Abstract
Purpose
Novel anthropometric measures are simple, applicable, and inexpensive tools for cardiovascular risk assessment. This study evaluates the association of lipid accumulation product (LAP) with hypertension, type 2 diabetes mellitus (T2DM), and all-cause mortality, and compares it with other anthropometric measures.
Methods
PubMed, Web of Science, EMBASE, and Scopus were systematically searched for articles published until May 15, 2021. We included all the studies that had measured LAP predictability for T2DM, all-cause mortality, and hypertension with no limitation in comorbidities and follow-up duration. We assessed the predictability measures of LAP for the aforementioned outcomes. We also performed a meta-analysis on four articles on mortality using an inverse variance method by the “meta” package in R software.
Results
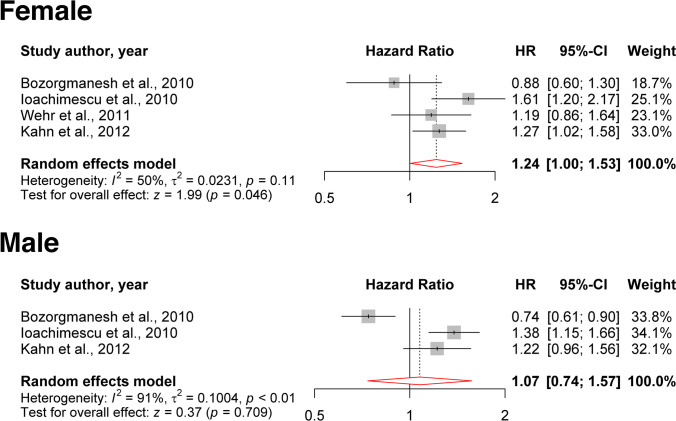
Twenty-nine studies were included in the review after applying the eligibility criteria. The hazard ratio for all-cause mortality per one standard deviation increment of LAP was 1.24 (95% confidence interval [CI]: 1.00–1.53; P = 0.0463) in females, and 1.07 (95% CI: 0.74–1.57; P = 0.709) in males. All included studies found a direct association between LAP with T2DM and hypertension. However, studies used different cut-off points for LAP. Most studies found that LAP was superior in predicting T2DM and hypertension compared to conventional indices, e.g., body mass index and waist circumference. We found that LAP may have higher prognostic significance in females compared to males.
Conclusion
LAP is an inexpensive method to evaluate the risk of all-cause mortality, T2DM, and hypertension, and could outperform conventional anthropometric indices in this regard.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01114-z.
Keywords: Lipid accumulation product, LAP, Type 2 diabetes mellitus, Hypertension, Mortality, Anthropometric measure
Introduction
Obesity and overweight are among the principal modifiable risk factors for coronary heart disease (CHD), ischemic stroke, hypertension, metabolic syndrome, and type 2 diabetes mellitus (T2DM) [1, 2]. According to World Health Organization (WHO), 39% of adults were overweight, as 11% of males and 15% of females were obese in 2016 globally; therefore, more than half a billion adults suffer from obesity and overweight worldwide [1]. Moreover, hypertension and T2DM are global health concerns since their global prevalence has an increasing trend [3, 4]. Screening and early detection of the high-risk populations for chronic diseases could contribute to controlling their morbidity and mortality [5].
Anthropometric measures are applicable tools for screening and early detection of weight-related disorders, having the advantages of simplicity. Notable among these are body mass index (BMI) [5], waist circumference (WC), hip circumference (HC), waist-to-hip ratio (WHR; the ratio of WC to HC), and waist to height ratio (WHtR; the ratio of WC to height) [6]. While BMI is the best known and most widely used anthropometric index, it has major limitations for the determination of body fat mass. For instance, BMI is not able to show fat distribution and is affected by age and sex; therefore, there is a need to investigate more powerful indices [7, 8].
Several new anthropometric measures have recently been recommended, and different studies have evaluated their performances in predicting chronic diseases. Abdominal volume index (AVI), body adiposity index (BAI), body shape index (ABSI), body roundness index (BRI), and lipid accumulation product (LAP) are a few examples that are associated with CHD [9, 10]. LAP was introduced by Kahn in 2005 [11]. Kahn suggested that “BMI may not be the best marker for estimating the risk of obesity-related disease”, and LAP could be a better predictor of the incidence of cardiovascular diseases than BMI. Studies have reported a correlation between LAP and insulin resistance [12]. Moreover, the accuracy of LAP for predicting metabolic syndrome has been validated, and it was demonstrated that LAP is superior to other indices in this regard [13]. There is evidence that LAP can be used in predicting long-term cardiometabolic diseases among females with higher accuracy than other anthropometric and central obesity markers [14]. Furthermore, the results of a retrospective study revealed that LAP is associated with mortality, but that in some cases like diabetic patients, this association is not present [15].
Although many studies have evaluated and compared the predictability of different anthropometric measures, contradictory findings are confusing and complicate choosing an appropriate obesity index for practice and screening. Systematic reviews and meta-analyses might help us to determine which anthropometric measures are more appropriate for screening a specific subgroup of the population. We aimed to systematically review the literature and perform a meta-analysis to establish the correlation between LAP and T2DM, mortality, and hypertension.
Methods
This review was conducted in compliance with the review protocol registered on PROSPERO, PROSPERO 2019 CRD42019142239 [16]. It is reported according to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement [17].
Study eligibility criteria
Studies were included if: (1) they were conducted among adults above 18 years of age; (2) used LAP (calculated by the following formula: male LAP = [WC (cm) − 65] × Triglyceride (TG) concentration (mmol/l) and female LAP = [WC (cm) − 58] × TG concentration (mmol/l)) as an exposure variable; (3) described the desired outcomes: hypertension, T2DM, and mortality; (4) studies that have evaluated the predictability of LAP for the above-mentioned health outcomes; (5) published in the English language; (6) published in peer-reviewed journals before May 15, 2021 (search date). The following studies were excluded: (1) case reports, letters, editorials, commentary articles, review articles, abstracts, and protocols; (2) articles that have reported no health outcome related to LAP. The selected studies were not limited due to comorbidities and follow-up duration.
Search strategy
Two authors (S.K. and A.A.) systematically and independently searched the electronic databases PubMed, Web of Science, EMBASE, and SCOPUS for related studies from inception to May 15, 2021. We developed our search strategy in PubMed and subsequently searched other databases through the following medical subject headings (MeSH) terms and free keywords: “Lipid Accumulation Product”, “Hypertension”, “Blood Pressure”, “Diabetes Mellitus”, “Diabetes Mellitus Type 2”, and “Mortality”. The search strategy is provided in the Supplementary Material. All records were transferred to EndNote software, and the duplicates were removed.
Data extraction and preparation
Three authors (S.K., H.T., and A.A.) independently screened the titles and abstracts to apply inclusion/exclusion criteria. The full text was reviewed thoroughly if any article’s admissibility remained unclear. Following the selection of eligible studies, a comprehensive full-text review and data extraction were conducted by two authors (S.K. and H.T.) independently. Standardized data extraction forms were used to compile the variables comprising of methodological features (first author and year of publication, country, study type, source of data, population size, percentage of females, comorbidities, age of population, follow-up duration, method of LAP determination, statistical analysis, adjustment for confounders), outcome (T2DM, hypertension, and mortality), predictability measure (odds ratio [OR], area under receiver operating characteristic curve [AUC], hazard ratio [HR], relative risk [RR], and Poisson regression) and predictability of other anthropometric measures (BMI, WC, WHR, WHtR, VAI, BAI, and others). Disagreements in any of the steps were resolved through discussion and a third author’s opinion.
Quality assessment
Study quality was evaluated with the National Institutes of Health’s (NIH) Quality Assessment Tool for Observational Cohort and Cross-Sectional Studies [18]. This tool has 14 criteria to evaluate each study, and each criterion should be answered with “Yes”, “No”, or “Other” (cannot determine, not applicable, not reported). After determining the answer to each question, each study was scored as good, fair, or poor. Two authors (S.K. and H.T.) independently rated included articles according to the NIH checklists. The quality assessment was not used to exclude studies but made the robustness of the evidence clear. Discordance in ratings was resolved through discussion or arbitration by a third author.
Statistical analyses
Meta-analysis was performed to assess the predictability of LAP for the desired outcomes if two or more studies reported the same outcome measure. According to sex differences in LAP, the meta-analysis was done for each sex separately. The meta-analysis was done on mortality papers using an inverse variance method, and the random-effects model was reported. Heterogeneity was evaluated by I2 and τ2 tests with a P < 0.1 as evidence of heterogeneity. We used R statistical software version 4.0.3 and the “meta” package, including “metagen” command for this purpose.
Results
Study selection
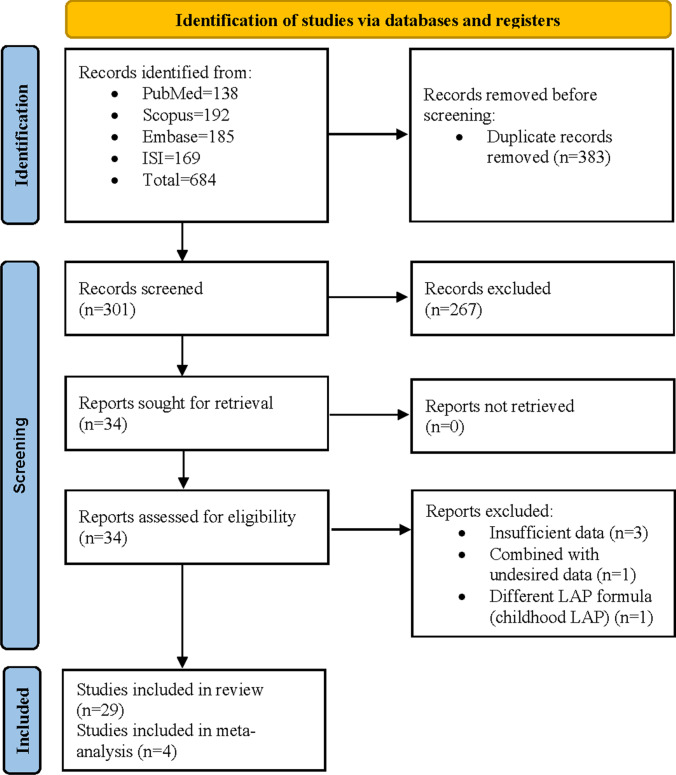
Our search identified 684 publications, including 185 articles from Embase, 169 articles from Web of Science, 138 articles from PubMed, and 192 articles from Scopus. After removing duplicates, 301 records were screened through title and abstract, and 267 citations were removed. We reviewed the full-text of 34 articles, and five articles were excluded due to the following reasons: (1) Insufficient data (three articles), (2) Combination with undesired data (one article), and (3) Different LAP formula (one article). Finally, 29 articles were included in our study (Table 1). Figure 1 shows a flow diagram of study selection.
