Abstract
Purpose
We aimed to provide a scientometric assessment of global research in stem cell therapy (SCT) for type 1 diabetes (T1D) during 1999–2020.
Methods
The published data on SCT in T1D were retrieved from Elsevier’s Scopus database and analyzed using select bibliometric tools. We used VOSviewer software and the Biblioshiny app to construct and visualize bibliometric networks.
Results
The global yield totaled 1806 publications in the 22-year study period, registering a 17.7% annual growth peaking at 196.9% in the last 11 years. The average citations per publication (CPP) decreased from 62.0 during 1999–2009 to 24.3 during 2010–2020. The funded publications were 727 (40.2%). Randomized controlled trials (RCTs) were only 2.4% (45). Amongst 70 participating countries, the USA led with a 38.6% share. Of the 388 global organizations, Harvard Medical School, USA, San Raffaele Scientific Institute, Italy, and the University of Florida, USA were the topmost contributors. Florina, Couri, and Trucco were the top productive authors, whereas Melton, Abdi, and Simoes were the most impactful. Only 129 (3.1%) publications were highly-cited; their total and average CPP were 31,228 and 214.0 (range 101–1841), respectively.
Conclusions
The quantity of research in SCT for T1D has increased during the last two decades while the quality has dipped. The research landscape is dominated by high-income North-American and Western-European countries. There is a need for conducting large-scale RCTs and promoting research collaborations between high- and low-income countries for long-term sustainability and global impact.
Keywords: Bibliometrics, Global publications, Scientometrics, Stem cell therapy, Treatment, Type 1 diabetes
Introduction
Type 1 diabetes (T1D) is a chronic metabolic disorder characterized by autoimmune destruction of insulin-producing pancreatic β-cells resulting in life-long insulin dependency and is associated with significant morbidity and mortality due to acute and chronic complications. The worldwide incidence of T1D has been increasing steadily; 5–10% of the estimated 425 million people with diabetes have T1D. The increase in average annual incidence has been steeper in countries with previously low incidence. For example, India has recently surpassed the USA in the number of incident cases of T1D [1]. Thus the global disease burden due to T1D remains high. Consequently, there also remains an urgent need for more effective therapies for T1D despite intense overall research in this field [2].
The past few decades have witnessed significant progress in therapeutic options for T1D, such as newer insulin analogs, smart insulins, oral and weekly insulins, artificial pancreas, durable human β-cell replacement, and selective immune manipulation to preserve β-cell function [3, 4]. Of all these therapies, biological approaches involving functional β-cells obtained from stem cells, even though very challenging, offer the biggest hope for patients with T1D [5]. Several experimental and clinical studies conducted in the last decade suggest that stem-cell therapy (SCT) is a promising therapeutic modality for treating T1D [6–8]. Two recent meta-analyses concluded that SCT has beneficial effects on T1D and is safe [9, 10]. However, there are several aspects of SCT that still need to be evaluated. For example, there is considerable uncertainty about which mechanism works for the therapeutic effect, the duration of therapeutic effect, and the selection of T1D patients most likely to benefit from SCT [10]. The recent meta-analyses recognize the need to address the gaps in SCT research through multiple high-quality, large-scale randomized controlled trials (RCTs) [9, 10]. However, large-scale research requires extensive collaboration between organizations and researchers located in several countries [11]. The first step for international collaboration is to identify researchers, organizations, and funding agencies that share research interests and is often achieved through scientometric or bibliometric studies [12]. Additionally, scientometric analysis is essential for assessing the quantity and quality of the published research in any field. There is thus a need for conducting a bibliometric evaluation of research output in the field of SCT in T1D.
Several previous bibliometric studies have analyzed the research yield in SCT. However, the focus of these studies was either on research competencies, trends, or the use of SCT in diabetes and Parkinson’s disease [13–17]. Similarly, the bibliometric studies on T1D did not analyze the SCT separately [2, 18, 19]. A recent bibliometric assessment of SCT in type 2 diabetes (T2D) analyzed the research output of only China and the USA [20]. The present study was thus planned to provide a comprehensive evaluation of global research output in the field of SCT in T1D. We aimed to evaluate the publication types, annual and cumulative growth, and citation impact of published research in SCT for T1D and identify the most productive countries, organizations, authors, journals, and highly-cited publications (HCP) on this topic.
Methods
Data on SCT for T1D was retrieved from Elsevier’s Scopus database (http://www.scopus.com) using a defined search strategy with keywords “Stem Cell” and “Type 1 Diabetes” tagged to field tags “Keyword” and “Title” (Article Title), and confining output to the period ‘1999–2020’. The search strategy was similar to our recent bibliometric studies [21]. The details of data collection and analysis are shown in Fig. 1.
Fig. 1.
Flow chart of data collection and analysis
The research was quantified by the number of publications using the complete counting technique, i.e., every contributing author or organization included in multiple authorship papers was fully counted and received equal credit. We used several indicators of quality such as citations per paper (CPP), relative citation index (RCI), and h-index (HI). The CPP is the total number of citations divided by the total number of papers. The RCI refers to the influence of a publication and is calculated by the number of citations divided by the average number of citations that a publication usually receives in that same field [22]. H-index, or Hirsch index, is defined as the maximum value of h such that the given author/journal has published h papers that have each been cited at least h times. Publications that had received more than 100 citations were considered HCPs. The VOSviewer and Biblioshiny app for Bibliometrix were used to evaluate and visualize the interactions among countries, organizations, authors, and keywords. To understand changes in publications’ growth and metrics over time, the study period was divided into two 11-year time periods. The citations were counted from the date of publication till February 5, 2021.
