Abstract
Purpose
In people with diabetes, one of the problems for patients is muscle wasting and inhibition of the protein synthesis pathway. This study aimed to evaluate the effects of HIIT on protein expression in two skeletal muscles, flexor hallucis longus (FHL) and soleus (SOL) in rats with type 2 diabetes mellitus (T2DM).
Materials and methods
Diabetes initially was induced by streptozotocin (STZ) and nicotinamide. Rats with type 2 diabetes were randomly and equally divided into control (n = 6) and HIIT groups (n = 6). After 8 weeks of training, the content of total and phosphorylated proteins of serine/threonine-protein kinases (AKT1), mammalian target of rapamycin (mTOR), P70 ribosomal protein S6 kinase 1 (P70S6K1), and 4E (eIF4E)-binding protein 1 (4E-BP1) in FHL and SOL muscles were measured by Western blotting. While body weight and blood glucose were also controlled.
Results
In the HIIT training group, compared to the control group, a significant increase in the content of AKT1 (0.003) and mTOR (0.001) proteins was observed in the FHL muscle. Also, after 8 weeks of HIIT training, protein 4E-BP1 (0.001) was increased in SOL muscle. However, there was no significant change in other proteins in FHL and SOL muscle.
Conclusions
In rats with type 2 diabetes appear to HIIT leading to more protein expression of fast-twitch muscles than slow-twitch muscles. thus likely HIIT exercises can be an important approach to increase protein synthesis and prevent muscle atrophy in people with type 2 diabetes.
Keywords: Exercise, Muscle atrophy, Protein expression, Type 2 diabetes (T2D)
Introduction
Skeletal muscle is the largest organ in the human body and it forms more than 30–40% of the total body mass in healthy adults. Atrophy is one of the common consequences of diabetes. In people with diabetes, protein breakdown increases, and protein synthesis decreases [1]. Muscle atrophy of type I and type II fibers has different reactions in people with diabetes. For example, type I muscle fibers are more resistant to protein synthesis and degradation than type II muscle fibers. Type II muscle fibers have also been shown to be more vulnerable to cancer, diabetes, and aging than type I muscle fibers. The cause of muscle protein loss in diabetes may be low levels of insulin and insulin-like growth factor 1 (IGF-1) along with elevated glucocorticoid levels [2–5] because insulin is a potent anabolic hormone for protein synthesis [6]. However, insulin concentration should be sufficient to activate insulin signaling to enhance muscle protein synthesis because the mechanism for protein synthesis in skeletal muscle through increased mTORC1 activity is the insulin/IGF-1 pathway [7, 8]. Stimulation of muscle cells with insulin or IGF-1 activates phosphoinositide-3-kinase (PI3K), which results in the production of the phosphatidylinositol [3–5]-triphosphate (PIP3) messenger. Then, PIP3 in the membrane affects the use of the pyruvate dehydrogenase kinase 1 (PDK1). This activity leads to phosphorylation and activation of AKT [9]. Activation of mTORC1 with AKT is then performed by inactivating the tuberous sclerosis complex 1/2 (TSC1/2) [10]. The AKT protein directly phosphorylates TSC2 and then activates mTORC1 [11]. The role of mTOR is also very important in muscle protein synthesis [10]. The major substrates of mTORC1 are downstream proteins such as the p70 ribosomal S6 kinase (P70S6K1) and 4E binding protein 1 (4E-BP1) [12], which result in protein synthesis and muscle hypertrophy [13]. On the other hand, chronic inhibition of the mTOR pathway can also induce diabetes [14], and deficiency in S6K1 protein has been shown to lead to glucose intolerance, and a decrease in β cell size [15]. Thus, the mTORC1 / S6K1 signaling impairment appears to result in increased insulin resistance and T2DM development [16]. In skeletal muscle cells, insulin resistance disrupts AKT and mTOR signaling, reducing glucose transport across the plasma membrane [17].
In contrast, regular physical activity is an effective non-pharmacological intervention to control and treat diabetes and improve insulin sensitivity [18]. There is also a very strong correlation between the mechanical load from physical activity and the mTORC1 activation. The mechanical load to activate mTOR is sufficient and this activation can occur from the PI3K-AKT pathway [13]. Therefore, mTORC1 is known as a key regulator in the control of skeletal muscle mass after contraction and hypertrophy due to mechanical load [19]. In this way, it seems regular physical activity (PA) can increase the synthesis of proteins in the skeletal muscles [20]. Sports activities are strong stimulations that can cause changes in signal transduction and cellular metabolism that vary depending on the intensity, type, and timing of exercise. Therefore, the selection of exercise activities with different conditions (severity, type, and time) is important for biochemical and morphological adaptation in skeletal muscle [21]. Among these, an important issue is the volume and intensity of exercises [18]. High-Intensity Interval Training (HIIT) is the repetitive levels of short, high-intensity, or near-peak VO2 activity. A HIIT session may last from a few seconds to a few minutes. The various stages of high-intensity training are separated by a few minutes of rest or low-intensity activity [22]. HIIT is a viable option for controlling and improving hyperglycemia, body composition, regulating blood pressure, and improving lipid profile in people with type 2 diabetes [23]. HIIT has been reported to lead to changes in protein expression associated with protein synthesis and skeletal muscle hypertrophy [24]. An exercise study has shown that HIIT increases the levels of AKT and P-AKT proteins after 8 weeks. While there was no significant difference between HIIT and control groups in the expression of mTOR, P-mTOR, P70S6K, and P-P70S6K. This study stated that HIIT increases muscle hypertrophy by improving the signal transduction pathways [25]. In another study, after 3 and 6 weeks of resistance training, no significant differences were reported in mTOR, p70S6K1, 4E-BP1, and AKT proteins [26]. In general, it appears HIIT training with high speed and volume is the same as endurance and resistance training [27]. For example, resistance training has been reported to increase the protein content of the mTORC1 pathway in fast-twitch muscle tissue. On the other hand, endurance training has been reported to inactivate the mTORC1 cell pathway. In addition, fast-twitch (FHL) and slow-twitch (SOL) muscles, are different and respond differently to exercise [28]. In other words, fast-twitch muscles respond better to high-intensity exercise due to their higher rate of contraction and higher energy production [28]. While slow-twitch muscles are more efficient than fast-twitch muscles due to higher myoglobin concentrations, more capillaries, and higher mitochondrial enzyme activity [28–30]. Therefore, the researchers of the present study investigated the effect of mTORC1 pathway proteins on fast-twitch and slow-twitch skeletal muscle because HIIT training can have a dual nature (resistance-endurance). Since type 2 diabetes causes disorders in the mTORC1 pathway and on the other hand, exercise can regulate this pathway, a study in this field can be useful. In addition, the effect of HIIT on the mTOR signaling pathway in diabetic patients is not well understood, and sports studies have reported conflicting results, so it is important to study this pathway. finally, the purpose of this study was to investigate the effect of 8 weeks HIIT on the content of AKT1, mTOR, P70S6K1, and 4E-BP1 proteins expression in Flexor Hallucis longus (FHL) and Soleus (SOL) muscles in rats with type 2 diabetes.
