Abstract
Background
Peripheral sensory and motor nerves are often affected in people with diabetes mellitus (DM), and balance problems are widespread. Diabetic peripheral neuropathy is common. This study investigated the impact of exercise treatment on balance parameters in diabetic peripheral neuropathy patients.
Method
Electronic databases, such as PubMed, Web of Science, and Science Direct, were used to perform a search of accessible papers. The search strategy was (exercise therapy OR physical activity) AND (balance OR equilibrium OR postural control OR fall OR fall risk OR static balance OR dynamic balance OR functional balance) AND diabetic peripheral neuropathy. The Physiotherapy Evidence Database (PEDro) scale was used to rate the research in this review for its quality and reliability. Scores ranged from 5 to 9 on the PEDro scale.
Result
According to databases, about 9,103 articles were found in August 2021 from March 1984 to February 2020. From 9103 articles, 3872 were deleted for different reasons, including duplicate, non-randomized controlled trial (RCT) articles and after reviewing the title and abstract. About 5,231 articles were found in free full text. In the end, 12 submissions were approved. These studies investigated the effects of exercise treatment on static, dynamic, and functional balance parameters in diabetic peripheral neuropathy patients.
Conclusions
This study showed the positive effects of balance and strengthening exercise on static balance indices in patients with diabetic peripheral neuropathy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01077-1.
Keywords: Type 2 diabetic neuropathy, Exercise therapy, Balance, Physical activity, Postural control
Introduction
The prevalence of diabetes mellitus (DM) is rising worldwide [1, 2]. As many as 220 million people throughout the globe deal with diabetes, which is anticipated to double by 2030 [3]. Furthermore, 12 to 50% of them suffer from DPN [4, 5]. As diabetes progresses, one of the most prevalent and debilitating complications is diabetic peripheral neuropathy (DPN) [6]. Patients with DPN have bilateral distal deterioration of the peripheral nerves that causes pain and loss of feeling. DPN is a very complicated and progressive condition [7]. As a result of this disease, people suffer from foot ulcers, amputations, and a general decline in health [8, 9]. Consequently, people with diabetes have postural instability and alterations in their peripheral nerve function [10].
Postural instability and imbalance are common findings in DPN [11]. Lack of sensory nerve function leads to decreased sensory input from the limbs, while lack of motor axon activity leads to muscle weakness. Lack of proprioception and motor nerve disorders lead to impaired muscle activity and imbalance. Balance and gait result from complex neural and muscular activities coordinated with musculoskeletal functions [12]. Reduced ankle, foot, and plantar flexor muscle strength and range of motion are associated with impaired balance and mobility in people with DPN [13]. People with DPN may be at greater risk of falling because of their diminished sense of balance [14]. Both balance and gait are complex interactions of neuromuscular activity coordinated with skeletal functions [12]. Reduced balance can also lead to impaired function, which has a detrimental impact on patients live quality [14]. Physical exercise improves physical fitness [15, 16], quality of life [17], anatomic modulation [18], and neuropathic symptoms [19] in patients with type 2 diabetes mellitus.
Inflammatory indicators such as interleukins (IL) and tumor necrotic factor-alpha (TNF-) are more significant in diabetic patients compared to healthy controls persons, and exercise therapy has been shown to diminish these markers and ameliorate the patient’s physiological state [20]. Strategies to enhance balance in DPN patients may reduce postural impairment and the possibility of falling [4]. The interventions used in the management of diabetes are exercise, diet and drugs [21]. The beneficial impact of physical activity and exercise training on cardiovascular risk factors, glucose management, and lipid metabolism in diabetes individuals have been extensively established [22]. Exercise reduces HbA1c levels, and muscle contraction increases membrane permeability to glucose due to increased glucose transporters in the plasma membrane. Exercise promotes insulin action on glucose metabolism and can lower HbA1c levels via increasing glucose carriers [23].
Nevertheless, little is documented regarding the impact of exercise on neuropathy-related alterations in periphery sensation and equilibrium in diabetes individuals [24]. Some researchers urged their patients to engage in strength, cardio, stability, and stretching exercise [25]. The advantage of these exercises is improving the patient’s level of function [24]. Changes in sensory-motor signaling may explain why people with diabetes have a greater risk of falling than non-diabetic persons [26]. Physical activity may improve cutaneous neuron regeneration and decrease the development of neuropathy [27]. In a randomized controlled trial (RCT) study, 78 patients with DPN who participated in a 4-year exercise program reported less significant sensory and motor neuropathy than control [28]. Diabetic patients are less presumably to participate in an exercise regimen than their healthy counterparts [28]. A practical and safe exercise intervention is needed to help patients with DPN practice for longer lengths of time [29].
Lack of glucose management and impaired motor conduction are significant contributors to type 2 diabetes-related physical disability [30]. Peripheral neuropathy is not a condition that affects everyone with type 2 diabetes. At diagnosis, 10% of people with diabetes have neuropathy, and 50% will acquire it within 25 years. However, identifying risk factors and accelerators of the diabetic neuropathy process and controlling them can effectively prevent diabetic neuropathy [31]. Patients with diabetes have elevated blood glucose levels that include balance-sensory-motor receptors [26]. People with DPN are encouraged to participate in exercise programs to reduce the risk of falling, which effectively improves balance and maintains independence [25]. Whole-body vibration (WBV) has been shown to enhance balance indices in individuals with DPN [32]. Based on the prior research and the information we found when searching for studies on DPN patients and their balance indices. To find out if exercise may help individuals with diabetic peripheral neuropathy improve their balance, the researchers investigated the impact of exercise programs on balance indicators.
Method
Eligibility criteria
Studies that satisfied the following criteria were considered for inclusion: (1 Studies designed as randomized control trials. 2) Participants who have DPN. 3) Research that used exercise therapy as an intervention and a control group that did not get any treatment. 4) Trials whose variables include static, dynamic, and functional balance indices. 5) Both sexes have been included in the study. 6) Human study.7) Studies whose results are presented quantitively. 8) Studies in English have been included in the study.