Table 1.
Excluded articles after full-text evaluation
| Author/Year | Title | Reason of exclusion |
|---|---|---|
| H. S. Kahn, 2006 [19] | The Lipid Accumulation Product Is Better Than BMI For Identifying Diabetes: A Population-Based Comparison | Insufficient data |
| Hamsaveena, 2014 [20] | Lipid Accumulation Product As A Novel Index To Predict Diabetes In Women | Insufficient data |
| Wanderley Rocha, 2017 [21] | Visceral Adiposity Measurements, Metabolic and Inflammatory Profile in Obese Patients with and Without Type 2 Diabetes Mellitus: A Cross-sectional Analysis | Insufficient data |
| N. Ahn, 2019 [22] | Visceral Adiposity Index (VAI), Lipid Accumulation Product (LAP), And Product Of Triglycerides And Glucose (Tyg) To Discriminate Prediabetes And Diabetes | Combined with undesired data (Diabetic and prediabetic patients were not separated) |
| Y. Wang, 2020 [23] | A Novel Indicator, Childhood Lipid Accumulation Product, Is Associated With Hypertension In Chinese Children And Adolescents | Different LAP formula (childhood LAP) |
Fig. 1.
PRISMA flowchart
Study characteristics
The baseline characteristics of included records are illustrated in Table 2. Studies have been conducted in 14 countries (China = 12, Iran = 3, Korea = 2, Brazil = 1, Serbia = 1, USA = 2, Italy = 1, Romania = 1, Japan = 2, Mongolia = 1, Germany = 1, Poland = 1, Thailand = 1, Netherlands = 1). Eighteen studies were cross-sectional, and 12 studies were prospective or retrospective cohorts. The sample size in the studies varied from 264 to 215,651, and the range of follow-up duration in cohort studies was from 5 to 18.1 years. The sample of 26 studies was the general population, and the others had evaluated people with specific conditions like menopausal women, post-menopausal women, and people with hypertension. In all the studies, LAP was measured objectively, and none of them were self-report. The minimum age in the studies was 18. All of the studies were adjusted for some health-related items (e.g., age; smoking; systolic blood pressure; family history of premature CVD; diabetes; antihypertensive drug use; HDL and non-HDL cholesterol; FPG (fasting plasma glucose); 2hPCPG (2 h post-challenge plasma glucose); socioeconomic status (rural/urban setting; region; education level; family income); alcohol use; ALT; Apo-lipoprotein A1; Apo-lipoprotein B; uric acid; bilirubin, creatinine, eGFRMDRD (continuous variables); VAI; hsCRP, WC, TG, WHR, Hypolipemics, BMI, physical activity, SHBG, physical activity level, race, marriage status, eGFR, and antidiabetic treatment).
Table 2.
Baseline characteristics of the included studies
| Study ID | Author, year | Country | Study design | Data source | Sample size (%F) | Specific subgroup | Age range | Age (Mean ± SD) | Follow-up (years) | LAP determination | Statistical analysis | Adjustment |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Bozorgmanesh, 2010 | Iran | Cross-sectional and prospective cohort | Tehran lipid and glucose study | 8671 (57.2) for cross-sectional and 3242 (57.8) for longitudinal | ≥ 20 | 42.9 ± 15(cross sectional), 41.6 ± 13.2(longitudinal) | 6 | Objectively | Linear and logistic regression | Baseline mean arterial pressure, family history of DM | |
| 2 | Bozorgmanesh, 2010 | Iran | Prospective cohort | Tehran lipid and glucose study | 6751(56.1) | ≥ 30 | NA | 8.6 | Objectively | General linear model, Cox's proportional hazards regression | Age, smoking, SBP, family history of premature CVD, DM, antihypertensive drug use, HDL and non-HDL-C, FPG, 2hPCPG, Tehran Lipid and Glucose (TLGS) intervention measures (whether a patient was or was not assigned to lifestyle intervention measures in the TLGS study) | |
| 3 | Gao, 2013 | Mongolia, china | Cross-sectional | Originally designed | 2589(58.9) | 20–84 | HTN = 52.03 ± 12.05, Non-HTN = 43.21 ± 11.46 | N/A | Objectively | Student’s t-tests, χ2-test, Logistic regression, Wilcoxon rank-sum test | Age, current cigarette smoking, alcohol consumption, family history of HTN, FPG | |
| 4 | Ioachimescu, 2010 | USA | Retrospective cohort | preCIS database (Preventive Cardiology Information System) | 5924(39.2) | NA | 55 ± 13 | 5.3 | Objectively | Cox's proportional model | Age, sex, smoking status, history of DM, SBP, DBP, and fasting LDL-C and HDL-C | |
| 5 | Kavaric, 2018 | Serbia | Cross-sectional | Originally designed | 299(58.5) | NA | Control = 55.0, DM = 63.0 | N/A | Objectively | Mann–Whitney U test, Student’s t-tests, χ2-test, Spearman’s correlation analysis, Logistic regression | Age, LAP, hsCRP, ALT, GGT, uric acid, bilirubin, creatinine, eGFRMDRD, gender, smoking status, hypolipemics, and antihypertensive therapies | |
| 6 | Kim, 2018 | Korea | Prospective cohort | AnsungAnsan cohort database | 7643(52.9) | 40–69 | 51.7 ± 8.8 | 10 | Objectively | De Long’s test, Cox's proportional hazards regression | Age, sex, BMI, smoking, HTN, physical activity, energy intake | |
| 7 | Lee, 2018 | Korea | Prospective cohort | Korean Genome and Epidemiology Study | 7708(52.8) | 40–69 | 51.4 ± 8.6(M), 52.0 ± 8.9(F) | 10 | Objectively | χ2-test, Student’s t-tests, Multiple logistic regression | Age, BMI, HTN, family history of DM, current smoking and alcohol consumption status, and regular exercise | |
| 8 | Malavazos, 2015 | Italy | Cross-sectional | Originally designed | 381(77) | 18–70 | 41.3 ± 12.5 | N/A | Objectively | ANOVA, Kruskal–Wallis test, Logistic regression | Age, smoking status | |
| 9 | Marcadenti, 2017 | Brazil | Cross-sectional | Originally designed | 430(66.3) | HTN | 18–80 | 58.3 ± 11.7 | N/A | Objectively | Student’s t-tests, Pearson's χ2-test, Shapiro-Wilks, Levene, C-statistics, Poisson regression | Gender, age, physical activity, smoking, and BMI |
| 10 | Namazi Shabestari, 2016 | Iran | Cross-sectional | Originally designed | 264(100) | Menopausal women | ≥ 40 | 53.98 ± 5.57 | N/A | Objectively | Student’s t-tests, Man-Whitney U test, Pearson's correlation, Kolomogrov-Smirnov test | Age |
| 11 | Song, 2018 | China | Cross-sectional | Originally designed | 1777(57.9) | NA | Non-HTN = 60.33 ± 11.38, HTN = 62.31 ± 10.64 | N/A | Objectively | Wilcoxon rank-sum test, Student’s t-tests, Kruskal–Wallis H, χ2-test, Multivariate logistic regression, Statistic of Z | Age, BMI, WHtR, smoking status, family history of HTN, educational level, marital status, and family income | |
| 12 | Wakabayashi, 2014 | Japan | Cross-sectional | Originally designed | 54,477 (34.5) | 35–70 | 48.5 ± 7.7 | N/A | Objectively | χ2-test, Student’s t-tests, ANOVA, Sheffe F test, Kruskal–Wallis H, Post-hoc test, Mann–Whitney U test, Steel–dwass, Jonckheere-terpstra, Pearson's correlation | History of smoking, alcohol drinking, regular exercise, and drug therapy for DM | |
| 13 | Wakabayashi, 2014 | Japan | Cross-sectional | Originally designed | 10,170(32.1) | 35–40 | 37.5 ± 1.8(F), 37.4 ± 1.7(M) | N/A | Objectively | Mann–Whitney U test, Student’s t-tests, χ2-test, Logistic regression | Age, smoking, alcohol consumption, regular exercise | |
| 14 | Wang, B., 2018 | China | Prospective cohort | Originally designed | 11,113(61.6) | ≥ 18 | 50 ± 9 | 6 | Objectively | Wilcoxon rank-sum test, Cox's proportional hazards regression | Age, family history of DM, family history of HTN, education level, marital status, smoking, alcohol consumption, physical activity, SBP | |
| 15 | Wang, H., 2018 | China | Cross-sectional | Originally designed | 11,258(54.0) | ≥ 35 | 54 | N/A | Objectively | Mann–Whitney U test, Student’s t-tests, χ2-test, Linear regression | Age, race, educational status, family income, salt intake, cigarette smoking, alcohol consumption, and physical activity, FPG, eGFR, history of CVD, and any medication used | |
| 16 | Wehr, 2011 | Germany | Prospective cohort | LUdwigshafen RIsk and Cardiovascular Health (LURIC) study | 875(100) | Post-menopausal women (who were scheduled for coronary angiography or presenting with coronary angiography) | NA | NA | 7.7 | Objectively | Kolmogorov–Smirnov test, ANOVA, χ2-test | Age, BMI, DM, arterial HTN, HDL, LDL, active smoking, CRP, and lipid-lowering medication |
| 17 | Rotter, 2017 | Poland | Cross-sectional | Originally designed | 313(0) | 50–75 | 61.3 ± 6.3 | N/A | Objectively | Shapiro–Wilk test, Mann–Whitney U test, ANOVA, Pearson's correlation, Logistic regression, Linear regression | Age, SHBG, HOMA-IR | |
| 18 | Bala, 2019 | Romania | Cross-sectional | Originally designed | 1730(53.4) | 18–80 | Non-HTN = 41.3 ± 16.2, HTN = 54.4 ± 16.0 | N/A | Objectively | Student’s t-tests, Mann–Whitney U test, χ2-test, Logistic regression | Age, gender, smoking, drinking, and sedentary lifestyle, eGFR, urinary sodium (spot), urinary albumin creatinine ratio | |
