 24.8 | <0.001 |
| No | 77.9 | 75.2 | ||
| Marital status | Not single | 68.8 | 88.5 | <0.001 |
| Single | 31.2 | 11.5 | ||
| Ethnicity | While | 74.5 | 85.2 | <0.001 |
| Aboriginal | 1.7 | 3 | ||
| Others | 23.8 | 11.8 | ||
| Smoking status | Current smokers | 21.6 | 17.4 | <0.001 |
| Ex-smokers | 24.4 | 39 | ||
| Non-smokers | 54 | 43.6 | ||
| Drinking status | Current drinkers | 83.3 | 81.1 | <0.001 |
| Ex-drinkers | 9.9 | 13.2 | ||
| Non-drinkers | 6.8 | 5.7 | ||
Aspartate aminotransferase (AST); Gamma-glutamyl transferase (GGT); Alkaline phosphatase (ALKP)
Based on univariable logistic regression, single associations of each predictor variable with the presence/absence of MetS reveal that GGT, AST (with a dose-response relation), ALKP (with a dose-response relation), age (with a dose-response relation), BMI (with a dose-response relation), education, income, ethnicity (White, Aboriginal, and others), marital status, sedentary lifestyle, and smoking were significant predictors of MetS prevalence in the Canadian population cross-section analyzed by this study.
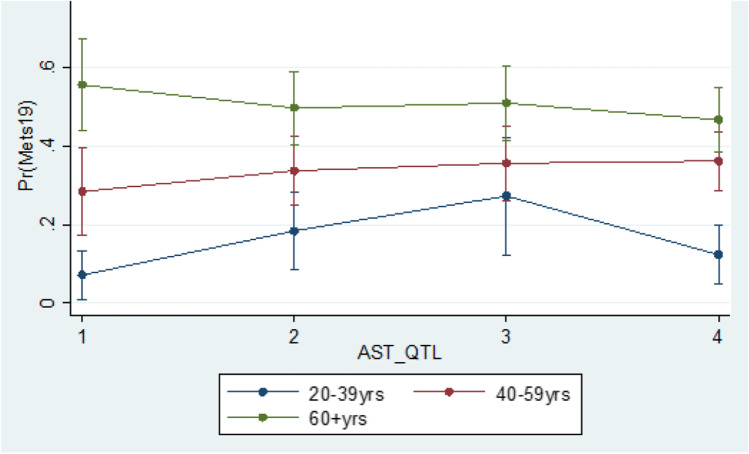
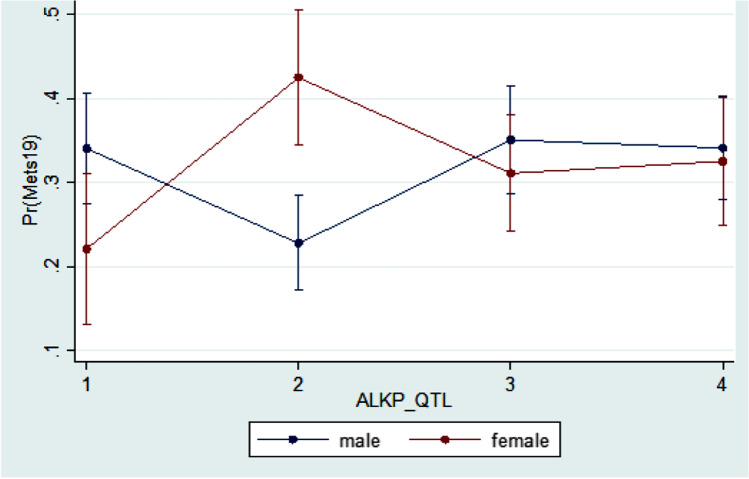
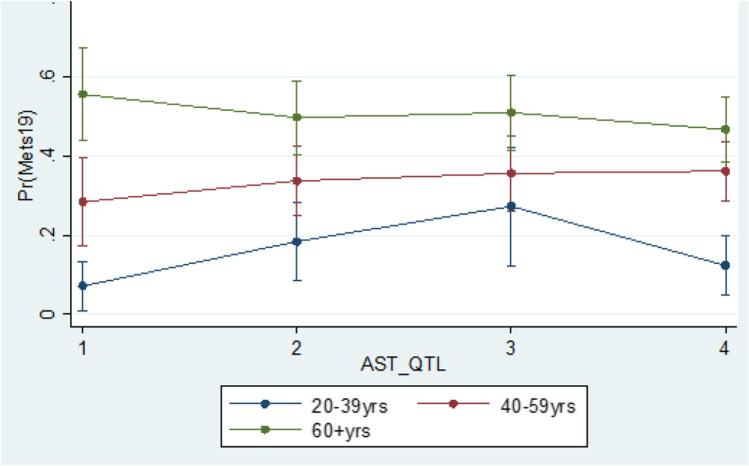
Based on multivariable logistic regression, we observe that education (OR = 2.14 (1.23–3.73); referent category (RC): Post-secondary graduate) was significantly associated with MetS prevalence. BMI was found to be an effect modifier in the relationship between GGT and MetS prevalence, while gender was an effect modifier in the relationship between ALKP and MetS prevalence; and age was an effect modifier in the relationship between AST and MetS prevalence (Figs. 1, 2 and 3).
Fig. 1.
Interaction between BMI and GGT based on the logistic regression of the MetS prevalence in the Canadian population age 20 and older
Fig. 2.
Interaction between sex and ALKP based on the logistic regression of the MetS prevalence in the Canadian population age 20 and older
Fig. 3.
Interaction between age and AST based on the logistic regression of the MetS prevalence in the Canadian population age 20 and older
Discussion