Ethical considerations
We used secondary data in this study that does not require approval from the ethics committee for research on humans. However, all the ethical principles recommended for such analysis were followed by respecting ideas and citations and referencing authors and their publications.
Results
Citations and funding of research
There were 1806 publications in the 22-year study period, an average of 82.0 publications per year. The research registered a 17.7% annual growth, with a peak of 196.9% in the last 11 years (Table 1). The average CPP was 33.8 but showed a decrease from 62.0 during 1999–2009 to 24.3 during 2010–2020. 727 (40.2%) publications were funded by more than 100 national and international funding agencies. The number of funded papers increased by more than fourfold during the second half of the study period (Table 1). However, the average CPP of funded publications (38.4) was only marginally better than that of all publications (33.8). The leading funding agencies were the National Institute of Health, USA (357 papers), US Department of Health & Human Service (343 papers), National Institute of Diabetes & Digestive and Kidney Diseases (198 papers), and National Natural Science Foundation of China (77 papers).
Table 1.
Number of yearly publications on stem cell therapy in type 1 diabetes, their citations and funding during 1999–2020
| Year | Number of publications | Citations | Citations per paper | Funded papers |
|---|---|---|---|---|
| 1999 | 7 | 189 | 27.0 | 4 |
| 2000 | 11 | 867 | 78.8 | 0 |
| 2001 | 17 | 1302 | 76.59 | 4 |
| 2002 | 26 | 1262 | 48.5 | 4 |
| 2003 | 26 | 2681 | 103.1 | 6 |
| 2004 | 44 | 2943 | 66.8 | 10 |
| 2005 | 48 | 1895 | 39.4 | 17 |
| 2006 | 49 | 5119 | 104.4 | 13 |
| 2007 | 56 | 3854 | 68.8 | 14 |
| 2008 | 81 | 3942 | 48.6 | 36 |
| 2009 | 90 | 4173 | 46.3 | 29 |
| 2010 | 109 | 4507 | 41.3 | 34 |
| 2011 | 88 | 4331 | 49.2 | 25 |
| 2012 | 118 | 4752 | 40.2 | 41 |
| 2013 | 104 | 3825 | 36.7 | 38 |
| 2014 | 108 | 3073 | 28.4 | 36 |
| 2015 | 121 | 2997 | 24.7 | 44 |
| 2016 | 137 | 2978 | 21.7 | 59 |
| 2017 | 127 | 2488 | 19.5 | 70 |
| 2018 | 156 | 2100 | 13.4 | 89 |
| 2019 | 143 | 1324 | 9.2 | 79 |
| 2020 | 140 | 487 | 3.4 | 75 |
| 1999–09 | 455 | 28,227 | 62.0 | 137 |
| 2010–20 | 1351 | 32,862 | 24.3 | 590 |
| 1999–2020 | 1806 | 61,089 | 33.8 | 727 |
The retrieved publications were classified as articles (58.4%), reviews (20.3%), notes (2.4%), editorials (2.2%), conference papers (2.0%), book chapters and short surveys (1.8% each), letters (0.6%), erratum (0.1%) and undefined (0.1%). Only 238 publications were clinical studies; the proportion of clinical to non-clinical studies showed an increase during the second 12-year period of the study (15/455, 3.3% versus 223/1351, 16.5%). Forty-five publications were RCTs. According to the type of stem cells used, the distribution of retrieved publications was as follows: Mesenchymal (452, 25.0%), Hematopoietic (302, 16.7%), Pluripotent (283, 15.6%), Embryonic (237, 13.1%), Multipotent (54, 2.9%) and Totipotent (5, 0.2%). Publications on multipotent stem cells recorded the highest average CPP of 42.2 followed by mesenchymal (39.8), hematopoietic (38.9), embryonic (29.3), pluripotent (26.0) and totipotent (22.6) stem cells.
Research hot spots
Thirty-eight significant keywords were identified from the global literature on SCT in T1D that denote hot spots and trends in this domain. The frequency of their occurrence was the maximum (1098) for T1D, followed by insulin-dependent diabetes mellitus (1029), insulin (741), stem cells (676), pancreas islet beta cells (579), metabolism (560), stem cell transplantation (397) (Fig. 2).
Fig. 2.
WorldCloud sketch of the top 50 keywords. The significance of every tag is displayed with text dimension or shading with the bigger term implying more significance
Most productive countries
Of the 70 participating countries, the top 12 contributed 98.1% to the global publication output. The USA was the leading contributor with a 38.6% share. The USA, Canada, and Italy registered their RCI above the group average of 1.1 and were considered more impactful than others (Table 2). The average collaboration of the top 12 countries was 39.7% and varied from 20.8% to 58.5%; the leading collaborating country pairs were the USA-China, the USA-Italy, USA-Japan, USA-Germany, and USA-Canada with 60, 58, 24, 22, and 21 collaborative linkages respectively (Fig. 3).
Table 2.
