Abstract
Presently on a global scale, one of the major concerns is to find effective strategies to manage the agricultural waste to protect the environment. One strategy that has been drawing attention among the researchers is the development of biocompatible materials from agricultural waste. This strategy implies successful conversion of agricultural waste products (e.g.: cellulose, eggshell etc.) into building blocks for biomaterial development. Some of these wastes contain even bioactive compounds having biomedical applications. The replacement and augmentation of human tissue with biomaterials as alternative to traditional method not only bypasses immune-rejection, donor scarcity, and maintenance; but also provides long term solution to damaged or malfunctioning organs. Biomaterials development as one of the key challenges in tissue engineering approach, resourced from natural origin imparts better biocompatibility due to closely mimicking composition with cellular microenvironment. The “Garbage In, Biomaterials Out (GIBO)” concept, not only recycles the agricultural wastes, but also adds to biomaterial raw products for further product development in tissue regeneration. This paper reviews the conversion of garbage agricultural by-products to the biocompatible materials for various biomedical applications.
Graphical abstract
The agro-waste biomass processed, purified, modified, and further utilized for the fabrication of biomaterials-based support system for tissue engineering applications to grow living body parts in vitro or in vivo.
Keywords: Agricultural waste, Biomaterials, Tissue engineering, Biomedical engineering, Value added products, Green method
Introduction
The quality of our environment is steadily declining due to the prevailing increase in waste generation and accumulation. As a result of these existing conditions of environmental degradation, the consciousness for the protection of environment as well decoding the green resources and technologies has exponentially increased since last decade. Various research groups across the world are attempting to harness green, non-toxic and recyclable materials such as agricultural wastes, industrial wastes, and biodegradable fibres as starting material to develop biomaterials [1, 2]. The potential of waste materials to be recycled into valuable biomaterials along with the increasing awareness about environmental issues is serving as the primary driving force to this approach. Advancement in modern agricultural practices for production has contributed to enormous agricultural production, representing an incredible risk to the environment in the form of increased waste production. Agricultural waste is generated from different farming processes in accumulative concentration. Any waste in the environment when its concentration is in excess can become a perilous factor for human, animal, and vegetation health [3]. The type, quality, and quantity of agricultural waste generated varies from country to country. The agricultural wastes management is obligatory and is central to the global waste management strategy. The proper and effective way to utilize agricultural waste will reduce environmental issues caused by irresponsible waste disposal and help protect the environment and the health quality. Today’s priority is utilization of the waste to achieve sustainable development [4], and attempts are being made to channelize the reuse and recycling of waste products for its valorization.
Across the globe, hundreds of mega tonnes of waste whose constituents could be a potential resource and thus bio-based economic development is the need of era. Due to its abundant availability, cheapness and renewability, most research groups have mainly focused on its energy potentials, chemical feedstock, and as renewable raw materials [5–8]. The large quantity of biomass produced from agricultural waste that are classified as natural fibers have until now only have 10% utility as alternative raw materials for several industries, such as automobiles, biomedical, and others. The unveiled potential of agricultural waste fibers has ignited a lot of research to utilize these natural fibers as a material to replace synthetic fibers for developing safe and environmentally friendly products. The transformation of these undervalued natural agricultural fiber needs a longstanding plan. Agriculture waste can be obtained from plant sources such as oil palm, bagasse, corn stalks, coir, bamboo, pineapple, banana as well as rice husk which extracted on their part of plant (stem, leaf, seed, fruit, stalk and grass/reed) [9] (Fig. 1). The main fiber wastes produced from the agricultural activity is Cellulose Fibers (CF) having potential as reinforcing materials due to high strength, environmentally friendly nature, low cost, availability and sustainability [9–11]. Eggshells (membrane bound), Feathers (keratin), Skins (gelatine), Bones, Fish Scales are few among by-products of agricultural animal resources being unveiled towards sustainable development. Waste by-products from the poultry processing and egg production must be resourcefully dealt, as not only these industries growth depends on their waste management, but also the by-products could be processed into value added products. Many reports are available on utilization of processed fish scales and bones for animal feed, energy production, natural color and cosmetics (collagen). In this regard, the attempts are being made for the valorisation of agro-waste materials for the extraction, purification, and/or development of biomaterials with a recently introduced concept: Garbage In Biomaterials Out (GIBO).
Fig. 1.
Garbage from living resources as promising biomaterials for biomedical applications
Agricultural waste as source for biomass is one of the important substitutes for the production of bio-composite materials [12, 13]. This has become possible because some agro waste derived active compounds have applications as biomaterials used in medical surgery and therapeutics. For example, agricultural waste has been proven as alternative source for developing biomaterials in therapies that replaces bone for the proliferation of osteoblasts [4]. The present paper reviews and establish the necessity of agricultural waste management, their applicability as resource for biomaterials, advancement in their processing techniques to extract bio-macromolecules, and further possible utilization as biocompatible value-added products for tissue engineering and biomedical applications.
Present global scenario of agricultural waste
Agricultural wastes which are residues from the harvesting and processing of raw agricultural products can be categorized as primary residues (field based post-harvest yield) and secondary residues (process based post-harvest yield) [14]. For example, rice straw is primary residue whereas rice husk is secondary residue. The agricultural wastes which can be further categorized based on agricultural activities, can be either solid, liquid, or slurries.
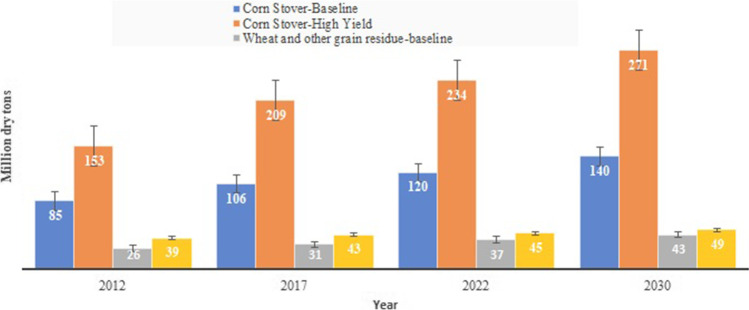
The largest contributor is carbohydrates and lignin from lignocellulosic biomass residues estimated to be thousands of tonnes every year worldwide. Out of this, the major sources are rice husk and sugarcane bagasse. It is estimated, every 25% of rice weight harvested is produced as husk. For sugarcane processing for sugar production, 13% weight is produced as baggage. The recent Indian government survey estimated that about 500 metric tons of crop residues are generated every year in country [15]. Figure 2 summarizes the extent of agricultural waste production in the US along with future projection which indicates the extent of possible environmental damage.
Fig. 2.
Agricultural waste production (in million tons) in the US as per US Department of Energy update (https://www1.eere.energy.gov/bioenergy/pdfs/btu_crop_residues.pdf)
Assuming that 40% of the production by weight is available as waste and out of that if at least 10% can be obtained as fibre, millions of metric tons of fibres is available every year and it is increasing with each passing day. These wastes could be the potential resources for production of useful compounds in the field of bioenergy, bio refineries, food industries, and reinforcing agents in preparation of bio-composites for application into biomedical engineering. The utilization of such resources will not only provide the sustainable and less expensive material but at the same time will contribute towards the waste disposal management for overcoming environmental problems. For example, as a sign of the changing times, the bio-based market review reports that about 2.05 million tonnes of bio plastics have been produced globally in the year 2017 [16].
The quality of fibre is affected by a variety of factors and conditions that a plant may undergo such as growth conditions, harvesting and extraction processes [17]. Therefore, a better understanding of the fundamental properties of these agricultural wastes is of indispensable importance. The conventional rich sources of fibre such as coconut, oil palm, pineapple, and bagasse have been technically and economically viable for paper production and composite engineered construction materials [18]. Tropical region is gifted with a variety of biomass fibre resources. Malaysia and Indonesia are found to be the world’s largest producer of agricultural industry waste especially oil palm [19]. Ananas comosus (Pineapple leaf), Zea mays (corn stalk) and Pennisetum purpureum (Napier grass) are rich sources of agricultural waste material in Malaysia. Since the last decade, increasing population and technological advancement have elevated the volume of waste generation. The elevated levels of waste generation need stringent monitoring and development of a framework to ensure effective management of waste. The concept of waste to wealth took place in various industrial processes as ethanol production, biomaterial synthesis, organic manure extraction from agricultural waste along with 3R’s approach i.e. Reduce, Recycle and Reuse has been implemented.
Agro-waste classification and structure
Because the sources of waste are so diverse, it is convenient to consider the chemistry in terms of four source-independent categories: polysaccharides, lignin, triglycerides (from fats and oils), and proteins.
Polysaccharide based biomass
Agro-waste polysaccharides basically include starch, cellulose, lignin, and hemicellulose and can be obtained from various agricultural residues such as potato, wheat, maize, rice, corn, cotton etc. The lignocellulosic chemical composition in various agro-waste materials is summarized with Table 1. They have different extraction methods and can be blended with other natural or synthetic polymers to be used for scaffold preparation for tissue engineering [20]. Since, these wastes are inexpensive, renewable, abundant, these are utilized for cost-effective industrial bio-energy production.
Table 1.
| S. No | Agro-Waste | Cellulose (%) | Hemicellulose (%) | Lignin (%) |
|---|---|---|---|---|
| 1 | Wheat straw | 35.0–39.0 | 23.0–30.0 | 12.0–16.0 |
| 2 | Barley straw | 36.0–43.0 | 24.0–33.0 | 6.3–9.8 |
| 3 | Rice straw | 29.2–34.7 | 23.0–25.9 | 17.0–19.0 |
| 4 | Rice Husks | 28.7–35.6 | 12.0–29.3 | 15.4–20.0 |
| 5 | Corn cobs | 33.7–41.2 | 31.9–36.0 | 6.1–15.9 |
| 6 | Sweet sorghum | 45.0 | 27.0 | 21.0 |
| 7 | Sugarcane bagasse | 25.0–45.0 | 28.0–32.0 | 15.0–25.0 |
| 8 | Hardwood | 40.0–55.0 | 24.0–40.0 | 18.0–25.0 |
| 9 | Softwood | 45.0–50.0 | 25.0–35.0 | 25.0–35.0 |
| 10 | Grasses | 25.0–40.0 | 25.0–50.0 | 10.0–30.0 |
It is noticeable that a huge amount of such lignocellulosic biomass is disposed of by burning in developing countries like India, which is a major contributing factor for pollution. Such lignocellulosic biomass attracted researchers due to its renewable nature for its diverted products development [21, 22]. Such value-added products include biogas, biodiesel, energy source for microbial fermentation and enzyme production, cellular metabolites, pigments, cosmetics, etc. [21, 23].
Natural fibres are abundantly available providing cost effective biomaterials and can be extracted from seeds, Stems (Flax, hemp, and jute), leaf, core fibre, grass and reed and other like wood, roots. These renewable resources provide a rich source of polysaccharides and proteins that can be used as suture material. Various extraction methods have been studied along with their characterization, physical and mechanical properties to assess the suitability of the different agricultural wastes to be used in variety of applications [9, 11, 24].
Physico-chemical and mechanical properties
The wide range of properties including size, structure, and composition of agricultural waste fibre makes it an eligible candidate for a wide array of applications [25]. The structural components of plant fibre mainly comprised of cellulose, hemicellulose, and lignin along with small amount of pectin [26], as termed as lignocellulosic or cellulosic fibres [27]. The composition range of these components varies with the cultivation techniques, climatic changes and extraction methods [25]. High degree of polymerization increasing length of cellulose improves its mechanical properties [28, 29]. Orientation of middle layer of secondary cell wall and its thickness determines tensile strength and Young’s modulus of the fibre [30]. Microfibrillar angles also play vital role in regulating the mechanical properties of fibre[28, 29].
Starch
Starch is a polysaccharide found richly in principal food crops as wheat, potatoes, rice, maize, barley, rye, beans, peas, sorghum, tapioca (or cassava), sweet potatoes, avocados, arrowroot, taro, bananas, mango, pineapple, sago (palm starch) and so forth. Starch obtained from different sources show different morphologies. It has granular and crystalline forms which can be studied through microscopy, X-ray scattering and X-ray diffraction techniques. Starch is a homopolysaccharide comprising of amylose and amylopectin bonded together by α 1, 4 and α 1, 6 glycosidic linkages of glucose. Amylose is mostly linear α-D glucan with 1, 4 glycosidic bonding and occur in low amount as compared to amylopectin which is highly branched structure. Its content determines the viscosity and strength of starch gel. Higher the amylose content higher will be the strength and viscosity of the starch paste [31].
Physio-Chemical Properties of Starch
Starch is second important constituent of polysaccharide biomass after cellulose. The major constituents are amylose and amylopectin, whereas lipids, proteins and minerals are also present in small traces [32]. Gelatinization has been observed due to which ordered state of starch changes to disordered state approximately at 25% w/w starch to moisture ratio and temperature range of 60–70% [33]. The non-toxic, renewability and biodegradability makes it vital candidate for the biomedical applications. Nevertheless various chemical, physical and enzymatic modifications or blending with more appropriate material has been used to enrich the properties of starch [34].