In this study, we explored the relationship between liver enzymes (GGT, AST, and ALKP), individual (age, sex, obesity, etc.) and contextual (income, education, etc.) factors, and MetS. We found that the overall prevalence of MetS – defined as the Joint Interim Statement criteria - was 32.3%. We also observed the pattern of the associations between elevated liver enzymes and MetS, with BMI, age, and sex to be significant effect modifiers Table 4.
Table 4.
Unadjusted (ORunadj) and adjusted odds ratios (ORadj) and their robust (BRR) 95% confidence interval (95% CI) based on logistic regression of the prevalence of MetS
| ORunadj (BRR 95% CI) | ORadj (BRR 95% CI) | p value* | ||
|---|---|---|---|---|
| AST | <Q1 | ref | ref | |
| Q1-Q2 | 1.56 (1.03–2.37) | 3.63 (1.23–10.63) | 0.019 | |
| Q2-Q3 | 2.27 (1.55–3.32) | 7.05 (1.64–30.27) | 0.009 | |
| >Q3 | 2.12 (1.40–3.21) | 2.03 (0.47–8.79) | 0.339 | |
| GGT | <Q1 | ref | ref | |
| Q1-Q2 | 2.00 (1.31–3.06) | 1.73 (0.56–5.39) | 0.338 | |
| Q2-Q3 | 3.43 (2.39–4.92) | 3.64 (1.36–9.72) | 0.01 | |
| >Q3 | 5.17 (3.98–6.72) | 2.33 (0.71–7.61) | 0.158 | |
| ALKP | <Q1 | ref | ref | |
| Q1-Q2 | 1.72 (1.10–2.69) | 0.43 (0.23–0.80) | 0.008 | |
| Q2-Q3 | 2.19 (1.28–3.73) | 1.07 (0.60–1.91) | 0.816 | |
| >Q3 | 3.42 (2.35–4.97) | 1.00 (0.58–1.72) | 0.99 | |
| Sex | Male | ref | ref | |
| Female | 0.93 (0.75–1.14) | 0.40 (0.15–1.07) | 0.07 | |
| Age groups | 20–39 years | ref | ref | |
| 40–59 years | 4.14 (2.59–6.61) | 7.59 (1.76–32.73) | 0.007 | |
| 60+ years | 9.45 (5.98–14.92) | 38.23 (8.83–165.58) | <0.001 | |
| Education | Less than secondary | 3.80 (2.70–5.34) | 2.14 (1.23–3.73) | 0.007 |
| Secondary grad/other post-secondary | 1.19 (0.81–1.75) | 1.03 (0.66–1.63) | 0.865 | |
| Post-secondary graduate | ref | ref | ||
| Ethnicity | White | 2.30 (1.54–3.44) | 1.43 (0.85–2.40) | 0.171 |
| Aboriginal | 3.62 (1.28–10.22) | 1.72 (0.48–6.09) | 0.397 | |
| Others | ref | ref | ||
| Total number of hours per week spent in sedentary activities | <=14 hours | ref | ref | |
| 15–29 hours | 1.10 (0.66–1.84) | 1.12 (0.60–2.09) | 0.712 | |
| > = 30 hours | 1.78 (1.14–2.78) | 1.72 (0.98–3.01) | 0.059 | |
| Body Mass Index (BMI)-kg/m2 | Neither overweight nor obese | ref | ref | |
| Overweight | 4.53 (3.20–6.41) | 6.00 (2.54–14.15) | <0.001 | |
| Obese | 13.08 (9.30–18.39) | 9.83 (2.98–32.42) | <0.001 | |
| Immigrant | Yes | ref | ||
| No | 0.86 (0.64–1.16) | |||
| Marital status | Not single | ref | – | |