Researchers excluded articles with the following scales in the present study: (1) Studies with unrelated intervention or non-physiotherapy treatments. (2) Studies that do not define a suitable measurement tool for the variable. (3) Studies that do not include the study population of diabetes or the control group of patients with diabetes. (4) Studies in which the 9 section has a qualitative measurement.
Information sources
The initial search for relevant papers for our systematic review was conducted between March 1984 and February 2020. To locate studies on the effects of exercise treatment on balance in patients with DPN. They include Science Direct, Web of Science and Scielo in the list of databases.
Search strategy
For this investigation, we utilized the PICO technique (populations, interventions, controls, and outcomes). An initial search method was devised and then used to search additional databases, such as PubMed (Supplemental Appendix 1). A researcher searched (N.JA); keywords related to this disease were diabetic peripheral neuropathy. The terms exercise therapy and physical activity were searched in terms of intervention. The words balance, equilibrium, position control, fall, fall risk, static equilibrium, dynamic equilibrium, and functional were searched for variable measurement. Two researchers (N.JA. and SS.N.) independently assessed the titles and abstracts for eligibility before acquiring the full texts of the publications after deleting duplicates. The article selection procedure was launched by two researchers (N.JA and SS.N).
Study selection
Ordering to assign whether or not a title or abstract fulfilled the inclusion or exclusion criteria, two reviewers (N.JA and SS.N) independently reviewed them. Finally, full-text versions of articles that qualified as eligible for inclusion were analyzed and selected (N.JA and SS.N). Discussion between the two authors (N.JA and SS.N) resolved any disagreement in choosing the appropriate article.
Quality assessment
The PEDro scale was used to evaluate the quality of each article. The authors were familiar with the PEDro scale from earlier research [33] and had received training in its usage. There has never been any disagreement during the research between these two authors. The quality of randomized clinical trials may be assessed using the PEDro scale, including 11 elements. The PEDro scale is a valid and reliable scale for evaluating articles for a systematic review in physiotherapy [34, 35]. Researchers have good validity for the PEDro scale direction evaluation of review articles in physiotherapy (0.76, ICC = 0.68 and 95% -CI = 0.57 ([35]. ‘Yes,‘ or ‘no’ answers were given to each question on the scale. It was then added to get an overall score of 11 for each item marked “yes” (except the first item, which deals with external validity). Quality is indicated with a high score [34].
Outcome measures
Several tools and tests were used to measure balance indices in this study. Balance indices included static, dynamic, and functional indices. Biodex was used to determine static balancing indices such as total, anterior-posterior, and mediolateral [6, 12, 36, 37]. Other associated parameters with the De Morton mobility index [38], balance platform tool [8], single-leg stance [28, 39], the center of pressure displacement [25, 27], functional balance indexes including five times sit-to-stand (FTSTS) [8], Chair-stand [10], Timed Up and Go test (TUG) [6, 8, 10, 39], Berg Balance Scale(BBS) [6, 39, 40], Activities-specific balance confidence (ABC) [24], the Fullerton Advanced Balance (FAB) scale [41], functional reach test [38] were also studied.
Results
In the initial search, 9103 articles were identified on electronic sources. After reading the entire text, about 5231 free full-text articles were chosen. Two authors (N.JA and SS.N) reviewed this number of articles. 3872 articles were deleted for title, abstract, duplicate articles, and other factors (review articles, non-RCT articles, and unassociated publications). Also, among 5231 articles, articles in which the intervention without exercise therapy, articles that missed a balance variable or articles whose statistical population was not diabetic patients with diabetic neuropathy or a combination with other diseases were removed. Also, articles whose control group was healthy people have been removed from the list of the reviewed articles. Finally, 12 articles were selected; and they were eligible for our study (Fig. 1). The sample size in 12 studies was between 16 and 143 people, and 580 people participated. The results of the studies showed that the PEDro scale scores were between 5 and 9. Balance indices studied in these studies include static, dynamic, and functional balance indices. According to the search studies, nine studies investigated exercise therapy’s effect on static balance indices [6, 8, 12, 25, 26, 36–39], and two studies the effect of exercise therapy on dynamic balance indices investigated. [37, 38], and in six studies, the effect of exercise therapy on functional balance indices was investigated [6, 8, 10, 39–41] (Table 1).
Fig. 1.
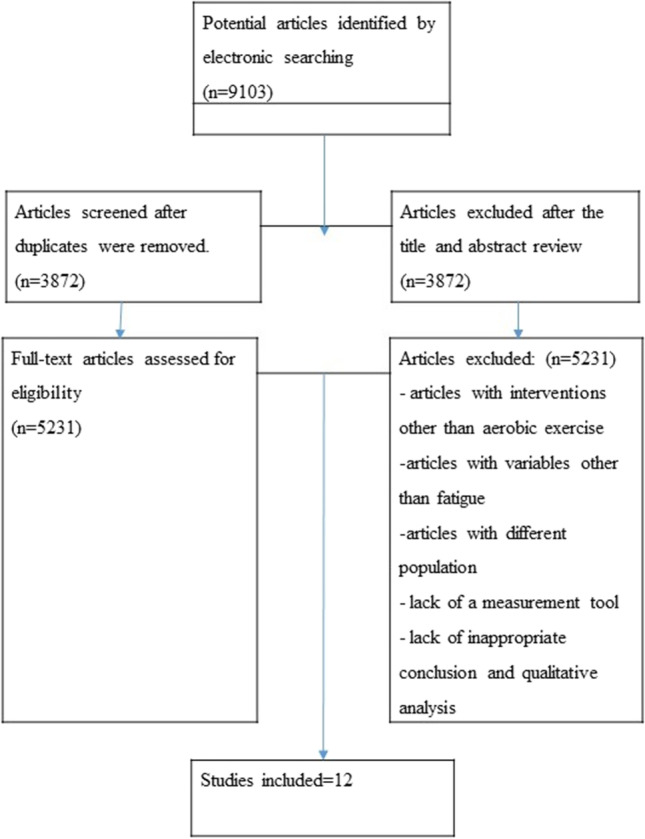
Flow chart of the study
Table 1.
