Abstract
Background
One of the most common metabolic diseases, Type 2 Diabetes Mellitus (T2DM), is caused by a combination of two basic factors: insufficient insulin secretion by pancreatic -cells or a failure of insulin-sensitive tissues to respond adequately to insulin. The aim of this paper was to assess the diagnostic accuracy of serum nesfatin-1 in type 2 diabetes mellitus (T2DM).
Methods
Sixty patients with T2DM were recruited from (Baquba Teaching Hospital) in Iraq during the period (from November 2021 - March 2022). T2DM group was classified into 30 newly diagnosis patients (without treatment) and 30 ongoing diabetic patients (with treatment) for comparison, as well 30 healthy individuals were included as a control. The ELISA Kit was used to measure serum nesfatin-1 and serum insulin, fasting serum glucose, and lipid profile test were measured through spectrophotometric, instead of HbA1c was determined using HPLC method.
Results
The concentration of serum nesfatin-1 in the T2DM group was significantly lower than that of the healthy subjects (p < 0.05). There was a significant difference in the serum nestafin-1 concentrations between newly diagnosis and ongoing T2DM patients. There were substantial negative connections between serum Nesfatin-1 concentration and HOMA-IR, as well as strong negative correlations between serum nesfatin-1 and serum insulin level. The concentration of serum Nesfatin-1, on the other hand, had no significant association with the anthropometries measurements and biochemical parameters. The area under the curve was excellent (AUC = 0.827, p = 0.0001), with high diagnostic accuracy (86.2) in differentiating newly diagnosis T2DM from the healthy subject group.
Conclusion
Nesfatin-1 levels in the sera of diabetic patients was shown to be lower and nesfatin-1 levels were shown to be significantly linked to newly diagnosed patients.
Keywords: Nesfatin-1, Type 2 diabetes mellitus, HOMA-IR, Serum insulin
Highlight
• Nesfatin-1 is a novel biomarker candidate in type 2 diabetes mellitus.
• Nesfain-1 can be used to differentiate T2DM from healthy subject.
• The nesfatin-1 change depends on the severity of diseases (disease severity depended on increased HOMA-IR) of T2DM.
Introduction
Diabetes mellitus is one of the most difficult health problems of the twenty-first century [1]. High blood glucose levels cause considerable damage to various organs, including the heart, kidney, liver, nerve, eyes, and arteries [2]. Moreover, insulin resistance is a metabolic defect of diabetes type 2 that has emerged as a new global reaction to the epidemic [3]. Furthermore, the function of adipocytes as protein-secreting cells has long been recognized, and the bulk of these proteins, known as adipokines, have biological significance [4]. Only a few adipokines, on the other hand, have been proven to influence insulin sensitivity, either directly or indirectly. The adipokine nesfatin-1 regulates insulin sensitivity [5].
Nesfatin-1may reveal a mechanistic link between Insulin resistance and T2DM, the adipokine nesfatin-1 affects glucose metabolism, phosphorylation of particular signaling proteins via AMP-activated protein kinase, and increases liver insulin sensitivity, all of which help to regulate hunger and body fat storage [6]. This adipokine is found in brain regions that are involved with metabolic regulation and eating behavior [7]. Nesfatin-1 has also been shown to have an anti-hyperglycemic impact in glucose homeostasis in recent studies [8]. According to numerous studies, nesfatin-1 regulates insulin sensitivity in the brain [9]. Furthermore, nesfatin-1 has been shown to stimulate insulin release in beta cells under hyperglycemic conditions [8], and it can cross the brain-blood barrier bidirectional in a non-saturable way [10]. These findings suggest that circulating nesfatin-1 plays an important role in energy balancing, although the potential explanations of these contradictory results have been poorly characterized. The results of studies on the association between diabetes and nesfatin-1 have been contradictory. In certain investigations, patients with T 2 DM had low nesfatin-1 levels, while individuals with Type 1 DM had high nesfatin-1 levels [11]. Other studies, on the other hand, have found increased nesfatin-1 levels in people with Type 2 DM and IGT [12]. To our knowledge, no extensive research in patients with newly diagnosed (without treatment) and nesfatin-1 levels have been done. It’s crucial to figure out if nesfatin-1 can be utilized as a supplement in the diagnosis and treatment of T2DM. The goal of this study was to clarify the association serum nesfatin-1 levels and T2DM by compared the levels of nesfatin-1 in patients with newly diagnosed (without treatment), ongoing ((with treatment ) T2 DM and healthy people.
