Abstract
In this investigation, the concentration of some organochlorine pesticides (OCPs) was monitored in the Karkheh River and the risk assessment of exposure to these pesticides residue through the water supply system was calculated. The mean concentrations of Lindane, Heptachlor, Chlordane, Dieldrin, Endrin, DDT were 0.135, 0.123, 0.077, 0.081, 0.076, 0.01 µg/L, respectively. The average risk of Lindane, Heptachlor, Chlordane, Dieldrin, DDT, and Endrin for adults was 1.2 E-6, 1.1E-6, 7E-7, 7.6E-7, 9E-8, 7E-7 or non-carcinogenic risks to adults decreased in Dieldrin > Chlordane > Heptachlor > Endrine > DDT > lindane. The hazard index for all organochlorine pesticides was less than 1. These results did not raise concerns about the health of people exposed to studied pesticides. Total concentrations of all OCPs in the Karkheh River were below guidelines for individual pesticides. The hazard quotient showed that the consumption of treated water from the Susangard drinking water treatment plant has no non-cancerous effects. The HI was less than 1 that indicating the risk of exposure to a mixture of OCPs was not significant. Developing policies to reduce the use of pesticides and the use of suitable management practices could be implemented to lower the pesticide levels in the Karkheh River.
Keywords: Risk assessment, Organochlorine pesticides, Water quality, Karkheh River, Iran
Introduction
In recent years, due to population growth and the need for more food production, the use of pesticides for the enhancement of productivity in modern agriculture has increased [1, 2]. The widespread use of pesticides for different purposes in agriculture has resulted in their residues entry into the environment [3, 4]. Drainage caused by irrigation of agricultural lands, wastewater effluents, and surface runoff causes the washing of these pesticides and directs them from different layers of the soil into aquatic environments [5]. The physicochemical attributes of these compounds, particularly relevant to their solubility in organic solvents and water, determine their character of leaching into different sources of water, and therefore these resource quality is a subject to be concerned [3, 6]. Depending on their chemical stability, these substances may undergo decomposition processes, and their metabolites can be also pollutants [7, 8]. Pesticide exposure through environments such as atmospheric air, soil, variety of food products, and surface and groundwater has become a concern [9, 10]. Recently, toxicological interactions of pesticides have also been identified (immunomodulant effects, mutagenicity, carcinogenicity, hormone modulate effects of environmental endocrine disruptor chemicals), they are toxic substances for human health and other organisms in the environment [11]. Classification of pesticides is mainly based on target organisms or targeted use (insecticide, herbicide, fungicide, etc.), application requirement (agriculture, public health, domestic), or chemical nature (organochlorines, organophosphates, etc.). The entry of agricultural, urban, and industrial wastewater into rivers increases the level of pesticides in these water resources [12, 13]. The presence of these compounds in water is regulated through two different directives, including the drinking water directive [14], groundwater directive [15], and water framework directive [16–18]. According to these directives, the maximum allowed concentration is 500 ng/L for total pesticide residues and 100 ng/L for individual pesticides and their degradation products respectively in surface and drinking water samples [19]. Organochlorines (OC) are widely used as pesticides which are a group of chlorinated compounds. These chemicals are high persistence in the environment that belongs to the class of persistent organic pollutants (POPs). Chlorinated insecticides are used in the control of typhus and malaria successfully, but in most advanced countries, these compounds are banned [20]. OCPs constitute about 40% of the total consumption of pesticides [21, 22]. Chronic exposure to pesticides has been associated with negative health effects such as neurological disorders, hormone disturbance, cardiorespiratory symptoms, reproductive aberrance, and cancer [23]. Karkheh River originates from the Zagros Mountains and after passing about 900 km in Lorestan and Khuzestan provinces, reaches to Hoor al-Azim wetland. Karkheh River is the longest river and has the third inflow rate in Iran. In addition, the Karkheh River is used for irrigation of vast agricultural lands as well as an application for water supply systems. Due to its important role in agriculture and water supply systems, Karkheh River was selected for monitoring water quality in terms of OCPs. So far, various studies have investigated different pollutants in Karkheh River, but no study has been done on the survey of OCPs in this River. This investigation aimed to monitor the OCPs in Karkheh River, OCPs in Susangerd water treatment plant in OCPs removal, and the risk assessment of exposure to OCPs through the water supply system in Karkheh River, Khuzestan province for the first time.
