Abstract
Background
This investigation was performed to assess the effects of alpha-lipoic acid (ALA) supplementation on psychological status and markers of inflammation and oxidative damage in patients with type 2 diabetes mellitus (T2DM) and coronary heart disease (CHD).
Methods
This randomized, double-blind, placebo-controlled trial was performed in 60 patients with T2DM and CHD, aged 45–85 years. Patients were randomized into two groups to receive either 600 mg/day ALA (n = 30) or placebo (n = 30) for 12 weeks.
Results
ALA supplementation significantly decreased Beck Depression Inventory index (BDI) (-5.1 ± 3.5 vs. -1.1 ± 4.8, P = 0.001) when compared with the placebo. ALA supplementation resulted also in a significant reduction of serum high sensitivity C-reactive protein (hs-CRP) (-0.8 ± 1.4 vs. +0.5 ± 0.6 mg/L, P < 0.001) and malondialdehyde (MDA) (-0.3 ± 0.2 vs. -0.1 ± 0.3 µmol/L, P = 0.003), and a significant increase in plasma total antioxidant capacity (TAC) levels (+ 26.8 ± 36.0 vs. -4.6 ± 43.4 mmol/L, P = 0.007) when compared with the placebo. ALA intake upregulated transforming growth factor beta (TGF-β) (P = 0.03) and downregulated gene expression of interleukin-1 (IL-1) (P = 0.001) in peripheral blood mononuclear cells of patients with T2DM and CHD as well.
Conclusions
ALA supplementation for 12 weeks in patients with T2DM and CHD had beneficial effects on BDI, hs-CRP, TAC, MDA values, and gene expression of IL-1 and TGF-β.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01031-1.
Keywords: Alpha-lipoic acid, Mental health, Oxidative stress, Type 2 diabetes mellitus, Coronary heart disease
Introduction
Diabetes mellitus (DM) is a major public health challenge, accounting for 5% of causes of death globally [1]. Based on prior reports, patients with diabetes when compared with healthy people have six-fold higher risk of developing coronary heart disease (CHD) [2, 3]. It has been shown that increased inflammatory state and its constant companion, oxidative damage may play pivotal roles in the occurrence of diabetes complications such as atherosclerotic CHD [4]. Both conditions have in common an impairment of the cellular antioxidant system due to increased free radicals resulting from disturbances in glucose and lipoprotein metabolism [5]. Moreover, psychological disturbances such as depression and anxiety are prevalent in patients with metabolic diseases, including diabetes, dyslipidemia and CHD [6–8].
Interestingly, increased levels of inflammatory cytokines have been associated with depression in individuals with or without CHD [9, 10]. Furthermore, mental disorders and metabolic diseases usually occur together and may cause a vicious cycle [6]. It was reported that there are metabolic disturbances in patients with metabolic disorders, and nutritional and antioxidant supplements have beneficial effects in these patients [11–15]. Alpha-lipoic acid (ALA), an eight-carbon fatty acid with a reductive potential that is required for catalysis by mitochondrial 2-ketoacid dehydrogenases and participates in stabilization and redox-dependent regulation of these multienzyme complexes. Therefore, it is essential for cell growth, oxidation of carbohydrates, amino acids, and regulation of mitochondrial redox balance. ALA has gained considerable attention as an antioxidant for use in managing complications of diabetes mellitus [16]. It has been reported that ALA can directly scavenge free radical species, decrease lipid peroxidation, regenerate other antioxidants, chelate metal ions, repair oxidized proteins, regulate gene transcription, inhibit the activation of NF-κB and stimulate the activity of superoxide dismutase and catalase enzymes [17]. Earlier meta-analyses suggested that supplementation with ALA might have some favorable impact on inflammatory markers, serum lipoproteins and glucose metabolism in patients with metabolic disorders [18–20].
Regarding the antioxidative and anti-inflammatory effects of ALA, we assumed that supplementation with ALA might be beneficial in patients with T2DM and CHD. To our knowledge, data on the effects of ALA intake on mental health status and gene expression related to inflammation, insulin and lipoprotein metabolism in patients with T2DM and CHD are limited. In addition, data on the effects of ALA supplementation on metabolic profiles and mental health status are conflicting. Therefore, we tried to assess the effects of ALA administration on mental health status, biomarkers of inflammation and oxidative damage, and gene expression related to inflammation, insulin and lipoprotein metabolism in these patients.
Subjects and methods
Participants and ethics statements
This randomized, double-blind, placebo-controlled trial, registered in the Iranian registry of clinical trials (http://www.irct.ir: IRCT20170513033941N49), was performed at a cardiology clinic affiliated to Kashan University of Medical Sciences (KAUMS), Kashan, Iran, between January 2019 and May 2019 (recruitment date: January 2019 until February 2019 and intervention date: February 2019 until May 2019). Patients with T2DM, aged 45–85 years with 2- and 3-vessel CHD were included. Diagnosis of T2DM and CHD was made based on the criteria of the American Diabetes Association and American Heart Association, respectively. Patients with thyroid disorders, severe renal insufficiency hepatic failure, those experiencing an acute myocardial infarction within the past 3 months, and patients undergoing cardiac surgery within the past 3 months were not included in this study. This study was done according to the principals of the Declaration of Helsinki. The study was approved by the ethics committee of National Institute for Medical Research Development of Iran (NIMAD) (ethics committee reference number. IR.NIMAD.REC.1397.323). Written informed consent was obtained from all participants prior to the initiation of the trial. Type one (α) and type two errors (β) were defined as 0.05, and 0.20 (power = 80%), respectively. According to our previously published trial [21], we used 0.35 nmol/mL as the SD and 0.28 nmol/mL as the change in mean (d) of MDA as a primary outcome parameter. Based on the power analysis, we needed 25 people in each group; after allowing for 5 dropouts in each group, the final sample size was 30 persons in each group.
