Abstract
Objective
Alterations in the serotonergic system were verified to act a role in the pathogenesis of altered neurological and psychiatric diseases. In recent years, Tryptophan (Trp) and serotonin (5-HT) levels have been considered potent biomarkers of diabetes mellitus (DM).
Method
The different Trp metabolism may also play roles in the pathogenesis of DM and mounting risk of complications. The whole blood (WB) 5-HT level was mainly lower among diabetic patients compared to others. That is mostly derived from a lower platelet concentration of 5-HT in these patients.
Results
Indeed, 5-HT level can be considered a potent biomarker for early detection of DM complications. Besides, it was proved that outside the digestive and central nervous systems, 5-HT was discovered in beta cells, and scientists have been attempting to realize its mechanism of action ever since. Towards to end, the determination methods, biomarker’s role, and approaches of 5-HT and Trp levels were thoroughly investigated in both healthy and diabetic patients with or without complications. Moreover, the association between insulin and 5-HT has been specifically discussed.
Conclusions
Our study concluded that Trp and 5-HT levels could be exclusively applied for early diagnosis of DM complications as well as many other complications.
Graphical abstract
Keywords: Tryptophan, Serotonin, Diabetes mellitus, Insulin, Biomarker role, Complications
Introduction
Alteration of tryptophan and serotonin
Tryptophan (Trp) is a vital amino acid causing clinical implications in the human body and biological specimens [1]. Moreover, Trp is recommended to be used as a medication for disorders such as insomnia, anxiety, addiction, and obesity [2]. Recently, Trp level has been considered as a biomarker for diabetes discovery.
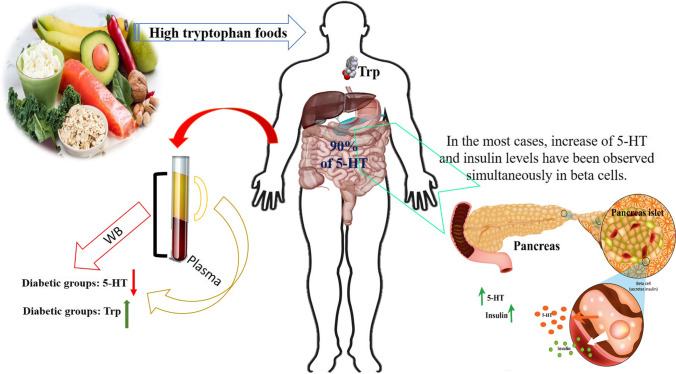
Serotonin (5-HT, feel-good hormone) is well-recognized for its biological activities in the brain, and as a familiar neurotransmitter, it regulates a variety of neuropsychological processes [3]. Alterations in serotonergic system were proved to provide a role in pathogenesis of different neurological and psychiatric diseases [3]. Recently, 5-HT dysregulation was linked to the pathogenesis of diabetes mellitus (DM). In addition, this was found in beta cells and some previous studies showed that 5-HT signaling is significant in insulin release, and the absence of 5-HT can consequently lead to diabetes [4]. Besides, several studies suggested that the whole blood (WB) 5-HT level is lower among diabetic patients, especially in those with DM complications such as retinopathy [5], nephropathy [6], and peripheral vascular diseases [7]. Besides, the WB 5-HT level was mainly lower among diabetic patients. Accordingly, this is mostly derived from a lower platelet concentration of 5-HT in these patients [5]. Indeed, 5-HT level may be considered as a useful biomarker for early detection of DM complications [6].
Determination methods
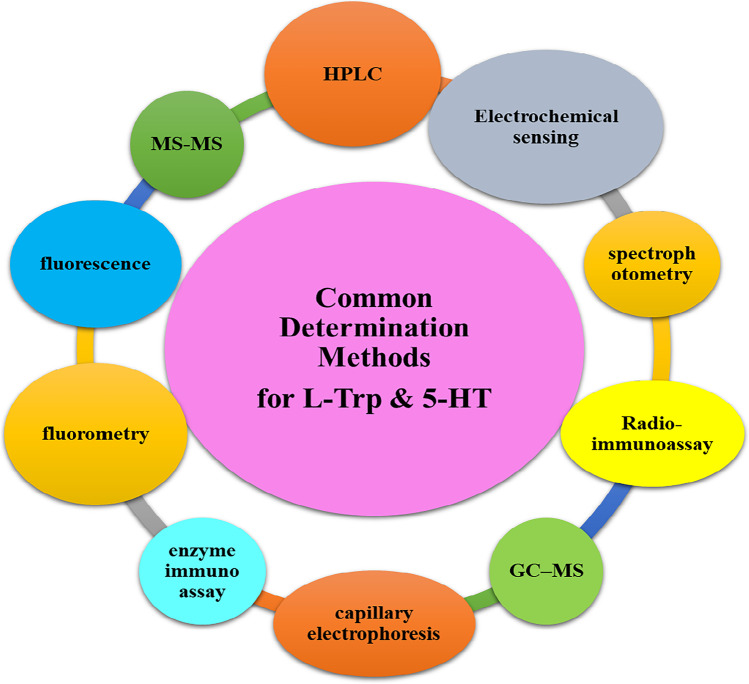
Up to now, several methods such as high performance liquid chromatography (HPLC), tandem mass spectrometry (MS–MS), fluorescence, spectrophotometry, fluorometry, gas chromatography-mass spectrometry (GC–MS), and electrochemical sensing, have been applied to measure 5-HT and Trp, as well as some other analytes with similar chemical structures [8, 9]. Figure 1 represents an outline of the most common methods used for the detection of both Trp and 5-HT. Accordingly, among these methods, electrochemical sensing approaches have received much attention due to their easiness, high sensitivity, selectivity, and inexpensive determination of medical biomolecules [10]. As well, it is believed that the electrochemical method can be applied as a reliable method for the detection of medical analytes such as amino acids and neurotransmitters in the clinical lab [11]. In addition, nanomaterials-based electrochemical sensing of both Trp and 5-HT have been exclusively investigated to determine their levels in both animal models and biological fluid of the human body.
Fig. 1.
An outline of 5-HT and L-Trp levels determination methods
Biomarker role of 5-HT and Trp
It was shown that 5-HT, as a biomarker, plays noteworthy roles in some main diseases such as autism spectrum disorder (ASD), attention-deficit hyperkinetic disorders (AD-HKD), coronary microvascular dysfunction (CMD), atherosclerotic cardiovascular disease (ASCVD), chronic heart failure (CHF), and vascular complications in DM [12–14]. In this regard, the WB 5-HT level was also found to be mainly lower among diabetic patients. That is mostly derived from a lower platelet concentration of 5-HT in these patients. Indeed, 5-HT level may be considered as a useful biomarker for early detection of DM complications. Besides, it was proved that outside the digestive and central nervous systems, 5-HT can be discovered in beta cells, and found that it acts as a potent tool to regulate insulin release.
