Abstract
Background
Fasting during Ramadan is mandatory for all adult healthy Muslims. International studies found that most Muslims with diabetes mellitus fast during Ramadan. The main risk factors are hypoglycemia, Hyperglycemia, diabetic ketoacidosis, and dehydration during fasting. Therefore, stratification of the risks for severe acute diabetes complications needs to be considered for each individual and strategies personalized to advert these complications. The advent of new diabetes medications which are effective yet with a better safety profile and monitoring of blood glucose levels during the day are important to reduce the risk of untoward effects of hypoglycemia and hyperglycemia during Ramadan fasting. Here we review the safety and effectiveness of the newer diabetes medications for Ramadan fasting and whether it is safe to perform fasting after bariatric surgery.
Methods
An extensive literature search using PubMed and Google Scholar was done using different search terms. The eligible studies were 48 randomized controlled trials, prospective observational studies, and reviews from January 2008 to June 2022 which were conducted in individuals living with diabetes.
Results and Conclusions
The newer diabetes medications such as GLP-1 agonists, DPP-4 inhibitors, SGLT-2 inhibitors, and new Insulin therapy are thought to be safe and effective during fasting of Ramadan. These medications are associated with a reduction in HbA1c, body weight, systolic blood pressure and risk of hypoglycemia during Ramadan fasting. However, further studies with larger sample size are needed to confirm the efficacy and safety of these newer medications during Ramadan fasting. Individuals with Bariatric surgery should seek advice and approval to fast from the bariatric dietician, physician, and surgeon before the beginning of the month of Ramadan.
Keywords: Ramadan, Diabetes mellitus, Hypoglycemia, Hyperglycemia, Safety, Anti-diabetic medications
Introduction
Fasting during the month of Ramadan is mandatory for all healthy post-pubertal Muslims. As fasting starts from dawn and ends at dusk and the Islamic calendar differs from the Gregorian calendar, the duration of the daily fast varies from a few hours to more than twenty hours in different regions across the globe and from year to year. Muslims fasting during Ramadan must abstain from any oral consumption, including drinking, eating, smoking, sexual practice, and intake of oral medications from predawn to after sunset. For most who fast during Ramadan, two meals are consumed daily, at Suhoor (predawn) prior to the start of the fast and at Iftar (sunset) during the breaking of the fast [1]. Although the ill can choose not to fast during Ramadan, international studies found that most Muslims with diabetes mellitus fast during Ramadan [2].
As safety during fasting is paramount, stratification of the risks for severe acute diabetes complications needs to be considered for each individual and personalized strategies [3] to advert these complications. Fasting during Ramadan has been associated with multi-fold increased risks of hyperglycemia, diabetic ketoacidosis, and hypoglycemia [4]. Factors that increase these risks should be considered during risk stratification. These Factors include the type of diabetes, type of medication, frequency and severity of hypoglycemia, pre-existing diabetes complications, medical comorbidities which increase these risks or reduces risk of response, social and work habits, as well as previous experience during fasting. During Ramadan, there are lifestyle changes with altered sleep and meal timings and patterns [4]. The decreased intake of food during daytime is associated with higher risk of hypoglycemia, which may lead to increased mortality, with more pronounced risk in type 1 when compared to type 2 diabetes mellitus [5, 6]. In parallel with suitable and safe anti-diabetic medications use during fasting, diabetes education remains of essence to ensure that individuals recognize symptoms of hypoglycemia as well as when to break their fast, the attending risks of fasting, the need for regular blood glucose monitoring prior to developing symptoms, adequate fluids and dietary intake to advert complications, the monitoring needed to exercise safely, as well as medication adjustment as advised by their healthcare providers (HCPs) [3, 7, 8]. Here we discuss history and physiology of incretins, safety and effectiveness of GLP-1 RA, Dipeptidyl peptidase-4 inhibitors, SGLT-2 inhibitors, new insulins and technologies during fasting of Ramadan and whether it is safe to perform fasting of Ramadan after bariatric surgery.
Methods
This research project was conducted as a review article. The authors searched the literature using the following databases: PubMed and Google Scholar. These databases were searched using the keywords: Ramadan, GLP-1 RA, Dipeptidyl peptidase-4 inhibitors, SGLT-2 inhibitors, new insulins, technologies and bariatric surgery. The search is based on studies published in English from January 2008 to June 2022. The abstracts and the articles were then screened. Total number of publications found in the initial search was 136. Total number of publications selected for the review was 48 (Fig. 1). The selected studies included 15 randomized controlled trials, 19 prospective observational studies and 14 reviews. Articles were scanned and read; further relevant references in the reference lists are also included. Information from these articles was summarized in relation to study design, duration of study, number of participating patients, medications used, assessment criteria for medication safety and effectiveness, and final conclusions. The following criteria were used to select the articles:
-
Inclusion criteria:
Only randomized controlled trials, prospective observational studies and reviews were included in the study. Only English documents were eligible. Based on the topics, all studies about Ramadan fasting or new antidiabetic medications or fasting after bariatric surgery were included.
-
Exclusion criteria:
Commentaries, letters to the editor, case reports, book, protocols, news, opinions, theses, notes, short surveys, conference abstracts, repeated studies, and papers written in other languages than English were removed.
Fig. 1.
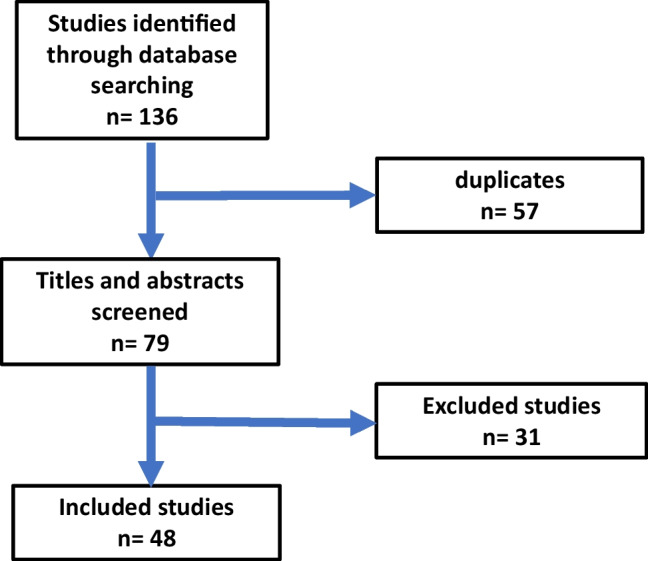
Flow chart for selection of articles included in the review
All the retrieved manuscripts were imported into EndNote software to remove the duplicates. Then, the titles and abstracts of the studies were screened based on the eligibility criteria by two research team members. The flow chart for the selection of the publications is shown in Fig. 1.
History and physiology of incretins
It was discovered that ingestion of nutrients stimulates the release of intestinal factors from the pancreas, which decreases plasma glucose levels. The term incretin was later coined for these intestinal factors and defined as hormones secreted from the digestive tract in response to nutrient ingestion resulting in potentiation of glucose-stimulated insulin secretion. The incretin effect has been shown to be responsible for more than 50% of insulin secreted after administration of oral glucose [9]. As the incretin effect of this hormone occurred at physiological concentrations, it was renamed glucose-dependent insulin-tropic polypeptide (GIP). It has been shown that GIP alone is partially responsible for the incretin effect in vivo [9, 10]. Glucagon-like peptide-1 (GLP-1) was the second incretin hormone discovered following cloning and sequencing of mammalian proglucagon genes and complementary DNAs (cDNAs). GLP-1 is a peptide composed of thirty amino acids released from enteroendocrine L-cells [11, 12]. The stimulus responsible for the release of GLP-1 is the ingestion of nutrients. Many studies were conducted to investigate the effects and the efficacy of GLP-1 [13]. The first conducted studies showed the effect of GLP-1 as an incretin hormone, potentiating glucose-stimulated insulin secretion from the pancreas in response to nutrient ingestion [14]. Subsequent research studies found that GLP-1 stimulates the proliferation and survival of pancreatic β cell and inhibits pancreatic α cells and secretion of glucagon [15]. Activation of the GLP-1 receptor inhibits both stomach emptying and small intestinal motility leading to slow absorption of nutrients [16]. Acute and sustained GLP-1 receptor signaling leads to decreased appetite and food consumption, leading to weight loss [17]. Further studies proposed that GLP-1 has anti-inflammatory and cardioprotective effects [18].
