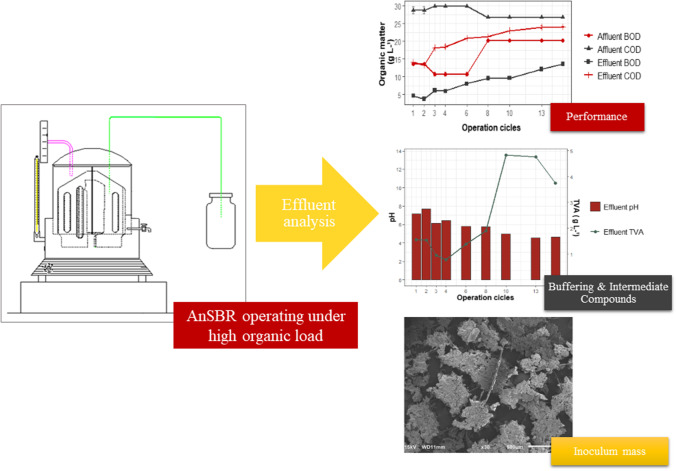
Abstract
Studies reporting the performance of anaerobic sequencing batch reactor (AnSBR) operating with high organic loadings are scarce. This study aimed to contribute to the technical and scientific literature by reporting the experience obtained when biodiesel wastewater was treated in an AnSBR applying organic loading rates (OLR) above those commonly used in batch reactor projects. For this, physicochemical and chromatographic analysis of the effluent were carried out. Further, the biomass was assessed chemically and morphologically, along with bacterial diversity characteristics. Supported by these analyses, the system performance was discussed in terms of COD remotion efficiency and buffering capacity. The AnSBR reached 10% of COD removal at the steady-state, which caused the biomass defragmentation and facilitated washout. This suggests that the startup and operation of AnSBR under optimized conditions with an average applied OLR of 11.3 gCOD L−1 d−1 worked as a pressure for the microbiota selection, stimulating the production of total volatile acids, which promoted system reduction efficiency and souring. In this context, food/microorganism ratios above 1.0 gCOD gTVS−1 d−1 can favor acidogenic activity, and total volatile acids/bicarbonate alkalinity concentration ratios above 1.9 may indicate acidification. The addition of support material for immobilizing/increasing biomass retention and/or operation under two-stage may be interesting alternatives for increasing AnSBR efficiencies under high OLRs.
Graphical abstract
Keywords: Biomass washout, Biomass shear, Microbiota selection, Glycerol, Overload, Acidification
Introduction
Due to increasing concerns related to climate change, renewable energy matrices such as biodiesel emerge as a potential substitute for fossil fuel use. The latest growth projections predict a growth in the global biodiesel market corresponding to 218.7 billion dollars by 2022, with emphasis on the expansion of the Brazilian market [1]. At least 10% of the volume of the total biodiesel produced corresponds to glycerol, which is generated as a byproduct of the transesterification reaction [2]. Biodiesel agroindustry wastewater (BW) is therefore composed of a large amount of glycerol that is produced but not processed, together with other aqueous byproducts from the biodiesel industry.
Research has been conducted to investigate the operational parameters and types of anaerobic reactors suitable for BW treatment. With this focus, anaerobic digestion has been widely studied as an alternative for the BW treatment due to its rich carbonaceous chain [3–5]. Among the alternatives, the anaerobic sequencing batch reactor (AnSBR) offers advantages concerning continuous treatment due to the great process flexibility and lesser need for segregated clarifiers from the unit [6].
Seifert et al. [7] studied anaerobic digestion of glycerol for H2 production, concluding that glycerol concentration in BW should not exceed 30 g L−1 in batch anaerobic reactors, i.e. the maximum permissible COD in BW would be 36 gCOD L−1. Furthermore, these authors found that the biomass concentration directly influenced organic matter conversion efficiency. According to Seifert et al. [7], the optimized values for the operation of anaerobic batch reactors would be BW with 10 g L−1 glycerol (or 12 gCOD L−1) and a minimum biomass concentration of 1.16 gVSS L−1 volatile suspended solids (VSS). Their study was cited in several further studies that investigated anaerobic digestion of BW containing glycerol.
Lovato et al. [8] investigated an anaerobic sequencing batch biofilm reactor (AnSBBR) in treatment of BW with 3.1 gCOD L-1, biomass concentration of 36.7 gTVS L−1 total volatile solids (TVS), applied organic loading rate (OLR) of 4.5 gTVS L−1 d−1 and 6 h cycle time, in which they obtained COD removal efficiencies of 83%, higher than values previously reported.
Bravo et al. [9] operated an AnSBBR with biomass in the range of 0.9 to 2.1 gTVS L−1 for treatment of BW with COD ranging from 3 to 5 gCOD L−1, OLR from 7.6 to 12.9 gCOD L−1 d−1 and 3 and 4 h cycles, and obtained COD removal efficiencies between 19 and 26%.
Pereira et al. [10] analyzed the synergic effects of six factors on anaerobic biodegradation of glycerol during the treatment of BW in an AnSBR and determine that that temperature, mixing speed, mass of inoculum, and reaction time were the most influent factors on COD removal efficiency from BW. Tangkathitipong et al. [5] tested the hydrogen and methane production separately and spontaneously from biodiesel wastewater with added glycerin using a two-stage AnSBR system and found that the use of mixed gas produced from a two-stage anaerobic system can provide a higher heating value than the biogas generated from any single anaerobic unit, besides of better performance of both gas production compared to a unique stage. Pereira et al. [6] optimize the factors reaction time, inoculum mass and reactor operating temperature, as well as developed a mathematical model for simulation and control of the BW in AnSBR treatment process. Also, according to the authors, inoculum mass and applied OLR are important for the start and operation of AnSBR, as it affects the relationship between the food to microorganism (F/M) ratio. Pereira et al. [3] pointed out that values of aOLR above 5.0 gCOD L−1 d−1 and F/M ratio above 0.4 gCOD gVSS−1 d−1 have the potential, under inadequate conditions of alkalinity, to inhibit the growth of methanogenic archaea, due to accumulation of TVA promoting souring of AnSBR during the treatment of biodiesel wastewater.
From an analysis of recent literature in the matter of BW degradation by anaerobic batch reactors [3, 5, 6, 10], the following gaps are found: (1) few studies have reported the effect of organic shocks by progressive increased organic loading rate on BW degradation kinetics and microbial diversity in AnSBRs; (2) further studies must be conducted to analyze the effects of initial pH and influent COD on the souring process caused by excessive alkalinity consumption; (3) no study have explored the single stage AnSBR capacity of BW treatment operating with OLR above 9.3 gCOD L−1 d−1 and under optimal conditions.
Although these works indicated, preliminary, that OLR values above 7.6 gCOD L−1 d−1 are too high for use in batch reactors for reaction times of up to 6 h in BW treatment because of the high accumulation of volatile acids in the reactor, the capacity of operating in higher loads and, in consequence, greater reaction time, must be further tested with AnSBR in the steady-state operating under optimized conditions.
With the increase in biodiesel production and residual glycerol production, studies focusing on BW treatment operating under optimal conditions and high loads and, consequently, smaller net volumes are significant for the environmental engineering. Therefore, the objective of this study was to evaluate an anaerobic sequencing batch reactor (AnSBR) operating under optimized conditions at organic loading above those established in the literature for batch reactors and investigate the effect of the high organic loading on COD removal, production of intermediate anaerobic degradation products and on anaerobic biomass morphology and bacterial diversity. We chose the anaerobic sequencing batch reactor because it offers high efficiency of organic matter removal, better control of the effluent quality, and simple and stable operation in the industry [11].
