Abstract
Bioremediation of hydrocarbons-contaminated soils, using enzymes, is considered an alternative technology for soil remediation, obtaining shorter remediation times, greater removal efficiencies, and less waste generation. The lipases from invasive plants such as castor bean (Ricinus Communis L.) could represent an opportunity for its application in this purpose. This paper reports the results of evaluating enzymatic treatment at different conditions for the remediation of used lubricating oil-contaminated soils. Four assays were performed for the removal of the contaminant in a soil sample: (1) natural attenuation and (2) biostimulation with urea (10% w/v), both used as blanks, (3) enzymatic treatment with lipases at ambient conditions (room temperature, soil pH) and (4) enzymatic treatment with lipases at ideal conditions (temperature 37 °C, pH 4.5). After seven weeks of treatment, removal percentages of 14.23 ± 1.92%, 35.71 ± 5.17%, 14.11 ± 6.71%, and 94.26 ± 1.91%, respectively, were obtained. The degradation of the contaminant was analyzed by Fourier-transform Infrared spectroscopy (FTIR) for each assay. Results show the potential of the lipases for catalyzing the degradation of this contaminant in the soil at ideal conditions, representing an alternative technology to be applied as treatment ex-situ. This paper is the first study known to show the utilization of castor bean lipase for the remediation of hydrocarbons-contaminated soils.
Keywords: Bioremediation, Castor bean, Enzymatic treatment, Hydrocarbons-contaminated soils, Invasive plants, Lipase.
Introduction
Crude oil and its derivatives are indispensable compounds for human development, but they are also one of the most important sources of pollution (Imam et al. 2019; Khan et al. 2018). Each year, from global oil production, approximately 8 million tons are produced during oil exploitation and lost during refining, transporting, and draining of oily wastewater (Hewelke and Gozdowski 2020; Zhang et al. 2019). This lost oil slowly leaches into the soil and groundwater (Wang et al. 2015; Xu et al. 2017). Once these compounds reach the soil surface, they tend to adhere to soil particles or the roots of vegetation and thus join the food chain and, finally, the human body, harming human health (Adipah 2018; US EPA 2011; Zhang et al. 2019).
In Mexico, lubricating oil is a derivative of petroleum and, once converted into waste (used lubricating oil), is considered hazardous waste due to its characteristics established by the General Law for the Prevention and Comprehensive Management of Waste (LGPGIR by its Spanish acronym), and by the Official Mexican Standard NOM-052-SEMARNAT-2005. This hazardous waste had the highest generation in the country during the 2017–2018 period, with 210 thousand tons, and was the main waste generated within the oil sector (Security, Energy and Environment Agency 2019).
Some biological, physical, chemical, and thermal technologies have been employed to remove used lubricating oil from the soil. However, their advantages and disadvantages are defined by removal efficiencies, treatment times, applicability, and costs. This situation suggests searching for alternative technologies that reduce such inconvenience and perform better (Dominguez-Rodriguez et al. 2020; Lim et al. 2016).
On the other hand, bioremediation is a set of techniques that use biological agents such as plants and microorganisms or products that come from them to remove an undesirable external agent from a specific site. However, most techniques that use microorganisms or plants require long periods, and their by-products are sometimes more toxic (Brown et al. 2017; Hlihor et al. 2017; Imam et al. 2019; Kumar and Bharadvaja 2019). The enzymatic treatment is a bioremediation technique that seeks to compensate for the main disadvantages of the use of microorganisms (biodegradation) and plants (phytoremediation) (Demarche et al. 2012; Kumar and Bharadvaja 2019). The technique uses enzymes produced from microorganisms, plants, and animals to eliminate toxic compounds in contaminated sites (Karigar and Rao 2011; Kumar and Bharadvaja 2019; Sutherland et al. 2004).
Some authors reported extensively on the role of various enzymes in the degradation pathways of various pollutants, including lipases. Lipases (E.C. 3.1.1.3) are reported ubiquitous enzymes from plants, animals, and microbial sources. Also called triacylglycerol ester hydrolases, they catalyze the hydrolysis of triglycerides to glycerol and free fatty acids on an oil-water interface. However, they have also recently attracted attention due to their catalytic activity in soils contaminated with hydrocarbons and their use in the degradation of hexane, hexadecane, motor oil, and heavy oil (Alhamdani and Alkabbi 2016; Ghafil et al. 2016; Kadri et al. 2018; Okino-Delgado et al. 2017; Sharma et al. 2018; Singh et al. 2019; Verma et al. 2012).
Although the lipases obtained from microorganisms have been widely applied in the bioremediation of hydrocarbons-contaminated soils, from the practical and environmental point of view, to use lipases of plant origin attracts more attention because they are widely distributed in nature, they are stable in the pH range of 4.0 to 9.0, temperature range of 25 to 60 °C, and have a variable molecular weight of 19 to 270 kDa (Barros et al. 2010; Villeneuve 2003). Castor bean (Ricinus Communis L.) is an invasive species declared in Mexico in the Agreement that determines the list of Invasive Exotic Species of Mexico, representing a risk towards native biodiversity. In addition, unlike other oilseeds, castor seeds have catalytic activity when it is inactive or at rest and not only in a state of germination. The use of castor bean, like lipase source, is a viable option to treat two environmental problems, hydrocarbons-contaminated soils and control or eradication of the population of this plant (Ory et al. 1962; Ministry of the Environment and Natural Resources 2016).
Therefore, the present work aims to evaluate the potential of Ricinus Communis L. lipases in the remediation of used lubricating oil-contaminated soils as an alternative to conventional technologies. This paper is the first study known to show the utilization of castor bean lipase for the remediation of hydrocarbons-contaminated soils.
Materials and methods
Obtaining and treatment of feedstock
Castor bean seeds (Ricinus communis L.) were used as feedstock to obtain lipases. The samples of plants were collected in the city of Jerez (Zacatecas, Mexico), with geographical coordinates: 22°38’ N, 102°59’ W, in public lands where they grow wild without being necessary any permissions or regulation applicable in Mexico for their collection since these are considered invasive exotic plants in the country and without any use in the state of Zacatecas (Mexico). The plant samples were identified as Ricinus communis in the herbal by researchers of the Biology Department of the Basic Science Center of the Autonomous University of Aguascalientes (Aguascalientes, Mexico). Clusters of plants containing seeds, which were not infected by insects or affected by other damage types and ripe fruit, were selected. These clusters were cut by pruning shears and stored in black plastic, and then they were sun-dried until the seeds were exposed.
Representative soil samples were used as feedstock for performing remediation assays. These were taken from an agricultural area (geographical coordinates: 23°49’ N 103°03’ W), ensuring that it was not contaminated with used lubricating oil. Sampling was made according to Mexican standard NOM-021-SEMARNAT-2000 guidelines. Random zig-zag sampling was performed, taking 1 kg soil simple samples on 13 points in a terrain of 0.13 hectares. A soil composite sample of 13 kg was made from 13 simple samples. The soil composite sample was sun-dried, sieved with a 2 mm mesh and placed in a Ziploc bag at room temperature until use (Ministry of the Environment and Natural Resources 2002).
1 L of used lubricating oil was provided by a mechanical workshop and used as a contaminant in remediation assays.
Physic-chemical characterization of soil
The soil composite sample was characterized physic-chemically according to Mexican standard NOM-021-SEMARNAT-2000 guidelines. The evaluated physic-chemical parameters and analysis methods correspond to pH (Method AS-02), bulk density (Method AS-03), water content and water holding capacity (Method AS-06), organic matter content (Method AS-07), inorganic nitrogen (Method AS-08), and soil texture (Method AS-09). These parameters were evaluated to analyze their influence on remediation processes (Ministry of the Environment and Natural Resources 2002).
Lipase extract powder from castor bean seeds
The extract was obtained according to the methodology described by Avelar et al. (2013) with slight modifications. Initially, the endosperm tissues were carefully removed, and the shells of the seedling were discarded. 40 g of endosperms were then triturated using a commercial blender for 10 min by adding 10 mL acetone. The samples were mixed with chilled acetone with a ratio of 1:5 w/v at 4 ºC for 16 h under agitation (150 rpm). Then, the suspension was filtered under vacuum via a Buchner funnel and washed with acetone in excess. Once the filtered product was obtained, it was placed in aluminum trays at room temperature for 24 h to evaporate the solvent completely. The delipidated extract from seeds was sieved to obtain particles size less than 1 mm. The product was defined as lipase extract powder, which was stored and kept in a sterile place at 4 ºC until used in hydrolysis and remediation assays.
Catalytic activity of lipase extract powder
The catalytic activity of the lipase extract powder from castor bean seeds was determined on the hydrolysis of emulsified vegetable oil, according to the methodology described by Avelar et al. (2013) and Soares et al. (1999), with slight modifications. The substrate was prepared by mixing 50 g commercial olive oil and 150 g gum Arabic solution (30 g/L).