Materials and methods
The present study is an experimental-fundamental study performed on experimental groups with type 2 diabetes. Male sprague-dawley rats (2 months old) with an average weight of 260 ± 20 g were kept in the animal room of shiraz university of medical sciences. Room temperature was 22 ± 2 °C with light-dark cycle 12:12 h with 50% humidity. Rat had free access to water and food. The study protocol was reviewed and approved by the ethics committee of shiraz university of medical sciences (IR.SUMS.REC.1396.S1062). All methods of keeping and using laboratory animals had approved by shiraz university of medical sciences.
Induction of type 2 DM
For the development of type 2 diabetes in rats, streptozotocin (STZ) solution (solubilized in citrate buffer 0.1 m with pH = 4.5) was injected intraperitoneally and only once at a dose of 60 mg/kg body weight. After 15 min, nicotinAmide (NA) was injected at a dose of 110 mg/kg body weight [31]. Three days later, the blood glucose levels of rats were measured with glucometers (Accu-check ® active, Germany) and animals with blood glucose levels more than 130 mg/dl were considered as having diabetes type 2 [32]. A total of 12 diabetic rats were then randomly assigned into two groups the control group (n = 6) and the HIIT-trained group (n = 6).
Exercise protocol
In the first week, the HIIT experimental group became familiar with the training program by running on a treadmill at a speed of 5–10 m per second. The HIIT group underwent this procedure for 8 weeks (4 sessions per week). Each session was forty-four minutes, including six minutes of warm-up (10–12 m / min), five high-intensity workouts (70–95% of maximum speed for four minutes), four low-intensity workouts (50–60% maximum speed for three minutes). Finally, a six-minute recovery exercise (10–12 m/min) was performed with a zero treadmill incline. During 8 weeks, this exercise did not change [33]. The maximum speed measurement test was started at a speed of 5 m per minute. Every 3 min, the speed of the treadmill increased by 5 m per minute until the rats became tired (the rats clung to the end of the treadmill). The speed at which the rats became tired was considered the maximum speed [34].
Laboratory methods
The control group did not receive any exercise or insulin therapy interventions. Twenty-four hours after the last exercise session, the rats’ body weight and blood glucose were measured. Then, they were anesthetized with ethical guidelines and intraperitoneal injection of a combination of ketamine (30 to 50 mg/kg body weight) and xylazine (3 to 5 mg/kg body weight). Then in an anesthetic state, sections from muscles of FHL and SOL were removed from the animal body and washed in the physiologic serum. It was then rapidly frozen by immersing in liquid nitrogen (obtained from the pharmacology department of shiraz university of medical sciences). These samples were kept at -80 °C for further investigations (AFR-80, ARMINCO, Iran).
Western blot analysis
Changes in protein expression were assessed using western blot procedures. Initially, FHL and SOL tissues were homogenized in RIPA lysis buffer (Sigma) containing an anti-protease cocktail. Centrifugation was then performed at 12,000 rpm to precipitate the sample. Proteins were separated with electrophoresis (Vertical Model, BioRad, USA) into acrylamide gel containing Sodium dodecyl sulfate (SDS). Then, the protein bands on the transfected membrane (polyvinylidene difluoride (PVDF) membrane sigma) were blocked by 3% bovine albumin solution for 1 h at room temperature. They were, then, probed overnight at 4 ° C with the primary antibodies from rabbit, against phosphorylated forms of protein including anti-AKT1 (sc-52,940), anti-mTOR (Sc-293,133), anti-P70S6K1 (Sc-11,759), and anti-4E-BP1 (#-2855) diluted (1: 500) in a blocking solution. The same procedure was performed for total forms of protein including anti-AKT1 (sc-135,829), anti-mTOR (Sc-1550-R), anti-P70S6K1 (Sc-230), and anti-4E-BP1 (Sc-9977). After washing with PBS-T (8 mmol/L phosphate saline buffer (pH 7.4), 3% BSA, 0.1% Tween 20), for 3 times, they were incubated with horseradish peroxidase (HRP) conjugated goat anti-rabbit antibody (diluted 1:10000) (ab6721, Abcam, UK) for 1 h at 25 °. Luminol/enhancer solution (Bio-Rad, cod. 102,030,394) was used to visualize the protein bands. Image J software (1/8/0/112 version) was applied to measure the density of the protein bands. The results were presented after the normalization against control loadings (beta-actin) [35].
Statistical analysis
SPSS 19 was used to perform the statistical analyses. After the confirmation of distribution normality of the data by one-sample Kolmogorov-Smirnov test, the independent T-test was used to compare the content of AKT1, mTOR, P70S6K1, and 4E-BP1 proteins between the two groups. Paired t-test was used to compare weight and blood glucose factors before and after exercise. Statistical significance was set at p < 0.05.
Results
The results of our study showed that the weight (gr) of rats in the control group was increased significantly in the 8th week compared to the first week (p < 0.002). Additionally, the HIIT group’s body weights were increased after 8 weeks in comparison to the first week (p < 0.001). Furthermore, blood glucose (mg/dl) in the control group was significantly higher in the 8th week than in the first week (p < 0.006). However, there were no significant changes in blood glucose of HIIT groups after 8 weeks of training compared to the first week (p < 0.12) (Table 1).
Table 1.