GLP-1 RA
The development of GLP-1 receptor agonists (RAs), known as GLP-1 analogs, started twenty years ago [19]. The known GLP-1 analogs are liraglutide, lixisenatide, dulaglutide, albiglutide, and semaglutide. In type 2 diabetes mellitus (DM), there is impaired GLP-1 secretion and almost complete loss of incretin effect [20]. Therefore, GLP-1 RAs had been introduced as a therapeutic agent in patients with poorly controlled type 2 DM. GLP-1 RAs are injectable peptides structurally and functionally resemble endogenous GLP-1, though with a prolonged half-life as they are not deactivated by DPP-4 enzyme [21]. This group of drugs includes the short-acting Exenatide and Lixisenatide administered once daily, the intermediate-acting Liraglutide administered daily, and the long-acting Exenatide QW Albiglutide, and Dulaglutide administered weekly [22, 23]. While short-acting drugs mainly lower postprandial blood glucose by slowing gastric emptying, long-acting drugs mostly control fasting blood glucose levels through their insulin-augmenting and glucagon-lowering effects [22]. GLP-1 RA are potent glucose-lowering drugs with a low risk of hypoglycemia when used alone or in combination with other medications. This is due to the glucose-dependent insulinotropic actions with insulin secretion induced when blood glucose levels are elevated [24–26]. A large meta-analysis of randomized controlled trials which included 9771 participants, compared the use of GLP-1 RA to placebo and found that a large proportion of those with type 2 diabetes mellitus was able to achieve HbA1c goal of < 7% on GLP-1 agonists compared to placebo or other diabetes medication [27]. Nausea, vomiting, and diarrhea are the commonest side effects associated with GLP-1 RA use [28]. GLP-1 RA was also found to improve the cardiovascular risk factors associated with type 2 DM due to weight reduction, improved systolic blood pressure, and improved lipid profile [24, 29]. The American Diabetes Association and the European Association for the Study of Diabetes suggested that GLP-1 RA therapy can be considered a treatment option for patients with diabetes without established cardiovascular disease with high-risk indicators to reduce the risk of a major adverse cardiovascular event (MACE) [30]. Regarding the efficacy and tolerability, many studies had been conducted to compare between agents within the class, the main end-points in the studies were glycemic control, HbA1c control, body weight reduction, hypoglycemic episodes and gastrointestinal adverse effects [31].
Tirzepatide as monotherapy
Tirzepatide is recently approved, under the brand name Mounjaro, by the United States Food and Drug Administration (FDA) in May 2022. This drug is a dual glucose-dependent insulinotropic peptide (GIP) and glucagon-like peptide-1 (GLP-1) receptor agonist that reduces the blood glucose levels and body weight by more than 20% and HbA1c and improve insulin sensitivity and metabolism of lipid. The route of administration of the drug dose is subcutaneous injection once weekly. The half-life of the drug is about 5 days. Patient dose adherence and compliance are favored because Tirzepatide has the advantage of a once-per-week dose administration. Tirzepatide is a promising drug in the treatrment of type 2 diabetes mellitus and obesity [32].
GLP-1 RA use in individuals with diabetes during fasting month of Ramadan
The literature search showed that Liraglutide followed by Lixisenatide are the mostly studied GLP-RAs during Ramadan Fasting.
Liraglutide as add-on therapy
LIRA-Ramadan is an active‐controlled trial included 343 study participants comparing the effects of Liraglutide and sulphonylurea combined with Metformin in patients with type 2 diabetes during Ramadan. The study showed that glycemic control assessed using fructosamine levels was the same for the two groups and the use of Liraglutide was associated with fewer episodes of hypoglycemia, greater body weight reduction and greater HbA1c control [33]. Treat 4 Ramadan Trial is another randomized control trial included 99 adults which compared Liraglutide to sulphonylurea in combination with Metformin conducted in 2 centers in the UK by Brady EM et al. with findings supporting the aforementioned study. They also reported improved diastolic blood pressure and significantly lower episodes of hypoglycaemia in Liraglutide group. Hence, the authors found that Liraglutide was well tolerated and could be effective as therapy in combination with Metformin during Ramadan [34]. Another study conducted in the Middle East included 111 participants to assess the safety of Liraglutide as add-on to existing diabetes medications during Ramadan found that adding Liraglutide to the existing treatment regimen is not associated with increased risk of hypoglycemia. The frequency of the hypoglycemic episodes was related to the duration of diabetes with fewer episodes in newly diagnosed patients. Furthermore, they also emphasized the importance of pre-Ramadan structured education in reducing the frequency of hypoglycemia [35]. A significant positive effect on weight using Liraglutide was reported in a study that analysed 9 randomized controlled trials and 7 observational studies comparing non‐insulin glucose‐lowering drugs in patients with type 2 diabetes fasting during Ramadan [36].
Lixisenatide as add-on therapy
Other than Liraglutide, Lixisenatide which effectively improves blood glucose levels had also been studied. LixiRam is a recent phase 4 randomized trial that compared the efficacy and safety of Lixisenatide to sulphonylurea as add-on to basal insulin during Ramadan. Results showed decreased hypoglycemic events, reduced HbA1c readings, reduced weight, and few gastrointestinal adverse events during fasting in Lixisenatide group, making Lixisenatide a suitable option for diabetes intending to fast during Ramadan [37, 38].
The South Asian Consensus Guideline was updated to include the use of GLP-1 RA during Ramadan and highlighted the importance of pre-Ramadan planning including stating that GLP-1 analogues have good glycaemic efficacy without causing hypoglycemia, hence provides a very useful choice for Ramadan fasting either alone or in combination with other diabetes drugs [39].
A recent update for the management of diabetes in Ramadan released in 2020 applying the principal of the American Diabetes Association and European Association for the Study of Diabetes (ADA/EASD) had supported the aforementioned effectiveness of GLP-1 RA in improving glycaemic control with low risk of hypoglycaemia. It also added that these drugs are a preferred option for fasting patients with weight problems or high risk for cardiovascular disease. Nausea and vomiting with the concomitant risk of dehydration were the most commonly reported adverse effects, and they recommended starting and adjusting the dose at least 4–8 weeks before Ramadan [40]. Incretin-based therapy, including GLP-1 RA, is considered the drug to avoid hypoglycemia in patients with diabetes in general and especially for those vulnerable to hypoglycemia such as those with renal impairment and the elderly [25]. Patients with diabetes fasting during Ramadan can be included in this vulnerable group. More studies are needed to assess the efficacy and safety of other GLP-1 RA drug use in patients with diabetes during Ramadan fasting (Table 1).
Table 1.