Study design
Participants were randomized into two groups to take either 600 mg/day ALA (n = 30) or placebo (n = 30) for 12 weeks. ALA and its placebo (starch) were produced by Support Nutrition Pharmaceutical Company (Lowa, USA) and Barij Essence Pharmaceutical Company (Kashan, Iran), respectively. Both ALA supplements and placebo capsules had the same packaging and neither patients nor the investigators were aware of the content of the package until the end of study. Although the capsules were prepared by different companies, they were similar in appearance, color, shape, size, odor and packaging. In addition, supplements and placebos were put in identical coded containers. Randomization was made using computer-generated random numbers by a trained staff at the cardiology clinic. Participants received a daily reminder message on their cell phones to take their supplements regularly. All participants completed three dietary records (two week days and one weekend) at baseline, week 6 and 12 of the trial. To obtain nutrient intakes of patients according to three-day food records, we applied Nutritionist IV software (First Databank, San Bruno, CA) adopted for the Iranian food pattern. In the current study, physical activity was described as metabolic equivalents (METs) in hours per day. To determine the METs for each patient, we multiplied the times (in hour per day) reported for each physical activity by its related METs coefficient by standard Table [22].
Assessment of anthropometric measures
Weight and height (Seca, Hamburg, Germany) were measured at the beginning of the study and after intervention without shoes in a minimal clothing condition in the cardiology clinic by a trained nutritionist. BMI was calculated as weight in kg divided by height in meters squared.
Assessment of outcomes parameters
Malondialdehyde (MDA) was considered as the primary outcome parameter and psychological status and other markers of inflammation and oxidative damage were defined as the secondary outcomes. Fifteen milliliter fasting blood samples were collected at the beginning and after the 12-week intervention at Kashan reference laboratory, Kashan, Iran. Plasma total antioxidant capacity (TAC) was determined using the method of ferric reducing antioxidant power developed by Benzie and Strain [23], total glutathione (GSH) using the method of Beutler et al.[24] and MDA concentrations by the thiobarbituric acid reactive substances spectrophotometric test [25] with coefficient of variations (CVs) lower than 5%. Serum high-sensitivity C-reactive protein (hs-CRP) levels were assessed by commercial ELISA kit (LDN, Nordhorn, Germany) with inter- and intra-assay CVs below 7%. Plasma total nitrite levels were determined using Griess method [26] with coefficient of variations (CVs) lower than 5%.
Clinical assessment
Beck Depression Inventory (BDI) was assessed using a modified questionnaire [27]. Anxiety was measured by Beck Anxiety Inventory (BAI) developed by Beck et al. [28]. Quality of sleep was determined using Pittsburgh Sleep Quality Index (PSQI) [29].
Isolation of lymphocyte cells
Lymphocyte cells were extracted from blood samples of T2DM patients with CHD with a 50% percoll (Sigma-Aldrich, Dorset, UK). Samples were taken for cell count and viability testing using trypan blue, RNA and DNA extraction [30].
RNA extraction and real-time PCR
Following extraction of the total RNAs from each sample, the RNA quantification was evaluated by the UV spectrophotometer. Samples OD 260/280 ratio was standardized between 1.7 and 2.1 showing no contamination for both protein and DNA [30]. Gene expressions of IL-1, TNF-α, transforming growth factor beta (TGF-β) and vascular endothelial growth factor (VEGF) were performed on mononuclear cells (PBMCs) from peripheral blood, using SYBR green detection and Amplicon Kit and applying quantitative RT-PCR and Light Cycler technology (Roche Diagnostics, Rotkreuz, Switzerland) (Table 1).
Table 1.
Specific primers used for real-time quantitative PCR
| Gene | Primer | Product size (bp) | Annealing temperature (C) |
|---|---|---|---|
| GAPDH | F: AAGCTCATTTCCTGGTATGACAACG | 126 | 61.3 |
| R: TCTTCCTCTTGTGCTCTTGCTGG | |||
| IL-1 | F: GCTTCTCTCTGGTCCTTGG | 174 | 56 |
| R: AGGGCAGGGTAGAGAAGAG | |||
| TNF-α |
F: GTCAACCTCCTCTCTGCCAT R: CCAAAGTAGACCTGCCCAGA |
188 | 52 |
| TGF-β | F: TTGAGACTTTTCCGTTGCCG | 227 | 56 |
| R: CGAGGTCTGGGGAAAAGTCT | |||
| VEGF | F: CTTCTGAGTTGCCCAGGAGA | 216 | 54 |
| R: CTCACACACACACAACCAGG |
GAPDH, glyceraldehyde-3-Phosphate dehydrogenase; IL-1, interleukin-1; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor
Statistical methods
The Kolmogorov-Smirnov test was performed to determine the normality of data. Differences in anthropometric measurements and study outcomes between the groups were made using independent-sample t-tests. Differences in proportions were evaluated by Fisher’s exact test. P-values < 0.05 were considered statistically significant. All statistical analyses were done using the Statistical Package for Social Science version 18 (SPSS Inc., Chicago, Illinois, USA).