Characteristics of studies included in systematic review
| Study | Design | Participant details | Intervention group | Comparison group | Adherence rate | Dependent variables | PEDro score |
|---|---|---|---|---|---|---|---|
| Eftekhar-Sadat et al. [6] | RCT |
Mean± (SD) age: 6.97 ± 58.94 years. No of participants: 34, 22(f)/12(m) Dropouts:0 |
N = 17, outcome improvement (before to after): TUG (before: 11.18 [9.53 to 12.46], after: 10.97 [9.43 to 12.38]), Berg Balance Scale (before: 52.58 [52.00-55.50], after: 53.00 [52.50 to 55.50]), API (before: 0.51 [0.20 to 0.80], after: 0.24 [0.20 to 0.25]), MLI (before: 0.28 [0.10 to 0.40], after: 0.14 [0.10 to 0.20]), OSI (before: 0.65 [0.20 to 1.00], after: 0.32 [0.20 to 0.40]). |
N = 17, outcome improvement (before to after): TUG (before: 10.80 [9.21 to 11.78], after: 10.80 [9.21 to 11.76]), Berg Balance Scale (before: 53.00 [49.50 to 56.00], after: 53.05 [50.00 to 56.00]), API (before: 0.59 [0.40 to 0.80], after: 0.54 [0.25 to 0.65]), MLI (before: 0.33 [0.10 to 0.45], after: 0.44 [0.10 to 0.50]), OSI (before: 0.75 [0.40 to 0.90], after: 0.82 [0.35 to 0.85]). |
100% of participants completed the program | TUG, Berg Balance Scale, API, MLI, OSI | 9 |
| Venkataraman et al. [8] | RCT |
Mean± (SD) age: 62 years . No of participants: 80(f)/63 (m) Dropouts:14 |
N = 70, outcome improvement (mean(SD)): TUG test(10.9 (3.9)), FTSTS test (14.4 (4.0)), Total ABC score (76.3 (20.5)), Body sway velocity, eyes closed (1.6 (1.3)). | N = 73, outcome improvement (mean(SD)): TUG test (12.2 (4.8)), FTSTS test (15.6 (5.8)), Total ABC score (73.3 (22.6)), Body sway velocity, eyes closed (1.6 (1.1)). | 90/20%Participant s completed | TUG test, FTSTSa test, Total ABC score, Body sway velocity, eyes closed. | 8 |
| Sartor et al. [36] | RCT |
Mean± (SD) age: 59 (4) years in the intervention group and 60 (12) years in the control group. . No of participants: 26(f)/29 (m) Dropouts:0 |
N = 26, outcome improvement (mean(SD)): Center of pressure – mean velocity: (baseline: 0.4 (0.1), 12 weeks: 0.3 (0.0), 24 weeks: 0.3 (0.0)). | N = 29, outcome improvement (mean(SD)): Center of pressure – mean velocity: (baseline: 0.3 (0.05), 12 weeks: 0.4 (0.04). | 100% of Participants completed. | Center of pressure. | 9 |
| Ahn et al. [28] | RCT |
Mean± (SD) age: 65 years . No of participants: 19(f)/20 (m) Dropouts:20. |
N = 20, outcome improvement (mean(SD)): Balance (Single leg stance): (Posttest: 30.02 (28.08), Pre-post difference: 7.65 (16.78)). | N = 19, outcome improvement (mean(SD)): Balance (Single leg stance): (Posttest: 14.27 (16.31), Pre-post difference: 1.44 (9.97)). | 66% Participant s completed. | Balance (Single leg stance) | 5 |
| Nadi et al. [38] | RCT |
Mean± (SD) age: 55.46 ± 3.06years . No of participants: 45(f), Dropouts:0. |
Resistance group (N = 15), outcome improvement (mean(SD)): static balance: (pretest: 2.33 ± 1.23, posttest: 2.73 ± 0.79), dynamic balance: (pretest: 0.67 ± 0.48, posttest: 2.13 ± 0.64), EPN group (N = 15), outcome improvement (mean(SD)): static balance:(pretest: 1.73 ± 0.88, posttest: 3.20 ± 0.77), dynamic balance: (pretest: 0.93 ± 0.70, posttest: 2.20 ± 0.67). |
N = 15, outcome improvement (mean(SD)): static balance: (pretest: 2.00 ± 1.13, posttest: 2.00 ± 1.12), dynamic balance: (pretest: 1.00 ± 0.65, posttest: 0.67 ± 0.72). | 100% of participants completed. | .static balance, dynamic balance. | 7 |
| Cavegn et al. [25] | RCT |
Mean± (SD) age: referent group (63.8 ± 5.7), Diabetic group (65.5 ± 7.4) . No of participants: 12(f),4(m), Dropouts:0 |
N = 8, outcome improvement (mean(SD)): Sway area: (base line: 2.10 ± 1.73, after: 1.79 ± 1.22), COP anterior-posterior: (base line: 3.00 ± 0.97, after: 2.94 ± 1.29), COP medial-lateral: (base line: 1.55 ± 0.64, after: 1.31 ± 0.37). | N = 8, outcome improvement (mean(SD)): Sway area: (1.53 ± 1.10), COP anterior-posterior: (2.35 ± 0.79), COP medial-lateral: (1.51 ± 0.77). | 100% of participants completed. | Center of pressure displacement | 6 |
| Muñoz et al. [10] | RCT |
Mean± (SD) age: 40 and 85 years. No of participants: |
N = 45, outcome improvement (mean (SD)): TUG: (pre: 8.29 ± 2.28, post: 7.42 ± 1.72), Chair-stand test (rep): (pre: 11.91 ± 2.24, post: 12.96 ± 2.24). |
N: 45, outcome improvement (mean (SD)): TUG: (pre: 7.96 ± 1.71, post: 7.49 ± 1.41), Chair-stand test (rep): (pre: 11.11 ± 2.21, post: 11.70 ± 2.31). | 100% of participants completed. | TUG, Chair-stand test (rep). | 7 |
| Ahmad et al. [12] | RCT |
Mean± (SD) age: Intervention group: (60.33 ± 8.48), Control group: (57.24 ± 8.85), No of participants: 12(f), 25(m), Dropouts:6. |
N: 21, outcome improvement (mean (SD)): Front proprioception: (base line: 6.50 ± 3.42, after 8 weeks: 4.46 ± 2.27), back proprioception: (base line: 10.41 ± 5.41, after 8 weeks: 7.06 ± 3.84), left proprioception: (base line: 10.07 ± 5.81, after 8 weeks: 5.31 ± 3.29), right proprioception: (base line: 8.75 ± 5.17, after 8 weeks: 4.60 ± 2.81). |