The role of Trp metabolism in inflammation disorders was also observed; however, there is limited evidence on the determination of Trp yet [15]. Observational studies have previously proposed that Trp metabolism could be exploited as a biomarker for inflammation disorders [16]. In the case of DM, the altered Trp metabolism may play roles in the pathogenesis of DM and developing the risk of complications. Based on recent data regarding this, the Trp metabolic pathway could be considered as an appropriate marker for the progression of novel antidiabetic drugs. Besides, it was found that Trp metabolism could be applied as therapeutic targets to control age-related diseases related to inflammation and probably even to extend lifespan.
It is believed that the Trp and 5-HT levels can be potentially applied as biomarkers for early diagnosis of DM as well as many other disorders [17, 18]. Herein, we attempted to provide a comprehensive summary on the relationship between Trp and 5-HT with DM as well as finding the existent mechanisms between insulin and 5-HT.
5-HT levels in DM
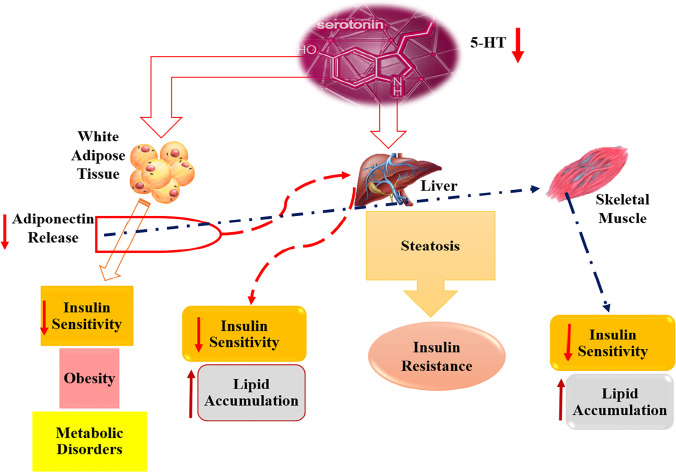
5-HT level could be considered as a marker for early detection of DM complications. Several studies have previously suggested that the WB 5-HT level is lower among diabetic patients, especially in those with DM complications such as retinopathy, nephropathy, and peripheral vascular diseases [5, 7, 19]. Due to the significance of 5-HT level and its relationship with DM complications, it is exclusively represented as a potent diabetes tool in this section. A schematic was also provided to demonstrate the direct effect of the reduced 5-HT level on the stimulation of liver, and adipose tissue; however, skeletal muscle can be provoked indirectly. As well, it seems that different mechanisms could lead to obesity, metabolic disorders, lipid accumulation, insulin sensitivity, and resistance in the human body (Fig. 2).
Fig. 2.
The relationship between 5-HT alteration and the probability of the T2D pathogenesis in different tissues
In a previous study, 5-HT level was successfully measured as a novel diabetes biomarker using a simple nanocomposite comprising of the reduced graphene oxide (rGO), gold nanoparticles (AuNPs), and 18-crown-6 (18.Cr.6) by HPLC method in zebrafish, as a novel animal model. As a result, the most diminution in 5-HT level was observed in diabetic zebrafish compared to the normal group. Moreover, this study verified that the 5-HT level can be employed as a potent biomarker used for the early diagnosis of DM [18].
The directed and undirected metabolomics’ procedures were used to examine plasma and urine samples of pregnant women with and without gestational DM (GDM). This study displayed that 5-HT and the associated metabolites significantly varied among the controls and patients, approving the relationship of 5-HT metabolism in GDM [20].
Saito et al. in their study registered 165 Type 2 diabetes (T2D), including 106 normoalbuminuric and 59 microalbuminuric patients, in order to investigate multivariate analyses. Thus, it was indicated that the increased plasma 5-hydroxyindole-3-acetic acid (5-HIAA) levels are involved in the pathogenesis of impaired blood flow in lower extremities and renal insufficiency among diabetic patients with microalbuminuria [21].
In an exploratory study, the associations amongst cortisol parameters, platelet 5-HT content, and platelet activity markers were testified in patients with major depression (MD) and/or T2D. Finally, the results showed that augmented platelet activity in T2D could play a role in the relationship amongst diabetes, depression, and coronary artery disease (CAD) [22].
Hara et al. in their study evaluated plasma 5-HT levels in patients with the DM complicated with chronic kidney disease disposed to atherosclerosis. The results proved that platelets are stimulated to release 5-HT into plasma among patients with moderate diabetes and the damaged renal role. Moreover, platelets can be over-activated to release 5-HT in patients with severe diabetes [19].
An observational study was conducted on 162 male patients with T2D, in order to examine if plasma 5-HIAA level, as a derivative final version of 5-HT, may be considered as an interpreter for decline of urinary albumin expulsion. So, for this purpose, the association between baseline plasma 5-HIAA level and alterations in urinary albumin was inspected for a 24-month duration. This observational study proved that plasma 5-HIAA concentration is associated with alterations in urinary albumin excretion, pointing out causality in complicated diabetic nephropathy with T2D and a high plasma 5-HIAA concentration [23].
In another study, the Framingham 10-year risk scores (FRS) of 30–74 years old issues were considered, and some clinical characteristics such as age, sex, blood pressure, smoking, and diabetes status were then combined into the measurement. Plasma 5-HT levels were found to be significantly correlated with FRS. The association of the declined brachial-ankle pulse wave velocity (baPWV) in diabetic patients with the promotion of plasma 5-HT levels was successfully obtained, describing an important association between plasma 5-HT and atherosclerosis [24].
As alluded to the above-mentioned statements, it is believed that 5-HT/5-HIAA levels in plasma and WB are valuable biomarkers for predicting the risk of developing vascular complications and depression in patients with DM. In conclusion to this section, in the current study, we explored that 5-HT/5-HIAA levels could be exploited as a potential biomarker in patients at early stages of DM complications. The determination method, sample type, and the main finding obtained from 5-HT/5HIAA levels in the patients with DM are shown in Table 1.
Table 1.