Paired t-test for weight (g) and blood glucose (mg/dl)
| variable | Group | Mean | SD | t | P-value |
|---|---|---|---|---|---|
| Weight (g) | Control (1th week) | 259.20 | 11.07 | 7.35 | 0.002 ‡ |
| Control (8th week) | 296.20 | 12.19 | |||
| HIIT (1th week) | 251.80 | 9.70 | 8.84 | 0.001# | |
| HIIT (8th week) | 263.20 | 11.69 | |||
| Blood glucose (mg/dl) | Control (1th week) | 222.00 | 33.25 | 5.31 | 0.006 † |
| Control (8th week) | 292.00 | 15.34 | |||
| HIIT (1th week) | 236.20 | 39.95 | 1.95 | 0.12 ns | |
| HIIT (8th week) | 251.00 | 30.04 |
Data are expressed as mean ± SD. HIIT: High-Intensity Interval Training. ns: non-significant
‡ Rats’ weight (g) in the control group was significantly increased after 8 weeks (p < 0.002)
# HIIT group’s weight was increased after 8 weeks (p < 0.001)
† Blood glucose (mg/dl) in the control group was significantly higher in the 8th week than in the first week (p < 0.006)
Blood glucose of HIIT groups after 8 weeks wasn’t changed significantly (p < 0.12)
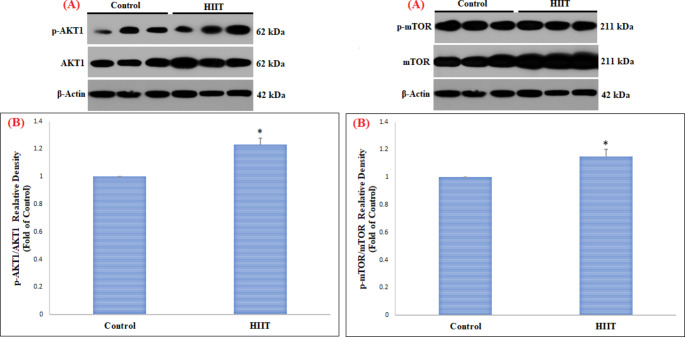
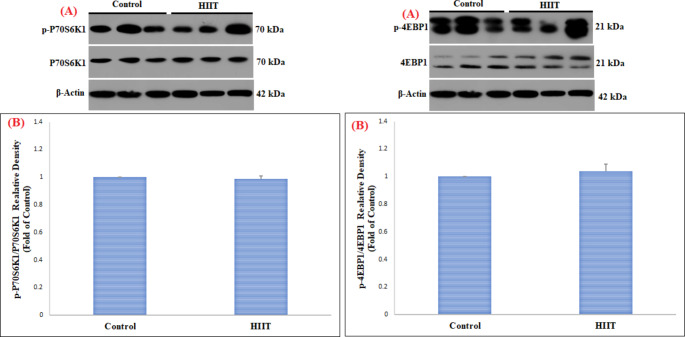
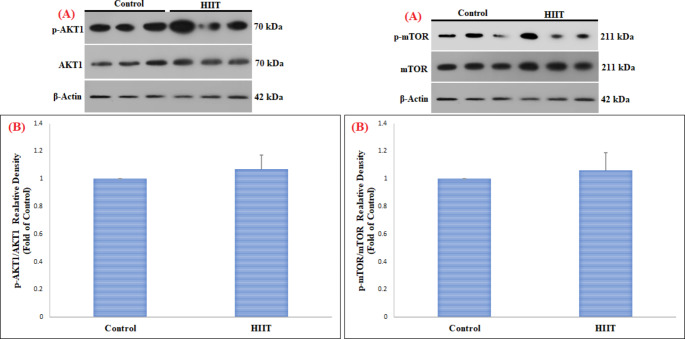
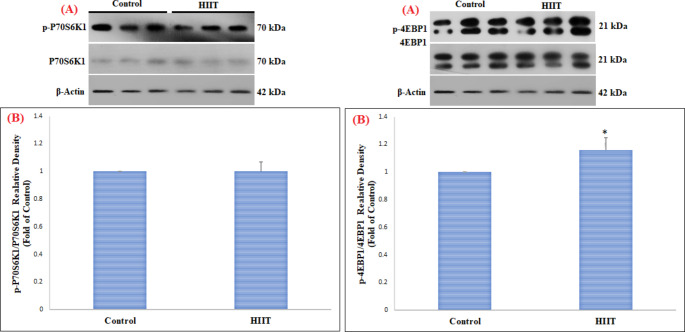
This study in FHL muscle showed a significant increase in pAKT1ser473/AKT1 (p = 0.003) and pmTORser2448/mTOR (p = 0.01) proteins in the HIIT group compared to the control group (Table 2) (Fig. 1). However, changes in pP70S6K1Thr389/P70S6K1 (p = 0.86) and p4EBP1Thr37/46/4EBP1 (p = 0.24) proteins in FHL muscle were not significant (Table 2) (Fig. 2). On the other hand, in SOL muscle, only p4EBP1Thr37/46/4EBP1 (Table 2) (p = 0.01) protein was significantly increased, while no significant change was observed in pAKT1ser473/AKT1 (p = 0.19), pmTORser2448/mTOR (p = 0.38), and pP70S6K1Thr389/P70S6K1 (p = 0.95) proteins (Table 2) (Figs. 3 and 4).
Table 2.
Independent t-test for variables in Control and HIIT groups
| Muscle | variable | Group | Mean | SD | t | P-value |
|---|---|---|---|---|---|---|
| FHL | pAKT1ser473/AKT1 | Control | 1.00 | 0.00 | 6.49 | 0.003∗ |
| HIIT | 1.23 | 0.05 | ||||
| pmTORser2448/mTOR | Control | 1.00 | 0.00 | 4.44 | 0.01∗ | |
| HIIT | 1.15 | 0.05 | ||||
| pP70S6K1Thr389/P70S6K1 | Control | 1.00 | 0.00 | 0.18 | 0.86 | |
| HIIT | 0.99 | 0.02 | ||||
| p4EBP1Thr37/46/4EBP1 | Control | 1.00 | 0.00 | 1.35 | 0.24 | |
| HIIT | 1.04 | 0.05 | ||||
| SOL | pAKT1ser473/AKT1 | Control | 1.00 | 0.00 | 1.45 | 0.19 |
| HIIT | 1.07 | 0.01 | ||||
| pmTORser2448/mTOR | Control | 1.00 | 0.00 | 0.94 | 0.38 | |
| HIIT | 1.06 | 0.13 | ||||
| pP70S6K1Thr389/P70S6K1 | Control | 1.00 | 0.00 | 0.05 | 0.95 | |
| HIIT | 1.00 | 0.07 | ||||
| p4EBP1Thr37/46/4EBP1 | Control | 1.00 | 0.00 | 3.55 | 0.01∗ | |
| HIIT | 1.16 | 0.09 |
*Significant differences between HIIT and Control groups
Fig. 1.