Profile of most productive and most impactful countries in research on stem cells therapy for type 1 diabetes during 1999–2020
| S.no | Country | Number and (% share) of papers | TC | CPP | ICP (%) | RCI | ||
|---|---|---|---|---|---|---|---|---|
| 1999–2009 | 2010–2020 | 1999–2020 | 1999–2020 | |||||
| 1 | USA | 196 (43.1) | 501 (37.1) | 697 (38.6) | 34,483 | 49.5 | 260 (37.3) | 1.5* |
| 2 | China | 25 (5.5) | 242 (17.9) | 267 (14.8) | 5032 | 18.9 | 83 (31.1) | 0.6 |
| 3 | Italy | 23 (5.1) | 117 (8.7) | 140 (7.8) | 5673 | 40.5 | 82 (58.6) | 1.2* |
| 4 | U.K | 34 (7.5) | 98 (7.3) | 132 (7.3) | 4623 | 35.0 | 65 (49.2) | 1.0 |
| 5 | Germany | 29 (6.4) | 66 (4.9) | 95 (5.3) | 3392 | 35.7 | 50 (52.6) | 1.1* |
| 6 | Japan | 26 (5.7) | 64 (4.7) | 90 (5.0) | 3284 | 36.5 | 32 (35.6) | 1.1* |
| 7 | Canada | 31 (6.8) | 58 (4.3) | 89 (4.9) | 3824 | 43.0 | 32 (36.0) | 1.3* |
| 8 | Australia | 16 (3.5) | 42 (3.1) | 58 (3.2) | 1504 | 25.9 | 23 (39.7) | 0.8 |
| 9 | France | 16 (3.5) | 40 (3.0) | 56(3.1) | 2019 | 36.1 | 30 (53.6) | 1.1* |
| 10 | S. Korea | 5 (1.1) | 51 (3.8) | 56 (3.1) | 1451 | 25.9 | 19 (33.9) | 0.8 |
| 11 | India | 3 (0.7) | 45 (3.3) | 48 (2.7) | 785 | 16.4 | 10 (20.8) | 0.5 |
| 12 | Brazil | 10 (2.2) | 35 (2.6) | 45 (2.5) | 1499 | 33.3 | 18 (40.0) | 1.0 |
| Total | 414 (91.0) | 1359 (100.6) | 1773 (98.2) | 67,569 | 38.1 | 704 (39.7) | 1.1 | |
| World total | 455 (100.0) | 1351 (100.0) | 1806 (100.0) | 61,089 | 33.8 | –- | –- | |
*Impactful countries
Abbreviations: TC, total citations; CPP, citations per publication; ICP, international collaborative publications; RCI, relative citation index
Fig. 3.
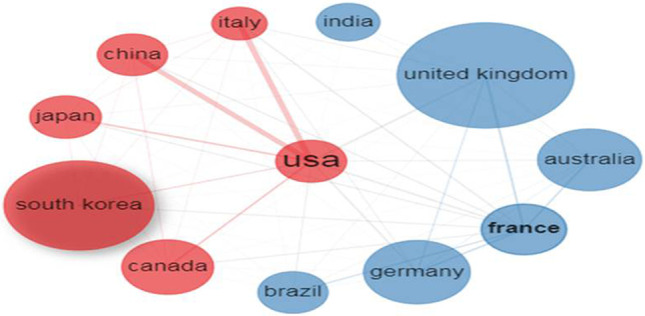
Collaboration network of the top 12 countries generated using the Biblioshiny app. The countries with the same colour belong to a single cluster, the thickness of the linking lines and the distance between countries represents the degree of collaborative relationships. The diameter and font size of the node represents the value of a country in research collaboration
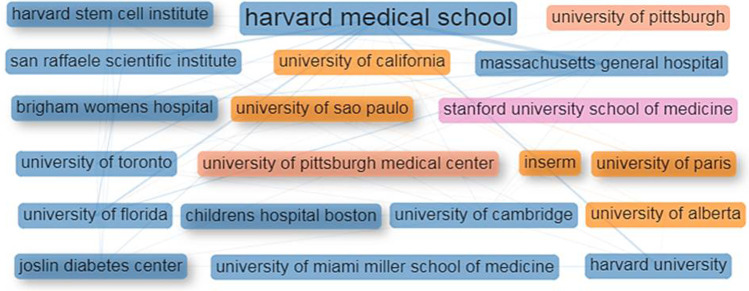
Most productive organizations
Three hundred eighty-eight organizations participated in the SCT research. The publication output of 213 organizations was 1–5 papers each, 112 organizations 6–10 papers each, 46 organizations 11–20 papers each, 15 organizations 21–50 papers each, and one organization 84 papers. Thirteen of the top 20 most productive organizations were from the USA, two each from Canada and France and one each from Italy and the UK. Six organizations registered their productivity above the group average of 29.6. Ten organizations that reported CPP and RCI above their group average of 58.9 and 1.7 were considered most impactful (Table 3). The research collaboration between the top 20 most productive organizations was high; their collaborative linkages varied from 1 to 26 (Fig. 4).
Table 3.