The reaction mixture containing 5 g emulsion, 45 mL 100 mmol/L phosphate buffer (pH 4.5), and 2 g of lipase extract powder was incubated at 25 ºC for 3 h under agitation (1,100 rpm). Samples of 1 ± 0.15 g were periodically removed from the reactor via a Pasteur pipette, weighed, and transferred to 125 mL Erlenmeyer flasks at intervals of 20 min. 10 mL of commercial ethanol were added to the sample to stop the reaction and denaturalize the enzyme, thus effectively freezing the reaction. The free fatty acids (FFA) formed were titrated against standard 20 mmol/L sodium hydroxide solution in the presence of phenolphthalein as an indicator.
The hydrolysis degree was defined as the percentage weight of FFA in the sample divided by the maximum theoretical amount and calculated using Eq. (1).
 |
1 |
Where VNaOH is the volume of sodium hydroxide solution (NaOH) required for titration; MNaOH is the NaOH concentration (20 mmol/L); MM is the average molecular mass of fatty acids in commercial olive oil (279.6 g/mol); Wt is the weight of the sample taken, and f is the fraction of oil at the start of reaction (0.11) (Avelar et al. 2013).
The maximum activities of lipases were defined as 100% relative activity.
Catalytic activity of lipase in contaminated soil
The catalytic activity of the lipase in soil was determined using Tween 20 as a substrate according to the methodology described by Sakai et al. (2002). A test tube was filled with l.0 g of soil, 0.2 mL of toluene, 0.6 mL of Tween 20, 1.15 mL of distilled water, and 0.2 mol/L sodium acetate buffer solution. It was incubated at 30 ºC for 18 h under agitation (240 rpm).
After 18 h, 8 mL of ethanol was added to stop the reaction, the test tube was swirled for 10 s, and the soil suspension was centrifuged at 3,000xG for 10 min. After centrifugation, the supernatant was transferred to another 50 mL Erlenmeyer flask and titrated with a 0.02 mol/L NaOH using 0.375 mL phenol red indicator (0.02 g/L) in alcohol.
The lauric acid content of the supernatant was calculated by referencing a calibration curve plotted from the results obtained with standards containing lauric acid.
The calibration curve was plotted using a lauric acid solution (100 mg/L) in ethanol (stock solution), dilutions containing 0, 0.1, 0.4, 0.6, and 1.0 µmol of lauric acid were made from a stock solution. The samples were subsequently titrated with a 20 mmol NaOH solution, and their absorbance was measured at 205 nm (Fig. 1).
Fig. 1.
Calibration curve of lauric acid
The net activity of all the enzyme assays was determined by subtracting the values of two controls, either without soil or without substrate, and was expressed as nmol of lauric acid equivalents released per g dry weight of soil per minute (mU/g). One unit of the enzyme was defined as the amount that releases 1 µmol of lauric acid per min at 30 ºC and in 0.2 mol/L sodium acetate buffer solution. This analysis was performed for each assay type in the first four weeks of treatment.
Remediation assays
The remediation assays were performed according to the methodology described by Margesin et al. (1999) with slight modifications. 10,000 mg of used lubricating oil were added per 1 kg of soil for each assay. The oil and soil were homogenized using a V Type Powder Homogenizer. Four remediation assays in triplicate were performed in the absence of sunlight. (1) Natural attenuation. (2) Biostimulation with urea (10% w/v), both used as blanks. (3) Enzymatic treatment with lipase extract powder from castor bean seeds at ambient conditions (room temperature, soil pH). (4) Enzymatic treatment with lipase extract powder from castor bean seeds at ideal conditions (temperature 37 °C, pH 4.5). The enzyme extract was added using 100 mL of a 3% (w/v) solution for both assays (Kadri et al. 2018; Margesin et al. 1999).
Soil sub-samples of 10 ± 0.1 g were periodically removed and weighed per week. 10 g anhydrous sodium sulfate was added to the sub-sample and transferred into an extraction cartridge of filter paper of 20-micron. The amount of used lubricating oil was determined by the Soxhlet extraction method using 120 mL per sub-sample of dichloromethane as the solvent, according to the method EPA 9071B stipulated by Mexican standard NMX-AA-134-SCFI-2006 guidelines, with slight modifications. The extraction process was performed at 40 ºC for 4 h.
The removal percentage of used lubricating oil was calculated using Eq. (2).
 |
2 |
Where C0 is the mg initial of the contaminate per mg of contaminated soil, Ci is the mg of the contaminate extracted per mg of contaminated soil, Me is the mg of removable material in non-contaminated soil per mg of non-contaminated soil. The amount of this material was determined by heating non-contaminated soil in an oven at 70 °C for 24 h and carrying out the extraction described above, using the same solvent and operating conditions.
Fourier transform infrared (FTIR) spectroscopy
The analysis FTIR was performed according to method EPA 418.1 with slight modifications described by Schwartz et al. (2012). When the assays were stopped, samples of 10 g of soil were taken per assay type. Samples were subjected to Soxhlet extraction under the same conditions described in the previous section, adding 5 g of anhydrous sodium sulfate. Then 30 mL dichloromethane was added to each extract. This mixture was kept in a sealed glass vial capped with a PTFE cap and placed in a sonic bath to assist and hasten the extraction process for 30 min. Silica gel was added to the mixture to absorb any polar hydrocarbon (nonfuel-related soil organic matter and fatty acids). This substance was mixed well and filtered. The filtered extract was measured in an FTIR spectrometer. The spectra were collected in 32 scans at 4 cm-1 in the mid-IR range 4000 − 400 cm-1 with automatic signal gain and rationed against a background spectrum recorded from the clean empty cell at 25 °C. Spectral data analysis was performed using the OPUS 3.0 data collection software program.
Results and discussion
Physic-chemical parameters of soil
The results of physic-chemical characterization of soil samples used for remediation assays are shown in Table 1.
Table 1.
Physic-chemical characterization of soil used for remediation assays
| Physic-chemical parameter | Value | Unit | |
|---|---|---|---|
| Texture | Clay content | 35.1 | % |
| Silt content | 20.0 | % | |
| Sand content | 47.2 | % | |
| Bulk density | 1.4 | g/ml | |
| pH | 8.2 | - | |
| Water content | 2.1 | % | |
| Water holding capacity | 35.4 | % | |
| Organic matter content | 1.6 | % | |
| Inorganic nitrogen | 11.7 | mg/kg | |
Texture and bulk density
The texture is an essential parameter in the availability of the used lubricating oil to microorganisms in the soil since it determines the mobility of the contaminant. Previous studies have shown that the nature of horizons influences this mechanism, but the variety of used lubricating oil concentrations in sandy soils is minimal at 10, 20, and 40 cm of depth from the superficial layer (130, 125.58, and 125.58 g/kg, respectively) (Abdel-Moghny et al. 2012). In contrast, the contaminant concentration in clayey soils is higher in superficial soil layers (0–10 cm) than in the horizons (Kogbara et al. 2016). In this case study, soil texture is mainly sandy (47.2% sand); therefore, the used lubricating oil was homogeneously distributed in the soil during the assays, reducing the bias in the determination of the contaminant in the Soxhlet extraction processes performed. Furthermore, in bioremediation processes directly mediated by microorganisms, the textural class also conditions the transport of water, oxygen, and nutrients and, consequently, the remediation rate, since a high content of fine particles such as clays will decrease it.
On the contrary, a higher percentage of sands will stimulate the remediation processes, favoring this physic parameter to the remediation assays performed in this work (Akbari and Ghoshal 2015; Kogbara et al. 2016). Bulk density refers to the mass of solids in the soil by the total volume. The value obtained corresponds to “sandy mineral soils,” according to the classification of the Mexican standard. Thus, it confirms the information analyzed of soil texture.
pH
Soil pH is one of the critical environmental parameters that influence the bioavailability of contaminants, the availability of other nutrients, and the activity of biological processes (Al-Hawash et al. 2018). Soil pH effect in different types of hydrocarbons (gasoline, diesel, and fuel oil) with concentrations in the soil from 100 to 150,000 mg/kg has been studied. It was found to have a minimal effect since the pH value had a change of less than one unit (Ajoku and Oduola 2013). This argument is important because an optimal pH is required for microorganisms and enzymes, which play an indispensable role in the present study.
The pH value obtained was 8.2. However, in situ and ex situ bioremediation tests have shown that a pH in the range 6–8 is sufficient for the optimal growth of hydrocarbon-degrading bacteria, so soil pH is favorable for the degradation of hydrocarbons by this type of microorganisms Ajoku and Oduola 2013; Al-Hawash et al. 2018; Martinez 2001).
In contrast, hydrocarbons biodegradation by microorganisms implicates enzymatic activities that are also affected by changes in pH. Several lipases have a high catalytic activity at optimal pH in the range 7–9. However, others have catalytic activity in a broader range 4–10, such as the case of lipases from Ricinus Communis L., which has a higher catalytic activity when the pH is acidic (4.5) (Santos et al. 2013). Thus, considering this issue, two enzymatic assays for remediation soil were proposed, one with ideal pH conditions for lipases (pH 4.5) and another without modifying soil pH (pH 8.2).