Findings of 5-HT/5HIAA levels in different studies including animal and human studies with DM
| 5-HT metabolite level in the diabetic and normal group (µg L−1) |
Determination Method | Types of diabetes or complications | Sample | Main finding | Refs |
|---|---|---|---|---|---|
| Diabetic: 1.93 ± 0.07 and healthy: 8.70 ± 0.30 | Electrochemical sensing and HPLC | T2D | GI of ZF | Significant reduction in the 5-HT level in diabetic ZF | [18] |
| NA | A stable isotope dilution direct-infusion method (SIDE-MS assay) | GDM | Plasma and urine | Displaying higher 5-HT levels about disrupted metabolisms, like GDM | [20] |
|
5-HIAA as a mediator of 5-HT 5.2 ± 2.0 and 4.1 ± 1.4 |
HPLC | T2D/ normoalbuminuric and microalbuminuric patients | WB | Increasing plasma 5-HIAA concentrations associated with impaired systolic and late diastolic, and increasing the resistance of peripheral vascular in diabetic patients | [21] |
|
358.5 and 379.5 (ng/109) |
HPLC |
T2D and depression |
PPP |
Finding association between cortisol or 5-HT and platelet markers |
[22] |
|
10.2, 6.2, and 8.5 for diabetic groups and 4.8 for the healthy group in PPP 657,485, and 582 for diabetic groups and 713 for the healthy group in WB |
HPLC | T2D complicated with chronic kidney (divided into 3 groups and 1 healthy group | PPP, WB | Reduction of the 5-HT level in the plasma and raising 5-HT level in WB, perhaps by suppressing platelet aggregation by administration of iloprost | [19] |
|
5-HIAA 6.9 ± 5.4 Low plasma 3.5 ± 0.8 Moderate plasma 5.7 ± 0.7 High plasma 11.6 ± 7.3 |
HPLC | T2D- nephropathy | Plasma | Demonstrating relation between plasma 5-HIAA concentration and urinary albumin excretion | [23] |
| Qualitative consideration | HPLC | ACD- T2D | Plasma | Presenting a major association between plasma 5-HT and atherosclerosis in diabetic patients | [24] |
HPLC High performance liquid chromatography; T2D Type 2 diabetes; WB Whole blood; PPP Platelet-rich plasma; 5-HIAA 5-Hydroxyindoleacetic acid; ACD Atherosclerotic cardiovascular disease; GI Gastrointestinal; ZF Zebrafish; GDM Gestational diabetes mellitus
Trp levels in DM
Accumulating data showed that the changes in metabolism of Trp and its active metabolites play essential roles in both the pathogenesis and complications of diabetes [25]. The altered L-Trp metabolism may play a role in the pathogenesis of DM and developing the risk of complications. Some previous studies have suggested that Trp decreased and its metabolism up-regulates in diabetic patients [26, 27]. Based on studies performed on the relationship between Trp and diabetes, the related studies on human and animal samples have been selected, the details of which are explained below:
In another study, L- Trp level was assessed in diabetic and normal serum samples in human using the reduced graphene oxide/gold nanoparticles/18-crown-6. Based on the square wave voltammetry (SWV) results, a low limit of detection (LOD) was calculated as about 0.48 μM and 0.61 μM for diabetic and normal samples, respectively. It seems that the nanocomposite could be known as a good choice for L-Trp determination in human serum [17]. In another study, C-mannosyl tryptophan (C-Man-Trp) was measured in different tissues obtained from normal or diabetic mice. The increased excretion of C-Man-Trp level was also observed in urine and kidney tissue; however, C-Man-Trp levels reduced in the liver of diabetic mice. Correspondingly, these results suggested that C-Man-Trp metabolism is greatly affected by diabetes [28].
Chou et al. in their study evaluated serum levels of various metabolites among diabetic patients at various stages of chronic kidney disease. It was shown that Lower Trp levels are associated with a rapid drop in the estimated glomerular filtration rate (eGFR). Moreover, this study exhibited that Trp level might be considered as a potential biomarker for diabetic nephropathy [29].
In the Rebnord et al.’s study, the associations of the kynurenine (KYN): Trp ratio (KTR) to the occurrence of T2D, was considered. Thereafter, the plasma and urine samples of studied individuals with coronary artery disease were obtained, and the levels of KYN and Trp were then measured. Although the results showed no significant relationship between KTR and T2Din Plasma samples, a strong positive association was found between KTR and T2Din urine samples [30].
Chen et al. in their study evaluated the role of Trp, as a predicting risk factor for T2D outcomes, in healthy and diabetic Chinese men. Accordingly, their results showed that serum Trp level was significantly higher among diabetic patients, and Trp biomarker was associated with DM onset risk positively and independently. It was indicated that the higher Trp levels in these patients can make higher levels of both insulin resistance and secretion. These findings revealed the potential of Trp as a new biomarker of diabetes risk in the Chinese population [31].
Trp level in diabetes of elderly people
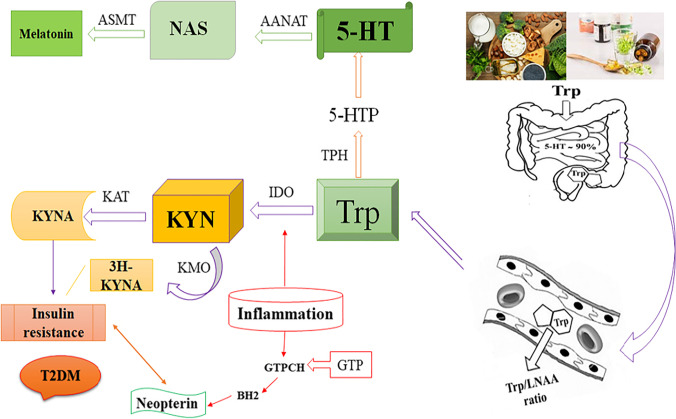
It was found that aging changes the composition and function of adipose tissue, and consequently leads to insulin resistance and fat storage in the ectopic body part [32]. The cellular changes that converge during the aging process are dysfunction in mitochondria, antioxidant deficiency, inflammation, and the decreased immune response. Accordingly, these changes affect the KYN pathway (KP) (Fig. 3), which is known as a major pathway for Trp catabolism [33].
Fig. 3.
The relationship of Trp-KYN metabolic pathways with T2D. Abbreviations: KYN, Kynurenine; IDO, indoleamine 2,3-dioxygenase; KAT, KYN-aminotransferase; KMO, KYN-3-monooxygenase; 3H-KYNA, 3-hydroxyKYN acid (xanthurenic acid); IR, insulin resistance; TpH, Tryptophan Hydroxylase; AAAD, Aromatic Amino Acid Decarboxylase; AANAT, Aralkylamine N-acetyltransferase; ASMT, Acetylserotonin O-methyltransferase; GTP, guanosine triphosphate; GTPCH, GTP cyclohydrolase I; BH2, 7,8-dihydroneopterin
Some studies have previously reported that Trp metabolites play a key role as a potential biological mediator for T2D [31, 34, 35]. Trp metabolism is thought to be altered due to various physiological and psychological pressures such as self-care in diabetic patients [36]. These findings exhibited the possible role of Trp in geriatric diabetic patients.
In a study, Trp metabolites were assessed in healthy and diabetic adult men. The results confirmed that 5-hydroxytryptophan (5-HTP) levels were higher in diabetic patients compared to healthy adult men [37].
Calvani et al. in their study determined the circulating amino acids in diabetic frail adults, in order to detect the concentrations of circulating 37 amino acids. They reported high Trp levels in serum samples of diabetic adults compared to their control participants [38]. In another study, the measurement of Trp, KYN, and neopterin levels as immune activation markers in volunteers aged ≥ 65 and < 65 years old, was performed. The geriatric groups had low Trp levels compared to the young groups. Conversely, Trp and KYN/Trp levels were found to be significantly higher than adults without DM [39].