Scientometric profile of the most productive and impactful organizations in stem cell therapy for type 1 diabetes during 1999–2020
| S.no | Organization | TP | TC | CPP | HI | ICP | ICP (%) | RCI |
|---|---|---|---|---|---|---|---|---|
| Most productive organizations | ||||||||
| 1 | Harvard Medical School, USA | 84 | 4964 | 59.1 | 28 | 45 | 53.6 | 1.8 |
| 2 | IRCCS San Raffaele Scientific Institute, Italy | 45 | 2401 | 53.4 | 32 | 7 | 8.3 | 1.6 |
| 3 | University of Florida, USA | 43 | 1995 | 46.4 | 15 | 4 | 4.8 | 1.4 |
| 4 | INSERM, France | 34 | 1073 | 31.6 | 20 | 3 | 3.6 | 0.9 |
| 5 | University of Sao Paulo, Brazil | 31 | 1346 | 43.4 | 14 | 3 | 3.6 | 1.3 |
| 6 | Massachusetts General Hospital, USA | 30 | 2316 | 77.2 | 15 | 4 | 4.8 | 2.3 |
| 7 | Brigham & Women’s Hospital, USA | 28 | 2568 | 91.7 | 14 | 5 | 6.0 | 2.7 |
| 8 | Children’s Hospital, Boston, USA | 28 | 2620 | 93.6 | 22 | 6 | 7.1 | 2.8 |
| 9 | University of California, San Francisco, USA | 27 | 2765 | 102.4 | 7 | 3 | 3.6 | 3.0 |
| 10 | University of Alberta, Canada | 26 | 1775 | 68.3 | 5 | 3 | 3.6 | 2.0 |
| Most impactful organizations | ||||||||
| 1 | University of California, San Fransico, USA | 27 | 2765 | 102.4 | 7 | 3 | 3.6 | 3.0 |
| 2 | Stanford University School of Medicine, USA | 19 | 1941 | 102.2 | 12 | 6 | 7.1 | 3.0 |
| 3 | Children’s Hospital, Boston, USA | 28 | 2620 | 93.6 | 22 | 6 | 7.1 | 2.8 |
| 4 | Brigham & Women’s Hospital, USA | 28 | 2568 | 91.7 | 14 | 5 | 6.0 | 2.7 |
| 5 | Massachsetts General Hospital, USA | 30 | 2316 | 77.2 | 15 | 4 | 4.8 | 2.3 |
| 6 | Harvard University, USA | 25 | 1884 | 75.4 | 5 | 6 | 7.1 | 2.2 |
| 7 | University of Alberta, Canada | 26 | 1775 | 68.3 | 5 | 3 | 3.6 | 2.0 |
| 8 | University of Pittsburg, School of Medicine, USA | 23 | 1548 | 67.3 | 8 | 5 | 6.0 | 2.0 |
| 9 | Harvard Medical School, USA | 84 | 4964 | 59.1 | 28 | 45 | 53.6 | 1.8 |
| 10 | Harvard Stem Cell Institute, USA | 22 | 1307 | 59.4 | 8 | 4 | 4.8 | 1.8 |
Abbreviations: TP, total publications; TC, total citations; CPP, citations per publication; HI, Hirsch Index; ICP, international collaborative publications; RCI, relative citation index
Fig. 4.
Collaboration network of the prime organizations in research on stem cell therapy for type 1 diabetes. The box size and text dimension of each hub are relative to the organization’s research yield
Most productive authors
A total of 526 authors were involved in research on SCT in T1D during 1999–2020. Of these, 447 authors published 1–5 papers each, 63 authors 6–10 papers each, and 16 authors 11–22 papers each. Ten of the top 20 authors were from the USA, whereas three each were from Brazil and Italy, two from Poland, and one was from India. The top 20 together contributed 15.4% (278 publications) of global output and 20.9% (12,799) of total citations. The scientometric profile of the most productive and most impactful authors is presented in Table 4. The research collaborations between top authors varied from 6–35; the highest linkages (14 each) on a one-to-one basis were noted between C.E.B. Couri and J.C. Voltarelli, G.P. Fadini, and A. Avogaro and A. Avogaro and M. Albiero (Fig. 5).
Table 4.
Scientometric profiles of the most productive and impactful authors in research on stem cell therapy for type 1 diabetes during 1999–2020
| S.no | Author | Affiliation | TP | TC | CPP | HI | ICP (%) | RCI |
|---|---|---|---|---|---|---|---|---|
| Most productive authors | ||||||||
| 1 | P. Florina | Harvard Medical School, USA | 22 | 1239 | 56.3 | 14 | 21 (95.5) | 1.7 |
| 2 | C.E.B. Couri | University of Sao Paulo, Brazil | 19 | 978 | 51.5 | 11 | 7 (36.8) | 1.5 |
| 3 | M. Trucco | University of Pittsburg Medical Center, Children’s Hospital, USA | 18 | 374 | 20.8 | 10 | 1 (5.6) | 0.6 |
| 4 | J.C. Voltarelli | University of Sao Paulo, Brazil | 16 | 961 | 60.1 | 10 | 5 (31.3) | 1.8 |
| 5 | G.P. Fadini | Universita degli Studi di Padova, Italy | 16 | 938 | 58.6 | 13 | 6 (37.5) | 1.7 |
| 6 | C. Ricordi | Diabetes Research Unit, Miami, USA | 15 | 393 | 26.2 | 12 | 10 (66.7) | 0.8 |
| 7 | A.M.J. Shapiro | University of Alberta, Canada | 15 | 360 | 24.0 | 8 | 3 (20.0) | 0.7 |
| 8 | A. Avogaro | Universita degli Studi di Padova, Italy | 14 | 895 | 63.9 | 12 | 5 (35.7) | 1.9 |
| 9 | R.T. Lakey | University of California, Irvine, USA | 13 | 371 | 28.5 | 9 | 9 (69.2) | 0.8 |
| 10 | M. Ben Nasr | Harvard Medical School, USA | 12 | 162 | 13.5 | 6 | 12 (100.0) | 0.4 |
| Most impactful authors | ||||||||
| 1 | D.A. Melton | Harvard University, USA | 11 | 1162 | 105.6 | 8 | 2 (18.2) | 3.1 |
| 2 | R. Abdi | Harvard Medical School, USA | 10 | 918 | 91.8 | 8 | 6 (60.0) | 2.7 |
| 3 | B.P. Simoes | University of Sao Paulo, Brazil | 12 | 890 | 74.2 | 8 | 7 (58.3) | 2.2 |
| 4 | M. Albiero | Universita degli Studi di Padova, Italy | 10 | 699 | 69.9 | 10 | 5 (50.0) | 2.1 |
| 5 | A. Avogaro | Universita degli Studi di Padova, Italy | 14 | 895 | 63.9 | 12 | 5 (35.7) | 1.9 |
| 6 | M.A. Atkinson | University of Florida, USA | 20 | 1223 | 61.2 | 13 | 7 (35.0) | 1.8 |
| 7 | J.C. Voltarelli | University of Sao Paulo, Brazil | 16 | 961 | 60.1 | 10 | 5 (31.3) | 1.8 |
| 8 | G.P. Fadini | Universita degli Studi di Padova, Italy | 16 | 938 | 58.6 | 13 | 6 (37.5) | 1.7 |
| 9 | P. Florina | Harvard Medical School, USA | 22 | 1239 | 56.3 | 14 | 21 (95.5) | 1.7 |
| 10 | C.E.B. Couri | University of Sao Paulo, Brazil | 19 | 978 | 51.5 | 11 | 7 (36.8) | 1.5 |
Abbreviations: TP, total publications; TC, total citations; CPP, citations per publication; HI, Hirsch Index; ICP, international collaborative publications; RCI, relative citation index
Fig. 5.