Studies primarily focusing on GLP-1 RA use in Ramadan. The number in the parenthesis is reference
| No | Drug GLP-1 RA | Study | Main findings |
|---|---|---|---|
| 1. | Liraglutide [33] | LIRA-Ramadan comparing the effects of Liraglutide and sulphonylurea combined with Metformin in patients with type 2 diabetes during Ramadan | the use of Liraglutide was associated with fewer episodes of hypoglycemia, greater body weight reduction and greater HbA1c control |
| 2. | Liraglutide [34] | Treat 4 Ramadan Trial compared Liraglutide to sulphonylurea in combination with Metformin conducted in 2 centers in the UK | improved diastolic blood pressure and significantly lower episodes of hypoglycaemia in Liraglutide group |
| 3. | Liraglutide [35] | Liraglutide as add-on to existing diabetes medications during Ramadan taking into consideration the different eating habits in the Middle East during Ramadan | adding Liraglutide to the existing treatment regimen is not associated with increased risk of hypoglycemia |
| 4. | Liraglutide [36] | Systematic review and meta-analysis analysed 9 randomized controlled trials and 7 observational studies comparing non‐insulin glucose‐lowering drugs in patients with type 2 diabetes fasting during Ramadan | using Liraglutide was associated significant positive effect on weight |
| 5. | Lixisenatide [37, 38] | LixiRam is a recent phase 4 randomized trial that compared the efficacy and safety of Lixisenatide to sulphonylurea as add-on to basal insulin during Ramadan | decreased hypoglycemic events, reduced HbA1c readings, reduced weight, and few gastrointestinal adverse events during fasting in Lixisenatide group |
Use of Dipeptidyl peptidase-4 inhibitors (DPP-4i) in Ramadan fasting
Ten studies conducted in India, Egypt, Lebanon, Middle east, France, London, United Kingdom (UK), (16 countries in Middle east, Europe and Asia) showed that vildagliptin reduced HbA1c levels during Ramadan [41–50], and 8 studies showed that vildagliptin reduced body weight during Ramadan [41, 42, 44–46, 48–50]. Seven studies [44–50] reported association of vildagliptin with hypoglycemia. Hassoun et al. found about 5% of those on vildagliptin develop hypoglycemia during Ramadan fasting [44]. In contrast, Devendra et al. found vildagliptin was associated with reduced incidence of hypoglycemia from pre- to post-Ramadan [46]. In addition, studies conducted by Hassanein et al. found that vildagliptin was associated with lower incidence of hypoglycemia during Ramadan [47, 48]. Hassoun et al. similarly found 3.7% (11 of 300) patients [49] whilst Al-Arouj et al. found 5.4% (36 of 669) receiving vildagliptin had hypoglycemia during Ramadan [50], whereas Halimi et al. found 34.2% develop hypoglycemia for vildagliptin use during Ramadan [45] (Table 2).
Table 2.
Studies primarily focusing on Dipeptidyl peptidase-4 inhibitors (DPP-4i) use in Ramadan. The number in the parenthesis is reference
| No | Drug DPP-4i | Trial | Main findings |
|---|---|---|---|
| 1. | Vildagliptin [41] | Vildagliptin vs sulfonylurea in Indian Muslim diabetes patients fasting during Ramadan | Higher percentage of vildagliptin-treated patients achieved HbA1c compared with sulfonylurea. Mean decrease in the body weight was 1.2 kg and 0.03 kg, respectively (P < 0.001) |
| 2. | Vildagliptin [42] | Effect of Vildagliptin Versus Sulfonylurea in Muslim Patients with Type 2 Diabetes Fasting During Ramadan in Egypt | Treatment with vildagliptin was associated with lower incidence of hypoglycemia compared with SU and showed good glycemic and weight control in patients with T2DM fasting during Ramadan |
| 3. | Vildagliptin [43] | Glycemic effects of vildagliptin in patients with type 2 diabetes before, during and after the period of fasting in Ramadan | Change in hemoglobin A1c from baseline to last visit was similar for both groups. The incidence of hypoglycaemia during Ramadan was higher in the control. This result was not statistically significant. However, the number of patients who dropped out from the was higher in the control group |
| 4. | Vildagliptin [44] | Effects of Vildagliptin relative to sulfonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: influence of age and treatment with/without metformin in the VIRTUE study | A few patients experienced hypoglycemic episodes with vildagliptin vs SUs. Vildagliptin ± metformin was also associated with good glycemic and weight control and was well tolerated. Vildagliptin might be a useful treatment option for patients with type 2 diabetes mellitus, particularly high-risk populations such as the elderly fasting during Ramadan |
| 5. | Vildagliptin [45] | Experience with Vildagliptin in Type 2 Diabetic Patients Fasting During Ramadan in France: Insights from the VERDI Study | Although the overall frequency of malaise suggestive of hypoglycemia was high, which would be expected with prolonged fasting in a well-controlled T2DM population during hot summer days, the incidence of more severe and better-documented episodes were much lower, with consistently less events with vildagliptin therapy |
| 6. | Vildagliptin [46] | Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan | The addition of vildagliptin to metformin therapy during Ramadan in Muslim patients with type 2 diabetes was associated with a reduction in the incidence of hypoglycaemia |
| 7. | Vildagliptin [47] | Comparison vildagliptin and the sulphonylurea gliclazide in combination with metformin, in Muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the VECTOR study | Vildagliptin caused no hypoglycaemia, was well adhered to and improved HbA 1c, making it a suitable treatment option for managing fasting |
| 8. | Vildagliptin [48] | A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study | vildagliptin was shown to be an effective, safe, and well-tolerated treatment in patients with T2DM fasting during Ramadan, with a consistently low incidence of hypoglycemia across studies, accompanied by good glycemic and weight control |
| 9. | Vildagliptin [49] | The effect of vildagliptin relative to sulfonylurea as dual therapy with metformin (or as monotherapy) in Muslim patients with type 2 diabetes fasting during Ramadan in the Middle East: the VIRTUE study | Anti-hyperglycemic treatment with vildagliptin led to significantly fewer hypoglycemia events compared and good weight control compared to sulfonylurea treatment among Muslim diabetic patients who fast during Ramadan |
| 10. | Vildagliptin [50] | The effect of vildagliptin relative to sulphonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: the VIRTUE study | vildagliptin was well tolerated and associated with significantly fewer hypoglycaemic episodes compared with SU therapy |
Use of Sodium-glucose cotransporter-2 (SGLT2) inhibitors in Ramadan fasting
Five studies were conducted in different regions, which are Malaysia [51], the Middle East and UAE [52, 53], and Singapore [54], with a total of 1011 participants. One randomized study [51] compared against sulphonylureas. Four of the studies [51–53, 55] reported a reduction in HbA1c with SGLT2i post-Ramadan, ranging from 0.05% to 0.7%, with only 1 study reporting a significant reduction [53], especially in the group treated with insulin concurrently. Four of the studies [52–55] reported a weight reduction ranging from 0.1 to 1.8 kg, with only 1 reporting a significant reduction [53]. Three studies [52–54] reported a non-significant reduction in blood pressure while one [5] reported a non-significant mild elevation of blood pressure. Four studies [51, 52, 54, 55] showed a reduction in hypoglycemia. One of these four studies [51] reported a significant reduction compared to sulphonylureas.
Adverse effect like dehydration, Increased volume depletion effects [52], and thirst [51, 53] was reported in three studies, with one study [51] reporting higher incidence of postural hypotension. Two studies [51, 52] reported a non-significant increase in urinary tract infections. Limitations of these studies were related to data on treatment adherence [51], study design [51] due to unblinded nature and small sample size [51, 54], generalizability due to geographic restriction, and exclusion of those with a high risk of hypoglycemia [52] and lack of data for confounding factors for hypoglycemia [53] and dehydration [53, 54] (Tables 3 and 4).
Table 3.
Studies primarily focusing on SGLT2 inhibitor (SGLT2i) use in Ramadan. The number in the parenthesis is reference
| No | Drug (SGLT2 inhibitor) | Trial | Main findings |
|---|---|---|---|
| 1. | Dapagliflozin [51] | Switching from SU to SGLT2i in Ramadan fasting. Both arms have metformin | Fewer patients exhibited hypoglycaemia for dapagliflozin vs SU |
| 2. | Canagliflozin [52] | Canagliflozin in Ramadan Tolerance Observational Study | Support use of canagliflozin for T2D adults during Ramadan fasting as less episodes of hyopglycaemia |
| 3. | Canagliflozin 100 mg or Dapagliflozin 10 mg [53] | Safety SGLT2i during Ramadan for Muslim T2D |
Use of insulin with SGLT2i increases hypoglycaemia risk during Ramadan Mild hypo not needing hospitalisation Careful monitoring during prolonged fasting No effect on renal function |
| 4. | Any drug and dose of SGLT2 inhibitor [54] | The effect of Ramadan fasting and continuing SGLT2i on ketonemia, BP and renal function in Muslim patients with T2D | non- significant changes in weight, BP and eGFR regardless of SGLT2i with no increase in ketonemia, eGFR deterioration or hypoglycemia |
| 5. | Drug not specified [55] | Use of Flash Glucose Monitoring System (FGMS) in SGLT2i during Ramadan fasting in high risk insulin treated T2D |
Use of SGLT2i with insulin during Ramadan using FGMS in high-risk patients with T2D under optimal care There was minimal interruption of fasting, significant improvement in glycemic control and no significant change in kidney function after Ramadan |
SU = sulphonylurea
Table 4.