| Single | 0.28 (0.17–0.46) | |||
| Smoking status | Current smokers | 0.99 (0.64–1.53) | – | |
| Ex-smokers | 1.98 (1.28–3.05) | |||
| Non-smokers | ref | |||
| Drinking status | Current drinkers | 1.16 (0.70–1.91) | – | |
| Ex-drinkers | 1.58 (0.85–2.94) | |||
| Non-drinkers | ref | |||
| Household Income | Low income | ref | – | |
| Middle income | 1.13 (0.52–2.43) | |||
| High income | 1.15 (0.62–2.15) | |||
| Environmental tobacco smoking (ETS) exposures | No | ref | – | |
| Yes | 0.90 (0.60–1.34) | |||
| Interaction (See Figs. 1, 2 and 3) | p value | |||
| ALKP quartiles*Sex | <0.05 | |||
| AST quartiles*Age groups | <0.05 | |||
| GGT quartiles*BMI | <0.05 |
*based on multivariate analyses
“- “not included in the multivariate analyses
Our findings based on the cross-section of the Canadian population analyzed in this study suggested that there is an increasing trend in MetS prevalence in adults. Data from Cycle 1 of the Canadian Health Measures Survey (2007–2009) showed that the estimated prevalence of MetS was 19.1% overall [3]. Another report using the same dataset in 2012–2013 [4] indicated that approximately 21% of adults aged 18 to 79 had metabolic syndrome, which is comparable to the observations reported in our study. Furthermore, our findings were also comparable to one US study that used data from the 2001–2012 Health and Nutrition Examination Survey (NHANES). This study revealed MeS prevalence among 33,035 adults aged 18 years and older was 32.1% [33].
A higher prevalence of MetS (41.9%) [17] was found in a multicenter, cross-sectional study conducted in rural areas of China between 2012 and 2013 as part of the Northeast China Rural Cardiovascular Health Study (NCRCHS) that considered 11,573 adults (5357 men and 6216 women) aged ≥35 years. However, in another study from Korea [13] conducted from 2013 to 2015 in a group of 11,587 adults ≥30 years of age, MetS was prevalent in 26.9% of subjects, which is higher than that of our current finding. A cross-sectional study (2006–2007) comprised of 1391 Thai participants receiving annual health check-ups reported that the prevalence of MetS was 13.5% [11]. These studies showed that it is challenging to compare results among studies in different populations with a slight difference in MetS definition.