Comparison of the contents of pAKT1 ser473 /AKT1 and pmTOR ser2448 /mTOR proteins in FHL skeletal muscle among the control and HIIT groups
(A) Western blotting of pAKT1ser473/AKT1 and pmTORser2448/mTOR proteins, with β-actin as an internal control in FHL skeletal muscle tissue
(B) The column graph (mean and standard deviation) represents the quantity of pAKT1ser473/AKT1 and pmTORser2448/mTOR protein levels versus internal control (β-actin), which are presented after the normalization against the control group. (*indicating a significant difference between 8 weeks of HIIT training compared to the control group)
Fig. 2.
Comparison of the contents of pP70S6K1 Thr389 /P70S6K1 and p4EBP1 Thr37/46 /4EBP1 proteins in FHL skeletal muscle among the control and HIIT groups
(A) Western blotting of pP70S6K1Thr389/P70S6K1 and p4EBP1Thr37/46/4EBP1 proteins, with β-actin as an internal control in FHL skeletal muscle tissue
(B) The column graph (mean and standard deviation) represents the quantity of pP70S6K1Thr389/P70S6K1 and p4EBP1Thr37/46/4EBP1 protein levels versus internal control (β-actin), which are presented after the normalization against the control group
Fig. 3.
Comparison of the contents of pAKT1 ser473 /AKT1 and pmTOR ser2448 /mTOR proteins in SOL skeletal muscle among the control and HIIT groups
(A) Western blotting of pAKT1ser473/AKT1 and pmTORser2448/mTOR proteins, with β-actin as an internal control in SOL skeletal muscle tissue
(B) The column graph (mean and standard deviation) represents the quantity of pAKT1ser473/AKT1 and pmTORser2448/mTOR protein levels versus internal control (β-actin), which are presented after the normalization against the control group
Fig. 4.
Comparison of the contents of pP70S6K1 Thr389 /P70S6K1 and p4EBP1 Thr37/46 /4EBP1 proteins in SOL skeletal muscle among the control and HIIT groups
(A) Western blotting of pP70S6K1Thr389/P70S6K1 and p4EBP1Thr37/46/4EBP1 proteins, with β-actin as an internal control in SOL skeletal muscle tissue
(B) The column graph (mean and standard deviation) represents the quantity of pP70S6K1Thr389/P70S6K1 and p4EBP1Thr37/46/4EBP1 protein levels versus internal control (β-actin), which are presented after the normalization against the control group. (* indicating a significant difference between 8 weeks of HIIT training compared to the control group)
Discussion
The results of this study showed that 8 weeks of HIIT resulted in a significant change in the protein content of pAKT1ser473/AKT1 and pmTORser2448/mTOR in FHL muscle, but this change was not significant in the protein content of p4EBP1Thr37/46/4EBP1 and pP70S6K1Thr389/P70S6K1. On the other hand, HIIT has been shown to lead to significant changes in the content of p4EBP1Thr37/46/4EBP1 proteins in SOL muscle. However, this change in the protein content of pAKT1ser473/AKT1, pmTORser2448/mTOR, and pP70S6K1Thr389/P70S6K1 was not significant. Before these results, the effect of HIIT on these proteins was less reported in people with type 2 diabetes. Only a few studies have examined the direct effect of different training on AKT1, mTOR, P70S6K1, and 4E-BP1 proteins in type 2 diabetics. Most studies have focused on endurance and resistance training. In a study designed and carried out by Luciano et al. (2017), The reaction of skeletal muscle hypertrophy of rats was examined in response to different models of resistance training. Three types of resistance training such as hypertrophy resistance training (HRT), strength resistance training (SRT), and endurance resistance training (ERT) were performed. The results demonstrated a significant difference in AKT, mTOR, and 4E-BP1 levels of protein in three training groups compared to the control group within rats’ Quadriceps muscles. The results of this study showed an increase in the levels of proteins involved in the mTORC1 pathway and stated that the mTORC1 signaling pathway and key proteins in this path such as AKT1, mTOR, P70S6K1, and 4E-BP1 are very important in protein synthesis [36]. One of the differences between this study and the present study was the duration of training because this study had 12 weeks of training while the present study had 8 weeks of training. Therefore, it seems that the duration of exercise can affect the factors related to protein synthesis. Therefore, it seems that HIIT and resistance training with increasing 4E-BP1, mTOR, AKT lead to protein synthesis and consequently decrease insulin resistance because the AKT protein directly phosphorylates TSC2, which in turn activates mTORC1 [11].
Fig. 5.