Materials and methods
Experimental apparatus and BW
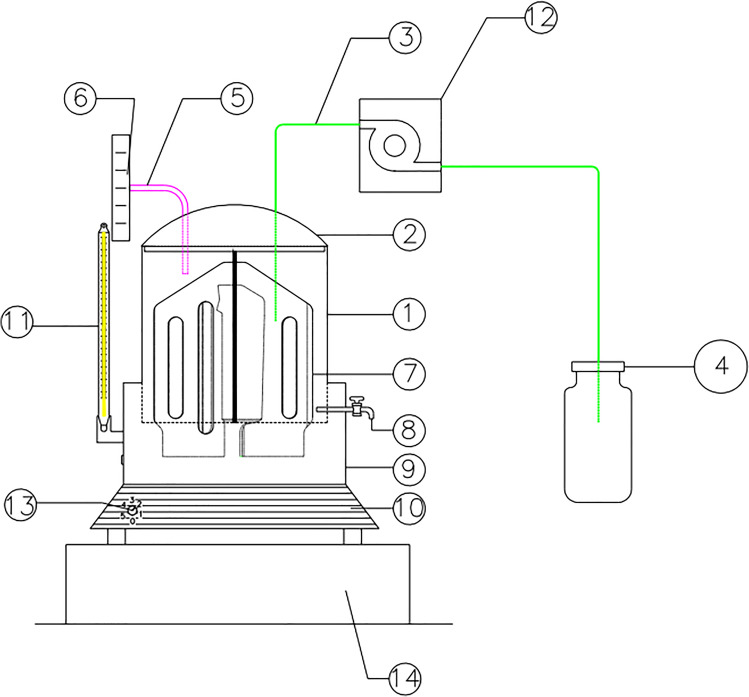
The AnSBR used in the experiment (Fig. 1) was constructed in acrylic, with a thermostatic bath for temperature control (10 to 90 °C), regulator for mixing speed (0 to 40 rpm) and sludge disposal valve installed in the reactor base. The reactor had a total volume of 6.8 L, including a 5.0 L working volume and 1.8 L (headspace) for biogas storage. Two valves were installed on the upper part of the reactor cover, one for release of biogas through a gasometer for flow measurement and another for feeding the reactor with synthetic BW and withdrawing the treated BW after each batch cycle, using a positive displacement peristaltic pump. An acrylic impeller, developed by Pereira et al. [10], was used to provide a completely-mixed hydraulic condition in the reactor.
Fig. 1.
AnSBR Reactor used for the treatment of the BW. Source: [10]. 1- Reactor working volume. 2- Reactor cover. 3- Piping for the influent BW pumping and suction of treated BW (supernatant) after decantation. 4- Recipient for storage of influent and treated BW. 5- Piping for biogas conduction to the gasometer. 6- Gasometer. 7- AnSBR reactor impeller. 8- Valve for biomass disposal. 9- Thermostatic bath for temperature maintenance. 10- Region with electrical devices used for temperature control and agitation of the impeller. 11- Digital thermometer connected to the thermostatic bath for continuous temperature recording. 12-Pump used for influent suction and effluent pumping. 13- Temperature control. 14- Holder for the reactor
The AnSBR operated in batch mode, with fixed feeding (FT), reaction (RT), sedimentation (ST) and withdrawal (WT) times of 0.5; 20; 3 and 0.5 h, respectively, totalizing a 24 h cycle time (CT), that correspond to the total time of batch operation. The values adopted were based on studies presented by Pereira et al. [6].
The synthetic BW used throughout the experiment was prepared with commercial glycerol (sole source of organic matter) diluted in distilled water. COD of the BW used throughout the experiment was fixed at 30 gCOD L−1. Concentrations of nitrogen (from urea) and phosphorus (from a mixture of phosphate salts) were determined to maintain a COD:N:P ratio of 1000:5:1. Sodium bicarbonate was added to supplement alkalinity and other nutrients (FeSO4; FeCl3; CaCl2; CoCl2; SeO2 and MgCl2) also added, obeying quantities indicated by Silva et al. [12] and Lovato et al. [13]. The mixture of phosphates came from monobasic potassium phosphate (KH2PO4), dibasic potassium phosphate (K2HPO4) and dibasic sodium phosphate (Na2HPO4), contained each 31 g of P and totaling 93 g of phosphorus.
AnSBR start-up and monitoring of the experiment
The biomass used to inoculate the AnSBR was collected from an upflow anaerobic sludge blanket (UASB) reactor used in the treatment of municipal wastewater. The inoculum presented 74.6 ± 0.6 gTS L−1 total solids (TS), 43.6TVS ± 0.4 g L−1 total volatile solids (TVS) and 31.0 ± 0.3 gTFS L−1 total fixed solids (TFS). AnSBR operation was initiated with a mixture of 2.34 L of biomass inoculum and 2.66 L of BW, corresponding to a 20.4 gVSS L−1, as suggested by Pereira et al. [6] for AnSBR startup in treatment of BW. No further biomass was added to the reactor throughout the remainder of the study.
The reactor thermostat was adjusted to 36 °C (maintained constant in all cycles) and at the end of the FT, the BW-biomass mixture was in thermal equilibrium. As soon as the reactor was filled, the impeller was turned on to 40 rpm for the duration of the RT. At the end of this stage, the impeller was turned off and the biomass sedimentation stage (ST) began. After the ST, 2 L of the supernatant was withdrawn and stored for physical and chemical analysis as described in Table 1. After the withdrawal of the treated BW, 2 L of raw BW was added to the reactor to initiate a new cycle.
Table 1.
Analytical methods used to characterize biomass inoculum and BW (influent and effluent)
| Parameter | Method | Reference |
|---|---|---|
| pH | Laboratory electrode method: 4500—H + B | APHA, AWWA, WPCF [14] |
| Alkalinities: total (TA) and bicarbonate (BA) | Potentiometric Titration | Ripley et al. [15] e Jenkins et. al [16] |
| Chemical Oxygen Demand: Total and Soluble | 5220-D: Colorimetric Method with digestion in closed reflux | APHA, AWWA, WPCF [14] |
| Total Solids (TS), Fixed (TFS), Volatile (TVS) and Total Suspended Solids (TSS), Fixed (FSS) and Volatile (VSS) of sludge and BW | 2540-B; 2540-D; 2540-E | |
| Total Volatile Acids (TVA) | Potentiometric Titration | Dilallo and Albertson [17] |
| BW temperature (operating) | Laboratory method with digital thermometer: 2550-B | APHA, AWWA, WPCF [14] |
| Biochemical Oxygen Demand (BOD) at 5d and 20ºC: Total and Soluble | Incubation: 5210- B. Determination of dissolved oxygen by the electrode method: 4500—O G | APHA, AWWA, WPCF [14] |
| Biomass proteins | Colorimetric method with spectrophotometer reading | Bradford [18] |
| Biomass polysaccharides | Colorimetric method with spectrophotometer reading | Dubois et al. [19] |
The applied volumetric organic loading values (OLR), food to microorganism ratio (F/M) and the organic matter removal efficiency in terms of COD were determined using Eqs. 1, 2, and 3, respectively.
| 1 |
| 2 |
| 3 |
where
- influent CODT
is the total COD of the influent BW (gCOD L−1),
- effluent CODS
is the soluble COD of the effluent BW (gCOD L−1),
- VBW
is the BW volume used in the feeding of AnSBR (2 L),
- VT
is the reactor useful total volume (L),
- MS
is the sludge mass present in the AnSBR (gVSS),
- OLR
is the applied organic loading rate (gCOD L−1 d−1),
- F/M
is the food/microorganism ratio (gCOD gVSS−1),
- RT
is the reaction time (d),
- E (%)
is the COD removal efficiency (%).
