Abstract
Aim
The present study was designed to investigate the effect of methanolic extract of Costus pictus (MECP) per se and in combination with drugs (Metformin and Enalapril) used in clinical practice in streptozotocin (STZ) induced diabetic nephropathy (DN) in rats.
Methods
Diabetes was induced in male Wistar rats by a single injection of STZ (50 mg/kg i.p.). After 28 days diabetic rats were divided into six groups. Two groups were treated with MECP (200 mg/kg p.o.), MECP (400 mg/kg p.o.) respectively; one group was treated with metformin (225 mg/kg), enalapril (3.2 mg/kg) combination; and two groups were treated with a combination of metformin, enalapril and MECP (200 mg/kg) and combination of metformin, enalapril and MECP (400 mg/kg) respectively. One group was kept as diabetic control. At the end of the study, body weight, kidney weight, and kidney hypertrophy index were evaluated. Biochemical and antioxidant parameters were evaluated. TGF-β levels in serum were estimated. Histopathology of the kidney was also studied.
Results
The combination therapy showed a significant increase in the body weight, lowered blood glucose levels and ameliorated kidney hypertrophy index in STZ induced diabetic nephropathy in rats. It also normalized the altered levels of serum and urine parameters. Histopathological evaluation revealed that combination therapy reduced the vacuolar degeneration of tubules.
Conclusions
The results indicate that combination therapy of metformin, enalapril, and MECP has beneficial effects in management of diabetic nephropathy.
Keywords: Diabetic Nephropathy, Streptozotocin, Costus pictus, Metformin, Enalapril
Introduction
Diabetic Nephropathy (DN) as a microvascular disease represents a major long-term complication of diabetes mellitus. It is the leading cause of end-stage renal disease (ESRD) and accounts for about 30–35% of cases of renal replacement therapy worldwide. The role of hyperglycemia in the pathogenesis of diabetic nephropathy has been well established in various experimental animal models. Hyperglycemia is also known to promote oxidative stress and hence involved in the generation of reactive oxygen species (ROS) which play a crucial role in the pathogenesis of diabetic nephropathy[1]. Therefore, controlling hyperglycemia and improving antioxidant capacity will be beneficial in curbing the progression of diabetic nephropathy. TGF- β has a key role in the progression of DN along with the fact that almost all the mechanisms responsible for the progression of nephropathy have involvement of TGF- β at some stage[2].
Traditional herbal medicines are widely used to treat diabetes and its complications. The role of medicinal plants needs to be systematically explored to get effective option for management diabetic nephropathy. Herbs being the storehouse of many therapeutically potent phytoconstituents, act via an array of pharmacological targets and hence can be used efficiently to synergize effects of allopathic medicines acting on only one specific target. Herbal medicines also offer the advantage of overcoming various side effects of allopathic medicines if used in combination[3].
Hence, traditionally used herbal drugs in combination with allopathic medicines may prove beneficial in management of diabetes induced renal damage.
Costus pictus (CP) belonging to family costaceae is commonly known as the insulin plant. It is reported for its antidiabetic, hypolipidemic, diuretic, antioxidant, antimicrobial, anticancer and antifungal effects [3, 4] Phytoconstituents reported to be present in CP are carbohydrates, triterpenoids, proteins, alkaloids, tannins, saponins, flavonoids, steroids, and appreciable amounts of trace elements[4]. Literature survey reveals the presence of flavonoids and phenolic acids [5]. Quercetin is reported to have antidiabetic activity and significant effects in diabetic nephropathy[6]. P-coumaric acid has a reno-protective effect in diabetes[7].
In routine clinical practice, combination of metformin with enalapril is commonly prescribed for the treatment of diabetic nephropathy. So the present study was designed to observe the effects of extract MECP and its combination with metformin and enalapril in experimentally induced diabetic nephropathy in rats.
Materials and methods
Drugs and chemicals
Streptozotocin (STZ), Di- thiobisnitrobenzoic acid (DTNB), 2, 4-dinitrophenyl hydrazine, Nicotine amide adenine dinucleotide phosphate (NADP), Nitro blue tetrazolium (NBT), Phenazinemethosulphate and Reduced glutathione (GSH) were procured from Sisco Research Laboratories Pvt. Ltd., India. Metformin (Okamet – 500, Cipla Ltd) and enalapril (ENAM™ 5, Dr. Reddy’s laboratories Pvt Ltd.) were purchased from a local medical shop. Quercetin and P-coumaric acid were purchased from Sisco Research Laboratories Pvt. Ltd. and Sigma-Aldrich (St. Louis, MO, USA), respectively.
Plant material
The shade dried leaves of Costus pictus D. Don were procured from Aditi Herbals Pvt. Ltd, Karnataka, India. The plant material was authenticated at Blatter herbarium, St. Xavier’s College, Mumbai (voucher specimen No. AM-1). The leaves were powdered and subjected to Soxhlet extraction using methanol as solvent. The excess of methanol was evaporated to obtain a methanolic extract of Costus pictus D. Don. (MECP). The extract was stored in airtight container at 2–4°C until further use.