Literature has suggested that high levels of GGT, even within the normal range, are an important predictor of increased risk of cardiovascular diseases and may reflect the development of MetS [15, 16, 22, 28, 34, 35]. Baseline data from a current large population-based study (N = 211,725) in 2019 in Korea showed that GGT level was significantly higher in subjects with MetS compared to normal subjects (37.92 ± 48.20 mg/dL vs. 25.62 ± 33.56 mg/dL) [28]. Compared to the lowest GGT quartile, the odds ratio increased with increasing GGT quartile. A high level of GGT was found to be positively associated with clustered components of MetS in another population-based study in Beijing, China in 2012 that considered10,553 adults aged 20–65 years [15]. Our results, based on univariate analysis, support these published findings. However, in the multivariable analysis, BMI was found to be an effect modifier in the relationship between GGT and MetS prevalence (Fig. 1). Furthermore, overweight and obese people had a significantly higher prevalence of MetS compared to neither overweight nor obese people for the first through the fourth quartiles, except for the overweight group with inconsistent trend through the quartiles.
Several studies have demonstrated the association of MetS with elevated AST levels [36–38], while others did not find a statistically significant relationship [11, 17]. The inconsistency in findings may partially be explained to be the characteristics of AST. AST is a less specific marker of liver injury because it also exists in other organs and cells, such as the heart, kidneys, and lungs [25, 39]. In our analyses, despite the observation that AST levels were found to be associated with MetS risk, the multivariate analyses demonstrated that age was an effect modifier in this relationship. In the multivariate analyses, there was a trend of higher probabilities of being diagnosed with MetS with increasing quartiles of AST among individuals in the older groups (40+ years old). However, this trend did not occur in the youngest age group (20–39 years old). It is unclear what the reasons for this phenomenon are, and a more in depth investigation on the effect of age in the association between elevated AST and MetS in adults is required.
With regard to the relationships between serum ALKP activity and MetS, inconsistent and conflicting results have been reported previously. Several observational studies [26, 40, 41] have suggested that serum ALKP activity may be higher in individuals with MetS versus in those without MetS, while others [42] did not find any statistically significant relationship. Hanley et al. reported that subjects in the upper quartile of ALKP had a 2.88-fold increased risk of incident metabolic syndrome compared with those in the lowest quartile (95%CI 1.24–4.20) [26]. In our current study, we reported similar results in the univariate analyses. Multivariate analyses revealed that sex was an effect modifier in the relationship between ALKP levels and Mets, with males being more likely to have MetS compared to females in the first, the third, and the fourth ALKP quartile, but not in the second quartile (Fig. 3). Our findings are in contrast to those of a Korean National Health and Nutrition Examination Survey conducted in Korea between 2008 and 2011, and which involved 7101 men and 8873 women aged 19 to 75 years [41]. In that cross-sectional study [41], Kim et al. reported that in comparison with those of individuals in the lowest quartile, the OR (95% CI) for MetS in the highest quartile was 1.32 (1.05–1.64) in men and 1.99 (1.42–3.81) in women. The authors concluded that serum ALKP level was positively and independently associated with MetS in men and women. While exact causes for the inconsistency in results between our current study and that by Kim et al. [41] are not known, it has been suggested that sex differences in the distribution of total body adipose tissue and sex hormones may play a role [43].
Regardless of interaction effects, our findings are consistent with that of current studies. A study among men and women aged ≥19 years participating in the sixth examination of Tehran Lipid and Glucose study (TLGS) in 2020 found similar trend that we observed in our current study. The TLGS study found that subjects in the third tertile of ALT, AST were found with a 3.8-, 1.52-, and 3.08-fold increased risk for MetS compared with those with concentrations in the first tertile (OR = 3.80, 95% CI = 2.46–5.87 for ALT, OR = 1.52, 95% CI = 1.04–2.23 for AST). The OR of MetS was 2.71 (95% CI = 1.80–4.09) in subjects with elevated levels of GGT compared with the subjects in the first tertile. Also, the OR of MetS was 1.64 (95% CI = 1.12–2.38) in elevated ALKP levels compared with the subjects in the first tertile [44]. Data from the baseline phase of the Ravansar noncommunicable disease (RaNCD) cohort among residents of Ravansar aged 35–65 years in Iran (2021) showed that all enzymes were positively associated with MetS. Subjects in the fourth quartile for GGT, ALT, ALKP, and AST had 3.29-, 2.45-, 2.00-, and 1.19-fold increased risk for MetS compared with subjects in the first quartile [45]. We observed the similar trend in our current study.