N:17, outcome improvement (mean (SD)): Front proprioception: (base line: 9.07 ± 3.51, after 8 weeks: 7.32 ± 4.31), back proprioception: (base line: 9.42 ± 6.96, after 8 weeks: 9.21 ± 6.49), left proprioception: (base line: 9.55 ± 4.71, after 8 weeks: 9.57 ± 6.69), right proprioception: (base line: 7.87 ± 6.18, after 8 weeks: 7.10 ± 5.24). |
84.21% of participants completed. | proprioception | 8 |
| Ghazal et al. [40] | RCT |
Mean± (SD) age: 49 ± 6.79, No of participants: 12(f), 6(m), Dropouts: 0 |
N: 8, outcome improvement (mean (SD)): Berg balance Scale: (base line: 46.75 ± 2.12, 4 weeks: 50.25 ± 1.66, 8 weeks: 54.88 ± 1.12). | N:10, outcome improvement (mean (SD)): Berg balance Scale: (base line: 47.60 ± 1.84, 4 weeks: 49.20 ± 1.33, 8 weeks: 52.0 ± 3.36). | 100% of participants completed. | Berg Balance Scale, functional reach test | 8 |
| Schmid et al. [41] | RCT |
Mean± (SD) age: 54.94 ± 9.94 years, No of participants: 12(f), 6(m), Dropouts: 0 |
N: 9, outcome improvement (mean (SD)): balance:(before: 14.2 ± 14.07, after: 20.4 ± 13.48). |
N:9, outcome improvement (mean (SD)): balance:(before: 27.1 ± 9.92, after: 21.7 ± 13.36). |
100% of participants completed. | FABb | 8 |
| Kruse et al. [39] | RCT |
Mean± (SD) age: Intervention group: 66.3 ± 10.6, control group: 64.8 ± 9.4, No of participants: 40(f), 39(m), Dropouts: 0 |
N: 41, outcome improvement (mean (SD)): Berg Balance Scale score: (Baseline: 48.1 (46.0–50.3), 6 month: 48.1 (45.1–51.1), 12 month: 47.1 (43.4–50.8), One-leg stance test with eyes open: (base line: 10.1 (6.0–14.2), 6 month: 15.7 (8.7–22.7), 12 month: 14.6 (8.4–20.7), One-leg stance test with eyes open, ≥ 5 s: base line: 20 (48.8), 6 month: 18 (48.6), 12 month: 21 (60.0), One-leg stance test with eyes closed: base line: 1.5 (0.6–2.3), 6 month: 2.8 (1.1–4.4), 12 month: 1.9 (1.1–2.8), One-leg stance test with eyes closed, ≥ 2 s: base line: 10 (24.4), 6 month: 16 (43.2), 12 month: 14 (41.2), Timed “Up & Go” Test: base line: 12.8 (11.4–14.3), 6 month: 12.8 (11.4–14.3), 12 month: 13.8 (10.6–17.1). | N: 38, outcome improvement (mean (SD)): Berg Balance Scale score: (Baseline: 49.1 (47.3–50.8), 6 month: 49.9 (48.0–51.8), 12 month: 47.9 (45.5–50.4), One-leg stance test with eyes open: (base line: 9.5 (4.3–14.7), 6 month: 11.6 (6.0–17.1), 12 month: 10.8 (5.4–16.2), One-leg stance test with eyes open, ≥ 5 s: base line: 14 (36.8), 6 month: 18 (50.0), 12 month: 13 (38.2), One-leg stance test with eyes closed: base line: 1.0 (0.5–1.5), 6 month: 1.7 (1.1–2.3), 12 month: 1.0 (0.4–1.5), One-leg stance test with eyes closed, ≥ 2 s: base line: 10 (26.3), 6 month: 13 (36.1), 12 month: 7 (20.6), Timed “Up & Go” Test: base line: 12.3 (11.2–13.5), 6 month: 12.2 (11.3–13.2), 12 month: 13.2 (10.1–16.3). | 100% of participants completed. | Berg Balance Scale score, One-leg stance test with eyes open, One-leg stance test with eyes open, ≥ 5 s, One-leg stance test with eyes closed, One-leg stance test with eyes closed, ≥ 2 s, Timed “Up & Go” Test. | 8 |
| Hedayati et al. [37] | RCT |
Mean± (SD) age: Intervention group: 51.9 ± 4.3, control group: 48 ± 6.57, Dropouts: 0 |
N:10, outcome improvement (mean (SD)):static balance :(before: 1/09 ± 0/81, after: 0/05 ± 0/32), dynamic balance: before: 26/7 ± 10/44, after: 34/7 ± 11/73). | N:10, outcome improvement (mean (SD)):static balance :(before: 0/74 ± 0/36, after: 0/74 ± 0/21, dynamic balance: before: 34/6 ± 12/62, after: 35/8 ± 9/02). | 100% of participants completed. | Static balance. Dynamic balance. | 6 |
afive times sit-to-stand
bFullerton Advanced Balance scale
The effect of exercise therapy on static balance indices
Static balance indices were measured in 9 studies, and seven studies reported significant improvement in balance indices [6, 8, 12, 28, 36–38], while there was no significant improvement in balance indexes in two investigations [25, 39]. A biodex device was used to evaluate the static balance indices in two studies, and a significant improvement was reported in both studies [6, 37]. In 3 studies, strengthening, balance, and stretching exercises were evaluated in patients with DPN [8, 36, 39]. In one study to evaluate the static balance indices from the balance platform tool [8], a study measured the static balance indices from the center of pressure (COP) mean velocity [36]. In another study, one leg stance was used [39]. Two studies reported significant improvement [8, 36], while one study did not report significant improvement [39]. Two studies investigated the effect of tai chi exercises on DPN patients [25, 28]. In one study, a single leg stance was used to assess static balance indices [28], and in another study, the degree of displacement of the COP was used [25]. A significant improvement was reported [28], while another study found no significant improvement [25].