Modifications of Starch
Various physical and chemical modifications have been reported to meet the demands of required properties of the material to be used in variety of applications. Physical modifications have been done to enhance water solubility and alter the particle size. Granular cold-water-soluble-starch (GCWS) has been prepared by injection or novel spray drying which improves viscosity and other properties [31]. Different chemical modification involves cross linking, grafting, esterification, etherification, oxidization and others. These chemical modifications improve stability, thermodynamic properties, water permeability, absorbency and biodegradability [34]. To improvise the mechanical strength and biodegradability of starch, different blends have been reported, prepared through numerous methods as solvent casting, particulate leaching, membrane lamination, fiber bonding, microwave baking, and expansion [35].
Water Hyacinth as rich agro-weed
One of the emerging lignocellulosic waste for biocomposite synthesis and thus a prime candidate for use as a reinforcement agent is Water Hyacinth. Originally Eichhornia crassipes (water hyacinth) is a hydrophyte weed and affects most of the water bodies like lakes, ponds and can survive in extremities. In addition, it can reproduce both sexually and clonally due to which it shows rapid growth under favourable conditions. The growth rate of water hyacinth has been estimated to be 0.26 ton dry biomass per day per hectare under normal conditions whereas in favourable conditions it doubles its size and yield up to 17.5 tons per hectare per day [36]. The various applications have been discussed by Guna and co-workers as sorption of dyes, controlled drug delivery, fuel applications, supercapacitors and reinforcement agents in bio composite preparation. It has been reported that Polyethylene and natural rubber that has been reinforced by pre-treated hyacinth showed improved tensile strength [36].
Protein based biomass
Another plant origin biomaterial used in biomaterial fabrication is protein based. They have derived great attention from the scientists due to their exquisite properties of biocompatibility, biodegradability, and cost effectiveness [37, 38]. Plant proteins can be used in the form of fibres and films where fibres show good mechanical strength as compared to films. Plant proteins possess higher net negative charge so can suitably be used in delivering of drugs exhibiting positive charge [39]. Furthermore, proteins derived from plant sources as soy and zein are inexpensive as well as conveniently available in contrast to animal-origin protein which showed immunogenicity and disease transmission issues.
Silk
Silk is produced by spiders of class Arachnida and worms such as mites, butterflies and moths belonging to Lepidoptera order. The fibrous protein silk is synthesized within epithelial cells followed by secretion into lumen of specialized glands where proteins get stored in these organisms [37, 40]. Silk varies in composition, structure and properties depending on their specified sources. Silkworms Silk has been widely classified into mulberry and non-mulberry silk as per available food for the worm. The most popular and domesticated mulberry silkworm is Bombyx mori (B. mori) belonging to family Bombycidae. Others non-mulberry silkworms includes Antheraea mylitta (A. mylitta), muga silkworm Antheraea assamensis (A. assamensis), oak silkworm Antheraea pernyi (A. pernyi) and Philosamia ricini (P. ricini) or Samia cynthia ricini (S. cynthia ricin) belonging to Saturniidae Family [41]. A wide range of silk fibroin and silk sericin based biomaterials have been developed in different forms and for different tissue engineering and biomedical applications [37, 38].
Silk is composed of two major proteins viz. globular Sericin and Fibrous protein. Silk fibrils comprises of core of two fibroin filaments and glue-like sericin shell that surrounds it. In case of fibrils of spider web, spidroin core has been found with a shell of glycoprotein and lipids [42]. Sericin consists of 18 different amino acids chiefly glycine, alanine and serine. In comparison to fibroin protein obtained from spiders, glycine and alanine are present abundantly. In addition, the mechanical properties differ broadly with respect to source of silk as well its amino acid sequences [43]. In general, silk possess β- pleated secondary structure privileged by tight packing of sheets of hydrogen bonded anti-parallel chains [40]. Fibroin fibre is a glycoprotein composed of Heavy (~ 350 kDa) and light (~ 25 kDa) chains are interconnected by disulphide bond and released into lumen of posterior gland.
Physio-Chemical Properties
A thermochemical treatment of silk has been done to remove sericin by a process called degumming. Sericin separated from cocoon can be further can be used in treating cancer and wound healing purposes. Various modifications have been done to expand the functionalities of potential fibres using physical adsorption, encapsulation or covalent immobilization. Different active molecules as growth factors or antibiotics have been used to amend the silk fibres which improve cell and tissue adhesion and performance [44]. Numerous methods as force reeled silk fibres or stepwise twisting have been enforced to foster the mechanical strength of the material [43]. In a reported study from our group, silk fibroid extracted from Bombyx mori silk cocoon using Na2CO3 aqueous solution along with LiBr were utilized for silk degumming. The process condition optimized for the production of silk fibroin protein with maximum concentration without denaturation using degumming of silk fibre at 70℃ followed by production of silk fibroin at 0.02 M of Na2CO3 [45]. A versatile approach has been introduced to develop silk biomaterials with tunable mechanical, chemical and biodegradation properties by photo-crosslinking [46]. Many research groups are presently working on its sericin and fibroin components utilization for spectral tissue engineering applications [37, 47].
Zein
It has been estimated that worldwide grain production in the year 2017/18, about 1036 million metric tons of corn have been produced as compared to approximately 759 million metric tons of wheat. Corn or maize is the major reservoir of zein protein which comprises 40–50% of endosperm. Zein, approximately of 44 kDa molecular weight, is a heterogenous mixture of hydrophobic amino acids including leucine, proline, alanine linked by disulphide bond. Zein belongs to prolamines family of proteins and possesses a unique property of solubility in alcohol-water mixtures depending upon their amino acid composition [48].
Physio-Chemical Properties
Zein and its resins bear superlative properties to form tough, hydrophobic grease proof coatings and resistance to microbes make it a potential aspirant for a variety of applications as biodegradable films, plastics, fillers in scaffold designing, textiles, chewing gum and in antioxidant activities [49]. Various approaches have been used to boost the properties of Zein films. Zein films prepared in acetone found to have more tensile strength in comparison to be prepared in ethanol [50]. Plasticization has been enforced to modify glass transition temperature, polymer mobility and rheological properties. With the addition of plasticizers as glycerol, polyols, lipid and its derivatives, flexibility and extendibility have been hiked up leading to reduction in brittleness and glass transition temperature [51]. Different processing techniques have been applied for different geometry of zein protein into chains, layers or foams [52]. Different methods have been implemented in order to achieve higher mechanical strength of Zein based materials. These improved mechanical behaviours enhance the functionality of material and expands its horizons to different approaches. Different chemical methods have been reported to improve mechanical strength of the Zein based materials. The addition of cross-linking agents as transglutaminase, glutaraldehyde, formaldehyde and other found to be promising cross linkers for improving the mechanical properties of Zein. The physical, mechanical and antimicrobial activities have been improved as apprised on cross linking with Succinic anhydride, Eugenol and citric acid [53].
Application: Zein has been reported as successful coating agents in the food and pharmaceutical industries. It has been used as adhesives, as biodegradable plastics, in cosmetic powders and inks [54]. It has also shown biocompatibility with human liver cells and umbilical veins which makes it potential candidate for scaffold designing and drug targeting [49, 55].
Soy protein
According to the Soyabean Processors Association of India (SOPA), the total world soybean production is 346.919 million metric tonnes in the year 2017–18, among which US is the largest producer followed by Brazil. Soybean contains approximately 38% proteins, 30% carbohydrates, 18% oils and 14% oils and minerals. Soy protein can be extensively obtained from soyabean and exhibit globular structure majorly composed of two main subunits 7S as conglycinin and 11S as glycinin [56]. Both the subunits comprise of aspartic and glutamic acid as non-polar, alanine, Valine and leucine as basic amino acids and uncharged polar residues [57]. Basically, a soy protein is available in three forms specifically as soy flour, soy protein concentrates and soy protein isolate (SPI).
Physio-chemical properties
Various modifications have been done to overcome the hydrophilic nature of soy protein due to which it showed low resistance to moisture. Either mixing with hydrophobic compounds as lipids or cross-linking using physical, chemical or enzymatic techniques has been practiced overcoming this barrier [58]. Soy structures have also been fabricated through thermal and chemical modifications as heat generated thermoplasticity which leads to formation of desired shapes [59]. A major drawback of soy protein of being highly sensitive to moisture makes it a poor option to be used commercially. But various bulk and surface modifications including fractionation, pH value manipulation, blending using plasticizers, surfactants, polymers, and radiation modifications has been precisely reported to overcome the high hydrophilic character and to improvise other characteristics of soy protein [60].
Collagen
Collagen is one of the vital components of the extracellular matrix in the various connective tissues usually in fibrous tissues such as tendons, ligaments and skin. Five most common types of collagens have been expanded in different tissues as skin, tendons, cartilages, placenta, hairs and basements membrane. It is also the main constituent of double layered eggshell membrane [61].
Physio-chemical properties
Collagen protein is hydrophilic in nature and possesses amino acid triplet primary structure inclusive of proline rich Gly-X–Y polypeptide [62]. About 29 distinct collagen types have been characterized up to date and collagen fibers have been formed by Collagen types I, II, III, V and XI.
Degradation of collagen in vivo has been done through collagenases undergoing hydrolysis at different rates. Mammalian collagenases including matrix metalloproteinases, MMP-1, MMP-2, MMP-8, MMP-13, and MMP-14 have the capacity to hydrolyse collagen type I to III [63]. Porcine, bovine collagen has been used for wide applications in food, biomedical and cosmetics industries. Atelocollagen is one of the processed natural biomaterials composed of type I collagen obtained from bovine showed enhanced biocompatibility and biodegradability [64]. Cross- linking of collagen is done by using various chemical agents as aldehydes such as glutaraldehyde, carbodimides, polyepoxy, etc. or by physical methods in order to enhance the chemical and mechanical properties of collagen. Various chemical, natural (hydroxylation and glycation) and physical methods of crosslinking including radiation, DE hydrothermal method have been reported [65]. Recently, the valorization of discarded marine Eel fish skin for the isolation of collagen and potential application for skin tissue engineering by incorporating into alginate hydrogel to fabricate scaffolds using extrusion-based 3D printing technology was reported [66]. Table 2 illustrates the possible application of derived collagen as constituent of bio-composite variants for tissue regeneration in different supporting forms made possible with different manufacturing techniques.
Table 2.
Collagen derived biocomposites for spectrum of tissue regeneration and biomedical applications in different forms
| S. No | Collagen composites | Form of application | Tissue Engineering application | References |
|---|---|---|---|---|
| 1 | Chitosan/Collagen/β-glycerophosphate | Hydrogel | Tissue regeneration | [67] |
| 2 | PCL-Phlorotannin/ Fish Collagen-alginate PCL-Phlorotannin/ Porcine Collagen-alginate | Scaffold | Hard tissue regeneration | [68] |
| 3 | Fish Collagen/ alginate/ Chitooligosaccharides | Scaffold | Skin regeneration | [69] |
| 4 | Bovine dermis Collagen | Sponges | Drug delivery system for wound healing | [70] |
| 5 | Collagen-Silver Nanoparticles | Scaffold | Anti-infective and Skin regeneration | [71] |
| 6 | Insulin loaded PLGA microbead- Collagen | Scaffold | Controlled Insulin release for cartilage tissue regeneration | [72] |
| 7 | Collagen-human bone marrow-derived mesenchymal stem cells (hMSCs) | Scaffold | Traumatic brain injury in rats | [73] |
| 8 | 3D printed CaP/Collagen | Composite | Bone regeneration | [74] |
| 9 | Collagen binding domain- Vascular endothelial growth factors | Scaffold | Urethral tissue regeneration | [75] |
| 10 | Collagen/Hydroxyapatite | Scaffold | Bone regeneration | [76] |
| 11 | Collagen/PVA | Scaffold | Corneal regeneration | [77] |
| 12 | Collagen seeded with mesenchymal dental pulp stem cells (DPSC) | Gel Scaffold | Craniofacial bone regeneration | [78] |
| 13 | Chitosan/Collagen | Scaffold | Bone regeneration | [79] |
| 14 | human-like collagen (HLC)/nano-hydroxyapatite | 3D Scaffold | Bone defects | [80] |
| 15 | Gelatin/Collagen/ Polycaprolactone | Biocomposite | Skin regeneration | [81] |
| 16 | Collagen/Silk fibroin | Membrane | Corneal regeneration | [82] |
| 17 | Silver carp skin collagen / chitosan / chondroitin sulfate / PLGA | Microspheres | Growth factors delivery | [83] |
| 18 | Synodontidae (Lizardfish) fish scale collagen / calcium alginate | Nanoparticles | Calcium Delivery | [84] |
| 19 | Eel skin collagen | Gel / Films | Antimicrobial drugs delivery | [85] |
| 21 | Marine sponge collagen | Powder | L-cysteine hydrochloride delivery | [86] |
| 22 | Type I Collagen | Gel-Matrix | Biosensors for pathogen and toxin detection | [87] |
| 23 | Collagen-ZnO nano-particles composite | Thin film | Biosensors for glucose detection | [88] |
Mineralized by-product
Various minerals involving calcium, phosphorous, magnesium and carbonates plays vital role in functioning of a cell. Human bone is the rich reservoir of various minerals and composites that act synergistically in osteoinduction and osteoconduction during bone tissue engineering. Basically, bone is composed of calcium phosphate (69–80% mainly hydroxyapatite), collagen (17–20%) by weight and other components (water, proteins, etc.) [25], due to which various forms of calcium phosphates as BCP, TCP, HA have been studied broadly and used in different forms as nanoparticles, coatings, cements for the synthesis of biomaterial for the application into tissue engineering [89]. Bioactivity, biodegradability, osteoconductivity and osteoinductivity are some of the distinguished properties of BCP- BiCalcium phosphate that marked it as gold standard for the bone substitution [90]. Multi phasic calcium phosphate obtained from eggshells have been 3D printed and fabricated through coagulation assisted extrusion-based printing showed improved bioactivity [91]. Numerous scaffolds have been reported showing enhanced mechanical properties. Chitosan along PLLA have been fabricated using nano calcium phosphate through freeze casting method found promising for bone tissue engineering [92]. Another scaffold composing of chitosan, nano hydroxyapatite and beta-tricalcium phosphate have been prepared successfully possessing improved mechanical properties and thermal stability but lower degradation and water swelling and retention. Waste mussel shells have been used to extract TCP and nano hydroxyapatite [93]. In addition, PCL has been reinforced by TCP via electrospinning used in bone regeneration [94]. Among other bioactive ceramics are calcium silicates and bioactive glass having outstanding trait of direct bonding make it eligible for bone tissue engineering. A three-dimensional Nano fibrous calcium carbonate has been obtained from hen’s eggshell via high repetition femtosecond laser irradiation with improved cell interactions and suitable candidate for biomaterial [95]. Numerous methods have been discussed for the fabrication of bioceramics scaffolds including foaming methods, sol–gel foaming, H2O2 foaming, sponge replication, starch consolidation and others with their pros and cons [96]. Calcium silicates along with hydroxyapatite whiskers have been prepared using selective laser sintering and tested, reporting improved mechanical and biodegradability on increasing addition of hydroxyapatite whiskers [97]. Calcium silicates and polycaprolactone have been blended to form 3D scaffold with improved properties and application into bone tissue engineering [98]. Various bioceramics have also been extracted from plant and garbage waste to be used in biomaterial preparation. Graphene oxide found to be commendable filler which has been described to obtain from sugarcane baggage through SOMA method – (Sugarcane oxidized under muffled atmosphere graphene oxide) [99]. On the other hand, Calcium has been used extensively in various forms as calcium nanoparticles extracted from green tea and papaya [100], formation of scaffolds for tissue engineering or calcium phosphates or carbonates which can be obtained from different types of waste. Hydroxyapaptite is a remarkable bioceramic which has been imprinted in the field of bone tissue engineering can be obtained from various plant sources as fruit waste [101] and chicken eggshells [102]. Calcium carbonates exist as different forms as calcite, vaterite and aragonite and these can be blended and used as filler in composite preparation [103, 104].