Physicochemical and chromatographic analyses of biomass and BW
Table 1 presents the physicochemical analyses and methodological procedures used in monitoring the AnSBR influent and effluent and the biomass used as inoculum. The analysis in Table 1 was conducted in triplicates with subsequent mean and standard deviation calculation. The mass spectra (m/z) of the anaerobic metabolism intermediate compounds produced by biomass identified by chromatography were obtained based in duplicates.
Samples were filtered through glass microfiber filters (0.6 µm pore size) prior to analysis of soluble COD and BOD, TSS, FSS and VSS, polysaccharides and proteins. An industrial inoculum (Polyseed®, Interlab®) was used for the determination of BOD. The inoculum was activated in distilled water at 20 °C under aeration for 1 h and 4 mL of this solution were used in each BOD flask.
The AnSBR was considered in steady-state when variation in COD removal efficiency between cycles was less than 15%. When this situation occurred, t test was conducted at 5% of significance in order to confirm the inexistence of a significant difference between cycles. After the operation reach the steady-state, the effluent samples were collected and analyzed by gas chromatography-mass spectrometry (Shimadzu GCMS-QP2010, Shimadzu, Kyoto, Japan) for identification of intermediate anaerobic degradation products and quantification of volatile acids. Samples were prepared and standards extracted for calibration using the methodology adapted by Adorno et al. [20]. Four mL of filtered sample (0.6 µm membrane) or standard volatile acid solution were transferred to 22 mL flasks suitable for headspace analysis, equipped with Teflon tape and septa. Two g NaCl and 400 µL of a 2 M H2SO4 solution were added to the flasks that were then heated to transfer volatile compounds to the gas phase (headspace). The gas phase compounds were transferred to the GC–MS system for analysis using a gastight syringe. The GC–MS system operated in a full scanning mode (mass acquisition range m/z 30–400) with injector temperature of 250 °C. The GC was equipped with a RTX-5MS capillary column (30 m × 0.25 mm × 0.25 μm) operated under the following conditions: initial temperature 60 °C, heating rate 8 °C min−1 to 200 °C, held for 5 min. Helium at a flowrate of 1.37 mL min−1 was used as carrier gas. Solvent cutoff time was 1.5 min. The injected sample volume was 1.0 μL and the data acquisition time was 22.5 min. The detector voltage was 1.2 kV, and the electron impact (EI) model was selected for compound ionization at 70 eV. Compound identification was performed using spectral matching (similarity analysis), comparing experimental mass spectra with the mass spectra of compounds catalogued by the National Institute of Standards and Technology (NIST), with catalogued compounds stored in NIST mass spectral libraries 8, 8 s, 11 and 11 s. Quantification of volatile acids was conducted in this same GC–MS system using the same operating conditions, after calibration with a standard solution made with analytical grade reagents (> 98%).
Influent and effluent COD and volatile acids concentrations were used to calculate masses and yields described in Eqs. 4 to 9.
| 4 |
| 5 |
| 6 |
| 7 |
| 8 |
| 9 |
where
- CVA
is the concentration of each volatile acid in the AnSBR effluent (g L−1),
- VD
is the volume withdrawn from the AnSBR in each cycle (2 L),
- Eq-COD
is the COD equivalent of each quantified volatile acid (1 g of HAc = 1.067 g of COD; 1 g HPr = 1.514 g of COD; 1 g HBu = 1.818 g of COD),
- effluent CODS
is the soluble COD of the effluent from the AnSBR (g L−1),
- M CODVA
is the effluent COD mass in volatile acid form (g),
- M CODR
is the effluent COD mass not converted into volatile acids (g),
- M CODS
is the soluble effluent COD mass (g),
- M CODT
is the total COD mass applied to the AnSBR (g),
- influent CODT
is total COD of the effluent to the AnSBR,
- the YVA
is the CODT fraction applied to the AnSBR that was converted into volatile acids,
- YR
is the CODT fraction applied that was not transformed into volatile acids.
Biomass assessment: chemical, morphological characteristics and bacterial diversity
Assessment of biomass chemical characteristics and microbial diversity was conducted using 50 mL of AnSBR sludge inoculum (sample 1) and of sludge removed from the AnSBR after achieving stability (sample 2). Samples preparation were conducted following the methodology proposed by Pereira et al. [21]. Analyses were performed using scanning electron microscopy/silicon drift detector energy-dispersive X-ray spectrometry (SEM/SDD-EDS) (JEOL, JSM-6010LA) with scanning from 500 V to 20 kV, 4 nm (20 kV) resolution and 8X to 300 000X magnification.
Analysis of bacterial diversity was performed by denaturation gradient gel electrophoresis (DGGE). DNA extraction of the anaerobic bacteria and methanogenic archea of both sludge samples was conducted using PowerSoil® DNA Isolation Kit (MoBio Laboratories Inc.).
For the analysis of anaerobic bacterial and methanogenic archaea in the sludge samples, primers R1492 (5’-TACCTTGTTACGACTT-3’) and F27 (5’-AGAGTTTGATCMTGGCTCAG-3’) [22] were used for the amplification of the V1 to V9 regions of 16S rDNA. The reactions were conducted in a total volume of 25 μL containing 10 ng of the DNA mold, 0.2 μM of each primer, 0.2 mM of dNTPs, GoTaq Buffer (Promega), 3.0 mM of MgCl2, 0.2 mg.mL−1 of BSA and 1 U of GoTaq DNA polymerase (Promega). The amplifications were conducted in the Mastercycler (Eppendorf) thermocycler using an initial denaturation cycle at 94 °C for 4 min, followed by 35 cycles of 94 °C for 30 s, 55 °C for 1 min and 72 °C for 1.5 min.
A final extension cycle at 72 °C was conducted for 7 min. PCR products (~ 1400 pb) were analyzed in agarose gel 2.0% (m.v−1). The product of the first PCR reaction was used as a template for the amplification of the V6 to V9 regions of the 16S rDNA using the R1492 and F984 primers (5’-AACGCGAAGAACCTTAC-3’) [23] containing a GC clamp (5’-CGC CCG CGC CCC GCG CCC GTC CCG CCC CCG CCC G-3’) [24]. The reactions were carried out in a total volume of 25 μL containing 10 ng of the DNA mold, 0.2 μM of each primer, 0.2 mM of dNTPs, GoTaq Buffer (Promega), 3.0 mM of MgCl2, 0.2 mg.mL−1 of BSA and formamide 1% (v.v−1 and 1 U of GoTaq DNA polymerase (Promega).
The amplification reaction consisted of an initial denaturation cycle at 95 °C for 3 min, followed by 35 cycles of 94 °C for 30 s, 57 °C for 30 s and 72 °C for 30 s. A final extension cycle at 72 °C was conducted for 1 min. The PCR products (~ 500 pb) were analyzed in agarose gel 2.0% (m.v−1).
