Abstract
Esophageal cancer has always been associated with poor prognosis and a low five-year survival rate. Chalcone, a flavonoid family member, has shown anti-tumor property in several types of cancer. However, few studies reported the potency and mechanisms of action of synthetic Chalcone derivatives against esophageal squamous cell carcinoma. In this study, we designed and synthesized a series of novel chalcone analogs and Ch-19 was selected for its superior anti-tumor potency. Results indicated that Ch-19 shows a dose- and time-dependent anti-tumor activity in both KYSE-450 and Eca-109 esophageal cancer cells. Moreover, treatment of Ch-19 resulted in the regression of KYSE-450 tumor xenografts in nude mice. Furthermore, we investigated the potential mechanism involved in the effective anti-tumor effects of Ch-19. As a result, we observed that Ch-19 treatment promoted ROS accumulation and caused G2/M phase arrest in both Eca-109 and KYSE-450 cancer cell lines, thereby resulting in cell apoptosis. Taken together, our study provided a novel synthetic chalcone derivative as a potential anti-tumor therapeutic candidate for treating esophageal cancer.
Supplementary Information
The online version contains supplementary material available at 10.1007/s12192-022-01302-z.
Keywords: Esophageal squamous cell carcinoma, Chalcones, Reactive oxygen species, Apoptosis, Anti-tumor effect
Introduction
Esophageal squamous cell carcinoma (ESCC) is the sixth most common malignant tumor and the fourth leading cause of cancer mortalities in China (Cao et al. 2021). Current treatment strategies for patients with esophageal cancer include surgery followed by radiation and chemotherapeutic drug therapy; however, the prognosis of esophageal cancer is poor with a 5-year survival rate of less than 10% (Yang et al. 2018). Therefore, more promising therapeutic approaches or agents are urgently needed for development for improving the survival of patients with esophageal cancer.
Chalcone is a precursor of numerous open-chain flavonoids and isoflavonoids found in plants (Jandial et al. 2014). Both natural and synthetic chalcones show cytotoxic effects in a variety of human tumor cells, resulting in cell cycle arrest, which might inhibit cell growth and/or induce apoptosis (Silva et al. 2015; Karthikeyan et al. 2015; Kim et al. 2014; Sahu et al. 2012; Szliszka et al. 2010; Tsai et al. 2014; Xiao et al. 2011). Besides, synthetic chalcones showed stronger anti-tumor activity than natural chalcones (Mai et al. 2014; Shankaraiah et al. 2017; Srinivasan et al. 2009). Although the direct targets of chalcones in tumors have not been identified, some nonspecific mechanisms regulated by chalcones have been reported, including tubulin polymerization inhibition, cell cycle arrest, and apoptosis (Jandial et al. 2014). Compared with synthetic chalcones, the utility of natural chalcones is limited by the poor performance of lead compounds, low pharmacokinetic properties, and toxic side effects. Hence, chalcone within specific functional groups was synthesized to improve its water solubility and reduce potentially associated toxicity may enhance its activity. Developing structural analogs of chalcone and synthesizing “man-made” chalcone analogs may be an effective means to improve its pharmacokinetic properties.
In our study, we designed, synthesized, and evaluated the biological activities of twenty chalcone analogs in superior yields by reacting different aromatic ketones with aromatic aldehydes in treating ESCC. In screening, we observed that Ch-19 exerted superior anti-tumor effects in both Eca-109 and KYSE-450 ESCC cell lines. Moreover, Ch-19 treatment resulted in a significant tumor regression in KYSE-450 xenografts. Furthermore, we observed G2/M arrest in ESCC cell lines treated with Ch-19. Then apoptosis was also initiated in both Eca-109 and KYSE-450 ESCC cell lines. We then examined the mechanism involved in the apoptosis induced by Ch-19 in detail by detecting ROS level and apoptosis-related protein expression. Our results revealed that Ch-19 markedly triggered ROS production and upregulated the expression of cleaved-PARP, Bad, Bim, PUMA, and BAX expressions which suggested apoptosis was initiated in ESCC cells both in vitro and in vivo. Hence, it is considered that Ch-19 might be a promising clinical therapeutic candidate for treating ESCC in the future.
Materials and methods
Synthesis of chalcones derivatives
Twenty novel chalcone derivatives were prepared by the base-catalyzed Claisen-Schmidt condensation using aromatic aldehydes and corresponding acetophenones in methanol. A mixture of aromatic acetophenone (10 mmol) and different substituted benzaldehydes (10 mmol) in methanol (20 mL) was stirred at room temperature, and 40% (w/v) NaOH aqueous solution (3 mL) was added slowly. The reaction mixture was stirred overnight and monitored by TLC. After completion, the precipitate was isolated, washed with water and cold methanol, and dried to give chalcone derivatives 1–20 in 56 ~ 94% yield by crystallizing from EtOH. The structure of the compounds was confirmed by 1H and 13C nuclear magnetic resonance (NMR). Samples with a purity ≥ 95% were used for the bioassays.
Cell lines and reagents
Human ESCC cell lines, KYSE-450 and Eca-109 were purchased from the Type Culture Collection of the Chinese Academy of Sciences (Shanghai, China). Cell lines were cultured in RPMI-1640 medium with 10% (V/V) fetal bovine serum (FBS), penicillin (100 units/mL) and streptomycin (100 μg/mL). All culture cells were maintained at 37 °C under a humidified atmosphere containing 5% CO2 according to the standard protocols. N-acetyl-L-cysteine (NAC) was purchased from Sigma-Aldrich Inc. (Mo, USA). The antibodies were utilized as follows: PARP (1:1,000; cat. no. 9532 T; Cell Signaling Technology, Inc.), Bim (1:5,000; cat. no. CY5307; Abways, Inc.), Bad (1:1,000; cat. no. WL02304; Wanlei, Inc.), PUMA (1:2,000; cat. no. 55120–1-AP; ProteinTech Group, Inc.), Bax (1:3,000; cat. no. 50599–2-Ig; ProteinTech Group, Inc.), Bcl-2 (1:2,000; cat. no. 26593–1-AP; ProteinTech Group, Inc.), and β-Actin (1:3,000; cat. no. 20536–1-AP; ProteinTech Group, Inc.).
CCK-8 assay
ESCC cells were seeded onto 96-well plates (5 × 103/well) and incubated with the twenty chalcones derivatives for 24 h. Meanwhile, cells were treated with the increasing concentrations of the chosen derivatives (5 ~ 20 µM) at different time points (12, 24, or 36 h), and the cells were assessed using the CCK-8 assay (Beyotime, China). Microplate auto-reader was used to determine the optical density value at 450 nm. We used the OD value of 24 h after the chalcones derivatives were treated to calculate the IC50 value. The IC50 values were automatically calculated by using GraphPad Prism software.
Hoechst 33,258 staining
ESCC cells (5 × 104/well) were seeded into 6-well plates and maintained overnight and treated with 0.1% DMSO or Ch-19 (5, 10, 15, 20 μM) for 24 h. Then cells were stained with 10 mg/mL Hoechst 33,258 and avoided the light for 30 min at 37 °C. Cells were washed twice with phosphate-buffered saline (PBS) for then photographed using Nikon A1R confocal microscope.
