Abstract
Background
Dyslipidemia is the major contributor to the global disease burden. Earlier epidemiologic research has linked early-life famine exposure to dyslipidemia and altered lipid profiles in adulthood, but a uniform perspective has yet to be established. In response, this systematic review and meta-analysis is aimed to investigate the association of early life famine exposure and dyslipidemia in adults.
Methods
Scopus, Medline and Google scholar databases were searched for articles published until October 2020. Studies of famine exposure during prenatal and early postnatal life and their association with dyslipidemia and lipid profiles in adults were included. Random effect model in the Meta-analysis and Mantel– Haenszel model was used to calculate odds ratios and their 95% confidence intervals to evaluate the strength of association between famine exposure and dyslipidemia. The lipid profiles of the exposed and non-exposed groups were compared using the standardized mean difference (SMD). Heterogeneity between studies were assessed using I2 values.
Results
We identified 17 studies for assessing the association between early life famine exposure and risk of dyslipidemia in adults. About 11 studies were included for meta-analysis. Prenatal exposure to famine was associated with increased risk of dyslipidemia [OR = 1.74 (95% CI: 1.31, 2.31)], total cholesterol [SMD = 2.07 (95% CI: 1.40, 2.74)], LDL-cholesterol [SMD = 1.16 (95% CI: 0.25, 0.26)] and decreased HDL-cholesterol [SMD = −0.05 (95% CI: −0.10, −0.01)]. Likewise, famine exposure during early postnatal period was associated with increased risk of total cholesterol [SMD = 0.18 (95% CI: 0.11, 0.25), I2 = 29%] and LDL-cholesterol [SMD = 0.15 (95% CI: 0.07, 0.23), I2 = 61%].
Conclusions
Famine exposure in early life was found to have an association with increased risk of dyslipidemia and altered lipide profile during adulthood. Our findings highlight the need for promoting better nutrition during pregnancy and infancy to prevent dyslipidemia during adulthood.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01062-8.
Keywords: Famine exposure, Dyslipidemia, Systematic review, meta-analysis, Adulthood
Introduction
Dyslipidemia is characterized by aberrant lipoprotein metabolism, which manifests as high total cholesterol (TC), low-density lipoprotein cholesterol (LDL-C), triacylglycerol (TAG), and/or low high-density lipoprotein cholesterol (HDL-C) (HDL-C). Elevated TC, LDL-C, and TAG levels, as well as lower HDL-C levels, are all risk factors for cardiovascular disease [1]. Although it is believed that more than half of the adult population worldwide has dyslipidemia, the prevalence of dyslipidemia differs widely [2].
The available evidences indicated that adverse health outcomes during adulthood have a multifactorial etiology, including genetic predisposition and lifestyle factors such as sedentary life, alcohol drinking, cigarette smoking and dietary factors [3–5]. However, recent evidences are mounting on the impact of early life adversaries including nutritional deprivation on adulthood health and diseases [6, 7]. According to the Developmental Origins of Health and Disease (DOHaD) hypothesis, exposure to nutritional deprivation during intrauterine or early postnatal life leads to structural and functional changes and increases the risk of developing dyslipidemia and altered lipide profile later in life [8–10]. These periods are exceptional periods where the body employs reductive adaptive mechanisms to sustain life at the expense of shaping the future adulthood for the worst [11, 12].
In order to generate the best available evidence on the long-term impact of early life famine exposure on adulthood health, a natural study setting is required where the exposure was a natural phenomenon, such as historical global famine. Famine can serve as a natural experimental setting which can provide unique insights into the effect of earl life undernutrition on the development adulthood diseases [13–15].
Previous epidemiological studies have shown that exposure to starvation during early life has increased the risk of adult disease [15–32]. However, the findings were inconsistent and not summarized. Hence, a systematic review and meta-analysis was carried out to summarize and quantify the long-term consequences of prenatal and early postnatal famine exposure and the risk dyslipidemia and altered lipid profile.
Methods
Search strategy and study selection
This review looked at both published and unpublished research to assess if there was a link between early life famine experience and anthropometric measurements in adults. Manual and electronic searches were used to locate the studies. The following databases were used to conduct an electronic search. An electronic search was conducted on Scopus, Medline, and Google Scholar databases. Gray literatures were retrieved using Google. Moreover, a manual search was performed to locate papers from previous studies. The Preferred Reporting Items of Systematic Review and Meta-Analysis (PRISMA) 2020 guideline was used [33], and the following keywords were used to search terms: “Famine” OR “Malnutrition” OR” Starvation“ OR “Hunger” AND “early life” OR “Pregnancy “OR “Fetus “OR “Infant” OR “Child, Preschool” OR “Child” AND “dyslipidemia “OR “Lipid profile” OR “Plasma lipid profiles” OR “Total Cholesterol” OR “Triglycerides” OR “ High-density lipoprotein cholesterol” OR “Low-density lipoprotein cholesterol” AND “Adults” OR “Middle Aged “OR “Aged”. In addition, we searched the reference lists of retrieved articles manually.