In a research, Shimizu et al. evaluated the Trp metabolites in plasma samples obtained from both young and old participants with and without T2D. Plasma Trp metabolites levels in young women and old men were found to be higher than young men and old women. Except the KYN and indole butyric acid, the plasma levels of Trp metabolites were higher in diabetic patients than adult men [36]. Furthermore, Matsuoka et al. presented various Trp metabolites in diabetic and healthy men. Accordingly, their results showed that the plasma level of Trp in diabetic adults was lower than that of healthy subjects, but it was not statistically significant. As well, 5-HTP concentration and the other related Trp metabolites were statistically higher in diabetic adults [27].
As shown in most of the results, in human serum and plasma samples, the amount of Trp was higher in diabetic peoples compared to healthy ones. It seems that T2D is characterized by low-grade systemic inflammation, which consequently affects adipose tissue, the liver, pancreas, and kidneys [40]. On the other hand, degradation of Trp also appears to be closely related to the immune system activation [41]. Besides, KYN pathway activity significantly increased in chronic stress [42]. Correspondingly, all these factors manifested themselves by growing the Trp degradation in a patient's serum. Altogether, our observations indicated that the Trp levels have been previously evaluated in different samples, participants, and disease stages in various studies. Unfortunately, there was a few studies on determining the role of Trp in diabetic patients; therefore, it is hard to speak about the exact changes of Trp levels and their metabolites in different study samples. More information on methodology and the obtained results are mentioned in Table 2.
Table 2.
Findings of Trp levels in different studies including animal and human studies with DM
| L-Trp metabolite level in the diabetic and normal group (µg L−1) |
Determination Method | Types of diabetes or complications | Sample | Main finding | Refs | |
|---|---|---|---|---|---|---|
| Trp in General Population |
Proposed sensor: Diabetic: 1.97 Healthy: 2.03, and HPLC: Diabetic: 2.10 Healthy: 2.21 |
electrochemical sensor and HPLC | T2D | HS | Providing a suitable sensor for L-Trp determination | [17] |
|
Plasma: Male: 0.166 ± 0.030 Female: 0.145 ± 0.024 |
Hydrophilic interaction LC | T2D | Different tissues (Brain, spleen, lungs, bladder, and testes) and Plasma of mice | The C-Man-Trp metabolism is greatly affected by diabetes | [28] | |
| Targeted quantitative metabolomics approach | LC | T2D/ Nephropathy | HS | There was a relationship between tryptophan (Trp) and rapid decrease in eGFR | [29] | |
|
Plasma: Diabetic: 72.5, Non Diabetic: 70.1 |
MS/MS and GC–MS/MS |
T2D/ Coronary artery disease |
HP and HU |
No relationship between KTR and T2D in Plasma There was Strong association between KTR and T2D in urine |
[30] | |
|
Diabetic: 0.5 ± 0.02 Non Diabetic: 0.1 ± 0.02 |
UPLC-TQ/MS | T2D | HS |
Serum Trp level was significantly higher in diabetic patients Trp associated with diabetes onset risk in future |
[31] | |
| Trp in Geriatric patients | Trp: Diabetic: 53.4 ± 13.1, Non Diabetic: 59.0 ± 9.2 and 5-HTP: Diabetic 2000 ± 1000, Non Diabetic 622 ± 100 | UH-HPLC | T2D | HP |
The plasma levels of Trp were lower in diabetic older adults The plasma levels of 5-HTP were higher in diabetic older adults |
[27] |
|
Diabetic: 2000 ± 1000 Healthy old men:630 ± 100 |
UHS-LC–MS/MS | T2D | HP | The plasma levels of 5-HTRP were higher in diabetic patients, and | [36] | |
|
Diabetic: 0.048 ± 0.038 Non Diabetic: 0.014 ± 0.029 |
LC–MS/MS | T2D | Fasting HP | the level of 5-HTRP were higher in plasma | [37] | |
|
Diabetic: 66.2 ± 23.4 Non Diabetic: 62.0 ± 13.1 |
PLS-DA | Frail older adults with T2D | HS | The level of Trp was higher in serum of diabetic patients | [38] | |
|
Diabetic: 51.7 ± 2.6 Non F = Diabetic: 58.3 ± 1.6 |
HPLC | T2D | HS | The levels of Trp and Kyn/Trp were higher than healthy older adults | [39] |
HS Human serum; HP Human plasma; HU Human urine; C-Man-Trp, UHS Ultrahigh speed; C-mannosyl-tryptophan; eGFR Estimated glomerular filtration rate; KTR Kynurenine/tryptophan ratio; 5-HTP 5-hydroxytryptophan; PLS-DA Partial least squares-discriminant analysis; LC Liquid chromatography; MS/MS Tandem mass spectrometry; UPLC-TQ/MS Ultra-performance liquid chromatography triple quadruple mass spectrometry; PLS-DA Partial least squares-discrimination analysis
Relationship between 5-HT and insulin: Role in DM
It was verified that outside the digestive and central nervous systems, 5-HT can also be discovered in pancreatic beta cells, and scientists have been attempting to realize its mechanism of action ever since [43, 44]. 5-HT is synthesized and then stored in pancreatic beta cells and co-released with insulin by glucose stimulation of beta cells [45, 46].
Several studies indicated that 5-HT is essential for glucose-dependent insulin secretion and the lack of 5-HT in pancreatic beta cells consequently causes hyperglycemia and DM [47–50]. In this regard, several mechanisms have been proposed to explain the 5-HT role in the regulation of both insulin secretion and glucose hemostasis. Moon et al. in their study indicated that growth hormone (GH) could mediate expression of tryptophan hydroxylase 1, which is a key enzyme in the synthesis of 5-HT, and consequently the production of 5-HT can be stimulated in pancreatic beta cells during the perinatal period. Accordingly, in perinatal period, 5-HT acts as a paracrine/autocrine regulator in inducing the proliferation of beta cells through the 5-HT2B receptor.
The amount of cell proliferation achieved during the perinatal period, is one of the major determinants of adult beta-cell mass, in such way that those with lower beta-cell mass have a higher susceptibility to diabetes [51]. Additionally, Ohara-Imaizumi et al. in their study showed that 5-HT can lower glucose threshold for insulin release and also increase glucose-stimulated insulin secretion from beta cells via the 5-HT3 receptor in an autocrine/paracrine manner during pregnancy. Therefore, the authors suggested that 5-HT may play a role in the pathogenesis of gestational diabetes [52]. Other reports have also confirmed the regulatory effects of 5-HT on beta-cell proliferation and insulin secretion through both 5-HT2B and 5-HT3 receptors [4, 53–55]. Moreover, it was shown that 5-HT secreted from beta cells acts on neighboring alpha cells in a paracrine manner through the 5-HT1F receptor and subsides glucagon secretion, which consequently enhances insulin release [56]. Besides the receptor-dependent effects of 5-HT on beta cells, Paulmann et al. in their study suggested that intracellular 5-HT modulates the secretion of insulin through the serotonylation of particular small GTPases (Rab family). Notably, Serotonylation, as a covalent linkage of 5-HT to target proteins, which is catalyzed by the transglutaminase enzymes, could activate GTPases and trigger exocytosis of insulin vesicles [48].