Standardisation of MECP by HPTLC
HPTLC chromatography was performed on a 20 × 10 cm plate precoated with silica gel F254 (E.Merck, Darmstadt, Germany). MECP (10 µl), quercetin (600 ng/band) and p-coumaric acid (600 ng/band) was applied as bands with linear ascending development with Toulene: Ethyl Acetate: Formic acid (6:4:1) and Cyclohexane: Ethyl acetate: Formic acid (4:6:1) as a mobile phase for quercetin and p-coumaric acid respectively was performed in a 20 × 10 cm twin -trough glass chamber (CAMAG) previously saturated with mobile phase for 15 min at room temperature. HPTLC analysis was performed at 270 nm in a reflectance mode with Camage TLC scanner III operated by WinCATS software. The slit dimensions were 5 × 0.45 mm and scanning speed of 20 mm/sec.
Experimental animals
Male albino Wistar rats weighing between 250- 300 gm were procured from Bharat Serum and Vaccines Private Ltd, Thane. The experimental protocol was approved by Institutional Animal Ethics Committee (Approval no. KMKCP/IAEC/161704).
Streptozotocin induced diabetic nephropathy
Induction of diabetes and treatment
Diabetes was induced in overnight fasted male rats by single i.p. injection of freshly prepared STZ (50 mg/kg dissolved in 0.1 M cold citrate buffer, pH 4.4). Three days after STZ injection, blood samples were collected through retro-orbital plexus and blood glucose levels were measured using a diagnostic kit. Animals with blood glucose levels ≥ 250 mg/dl were selected for the study. After four weeks, diabetic animals were divided into six groups containing six animals in each and treatment was given for the next four weeks[8] as follows.
Group 1: Diabetic control (DC): The rats were treated with 0.1% w/v carboxy methyl cellulose (CMC), p.o..
Group 2
The rats were treated daily with a standard drug combination of metformin (225 mg/kg p.o.) and enalapril (3.2 mg/kg p.o.) [M + E].
Group 3
The rats were treated daily with MECP (200 mg/kg, p.o.)
Group 4
The rats were treated daily with MECP (400 mg/kg, p.o.)
Group 5
The rats were treated daily with standard drugs (M + E) and MECP (200 mg/kg. p.o.)
Group 6
The rats were treated daily with standard drugs (M + E) and MECP (400 mg/kg p.o.)
One group of normal animals was treated with 0.1% w/v CMC.
Parameters evaluated
Body weight
The body weight of all animals was recorded at the end of the study.
Estimation of biochemical parameters
Serum levels of glucose, total protein, albumin, creatinine, and blood urea nitrogen (BUN) were estimated at the end of the study by using commercially available diagnostic kits (Transasia Biomedicals Ltd., India).
Estimation of TGF β1
A commercial enzyme-linked immunosorbent assay kit (Elabscience, USA) was used to quantify TGF-β1 levels with the help of Microplate Reader ELx 800 (BIOTEK Instruments) at the end of the study.
Estimation of urine parameters
The urine was collected at the end of the study and urinary volume, total protein, albumin, glucose, and creatinine were estimated using commercially available diagnostic kits (Transasia Biomedicals Ltd., India).
Creatinine clearance (Ccr) was calculated by using following formula [9].
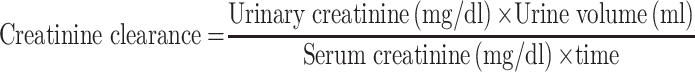 |
Kidney weight
All animals were weighed and sacrificed at the end of the study and their kidneys were isolated and weighed and the renal index was calculated by using following formula.
 |
Estimation of oxidative stress parameters
Homogenate of kidney tissue (10% w/v) was prepared in ice-cold phosphate buffer (pH 7.4) and an aliquot was used for lipid peroxidation (LPO) estimation and the remaining portions of homogenates were centrifuged at 4000 rpm for 10 min and the supernatant obtained was used for estimation of Superoxide dismutase (SOD), Catalase (CAT) and reduced glutathione (GSH) [10, 11].
Histopathology of kidney tissue
The kidney tissues were stained using three stains viz. hematoxylin and eosin (H&E), trichome masson and periodic acid schiff (PAS). Stained sections of kidney were then examined under a light microscope for histopathological changes.
Statistical analysis
The data are expressed as mean ± S.E.M . Results were statistically analyzed using one-way ANOVA followed by the Turkey-Kramer post-test; p < 0.05 was considered significant. GraphPad Prism was used for statistical analysis.
Results
Standardisation of MECP by HPTLC
The amount of quercetin and p-coumaric acid was found to be 0.44% w/w and 3.014%w/w respectively in MECP (Supporting information Figs. 1 and 2).