The research question was defined by the Participants, Interventions, Comparisons, Outcomes, and Study design (PICOS) criteria. In order to avoid double counting, only the article with the most relevant was included if several articles reported data from the same study population.
Famine exposure status
Prenatal exposure was described as being exposed to famine while still in the womb. Famine exposure during the first two years of life was considered early postnatal exposure. Famine was described as a continuous period of food scarcity, famine, or calorie restriction induced by a natural or man-made disaster. Individuals who had never been exposed to famine while in the womb or during the postnatal period were classified as unexposed.
Inclusion and exclusion criteria
The inclusion criteria were as follows: [1] The original study was an observational one [2] the exposure of interest was famine. [3] the outcome of interest was dyslipidemia, lipid profiles (TC, TG, HDL-C, LDL-C [4] both published and unpublished studies conducted among early life famine exposed adults (aged ≥19 years) in any setting across the world [5] articles published until October 30,2020. Articles were excluded based on the following criteria: [1] animal experiment [2] editorials, letters, reviews, commentaries or interviews. [3] duplicate articles [4] irrelevant for famine exposure and dyslipidemia, lipid profiles [5] undefined famine exposure time [6] articles written in languages other than English [7]. Moreover, the studies that did not fully accessed after accessing abstracts were excluded after at least two email contacts with the primary author. Two investigators independently assessed all listed studies to see if they met the study’s inclusion criteria.
Data extraction and quality assessment
Data were extracted by two independent reviewers using the standardized data extraction tool [34]. We extracted specific details about participant characteristics such as age, study design, sample size, first author, publication year, country of origin, outcome, main findings between famine exposure in early life and adulthood, dyslipidemia/lipid profiles and authors conclusions (Supplementary file 1). The quality of the studies was assessed by Newcastle-Ottawa Quality Assessment Scale on three aspects, including the selection of participants, the comparability of the participating groups, and the outcome assessment [35].
Statistical analysis
Statistical analysis was performed using the Rev. Man 5.3 software (Rev Man 5.3) [36]. Odds Ratio (OR) pooled with 95% CI was determined to assess the strength of the association between exposure to famine and the risk of dyslipidemia. Standardized mean difference (SMD) was used to compare lipide profile difference (TC, LDL-C, TG and HDL-C between exposed and nonexposed groups. The I square value (I2) was used to assess the heterogeneity between studies and the Random Effects Model (REM) was used as a pooling method. The I2 values of 0, 25, 50 and 75%, respectively, represent no, low, moderate, and high heterogeneity, while the P-values of chi-square statistics <0.05 represent significant heterogeneity. Sensitivity analysis was performed by sequential failure of individual studies to further evaluate the source of heterogeneity [37].
Results
The studies’ characteristics
During the literature search, 89 papers were discovered. About 43 articles were duplicated and eliminated, leaving 43 for further research. Eight were eliminated after an assessment of the titles and abstracts. The complete text of the remaining 27 studies was downloaded for a thorough review, and 10 were eliminated. In the current systematic review, the remaining 17 studies have been included. A total of 11 papers were included in the meta-analysis to determine the link between early-life hunger and adult dyslipidemia. Table 1 lists the studies’ specific characteristics in detail. Figure 1 shows flow chart diagrams to demonstrate the process of selecting papers for a systematic review and meta-analysis. The results of the studies quality evaluation were shown in supplementary file 2.
Table 1.