Measurement reactive oxygen species (ROS) detection
The production of ROS in solution was routinely detected with 2′, 7′-dichlorodihydrofluorescein diacetate (DCFH-DA) (Sigma–Aldrich) according to the method described previously (Yang et al. 2020) with some modifications. Briefly, ESCC cells (5 × 104/well) were treated with 0.1% DMSO or Ch-19 (5, 10, 15, 20 μM) at 37 °C for 24 h. Then, 1 mM DCFH-DA was added to the cells (final concentration of DCFH-DA will be 10 μM). After incubation for 30 min, the cells were washed with phosphate buffer saline (PBS) and collected. Meanwhile, intracellular ROS level in ESCC cells treated with Ch-19 (10 μM) was also measured for different time points (0 min, 15 min, 30 min, 45 min, or 60 min). Fluorescence signal intensities indicating ROS levels were recorded by flow cytometer (BD Biosciences) using excitation and emission spectra of 488/525 nm. All cells were photographed by utilizing an Olympus (Japan) BH-2fluorescence microscope and analyzed by using FlowJo software.
Apoptosis analysis
The cell apoptosis was analyzed by flow cytometry following our established procedures (Tian et al. 2022; Yang et al. 2020) using double labeling (Annexin-V/propidium iodide), with slight modifications. Briefly, ESCC cells (5 × 104 cells/well) were seeded into 6-well plates, followed by treatment with 0, 5, 10, or 20 μM Ch-19 in media with 10% FBS for 24 h. After incubation for the indicated dose of Ch-19, the cells were treated with 0.25% trypsin without EDTA. Adherent and floating cells were collected together, and an apoptosis assay was conducted according to the manufacturer’s protocol. The cells were incubated with Annexin V-FITC and propidium iodide (PI) (Beyotime, China) for 15 min at room temperature (RT) in the dark. Apoptotic cells were analyzed by fluorescent-activated cell sorting (FACS) flow cytometer (BD Bioscience, USA) and analyzed by FlowJo software.
Cell cycle analysis
ESCC cells (5 × 104 cells/well) were seeded into 6-well plates, cultured overnight, and then were exposed to 0.1% DMSO or Ch-19 (5, 10, 20 μM) for different times (24, 48, or 72 h). Cells were washed and fixed and suspended in flow cytometry staining buffer, then propidium iodide (PI) reagent was added (Beyotime, China). The samples were sorted by flow cytometry and analyzed by using FlowJo software. In another experiment, the cells were treated with 10 μM Ch-19 or 10 μM Ch-19 plus 10 mmol/L N-acetylcysteine (NAC) (Beyotime, China) for 24 h, then stained with Annexin V-FITC/PI or PI.
Western blot analysis
Western blot was performed using established procedures (Tian et al. 2022; Xu et al. 2015). Cells were lysed in lysis buffer (Dingguo, China), incubated on ice for 30 min, and centrifuged for 20 min to remove cell debris. Total cell lysates were subjected to SDS–polyacrylamide electrophoresis and fractionated proteins immunoblotted with primary antibodies and HRP-conjugated secondary antibodies. After another wash of the membrane, the bands were detected using a super-sensitive ECL solution (Boster, China), and visualized using an Amersham imager 600 (GE Healthcare Life Sciences, Fairfield, CT, United States), and quantified using Image J software.
In vivo therapy study
Four-week-old female BALB/c nude mice were purchased from the Beijing Vital River Laboratory Animal Technology (Beijing, China) and kept under SPF-level conditions. All experimental protocols were approved by the Animal Experimentation Ethics Committee of Xinxiang Medical University and all efforts were made to minimize animal suffering and reduce the number of animals used. Animals were treated in accordance with the guideline of the Animal Care and Use Committee of Xinxiang Medical University.
KYSE-450 cells (5 × 106 cells in 200 μL PBS per mouse) were inoculated subcutaneously into the right flank of female BALB/c nude mice. When the tumor grew to 50 mm3, the nude mice were randomly divided into three groups, namely the control group, Ch-19 low dosage group, and Ch-19 high dosage group. Both Ch-19 groups were intraperitoneally injected with 50 mg/kg or 100 mg/kg Ch-19 three times every week. The control mice were intraperitoneally injected with physiological saline. Body weight and tumor size of the nude mice were recorded every 2 days. The size of the tumor was calculated according to the formula: volume = [Length × (Width)2]/2. At day 26 post-drug injection, the nude mice were sacrificed and the tumor tissues were isolated for further analysis.
Statistical analysis
Quantitative data are expressed as mean ± standard deviation (SD). Comparisons were analyzed by Student’s t-test. Data are the results of at least three independent experiments.
Results
Novel chalcone analog Ch-19 effectively inhibited ESCC proliferation in vitro
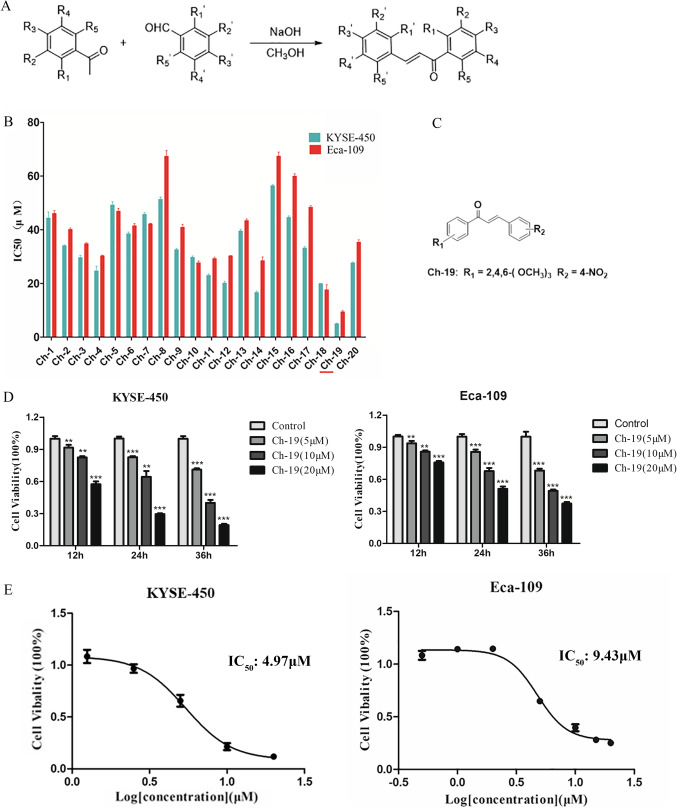
A series of 2,4,6-trimethoxy-4′-nitrochalcone were designed and synthesized, and all target products were obtained by crystallization or by silica gel flash chromatography (Fig. 1A). Table 1 showed all the chemical structures and the yields of the synthesized chalcone analogs. CCK-8 assay demonstrated that mean IC50 values of the twenty compounds against both ESCC cells varying from 4.97 to 56.39 µM and 9.43 to 67.40, respectively (Fig. 1B). Notably, Ch-19 exerted the strongest anti-tumor activity against ESCC cells (Fig. 1C), which may be attributed to the substitution of the methoxy group of aromatic ketones and the 4-substitution nitro group of aromatic aldehydes. Moreover, the cell viability was significantly reduced after Ch-19 treatment in both ESCC cells in a dose and time-dependent manner (Fig. 1D-E). The mean IC50 values in KYSE-450 and Eca-109 cells were 4.97 µM and 9.43 µM, respectively.