DGGE analysis was conducted using the DCode Universal Mutation System (Bio-Rad, HERCULES, CA, USA). Thirty microliters of the PCR products from the last reaction (F984-GC and R1492 primers) were applied to a polyacrylamide gel (acrylamide: bisacrylamide) at 8% (m.v−1) in TAE buffer (Tris base 40 mM; glacial acetic acid 20 mM; EDTA 10 mM, pH 8.0) prepared with 40 to 55% denaturant gradient, from 100% denaturant solution [7 M of urea and 40% (v.v−1) of formamide, and 0% denaturant. Electrophoresis was conducted for 20 h under 60 V and 60 °C. At the end of the run, the gel was stained for 20 min with SYBR Gold solution (Invitrogen™).
DNA was revealed and the image digitized using a photo-documentation imager Fire Reader XS (Uvitec). The images were processed in the BioNumerics version 6.0 program (Applied Maths, Inc.) for comparing DGGE band patterns. The analyses were conducted based on the Dice similarity coefficient and the unweighted pair group method with arithmetic mean (UPGMA). The diversity and richness analyses were conducted using the PAST software [25].
Results and discussion
Operational parameters, removal of organic matter and solids in the system
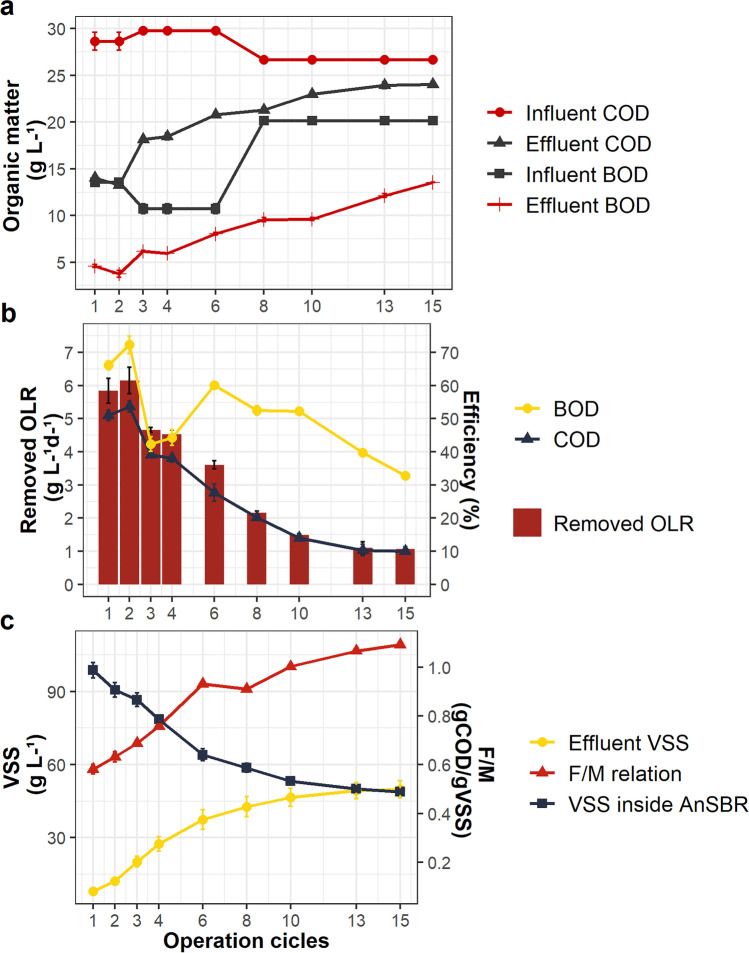
The OLR was maintained at 11.3 ± 0.6 g L−1 d−1 COD until COD removal efficiency stabilizes. The effects of the high OLR can be seen in Fig. 2, that includes influent and effluent COD and BOD levels (Fig. 2a), removed OLR (OLRR) and removal efficiency (Fig. 2b), and solids and F/M ratio in the reactor (Fig. 2c).
Fig. 2.
Influent and effluent organic matter concentrations in terms of COD and BOD (a), removal efficiency values and OLRR (b) and VSS concentration in the effluent (accumulated) and reactor, as well as the food/microorganism ratio (F/M) (c) from the start to the steady-state
Influent COD and BOD were 28.1 ± 1.3 gCOD L−1 and 15.5 ± 4.2 gBOD L−1, respectively throughout the experiment. Removal efficiencies in the first cycle were 54% for COD and 70% for BOD, however after 15 cycles of operation values of COD removal efficiency fell to 10% and BOD removal fell to 30% (Fig. 2b), indicating process collapse. Due to the reduction in the COD removal efficiency over time (Fig. 2b), the OLRR value decreased in the same intensity, with average OLRR values of 6.0 ± 0.2 g L−1 d−1 at the beginning and 1.1 ± 0.1 g L−1 d−1 at the end of the experiment.
No significant differences (p > 0.05) were found in COD removal efficiencies from the 13th to 15th cycles (Fig. 2a and b), indicating that the AnSBR reached steady-state within 15 cycles of operation.
The influent BW contained 6.7 ± 0.5 gTS L−1, 5.3 ± 0.4 gTVS L−1, 0.5 ± 0.1 gTSS L−1, and 0.2 ± 0.04 gVSS L−1, with a much higher proportion of dissolved solids than suspended solids. However, effluent VSS were consistently higher than influent VSS. Furthermore, there was a decrease in sludge VSS content, probably due loss of solids from the reactor (washout).
Organic matter removal efficiency (Fig. 2b) decreased with the loss of biomass (Fig. 2c) from the AnSBR. Thus, in the 15th cycle, after the continuous loss of biomass, the lowest organic matter removal efficiency of 10% was obtained. Lovato et al. [13], utilizing AnSBBR for treating whey and glycerol, applied an OLR 7.5 gCOD L−1 d−1 and obtained a performance of 89% of COD removal efficiency. Pereira et al. [3] obtained 54% of efficiency at aOLR of 1.3 gCOD L−1 d−1.
The inoculum mass was shown to be the most influential factor in organic matter removal efficiency [6], which can explain the lower efficiency found in the present work related to others, making the F/M ratio a parameter of great importance to process efficiency. This is linked to the fact that the rates of hydrolysis, production of volatile acids and methane directly depend on the availability of food and the population of microorganisms.
Bezerra et al. [26], treating BW in AnSBBR, used F/M ratios from 0.03 to 0.12 gCOD gTVS-1 d-1 for methane production and achieved COD removal efficiencies of 67 to 92%. Souza et al. [27], in order to promote methanogenic inhibition and produce hydrogen in AnSBR, used F/M ratios of 1 to 3 gCOD gTVS-1 d-1 and obtained COD removal efficiencies of 14 to 21%.
Pereira et al. [3], treating BW in AnSBR, used aOLR ranging from 1.3 to 9.3 gCOD L−1 d−1 at 36ºC and F/M ratio from 0.1 to 0.6 gCOD gVSS−1 d−1, found a statistically significant difference in COD removal efficiency with aOLR values higher 5.0 gCOD L−1 d−1 and affirmed that values of aOLR above this limit and F/M above 0.4 gCOD gVSS−1 d−1 have the potential to inhibit methanogenic archae growth and to accumulate of TVA produced by acidogenic fermentative bacteria, promoting the souring of the system.
Furthermore, the results of the work of Tangkathitipong et al. [5], which obtained a 77% remotion efficiency treating BW with addiction of crude glycerol at 37º, cycle time of 4 h at maximum, aOLR of 9.6 gCOD L−1 d−1 (a close aOLR compared to present work), indicate preliminary that an increase in the reaction time to 20 h (adopted in this work) is not enough and a two stage ASBR is more recommended for reaching satisfactory performance of COD remotion.