| 19 | Ngoc, 2019 | Thailand | Cross-sectional | National Health Examination Survey 2009 | 15,842(52.6) | ≥ 35 | 59.3 ± 13.2 | N/A | Objectively | Student’s t-tests, χ2-test, Man-Whitney U test, Linear regression | Age, living area, education background, cigarette smoking within 12 months and regular smoking, alcohol consumption, alcohol consumption level, and physical activity, log of FPG, HDL-C, and LDL-C level | |
| 20 | Kahn, 2012 | USA | Cohort | Third National Health and Nutrition Examination Survey | 11,437(51.79) | 18–64 | 38.1 ± 0.3 | Up to 18.1 | Objectively | Cox's proportional model, χ2-test | Age, black ancestry, tobacco exposure, and socioeconomic position | |
| 21 | Brahimaj, 2019 | Netherlands | Prospective cohort | Rotterdam study | 9564(58.3) | ≥ 55 | 65.1 ± 10.3(F), 64.3 ± 9.5(M) | 6.5 | Objectively | χ2 test, Cox's proportional hazards models | Age, cohort, BMI, SBP, treatment for HTN, smoking and prevalent CVD, HDL-C, TG and serum lipid-reducing agents, FPG | |
| 22 | Shi, 2018 | China | Cross-sectional | Originally designed | 11,478(53.8) | ≥ 35 | NA | N/A | Objectively | Student’s t-tests, Mann–Whitney tests, χ2-test, Wilcoxon rank-sum tests | Age, race, education levels, income levels, and physical activity | |
| 23 | Sun, 2019 | China | Cross-sectional | Originally designed | 9496(71.65) | ≥ 40 | 55.9 ± 8.1 | N/A | Objectively | χ2-test, ANOVA, Linear regression, Pearson’s correlation | Age, sex, current smoking and drinking status, physical activity level, SBP, LDL-C, γ-GGT, eGFR, and antidiabetic treatment | |
| 24 | Wang, 2019 | China | Retrospective cohort | Originally designed | 687(41.92) | NA | 48.1 ± 6.2(1992), 63.1 ± 6.2(2007) | 15 | Objectively | Mann–Whitney U test, Student’s t-tests, χ2-test, Cox's proportional regression | Age, gender, cigarette consumption, alcohol intake, log10-SBP, log10-total cholesterol, and log10-TG | |
| 25 | Yan, 2019 | China | Retrospective cohort | Originally designed | 4508(45.9) | > 18 | 42 | 5 | Objectively | ANOVA | Baseline age, gender, race, current smoking, current alcohol drinking, and married status, baseline diagnosis of HTN, use of antihypertensive, total cholesterol, HDL, hemoglobin, creatinine, and uric acid, Baseline blood glucose, and family history of DM | |
| 26 | Huang, 2019 | China | Cross-sectional | Originally designed | 2079(51.8) | NA | 41.06 | N/A | Objectively | Kruskal–Wallis H, χ2-test, Multivariate logistic regression | Age, sex, marital status and educational level, physical activity, smoker, drinker, BMI, WHR, FPG, family history of HTN | |
| 27 | Tian Tian, 2020 | China | Cross-sectional | National Physical Examination Project | 215,651(55.86) | NA | 50.02 | N/A | Objectively | Pearson’s χ2, Student’s t-tests, Logistic regression | Age, gender, education, smoking, and alcohol consumption | |
| 28 | Xu, 2020 | China | Prospective cohort | Originally designed | 15,717(58.2) | > 35 | 52.70 ± 11.58 | 7.77 | Objectively | Cox’s proportional regression | Age, sex, smoking status, drinking status, physical activity, family history of DM, family income, and education | |
| 29 | Wang, 2021 | China | Cross-sectional | Chinese National Stroke Prevention Project | 162,880(54.47) | ≥ 40 | 59.24 ± 11.04 | N/A | Objectively |
χ2 test, two-level logistic regression model, Student’s t-tests |
Age, physical exercise, smoking, alcohol consumption, BMI, WC, LAP, VAI, and BAI |
2hPCPG, 2 h post-challenge plasma glucose; ALT, Alanine transaminase; BAI, Body adiposity index; BMI, Body mass index; CRP, C-reactive protein; CVD, cardiovascular diseases; DBP, Diastolic blood pressure; DM, Diabetes mellitus; eGFR, Estimated glomerular filtration rate; FPG, Fasting plasma glucose; GGT, Gamma-glutamyl transferase; HDL, High-density lipoproteins; HDL-C, HDL cholesterol; HOMA-IR, Homeostatic Model Assessment for Insulin Resistance; hsCRP, high-sensitivity C-reactive protein; HTN, Hypertension; LAP, Lipid accumulation product; LDL, Low-density lipoproteins; LDL-C, LDL cholesterol; SBP, Systolic blood pressure; SHBG, Sex hormone binding globulin; TG, Triglyceride; VAI, Visceral adiposity index; WC, Waist circumference; WHR, Waist-hip ratio; WHtR, Waist-to-height ratio;
Table 3 illustrates study outcomes with their statistical measures. Study outcomes are hypertension, mortality, and diabetes. Statistical measures include OR, AUC, HR, RR, and Poisson regression.
Table 3.
Reported outcomes and measures of the included studies
| Study ID | Study outcome | Outcome assessment | LAP | BMI | WC | WHR | WHtR | VAI | BAI | Other |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | DM prevalence | AROC |
M (20–49 years): 0.75 M(≥ 50 years): 0.81 F(20–49 years): 0.81 F(≥ 50 years): 0.72 |
M(20–49 years): 0.7 M(≥ 50 years): 0.76 F(20–49 years): 0.76 F(≥ 50 years): 0.65 |
M(20–49 years): 0.74 M(≥ 50 years): 0.78 F(20–49 years): 0.78 F(≥ 50 years): 0.68 |
M(20–49 years): 0.74 M(≥ 50 years): 0.79 F(20–49 years): 0.79 F(≥ 50 years): 0.68 |
||||
| DM incidence | AROC |
M(20–49 years): 0.66 M(≥ 50 years): 0.71 F(20–49 years): 0.78 F(≥ 50 years): 0.65 |
M(20–49 years): 0.66 M(≥ 50 years): 0.69 F(20–49 years): 0.76 F(≥ 50 years): 0.63 |
M(20–49 years): 0.67 M(≥ 50 years): 0.70 F(20–49 years): 0.77 F(≥ 50 years): 0.64 |
M(20–49 years): 0.66 M(≥ 50 years): 0.69 F(20–49 years): 0.79 F(≥ 50 years): 0.65 |
|||||
| DM prevalence | OR(95%CI) |
M(20–49 years): 1.4[1.2–1.6] M(≥ 50 years): 1.5[1.3–1.8] F(20–49 years): 2.1[1.8–2.5] F(≥ 50 years): 1.5[1.3–1.8] |
M(20–49 years): 1.3 [1.1–1.5] M(≥ 50 years): 1.6 [1.3–1.9] F(20–49 years): 1.6 [1.5–1.9] F(≥ 50 years): 1.3 [1.1–1.4] |
M(20–49 years): 1.7 [1.4–2.1] M(≥ 50 years): 1.6 [1.3–1.9] F(20–49 years): 1.8 [1.6–2.1] F(≥ 50 years): 1.1 [1.0–1.3] |
M(20–49 years): 1.5 [1.3–1.8] M(≥ 50 years): 1.6 [1.3–1.9] F(20–49 years): 1.9 [1.3–2.1] F(≥ 50 years): 1.3 [1.1–1.5] |
|||||
| DM incidence | OR(95%CI) |
M(20–49 years): 1.7[1.2–2.5] M(≥ 50 years): 1.7[1.1–2.6] F(20–49 years): 2.6[1.9–3.6] F(≥ 50 years): 2.1[1.3–3.3] |
M(20–49 years): 1.3 [0.9–1.8] M(≥ 50 years): 1.5 [1.0–2.2] F(20–49 years): 1.9 [1.5–2.4] F(≥ 50 years): 1.5 [1.1–2.1] |
M(20–49 years): 1.7 [1.0–2.7] M(≥ 50 years): 1.5 [0.9–2.4] F(20–49 years): 2.2 [1.7–2.9] F(≥ 50 years): 1.6 [1.1–2.3] |
M(20–49 years): 1.4 [1.0–2.1] M(≥ 50 years): 1.5 [1.0–2.3] F(20–49 years): 2.3 [1.8–3.0] F(≥ 50 years): 1.9 [1.3–2.8] |
|||||
| 2 | All-cause mortality | HR(95% CI) |
M: 0.74 [0.61–0.90] F: 0.88 [0.60–1.30] |
|||||||
| 3 | Hypertension | OR(95%CI) |
M Q1: Ref Q2: 1.85 [1.23–2.79] Q3: 2.20 [1.47–3.28] Q4: 4.21 [2.78–6.38] F Q1: Ref Q2: 1.90 [1.28–2.81] Q3: 2.29 [1.56–3.36] Q4: 3.33 [2.26–4.89] |
F Q1: Ref Q2: 2.06 [1.40–3.03] Q3: 2.03 [1.39–2.96] Q4: 4.51 [3.10–6.55] |
F Q1: Ref Q2: NS Q3: 1.84 [1.28–2.65] Q4: 2.89 [2.03–4.13] |
|||||
| 4 | All-cause mortality | HR(95% CI) |
M: 1.38 [1.15–1.66] F: 1.61 [1.19–2.16] |
|||||||
| 5 | DM | AUC(95% CI) | 0.716 [0.657–0.776] | 0.667 [0.603–0.732] | 0.715 [0.653–0.777] | 0.707 [0.647–0.776] | ||||
| DM development | OR(95%CI) | 1.016 [1.010–1.021] | 1.140 [1.079–1.205] | 1.068 [1.046–1.091] | 1.292 [1.133–1.474] | |||||
| 6 | DM | AUC(95% CI) | 0.642 [0.625–0.658] | 0.622 [0.605–0.639] |
TyG index: 0.672 [0.656–0.687] |
|||||
| DM | HR(95% CI) | 1.87[1.64–2.14] | 1.75 [1.55–1.96] |
TyG index: 2.17 [1.92–2.45] |
||||||
| 7 | DM | AUC(95% CI) |
M: 0.602 [0.586–0.618] F: 0.623 [0.607–0.637] |
M: 0.579 [0.563–0.595] F: 0.576 [0.561–0.592] |
TyG index M: 0.623 [0.607–0.638] F: 0.644 [0.629–0.659] |
|||||
| DM incidence | OR(95%CI) |
M Q1: ref Q2: 1.04 [0.79–1.36] Q3: 1.70 [1.28–2.25] Q4: 2.47 [1.82–3.34] F Q1: ref Q2: 1.26 [0.97–1.64] Q3: 1.35 [1.03–1.78] Q4: 2.44 [1.82–3.26] |
M Q1: ref Q2: 1.07 [0.81–1.40] Q3: 1.35 [1.00–1.83] Q4: 1.64 [1.13–2.38] F Q1: ref Q2: 1.14 [0.88–1.48] Q3: 1.27 [0.95–1.69] Q4: 1.17 [0.83–1.65] |
TyG index: M Q1: ref Q2: 1.26 [0.97–1.64] Q3: 1.82 [1.41–2.36] Q4: 2.79 [2.16–3.60] F Q1: ref Q2: 1.19 [0.91–1.55] Q3: 1.97 [1.53–2.53] Q4: 2.85 [2.22–3.66] |
||||||
| 8 | DM | AUC(95% CI) | 0.77 [0.72–0.81] | 0.66 [0.61–0.71] | ||||||
| DM identifying abnormalities | OR(95%CI) | 3.17 [1.75–5.77] | 1.33 [0.83–2.15] | |||||||
| 9 | DM | Poisson regression (95% CI) |
M Q1: 1.07 [0.47–2.41] Q2: 0.69 [0.33–1.42] Q3: 1.42 [0.85–2.37] Q4: 1 F Q1: 0.34 [0.19–0.62] Q2: 0.53 [0.34–0.82] Q3: 0.55 [0.35–0.85] Q4: 1 |
M(< P75): 1 M(> P75): 1.61 [1.04–2.49] F(< P75): 1 F(> P75): 0.89 [0.62–1.30] |