While our study provides novel insights as to the possible contribution of certain liver enzyme activities to MetS and associated CVD risk, several limitations need to be acknowledged. First, our study design was cross-sectional; therefore, causal relationship between liver enzymes and MetS could not be elucidated. However, evidence from the literature has suggested the direction of elevated liver enzymes and an increased risk of MetS as discussed above. Also, several prospective studies have confirmed that longitudinal increases in liver enzymes are positively associated with MetS [46, 47]. Further studies are required, however, to confirm the hypothesis postulated by this study that liver enzyme activity patterns are indicative of MetS risk. Second, the sample size we used for the current paper was smaller than the overall survey samples from Cycle 1 to Cycle 5, because we included only those with available information on fasting blood samples. However, we applied separate weights that were provided by Statistics Canada to ensure the generalizability of the findings.
Regardless of these remaining limitations, to our best knowledge, this is the first national study to examine the association between liver enzymes and MetS in Canadian adults. This study was conducted using Statistics Canada survey data that is both high quality and representative of 96% of Canadians. Therefore, the current findings may be generalized to the adult’s Canadian population.
Conclusions
These representative data of the Canadian general population suggested that elevated levels of GGT, AST, and ALKP were significantly associated with an increased prevalence of MetS. Gender, age, and BMI acted as effect modifiers in these associations. Further epidemiologic investigations using longitudinal designs are necessary to understand these associations.
Acknowledgments
“This research was supported by funds to the Canadian Research Data Centre Network (CRDCN) from the Social Science and Humanities research Council (SSHRC), the Canadian Institute for Health Research (CIHR), the Canadian Foundation for Innovation (CFI) and Statistics Canada”. “Although the research and analysis are based on data from Statistics Canada, the opinions expressed do not represent the views of Statistics Canada or the Canadian Research Data Centre Network (CRDCN).”
Abbreviations
- GGT
- gamma-glutamyltransferase
- ALT
- alanine aminotransferase
- AST
- aspartate aminotransferase
- ALKP
- alkaline phosphatase
- CVD
- cardiovascular disease
- CHMSs
- Canadian Health Measures Surveys.
- HDL
- high-density lipoproteins
- MetS
- metabolic syndrome
- BMI
- Body Mass Index
- OR
- Odds Ratio
- CI
- Confidence interval
- BRR
- Balanced Repeated Replication
Author contributions
Conceptualization, Punam Pahwa and Luan Manh Chu; Statistical analysis, Luan Manh Chu; Funding acquisition, Punam Pahwa, Chandima Karunanayake, Palok Aich, Markus Hecker; Methodology, Punam Pahwa and Luan Manh Chu; Project administration, Punam Pahwa; Supervision, Punam Pahwa and Chandima Karunanayake; Writing – original draft, Luan Manh Chu; Writing – review & editing, Luan Manh Chu, Punam Pahwa, Chandima Karunanayake, Palok Aich, Markus Hecker.
Funding
This research was supported by 2018 CoMRAD grant.
Data availability
Contact Statistics Canada regarding availability of data.
Code availability
Contact Statistics Canada regarding code availability.
Declarations
Conflict of interest
The authors declare no competing interests.
The authors declare no conflict of interest. The funders had no role in the collection, analyses, or interpretation of data, or in the writing of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Alberti KG, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.Saklayen MG. The Global Epidemic of the Metabolic Syndrome. Curr Hypertens Rep. 2018;20(2):12. doi: 10.1007/s11906-018-0812-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Riediger ND, Clara I. Prevalence of metabolic syndrome in the Canadian adult population. Can Med Assoc J. 2011;183(15):E1127–E1134. doi: 10.1503/cmaj.110070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Canada S. Metabolic syndrome in adults, 2012 to 2013. [Internet]. 2015. https://www150.statcan.gc.ca/n1/pub/82-625-x/2014001/article/14123-eng.htm. Accessed 10 Sept 2020.