Materials and methods
T2DM patients
Sixty diabetic patients, ranging in age from (37–70 years) were divided into two groups: (30) newly diagnosed diabetic with mean age (45.81 ± 8.16) years and (30) ongoing diabetic patients with mean age (49.33 ± 6.64) years. Type I DM, metabolic syndrome, POC, pregnant women, smokers, liver disease, kidney disease, and hypertensive were all excluded from this study.
Healthy subjects
The study included thirty healthy control subjects (m = 10, f = 20) who were comparable to type 2 diabetes mellitus patients in terms of age (37–70 years) and gender (male and female). The controls were chosen based on the following physician criteria: Non-diabetic, non-hypertensive, and clear of acute disease, with no history of alcohol consumption or smoking.
Sample collection
Ten ml of blood have been collected from each patient and control. The samples were taken between 8.00 and 11.00 A.M after 12–15 h of fasting each blood sample divided into two parts. For the first part, ethylene di amine tetra acetic acid (EDTA) (1.5 mg/ml) was used to estimate HbA1c in less than three hours, while the second part was utilized to collect serum by distributing the sample in a simple tube and letting it coagulate at room temperature (22°c). Then the serum was collected after centrifuging the sample at 3000r.p.m. The collected serum was divided into several parts each containing 500 µl in eppendorf tubes and stored in the freezer (-20° c) until use.
Anthropometries measurements
Anthropometric measurement including age, weight, height, waist circumference (WC) and waist circumference to height ratio (WHtR) was determined
The Body Mass Index (BMI) is determined using a formula that contains the basic equation of weight divided by the square of height. There are various abdominal adiposity markers, one of which is the waist to height ratio, which is thought to be a better indication of metabolic and cardiovascular disease than BMI. The WHtR = waist in centimeters divided by height in (centimeters) (WHtR = Waist/Height). The value of this ratio of ≥ 0.55 highlights cardiovascular disease risk [13].
Assessment of the Homeostasis Model Assessment (HOMA-IR)
Insulin resistance (IR) was measured using a variety of approaches, the most frequent of which included calculating the homeostasis model assessment (HOMA) using fasting insulin (U/ml) and glucose (mg/dl), as indicated in the equation below. Insulin resistance is a critical topic to investigate since it affects the balance of various metabolic pathways [14].
Measurement of Atherogenic Index of Plasma (AIP)
It was calculated from the molar ratio of the logarithm of triglyceride to the high-density lipoprotein [15].
Clinical laboratory analysis of groups
An ELISA plate reader was used to determine the quantities of Nestafin-1 (My Bio Source Inc., San Diego, CA, USA) and Insulin (Demeditec Diagnostic GmbH, Germany) (human reader HS and comb wash from Human GMBh, Germany). The serum fasting blood sugar and lipid profile tests were assessed using an automated chemical analyzer COBSe 411, Germany).
Ethical approval
The Baquba Teaching Hospital Committee of Ethics, and the Iraqi Ministry of Health's National Centre for Training and Human Development, all gave their permission to this study. All participants gave informed written permission before being included in the study, and the research was carried out in accordance with the Helsinki Declaration.