In contrast to the above-mentioned reports, some other studies reported that 5-HT or its agonists can decrease insulin secretion, and it also has the ability to induce hyperglycemia [44, 57]. Some of these variations in the results of previous studies may be due to different study protocols, samples, cell lines, and animal models used in them. Another reason for these discrepancies may be due to complicated 5-HT and its receptors interactions.
At least 15 subtypes of 5-HT receptors which are grouped in 7 different families play roles in 5-HT action and stimulation of different 5-HT receptors and they could provide augmentation to distinct cellular responses [58]. Recently, mRNA of all 5-HT receptor families (5-HTR1-7) were identified in human islets [58]. Furthermore, it was indicated that the response to metabolic status may be altered by the expression of particular receptors, and this event complicates the situation. For example, Kim et al. indicated that the expression of the 5-HT2B receptor increases during mid-gestation, in order to provoke β-cell proliferation and elevate the β-cell mass, while 5-HT1D receptor expression boosts at the end of gestation to lessen the β-cell mass [54]. In another study, Bennet et al. demonstrated that both 5-HT1D and 5-HT2A receptors, which stimulate and inhibit insulin secretion, respectively, showed over-expression in islets tissue from T2D donors compared to normal group [59].
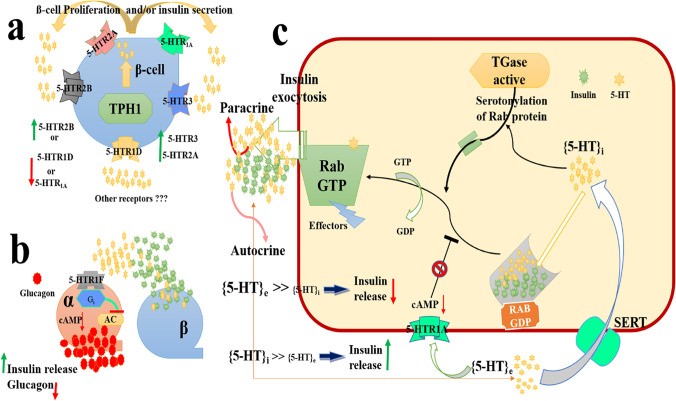
Finally, a recent theory suggested that high extracellular 5-HT concentrations [5-HT]e could diminish extra insulin release through 5-HT1A receptors. In contrast, when intracellular 5-HT [5-HT]i attains much higher levels compared to [5-HT]e in beta cells, insulin secretion can be made by serotonylation. These findings suggested that both the inhibition and stimulation of 5-HT-dependent cycling could contribute to the recognized oscillating landscape of insulin exocytosis from glucose-stimulated β-cells [48]. Figure 4 represents the possible mechanisms that could be involved in the regulation of insulin by different 5-HT receptors.
Fig. 4.
Schematic illustration of a) Effect of the main 5-HT receptors classes on β-cell proliferation, b) Effect of 5-HTR1F on glucagon in α-cell, and c) The relationship between the reduction and augmentation of insulin release depending on [5-HT]e and [5-HT]i. Abbreviations: 5-HTi, 5-Hydroxytryptophan intracellular; 5-HTe, 5-Hydroxytryptophan extracellular; SERT, 5-HT Transporter; cAMP, Cyclic Adenosine Monophosphate; GDP, Guanosine diphosphate; GTP, Guanosine 5'-Triphosphate; T Gase, Transglutaminase; TPH1, Tryptophan Hydroxylase 1; 5HTR2B, 5-Hydroxytryptamine receptor 2B; 5HTR3, 5-Hydroxytryptamine Receptor 3; 5-HTR1D, 5-Hydroxytryptamine Receptor 1D; AC, adenylate cyclase; Gi, inhibitory G protein
Taken together, 5-HT plays significant roles in insulin secretion and glucose hemostasis. Moreover, dysregulations of 5-HT pathway may be involved in diabetes pathogenesis, so it should be considered as a potential target for novel antidiabetic drugs. In this regard, further studies are needed to clear the exact roles of 5-HT and its receptors in both insulin secretion and glucose hemostasis.
Boundaries, future outlook, and conclusions
Based on the evaluated studies, it is difficult to express the exact alteration of 5-HT and Trp levels in plasma, serum or even tissue of human and animal samples due to the multi-dimensional nature of T2D. However, the results show that changes in 5-HT and Trp levels in different samples trigger variations in cell levels leading to the reduced insulin sensitivity as well as the increased insulin resistance, fat accumulation, and the development of metabolic syndromes. It seems that 5-HT and Trp may be considered as the markers of pathological changes of diabetes in cell’s level.
The effects of 5-HT and Trp, which are present in the body, on the regulation of behavior and physiology have been in the center of scientific attention for decades. Although there have been reports on the involvement of both 5-HT and Trp metabolites in relation to diabetes, no systematic research on the roles of 5-HT and Trp in the pathogenesis of DM has been conducted yet.
Identification of T2D biomarkers due to the heterogeneity of the nature of T2D, is a big challenge. Another point to be considered is clinical phenotypes of disease comprising of age and BMI, as well as considering biochemical features, including insulin resistance and the effect of various environmental exposures. Accordingly, all of them lead to disease variability. In these circumstances, the findings of recent studies could not help us in clarifying how a group of biomarkers may interact in other biological pathways or interact with biomarkers, in order to intensify diabetes progress. Therefore, to overcome such challenges, some studies have suggested that the use of data clustering methods can affect DM to control the role of underlying variables in relevant studies. So, a well-organized of these variables could provide a suitable way for biomarker determination in DM. Another suggestion for biomarker research studies is the use of the network analysis to evaluate the cumulative effect of the different biomarkers on the enhancement of early diagnosis of DM complications.
Up to now, no systematic reviews/clinical trials have been conducted by considering Trp and 5-HT levels as trigger biomarkers for DM complications. Recently obtained results from Trp and 5-HT determination supported the clinical significance of both Trp and 5-HT levels in human metabolism; however, most of these studies had small sample sizes. Moreover, they have not considered all required items for the identification of the direct relationship between Trp and 5-HT levels in DM patients. Therefore, to improve the understanding on the roles of 5-HT and Trp in metabolism and to offer real diagnostic and therapeutic applications of 5-HT, more systematic studies are needed to control the serotonergic system, and genetic studies are required to explore the associations between 5-HT and Trp in DM with or without complications. In addition, it is necessary to figure out the precise evaluation of the effect of 5-HT on insulin resistance. We hope that the observation from this study helps scientists and researchers to provide a suitable route for early diagnosis of DM with or without complications in each step.
Acknowledgements
This work was nonfinancially supported by Endocrinology and Metabolism Research Institute, Tehran University of Medical Sciences.