Water holding capacity and water content
Water holding capacity refers to the amount of water that a soil can hold after being saturated and allowed to drain for 1–2 days. This parameter and water content are essential factors in bioremediation processes since water constitutes from 80 to 90% of the molecular composition of bacterial cells and is its primary nutrient (Nyer 2000).
The water holding capacity value obtained was 35.4%. Various authors have reported that percentages between 25 and 85% are adequate for soil hydrocarbons degradation so this parameter would favor used lubricating oil degradation by microorganisms present in the soil (Lemos et al. 2013; Masciandaro et al. 2013).
Soil water content must be optimal since low levels of water decrease microbial activity, and water in excess generates resistance to oxygen transfer and can also lead to undesirable leachate (Schjønning et al. 2011). The optimal value of soil water content for remediation processes in the literature is scarce. However, optimal microbial activity is achieved through maximum water content that does not restrict oxygen diffusion (Haghollahi et al. 2016). It has been reported that a soil water content of at least 5% results favorable for removing contaminants in bioremediation processes, but higher removal percentages have been obtained in soil water content above 5% (Acuña et al. 2012; Lahel et al. 2016; Ordaz et al. 2011). This work obtained an initial water content of 2.16%, limiting the bioremediation processes performed.
Furthermore, soil water content influences the catalytic activity of lipases since it depends on the amount of water present in the reaction medium. These enzymes can catalyze degradation reactions such as hydrolysis or synthesis reactions such as the production of esters by an esterification reaction (Farnet et al. 2013; Smyth et al. 2011). In the present work, hydrolysis or transesterification reactions were not studied. A previous study reported that the water available for enzymes significantly influences their activity. They modified soil water content in a range of 10–60%, evaluating rates of hydrolysis and transesterification. It was found that both reactions occurred simultaneously and in the same proportion as the water content increased, showing lower reaction rates at both extremes (10 and 60% humidity) (Farnet et al. 2013). However, these reactions continue to be carried out despite low water content. This issue is possible because the activity of the enzymes also depends on the amount of substrate (Chowdary and Prapulla 2002).
Organic matter content and inorganic nitrogen
Organic matter and nitrogen are considered nutrients for the soil biota. An organic matter content of less than 2% in soils decreases the bioavailability of polyaromatic hydrocarbons, forming aggregates with the soil particles. The soil analyzed in this study presented an organic matter content of 1.6%. Therefore, this parameter could interfere with the activity of the microorganisms on this type of hydrocarbon. However, it does not influence the activity of the enzymes (Nam et al. 1998).
Catalytic activity of castor bean lipases on commercial olive oil hydrolysis
The percentage of catalytic activity for lipase extract powder from castor bean seeds is shown in Fig. 2. The determination of catalytic activity was performed on the hydrolysis of commercial olive oil, and the optimal activity was defined as 100% relative activity.
Fig. 2.
Catalytic activity of lipases from castor bean seeds
As shown in Fig. 2, castor bean lipases displayed maximum catalytic activity (99.78 ± 0.5%) after 2.5 h of incubation. Other authors have reported a maximum catalytic activity of 83% for castor bean lipase at pH 4.5 after 2 and 3 h of incubation under agitation (1,000 rpm) (Avelar et al. 2013; Santos et al. 2013). The high specificity of castor beans lipases on the triacylglycerols of the commercial olive oil used can be verified in these results. Studies have reported that oilseed lipases, such as the castor bean, show more significant catalytic activity on triacylglycerols that contain the fatty acids found in higher percentages in the seed (Barros et al. 2010; Hellyer et al. 1999). Castor bean seeds are composited by ricinoleic (74.10%), linoleic (10.32%), and oleic (7.55%) acids. The last one is the fatty acid found in a higher proportion in olive oil (55–83%), so the hydrolysis of triacylglycerols in olive oil by the action of castor bean lipases is marked (Boskou et al. 2006; Salimon et al. 2010).
The difference between maximum catalytic activities obtained in this work and that reported by other authors can be attributed to the variety of castor bean seed used or agitation conditions (Avelar et al. 2013; Santos et al. 2013). Emulsifying agents are usually added in practice to reduce the surface tension that exists at the biphasic interface (lipid/aqueous medium), which results in greater availability of the oil towards the lipase, or otherwise, that the lipase can take couple efficiently to the substrate. However, the last condition can be achieved with mechanical agitation due to the shear between the oil droplets, which reduces their size and therefore increase the interfacial area between the aqueous medium and the hydrophobic medium, increasing the conversion rate of triglycerides into di- and monoglycerides, which is reflected in the percentage of hydrolysis (Al-Zuhair et al. 2003; Avelar et al. 2013; Tiss et al. 2002). This slight increase from 1,000 to 1,100 rpm could be a favorable factor in the results obtained in this study.
Catalytic activity of castor bean lipases on used lubricating oil-contaminated soils
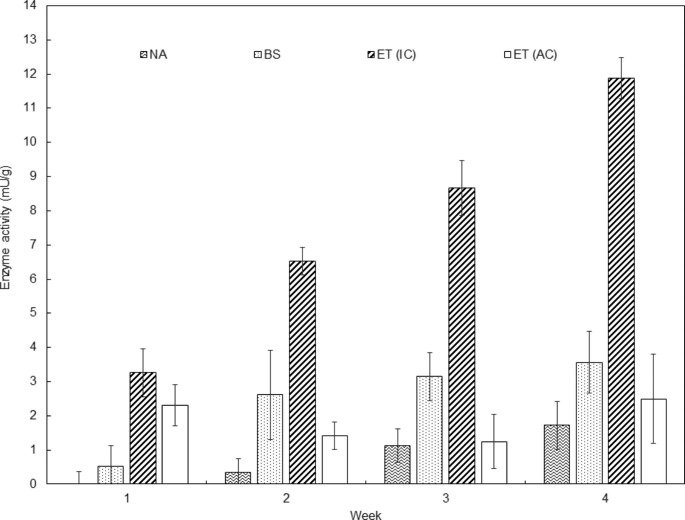
The catalytic activity of castor bean lipases during the first four weeks of the bioremediation treatment for the four types of assays is shown in Fig. 3.
Fig. 3.
Catalytic activity of castor bean lipases during the first four weeks of bioremediation assays. NA: Natural attenuation; BS: Biostimulation with urea assays; ET (IC): Enzymatic treatment with lipases at ideal conditions (temperature 37 °C, pH 4.5); ET (AC): Enzymatic treatment with lipases at ambient conditions (room temperature, soil pH)
The enzymatic treatment with castor bean lipases at ideal conditions (temperature 37 °C, pH 4.5) showed the highest catalytic activity (11.8 ± 0.6 mU/g), followed by the activity in the assays of biostimulation with urea (3.56 ± 0.9 mU/g), enzymatic treatment at ambient conditions (room temperature, soil pH) (2.49 ± 1.3 mU/g) and natural attenuation (1.72 ± 0.7 mU/g). This trend was expected as microorganisms are sensitive to any environmental disturbance, and their diversity and activity are rapidly altered in contaminated soil (Babin et al. 2014).
It has been verified that microorganisms release extracellular enzymes for mineralizing organic compounds into elemental minerals. These enzymes attached to external microbial cells initiate the hydrolysis and oxidation from high molecular weight substrates, such as hydrocarbons, to mineral elements (Nannipieri et al. 2002; Otitoju et al. 2017).
The incidence of a degradable substrate (contaminant) induces the enzymes to metabolize the xenobiotic to harmless products or even to a more toxic intermediate, while the enzyme represses and disappears when its substrate is depleted. This type of metabolic control of microbial cells regarding enzyme induction and repression optimizes their internal biochemistry because of the introduction of contaminants into the environment (Otitoju et al. 2017).
Removal of used lubricating oil in the soil
The removal percentages of used lubricating oil in the soil in the remediation assays performed are shown in Fig. 4.
Fig. 4.
Removal percentage of used lubricating oil in the soil in the remediation assays performed per 7 weeks (49 days) NA: Natural attenuation; BS: Biostimulation with urea; ET (IC): Enzymatic treatment with lipases at ideal conditions (temperature 37 °C, pH 4.5); ET (AC): Enzymatic treatment with lipases at ambient conditions (room temperature, soil pH)
As shown in Fig. 4, adding the enzymatic extract of castor bean lipases in solution (3% w/v) to the soil subjected to treatment favored the removal of used lubricating oil. The soil properties are altered when an external agent is introduced. One of these properties is the microbial activity that, at high concentrations of a particular contaminant, can strongly inhibit microbial growth and even cause its death, resulting in low biodegradation efficiency, as can be observed in natural attenuation and biostimulation assays during the first 14 days of treatment. The enzymatic treatment at ambient conditions showed better removal efficiencies in the first 14 days than natural attenuation and biostimulation. This difference can be attributed to the presence of the enzyme extract since one of the advantages of the enzymes is their resistance to high concentrations of contaminants without requiring a phase of adaptation to a new environment, as seen with microorganisms (Khudur et al. 2015; Kumar and Bharadvaja 2019; Ma et al. 2015; Truskewycz et al. 2019; Yan et al. 2016).