Effects SGLT2i use pre and post-Ramadan
| No | Study | HbA1c (%) | Weight (kg) | BP | Hypoglycemia | Dehydration | GU infections | Hyperglycemia | Others |
|---|---|---|---|---|---|---|---|---|---|
| 1. | Wan Seman WJ, et al |
7.7 in dapagliflozin vs 7.6 in SU With reduction of 0.05% in dapagliflozin vs 0.32% in SU |
No comparison | No comparison |
Significant reduction reported symptomatic 24.1% to 3.4% in 4 week Ramadan for SGLT2i. No significant changes for SU RR 0.24 (CI 0.09–0.68) Documented hypoglycemia 7.3% in dapagliflozin vs 27.1% for SU Fewer hypoglycemia in dapagliflozin 5 events vs 20 events for SU |
Thirst 3.5% | More urinary tract infections 10.3% in dapagliflozin vs 3.8% (p = 0.277) but not significant | No comparison |
No difference in overall adverse events Others include polyuria 3.5%, itching 1.7%, dry skin 1.7%, nausea 1.7% and lethargy 1.7% More postural hypotension in dapagliflozin 13.8% vs 5.8% for SU |
| 2. | Hassanein M et al | 7.2 ± 0.8 to 7.1 ± 0.7 for SU vs 7.3 ± 0.8 to 6.9 ± 0.8 for canagliflozin | 82.1 ± 14.1 to 81.5 ± 14.0 in SU vs 87.1 ± 14.8 to 85.31 ± 14.3 for canagliflozin; 3 more for SGLT2i with weight loss (-2.4 vs -0.5 kg), not significant | 129.8 ± 12.0 to 130.7 ± 12.1 for SU vs 129.0 ± 11.7 to 127.9 ± 10.2 for canagliflozin |
3.7% reported ≥ 1 symptomatic hypo vs 13.2% for SU (adj OR 0.273) 2 of 6 canagliflozin vs 27 of 37 for SU confirmed with blood glucose < 3.9 mmol/L; none for canagliflozin vs 6 in SU with glucose < 3.0mmmol/L. None for canagliflozin vs 47.6% for SU reported > 1 event |
Volume depletion events 16.1% in SGLT2i compared to 5% for SU (adj OR 3.5) | 0.6% for canagliflozin vs none for SU | No comparison |
eGFR 88.7 ± 17.6 to 89.9 ± 19.9 9.3% adverse effects related to study drug for canagliflozin vs 8.8% for SU |
| 3. | Bashier A et al |
Significantly reduced 8.3 ± 1.7 to 7.8 ± 1.3; significantly higher for Insulin treated with 8.9 ± 1.7 to 8.2 ± 1.5 vs Oral medication with 7.8 ± 1.5 to 7.4 ± 1.4 |
Significantly reduced from 83.9 ± 17.0 to 83.8 ± 16.6 | No comparison |
Hypoglycemia symptoms 27.0%; 82.3% checked glucose, of which 83.8% confirmed hypoglycemia, of which 48.7% broke their fast 13.6% adjusted their insulin or oral medication during Ramadan 1 hospital admission with hypoglycemia symptoms Event in evening 64.4%, afternoon 24.0%, morning 15.4% and after breaking fast 15.4%. Significantly more for SGLT2i with insulin 37.8 vs 18.0% SGLT2i with oral |
Extreme and unusual thirst 9.3%; significantly more for SGLT2i with insulin 13.15% vs 6.16% SGLT2i with orals | No comparison | No comparison | No comparison |
| 4. | Shao Y et al | Not reported. Baseline 8.7 ± 1.6 for non-SGLT2i vs 9.3 ± 1.9 for SGLT2i | More reduction, -1.8 vs 1.1, not significant | Similar reduction of sitting systolic BP-8.1 vs -10.4 and sitting diastolic BP -3.7 vs -3.5, not significant. 30.3% study vs 3.7.39% control reduced sitting systolic BP ≥ 20 mmHg. No increased in symptoms of postural giddiness or hypotension | Proportion with at least 1 hypoglycemia event decreased in study from 22.9 to 15.4%, not significant vs increased in control 12.1 to 20.7%, not significant | No comparison | No comparison |
Higher fasting plasma glucose in study 0.35 vs -1.17 mmol/L control, not significant Mean fasting plasma glucose higher in study 9.9 vs 8.5 mmol/L in control, but not significant |
Not significantly higher B-hydroxybutyrate in study 0.31 vs 0.24 mmol/L; no significant change -0.01 vs -0.02 and bicarbonate 0.52 vs -0.05, 1 increased 0.53 to 0.69 at Ramadan but well No significant difference in eGFR -6.0 vs -4.2 ml/min/1.73m2 Hyperkalaemia 12.3% in study vs 10.3% in control, not significant Increase in serum K positively correlated with eGFR reduction (r = 0.358, P = 0.004) |
| 5. | Abdelgadir E et al | HbA1c from 7.3 ± 1.5 to 6.8 ± 1.1 for SGLT2i vs 8 ± 1.6 to 7.7 ± 1.5 for non-SGLT2i | Reduced weight 82 ± 13.9 to 81.9 ± 15.5 for SGLT2i vs 85 ± 11.1 to 83 ± 12.1, both not significant |
Mild elevated systolic and diastolic BP, with systolic BP 131 ± 17.1 to 135 ± 15.4 mmHg for SGLT2i vs 122.5 ± 17.8 to 124 ± 16.1 mmHg for control, and diastolic BP from 70 ± 10.6 to 71 ± 8.9 mmHg for SGT2i vs 71.5 ± 9.4 to 72 ± 10.5 mmHg for control All not significant |
Both groups reduced in number and duration, not significant Timing increased hypoglycemia 1200-1800H for SGLT2i reduced in all other timings with no change for control |
No comparison | No comparison | No comparison | Serum creatinine improvement 0.8 ± 0.2 to 0.7 ± 0.2 mg/dL for SGLT2i, not significant |
According to guidelines by International Diabetes Federation in collaboration with Diabetes and Ramadan International Alliance [3], SGLT2 inhibitors can be used with caution in some patients, with no dose adjustment is required, and it is to be taken with iftar during Ramadan. With the latest data on renal and cardiac benefits, consideration of its use should consider these indications [56]. Because of the risk of dehydration, Some guidelines prohibit the prescription of SGLT2 inhibitors in patients with frequent diarrhoea or vomiting, and those who use diuretics and angiotensin converting enzyme inhibitors [7, 57].