In contrast with the limit found by the work of Pereira et al. [3] and in consonance with the results of Souza et al. [27], our results, presented in Fig. 2c suggest that for batch reactors, F/M ratios above 1 gCOD gTVS−1 d−1 can favor acidogenic activity and decrease COD removal efficiency. Under the F/M ratio conditions reigning in the reactor from 7th cycle onward (Fig. 2c), the accumulation of volatile organic and alcohols could be expected to occur, leading to an increase in effluent TVA and a drop in pH, indicating imbalance in the anaerobic biodegradation process [9, 27].
Buffering and intermediate compounds of anaerobic metabolism
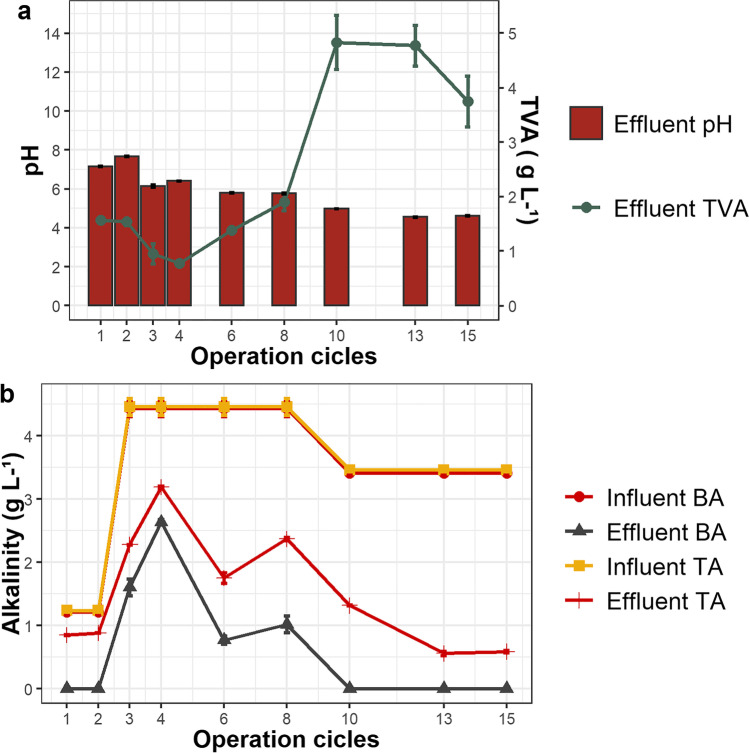
Figure 3 shows the behavior of total and bicarbonate alkalinities, TVA concentration and pH variation over 15 cyles of AnSBR operation.
Fig. 3.
Concentrations of total volatile acids (TVA) and pH variation effluent to AnSBR (a). Values of bicarbonate (BA) and total (TA) alkalinities over the 15 cycles of influent (BW) and effluent to AnSBR (b)
The BW contained 0.052 ± 0.017 g L−1 TVA, however, TVA in the AnSBR effluent ranged from 1.5 to 2.0 g L −1 through the eighth cycle, and thereupon increased progressively, reaching maximum values of 5.0 g L−1. Although raw BW pH was maintained between 8 ± 0.2, pH of the treated effluent fluctuated between 7.2 and 4.6 over the period of operation (Fig. 3a). From the 6th cycle, the pH was 5.8, which is inadequate for the growth of methanogenic archaea, but suitable for growth of acidogenic fermentative bacteria. The low pH suggests that TVA accumulated in the reactor, causing souring of the medium and reduction in COD removal efficiency (Fig. 2b).
Although effluent alkalinity decreased from the initial values, until the 6th cycle the system still had 1.75 g L−1 TA and 0.77 g L−1 BA, indicating buffering capacity was maintained with a minimum pH of 6 until the 6th cycle (Fig. 3b). From the 10th cycle onwards, BA was completely consumed and TA decreased from of 1.32 ± 0.008 to 0.59 ± 0.020 g L−1 from the 10th to the 15th cycle, evidence of reactor souring [6]. Before souring started (8th cycle), concentrations of TVA (Fig. 3a) and BA (Fig. 3b) were 1.9 g L−1 and 1.0 g L−1, respectively. Thus, the TVA concentration can be up to 1.9 times higher than that of BA and higher ratios between the two factors may indicate souring of the environment.
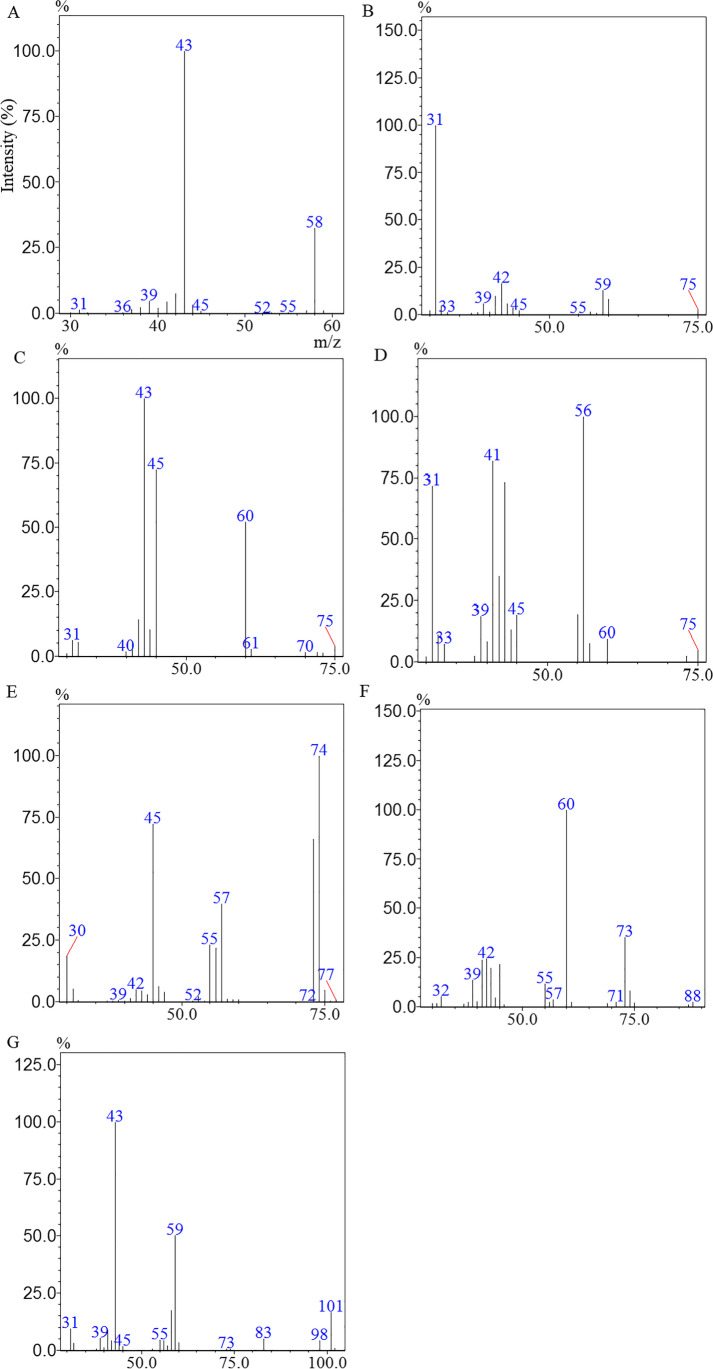
The increase in TVA concentration observed (Fig. 3a) was accompanied by a decrease in COD removal efficiency (Fig. 2b), suggesting that there was an accumulation of volatile acids which resulted in the souring of the AnSBR. The mass spectra (m/z) of the anaerobic metabolism intermediate compounds produced by biomass under the souring condition are shown in Fig. 4.