Statistical analysis
SPSS was used to conduct the statistical analysis (version 25). For normally distributed numerical variables, the mean and standard deviation were used, and for categorical variables, the frequency/percentage was used. The significance of the difference between the typically numerical variables was further tested using an independent t-test and an ANOVA test. The significance level was chosen at p 0.05, and the Pearson correlation was determined using the t-test to examine the significance of correlation for the link between the two quantitative variables. The utilization of Nesfatin-1 as a diagnostic marker or disease-screening tool was evaluated using a receiver operating characteristic (ROC) curve approach, as well as the best value of the serum Nesfatin-1 concentration cut-off that has the best specificity and sensitivity for diagnosing a disease. The “area under the curve” (AUC) description of the ROC area was as follows: 0.90 (“Perfect”), 0.80 (“Good”), 0.70 (“Fair”), 0.60 (“Poor”), 0.60 (“Failure”) [16].
Anthropometries and biochemical parameters of T2DM patient groups and healthy subjects
Table 1 shows the average age distribution of T2DM patients (43.5 ± 10.7) and healthy subjects (43.6 ± 4.7), with p 0.05 for both groups. The mean BMI of T2DM patients (28.7 ± 3.76 kg/m2) was similar to that of healthy subjects (29.2 ± 3.7 kg/m2), with no statistically significant difference (p > 0.05). Table 1 summarizes other sociodemographic and distinctive aspects of the T2DM and healthy subject groups. The results from the subgroups consist of 30 newly diagnosis T2DM and 30 ongoing diabetics patients as shown in Table 2.
Table 1.
Anthropometries and bio chemical parameters of T2DM patient groups and healthy subjects
| Variables | T2DM patients (n = 60) | Healthy subject (n = 30) | p- Value |
|---|---|---|---|
| Age mean ± SE | 49.295 ± 2.262 | 48.18 ± 3.60 | 0.876 |
| Sex n. (%) | |||
|
Male Female |
31(51.67% 29(48.33%) |
18(60%) 12(40%) |
0.321 0.211 |
| BMI mean ± SE | 28.88 ± 0.67 | 27.43 ± 0.90 | 0.170 |
| WHR | 1.09 ± 0.05 | 0.89 ± 0.03 | 0.538 |
| WHtR | 0.66 0.08± | 0.56 ± 0.02 | 0.547 |
| Disease duration (years) | 7.98 ± 7.12 | - | - |
| FSB | 216.3 ± 15.25 | 96.43 ± 6.10 | 0.000 |
| HbA1C% | 8.215 ± 0.12 | 5.72 ± 0.29 | 0.000 |
|
Insulin Median(Geometric mean) |
20.4949 ± 2.5 | 14.21 ± 2.12 | 0.000 |
|
HOMA-IR Median (Geometric mean) |
12.60 ± 1.765 | 3.83 ± 0.709 | 0.001 |
| Cholesterol | 194.1 ± 4.8 | 180.07 ± 8.96 | 0.136 |
| Triglyceride | 204.13 ± 12.1 | 178.27 ± 19.8 | 0.246 |
| HDL-C | 42.8 ± 1.02 | 42.56 ± 1.5 | 0.890 |
| LDL-C | 115.9 ± 5.6 | 103.0 ± 7.5 | 0.182 |
| VLDL-C | 39.71 ± 2.10 | 35.47 ± 3.94 | 0.299 |
The collected data were analyzed by either mean ± SD, median (25th and 75th Percentiles) and no (%)
Age and period of diseases in years
Table 2.