Effect on body weight and kidney weight
During the 4-week study period, STZ induced diabetic rats exhibited significant weight loss when compared with normal rats. At the end of 4 weeks of treatment, all the treatment groups showed a significant (p < 0.001) rise in the body weight of rats as compared withdiabetic rats (Supporting information Fig. 3). The kidney hypertrophy index of diabetic rats was significantly increased as compared to normal control group. Kidney hypertrophy index in all treatment groups was significantly (p < 0.001) reduced when compared with diabetic control group (Supporting information Fig. 4).
Effect on serum and urine biochemical parameters
The results of treatment on urine and serum biochemical parameters are presented in Figs. 1 and 2. A significant rise in serum glucose level was found in diabetic rats as compared to the rats in normal control group (p < 0.001). Treatment with extract and combination, significantly (p < 0.001) decreased the serum glucose level in diabetic animals. The effect was more prominent with a dose of M + E + MECP 400 mg/kg .
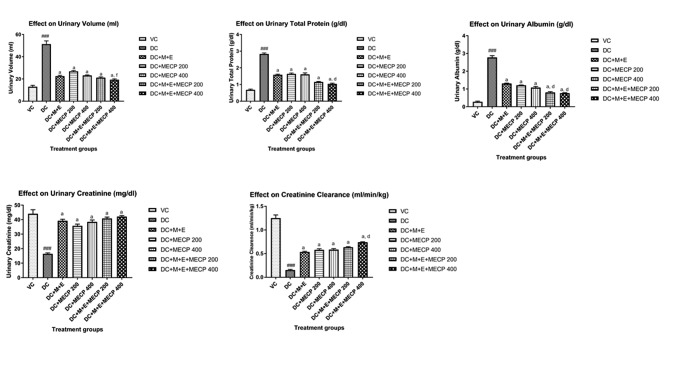
Fig. 1.
Effect on urine biochemical parameters in STZ induced diabetic nephropathy in rats. The data are presented as Mean ± S.E.M. (n = 6). ###p < 0.001 as compared to vehicle control group; a(p < 0.001), b(p < 0.01) as compared to diabetic control group; d(p < 0.001), and f (p < 0.05) as compared to standard treatment (M + E) group. Data analysed by a one-way analysis of variance (ANOVA) followed by Turkey’s Kramer multiple test for comparison
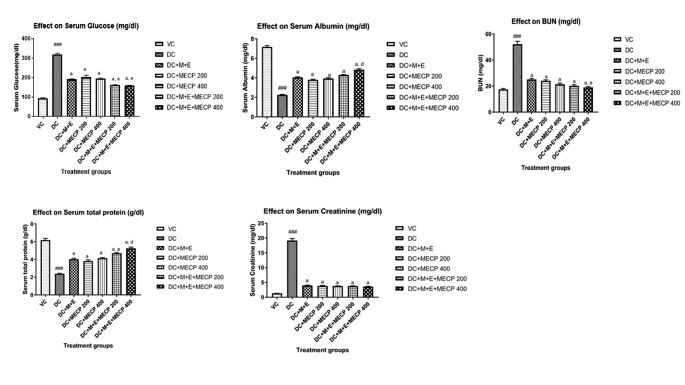
Fig. 2.
Effect on serum biochemical parameters in STZ induced diabetic nephropathy in rats. The data are presented as Mean ± S.E.M. (n = 6). ###p < 0.001 as compared to vehicle control group; a(p < 0.001), b(p < 0.01) as compared to diabetic control group; d(p < 0.001), e(p < 0.01) as compared to standard treatment (M + E) group. Data analysed by a one-way analysis of variance (ANOVA) followed by Turkey’s Kramer multiple test for comparison.
The total protein and albumin levels were significantly (p < 0.001) decreased in the serum and increased in the urine of the diabetic control group as compared to the normal control group. The diabetic rats showed a significant (p < 0.001) decrease in the level of creatinine in urine with a significant increase in serum as compared to the normal control group . The BUN level was significantly (P < 0.001) increased in diabetic rats as compared to normal control rats. Diabetic rats also exhibited a significant (p < 0.001) rise in the urinary volume level. All the treatment groups showed significant (p < 0.001) rise in serum total protein level whereas urinary total protein level was decreased as compared to the diabetic control group, the effect was more prominent with the treatment group M + E + MECP200 and M + E + MECP400 (Figs. 1 and 2).
All the treatment groups showed significant (p < 0.001) rise in serum albumin level whereas urinary albumin level was decreased as compared to the diabetic control group, more effective results were observed with treatment group M + E + MECP200 and M + E + MECP400. There was significant (p < 0.001) decline in the serum creatinine levels in the treatment groups as compared to the diabetic control group. OAll the treatment groups exhibited significant (p < 0.001) elevation in the urinary creatinine levels as compared to the diabetic control group. There was significant (p < 0.001) decline in the BUN levels in the treatment groups as compared to the diabetic control group. There was a significant (p < 0.001) rise in the serum creatinine levels in the treatment groups as compared to the diabetic control group (Supporting information Tables 1 and 2).