Fig. 1.
Inhibitory effect of 20 chalcone derivatives against ESCC cells in vitro. A Overview of chalcones synthetic route. B ESCC cells were treated with twenty chalcone derivatives (Ch-1 ~ Ch-20) for 24 h. CCK-8 assay was utilized to examine the cell viability, and IC50 was calculated by GraphPad Prism software. C Structure of 2,4,6-trimethoxy-4′-nitrochalcone (Ch-19). D KYSE-450 (left panel) and Eca-109 (right panel) were treated with increasing dose Ch-19 (0, 5, 10, or 20 µM) for 12, 24 or 36 h. Cell viability was determined using CCK-8 assay. The results represent the means (n = 3) ± SD. Significant differences were evaluated using Student’s t-test, **p < 0.01; ***p < 0.001. (E) CCK-8 assays were used to evaluate the inhibitory effects of Ch-19 on KYSE-450 (left panel) and Eca-109 (right panel) cell growth. Points, mean of 3 independent CCK-8 assays
Table 1.
Chemical structures of the synthesized chalcone analogues
| Chalcones | Acetophenone | Aromatic aldehydes | Yield (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| NO | R1 | R2 | R3 | R4 | R5 | R1′ | R2′ | R3′ | R4′ | R5′ | |
| 1 | H | H | NO2 | H | H | H | CH3O | CH3OCH2O | H | H | 82 |
| 2 | H | H | NO2 | H | H | H | CH3O | OH | H | H | 56 |
| 3 | H | CH3O | CH3O | H | H | H | CH3O | OH | H | H | 88 |
| 4 | H | H | (CH3)2 N | H | H | H | CH3O | OH | H | H | 75 |
| 5 | H | H | (CH3)2 N | H | H | H | CH3O | CH3O | CH3O | H | 92 |
| 6 | H | H | NO2 | H | H | H | CH3O | CH3O | CH3O | H | 64 |
| 7 | H | H | (CH3)2 N | H | H | H | CH3O | CH3O | H | H | 78 |
| 8 | H | H | (CH3)2 N | H | H | H | H | (CH3)2 N | H | H | 94 |
| 9 | H | H | NO2 | H | H | H | H | CH3O | H | H | 60 |
| 10 | H | CH3O | CH3O | H | H | H | H | CH3O | H | H | 84 |
| 11 | H | H | NO2 | H | H | H | H | (CH3)2 N | H | H | 58 |
| 12 | H | CH3O | CH3O | H | H | H | H | NO2 | H | H | 88 |
| 13 | H | H | NO2 | H | H | CH3O | H | CH3O | H | CH3O | 94 |
| 14 | H | H | NO2 | H | H | H | CH3O | CH3O | H | H | 67 |
| 15 | H | H | (CH3)2 N | H | H | CH3O | H | CH3O | H | CH3O | 91 |
| 16 | H | H | CH3 | H | H | H | H | NO2 | H | H | 60 |
| 17 | H | H | CH3O | H | H | H | H | NO2 | H | H | 75 |
| 18 | H | H | (CH3)2 N | H | H | H | H | NO2 | H | H | 94 |
| 19 | CH3O | H | CH3O | H | CH3O | H | H | NO2 | H | H | 83 |
| 20 | CH3O | H | CH3O | H | CH3O | H | CH3O | CH3OCH2O | H | H | 90 |
Enhancement of ROS accumulation and apoptosis induction may explain the superior anti-tumor effects of Ch-19 in ESCC Cells
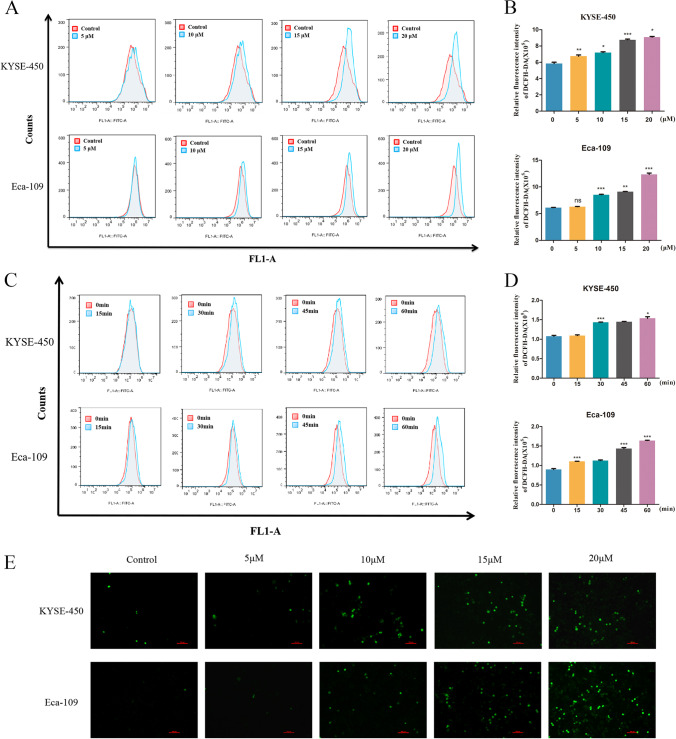
Many anti-tumor agents are known to cause cancer cell death through promoting ROS accumulation (Lin et al. 2019). At a lower level, ROS promotes carcinogenesis through the regulation of proliferation, angiogenesis, and metastasis; while a high level of ROS induces DNA toxicity, resulting in cellular apoptosis and cell death, which leads to anti-tumor effects (Li et al. 2020). Next, we examined the total ROS levels in ESCC cells after treatment with Ch-19 by flow cytometry. Data indicated that Ch-19 treatment (5, 10, 15, 20 µM) remarkably elevated ROS levels in a dose-dependent manner in KYSE-450 and Eca-109 cells (Fig. 2A, B), which was confirmed via DCF-DA staining (Fig. 2E). Unexpectedly, we also found that Ch-19 treatment significantly induced ROS products in ESCC cell lines from 15 min to 1 h (Fig. 2C, D). Collectively, the data above suggested that compound Ch-19 was capable of stimulating the generation of intracellular ROS.
Fig. 2.