Anthropometries and biochemical parameters of the subgroups of T2DM patients and healthy subjects
| Variables | newly diagnosed T2DM (n = 30) | ongoing T2DM (n = 30) | Healthy subject (n = 30) | p- Value |
|---|---|---|---|---|
| Age mean ± SE | 44.36 ± 2.39 | 54.23 ± 2.13438 | 48.18 ± 3.60 | 0.876 |
| Sex n. (%) | ||||
|
Male Female |
19 (63%) 11(36%) |
18(60%) 12(40%) |
18(60%) 12(40%) |
0.321 0.211 |
| BMI mean ± SE | 29.13 ± 0.68 | 28.63 ± 0.67 | 27.43 ± 0.90 | 0.343 |
| WHR | 1.09 ± 0.05 | 1.10 ± 0.074 | 0.89 ± 0.03 | 0.828 |
| WHtR | 0.66 ± 0.08 | 0.53 ± 0.02 | 0.56 ± 0.02 | 0.177 |
| FSB |
175.40 ± 13.31 b** |
257.20 ± 17.50 a*** c*** |
96.43 ± 6.10 | 0.000 |
| HbA1C | 7.30 ± 0.33 b** |
9.13 ± 0.42 a*** c** |
5.72 ± 0.29 | 0.000 |
| Insulin | 20.21 ± 3.30 |
26.53 ± 3.90 a** |
10.64 ± 1.23 | 0.014 |
| HOMA-IR |
10.10 ± 2.21 b* |
17.25 ± 2.97 a* |
2.53 ± 0.32 | 0.000 |
| Cholesterol | 193.86 ± 6.54 | 194.20 ± 6.32 | 168.37 ± 11.74 | 0.331 |
| Triglyceride | 182.11 ± 11.35 | 197.30 ± 13.24 | 139.06 ± 40.05 | 0.445 |
| HDL | 43.40 ± 1.07 | 43.60 ± 1.41 | 39.25 ± 2.51 | 0.746 |
| LDL | 115.43 ± 5.82 | 115.63 ± 9.01 | 93.91 ± 9.90 | 0.412 |
| VLDL | 36.84 ± 2.18 | 39.93 ± 2.56 | 39.25 ± 7.68 | 0.583 |
* p < 0.05. * p < 0.001 *** p <0.0001
a indicate significant between diabetic patients and control groups
b indicate significant between newly patients and control groups
c indicate significant between diabetic patients and newly groups
Serum Nesfatin-1 in T2DM groups and healthy subjects
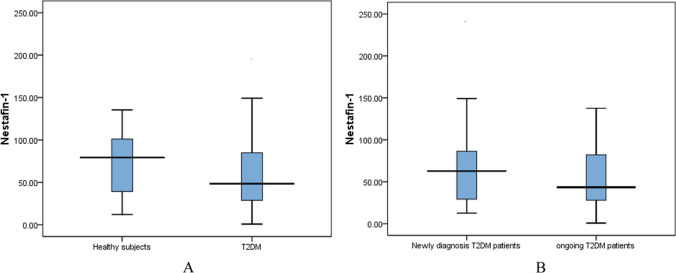
The level of serum Nesfatin-1 was significantly lower in T2DM patients (58.31]) ng/ml compared to the healthy subject group (82.53 ± 13.16 ng/ml) (p < 0.05), also the level of serum Nesfatin-1 was significantly lower in ongoing T2DM patients (51.26 ± 7.05) ng/ml compared to the newly diagnosis patients group (67.08 ± 6.59] ng/ml) (p < 0.05) as shown in Fig. 1A, B.
Fig. 1.
A Comparison of serum Nesfatin-1 among T2DM (n = 60) patients and healthy subject (n = 30). B The serum Nesfatin-1among newly diagnosis (n = 30) and ongoing T2DM (n = 30). Value is median (25th and 75th percentiles)
Correlation of serum Nesfatin-1 and severity of disease
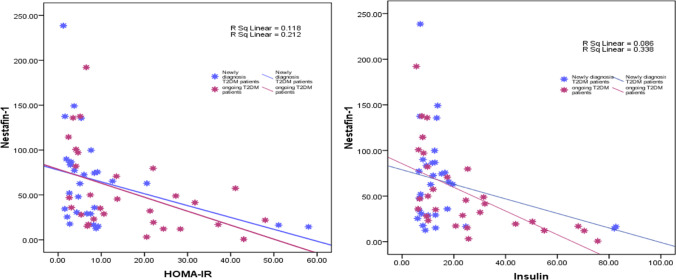
There was a significant negative connection between serum Nestafin-1 concentration and HOMA-IR (R = 0.471, p = 00.01) and serum Insulin (R = 00.407, p = 00.028), as shown in Fig. 2A, B. There were no significant relationships between serum Nesfatin-1 and biochemical parameters such as serum FSG or lipid profile (p > 0.05).