Extraction and purification techniques of various materials
Polysaccharide material
The chemical composition of polysaccharide-based material varies from plant to plant and thus the extraction process also differs as such [105]. The basic principle for all the extraction processes is to extract a particular content. For instance, to extract the cellulose, it is necessary to remove all the other non-cellulosic components such as lignin, hemicellulose and pectin. A variety of sources are available for polysaccharides and can be obtained as per the requirement and availability of the plant. Kenaf, banana, oil pal, jute, kapok, sisal etc. are the richest sources of cellulose fibres [106]. There are different extraction processes including various chemical and mechanical methods. The retting process is one of the simplest extraction methods. This gave rise to delicate, thin and color adequate quality of material [107]. Retting process can be conducted in five ways as, dew, water, enzymatic, mechanical, and chemical retting [107, 108]. The crude fibres obtained from retting process are further purified by mechanical or chemo mechanical processes as high-pressure homogenization, microfluidization, ultrasonication, electrospinning and steam explosion [107, 108]. A combination of interdisciplinary processes leads to extraction of agro-waste by-products in more cost-effective manner with high yield [109].
Chemical treatment
Various chemical treatments have been applied to extract cellulose. These treatments involve chlorite bleaching, alkali treatment and acid hydrolysis [110]. Extraction of cellulose has been reported from rice husk and jute using Sulfuric acid hydrolysis [111, 112] and using hydrochloric acid (HCl) [113].
Mechanical treatment
Electrospinning, ultrasonication and homogenization are several mechanical processes used for the isolation of cellulosic fibres. In addition, refining [63], cryocrushing [114], grinding [115] and microfluidization [116] have also been reported as mechanical treatments. The combination of methods has also been applied for the extraction process. A combination of ultrasonication and acid hydrolysis [117] or ultrasonic and homogenization [118] have been studied so as to enhance the quality of the fibres obtained.
Chemo-mechanical treatment
A combination of chemical and mechanical process has been used together. Chemical treatment has been done prior to mechanical processes which involves cryo-crushed and homogenization using high pressure so as to get fine fibres [112, 113]. Cellulose nanofibers have been extracted from oil palm using sulfuric acid treatment followed by high pressure homogenization (HPH) [119].
Protein extraction
Decellularization process is a multiple-step process to eliminate all the cell components from tissue or organ without altering the Extracellular Matrix (ECM). Various methods have been reported for decellularization as physical methods viz. snap freezing, mechanical agitation, chemical methods (ionic and non-ionic detergents, and enzymatic) have been studied extensively [120]. Different physical and enzymatic processes have been implemented for the extraction of various proteins obtained from rice bran reported by Fabian and group [121].
Protein based material like silk is degummed for the application into tissue engineering, textiles, cosmetics and food industries. Degumming can be achieved from silk cocoons using different chemical treatment, boiling in water and enzymatic treatments. The process of degumming has been studied precisely using a mixed solution of calcium chloride, ethanol and water and then centrifuged to obtain silk protein and eliminating the glue sericin content [40, 45].
Biomaterials development and applications
Different forms of biomaterials
The different forms of biomaterials are desirable for different tissue engineering applications. While the hydrogel form is more suited for corneal tissue regeneration, the scaffold is desirable for bone regeneration for loading patient derived stem cells. The nano-fibrous matrices developed with electrospinning are more approachable for wide range of cells, the thin film form is suited particularly for skin regeneration. As a whole, the highly specialized and designed constructs with the well-interconnected network from agro-derived biomaterials, such as complex sugars (hyaluronan and chitosan), and other inorganics (hydroxyapatite) should be maintained. The different forms of biomaterials are classified either based on their structure or functions. The objectives of developing into different forms is to mimic highly organized tissue and organ architectures, to channelize mass transfer, and other critical biological functions. This is always considered important tissue regeneration. The form of biomaterials affects its chemical-physical properties such as surface morphology, pore stiffness, and mechanical strength that ultimately affect how the cells interact with biomaterial in living system. It is expressed in terms of altered biomaterials behaviour such as biocompatibility, bioactivity, and biodegradation. [61, 122]. The Fig. 3 depicts the porous scaffold for bone tissue regeneration developed from eggshell by our research group.
Fig. 3.
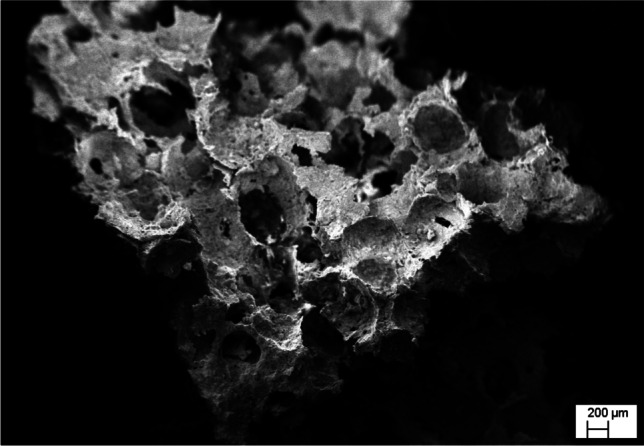
Porous scaffold developed from avian eggshell [102]
Different possible bio-composites
Different types of biocomposites have been developed so far. In the past few decades, blending the materials and fabricating them in search of attaining compatibility in physical, mechanical and biodegradability of the material has marked a milestone in field of biomedical engineering. As aforesaid, many of the polymeric, metallic and ceramic based materials are commercially being used in implants or other categories. Ceramics and metallic biomaterials showed stress shielding which lowers the density of the host bone due to stress by the implants [123]. This can be overcome by use of polymers and modifying it with different fillers that improve its physical and mechanical strength. With the advancement in the technology researchers are aiming to develop a biomaterial which is bioactive as well as bioresorbable with ease of manufacturing and effective healing. Polymeric materials are usually biocompatible and can be easily fabricated. One of the accomplished candidates to substitute metallic and ceramics biomaterial is polymeric biomaterial. There are two types of polymers used in bone tissue engineering classified as Natural and Synthetic on the basis of their source. Natural polymers include chitosan, elastin, gelatin, fibrinogen, keratin, actin, collagen, silk and polynucleotides as DNA and RNA. Man-made polymers can be biodegradable or non-biodegradable. In bone tissue engineering commonly used synthetic polymers are Poly (lactic acid) (PLA), Polycaprolactone (PCL), Poly (glycolic acid) (PGA) and Polyhydroxybutyrate (PHB), poly (hydroxybutyrate-co-hydroxyvalerate) (PHBV) and copolymer poly(lactic acid-co-glycolic acid) (PLGA). These Synthetic polymers produced industrially and have long shelf life and improved properties. There are various factors that drive the synthesis of most of the biocomposites as biocompatibility, biodegradability, bioactivity and structural requirements including physical and mechanical properties. There are the various fabrication methods which have been used differently as per the need and ease of the manufacturing. The shapes of desired biocomposite can be done using different moulding techniques inclusive of Compression moulding, Hot pressing, Injection moulding and Resin transfer technique [124] whereas there are different fabrication methods used to manufacture of scaffolds including Selective Laser Sintering, Rapid Prototyping, 3D printing, Selective Laser Sintering, Electrospinning, Solvent casting, Particulate leaching, Freeze-drying and others [125, 126] (Table 3).
Table 3.
Different combination of agro-waste based biomaterials with natural and/or synthetic polymeric/ceramic materials developed in usable form with different fabrication methods and relevant tissue engineering applications
| S. No | Bio-composites | Fabrication Method | Application | References |
|---|---|---|---|---|
| 1 | PCL/Keratin | Electrospinning | Tissue engineering and regenerative medicine | [127] |
| 2 | PVA/Cellulose | Degassing | Reinforcement agent for biocomposites | [128] |
| 3 | Gelatin/PCL and Collagen/PLCL | Electrospinning | Vascular tissue engineering | [129] |
| 4 | PCL- PLA/Collagen I/ Collagen III | Electrospinning | Nerve tissue engineering | [130] |
| 5 | Injectable Chitin-PCL/nHA | Solvent regeneration method | Bone tissue engineering | [131] |
| 6 | Nanocellulose fibre/Collagen | Fibrillation | Wound dressing | [132, 133] |
| 7 | Chitosan/Carbon Nanofibres | Precipitation | Cardiac tissue engineering | [134] |
| 8 | Starch/Cellulose | Salt Leaching | Tissue engineering | [135] |
| 9 | Starch/ PCL | 3D Bioprinting | Spinal Cord Injury (SCI) Regeneration | [136] |
| 10 | Elastin/ Collagen | Electrospinning | Dermal tissue engineering | [137] |
| 11 | PEG-Cellulose/PLA | Electrospinning | Bone tissue engineering | [138] |
| 12 | PCL/Silk/Chitosan | Electrospinning | Urethral tissue engineering | [139] |
| 13 | PCL/chitosan | Electrospinning | Liver tissue engineering | [140] |
| 14 | Chitosan/HA | Magnetic Stirring and Sonication | Bone tissue engineering | [141] |
| 15 | PVA/Chitosan/ CaCO3 | Electrospinning | Cardiac tissue engineering | [142, 143] |
In the table above, various possible blends have been made using different fabrication methods and have been studied extensively in-vitro and in-vivo conditions for application into different tissue engineering. Silk based, injectables and hydrogels have been described by many researchers along with their applications in bone tissue engineering [144, 145]. A silk and Forsterite blend has also been studied and tested for cytocompatibility and mechanical properties [146]. A lot of composites have been described using ceramics as glass, hydroxyapatite and other calcium phosphates along their fabrication methods [147]. In addition, collagen has diversified applications in various fields as vascular diseases, urogenital system, dermal and drug delivery systems and a profuse amount of biocomposites has been extensively studied by Parenteau-Bareil and co-workers in 2010 [148].
Modifications of biocomposites
The basic requirements for the scaffold preparation to be used in tissue engineering are biocompatibility, biodegradability, non-toxicity, non-immunogenicity, adequate porosity and mechanical strength. The scaffold should possess improved qualities due to which either matrix or the reinforcing agents need to be modified so that a reformed scaffold can be prepared. For instance, Natural fibres do not possess good adhesion properties with the polymeric matrices, to overcome it various surface modification have been applied to reduce the moisture absorption and enhancing the wettability of the fibres with the polymer matrixes. Numerous methods have been attempted for the surface modification of the fibres including Physical, Chemical, mechanical or a combination of any of these methods.
Physical treatment methods
Physical treatments involve thermal treatments and non-thermal treatments. Steam explosion treatment, and high energy ray radiation processing and autoclave treatment are thermal treatments whereas Corona Treatment, Dielectric barrier technique, Ultrasound and Ultraviolet Treatments. Cleaning, sterilization and surface etching can be done by plasma treatment usually in packaging applications. Plasma treatment can be done either in Vaccum or in situ i.e. atmospheric. On the other hand, Steam explosion has been applied to achieve dispersibility and adhesion properties using heating of lignocellulosic materials at high temperatures and pressures pursued by mechanical disruption [149, 150].