Characteristics of studies reporting the long-term consequences of early life famine exposure on dyslipidemia in adults
| Author/Year | Location | Famine year /duration | Age at enrollment (years) | Famine exposure status | Sample size | Conditions studied | Adjustment for covariates |
|---|---|---|---|---|---|---|---|
| [38] | South Korea | 1950–53/4 year |
Exposed ~59–73 Unexposed ~50–55 |
Prenatal and Postnatal exposed | 25,708 |
Lipide profiles (TC (mmol/l) HDL-c (mmol/l) |
Age; household income, smoking status, drinking status, exercise status |
| [39] | China | 1959–61/3 year |
Exposed ~46–51 Unexposed ~44 |
Prenatal and Postnatal exposed | 4040 |
TC (mmol/l) HDL-c (mmol/l) Dysglycemia Dyslipidemia |
Age, gender |
| [40] | Israel | 1940–44/4 year |
Exposed ~69.4 Unexposed ~69.2 |
Prenatal exposed | 1086 | Dyslipidemia | Adjustment |
| [41] | Dutch | 1944–45/6 months |
Exposed = 58 Unexposed = 57 |
Prenatal exposed | 208 |
Dyslipidemia Lipide profiles (TC (mmol/l) HDL-c (mmol/l) |
Sex, social class and size at birth |
| [42] | Dutch | 1944–45/6 months |
Exposed = 58 Unexposed = 57 |
Prenatal exposed |
Lipid profiles (TC (mmol/l) HDL-c (mmol/l) |
sex, BMI, socioeconomic status, and lipid-lowering medication and fat intake | |
| [43] | Dutch | 1944–45/6 months |
Exposed = 58 Unexposed = 57 |
Prenatal exposed | Plasma lipid profiles | Sex | |
| [44] | China | 1959–61/3 year |
Exposed = 50–55 Unexposed = 47 |
Prenatal and Postnatal exposed | Dyslipidemia | sex and current family economic status | |
| [45] | China | 1959–61/3 year |
Exposed = 53–54 Unexposed = 52 |
Prenatal and Postnatal exposed | Dyslipidemia | sex, smoking, drinking, BMI, hypertension, diabetes, education | |
| [46] | China | 1959–61/3 year |
Exposed = 50–55 Unexposed = 47/1959–1961 |
Prenatal and Postnatal exposed | Dyslipidemia | gender, diabetes, hypertension, BMI, occupation, education, smoking, alcohol drinking, exercise frequency, sleeping time, birth weight, feeding methods, waist-to-hip ratio, and dietary pattern | |
| [47] | Leningrad | 1941–44/ 6 months |
Exposed = 52–53 Unexposed = 52.8 |
Prenatal exposed |
TC HDL-cholesterol LDL-cholesterol Triglyceride |
season of birth, sex | |
| [48] | China | 1959–61/3 year |
Exposed = 50–57 Unexposed = 47 |
Prenatal and Postnatal exposed |
Dyslipidemia Total cholesterol HDL (mg/dl), LDL (mg/dl), TG (mg/dl) |
sex, smoking, drinking, BMI, education | |
| [49] | Dutch | 1944–45/6 months |
Exposed ~58.7 Unexposed ~58.6 |
Prenatal exposed | Dyslipidemia | age at enrollment, socioeconomic status, and cigarette smoking habits | |
| [50] | Israel | 1940–44/4 year |
Exposed ~69.4 Unexposed ~69.2 |
Prenatal exposed | Dyslipidemia | Unadjusted | |
| [51] | China | 1959–61/3 year |
Exposed = 52–93 Unexposed = 40–51 |
Prenatal and Postnatal exposed |
Lipid profiles (TC, HDL-cholesterol LDL-cholesterol, Triglyceride) |
age, smoking, rural/urban residence, economic status, diabetes and hypertension | |
| [52] | Dutch | 1944–45/6 months |
Exposed = 58 Unexposed = 57 |
Prenatal exposed |
Lipid profiles (TC, HDL-cholesterol LDL-cholesterol) |
Age, sex, BMI, waist circumference, education, current smoking habit, alcohol use, and prevalent hypertension | |
| [53]/ China | China | 1959–61/3 year |
Exposed = 50–51 Unexposed = 48 |
Prenatal and Postnatal exposed | 1529 |
Lipid Profiles (TG, mmol/L, HDL-C, mmol/L, TC/HDL-C, TG/HDL-C) |
sex, smoking, drinking, BMI, socio-economic status, education |
Prenatal exposed: Exposed to famine in the intrauterine life
Postnatal exposed: Exposed during the first two years of life (1–2 years during famine)
AOR adjusted odds ratio, TC Total cholesterol, HDL-c High density lipoprotein – cholesterol, LDL low density lipoprotein cholesterol
Fig. 1.
Flow diagram of studies included in the systematic review and meta-analysis of famine exposure in early life and dyslipidemia in adults
Meta-analysis
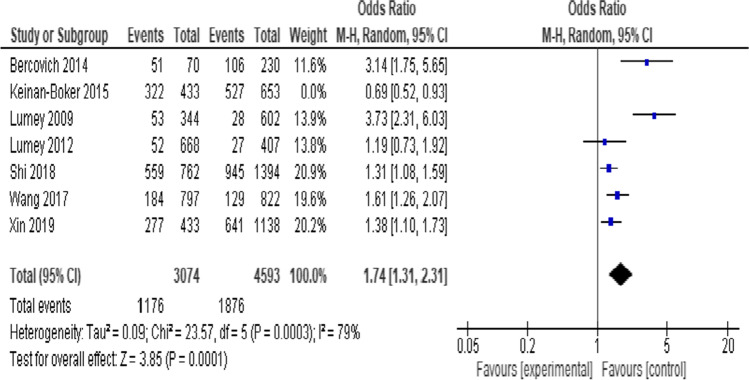
Prenatal exposure to famine was associated with higher odds of dyslipidemia [OR = 1.54 (95% CI: 1.11, 1.2.14), I2 = 87%] (Supplementary file 3). In order to explore the source of heterogeneity, a sensitivity analysis was performed. The I2 remained high after removal of Keinan-Boker et al., (2015) [40] with a minimal increase in the effect measure [OR = 1. 74 (95% CI: 1.31, 2.31), I2 = 79%] (Fig. 2). Similary, prenatal exposure was associated with higher TC [SMD = 2.07 (95% CI: 1.40, 2.74), I2 = 100%)] (Fig. 3a), HDL-C [SMD = −0.05 (95% CI: −0.10, −0.01), I2 = 0%] (Fig. 3b), LDL-C [SMD = 1.16 (95% CI: 0.25, 2.06), I2 = 100%] (Fig. 3c), and but not with TG [SMD = 0.12 (95% CI: −0.03, 0.27), I2 = 96%] (Fig. 3d). These results were stable after sensitivity analyses with no change in heterogeneity level.