Results
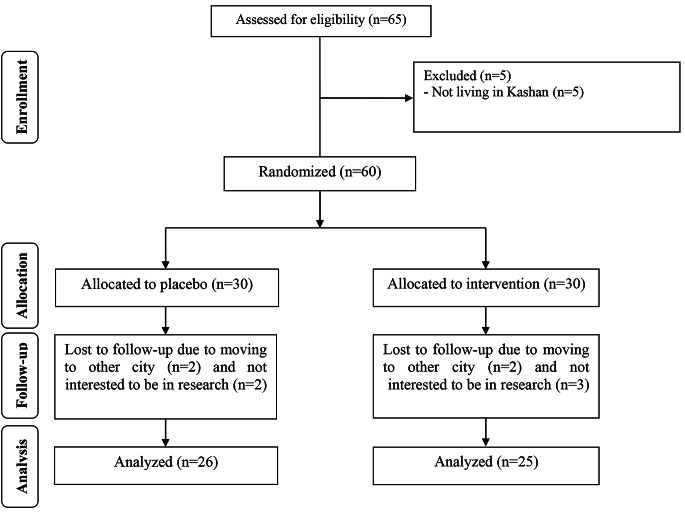
Five subjects in the group taking ALA and 4 in the placebo group dropped out for personal reasons (Fig. 1). The compliance ranged between 90 and 100% throughout the study in both groups. To determine the compliance of remaining supplements were counted and subtracted from the amount of supplements provided to the participants.
Fig. 1.
Summary of patient flow diagram
There were no significant differences between the two groups in terms of mean age, anthropometric measurements and METs (Table 2).
Table 2.
General characteristics of study participants at baseline
| Placebo group (n = 26) |
Alpha-lipoic acid group (n = 25) |
P1 | |
|---|---|---|---|
| Age (y) | 62.2 ± 10.1 | 66.1 ± 9.1 | 0.15 |
| Gender | |||
| Female | 12 (46.2%) | 14 (56.0%) | |
| Male | 14 (53.8%) | 11 (44.0%) | 0.57† |
| Height (m) | 159.1 ± 7.0 | 157.3 ± 9.3 | 0.44 |
| Weight-baseline (kg) | 69.7 ± 9.4 | 71.1 ± 10.9 | 0.63 |
| Weight at the end of trial (kg) | 69.5 ± 9.5 | 70.7 ± 10.6 | 0.68 |
| Body weight change (kg) | -0.2 ± 0.7 | -0.4 ± 1.4 | 0.55 |
| BMI- baseline (kg/m2) | 27.6 ± 3.5 | 28.8 ± 4.3 | 0.27 |
| BMI at the end of trial (kg/m2) | 27.5 ± 3.6 | 28.6 ± 4.1 | 0.29 |
| BMI change (kg/m2) | -0.1 ± 0.3 | -0.2 ± 0.6 | 0.47 |
| MET-h/day at study baseline | 25.1 ± 1.6 | 24.78 ± 1.6 | 0.42 |
| MET-h/day at end-of-trial | 25.3 ± 1.6 | 24.81 ± 1.6 | 0.31 |
| MET-h/day change | 0.1 ± 0.3 | 0.03 ± 0.3 | 0.31 |
Data are means ± SDs. METs, metabolic equivalents
1 Obtained from independent samples t-test
† Obtained from Fisher’s exact test
No significant difference in dietary macronutrient and total dietary fiber intake was observed between the two groups (Table 3).
Table 3.
Dietary intakes of study participants throughout the study
| Placebo group (n = 26) |
Alpha-lipoic acid group (n = 25) |
P1 | |
|---|---|---|---|
| Energy (kcal/d) | 1997 ± 217 | 2072 ± 229 | 0.23 |
| Carbohydrates (g/d) | 277.4 ± 48.7 | 283.7 ± 46.6 | 0.64 |
| Protein (g/d) | 71.4 ± 10.6 | 76.1 ± 9.5 | 0.10 |
| Fat (g/d) | 70.7 ± 9.6 | 72.6 ± 13.9 | 0.57 |
| SFA (g/d) | 20.9 ± 3.8 | 22.5 ± 4.7 | 0.18 |
| MUFA (g/d) | 18.3 ± 4.0 | 20.7 ± 6.2 | 0.11 |
| PUFA (g/d) | 22.6 ± 5.2 | 22.1 ± 6.2 | 0.76 |
| TDF (g/d) | 16.4 ± 4.0 | 17.1 ± 4.1 | 0.56 |
Data are means ± SDs
1 Obtained from independent t test
SFA, saturated fatty acid; MUFA, monounsaturated fatty acid; PUFA, polyunsaturated fatty acid; TDF, total dietary fiber
After the 12-week, ALA supplementation significantly decreased BDI (-5.1 ± 3.5 vs. -1.1 ± 4.8, P = 0.001) when compared with the placebo (Table 4). ALA supplementation resulted also in a significant reduction of serum hs-CRP (-0.8 ± 1.4 vs. +0.5 ± 0.6 mg/L, P < 0.001) and MDA (-0.3 ± 0.2 vs. -0.1 ± 0.3 µmol/L, P = 0.003), and a significant increase in plasma TAC levels (+ 26.8 ± 36.0 vs. -4.6 ± 43.4 mmol/L, P = 0.007) when compared with the placebo. Supplementation with ALA had no significant effect on other metabolic parameters when compared with the placebo
Table 4.