Serum TGF-β1 levels
TGF-β1 level was found to be significantly increased in diabetic rats as compared to rats in the normal control group. Treatment with standard, MECP200 and MECP 400 showed significant (p < 0.01) decline in the level of serum TGF-β. M + E + MECP200 and M + E + MECP400 also decreased level of TGF-β. (Fig. 3)
Fig. 3.
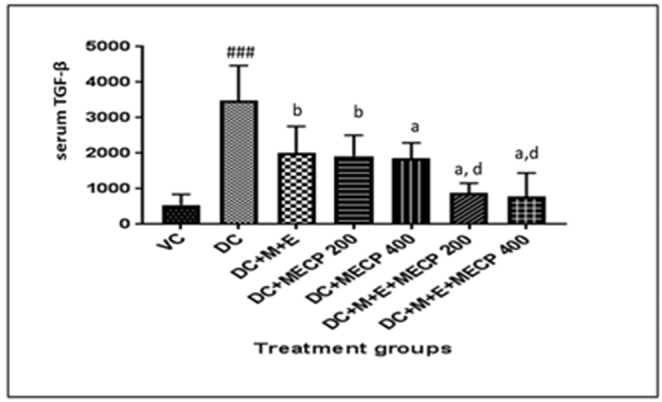
Effect on TGF-β in STZ induced diabetic nephropathy in rats. The data are presented as Mean ± S.E.M. (n = 6). ###p < 0.001 as compared to vehicle control group; a(p < 0.001), b(p < 0.01) as compared to diabetic control group; d(p < 0.001) as compared to standard treatment (M + E) group. Data analysed by a one-way analysis of variance (ANOVA) followed by Turkey’s Kramer multiple test for comparison.
Effect on oxidative stress parameters
The levels of GSH, SOD and Catalase were significantly (P < 0.001) decreased in diabetic rats as compared to normal control rats. Increased LPO levels were found in diabetic rats as compared to normal control rats. However, treatment with M + E + MECP400 the levels of GSH, SOD, CAT and LPO were found to be restored (Supporting information Table 3).
Histopathological evaluation
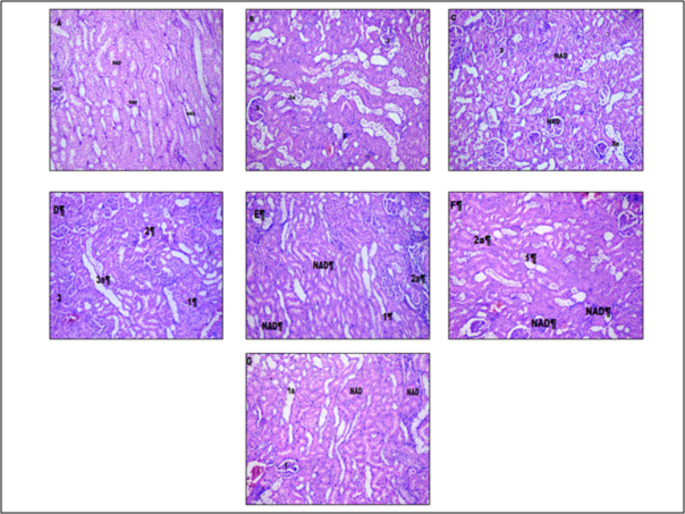
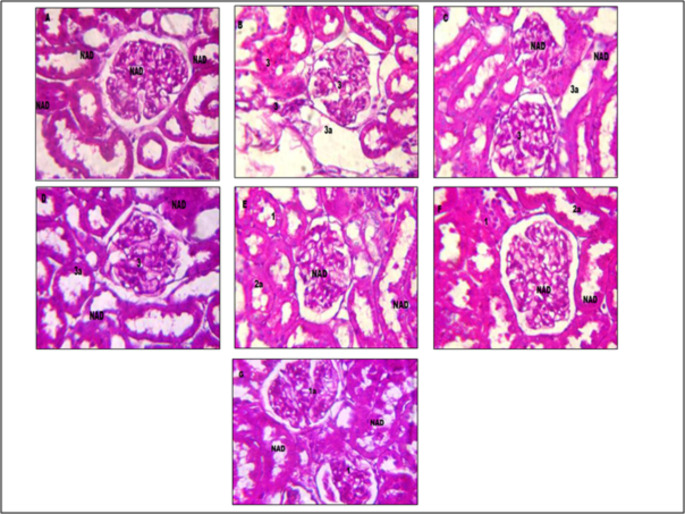
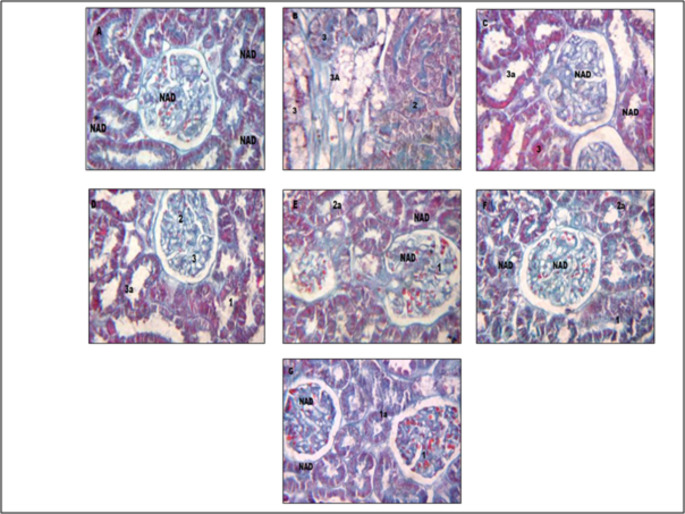
Effect of M +E, and M + E + MECP treatment on histopathological changes in kidney of treatment groups is presented in Figs. 4 and 5, and 6. Kidneys of normal control rats did not show any abnormal morphological changes. The kidney of diabetic control rats demonstrated severe degeneration of proximal convoluted tubules, necrosis and hyalinization of arterioles. The histopathological evaluation confirms the ameliorating effect of M + E + MECP on kidneys of diabetic rats.