NC: M Q1: 1 Q2: 1.07 [0.55–2.07] Q3: 1.23 [0.62–2.44] Q4: 1.44 [0.69–3.03] F Q1: 1 Q2: 1.51 [0.82–2.79] Q3: 1.67 [0.90–3.11] Q4: 3.30 [1.78–6.14] |
|||||
| 10 | Hypertension | OR(95%CI) | 2.07 [1.24–3.47] | |||||||
| 11 | Hypertension risk | AUC(95% CI) |
M: 0.66 [0.62–0.69] F: 0.70 [0.67–0.73] |
M: 0.61 [0.57–0.64] F: 0.63 [0.60–0.66] |
M: 0.67 [0.63–0.70] F: 0.66 [0.63–0.69] |
|||||
| Hypertension risk | OR(95%CI) |
Q1: ref Q2: 1.91 [1.26–2.90] Q3: 2.32 [1.44–3.74] Q4: 3.31 [1.76–6.25] |
||||||||
| 12 | DM prevalence | OR(95%CI) |
M(35–39 years): 6.36 [4.11–9.82] M(40–49 years): 3.43 [2.84–4.15] M(50–59 years): 2.05 [1.81–2.34] M(60–70 years): 1.53 [1.28–1.82] F(35–39 years): 7.00 [4.44–11.04] F(40–49 years): 5.33 [4.42–6.42] F(50–59 years): 2.99 [2.63–3.40] F(60–70 years): 1.89 [1.47–2.41] |
|||||||
| 13 | DM prevalence | OR(95%CI) |
M: 7.40 [5.10–10.75] F: 19.09 [6.57–55.50] |
|||||||
| Hypertension | OR(95%CI) |
M: 7.31 [6.20–8.62] F: 10.66 [7.77–14.63] |
||||||||
| 14 | DM | AUC(95% CI) |
M: 0.653 [0.638–0.667] F: 0.693 [0.682–0.704] |
M: 0.654 [0.640–0.669] F: 0.669 [0.657–0.680] |
M: 0.622 [0.607–0.636] F: 0.654 [0.642–0.665] |
TyG index: M: 0.625 [0.610–0.639] F: 0.669 [0.657–0.680] |
||||
| DM | HR(95% CIs) |
M Q1: 1 Q2: 1.59 [0.84–3.01] Q3: 2.12 [1.15–3.91] Q4: 5.02 [2.85–8.85] F Q1: 1 Q2: 2.42 [1.23–4.74] Q3: 3.65 [1.92–6.92] Q4: 6.49 [3.48–12.12] |
M Q1: 1 Q2: 1.06 [0.58–1.94] Q3: 1.49 [0.82–2.69] Q4: 4.25 [2.51–7.21] F Q1: 1 Q2: 1.74 [0.96–3.16] Q3: 1.97 [1.09–3.56] Q4: 4.07 [2.36–7.03] |
M Q1: 1 Q2: 1.65 [0.94–2.89] Q3: 1.49 [0.84–2.64] Q4: 2.89 [1.72–4.87] F Q1: 1 Q2: 1.75 [0.99–3.10] Q3: 2.13 [1.22–3.74] Q4: 4.40 [2.61–7.42] |
TyG index: M Q1: 1 Q2: 1.59 [0.88–2.88] Q3: 2.22 [1.27–3.88] Q4: 3.54 [2.08–6.03] F Q1: 1 Q2: 2.50 [1.36–4.60] Q3: 3.12 [1.72–5.67] Q4: 6.15 [3.48–10.85] |
|||||
| 15 | Hypertension | AUC(95% CI) |
M: 0.627 [0.614–0.641] F: 0.678 [0.666–0.690] |
M: 0.620 [0.607–0.634] F: 0.637 [0.625–0.649] |
M: 0.638 [0.625–0.652] F: 0.655 [0.643–0.667] |
M: 0.564 [0.550–0.577] F: 0.621 [0.608–0.633] |
M: 0.639 [0.625–0.652] F: 0.654 [0.642–0.666] |
CMI: M: 0.574 [0.560–0.587] F: 0.635 [0.622–0.647] |
||
| Hypertension | OR(95%CI) |
M Q1: ref Q2: 1.643 [1.385–1.949] Q3: 2.302 [1.934–2.741] Q4: 3.892 [3.238–4.677] per SD: 1.651 [1.503–1.813] F Q1: ref Q2: 1.562 [1.325–1.841] Q3: 2.264 [1.919–2.670] Q4: 3.548 [2.985–4.217] per SD: 1.631 [1.501–1.771] |
M Q1: ref Q2: 1.673 [1.412–1.982] Q3: 2.420 [2.039–2.873] Q4: 3.288 [2.754–3.927] per SD: 1.528 [1.427–1.637] M Q1: ref Q2: 1.636 [1.390–1.926] Q3: 2.130[1.808–2.508] Q4: 3.004 [2.537–3.557] per SD: 1.555 [1.454–1.662] |
CMI: M Q1: ref Q2: 1.024 [0.864–1.214] Q3: 1.420 [1.197–1.685] Q4: 2.200 [1.838–2.635] per SD: 1.310 [1.204–1.425] F Q1: ref Q2: 1.279 [1.087–1.504] Q3: 1.641 [1.394–1.932] Q4: 2.318 [1.956–2.745] per SD: 1.356 [1.259–1.459] |
||||||
| 16 | All-cause mortality | HR (95% CI) |
F T1: 1 T2: 1.23 [0.82–1.84] T3: 1.43 [0.91–2.25] per SD: 1.19 [0.86–1.64] |
|||||||
| DM | OR(95%CI) |
M T1: 1 T2: 1.39 [1.09–1.78] T3: 2.16 [1.66–2.81] F T1: 1 T2: 2.29 [1.50–3.50] T3: 5.03 [3.21–7.89] |
||||||||
| 17 | DM | OR(95%CI) | 1.012 [1.006–1.017] | |||||||
| Hypertension | OR(95%CI) | 1.014 [1.007–1.020] | ||||||||
| 18 | Hypertension | OR(95%CI) | 2.09 [1.60–2.73] | 1.94 [1.48–2.53] |
TyG index: 1.83 [1.39–2.41] |
|||||
| 19 | Hypertension | AUC(95% CI) |
M: 0.632 [0.620–0.645] F: 0.646 [0.634–0.658] total: 0.636 [0.627–0.645] M(35–49 years): 0.660 [0.631–0.689] M(50–64 years) 0.665 [0.644–0.687] M(≥ 65) 0.647 [0.628–0.667] F(35–49 years) 0.707 [0.681–0.733] F(50–64 years) 0.646 [0.625–0.666] F(≥ 65 years) 0.609 [0.589–0.629] total(35–49 years): 0.681 [0.661–0.701] total(50–64 years): 0.653 [0.638–0.668] total(≥ 65): 0.630 [0.616–0.644] |
M: 0.624 [0.611–0.637] F: 0.591 [0.579–0.604] total: 0.603 [0.594–0.612] M(35–49 years): 0.653 [0.622–0.683] M(50–64 years): 0.674 [0.652–0.695] M(≥ 65 years): 0.654 [0.635–0.674] F(35–49 years): 0.685 [0.657–0.712] F(50–64 years): 0.638 [0.617–0.658] F(≥ 65 years): 0.616 [0.597–0.636] total(35–49 years): 0.657 [0.636–0.678] total(50–64 years): 0.651 [0.636–0.666] total(≥ 65 years): 0.636 [0.622–0.650] |
M: 0.651 [0.638–0.664] F: 0.615 [0.603–0.628] total: 0.633 [0.624–0.641] M(35–49 years): 0.660 [0.630–0.689] M(50–64 years): 0.683 [0.662–0.704] M(≥ 65 years): 0.658 [0.638–0.677] F(35–49 years): 0.689 [0.662–0.717] F(50–64 years): 0.632 [0.612–0.653] F(≥ 65 years): 0.608 [0.588–0.628] total(35–49 years): 0.677 [0.656–0.697] total(50–64 years): 0.656 [0.642–0.671] total(≥ 65 years): 0.632 [0.618–0.645] |
M: 0.650 [0.637–0.662] F: 0.605 [0.593–0.618] total: 0.620 [0.611–0.629] M(35–49 years): 0.652 [0.622–0.681] M(50–64 years): 0.663 [0.641–0.684] M(≥ 65 years): 0.623 [0.604–0.643] F(35–49 years): 0.649 [0.620–0.677] F(50–64 years): 0.590 [0.569–0.612] F(≥ 65 years): 0.568 [0.547–0.588] total(35–49 years): 0.657 [0.637–0.677] total(50–64 years): 0.612 [0.597–0.627] total(≥ 65 years): 0.585 [0.570–0.599] |
M: 0.658 [0.646–0.671] F: 0.632 [0.620–0.644] total: 0.640 [0.631–0.649] M(35–49 years): 0.662 [0.633–0.692] M(50–64 years): 0.680 [0.659–0.701] M(≥ 65 years): 0.651 [0.631–0.670] F(35–49 years): 0.686 [0.659–0.714] F(50–64 years): 0.635 [0.614–0.656] F(≥ 65 years): 0.610 [0.590–0.630] total(35–49 years): 0.661 [0.640–0.681] total(50–64 years): 0.653 [0.638–0.667] total(≥ 65 years): 0.632 [0.618–0.645] |
M: 0.555 [0.542–0.569] F: 0.618 [0.606–0.630] total: 0.586 [0.577–0.595] M(35–49 years): 0.594 [0.564–0.625] M(50–64 years): 0.564 [0.542–0.587] M(≥ 65 years): 0.557 [0.537–0.578] F(35–49 years): 0.650 [0.622–0.677] F(50–64 years): 0.589 [0.568–0.610] F(≥ 65 years): 0.551 [0.531–0.572] total(35–49 years): 0.623 [0.602–0.644] total(50–64 years): 0.577 [0.562–0.593] total(≥ 65 years): 0.559 [0.545–0.574] |
M: 0.614 [0.601–0.627] F: 0.607 [0.595–0.620] total: 0.578 [0.569–0.587] M(35–49 years): 0.618 [0.587–0.648] M(50–64 years): 0.628 [0.606–0.650] M(≥ 65 years): 0.617 [0.597–0.637] F(35–49 years): 0.640 [0.610–0.670] F(50–64 years): 0.616 [0.595–0.637] F(≥ 65 years): 0.599 [0.579–0.619] total(35–49 years): 0.560 [0.538–0.582] total(50–64 years): 0.593 [0.578–0.609] total(≥ 65 years): 0.597 [0.583–0.611] |
CI: M: 0.649 [0.636–0.662] F: 0.614 [0.601–0.626] total: 0.630 [0.621–0.639] M(35–49 years): 0.637 [0.607–0.667] M(50–64 years): 0.646 [0.624–0.668] M(≥ 65 years): 0.617 [0.597–0.637] F(35–49 years): 0.624 [0.594–0.654] F(50–64 years): 0.588 [0.567–0.610] F(≥ 65 years): 0.556 [0.535–0.576] total(35–49 years): 0.634 [0.614–0.655] total(50–64 years): 0.614 [0.599–0.629] total(≥ 65 years): 0.584 [0.570–0.599] |
| Hypertension | OR(95%CI) |
Q1: 1 ref Q2: 1.804 [1.621–2.008] Q3: 2.704 [2.425–3.015] Q4: 4.251 [3.792–4.765] per SD: 1.602 [1.535–1.671] cutoff > 24.44: 2.461 [2.277–2.660] |
Q1: 1 ref Q2: 1.527 [1.375–1.697] Q3: 2.289 [2.060–2.544] Q4: 3.742 [3.355–4.174] per SD: 1.688 [1.623–1.756] cutoff > 81.58: 2.360 [2.191–2.542] |
Q1: 1 ref Q2: 1.616 [1.453–1.797] Q3: 2.343 [2.105–2.607] Q4: 3.525 [3.162–3.931] per SD: 1.629 [1.567–1.694] cutoff > 0.52: 2.170 [2.016–2.336] |
CI: Q1: 1 ref Q2: 1.251 [1.129–1.387] Q3: 1.705 [1.540–1.888] Q4: 2.140 [1.929–2.373] per SD: 1.343 [1.293–1.394] cutoff > 1.21: 1.693 [1.576–1.818] |
|||||
| 20 | All-cause mortality | HR(95%CI) |
M per SD linear: 1.22 [0.95–1.55] M(at p25): 1.03 [0.72–1.49] M(at p75): 1.11 [0.66–1.85] F per SD linear: 1.27 [1.02–1.57] F(at p25): 1.26 [0.75–2.15] F(at p75): 1.48 [0.90–2.43] |
|||||||
| 21 | DM incidence | HR(95% CI) |
F: 1.08 [0.93- 1.26] M: 0.96 [0.81- 1.15] |
|||||||
| 22 | discriminate DM | AUC(95% CI) |
F: 0.717 [0.706–0.729] M: 0.683 [0.670–0.696] |
|||||||
| 23 | DM prevalence | AUC(95% CI) | 0.658 [0.645–0.671] | |||||||
| 24 | DM incidence | HR(95% CI) |
univariate per SD: 2.16 [1.65–2.84] Q1: 1 ref Q2: 1.11 [0.45–2.74] Q3: 1.71 [0.75–3.91] Q4: 4.98 [2.42–10.26] multivariate per SD: 2.06 [1.56–2.73] Q1: 1 ref Q2: 1.17 [0.47–2.89] Q3: 1.66 [0.72–3.83] Q4: 4.70 [2.20–9.952] |
|||||||
| 25 | DM incidence | RR(95% CI) |
T1: (< 12.7): ref T2: (12.7 ≤ ~ < 29.3): 1.03 (0.52–2.03) T3: (≥ 29.3):1.91 (0.97–3.74) |
|||||||
| 26 | Hypertension | AUC(95% CI) |
M: 0.677 [0.640–0.713] F: 0.721 [0.680–0.761] |
M: 0.707 [0.672–0.742] F: 0.698 [0.658–0.737] |
M: 0.734 [0.700–0.769] F: 0.725 [0.686–0.766] |
|||||
| Hypertension | OR(95% CI) |
M Q1: ref Q2: 1.61 [0.89–2.94] Q3: 1.75 [0.94–3.26] Q4: 2.79 [1.43–5.44] F Q1: ref Q2: 1.015 [0.51–2.03] Q3: 1.19 [0.60–2.38] Q4: 3.15 [1.56–6.39] |
||||||||
| 27 | DM prevalence | AUC(95% CI) |
total: 0.655 [0.652–0.658] M: 0.625 [0.621–0.630] F: 0.679 [0.674–0.684] |