Fig. 2.
Sensitivity analysis of prenatal famine exposure and risk of dyslipidemia, 2021
Fig. 3.
Forest plot of prenatal famine exposure and risk of (a) total cholesterol (b) HDL-cholesterol (c), LDL-cholesterol (d) triglyceride, 2021
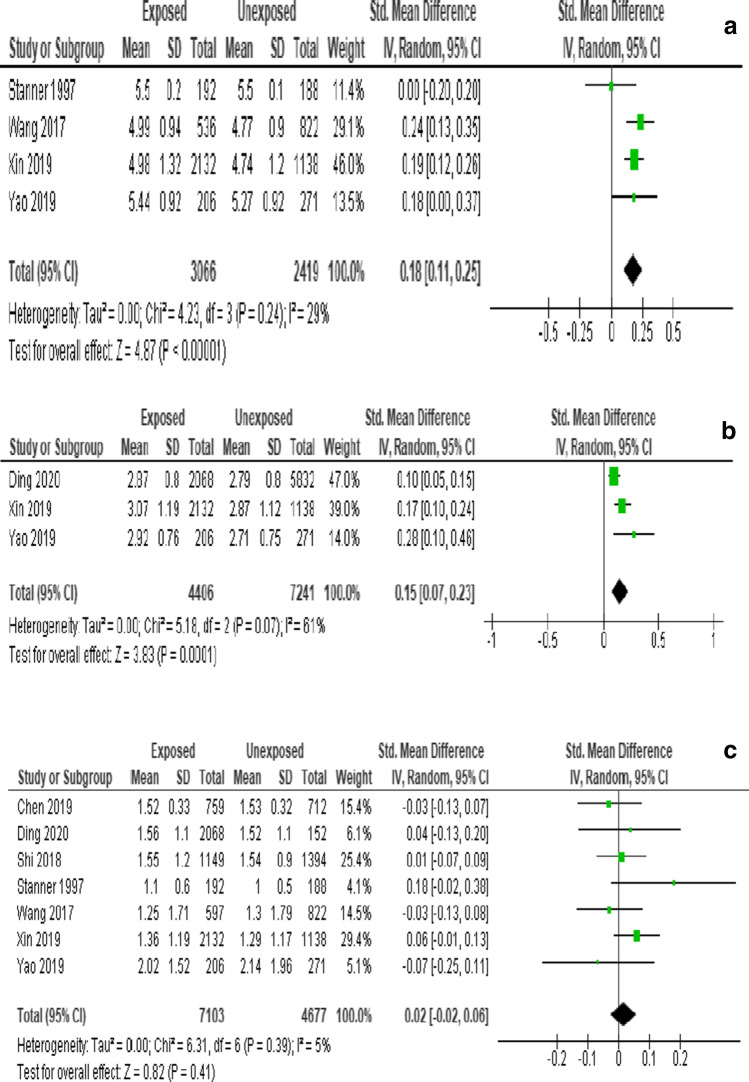
Postnatal famine exposure was associated with increased risk of TC [SMD = 0.18 (95% CI: 0.11, 0.25), I2 = 29%] (Fig. 4a) and LDL-C [SMD = 0.15 (95% CI: 0.07, 0.23), I2 = 61%] (Fig. 4b). However, no association was observed between Postnatal exposure and TG [SMD = 0.02 (95% CI: −0.02, 0.06), I2 = 5%] (Fig. 4c).
Fig. 4.
Forest plot of childhood famine exposure and risk of (a) Total cholesterol (b) LDL-cholesterol (c) triglyceride, 2021
Publication bias
Publication bias was evaluated by funnel plots. As the study sourced out all quality gray literatures, less publication bias was reported for the analysis of dyslipidemia, TC and TG (supplementary file 4).