Fig. 4.
Effect on histopathology of kidney tissue (H& E staining). Figure A: kidney tissue of vehicle control rat. Figure B: kidney of tissue of diabetic control rat. Figure C:kidney tissue of rat treated with standard metformin (225 mg/kg) and Enalapril (3.2 mg/kg) (M+E). Figure D: kidney tissue of rat treated with MECP (200 mg/kg)(MECP 200). Figure E: kidney tissue of rat treated with MECP (400 mg /kg) (MECP 400). Figure F: kidney tissue of rat treated with metformin (225 mg/kg), Enalalpril (3.2 mg/kg) and MECP (200 mg/kg) (M + E + MECP 200). Figure G: kidney tissue of rat treated with metformin (225 mg/kg), Enalalpril (3.2 mg/kg) and MECP (400 mg/kg) (M + E + MECP 400). Lesion Grading: Minimal (1), mild (2), moderate (3), and marked (4) Distribution of Lesions: Focal (a), Multifocal (b), Diffuse (c) No abnormalities detected: NAD
Fig. 5.
Effect on histopathology of kidney tissue (Masson’s trichome staining). Figure A: kidney tissue of vehicle control rat. Figure B: kidney tissue of diabetic control rat. Figure C: kidney tissue of rat treated with standard metformin (225 mg/kg) and enalalpril (3.2 mg/kg) (M + E). Figure D: kidney tissue of rat treated with MECP (200 mg/kg) (MECP 200). Figure E: kidney tissue of rat treated with MECP (400 mg /kg) (MECP 400). Figure F: kidney tissue of rat treated with metformin (225 mg/kg), enalalpril (3.2 mg/kg) and MECP (200 mg/kg) (M + E + MECP 200). Figure G: kidney tissue of rat treated with metformin (225 mg/kg), enalalpril (3.2 mg/kg) and MECP (400 mg/kg) (M + E + MECP 400). Lesion Grading: Minimal (1), mild (2), moderate (3), and marked (4). Distribution of Lesions: Focal (a), Multifocal (b), Diffuse (c), No abnormalities detected: NAD
Fig. 6.
Effect on histopathology of kidney tissue (periodic acid schiff staining). Figure A: kidney tissue of normal control rat. Figure B: kidney tissue of diabetic control rat. Figure C: kidney tissue of rat treated with standard metformin (225 mg/kg) and enalapril. (3.2 mg/kg) (M + E). Figure D: kidney tissue of rat treated with MECP (200 mg/kg) (MECP 200). Figure E: kidney tissue of rat treated with MECP (400 mg /kg) (MECP 400). Figure F: kidney tissue of rat treated with metformin (225 mg/kg), enalapril (3.2 mg/kg). and MECP (200 mg/kg) (M + E + MECP 200). Figure G: kidney tissue of rat treated with metformin (225 mg/kg), enalapril (3.2 mg/kg) and MECP (400 mg/kg) (M + E + MECP 400). Lesion Grading: Minimal (1), mild (2), moderate (3) and marked (4). Distribution of Lesions: Focal (a), Multifocal (b), Diffuse (c) No abnormalities detected: NAD
Discussion
The present study was designed to study the protective effect of combination of standard drugs in with herbal extract in STZ induced diabetic nephropathy in rats.
Production of ROS during hyperglycemia plays a vital role in the pathogenesis of diabetic complications[12]. Excessive production of ROS leads to the progression of various inflammatory cytokines like TGF-β which develops oxidative stress in tissue[13]. Besides this diabetes is also associated with over-expression of the advanced glycation end product which alters renal architecture leading to loss of renal function by altering cross-linking between cellular matrix and developing aberrant cell protein and cell-matrix interaction which have a role in diabetic glomerulosclerosis[14]. The Renin-angiotensin system also plays an important role in the development of diabetic nephropathy[15]. Angiotensin-II is responsible for the over-expression of TGF-β which has a crucial role in the development of fibrosis[16]. Various scientific reports have shown that down-regulation of TGF-β is beneficial in controlling the development of fibrotic renal disorders.