Material and method
Study area
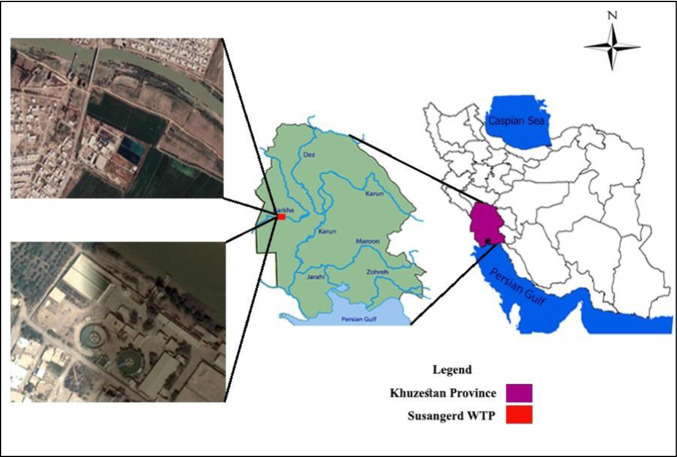
As shown in Fig. 1, Khuzestan province is a vast plain in which the biggest rivers of Iran are located in this region, where the drinking water of people is supplied by the Karkheh River (31° 33′ 0″ and 48° 10′ 12″). The intake and water treatment plant of Susangerd city with a population of 52,000 people, was subjected to sampling.
Fig. 1.
The location of sampling points in the study area
Sampling
A period of one year in 2019 with intervals of one month was considered for sampling from the inlet and outlet of the WTPs. Samples of each station were collected in 2.5 L glass bottles according to standard methods for the examination of water and wastewater [24]. In this study, six OCPs including Lindan, Heptachlor, Chlordane, Dieldrin, Endrin, and DDT were investigated.
OCPs analysis
Acetone, carbon tetrachloride, De-ionized water, and methanol were purchased from Sigma–Aldrich (pesticide- Silanized). Methanol was used to prepare the stock standard solutions. Working solutions were prepared by dilution of the standard stock. To prevent pesticide degradation, the stock solution was stored at 4 ˚C. The working solutions with the concentrations range of 0.01 and 0.6 µg/L were prepared by dilution of the stock solution, and to diminish the possible errors, the diluted solutions were prepared daily. Samples were analyzed by model Agilent 7890 gas chromatography-negative-ion-chemical ionization mass spectrometry. First, 5 mL of each considered sample was poured into a 10 mL-falcon tube and spiked with each pesticide at a concentration of 10 µg/L. Next, an appropriate mixture of the solvent extraction and dispensing solvent was injected into the falcon tube (10 µL of a mixture of organic solvents containing 10 µL of tetrachloroethylene, as extraction solvent, and 1000 µL of acetone as disperser solvent). Then, the mixture was gently shaken for 30 min until the formation of the cloud solution in the test tube. The resulting solution was centrifuged at 3000 rpm for 3 min. Then, a specific volume of the settling phase was injected into the GC–MS system for analysis of the analytes of interest [25]. In this study, a GC column (HP-5, 30 m L × 0.25 mm ID and 0.25 μm) was used to separate the analytes of interest. The oven temperature was started at 80 ˚C and kept at this temperature for 1 min. Then, the temperature was increased to 175 ˚C with a ramp of 30 ˚C/min and then kept for 4 min. Next, the oven temperature was increased to 225 ˚C with a ramp of 3 ˚C/min and kept at this final temperature for 10 min to remove all remaining analytes inside the column.
Quality control and assurance (QC/QA)
Throughout the investigation, to increase the accuracy of the results, an analytical quality control procedure was used. all glassware related to sampling and analysis of collected water samples was washed with deionized water several times. High purity reagents and chemicals were used for experiments. All evaluations were performed triplicate to ensure that the results were within the range. The limit of detection (LOD) and Limit of quantification (LOQ) was used to determine the sensitivity of the analytical methods used in the study [26]. The LOD for Lindan, Heptachlor, Chlordane, Dieldrin, Endrin, and DDT was 0.512, 0.496, 0.264, 0.257, 0.27, and 0.025 µg/L respectively. The LOQ for Lindan, Heptachlor, Chlordane, Dieldrin, Endrin, and DDT was 1.63, 1.58, 0.84, 0.82, 0.86, and 0.8 µg/L respectively.