Discussion
The present review indicates that dyslipidemia and lipid profiles such as TC, LDL-C, TG, or a decreased serum HDL-C were more prevalent among adults exposed to some form of famine in their prenatal or early postnatal years.
The mechanisms of famine exposure during early life and risk of dyslipidemia and altered lipid profiles in adult could be due to changes in liver function. Another potential pathway may be through differences in eating behavior. In Dutch famine study, participants exposed to famine in early gestation demonstrated a preference for fatty foods, which fits with the finding of a more atherogenic lipid profile in this group [42].
The contemporary relevance of our finding indicate the long term effects of earlier famine and undernutrition are far from over [54, 55]. Furthermore, the findings indicated that the association of famine exposure during early life and risk of dyslipidemia could be modified by nutrition transition occurring in many countries which had history of great famines [55]. The shift to excess caloric intake further worsens by sedentary and obesogenic environment which may likely potentiate the effect the impact of childhood adverse nurturing [56].
The study could potentially reveal a possible gender difference in the link between early life famine experience and adult dyslipidemia. Parents in several parts of the world, notably in Asia and Africa, prefer to prioritize sons above daughters [57, 58]. These preferences may result in poor health, increasing their risk of having dyslipidemia later in life. Furthermore, the sex-difference effect could be explained in part by mortality selection, where men died at higher rates than women during famines [58, 59].
Overall, the results of this systematic review and meta-analysis demonstrate that famine exposure during childhood has an impact on long-term dyslipidemia risk. Given the developing metabolic dysfunction in low- and middle-income countries (LMICs), where severe maternal and pediatric malnutrition remains a major problem, this is of worldwide public health importance [60]. As a result, evidence linking early-life nutrition to dyslipidemia and changed lipid profiles in adulthood is a cornerstone of health promotion and public-health nutrition programs [61].
The potential limitations of this study include: First, due to the lack of available data from the original articles, this meta-analysis was unable to analyze the severity of exposure to famine and the risk of dyslipidemia. Second, the duration of famine was not consistent across all of the included studies, ranging from 1 to 4 years, which could influence the stability of our results. Thus, it was likely to be a source of heterogeneity. Third, only published studies have been included in this meta-analysis, so the bias of publication may have occurred despite the lack of bias in the publication of the funnel plot. Fourth, our study was unable to quantify the gender-specific effects of early life famine exposure on adulthood dyslipidemia since the individual study reports were available to the general population. Moreover, this study acknowledges the negative points of meta-analysis, namely the low quality of included studies, heterogeneity among meta-analyzed studies, and failure to address publication bias. These limitations can lead to misleading results, which is critical if policy and practice decisions are based on systematic reviews and meta-analyses.
Conclusions
Findings from the review suggest that undernutrition in early life particularly fetal exposure play an important role in development of dyslipidemia in adulthood. The finding highlights the fundamental importance of ensuring sufficient nutrition during the earliest stages of human development as a prevention strategy of adulthood diseases.
Supplementary Information
Below is the link to the electronic supplementary material.
Authors’ contributions
GA and KHA wrote the manuscript text and TB and DT perform data extraction and prepare figures. All authors have read and approved the final manuscript.
Data availability
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Conflict of interest
The authors have no conflicts of interest to declare.
Competing interests
The author declares that he has no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, et al. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. Jama. 2007;298(7):776–785. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 2.Mendis S, Davis S, Norrving B. Organizational update: the world health organization global status report on noncommunicable diseases 2014; one more landmark step in the combat against stroke and vascular disease. Stroke. 2015;46(5):e121–e1e2. doi: 10.1161/STROKEAHA.115.008097. [DOI] [PubMed] [Google Scholar]
- 3.Collaborators GRF. Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the global burden of disease study 2013. Lancet (London, England) 2015;386(10010):2287. doi: 10.1016/S0140-6736(15)00128-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Azizi F, Ghanbarian A, Momenan AA, Hadaegh F, Mirmiran P, Hedayati M, et al. Prevention of non-communicable disease in a population in nutrition transition: Tehran lipid and glucose study phase II. Trials. 2009;10(1):5. doi: 10.1186/1745-6215-10-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Frayling TM. Genome–wide association studies provide new insights into type 2 diabetes aetiology. Nat Rev Genet. 2007;8(9):657–662. doi: 10.1038/nrg2178. [DOI] [PubMed] [Google Scholar]
- 6.Gluckman PD, Hanson MA. Living with the past: evolution, development, and patterns of disease. Science. 2004;305(5691):1733–1736. doi: 10.1126/science.1095292. [DOI] [PubMed] [Google Scholar]
- 7.Gluckman PD, Hanson MA, Low FM. The role of developmental plasticity and epigenetics in human health. Birth Defects Res C Embryo Today Rev. 2011;93(1):12–18. doi: 10.1002/bdrc.20198. [DOI] [PubMed] [Google Scholar]
- 8.Barker D. Developmental origins of adult health and disease. J Epidemiol Community Health. 2004;58(2):114. doi: 10.1136/jech.58.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silveira PP, Portella AK, Goldani MZ, Barbieri MA. Developmental origins of health and disease (DOHaD) J Pediatr. 2007;83(6):494–504. doi: 10.2223/JPED.1728. [DOI] [PubMed] [Google Scholar]
- 10.McMillen IC, Robinson JS. Developmental origins of the metabolic syndrome: prediction, plasticity, and programming. Physiol Rev. 2005;85(2):571–633. doi: 10.1152/physrev.00053.2003. [DOI] [PubMed] [Google Scholar]
- 11.Levinson DJ. A conception of adult development. American psychologist. 1986;41(1):3.