Chemical treatment methods
Chemical modifications inclusive of Silanization, Alkalization, Acetylation, cyanoethylation, benzoylation, isocyanation, dewaxing, esterification, etherification, and graft copolymerization. In addition to these treatments cross linking has also been applied using formaldehyde, p-phenylenediamine and phthalic anhydride; nitration; dinitrophenylation and transesterification.
Alkaline treatment usually by NaOH has been used to reinforce thermoplastics and thermosets by removing the lignin, hemicellulose, wax or oils covering the surface of the fibers. These modifications enhance thermal stability, biodegradability, moisture resistant and strength [34].
Biological treatment methods
Protein derived scaffolds have been modified by physical adsorption, entrapment and chemical modifications. These modifications enhance the functionalization of protein-based biomaterials so as to be used as growth factors, antibiotics and reinforcement. Immobilization can be achieved either by physical adsorption or by encapsulation. UV light, Ion bombardment and plasma modifications have been used to disrupt bonding and generating radicals which react with oxygen, improving hydrophilicity and reactivity [44].
Biomaterials from agricultural resource for biomedical applications
The idea of zero waste standards dwelled the path of using waste material into a beneficial one. Garbage in, biomaterial out concept has been enforced in the past years. Natural fibers are the rich sources for the application into biomedical field due to their impressive properties of biocompatibility. It has been used as reinforcement agent for the synthesis of bio composites. These natural fibers have been obtained from either plant extract or the waste of it. The potential scaffolds have been found and many of them are commercially available for different applications as wound healing, skin grafts, stents, bone replacements and nerve tissue engineering [12, 151]. The very promising range of agro-waste available for possible biocomposites development is summarized in Table 4.
Table 4.
Notable biomaterials developed from agro waste and their applications
| S. No | Biomaterial | Agro Source | Application | References |
|---|---|---|---|---|
| 1 | Cellulose |
Grapewine Gluconacetobacter hansenii |
Tissue Engineering | [152] |
| 2 | Silk | Sericin-waste of textile industry | Cosmetics and pharmaceuticals | [153] |
| 3 | Zein Scaffold | Zein | Bone Tissue Regeneration | [48] |
| 4 | Mucilage | Abelmoscus esculentus- Okra Plant | Food & pharmaceutical industries | [154] |
| 5 | Biogenic Silica Nanoparticles | Rice husk | Bone tissue engineering | [155] |
| 6 | Chitosan/ Silk fibroin | Chitosan from Crab shells, Silk Fibroin from silkworm farm | Cartilage tissue engineering | [156] |
| 7 | Collagen/ Gulmohar seeds | Gulmohar seeds (Delonix regia) from trimmed waste of cowhide and Cinnamon bark as cross-linking agent | Antimicrobial wound dressing | [157] |
| 8 | Cellulose Nanofibrils | Sugarcane bagasse | Biomedical devices, Tissue engineering | [158] |
| 9 | Chitosan acetate bandage | Shrimp waste | Skin regeneration | [159] |
| 10 | Silk Scaffolds | Bombyx mori | Bone repair | [160] |
| 11 | Cellulose hydrogel film |
Agave Tequilana Weber Bagasse |
Tissue engineering | [161] |
| 12 | Cellulose Nanocrystals | Sugarcane bagasse | Biomedical and tissue engineering | [162] |
| 13 | Cellulose Nanofibrils | Pineapple leaf | Surgical Wounds, Tissue engineering & medical implants | [163] |
| 14 | Cellulose film | Microbial Cellulose (MC) produced by Acetobacter Xylinum | Wound dressing, Skin Substitute | [164] |
| 15 | Collagen fibrils | Skin of Atlantic salmon | Bone tissue engineering | [165] |
| 16 | Collagen/Chitosan/Aloe Vera Scaffold | Collagen from Tachysurus maculatus- marine cat fish | Tissue engineering | [166] |
| 17 | Cellulose Hydrogel Film | Sugarcane bagasse | Biomedical implants | [167] |
| 18 | Cellulose nanocrystals |
Corncob Pineapple leaf |
Reinforcing agent in nanocomposites |
[168] [169] |
| 19 | Nano-Hydroxyapatite | Eggshells and Fruit waste (grape, sweet potato, and pomelo peels) | Bioimplants | [101] |
| 20 |
Natural polysaccharides (Agar, Carragenan,Alginate, Fucoidan) |
Seaweeds: Gelidium amansii, Chondrus crispus |
Medical, pharmaceutical, cosmetics, Hormones and industrial applications | [170] |
| 21 | Highly Pure Graphite (HPG) | Caronaceous waste material (wood, leaf, bagasse, fruit, newspaper, Bone, cowdung) | Tissue engineering | [171] |
| 22 | Cellulose Nanocrystals | Potato Peel waste | Reinforcement agent in biocomposites | [172] |
| 23 | Starch scaffold | Corn starch | Bone tissue-engineering | [173] |
| 24 | nHydroxyapatite/polyurethane | Castor oil | Knee joint & articular cartilage | [174] |
| 25 | MWCNTs and graphene nanosheets | Sesame oil | Tissue engineering | [162] |
| 26 | Microfibrillated cellulose | Bleached sulfite softwood | Reinforcement in bionanocomposite | [175] |
| 27 | Chitosan composites |
Marine Crustacean Shells Thunnus obesus Fish Bone |
Bone tissue engineering | [176, 177] |
| 28 | Soy protein scaffolds |
Soy protein isolates Soybean oil epoxidized acrylate |
Tissue Engineering Tissue Engineering |
[59] [178] |
| 29 | Graphene Oxide | Sugarcane bagasse | Bone Tissue Engineering | [99, 179] |
Commercialization of natural biomaterials
Natural biomaterials have created a milestone by their commercial availability. Easy modification, biodegradability, bioresorbability among many other traits make them a potential filler and have been already being used in scaffold preparation for various applications as bone tissue engineering, nerve and brain, ocular and skin implants, sutures, ins and stents.
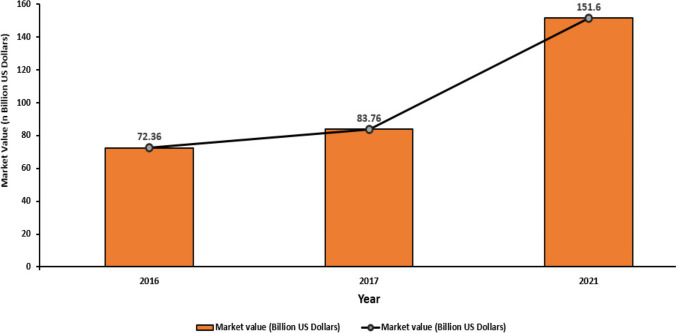
There are so many natural polymers which have been already available commercially PHAs-Polyhydroxyalkanoates as PHB, PHH, PHV have been used for synthesis of biocomposites for implants in various tissue engineering applications [180]. Bioimplants showed diversity in various biomedical applications including brain and sensory implants, organ, dental, cosmetic and structural implants as stents, braces, rods, heart valves, bones, and pins [181, 182]. There are various implants derived with constituents from agro-waste and are available in the market for different applications as dental implants, eyeball implant, cardiac valves and stent, Knee or hip replacements, pins, sutures (Table 5). These biomaterials, after successfully completing the clinical trials, were marketed as implants for the use. It has been estimated, the global market value of biomaterials was 72.36 billion US dollars which gradually hiked up in 2017 and found to be 83.76 and it is forecasted to increase to nearly 152 billion U.S. dollars in 2021 (Fig. 4).
Table 5.
Commercially available biomaterials from agro-waste
| S. No | Product | Constituents | Used For | Company | References |
|---|---|---|---|---|---|
| 1 | Granugel | Pectin, carboxymethylcellulose and propylene glycol | Wound Dressing | Convatec Co | [183, 184] |
| 2 | Chitipack S | Sponge-like chitin from squid and dispersed | Treatment of traumatic wounds and surgical tissue defects in animals | Eisai Co | [185] |
| 3 | Chitipack P | Swollen chitin supported on poly(ethylene therephthalate) | Dressing of large skin defects in animals | Eisai Co | [185] |
| 4 | HemCon | Freeze-dried chitosan acetate salt | Fatal infections in civilian emergency medical services and as antimicrobial dressings on infected burns, | HemCon Medical Technologies Inc | [185–187] |
| 5 | OTA Collagen Biomaterial (Patent) | Collagen dental membrane | Dental implants | Osseous Technologies of America, Inc | [188] |
| 6 | Bioseed-S | Keratinocytes suspended in fibrin glue | Epidermal Skin substitute, Venous leg ulcers | BioTissue technologies, Freidburg | [188] |
| 7 | EZ Derm | Cross-linked porcine collagen | Dermal Skin substitute for partial thickness burns | Genzyme Corp. Cambridge, Mass | [189] |
| 8 | Hyalograft 3D | Esterified hyaluronic acid matrix seeded with autologous fibroblasts | Dermal Skin substitute for full and partial thickness wounds, Scleroderma | Fidia Advanced biopolymers padova, Italy | [190] |
| 9 | Integra | Temporary silicone epidermal substitute over a dermal scaffold comprise of collagen and chondroitin-6-sulfate | Dermal skin burns, post-surgical wounds, diabetic | Integra LifeSciences Corp., Plainsboro, NJ | [191] |
| 10 | Alginate matrices as cardiac patches | Alginate matrices, seeded with cardiomyocytes | Cardiac patches | Childrens Medical Center Corp Massachusetts Institute of Technology | [192] |
| 11 | Chitosan/ hyaluronan | Chitosan or hyaluronan-based anisotropic scaffold prepared by electrospinning | Cardiac tissue engineering | Drexel University, Philadelphia, USA | [193, 194] |
| 12 | TriVisc (FDA approved) | Sterile sodium hyaluronate solution | Osteoarthritis of the knee | OrthogenRx, Inc | [195] |
| 13 | Edwards SAPIEN 3 Transcatheter Heart Valve | Cow tissue attached to a balloon-expandable, cobalt-chromium frame for support | Implant the valve without open-heart surgery | Edwards Lifesciences LLC | [196] |
| 14 | Endurant II/Endurant IIs Stent Graft System | Prosthetic endovascular graft, flexible fabric tube supported by a metal framework | Abdominal aortic aneurysm (AAA) | Medtronic Vascular, Santa Rosa, California | [197] |
| 15 | NeuraGen® NeuroFlex™ | Semipermeable, fibrillar structure of the collagen type 1 | Nerve tissue engineering | Integra Life Sciences Co, Plainsboro, NJ, USA; Collagen Matrix Inc., Franklin Lakes, NJ, USA | [198] |
Fig. 4.
Global growth profile of biomaterials market value from 2016 to 2021 (in billion U.S. dollars) (https://www.statista.com/statistics/800209/global-biomaterials-market-value/)
Conclusion and future outlook
Utilization of agricultural waste has been attempted several ways and its transformation for the development of biomaterials have given rise to value added products. These agro-waste derived biomaterials have attained place for application in tissue regeneration individually or in combination of other natural and synthetic biomaterials. In a way, it paves the way to agricultural waste management for commercially important products. Biomaterials extracted, purified, modified, and transformed can be employed for a myriad of biomedical applications. These agro based by-products are very much biocompatible, cost-effective, renewable, and sustainable source of polymers and minerals which could become integral part of living system. A wide range of commercial products are available being employed as biomaterials and having ingredients present even in agro-waste. Still the search for natural novel biomaterials is one of the key concern of biomaterials scientists. The challenge persists to extract the natural ingredients in pure form and in appreciable quantity for biomaterials development so as to achieve the goal of in vitro and in vivo tissue support and microenvironment mediated cell differentiation. The agro-waste is mine for the biomaterials constituent and further efforts are required for developing cost effective techniques for its transformation to biomaterials with commercial value while maintaining its high quality.
Acknowledgements
Authors are thankful to MHRD (Govt. of India) for motivating to contribute towards Clean India and Wealth from Waste initiatives. Also, authors express their gratitude towards continuous support from the Institutes (Indian Institute of Technology, Hyderabad and Dr. B. R. Ambedkar National Institute of Technology Jalandhar, Punjab, India).
Declarations
Conflict of Interest
The authors declare that there is no conflict of interest in any manner regarding the publication of this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Singha AS, Thakur VK. Physical, Chemical and Mechanical Properties of Hibiscus sabdariffa Fiber/Polymer Composite. Int J Polym Mater Polym Biomater. 2009;58:217–28 . [Google Scholar]
- 2.Thakur VK, Singha AS, Thakur MK. Biopolymers Based Green Composites: Mechanical, Thermal and Physico-chemical Characterization. J Polym Environ. 2012;20:412–421. [Google Scholar]
- 3.Seadi TA, Holm-Nielsen JB., III 2 Agricultural wastes. Waste Manag Ser. 2004;4:207–15. [Google Scholar]
- 4.Martin-Luengo MA, Yates M, Ramos M, Salgado JL, Aranda RMM, Plou F, et al. Renewable Raw Materials for advanced applications. 2011 World Congr Sustain Technol WCST. 2011. p. 19–22
- 5.Guo YP, Yang SF, Zhao JZ, Wang ZC, Zhao MY. Preparation of active carbon with high specific surface area from rice husks. Chem J Chinese Univ-Chinese. 2000;21(3):335–338.
- 6.Ling IH, Teo DCL. Lightweight concrete bricks produced from industrial and agricultural solid waste. 2011 World Congr Sustain Technol WCST. IEEE. 2011;148–52.