Statistical analysis
All measured data were analyzed using Excel, SPSS v.23 software for windows. Descriptive statistics including the means, standard deviation, max, and min values were determined first. The original concentration data normality was evaluated using the Kolmogorov-Smirnov test (significance level was considered as P value ≤ 0.05) [27]. Analysis of variance was used to compare the effect of time on concentration difference of measured OCPs, especially in terms of before and after cultivation period. Spearman correlation analysis was used to assess the linear correlation between OCPs. Kruskal Wallis test was used to determine the significance of the mean difference of each OCP [28]. Man Whitney test was used to compare the mean concentrations of OCPs at the inlet and effluent of the WTPs [29].
Health risk assessment
The assessment model used by USEPA was applied to calculate the carcinogenic and non-carcinogenic risks for adults and children consuming the water. The chronic daily intake (CDI) is used to estimate human exposure to OCPs [9], which was calculated using the following formula [30]:
| 1 |
Where CDI is the chronic daily intake from the oral exposure route (µg kg− 1per day), C represents the concentration of the target pollutants (µg L− 1); IR is the water intake rate (L day− 1) (for children: IR = 1.0; for adults: IR = 2.0); EF represents the exposure frequency (350 days year− 1); ED is exposure duration (year) (for children: ED = 6; for adults: ED = 70); BW is body weight (kg) (for children: BW = 14; for adults: BW = 60); AT is average lifespan (days) (for children: AT = 2190; for adults: AT = 25,550). According to the USEPA exposure factors handbook [10], carcinogenic risk (LCR) is calculated as follows [30]:
| 2 |
Where SF is the slope factor of the contaminant via oral exposure route [(µg kg− 1 per day) −1]. Therefore, the following equation was used to evaluate the non-carcinogenic risk, hazard quotient (HQ) [31]:
| 3 |
Where RfD (µg kg− 1 per day) is the reference dose of the contaminant via oral exposure route. The rates of SF and RfD (Table 1) for OCPs are prepared from the USEPA Integrated Risk [32].
Table 1.
Toxicological parameter of studied OCPs
| OCP | SF [(µg kg− 1 per day)−1] | RFD (µg kg− 1 per day) |
|---|---|---|
| Lindane | 1.3 | 3 .0E -04 |
| Heptachlor | 1.3E -05 | 1.7E 01 |
| Chlordane | 5 E -04 | 3.5E 0–1 |
| Dieldrin | 5 E -05 | 1.6E 01 |
| DDT | 5 E -04 | 3.4 E -01 |
| Endrine | 5 E -04 | 1 |
Results and discussion
OCPs concentration in Karkheh River
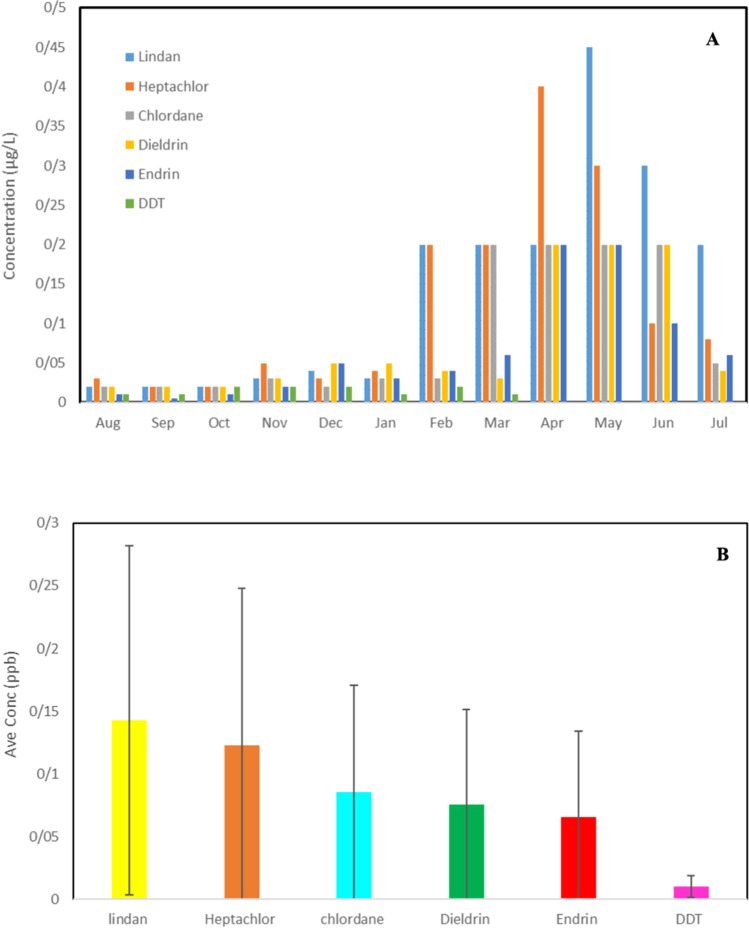