New Insulins and technology in the management of diabetes during fasting
The new insulins used during Ramadan are insulin glargine-300 and insulin degludec. For instance, in a prospective, observational study across 11 countries, It was shown that administration of insulin glargine-300 pre-Ramadan, during Ramadan, and post-Ramadan periods in individuals with type 2 diabetes was associated with low risk of severe/symptomatic hypoglycemia and improved glycaemic control [58]. A multinational, randomized, treat-to-target trial in individuals with type 2 diabetes showed that insulin degludec/insulin aspart is a suitable therapeutic agent for patients who need insulin for sustained glucose control before, during and after Ramadan fasting, with a significantly lower risk of hypoglycaemia in comparison with biphasic insulin aspart 30 [59]. Aldibbiat et al. showed that automated insulin dosing systems in fasting individuals with type 1 diabetes were also safe and effective in managing diabetes during fasting [60]. In addition, the safety and effectiveness of insulin pump during Ramadan fasting was also shown in different studies in children and adults with diabetes [61–63]. Therefore, using technology to manage diabetes during Ramadan fasting is associated with excellent clinical outcomes. For instance, free style libre (continuous glucose monitoring) was also found to be associated with excellent clinical outcomes and decreased risk of hypoglycemia. For instance, Elhadd et al. showed that the use of freestyle libre in association with patient education and medication adjustment effectively decreased the risk of hypoglycemia during Ramadan fasting [64]. Importantly, freestyle libre also helped in detecting prolong hypoglycemia with stable chronic kidney disease and coronary heart disease [65, 66].
Ramadan fasting and bariatric surgery
Unfortunately, a limited number of studies were published on the effect of Ramadan Fasting on bariatric surgery [67]. Kermansaravi et al. formed Delphi consensus about bariatric surgery and Ramadan fasting from 61 well-known surgeons and metabolic physicians in bariatric surgery. Their conclusion is that fasting needs special nutritional support in patients who underwent bariatric surgery, and prior to fasting, patient will need to discuss this with the surgeon and dietician. Fasting also can be started at least 6–12 months after the operation (combined and malabsorptive procedures). Proton pump inhibitors were recommended in those with intention to fast in less than 6 months after the operation. The committee also recommended to stop fasting in the presence of persistent symptoms of intolerance [68]. A study conducted by Al-Ozairi et al. in Kuwait to investigate nutrient and calorie consumption, satiety, appetite, and lifestyle behaviors in patients who had bariatric surgery and were fasting Ramadan for about one year and 2 months showed a slight loss of weight after fasting Ramadan in 52.7% of the study participants, weight gain in about 18% of the study participants and no change in weight after Ramadan in more than 22% of the study participants. About 90% of study participants were compliant with their medications. There was no significant difference in the intake of fluid during fasting in comparison with the non-fasting period. Intake of protein was low in the participants and the satiety in females was greater than men. There was no significant difference in occurrence of complications such as hypoglycemia, abdominal pain, diarrhea, or constipation during fasting compared with non-fasting period. The study concluded that fasting Ramadan after bariatric surgery is well tolerated but is important to educate patients on the importance of medication adherence and protein intake [69]. Another study conducted by Tat et al. in United Arab Emirates to assess the perioperative outcomes of bariatric surgeries carried out before or during Ramadan and after Ramadan. The study included 542 patients who performed bariatric surgeries from September 2015 to July 2019. The study showed no difference in the outcomes among patients who perform bariatric surgeries before or during Ramadan and after Ramadan. The authors concluded that there is no high risk in performing bariatric surgery before and during Ramadan [70].
based on the limited number of studies focusing on fasting after bariatric surgery, fasting after bariatric surgery might be safe, but there are surgery-related and nutritional risks. Fasting should be delayed within the postoperative period from six to eighteen months to minimize these risks [68, 71]. Moreover, Patients who underwent bariatric surgery and wish to fast Ramadan should be educated, monitored, and evaluated by bariatric surgery team and physicians.
Limitations of the study
This review has some limitations. Free search engines were used for literature search and the inability to fully retrieve some articles. We only included English-language Studies, but due to the number of studies included in our review, a limited number of studies missed for this reason would likely have minimal impact on our results.
Conclusions
A pre-Ramadan assessment should be the initial step for every patient with diabetes wishing to fast during Ramadan. It is also important to educate healthcare providers and patients with diabetes to ensure safe fasting during Ramadan. Education and diabetes treatment program during Ramadan for patients with diabetes who wish to fast help these patients to self-manage diabetes, lose weight, improve glycemic control, and avoid other complications of fasting during Ramadan. Early and effective counseling at least one to two months prior to Ramadan on blood glucose measurement, information about signs and symptoms of hyperglycemia and hypoglycemia, changes in meal pattern, physical activity, and medications is recommended according to the risk stratification of the patients with diabetes willing to fast to avoid complications during Ramadan. In addition, expert nutritionist advice can be provided to patients with diabetes to stay properly nourished and not to experience any issues related to their diets during or after Ramadan. The newer diabetes medications such as, GLP-1 agonists, DPP-4 inhibitors, SGLT-2 inhibitors and new Insulin therapy are thought to be safe and effective during fasting of Ramadan. These medications are associated with a reduction in HbA1c, body weight, systolic blood pressure, and risk of hypoglycemia during Ramadan fasting. However, further studies with a larger sample size are needed to confirm the efficacy and safety of these newer medications during Ramadan fasting. The use of new technology like freestyle libre can help decrease the risk of hypoglycemia and hyperglycemia. Individuals with bariatric surgery surgery should seek the advice and approval to fast from the bariatric dietician, physician, and surgeon before the beginning of the month of Ramadan and make adjustment for their medications during fasting. These patients should be advised to drink adequate fluids, eat hydrated food, avoid salty and excessive spicy food, and limit taking sweets when they break their fast to avoid the risk of dehydration and dumping syndrome. In addition, these patients should receive proton pump inhibitors daily to avoid GIT complaints during Ramadan (Table 5).
Table 5.