- 5.Meisinger C, Döring A, Schneider A, Löwel H, Group KS Serum γ-glutamyltransferase is a predictor of incident coronary events in apparently healthy men from the general population. Atherosclerosis. 2006;189(2):297–302. doi: 10.1016/j.atherosclerosis.2006.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Schindhelm RK, Dekker JM, Nijpels G, et al. Alanine aminotransferase predicts coronary heart disease events: a 10-year follow-up of the Hoorn study. Atherosclerosis. 2007;191(2):391–396. doi: 10.1016/j.atherosclerosis.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 7.Mottillo S, Filion KB, Genest J, et al. The metabolic syndrome and cardiovascular risk: a systematic review and meta-analysis. J Am Coll Cardiol. 2010;56(14):1113–1132. doi: 10.1016/j.jacc.2010.05.034. [DOI] [PubMed] [Google Scholar]
- 8.Alberti K, Eckel RH, Grundy SM, et al. Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention; national heart, lung, and blood institute; American heart association; world heart federation; international atherosclerosis society; and international association for the study of obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 9.Park EY, Lim MK, Oh J-K, et al. Independent and supra-additive effects of alcohol consumption, cigarette smoking, and metabolic syndrome on the elevation of serum liver enzyme levels. PLoS One. 2013;8(5):e63439. doi: 10.1371/journal.pone.0063439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steinvil A, Shapira I, Ben-Bassat OK, et al. The association of higher levels of within-normal-limits liver enzymes and the prevalence of the metabolic syndrome. Cardiovasc Diabetol. 2010;9(1):30. doi: 10.1186/1475-2840-9-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera S, Lohsoonthorn V, Jiamjarasrangsi W, Lertmaharit S, Williams MA. Association between elevated liver enzymes and metabolic syndrome among Thai adults. Diabetes Metab Syndr Clin Res Rev. 2008;2(3):171–178. doi: 10.1016/j.dsx.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang L, Ma X, Jiang Z, et al. Liver enzymes and metabolic syndrome: a large-scale case-control study. Oncotarget. 2015;6(29):26782. doi: 10.18632/oncotarget.5792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim HR, Han MA. Association between serum liver enzymes and metabolic syndrome in Korean adults. Int J Environ Res Public Health. 2018;15(8):1658. doi: 10.3390/ijerph15081658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang S, Zhang J, Zhu L, et al. Association between liver function and metabolic syndrome in Chinese men and women. Sci Rep. 2017;7:44844. doi: 10.1038/srep44844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tao L, Li X, Zhu H, et al. Association between γ-glutamyl transferase and metabolic syndrome: a cross-sectional study of an adult population in Beijing. Int J Environ Res Public Health. 2013;10(11):5523–5540. doi: 10.3390/ijerph10115523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu CF, Zhou WN, Fang NY. Gamma-glutamyltransferase levels and risk of metabolic syndrome: a meta-analysis of prospective cohort studies. Int J Clin Pract. 2012;66(7):692–698. doi: 10.1111/j.1742-1241.2012.02959.x. [DOI] [PubMed] [Google Scholar]