Nadi et al. investigated the impact of resistance training and exercises for peripheral neuropathy (EPN) in diabetic neuropathy patients. The De Morton mobility index was employed to measure static balance indices in this study, and the results showed that in the EPN group, the static balance was enhanced [38]. Ahmad et al. investigated sensorimotor and gait training in patients with DPN. After the intervention, the results showed a significant temporal effect on proprioception in patients [12]. Hedayati et al. studied resistance training’s effect on DPN patients. In this study, a biodex device was used to measure static balance, and the results showed that static balance had a significant difference between the intervention and control groups [37].
The effect of exercise therapy on functional balance indices
Six studies investigated how exercise therapy affected functional balance indices in diabetic peripheral neuropathy patients; five studies showed significant improvement [6, 8, 10, 40, 41], while one study did not show significant improvement [39]. Two studies used BBS and TUG to evaluate performance balance indices [6, 39], while one study reported significant improvement [6], and the other reported no significant improvement [39]. Venkataraman et al. used TUG, ABC score, and FTSTS to evaluate balance. The results showed a significant improvement in TUG, FTSTS, and ABS scores [8]. In the study of Munoz et al., the TUG and chair stand test was used to evaluate the performance balance indices, and the results found increases in the TUG; both the WBV and placebo groups performed the chair stand test [10].
Ghazal et al. used BBS and functional reach test to evaluate functional balance indices. Results showed a significant improvement for the task-oriented balance exercise group after eight weeks of treatment compared to the traditional balance exercise group [40]. Schmid et al. used the FAB scale to assess functional balance and reported that balance scores improved in the intervention group, while they deteriorated significantly in the control group [41].
The effect of exercise therapy on dynamic balance indices
Dynamic balance indices were examined in two studies, and both studies reported significant improvement [37, 38]. In the study of Nadi et al., the De Morton mobility index was employed to assess dynamic balance, and the findings of the study revealed that the EPN group improved significantly [38]. Hedayati and colleagues used a biodex device to assess the dynamic balance in their research. The findings revealed a considerable rise in the intervention group compared to the control group [37].
Discussion
DPN is one of the most frequent long-ranged microvascular issues, resulting in considerable immobility and mortality in patients [28, 42]. DPN is the most common DM-related problem affecting peripheral nerves, sensory and motor [12]. The sensitivity of sensory inputs is reduced when sensory nerve function is impaired [43]. In comparison, the lack of motor axons is associated with inadequate re-denervation associated with muscle weakness and atrophy of the lower limb muscles [44]. However, little is known regarding the impact of exercise on peripheral sensation and balance abnormalities in diabetes patients with neuropathy [24]. This research aimed to realize how exercise therapy affected balance indices in DPN patients.
Among these articles, nine studies investigated the effect of exercise therapy on static balance indices, six studies on functional balance indices, and two studies on dynamic balance indices. According to PEDro scale scores to assess the quality of this research, the majority of those that examined the influence of balance and strengthening exercises, especially on static balance indices, had PEDro scale scores between 7 and 9, which indicates the high quality of these studies. This finding indicates that balance and strengthening exercises are more effective than other interventions on balance indices, primarily static balance.
According to a study by Eftekhar Sadat et al. [6], Venkataraman et al. [8], and the study by Ahmad et al. [12], balance indices were improved following balance exercises. Improved proprioception and muscle strength were credited with this improvement [6]. Balance exercises stimulate the mechanoreceptors in the spindle, GTO, and responsive joint capsule, increasing sensory inputs from the foot, ankle, and trunk [12].
According to Sartor et al. [36], the group of interventions had a lower center of pressure (COP) tragedy in midfoot in the midstance phase. DPN patients had direct COP tragedy from heel to heel forefoot with short COPs performed internally-externally. Improvement in the foot flattening phase was supported by reduced ankle extensor torque, improved ankle dorsiflexion and the tibialis anterior muscle function [36]. Ahn et al. [45], following tai chi exercises, reported a considerable improvement in their balance. However, this could be attributed to increased cutaneous vascularity conduction and conduction through peripheral nerve velocities better than bilateral tibial nerve [45]. The study by Nadi et al. [38] reported improvements in the EPN group; there is a static and dynamic balance. However, exercise and physical exercise can improve patients’ complaints by reducing diabetes patients’ inflammation and glycemic conditions [38].