Ch-19 treatment effectively enhanced ROS accumulation in ESCC cells. A KYSE-450 and Eca-109 cells were treated with DMSO or Ch-19 (5, 10, 15, or 20 μM) for 24 h, and flow cytometry was used to analyze the levels of ROS in cells after DCFH-DA staining. B Bar graphic representations of the fluorescence intensity upon Ch-19 treatment in ESCC cells. C The level of ROS accumulation in KYSE-450 (up panel) and Eca-109 (down panel) cells were analyzed after treatment with Ch-19 (10 μM) for different time point (0 min, 15 min, 30 min, 45 min, or 60 min). D Bar graphic representations of the fluorescence intensity upon Ch-19 treatments in ESCC cells. Data showed the mean ± S.D. (3 independent experiments); **p < 0.01; ***p < 0.001. E The level of ROS was detected after the cells were treated with Ch-19 (5, 10, 15, or 20 μM) for 24 h (magnification × 200). Fluorescent image of KYSE-450 (up panel) and Eca-109 (down panel) cells stained by DCHF-DA. Scale bar: 10 µm
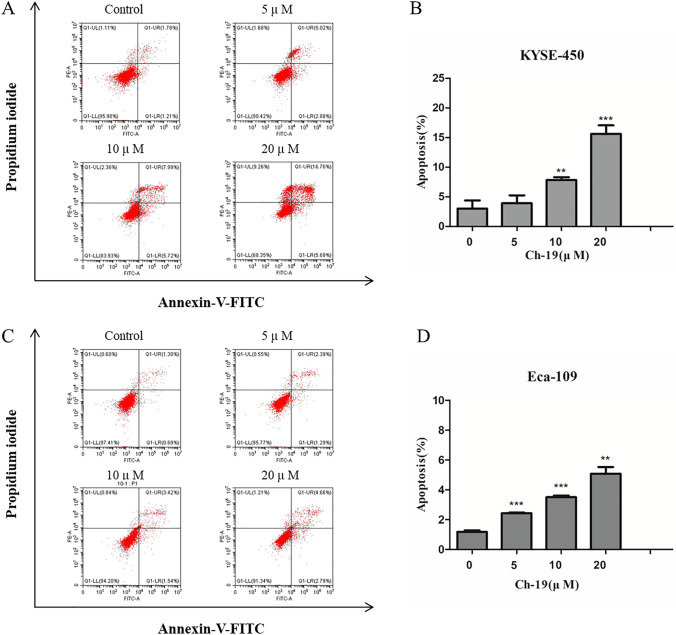
To address the question of whether the anti-proliferative effect of Ch-19 was associated with the induction of apoptotic cell death, ESCC cells were treated with the indicated concentration of Ch-19 for 24 h, stained with Annexin V and propidium iodide, and then analyzed by fluorescence-activated cell sorting (FACS). Data indicated that Ch-19 markedly induced apoptosis in a dose-dependent manner in both KYSE-450 (Fig. 3A) and Eca-109 (Fig. 3C) cells. As shown in Fig. 3B and D, the percentage of apoptotic cells was significantly increased in the Ch-19-treated cells compared to the control group. To further confirm the apoptosis effects, we also performed Hoechst 33,258 staining assay. Data suggested that Ch-19 could notably trigger apoptosis in KYSE-450 and Eca-109 cells through inducing chromatin condensation and nuclear fragmentation formation (Fig. S1).
Fig. 3.
Ch-19 induced cell apoptosis in ESCC cells. A Induction of apoptosis of KYSE-450 cells after treatment with DMSO or Ch-19 (5, 10, or 20 μM) for 24 h. B Apoptosis ratios were measured by flow cytometry; Data was shown with mean ± SD of three independent experiments. **p < 0.01; ***p < 0.001. C Induction of apoptosis of Eca-109 cells after treatment with DMSO or Ch-19 (5, 10, or 20 μM) for 24 h. D Apoptosis ratios were measured by flow cytometry. Data was shown with mean ± SD of three independent experiments. **p < 0.01; ***p < 0.001
Next, we verified key apoptosis-relevant proteins, including cleaved poly (ADP-ribose) polymerase (PARP), cleaved caspase 3, B-cell lymphoma 2 (Bcl-2), Bcl-2 associated X protein (Bax), Bad, Bim and PUMA. Data indicated that Ch-19 induced the cleavage of PARP and caspase 3, and increased the levels of Bad, Bim, PUMA, and BAX, while decreasing the anti-apoptotic protein, Bcl-2 level in a dose-dependent manner (Fig. 4A). Moreover, we also detected the level of apoptosis-relevant proteins at different time point (24 h, 48 h, or 72 h). Results revealed that Ch-19 treatment significantly induced the cleavage of PARP and caspase 3, as well as increased the levels of Bad, Bim, PUMA, and BAX, and decreased Bcl-2 expression in a time-dependent manner (Fig. 4B). Taken together, these results confirmed that Ch-19 may exert its anti-tumor activity through inducing ROS accumulation and initiating apoptosis in ESCC cells.
Fig. 4.
The effects of Ch-19 on the level of apoptosis-related proteins. A The levels of cleaved-PARP, Bad, Bim, PUMA, Bax, and Bcl-2 in KYSE-450 and Eca-109 cells were determined after treatment with Ch-19 (5, 10, 15, or 20 μM) for 24 h. B Quantification of Western blotting signal intensity analysis was expressed relative to the β-actin loading control by using Image J software. Data show the mean ± SD (three independent experiments); *p < 0.05, **p < 0.01, ***p < 0.001. C The levels of cleaved-PARP, Bad, Bim, PUMA, Bax, and Bcl-2 in KYSE-450 and Eca-109 cells were determined after treatment with Ch-19 (10 μM) for different time point (0, 12, 24, 36, or 48 h). D Quantification of Western blotting signal intensity analysis was expressed relative to the β-actin loading control by using Image J software. Data show the mean ± SD (three independent experiments); *p < 0.05, **p < 0.01, ***p < 0.001
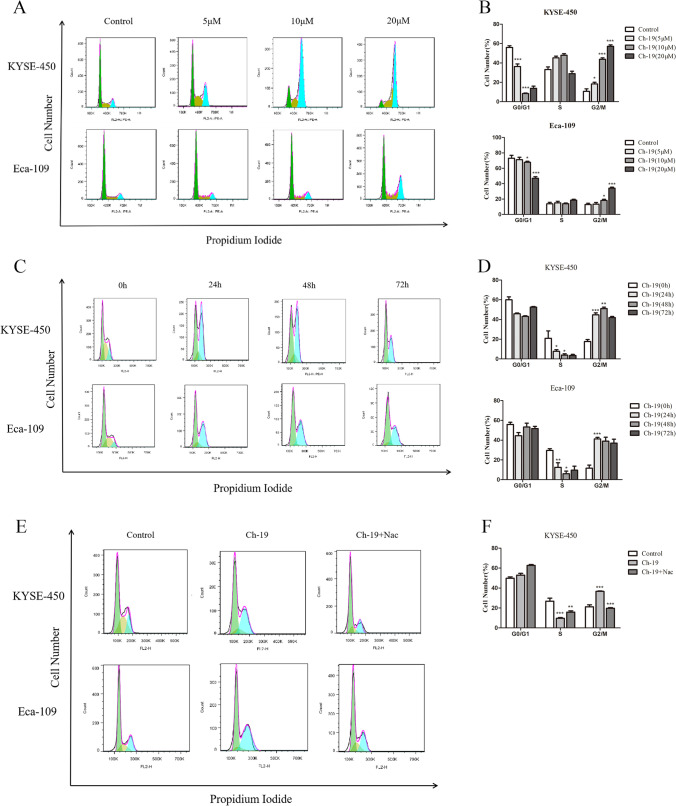
Ch-19 induced cell cycle arrest at G2/M phase by increasing ROS accumulation
To identify the possible molecular mechanism of Ch-19 for anti-tumor activity, we verified whether cell cycle and cell apoptosis were regulated by this compound via ROS accumulation. Here, we showed that this compound induced cell cycle arrest at the G2/M phase and promoted apoptosis. As shown in Fig. 3A–D, the cell cycle was arrested in the G2/M phase in a dose- and time-dependent manner after Ch-19 treatment in ESCC cells. Then we detected the anti-arrest cell cycle effect of N-acetyl-L-cysteine (NAC) in Ch-19-induced cell death by flow cytometry. The G2/M phase arrest was reversed partly to control levels in KYSE-450 and Eca-109 cells co-treated with NAC (10 mM) and Ch-19 (Fig. 5E, F). Taken together, these data suggested that Ch-19 treatment raises ROS products and induces cell cycle arrest and apoptosis in ESCC cells.
