Dear Editor,
Alzheimer’s disease (AD) is one of the most devastating neurodegenerative disorders and the most common form of dementia. Synaptic loss is a hallmark of AD pathology and exacerbates cognitive impairment [1]. Synaptic loss, unlike neuronal loss, is reversible due to the highly dynamic properties of synapses. Thus, therapeutics targeting synaptic loss and dysfunction are practical and beneficial for treating neurodegenerative diseases. Most importantly, the window for the treatment of synapses is longer than that of clearing toxic proteins and preventing neuronal death. Treatment targeting synapses delivered at a relatively late stage still slows the progression of AD [2]. Evidence supporting a hypothesis for targeting the excitation-inhibition (EI) synaptic balance provides a therapeutic approach for preventing neurodegeneration in patients with AD [3].
Brain-derived neurotrophic factor (BDNF), a neurotrophic factor family member, has prominent effects on synaptic development and function, neuronal survival and differentiation, and cognition. As a synaptogenic modulator that improves synaptic function and plasticity, BDNF is sufficient to induce synapse formation and enhance synaptic transmission [4]. In patients with AD, the level of BDNF protein in the entorhinal cortex is reduced [5]. BDNF has an extensive protective action in AD animal models, aged rats, and adult primates. The TrkB (tropomyosin-related kinase B, BDNF receptor) agonist, AS86, reverses the cognitive function deficit in the amyloid-β precursor protein/presenilin 1 (APP/PS1) mouse model [6], implying that BDNF is a beneficial therapy for AD [2]. However, the development of BDNF-based drugs is still challenging. Due to its intrinsic biochemical properties, exogenous BDNF protein directly delivered into the blood or cerebrospinal fluid is quickly metabolized, making it difficult to reach the target region. Diffusion of exogenous BDNF protein in the brain parenchyma is difficult due to the binding of BDNF to its high-affinity receptor TrkB. Previous findings indicate that BDNF release is dependent on neuronal activity and high-frequency stimulation is sufficient to induce BDNF release [7]. Therefore, it is urgent to develop novel therapies to increase endogenous BDNF levels in the target location by stimulating BDNF-secreting neurons.
Several studies have suggested that the entorhinal cortex (EC) is a key region in the pathophysiology of the early stages of AD [8, 9]. Metabolic dysfunction in the EC has been reported using cerebral blood volume-functional magnetic resonance imaging (CBV-fMRI) in patients and mice [10]. AD is characterized by a series of pathological susceptibilities, the most prominent of which are amyloid and tau protein abnormalities in the EC in the early stage, resulting in short-term memory impairment [11]. A reduction in interneurons and inhibitory axon terminals has also been reported in the EC; this is consistent with synaptic dysfunction, and suggests that resolving synaptic imbalance in the EC may provide therapeutic benefit in the early stages of AD [3]. EC also relays and processes social signals associated with social cognition in human and animal models. A study has shown that optogenetic stimulation of the lateral entorhinal cortex (LEC) to the dentate gyrus (DG) facilitates social memory, providing insight into the improvement of social memory in brain diseases [12]. Thus, in the 5×FAD (familial Alzheimer’s disease) mouse model, the LEC could serve as a potential area for the treatment of social memory deficits.
Based on the evidence above, we hypothesized that endogenous BDNF release into the LEC via stimulation of a source region could protect against AD-related pathological changes and ameliorate cognitive impairments. To determine the regions upstream of the LEC, we injected retroAAV-syn-EYFP into the LEC of adult wild-type (WT) mice. Retrogradely-labeled cells were found in the paraventricular thalamus (PVT) [13, 14], anteromedial thalamic nucleus (AM), piriform cortex (Pir), and amygdala (Fig. 1A, B), suggesting that these regions send monosynaptic inputs to the LEC. Fluorescent in situ hybridization revealed the presence of BDNF messenger RNA (mRNA) in the PVT (Fig. 1C), implying that it is a source of BDNF delivery into the LEC. We confirmed this connection by using another retrograde tracer CTB (cholera toxin subunit B)-488 and found that CTB-labeled neurons were distributed from anterior to posterior in the PVT. Careful examination revealed that the number of CTB+ neurons in the anterior PVT was higher than that in the posterior PVT (Fig. S1A) and CTB+ neurons were co-localized with BDNF in the PVT (Fig. S1B). Therefore, we focused on the PVT-LEC circuit.