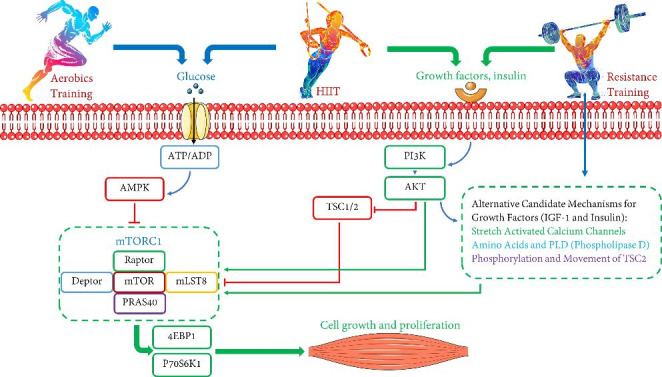
Schematic of the effect of HIIT on protein expression. HIIT can act as a double-edged sword. HIIT, like resistance and endurance training, can activate and inactivate the mTOR pathway. Factors such as severity, duration, frequency, and the number of HIIT sessions are very influential. Activation of the mTORC1 pathway can activate factors such as P70S6K1 and 4E-BP1, which can increase protein synthesis, especially in diabetics
On the other hand, Gibala et al. (2009) investigated the effect of interval exercise with high- intensity on human skeletal muscle in young men. The six young men performed four 30-second workouts with 4 min of rest, totaling less than 80 kJ. The results showed that phosphorylation of AKT in Thr308 and Ser473 tended to decrease immediately after exercise while P70S6K and 4EBP1 remained unchanged after exercise and recovery [37]. One of the differences between this study and our study is that in this study, four 30-second exercises with 4 min of rest were performed, while in the present study, 4 min of high-intensity training and 3 min of low-intensity exercise were performed. Another difference between this study and the present study is the duration of protein measurements. In the present study, measurement was performed 48 h after the last exercise session, while in this study, measurement was performed immediately after exercise. It seems immediately after exercise, more protein breakdown occurs than protein synthesis. In another study, it was reported that the effects of HIIT and MICT (continuous moderate-intensity exercise) at 10 weeks (5 sessions per week) on AKT protein in the skeletal muscle of diabetic rats were investigated. They showed a significant increase in AKT protein expression in Gastrocnemius and Soleus muscles with HIIT exercise [38]. These results are both consistent and inconsistent with our results because the AKT protein was significantly increased in the FHL muscle but not significantly altered in the SOL muscle. One reason for this discrepancy could be the 20-degree slope, which could cause a resistance load, whereas the treadmill slope in the present study was 0 degrees. It has been suggested that overload-induced muscle hypertrophy may activate the PI3K / Akt pathway by directly inducing IGF-1 expression [39, 40] because One of the suggested pathways could be IGF-1/PI3K/AKT [41]. Other differences were 5 training sessions per week for 10 weeks, while in the present study there were 4 training sessions per week for 8 weeks. On the other hand, in HIIT, fast-twitch fibers are used faster than slow-twitch fibers. Therefore, it seems that in HIIT, the Akt protein is activated faster in FHL than in SOL. In the present study, a significant increase in 4E-BP1 was observed in the SOL muscle, while AKT1 and mTOR did not change significantly. In this regard, we can refer to mechanotransduction theory, or “THE HUNT FOR THE ELUSIVE MECHANOSENSOR”, which suggests that protein synthesis and hypertrophy may occur in response to overload, independent of growth pathway (insulin and IGF-1) [42]. Therefore, HIIT appears to activate 4E-BP1 independent of the AKT1 / mTOR pathway [43, 44]. These results are consistent with the study by Cui et al. (2019) because it was shown that HIIT training increases the protein content of P70S6K1 and 4EBP1 but has no effect on AKT protein content [45].
On the other hand, the pathway of protein expression seems to be different in different muscle fibers because the level of 4E-BP1 protein is activated by HIIT in slow-twitch fibers while AKT and mTOR are activated in fast-twitch fibers. Kwon et al. (2017) evaluated 7 weeks of resistance exercise for muscle hypertrophy in mice. The results of their study showed that resistance training leads to an increase in AKT, mTOR, and p70S6K factors in flexor digitorum profundus (FDP) [46]. These results are consistent with the present study because HIIT protocol in the FHL muscle increased the levels of AKT and mTOR proteins. Therefore, it can be concluded that HIIT in fast-twitch muscles is similar to resistance training to activate the AKT and mTOR pathways. Because FHL muscle is more damaged than SOL muscle, it seems that HIIT increases protein synthesis by increasing the AKT / mTOR pathway proteins. In addition, in the SOL muscle, we observed that the 4E-BP1 protein was increased while AKT1 and mTOR were not significantly altered. The increase in 4E-BP1 appears to be independent of the AKT1 and mTOR pathways in the SOL muscle [47]. It seems in addition to the mTORC1 pathway, there are other important pathways involved in protein synthesis and muscle hypertrophy that HIIT can activate. In contrast, HIIT can inactivate the atrophic, apoptotic, and autophagic pathways. Usually, these pathways become overactive in diseases and this can lead to a decrease in muscle mass [48, 49]. In recent years, HIIT training has been introduced as an effective treatment that has the same benefits as pharmacotherapy. For example, it is reported that HIIT training leads to beneficial effects on metabolic adaptations in skeletal muscle, cardiovascular, body mass index, and improved glycemic control [50, 51]. In addition, HIIT exercise has been shown to reduce fasting serum glucose, improve glucose tolerance, and improve aerobic capacity in patients. HIIT exercise has also been reported to be more effective in treating type 2 diabetes in the afternoon than in the morning [52–55]. Therefore, the results of other studies are in line with the present study because we saw control of blood glucose and body weight as in other studies. It is worth mentioning that in the present study, in the group with type 2 diabetes combined with exercise, an excessive increase in blood glucose and body weight were prevented compared to the control group. However, it seems that various factors such as intensity, duration, and type of exercise can affect blood glucose and body weight. In confirmation of this issue, the American Diabetes Association recommends that children and adults with diabetes have at least 60 min of moderate to vigorous daily activity. Adults are also advised to exercise at least 150 min a week for 3 to 7 days a week with moderate to vigorous intensity (50 to 70% of maximum heart rate; 50 to 85% of maximal oxygen consumption) [56, 57].
Conclusions
We have shown that HIIT may activate the mTOR cellular pathway in skeletal muscle in type 2 diabetics by increasing AKT and mTOR in FHL muscle and 4E-BP1 protein in SOL muscle. In type 2 diabetes, FHL muscles are more prone to injury than SOL, so HIIT exercises have a greater effect on FHL. However, factors such as weekly training duration, training session duration, treadmill slope, muscle fiber type, and protein measurement time can affect the results of protein expression that should be considered in future research. On the other hand, HIIT may be an important factor in insulin sensitivity by increasing muscle volume, which leads to the regulation of blood glucose in people with type 2 diabetes. Finally, it can be suggested that using HIIT as part of the management of type 2 diabetes may be beneficial in maintaining muscle health.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgements
This study was conducted at shiraz university of medical sciences and shiraz university. We appreciate the people working in the laboratory of these two centers who helped us implement the research.