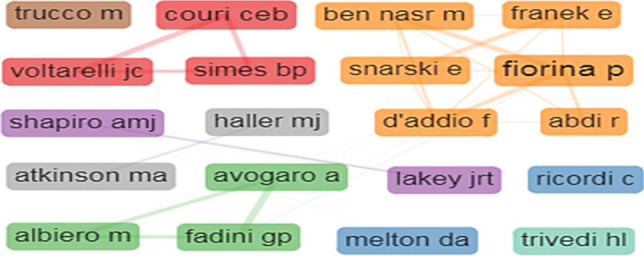
The author collaboration network on stem cell therapy for type 1 diabetes. The top 20 authors are grouped into eight clusters; cluster 1 consists of 6 authors, clusters 2 and 3 of 3 authors each, clusters 4, 5 and 6 of 2 authors each, and clusters 7 and 8 of one author each
Top journals
96.4% (1742 articles) of the total publications appeared in 692 journals; 2.0% (37 papers) in book series, and 0.3% (5 publications) each as conference proceedings and undefined. The top 20 journals accounted for a 22.2% share of the global output; the most impactful journal was Proceedings of the National Academy of Sciences of USA, with a CPP of 149.4 (Table 5).
Table 5.
The most productive journals in stem cell therapy for type 1 diabetes during 1999–2020
| S.no | Journal | TP | TC | CPP |
|---|---|---|---|---|
| 1 | Diabetes | 56 | 1032 | 18.4 |
| 2 | Diabetologia | 32 | 1455 | 45.5* |
| 3 | PLOS One | 31 | 1241 | 40.0* |
| 4 | Stem Cell Research & Therapy | 26 | 411 | 15.8 |
| 5 | Current Diabetes Report | 25 | 282 | 11.3 |
| 6 | Cell Transplantation | 18 | 253 | 14.1 |
| 7 | International Journal of Molecular Sciences | 18 | 310 | 17.2 |
| 8 | Stem Cells | 18 | 2690 | 149.4 |
| 9 | Stem Cell Transplantation Medicine | 18 | 475 | 26.4 |
| 10 | Advances in Experimental Medicine & Biology | 17 | 184 | 10.8 |
| 11 | Pediatric Diabetes | 16 | 118 | 7.4 |
| 12 | Proceedings of National Academy of Sciences of USA | 16 | 2502 | 156.4* |
| 13 | Diabetes Research & Clinical Practice | 14 | 191 | 13.6 |
| 14 | Diabetes Care | 13 | 830 | 63.9* |
| 15 | Diabetes Metabolism Research & Review | 13 | 358 | 27.5* |
| 16 | American Journal of Transplantation | 12 | 258 | 21.5 |
| 17 | Cell Stem Cell | 12 | 238 | 19.8 |
| 18 | Frontiers in Immunology | 11 | 139 | 12.6 |
| 19 | Regenerative Medicine | 11 | 600 | 54.6* |
| 20 | Science | 11 | 854 | 77.6* |
*impactful journals
Abbreviations: TP, total publications; TC, total citations; CPP, citations per publication
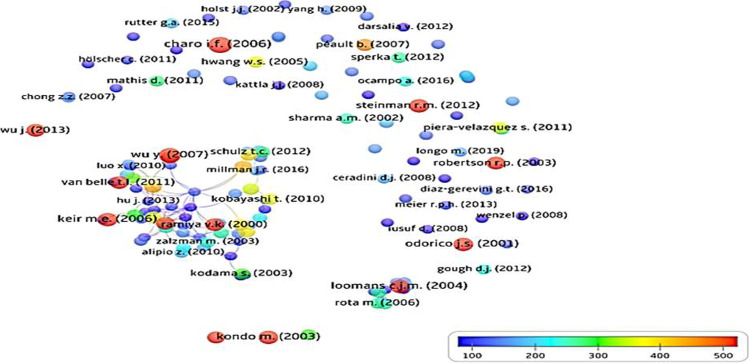
Highly-cited publications
Only 129 (3.1%) publications were HCPs; their total and average CPP were 31,228 and 214.0 (range 101–1841), respectively (Fig. 6). The USA contributed the most HCPs (76 publications), followed by Italy (14 papers), the UK (13 papers), Japan (9 papers), Germany (7 papers), China (6 papers), etc. Harvard Medical School, USA, San Raffaele Scientific Institute, Italy, Children’s Hospital, Boston, USA, Howard Hughes Medical Institute, USA contributed 11, 7, and 6 HCPs, respectively. Of the 83 journals that published 129 HCPs, Diabetes published the maximum numbers (9 papers) followed by Proceedings of the National Academy of Sciences of USA (7 papers), Circulation and Diabetologia (4 papers each), etc.