The variations of OCPs residues (µg/L) in raw water samples are presented in Table 2. As can be seen from Table 2, the mean and max concentrations of all studied OCPs were less than national standards. Among the measured OCPs, the highest residue concentrations were related to lindane and heptachlor, which were observed in early summer (July). However, the Kruskal Wallis test showed that due to the trend of changes in the concentrations of OCPs throughout the year, no significant difference was observed for the concentration of each pesticide in different months of the year (Table 3). Variations of studied OCPs and the average cumulative concentrations of each OCP are presented in Fig. 2. Compared with other regions around the world, the levels of heptachlor and lindane in the Karkheh River were upper than those in the Weihe River, china (0.00089 and 0.00385 µg/L, respectively) [33]. Lindane levels in Lake Bosomtwi (0.071 µg/L) were lower than what was observed at Karkheh River in this study with a mean concentration of 0.135 µg/L [34]. Adeel Mahmood et al. (2014) Reported heptachlor and lindane levels of 0.00016 and 0.00085 µg/L in Chenab River, and this value is lower than what was observed at Karkheh River in this study (0.135, 0.123 µg/L respectively) [35]. Studies have shown that the concentration of pesticides in different water sources varies between 0.1 and 107 mg/L [36, 37] This result was supported by our study. Akan JC et al. (2014) reported that the water samples within the river Challawa in Nigeria were contaminated by some OCPs and their concentrations were higher than the set EU maximum residue limit (MRL) [38]. In the study area, according to the climatic and geographic conditions, during the year, agricultural activities are longer than in other areas, starting in mid-winter and continuing until early autumn, which leads to the consumption of various pesticides, because of their low cost, and finally the entry of these pesticides through the runoff from spring rains into Karkheh River.
Table 2.
Descriptive statistics of individual OCPs in intake of Karkheh River
| OCP | Mean concentration (µg/L) | Stdev | Min | Max | National standard (µg/L) |
|---|---|---|---|---|---|
| Lindan | 0.135 | 0.163 | 0.02 | 0.5 | 2 |
| Heptachlor | 0.123 | 0.158 | 0.022 | 0.5 | - |
| Chlordane | 0.077 | 0.084 | 0.02 | 0.2 | 200 |
| Dieldrin | 0.081 | 0.082 | 0.02 | 0.2 | 0.3 |
| Endrin | 0.076 | 0.086 | 0.02 | 0.2 | 0.6 |
| DDT | 0.01 | 0.008 | 0.01 | 0.02 | 1 |
Table 3.
Analysis of difference of OCPs concentrations between months using Kruskal Wallis test
| Lindan | Heptachlor | Chlordane | Dieldrin | Endrin | DDT | |
|---|---|---|---|---|---|---|
| Chi-Square | 5.636 | 10.047 | 4.872 | 7.637 | 7.061 | 3.668 |
| df | 5 | 5 | 5 | 5 | 5 | 5 |
| Asymp. Sig. | 0.343 | 0.074 | 0.432 | 0.177 | 0.216 | 0.598 |
Fig. 2.