In this study, HPTLC Quantification has revealed the presence of flavonoids (Quercetin) and phenolic (P-coumaric acid ) phytoconstituents in extract MECP. Literature study reports the protective effect of these phytoconstituents in kidney injury. Thus, supporting our findings of the nephroprotective potential of MECP in the present study. STZ induced diabetes is characterized by severe loss of body weight possibly due to hyperglycemia induced dehydration resulting in hyperuricemia, also an increase in muscle wasting and catabolism of fats and proteins[17] contributes to resultant fall in body weight. Treatment with M + E + MECP was found to prevent this fall in body weight. The whole kidney weight of STZ induced diabetic rats was found to be increased which may be due to enlargement of lining cells of tubules, fatty infiltration and large hemorrhagic areas[18]. The reduced whole kidney weight in groups treated with M + E + MECP is an indication of minimal nephropathy.
Normally, urine volume is maintained by Na+K+ ATPase pump in the basolateral membrane of nephrons. The increase in Na+K+ATPase activity in diabetic rats results in increased urine flow[19]. Progression in kidney malfunctioning was indicated in this study with a graded increase in urine volume over an experimental period in diabetic control rats however, this effect was found to be decreased on treatment with M + E + MECP.
Hyperglycemia occurring during diabetes is the principal factor responsible for the development of structural alterations in the renal tissues[20]. In this study, successful induction of diabetes by STZ was evident by the observed consistent rise in the serum glucose levels over the experimental period in the diabetic control group. Studies have proved that improved glycemic control can significantly decrease the development and progression of diabetic nephropathy Treatment with M + E + MECP exhibited more significant glycemic control as compared to standard treatment, M + E suggesting potentiation of anti-hyperglycemic effect of allopathic medicines in combination with herbal preparation which could be one of the contributing factors in delaying the progression of DN in treatment groups.
The elevated serum levels of urea and creatinine accompanied with diabetic hyperglycemia are significant markers of renal dysfunction and are indicators of decline in the glomerular filtration rate which was evident in this study by decrease in the creatinine clearance levels [21] and rise in levels of serum urea and creatinine in diabetic rats, however, on treatment with M + E + MECP prevented the rise in levels of serum urea and creatinine indicating improvement in the glomerular filtration rate. Elevated levels of urinary proteins, and declined levels of serum proteins observed in STZ induced diabetic rats are indicators of changes in the capillary filtration barrier leading to increased permeability of the glomerular basement membrane which on treatment with M + E + MECP were significantly reversed. Thus these results ensure improvement in renal functioning by minimizing nephropathy on treatment with the herbal combination.
Diabetic Nephropathy is also characterized by persistent albuminuria and is more commonly clinically diagnosed by urinary excretion of more than 300 mg/24 hours. It is another indicator of diabetes induced increased glomerular capillary permeability[22]. In the present study, the excretion of albumin in urine was increased with a parallel decrease in the levels of serum albumin in diabetic control rats reflecting glomerular hyperfiltration which was reversed on treatment with M + E + MECP supporting earlier indications of nephroprotective effect.
Hyperglycemia due to diabetes is also responsible for inducing oxidative stress in the kidney which triggers apoptosis, ultimately leading to the development of diabetic nephropathy. Reactive oxygen species like superoxide anions, and hydroxyl radicals formed due to oxidative stress enhance the lipid peroxidation which damages the cell membrane and disturbs the membrane integrity of kidney tissues causing kidney malfunctioning. During oxidative stress, endogenous key antioxidants like SOD, CAT and GSH, play an important role in scavenging these toxic free radicals and hence get used up rapidly by tissue [23]. Our study supports this hypothesis as levels of SOD, CAT and GSH were found to be declined in the diabetic control group ensuring induction of oxidative kidney damage. Treatment with M + E + MECP to STZ induced diabetic rats was found to restore levels of antioxidant enzymes and prevented lipid peroxidation thereby exerting cytoprotective effect against oxidative stress. Supplementation of herbal preparation in our study facilitates enrichment of antioxidant content of tissue which might be responsible to prevent lipid peroxidation and oxidative damage by free radicals in kidneys.
An important regulator involved in the accumulation of extra cellular matrix (ECM) is TGF-β1. Literature reports progressively increased expression of TGF-β1 accompanied with ECM accumulation and GBM thickening[22].In our study overexpression of TGF- β1 was observed in diabetic control rats whereas treatment with M + E + MECP significantly attenuated overexpression of TGF-β1. The observed increased levels of TGF-β1 along with the excessive oxidative stress provide evidence for the conjecture that oxidative stress may induce ECM accumulation through regulating TGF-β1 expression. Thus, the observed decline in the TGF-β1 levels on supplementation of M + E + MECP might have resulted due to blockade of overexpression of TGF-β1. Aforesaid antioxidant potential of herbal combination might also be contributing to attenuating the release of TGF- β1 hence supplemented with allopathic medicines. Hyperglycemia induced oxidative stress and overexpression of inflammatory mediator TGF-β1 leads to alteration in morphological characters of the kidney. HE staining, PAS staining and trichrome staining indicated that animals in the group of diabetic control have suffered from kidney damage.