Although both enzymatic treatments showed better biodegradation efficiency than natural attenuation and biostimulation during the first 21 days of treatment, in the following days, the performance of the enzymatic treatment at ideal conditions of the castor bean lipase was better than at ambient conditions. It was possible due to the optimal conditions of temperature (37 ºC) and pH (4.5), in which castor been lipases have the higher catalytic activity, favoring the used lubricating oil biodegradation. Furthermore, the increment of temperature from 20 ºC approximately (room temperature) to 37 ºC raised the microbial growth with capacities for degradation of hydrocarbons (Cavalcanti et al. 2007; Santos et al. 2013; Zekri and Chaalal 2005).
The pH can significantly affect microbial activity and bioremediation rate. Studies have shown that a value between 6.5 and 7.5 is sufficient for the optimal growth of hydrocarbon-degrading microorganisms. However, some microbial populations still have a degradation capacity in acidic or alkaline soils. Microbial activity is generally inhibited at extreme pH (Ajoku and Oduola 2013; Li et al. 2016).
Similarly, pH is a parameter that influences the enzyme functionality since they can be denatured by extreme levels of hydrogen ions (either high or low); any change in pH, even a minor one, alters the degree of ionization of the acidic and basic side groups of an enzyme and the components of the substrate as well. The ionizable side groups located at the active site must have a specific charge for the enzyme to bind to its substrate. The neutralization of one of these charges alters the catalytic activity of an enzyme (Bearne 2014; Pawar 2015).
A soil pH of 8.29 was constant during remediation assays, being likely the adequate pH for the autochthon soil microorganisms. However, when the soil pH was modified to 4.5 for the remediation assay at ideal conditions for the enzyme, its effect is evident in the results of lipase catalytic activity and removal of used lubrication oil in the soil. Removal percentages of 14.11 ± 6.71% and 94.26 ± 1.91% were observed for enzymatic treatment at ambient and ideal conditions per 49 days, respectively. Castor bean lipases significantly reduce its catalytic capacity by almost 90% at pH different to 4.5 (Santos et al. 2013) since all enzymes will have a maximum activity at a specific pH (optimal pH for the enzyme) if the pH were higher or lower than optimal pH, the activity of the enzyme would decrease (Bender 2002). These conditions can be reflected in the results of remediation assays.
An enzymatic treatment from castor bean lipases in situ is not recommended. However, it could be tried out in soils with pH between five and seven since several enzymes still have catalytic activity in two or three units below their optimal pH (Bender 2002). Positive results could be an alternative to ex-situ treatment. Another alternative would be to add a surfactant (gum Arabic or Triton X-100) to reduce the interfacial tension at the water-oil interface, facilitating the formation of micelles in the substrate and being more available for the enzyme (Avelar et al. 2013; Santos et al. 2013).
Figure 5 Concentration of used lubricating oil in soil (mg/kg) in the remediation assays performed per 7 weeks (49 days) NA: Natural attenuation; BS: Biostimulation with urea; ET (IC): Enzymatic treatment with lipases at ideal conditions (temperature 37 °C, pH 4.5); ET (AC): Enzymatic treatment with lipases at ambient conditions (room temperature, soil pH); LC: Limit concentration of used lubricating oil in the soil established by the Mexican standard NOM-138-SEMARNAT/SSA1-2012.
Fig. 5.
shows the remaining concentration of used lubricating oil in soil (mg/Kg) during treatment for each remediation assay. The Mexican standard NOM-138-SEMARNAT/SSA1-2012 stipulates a maximum concentration of 3,000 mg/kg of this contaminant in agricultural soils (Ministry of the Environment and Natural Resources and Ministry of Health 2013). It can also be appreciated in it
After seven weeks of treatment, the concentrations of used lubricating oil in soil were 8,576.39 ± 26.58, 6,428.45 ± 184.49, 8,588.91 ± 94.82, and 573.88 ± 17.25 mg/kg for natural attenuation, biostimulation, enzymatic treatment at ambient conditions, and enzymatic treatment at ideal conditions, respectively. The concentration of used lubricating oil in the soil in the assays of enzymatic treatment at ideal conditions of castor bean lipase was inferior to the limit stipulated by the Mexican standard. This condition was already complied with Even between weeks 6 and 7, being an alternative for remediation of used lubricating oil-contaminated soils. Table 2 shows a comparison of the efficiency and treatment time of remediation technologies applied in the removal of used lubricating oil in the soil.
Table 2.
Remediation technologies applied in the removal of used lubricating oil in the soil
| Remediation technology | Used lubricating oil concentration (mg/kg) | Treatment time (days) |
Removal efficiency (%) | Reference |
|---|---|---|---|---|
| Enzymatic bioremediation (castor beans lipases) at 37 °C and pH 4.5 | 10,000 | 49 | 94.3 | This study |
| Enzymatic bioremediation (orange garbage enzymes) | 52,630 | 42 | 62.0 |
Bulai et al. |
| Enzymatic bioremediation (watermelon garbage enzymes) | 52,630 | 42 | 45.0 |
Bulai et al. |
| Biostimulation (Rhodococcus qingshengii KAG C) and Extracellular Organic Matter (Micrococcus luteus) | 52,500 | 60 | 37.0 |
Bodor et al. |
| Bioaugmentation (R. erythropolis PR) and Extracellular Organic Matter (Micrococcus luteus) | 52,500 | 60 | 45.0 | Bodor et al. 2021 |
| Phytoremediation (Crotalaria retusa L.) | 10,000 | 90 | 52.6 | Gamage et al. 2021 |
| Phytoremediation (Impatiens balsamina) | 10,000 | 90 | 46.2 | Gamage et al. 2021 |
| Biodegradation fungal (Purpureocillium lilacinum) | 25* | 40 | 14.4 | Benguenab and Chibani 2020 |
| Biodegradation fungal (Aspergillus ustus) | 25* | 40 | 16.0 | Benguenab and Chibani 2020 |
*The amount corresponds to ml/kg
It can be noted in Table 2 that the removal efficiency of used lubricating oil in soil using enzymatic bioremediation with castor beans lipases at 37 °C and pH 4.5 is 1.52 and 2.09 times higher than watermelon garbage and orange garbage enzymes, respectively. Nevertheless, one week more of treatment was required. Likewise, the technology studied in this work showed a removal efficiency of 2.09 and 2.55 times higher than biostimulation and bioaugmentation technologies reported by Bodor et al. (2021), even in a shorter time (Table 2). However, these authors used a concentration of used lubricating oil 5.25 higher than this study, which could be a disadvantage. Although the performance of the castor bean lipase could be evaluated in the future, using higher concentrations of used lubricating oil in the soil.
Gamage et al. (2021) reported removal efficiencies of used lubricating oil in soil using phytoremediation technology with Crotalaria retusa L. and Impatiens balsamina. They reported efficiencies 1.79 and 2.04 lower than this study (Table 2). Furthermore, phytoremediation technology required more than twice the time used in the enzymatic bioremediation with castor beans lipases. Finally, the biodegradation of used lubricating oil in soil using fungi (Purpureocillium lilacinum and Aspergillus ustus) shows efficiencies 6.54 times lower than this study (Table 2). Hence, the enzymatic bioremediation with castor beans lipases at 37 °C and pH 4.5 could be competitive regard other remediation technologies. However, a cost-benefit analysis would have to be performed since, due to the pH and temperature conditions of the castor bean lipase, a treatment ex-situ would have to be operated.
Analysis of Fourier transform infrared (FTIR)
The results at a molecular level of the remediation assays performed per 7 weeks are shown in Fig. 6.
Fig. 6.
FTIR analysis of contaminated soil after remediation assays performed per 7 weeks (49 days) and its comparison with non-contaminated soil (a) FTIR spectra of soil contaminated after treatment of natural attenuation (NA); (b) FTIR spectra of soil contaminated after treatment of biostimulation with urea (BS); (c) FTIR spectra of soil contaminated after enzymatic treatment with lipases at ideal conditions (temperature 37 °C, pH 4.5) (ET (IC)); (d) FTIR spectra of soil contaminated after enzymatic treatment with lipases at ambient conditions (room temperature, soil pH) (ET (AC))
The main compounds of the petroleum derivatives are hydrocarbons, and the used lubricating oil contains mostly alkanes that have at least one fragment or an aliphatic center. FTIR’s hydrocarbon analysis was identified through the C-H stretching vibrations for methyl (CH3) and methylene (CH2). It can be observed in Fig. 6 the prominent peaks in wave numbers 2922.60 and 2853.76 cm-1 for each treatment associated with the presence of the remaining oil, although other types of hydrocarbons such as cycloalkanes, alkenes, and alkynes can also be identified (Coates 2006; Ganesh Kumar et al. 2014; Ghafil et al. 2016; Imam et al. 2019; Mahmood et al. 2017).