A Variations of studied OCPs during different months of sampling and B The average cumulative concentrations of each OCP
OCPs removal efficiency
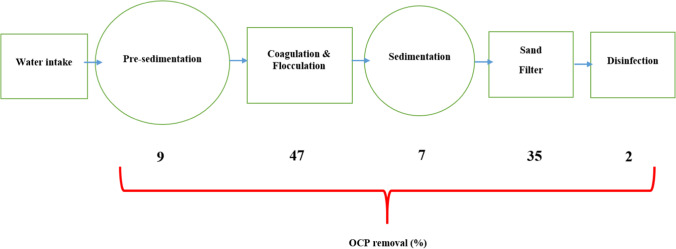
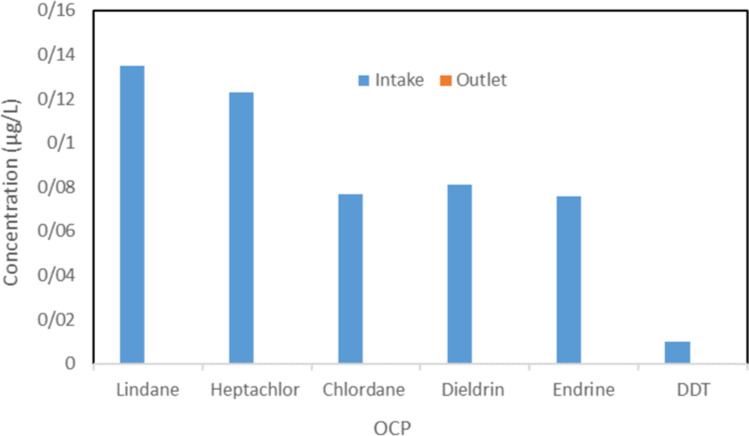
The removal of studied OCPs in the Susangerd water treatment plant was investigated to determine the operational function of unit operations and processes. The flow diagram of the Susangerd water treatment plant is presented in Fig. 3. which demonstrates a conventional treatment chain [39]. The OCPs removal occurred in each unit is also presented in Fig. 3. which shows that the concentration of these pesticides after the treatment process ranged between 0 and 0.0003 µg/L for Lindane, Heptachlor, Chlordane, Dieldrin, Endrin, DDT. The most percentage of OCPs removal is related to coagulation & flocculation, and sand filter units, 47 and 35%, respectively. The result was supported by Nurulizani Elfikrie et al. (2020), pesticide removal efficiencies in the conventional drinking water treatment plant were between 77 and 100% in Tengi River Basin, Malaysia [40]. A study conducted by M.P. Ormad. (2007) in the Ebro River Basin, Spain, has presented by using different methods in the drinking water treatment plant, 60 to more than 90% of pesticide removal can be reached [41]. In addition, the comparison of the OCPs concentration in intake and outlet is presented in Fig. 4.
Fig. 3.
The OCPs removal occurred in each unit operation or process of Susangerd water treatment plant, Iran
Fig. 4.
The Comparison of OCPs concentration between raw and treated water
Health risk assessment
Lifetime cancer risk (LCR) is used to estimate carcinogenicity. It is introduced as an indicator of the probability of increasing the incidence of cancer due to specific exposure (Eq. 2). In addition, the hazard ratio relationship is used to estimate the risk of non-carcinogenic exposure The relationship of the hazard ratio (Hazard Quotient or HQ) is the ratio of exposure to a substance than that of the substance without any adverse effects [42]. The non-carcinogenic human health risks posed by exposure to OCPs in the Karkheh River are given as HQs in Table 4. The slope factor is an acceptable range where the probability of a response to the consumption of a chemical is present during the lifetime. The presence of pesticide residues in the water indicates an environmental and health risk due to reuse for agricultural purposes. The entry of toxins into drinking water sources due to their extreme resistance to environmental factors, water solubility, and toxicity to living things can have detrimental effects on human health and the environment. Various studies show that residues of pesticides in water resources are directly related to the amount of pesticides consumed in these areas and if these pesticides are not controlled, these persistent pollutants pose a serious threat to consumers’ health [43]. The adult’s health risks (HI) were calculated according to the assessment model. The average risk of Lindane, Heptachlor, Chlordane, Dieldrin, DDT, and Endrin for adults was 1.2 E-6, 1.1E-6, 7E-7, 7.6E-7, 9E-8, 7E-7 or non-carcinogenic risks to adults decreased in Dieldrin > Chlordane > Heptachlor > Endrine > DDT > lindane. Heptachlor had the highest risk index, and Chlordane had the lowest risk index among the listed OCPs. The World Health Organization has accepted the LCR in the range of 10E-5–10E-6 and below that value [44]. According to previous studies, the value of 10E-4 has been determined as (Definite Risk), Probable Risk (10E-4 to 10E-5), and Possible Risk in the range of 10E-5–10E-6. Comparison of risk level of OCPs with a recommended limit of WHO (range between 10E-5 Up to 10E-6 and below the rate) the acceptable risk of OCPs was acceptable and these results did not raise concerns about the health of people exposed to OCPs. The cumulative risk in OCPs (non-carcinogenic) was calculated by summing the total HQ of each toxin (Eq. 4). Risk index below one is safe and above one is unsafe.