Authorship contribution statement
KK: Conceptualization, Investigation, Methodology, Design, Writing-review &editing. MCG: Investigation, Methodology, Design, Writing-review &editing. SMSJ: Investigation, Methodology, Writing-review &editing. AMM: Investigation, Writing-review &editing.
Funding
Private funds were applied to carry out this study.
Data availability
Not Applicable.
Declarations
Ethics approval and consent to participate
Not Applicable.
Consent for publication
All the authors have approved this article and agreed with submission.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Kamyar Khoshnevisan and Maryam Chehrehgosha are equally contributed as first authors.
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Kamyar Khoshnevisan, Email: kamyar.khoshnevisan@gmail.com.
Sayed Mahmoud Sajjadi-Jazi, Email: mahmood.sajadi@gmail.com.
References
- 1.Comai S, Bertazzo A, Brughera M, Crotti S. Chapter Five - Tryptophan in health and disease. In: Makowski GSBT-A in CC, editor. Elsevier; 2020. p. 165–218. Available from: https://www.sciencedirect.com/science/article/pii/S0065242319300745. [DOI] [PubMed]
- 2.Khoshnevisan K, Chehrehgosha M, Conant M, Meftah AM, Baharifar H, Ejtahed H-S, et al. Interactive relationship between Trp metabolites and gut microbiota: The impact on human pathology of disease. J Appl Microbiol [Internet] 2022;132:4186–207. doi: 10.1111/jam.15533. [DOI] [PubMed] [Google Scholar]
- 3.Berger M, Gray JA, Roth BL. The Expanded Biology of Serotonin. Annu Rev Med [Internet] 2009;60:355–66. doi: 10.1146/annurev.med.60.042307.110802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim K, Oh C-M, Ohara-Imaizumi M, Park S, Namkung J, Yadav VK, et al. Functional Role of Serotonin in Insulin Secretion in a Diet-Induced Insulin-Resistant State. Endocrinology [Internet] 2015;156:444–52. doi: 10.1210/en.2014-1687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pietraszek MH, Takada Y, Takada A, Fujita M, Watanabe I, Taminato A, et al. Blood serotonergic mechanisms in type 2 (non-insulin-dependent) diabetes mellitus. Thromb Res [Internet] 1992;66:765–74. doi: 10.1016/0049-3848(92)90052-C. [DOI] [PubMed] [Google Scholar]
- 6.Hara K, Hirowatari Y, Shimura Y, Takahashi H. Serotonin levels in platelet-poor plasma and whole blood in people with type 2 diabetes with chronic kidney disease. Diabetes Res Clin Pract [Internet]. 2011;94:167–71. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0168822711003421. [DOI] [PubMed]
- 7.Barradas MA, Gill DS, Fonseca VA, Mikhailidis DP, Dandona P. Intraplatelet serotonin in patients with diabetes mellitus and peripheral vascular disease. Eur J Clin Invest [Internet] 1988;18:399–404. doi: 10.1111/j.1365-2362.1988.tb01030.x. [DOI] [PubMed] [Google Scholar]
- 8.Khoshnevisan K, Maleki H, Honarvarfard E, Baharifar H, Gholami M, Faridbod F, et al. Nanomaterial based electrochemical sensing of the biomarker serotonin: a comprehensive review. Microchim Acta [Internet]. 2019;186:49. Available from: http://link.springer.com/10.1007/s00604-018-3069-y. [DOI] [PubMed]
- 9.Danaceau JP, Anderson GM, McMahon WM, Crouch DJ. A Liquid chromatographic-tandem mass spectrometric method for the analysis of serotonin and related indoles in human whole blood. J Anal Toxicol. 2003;27:440–4. Available from: https://academic.oup.com/jat/article-lookup/doi/10.1093/jat/27.7.440 [DOI] [PubMed]
- 10.Labib M, Sargent EH, Kelley SO. Electrochemical Methods for the Analysis of Clinically Relevant Biomolecules. Chem Rev [Internet] 2016;116:9001–90. doi: 10.1021/acs.chemrev.6b00220. [DOI] [PubMed] [Google Scholar]
- 11.Khoshnevisan K, Honarvarfard E, Torabi F, Maleki H, Baharifar H, Faridbod F, et al. Electrochemical detection of serotonin: A new approach. Clin Chim Acta [Internet]. 2020;501:112–9. Available from: https://linkinghub.elsevier.com/retrieve/pii/S0009898119320947. [DOI] [PubMed]
- 12.Huang H, Chen Z, Yan X. Simultaneous determination of serotonin and creatinine in urine by combining two ultrasound-assisted emulsification microextractions with on-column stacking in capillary electrophoresis. J Sep Sci [Internet] 2012;35:436–44. doi: 10.1002/jssc.201100778. [DOI] [PubMed] [Google Scholar]
- 13.Rognum IJ, Tran H, Haas EA, Hyland K, Paterson DS, Haynes RL, et al. Serotonin metabolites in the cerebrospinal fluid in sudden infant death syndrome. J Neuropathol Exp Neurol [Internet]. England; 2014;73:115–22. Available from: https://academic.oup.com/jnen/article-lookup/doi/10.1097/NEN.0000000000000034. [DOI] [PMC free article] [PubMed]
- 14.Runions KC, Morandini HAE, Rao P, Wong JWY, Kolla NJ, Pace G, et al. Serotonin and aggressive behaviour in children and adolescents: a systematic review. Acta Psychiatr Scand [Internet] 2019;139:117–44. doi: 10.1111/acps.12986. [DOI] [PubMed] [Google Scholar]
- 15.O’Mahony SM, Clarke G, Borre YE, Dinan TG, Cryan JF. Serotonin, tryptophan metabolism and the brain-gut-microbiome axis. Behav Brain Res [Internet]. Alimentary Pharmabiotic Centre, University College Cork, Cork, Ireland: Elsevier; 2015;277:32–48. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-84912141027&doi=10.1016%2Fj.bbr.2014.07.027&partnerID=40&md5=151e9873ec34b130d9c81dcc5ce418d5. [DOI] [PubMed]
- 16.Sorgdrager FJH, Naudé PJW, Kema IP, Nollen EA, Deyn PP De. Tryptophan Metabolism in Inflammaging: From Biomarker to Therapeutic Target. Front Immunol [Internet]. 2019;10:2565. Available from: https://www.frontiersin.org/article/10.3389/fimmu.2019.02565. [DOI] [PMC free article] [PubMed]