Fig. 5.
Ch-19 induced G2/M phase arrest in ESCC cells. A KYSE-450 and Eca-109 cells were treated with DMSO or Ch-19 (5, 10, or 20 μM) for 24 h, and flow cytometry was used to analyze the cell cycle in cells after propidium iodide staining. Representative FACS analysis images are shown. B Statistics of the cell cycle distribution. Data were shown as percent over control. Data are shown as mean ± SD; *p < 0.05; ***p < 0.001, compared to control. (n ≥ 3). C KYSE-450 and Eca-109 cells were treated with DMSO or Ch-19 (10 μM) for different time (24, 48, or 72 h), followed by propidium iodide staining. Representative FACS analysis images are shown. D Statistics of the cell cycle distribution. Data are shown as mean ± SD; *p < 0.05; **p < 0.01; ***p < 0.001, compared to control. (n ≥ 3). E KYSE-450 and Eca-109 cells were treated with DMSO, Ch-19 (10 μM) or Ch-19 (10 μM) plus NAC (10 mM) for 24 h, followed by propidium iodide staining. Representative FACS analysis images are shown. F Statistics of the cell cycle distribution. Data were shown as percent over control. Data are shown as mean ± SD; **p < 0.01; ***p < 0.001, compared to control. (n ≥ 3)
Ch-19 inhibited ESCC cell growth in the KYSE-450 xenograft model
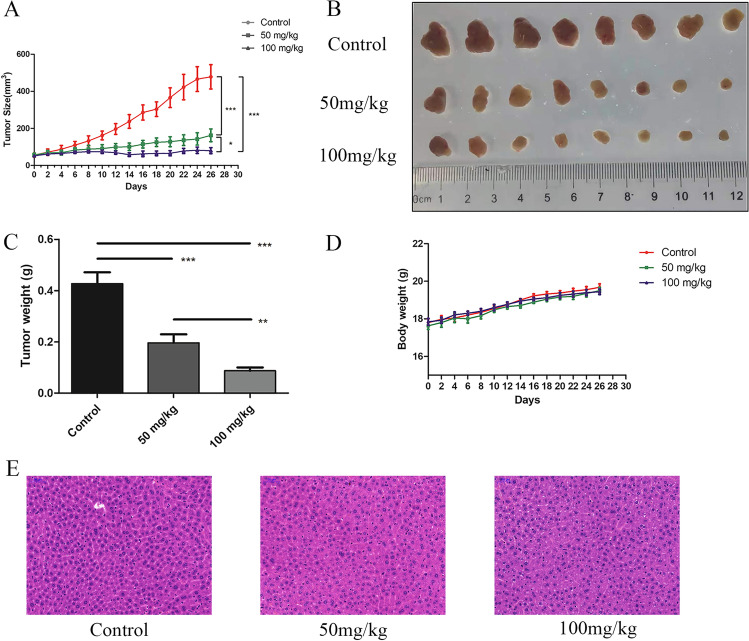
To study the effects of Ch-19 on ESCC cell growth in vivo, we generated a KYSE-450 xenograft model. Consistent with previous results in vitro, Ch-19 treatment reduced tumor burden at both low (50 mg/kg) and high (100 mg/kg) dosages (Fig. 6A–C) but did not affect body weight (Fig. 6D), indicating that this compound has low toxicity. Moreover, hematoxylin and eosin (H&E) staining also showed that no marked liver toxicity was observed in KYSE-450 tumor-bearing mice after Ch-19 treatment in vivo (Fig. 6E).
Fig. 6.
Anti-tumor effect of Ch-19 in the KYSE-450 xenograft tumor model. A Mean tumor volume of KYSE-450 xenografts after injection with control (PBS), Ch-19 (50 mg/kg), or Ch-19 (100 mg/kg). B On day 26 post first injection, xenograft tumors from each group were removed and photographed. Representative tumors in each group were shown. C After xenograft tumors were removed, these tumor masses were weighted. D Effects of agents on tumor-bearing mice body weight were determined using KYSE-450 tumor-bearing nude mice. Mice were weighed at regular intervals during the whole period to monitor unspecific toxicity. Data are shown as mean ± SD. (n = 8 mice, each group); ∗ ∗ p < 0.01; ∗ ∗ ∗ p < 0.001. E Histological examination was conducted in KYSE-450 tumor-bearing mice treated with Ch-19. The results are obtained using representative images (magnification, × 200) of livers from nude mice (n = 8) after injection with the indicated agents and stained with hematoxylin and eosin. Scale bars, 50 μm
Discussion
Esophageal cancer is one of the most serious tumor diseases around the world. Particularly, the northern region in the Henan Province of China has the highest incidence of esophageal squamous cell carcinoma (ESCC). Natural products and their synthetic analogs are characterized by low cytotoxicity and antitumor activity, which have been a concern for the development of antitumor lead candidates (Kingston 2011; Koehn and Carter 2005). Among these natural products, chalcone exhibits diverse biological activities, including anti-tumor effects, on breast cancer, ovarian cancer, and pancreatic cancer (Kwak et al. 2020; Li et al. 2020; Zhuang et al. 2017).