Table 4.
Human health risk assessment results (HQ) for selected OCPs
| OCP | SF [(µg kg− 1 per day)−1] | RFD (µg kg− 1 per day) | CDI | HQ | LCR |
|---|---|---|---|---|---|
| Lindane | 1.3 | 3E -4 | 1.2 E-6 | 4E-3 | 1.5E-6 |
| Heptachlor | 1.3E -5 | 1.7E 1 | 1.1E-6 | 6E-8 | 1E-5 |
| Chlordane | 5 E -4 | 3.5E -1 | 7E-7 | 1.4E-3 | 2.4E-8 |
| Dieldrin | 5 E -5 | 1.6E 1 | 7.6E-7 | 1.5E-2 | 1.2E-5 |
| DDT | 5 E -4 | 3.4 E -1 | 9E-8 | 1.8E-4 | 3E-6 |
| Endrine | 5 E -4 | 1 | 7E-7 | 2.3E-3 | 7E-7 |
| 4 |
HQ of less than 1 indicates that the OCP concentration is lower than the threshold concentration to produce harmful effects and does not cause any harm to individuals [42].
Conclusion
During this study, we detected a variety of OCPs at µg/L levels in the Karkheh River that supplies drinking water and treated drinking water of large communities such as Susngerd city, situated in Khuzestan province. The water treatment plant of Susangerd city reduced OCPs concentrations significantly. Generally, total concentrations of all OCPs in the Karkheh River were below guidelines for individual pesticides. The hazard quotient showed that the consumption of treated water from the Susangard drinking water treatment plant has no non-cancerous effects. The HI was less than 1 that indicating the risk of exposure to a mixture of OCPs was not significant. Management methods are recommended to reduce the concentration of pesticides in the Karkheh River. This requires the cooperation of landowners working around agricultural resources, which can include the creation of buffer strips of natural vegetation along field margins, the development of wildlife habitat along reservoir margins, and organic farming. Developing policies to reduce the use of pesticides and the use of suitable management practices could be implemented to lower the pesticide levels in reservoirs and thereby improve the water quality and safety of the water.
Acknowledgements
We would like to thank ABFA Corporation for its financial support. Also, the researchers express their heartfelt gratitude to everyone involved in this study.
Author contribution
Neematollah Jaafarzadeh Haghighi Fard: conceptualized, and designed the experiments, Masoud Panahi Fard: performed the experiments, Sadegh Haghighipur: writing-review, and editing. Ebrahim Sharifi: writing–original draft, Sahand Jorfi: writing-review, and editing.
Funding
This research was financially supported by ABFA Corporation, Khuzestan.
Data availability
All relevant data during the current study are within the manuscript and available from the corresponding author on reasonable request.
Code availability
Not applicable.
Declarations
Ethical approval
Not applicable.
Consent for publication
Not applicable.
Conflict of interest
The authors declare that they have no conflict of interest.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kılıç O, Boz İ, Eryılmaz GA. Comparison of conventional and good agricultural practices farms: A socio-economic and technical perspective. J Clean Prod. 2020;258:120666. doi: 10.1016/j.jclepro.2020.120666. [DOI] [Google Scholar]
- 2.Hemathilake D, Gunathilake D (2022) High-productive agricultural technologies to fulfill future food demands: Hydroponics, aquaponics, and precision/smart agriculture, in Future Foods. Elsevier, pp 555–67.
- 3.MacBean CE. The Pesticide Manual. 16th ed. Brighton: British Crop Production Council; 2015.
- 4.Medić Pap S, Popović B, Stojić N, Danojević D, Pucarević M, Červenski J, Šperanda M. The environmental issue of pesticide residues in agricultural soils in Serbia. Int J Environ Sci Technol. 2022;1–14.