- 12.Langley-Evans SC. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet. 2015;28(Suppl 1):1–14. doi: 10.1111/jhn.12212. [DOI] [PubMed] [Google Scholar]
- 13.Currie J, Vogl T. Early-life health and adult circumstance in developing countries. Annu Rev Econ. 2013;5(1):1–36. [Google Scholar]
- 14.Roseboom T, de Rooij S, Painter R. The Dutch famine and its long-term consequences for adult health. Early Hum Dev. 2006;82(8):485–491. doi: 10.1016/j.earlhumdev.2006.07.001. [DOI] [PubMed] [Google Scholar]
- 15.Meng X, Qian N. The long term consequences of famine on survivors: evidence from a unique natural experiment using China's great famine. National Bureau of Economic Research; 2009.
- 16.de Rooij SR, Painter RC, Holleman F, Bossuyt PM, Roseboom TJ. The metabolic syndrome in adults prenatally exposed to the Dutch famine. Am J Clin Nutr. 2007;86(4):1219–1224. doi: 10.1093/ajcn/86.4.1219. [DOI] [PubMed] [Google Scholar]
- 17.Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, et al. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017;36(1):253–259. doi: 10.1016/j.clnu.2015.11.010. [DOI] [PubMed] [Google Scholar]
- 18.Finer S, Iqbal MS, Lowe R, Ogunkolade BW, Pervin S, Mathews C, Smart M, Alam DS, Hitman GA. Is famine exposure during developmental life in rural Bangladesh associated with a metabolic and epigenetic signature in young adulthood? A historical cohort study. BMJ open. 2016;6(11):e011768. [DOI] [PMC free article] [PubMed]
- 19.Hult M, Tornhammar P, Ueda P, Chima C, Bonamy EA-K, Ozumba B, et al. 12 hypertension, diabetes and overweight: looming legacies of the Biafran famine. Pediatr Res. 2010;68(1):8–9. doi: 10.1371/journal.pone.0013582. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koupil I, Shestov DB, Sparén P, Plavinskaja S, Parfenova N, Vågerö D. Blood pressure, hypertension and mortality from circulatory disease in men and women who survived the siege of Leningrad. Eur J Epidemiol. 2007;22(4):223–234. doi: 10.1007/s10654-007-9113-6. [DOI] [PubMed] [Google Scholar]
- 21.Neelsen S, Stratmann T. Effects of prenatal and early life malnutrition: evidence from the Greek famine. J Health Econ. 2011;30(3):479–488. doi: 10.1016/j.jhealeco.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 22.Koupil I, Plavinskaja S, Parfenova N, Shestov DB, Danziger PD, Vågerö D. Cancer mortality in women and men who survived the siege of Leningrad (1941–1944) Int J Cancer. 2009;124(6):1416–1421. doi: 10.1002/ijc.24093. [DOI] [PubMed] [Google Scholar]
- 23.Vaiserman A. Early-life nutritional programming of type 2 diabetes: experimental and quasi-experimental evidence. Nutrients. 2017;9(3):236. doi: 10.3390/nu9030236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang Y, Ying Y, Zhou L, Fu J, Shen Y, Ke C. Exposure to Chinese famine in early life modifies the association between hyperglycaemia and cardiovascular disease. Nutr Metab Cardiovasc Dis. 2019;29(11):1230–1236. doi: 10.1016/j.numecd.2019.07.004. [DOI] [PubMed] [Google Scholar]
- 25.Li C, Miles T, Shen L, Shen Y, Liu T, Zhang M, et al. Early-life exposure to severe famine and subsequent risk of depressive symptoms in late adulthood: the China health and retirement longitudinal study. Br J Psychiatry. 2018;213(4):579–586. doi: 10.1192/bjp.2018.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.van Abeelen AF, Elias SG, Roseboom TJ, Bossuyt PM, van der Schouw YT, Grobbee DE, et al. Postnatal acute famine and risk of overweight: the dutch hungerwinter study. Int J Pediatr. 2012;2012. [DOI] [PMC free article] [PubMed]
- 27.Lumey L, Khalangot MD, Vaiserman AM. Association between type 2 diabetes and prenatal exposure to the Ukraine famine of 1932–33: a retrospective cohort study. Lancet Diabetes Endocrinol. 2015;3(10):787–794. doi: 10.1016/S2213-8587(15)00279-X. [DOI] [PubMed] [Google Scholar]