A summary of the safety and efficacy of the new drugs/technology and surgery during the month of Ramadan. The number in the parenthesis is reference
| Medicine/procedure | Example | Main effect |
|---|---|---|
| GLP-1 RA [33–38] | Liraglutide, Lixisenatide | Safe and effective during Ramadan. these drugs are a preferred option for fasting patients with weight problems or high risk for cardiovascular disease |
| DPP4 inhibitors [41–50] | Vildagliptin | vildagliptin was shown to be an effective, safe, and well-tolerated treatment in patients with T2DM fasting during Ramadan, with a consistently low incidence of hypoglycemia across studies, accompanied by good glycemic and weight control. Can be used with metformin during Ramadan |
| SGLT2 inhibitors [51–55] | Dapagliflozin, Canagliflozin | Safe to use in Ramadan less risk of hypoglycemia in comparison with SU. High risk of hypoglycemia if combined with insulin |
| Insulin [58, 59] | Insulin glargine-300 and insulin degludec | Both are associated with low risk of severe/symptomatic hypoglycemia and improved glycemic control |
| Technology in delivery of insulin [60–63] | Insulin pump and automated insulin dosing system | Both are safe and effective in Ramadan |
| Flash glucose monitoring system [64–66] | Free style Libre | Effective in monitoring glycemic control |
| Bariatric surgery [67–71] | Gastric bypass surgery | advice and approval to fast from the bariatric dietician, physician, and surgeon before the beginning of the month of Ramadan is needed |
SU = sulphonylurea
Declarations
Conflict of interest
The authors declare that they do not have a conflict of interest.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Hassanein M, Afandi B, YakoobAhmedani M, Mohammad Alamoudi R, Alawadi F, Bajaj HS, et al. Diabetes and Ramadan: Practical guidelines 2021. Diabetes Res Clin Pract. 2022;185:109185. doi: 10.1016/j.diabres.2021.109185. [DOI] [PubMed] [Google Scholar]
- 2.Jabbar A, Hassanein M, Beshyah SA, Boye KS, Yu M, Babineaux SM. CREED study: Hypoglycaemia during Ramadan in individuals with Type 2 diabetes mellitus from three continents. Diabetes Res Clin Pract. 2017;132:19–26. doi: 10.1016/j.diabres.2017.07.014. [DOI] [PubMed] [Google Scholar]
- 3.Hassanein M, Al-Arouj M, Hamdy O, Bebakar WMW, Jabbar A, Al-Madani A, et al. Diabetes and Ramadan: Practical guidelines. Diabetes Res Clin Pract. 2017;126:303–316. doi: 10.1016/j.diabres.2017.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Roky R, Chapotot F, Hakkou F, Benchekroun MT, Buguet A. Sleep during Ramadan intermittent fasting. J Sleep Res. 2001;10(4):319–327. doi: 10.1046/j.1365-2869.2001.00269.x. [DOI] [PubMed] [Google Scholar]
- 5.Ahmad J, Pathan MF, Jaleel MA, Fathima FN, Raza SA, Khan AK, et al. Diabetic emergencies including hypoglycemia during Ramadan. Indian J Endocrinol Metab. 2012;16(4):512–515. doi: 10.4103/2230-8210.97996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Salti I, Benard E, Detournay B, Bianchi-Biscay M, Le Brigand C, Voinet C, et al. A population-based study of diabetes and its characteristics during the fasting month of Ramadan in 13 countries: results of the epidemiology of diabetes and Ramadan 1422/2001 (EPIDIAR) study. Diabetes Care. 2004;27(10):2306–2311. doi: 10.2337/diacare.27.10.2306. [DOI] [PubMed] [Google Scholar]
- 7.Tootee A, Larijani B. Ramadan fasting and diabetes, latest evidence and technological advancements: 2021 update. J Diabetes Metab Disord. 2021;20(1):1003–1009. doi: 10.1007/s40200-021-00804-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hassanein M, Abdelgadir E, Bashier A, Rashid F, Saeed MA, Khalifa A, et al. The role of optimum diabetes care in form of Ramadan focused diabetes education, flash glucose monitoring system and pre-Ramadan dose adjustments in the safety of Ramadan fasting in high risk patients with diabetes. Diabetes Res Clin Pract. 2019;150:288–295. doi: 10.1016/j.diabres.2018.12.013. [DOI] [PubMed] [Google Scholar]
- 9.Baggio LL, Drucker DJ. Biology of incretins: GLP-1 and GIP. Gastroenterology. 2007;132(6):2131–2157. doi: 10.1053/j.gastro.2007.03.054. [DOI] [PubMed] [Google Scholar]
- 10.Lauritsen KB, Moody AJ, Christensen KC, Lindkaer JS. Gastric inhibitory polypeptide (GIP) and insulin release after small-bowel resection in man. Scand J Gastroenterol. 1980;15(7):833–840. doi: 10.3109/00365528009181538. [DOI] [PubMed] [Google Scholar]
- 11.Mojsov S, Weir GC, Habener JF. Insulinotropin: glucagon-like peptide I (7–37) co-encoded in the glucagon gene is a potent stimulator of insulin release in the perfused rat pancreas. J Clin Investig. 1987;79(2):616–619. doi: 10.1172/JCI112855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kreymann B, Williams G, Ghatei MA, Bloom SR. Glucagon-like peptide-1 7–36: a physiological incretin in man. Lancet. 1987;2(8571):1300–1304. doi: 10.1016/s0140-6736(87)91194-9. [DOI] [PubMed] [Google Scholar]
- 13.Holst JJ. The physiology of glucagon-like peptide 1. Physiol Rev. 2007;87(4):1409–1439. doi: 10.1152/physrev.00034.2006. [DOI] [PubMed] [Google Scholar]
- 14.Drucker DJ, Nauck MA. The incretin system: glucagon-like peptide-1 receptor agonists and dipeptidyl peptidase-4 inhibitors in type 2 diabetes. Lancet. 2006;368(9548):1696–1705. doi: 10.1016/S0140-6736(06)69705-5. [DOI] [PubMed] [Google Scholar]
- 15.Campbell JE, Drucker DJ. Pharmacology, physiology, and mechanisms of incretin hormone action. Cell Metab. 2013;17(6):819–837. doi: 10.1016/j.cmet.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 16.Nauck MA, Kemmeries G, Holst JJ, Meier JJ. Rapid tachyphylaxis of the glucagon-like peptide 1-induced deceleration of gastric emptying in humans. Diabetes. 2011;60(5):1561–1565. doi: 10.2337/db10-0474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Naslund E, King N, Mansten S, Adner N, Holst JJ, Gutniak M, et al. Prandial subcutaneous injections of glucagon-like peptide-1 cause weight loss in obese human subjects. Br J Nutr. 2004;91(3):439–446. doi: 10.1079/BJN20031064. [DOI] [PubMed] [Google Scholar]
- 18.Marx N, Libby P. Cardiovascular Benefits of GLP-1 Receptor Agonism: Is Inflammation a Key? JACC Basic Translat Sci. 2018;3(6):858–860. doi: 10.1016/j.jacbts.2018.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Eng J, Kleinman WA, Singh L, Singh G, Raufman JP. Isolation and characterization of exendin-4, an exendin-3 analogue, from Heloderma suspectum venom. Further evidence for an exendin receptor on dispersed acini from guinea pig pancreas. J Biol Chem. 1992;267(11):7402–5. [PubMed] [Google Scholar]
- 20.Richter B, Bandeira‐Echtler E, Bergerhoff K, Lerch C. Dipeptidyl peptidase‐4 (DPP‐4) inhibitors for type 2 diabetes mellitus. Cochrane Database Syst Rev. 2008;2008(2):CD006739. [DOI] [PMC free article] [PubMed]
- 21.Sfairopoulos D, Liatis S, Tigas S, Liberopoulos E. Clinical pharmacology of glucagon-like peptide-1 receptor agonists. Hormones. 2018;17(3):333–350. doi: 10.1007/s42000-018-0038-0. [DOI] [PubMed] [Google Scholar]
- 22.Meier JJ. GLP-1 receptor agonists for individualized treatment of type 2 diabetes mellitus. Nat Rev Endocrinol. 2012;8(12):728–742. doi: 10.1038/nrendo.2012.140. [DOI] [PubMed] [Google Scholar]
- 23.Hinnen D. Glucagon-Like Peptide 1 Receptor Agonists for Type 2 Diabetes. Diabetes Spectrum. 2017;30(3):202–210. doi: 10.2337/ds16-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyle James G, Livingstone R, Petrie JR. Cardiovascular benefits of GLP-1 agonists in type 2 diabetes: a comparative review. Clin Sci. 2018;132(15):1699–1709. doi: 10.1042/CS20171299. [DOI] [PubMed] [Google Scholar]