- 17.Chen S, Guo X, Yu S, Zhou Y, Li Z, Sun Y. Metabolic syndrome and serum liver enzymes in the general chinese population. Int J Environ Res Public Health. 2016;13(2):223. doi: 10.3390/ijerph13020223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park Y-W, Zhu S, Palaniappan L, Heshka S, Carnethon MR, Heymsfield SB. The metabolic syndrome: prevalence and associated risk factor findings in the US population from the third National Health and nutrition examination survey, 1988-1994. Arch Intern Med. 2003;163(4):427–436. doi: 10.1001/archinte.163.4.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tran BT, Jeong BY, Oh J-K. The prevalence trend of metabolic syndrome and its components and risk factors in Korean adults: results from the Korean National Health and nutrition examination survey 2008–2013. BMC Public Health. 2017;17(1):71. doi: 10.1186/s12889-016-3936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanley AJG, Williams K, Festa A, et al. Elevations in markers of liver injury and risk of type 2 diabetes. Insulin Resist Atherosclerosis Stud. 2004;53(10):2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 21.Lee D-H, Jacobs DR, Jr, Gross M, Steffes M. Serum γ-Glutamyltransferase was differently associated with microalbuminuria by status of hypertension or diabetes: the coronary artery risk development in young adults (CARDIA) study. Clin Chem. 2005;51(7):1185–1191. doi: 10.1373/clinchem.2004.045872. [DOI] [PubMed] [Google Scholar]
- 22.Hwang A-C, Lin Y-C, Liu P-T, Kao Y-M, Chen J-D. Synergistic effect of gamma glutamyltransferase and obesity on metabolic syndrome, independent of hepatic steatosis. Ann Epidemiol. 2012;22(12):876–880. doi: 10.1016/j.annepidem.2012.09.012. [DOI] [PubMed] [Google Scholar]
- 23.Goessling W, Massaro JM, Vasan RS, D'Agostino RB, Sr, Ellison RC, Fox CS. Aminotransferase levels and 20-year risk of metabolic syndrome, diabetes, and cardiovascular disease. Gastroenterology. 2008;135(6):1935–1944. doi: 10.1053/j.gastro.2008.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patel DA, Srinivasan SR, Xu J-H, Chen W, Berenson GS. Persistent elevation of liver function enzymes within the reference range is associated with increased cardiovascular risk in young adults: the Bogalusa heart study. Metabolism. 2007;56(6):792–798. doi: 10.1016/j.metabol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 25.Pratt DS, Kaplan MM. Evaluation of abnormal liver-enzyme results in asymptomatic patients. N Engl J Med. 2000;342(17):1266–1271. doi: 10.1056/NEJM200004273421707. [DOI] [PubMed] [Google Scholar]
- 26.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Haffner SM. Liver markers and development of the metabolic syndrome: the insulin resistance atherosclerosis study. Diabetes. 2005;54(11):3140–3147. doi: 10.2337/diabetes.54.11.3140. [DOI] [PubMed] [Google Scholar]
- 27.Koskinen J, Magnussen CG, Kähönen M, et al. Association of liver enzymes with metabolic syndrome and carotid atherosclerosis in young adults. The cardiovascular risk in young Finns study. Ann Med. 2012;44(2):187–195. doi: 10.3109/07853890.2010.532152. [DOI] [PubMed] [Google Scholar]
- 28.Lee MY, Hyon DS, Huh JH, et al. Association between Serum Gamma-Glutamyltransferase and prevalence of metabolic syndrome using data from the Korean Genome and Epidemiology Study. Endocrinol Metab. 2019;34(4):390–397. doi: 10.3803/EnM.2019.34.4.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bryan S, St-Denis M, Wojtas D. Canadian health measures survey: clinic operations and logistics. Health Rep. 2007;18:53–70. [PubMed] [Google Scholar]
- 30.Tremblay M, Wolfson M, Gorber SC. Canadian health measures survey: rationale, background and overview. Health Rep. 2007;18:7–20. [PubMed] [Google Scholar]
- 31.Czepiel SA. Maximum likelihood estimation of logistic regression models: theory and implementation. 2002. http://czep.net.
- 32.Pitblado J. Survey data analysis in Stata. Stata Users Group, DC09 Stata Conference. 2009.