- 28.Thurner S, Klimek P, Szell M, Duftschmid G, Endel G, Kautzky-Willer A, et al. Quantification of excess risk for diabetes for those born in times of hunger, in an entire population of a nation, across a century. Proc Natl Acad Sci. 2013;110(12):4703–4707. doi: 10.1073/pnas.1215626110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang N, Ning Z, Xia F, Chen C, Cheng J, Chen Y, et al. Exposure to famine in early life and chronic kidney diseases in adulthood. Nutr Diabetes. 2018;8(1):1–7. doi: 10.1038/s41387-017-0014-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Shi Z, Zhang C, Zhou M, Zhen S, Taylor AW. Exposure to the Chinese famine in early life and the risk of anaemia in adulthood. BMC Public Health. 2013;13:904. doi: 10.1186/1471-2458-13-904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang W, Luan R. Early-life exposure to the Chinese famine of 1959-61 and risk of hyperuricemia: results from the China health and retirement longitudinal study. BMC Public Health. 2020;20(1):15. doi: 10.1186/s12889-019-8017-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Elias SG, Peeters PH, Grobbee DE, van Noord PA. The 1944-1945 Dutch famine and subsequent overall cancer incidence. Cancer Epidemiol Prev Biomark. 2005;14(8):1981–1985. doi: 10.1158/1055-9965.EPI-04-0839. [DOI] [PubMed] [Google Scholar]
- 33.Moher D, Liberati A, Tetzlaff J, Altman DG, Group P Reprint—preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Phys Ther. 2009;89(9):873–880. [PubMed] [Google Scholar]
- 34.Munn Z, Tufanaru C, Aromataris E. JBI's systematic reviews: data extraction and synthesis. Am J Nurs. 2014;114(7):49–54. doi: 10.1097/01.NAJ.0000451683.66447.89. [DOI] [PubMed] [Google Scholar]
- 35.Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010;25(9):603–605. doi: 10.1007/s10654-010-9491-z. [DOI] [PubMed] [Google Scholar]
- 36.Review Manager (RevMan)[Computer Program] Version 5.2. 3, The Nordic Cochrane Centre, Copenhagen; 2012.
- 37.Patsopoulos NA, Evangelou E, Ioannidis JP. Sensitivity of between-study heterogeneity in meta-analysis: proposed metrics and empirical evaluation. Int J Epidemiol. 2008;37(5):1148–1157. doi: 10.1093/ije/dyn065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Han C, Hong YC. Fetal and childhood malnutrition during the Korean War and metabolic syndrome in adulthood. Nutrition. 2019;62:186–93. [DOI] [PubMed]
- 39.Zheng X, Wang Y, Ren W, Luo R, Zhang S, Zhang J, ZENG Q. Risk of metabolic syndrome in adults exposed to the great Chinese famine during the fetal life and early<br>childhood. European journal of clinical nutrition. 2012;66:231. [DOI] [PubMed]
- 40.Keinan-Boker L, Shasha-Lavsky H, Eilat-Zanani S, Edri-Shur A, Shasha SM. Chronic health conditions in Jewish holocaust survivors born during world war II. Israel Med Assoc J. 2015;17(4):206–212. [PubMed] [Google Scholar]
- 41.de Rooij SR, Painter RC, Roseboom TJ, Phillips D, Osmond C, Barker D, et al. Glucose tolerance at age 58 and the decline of glucose tolerance in comparison with age 50 in people prenatally exposed to the Dutch famine. Diabetologia. 2006;49(4):637–643. doi: 10.1007/s00125-005-0136-9. [DOI] [PubMed] [Google Scholar]
- 42.Lussana F, Painter RC, Ocke MC, Buller HR, Bossuyt PM, Roseboom TJ. Prenatal exposure to the Dutch famine is associated with a preference for fatty foods and a more atherogenic lipid profile. Am J Clin Nutr. 2008;88(6):1648–1652. doi: 10.3945/ajcn.2008.26140. [DOI] [PubMed] [Google Scholar]
- 43.Roseboom TJ, van der Meulen JH, Osmond C, Barker DJ, Ravelli AC, Bleker OP. Plasma lipid profiles in adults after prenatal exposure to the Dutch famine. Am J Clin Nutr. 2000;72(5):1101–1106. doi: 10.1093/ajcn/72.5.1101. [DOI] [PubMed] [Google Scholar]
- 44.Wang N, Wang X, Li Q, Han B, Chen Y, Zhu C, Chen Y, Lin D, Wang B, Jensen MD, LU Y. The famine exposure in early life and metabolic syndrome in adulthood. Clin Nutr. 2017c;36:253–259. [DOI] [PubMed]