Fig. 6.
Network visualization of the citation counts of the 129 highly-cited publications in stem cell therapy for type 1 diabetes
Discussion
Our analysis shows that research in SCT for T1D showed an impressive growth during the twenty-first century, increasing by almost threefold in the second 11-year period of the study. In addition, the funding support increased by nearly fourfold. But even as the quantity increased, the quality of research dipped. This finding is consistent with a general decline in the quality of scientific research over the last decades, which has been attributed to several reasons such as an increase in the number of researchers, and linking the quantity of publications to academic promotions, job retention, job mobility, and professional development, which has led to competitive pressure to publish at all costs, sometimes compromising the quality of research publications [23, 24]. It is also perplexing to note that the quality of research in SCT for T1D declined despite increased funding support during the last decade; the quality of funded publications was only slightly better than non-funded publications. Funding is generally associated with improved research quality, as indicated by the citation impact of publications [24, 25]. Conversely, lack of funding support adversely affects the quality of research [26]. However, the increase in the growth of clinical studies during the last decade may indirectly indicate quality improvement in SCT research, as more researchers appear to now focus on the clinical application of research.
An important finding of our analysis was the dominance of the research landscape of SCT for T1D by high-income North-American and Western-European countries. Previous bibliometric studies have reported similar dominance by these countries in other research fields also [2, 27]. The quality and quantity of research in these countries appear to be driven by the availability of adequate infrastructure and funding support essential to conduct highly organized research activity in any field and their governments’ commitment to research [28]. The eminence of China in SCT research reflects the enhanced spending on biomedical research in general, which has resulted in an exponential growth in publications over the past few decades [28, 29]. However, the quality of research indicated by CPP and RCI has remained low, an observation also reported for other fields of medical research from China [29]. The inclusion of India in the top-performing countries is largely due to the research initiatives of a few dedicated organizations and researchers in SCT for T1D [30, 31] and T2D [32, 33]. There was no representation of low-income countries in the most productive or most impactful countries in SCT research for T1D. This is probably due to a meager investment in medical research and several other challenges of conducting biomedical research in low-resource countries [34]. We also noted a worrying trend of lack of collaboration between the high-income and low-income countries in SCT research for T1D. Most of the partnerships occurred amongst researchers and organizations located in high-income countries. However, the improvement in the long-term impact and sustainability of global research requires strengthening of collaborations between high- and low-income countries [35]. Thus, high-income countries need to foster research endeavors and capacity-strengthening initiatives in low and middle-income countries in the area of SCT for T1D.
The gold standard for measuring the effectiveness of any intervention or treatment is RCTs [36]. However, our data show a striking lack of RCTs on SCT in T1D; only 2.4% were RCTs. Recently published meta-analyses that used multiple databases have also highlighted the small number of RCTs in the field of SCT in T1D and suggested large-scale RCTs to confirm the efficacy and safety of SCT in T1D [9, 10].
Our analysis also revealed a lack of SCT studies on children and adolescents with T1D as the analyzed publications did not contain these keywords. The ethical issues and the complexity of the translational pathway probably did not allow younger age groups to be included in RCTs [10]. Only two previous RCTs on mesenchymal SCT probably included young adults with T1D as indicated by the participants’ mean age of 17.6 ± 8.7 and 19.67 ± 2.5 years mentioned in the reports [8, 37]. Thus, future studies should aim to include children and adolescents as T1D is mainly diagnosed during childhood and adolescence, and SCT may benefit this age group the most in the long term [30].
Our analysis had some limitations. Although we tried to address the issue of synonyms or homonyms in authors’ names by using other specific fields such as affiliations, some publications may still have remained uncaptured. Additionally, with the use of a single database compared to multiple databases, it is possible to miss some data [38]. We chose Scopus as it is considered the most authoritative and widely-used medical bibliographic database [39]. Its content coverage, search analysis tools, citation accuracy, and funding information are considered better than PubMed or Web of Science [38, 39]. A vast majority of bibliometric studies also use a single database [38, 40]. Notwithstanding the limitation of using a single database, we could accomplish our study’s stated objectives within its protocol and provide the first global architecture of research on SCT for T1D. The study also provides a framework for researchers, policymakers, organizations, and countries to develop more meaningful collaborations on future research in this field.
Author contributions
Conceptualization: Brij Mohan Gupta; Methodology: Brij Mohan Gupta; Formal analysis and investigation: Brij Mohan Gupta and Ghouse Modin Mamdapur; Writing—original draft preparation: Brij Mohan Gupta and Devi Dayal; Writing—review and editing: Pamali Nanda and Latika Rohilla; Writing manuscript and supervision: Devi Dayal.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.