In this study, we designed and synthesized 2,4,6-trimethoxy-4′-nitrochalcone, a promising therapeutic chalcone derivative for ESCC. Specifically, we employed NaOH as a catalyst to construct the compounds 1–20 by the Claisen-Schmidt reaction in methanol according to previous reports (Chiaradia et al. 2008). Different substituted acetophenone was dissolved in anhydrous methanol and followed with addition of NaOH, and the reaction was carried out at room temperature (RT) until completion, then the precipitate was filtered and washed with water and cold methanol. 1H NMR and 13C NMR were used to identify and characterize all chalcone derivatives. To observe the inhibitory effects of the twenty synthesized chalcone derivatives (Ch-1 ~ Ch-20) in vitro, we utilized 2 esophageal cancer cell lines, including KYSE-450 and Eca-109. Data indicated that almost all of them shows potent inhibitory effects on both cell lines. Among these derivatives, Ch-19 exhibited a significant effect on inhibiting ESCC cell proliferation. We further explored the mechanism of the anti-tumor effects of Ch-19.
Clearly, ROS plays a key role in a variety of cellular signaling pathways, including proliferation, metabolism, and apoptosis, among others (Chen et al. 2018; Costa et al. 2014; Liu et al. 2017). Excessive ROS production could disrupt oxidative balance, leading to cell damage and cell death in various cancers (Jin et al. 2019; Leanza et al. 2017; Scialò et al. 2017). Studies earlier have reported that numerous anti-tumor agents (Ai et al. 2016; Poprac et al. 2017; Raj et al. 2011; Wu et al. 2017), including chalcones, were able to induce the production of intracellular ROS, which influenced proliferation and apoptosis in various cancers (Ray et al. 2012). Thus, the detection of ROS is essential to illustrate the mechanism of action of a potential anti-tumor drug (Jutooru et al. 2014). Our results revealed that significant elevation of ROS accumulation in a dose- and time manner was verified in Ch-19-treated ESCC cells. Moreover, oxidative stress-dependent apoptosis and cell cycle arrest were also found when treated with Ch-19 with a longer period. Therefore, we speculated Ch-19 treatment effectively increased ROS accumulation and then induced apoptosis of KYSE-450 cells, which may also partly explain a stronger inhibitory effect of Ch-19 treatment on ESCC cells. However, the mechanisms may be related to induce ROS accumulation and induce cell apoptosis and cell cycle arrest, but still need further study to prove the conclusions.
Since apoptosis is a type I cell death, this complex process involves multiple steps with participation of diverse effector proteins. It physiologically showed plasma membrane shrinkage and nuclear fragmentation following death ligand binding (extrinsic pathway) and DNA damage/cell stresses (intrinsic pathway) [26]. Ch-19 was discovered to have anti-tumor effects by upregulating the expressions of pro-apoptotic signaling proteins including cleaved-PARP, cleaved-caspase 3, Bax, Bim, Bad, and PUMA, and downregulating the expression of anti-apoptotic-protein Bcl-2, leading to apoptosis. Interestingly, as a major member of the Poly (ADP-ribose) polymerases (Parps) family, PARP plays a key role in regulating DNA repair, especially cell death (Jiang et al. 2015). Besides, up-regulation of cleaved-PARP and caspase 3 is one of the principal features of apoptosis (Wu et al. 2016). Our data indicated that despite G2/M phase arrest-related DNA repair, PARP was cleaved after treatment with Ch-19, suggesting a dysfunctional DNA damage repair upon exposure to Ch-19.
Most of the chemotherapeutic drugs and radiotherapy drugs induce cytotoxicity and cell cycle arrest at G1- or G2/M-phase by increasing oxidative stress (Wang et al. 2016a). Cells could be prevented from entering mitosis at G2 checkpoint when DNA is damaged, ensuring the propagation of error-free copies of the genome into each daughter cell. The results revealed that cells at G2/M phases are increased, which means that the cell cycle does not proceed further for both ESCC cell lines. Additionally, G2/M arrest of ESCC cells upon Ch-19 treatment partly explained ESCC cell growth suppression. We also identified Ch-19 as a ROS inducer in ESCC cells. Importantly, NAC, the active oxygen scavenger, significantly induced Ch-19-induced cell apoptosis, and partly reversed the G2/M arrest. These results suggest that ROS is the critical mediator of inhibitory function of Ch-19 in ESCC cells.
Ch-19 mediates anti-proliferative and apoptotic effects in vitro, thus athymic nude mice orthotopically xenografted with KYSE-450 cells were used in vivo. Consequently, the results validated in vitro findings and we concluded that Ch-19 could be used as a potentially safe anti-tumor lead drug. Similarly, previous results reported a 57% reduction in tumor growth in KYSE-450 xenograft nude mice after being treated with a chalcone-based compound (Wang et al. 2016b). Data indicated that compared with the control group, Ch-19 administered at both doses by intraperitoneal injection significantly inhibited tumor growth, indicating its potential for tumor-preventative application. The results also suggested that the compound was associated with a good safety profile since there were no negative effects observed on mice’s health or caused any changes in their body weight during the experiment. Moreover, H&E staining showed that Ch-19 treatment did not induce any noticeable toxicity in nude mice. However, future toxicological studies should be carried out to confirm the safety of Ch-19.
Conclusions
After treatment with a series of synthesized 2,4,6-trimethoxy-4′-nitrochalcone compounds, we observed inhibition of cell growth in ESCC cell lines. Among the twenty chalcone derivatives, Ch-19 inhibited ESCC cell viability obviously in vitro and in vivo by inducing ESCC cell apoptosis and stopping the cell cycle in the G2/M phase through ROS-mediated signaling (Fig. 7). Our findings suggested that this compound might be considered as a potential lead chemotherapeutic candidate or a supplement drug for more research on human clinical trials.
Fig. 7.
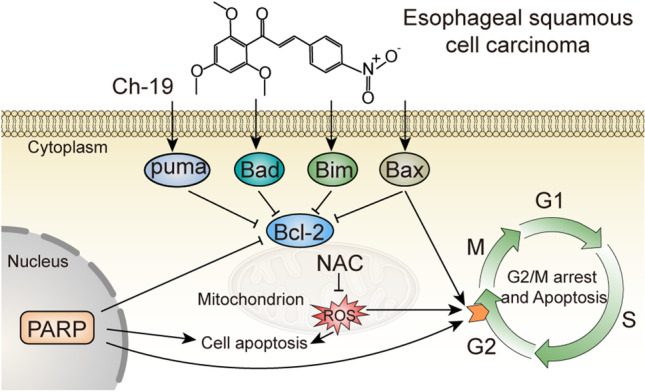
Schematic illustrations showing Ch-19-induced cell death. Ch-19 induces ROS accumulation and G2/M phase arrest, by inducing PARP cleavage and increased Bad, Bim, PUMA, and BAX expressions, whereas decreasing Bcl-2 expression, thus resulting in cell apoptosis. In addition, ROS accumulation also induces apoptosis directly
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
This work was supported by grants from the Program for Science&Technology Innovation Talents in Universities of Henan Province (22HASTIT049), Natural Science Foundation of Henan (202300410322 and 222300420266), Training Plan for Young Backbone Teachers in Universities of Henan Province (2020GGJS143), Key Scientific Research Project of Higher Education of Henan Province (22A310007 and 22B310011), Science & Technology Project for Young Talents of Henan Province (2020HYTP048 and 2022HYTP042), National College Students’ Innovation and Entrepreneurship Training Program (202110472008) and Innovation project of Graduate in Xinxiang Medical University (YJSCX202130Y), Open Program of International Joint Research Laboratory for Recombinant Pharmaceutical Protein Expression System of Henan (KFKTYB202204).