Biomarkers of oxidative stress and inflammation at baseline and after 12-week intervention with alpha-lipoic acid in patients with type 2 diabetes and coronary heart disease
| Placebo group (n = 26) | Alpha-lipoic acid group (n = 25) | |||||||
|---|---|---|---|---|---|---|---|---|
| Baseline | End-of-trial | Change | Baseline | End-of-trial | Change | P1 | P2 | |
| BDI | 21.5 ± 5.1 | 20.4 ± 5.8 | -1.1 ± 4.8 | 24.7 ± 4.2 | 19.6 ± 4.5 | -5.1 ± 3.5 | 0.01 | 0.001 |
| BAI | 16.3 ± 3.6 | 14.5 ± 4.3 | -1.8 ± 3.1 | 18.2 ± 4.7 | 15.4 ± 4.5 | -2.8 ± 1.8 | 0.11 | 0.17 |
| PSQI | 9.2 ± 2.2 | 8.4 ± 2.5 | -0.8 ± 2.7 | 8.5 ± 2.4 | 7.6 ± 2.0 | -0.9 ± 2.9 | 0.33 | 0.88 |
| TAC (mmol/L) | 617.9 ± 92.5 | 613.3 ± 74.7 | -4.6 ± 43.4 | 637.1 ± 81.7 | 663.9 ± 94.5 | 26.8 ± 36.0 | 0.43 | 0.007 |
| GSH (µmol/L) | 455.9 ± 57.7 | 460.0 ± 64.7 | 4.1 ± 23.7 | 474.1 ± 56.1 | 488.1 ± 77.6 | 14.1 ± 48.0 | 0.26 | 0.34 |
| MDA (µmol/L) | 2.7 ± 0.5 | 2.6 ± 0.4 | -0.1 ± 0.3 | 2.8 ± 0.4 | 2.5 ± 0.4 | -0.3 ± 0.2 | 0.51 | 0.003 |
| hs-CRP (mg/L) | 5.5 ± 3.2 | 6.0 ± 3.3 | 0.5 ± 0.6 | 6.5 ± 1.7 | 5.7 ± 1.6 | -0.8 ± 1.4 | 0.15 | < 0.001 |
| Total nitrite (µmol/L) | 32.7 ± 4.4 | 32.3 ± 4.1 | -0.4 ± 1.3 | 33.7 ± 4.5 | 34.1 ± 4.6 | 0.4 ± 2.2 | 0.40 | 0.11 |
Data are mean ± SDs
1 Independent sample Student’s t test (the comparison of the biochemical variables between the two groups at the baseline)
2 Obtained from independent samples t-test (the comparison of change between the two groups)
BDI, beck depression inventory; BAI, beck anxiety inventory; GSH, total glutathione; hs-CRP, high-sensitivity C-reactive protein; MDA, malondialdehyde; PSQI, Pittsburgh Sleep Quality Index; TAC, total antioxidant capacity
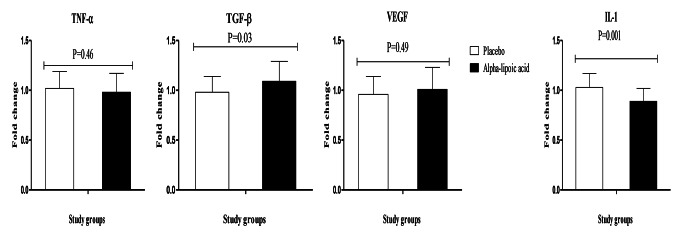
ALA upregulated TGF-β (P = 0.03) and downregulated gene expression for IL-1 (P = 0.001) in peripheral blood mononuclear cells of patients with T2DM and CHD, but did not influence TNF-α and VEGF expression (Fig. 2).
Fig. 2.
Change (means ± SDs) in gene expression levels of IL-1, TNF-α, TGF-β and VEGF in in type 2 diabetic patients with coronary heart disease who were received alpha-lipoic acid supplements and placebo. IL-1, interleukin-1; TNF-α, tumor necrosis factor alpha; TGF-β, transforming growth factor beta; VEGF, vascular endothelial growth factor
Discussion
To our knowledge, data regarding the effects of ALA on psychological symptoms, inflammatory factors, oxidative status, and gene expression related to metabolic profiles in patients with T2DM and CHD are limited. We demonstrated that administration of ALA to these patients has beneficial effects on BDI, hs-CRP, TAC, MDA values, and gene expression for IL-1 and TGF-β, but did not affect BAI, PSQI, plasma total nitrite, GSH, and mRNA expression for TNF-α and VEGF.