Fig. 4.
Mass spectra of the acetone (a), propanol (b), acetic acid (c), butanol (d), propionic acid (e), butyric acid (f) and 2-pentanone (g) found in samples effluent to AnSBR during cycles 13th, 14th and 15th
The BW (influent) contained glycerol as the only carbon source, while the mean of effluent samples from cycles 13–15 contained the intermediate anaerobic degradation products acetone (Fig. 4a), propanol (Fig. 4b), acetic acid (Fig. 4c), butanol (Fig. 4d), propionic acid (Fig. 4e), butyric acid (Fig. 4f), 2-pentanone (Fig. 4g). From this group of 7 anaerobic degradation intermediates, the 3 volatile acids produced were quantified (Table 2).
Table 2.
Average values of the concentration of bioproducts, CODVA, CODS, CODR, YVA and YR
| Parameter | CVA (g L−1) |
VD (L) |
Eq-COD (g/g) |
Mass (g) | M CODVA; M CODT; M CODS; M CODR (g) | YVA and YR |
|---|---|---|---|---|---|---|
| Acetic Acid | 1.1 | 2.0 | 1.067 | 2.3 | M CODVA = 19.1 | YVA = 0.36 |
| Propionic Acid | 5.3 | 2.0 | 1.514 | 16.1 | ||
| Butyric Acid | 0.2 | 2.0 | 1.818 | 0.7 | ||
| Influent CODT | 26.7 | 2.0 | - | - | M CODT = 53.4 | - |
| Effluent CODS | 24.0 | 2.0 | - | - | M CODS = 48.0 | - |
| CODR | - | - | - | - | M CODR = 28.9 | YR = 0.54 |
As can be seen in Table 2, 36% of total influent COD was converted to acetic acid, propionic acid and butyric acid, which, according to Seifert et al. [7], characterizes AnSBR operation under acidogenic conditions. In addition, 54% of the soluble effluent COD was in the form of unconverted glycerol, recalcitrant organic matter and anaerobic metabolism intermediates, such as propanol, butanol and 2-pentanone. The 10% of unaccounted COD may have been used for cell growth and/or production of gaseous products such as CH4 and H2. This coincides with the 10% of COD removal efficiency stabilized since the 13th cycle (Fig. 2b). The results presented in Table 2 suggest that the actual efficiency of glycerol conversion would be 46%.
Characterization of the inoculum mass in the AnSBR reactor
Anaerobic reactor biomass is composed of a mixture of microbial communities and their metabolic products, including exopolymeric substances (EPS). EPS composition depends on the type of biomass present in the reactor. Furthermore, the mass ratio between EPS constituents and sludge VSS is of fundamental importance for characterization of anaerobic biomass and its granulation conditions [28]. Thus, sludge EPS polysaccharide and protein concentrations and their mass ratios to VSS were determined in the sludge washed out from the AnSBR (Fig. 5).
Fig. 5.
EPS composition in the biomass found in the washout over the 15 operation cycles
In the first cycle of AnSBR operation, the average protein concentration (0.33 g L−1) was higher than that of carbohydrates (0.071 g L−1). However, over the course of operation, proteins decreased and carbohydrates increased, so that after the 15th cycle the mass of carbohydrates was approximately 6 times greater than of proteins (Fig. 5). The initial EPS characteristics were typical of methanogenic biomass, whereas after 15 cycles they were characteristic of acidogenic bacteria [29, 30], corroborating the increase in TVA production after the 8th cycle.
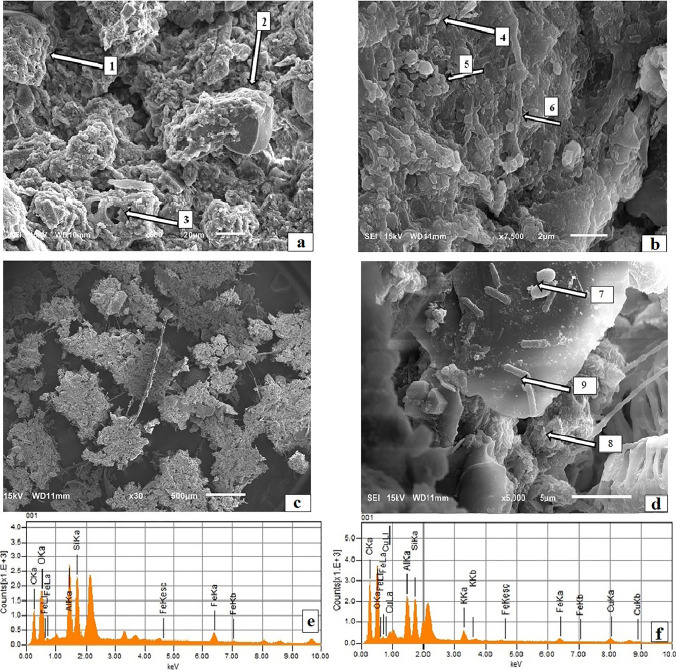
The continuous increase of the ratio exopolysaccharides by VSS represented in Fig. 5 indicates that, even though the biomass was continuously washed out, the EPS mainly composed of polysaccharide, was retained in the AnSBR, produced by acidogenic biomass that maybe was continuously colonizing the medium. To evaluate this hypothesis and the AnSBR reactor souring, an analysis of biomass used to inoculate the AnSBR reactor and that remained in the reactor after 15 cycles of operation was performed using scanning electron microscopy (SEM) (Fig. 6a-d) and energy dispersion X-ray spectroscopy (EDS) (Fig. 6e-f).
Fig. 6.
SEM micrograph of the inoculated biomass (a and b) and the biomass after 15 cycles of operation (c and d), in addition to the general profile of the EDS of the biomass used in the inoculation (e) and after 15 cycles of AnSBR operation (f)
Figure 6a shows the cohesion of biomass, with few empty spaces and well-defined granules (point 1). The structural differentiation of points 2 and 3 was performed through the analysis of EDS (Fig. 6e), which suggests a composition rich in carbon, oxygen and silicon for the amorphous material in point 3. Since the composition is rich in carbon, the organic helical structures (point 3) may be a vegetable compound, not bacteria or fungi, due to their 20 µm scale. The vegetable compound is believed to be cellulose, once Da Silva et al. [31] found structure with similar morphology in a SEM of coffee wastewater in endogeny, which is rich in cellulosic material. Once the material in point 2 is poor of carbon, it may be a mineral structure.
A loss of cohesion of the biomass (Fig. 6c), along with losses of amorphous material evidenced via EDS (Fig. 6f), after 15 cycles of operation was observed with defragmentation and no clearly defined granules observed. In the sludge inoculum (Fig. 6b) it is possible to perceive apparent microbial formations, with several agglomerates in the form of bacilli (point 4), cocci colonies (point 5) and cocobacilli linked to central cylindrical structures, possibly filamentous bacteria (point 6). Thus, it appears that the biomass used to inoculate the reactor was cohesive, with consistent granular aspects and had considerable morphological diversity. After the 15th cycle (Fig. 6d) fewer microbial formations were found and those that were detected were in the form of bacilli (point 7), bound cocobacilli (point 9) and, under the disperse amorphous material (point 8), cocci bacteria. Based on these analyses, it was possible to verify that after 15 cycles of operation, the anaerobic biomass, exhibited neither cohesion (Fig. 6c), nor morphological diversity. The reduction in the amount of biomass may have occurred due to its washout.