Author contribution
Yun Yang and Chunpo Ge designed the study, and Yan Yang and Yun Yang wrote the manuscript. Yan Yang, He Wu, and Xiao Zou performed almost all the experiments and data analysis. Yongye Chen and Runjia performed cell proliferation and cell apoptosis assays. Yibo Jin and Bei Zhou help in implementing in vivo assay. All authors read and approved the final manuscript.
Declarations
Conflicts of interest
The authors declare no competing interests.
Footnotes
Yan Yang, He Wu, and Xiao Zou contributed equally to this work and share the first authorship.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chunpo Ge, Email: gecp1987@xxmu.edu.cn.
Yun Yang, Email: jamesyangyun1@126.com.
References
- Ai Y, Zhu B, Ren C, Kang F, Li J, Huang Z, Lai Y, Peng S, Ding K, Tian J, Zhang Y. Discovery of new monocarbonyl ligustrazine-curcumin hybrids for intervention of drug-sensitive and drug-resistant lung cancer. J Med Chem. 2016;59:1747–1760. doi: 10.1021/acs.jmedchem.5b01203. [DOI] [PubMed] [Google Scholar]
- Cao J, Xu H, Li W, Guo Z, Lin Y, Shi Y, Hu W, Ba Y, Li S, Li Z. Nutritional assessment and risk factors associated to malnutrition in patients with esophageal cancer. Curr Probl Cancer. 2021;45:100638. doi: 10.1016/j.currproblcancer.2020.100638. [DOI] [PubMed] [Google Scholar]
- Chen L, Li Q, Weng B, Wang J, Zhou Y, Cheng D, Sirirak T, Qiu P, Wu J. Design, synthesis, anti-lung cancer activity, and chemosensitization of tumor-selective MCACs based on ROS-mediated JNK pathway activation and NF-κB pathway inhibition. Eur J Med Chem. 2018;151:508–519. doi: 10.1016/j.ejmech.2018.03.051. [DOI] [PubMed] [Google Scholar]
- Chiaradia LD, Santos RD, Vitor CE, Vieira AA, Leal PCS, Nunes RJ, Calixtob JOB, Yunes RA. Synthesis and pharmacological activity of chalcones derived from 2,4,6-trimethoxyacetophenone in RAW 264.7 cells stimulated by LPS: quantitative structure-activity relationships. Bioorg Med Chem. 2008;16:658–67. doi: 10.1016/j.bmc.2007.10.039. [DOI] [PubMed] [Google Scholar]
- Costa A, Scholer-Dahirel A, Mechta-Grigoriou F. The role of reactive oxygen species and metabolism on cancer cells and their microenvironment. Semin Cancer Biol. 2014;25:23–32. doi: 10.1016/j.semcancer.2013.12.007. [DOI] [PubMed] [Google Scholar]
- Jandial DD, Blair CA, Zhang S, Krill LS, Zhang Y-B, Zi X. Molecular targeted approaches to cancer therapy and prevention using chalcones. Curr Cancer Drug Targets. 2014;14:181–200. doi: 10.2174/1568009614666140122160515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang B-H, Tseng W-L, Li H-Y, Wang M-L, Chang Y-L, Sung Y-J, Chiou S-H. Poly(ADP-Ribose) Polymerase 1: cellular pluripotency, reprogramming, and tumorogenesis. Int J Mol Sci. 2015;16:15531–15545. doi: 10.3390/ijms160715531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J, Qiu S, Wang P, Liang X, Huang F, Wu H, Zhang B, Zhang W, Tian X, Xu R, Shi H, Wu X. Cardamonin inhibits breast cancer growth by repressing HIF-1α-dependent metabolic reprogramming. J Exp Clin Cancer Res. 2019;38:377. doi: 10.1186/s13046-019-1351-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jutooru I, Guthrie AS, Chadalapaka G, Pathi S, Kim K, Burghardt R, Jin U-H, Safe S. Mechanism of action of phenethylisothiocyanate and other reactive oxygen species-inducing anticancer agents. Mol Cell Biol. 2014;34:2382–2395. doi: 10.1128/MCB.01602-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karthikeyan C, Moorthy NSHN, Ramasamy S, Vanam U, Manivannan E, Karunagaran D, Trivedi P. Advances in chalcones with anticancer activities. Recent Pat Anticancer Drug Discov. 2015;10:97–115. doi: 10.2174/1574892809666140819153902. [DOI] [PubMed] [Google Scholar]
- Kim J-S, Park M-R, Lee S-Y, Kim DK, Moon S-M, Kim CS, Cho SS, Yoon G, Im H-J, You J-S, Oh J-S, Kim S-G. Licochalcone A induces apoptosis in KB human oral cancer cells via a caspase-dependent FasL signaling pathway. Oncol Rep. 2014;31:755–762. doi: 10.3892/or.2013.2929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston DGI. Modern natural products drug discovery and its relevance to biodiversity conservation. J Nat Prod. 2011;74:496–511. doi: 10.1021/np100550t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koehn FE, Carter GT. The evolving role of natural products in drug discovery. Nat Rev Drug Discov. 2005;4:206–220. doi: 10.1038/nrd1657. [DOI] [PubMed] [Google Scholar]
- Kwak A-W, Cho S-S, Yoon G, Oh H-N, Lee M-H, Chae J-I, Shim J-H. Licochalcone H synthesized by modifying structure of licochalcone C extracted from glycyrrhiza inflata induces apoptosis of esophageal squamous cell carcinoma cells. Cell Biochem Biophys. 2020;78:65–76. doi: 10.1007/s12013-019-00892-3. [DOI] [PubMed] [Google Scholar]
- Leanza L, Romio M, Becker KA, Azzolini M, Trentin L, Managò A. Direct pharmacological targeting of a mitochondrial ion channel selectively kills tumor cells in vivo. Cancer Cell. 2017;31:516–531. doi: 10.1016/j.ccell.2017.03.003. [DOI] [PubMed] [Google Scholar]
- Li K, Zhao S, Long J, Su J, Wu L, Tao J, Zhou J, Zhang J, Chen X, Peng C. A novel chalcone derivative has antitumor activity in melanoma by inducing DNA damage through the upregulation of ROS products. Cancer Cell Int. 2020;20:36. doi: 10.1186/s12935-020-1114-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Jiang M, Chen W, Zhao T, Wei Y. Cancer and ER stress: mutual crosstalk between autophagy, oxidative stress and inflammatory response. Biomed Pharmacother. 2019;118:109249. doi: 10.1016/j.biopha.2019.109249. [DOI] [PubMed] [Google Scholar]
- Liu X, Rothe K, Yen R, Fruhstorfer C, Maetzig T, Chen M, Forrest DL, Humphries RK, Jiang X. A novel AHI-1-BCR-ABL-DNM2 complex regulates leukemic properties of primitive CML cells through enhanced cellular endocytosis and ROS-mediated autophagy. Leukemia. 2017;31:2376–2387. doi: 10.1038/leu.2017.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mai CW, Yaeghoobi M, Abd-Rahman N, Kang YB, Pichika MR. Chalcones with electron-withdrawing and electron-donating substituents: anticancer activity against TRAIL resistant cancer cells, structureeactivity relationship analysis and regulation of apoptotic. Eur J Med Chem. 2014;77:378–387. doi: 10.1016/j.ejmech.2014.03.002. [DOI] [PubMed] [Google Scholar]