total: 0.604 [0.600–0.607] M: 0.580 [0.576–0.586] F: 0.618 [0.614–0.623] |
||||||
| DM prevalence | COR(95%CI) |
Q1: ref Q2: 1.28 [1.22–1.34] Q3: 1.86 [1.78–1.95] Q4: 4.67 [4.49–4.86] |
||||||||
| DM prevalence | AOR(95%CI) |
Q1: ref Q2: 0.97 [0.92–1.02] Q3: 1.28 [1.23–1.34] Q4: 3.24 [3.11–3.37] |
||||||||
| 28 | DM incidence | HR(95% CI) |
total Q1: ref Q2: 1.169 [0.857–1.595] Q3: 2.903 [2.226–3.784] Q4: 6.298 [4.911–8.077] M Q1: ref Q2: 1.123 [0.719–1.752] Q3: 1.839 [1.230–2.748] Q4: 4.773 [3.324–6.854] F Q1: ref Q2: 1.633 [1.073–2.485] Q3: 4.150 [2.865–6.013] Q4: 8.063 [5.645–11.516] |
|||||||
| 29 | Hypertension | OR(95%CI) |
Total: 1.289 [1.275–1.303] M: 1.316 [1.294–1.338] F: 1.294 [1.266–1.313] |
Total: 1.539 [1.514–1.566] M: 1.439 [1.413–1.465] F: 1.510 [1.479–1.543] |
Total: 1.389 [1.372–1.406] M: 1.733 [1.685–1.782] F: 1.435 [1.413–1.459] |
Total: 1.146 [1.133–1.159 M: 1.141 [1.120–1.162] F: 1.131 [1.115–1.147] |
Total: 1.317 [1.301–1.333] M: 1.297 [1.271–1.323] F: 1.343 [1.322–1.365] |
|||
| AUC(95%CI) |
Total: 0.679 [0.675–0.683] M: 0.670 [0.666–0.674] F: 0.688 [0.684–0.691] |
Total: 0.695 [0.690–0.699] M: 0.679 [0.675–0.683] F: 0.709 [0.706–0.713] |
Total: 0.696 [0.693–0.700] M: 0.693 [0.689–0.696] F: 0.698 [0.695–0.702] |
Total: 0.654 [0.650–0.658] M: 0.645 [0.641–0.649] F: 0.662 [0.659–0.666] |
Total: 0.675 [0.672–0.679] M: 0.661 [0.658–0.665] F: 0.689 [0.679–0.693] |
95%CI, 95% confidence interval; AOR, adjusted odds ratio; AUC, area under receiver operating characteristic curve; BAI, body adipose index; BMI, body mass index; CI, conicity index; CMI, cardiometabolic index; COR, crude odds ratio; DM, diabetes mellitus; F, female; HR, hazard ratios; LAP, lipid accumulation product; M, male; NC, neck circumference; OR, odds ratio; Q, quartile; T, tertile; TyG, triglyceride-glucose; VAI, visceral adiposity index; WC, waist circumference; WHR, waist-to-hip ratio; WHtR, waist-to-height ratio;
Study quality assessment
The result of the study quality assessments is summarized in Table 4. Overall, based on the NIH criteria, 16 studies scored as good, nine studies as fair, and three studies as poor. However, we decided to include all the studies.
Table 4.
Quality assessment of the included studies
| Study ID Criteria | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 | 14 | 15 | 16 | 17 | 18 | 19 | 20 | 21 | 22 | 23 | 24 | 25 | 26 | 27 | 28 | 29 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Was the research question or objective in this paper clearly stated? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 2. Was the study population clearly specified and defined? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 3. Was the participation rate of eligible persons at least 50%? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 4. Were all the subjects selected or recruited from the same or similar populations (including the same time period)? Were inclusion and exclusion criteria for being in the study prespecified and applied uniformly to all participants? | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | No | Yes | Yes | Yes | CD | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 5. Was a sample size justification, power description, or variance and effect estimates provided? | NR | No | No | No | NR | NR | NR | NR | Yes | NR | No | NR | NR | NR | Yes | NR | No | No | No | NR | NR | NR | NR | NR | NR | NR | NR | NR | NR |
| 6. For the analyses in this paper, were the exposure(s) of interest measured prior to the outcome(s) being measured? | Yes | Yes | No | Yes | No | Yes | Yes | No | No | No | No | No | No | Yes | No | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | No | No | Yes | No |
| 7. Was the timeframe sufficient so that one could reasonably expect to see an association between exposure and outcome if it existed? | Yes | Yes | No | CD | No | Yes | Yes | No | No | No | No | No | No | Yes | No | Yes | No | No | No | Yes | Yes | No | No | Yes | Yes | No | No | Yes | No |
| 8. For exposures that can vary in amount or level, did the study examine different levels of the exposure as related to the outcome (e.g., categories of exposure, or exposure measured as continuous variable)? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | No | Yes | No | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 9. Were the exposure measures (independent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 10. Was the exposure(s) assessed more than once over time? | No | No | No | No | No | Yes | No | No | No | No | No | No | No | No | No | No | No | Yes | No | No | No | No | No | Yes | No | No | No | No | No |
| 11. Were the outcome measures (dependent variables) clearly defined, valid, reliable, and implemented consistently across all study participants? | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes |
| 12. Were the outcome assessors blinded to the exposure status of participants? | NR | NR | NA | NR | NR | NR | NR | NR | NR | NA | NA | NR | NA | NR | NA | NR | CD | NA | NA | NR | NR | NR | NR | NR | NR | NR | NR | NR | Yes |
| 13. Was loss to follow-up after baseline 20% or less? | No | Yes | NA | Yes | Yes | Yes | Yes | Yes | Yes | NR | Yes | Yes | Yes | No | Yes | Yes | NR | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| 14. Were key potential confounding variables measured and adjusted statistically for their impact on the relationship between exposure(s) and outcome(s)? | No | Yes | Yes | Yes | Yes | CD | CD | No | Yes | No | Yes | No | No | Yes | Yes | Yes | No | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
| Result: Study Quality | Fair | Good | Fair | Good | Poor | Good | Good | Fair | Good | Poor | Good | Fair | Fair | Fair | Good | Good | Poor | Good | Good | Good | Good | Good | Good | Fair | Good | Fair | Fair | Good | Good |
*CD, Cannot determine; NA, Not applicable; NR, Not reported
Mortality
Four articles studied the association of LAP with all-cause mortality [15, 24–26]. Two of them also assessed other adiposity indicators to compare their predictability power for mortality with each other [24, 26]. One of the studies showed an inverse association between LAP and all-cause mortality after adjustment [24], while the others showed positive association only in specific subgroups [15, 25, 26]. Bozorgmanesh et al. (2010) evaluated the predictive performance of LAP for all-cause mortality and compared LAP with other anthropometric measures. They assessed HR to describe the contribution of LAP to the risk of all-cause mortality for one SD increment, and LAP was in natural logarithm transformed. The results surprisingly revealed that LAP after adjustment is inversely associated with all-cause mortality, which was only statistically meaningful for males. Besides, LAP was no better predictor in comparison with other anthropometric measures [24]. Ioachimescu et al. (2010) examined the association of LAP with all-cause mortality among patients with high cardiovascular risk and compared it with BMI. They assessed HR to describe the contribution of LAP to the risk of all-cause mortality for one SD increment, and LAP was in natural logarithm transformed. The results indicated that after adjustment, LAP is significantly associated with all-cause mortality. Moreover, LAP in nondiabetic subgroups showed a statistically meaningful association with all-cause mortality, and no strong association in diabetic groups was detected. Also, the results revealed that LAP is a better predictor for all-cause mortality than BMI (8.2 vs. 5.4% mortality at 6 years) [15]. Kahn et al. (2012) compared the power of different anthropometric measures for predicting all-cause mortality in non-elderly adults. They assessed quartiles and SD for their statistical analysis. In multiple adjusted models, in black females, LAP showed a positive association with mortality at p75. In addition, Tobacco exposure in both sexes showed the highest mortality risk for LAP at p75. It is worth mentioning that in this article, considering all the results, LAP had a weak association with all-cause mortality [26]. Wehr et al. (2011) studied the association of LAP with mortality in post-menopausal women and men. They measured HR for tertile, first tertile as a reference, and HR for one SD increase in LAP. In model 1 and model 3, LAP showed a statistically significant association with all-cause mortality in post-menopausal women. However, there was no significant association between LAP and all-cause mortality in men. Moreover, they did not detect any association between BMI and all-cause mortality at all [25].