- 7.Surip SN, Bonnia NN, Anuar H, Hassan NA, Yusof NM. Nanofibers from oil palm trunk (OPT): Preparation chemical analysis. 2012 IEEE Symp Bus Eng Ind Appl. 2012;809–12.
- 8.Zakari Z, Buniran S, Ishak MI. Nanopores activated carbon rice husk. 2010 Int Conf Enabling Sci Nanotechnol (ESciNano). 2010;1–2.
- 9.Jawaid M, Khalil HPSA. EFFECT OF LAYERING PATTERN ON THE DYNAMIC MECHANICAL PROPERTIES AND THERMAL DEGRADATION OF OIL PALM-JUTE FIBERS REINFORCED EPOXY HYBRID COMPOSITE. BioResources. 2011;6:2309–2322. [Google Scholar]
- 10.Joshi SV, Drzal LT, Mohanty AK, Arora S. Are natural fiber composites environmentally superior to glass fiber reinforced composites? Compos Part Appl Sci Manuf. 2004;35:371–376. [Google Scholar]
- 11.Kalia S, Kaith BS, Kaur I. Pretreatments of natural fibers and their application as reinforcing material in polymer composites—A review. Polym Eng Sci. 2009;49:1253–1272. [Google Scholar]
- 12.Namvar F, Jawaid M, Tanir PM, Mohamad R, Azizi S, Khodavandi A, et al. Potential Use of Plant Fibres and their Composites for Biomedical Applications. BioResources. 2014;9:5688–5706. [Google Scholar]
- 13.Ogah AO, Afiukwa JN, Nduji AA. Characterization and comparison of rheological properties of agro fiber filled high-density polyethylene bio-composites. Open J Polym Chem. 2014;4(1):12–19.
- 14.Murali S, Shrivastava R, Saxena M. Quantification of agricultural residues for energy generation - A case study. J IPHE. 2007;3:27–31. [Google Scholar]
- 15.Kumar A, Kumar N, Baredar P, Shukla A. A review on biomass energy resources, potential, conversion and policy in India. Renew Sustain Energy Rev. 2015;45:530–539. [Google Scholar]
- 16.Tsang YF, Kumar V, Samadar P, Yang Y, Lee J, Ok YS, et al. Production of bioplastic through food waste valorization. Environ Int Elsevier. 2019;127:625–644. doi: 10.1016/j.envint.2019.03.076. [DOI] [PubMed] [Google Scholar]
- 17.Dittenber DB, GangaRao HVS. Critical review of recent publications on use of natural composites in infrastructure. Compos Part Appl Sci Manuf. 2012;43:1419–1429. [Google Scholar]
- 18.Dungani R, Karina M, Subyakto, Sulaeman A, Hermawan D, Hadiyane A. Agricultural waste fibers towards sustainability and advanced utilization: a review. Asian J Plant Sci. 2016;15:42–55. [Google Scholar]
- 19.Abdul Khalil HPS, Firoozian P, Bakare IO, Akil HMd, Noor AMd. Exploring biomass based carbon black as filler in epoxy composites: Flexural and thermal properties. Mater Des. 2010;31:3419–25. [Google Scholar]
- 20.Malafaya PB, Silva GA, Reis RL. Natural–origin polymers as carriers and scaffolds for biomolecules and cell delivery in tissue engineering applications. Adv Drug Deliv Rev. 2007;59:207–233. doi: 10.1016/j.addr.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 21.Asgher M, Ahmad Z, Iqbal HMN. Alkali and enzymatic delignification of sugarcane bagasse to expose cellulose polymers for saccharification and bio-ethanol production. Ind Crops Prod. 2013;44:488–495. [Google Scholar]
- 22.Ofori-Boateng C, Lee KT. Sustainable utilization of oil palm wastes for bioactive phytochemicals for the benefit of the oil palm and nutraceutical industries. Phytochem Rev. 2013;12:173–190. [Google Scholar]
- 23.Iqbal HMN, Kyazze G, Keshavarz T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. BioResources. 2013;8:3157–3176. [Google Scholar]
- 24.Driemeier C, Santos WD, Buckeridge MS. Cellulose crystals in fibrovascular bundles of sugarcane culms: orientation, size, distortion, and variability. Cellulose. 2012;19:1507–1515. [Google Scholar]
- 25.ROWELL RM. Characterization and factors effecting fiber properties. Nat Polym Agrofibers Based Compos [Internet]. Embrapa Instrumentacao Agropecuaria; 2000 [cited 2021 Apr 5]; Available from: https://ci.nii.ac.jp/naid/10019393394/
- 26.Azwa ZN, Yousif BF, Manalo AC, Karunasena W. A review on the degradability of polymeric composites based on natural fibres. Mater Des. 2013;47:424–442. [Google Scholar]
- 27.Siqueira G, Bras J, Dufresne A. New Process of Chemical Grafting of Cellulose Nanoparticles with a Long Chain Isocyanate. Langmuir American Chemical Society. 2010;26:402–411 . doi: 10.1021/la9028595. [DOI] [PubMed] [Google Scholar]
- 28.Methacanon P, Weerawatsophon U, Sumransin N, Prahsarn C, Bergado DT. Properties and potential application of the selected natural fibers as limited life geotextiles. Carbohydr Polym. 2010;82:1090–1096. [Google Scholar]
- 29.John MJ, Thomas S. Biofibres and biocomposites. Carbohydr Polym. 2008;71:343–364. doi: 10.1016/j.carbpol.2014.03.071. [DOI] [PubMed] [Google Scholar]
- 30.Thygesen A, Thomsen AB, Daniel G, Lilholt H. Comparison of composites made from fungal defibrated hemp with composites of traditional hemp yarn. Ind Crops Prod. 2007;25:147–159. [Google Scholar]
- 31.Jane J-L, Kasemsuwan T, Leas S, Zobel H, Robyt JF. Anthology of Starch Granule Morphology by Scanning Electron Microscopy. Starch - Stärke. 1994;46:121–129. [Google Scholar]
- 32.Lindeboom N, Chang PR, Tyler RT. Analytical, Biochemical and Physicochemical Aspects of Starch Granule Size, with Emphasis on Small Granule Starches: A Review. Starch - Stärke. 2004;56:89–99. [Google Scholar]
- 33.Rosen S, Meade B, Fuglie K, Rada N. International Food Security Assessment, 2014–2024. 2.
- 34.Chen Q, Yu H, Wang L, Abdin Z ul, Chen Y, Wang J, et al. Recent progress in chemical modification of starch and its applications. RSC Adv. 2015;5:67459–74 . [Google Scholar]
- 35.Liu D, Qi Z, Zhang Y, Xu J, Guo B. Poly(butylene succinate) (PBS)/ionic liquid plasticized starch blends: Preparation, characterization, and properties. Starch - Stärke. 2015;67:802–809. [Google Scholar]
- 36.Guna V, Ilangovan M, Anantha Prasad MG, Reddy N. Water Hyacinth: A Unique Source for Sustainable Materials and Products. ACS Sustain Chem Eng. 2017;5:4478–90 . [Google Scholar]
- 37.Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003;24:401–416. doi: 10.1016/s0142-9612(02)00353-8. [DOI] [PubMed] [Google Scholar]
- 38.Sah MK, Pramanik K. Soluble-eggshell-membrane-protein-modified porous silk fibroin scaffolds with enhanced cell adhesion and proliferation properties. J Appl Polym Sci [Internet]. 2014 [cited 2021 Apr 6];131. Available from: https://onlinelibrary.wiley.com/doi/abs/10.1002/app.40138
- 39.Reddy N, Yang Y. Potential of plant proteins for medical applications. Trends Biotechnol. 2011;29:490–498. doi: 10.1016/j.tibtech.2011.05.003. [DOI] [PubMed] [Google Scholar]
- 40.Vepari C, Kaplan DL. Silk as a biomaterial. Prog Polym Sci. 2007;32:991–1007. doi: 10.1016/j.progpolymsci.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Naskar D, Nayak S, Dey T, Kundu SC. Non-mulberry silk fibroin influence osteogenesis and osteoblast-macrophage cross talk on titanium based surface. Sci Rep. 2014;4:4745. doi: 10.1038/srep04745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Humenik M, Scheibel T, Smith A. Spider Silk: Understanding the Structure–Function Relationship of a Natural Fiber. In: Howorka S, editor. Prog Mol Biol Transl Sci [Internet]. Academic Press; 2011 [cited 2021 Apr 6]. p. 131–85. Available from: https://www.sciencedirect.com/science/article/pii/B9780124159068000078 [DOI] [PubMed]
- 43.Kundu B, Kurland NE, Bano S, Patra C, Engel FB, Yadavalli VK, et al. Silk proteins for biomedical applications: Bioengineering perspectives. Prog Polym Sci. 2014;39:251–267. [Google Scholar]
- 44.Gomes S, Leonor IB, Mano JF, Reis RL, Kaplan DL. Natural and genetically engineered proteins for tissue engineering. Prog Polym Sci. 2012;37:1–17. doi: 10.1016/j.progpolymsci.2011.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sah MK, Pramanik K. Regenerated Silk Fibroin from B. mori Silk Cocoon for Tissue Engineering Applications. Int J Environ Sci Dev. 2010;404–8.
- 46.Maziz A, Leprette O, Boyer L, Blatché C, Bergaud C. Tuning the properties of silk fibroin biomaterial via chemical cross-linking. Biomed Phys Eng Express. 2018;4:065012. [Google Scholar]
- 47.Janani G, Kumar M, Chouhan D, Moses JC, Gangrade A, Bhattacharjee S, et al. Insight into Silk-Based Biomaterials: From Physicochemical Attributes to Recent Biomedical Applications. ACS Appl Bio Mater. 2019;2:5460–91 . doi: 10.1021/acsabm.9b00576. [DOI] [PubMed] [Google Scholar]
- 48.Tu J, Wang H, Li H, Dai K, Wang J, Zhang X. The in vivo bone formation by mesenchymal stem cells in zein scaffolds. Biomaterials. 2009;30:4369–4376. doi: 10.1016/j.biomaterials.2009.04.054. [DOI] [PubMed] [Google Scholar]
- 49.Dong J, Sun Q, Wang J-Y. Basic study of corn protein, zein, as a biomaterial in tissue engineering, surface morphology and biocompatibility. Biomaterials. 2004;25:4691–4697. doi: 10.1016/j.biomaterials.2003.10.084. [DOI] [PubMed] [Google Scholar]
- 50.Kim S, Xu J. Aggregate formation of zein and its structural inversion in aqueous ethanol. J Cereal Sci. 2008;47:1–5. [Google Scholar]
- 51.Ghanbarzadeh B, Musavi M, Oromiehie AR, Rezayi K, Razmi Rad E, Milani J. Effect of plasticizing sugars on water vapor permeability, surface energy and microstructure properties of zein films. LWT - Food Sci Technol. 2007;40:1191–1197. [Google Scholar]
- 52.Sousa FFO, Luzardo-Álvarez A, Blanco-Méndez J, Martín-Pastor M. NMR techniques in drug delivery: Application to zein protein complexes. Int J Pharm. 2012;439:41–48. doi: 10.1016/j.ijpharm.2012.09.046. [DOI] [PubMed] [Google Scholar]
- 53.Khalil AA, Deraz SF, Elrahman SA, El-Fawal G. Enhancement of Mechanical Properties, Microstructure, and Antimicrobial Activities of Zein Films Cross-Linked Using Succinic Anhydride, Eugenol, and Citric Acid. Prep Biochem Biotechnol Taylor & Francis. 2015;45:551–567. doi: 10.1080/10826068.2014.940967. [DOI] [PubMed] [Google Scholar]
- 54.Shukla R, Cheryan M. Zein: the industrial protein from corn. Ind Crops Prod. 2001;13:171–192. [Google Scholar]
- 55.Paliwal R, Palakurthi S. Zein in controlled drug delivery and tissue engineering. J Controlled Release. 2014;189:108–122. doi: 10.1016/j.jconrel.2014.06.036. [DOI] [PubMed] [Google Scholar]
- 56.Zhao X, Chen F, Xue W, Lee L. FTIR spectra studies on the secondary structures of 7S and 11S globulins from soybean proteins using AOT reverse micellar extraction. Food Hydrocoll. 2008;22:568–575. [Google Scholar]
- 57.Silva NHCS, Vilela C, Marrucho IM, Freire CSR, Neto CP, Silvestre AJD. Protein-based materials: from sources to innovative sustainable materials for biomedical applications. J Mater Chem B. 2014;2:3715–40 . doi: 10.1039/c4tb00168k. [DOI] [PubMed] [Google Scholar]
- 58.Burgos-Díaz C, Wandersleben T, Marqués AM, Rubilar M. Multilayer emulsions stabilized by vegetable proteins and polysaccharides. Curr Opin Colloid Interface Sci. 2016;25:51–57. [Google Scholar]
- 59.Chien KB, Shah RN. Novel soy protein scaffolds for tissue regeneration: Material characterization and interaction with human mesenchymal stem cells. Acta Biomater. 2012;8:694–703. doi: 10.1016/j.actbio.2011.09.036. [DOI] [PubMed] [Google Scholar]