In diabetic control rats morphological changes like moderate multifocal vacuolar and granular degeneration of tubular epithelium leading to cell infiltration, moderate glomerular contraction with mild hyalinization leading to accumulation of extracellular matrix in kidney tissues were observed[24]. Treatment with standard M + E only showed minimization of glomerular contraction and hyalinization in kidney tissues whereas no effect was observed in vacuolar and granular degeneration of tubular epithelium. Treatment with M + E + MECP showed marked improvement in morphological characteristics of kidney tissue which was evident by mild vacuolar and granular degeneration of tubular epithelium as well as minimal hyalinization which may be due to antioxidant activity of M + E + MECP and decreased expression of TGF-β1. Hence MECP in combination with standard drugs exhibits a significant protective effect in diabetic nephropathy.
Conclusion
Research findings of the experiment revealed that Costus pictus per se and with a combination of metformin and enalapril treatment ameliorates the condition of diabetic nephropathy and prevents kidney tissue damage by reducing levels of TGF-β. From all the results obtained, it can be concluded that Costus pictus per se and with metformin and enalapril can be effectively used for the management of diabetic nephropathy.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Authors’ contributions
MS and YK designed the experiment. GS performed the experiment. GS, MS and YK analysed the data and wrote the manuscript.
Declarations
Conflict of interest
Authors have no conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01065-5.
References
- 1.Laddha AP, Kulkarni YA. Tannins and vascular complications of Diabetes: An update [Internet]. Phytomedicine. Urban & Fischer; 2019 [cited 2019 Jan 14]. p. 229–45. Available from: https://www.sciencedirect.com/science/article/pii/S0944711318305464. [DOI] [PubMed]
- 2.Garud MS, Kulkarni YA. Gallic acid attenuates type I diabetic nephropathy in rats. Chem Biol Interact [Internet]. 2018 [cited 2018 Jun 26];282:69–76. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29331653. [DOI] [PubMed]
- 3.Article R, Ayurvedic I, Journal M. Role of Traditional Medicine in Improving the Socio-Economic. 1986 [cited 2019 Jan 14]; Available from: www.aims.in.
- 4.Majumdar M, Parihar PS. Antibacterial, antioxidant and antiglycation potential of Costus pictus from Southern region, India. Asian J Plant Sci Res [Internet] 2012;2:95–101. [Google Scholar]
- 5.Don D, Remya R, Daniel M. Phytochemical and pharmacognostic investigation of antidiabetic Costus. 2012 [cited 2019 Jan 14];3:30–9. Available from: https://www.nresearchgate.net/publication/265877259.
- 6.Kulkarni YA, Garud MS, Oza MJ, Barve KH, Gaikwad AB. Diabetes, diabetic complications, and flavonoids. Fruits, Veg Herbs Bioact Foods Heal Promot [Internet]. Academic Press; 2016 [cited 2019 Jan 14]. p. 77–104. Available from: https://www.sciencedirect.com/science/article/pii/B9780128029725000056.
- 7.Moneim AA, Abd El-Twab SM, Ashour MB, Yousef AI, Yousef AI. Hepato-renal protective effects of gallic acid and p-coumaric acid in nicotinamide/streptozotocin-induced diabetic rats. Int J Bioassays [Internet]. International Journal of Bioassays; 2016 [cited 2019 Jan 14];5:4641. Available from: http://ijbio.com/index.php/ijb/article/view/1020.
- 8.Garud MS, Kulkarni YA. Attenuation of renal damage in type I diabetic rats by umbelliferone – a coumarin derivative. Pharmacol Reports [Internet]. Elsevier; 2017 [cited 2019 Jan 14];69:1263–9. Available from: https://www.sciencedirect.com/science/article/abs/pii/S1734114017301366. [DOI] [PubMed]
- 9.Garud MS, Kulkarni YA. Eugenol ameliorates renal damage in streptozotocin-induced diabetic rats. Flavour Fragr J; 2017; 32:54–62. Available from: 10.1002/ffj.3357.
- 10.Wang GG, Lu XH, Li W, Zhao X, Zhang C. Protective effects of luteolin on diabetic nephropathy in STZ-induced diabetic rats. Evidence-based Complement Altern Med [Internet]. Hindawi; 2011 [cited 2019 Jan 14];2011:323171. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21584231. [DOI] [PMC free article] [PubMed]
- 11.Elberry AA, Harraz FM, Ghareib SA, Gabr SA, Nagy AA, Abdel-Sattar E. Methanolic extract of Marrubium vulgare ameliorates hyperglycemia and dyslipidemia in streptozotocin-induced diabetic rats. Int J Diabetes Mellit [Internet].; 2015 [cited 2019 Jan 14];3:37–44. Available from: https://www.sciencedirect.com/science/article/pii/S1877593411000051.