- 25.Farngren J, Ahrén B. Incretin-based medications (GLP-1 receptor agonists, DPP-4 inhibitors) as a means to avoid hypoglycaemic episodes. Metabolism. 2019;99:25–31. doi: 10.1016/j.metabol.2019.06.016. [DOI] [PubMed] [Google Scholar]
- 26.Nauck MA, Kleine N, Ørskov C, Holst JJ, Willms B, Creutzfeldt W. Normalization of fasting hyperglycaemia by exogenous glucagon-like peptide 1 (7–36 amide) in Type 2 (non-insulin-dependent) diabetic patients. Diabetologia. 1993;36(8):741–744. doi: 10.1007/BF00401145. [DOI] [PubMed] [Google Scholar]
- 27.Esposito K, Mosca C, Brancario C, Chiodini P, Ceriello A, Giugliano D. GLP-1 receptor agonists and HBA1c target of <7% in type 2 diabetes: meta-analysis of randomized controlled trials. Curr Med Res Opin. 2011;27(8):1519–1528. doi: 10.1185/03007995.2011.590127. [DOI] [PubMed] [Google Scholar]
- 28.Trujillo JM, Nuffer W. GLP-1 receptor agonists for type 2 diabetes mellitus: recent developments and emerging agents. Pharmacotherapy. 2014;34(11):1174–86. doi: 10.1002/phar.1507. [DOI] [PubMed] [Google Scholar]
- 29.Lorber D. GLP-1 receptor agonists: effects on cardiovascular risk reduction. Cardiovasc Ther. 2013;31(4):238–249. doi: 10.1111/1755-5922.12000. [DOI] [PubMed] [Google Scholar]
- 30.Buse JB, Wexler DJ, Tsapas A, Rossing P, Mingrone G, Mathieu C, et al. 2019 update to: Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetologia. 2020;63(2):221–8. doi: 10.1007/s00125-019-05039-w. [DOI] [PubMed] [Google Scholar]
- 31.Alexiadou K, Tan TM. Gastrointestinal Peptides as Therapeutic Targets to Mitigate Obesity and Metabolic Syndrome. Curr DiabRep. 2020;20(7):26. doi: 10.1007/s11892-020-01309-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chavda VP, Ajabiya J, Teli D, Bojarska J, Apostolopoulos V. Tirzepatide, a New Era of Dual-Targeted Treatment for Diabetes and Obesity: A Mini-Review. Molecules. 2022;27(13):4315. [DOI] [PMC free article] [PubMed]
- 33.Azar ST, Echtay A, Wan Bebakar WM, Al Araj S, Berrah A, Omar M, et al. Efficacy and safety of liraglutide compared to sulphonylurea during Ramadan in patients with type 2 diabetes (LIRA-Ramadan): a randomized trial. Diabetes Obes Metab. 2016;18(10):1025–1033. doi: 10.1111/dom.12733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Brady EM, Davies MJ, Gray LJ, Saeed MA, Smith D, Hanif W, et al. A randomized controlled trial comparing the GLP-1 receptor agonist liraglutide to a sulphonylurea as add on to metformin in patients with established type 2 diabetes during Ramadan: the Treat 4 Ramadan Trial. Diabetes Obes Metab. 2014;16(6):527–536. doi: 10.1111/dom.12249. [DOI] [PubMed] [Google Scholar]
- 35.Khalifa AA, El Rashid A, Bashier A. Safety and Efficacy of Liraglutide as an Add-On Therapy to Pre-Existing Anti-Diabetic Regimens during Ramadan, A Prospective Observational Trial. J Diabetes Metabolism. 2015;6(9):1–5. [Google Scholar]
- 36.Gray LJ, Dales J, Brady EM, Khunti K, Hanif W, Davies MJ. Safety and effectiveness of non-insulin glucose-lowering agents in the treatment of people with type 2 diabetes who observe Ramadan: a systematic review and meta-analysis. Diabetes Obes Metab. 2015;17(7):639–648. doi: 10.1111/dom.12462. [DOI] [PubMed] [Google Scholar]
- 37.Sahay R, Hafidh K, Djaballah K, Coudert M, Azar S, Shehadeh N, et al. Safety of lixisenatide plus basal insulin treatment regimen in Indian people with type 2 diabetes mellitus during Ramadan fast: A post hoc analysis of the LixiRam randomized trial. Diabetes Res Clin Pract. 2020;163:108148. doi: 10.1016/j.diabres.2020.108148. [DOI] [PubMed] [Google Scholar]
- 38.Hassanein MM, Sahay R, Hafidh K, Djaballah K, Li H, Azar S, et al. Safety of lixisenatide versus sulfonylurea added to basal insulin treatment in people with type 2 diabetes mellitus who elect to fast during Ramadan (LixiRam): An international, randomized, open-label trial. Diabetes Res Clin Pract. 2019;150:331–341. doi: 10.1016/j.diabres.2019.01.035. [DOI] [PubMed] [Google Scholar]
- 39.Pathan F, Latif ZA, Sahay RK, Zargar AH, Raza SA, Khan A, et al. South Asian consensus guideline: Use of GLP-1 receptor agonists during Ramadan: Update 2016 Revised Guidelines on the use of GLP-1A in Ramadan. J Pak Med Assoc. 2016;66(6):774–776. [PubMed] [Google Scholar]
- 40.Ibrahim M, Davies MJ, Ahmad E, Annabi FA, Eckel RH, Ba-Essa EM, et al. Recommendations for management of diabetes during Ramadan: update 2020, applying the principles of the ADA/EASD consensus. BMJ Open Diabetes Res Care. 2020;8(1):e001248. doi: 10.1136/bmjdrc-2020-001248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shete A, Shaikh A, Nayeem KJ, Rodrigues L, Ali MS, Shah P, et al. Vildagliptin vs sulfonylurea in Indian Muslim diabetes patients fasting during Ramadan. World J Diabetes. 2013;4(6):358–364. doi: 10.4239/wjd.v4.i6.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khattab M, Mahmoud K, Shaltout I. Effect of Vildagliptin Versus Sulfonylurea in Muslim Patients with Type 2 Diabetes Fasting During Ramadan in Egypt: Results from VIRTUE Study. Diabetes Ther. 2016;7(3):551–560. doi: 10.1007/s13300-016-0190-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Malha LP, Taan G, Zantout MS, Azar ST. Glycemic effects of vildagliptin in patients with type 2 diabetes before, during and after the period of fasting in Ramadan. Ther Adv Endocrinol Metab. 2014;5(1):3–9. doi: 10.1177/2042018814529062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hassoun AA, Pathan MF, Medlej RC, Alarouj M, Shaltout I, Chawla MS, et al. Effects of vildagliptin relative to sulfonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: influence of age and treatment with/without metformin in the VIRTUE study. Diabetes Metab Syndr Obes. 2016;9:225–231. doi: 10.2147/DMSO.S103692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Halimi S, Levy M, Huet D, Quere S, Dejager S. Experience with Vildagliptin in Type 2 Diabetic Patients Fasting During Ramadan in France: Insights from the VERDI Study. Diabetes Ther. 2013;4(2):385–398. doi: 10.1007/s13300-013-0038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Devendra D, Gohel B, Bravis V, Hui E, Salih S, Mehar S, et al. Vildagliptin therapy and hypoglycaemia in Muslim type 2 diabetes patients during Ramadan. Int J Clin Pract. 2009;63(10):1446–1450. doi: 10.1111/j.1742-1241.2009.02171.x. [DOI] [PubMed] [Google Scholar]
- 47.Hassanein M, Hanif W, Malik W, Kamal A, Geransar P, Lister N, et al. Comparison of the dipeptidyl peptidase-4 inhibitor vildagliptin and the sulphonylurea gliclazide in combination with metformin, in Muslim patients with type 2 diabetes mellitus fasting during Ramadan: results of the VECTOR study. Curr Med Res Opin. 2011;27(7):1367–1374. doi: 10.1185/03007995.2011.579951. [DOI] [PubMed] [Google Scholar]
- 48.Hassanein M, Abdallah K, Schweizer A. A double-blind, randomized trial, including frequent patient-physician contacts and Ramadan-focused advice, assessing vildagliptin and gliclazide in patients with type 2 diabetes fasting during Ramadan: the STEADFAST study. Vasc Health Risk Manag. 2014;10:319–326. doi: 10.2147/VHRM.S64038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hassoun AA, Al-Arouj M, Ibrahim M. The effect of vildagliptin relative to sulfonylurea as dual therapy with metformin (or as monotherapy) in Muslim patients with type 2 diabetes fasting during Ramadan in the Middle East: the VIRTUE study. Curr Med Res Opin. 2017;33(1):161–167. doi: 10.1080/03007995.2016.1243093. [DOI] [PubMed] [Google Scholar]