Abbreviations
- FHL
flexor hallucis longus
- SOL
soleus
- T2DM
type 2 diabetes mellitus
- STZ
streptozotocin
- AKT1
serine/threonine-protein kinases
- mTOR
mammalian target of rapamycin
- P70S6K1
P70 ribosomal protein S6 kinase 1
- 4E (eIF4E)
binding protein 1 (4E-BP1)
- IGF-1
insulin-like growth factor 1
- PI3K
phosphoinositide-3-kinase
- PIP3
phosphatidylinositol (3,4,5)-triphosphate
- PDK1
pyruvate dehydrogenase kinase 1
- TSC1/2
tuberous sclerosis complex 1/2
- HIIT
high-intensity interval training
Author contributions
M.SH.M has done executive work with animals. F.D has supervised the work. M.S has given advice. H.A.P has written and submitted the article.
Declarations
Competing Interests and Funding
The authors have no competing interests to declare that are relevant to the content of this article.
Statements and Declarations
The authors did not receive support from any organization for the submitted work.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Mohammad Sherafati-Moghadam, Email: m.sherafati@apadana.ac.ir.
Hamed Alizadeh Pahlavani, Email: ha.alizadeh@cfu.ac.ir.
Farhad Daryanoosh, Email: daryanoosh@shirazu.ac.ir.
Mohsen Salesi, Email: msalesi@shirazu.ac.ir.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40200-022-01091-3.
References
- 1.Kelleher AR, Fairchild TJ, Keslacy S. STZ-induced skeletal muscle atrophy is associated with increased p65 content and downregulation of insulin pathway without NF-κB canonical cascade activation. Acta Diabetol. 2010;47(4):315–23. doi: 10.1007/s00592-010-0209-1. [DOI] [PubMed] [Google Scholar]
- 2.Macpherson PC, Wang X, Goldman D. Myogenin regulates denervation-dependent muscle atrophy in mouse soleus muscle. J Cell Biochem. 2011;112(8):2149–59. doi: 10.1002/jcb.23136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schiaffino S, Reggiani C. Fiber types in mammalian skeletal muscles. Physiol Rev. 2011;91(4):1447–531. doi: 10.1152/physrev.00031.2010. [DOI] [PubMed] [Google Scholar]
- 4.von Walden F, Jakobsson F, EdstrÖm L, Nader GA. Altered autophagy gene expression and persistent atrophy suggest impaired remodeling in chronic hemiplegic human skeletal muscle. Muscle Nerve. 2012;46(5):785–92. doi: 10.1002/mus.23387. [DOI] [PubMed] [Google Scholar]
- 5.Wang Y, Pessin JE. Mechanisms for fiber-type specificity of skeletal muscle atrophy. Current opinion in clinical nutrition and metabolic care. 2013;16(3):243, doi: 10.1097/MCO.0b013e328360272d. [DOI] [PMC free article] [PubMed]
- 6.Sandri M, Sandri C, Gilbert A, Skurk C, Calabria E, Picard A, et al. Foxo transcription factors induce the atrophy-related ubiquitin ligase atrogin-1 and cause skeletal muscle atrophy. Cell. 2004;117(3):399–412. doi: 10.1016/S0092-8674(04)00400-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barrett EJ, Wang H, Upchurch CT, Liu Z. Insulin regulates its own delivery to skeletal muscle by feed-forward actions on the vasculature. Am J Physiology-Endocrinology Metabolism. 2011;301(2):E252-E63. doi: 10.1152/ajpendo.00186.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Suhara T, Baba Y, Shimada BK, Higa JK, Matsui T. The mTOR signaling pathway in myocardial dysfunction in type 2 diabetes mellitus. Curr Diab Rep. 2017;17(6):1–10. doi: 10.1007/s11892-017-0865-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hobert JA, Embacher R, Mester JL, Frazier TW, Eng C. Biochemical screening and PTEN mutation analysis in individuals with autism spectrum disorders and macrocephaly. Eur J Hum Genet. 2014;22(2):273–6. doi: 10.1038/ejhg.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Menon S, Dibble CC, Talbott G, Hoxhaj G, Valvezan AJ, Takahashi H, et al. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell. 2014;156(4):771–85. doi: 10.1016/j.cell.2013.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace MA, Hughes DC, Baar K. mTORC1 in the Control of Myogenesis and Adult Skeletal Muscle Mass. Molecules to Medicine with mTOR. Elsevier; 2016. pp. 37–56. 10.1016/B978-0-12-802733-2.00025-6.