Fig. 2.
Correlation between nesfatin-1 and HOMA-IR and nesfatin-1 and serum insulin. Spearman correlation coefficient was calculated, significant at p ≤ 0.05
ROC curve analysis T
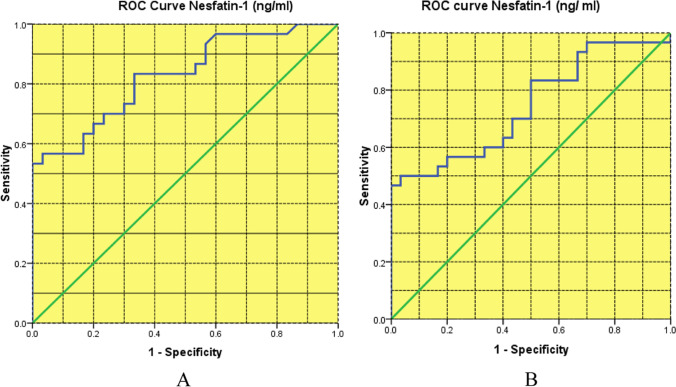
ROC curve analysis was used to examine the ability of serum Nesfatin-1 levels to discriminate newly diagnosed T2DM patients from healthy subjects (Table 3; Fig. 3A). The ROC curve for T2DM was significantly higher than the diagnostic test, implying greater validity (high sensitivity and high specificity). The AUC of the ROC curve for the presence of newly diagnosed T2DM patients was 0.827 (p 0.001), which was the optimal level of correct IR prediction (Table 3; Fig. 3A). ROC curve analysis was used to investigate the ability of serum Nesfatin-1 concentration to discriminate continuing T2DM patients from healthy subjects (Table 4; Fig. 3B). The ROC curve for T2DM was significantly higher than the diagnostic test, implying greater validity (high sensitivity and high specificity). The AUC of the ROC curve for the existence of T2DM diagnosis was 0.73 (p 0.001), which was a reasonable level (high probability) of correct IR prediction.
Table 3.
AUC and validity of nesfatin-1 to differentiate between newly diagnosis T2DM patients and the healthy subject group
| Variable | AUC | p-Value | Optimum cut off value | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Nestafin-1 | 0.827 | 0.001 | 20.7 | 70 | 70 | 70 | 70 | 70 |
Fig. 3.
A ROC curve analysis of blood nesfatin-1 concentration in newly diagnosed T2DM patients (n = 30) against healthy participants (n = 30) (AUC is 0.827; 95% CI 0.907–1.000), p 0.0001. B ROC curve analysis of blood nesfatin-1 concentrations in continuing T2DM patients (n = 30) against healthy participants (n = 30) (AUC is 0.73; 95% CI 0.592–0.869), p 0.003
Table 4.
AUC and validity of nesfatin-1 to differentiate between ongoing T2DM patients and the healthy subject group
| Variable | AUC | p-Value | Optimum cut off value | Sensitivity | Specificity | Accuracy | PPV | NPV |
|---|---|---|---|---|---|---|---|---|
| Nestafin-1 | 0.73 | 0.004 | 29.1 | 83 | 83 | 83 | 83 | 83 |
Discussion and conclusion
The FSG distribution (mean ± SE) of T2DM patients and normal healthy samples expressed in mg / dl as shown in Table 1. Results of FSG revealed that there was significant variation in a nova test (p < 0.001) between the three groups. Both patient groups showed a high significant increase (p < 0.001) as compared with control group. Also in the current study, both patient groups showed a high significant increase (p < 0.001) in levels of HbA1c as compared with control group. High levels of HbA1c are due to poor glycemic control in T2DM subjects. This finding concurs with that of Khan et al., who discovered that the levels of HbA1c are strongly correlated with FPG [17]. With regard to IR, T2DM is a type of diabetes that causes IR and beta-cell dysfunction. A compensatory increase in insulin secretion occurs at first, keeping glucose levels in the normal range. Beta cells shift as the disease advances, and insulin secretion is unable to maintain glucose homeostasis, resulting in hyperglycemia. The majorities of T2DM patients are obese or have a greater body fat percentage, which is primarily distributed in the abdominal area. This adipose tissue induces IR [18]. This finding can be clarified on the ground that insulin resistance is likely to be the first metabolic abnormality in DM type2.