- 17.Khoshnevisan K, Torabi F, Baharifar H, Sajjadi-Jazi SM, Afjeh MS, Faridbod F, et al. Determination of the biomarker L-tryptophan level in diabetic and normal human serum based on an electrochemical sensing method using reduced graphene oxide/gold nanoparticles/18-crown-6. Anal Bioanal Chem [Internet]. 2020;412:3615–27. Available from: http://link.springer.com/10.1007/s00216-020-02598-5. [DOI] [PubMed]
- 18.Khoshnevisan K, Baharifar H, Torabi F, Sadeghi Afjeh M, Maleki H, Honarvarfard E, et al. Serotonin level as a potent diabetes biomarker based on electrochemical sensing: a new approach in a zebra fish model. Anal Bioanal Chem [Internet]. 2021;413:1615–27. Available from: http://link.springer.com/10.1007/s00216-020-03122-5. [DOI] [PubMed]
- 19.Hara K, Hirowatari Y, Shimura Y, Takahashi H. Serotonin levels in platelet-poor plasma and whole blood in people with type 2 diabetes with chronic kidney disease. Diabetes Res Clin Pract [Internet]. Elsevier; 2011 [cited 2019 Nov 10];94:167–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/21775011. [DOI] [PubMed]
- 20.Leitner M, Fragner L, Danner S, Holeschofsky N, Leitner K, Tischler S, et al. Combined Metabolomic Analysis of Plasma and Urine Reveals AHBA, Tryptophan and Serotonin Metabolism as Potential Risk Factors in Gestational Diabetes Mellitus (GDM). Front Mol Biosci [Internet]. Frontiers Media SA; 2017 [cited 2019 Nov 13];4:84. Available from: http://www.ncbi.nlm.nih.gov/pubmed/29312952. [DOI] [PMC free article] [PubMed]
- 21.Saito J, Suzuki E, Tajima Y, Takami K, Horikawa Y, Takeda J. Increased plasma serotonin metabolite 5-hydroxyindole acetic acid concentrations are associated with impaired systolic and late diastolic forward flows during cardiac cycle and elevated resistive index at popliteal artery and renal insufficiency in type 2. Endocr J. 2016;63:69–76. doi: 10.1507/endocrj.EJ15-0343. [DOI] [PubMed] [Google Scholar]
- 22.Zahn D, Petrak F, Franke L, Hägele A-K, Juckel G, Lederbogen F, et al. Cortisol, Platelet Serotonin Content, and Platelet Activity in Patients With Major Depression and Type 2 Diabetes. Psychosom Med [Internet]. 2015;77:145–55. Available from: https://journals.lww.com/psychosomaticmedicine/Fulltext/2015/02000/Cortisol,_Platelet_Serotonin_Content,_and_Platelet.6.aspx. [DOI] [PubMed]
- 23.Fukui M, Shiraishi E, Tanaka M, Senmaru T, Sakabe K, Harusato I, et al. Plasma serotonin is a predictor for deterioration of urinary albumin excretion in men with type 2 diabetes mellitus. Metabolism [Internet] 2009;58:1076–9. doi: 10.1016/j.metabol.2009.03.009. [DOI] [PubMed] [Google Scholar]
- 24.Yanai H, Hirowatari Y. A significant association of plasma serotonin to cardiovascular risk factors and changes in pulse wave velocity in patients with type 2 diabetes. Int J Cardiol [Internet] 2012;157:312–3. doi: 10.1016/j.ijcard.2012.03.144. [DOI] [PubMed] [Google Scholar]
- 25.Takada A, Shimizu F, Masuda J, Matsuoka K. Plasma Levels of Tryptophan Metabolites in Patients of Type 2 Diabetes Mellitus. Bioact Food as Diet Interv Diabetes. Elsevier; 2019. p. 265–76.
- 26.Unluturk U, Erbas T. Diabetes and tryptophan metabolism. Cham: Humana Press; 2015. [Google Scholar]
- 27.Matsuoka K, Kato K, Takao T, Ogawa M, Ishii Y, Shimizu F, et al. Concentrations of various tryptophan metabolites are higher in patients with diabetes mellitus than in healthy aged male adults. Diabetol Int. 2017;8:69–75. doi: 10.1007/s13340-016-0282-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sakurai S, Inai Y, Minakata S, Manabe S, Ito Y, Ihara Y. A novel assay for detection and quantification of C-mannosyl tryptophan in normal or diabetic mice. Sci Rep. Nature Publishing Group; 2019;9. [DOI] [PMC free article] [PubMed]
- 29.Chou CA, Lin CN, Chiu DTY, Chen IW, Chen ST. Tryptophan as a surrogate prognostic marker for diabetic nephropathy. J Diabetes Investig. 2018;9:366–374. doi: 10.1111/jdi.12707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rebnord EW, Strand E, Midttun Ø, Svingen GFT, Christensen MHE, Ueland PM, et al. The kynurenine:tryptophan ratio as a predictor of incident type 2 diabetes mellitus in individuals with coronary artery disease. Diabetologia. 2017;60:1712–1721. doi: 10.1007/s00125-017-4329-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chen T, Zheng X, Ma X, Bao Y, Ni Y, Hu C, et al. Tryptophan predicts the risk for future type 2 diabetes. PLoS One. 2016;11. (Public Library of Science). [DOI] [PMC free article] [PubMed]
- 32.Mancuso P, Bouchard B. The impact of aging on adipose function and adipokine synthesis. Front Endocrinol. (Lausanne). Frontiers Media S.A.; 2019. [DOI] [PMC free article] [PubMed]
- 33.Ramos-Chávez LA, Roldán-Roldán G, García-Juárez B, González-Esquivel D, Pérez de la Cruz G, Pineda B, et al. Low serum tryptophan levels as an indicator of global cognitive performance in nondemented women over 50 years of age. Oxid Med Cell Longev. Hindawi Limited; 2018;2018. [DOI] [PMC free article] [PubMed]
- 34.Yu E, Papandreou C, Ruiz-Canela M, Guasch-Ferre M, Clish CB, Dennis C, et al. Association of tryptophan metabolites with incident type 2 diabetes in the PREDIMED trial: A case–cohort study. Clin Chem. 2018;64:1211–20. doi: 10.1373/clinchem.2018.288720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floegel A, Stefan N, Yu Z, Mühlenbruch K, Drogan D, Joost HG, et al. Identification of serum metabolites associated with risk of type 2 diabetes using a targeted metabolomic approach. Diabetes Diabetes. 2013;62:639–648. doi: 10.2337/db12-0495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.F S. Plasma Levels of Tryptophan Metabolites in Healthy Young and Old Men and Women, and Patients of Type 2 Diabetes Mellitus (T2DM). Obes Open Access. Sci Forschen, Inc.; 2018;4.