- 5.Holvoet KM, Seuntjens P, Vanrolleghem PA. Monitoring and modeling pesticide fate in surface waters at the catchment scale. Ecol Model. 2007;209(1):53–64. doi: 10.1016/j.ecolmodel.2007.07.030. [DOI] [Google Scholar]
- 6.Sheridan EA, et al. Plastic pollution fosters more microbial growth in lakes than natural organic matter. Nat Commun. 2022;13(1):1–9. doi: 10.1038/s41467-022-31691-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aizawa H. Metabolic maps of pesticides. vol.2: Amsterdam: Elsevier; 2012.
- 8.Moradnia M, et al. The relation of cancer risk with nitrate exposure in drinking water in Iran. Iran J Public Health. 2019;48(2):362–4. [PMC free article] [PubMed] [Google Scholar]
- 9.Samarghandi MR, et al. Pollution status of pesticide residues in food products in Iran: A mini-review within 2008–2018. Arch Hyg Sci. 2020;9(3):214–23. doi: 10.29252/ArchHygSci.9.3.214. [DOI] [Google Scholar]
- 10.Ahmadi M, et al. Monitoring and application of artificial neural network model for prediction of organophosphorus pesticides residue in Ahvaz water treatment plants. Biointerface Res Appl Chem. 2021;11(6):14032–43. doi: 10.33263/BRIAC116.1403214043. [DOI] [Google Scholar]
- 11.Jaafarzadeh N, et al. Carcinogenic risk assessment of nitrate contamination of drinking water resources in South Provinces of Iran. Int J Environ Anal Chem. 2021:1–10.
- 12.Mushtaq N, et al. Freshwater contamination: sources and hazards to aquatic biota. In: Fresh Water Pollution Dynamics and Remediation. Berlin: Springer; 2020. pp. 27–50.
- 13.Whelan MJ, et al. Is water quality in British rivers “better than at any time since the end of the Industrial Revolution”? Sci Total Environ. 2022:157014. [DOI] [PubMed]
- 14.Ljujic B, Sundac L. [[Council] Directive 98/83/EC [of 3 November 1998] on the quality of water intended for human consumption: review and intregral translation [from English into Serbian]]. Voda i sanitarna tehnika (Serbia and Montenegro).
- 15.Directive G. Directive 2006/118/EC of the European Parliament and of the Council of 12 December 2006 on the protection of groundwater against pollution and deterioration. Official J Eur Union L. 2006;372:19–31. [Google Scholar]
- 16.Commission E. Directive 2013/39/EU of the European Parliament and of the Council of 12 August 2013 amending Directives 2000/60/EC and 2008/105/EC as regards priority substances in the field of water policy. Off J Eur Union. 2013;226:1–17. [Google Scholar]
- 17.Parliament E. Directive 2008/105/EC of the European Parliament and of the Council of 16 December 2008 on environmental quality standards in the field of water policy, amending and subsequently repealing. Official J Eur Union. 2008;348:84–97. [Google Scholar]
- 18.Directive WF, Directive 2000/60/EC of the European Parliament and of the Council of 23 October 2000 establishing a framework for Community action in the field of water policy. Off J Eur Commun. 2000;22(12):2000.
- 19.Wang G, et al. Risk assessment of organophosphorus pesticide residues in drinking water resources: Statistical and Monte-Carlo approach. Chemosphere. 2022:135632. [DOI] [PubMed]
- 20.Aktar W, Sengupta D, Chowdhury A. Impact of pesticides use in agriculture: their benefits and hazards. Interdiscip Toxicol. 2009;2(1):1–12. doi: 10.2478/v10102-009-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamid ZA, et al. The association of nuclear abnormalities in exfoliated buccal epithelial cells with the health status of different agricultural activities farmers in Peninsular Malaysia. Genes Environ. 2016;38(1):7. doi: 10.1186/s41021-016-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sarkar UK, Pathak AK, Srivastava SM. Assessing organochlorine pesticide (OCP) residues in water and fish samples from a small perennial river and associated wetlands of Ganga Basin, India for sustainable management. Sustain Water Resour Manag. 2022;8(1):1–8. doi: 10.1007/s40899-022-00623-2. [DOI] [Google Scholar]
- 23.Gupta P. Pesticide exposure—Indian scene. Toxicology. 2004;198(1–3):83–90. doi: 10.1016/j.tox.2004.01.021. [DOI] [PubMed] [Google Scholar]
- 24.Federation WE, Association APH. Standard methods for the examination of water and wastewater. Washington, DC: American Public Health Association (APHA); 2005.