- 33.Campbell B, Aguilar M, Bhuket T, Torres S, Liu B, Wong RJ. Females, Hispanics and older individuals are at greatest risk of developing metabolic syndrome in the US. Diabetes Metab Syndr Clin Res Rev. 2016;10(4):230–233. doi: 10.1016/j.dsx.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 34.Shimizu Y, Imano H, Ohira T, et al. γ-Glutamyltranspeptidase and incident stroke among Japanese men and women: the circulatory risk in communities study (CIRCS) Stroke. 2010;41(2):385–388. doi: 10.1161/STROKEAHA.109.569061. [DOI] [PubMed] [Google Scholar]
- 35.Rantala A, Lilja M, Kauma H, Savolainen M, Reunanen A, Kesäniemi Y. Gamma-glutamyl transpeptidase and the metabolic syndrome. J Intern Med. 2000;248(3):230–238. doi: 10.1046/j.1365-2796.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 36.Esteghamati A, Jamali A, Khalilzadeh O, et al. Metabolic syndrome is linked to a mild elevation in liver aminotransferases in diabetic patients with undetectable non-alcoholic fatty liver disease by ultrasound. Diabetol Metab Syndr. 2010;2(1):65. doi: 10.1186/1758-5996-2-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kälsch J, Bechmann LP, Heider D, et al. Normal liver enzymes are correlated with severity of metabolic syndrome in a large population based cohort. Sci Rep. 2015;5:13058. doi: 10.1038/srep13058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Schindhelm R, Dekker J, Nijpels G, et al. Alanine aminotransferase and the 6-year risk of the metabolic syndrome in Caucasian men and women: the Hoorn study. Diabet Med. 2007;24(4):430–435. doi: 10.1111/j.1464-5491.2007.02100.x. [DOI] [PubMed] [Google Scholar]
- 39.Lee DH, Silventoinen K, Jacobs DR, Jr, Jousilahti P, Tuomileto J. Gamma-Glutamyltransferase, obesity, and the risk of type 2 diabetes: observational cohort study among 20,158 middle-aged men and women. J Clin Endocrinol Metab. 2004;89(11):5410–5414. doi: 10.1210/jc.2004-0505. [DOI] [PubMed] [Google Scholar]
- 40.Kim MK, Baek KH, Kang MI, et al. Serum alkaline phosphatase, body composition, and risk of metabolic syndrome in middle-aged Korean. Endocr J. 2012;60:EJ12–0331. doi: 10.1507/endocrj.ej12-0331. [DOI] [PubMed] [Google Scholar]
- 41.Kim J-H, Lee HS, Park H-M, Lee Y-J. Serum alkaline phosphatase level is positively associated with metabolic syndrome: a nationwide population-based study. Clin Chim Acta. 2020;500:189–194. doi: 10.1016/j.cca.2019.10.015. [DOI] [PubMed] [Google Scholar]
- 42.Bae S, Choe J, Chung Y, et al. The association between serum osteocalcin levels and metabolic syndrome in Koreans. Osteoporos Int. 2011;22(11):2837–2846. doi: 10.1007/s00198-010-1504-y. [DOI] [PubMed] [Google Scholar]
- 43.Geer EB, Shen W. Gender differences in insulin resistance, body composition, and energy balance. Gender Med. 2009;6:60–75. doi: 10.1016/j.genm.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gaeini Z, Bahadoran Z, Mirmiran P, Azizi F. The association between liver function tests and some metabolic outcomes: Tehran lipid and glucose study. Hepat Mon. 2020;20(5):e98535. [Google Scholar]
- 45.Kohsari M, Moradinazar M, Rahimi Z, Pasdar Y, Shakiba E. Liver enzymes and their association with some Cardiometabolic diseases: evidence from a large Kurdish cohort. Biomed Res Int. 2021;2021(5584452):8. doi: 10.1155/2021/5584452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS. Persistent elevation of liver function enzymes within the reference range is associated with increased cardiovascular risk in young adults: the Bogalusa heart study. Metabolism. 2007;56(6):792–798. doi: 10.1016/j.metabol.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 47.Han K-S, Cho D-Y, Kim Y-S, Kim K-N. Serum gamma-glutamyl transferase concentration within the reference range is related to the coronary heart disease risk prediction in Korean men: analysis of the Korea National Health and nutrition examination survey (V-1, 2010 and V-2, 2011) Chin Med J. 2015;128(15):2006–2011. doi: 10.4103/0366-6999.161343. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Contact Statistics Canada regarding availability of data.
Contact Statistics Canada regarding code availability.