Effects on psychological status
We found that ALA supplementation to patients with T2DM and CHD remarkably improved their BDI, but did not influence BAI and PSQI scores. Chronic systemic inflammation and its constant companion, and oxidative damage are considered as the important mechanisms in depression and metabolic diseases, including T2DM and atherosclerotic CVD but direct evidence of their associations is lacking because of lack of longitudinal studies that focus on the pathobiology of both T2DM and depression [31]. Previous meta-analyses have indicated that patients with depression have a 41% increased risk for developing diabetes mellitus and a 32% (even 37%) increased risk for developing T2DM but the mechanisms underlying this relationship are still unclear [32, 33]. On the other side, according to a most recent meta-analysis which included twenty high-quality cohort studies diabetes is an independent risk factor for depression [34]. Thus, mental disorders and metabolic diseases may cause a vicious cycle and exacerbate each other [6]. In a study, it was reported that short-term supplementation of 1000 mg vitamin C for 6 weeks significantly reduced anxiety levels in diabetic patients through alleviating oxidative damage [35]. In addition, ALA supplementation for 3 months at a dosage 300 mg twice a day had beneficial effects on improving the oxidative, inflammatory, and mood conditions in patients suffering from episodic migraine [36]. Antioxidant supplements like ALA may decrease oxidative damage markers and inflammatory factors, so they can break this vicious cycle and improve mental disorders, metabolic disturbances, as well as the patient’s quality of life [37]. The most probable major mechanisms by which ALA may improve metal health parameters include an increase of neurotransmitters such as dopamine and norepinephrine in the brain [38], dopamine receptor blockage [39], alteration of brain acetylcholinesterase activity [40], and modulation of oxidative stress and inflammation [37].
Effects on antioxidative defense system and inflammatory status
This study showed that taking ALA supplement for 3 months by patients with T2DM and CHD improved hs-CRP, MDA and TAC, but did not influence total nitrite and GSH concentrations. ALA also down-regulated mRNA expression of IL-1 and up-regulated TGF-β in patients with T2DM and CHD, but did not influence gene expression for TNF-α and VEGF. Diabetes is not only significantly correlated with elevated oxidative damage and inflammatory markers, which is either the consequence of metabolic disturbances, decreased antioxidant defense, or increased free radical’s production but it has been shown that even with acceptable glycemic control there is a significantly elevated level of DNA damage within neutrophils of patients with diabetes in vivo. [41]. Furthermore, increased oxidation and inflammation play a crucial role in the development of atherosclerosis and CVD [42]. On the basis of earlier reports, ALA administration might have a promising effect on antioxidative defense and inflammation. In a recent trial by Amirkhizi et al.[21], daily consumption of 1,200 mg/day ALA during 3 months improved oxidative stress-related parameters such as MDA, TAC, and glutathione peroxidase levels in patients with fatty liver disease. In another study, Khabbazi et al.[43] reported that ALA (600 mg/day) for 8 weeks had a significant effect on CRP concentrations in hemodialysis patients. Treatment with 300 mg/day ALA for 4 weeks was correlated with significant reductions in IL-6 and 8-isoprostane levels in subjects with metabolic syndrome [44]. However, Ahmadi et al.[45] could not find any significant changes hs-CRP and MDA values in patients with end-stage renal disease after supplementation with 600 mg ALA once a day for 2 months. In another study ALA supplementation to patients with chronic kidney disease did not influence pro-inflammatory cytokines such as TNF-α and IL-8 levels [46]. The discrepancy between the results of various trials could be caused by the specific characteristics of participants, different duration of the trials, different dosage of ALA used, as well as differences in studies design. A high-dose supplementation with ALA or a longer intervention period in patients with T2DM and CHD might improve GSH, total nitrite, BAI, PSQI, and other gene expression related to metabolic profiles. The anti-inflammatory and antioxidative effects of ALA may be due to scavenging free radical species, reducing lipid peroxidation, inhibiting of nuclear factor-κB, and down-regulating pro-inflammatory cytokines [47–49].
Our study has some limitations including the short intervention period, small sample size, and the fact that we did not evaluate other metabolic and genetic biomarkers of inflammation and oxidative damage. Further trials including more patients, a higher dose of ALA, and longer intervention period might show more benefits of ALA supplementation. Supplements and their placebos were provided by different companies. These should be considered in the interpretation of our findings.
Conclusions
ALA supplementation for 12 weeks in patients with T2DM and CHD had beneficial effects on BDI, hs-CRP, TAC, MDA values, and gene expression for IL-1 and TGF-β, but did not affect BAI, PSQI, total nitrite, GSH, and mRNA expression for TNF-α and VEGF.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Acknowledgements
Not applicable.