- 37.Takada A, Shimizu F, Takao T, Masuda J. Measurement of tryptophan metabolites in healthy old men and patients of Type 2 Diabetes Mellitus (T2DM) Food Nutr Sci. 2018;09:1206–20. [Google Scholar]
- 38.Calvani R, Rodriguez-Mañas L, Picca A, Marini F, Biancolillo A, Laosa O, et al. Identification of a circulating amino acid signature in frail older persons with type 2 diabetes mellitus: Results from the metabofrail study. Nutrients. MDPI AG; 2020;12. [DOI] [PMC free article] [PubMed]
- 39.Sipahi H, Girgin G, Inanici F, Ariogul S, Sahin G, Baydar T. Tryptophan degradation and neopterin levels by aging. Pteridines. 2013;24:33–39. doi: 10.1515/pterid-2013-0008. [DOI] [Google Scholar]
- 40.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. Available from: https://www.nature.com/articles/nri2925. [DOI] [PubMed]
- 41.Oxenkrug GF. Metabolic syndrome, age-associated neuroendocrine disorders, and dysregulation of tryptophan - Kynurenine metabolism. Ann N Y Acad Sci. Blackwell Publishing Inc.; 2010; 1–14. [DOI] [PubMed]
- 42.Fuertig R, Azzinnari D, Bergamini G, Cathomas F, Sigrist H, Seifritz E, et al. Mouse chronic social stress increases blood and brain kynurenine pathway activity and fear behaviour: Both effects are reversed by inhibition of indoleamine 2,3-dioxygenase. Brain Behav Immun. Academic Press Inc.; 2016;54:59–72. [DOI] [PubMed]
- 43.Robinson R. Serotonin's role in the pancreas revealed at last. PLoS Biology. 2009;7(10):e1000227. [DOI] [PMC free article] [PubMed]
- 44.Zhang Q, Zhu Y, Zhou W, Gao L, Yuan L, Han X. Serotonin Receptor 2C and Insulin Secretion. PLoS One [Internet] 2013;8:e54250. doi: 10.1371/journal.pone.0054250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mohamed RA, Galal O, Mohammed AR, El-Abhar HS. Tropisetron modulates peripheral and central serotonin/insulin levels via insulin and nuclear factor kappa B/receptor for advanced glycation end products signalling to regulate type-2 diabetes in rats. RSC Adv [Internet] 2018;8:11908–20. doi: 10.1039/C7RA13105D. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gylfe E. Association Between 5-Hydroxytryptamine Release And Insulin Secretion. J Endocrinol [Internet]. Bristol, UK: Bioscientifica Ltd; 78:239–48. Available from: https://joe.bioscientifica.com/view/journals/joe/78/2/joe_78_2_010.xml. [DOI] [PubMed]
- 47.Sugimoto Y, Kimura I, Yamada J, Watanabe Y, Takeuchi N, Horisaka K. Effects of serotonin on blood glucose and insulin levels of glucose and streptozotocin-treated mice. Jpn J Pharmacol. 1990;54:93–6. doi: 10.1254/jjp.54.93. [DOI] [PubMed] [Google Scholar]
- 48.Paulmann N, Grohmann M, Voigt J-P, Bert B, Vowinckel J, Bader M, et al. Intracellular Serotonin Modulates Insulin Secretion from Pancreatic β-Cells by Protein Serotonylation. PLOS Biol [Internet] 2009;7:e1000229. doi: 10.1371/journal.pbio.1000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Malyszko J, Urano T, Knofler R, Taminato A, Yoshimi T, Takada Y, et al. Daily variations of platelet aggregation in relation to blood and plasma serotonin in diabetes. Thromb Res [Internet]. 1994;75:569–76. Available from: https://www.sciencedirect.com/science/article/pii/0049384894902313. [DOI] [PubMed]
- 50.Marti´n FJ, Mi´guez JM, Aldegunde M, Atienza G. Effect of streptozotocin-induced diabetes mellitus on serotonin measures of peripheral tissues in rats. Life Sci [Internet]. 1994;56:51–9. Available from: https://www.sciencedirect.com/science/article/pii/002432059400407J. [DOI] [PubMed]
- 51.Moon JH, Kim YG, Kim K, Osonoi S, Wang S, Saunders DC, et al. Serotonin Regulates Adult β-Cell Mass by Stimulating Perinatal β-Cell Proliferation. Diabetes [Internet]. 2020;69:205 LP – 214. Available from: http://diabetes.diabetesjournals.org/content/69/2/205.abstract. [DOI] [PMC free article] [PubMed]
- 52.Ohara-Imaizumi M, Kim H, Yoshida M, Fujiwara T, Aoyagi K, Toyofuku Y, et al. Serotonin regulates glucose-stimulated insulin secretion from pancreatic β cells during pregnancy. Proc Natl Acad Sci [Internet]. 2013;110:19420 LP – 19425. Available from: http://www.pnas.org/content/110/48/19420.abstract. [DOI] [PMC free article] [PubMed]
- 53.Bennet H, Mollet IG, Balhuizen A, Medina A, Nagorny C, Bagge A, et al. Serotonin (5-HT) receptor 2b activation augments glucose-stimulated insulin secretion in human and mouse islets of Langerhans. Diabetologia [Internet]. Springer; 2016;59:744–54. Available from: http://link.springer.com/10.1007/s00125-015-3847-6. [DOI] [PubMed]
- 54.Kim H, Toyofuku Y, Lynn FC, Chak E, Uchida T, Mizukami H, et al. Serotonin regulates pancreatic beta cell mass during pregnancy. Nat Med [Internet] 2010;16:804–8. doi: 10.1038/nm.2173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma N, Ma X. Dietary Amino Acids and the Gut-Microbiome-Immune Axis: Physiological Metabolism and Therapeutic Prospects. Compr Rev Food Sci Food Saf [Internet]. State Key Laboratory of Animal Nutrition, College of Animal Science and Technology, China Agricultural Univ., Beijing, 100193, China: Blackwell Publishing Inc.; 2019;18:221–42. Available from: https://www.scopus.com/inward/record.uri?eid=2-s2.0-85058850635&doi=10.1111%2F1541-4337.12401&partnerID=40&md5=76abf6fd4e8ba1296e5dd585e7064d8b. [DOI] [PubMed]
- 56.Almaça J, Molina J, Menegaz D, Pronin AN, Tamayo A, Slepak V, et al. Human Beta Cells Produce and Release Serotonin to Inhibit Glucagon Secretion from Alpha Cells. Cell Rep [Internet]. 2016;17:3281–91. Available from: https://www.sciencedirect.com/science/article/pii/S2211124716316461. [DOI] [PMC free article] [PubMed]
- 57.Heimes K, Feistel B, Verspohl EJ. Impact of the 5-HT3 receptor channel system for insulin secretion and interaction of ginger extracts. Eur J Pharmacol [Internet]. 2009;624:58–65. Available from: https://www.sciencedirect.com/science/article/pii/S0014299909008334. [DOI] [PubMed]
- 58.Cataldo Bascuñan LR, Lyons C, Bennet H, Artner I, Fex M. Serotonergic regulation of insulin secretion. Acta Physiol [Internet] 2019;225:e13101. doi: 10.1111/apha.13101. [DOI] [PubMed] [Google Scholar]
- 59.Bennet H, Balhuizen A, Medina A, Dekker Nitert M, Ottosson Laakso E, Essén S, et al. Altered serotonin (5-HT) 1D and 2A receptor expression may contribute to defective insulin and glucagon secretion in human type 2 diabetes. Peptides [Internet]. 2015;71:113–20. Available from: https://www.sciencedirect.com/science/article/pii/S0196978115002041. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not Applicable.