The meta-analysis was done for four studies [15, 24–26] in females and three studies in males [15, 24, 26] (Fig. 2). We found that the HR of all-cause mortality per one SD increment in LAP in females is 1.24 (95% CI [1.00–1.53]; P = 0.0463). We found a marginally non-significant heterogeneity between the four included studies (I2 = 50%, τ2 = 0.0231; P = 0.11) [15, 24–26]. Except for one study [24], others found a positive association between LAP increments and all-cause mortality in females. In the male subgroup, three studies [15, 24, 26] were included, and we found that one SD increment in LAP non-significantly increases the hazard of all-cause mortality (HR: 1.07; 95% CI [0.74–1.57]; P = 0.709); however, significant heterogeneity was detected (I2 = 91%, τ2 = 0.1004; P < 0.01). Similar to females, except for one study, others reported a positive association between LAP and all-cause mortality in males.
Fig. 2.
The HR of all-cause mortality per one SD increment of LAP in females and males
Hypertension
Ten studies evaluated the association of LAP with hypertension [27–36]. All of them found a positive and significant association between LAP and hypertension. All included studies measured OR for LAP. In addition, five articles also analyzed AUC for the association of anthropometric measures with hypertension [30, 32, 34–36].
For the association of LAP and hypertension, Song et al. reported the highest OR for the fourth quartile vs. The first quartile in both sexes (unadjusted OR: 6.35; 95% CI [4.39–9.12]) and for Q4 vs. Q1. Huang et al. similarly reported the highest OR for males (OR: 17.82; 95% CI (9.21–34.46]) and for females (Model 1 OR: 20.06 95% CI [11.37—35.38]) [28, 32, 34, 35]. The lowest OR for Q4 vs. Q1, for the association of LAP with hypertension, was reported by Huang et al. for males (Model 3 OR: 2.79; 95% CI [1.43—5.44]) and females (Model 3 OR: 3.15; 95% CI [1.56—6.39]) [35]. Ngoc et al. reported the lowest OR for 1 SD increase in both sexes (Model 3 OR: 1.602; 95% CI [1.535–1.671]). Ngoc et al. also analyzed AUC in each sex and different age subgroups. They reported the highest AUC for 35–49 years old females (AUC: 0.707; 95% CI [0.681–0.733]) and the lowest AUC for over 65 females (AUC:0.609; 95% CI [0.589–0.629]) [30]. Although the results of Gao (2013) et al. (in males) and Song (2018) et al. (in both sexes) studies indicated that LAP has a stronger association with hypertension in comparison to BMI, Bala et al. (2019) revealed that LAP has no better power than BMI and WC [27, 28, 32]. Song (2018) et al. demonstrated that LAP is a stronger index for hypertension than WHtR in females [32]. However, Ngoc (2019) et al. indicated that WHtR has a stronger association with hypertension (AUC: 0.640 95% CI [0.631– 0.649]) in comparison with other anthropometric measures (BMI, WC, WHR, VAI, BAI, CI, LAP) and LAP (AUC: 0.636; 95% CI [0.627–0.645]) is the second strong index in association with hypertension [30]. Moreover, another study revealed that LAP is a stronger index for hypertension than WC and VAI in females [34]. It is also worth mentioning that Song et al. found an association between LAP and hypertension family history [32].
Except for two papers, the others had a good or fair quality based on our quality assessment. Considering all the included papers, LAP is an appropriate predictor of hypertension in both males and females, but it seems that it has better predictability for hypertension in females compared with males. Additionally, most studies reported that LAP is a better predictor of hypertension than other anthropometric measures in at least one sex. LAP also has interactive effects with smoking and a family history of hypertension.
Diabetes mellitus
Eighteen articles assessed the association of LAP with incidence or prevalence of T2DM, and all of them revealed that LAP has a significantly positive association with T2DM [3, 25, 33, 37–51], with the exception of one study which demonstrated that LAP has a statistically meaningful association with diabetes only in hypertensive female groups [51]. The lowest OR for the association of LAP with the prevalence of diabetes was 1.012 (95% CI [1.006–1.017]) [43]. Lee et al. reported the lowest OR for Q4 vs. Q1 for females (adjusted OR: 2.44; 95% CI [1.82–3.26]) and males (adjusted OR: 2.47; 95% CI [1.82–3.34]) [41]. Wakabayashi et al. reported the highest OR in the prevalence of diabetes in females (adjusted OR:19.09; 95% CI [6.57–55.50]) [33]. Wakabayashi et al. and Bozorgmanesh et al. assessed the association of LAP with the prevalence of diabetes in specific age subgroups. In both studies, ORs were highest in the youngest age sub-groups in females. The OR for females in the first study is 7.00 (95% CI: 4.44–11.04) and 2.1 (95% CI: 1.8–2.5) in the latter. Also, OR in males was higher in younger age subgroups in the first study (crude OR: 6.85; 95% CI [4.45–10.56]) [37, 46]. Five studies evaluated HR for predicting diabetes with LAP [38, 40, 47, 48, 50]. The lowest HR in males and females was 0.96 (Model 5; 95% CI [0.81, 1.15]) and 9.058 (unadjusted; 95% CI [6.377–12.867]), respectively [38, 50]. In AUC analysis, the highest AUC for incidence of diabetes in females was 0.78 in the 20–49 years old age sub-group, and the highest prevalence of diabetes in males was 0.81 in the ≥ 50 years old age sub-group [37]. Three studies compared anthropometric measures with each other, and all of them revealed that the triglyceride glucose (TyG) index is the strongest index for predicting diabetes [38, 40, 41]. Seven articles found that LAP has a stronger association with diabetes in females in comparison with males [25, 33, 38, 42, 44, 46, 48]. Four articles reported that LAP is a stronger index than WC in association with diabetes, whereas Kavaric et al. analyses suggest that LAP and VAI are not better than WC and HDL-c, and Wang B et al. reported that AUC for LAP and WC is similar [39, 41, 44, 45, 47, 48]. Different articles reported that LAP has a stronger association with the incidence or prevalence of diabetes in comparison to HOMA-IR, BMI, CMI, BAI, VAI, WHtR, and WHR [42, 44, 45]. Meanwhile, it is worth mentioning that Bozorgmanesh et al. suggested that LAP is only better for the prevalence of diabetes in females in comparison to BMI, WHtR, and WHpR. In contrast to BMI, WHR, and WHtR, LAP showed only a statistically stronger positive association with the incidence and prevalence of diabetes in males in compere to BMI [37].
Except for two studies, the others had good or fair quality based on our quality assessment. Considering all the studies, LAP is positively and significantly associated with the incidence and prevalence of T2DM. It appears that LAP is a better predictor of T2DM in females than males. Most of the studies confirm the superiority of LAP over traditional anthropometric measures, such as BMI and WC, in predicting T2DM.
Discussion
This systematic review evaluated the predictability of LAP for T2DM, hypertension, and all-cause mortality. We also conducted a meta-analysis on the correlation of LAP with all-cause mortality. Our result showed that LAP is an appropriate predictor of all-cause mortality, hypertension, and T2DM with different predictabilities per sex. Most of the studies reported higher predictability measures for LAP in females in comparison with males. Although there are contradictory findings regarding the superiority of LAP over traditional anthropometric measures, evidence shows that LAP could be a better predictor of hypertension and T2DM than other indices like BMI, WC, CMI, etc. LAP could be used as an inexpensive method to determine the risk of developing T2DM and hypertension.
The ability of LAP to predict T2DM and hypertension has several reasons. LAP considers both anatomic and physiologic changes since it has WC and TG in its formula. LAP is an indicator of visceral adipose tissue which is correlated with insulin resistance [50]. Therefore, LAP as a predictor of insulin resistance is associated with the development of T2DM [50]. “Ectopic” lipid accumulation (e.g., liver, blood vessels, and heart) alters the metabolism of the human body. Insulin resistance as a result can lead to the development of T2DM [11]. TG in the LAP formula is an independent risk factor for T2DM [3]. Moreover, LAP is also a good indicator of hypertension. As mentioned before, TG, and therefore LAP, is associated with visceral adipose tissue that has more harmful effects than subcutaneous fat tissue. Adipocytokines secreted from adipose tissue can alter endothelial cells, consequently increasing the risk of hypertension [28, 32]. Considering both abdominal fat and visceral fat tissues in its formula, LAP can be a strong predictor of T2DM and hypertension.