Author contributions
ZA contributed in conception, design, statistical analysis and drafting of the manuscript. VO and FR contributed in conception, data collection and manuscript drafting. The final version was confirmed by all authors for submission.
Funding
Research reported in this publication was supported by Elite Researcher Grant Committee under award number (976890) from the National Institutes for Medical Research Development (NIMAD), Tehran, Iran.
Availability of data and material
The primary data for this study is available from the authors on direct request.
Declarations
Competing interests
The authors declare no conflict of interest.
Ethics approval and consent to participate
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments.
Consent for publication
Not applicable.
Clinical trial registration number
http://www.irct.ir: IRCT20170513033941N49
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Vahidreza Ostadmohammadi, Email: vrom.1993@gmail.com.
Fariba Raygan, Email: Raygan11@gmail.com.
Zatollah Asemi, Email: asemi_r@yahoo.com.
References
- 1.Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239–51. doi: 10.1016/S0140-6736(17)30058-2. [DOI] [PubMed] [Google Scholar]
- 2.Juutilainen A, Lehto S, Ronnemaa T, Pyorala K, Laakso M. Type 2 diabetes as a “coronary heart disease equivalent”: an 18-year prospective population-based study in Finnish subjects. Diabetes Care. 2005;28:2901–7. doi: 10.2337/diacare.28.12.2901. [DOI] [PubMed] [Google Scholar]
- 3.Catapano AL, Graham I, De Backer G, Wiklund O, Chapman MJ, Drexel H, et al. 2016 ESC/EAS Guidelines for the Management of Dyslipidaemias: The Task Force for the Management of Dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS) Developed with the special contribution of the European Assocciation for Cardiovascular Prevention & Rehabilitation (EACPR) Atherosclerosis. 2016;253:281–344. doi: 10.1016/j.atherosclerosis.2016.08.018. [DOI] [PubMed] [Google Scholar]
- 4.Tatsch E, De Carvalho JA, Hausen BS, Bollick YS, Torbitz VD, Duarte T, et al. Oxidative DNA damage is associated with inflammatory response, insulin resistance and microvascular complications in type 2 diabetes. Mutat Res. 2015;782:17–22. doi: 10.1016/j.mrfmmm.2015.10.003. [DOI] [PubMed] [Google Scholar]
- 5.Maritim AC, Sanders RA, Watkins JB. Diabetes, oxidative stress, and antioxidants: a review. J Biochem Mol Toxicol. 2003;17:24–38. doi: 10.1002/jbt.10058. [DOI] [PubMed] [Google Scholar]
- 6.Ali S, Stone MA, Peters JL, Davies MJ, Khunti K. The prevalence of co-morbid depression in adults with Type 2 diabetes: a systematic review and meta-analysis. Diabet Med. 2006;23:1165–73. doi: 10.1111/j.1464-5491.2006.01943.x. [DOI] [PubMed] [Google Scholar]
- 7.Smith KJ, Beland M, Clyde M, Gariepy G, Page V, Badawi G, et al. Association of diabetes with anxiety: a systematic review and meta-analysis. J Psychosom Res. 2013;74:89–99. doi: 10.1016/j.jpsychores.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Jakovljevic M, Reiner Z, Milicic D. Mental disorders, treatment response, mortality and serum cholesterol: a new holistic look at old data. Psychiatr Danub. 2007;19:270–81. [PubMed] [Google Scholar]
- 9.Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, et al. Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 10.Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, et al. Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry. 2001;158:1252–7. doi: 10.1176/appi.ajp.158.8.1252. [DOI] [PubMed] [Google Scholar]
- 11.Ostadmohammadi V, Jamilian M, Bahmani F, Asemi Z. Vitamin D and probiotic co-supplementation affects mental health, hormonal, inflammatory and oxidative stress parameters in women with polycystic ovary syndrome. J Ovarian Res. 2019;12:5. doi: 10.1186/s13048-019-0480-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raygan F, Ostadmohammadi V, Asemi Z. The effects of probiotic and selenium co-supplementation on mental health parameters and metabolic profiles in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Clin Nutr. 2019;38:1594–1598. [DOI] [PubMed]
- 13.Shabani A, Foroozanfard F, Kavossian E, Aghadavod E, Ostadmohammadi V, Reiter RJ, et al. Effects of melatonin administration on mental health parameters, metabolic and genetic profiles in women with polycystic ovary syndrome: A randomized, double-blind, placebo-controlled trial. J Affect Disord. 2019;250:51–6. doi: 10.1016/j.jad.2019.02.066. [DOI] [PubMed] [Google Scholar]