- 12.Showkat M, Beigh MA, Andrabi KI. mTOR signaling in protein translation regulation: implications in cancer genesis and therapeutic interventions. Molecular biology international. 2014;2014. [DOI] [PMC free article] [PubMed]
- 13.Klossner S, Durieux A-C, Freyssenet D, Flueck M. Mechano-transduction to muscle protein synthesis is modulated by FAK. Eur J Appl Physiol. 2009;106(3):389–98. doi: 10.1007/s00421-009-1032-7. [DOI] [PubMed] [Google Scholar]
- 14.Xie J, Herbert TP. The role of mammalian target of rapamycin (mTOR) in the regulation of pancreatic β-cell mass: implications in the development of type-2 diabetes. Cell Mol Life Sci. 2012;69(8):1289–304. doi: 10.1007/s00018-011-0874-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pende M, Kozma SC, Jaquet M, Oorschot V, Burcelin R, Marchand-Brustel L, et al. Hypoinsulinaemia, glucose intolerance and diminished β-cell size in S6K1-deficient mice. Nature. 2000;408(6815):994–7. doi: 10.1038/35050135. [DOI] [PubMed] [Google Scholar]
- 16.Shigeyama Y, Kobayashi T, Kido Y, Hashimoto N, Asahara S-i, Matsuda T, et al. Biphasic response of pancreatic β-cell mass to ablation of tuberous sclerosis complex 2 in mice. Mol Cell Biol. 2008;28(9):2971–9. doi: 10.1128/MCB.01695-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang S-B, Lee HY, Young DM, Tien A-C, Rowson-Baldwin A, Shu YY, et al. Rapamycin induces glucose intolerance in mice by reducing islet mass, insulin content, and insulin sensitivity. J Mol Med. 2012;90(5):575–85. doi: 10.1007/s00109-011-0834-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Colberg SR, Sigal RJ, Fernhall B, Regensteiner JG, Blissmer BJ, Rubin RR, et al. Exercise and type 2 diabetes: the American College of Sports Medicine and the American Diabetes Association: joint position statement. Diabetes Care. 2010;33(12):e147-e67. doi: 10.2337/dc10-9990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yoon M-S. mTOR as a key regulator in maintaining skeletal muscle mass. Front Physiol. 2017;8:788. doi: 10.3389/fphys.2017.00788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hansen D, De Strijcker D, Calders P. Impact of endurance exercise training in the fasted state on muscle biochemistry and metabolism in healthy subjects: can these effects be of particular clinical benefit to type 2 diabetes mellitus and insulin-resistant patients? Sports Med. 2017;47(3):415–28. doi: 10.1007/s40279-016-0594-x. [DOI] [PubMed] [Google Scholar]
- 21.Coffey VG, Jemiolo B, Edge J, Garnham AP, Trappe SW, Hawley JA. Effect of consecutive repeated sprint and resistance exercise bouts on acute adaptive responses in human skeletal muscle. Am J Physiology-Regulatory Integr Comp Physiol. 2009;297(5):R1441-R51. doi: 10.1152/ajpregu.00351.2009. [DOI] [PubMed] [Google Scholar]
- 22.Cassidy S, Thoma C, Houghton D, Trenell MI. High-intensity interval training: a review of its impact on glucose control and cardiometabolic health. Diabetologia. 2017;60(1):7–23. doi: 10.1007/s00125-016-4106-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wormgoor SG, Dalleck LC, Zinn C, Harris NK. Effects of high-intensity interval training on people living with type 2 diabetes: a narrative review. Can J diabetes. 2017;41(5):536–47. doi: 10.1016/j.jcjd.2016.12.004. [DOI] [PubMed] [Google Scholar]
- 24.Lane MT, Herda TJ, Fry AC, Cooper MA, Andre MJ, Gallagher PM. Endocrine responses and acute mTOR pathway phosphorylation to resistance exercise with leucine and whey. Biology of sport. 2017;34(2):197. doi: 10.5114/biolsport.2017.65339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Biglari S, Afousi AG, Mafi F, Shabkhiz F. High-intensity interval training-induced hypertrophy in gastrocnemius muscle via improved IGF-I/Akt/FoxO and myostatin/Smad signaling pathways in rats. Physiol Int. 2020;107(2):220–30. doi: 10.1556/2060.2020.00020. [DOI] [PubMed] [Google Scholar]
- 26.Brook MS, Wilkinson DJ, Mitchell WK, Lund JN, Szewczyk NJ, Greenhaff PL, et al. Skeletal muscle hypertrophy adaptations predominate in the early stages of resistance exercise training, matching deuterium oxide-derived measures of muscle protein synthesis and mechanistic target of rapamycin complex 1 signaling. FASEB J. 2015;29(11):4485–96. doi: 10.1096/fj.15-273755. [DOI] [PubMed] [Google Scholar]
- 27.Vissing K, McGee S, Farup J, Kjølhede T, Vendelbo M, Jessen N. Differentiated mTOR but not AMPK signaling after strength vs endurance exercise in training-accustomed individuals. Scand J Med Sci Sports. 2013;23(3):355–66. doi: 10.1111/j.1600-0838.2011.01395.x. [DOI] [PubMed] [Google Scholar]
- 28.Callahan MJ, Parr EB, Hawley JA, Camera DM. Can high-intensity interval training promote skeletal muscle anabolism? Sports Med. 2021;51(3):405–21. doi: 10.1007/s40279-020-01397-3. [DOI] [PubMed] [Google Scholar]
- 29.Ogasawara R, Kobayashi K, Tsutaki A, Lee K, Abe T, Fujita S, et al. mTOR signaling response to resistance exercise is altered by chronic resistance training and detraining in skeletal muscle. J Appl Physiol. 2013;114(7):934–40. doi: 10.1152/japplphysiol.01161.2012. [DOI] [PubMed] [Google Scholar]
- 30.Luo L, Lu A-M, Wang Y, Hong A, Chen Y, Hu J, et al. Chronic resistance training activates autophagy and reduces apoptosis of muscle cells by modulating IGF-1 and its receptors, Akt/mTOR and Akt/FOXO3a signaling in aged rats. Exp Gerontol. 2013;48(4):427–36. doi: 10.1016/j.exger.2013.02.009. [DOI] [PubMed] [Google Scholar]
- 31.Safhi MM, Anwer T, Khan G, Siddiqui R, Sivakumar SM, Alam MF. The combination of canagliflozin and omega-3 fatty acid ameliorates insulin resistance and cardiac biomarkers via modulation of inflammatory cytokines in type 2 diabetic rats. Korean J Physiol Pharmacol. 2018;22(5):493–501. doi: 10.4196/kjpp.2018.22.5.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Khalili A, Nekooeian AA, Khosravi MB. Oleuropein improves glucose tolerance and lipid profile in rats with simultaneous renovascular hypertension and type 2 diabetes. J Asian Nat Prod Res. 2017;19(10):1011–21. doi: 10.1080/10286020.2017.1307834. [DOI] [PubMed] [Google Scholar]
- 33.Fallahi A, Gaeini A, Shekarfroush S, Khoshbaten A. Cardioprotective effect of high intensity interval training and nitric oxide metabolites (NO2–, NO3–) Iran J public health. 2015;44(9):1270. [PMC free article] [PubMed] [Google Scholar]