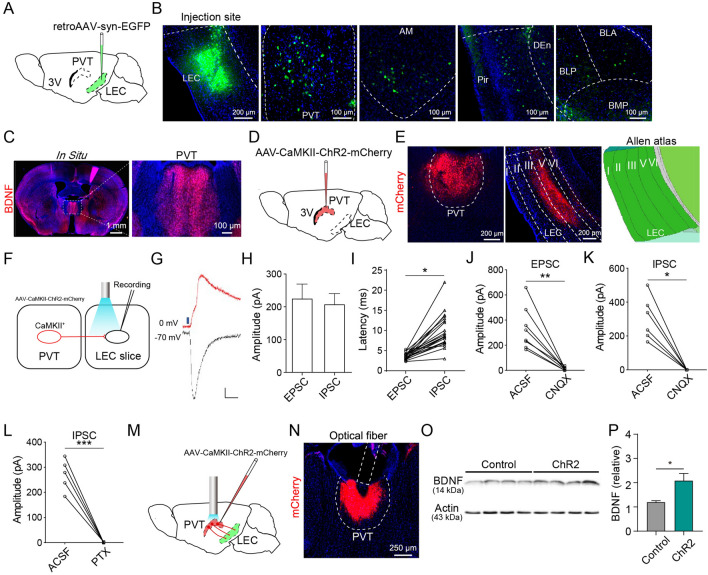
Fig. 1.
Excitatory neurons in the PVT release BDNF release into the LEC. A Schematic of retroAAV injection into the LEC. B Location of an injection site and fluorescence images of EYFP-labeled neurons in the PVT, AM, Pir, and BLA. C Fluorescent in situ hybridization for BDNF mRNA in the PVT. D Schematic of AAV-CaMKII-ChR2-mCherry injection to the PVT. E Fluorescence images of viral expression in the PVT and the distribution of axonal fibers in the LEC. F Procedures for optogenetic manipulation and electrophysiological recording. G Example traces of light-induced EPSCs and IPSCs (scale bars, 50 pA, 50 ms). H Amplitudes of EPSCs and IPSCs recorded in the LEC (n = 21 cells). I Latency of EPSCs and IPSCs recorded in the LEC (n = 21 cells; *P <0.05, paired Student’s t-test). J Amplitude of EPSCs recorded in the LEC after application of CNQX (10 μmol/L; n = 8 cells; **P <0.01, paired Student’s t-test). K Amplitude of IPSCs recorded in the LEC after application of CNQX (10 μmol/L; n = 6 cells; *P <0.05, paired Wilcoxon test). L Amplitude of IPSCs recorded in the LEC after application of picrotoxin (100 μmol/L; n = 5 cells; ***P <0.001, paired Student’s t-test). M Procedure for optogenetic manipulation. N Location of viral expression and optic fiber placement. O, P Western blots and quantification of BDNF protein levels in the LEC (n = 4/group; *P <0.05, unpaired Student’s t-test). 3V, 3rd ventricle; ACSF, artificial cerebrospinal fluid; AM, anteromedial thalamic nucleus; BDNF, brain-derived neurotrophic factor; BLA, basolateral amygdaloid nucleus, anterior part; BLP, basolateral amygdaloid nucleus, posterior part; BMP, basomedial amygdaloid nucleus, posterior part; CaMKII, calmodulin-dependent protein kinase II; DEn, dorsal endopiriform nucleus; EPSC, excitatory postsynaptic current; IPSC, inhibitory postsynaptic current; LEC, lateral entorhinal cortex; Pir, piriform cortex; PTX, picrotoxin; PVT, paraventricular thalamus.