- Poprac P, Jomova K, Simunkova M, Kollar V, Rhodes CJ, Valko M. Targeting free radicals in oxidative stress-related human diseases. Trends Pharmacol Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- Raj L, Ide T, Gurkar AU, Foley M, Schenone M, Li X, Tolliday NJ, Golub TR, Carr SA, Shamji AF, Stern AM, Mandinova A, Schreiber SL, Lee SW. Selective killing of cancer cells by a small molecule targeting the stress response to ROS. Nature. 2011;475:231–234. doi: 10.1038/nature10167. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Ray PD, Huang B-W, Tsuji Y. Reactive oxygen species (ROS) homeostasis and redox regulation in cellular signaling. Cell Signal. 2012;24:981–990. doi: 10.1016/j.cellsig.2012.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahu NK, Balbhadra SS, Choudhary J, Kohli DV. Exploring pharmacological significance of chalcone scaffold: a review. Curr Med Chem. 2012;19:209–225. doi: 10.2174/092986712803414132. [DOI] [PubMed] [Google Scholar]
- Scialò F, Fernández-Ayala DJ, Sanz A. Role of mitochondrial reverse electron transport in ROS signaling: potential roles in health and disease. Front Physiol. 2017;8:428. doi: 10.3389/fphys.2017.00428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva G, Marins M, Fachin AL, et al. Anti-cancer activity of trans-chalcone in osteosarcoma: involvement of Sp1 and p53. Mol Carcinog. 2015;55:1438–48. doi: 10.1002/mc.22386. [DOI] [PubMed] [Google Scholar]
- Shankaraiah N, Nekkanti S, Brahma UR, Kumar NP, Deshpande N, Prasanna D, Senwar KR, Lakshmi UJ. Synthesis of different heterocycles-linked chalcone conjugates as cytotoxic agents and tubulin polymerization inhibitors. Bioorg Med Chem. 2017;25:4805–4816. doi: 10.1016/j.bmc.2017.07.031. [DOI] [PubMed] [Google Scholar]
- Srinivasan B, Johnson TE, Rahul Lad CX. Structure-activity relationship studies of chalcone leading to 3-hydroxy-4,3′,4′,5′-tetramethoxychalcone and its analogues as potent nuclear factor kappaB inhibitors and their anticancer activities. J Med Chem. 2009;52:7228–7235. doi: 10.1021/jm901278z. [DOI] [PubMed] [Google Scholar]
- Szliszka E, Czuba ZP, Mazur B, Paradysz A, Krol W. Chalcones and dihydrochalcones augment TRAIL-mediated apoptosis in prostate cancer cells. Molecules. 2010;15:5336–5353. doi: 10.3390/molecules15085336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Z, Yang Y, HeWu CY, Jia H, Zhu L, He R, Jin Y, Zhou B, Ge C, Sun Y, Yang Y. The Nrf2 inhibitor brusatol synergistically enhances the cytotoxic effect of lapatinib in HER2-positive cancers. Heliyon. 2022;8:e10410. doi: 10.1016/j.heliyon.2022.e10410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai J-P, Hsiao P-C, Yang S-F, Hsieh S-C, Bau D-T, Ling C-L, Pai C-L, Hsieh Y-H. Licochalcone A suppresses migration and invasion of human hepatocellular carcinoma cells through downregulation of MKK4/JNK via NF-kB mediated urokinase plasminogen activator expression. PLoS ONE. 2014;9:e86537. doi: 10.1371/journal.pone.0086537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhang T, Sun W, Wang Z, Zuo D, Zhou Z, Li S, Xu J, Yin F, Hua Y, Cai Z. Erianin induces G2/M-phase arrest, apoptosis, and autophagy via the ROS/JNK signaling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2016;7:e2247. doi: 10.1038/cddis.2016.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L-H, Jiang X-R, Chen G-L, Guo W, Zhang J-Y, Cui L-J, Li H-H, Li M, Liu X, Yang J-Y, Wu C-F. Anti-tumor activity of SL4 against breast cancer cells: induction of G2/M arrest through modulation of the MAPK-dependent p21 signaling pathway. Sci Rep. 2016;6:36486. doi: 10.1038/srep36486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu J, Wu S, Shi L, Zhang S, Ren J, Yao S, Yun D, Huang L, Wang J, Li W, Wu X, Qiu P, Liang G. Design, synthesis, and evaluation of asymmetric EF24 analogues as potential anti-cancer agents for lung cancer. Eur J Med Chem. 2017;125:1321–1331. doi: 10.1016/j.ejmech.2016.10.027. [DOI] [PubMed] [Google Scholar]
- Wu W-S, Chien C-C, Chen Y-C, Chiu W-T. Protein kinase RNA-like endoplasmic reticulum kinase-mediated Bcl-2 protein phosphorylation contributes to evodiamine-induced apoptosis of human renal cell carcinoma cells. PLoS ONE. 2016;11:e0160484. doi: 10.1371/journal.pone.0160484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao X-y, Hao M, Yang X-y, Ba Q, Li M, Ni S-j, Wang L-S, Du X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011;302:69–75. doi: 10.1016/j.canlet.2010.12.016. [DOI] [PubMed] [Google Scholar]
- Xu S, Chen M, Chen W, Hui J, Ji J, Hu S, Zhou J, Wang Y, Liang G. Chemopreventive effect of chalcone derivative, L2H17, in colon cancer development. BMC Cancer. 2015;15:870. doi: 10.1186/s12885-015-1901-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tian Z, Ding Y, Li X, Zhang Z, Yang L, Zhao F, Ren F, Guo R. EGFR-targeted immunotoxin exerts antitumor effects on esophageal cancers by increasing ROS accumulation and inducing apoptosis via inhibition of the Nrf2-keap1 pathway. J Immunol Res. 2018;2018:1090287. doi: 10.1155/2018/1090287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Tian Z, Guo R, Ren F. Nrf2 inhibitor, brusatol in combination with trastuzumab exerts synergistic antitumor activity in HER2-positive cancers by inhibiting Nrf2/HO-1 and HER2-AKT/ERK1/2 pathways. Oxid Med Cell Longev. 2020;2020:9867595. doi: 10.1155/2020/9867595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang C, Zhang W, Sheng C, Zhang W, Xing C, Miao Z. Chalcone: a privileged structure in medicinal chemistry. Chem Rev. 2017;117:7762–7810. doi: 10.1021/acs.chemrev.7b00020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.