The improvement achievements were more significant in the EPN group than in another group. These excellent outcomes are likely due to the exercises’ concentration on the lower limbs and the patient’s comfort during the exercises [38]. Cavegn et al. [25] showed no significant change in balance, despite an achievement in ankle proprioception. Several theories explain that tai chi activities positively influence ankle proprioception. First, tai chi exercise may intensify receptor sensitivity, rely on afferent signals, and promote peripheral sensory receptor sensitivity [46]. Therefore, it provides the sensory receptors with vital oxygen and energy. Improved proprioception and re-sensitization of peripheral sensory receptors and feeling of lower limb tissues may coexist [25].
Study Munoz et al. [10] achieved an intragroup improvement in TUG and the chair stand test. On the other hand, WBV had a considerable impact. After eight weeks, most study variables improved in both placebo and intervention groups, and two reasons could explain this improvement. First, physical inactivity is common among persons with type 2 diabetes. Both groups performed at the primary care facility three times a week. Therefore, participants were more active after the intervention than before. Second, taking part in a physical exercise intervention had an excellent social effect on even the placebo group [10].
In the study by Kruse et al. [39], the intervention group’s lower limb strength and balance did not improve. Traditional measurements of strength and balance in those with injured legs may not be sensitive enough to detect changes. On the other hand, the intervention was not harsh enough to increase balance indices and lower limb strength [39]. Ghazal et al. [40] found that task-oriented balance exercises were more effective at improving balance than traditional balance exercises. This study trained in motor and cognitive tasks simultaneously over four weeks. The task-oriented balance exercises significantly improved balance and reduced the risk of falling; because those exercises are specific goal-oriented activities related to daily activities [40]. Schmid and colleagues conducted a study [41] and noted that their balance had improved significantly. Participants were sedentary, and moving from a sedentary lifestyle to a weekly yoga session could result in various benefits [41]. According to Hedayati et al. [37], enhancing the balance following strengthening exercises was attributed to the facilitation of large and fast-twitch muscles and stimulation of muscle spindles. It increased the coordination of muscles involved in co-contractile activity [37].
Our findings showed that balance and strengthening exercises as part of the intervention or control group treatment program are more effective in improving balance indices in patients with DPN. Among balance indices, they led to further improvement in functional and static balance indices, and they are more effective on static balance than functional balance indices. Although yoga, tai chi, and other forms of exercise as well as walking exercises have been studied, there are few investigations in this area, and more studies are needed.
Limitations
One limitation of this study was that we only reviewed studies in English. On the other hand, the studies used different measurement tools, so it is better to use the same measurement tools in future studies to quickly compare the results of different studies. The next finitude of the present investigation was the small sample size in most of the studies studied, and it is better to use more sample sizes in future studies. Only the effect of exercise therapy was studied; it is better to study the role of other physiotherapy interventions in studies. Various symptoms have been reported, and it is better to measure the effect of exercise therapy in diabetic individuals on these symptoms and their relationship with balance in future studies. The meta-analysis study was not possible due to the heterogeneity of the studies.
Clinical implication
This study showed that exercise therapy improves balance indices. Due to the improvement in balance indices following exercise therapy, it is expected that the risk of falling and gait parameters will also improve. This systematic review shows that exercise therapy can be considered a rehabilitation program since exercise therapy is a feasible, safe, and valuable treatment with no side effects.
Conclusions
Studies have shown that balance and strengthening exercises are more effective on predominantly static balance indices. On the other hand, according to various findings, factors affecting diabetes individuals’ postural stability are complicated. It is challenging to determine practical exercises on balance indices, so it is better to do more studies on this field.
Supplementary Information
Below is the link to the electronic supplementary material.
(DOCX 12.7 KB)
Acknowledgements
This study is related to project NO 1399/63677 and the code of ethics IR.SBMU.RETECH. REC.1399.1076 From the Student Research Committee, Shahid Beheshti University of Medical Sciences, Tehran, Iran. We also appreciate the “Student Research Committee” and “Research & Technology Chancellor” at Shahid Beheshti University of Medical Sciences for their financial support of this study.
Authors’ contributions
NJA advised on conception and design. SSN did planning and conception. SSN and NJA contributed to the execution, acquisition of data, interpretation and writing of the article.
Funding
Shahid Beheshti University of Medical Sciences sponsors this article.
Declarations
Conflict of interest
The corresponding author states no conflict of interest on behalf of all authors.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Javadzadeh A, Ghorbanihaghjo A, Adl FH, Andalib D, Khojasteh-Jafari H, Ghabili K. Calcium dobesilate reduces endothelin-1 and high-sensitivity C-reactive protein serum levels in patients with diabetic retinopathy. Mol Vis. 2013;19:62. [PMC free article] [PubMed] [Google Scholar]