According to Fig. 6e, the inoculum sludge was composed of carbon (C), oxygen (O), sodium (Na), aluminum (Al), silicon (Si), potassium (K), calcium (Ca), iron (Fe) and copper (Cu) with the following percentages 27 ± 9%; 45 ± 9%; 1 ± 0%; 8 ± 2%; 9 ± 0%; 2 ± 1%; 0.3 ± 0.3%; 5 ± 0% and 3 ± 0%, respectively. However, a 51% loss in carbon mass was detected after the 15 cycles of operation, which probably led to reduced cohesion of the biomass. In addition, enrichment of inorganic elements was noted, probably due to the precipitation of these nutrients from the BW.
EPS of the biomass used as reactor inoculum had characteristics of methanogenic archaea. However, the decrease in COD removal efficiency (Fig. 2b), the accumulation of TVA (Fig. 3a), the increase in EPS polysaccharides and decrease in proteins (Fig. 5), in addition to the decrease in density of bacteria in the biomass after 15 cycles of operation (Fig. 6d), all suggest modification in microbial ecology and diversity of microorganisms, which is corroborated by the change of biomass with methanogenic characteristics to acidogenic microbiota.
To confirm the assumptions regarding the change in microbial diversity caused by souring of the AnSBR reactor during BW treatment, DGGE analysis was performed comparing the biomass used as inoculum with the biomass sample maintained in the AnSBR reactor after 15 cycles of operation, already adapted to BW (Fig. 7).
Fig. 7.
Electrophoretic profiles and PCR-DGGE cluster analysis results generated from biomass samples using primers that target the coding sequences of regions V6 to V9 of the 16S rDNA. Sample 1—sample of the biomass used in the inoculation. Sample a—sample of the biomass acclimated to BW and collected after the souring of the AnSBR reactor
Visual analysis of the DGGE gel (Fig. 7) allowed the identification of some dominant operational taxonomic units (OTUs) present in both sample 1 and sample a, and unique OTUs in each sample. The position equivalence among the DGGE bands indicates that some bacteria or archaea present in the biomass used as inoculum (sample 1) were resistant to the souring of the AnSBR reactor, and became dominant members of the microbial community. However, the OTUs present in sample 1 and not observed in sample a indicate that some bacteria in the inoculum could not establish themselves as members of the microbial community in sample a, suggesting that they were not able to cope with interspecificcompetition or with reactor souring.
The presence of some OTUs in sample a but not in sample 1 indicates that some species present at low numbers in the inoculum became dominant in sample a. This, together with the results of EPS analysis (Fig. 5), indicates that most of the prokaryote populations in sample 1 were methanogens, but that from the 8th cycle of operation, the microbial community started to be progressively dominated by acidogenic microorganisms, which prevailed in biomass due to souring. Pereira et al. [3], studying BW degradation in AnSBR at 37ºC with an applied OLR of 9.3 gCOD L−1 d−1, also found the appearing of new OTUs in acid media with lower OLR conditions compared to the rate applied in this study, along with decrease of AnSBR performance. The authors also attributed to dominance of acidogenic microorganisms.
As can be seen in the cluster analysis (Fig. 7) the level of prokaryotic community similarity based on the Dice coefficient between sample 1 and sample a is approximately 55%. This indicates that after 15 cycles of operation, the microbial communities were significantly altered. To analyze the change in composition, the diversity and richness indices of OTUs were determined in samples 1 and a (Table 3).
Table 3.
Diversity and richness indexes of OTUs
| Biomass sample | ||
|---|---|---|
| 1 | a | |
| Richness (R) | 10 | 13 |
| Shannon (H) | 2.303 | 2.565 |
| Gini-Simpson (1-D) | 0.9 | 0.923 |
Sample 1—sample of the biomass used as inoculum. Sample a—sample of biomass acclimated to BW and collected after souring of the AnSBR reactor
The richness index (R) (Table 3) refers only to the amount of OTUs present in anaerobic biomass. The Shannon (H) and Gini-Simpson (1-D) biological diversity indices refer to both the number (richness) of different OTUs and the relative abundance of these categories.
Richness was higher in sample a than in sample 1 (Table 3). The values of the Shannon (H) and Gini-Simpson (1-D) diversity indices reveal that the microbial community established after 15 days in the treatment of BW (sample a) was more complex than the biomass used in inoculation. Therefore, the results suggest that the reactor souring led to changes in the diversity of microorganisms, turning the biomass present in the AnSBR reactor different from that used in the inoculation.
Conclusions
The analysis of exopolysaccharides, total volatile acids and intermediate compounds of anaerobic metabolism indicated that the anaerobic sequencing batch reactor operated under acidogenic conditions, reaching 10% of COD remotion at the wa, which caused the biomass defragmentation and facilitated anaerobic sequencing batch reactor washout. This suggests that startup and operation of the anaerobic sequencing batch reactor under optimized conditions with an average applied organic loading rate of 11.3 g L−1 d−1 worked as a pressure for the microbiota selection, stimulating the production of total volatile acids, which promoted system reduction efficiency and souring. In this context, food/microorganism ratios above 1.0 gCOD gTVS d−1 can favor acidogenic activity, and total volatile acids/bicarbonate alkalinity concentration ratios above 1.9 may indicate souring of the environment.
Therefore, a two-stage anaerobic sequencing batch reactor may be recommended for increase system efficiency to satisfactory levels at 11.3 gCOD L−1 d−1, or in case of operation under acidogenic conditions in BW treatment, the working volume of the reactor should contain support material for immobilizing biomass to increase its retention. Given the above, the use of anaerobic sequencing batch reactor that has mechanical agitation and operates with non-immobilized biomass is not recommended for this case, with the anaerobic sequencing batch biofilm reactors being more suitable for this purpose.
Funding
This work was funded by National Council for Scientific and Technological Development (CNPq 144783/2019–3) and by the Coordination for the Improvement of Higher Education Personnel (CAPES Finance Code 001).
Declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Zion Market Research. Biofuels market to drive swiftly and reach USD 218.7 769 billion in 2022. 2018. https://www.zionmarketresearch.com/news/biofuels-market Accessed 4 March 2020.