- 25.WEF AA. Standard Methods for the Examination of Waterand Wastewater. American Public Health Association. 21st ed. Washington DC: AmericanWater Works Association, Water Environmental Federation; 2005.
- 26.Long GL, Winefordner JD. Limit of detection. A closer look at the IUPAC definition. Anal Chem. 1983;55(7):712A–724A. [Google Scholar]
- 27.Myers L, Sirois MJ. Spearman correlation coefficients, differences between. Encyclopedia of statistical sciences. 2004;12.
- 28.Lee AF, Gurland J. One-sample t-test when sampling from a mixture of normal distributions. Ann Stat. 1977:803–7.
- 29.Hart A. Mann-Whitney test is not just a test of medians: differences in spread can be important. BMJ. 2001;323(7309):391–3. doi: 10.1136/bmj.323.7309.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guo W, Feng Y. Health risk assessment of organochlorine pesticides in a shallow freshwater lake, China. In: Advanced Materials Research. Trans Tech Publ; 2014.
- 31.Jaafarzadeh N, et al. Non-carcinogenic risk assessment of Cr and Pb in vegetables grown in the industrial area in the southwest of Iran using Monte Carlo Simulation approach. Int J Environ Res. 2022;16(2):1–10. doi: 10.1007/s41742-022-00396-8. [DOI] [Google Scholar]
- 32.EPA U. Integrated risk information system (IRIS) Washington, DC: EPA; 1999. [Google Scholar]
- 33.Wang D, et al. Residues and distributions of organochlorine pesticides in China’s Weihe River. Pol J Environ Stud. 2016;25(3).
- 34.Darko G, Akoto O, Oppong C. Persistent organochlorine pesticide residues in fish, sediments and water from Lake Bosomtwi, Ghana. Chemosphere. 2008;72(1):21–4. doi: 10.1016/j.chemosphere.2008.02.052. [DOI] [PubMed] [Google Scholar]
- 35.Mahmood A, et al. Levels, distribution pattern and ecological risk assessment of organochlorines pesticides (OCPs) in water and sediments from two tributaries of the Chenab River, Pakistan. Ecotoxicology. 2014;23(9):1713–21. doi: 10.1007/s10646-014-1332-5. [DOI] [PubMed] [Google Scholar]
- 36.Goodwin L, et al. Treatment options for reclaiming wastewater produced by the pesticide industry. 2017.
- 37.Rodriguez-Narvaez OM, et al. Treatment technologies for emerging contaminants in water: a review. Chem Eng J. 2017;323:361–80. doi: 10.1016/j.cej.2017.04.106. [DOI] [Google Scholar]
- 38.Akan J, et al. Determination of organochlorine, organophosphorus and pyrethroid pesticide residues in water and sediment samples by high performance liquid chromatography (HPLC) with UV/visible detector. J Anal Bioanal Tech. 2014;5(6).
- 39.Hamilton D, et al. Regulatory limits for pesticide residues in water (IUPAC Technical Report). Pure Appl Chem. 2003;75(8):1123–1155.
- 40.Elfikrie N, et al. Occurrence of pesticides in surface water, pesticides removal efficiency in drinking water treatment plant and potential health risk to consumers in Tengi River Basin, Malaysia. Sci Total Environ. 2020;712:136540. doi: 10.1016/j.scitotenv.2020.136540. [DOI] [PubMed] [Google Scholar]
- 41.Ormad MP, et al. Pesticides removal in the process of drinking water production. Chemosphere. 2008;71(1):97–106. doi: 10.1016/j.chemosphere.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 42.Rahimnejad S, et al. Quantitative risk assessment of occupational exposure to Volatile Organic Compounds in the oil-dependent chemical industry. J Sabzevar Univ Med Sci. 2014;21(4):829–41. [Google Scholar]
- 43.Mendes KF, et al. Water resource pollution by herbicide residues. In: Organic Pollutants. IntechOpen; 2019.
- 44.Agency UEP. Methods for derivation of inhalation reference concentrations and application of inhalation dosimetry. Washington, DC: US EPA; 1994.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data during the current study are within the manuscript and available from the corresponding author on reasonable request.
Not applicable.