Slight changes were observed in the results of natural attenuation, biostimulation, and enzymatic treatment at ambient conditions (Fig. 6 (a), (b), and (d)). However, soil analysis subjected to the enzymatic treatment at ideal conditions showed considerable changes after the remediation assay (Fig. 6 (c)). The peaks corresponding to hydrocarbons were attenuated, which translates to a decrease in the concentration of used lubricating oil in the soil. Some absorption bands were appreciated in 1732.79 and 1707.98 cm-1 attributed to aldehydes and fatty acids. The last ones are intermediaries in the main alkane degradation pathway, mainly by autochthonous microorganisms present in soil and usually found in biosurfactants (Pacwa-Płociniczak et al. 2014). Furthermore, the attenuation of the bands corresponding to the stretching vibration of O-H attributed to alcohols suggests a mechanism of action by lipases in the structure of the used lubricating oil Abbasian et al. 2015; Carvalho-Gonçalves and Gorlach-Lira 2018; Coates 2006; Colla et al. 2010; de Paula et al. 2005; Ganesh Kumar et al. 2014).
To date, there are few reports on the role of lipases in the degradation of petroleum-derived compounds and the potential application of enzyme cocktails with lipase to improve the removal of hydrocarbons from the environment. Furthermore, the authors of this study do not know if there is information on the degradation mechanisms of petroleum-derived hydrocarbons by the action of lipases. However, lipases belong to the family of α/β hydrolases, so they exhibit diverse catalytic activities such as carboxylic acid esterases, thioesterases, peptidases, dehalogenases, epoxide hydrolases, halo peroxidases, and C-C bond breaking (Bhandari et al. 2021; Carr et al. 2009; Lenfant et al. 2013). In addition, they can also catalyze synthetic reactions if the chemical equilibrium is shifted to synthesis. The above is demonstrated in applications for biocatalysis of multicomponent reactions, transacylation, C-C, C-N, and C-S bond formation, and oxygenated compounds (Reetz 2002; Yang et al. 2014). Likewise, they are considered promiscuous enzymes concerning substrates. For example, they can catalyze more than one different chemical transformation at the same active site due to their broad specificity (Hult and Berglund 2007). Thus, it is likely that lipases, like enolases, when exposed to substrates and conditions absent in vivo, are forced to catalyze reactions for which they were not created (Gerlt et al. 2005). It has been reported that the native catalytic activity of lipases is due to an active site composed of serine, histidine, and aspartate or glutamate. This site forms an oxyanion hole that facilitates the transformation of carbonyl substrates by hydrolysis, esterification, transesterification, interesterification, and aminolysis reactions (Stergiou et al. 2013).
In this work, some signs of carbonyl compounds were observed at the end of the enzymatic treatment (Fig. 6 (c)). This result implies the presence of oxidation-reduction reactions in the initial stages of this study and agrees with the increase of lipase activity as the treatment progresses. Some reports indicate that the presence of oxide-reducing enzymes joins efforts with lipases to transform hydrocarbons to oxygenated organic compounds of lower toxicity. For example, monooxygenases, dioxygenases, and hydroxylases incorporate oxygen atoms into alkyl or aryl substrates and convert them into alcohols, aldehydes, and fatty acids (Ji et al. 2013). Dehydrogenases similarly oxidize alcohols producing aldehydes, ketones, and carboxylic acids, depending on the substrate (Karigar and Rao 2011) (Fig. 7). Castor bean seed contains oxide-reducing enzymes, so it is likely, that a certain amount of these is still active in the obtained enzymatic extract and contributes to the bioconversion of hydrocarbons followed by lipases degradation.
Fig. 7.

Degradation of hydrocarbons by lipases aided with oxide-reducing enzymes
On the other hand, considering the promiscuous activity reported for lipases (Hult and Berglund 2007). The transformation of hydrocarbons to fewer toxic compounds is very complex since the diversity of substrates faced by lipases, once enzymatic biodegradation is initiated, is very wide. The lipases might be able to carry out a series of oxidation, reduction, condensation, and addition reactions (Dwivedee et al. 2018) (Fig. 8). Hence, it is considered that they modify the initial substrate to less hazardous substances under the conditions evaluated in this work. A proposed mechanism for removing used lubricating oil in soil using castor bean lipases is shown in Fig. 9.
Fig. 8.
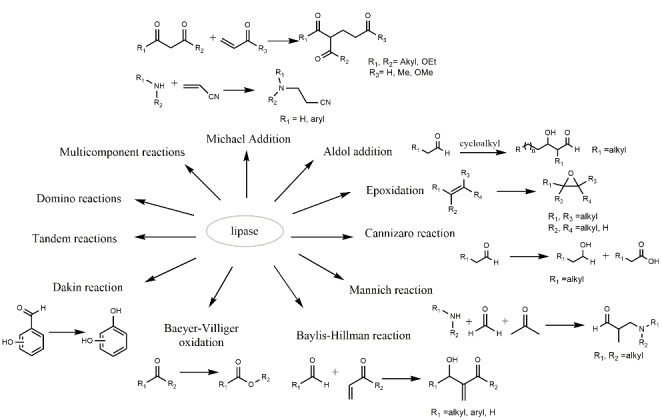
Promiscuous catalytic activity of lipases for modification of oil substrates
Fig. 9.

Proposed mechanism for removing used lubricating oil in soil using castor bean lipases
Finally, it is necessary to consider a technique to propose a degradation pathway, as in the study by Pourfadakari et al. (2021), who used a GC-MS analysis for the phenanthrene degradation pathway. However, in the present study, FTIR spectroscopy allowed the analysis of the products and by-products of the degradation of used lubricating oil in the soil using an enzymatic treatment with castor bean lipases, confirming the degradation of the contaminant, and proposing a removal mechanism.
Conclusions
This paper is the first study showing the utilization of castor bean lipase for the remediation of hydrocarbons-contaminated soils. The lipase extract powder from castor bean seeds showed high efficiency (94.26 ± 1.91) in the removal of used lubricating oil in soil (10,000 mg/kg) at ideal conditions for the lipase in terms of its maximum catalytic activity (temperature 37 ºC, pH 4.5). This study confirms the possibility of using castor bean lipase for catalyzing contaminant biodegradation and being an alternative as treatment ex-situ for bioremediation. The results open new avenues for investigating and applying this lipase to remediate hydrocarbons-contaminated soils.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
The authors would like to thank the ‘Laboratory of Environmental Engineering’ technical staff at the ‘Interdisciplinary Professional Unit of Engineering, Zacatecas campus (UPIIZ)’ of the ‘Instituto Politécnico Nacional (IPN)’ for the technical and analytical support. The authors wish to thank the ‘Environmental Science Laboratory’ of the ‘Autonomous University of San Luis Potosi’ for allowing us to perform FTIR analysis, and to the ‘Laboratory of the Biology Department’ of the ‘Basic Science Center’ of the ‘Autonomous University of Aguascalientes’ for the technical support in the identification of the plants’ samples collected.
Authors’ contributions
Conceptualization: Manuel Alexis Sánchez Castro; Methodology: Manuel Alexis Sánchez Castro, Hans Christian Correa Aguado, Jésica García Torres; Formal analysis and investigation: Manuel Alexis Sánchez Castro; Writing - original draft preparation: Miguel Mauricio Aguilera Flores; Writing - review and editing: Miguel Mauricio Aguilera Flores, Verónica Ávila Vázquez, Manuel Alexis Sánchez Castro, Hans Christian Correa Aguado, Jésica García Torres; Funding acquisition: Miguel Mauricio Aguilera Flores, Verónica Ávila Vázquez; Resources: Verónica Ávila Vázquez, Miguel Mauricio Aguilera Flores, Hans Christian Correa Aguado, Jésica García Torres; Supervision: Miguel Mauricio Aguilera Flores.
Funding
This work was supported by the ‘Instituto Politécnico Nacional (IPN)’ (Grant number SIP project-20200995).
Data availability
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40201-022-00806-1.