To characterize the synaptic connection between the PVT and the LEC, we injected AAV-CaMKII-ChR2-mCherry into the PVT and made patch-clamp recordings from LEC neurons in slices prepared 4 weeks later (Fig. 1D). Dense mCherry fluorescent signals were detected in layers III and V of the LEC (Fig. 1E), suggesting that the PVT mainly innervates deep layers of the LEC. In acute LEC slices containing ChR2-expressing terminals from the PVT (Fig. 1F), brief blue light stimulation (1 ms) elicited reliable excitatory postsynaptic currents (EPSCs) and inhibitory postsynaptic currents (IPSCs) in LEC neurons (Fig. 1G, H). The latency of the picrotoxin-sensitive IPSCs was significantly longer than that of the EPSCs, and extended beyond the duration of monosynaptic transmission (Fig. 1I, L). Because most PVT neurons are glutamatergic, we suspected that the light-evoked IPSCs were mediated by multisynaptic feedforward inhibition. Indeed, both EPSCs and IPSCs were blocked by the α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid (AMPA) receptor antagonist CNQX (10 µmol/L) (Fig. 1J, K), suggesting that PVT sends feedforward inhibition via local LEC interneurons. To examine the LEC cell types that were directly innervated by PVT, we made targeted patch-clamp recordings from glutamatergic neurons in the LEC of vGluT1::Ai14 mice in which glutamatergic neurons were labeled with the red fluorescence protein tdTomato. Brief light stimulation evoked robust EPSCs in 90% (9/10) of tdTomato+ glutamatergic neurons in the LEC (Fig. S1C). To determine whether PVT terminals also synapse on gamma-aminobutyric acid-ergic (GABAergic) neurons in the LEC, we made targeted recordings from GABAergic neurons in the LEC after labeling GABAergic neurons by injecting AAV2-dlx5/6-GFP into the LEC of WT mice [15]. We found that light-evoked EPSCs could be recorded from 40% (2/5) of GABAergic neurons (Fig. S1C). Overall, these results suggest that PVT neurons make synaptic connections with both glutamatergic and GABAergic neurons in the LEC, and the connection probability is higher with glutamatergic neurons.
To determine the activity-dependent release of BDNF from the PVT neurons into the LEC, we optogenetically activated PVT neurons (Fig. 1M, N) for 15 min (20 Hz, 15 mW) and collected LEC tissue for Western blotting to measure the BDNF protein. The BDNF protein level in the LEC was significantly higher in the ChR2 group than in the control group (Fig. 1O, P), indicating that the activation of PVT neurons is sufficient to release BDNF into the LEC.
Massive synaptic loss is a hallmark of the early stages of AD. To determine whether endogenous BDNF could rescue synaptic loss in AD, we applied chemogenetic tools to chronically activate the PVT neurons in 5×FAD mice. The utility of the chemogenetic approach in activating PVT and LEC neurons were validated by c-fos immunostaining (Fig. S2A–D). We then transduced PVT neurons with Gq-coupled human M3 muscarinic receptor (hM3Dq) in 5×FAD mice at 7 months old and chronically activated PVT neurons by daily injections of clozapine-N-oxide (CNO) (0.6 mg/kg) for 2 months (Fig. 2A). The animals were sacrificed at 10 months of age, and Golgi-Cox staining was used to evaluate the density and morphology of spines in the LEC. We found that the reduction in the density of all spines and mushroom spines in the AD mice was largely rescued by the chemogenetic activation of PVT neurons (Fig. 2B–D). Moreover, immunostaining of the presynaptic marker synaptophysin also showed a significant increase in the number of synaptophysin+ puncta in the hM3Dq group of AD mice compared to that of mCherry controls (Fig. 2E, F). Nonetheless, we found no difference in the number of NeuN+ cells in the LEC among the three groups, hinting that neuronal loss was not reversed by the activation of PVT neurons (Fig. 2E, G). Overall, these results indicated that chronic activation of the PVT is sufficient to prevent synaptic loss in AD mice. Following that, amyloid β (Aβ) immunostaining in the LEC showed that there was no significant difference in the number of Aβ plaques between the hM3Dq and mCherry groups (Fig. S2K, L), which suggests that chronic activation of the PVT has no effect on clearing Aβ.
Fig. 2.