Declarations
Competing interests
None.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Patterson CC, Karuranga S, Salpea P, Saeedi P, Dahlquist G, Soltesz G, et al. Worldwide estimates of incidence, prevalence and mortality of type 1 diabetes in children and adolescents Results from the International Diabetes Federation Diabetes Atlas 9th edition. Diabetes Res Clin Pract. 2019;157:107842. doi: 10.1016/j.diabres.2019.107842. [DOI] [PubMed] [Google Scholar]
- 2.Gupta BM, Dayal D. Pediatric Type 1 Diabetes Research in the 21st Century: A Scientometric Review. Pediatr Endocrinol Diabetes Metab. 2020;26:132–139. doi: 10.5114/pedm.2020.98165. [DOI] [PubMed] [Google Scholar]
- 3.Drucker DJ. Transforming type 1 diabetes: the next wave of innovation. Diabetologia. 2021;64:1059–1065. doi: 10.1007/s00125-021-05396-5. [DOI] [PubMed] [Google Scholar]
- 4.Paul M, Dayal D, Bhansali A, Dhaliwal L, Sachdeva N. In vitro assessment of cord blood-derived proinsulin-specific regulatory T cells for cellular therapy in type 1 diabetes. Cytotherapy. 2018;20:1355–1370. doi: 10.1016/j.jcyt.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Boscari F, Avogaro A. Current treatment options and challenges in patients with Type 1 diabetes: Pharmacological, technical advances and future perspectives. Rev Endocr Metab Disord. 2021;22:217–240. doi: 10.1007/s11154-021-09635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li L, Li F, Gao F, Yang Y, Liu Y, Guo P, Li Y. Transplantation of mesenchymal stem cells improves type 1 diabetes mellitus. Cell Tissue Res. 2016;364:345–355. doi: 10.1007/s00441-015-2330-5. [DOI] [PubMed] [Google Scholar]
- 7.Carlsson PO, Schwarcz E, Korsgren O, Le Blanc K. Preserved β-cell function in type 1 diabetes by mesenchymal stromal cells. Diabetes. 2015;64:587–592. doi: 10.2337/db14-0656. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, Yu X, Wang Z, Wang F, Wang L, Gao H, et al. Long-term effects of the implantation of Wharton’s jelly-derived mesenchymal stem cells from the umbilical cord for newly-onset type 1 diabetes mellitus. Endocr J. 2013;60:47–357. doi: 10.1507/endocrj.EJ12-0343. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Wang F, Liang H, Tang D, Huang M, Zhao J, Yang X, Liu Y, Shu L, Wang J, He Z, Liu Y. Efficacy of mesenchymal stem cell transplantation therapy for type 1 and type 2 diabetes mellitus: a meta-analysis. Stem Cell Res Ther. 2021;12:273. doi: 10.1186/s13287-021-02342-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He J, Kong D, Yang Z, Guo R, Amponsah AE, Feng B, Zhang X, Zhang W, Liu A, Ma J, O’Brien T, Cui H. Clinical efficacy on glycemic control and safety of mesenchymal stem cells in patients with diabetes mellitus: Systematic review and meta-analysis of RCT data. PLoS ONE. 2021;16:e0247662. doi: 10.1371/journal.pone.0247662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yao B. International Research Collaboration: Challenges and Opportunities. J Diagn Med Sonogr. 2021;37:107–108. doi: 10.1177/8756479320976130. [DOI] [Google Scholar]
- 12.Cooper ID. Bibliometrics basics. J Med Libr Assoc. 2015;103:217–218. doi: 10.3163/1536-5050.103.4.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li LL, Ding G, Feng N, Wang MH, Ho YS. Global stem cell research trend: Bibliometric analysis as a tool for mapping of trends from 1991 to 2006. Scientometrics. 2009;80:39–58. doi: 10.1007/s11192-008-1939-5. [DOI] [Google Scholar]
- 14.Watatani K, Xie Z, Nakatsuji N, Sengoku S. Global competencies of regional stem cell research: bibliometrics for investigating and forecasting research trends. Regen Med. 2013;8:659–668. doi: 10.2217/rme.13.51. [DOI] [PubMed] [Google Scholar]
- 15.Zhao J, Yu G, Cai M, Lei X, Yang Y, Wang Q, Zhai X. Bibliometric analysis of global scientific activity on umbilical cord mesenchymal stem cells: a swiftly expanding and shifting focus. Stem Cell Res Ther. 2018;9:32. doi: 10.1186/s13287-018-0785-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin CL, Ho YS. A bibliometric analysis of publications on pluripotent stem cell research. Cell J. 2015;17:59–70. doi: 10.22074/cellj.2015.512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang C, Wang X, Tang X, Wang R, Bao X. Stem-Cell Research of Parkinson Disease: Bibliometric Analysis of Research Productivity from 1999 to 2018. World Neurosurg. 2020;134:e405–e411. doi: 10.1016/j.wneu.2019.10.087. [DOI] [PubMed] [Google Scholar]
- 18.Dayal D, Gupta BM, Gupta S. Quantitative and qualitative assessment of Indian research yield in type 1 diabetes during 1996–2019. J Diabetol. 2021;12:28–35. [Google Scholar]
- 19.Dayal D, Gupta BM, Gupta S, Gupta A. Type 1 Diabetes in Children: A Scientometric Assessment of Indian Research Output from 1990 to 2019. Int J Diabetes Dev Ctries. 2021;41:404–411. doi: 10.1007/s13410-021-00919-7. [DOI] [Google Scholar]
- 20.Editorial Office of Chinese Journal of Tissue Engineering Research Hot-spots and prospects of stem cell therapy for type 2 diabetes: bibliometric and visual analysis based on publications, clinical registries, drug approval information, and patent information in China and the United States. Clin Trials Degener Dis. 2020;5:5–17. [Google Scholar]