- 2.Rahbar S, Naimi SS, Reza Soltani A, Rahimi A, Akbarzadeh Baghban A, Rashedi V, et al. Improvement in biochemical parameters in patients with type 2 diabetes after twenty-four sessions of aerobic exercise: A randomized controlled trial. Iran Red Crescent Med J. 2017;19(7):2. [Google Scholar]
- 3.Wild S, Roglic G, Green A, Sicree R, King H. Global prevalence of diabetes: estimates for the year 2000 and projections for 2030. Diabetes Care. 2004;27(5):1047–53. doi: 10.2337/diacare.27.5.1047. [DOI] [PubMed] [Google Scholar]
- 4.Shrivastava SR, Shrivastava PS, Ramasamy J. Role of self-care in management of diabetes mellitus. J Diabetes Metabolic Disorders. 2013;12(1):1–5. doi: 10.1186/2251-6581-12-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dyck PJ, Kratz K, Karnes J, Litchy WJ, Klein R, Pach J, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: the Rochester Diabetic Neuropathy Study. Neurology. 1993;43(4):817. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 6.Eftekhar-Sadat B, Azizi R, Aliasgharzadeh A, Toopchizadeh V, Ghojazadeh M. Effect of balance training with Biodex Stability System on balance in diabetic neuropathy. Ther Adv Endocrinol Metab. 2015;6(5):233–40. doi: 10.1177/2042018815595566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28(4):956–62. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 8.Venkataraman K, Tai BC, Khoo EY, Tavintharan S, Chandran K, Hwang SW, et al. Short-term strength and balance training does not improve quality of life but improves functional status in individuals with diabetic peripheral neuropathy: a randomised controlled trial. Diabetologia. 2019;62(12):2200–10. doi: 10.1007/s00125-019-04979-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sohrabzadeh E, Kalantari KK, Naimi SS, et al. The immediate effect of a single whole-body vibration session on balance, skin sensation, and pain in patients with type 2 diabetic neuropathy. J Diabetes Metab Disord. 2021 doi: 10.1007/s40200-021-00933-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Domínguez-Muñoz FJ, Villafaina S, García-Gordillo MA, Hernández-Mocholi M, Collado-Mateo D, Adsuar JC, et al. Effects of 8-week whole-body vibration training on the HbA1c, quality of life, physical fitness, body composition and foot health status in people with T2DM: A double-blinded randomized controlled trial. Int J Environ Res Public Health. 2020;17(4):1317. doi: 10.3390/ijerph17041317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Said G. Diabetic neuropathy—a review. Nat Clin Pract Neurol. 2007;3(6):331–40. doi: 10.1038/ncpneuro0504. [DOI] [PubMed] [Google Scholar]
- 12.Ahmad I, Verma S, Noohu MM, Shareef MY, Hussain ME. Sensorimotor and gait training improves proprioception, nerve function, and muscular activation in patients with diabetic peripheral neuropathy: A randomized control trial. J Musculoskel Neuronal Interact. 2020;20(2):234. [PMC free article] [PubMed] [Google Scholar]
- 13.Menz HB, Lord SR, St George R, Fitzpatrick RC. Walking stability and sensorimotor function in older people with diabetic peripheral neuropathy. Arch Phys Med Rehabil. 2004;85(2):245–52. doi: 10.1016/j.apmr.2003.06.015. [DOI] [PubMed] [Google Scholar]
- 14.Lau C-Y, Qureshi A, Scott S. Association between glycaemic control and quality of life in diabetes mellitus. J Postgrad Med. 2004;50(3):189. [PubMed] [Google Scholar]
- 15.Balducci S, Iacobellis G, Parisi L, Di Biase N, Calandriello E, Leonetti F, et al. Exercise training can modify the natural history of diabetic peripheral neuropathy. J Diabetes Complicat. 2006;20(4):216–23. doi: 10.1016/j.jdiacomp.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 16.Allet L, Armand S, De Bie R, Golay A, Monnin D, Aminian K, et al. The gait and balance of patients with diabetes can be improved: a randomised controlled trial. Diabetologia. 2010;53(3):458–66. doi: 10.1007/s00125-009-1592-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Van der Heijden M, van Dooren FE, Pop V, Pouwer F. Effects of exercise training on quality of life, symptoms of depression, symptoms of anxiety and emotional well-being in type 2 diabetes mellitus: a systematic review. Diabetologia. 2013;56(6):1210–25. doi: 10.1007/s00125-013-2871-7. [DOI] [PubMed] [Google Scholar]
- 18.Teixeira-Lemos E, Nunes S, Teixeira F, Reis F. Regular physical exercise training assists in preventing type 2 diabetes development: focus on its antioxidant and anti-inflammatory properties. Cardiovasc Diabetol. 2011;10(1):1–15. doi: 10.1186/1475-2840-10-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kluding PM, Pasnoor M, Singh R, Jernigan S, Farmer K, Rucker J, et al. The effect of exercise on neuropathic symptoms, nerve function, and cutaneous innervation in people with diabetic peripheral neuropathy. J Diabetes Complicat. 2012;26(5):424–9. doi: 10.1016/j.jdiacomp.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hopps E, Canino B, Caimi G. Effects of exercise on inflammation markers in type 2 diabetic subjects. Acta Diabetologia. 2011;48(3):183–9. doi: 10.1007/s00592-011-0278-9. [DOI] [PubMed] [Google Scholar]
- 21.Praet S, Van Rooij E, Wijtvliet A, Boonman-de Winter L, Enneking T, Kuipers H, et al. Brisk walking compared with an individualised medical fitness programme for patients with type 2 diabetes: a randomised controlled trial. Diabetologia. 2008;51(5):736–46. doi: 10.1007/s00125-008-0950-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Amini Najafabadi B, Keshavarz S, Asgary S. The 8-week aerobic exercise improves blood sugar، HbA1c and lipid profile in women with type 2 diabetes: A Controlled Randomized Clinical Trial. Jorjani Biomed J. 2020;8(3):44–57. [Google Scholar]
- 23.Sigal RJ, Kenny GP, Wasserman DH, Castaneda-Sceppa C. Physical activity/exercise and type 2 diabetes. Diabetes Care. 2004;27(10):2518–39. doi: 10.2337/diacare.27.10.2518. [DOI] [PubMed] [Google Scholar]