Our findings suggested that LAP is significantly associated with all-cause mortality in females; however, it failed to reach statistical significance in males. As mentioned before, LAP can predict many diseases, such as T2DM, insulin resistance, metabolic syndrome, hypertension, cardiovascular diseases, and chronic kidney disease [25, 52, 53]. Considering the fact that people with higher LAP have an increased risk of developing metabolic disorders and cardiovascular disease, the association of LAP with all-cause mortality could be explained [15, 25]. Different predictability power of LAP for males and females could be explained by different patterns of lipid over-accumulation in each sex with aging [15] and scarcity of data on the association between LAP and all-cause mortality.
The higher strength of LAP in predicting T2DM and hypertension than BMI, WC, etc., can have several explanations. Unlike LAP, the traditional anthropometric measures like BMI and WC only assess obesity, and they are unable to distinguish between visceral adipose tissue and subcutaneous adiposity tissue. Visceral adipose tissue is more harmful than subcutaneous tissue. Thus, fat distribution plays an important role in the risk of diseases, such as hypertension and T2DM [30, 49, 50]. Also, BMI is unable to differentiate between adipose tissue and lean mass. For instance, there are some patients with high LAP that still have a normal BMI. TG and WC are both independent risk factors for T2DM and hypertension. Combining TG and WC in the LAP formula can increase our insight regarding the fat distribution of the patients and the risk of developing diabetes or hypertension [30, 49, 50]. Since LAP considers both, it can be a better predictor for T2DM and hypertension in comparison with common anthropometric measures.
Discrepancies in the prediction power of LAP and different cut-off values could be due to the differences in the mean age, ethnicity of the study population, or sample size between the included articles. Additionally, most of the studies reported a stronger association of LAP with T2DM and hypertension in females than males [30, 35, 50, 54], but there are other studies that had different results [28, 32]. The outperformance of traditional anthropometric measures by LAP has been proved in several studies [32, 45, 55] but not all of them [56–58]. Different TG levels, WC, sample size, ethnicity, disease status, and confounding bias could explain the contradictory findings.
To the best of our knowledge, our study is the first systematic review and meta-analysis on the association of LAP with hypertension and all-cause mortality. We have compared the prediction power of LAP for T2DM, hypertension, and all-cause mortality by sex and age. Another strength of our study is the comparison of LAP with other anthropometric measures. However, our study has several limitations. Due to different cut-off values, we were unable to conduct a meta-analysis on T2DM and hypertension papers. Studies had a different adjusted model that complicates the pooling of studies. Some of the included studies had poor quality, and we cannot ignore the probability of confounding bias or poor methodology. Moreover, some of the studies were conducted on populations with a specific condition, such as post-menopausal women, which may call for caution in generalizing the findings of this study. Besides, most of the LAP measurements were done once in the follow-up years. Not all the studies had reported the predictability measures by sex.
Conclusion
In conclusion, LAP is associated with all-cause mortality, T2DM, and hypertension. The result of the meta-analysis showed that LAP is directly correlated with all-cause mortality in females; however, this association was not significant in males, probably due to scarcity of data. LAP is positively associated with T2DM and hypertension. Most of the studies showed that LAP is a better predictor of T2DM and hypertension in comparison to traditional anthropometric measures, such as BMI, WC, and WHR, especially in females. Overall, LAP has a higher prognostic significance in females compared to males. It also has interactive effects with smoking and a family history of hypertension. LAP is a cheap method to determine the risk of chronic diseases, such as hypertension, T2DM, or cardiovascular diseases. Different cut-off values in studies complicate using LAP in population-level health surveillance. Therefore, further studies are required to determine specific cut-off values for sexes, age sub-groups, and different populations.
Supplementary Information
Below is the link to the electronic supplementary material.
Author Contributions
SK was responsible for designing the review protocol, screening eligible studies, extracting data, and writing the primary draft. HT was responsible for designing the review protocol, writing the review protocol, screening the eligible studies, extracting data, and writing the draft. AA was responsible for designing the review protocol, conducting meta-analysis, interpreting results, and writing the draft. YR was responsible for conducting meta-analysis, interpreting results, and writing the draft. HA contributed to designing the review protocol and revising the draft. AV was responsible for designing the review protocol, conceptualization, revising the draft and providing feedback on the review. All the authors approved the final version of the manuscript.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Conflicts of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Noncommunicable diseases: Risk factors. World Health Organization. https://www.who.int/data/gho/data/themes/topics/topic-details/GHO/ncd-risk-factors. Accessed Feb 2021.
- 2.De Oliveira CC, Roriz AKC, Ramos LB, Gomes NM. Indicators of Adiposity Predictors of Metabolic Syndrome in the Elderly. Ann Nutr Metab. 2017;70(1):9–15. doi: 10.1159/000455333. [DOI] [PubMed] [Google Scholar]
- 3.Tian T, Pei H, Chen Z, Hailili G, Wang S, Sun Y, Yao H, Jianghong D. Comparison of lipid accumulation product and body mass index as indicators of diabetes diagnosis among 215,651 Chinese adults. PeerJ. 2020;8:e8483. 10.7717/peerj.8483. [DOI] [PMC free article] [PubMed]
- 4.Dai H, Bragazzi NL, Younis A, Zhong W, Liu X, Wu J, et al. Worldwide Trends in Prevalence, Mortality, and Disability-Adjusted Life Years for Hypertensive Heart Disease From 1990 to 2017. Hypertension. 2021;77(4):1223–1233. doi: 10.1161/HYPERTENSIONAHA.120.16483. [DOI] [PubMed] [Google Scholar]
- 5.Ji M, Zhang S, An R. Effectiveness of A Body Shape Index (ABSI) in predicting chronic diseases and mortality: a systematic review and meta-analysis. Obes Rev. 2018;19(5):737–759. doi: 10.1111/obr.12666. [DOI] [PubMed] [Google Scholar]
- 6.Goh LGH, Dhaliwal SS, Welborn TA, Lee AH, Della PR. Anthropometric measurements of general and central obesity and the prediction of cardiovascular disease risk in women: a cross-sectional study. BMJ Open. 2014;4(2):e004138. doi: 10.1136/bmjopen-2013-004138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nuttall FQ. Body Mass Index: Obesity, BMI, and Health: A Critical Review. Nutr Today. 2015;50(3):117–128. doi: 10.1097/NT.0000000000000092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abolhasani M, Maghbouli N, Karbalai Saleh S, Aghsaeifar Z, Sazgara F, Tahmasebi M, et al. Which anthropometric and metabolic index is superior in hypertension prediction among overweight/obese adults? Integr Blood Press Control. 2021;14:153–161. doi: 10.2147/IBPC.S340664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Biyik Z, Guney I. Lipid accumulation product and visceral adiposity index: two new indices to predict metabolic syndrome in chronic kidney disease. Eur Rev Med Pharmacol Sci. 2019;23(5):2167–2173. doi: 10.26355/eurrev_201903_17262. [DOI] [PubMed] [Google Scholar]
- 10.Wang F, Chen Y, Chang Y, Sun G, Sun Y. New anthropometric indices or old ones: which perform better in estimating cardiovascular risks in Chinese adults. BMC Cardiovasc Disord. 2018;18(1):14. doi: 10.1186/s12872-018-0754-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kahn HS. The "lipid accumulation product" performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mirmiran P, Bahadoran Z, Azizi F. Lipid accumulation product is associated with insulin resistance, lipid peroxidation, and systemic inflammation in type 2 diabetic patients. Endocrinol Metab. 2014;29(4):443–449. doi: 10.3803/EnM.2014.29.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Motamed N, Razmjou S, Hemmasi G, Maadi M, Zamani F. Lipid accumulation product and metabolic syndrome: a population-based study in northern Iran. Amol J Endocrinol Invest. 2016;39(4):375–382. doi: 10.1007/s40618-015-0369-5. [DOI] [PubMed] [Google Scholar]
- 14.Abulmeaty MM, Almajwal AM, Almadani NK, Aldosari MS, Alnajim AA, Ali SB, et al. Anthropometric and central obesity indices as predictors of long-term cardiometabolic risk among Saudi young and middle-aged men and women. Saudi Med J. 2017;38(4):372–380. doi: 10.15537/smj.2017.4.18758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ioachimescu AG, Brennan DM, Hoar BM, Hoogwerf BJ. The lipid accumulation product and all-cause mortality in patients at high cardiovascular risk: a PreCIS database study. Obesity (Silver Spring) 2010;18(9):1836–1844. doi: 10.1038/oby.2009.453. [DOI] [PubMed] [Google Scholar]
- 16.Khanmohammadi S, Tavolinejad H, Aminorroaya A, Vasheghani-Farahani A. Efficacy of lipid accumulation product (LAP) in predicting cardiovascular risk modifiers, cardiovascular disease, and mortality: a systematic review and meta-analysis. PROSPERO 2019 CRD42019142239; 2019. https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=142239.
- 17.Page MJ, McKenzie JE, Bossuyt PM, Boutron I, Hoffmann TC, Mulrow CD, et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. J Clin Epidemiol. 2021;134:178–189. doi: 10.1016/j.jclinepi.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 18.NIH. Study Quality Assessment Tools 2021 [updated 7/2021. Available from: https://www.nhlbi.nih.gov/health-topics/study-quality-assessment-tools. Accessed Feb 2021.