- 14.Raygan F, Ostadmohammadi V, Bahmani F, Asemi Z. The effects of vitamin D and probiotic co-supplementation on mental health parameters and metabolic status in type 2 diabetic patients with coronary heart disease: A randomized, double-blind, placebo-controlled trial. Prog Neuro-psychopharmacol Biol Psychiatry. 2018;84:50–5. doi: 10.1016/j.pnpbp.2018.02.007. [DOI] [PubMed] [Google Scholar]
- 15.Cicero AFG, Colletti A, Bajraktari G, Descamps O, Djuric DM, Ezhov M, et al. Lipid-lowering nutraceuticals in clinical practice: position paper from an International Lipid Expert Panel. Nutr Rev. 2017;75:731–67. doi: 10.1093/nutrit/nux047. [DOI] [PubMed] [Google Scholar]
- 16.Solmonson A, DeBerardinis RJ. Lipoic acid metabolism and mitochondrial redox regulation. J Biol Chem. 2018;293:7522–30. doi: 10.1074/jbc.TM117.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Golbidi S, Badran M, Laher I. Diabetes and alpha lipoic Acid. Front Pharmacol. 2011;2:69. doi: 10.3389/fphar.2011.00069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kucukgoncu S, Zhou E, Lucas KB, Tek C. Alpha-lipoic acid (ALA) as a supplementation for weight loss: results from a meta-analysis of randomized controlled trials. Obes reviews: official J Int Association Study Obes. 2017;18:594–601. doi: 10.1111/obr.12528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Akbari M, Ostadmohammadi V, Lankarani KB, Tabrizi R, Kolahdooz F, Khatibi SR, et al. The effects of alpha-lipoic acid supplementation on glucose control and lipid profiles among patients with metabolic diseases: A systematic review and meta-analysis of randomized controlled trials. Metab Clin Exp. 2018;87:56–69. doi: 10.1016/j.metabol.2018.07.002. [DOI] [PubMed] [Google Scholar]
- 20.Akbari M, Ostadmohammadi V, Tabrizi R, Mobini M, Lankarani KB, Moosazadeh M, et al. The effects of alpha-lipoic acid supplementation on inflammatory markers among patients with metabolic syndrome and related disorders: a systematic review and meta-analysis of randomized controlled trials. Nutr Metab (Lond) 2018;15:39. doi: 10.1186/s12986-018-0274-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Amirkhizi F, Hamedi-Shahraki S, Hosseinpour-Arjmand S, Vaghef-Mehrabany E, Ebrahimi-Mameghani M. Effects of alpha-lipoic acid supplementation on oxidative stress status in patients with non-alcoholic fatty liver disease: A randomized, double blind, placebo-controlled clinical trial. Iran Red Crescent Med J. 2018;20. DOI:10.5812/ircmj.67615
- 22.Ainsworth BE, Haskell WL, Whitt MC, Irwin ML, Swartz AM, Strath SJ, et al. Compendium of physical activities: an update of activity codes and MET intensities. Med Sci Sports Exerc. 2000;32:498–504. doi: 10.1097/00005768-200009001-00009. [DOI] [PubMed] [Google Scholar]
- 23.Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–6. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 24.Beutler E, Gelbart T. Plasma glutathione in health and in patients with malignant disease. J Lab Clin Med. 1985;105:581–4. [PubMed] [Google Scholar]
- 25.Janero DR. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic Biol Med. 1990;9:515–40. doi: 10.1016/0891-5849(90)90131-2. [DOI] [PubMed] [Google Scholar]
- 26.Tatsch E, Bochi GV, Pereira Rda S, Kober H, Agertt VA, de Campos MM, et al. A simple and inexpensive automated technique for measurement of serum nitrite/nitrate. Clin Biochem. 2011;44:348–50. doi: 10.1016/j.clinbiochem.2010.12.011. [DOI] [PubMed] [Google Scholar]
- 27.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: psychometric properties. J Consult Clin Psychol. 1988;56:893–7. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- 29.Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 30.Dunkley PR, Jarvie PE, Robinson PJ. A rapid Percoll gradient procedure for preparation of synaptosomes. Nat Protoc. 2008;3:1718–28. doi: 10.1038/nprot.2008.171. [DOI] [PubMed] [Google Scholar]
- 31.van Sloten T, Schram M. Understanding depression in type 2 diabetes: a biological approach in observational studies. F1000Research. 2018;7. doi:10.12688/f1000research.13898.1 [DOI] [PMC free article] [PubMed]
- 32.Yu M, Zhang X, Lu F, Fang L. Depression and Risk for Diabetes: A Meta-Analysis. Can J diabetes. 2015;39:266–72. doi: 10.1016/j.jcjd.2014.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Knol MJ, Twisk JW, Beekman AT, Heine RJ, Snoek FJ, Pouwer F. Depression as a risk factor for the onset of type 2 diabetes mellitus. A meta-analysis. Diabetologia. 2006;49:837–45. doi: 10.1007/s00125-006-0159-x. [DOI] [PubMed] [Google Scholar]