Chronic activation of PVT neurons attenuates synaptic loss and ameliorates social memory in 5×FAD mice. A Experimental design for chemogenetic activation and staining. B Representative images of dendritic segments in the LEC (arrowheads represent mushroom spines). C Chronic activation of PVT neurons reduces total spine loss in the LEC (WT + mCherry: n = 43, AD + mCherry: n = 24, AD + hM3Dq: n = 24; **P <0.01, *P <0.05, one-way analysis of variance (ANOVA), Tukey’s post hoc test). D Chronic activation of PVT neurons prevents mushroom spine loss in the LEC (WT + mCherry: n = 43, AD + mCherry: n = 24, AD + hM3Dq: n = 24; ***P <0.001, *P <0.05, one-way ANOVA, Tukey’s post hoc test). E Representative immunofluorescence staining of synaptophysin and NeuN in the LEC. F Numbers of synaptophysin-positive puncta per mm2 in the LEC of the WT + mCherry (n = 6), AD + mCherry (n = 6), and AD + hM3Dq groups (n = 8) (**P <0.01, *P <0.05, one-way ANOVA, Dunn’s test). G Numbers of NeuN-positive cells per mm2 in the LEC of the WT + mCherry (n = 5), AD + mCherry (n = 5), and AD + hM3Dq groups (n = 5) (n.s., no significant difference, one-way ANOVA, Tukey’s post hoc test). H Experimental design for chemogenetic activation and behaviors. I Schematic of the injection of AAV-CaMKII-hM3Dq-mCherry into PVT (left) and the location of viral expression (right). J Protocol for the social discrimination test. K Total duration in the sniffing region during the social discrimination test (SDT) in the WT + mCherry (n = 28), AD + mCherry (n = 17), and AD + hM3Dq groups (n = 12) (***P <0.001, **P <0.01, n.s., no significant difference, paired Student’s t-test). FM, familiar mouse; NM, novel mouse. L Comparison of social discrimination index (SDI) for the WT + mCherry (n = 28), AD + mCherry (n = 17), and AD + hM3Dq groups (n = 12) (***P <0.001, *P <0.05, one-way ANOVA, Tukey’s post hoc test). M Total travel distance and speed in the open field for the WT + mCherry (n = 23), AD + mCherry (n = 10), and AD + hM3Dq groups (n = 9) (n.s., no significant difference, one-way ANOVA, Tukey’s post hoc test).
Next, we sought to determine whether chronic stimulation of the PVT is beneficial for the social memory deficits associated with AD. We transduced PVT neurons with hM3Dq in 5×FAD mice at 7 months old and chronically activated PVT neurons by daily injection of CNO (0.6 mg/kg) for different time periods. Control AD mice spent an equal amount of time sniffing the novel and familiar mice, which resulted in a low social discrimination index (SDI), indicating an impairment of social memory. Two months of CNO treatment (Fig. 2H, I) significantly increased the time spent in investigating the novel mice, and the SDI (Fig. 2J–L). To determine how long the CNO treatment is required for the improvement of social memory, we shortened the treatment duration to 1 month or 0.5 month. The one-month CNO treatment also significantly increased the time spent in sniffing novel mice and the SDI. The improvement effect was blocked by pretreatment with the TrkB antagonist K252a (1 μL and 1 μmol in 1% DMSO/artificial cerebrospinal fluid), demonstrating a crucial role of the BDNF–TrkB signaling pathway in mediating the memory improvement (Fig. S2H–J). AD mice with 0.5-month CNO treatment also exhibited a trend of increase in SDI, but this did not reach the significance level (Fig. S2E–G). These results suggest that one month of CNO treatment is required for the improvement of social memory.
We further investigated whether the activation of PVT neurons alters the locomotor activity of AD mice using the open field test. There was no significant difference among the three groups in total distance traveled and speed (Fig. 2M), suggesting that chronic PVT stimulation does not influence locomotion.
In summary, we demonstrated that activating PVT neurons results in the endogenous release of BDNF into the LEC. In line with a previous study in which optogenetic stimulation of the LEC–DG restores social memory deficits [12], chronic activation of PVT prevented synaptic loss, and was sufficient to attenuate social memory deficits. Another study has demonstrated that in the early stages of AD, an excitatory–inhibitory imbalance occurs in the LEC, and allosteric modulators restore synaptic balance [3], implying that regulating synaptic function in the LEC is a promising target for treating AD. Furthermore, our results also showed that the endogenous release of BDNF by manipulating neural circuits, which prevented synaptic loss and attenuated social memory deficits, could be a potentially important strategy in treating the symptoms of AD. Because of the longer window of treatment for synapses, this therapeutic strategy has a unique advantage over traditional treatments such as the clearance of toxic proteins.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by the Frontier Research Program of Bioland Laboratory (Guangzhou Regenerative Medicine and Health Guangdong Laboratory) (2018GZR110105006), the National Natural Science Foundation of China (31900735, 82171492, and 81922024), and the Science, Technology and Innovation Commission of Shenzhen Municipality (RCJC20200714114556103 and ZDSYS20190902093601675).
Conflict of interest
The authors declare no conflict of interest in this work.
Footnotes
Yun-Long Xu, Lin Zhu, and Zi-Jun Chen contributed equally to this work.