- 21.Dayal D, Gupta BM, Raviteja KV, Pal R, Dhawan SM. Research on type 2 diabetes in India during 1982 to 2019: a comprehensive bibliometric assessment. J Diabetol. 2021;12:472–9. [Google Scholar]
- 22.Surkis A, Spore S. The relative citation ratio: what is it and why should medical librarians care? J Med Libr Assoc. 2018;106:508–513. doi: 10.5195/jmla.2018.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Génova G, de la Vara JL. The Problem Is Not Professional Publishing, But the Publish-or-Perish Culture. Sci Eng Ethics. 2019;25:617–619. doi: 10.1007/s11948-017-0015-z. [DOI] [PubMed] [Google Scholar]
- 24.Sandström U, van den Besselaar P. Quantity and/or Quality? The Importance of Publishing Many Papers. PLoS ONE. 2016;11:e0166149. doi: 10.1371/journal.pone.0166149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gupta BM, Sikka P, Gupta S, et al. Indian Research in Gestational Diabetes Mellitus During the Past Three Decades: A Scientometric Analysis. J Obstet Gynaecol India. 2021;71:254–261. doi: 10.1007/s13224-021-01444-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lakhotia SC. Research Fund Crunch, Real or Created, is Hitting India’s Academia on the Wrong Side. Proc Indian Natn Sci Acad. 2018;84:545–547. [Google Scholar]
- 27.Dayal D, Gupta BM, Gupta A. Thyroid disorders in children and adolescents: systematic mapping of global research over the last three decades. Thyroid Res Pract. 2021;18:23–30. doi: 10.4103/trp.trp_5_21. [DOI] [Google Scholar]
- 28.Global Burden of Disease Health Financing Collaborator Network Past, present, and future of global health financing: a review of development assistance, government, out-of-pocket, and other private spending on health for 195 countries, 1995–2050. Lancet. 2019;393:2233–2260. doi: 10.1016/S0140-6736(19)30841-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu W. China’s SCI-Indexed Publications: Facts, Feelings, and Future Directions. ECNU Rev Educ. 2020;3:562–569. doi: 10.1177/2096531120933902. [DOI] [Google Scholar]
- 30.Dave SD, Vanikar AV, Trivedi HL, Thakkar UG, Gopal SC, Chandra T. Novel therapy for insulin-dependent diabetes mellitus: infusion of in vitro-generated insulin-secreting cells. Clin Exp Med. 2015;15:41–45. doi: 10.1007/s10238-013-0266-1. [DOI] [PubMed] [Google Scholar]
- 31.Khand BK, Bhonde RR. Can Functionally Mature Islet β-Cells be Derived from Pluripotent Stem Cells? A Step Towards Ready-To-Use β-Cells in Type 1 Diabetes. Curr Stem Cell Res Ther. 2021;16:231–237. doi: 10.2174/1574888X15666200621171726. [DOI] [PubMed] [Google Scholar]
- 32.Bhansali A, Asokumar P, Walia R, Bhansali S, Gupta V, Jain A, Sachdeva N, Sharma RR, Marwaha N, Khandelwal N. Efficacy and safety of autologous bone marrow-derived stem cell transplantation in patients with type 2 diabetes mellitus: a randomized placebo-controlled study. Cell Transplant. 2014;23:1075–1085. doi: 10.3727/096368913X665576. [DOI] [PubMed] [Google Scholar]
- 33.Bhansali S, Dutta P, Kumar V, Yadav MK, Jain A, Mudaliar S, Bhansali S, Sharma RR, Jha V, Marwaha N, Khandelwal N, Srinivasan A, Sachdeva N, Hawkins M, Bhansali A. Efficacy of Autologous Bone Marrow-Derived Mesenchymal Stem Cell and Mononuclear Cell Transplantation in Type 2 Diabetes Mellitus: A Randomized. Placebo-Controlled Comparative Study Stem Cells Dev. 2017;26:471–481. doi: 10.1089/scd.2016.0275. [DOI] [PubMed] [Google Scholar]
- 34.Rahman MM, Ghoshal UC, Ragunath K, Jenkins G, Rahman M, Edwards C, Hasan M, Taylor-Robinson SD. Biomedical research in developing countries: Opportunities, methods, and challenges. Indian J Gastroenterol. 2020;39:292–302. doi: 10.1007/s12664-020-01056-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Haregu TN, Byrnes A, Singh K, et al. A scoping review of non-communicable disease research capacity strengthening initiatives in low and middle-income countries. Glob Health Res Policy. 2019;4:31. doi: 10.1186/s41256-019-0123-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hariton E, Locascio JJ. Randomised controlled trials - the gold standard for effectiveness research: Study design: randomised controlled trials. BJOG. 2018;125:1716. doi: 10.1111/1471-0528.15199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu WL, Gao H, Yu XL, et al. Umbilical cord mesenchymal stem cells transplantation for newly-onset type 1 diabetes. J Clin Rehabilitative Tissue Eng Res. 2011;15:4363–4366. [Google Scholar]
- 38.AlRyalat SAS, Malkawi LW, Momani SM. Comparing Bibliometric Analysis Using PubMed, Scopus, and Web of Science Databases. J Vis Exp 2019;152:10.3791/58494. [DOI] [PubMed]
- 39.Baas J, Schotten M, Plume A, Côté G, Karimi R. Scopus as a curated, high-quality bibliometric data source for academic research in quantitative science studies. Quant Sci Stud. 2020;1:377–386. doi: 10.1162/qss_a_00019. [DOI] [Google Scholar]
- 40.Perryman CL. Mapping studies. J Med Libr Assoc. 2016;104:79–82. doi: 10.3163/1536-5050.104.1.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.