In this study, we discovered that serum nesfatin-1 levels were lower in T2DM patients than in healthy group .Because nesfatin-1 has been shown to alter glucose metabolism by enhancing insulin sensitivity and decreasing insulin resistance, lower nesfatin-1 levels in the T2DM group could explain the considerably higher insulin resistance in this group [19]. Furthermore, serum nesfatin-1 levels are inversely linked with HOMA-IR and serum insulin, according to our findings. The role of nesfatin-1 in glucose metabolism and its anorexigenic impact can explain these negative correlations, and this conclusion agrees with the findings of a study conducted by [20].
There are several positive aspects to our report. The first step was to age-match the three groups. Second, newly diagnosed T2DM patients were included, the majority of whom did not have access to oral hypoglycemic drugs. This could be explained by the fact that -cells only secrete normal levels of insulin and nesfatin-1 during the early stages of diabetes. Third, patients with T2DM were only given one type of treatment. Finally, the patients were divided into subgroups depending on the duration of disease.
There are some drawbacks to this study. To begin, the sample size was quite small. Second, examining nesfatin-1 levels in a detailed follow-up analysis from the time of diagnosis through the continuation of T2DM treatment could help researchers better understand the function of nesfatin-1 and its potential as a marker or therapeutic target.
In summary, our findings show that serum Nesfatin-1 levels are strongly linked to newly diagnosed patients. As a result, we propose that serum Nesfatin-1 be used as an early diagnostic test in diabetic patients, with (29.1) ng/ml as the best cutoff value for distinguishing patients from controls.
Declarations
Competing interest
The authors state that they have no known competing financial or personal interests that could have influenced the findings presented in this research.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Bushra Mussad Kadim, Email: Bushramussad@gmail.com.
Ekhlas Abdallah Hassan, Email: ekhlasbiochemistry@gmail.com.
References
- 1.Casqueiro J, Casqueiro J, Alves C. Infections in patients with diabetes mellitus: A review of pathogenesis. Indian J Endocrinol Metab. 2012;16(Suppl1):S27–S36. 10.4103/2230-8210.94253. [DOI] [PMC free article] [PubMed]
- 2.Singh A, Kukreti R, Saso L, Kukreti S. Mechanistic insight into oxidative stress-triggered signaling pathways and type 2 diabetes. Molecules. 2022;27(3):950. doi: 10.3390/molecules27030950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galicia-Garcia U, Benito-Vicente A, Jebari S, Larrea-Sebal A, Siddiqi H, Uribe KB, Ostolaza H, Martín C. Pathophysiology of type 2 diabetes mellitus. Int J Mol Sci. 2020;21(17):6275. doi: 10.3390/ijms21176275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perego S, Sansoni V, Ziemann E, Lombardi G. Another Weapon against Cancer and Metastasis: Physical-Activity-Dependent Effects on Adiposity and Adipokines. Int J Mol Sci. 2021;22(4):2005. [DOI] [PMC free article] [PubMed]
- 5.Recinella L, Orlando G, Ferrante C, Chiavaroli A, Brunetti L, Leone S. Adipokines: New potential therapeutic target for obesity and metabolic, rheumatic, and cardiovascular diseases. Front Physiol. 2020;11:578966. doi: 10.3389/fphys.2020.578966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saeidi, A., Haghighi, M. M., Kolahdouzi, S., Daraei, A., Abderrahmane, A. B., Essop,M. F., … Zouhal, H. The effects of physical activity on adipokines in individuals with overweight/obesity across the lifespan: A narrative review. Obes Rev. 2021;22(1):e13090. [DOI] [PubMed]