Contributor Information
Wei Xu, Email: freefresh2000@163.com.
Kui-Kui Zhou, Email: kk.zhou@siat.ac.cn.
Ying-Jie Zhu, Email: yj.zhu1@siat.ac.cn.
References
- 1.Zhang XM, Zhao F, Wang CF, Zhang J, Bai Y, Zhou F, et al. AVP(4–8) improves cognitive behaviors and hippocampal synaptic plasticity in the APP/PS1 mouse model of Alzheimer’s disease. Neurosci Bull. 2020;36:254–262. doi: 10.1007/s12264-019-00434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lu B, Nagappan G, Guan XM, Nathan PJ, Wren P. BDNF-based synaptic repair as a disease-modifying strategy for neurodegenerative diseases. Nat Rev Neurosci. 2013;14:401–416. doi: 10.1038/nrn3505. [DOI] [PubMed] [Google Scholar]
- 3.Petrache AL, Rajulawalla A, Shi AQ, Wetzel A, Saito T, Saido TC, et al. Aberrant excitatory-inhibitory synaptic mechanisms in entorhinal cortex microcircuits during the pathogenesis of Alzheimer’s disease. Cereb Cortex. 2019;29:1834–1850. doi: 10.1093/cercor/bhz016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ji YY, Lu Y, Yang F, Shen WH, Tang TTT, Feng LY, et al. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips HS, Hains JM, Armanini M, Laramee GR, Johnson SA, Winslow JW. BDNF mRNA is decreased in the hippocampus of individuals with Alzheimer’s disease. Neuron. 1991;7:695–702. doi: 10.1016/0896-6273(91)90273-3. [DOI] [PubMed] [Google Scholar]
- 6.Wang SD, Yao HY, Xu YH, Hao R, Zhang W, Liu H, et al. Therapeutic potential of a TrkB agonistic antibody for Alzheimer’s disease. Theranostics. 2020;10:6854–6874. doi: 10.7150/thno.44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Figurov A, Pozzo-Miller LD, Olafsson P, Wang T, Lu B. Regulation of synaptic responses to high-frequency stimulation and LTP by neurotrophins in the hippocampus. Nature. 1996;381:706–709. doi: 10.1038/381706a0. [DOI] [PubMed] [Google Scholar]
- 8.Dai ZJ, He Y. Disrupted structural and functional brain connectomes in mild cognitive impairment and Alzheimer’s disease. Neurosci Bull. 2014;30:217–232. doi: 10.1007/s12264-013-1421-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ying Y, Wang JZ. Illuminating neural circuits in Alzheimer’s disease. Neurosci Bull. 2021;37:1203–1217. doi: 10.1007/s12264-021-00716-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Khan UA, Liu L, Provenzano FA, Berman DE, Profaci CP, Sloan R, et al. Molecular drivers and cortical spread of lateral entorhinal cortex dysfunction in preclinical Alzheimer’s disease. Nat Neurosci. 2014;17:304–311. doi: 10.1038/nn.3606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kordower JH, Chu Y, Stebbins GT, DeKosky ST, Cochran EJ, Bennett D, et al. Loss and atrophy of layer II entorhinal cortex neurons in elderly people with mild cognitive impairment. Ann Neurol. 2001;49:202–213. doi: 10.1002/1531-8249(20010201)49:2<202::AID-ANA40>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 12.Leung C, Cao F, Nguyen R, Joshi K, Aqrabawi AJ, Xia ST, et al. Activation of entorhinal cortical projections to the dentate gyrus underlies social memory retrieval. Cell Rep. 2018;23:2379–2391. doi: 10.1016/j.celrep.2018.04.073. [DOI] [PubMed] [Google Scholar]
- 13.Zhu YJ, Nachtrab G, Keyes PC, Allen WE, Luo LQ, Chen XK. Dynamic salience processing in paraventricular thalamus gates associative learning. Science. 2018;362:423–429. doi: 10.1126/science.aat0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhu YJ, Wienecke CFR, Nachtrab G, Chen XK. A thalamic input to the nucleus accumbens mediates opiate dependence. Nature. 2016;530:219–222. doi: 10.1038/nature16954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dimidschstein J, Chen Q, Tremblay R, Rogers SL, Saldi GA, Guo LH, et al. A viral strategy for targeting and manipulating interneurons across vertebrate species. Nat Neurosci. 2016;19:1743–1749. doi: 10.1038/nn.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.