- 34.Garcia NF, Sponton AC, Delbin MA, Parente JM, Castro MM, Zanesco A, et al. Metabolic parameters and responsiveness of isolated iliac artery in LDLr-/-mice: role of aerobic exercise training. Am J Cardiovasc Dis. 2017;7(2):64. [PMC free article] [PubMed] [Google Scholar]
- 35.Khani M, Motamedi P, Dehkhoda MR, Dabagh Nikukheslat S, Karimi P. Effect of thyme extract supplementation on lipid peroxidation, antioxidant capacity, PGC-1α content and endurance exercise performance in rats. J Int Soc Sports Nutr. 2017;14(1):1–8. doi: 10.1186/s12970-017-0167-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luciano TF, Marques S, Pieri B, De Souza DR, Araújo L, Nesi R, et al. Responses of skeletal muscle hypertrophy in Wistar rats to different resistance exercise models. Physiol Res. 2017;66(2):317. doi: 10.33549/physiolres.933256. [DOI] [PubMed] [Google Scholar]
- 37.Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ, Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1α in human skeletal muscle. J Appl Physiol. 2009;106(3):929–34. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- 38.Chavanelle V, Boisseau N, Otero YF, Combaret L, Dardevet D, Montaurier C, et al. Effects of high-intensity interval training and moderate-intensity continuous training on glycaemic control and skeletal muscle mitochondrial function in db/db mice. Sci Rep. 2017;7(1):204. doi: 10.1038/s41598-017-00276-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Glass DJ. Skeletal muscle hypertrophy and atrophy signaling pathways. Int J Biochem Cell Biol. 2005;37(10):1974–84. doi: 10.1016/j.biocel.2005.04.018. [DOI] [PubMed] [Google Scholar]
- 40.Spangenburg EE, Le Roith D, Ward CW, Bodine SC. A functional insulin-like growth factor receptor is not necessary for load‐induced skeletal muscle hypertrophy. J Physiol. 2008;586(1):283–91. doi: 10.1113/jphysiol.2007.141507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Philp A, Hamilton DL, Baar K. Signals mediating skeletal muscle remodeling by resistance exercise: PI3-kinase independent activation of mTORC1. J Appl Physiol. 2011;110(2):561–8. doi: 10.1152/japplphysiol.00941.2010. [DOI] [PubMed] [Google Scholar]
- 42.Hornberger TA. Mechanotransduction and the regulation of mTORC1 signaling in skeletal muscle. Int J Biochem Cell Biol. 2011;43(9):1267–76. doi: 10.1016/j.biocel.2011.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Camera DM, Edge J, Short MJ, Hawley JA, Coffey VG. Early time course of Akt phosphorylation after endurance and resistance exercise. Med Sci Sports Exerc. 2010;42(10):1843–52. doi: 10.1249/mss.0b013e3181d964e4. [DOI] [PubMed] [Google Scholar]
- 44.Coffey VG, Pilegaard H, Garnham AP, O’Brien BJ, Hawley JA. Consecutive bouts of diverse contractile activity alter acute responses in human skeletal muscle. J Appl Physiol. 2009;106(4):1187–97. doi: 10.1152/japplphysiol.91221.2008. [DOI] [PubMed] [Google Scholar]
- 45.Cui X, Zhang Y, Wang Z, Yu J, Kong Z, Ružić L. High-intensity interval training changes the expression of muscle RING‐finger protein‐1 and muscle atrophy F‐box proteins and proteins involved in the mechanistic target of rapamycin pathway and autophagy in rat skeletal muscle. Exp Physiol. 2019;104(10):1505–17. doi: 10.1113/EP087601. [DOI] [PubMed] [Google Scholar]
- 46.Kwon I, Jang Y, Cho J-Y, Jang YC, Lee Y. Long-term resistance exercise-induced muscular hypertrophy is associated with autophagy modulation in rats. J Physiological Sci. 2018;68(3):269–80. doi: 10.1007/s12576-017-0531-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Glass DJ. PI3 kinase regulation of skeletal muscle hypertrophy and atrophy. Phosphoinositide 3-kinase in Health and Disease. 2010:267 – 78. [DOI] [PubMed]
- 48.Liu GY, Sabatini DM. mTOR at the nexus of nutrition, growth, ageing and disease. Nat Rev Mol Cell Biol. 2020;21(4):183–203. doi: 10.1038/s41580-019-0199-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yoshida T, Delafontaine P. Mechanisms of IGF-1-mediated regulation of skeletal muscle hypertrophy and atrophy. Cells. 2020;9(9):1970. doi: 10.3390/cells9091970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Little JP, Jung ME, Wright AE, Wright W, Manders RJ. Effects of high-intensity interval exercise versus continuous moderate-intensity exercise on postprandial glycemic control assessed by continuous glucose monitoring in obese adults. Applied physiology, nutrition, and metabolism. 2014;39(7):835–41, 10.1139/apnm-2013-0512. [DOI] [PubMed]
- 51.Hafstad AD, Lund J, Hadler-Olsen E, Höper AC, Larsen TS, Aasum E. High-and moderate-intensity training normalizes ventricular function and mechanoenergetics in mice with diet-induced obesity. Diabetes. 2013;62(7):2287–94. doi: 10.2337/db12-1580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kong Z, Sun S, Liu M, Shi Q. Short-term high-intensity interval training on body composition and blood glucose in overweight and obese young women. Journal of diabetes research. 2016;2016, 10.1155/2016/4073618. [DOI] [PMC free article] [PubMed]
- 53.Mancilla R, Torres P, Álvarez C, Schifferli I, Sapunar J, Diaz E. High intensity interval training improves glycemic control and aerobic capacity in glucose intolerant patients. Rev Med Chil. 2014;142(1):34–9. doi: 10.4067/S0034-98872014000100006. [DOI] [PubMed] [Google Scholar]
- 54.Savikj M, Gabriel BM, Alm PS, Smith J, Caidahl K, Björnholm M, et al. Afternoon exercise is more efficacious than morning exercise at improving blood glucose levels in individuals with type 2 diabetes: a randomised crossover trial. Diabetologia. 2019;62(2):233–7. doi: 10.1007/s00125-018-4767-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Codella R, Terruzzi I, Luzi L. Why should people with type 1 diabetes exercise regularly? Acta Diabetol. 2017;54(7):615–30. doi: 10.1007/s00592-017-0978-x. [DOI] [PubMed] [Google Scholar]
- 56.Chiang JL, Kirkman MS, Laffel LM, Peters AL, Authors TDS. Type 1 diabetes through the life span: a position statement of the American Diabetes Association. Diabetes Care. 2014;37(7):2034–54. doi: 10.2337/dc14-1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Colberg SR, Sigal RJ, Yardley JE, Riddell MC, Dunstan DW, Dempsey PC, et al. Physical activity/exercise and diabetes: a position statement of the American Diabetes Association. Diabetes Care. 2016;39(11):2065–79. doi: 10.2337/dc16-1728. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.