References
- Abbasian F, Lockington R, Mallavarapu M, Naidu R. A comprehensive review of aliphatic hydrocarbon biodegradation by bacteria. Appl Biochem Biotechnol. 2015;176(3):670–99. doi: 10.1007/s12010-015-1603-5. [DOI] [PubMed] [Google Scholar]
- Abdel-Moghny Th, Mohamed RSA, El-Sayed E, Mohammed Aly S, Snousy MG. Effect of soil texture on remediation of hydrocarbons-contaminated soil at El-Minia District, Upper Egypt. ISRN Chem Eng. 2012;2012:1–13. doi: 10.5402/2012/406598. [DOI] [Google Scholar]
- Acuña AJ, Tonin NL, Diaz V, Pucci GN, Pucci OH. Optimization of a laboratory-scale hydrocarbon bioremediation system. Eng Res Technol. 2012;13(1):105–12. [Google Scholar]
- Adipah S. Introduction of Petroleum Hydrocarbons Contaminants and its Human Effects. J Environ Sci Public Health. 2018;3(1):1–9. doi: 10.26502/jesph.96120043. [DOI] [Google Scholar]
- Ajoku GAO, Oduola MK. Kinetic model of pH effect on bioremediation of crude petroleum contaminated soil. Am J Chem Eng. 2013;1(1):6–10. doi: 10.11648/j.ajche.20130101.12. [DOI] [Google Scholar]
- Akbari A, Ghoshal S. Bioaccessible porosity in soil aggregates and implications for biodegradation of high molecular weight petroleum compounds. Environ Sci Technol. 2015;49(24):14368–75. doi: 10.1021/acs.est.5b03618. [DOI] [PubMed] [Google Scholar]
- Al-Hawash AB, Dragh MA, Li S, Alhujaily A, Abbood HA, Zhang X, Ma F. Principles of microbial degradation of petroleum hydrocarbons in the environment. Egypt J Aquat Res. 2018;44(2):71–6. doi: 10.1016/j.ejar.2018.06.001. [DOI] [Google Scholar]
- Al-Zuhair S, Hasan M, Ramachandran KB. Kinetics of the enzymatic hydrolysis of palm oil by lipase. Process Biochem. 2003;38(8):1155–63. doi: 10.1016/S0032-9592(02)00279-0. [DOI] [Google Scholar]
- Alhamdani MA, Alkabbi HJJ. Isolation and identification of lipase producing bacteria from oil-contaminant soil. J Biol Agric Healthcare. 2016;6(20):1–7. [Google Scholar]
- Avelar MHM, Cassimiro DMJ, Santos KC, Domingues RCC, de Castro HF, Mendes AA. Hydrolysis of vegetable oils catalyzed by lipase extract powder from dormant castor bean seeds. Ind Crops Prod. 2013;44:452–8. doi: 10.1016/j.indcrop.2012.10.011. [DOI] [Google Scholar]
- Babin D, Vogel C, Zühlke S, Schloter M, Pronk GJ, Heister K, Spiteller M, Kögel-Knabner I, Smalla K. Soil mineral composition matters: response of microbial communities to phenanthrene and plant litter addition in long-term matured artificial soils. PLoS ONE. 2014;9(9):e106865. doi: 10.1371/journal.pone.0106865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barros M, Fleuri LF, Macedo GA. Seed lipases: Sources, applications and properties - A review. Braz J Chem Eng. 2010;27(1):15–29. doi: 10.1590/S0104-66322010000100002. [DOI] [Google Scholar]
- Bearne SL. Illustrating the effect of pH on enzyme activity using Gibbs energy profiles. J Chem Educ. 2014;91(1):84–90. doi: 10.1021/ed400229g. [DOI] [Google Scholar]
- Bender DA. (2002) Introduction to Nutrition and Metabolism. London, UK.
- Benguenab A, Chibani A. Biodegradation of petroleum hydrocarbons by filamentous fungi (Aspergillus ustus and Purpureocillium lilacinum) isolated from used engine oil contaminated soil. Acta Ecol Sin. 2020;41(5):416–23. doi: 10.1016/j.chnaes.2020.10.008. [DOI] [Google Scholar]
- Bhandari S, Poudel DK, Marahatha R, Dawadi S, Khadayat K, Phuyal S, Shrestha S, Gaire S, Basnet K, Khadka U, Parajuli N. (2021) Microbial enzymes used in bioremediation. J Chem 2021:8849512. 10.1155/2021/8849512.
- Bodor A, Bounedjoum N, Feigl G, Duzs Á, Laczi K, Szilágyi Á, Rákhely G, Perei K. Exploitation of extracellular organic matter from Micrococcus luteus to enhance ex situ bioremediation of soils polluted with used lubricants. J Hazard Mater. 2021;417:125996. doi: 10.1016/j.jhazmat.2021.125996. [DOI] [PubMed] [Google Scholar]
- Boskou D, Blekas G, Tsimidou M. Olive Oil Composition. In: Boskou D, editor. Olive Oil: Chemistry and Technology. 2. Greece: Thessaloniki; 2006. pp. 41–72. [Google Scholar]
- Brown LD, Cologgi DL, Gee KF, Ulrich AC. Bioremediation of Oil Spills on Land. In: Fingas M, editor. Oil Spill Science and Technology. 1. Canada: Edmonton; 2017. pp. 699–729. [Google Scholar]
- Bulai IS, Adamu H, Umar YA, Sabo A. Biocatalytic remediation of used motor oil-contaminated soil by fruit garbage enzymes. J Environ Chem Eng. 2021;9(4):105465. doi: 10.1016/j.jece.2021.105465. [DOI] [Google Scholar]
- Carr PD, Ollis DL. α/β Hydrolase fold: an update. Protein Pept Lett. 2009;16(10):1137–48. doi: 10.2174/092986609789071298. [DOI] [PubMed] [Google Scholar]
- Carvalho-Gonçalves LCT, Gorlach-Lira K. Lipases and biosurfactants production by the newly isolated Burkholderia sp. Braz J Biol Sci. 2018;5(9):57–68. doi: 10.21472/bjbs.050906. [DOI] [Google Scholar]
- Cavalcanti EDC, Maciel FM, Villeneuve P, Lago RCA, Machado OLT, Freire DMG. Acetone powder from dormant seeds of Ricinus communis L: Lipase activity and presence of toxic and allergenic compounds. Appl Biochem Biotechnol. 2007;137–140(1–12):57–65. doi: 10.1007/s12010-007-9039-1. [DOI] [PubMed] [Google Scholar]
- Chowdary GV, Prapulla SG. The influence of water activity on the lipase catalyzed synthesis of butyl butyrate by transesterification. Process Biochem. 2002;38(3):393–7. doi: 10.1016/S0032-9592(02)00096-1. [DOI] [Google Scholar]
- Coates J. Interpretation of Infrared Spectra, a Practical Approach. Encycl Anal Chem. 2006 doi: 10.1002/9780470027318.a5606. [DOI] [Google Scholar]
- Colla LM, Rizzardi J, Pinto MH, Reinehr CO, Bertolin TE, Costa JAV. Simultaneous production of lipases and biosurfactants by submerged and solid-state bioprocesses. Bioresour Technol. 2010;101(21):8308–14. doi: 10.1016/j.biortech.2010.05.086. [DOI] [PubMed] [Google Scholar]
- De Paula AV, de Souza Barboza JC, de Castro HF. Study of the influence of solvent, carbohydrate and fatty acid in the enzymatic synthesis of sugar esters by lipases. Quim Nova. 2005;28(5):792–6. doi: 10.1590/S0100-40422005000500011. [DOI] [Google Scholar]
- Demarche P, Junghanns C, Nair RR, Agathos SN. Harnessing the power of enzymes for environmental stewardship. Biotechnol Adv. 2012;30(5):933–53. doi: 10.1016/j.biotechadv.2011.05.013. [DOI] [PubMed] [Google Scholar]
- Dominguez-Rodriguez VI, Adams RH, Vargas-Almeida M, Zavala-Cruz J, Romero-Frasca E. Fertility Deterioration in a Remediated Petroleum-Contaminated Soil. Int J Environ Res Public Health. 2020;17(2):382. doi: 10.3390/ijerph17020382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwivedee BP, Soni S, Sharma M, Bhaumik J, Laha JK, Banerjee UC. Promiscuity of Lipase-Catalyzed Reactions for Organic Synthesis: A Recent Update. ChemistrySelect. 2018;3(9):2441–66. doi: 10.1002/slct.201702954. [DOI] [Google Scholar]
- Farnet AM, Qasemian L, Gil G, Ferre E. The importance of water availability in the reaction equilibrium of hydrolases in forest litters from a Mediterranean area: A study on lipases: The influence of water activity on enzymes in litters. Eur J Soil Sci. 2013;64(5):661–6. doi: 10.1111/ejss.12069. [DOI] [Google Scholar]
- Gamage SSW, Masakorala K, Brown MT, Gamage SMKW. Comparative phytoremediation potentials of Impatiens balsamina L. and Crotalaria retusa L. for soil contaminated with used lubricating oil. Environ Adv. 2021;5:100095. doi: 10.1016/j.envadv.2021.100095. [DOI] [Google Scholar]
- Ganesh Kumar A, Vijayakumar L, Joshi G, Peter DM, Dharani G, Kirubagaran R. Biodegradation of complex hydrocarbons in spent engine oil by novel bacterial consortium isolated from deep sea sediment. Bioresour Technol. 2014;170:556–64. doi: 10.1016/j.biortech.2014.08.008. [DOI] [PubMed] [Google Scholar]