- 60.Song F, Tang D-L, Wang X-L, Wang Y-Z. Biodegradable Soy Protein Isolate-Based Materials: A Review. Biomacromolecules American Chemical Society. 2011;12:3369–3380. doi: 10.1021/bm200904x. [DOI] [PubMed] [Google Scholar]
- 61.Sah MK, Rath SN. Soluble eggshell membrane: A natural protein to improve the properties of biomaterials used for tissue engineering applications. Mater Sci Eng C. 2016;67:807–821. doi: 10.1016/j.msec.2016.05.005. [DOI] [PubMed] [Google Scholar]
- 62.Makareeva E, Leikin S. Chapter 7 - Collagen Structure, Folding and Function. In: Shapiro JR, Byers PH, Glorieux FH, Sponseller PD, editors. Osteogenes Imperfecta [Internet]. San Diego: Academic Press; 2014 [cited 2021 Apr 6]. p. 71–84. Available from: https://www.sciencedirect.com/science/article/pii/B9780123971654000071
- 63.Betancourt DE, Baldion PA, Castellanos JE. Resin-Dentin Bonding Interface: Mechanisms of Degradation and Strategies for Stabilization of the Hybrid Layer. Int J Biomater. 2019;2019:e5268342 . doi: 10.1155/2019/5268342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sato M, Asazuma T, Ishihara M, Kikuchi T, Masuoka K, Ichimura S, et al. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res A. 2003;64A:248–256. doi: 10.1002/jbm.a.10287. [DOI] [PubMed] [Google Scholar]
- 65.Rýglová Š, Braun M, Suchý T. Collagen and Its Modifications—Crucial Aspects with Concern to Its Processing and Analysis. Macromol Mater Eng. 2017;302:1600460. [Google Scholar]
- 66.Govindharaj M, Roopavath UK, Rath SN. Valorization of discarded Marine Eel fish skin for collagen extraction as a 3D printable blue biomaterial for tissue engineering. J Clean Prod. 2019;230:412–419. [Google Scholar]
- 67.Dang Q, Liu K, Zhang Z, Liu C, Liu X, Xin Y, et al. Fabrication and evaluation of thermosensitive chitosan/collagen/α, β-glycerophosphate hydrogels for tissue regeneration. Carbohydr Polym. 2017;167:145–157. doi: 10.1016/j.carbpol.2017.03.053. [DOI] [PubMed] [Google Scholar]
- 68.Im J, Hyun Choi C, Mun F, Lee J, Kim H, Jung W-K, et al. A polycaprolactone/fish collagen/alginate biocomposite supplemented with phlorotannin for hard tissue regeneration. RSC Adv Royal Society of Chemistry. 2017;7:2009–2018. [Google Scholar]
- 69.Chandika P, Ko S-C, Oh G-W, Heo S-Y, Nguyen V-T, Jeon Y-J, et al. Fish collagen/alginate/chitooligosaccharides integrated scaffold for skin tissue regeneration application. Int J Biol Macromol. 2015;81:504–513. doi: 10.1016/j.ijbiomac.2015.08.038. [DOI] [PubMed] [Google Scholar]
- 70.Ioan D-C, Rău I, Tihan GT, Zgârian RG, Ghica MV, Albu Kaya MG, et al. Piroxicam-Collagen-Based Sponges for Medical Applications. Int J Polym Sci. 2019;2019:e6062381 . [Google Scholar]
- 71.Alarcon EI, Udekwu KI, Noel CW, Gagnon LB-P, Taylor PK, Vulesevic B, et al. Safety and efficacy of composite collagen–silver nanoparticle hydrogels as tissue engineering scaffolds. Nanoscale. 2015;7:18789-98. [DOI] [PubMed]
- 72.Nanda HS, Chen S, Zhang Q, Kawazoe N, Chen G. Collagen Scaffolds with Controlled Insulin Release and Controlled Pore Structure for Cartilage Tissue Engineering. BioMed Res Int; 2014;2014:e623805. [DOI] [PMC free article] [PubMed]
- 73.Guan J, Zhu Z, Zhao RC, Xiao Z, Wu C, Han Q, et al. Transplantation of human mesenchymal stem cells loaded on collagen scaffolds for the treatment of traumatic brain injury in rats. Biomaterials. 2013;34:5937–5946. doi: 10.1016/j.biomaterials.2013.04.047. [DOI] [PubMed] [Google Scholar]
- 74.Inzana JA, Olvera D, Fuller SM, Kelly JP, Graeve OA, Schwarz EM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034. doi: 10.1016/j.biomaterials.2014.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jia W, Tang H, Wu J, Hou X, Chen B, Chen W, et al. Urethral tissue regeneration using collagen scaffold modified with collagen binding VEGF in a beagle model. Biomaterials. 2015;69:45–55. doi: 10.1016/j.biomaterials.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 76.Lin K-F, He S, Song Y, Wang C-M, Gao Y, Li J-Q, et al. Low-Temperature Additive Manufacturing of Biomimic Three-Dimensional Hydroxyapatite/Collagen Scaffolds for Bone Regeneration. ACS Appl Mater Interfaces. 2016;8:6905–16 . doi: 10.1021/acsami.6b00815. [DOI] [PubMed] [Google Scholar]
- 77.Huang D, Wu D. Biodegradable dendrimers for drug delivery. Mater Sci Eng C. 2018;90:713–727. doi: 10.1016/j.msec.2018.03.002. [DOI] [PubMed] [Google Scholar]
- 78.Chamieh F, Collignon A-M, Coyac BR, Lesieur J, Ribes S, Sadoine J, et al. Accelerated craniofacial bone regeneration through dense collagen gel scaffolds seeded with dental pulp stem cells. Sci Rep. 2016;6:38814 . doi: 10.1038/srep38814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Winias S, Ernawati DS, Ariani MD, Rahayu RP. Scaffold combination of chitosan and collagen synthesized from chicken feet induces osteoblast and osteoprotegerin expression in bone healing process of mice. Dent J Maj Kedokt Gigi. 2017;50:86. [Google Scholar]
- 80.Liu Y, Gu J, Fan D. Fabrication of High-Strength and Porous Hybrid Scaffolds Based on Nano-Hydroxyapatite and Human-Like Collagen for Bone Tissue Regeneration. Polymers. 2020;12:61 . doi: 10.3390/polym12010061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wei L-G, Chang H-I, Wang Y, Hsu S, Dai L-G, Fu K-Y, et al. A gelatin/collagen/polycaprolactone scaffold for skin regeneration. PeerJ. 2019;7:e6358 . doi: 10.7717/peerj.6358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Long K, Liu Y, Li W, Wang L, Liu S, Wang Y, et al. Improving the mechanical properties of collagen-based membranes using silk fibroin for corneal tissue engineering. J Biomed Mater Res A. 2015;103:1159–1168. doi: 10.1002/jbm.a.35268. [DOI] [PubMed] [Google Scholar]
- 83.Cao H, Chen M-M, Liu Y, Liu Y-Y, Huang Y-Q, Wang J-H, et al. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces. 2015;136:1098–1106. doi: 10.1016/j.colsurfb.2015.10.022. [DOI] [PubMed] [Google Scholar]
- 84.Guo H, Hong Z, Yi R. Core-shell collagen peptide chelated calcium/calcium alginate nanoparticles from fish scales for calcium supplementation. J Food Sci Wiley Online Library. 2015;80:N1595–N1601. doi: 10.1111/1750-3841.12912. [DOI] [PubMed] [Google Scholar]
- 85.Veeruraj A, Arumugam M, Ajithkumar T, Balasubramanian T. Isolation and characterization of drug delivering potential of type-I collagen from eel fish Evenchelys macrura. J Mater Sci Mater Med Springer. 2012;23:1729–1738. doi: 10.1007/s10856-012-4650-2. [DOI] [PubMed] [Google Scholar]
- 86.Nicklas M, Schatton W, Heinemann S, Hanke T, Kreuter J. Enteric coating derived from marine sponge collagen. Drug Dev Ind Pharm. 2009;35:1384–8 . doi: 10.3109/03639040902939239. [DOI] [PubMed] [Google Scholar]
- 87.Banerjee P, Lenz D, Robinson JP, Rickus JL, Bhunia AK. A novel and simple cell-based detection system with a collagen-encapsulated B-lymphocyte cell line as a biosensor for rapid detection of pathogens and toxins. Lab Invest Nature Publishing Group. 2008;88:196–206. doi: 10.1038/labinvest.3700703. [DOI] [PubMed] [Google Scholar]
- 88.Sastry TP. Collagen thin film glucose biosensor. Asian J Biomed Pharm Sci. 2014;4:11–14. [Google Scholar]
- 89.Bose S, Tarafder S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012;8:1401–1421. doi: 10.1016/j.actbio.2011.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Bouler JM, Pilet P, Gauthier O, Verron E. Biphasic calcium phosphate ceramics for bone reconstruction: A review of biological response. Acta Biomater. 2017;53:1–12. doi: 10.1016/j.actbio.2017.01.076. [DOI] [PubMed] [Google Scholar]
- 91.Dadhich P, Das B, Pal P, Srivas PK, Dutta J, Ray S, et al. A Simple Approach for an Eggshell-Based 3D-Printed Osteoinductive Multiphasic Calcium Phosphate Scaffold. ACS Appl Mater Interfaces. 2016;8:11910–24 . doi: 10.1021/acsami.5b11981. [DOI] [PubMed] [Google Scholar]
- 92.Jafarkhani M, Fazlali A, Moztarzadeh F, Moztarzadeh Z, Mozafari M. Fabrication and Characterization of PLLA/Chitosan/Nano Calcium Phosphate Scaffolds by Freeze-Casting Technique. Ind Eng Chem Res. 2012;51:9241–9 . [Google Scholar]
- 93.Shavandi A, Bekhit AE-DA, Ali A, Sun Z. Synthesis of nano-hydroxyapatite (nHA) from waste mussel shells using a rapid microwave method. Mater Chem Phys. 2015;149–150:607–16.
- 94.Baykan E, Koc A, Eser Elcin A, Murat Elcin Y. Evaluation of a biomimetic poly(ε-caprolactone)/β-tricalcium phosphate multispiral scaffold for bone tissue engineering: In vitro and in vivo studies. Biointerphases. 2014;9:0290113 . doi: 10.1116/1.4870781. [DOI] [PubMed] [Google Scholar]
- 95.Tavangar A, Tan B, Venkatakrishnan K. Synthesis of three-dimensional calcium carbonate nanofibrous structure from eggshell using femtosecond laser ablation. J Nanobiotechnology. 2011;9:1. doi: 10.1186/1477-3155-9-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Baino F, Vitale-Brovarone C. 8 - Bioactive glass and glass–ceramic foam scaffolds for bone tissue restoration. In: Netti PA, editor. Biomed Foams Tissue Eng Appl [Internet]. Woodhead Publishing; 2014 [cited 2021 Apr 6]. p. 213–48. Available from: https://www.sciencedirect.com/science/article/pii/B9780857096968500085
- 97.Feng P, Niu M, Gao C, Peng S, Shuai C. A novel two-step sintering for nano-hydroxyapatite scaffolds for bone tissue engineering. Sci Rep. 2014;4:5599 . doi: 10.1038/srep05599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lin Y-H, Chiu Y-C, Shen Y-F, Wu Y-HA, Shie M-Y. Bioactive calcium silicate/poly-ε-caprolactone composite scaffolds 3D printed under mild conditions for bone tissue engineering. J Mater Sci Mater Med. 2017;29:11. [DOI] [PubMed]
- 99.Somanathan T, Prasad K, Ostrikov K (Ken), Saravanan A, Krishna VM. Graphene Oxide Synthesis from Agro Waste. Nanomaterials. Multidisciplinary Digital Publishing Institute; 2015;5:826–34. [DOI] [PMC free article] [PubMed]
- 100.Anantharaman A. Green Synthesis of Calcium Oxide Nanoparticles and Its Applications. 2016;6:5. [Google Scholar]
- 101.Wu S-C, Tsou H-K, Hsu H-C, Hsu S-K, Liou S-P, Ho W-F. A hydrothermal synthesis of eggshell and fruit waste extract to produce nanosized hydroxyapatite. Ceram Int. 2013;39:8183–8188. [Google Scholar]
- 102.Roopavath UK, Sah MK, Panigrahi BB, Rath SN. Mechanochemically synthesized phase stable and biocompatible β-tricalcium phosphate from avian eggshell for the development of tissue ingrowth system. Ceram Int. 2019;45:12910–12919. [Google Scholar]
- 103.Neunzehn J, Szuwart T, Wiesmann H-P. Eggshells as natural calcium carbonate source in combination with hyaluronan as beneficial additives for bone graft materials, an in vitro study. Head Face Med. 2015;11:12. doi: 10.1186/s13005-015-0070-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Maleki S, Barzegar-Jalali M, Zarrintan MH, Adibkia K, Lotfipour F. Calcium carbonate nanoparticles; potential applications in bone and tooth disorders. Pharm Sci. Tabriz University of Medical Sciences; 20:175–82.