- 7.Navaneethan SD, Kirwan JP, Remer EM, Schneider E, Addeman B, Arrigain S, ... Sondheimer JH. Adiposity, physical function, and their associations with insulin resistance, inflammation, and adipokines in CKD. Am J Kidney Dis. 2021;77(1):44-55 [DOI] [PMC free article] [PubMed]
- 8.Kadoglou NP, Korakas E, Lampropoulos S, Maratou E, Kassimis G, Patsourakos N, … Lambadiari V. Plasma nesfatin-1 and DDP-4 levels in patients with coronary artery disease: Kozani study. Cardiovasc Diabetol. 2021;20(1):1–8. [DOI] [PMC free article] [PubMed]
- 9.Jafari-Maskouni S, Shahraki M, Daneshi-Maskooni M, Dashipour A, Shamsi-Goushki A, Mortazavi Z. Metabolic and clinical responses to Bunium Persicum (black caraway) supplementation in overweight and obese patients with type 2 diabetes: a double-blind, randomized placebo-controlled clinical trial. Nutr Metab. 2020;17(1):1–11. doi: 10.1186/s12986-020-00494-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Akbaba E, Sezgin B, Edgünlü T. The role of adropin, salusin-α, netrin-1, and nesfatin-1 in endometriosis and their association with insulin resistance. Turk J Obstet Gynecol. 2021;18(3):175. doi: 10.4274/tjod.galenos.2021.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li QC, Wang HY, Chen X, Guan HZ, Jiang ZY. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159(1–3):72–7. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 12.Zhang Z, Li L, Yang M, Liu H, Boden G, Yang G. Increased plasma levels of nesfatin-1 in patients with newly diagnosed type 2 diabetes mellitus. Exp Clin Endocrinol Diabetes. 2012;120(02):91–5. doi: 10.1055/s-0031-1286339. [DOI] [PubMed] [Google Scholar]
- 13.Kaur S, Sharma A, Singh HJ. Waist related anthropometric measures-simple and useful predictors of coronary artery disease in women. Int J Physiol Pathophysiol Pharmacol. 2014;6(4):216. [PMC free article] [PubMed] [Google Scholar]
- 14.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28(7):412-9. [DOI] [PubMed]
- 15.Zhu X, Yu L, Zhou H, Ma Q, Zhou X, Lei T, et al. Atherogenic index of plasma is a novel and better biomarker associated with obesity: a populationbased cross-sectional study in China. Lipids Health Dis. 2018;17(1):37. doi: 10.1186/s12944-018-0686-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoo ZH, Candlish J, Teare D. What is an ROC curve? Emerg Med J. 2017;34(6):357–359. 10.1136/emermed-2017-206735. [DOI] [PubMed]
- 17.Khan HA, Sobki SH, Khan SA. Association between glycaemic control and serum lipids profile in type 2 diabetic patients: HbA1c predicts dyslipidaemia. Clin Exp Med. 2007;7(1):24–9. doi: 10.1007/s10238-007-0121-3. [DOI] [PubMed] [Google Scholar]
- 18.Goyal R, Jialal I. Diabetes Mellitus Type 2.[Updated 2019 Dec 20]. StatPearls [Internet] Treasure Island: StatPearls Publishing; 2020. [Google Scholar]
- 19.Khalili S, Khaniani MS, Afkhami F, Derakhshan MS. NUCB2/Nesfatin-1: A potent meal regulatory hormone and its role in diabetes. EJMHG. 2017;18(2):105–9. [Google Scholar]
- 20.Mohammad NJ, Gallaly DQ. Serum Nesfatin-1 in patients with type 2 diabetes mellitus: A cross sectional study. Zanco J Med Sci. 2020;24(1):1–7. doi: 10.15218/zjms.2020.001. [DOI] [Google Scholar]