- 50.Al-Arouj M, Hassoun AA, Medlej R, Pathan MF, Shaltout I, Chawla MS, et al. The effect of vildagliptin relative to sulphonylureas in Muslim patients with type 2 diabetes fasting during Ramadan: the VIRTUE study. Int J Clin Pract. 2013;67(10):957–963. doi: 10.1111/ijcp.12243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wan Seman WJ, Kori N, Rajoo S, Othman H, Mohd Noor N, Wahab NA, et al. Switching from sulphonylurea to a sodium-glucose cotransporter2 inhibitor in the fasting month of Ramadan is associated with a reduction in hypoglycaemia. Diabetes Obes Metab. 2016;18(6):628–632. doi: 10.1111/dom.12649. [DOI] [PubMed] [Google Scholar]
- 52.Hassanein M, Echtay A, Hassoun A, Alarouj M, Afandi B, Poladian R, et al. Tolerability of canagliflozin in patients with type 2 diabetes mellitus fasting during Ramadan: Results of the Canagliflozin in Ramadan Tolerance Observational Study (CRATOS). Int J Clin Pract. 2017;71(10):e12991. [DOI] [PMC free article] [PubMed]
- 53.Bashier A, Khalifa AA, Abdelgadir EI, Al Saeed MA, Al Qaysi AA, Bayati MBA, et al. Safety of Sodium-Glucose Cotransporter 2 Inhibitors (SGLT2-I) During the Month of Ramadan in Muslim Patients with Type 2 Diabetes. Oman Med J. 2018;33(2):104–110. doi: 10.5001/omj.2018.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Shao Y, Lim GJ, Chua CL, Wong YF, Yeoh ECK, Low SKM, et al. The effect of Ramadan fasting and continuing sodium-glucose co-transporter-2 (SGLT2) inhibitor use on ketonemia, blood pressure and renal function in Muslim patients with type 2 diabetes. Diabetes Res Clin Pract. 2018;142:85–91. doi: 10.1016/j.diabres.2018.05.022. [DOI] [PubMed] [Google Scholar]
- 55.Abdelgadir E, Rashid F, Bashier A, Al Saeed M, Khalifa A, Alawadi F, et al. Use of flash glucose monitoring system in assessing safety of the SGLT2 inhibitors during Ramadan fasting in high risk insulin treated patients with type 2 diabetes. Diabetes Metab Syndr. 2019;13(5):2927–2932. doi: 10.1016/j.dsx.2019.07.055. [DOI] [PubMed] [Google Scholar]
- 56.Ekanayake P, Hupfeld C, Mudaliar S. Sodium-Glucose Cotransporter Type 2 (SGLT-2) Inhibitors and Ketogenesis: the Good and the Bad. Curr Diab Rep. 2020;20(12):74. doi: 10.1007/s11892-020-01359-z. [DOI] [PubMed] [Google Scholar]
- 57.Bajaj HS, Abouhassan T, Ahsan MR, Arnaout A, Hassanein M, Houlden RL, et al. Diabetes Canada Position Statement for People With Types 1 and 2 Diabetes Who Fast During Ramadan. Can J Diabetes. 2019;43(1):3–12. doi: 10.1016/j.jcjd.2018.04.007. [DOI] [PubMed] [Google Scholar]
- 58.Hassanein M, AkifBuyukbese M, Malek R, Pilorget V, Naqvi M, Berthou B, et al. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: The observational ORION study. Diabetes Res Clin Pract. 2020;166:108189. doi: 10.1016/j.diabres.2020.108189. [DOI] [PubMed] [Google Scholar]
- 59.Hassanein M, Echtay AS, Malek R, Omar M, Shaikh SS, Ekelund M, et al. Original paper: Efficacy and safety analysis of insulin degludec/insulin aspart compared with biphasic insulin aspart 30: A phase 3, multicentre, international, open-label, randomised, treat-to-target trial in patients with type 2 diabetes fasting during Ramadan. Diabetes Res Clin Pract. 2018;135:218–226. doi: 10.1016/j.diabres.2017.11.027. [DOI] [PubMed] [Google Scholar]
- 60.Aldibbiat A, Alqashami A, Hussain S. Use of automated insulin delivery systems in people with type 1 diabetes fasting during Ramadan: An observational study. J Diabetes Investig. 2021;4:647–651. doi: 10.1111/jdi.13720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.AlGhatam G, O'Keeffe D, Taha H. Effects of Alternate Insulin Pump Settings in Patients With Type 1 Diabetes During Ramadan: A Randomized Pilot Study. J Diabetes Sci Technol. 2021;19322968211059217. [DOI] [PMC free article] [PubMed]
- 62.Zabeen B, Ahmed B, Nahar J. Young people with type 1 diabetes on insulin pump therapy could fast safely during COVID-19 pandemic Ramadan: A telemonitoring experience in Bangladesh. J Diabetes Investig. 2021;12(6):1060–1063. doi: 10.1111/jdi.13449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mohamed K, Al-Abdulrazzaq D, Fayed A, El Busairi E, Al Shawaf F, Abdul-Rasoul M, et al. Fasting during the holy month of Ramadan among older children and adolescents with type 1 diabetes in Kuwait. J Pediatr Endocrinol Metab. 2019;32(8):843–849. doi: 10.1515/jpem-2019-0009. [DOI] [PubMed] [Google Scholar]
- 64.Elhadd T, Bashir M, Baager KA, Ali HA, Almohannadi DHS, Dabbous Z, et al. Mitigation of hypoglycemia during Ramadan using the flash glucose monitoring system following dose adjustment of insulin and sulphonylurea in patients taking multiple glucose-lowering therapies (The PROFAST-IT Study) Diabetes Res Clin Pract. 2021;172:108589. doi: 10.1016/j.diabres.2020.108589. [DOI] [PubMed] [Google Scholar]
- 65.Hassanein M, Rashid F, Elsayed M, Basheir A, Al Saeed M, Abdelgadir E, et al. Assessment of risk of fasting during Ramadan under optimal diabetes care, in high-risk patients with diabetes and coronary heart disease through the use of FreeStyle Libre flash continuous glucose monitor (FSL-CGMS) Diabetes Res Clin Pract. 2019;150:308–314. doi: 10.1016/j.diabres.2019.01.038. [DOI] [PubMed] [Google Scholar]
- 66.Alawadi F, Rashid F, Bashier A, Abdelgadir E, Al Saeed M, Abuelkheir S, et al. The use of Free Style Libre Continues Glucose Monitoring (FSL-CGM) to monitor the impact of Ramadan fasting on glycemic changes and kidney function in high-risk patients with diabetes and chronic kidney disease stage 3 under optimal diabetes care. Diabetes Res Clin Pract. 2019;151:305–312. doi: 10.1016/j.diabres.2019.03.015. [DOI] [PubMed] [Google Scholar]
- 67.Craggs-Dino L, El Chaar M, Husain FA, Rogers AM, Lima AG, Sadegh M, et al. American Society for Metabolic and Bariatric Surgery review on fasting for religious purposes after surgery. Surg Obes Relat Dis. 2022;18(7):861–871. doi: 10.1016/j.soard.2022.04.020. [DOI] [PubMed] [Google Scholar]
- 68.Kermansaravi M, Omar I, Mahawar K, Shahabi S, Bashir A, Haddad A, et al. Religious Fasting of Muslim Patients After Metabolic and Bariatric Surgery: a Modified Delphi Consensus. Obes Surg. 2021;31(12):5303–5311. doi: 10.1007/s11695-021-05724-z. [DOI] [PubMed] [Google Scholar]
- 69.Al-Ozairi E, Al Kandari J, AlHaqqan D, AlHarbi O, Masters Y, Syed AA. Obesity surgery and Ramadan: a prospective analysis of nutritional intake, hunger and satiety and adaptive behaviours during fasting. Obes Surg. 2015;25(3):523–529. doi: 10.1007/s11695-014-1373-0. [DOI] [PubMed] [Google Scholar]
- 70.Tat C, Barajas-Gamboa JS, Del Gobbo GD, Klingler M, Abdallah M, Raza J, et al. The Effect of Fasting during Ramadan on Outcomes after Bariatric Surgery at an Academic Medical Center in the Middle East. Obes Surg. 2020;30(11):4446–4451. doi: 10.1007/s11695-020-04844-2. [DOI] [PubMed] [Google Scholar]
- 71.Sherf Dagan S, Goldenshluger A, Globus I, Schweiger C, Kessler Y, Kowen Sandbank G, et al. Nutritional Recommendations for Adult Bariatric Surgery Patients: Clinical Practice. Adv Nutr. 2017;8(2):382–394. doi: 10.3945/an.116.014258. [DOI] [PMC free article] [PubMed] [Google Scholar]