- 34.Chireh B, Li M, D’Arcy C. Diabetes increases the risk of depression: A systematic review, meta-analysis and estimates of population attributable fractions based on prospective studies. Prev Med Rep. 2019;14:100822. doi: 10.1016/j.pmedr.2019.100822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mazloom Z, Ekramzadeh M, Hejazi N. Efficacy of supplementary vitamins C and E on anxiety, depression and stress in type 2 diabetic patients: a randomized, single-blind, placebo-controlled trial. Pak J Biol Sci. 2013;16:1597–600. doi: 10.3923/pjbs.2013.1597.1600. [DOI] [PubMed] [Google Scholar]
- 36.Rezaei Kelishadi M, Alavi Naeini A, Askari G, Khorvash F, Heidari Z. The efficacy of alpha-lipoic acid in improving oxidative, inflammatory, and mood status in women with episodic migraine in a randomised, double-blind, placebo-controlled clinical trial. Int J Clin Pract. 2021;75:e14455. doi: 10.1111/ijcp.14455. [DOI] [PubMed] [Google Scholar]
- 37.de Sousa CNS, da Silva Leite CMG, da Silva Medeiros I, Vasconcelos LC, Cabral LM, Patrocinio CFV, et al. Alpha-lipoic acid in the treatment of psychiatric and neurological disorders: a systematic review. Metab Brain Dis. 2019;34:39–52. doi: 10.1007/s11011-018-0344-x. [DOI] [PubMed] [Google Scholar]
- 38.Santos IM, Freitas RL, Saldanha GB, Tome Ada R, Jordan J, Freitas RM. Alterations on monoamines concentration in rat hippocampus produced by lipoic acid. Arq Neuropsiquiatr. 2010;68:362–6. doi: 10.1590/s0004-282x2010000300006. [DOI] [PubMed] [Google Scholar]
- 39.Deslauriers J, Desmarais C, Sarret P, Grignon S. alpha-Lipoic acid interaction with dopamine D2 receptor-dependent activation of the Akt/GSK-3beta signaling pathway induced by antipsychotics: potential relevance for the treatment of schizophrenia. J Mol neuroscience: MN. 2013;50:134–45. doi: 10.1007/s12031-012-9884-4. [DOI] [PubMed] [Google Scholar]
- 40.Arivazhagan P, Ayusawa D, Panneerselvam C. Protective efficacy of alpha-lipoic acid on acetylcholinesterase activity in aged rat brain regions. Rejuven Res. 2006;9:198–201. doi: 10.1089/rej.2006.9.198. [DOI] [PubMed] [Google Scholar]
- 41.Hannon-Fletcher MP, O’Kane MJ, Moles KW, Weatherup C, Barnett CR, Barnett YA. Levels of peripheral blood cell DNA damage in insulin dependent diabetes mellitus human subjects. Mutat Res. 2000;460:53–60. doi: 10.1016/s0921-8777(00)00013-6. [DOI] [PubMed] [Google Scholar]
- 42.Libby P. Inflammation in atherosclerosis. Arteriosclerosis, thrombosis, and vascular biology. 2012;32:2045-51. [DOI] [PMC free article] [PubMed]
- 43.Khabbazi T, Mahdavi R, Safa J, Pour-Abdollahi P. Effects of alpha-lipoic acid supplementation on inflammation, oxidative stress, and serum lipid profile levels in patients with end-stage renal disease on hemodialysis. J Ren Nutr. 2012;22:244–50. doi: 10.1053/j.jrn.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 44.Sola S, Mir MQ, Cheema FA, Khan-Merchant N, Menon RG, Parthasarathy S, et al. Irbesartan and lipoic acid improve endothelial function and reduce markers of inflammation in the metabolic syndrome: results of the Irbesartan and Lipoic Acid in Endothelial Dysfunction (ISLAND) study. Circulation. 2005;111:343–8. doi: 10.1161/01.CIR.0000153272.48711.B9. [DOI] [PubMed] [Google Scholar]
- 45.Ahmadi A, Mazooji N, Roozbeh J, Mazloom Z, Hasanzade J. Effect of alpha-lipoic acid and vitamin E supplementation on oxidative stress, inflammation, and malnutrition in hemodialysis patients. Iran J Kidney Dis. 2013;7:461–7. [PubMed] [Google Scholar]
- 46.Safa J, Ardalan MR, Rezazadehsaatlou M, Mesgari M, Mahdavi R, Jadid MP. Effects of alpha lipoic acid supplementation on serum levels of IL-8 and TNF-alpha in patient with ESRD undergoing hemodialysis. Int Urol Nephrol. 2014;46:1633–8. doi: 10.1007/s11255-014-0688-z. [DOI] [PubMed] [Google Scholar]
- 47.Silva MC, de Sousa CN, Gomes PX, de Oliveira GV, Araujo FY, Ximenes NC, et al. Evidence for protective effect of lipoic acid and desvenlafaxine on oxidative stress in a model depression in mice. Prog Neuro-psychopharmacol Biol Psychiatry. 2016;64:142–8. doi: 10.1016/j.pnpbp.2015.08.002. [DOI] [PubMed] [Google Scholar]
- 48.Chng HT, New LS, Neo AH, Goh CW, Browne ER, Chan EC. Distribution study of orally administered lipoic acid in rat brain tissues. Brain Res. 2009;1251:80–6. doi: 10.1016/j.brainres.2008.11.025. [DOI] [PubMed] [Google Scholar]
- 49.Bierhaus A, Chevion S, Chevion M, Hofmann M, Quehenberger P, Illmer T, et al. Advanced glycation end product-induced activation of NF-kappaB is suppressed by alpha-lipoic acid in cultured endothelial cells. Diabetes. 1997;46:1481–90. doi: 10.2337/diab.46.9.1481. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The primary data for this study is available from the authors on direct request.