- Gerlt JA, Babbitt PC, Rayment I. Divergent evolution in the enolase superfamily: the interplay of mechanism and specificity. Arch Biochem Biophys. 2005;433(1):59–70. doi: 10.1016/j.abb.2004.07.034. [DOI] [PubMed] [Google Scholar]
- Ghafil JA, Hassan SS, Zgair AK. Use of immobilized lipase in cleaning up soil contaminated with oil. World J Exp Biosci. 2016;4(1):53–7. [Google Scholar]
- Haghollahi A, Fazaelipoor MH, Schaffie M. The effect of soil type on the bioremediation of petroleum contaminated soils. J Environ Manage. 2016;180:197–201. doi: 10.1016/j.jenvman.2016.05.038. [DOI] [PubMed] [Google Scholar]
- Hewelke E, Gozdowski D. Hydrophysical properties of sandy clay contaminated by petroleum hydrocarbon. Environ Sci Pollut Res. 2020;27(9):9697–706. doi: 10.1007/s11356-020-07627-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hellyer SA, Chandler IC, Bosley JA. Can the fatty acid selectivity of plant lipases be predicted from the composition of the seed triglyceride? Biochim Biophys Acta. 1999;1440(2–3):215–24. doi: 10.1016/s1388-1981(99)00125-0. [DOI] [PubMed] [Google Scholar]
- Hlihor RM, Gavrilescu M, Tavares T, Favier L, Olivieri G. Bioremediation: An Overview on Current Practices, Advances, and New Perspectives in Environmental Pollution Treatment. Biomed Res Int. 2017;2017:1–2. doi: 10.1155/2017/6327610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hult K, Berglund P. Enzyme promiscuity: mechanism and applications. Trends Biotechnol. 2007;25(5):231–8. doi: 10.1016/j.tibtech.2007.03.002. [DOI] [PubMed] [Google Scholar]
- Imam A, Suman SK, Ghosh D, Kanaujia PK. Analytical approaches used in monitoring the bioremediation of hydrocarbons in petroleum-contaminated soil and sludge. TrAC Trends Anal Chem. 2019;118:50–64. doi: 10.1016/j.trac.2019.05.023. [DOI] [Google Scholar]
- Ji Y, Mao G, Wang Y, Bartlam M. Structural insights into diversity and n-alkane biodegradation mechanisms of alkane hydroxylases. Front Microbiol. 2013;4:58. doi: 10.3389/fmicb.2013.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadri T, Rouissi T, Magdouli S, Brar SK, Hegde K, Khiari Z, Daghrir R, Lauzon JM. Production and characterization of novel hydrocarbon degrading enzymes from Alcanivorax borkumensis. Int J Biol Macromol. 2018;112:230–40. doi: 10.1016/j.ijbiomac.2018.01.177. [DOI] [PubMed] [Google Scholar]
- Karigar CS, Rao SS. Role of Microbial Enzymes in the Bioremediation of Pollutants: A Review. Enzyme Res. 2011;2011:1–11. doi: 10.4061/2011/805187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan MAI, Biswas B, Smith E, Naidu R, Megharaj M. Toxicity assessment of fresh and weathered petroleum hydrocarbons in contaminated soil - A review. Chemosphere. 2018;212:755–67. doi: 10.1016/j.chemosphere.2018.08.094. [DOI] [PubMed] [Google Scholar]
- Khudur LS, Shahsavari E, Miranda AF, Morrison PD, Nugegoda D, Ball AS. Evaluating the efficacy of bioremediating a diesel-contaminated soil using ecotoxicological and bacterial community indices. Environ Sci Pollut Res. 2015;22(19):14809–19. doi: 10.1007/s11356-015-4624-2. [DOI] [PubMed] [Google Scholar]
- Kogbara RB, Ayotamuno JM, Worlu DC, Fubara-Manuel I. A Case Study of Petroleum Degradation in Different Soil Textural Classes. Recent Pat Biotechnol. 2016;9(2):108–15. doi: 10.2174/2211550105666151110203337. [DOI] [PubMed] [Google Scholar]
- Kumar L, Bharadvaja N. Enzymatic bioremediation: A smart tool to fight environmental pollutants. In: Bhatt P, editor. Smart Bioremediation Technologies. 1. India: Dehradun; 2019. pp. 99–118. [Google Scholar]
- Lahel A, Fanta AB, Sergienko N, Shakya M, Lopez ME, Behera SK, Rene ER, Park HS. Effect of process parameters on the bioremediation of diesel contaminated soil by mixed microbial consortia. Int Biodeterior Biodegrad. 2016;113:375–85. doi: 10.1016/j.ibiod.2016.05.005. [DOI] [Google Scholar]
- Lemos DA, Cardoso SL, Vieira PA, Cardoso VL. Bioremediation of Soil Contaminated with Biodiesel and Glycerin-Results of Soil Microbial Adaptation Through Evidence Contaminants Removal. Chem Eng Trans. 2013;32:463–8. doi: 10.3303/CET1332078. [DOI] [Google Scholar]
- Lenfant N, Hotelier T, Bourne Y, Marchot P, Chatonnet A. Proteins with an alpha/beta hydrolase fold: Relationships between subfamilies in an ever-growing superfamily. Chem Biol Interact. 2013;203(1):266–8. doi: 10.1016/j.cbi.2012.09.003. [DOI] [PubMed] [Google Scholar]
- Li J, Guo C, Lu G, Yi X, Dang Z. Bioremediation of Petroleum-Contaminated Acid Soil by a Constructed Bacterial Consortium Immobilized on Sawdust: Influences of Multiple Factors. Water Air Soil Pollut. 2016;227(12):444. doi: 10.1007/s11270-016-3117-3. [DOI] [Google Scholar]
- Lim MW, Lau EV, Poh PE. A comprehensive guide of remediation technologies for oil contaminated soil-Present works and future directions. Mar Pollut Bull. 2016;109(1):14–45. doi: 10.1016/j.marpolbul.2016.04.023. [DOI] [PubMed] [Google Scholar]
- Ma YL, Lu W, Wan LL, Luo N. Elucidation of fluoranthene degradative characteristics in a newly isolated Achromobacter xylosoxidans DN002. Appl Biochem Biotechnol. 2015;175(3):1294–305. doi: 10.1007/s12010-014-1347-7. [DOI] [PubMed] [Google Scholar]
- Mahmood MH, Yang Z, Thanoon RD, Makky EA, Rahim MHA. Lipase Production and Optimization from Bioremediation of Disposed Engine Oil. J Chem Pharm Res. 2017;9(6):26–36. [Google Scholar]
- Margesin R, Zimmerbauer A, Schinner F. Soil lipase activity - A useful indicator of oil biodegradation. Biotechnol Tech. 1999;13:859–63. doi: 10.1023/A:1008928308695. [DOI] [Google Scholar]
- Martinez VE, Lopez SM. Effect of hydrocarbons on the physical and chemical properties of clay soil. Terra Latinoam. 2001;19(1):9–17. [Google Scholar]
- Masciandaro G, Macci C, Peruzzi E, Ceccanti B, Doni S. Organic matter–microorganism–plant in soil bioremediation: A synergic approach. Rev Environ Sci Biotechnol. 2013;12(4):399–419. doi: 10.1007/s11157-013-9313-3. [DOI] [Google Scholar]
- Ministry of the Environment and Natural Resources. (2002) Mexican standard NOM-021-SEMARNAT-2002 establishes fertility, salinity, and soil classification specifications. Studies, sampling, and analysis. Published in the Official Gazette of the Federation on December 31, 2002. http://dof.gob.mx/nota_detalle_popup.php?codigo=791052#:~:text=NOM%2D021%2DSEMARNAT%2D2000,salinidad%20y%20clasificaci%C3%B3n%20de%20suelos.amp;text=Registro%20que%20acompa%C3%B1a%20a%20las,laboratorio%20de%20pruebas%20y%20an%C3%A1lisis. Accessed 22 September 2020 (in Spanish).
- Ministry of the Environment and Natural Resources. (2016) AGREEMENT by which the List of Invasive Alien Species for Mexico is determined. Published in the Official Gazette of the Federation on December 07, 2016. http://dof.gob.mx/nota_detalle.php?codigo=5464456&fecha=07/12/2016 Accessed 22 September 2020 (in Spanish).
- Ministry of the Environment and Natural Resources and Ministry of Health. (2013) Mexican standard NOM-138-SEMARNAT/SSA1-2012 establishes maximum permissible limits of hydrocarbons in soils and guidelines for sampling in the characterization and remediation specifications. Published in the Official Gazette of the Federation on September 10, 2013. http://www.dof.gob.mx/nota_detalle.php?codigo=5313544&fecha=10/09/2013. Accessed 22 September 2020 (in Spanish).
- Nam K, Chung N, Alexander M. Relationship between Organic Matter Content of Soil and the Sequestration of Phenanthrene. Environ Sci Technol. 1998;32(23):3785–8. doi: 10.1021/es980428m. [DOI] [Google Scholar]
- Nannipieri P, Kandeler E, Ruggiero P. (2002) Enzyme Activities and Microbiological and Biochemical Processes in Soil. New York, USA.
- Nyer EK. (2000) In situ treatment technology. London, UK.