- 19.Kahn HS. The lipid accumulation product is better than BMI for identifying diabetes: a population-based comparison. Diabetes Care. 2006;29(1):151–153. doi: 10.2337/diacare.29.1.151. [DOI] [PubMed] [Google Scholar]
- 20.Hamsaveena SM, Cariappa KB. Lipid accumulation product as a novel index to predict diabetes in women. Res J Phar, Biol Chem Sci. 2014;5(2):760–3. [Google Scholar]
- 21.Wanderley Rocha DRT, Jorge AR, Braulio VB, Arbex AK, Marcadenti A. Visceral adiposity measurements, metabolic and inflammatory profile in obese patients with and without type 2 diabetes mellitus: A crosssectional analysis. Curr Diabetes Rev. 2017;13(1):11–18. doi: 10.2174/1573399812666151015115924. [DOI] [PubMed] [Google Scholar]
- 22.Ahn N, Baumeister SE, Amann U, Rathmann W, Peters A, Huth C, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep. 2019;9(1):9693. doi: 10.1038/s41598-019-46187-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang Y, Liu W, Sun L, Zhang Y, Wang B, Yuan Y, et al. A novel indicator, childhood lipid accumulation product, is associated with hypertension in Chinese children and adolescents. Hypertens Res. 2020;43(4):305–312. doi: 10.1038/s41440-019-0366-8. [DOI] [PubMed] [Google Scholar]
- 24.Bozorgmanesh M, Hadaegh F, Azizi F. Predictive performances of lipid accumulation product vs. adiposity measures for cardiovascular diseases and all-cause mortality, 8.6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010;9:100. 10.1186/1476-511X-9-100. [DOI] [PMC free article] [PubMed]
- 25.Wehr E, Pilz S, Boehm BO, Marz W, Obermayer-Pietsch B. The Lipid Accumulation Product Is Associated With Increased Mortality in Normal Weight Postmenopausal Women. Obesity. 2011;19(9):1873–1880. doi: 10.1038/oby.2011.42. [DOI] [PubMed] [Google Scholar]
- 26.Kahn HS, Bullard KM, Barker LE, Imperatore G. Differences between adiposity indicators for predicting all-cause mortality in a representative sample of United States non-elderly adults. PLoS ONE. 2012;7(11):e50428. doi: 10.1371/journal.pone.0050428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bala C, Gheorghe-Fronea O, Pop D, Pop C, Caloian B, Comsa H, et al. The association between six surrogate insulin resistance indexes and hypertension: A population-based study. Metab Syndr Relat Disord. 2019;17(6):328–333. doi: 10.1089/met.2018.0122. [DOI] [PubMed] [Google Scholar]
- 28.Gao X, Wang GY, Wang AL, Xu T, Tong WJ, Zhang YH. Comparison of lipid accumulation product with body mass index as an indicator of hypertension risk among Mongolians in China. Obes Res Clin Pract. 2013;7(4):E308–E314. doi: 10.1016/j.orcp.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 29.Namazi Shabestari A, Asadi M, Jouyandeh Z, Qorbani M, Kelishadi R. Association of Lipid Accumulation Product with Cardio-Metabolic Risk Factors in Postmenopausal Women. Acta Med Iran. 2016;54(6):370–375. [PubMed] [Google Scholar]
- 30.Nguyen Ngoc H, Kriengsinyos W, Rojroongwasinkul N, Aekplakorn W. Association of adiposity indices with hypertension in middle-aged and elderly Thai population: National health examination survey 2009 (NHES-IV) J Cardiovasc Dev Dis. 2019;6(1):13. doi: 10.3390/jcdd6010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotter I, Ryl A, Szylinska A, Kaczmarczyk K, Laszczynska M. Can the lipid accumulation product index indicate sex hormone disorders in aging men? Andrology. 2016;4:74–75. [Google Scholar]
- 32.Song J, Zhao YY, Nie SM, Chen X, Wu XS, Mi J. The effect of lipid accumulation product and its interaction with other factors on hypertension risk in Chinese Han population: A cross-sectional study. PLoS ONE. 2018;13(6):15. doi: 10.1371/journal.pone.0198105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in Japanese women and men. J Atheroscler Thromb. 2014;21(3):282–288. doi: 10.5551/jat.20628. [DOI] [PubMed] [Google Scholar]
- 34.Wang HY, Chen YT, Sun GZ, Jia PY, Qian H, Sun YX. Validity of cardiometabolic index, lipid accumulation product, and body adiposity index in predicting the risk of hypertension in Chinese population. Postgrad Med. 2018;130(3):325–333. doi: 10.1080/00325481.2018.1444901. [DOI] [PubMed] [Google Scholar]
- 35.Huang J, Bao X, Xie Y, Zhang X, Peng X, Liu Y, et al. Interaction of lipid accumulation product and family history of hypertension on hypertension risk: a cross-sectional study in the Southern Chinese population. BMJ Open. 2019;9(11):e029253. doi: 10.1136/bmjopen-2019-029253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang C, Fu W, Cao S, Xu H, Tian Q, Gan Y, et al. Association of adiposity indicators with hypertension among Chinese adults. Nutr Metab Cardiovasc Dis. 2021;31(5):1391–1400. doi: 10.1016/j.numecd.2021.01.001. [DOI] [PubMed] [Google Scholar]
- 37.Bozorgmanesh M, Hadaegh F, Azizi F. Diabetes prediction, lipid accumulation product, and adiposity measures; 6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010;9:45. doi: 10.1186/1476-511X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brahimaj A, Rivadeneira F, Muka T, Sijbrands EJG, Franco OH, Dehghan A, et al. Novel metabolic indices and incident type 2 diabetes among women and men: the Rotterdam Study. Diabetologia. 2019;62(9):1581–1590. doi: 10.1007/s00125-019-4921-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kavaric N, Klisic A, Ninic A. Are visceral adiposity index and lipid accumulation product reliable indices for metabolic disturbances in patients with type 2 diabetes mellitus? J Clin Lab Anal. 2018;32(3):e22283. 10.1002/jcla.22283. [DOI] [PMC free article] [PubMed]
- 40.Kim B, Choi HY, Kim W, Ahn C, Lee J, et al. The cut-off values of surrogate measures for insulin resistance in the Korean population according to the Korean Genome and Epidemiology Study (KOGES). PLOS ONE. 2018;13(11):e0206994. 10.1371/journal.pone.0206994. [DOI] [PMC free article] [PubMed]
- 41.Lee JW, Lim NK, Park HY. The product of fasting plasma glucose and triglycerides improves risk prediction of type 2 diabetes in middle-aged Koreans. BMC Endocr Disord. 2018;18:33. doi: 10.1186/s12902-018-0259-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Malavazos AE, Cereda E, Ermetici F, Caccialanza R, Briganti S, Rondanelli M, Morricone L. The “lipid accumulation product” is associated with 2-hour postload glucose outcomes in overweight/obese subjects with nondiabetic fasting glucose. Int J Endocrinol. 2015;2015:836941. 10.1155/2015/836941. [DOI] [PMC free article] [PubMed]
- 43.Rotter I, Ryl A, Szylinska A, Pawlukowska W, Lubkowska A, Laszczynska M. Lipid Accumulation Product (LAP) as an Index of Metabolic and Hormonal Disorders in Aging Men. Exp Clin Endocrinol Diabetes. 2017;125(3):176–182. doi: 10.1055/s-0042-116071. [DOI] [PubMed] [Google Scholar]
- 44.Shi WR, Wang HY, Chen S, Guo XF, Li Z, Sun YX. Estimate of prevalent diabetes from cardiometabolic index in general Chinese population: a community-based study. Lipids Health Dis. 2018;17:9. doi: 10.1186/s12944-018-0886-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sun K, Lin D, Feng Q, et al. Assessment of adiposity distribution and its association with diabetes and insulin resistance: a population-based study. Diabetol Metab Syndr. 2019;11:51. doi: 10.1186/s13098-019-0450-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wakabayashi I. Influence of age and gender on lipid accumulation product and its relation to diabetes mellitus in Japanese. Clin Chim Acta. 2014;431:221–226. doi: 10.1016/j.cca.2014.02.002. [DOI] [PubMed] [Google Scholar]
- 47.Wang Z, He S, Chen X. Capacity of different anthropometric measures to predict diabetes in a Chinese population in southwest China: a 15-year prospective study. Diabet Med. 2019;36(10):1261–1267. doi: 10.1111/dme.14055. [DOI] [PubMed] [Google Scholar]
- 48.Wang BY, Zhang M, Liu Y, Sun XZ, Zhang L, Wang CJ, et al. Utility of three novel insulin resistance-related lipid indices for predicting type 2 diabetes mellitus among people with normal fasting glucose in rural China. J Diabetes. 2018;10(8):641–652. doi: 10.1111/1753-0407.12642. [DOI] [PubMed] [Google Scholar]
- 49.Yan GY, Li F, Elia C, Zhao YT, Wang JG, Chen ZH, et al. Association of lipid accumulation product trajectories with 5-year incidence of type 2 diabetes in Chinese adults: a cohort study. Nutr Metab. 2019;16(1):8. doi: 10.1186/s12986-019-0399-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu M, Huang M, Qiang D, Gu J, Li Y, Pan Y, Yao X, Xu W, Tao Y, Zhou Y, Ma H. Hypertriglyceridemic waist phenotype and lipid accumulation product: Two comprehensive obese indicators of waist circumference and triglyceride to predict type 2 diabetes mellitus in Chinese population. J Diabetes Res. 2020;2020:9157430. 10.1155/2020/9157430. [DOI] [PMC free article] [PubMed]
- 51.Marcadenti A, Fuchs FD, Moreira LB, Gus M, Fuchs SC. Adiposity phenotypes are associated with type-2 diabetes: LAP index, body adiposity index, and neck circumference. Atherosclerosis. 2017;266:145–150. doi: 10.1016/j.atherosclerosis.2017.09.022. [DOI] [PubMed] [Google Scholar]
- 52.Seong JM, Lee JH, Gi MY, Son YH, Moon AE, Park CE, Sung HH, Yoon H. Gender difference in the association of chronic kidney disease with visceral adiposity index and lipid accumulation product index in Korean adults: Korean National Health and Nutrition Examination Survey. Int Urol Nephrol. 2021;53(7):1417–1425. doi: 10.1007/s11255-020-02735-0. [DOI] [PubMed] [Google Scholar]
- 53.Taverna MJ, Aranguren F, Frechtel GD. Lipid accumulation product is strongly associated with surrogates of insulin resistance, metabolic syndrome and cardiovascular disease risk in healthy men. Diabetologia. 2009;52(S1):S214. [Google Scholar]
- 54.Wakabayashi I, Daimon T. A strong association between lipid accumulation product and diabetes mellitus in japanese women and men. J Atheroscler Thromb. 2014;21(3):282–288. doi: 10.5551/jat.20628. [DOI] [PubMed] [Google Scholar]
- 55.Bozorgmanesh M, Hadaegh F, Azizi F. Diabetes prediction, lipid accumulation product, and adiposity measures; 6-year follow-up: Tehran lipid and glucose study. Lipids Health Dis. 2010;9:45. doi: 10.1186/1476-511X-9-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bala C, Gheorghe-Fronea O, Pop D, Pop C, Caloian B, Comsa H, et al. The Association Between Six Surrogate Insulin Resistance Indexes and Hypertension: A Population-Based Study. Metab Syndr Relat Disord. 2019;17(6):328–333. doi: 10.1089/met.2018.0122. [DOI] [PubMed] [Google Scholar]
- 57.Kavaric N, Klisic A, Ninic A. Are visceral adiposity index and lipid accumulation product reliable indices for metabolic disturbances in patients with type 2 diabetes mellitus? J Clin Lab Anal. 2018;32(3). [DOI] [PMC free article] [PubMed]
- 58.Abolhasani M, Maghbouli N, Sazgara F, Karbalai Saleh S, Tahmasebi M, Ashraf H. Evaluation of Several Anthropometric and Metabolic Indices as Correlates of Hyperglycemia in Overweight/Obese Adults. Diabetes Metab Syndr Obes. 2020;13:2327–2336. doi: 10.2147/DMSO.S254741. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.