- 45.Xin X, Wang W, Xu H, Li Z, Zhang D. Exposure to Chinese famine in early life and the risk of dyslipidemia in adulthood. Eur J Nutr. 2019;58(1):391–398. doi: 10.1007/s00394-017-1603-z. [DOI] [PubMed] [Google Scholar]
- 46.Yao H, LI L. Famine exposure during the fetal period increased the risk of dyslipidemia in female adults. Lipids. 2019;54:301–309. [DOI] [PubMed]
- 47.Stanner SA, Bulmer K, Andres C, Lantseva OE, Borodina V, Poteen VV, Yudkin JS. Does malnutrition in utero determine diabetes and coronary heart disease in adulthood? Results from the Leningrad siege study, a cross sectional study. Bmj. 1997 Nov 22;315(7119):1342–8. [DOI] [PMC free article] [PubMed]
- 48.Shi Z, Nicholls SJ, Taylor AW, Magliano DJ, Appleton S, Zimmet P. Early life exposure to Chinese famine modifies the association between hypertension and cardiovascular disease. J Hypertens. 2018;36(1):54–60. doi: 10.1097/HJH.0000000000001496. [DOI] [PubMed] [Google Scholar]
- 49.Lumey L, Martini LH, Myerson M, Stein AD, Prineas RJ. No relation between coronary artery disease or electrocardiographic markers of disease in middle age and prenatal exposure to the Dutch famine of 1944–5. Heart. 2012;98:1653–1659. [DOI] [PubMed]
- 50.Bercovich E, Keinan-Boker L, Shasha SM. Long-term health effects in adults born during the holocaust. Israel Med Assoc J. 2014;16(4):203–207. [PubMed] [Google Scholar]
- 51.Chen C, Zhao L, Ning Z, Li Q, Han B, Cheng J, Chen Y, Nie X, Xia F, Wang N, Lu Y. Famine exposure in early life is associated with visceral adipose dysfunction in adult females. European journal of nutrition. 2019;58(4):1625–33. [DOI] [PubMed]
- 52.Lumey LH, Stein AD, Kahn HS, Romijn J. Lipid profiles in middle-aged men and women after famine exposure during gestation: the Dutch Hunger Winter Families Study. The American journal of clinical nutrition. 2009;89(6):1737-43. [DOI] [PMC free article] [PubMed]
- 53.Ding X-Y, Yang Z-Y, Zhao L-Y, Zhao W-H. Are lipid profiles in middle age associated with famine exposure during prenatal and early postnatal period? Nutrients. 2020;12(8):2266. doi: 10.3390/nu12082266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Popkin BM, Corvalan C, Grummer-Strawn LM. Dynamics of the double burden of malnutrition and the changing nutrition reality. Lancet. 2020;395(10217):65–74. doi: 10.1016/S0140-6736(19)32497-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Black RE, Alderman H, Bhutta ZA, Gillespie S, Haddad L, Horton S, et al. Maternal and child nutrition: building momentum for impact. Lancet. 2013;382(9890):372–375. doi: 10.1016/S0140-6736(13)60988-5. [DOI] [PubMed] [Google Scholar]
- 56.Popkin BM. Measuring the nutrition transition and its dynamics. Public Health Nutr. 2021;24(2):318–320. doi: 10.1017/S136898002000470X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mu R, Zhang X. Why does the great Chinese famine affect the male and female survivors differently? Mortality selection versus son preference. Econ Hum Biol. 2011;9(1):92–105. doi: 10.1016/j.ehb.2010.07.003. [DOI] [PubMed] [Google Scholar]
- 58.Hesketh T, Xing ZW. Abnormal sex ratios in human populations: causes and consequences. Proc Natl Acad Sci. 2006;103(36):13271–13275. doi: 10.1073/pnas.0602203103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lothe EA, Heggen K. A study of resilience in young Ethiopian famine survivors. J Transcult Nurs. 2003;14(4):313–320. doi: 10.1177/1043659603257161. [DOI] [PubMed] [Google Scholar]
- 60.Black RE, Victora CG, Walker SP, Bhutta ZA, Christian P, De Onis M, et al. Maternal and child undernutrition and overweight in low-income and middle-income countries. Lancet. 2013;382(9890):427–451. doi: 10.1016/S0140-6736(13)60937-X. [DOI] [PubMed] [Google Scholar]
- 61.Langley-Evans S. Nutrition in early life and the programming of adult disease: a review. J Hum Nutr Diet. 2015;28:1–14. doi: 10.1111/jhn.12212. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.