- Okino-Delgado CH, do Prado DZ do, Facanali R, Marques MMO, Nascimento AS, Fernandes CJ da Zambuzzi C, Fleuri WF LF (2017) Bioremediation of cooking oil waste using lipases from wastes. PLoS One 12(10):e0186246. 10.1371/journal.pone.0186246. [DOI] [PMC free article] [PubMed]
- Ordaz JA, Toledo AM, Morales FRR, Diaz LFS, Martinez AJ, Lopez JAT, Cuevas-Diaz MC. Bioremediation of soil contaminated with oil by using sugarcane bagasse with different particle sizes. Multisci. 2011;11(2):136–45. [Google Scholar]
- Ory RL, Angelo AJS, Altschul AM. The acid lipase of the castor bean. Properties and substrate specificity. J Lipid Res. 1962;3(1):99–105. doi: 10.1016/S0022-2275(20)40457-2. [DOI] [Google Scholar]
- Otitoju O, Udebuani A, Ebulue M, Onwurah I. Enzyme–based Assay for Toxicological Evaluation of Soil Ecosystem Polluted with Spent Engine Oil. J Agric Ecol Res Int. 2017;11(3):1–13. doi: 10.9734/JAERI/2017/27605. [DOI] [Google Scholar]
- Pacwa-Płociniczak M, Płaza GA, Poliwoda A, Piotrowska-Seget Z. Characterization of hydrocarbon-degrading and biosurfactant-producing Pseudomonas sp. P-1 strain as a potential tool for bioremediation of petroleum-contaminated soil. Environ Sci Pollut Res. 2014;21(15):9385–95. doi: 10.1007/s11356-014-2872-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawar RM. The Effect of Soil pH on Bioremediation of Polycyclic Aromatic Hydrocarbons (PAHS) J Biorem Biodegrad. 2015;6(03):291. doi: 10.4172/2155-6199.1000291. [DOI] [Google Scholar]
- Pourfadakari S, Jorfi S, Roudbari A, Javid A, Talebi SS, Ghadiri SK, Yousefi N. Optimization of electro-kinetic process for remediation of soil contaminated with phenanthrene using response surface methodology. Environ Sci Pollut Res. 2021;28:1006–17. doi: 10.1007/s11356-020-10495-8. [DOI] [PubMed] [Google Scholar]
- Reetz MT. Lipases as practical biocatalysts. Curr Opin Chem Biol. 2002;6(2):145–50. doi: 10.1016/s1367-5931(02)00297-1. [DOI] [PubMed] [Google Scholar]
- Sakai Y, Hayatsu M, Hayano K. Use of tween 20 as a substrate for assay of lipase activity in soils. J Soil Sci Plant Nutr. 2002;48(5):729–34. doi: 10.1080/00380768.2002.10409263. [DOI] [Google Scholar]
- Salimon J, Mohd Noor DA, Nazrizawati AT, Mohd Firdaus MY, Noraishah A. Fatty acid composition and physicochemical properties of Malaysian castor bean Ricinus communis L. seed oil. Sains Malays. 2010;39(5):761–4. [Google Scholar]
- Santos KC, Cassimiro DMJ, Avelar MHM, Hirata DB, de Castro HF, Fernandez-Lafuente R, Mendes AA. Characterization of the catalytic properties of lipases from plant seeds for the production of concentrated fatty acids from different vegetable oils. Ind Crops Prod. 2013;49:462–70. doi: 10.1016/j.indcrop.2013.05.035. [DOI] [Google Scholar]
- Schjønning P, Thomsen IK, Petersen SO, Kristensen K, Christensen BT. Relating soil microbial activity to water content and tillage-induced differences in soil structure. Geoderma. 2011;163(3):256–64. doi: 10.1016/j.geoderma.2011.04.022. [DOI] [Google Scholar]
- Schwartz G, Ben-Dor E, Eshel G. Quantitative Analysis of Total Petroleum Hydrocarbons in Soils: Comparison between Reflectance Spectroscopy and Solvent Extraction by 3 Certified Laboratories. Appl Environ Soil Sci. 2012;2012:1–11. doi: 10.1155/2012/751956. [DOI] [Google Scholar]
- Sharma B, Dangi AK, Shukla P. Contemporary enzyme based technologies for bioremediation: A review. J Environ Manage. 2018;210:10–22. doi: 10.1016/j.jenvman.2017.12.075. [DOI] [PubMed] [Google Scholar]
- Security E, Agency E. (2019) Estimated generation of hazardous waste by type of waste. Government of Mexico Web. https://datos.gob.mx/busca/dataset/generacion-estimada-de-residuos-peligrosos-por-tipo-de-residuo. Accessed 28 June 2020 (in Spanish).
- Singh RS, Singh T, Singh AK. (2019) Enzymes as Diagnostic Tools. In: Singh RS, Singhania RR, Pandey A, Larroche C, editor Advances in Enzyme Technology, 1st edn. pp 225–271.
- Smyth CE, Kurz WA, Trofymow JA. Including the effects of water stress on decomposition in the Carbon Budget Model of the Canadian Forest Sector CBM-CFS3. Ecol Modell. 2011;222(5):1080–91. doi: 10.1016/j.ecolmodel.2010.12.005. [DOI] [Google Scholar]
- Soares CMF, De Castro HF, De Moraes FF, Zanin GM. Characterization and Utilization of Candida rugosa Lipase Immobilized on Controlled Pore Silica. Appl Biochem Biotechnol. 1999;79(1–3):745–58. doi: 10.1385/abab:79:1-3:745. [DOI] [PubMed] [Google Scholar]
- Stergiou PY, Foukis A, Filippou M, Koukouritaki M, Parapouli M, Theodorou LG, Hatziloukas E, Afendra A, Pandey A, Papamichael EM. Advances in lipase-catalyzed esterification reactions. Biotechnol Adv. 2013;31(8):1846–59. doi: 10.1016/j.biotechadv.2013.08. [DOI] [PubMed] [Google Scholar]
- Sutherland TD, Horne I, Weir KM, Coppin CW, Williams MR, Selleck M, Russell RJ, Oakeshott JG. Enzymatic bioremediation: From enzyme discovery to applications. Clin Exp Pharmacol Physiol. 2004;31(11):817–21. doi: 10.1111/j.1440-1681.2004.04088.x. [DOI] [PubMed] [Google Scholar]
- Tiss A, Carrière F, Douchet I, Patkar S, Svendsen A, Verger R. Interfacial binding and activity of lipases at the lipid–water interface: Effects of Gum Arabic and surface pressure. Colloids Surf B. 2002;26(1):135–45. doi: 10.1016/S0927-7765(01)00315-0. [DOI] [Google Scholar]
- Truskewycz A, Gundry TD, Khudur LS, Kolobaric A, Taha M, Aburto-Medina A, Ball AS, Shahsavari E. Petroleum Hydrocarbon Contamination in Terrestrial Ecosystems-Fate and Microbial Responses. Mol. 2019;24(18):3400. doi: 10.3390/molecules24183400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- US EPA. (2011) The behavior of Effects of Oil Spill in Aquatic Environments. US EPA Archive Document Web. https://archive.epa.gov/emergencies/docs/oil/edu/web/pdf/chap1.pdf Accessed 24 July 2020.
- Verma S, Saxena J, Prasanna R, Sharma V, Nain L. Medium optimization for a novel crude-oil degrading lipase from Pseudomonas aeruginosa SL-72 using statistical approaches for bioremediation of crude-oil. Biocatal Agric Biotechnol. 2012;1(4):321–9. doi: 10.1016/j.bcab.2012.07.002. [DOI] [Google Scholar]
- Villeneuve P. Plant lipases and their applications in oils and fats modification. Eur J Lipid Sci Technol. 2003;105(6):308–17. doi: 10.1002/ejlt.200390061. [DOI] [Google Scholar]
- Wang J, Feng L, Steve M, Tang X, Gail TE, Mikael H. China’s unconventional oil: A review of its resources and outlook for long-term production. Energy. 2015;82:31–42. doi: 10.1016/j.energy.2014.12.042. [DOI] [Google Scholar]
- Xu J, Kong F, Song S, Cao Q, Huang T, Cui Y. Effect of Fenton pre-oxidation on mobilization of nutrients and efficient subsequent bioremediation of crude oil-contaminated soil. Chemosphere. 2017;180:1–10. doi: 10.1016/j.chemosphere.2017.03.087. [DOI] [PubMed] [Google Scholar]
- Yan L, Sinkko H, Penttinen P, Lindström K. Characterization of successional changes in bacterial community composition during bioremediation of used motor oil-contaminated soil in a boreal climate. Sci Total Environ. 2016;542:817–25. doi: 10.1016/j.scitotenv.2015.10.144. [DOI] [PubMed] [Google Scholar]
- Yang F, Wang Z, Wang H, Zhang H, Yue H, Wang L. Enzyme catalytic promiscuity: lipase catalyzed synthesis of substituted 2H-chromenes by a three-component reaction. RSC Adv. 2014;4(49):25633. doi: 10.1039/c4ra03367a. [DOI] [Google Scholar]
- Zekri A, Chaalal O. Effect of temperature on biodegradation of crude oil. Energy Sources. 2005;27(1–2):233–44. doi: 10.1080/00908310490448299. [DOI] [Google Scholar]
- Zhang W, Liu Y, Tan X, Zeng G, Gong J, Lai C, Niu Q, Tang Y. Enhancement of detoxification of petroleum hydrocarbons and heavy metals in oil-contaminated soil by using glycine-β-cyclodextrin. Int J Environ Res Public Health. 2019;16(7):1155. doi: 10.3390/ijerph16071155. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.