- 105.Siqueira G, Abdillahi H, Bras J, Dufresne A. High reinforcing capability cellulose nanocrystals extracted from Syngonanthus nitens (Capim Dourado) Cellulose. 2010;17:289–298. [Google Scholar]
- 106.Kalia S, Dufresne A, Cherian BM, Kaith BS, Avérous L, Njuguna J, et al. Cellulose-Based Bio- and Nanocomposites: A Review. Int J Polym Sci. . 2011;2011:e837875. [Google Scholar]
- 107.Akin DE, Condon B, Sohn M, Foulk JA, Dodd RB, Rigsby LL. Optimization for enzyme-retting of flax with pectate lyase. Ind Crops Prod. 2007;25:136–146. [Google Scholar]
- 108.Banik S, Basak MK, Paul D, Nayak P, Sardar D, Sil SC, et al. Ribbon retting of jute—a prospective and eco-friendly method for improvement of fibre quality. Ind Crops Prod. 2003;17:183–190. [Google Scholar]
- 109.Song B, Lin R, Lam CH, Wu H, Tsui T-H, Yu Y. Recent advances and challenges of inter-disciplinary biomass valorization by integrating hydrothermal and biological techniques. Renew Sustain Energy Rev. 2021;135:110370 . [Google Scholar]
- 110.Johar N, Ahmad I, Dufresne A. Extraction, preparation and characterization of cellulose fibres and nanocrystals from rice husk. Ind Crops Prod. 2012;37:93–99. [Google Scholar]
- 111.Jahan MS, Saeed A, He Z, Ni Y. Jute as raw material for the preparation of microcrystalline cellulose. Cellulose. 2011;18:451–459. [Google Scholar]
- 112.Qua EH, Hornsby PR, Sharma HSS, Lyons G. Preparation and characterisation of cellulose nanofibres. J Mater Sci. 2011;46:6029–6045. [Google Scholar]
- 113.Brinchi L, Cotana F, Fortunati E, Kenny JM. Production of nanocrystalline cellulose from lignocellulosic biomass: Technology and applications. Carbohydr Polym. 2013;94:154–169. doi: 10.1016/j.carbpol.2013.01.033. [DOI] [PubMed] [Google Scholar]
- 114.Alemdar A, Sain M. Isolation and characterization of nanofibers from agricultural residues – Wheat straw and soy hulls. Bioresour Technol. 2008;99:1664–1671. doi: 10.1016/j.biortech.2007.04.029. [DOI] [PubMed] [Google Scholar]
- 115.Hassan ML, Mathew AP, Hassan EA, El-Wakil NA, Oksman K. Nanofibers from bagasse and rice straw: process optimization and properties. Wood Sci Technol. 2012;46:193–205. [Google Scholar]
- 116.Ferrer A, Filpponen I, Rodríguez A, Laine J, Rojas OJ. Valorization of residual Empty Palm Fruit Bunch Fibers (EPFBF) by microfluidization: Production of nanofibrillated cellulose and EPFBF nanopaper. Bioresour Technol. 2012;125:249–255. doi: 10.1016/j.biortech.2012.08.108. [DOI] [PubMed] [Google Scholar]
- 117.Chen W, Yu H, Liu Y, Hai Y, Zhang M, Chen P. Isolation and characterization of cellulose nanofibers from four plant cellulose fibers using a chemical-ultrasonic process. Cellulose. 2011;18:433–442. [Google Scholar]
- 118.Wang S, Cheng Q. A novel process to isolate fibrils from cellulose fibers by high-intensity ultrasonication, Part 1: Process optimization. J Appl Polym Sci. 2009;113:1270–1275. [Google Scholar]
- 119.Fatah IYA, Khalil HPSA, Hossain MS, Aziz AA, Davoudpour Y, Dungani R, et al. Exploration of a Chemo-Mechanical Technique for the Isolation of Nanofibrillated Cellulosic Fiber from Oil Palm Empty Fruit Bunch as a Reinforcing Agent in Composites Materials. Polymers Multidisciplinary Digital Publishing Institute. 2014;6:2611–2624. [Google Scholar]
- 120.Ha TLB, Quan TM, Vu DN, Si DM. Naturally Derived Biomaterials: Preparation and Application. Regen Med Tissue Eng [Internet]. IntechOpen; 2013 [cited 2021 Apr 6]; Available from: https://www.intechopen.com/books/regenerative-medicine-and-tissue-engineering/naturally-derived-biomaterials-preparation-and-application
- 121.Fabian C, Ju Y-H. A Review on Rice Bran Protein: Its Properties and Extraction Methods. Crit Rev Food Sci Nutr. 2011;51:816–27 . doi: 10.1080/10408398.2010.482678. [DOI] [PubMed] [Google Scholar]
- 122.Sharma K, Mujawar MA, Kaushik A. State-of-Art Functional Biomaterials for Tissue Engineering. Front Mater [Internet]. Frontiers; 2019 [cited 2021 Apr 6];6. Available from: https://www.frontiersin.org/articles/10.3389/fmats.2019.00172/full
- 123.Ramesh N, Moratti SC, Dias GJ. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J Biomed Mater Res B Appl Biomater. 2018;106:2046–2057. doi: 10.1002/jbm.b.33950. [DOI] [PubMed] [Google Scholar]
- 124.Ho M, Wang H, Lee J-H, Ho C, Lau K, Leng J, et al. Critical factors on manufacturing processes of natural fibre composites. Compos Part B Eng. 2012;43:3549–3562. [Google Scholar]
- 125.Baino F, Novajra G, Vitale-Brovarone C. Bioceramics and Scaffolds: A Winning Combination for Tissue Engineering. Front Bioeng Biotechnol [Internet]. Frontiers; 2015 [cited 2021 Apr 6];3. Available from: https://www.frontiersin.org/articles/10.3389/fbioe.2015.00202/full [DOI] [PMC free article] [PubMed]
- 126.Pina S, Oliveira JM, Reis RL. Natural-Based Nanocomposites for Bone Tissue Engineering and Regenerative Medicine: A Review. Adv Mater. 2015;27:1143–1169. doi: 10.1002/adma.201403354. [DOI] [PubMed] [Google Scholar]
- 127.Edwards A, Jarvis D, Hopkins T, Pixley S, Bhattarai N. Poly(ε-caprolactone)/keratin-based composite nanofibers for biomedical applications. J Biomed Mater Res B Appl Biomater. 2015;103:21–30. doi: 10.1002/jbm.b.33172. [DOI] [PubMed] [Google Scholar]
- 128.Bulota M, Jääskeläinen AS, Paltakari J, Hughes M. Properties of biocomposites: influence of preparation method, testing environment and a comparison with theoretical models. J Mater Sci. 2011;46:3387–3398. [Google Scholar]
- 129.Fu W, Liu Z, Feng B, Hu R, He X, Wang H, et al. Electrospun gelatin/PCL and collagen/PLCL scaffolds for vascular tissue engineering. Int J Nanomedicine. 2014;9:2335–2344. doi: 10.2147/IJN.S61375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Kijeńska E, Prabhakaran MP, Swieszkowski W, Kurzydlowski KJ, Ramakrishna S. Electrospun bio-composite P(LLA-CL)/collagen I/collagen III scaffolds for nerve tissue engineering. J Biomed Mater Res B Appl Biomater. 2012;100B:1093–1102. doi: 10.1002/jbm.b.32676. [DOI] [PubMed] [Google Scholar]
- 131.Arun Kumar R, Sivashanmugam A, Deepthi S, Iseki S, Chennazhi KP, Nair SV, et al. Injectable Chitin-Poly(ε-caprolactone)/Nanohydroxyapatite Composite Microgels Prepared by Simple Regeneration Technique for Bone Tissue Engineering. ACS Appl Mater Interfaces. 2015;7:9399–409 . doi: 10.1021/acsami.5b02685. [DOI] [PubMed] [Google Scholar]
- 132.Lu T, Li Q, Chen W, Yu H. Composite aerogels based on dialdehyde nanocellulose and collagen for potential applications as wound dressing and tissue engineering scaffold. Compos Sci Technol. 2014;94:132–138. [Google Scholar]
- 133.Wu S, Applewhite AJ, Niezgoda J, Snyder R, Shah J, Cullen B, et al. Oxidized Regenerated Cellulose/Collagen Dressings: Review of Evidence and Recommendations. Adv Skin Wound Care. 2017;30:S1–18. doi: 10.1097/01.ASW.0000525951.20270.6c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Martins AM, Eng G, Caridade SG, Mano JF, Reis RL, Vunjak-Novakovic G. Electrically Conductive Chitosan/Carbon Scaffolds for Cardiac Tissue Engineering. Biomacromolecules American Chemical Society. 2014;15:635–643. doi: 10.1021/bm401679q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Nasri-Nasrabadi B, Mehrasa M, Rafienia M, Bonakdar S, Behzad T, Gavanji S. Porous starch/cellulose nanofibers composite prepared by salt leaching technique for tissue engineering. Carbohydr Polym. 2014;108:232–238. doi: 10.1016/j.carbpol.2014.02.075. [DOI] [PubMed] [Google Scholar]
- 136.Salgado AJ, Sousa RA, Fraga JS, Pego JM, Silva BA, Malva JO, et al. Effects of Starch/ Polycaprolactone-based Blends for Spinal Cord Injury Regeneration in Neurons/Glial Cells Viability and Proliferation. J Bioact Compat Polym. 2009;24:235–48. [Google Scholar]
- 137.Rnjak-Kovacina J, Wise SG, Li Z, Maitz PKM, Young CJ, Wang Y, et al. Electrospun synthetic human elastin:collagen composite scaffolds for dermal tissue engineering. Acta Biomater. 2012;8:3714–3722. doi: 10.1016/j.actbio.2012.06.032. [DOI] [PubMed] [Google Scholar]
- 138.Zhang C, Salick MR, Cordie TM, Ellingham T, Dan Y, Turng L-S. Incorporation of poly(ethylene glycol) grafted cellulose nanocrystals in poly(lactic acid) electrospun nanocomposite fibers as potential scaffolds for bone tissue engineering. Mater Sci Eng C. 2015;49:463–471. doi: 10.1016/j.msec.2015.01.024. [DOI] [PubMed] [Google Scholar]
- 139.Wei G, Li C, Fu Q, Xu Y, Li H. Preparation of PCL/silk fibroin/collagen electrospun fiber for urethral reconstruction. Int Urol Nephrol. 2015;47:95–99. doi: 10.1007/s11255-014-0854-3. [DOI] [PubMed] [Google Scholar]
- 140.Semnani D, Naghashzargar E, Hadjianfar M, Manshadi FD, Mohammadi S, Karbasi S, et al. Evaluation of PCL/chitosan electrospun nanofibers for liver tissue engineering. Int J Polym Mater Polym Biomater. 2017;66:149–57 . [Google Scholar]
- 141.Nazeer MA, Yilgör E, Yilgör I. Intercalated chitosan/hydroxyapatite nanocomposites: Promising materials for bone tissue engineering applications. Carbohydr Polym. 2017;175:38–46. doi: 10.1016/j.carbpol.2017.07.054. [DOI] [PubMed] [Google Scholar]
- 142.Sambudi NS, Sathyamurthy M, Lee GM, Park SB. Electrospun chitosan/poly(vinyl alcohol) reinforced with CaCO3 nanoparticles with enhanced mechanical properties and biocompatibility for cartilage tissue engineering. Compos Sci Technol. 2015;106:76–84. [Google Scholar]
- 143.Sambudi NS, Park SB, Cho K. Enhancing the mechanical properties of electrospun chitosan/poly(vinyl alcohol) fibers by mineralization with calcium carbonate. J Mater Sci. 2016;51:7742–7753. [Google Scholar]
- 144.Turnbull G, Clarke J, Picard F, Riches P, Jia L, Han F, et al. 3D bioactive composite scaffolds for bone tissue engineering. Bioact Mater. 2018;3:278–314. doi: 10.1016/j.bioactmat.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Ude AU, Eshkoor RA, Zulkifili R, Ariffin AK, Dzuraidah AW, Azhari CH. Bombyx mori silk fibre and its composite: A review of contemporary developments. Mater Des. 2014;57:298–305. [Google Scholar]
- 146.Teimouri A, Ghorbanian L, Najafi Chermahini A, Emadi R. Fabrication and characterization of silk/forsterite composites for tissue engineering applications. Ceram Int. 2014;40:6405–6411. [Google Scholar]
- 147.Tajbakhsh S, Hajiali F. A comprehensive study on the fabrication and properties of biocomposites of poly(lactic acid)/ceramics for bone tissue engineering. Mater Sci Eng C. 2017;70:897–912. doi: 10.1016/j.msec.2016.09.008. [DOI] [PubMed] [Google Scholar]
- 148.Parenteau-Bareil R, Gauvin R, Berthod F. Collagen-Based Biomaterials for Tissue Engineering Applications. Materials . 2010;3:1863–1887 . [Google Scholar]
- 149.Gurunathan T, Mohanty S, Nayak SK. A review of the recent developments in biocomposites based on natural fibres and their application perspectives. Compos Part Appl Sci Manuf. 2015;77:1–25. [Google Scholar]
- 150.Mukhopadhyay S, Fangueiro R. Physical Modification of Natural Fibers and Thermoplastic Films for Composites — A Review. J Thermoplast Compos Mater. 2009;22:135–62 . [Google Scholar]
- 151.Macocinschi D, Filip D, Vlad S, Butnaru M, Knieling L. Evaluation of polyurethane based on cellulose derivative-ketoprofen biosystem for implant biomedical devices. Int J Biol Macromol. 2013;52:32–37. doi: 10.1016/j.ijbiomac.2012.09.026. [DOI] [PubMed] [Google Scholar]