- 12.Vasavada N, Agarwal R. Role of oxidative stress in diabetic nephropathy [Internet]. Adv. Chronic Kidney Dis. 2005 [cited 2019 Jan 14]. p. 146–54. Available from: http://www.ncbi.nlm.nih.gov/pubmed/20939814. [DOI] [PubMed]
- 13.Gaedeke J, Noble NA, Border WA. Curcumin blocks multiple sites of the TGF-β signaling cascade in renal cells. Kidney Int [Internet]. 2004 [cited 2019 Jan 14];66:112–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/15200418. [DOI] [PubMed]
- 14.Ziyadeh FN, Hoffman BB, Han DC, Iglesias-de la Cruz MC, Hong SW, Isono M, et al. Long-term prevention of renal insufficiency, excess matrix gene expression, and glomerular mesangial matrix expansion by treatment with monoclonal antitransforming growth factor-beta antibody in db/db diabetic mice. Proc Natl Acad Sci [Internet]. 2000 [cited 2019 Jan 14];97:8015–20. Available from: http://www.ncbi.nlm.nih.gov/pubmed/10859350. [DOI] [PMC free article] [PubMed]
- 15.Oldfield MD, Bach LA, Forbes JM, Nikolic-Paterson D, McRobert A, Thallas V, et al. Advanced glycation end products cause epithelial-myofibroblast transdifferentiation via the receptor for advanced glycation end products (RAGE). J Clin Invest [Internet]. 2001 [cited 2019 Jan 14];108:1853–63. Available from: http://www.ncbi.nlm.nih.gov/pubmed/11748269. [DOI] [PMC free article] [PubMed]
- 16.Gaedeke J, Noble NA, Border WA. Angiotensin II and progressive renal insufficiency. Curr Hypertens Rep [Internet]. 2002 [cited 2019 Jan 14];4:403–7. Available from: http://www.ncbi.nlm.nih.gov/pubmed/12217260. [DOI] [PubMed]
- 17.Morsy MA, Ibrahim SA, Amin EF, Kamel MY, Abdelwahab SA, Hassan MK. Carvedilol ameliorates early diabetic nephropathy in streptozotocin-induced diabetic rats. Biomed Res Int [Internet]. Hindawi Limited; 2014 [cited 2019 Jan 14];2014:105214. Available from: http://www.ncbi.nlm.nih.gov/pubmed/24991534. [DOI] [PMC free article] [PubMed]
- 18.Abo-Salem OM, El-Edel RH, Harisa GEI, El-Halawany N, Ghonaim MM. Experimental diabetic nephropathy can be prevented by propolis: Effect on metabolic disturbances and renal oxidative parameters. Pak J Pharm Sci [Internet]. 2009 [cited 2019 Jan 14];22:205–10. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19339234. [PubMed]
- 19.Jin Y, Shi Y, Zou Y, Miao C, Sun B, Li C. Fenugreek prevents the development of STZ-induced diabetic nephropathy in a rat model of diabetes. Evidence-based Complement Altern Med [Internet]. 2014 [cited 2019 Jan 14];2014:1–11. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25057273. [DOI] [PMC free article] [PubMed]
- 20.Belhekar SN, Chaudhari PD, Pandhare RB, Pawar AR. Effect of polyherbal and allopolyherbal formulation on streptozotocin-nicotinamide induced diabetic nephropathy in rats. Int J Toxicol Pharmacol Res [Internet] 2016;8:138–45. [Google Scholar]
- 21.Mestry SN, Dhodi JB, Kumbhar SB, Juvekar AR. Attenuation of diabetic nephropathy in streptozotocin-induced diabetic rats by Punica granatum Linn. leaves extract. J Tradit Complement Med [Internet]. 2017 [cited 2018 Jun 26];7:273–80. Available from: http://www.ncbi.nlm.nih.gov/pubmed/28725620. [DOI] [PMC free article] [PubMed]
- 22.Kiran G, Nandini CD, Ramesh HP, Salimath PV. Progression of early phase diabetic nephropathy in streptozotocin-induced diabetic rats: Evaluation of various kidney-related parameters. Indian J Exp Biol [Internet]. 2012 [cited 2019 Jan 14];50:133–40. Available from: http://www.ncbi.nlm.nih.gov/pubmed/22670476. [PubMed]
- 23.Li X, Hu J, Zhang Q, Sun X, Li S. Urocortin 1 improves renal function in rats with streptozotocin-induced diabetes by inhibiting overproduction of TGF-Β1 and VEGF. Br J Pharmacol [Internet]. Wiley-Blackwell; 2009 [cited 2019 Jan 14];157:994–1003. Available from: http://www.ncbi.nlm.nih.gov/pubmed/19466989. [DOI] [PMC free article] [PubMed]
- 24.Braga Gomes K, Fontana Rodrigues K, Fernandes AP. The Role of Transforming Growth Factor-Beta in Diabetic Nephropathy. Int J Med Genet [Internet]. Hindawi; 2014 [cited 2019 Jan 14];2014:1–6. Available from: http://www.hindawi.com/archive/2014/180270/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.