- 2.He QS, McNutt J, Yang J. Utilization of the residual glycerol from biodiesel production for renewable energy generation. Renew Sustain Energy Rev. 2017;71:63–76. doi: 10.1016/j.rser.2016.12.110. [DOI] [Google Scholar]
- 3.Pereira EL, Borges AC, Silva GJ. Effect of the progressive increase of organic loading rate in an anaerobic sequencing batch reactor for biodiesel wastewater treatment. Water. 2022;14:223. doi: 10.3390/w14020223. [DOI] [Google Scholar]
- 4.Silva MCA, Monteggia LO, Alves Barroso Júnior JC, Granada CE, Giongo A. Evaluation of semi-continuous operation to hydrogen and volatile fatty acids production using raw glycerol as substrate. Renew Energy. 2020;153:701–710. doi: 10.1016/j.renene.2020.01.152. [DOI] [Google Scholar]
- 5.Tangkathitipong P, Intanoo P, Butpan J, Chavadej S. Separate production of hydrogen and methane from biodiesel wastewater with added glycerin by two-stage anaerobic sequencing batch reactors (ASBR) Renew Energy. 2017;113:1077–1085. doi: 10.1016/j.renene.2017.06.056. [DOI] [Google Scholar]
- 6.Pereira EL, Borges AC, Heleno FF, Oliveira KR, Silva GJ, Mounteer AH. Central composite rotatable design for startup optimization of anaerobic sequencing batch reactor treating biodiesel production wastewater. J Environ Chem Eng. 2019;7:103038. doi: 10.1016/j.jece.2019.103038. [DOI] [Google Scholar]
- 7.Seifert K, Waligorska M, Wojtowski M, Laniecki M. Hydrogen generation from glycerol in batch fermentation process. Int J Hydrogen Energy. 2009;34:3671–3678. doi: 10.1016/j.ijhydene.2009.02.045. [DOI] [Google Scholar]
- 8.Lovato G, Bezerra RA, Rodrigues JAD, Ratusznei SM, Zaiat M. Effect of feed strategy on methane production and performance of an AnSBBR treating effluent from biodiesel production. Appl Biochem Biotechnol. 2012;166:2007–2029. doi: 10.1007/s12010-012-9627-6. [DOI] [PubMed] [Google Scholar]
- 9.Bravo ISM, Lovato G, Rodrigues JAD, Ratusznei SM, Zaiat M. Biohydrogen production in an AnSBBR Treating glycerin-based wastewater: effects of organic loading, influent concentration, and cycle time. Appl Biochem Biotechnol. 2015;175:1892–1914. doi: 10.1007/s12010-014-1421-1. [DOI] [PubMed] [Google Scholar]
- 10.Pereira EL, Borges AC, Heleno FF, Costa THC, Mounteer AH. Factors influencing anaerobic biodegradation of biodiesel industry wastewater. Water Air Soil Pollut. 2017;228:213. doi: 10.1007/s11270-017-3395-4. [DOI] [Google Scholar]
- 11.Arreola-Vargas J, Jaramillo-Gante NE, Celis LB, Corona-González RI, González-Álvarez V, Méndez-Acosta HO. Biogas production in an anaerobic sequencing batch reactor by using tequila vinasses: effect of pH and temperature. Water Sci Technol. 2016;73:550–556. doi: 10.2166/wst.2015.520. [DOI] [PubMed] [Google Scholar]
- 12.Silva RC, Rodrigues JD, Ratusznei SM, Zaiat M. Anaerobic treatment of industrial biodiesel wastewater by an ASBR for methane production. Appl Biochem Biotechnol. 2013;170:105–118. doi: 10.1007/s12010-013-0171-9. [DOI] [PubMed] [Google Scholar]
- 13.Lovato G, Bravo ISM, Ratusznei SM, Rodrigues JAD, Zaiat M. The effect of organic load and feed strategy on biohydrogen production in an AnSBBR treating glycerin-based wastewater. J Environ Manage. 2015;154:128–137. doi: 10.1016/j.jenvman.2015.02.014. [DOI] [PubMed] [Google Scholar]
- 14.APHA, AWWA, WPCF . Standard Methods for the Examination of Water and Wastewater. Washington: American Public Health Association; 2017. [Google Scholar]
- 15.Ripley LE, Boyle WC, Converse JC. Improved alkalimetric monitoring for anaerobic digestion of highstrength wastes. Water Pollution Control Federation. 1986;58:406–411. [Google Scholar]
- 16.Jenkins SR, Morgan JM, Sawyer CL. Measuring anaerobic sludge digestion and growth by a simple alkalimetric titration. Water Pollution Control Federation. 1983;55:448–453. [Google Scholar]
- 17.Dilallo R, Albertson OE. Volatile acids by direct titration. J Water Pollut Control Fed. 1961;33:356–365. [Google Scholar]
- 18.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 19.Dubois M, Gilles A, Hamilton JK, Rebers PA, Smith F. Colorimetric method of determination of sugars and related substances. Anal Chem. 1956;28:350–356. doi: 10.1021/ac60111a017. [DOI] [Google Scholar]
- 20.Adorno MAT, Hirasawa JS, Varesche MBA. Development and validation of two methods to quantify volatile acids (C2–C6) by GC/FID: headspace (Automatic and Manual) and Liquid-Liquid Extraction (LLE) Am J Anal Chem. 2014;5:406–414. doi: 10.4236/ajac.2014.57049. [DOI] [Google Scholar]
- 21.Pereira EL, Campos CMM, Moterani F. Efeitos do pH, acidez e alcalinidade na microbiota de um reator anaeróbio de manta de lodo (UASB) tratando efluentes de suinocultura. Rev Ambiente Água. 2009;4:157–168. doi: 10.4136/ambi-agua.109. [DOI] [Google Scholar]
- 22.Lane DJ. 16S/23S rRNA sequencing. In: Stackebrandt E, Goodfellow M, editors. Nucleic acid techniques in bacterial systematics. New York: John Wiley & Sons; 1991. pp. 115–175. [Google Scholar]
- 23.Heuer H, Hartung K, Wieland G, Kramer I, Smalla K. Polynucleotide probes that target a hypervariable region of 16S rRNA genes to identify bacterial isolates corresponding to bands of community fingerprints. Appl Environ Microbiol. 1999;65:1045–1049. doi: 10.1128/AEM.65.3.1045-1049.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Myers RM, Fischer SG, Lerman LS, Maniatis T. Nearly all single base substitutions in DNA fragments joined to a GC-clamp can be detected by denaturing gradient gel electrophoresis. Nucleic Acids Res. 1985;13:3131–3145. doi: 10.1093/nar/13.9.3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hammer Ø, Harper DAT, Ryan PD. Paleontological statistics software package for education and data analysis. Palaeontol Electron. 2001;4:9–18. [Google Scholar]
- 26.Bezerra RA, Rodrigues JAD, Ratusznei SM, Canto CSA, Zaiat M. Effect of organic load on the performance and methane production of an AnSBBR treating effluent from biodiesel production. Appl Biochem Biotechnol. 2011;165:347–368. doi: 10.1007/s12010-011-9255-6. [DOI] [PubMed] [Google Scholar]
- 27.Souza LP, Lullio TG, Ratusznei SM, Rodrigues JAD, Zaiat M. Influence of organic load on biohydrogen production in an AnSBBR treating glucose-based wastewater. Appl Biochem Biotechnol. 2015;176:796–816. doi: 10.1007/s12010-015-1612-4. [DOI] [PubMed] [Google Scholar]
- 28.Zhou W, Imai T, Ukita M, Li F, Yuasa A. Effect of loading rate on the granulation process and granular activity in a bench scale UASB reactor. Biores Technol. 2007;98:1386–1392. doi: 10.1016/j.biortech.2006.05.035. [DOI] [PubMed] [Google Scholar]
- 29.Liu H, Fang HHP. Extraction of extracellular polymeric substances (EPS) of sludges. J Biotechnol. 2002;95:249–256. doi: 10.1016/S0168-1656(02)00025-1. [DOI] [PubMed] [Google Scholar]
- 30.Veiga MC, Jain MK, Wu WM, Hollingsworth RI, Zeikus JG. Composition and role of extracellular polymers in methanogenic granules. Appl Environ Microbiol. 1997;63:403–407. doi: 10.1128/AEM.63.2.403-407.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Da Silva VG, Campos CMM, Pereira EL, Da Silva JF. Characterization of the biomass of a hybrid anaerobic reactor (HAR) with two types of support material during the treatment of the coffee wastewater. Braz Arch Biol Technol. 2013;56:495–504. doi: 10.1590/S1516-89132013000300018. [DOI] [Google Scholar]