- 152.Rani MU, Rastogi NK, Anu Appaiah KA. Statistical Optimization of Medium Composition for Bacterial Cellulose Production by Gluconacetobacter hansenii UAC09 Using Coffee Cherry Husk Extract - an Agro-Industry Waste. J Microbiol Biotechnol. 2011;21:739–45 . doi: 10.4014/jmb.1012.12026. [DOI] [PubMed] [Google Scholar]
- 153.Aramwit P, Siritientong T, Srichana T. Potential applications of silk sericin, a natural protein from textile industry by-products. Waste Manag Res. 2012;30:217–24 . doi: 10.1177/0734242X11404733. [DOI] [PubMed] [Google Scholar]
- 154.Archana G, Sabina K, Babuskin S, Radhakrishnan K, Fayidh MA, Babu PAS, et al. Preparation and characterization of mucilage polysaccharide for biomedical applications. Carbohydr Polym. 2013;98:89–94. doi: 10.1016/j.carbpol.2013.04.062. [DOI] [PubMed] [Google Scholar]
- 155.Athinarayanan J, Periasamy VS, Alhazmi M, Alatiah KA, Alshatwi AA. Synthesis of biogenic silica nanoparticles from rice husks for biomedical applications. Ceram Int. 2015;41:275–281. [Google Scholar]
- 156.Bhardwaj N, Nguyen QT, Chen AC, Kaplan DL, Sah RL, Kundu SC. Potential of 3-D tissue constructs engineered from bovine chondrocytes/silk fibroin-chitosan for in vitro cartilage tissue engineering. Biomaterials. 2011;32:5773–5781. doi: 10.1016/j.biomaterials.2011.04.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Cheirmadurai K, Thanikaivelan P, Murali R. Highly biocompatible collagen–Delonix regia seed polysaccharide hybrid scaffolds for antimicrobial wound dressing. Carbohydr Polym. 2016;137:584–593. doi: 10.1016/j.carbpol.2015.11.015. [DOI] [PubMed] [Google Scholar]
- 158.Chinga-Carrasco G, Ehman NV, Pettersson J, Vallejos ME, Brodin MW, Felissia FE, et al. Pulping and Pretreatment Affect the Characteristics of Bagasse Inks for Three-dimensional Printing. ACS Sustain Chem Eng. 2018;6:4068–75 . [Google Scholar]
- 159.Li Q, Lu F, Zhou G, Yu K, Lu B, Xiao Y, et al. Silver Inlaid with Gold Nanoparticle/Chitosan Wound Dressing Enhances Antibacterial Activity and Porosity, and Promotes Wound Healing. Biomacromolecules . 2017;18:3766–3775 . doi: 10.1021/acs.biomac.7b01180. [DOI] [PubMed] [Google Scholar]
- 160.Mandal BB, Grinberg A, Gil ES, Panilaitis B, Kaplan DL. High-strength silk protein scaffolds for bone repair. Proc Natl Acad Sci. 2012;109:7699–704. doi: 10.1073/pnas.1119474109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Tovar-Carrillo KL, Sueyoshi SS, Tagaya M, Kobayashi T. Fibroblast Compatibility on Scaffold Hydrogels Prepared from Agave Tequilana Weber Bagasse for Tissue Regeneration. Ind Eng Chem Res. 2013;52:11607–13 . [Google Scholar]
- 162.Kumar A, Negi YS, Choudhary V, Bhardwaj NK. Characterization of Cellulose Nanocrystals Produced by Acid-Hydrolysis from Sugarcane Bagasse as Agro-Waste. J Mater Phys Chem. 2014;2:1–8 . [Google Scholar]
- 163.Cherian BM, Leão AL, de Souza SF, Costa LMM, de Olyveira GM, Kottaisamy M, et al. Cellulose nanocomposites with nanofibres isolated from pineapple leaf fibers for medical applications. Carbohydr Polym. 2011;86:1790–1798. [Google Scholar]
- 164.Czaja W, Krystynowicz A, Bielecki S, Brown RM. Microbial cellulose—the natural power to heal wounds. Biomaterials. 2006;27:145–151. doi: 10.1016/j.biomaterials.2005.07.035. [DOI] [PubMed] [Google Scholar]
- 165.Hoyer B, Bernhardt A, Heinemann S, Stachel I, Meyer M, Gelinsky M. Biomimetically Mineralized Salmon Collagen Scaffolds for Application in Bone Tissue Engineering. Biomacromolecules American Chemical Society. 2012;13:1059–1066. doi: 10.1021/bm201776r. [DOI] [PubMed] [Google Scholar]
- 166.Jithendra P, Rajam AM, Kalaivani T, Mandal AB, Rose C. Preparation and Characterization of Aloe Vera Blended Collagen-Chitosan Composite Scaffold for Tissue Engineering Applications. ACS Appl Mater Interfaces. 2013;5:7291–8 . doi: 10.1021/am401637c. [DOI] [PubMed] [Google Scholar]
- 167.Nakasone K, Ikematsu S, Kobayashi T. Biocompatibility Evaluation of Cellulose Hydrogel Film Regenerated from Sugar Cane Bagasse Waste and Its in Vivo Behavior in Mice. Ind Eng Chem Res. 2016;55:30–7 . [Google Scholar]
- 168.Silvério HA, Flauzino Neto WP, Pasquini D. Effect of Incorporating Cellulose Nanocrystals from Corncob on the Tensile, Thermal and Barrier Properties of Poly(Vinyl Alcohol) Nanocomposites. J Nanomater. 2013;2013:e289641 . [Google Scholar]
- 169.dos Santos RM, Flauzino Neto WP, Silvério HA, Martins DF, Dantas NO, Pasquini D. Cellulose nanocrystals from pineapple leaf, a new approach for the reuse of this agro-waste. Ind Crops Prod. 2013;50:707–714. [Google Scholar]
- 170.Gade R, Tulasi MS, Bhai VA. Seaweeds: a novel biomaterial. Int J Pharm Pharm Sci. 2013;5:975–1491. [Google Scholar]
- 171.Akhavan O, Bijanzad K, Mirsepah A. Synthesis of graphene from natural and industrial carbonaceous wastes. RSC Adv Royal Society of Chemistry. 2014;4:20441–20448. [Google Scholar]
- 172.Chen D, Lawton D, Thompson MR, Liu Q. Biocomposites reinforced with cellulose nanocrystals derived from potato peel waste. Carbohydr Polym. 2012;90:709–716. doi: 10.1016/j.carbpol.2012.06.002. [DOI] [PubMed] [Google Scholar]
- 173.Salgado AJ, Coutinho OP, Reis Rl. Novel starch-based scaffolds for bone tissue engineering: cytotoxicity, cell culture, and protein expression. Tissue eng. 2004;10(3-4):465–474. [DOI] [PubMed]
- 174.Chowdhury A, Anthony P. A review on biodegradable polymeric materials derived from vegetable oils for diverse applications. Int J Sci Res. 2016;5:1786–1791.
- 175.Lönnberg H, Larsson K, Lindström T, Hult A, Malmström E. Synthesis of Polycaprolactone-Grafted Microfibrillated Cellulose for Use in Novel Bionanocomposites-Influence of the Graft Length on the Mechanical Properties. ACS Appl Mater Interfaces. 2011;3:1426–33 . doi: 10.1021/am2001828. [DOI] [PubMed] [Google Scholar]
- 176.Venkatesan J, Kim S-K. Chitosan Composites for Bone Tissue Engineering—An Overview. Mar Drugs . 2010;8:2252–2266 . doi: 10.3390/md8082252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Venkatesan J, Qian Z-J, Ryu B, Ashok Kumar N, Kim S-K. Preparation and characterization of carbon nanotube-grafted-chitosan – Natural hydroxyapatite composite for bone tissue engineering. Carbohydr Polym. 2011;83:569–577. [Google Scholar]
- 178.Miao S, Zhu W, Castro NJ, Nowicki M, Zhou X, Cui H, et al. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci Rep. 2016;6:27226. doi: 10.1038/srep27226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Prasadh S, Suresh S, Wong R. Osteogenic Potential of Graphene in Bone Tissue Engineering Scaffolds. Materials. 2018;11:1430 . doi: 10.3390/ma11081430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Luef KP, Stelzer F, Wiesbrock F. Poly(hydroxy alkanoate)s in Medical Applications. Chem Biochem Eng Q. 2015;29:287–97 . doi: 10.15255/CABEQ.2014.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Soler-Botija C, Bagó JR, Llucià-Valldeperas A, Vallés-Lluch A, Castells-Sala C, Martínez-Ramos C, et al. Engineered 3D bioimplants using elastomeric scaffold, self-assembling peptide hydrogel, and adipose tissue-derived progenitor cells for cardiac regeneration. Am J Transl Res. 2014;6:291–301. [PMC free article] [PubMed] [Google Scholar]
- 182.Yahyavi-Firouz-Abadi N, Menias CO, Bhalla S, Siegel C, Gayer G, Katz DS. Imaging of Cosmetic Plastic Procedures and Implants in the Body and Their Potential Complications. Am J Roentgenol. 2015;204:707–15 . doi: 10.2214/AJR.14.13516. [DOI] [PubMed] [Google Scholar]
- 183.Williams C. Granugel: hydrocolloid gel. Br J Nurs Mark Allen Group. 1996;5:188–190. doi: 10.12968/bjon.1996.5.3.188. [DOI] [PubMed] [Google Scholar]
- 184.Caló E, Khutoryanskiy VV. Biomedical applications of hydrogels: A review of patents and commercial products. Eur Polym J. 2015;65:252–267. [Google Scholar]
- 185.Francesko A, Tzanov T. Chitin, Chitosan and Derivatives for Wound Healing and Tissue Engineering. In: Nyanhongo GS, Steiner W, Gübitz G, editors. Biofunctionalization Polym Their Appl [Internet]. Berlin, Heidelberg: Springer; 2011 [cited 2021 Apr 6]. p. 1–27. Available from: 10.1007/10_2010_93 [DOI] [PubMed]
- 186.Burkatovskaya M, Tegos GP, Swietlik E, Demidova TN, Castano PA, Hamblin MR. Use of chitosan bandage to prevent fatal infections developing from highly contaminated wounds in mice. Biomaterials. 2006;27:4157–64. doi: 10.1016/j.biomaterials.2006.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Huang L, Dai T, Xuan Y, Tegos GP, Hamblin MR. Synergistic Combination of Chitosan Acetate with Nanoparticle Silver as a Topical Antimicrobial: Efficacy against Bacterial Burn Infections. Antimicrob Agents Chemother. 2011;55:3432–8 . doi: 10.1128/AAC.01803-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Enoch S, Grey JE, Harding KG. Recent advances and emerging treatments. BMJ . 2006;332:962–965 . doi: 10.1136/bmj.332.7547.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Lawin PB, Silverstein P, Still JM. E-Z DERM™ A Porcine Heterograft Material. Am J Clin Dermatol. 2002;3:507–507. doi: 10.2165/00128071-200203070-00007. [DOI] [PubMed] [Google Scholar]
- 190.Pajardi G, Rapisarda V, Somalvico F, Scotti A, Russo GL, Ciancio F, et al. Skin substitutes based on allogenic fibroblasts or keratinocytes for chronic wounds not responding to conventional therapy: a retrospective observational study. Int Wound J. 2016;13:44–52. doi: 10.1111/iwj.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Varkey M, Ding J, Tredget EE. Advances in Skin Substitutes—Potential of Tissue Engineered Skin for Facilitating Anti-Fibrotic Healing. J Funct Biomater. 2015;6:547–63 . doi: 10.3390/jfb6030547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Dvir T, Kohane DS, Langer RS, Timko B. Nanowired three dimensional tissue scaffolds [Internet]. 2015 [cited 2021 Apr 6]. Available from: https://patents.google.com/patent/US9114009B2/en
- 193.Lelkes PI, Senel HG, Brookstein D, Govindaraj M. Textile-templated electrospun anisotropic scaffolds for tissue engineering and regenerative medicine [Internet]. 2017 [cited 2021 Apr 6]. Available from: https://patents.google.com/patent/US9668846B2/en [DOI] [PubMed]
- 194.Silva AKA, Juenet M, Meddahi-Pellé A, Letourneur D. Polysaccharide-based strategies for heart tissue engineering. Carbohydr Polym. 2015;116:267–277. doi: 10.1016/j.carbpol.2014.06.010. [DOI] [PubMed] [Google Scholar]
- 195.Note, I. Important. Hyaluronic Acid Derivatives: Durolane®, Gel-One®, GelSyn-3™, GenVisc 850®, Hyalgan™, Hymovis®, Monovisc®, Orthovisc™, Supartz/Supartz FX™, Synojoynt™, TriVisc™, VISCO-3™, Triluron™, sodium hyaluronate 1%. 2020;9.
- 196.Binder Ronald K., Rodés-Cabau Josep, Wood David A., Mok Michael, Leipsic Jonathon, De Larochellière Robert, et al. Transcatheter Aortic Valve Replacement With the SAPIEN 3. JACC Cardiovasc Interv. American College of Cardiology Foundation; 2013;6:293–300. [DOI] [PubMed]
- 197.Shih M, Chang J, Rhee R. Chapter 6 - Complications of the Medtronic Endurant Stent Graft. In: Dryjski ML, Harris LM, editors. Complicat Endovasc Surg [Internet]. Philadelphia: Elsevier; 2022 [cited 2021 Apr 6]. p. 39–44. Available from: https://www.sciencedirect.com/science/article/pii/B9780323554480000061
- 198.Du J, Chen H, Qing L, Yang X, Jia X. Biomimetic neural scaffolds: a crucial step towards optimal peripheral nerve regeneration. Biomater Sci. 2018;6:1299–311 . doi: 10.1039/c8bm00260f. [DOI] [PMC free article] [PubMed] [Google Scholar]