- 24.Richerson S, Rosendale K. Does Tai Chi improve plantar sensory ability? A pilot study. Diabetes Technol Ther. 2007;9(3):276–86. doi: 10.1089/dia.2006.0033. [DOI] [PubMed] [Google Scholar]
- 25.Cavegn EI, Riskowski JL. The effects of Tai Chi on peripheral somatosensation, balance, and fitness in Hispanic older adults with type 2 diabetes: a pilot and feasibility study. Evid Based Complement Alternat Med. 2015;2015:1–9. [DOI] [PMC free article] [PubMed]
- 26.Gurfinkel V, Ivanenko YP, Levik YS, Babakova I. Kinesthetic reference for human orthograde posture. Neuroscience. 1995;68(1):229–43. doi: 10.1016/0306-4522(95)00136-7. [DOI] [PubMed] [Google Scholar]
- 27.Singleton JR, Marcus RL, Lessard MK, Jackson JE, Smith AG. Supervised exercise improves cutaneous reinnervation capacity in metabolic syndrome patients. Ann Neurol. 2015;77(1):146–53. doi: 10.1002/ana.24310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ahn S, Song R. Effects of tai chi exercise on glucose control, neuropathy scores, balance, and quality of life in patients with type 2 diabetes and neuropathy. J Altern Complement Med. 2012;18(12):1172–8. doi: 10.1089/acm.2011.0690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ware JE., Jr SF-36 health survey update. Spine. 2000;25(24):3130–9. doi: 10.1097/00007632-200012150-00008. [DOI] [PubMed] [Google Scholar]
- 30.Park SW, Goodpaster BH, Strotmeyer ES, de Rekeneire N, Harris TB, Schwartz AV, et al. Decreased muscle strength and quality in older adults with type 2 diabetes: the health, aging, and body composition study. Diabetes. 2006;55(6):1813–8. doi: 10.2337/db05-1183. [DOI] [PubMed] [Google Scholar]
- 31.Vincent AM, Feldman EL. New insights into the mechanisms of diabetic neuropathy. Rev Endocrine Metab Disord. 2004;5(3):227–36. doi: 10.1023/B:REMD.0000032411.11422.e0. [DOI] [PubMed] [Google Scholar]
- 32.Robinson CC, Barreto RPG, Plentz RDM. Effects of whole-body vibration in individuals with diabetic peripheral neuropathy: a systematic review. J Musculoskel Neuronal Interact. 2018;18(3):382. [PMC free article] [PubMed] [Google Scholar]
- 33.Jahantigh Akbari N, Hosseinifar M, Naimi SS, Mikaili S, Rahbar S. The efficacy of physiotherapy interventions in mitigating the symptoms and complications of diabetic peripheral neuropathy: a systematic review. J Diabetes Metabolic Disorders. 2020;19(2):1995–2004. doi: 10.1007/s40200-020-00652-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.De Morton NA. The PEDro scale is a valid measure of the methodological quality of clinical trials: a demographic study. Aust J Physiother. 2009;55(2):129–33. doi: 10.1016/s0004-9514(09)70043-1. [DOI] [PubMed] [Google Scholar]
- 35.Maher CG, Sherrington C, Herbert RD, Moseley AM, Elkins M. Reliability of the PEDro scale for rating quality of randomized controlled trials. Phys Ther. 2003;83(8):713–21. [PubMed] [Google Scholar]
- 36.Sartor CD, Hasue RH, Cacciari LP, Butugan MK, Watari R, Pássaro AC, et al. Effects of strengthening, stretching and functional training on foot function in patients with diabetic neuropathy: results of a randomized controlled trial. BMC Musculoskelet Disord. 2014;15(1):1–13. doi: 10.1186/1471-2474-15-137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hedayati A, Rashidlamir A, Hashemi Javaheri A, Ehsaei MR. The effect of eight weeks of resistance training on static and dynamic balance as well as power of the foot muscles in diabetic women with peripheral neuropathy. SSU J. 2015;23(9):833–843. [Google Scholar]
- 38.Nadi M, Bambaeichi E, Marandi SM. Comparison of the effect of two therapeutic exercises on the inflammatory and physiological conditions and complications of diabetic neuropathy in female patients. Diabetes Metab Syndr Obes. 2019;12:1493. doi: 10.2147/DMSO.S206454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kruse RL, LeMaster JW, Madsen RW. Fall and balance outcomes after an intervention to promote leg strength, balance, and walking in people with diabetic peripheral neuropathy: “feet first” randomized controlled trial. Phys Ther. 2010;90(11):1568–79. doi: 10.2522/ptj.20090362. [DOI] [PubMed] [Google Scholar]
- 40.Ghazal J, Malik AN, Amjad I. Task-oriented training improves the balance outcome & reducing fall risk in diabetic population. Pakistan J Med Sci. 2016;32(4):983. doi: 10.12669/pjms.324.10092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schmid AA, Atler KE, Malcolm MP, Grimm LA, Klinedinst TC, Marchant DR, et al. Yoga improves quality of life and fall risk-factors in a sample of people with chronic pain and type 2 diabetes. Complement Ther Clin Pract. 2018;31:369–73. doi: 10.1016/j.ctcp.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 42.Rahbar S, Naimi SS, RezaSoltani A, Rahimi A, Baghban AA, Noori A, et al. Changes in vascular structure in diabetic patients after 8 weeks aerobic physical exercise: a randomized controlled trial. Int J Diabetes Dev Ctries. 2018;38(2):202–8. [Google Scholar]
- 43.Kraiwong R, Vongsirinavarat M, Hiengkaew V, von Heideken Wågert P. Effect of sensory impairment on balance performance and lower limb muscle strength in older adults with type 2 diabetes. Ann Rehabil Med. 2019;43(4):497. doi: 10.5535/arm.2019.43.4.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Andreassen C, Jakobsen J, Ringgaard S, Ejskjaer N, Andersen H. Accelerated atrophy of lower leg and foot muscles—a follow-up study of long-term diabetic polyneuropathy using magnetic resonance imaging (MRI) Diabetologia. 2009;52(6):1182–91. doi: 10.1007/s00125-009-1320-0. [DOI] [PubMed] [Google Scholar]
- 45.Hung J-W, Liou C-W, Wang P-W, Yeh S-H, Lin L-W, Lo S-K, et al. Effect of 12-week tai chi chuan exercise on peripheral nerve modulation in patients with type 2 diabetes mellitus. J Rehabil Med. 2009;41(11):924–9. doi: 10.2340/16501977-0445. [DOI] [PubMed] [Google Scholar]
- 46.Wang J-S, Lan C, Wong M-K. Tai Chi Chuan training to enhance microcirculatory function in healthy elderly men. Arch Phys Med Rehabil. 2001;82(9):1176–80. doi: 10.1053/apmr.2001.24305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX 12.7 KB)


