Abstract
Toxic metal(loid)s can lead to high damages on human. This work was conducted to investigate the levels of metal(loid)s in PM2.5 and a total of 123 male children’s (aged 6–9 years) blood chosen from different areas in Ahvaz and their association with the pre-inflammatory (Immunoglobulin E and cytokines: IgE, IL-4 and IL-13) responses in serum cells. Six metal(loid)s (arsenic, cadmium, chromium, mercury, nickel and lead) in three regions including industrial (Padad), vehicle traffic (Golestan) and reference (Kianpars) areas were studied. Results showed the concentrations of As, Cr, Cd, Ni and Hg in the ambient air of industrial area (Padad) (P < 0.001), and Pb in vehicle traffic area (Golestan) were higher (p < 0.001). Moreover, the mean levels of IgE (mean = 146.44 pg/200landa, P < 0.003), IL-4 (mean = 548.23 pg/200landa, P < 0.001) and IL-13 (mean = 53.21 pg/200landa, P < 0.001) in Padad were higher than Golestan and Kianpars. Our results suggest that living in industrial areas leads to accelerated synthesis of IgE, IL-4 and IL-13 in blood. The spatial distribution of children’s serum IgE, IL-4 and IL-13 concentrations showed an abnormal increase of 240 to 400 pg/200landa for IgE, 950 to 1400 pg/200landa for IL-4 and 90 to 128 pg/200landa for IL-13. Our results indicate children in the industrial area are prone to asthma, allergy, miRNA mutation, and other chronic diseases.
Keywords: Industrial area, Children, Pro-inflammatory factors (IgE IL-4 and IL-13), Spatial distribution. Allergy and asthma
Introduction
Factories and industrial areas emit large amounts of pollutants into the environment and reduce the quality of environmental health. Residing in industrial cities with poor environmental circumstances leads to human health concerns. Metals and metalloids enter the human body through drinking water, ingestion of food and supplements, inhalation, dermal absorption, and incidental ingestion [1, 2]. Several studies indicate that in the industrial cities, metalloids inhalation bound to the PM2.5 is increasing [3, 4]. Also, increase in exposure to inhalation of heavy metals had a significant relationship with lung function decrease in adults [3]. Allergic, atopic and other chronic diseases such as asthma are global illnesses that affect about 150 million children and adults worldwide [5]. Chronic diseases or syndromes are associated with an increase in pro- inflammatory biomarkers in humans. Children For some reasons are more sensitive than adults to the harmful health effects of air pollution [6]. Physiological and biological responses to the chemical in children are differing significantly from adults [7]. Children spend more time in outdoor and have more daily activities; such as soccer and other games, this group is breathing 20 to 50% more air and air pollutions than adults [8]. They don’t show similar symptoms even when exposed to higher concentrations of air pollution. It is not known clearly if they ignore or don’t feel the symptoms because of being preoccupied with physical activities. Human’s immune system is being developed in childhood; it is less poly-functional and produces fewer cytokines in comparison to maturity [9] In a cohort study carried out in New York City from 1998 to 2006 through 24 Months age children, it was reported that an increase in ambient air heavy metals (AAHM) was associated with increase in the probability of wheezing and coughing. However, there was no significant relation between total atmospheric PM2.5 and wheezing or coughing [10]. In a study conducted by Dunea et al. about the effect of heavy metals on Immunoglobulin-E (IgE), eosinophil’s, and wheezing in children diagnosed with respiratory diseases, showed that raising in the IgE production and lung disease is related to increasing atmospheric PM2.5 [11]. IgE is an antibody produced by the immune system, which causes allergic reactions in the nose, lungs, throat, or on the skin. There are some relationships between asthma and IgE that appear to be independent of allergen sensitization [12]. In addition, IL-4 and IL-13 secreted into plasma by Th2 lymphocytes and mast cells have some similar properties. These cytokines are overproduced in atopic, asthma and allergic reactions and play important roles by inducing IgE synthesis in naive B-lymphocytes [13]. Up to now, no study has been done about the effect of PM2.5-bound metal(loid)s in industrial area on the synthesis of pro-inflammatory IgE, IL-4 and IL-13. This work was conducted for the first time in Ahvaz as a highly industrialized city, to fulfill this gap. Present study aimed to investigate the relation of ambient air PM2.5-bound metal(loid)s concentration in industrial, vehicle traffic and reference areas and synthesis of IgE, IL-4 and IL-13 in the blood serum of children.
Methodology
Description of the study area
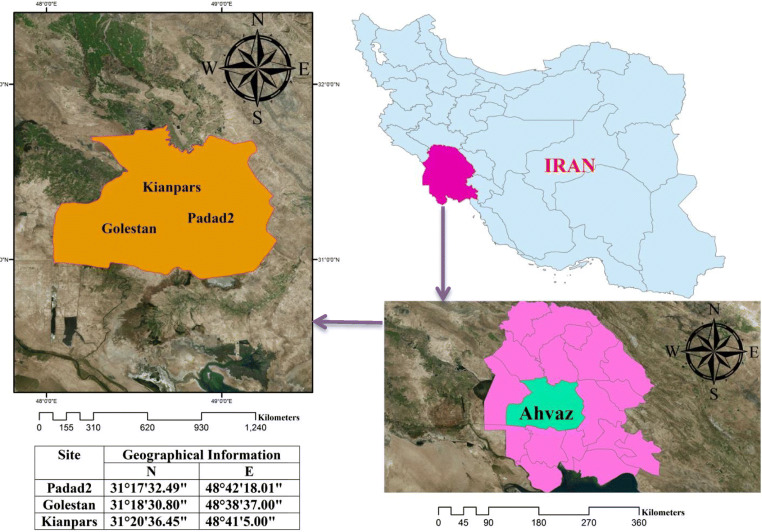
Ahvaz city is located in the south-west of Iran and has a tropical climate with a limited downfall period, high humidity and temperature during the year. Ahvaz has a surface area of about 220 km2 and a total population of about 1.3 million with an about density of 4815.73 people per square kilometer [14, 15]. Three locations in Ahvaz selected to ambient air metal(loid)s sampling were as follows: 1) Health center No. 18 in Padad region (industrial area) (31°17′32.49”N, 48°42′18.01″E) which is located in the east of Ahvaz and adjacent to steel, carbon black, pipeline manufacturing industries and Karun industrial area, 2) Department of Environmental Protection of Khuzestan in Golestan region (vehicle traffic area) (31°18′30.80”N, 48°38′37.00″E) located in southwest of Ahvaz, 3) Rajaei dormitory located in Kianpars region (reference area) (31°20′36.45”N, 48°41′5.00″E). Figure 1 shows the location of areas where the study was conducted.
Fig. 1.
Map of location study areas
Study participants description
A total of 123 children aged 6 to 9 years old were blood sampled. All of the participants based on income level belonged to the middle-class family and within 1000-m radius areas where the study was conducted. Students were selected from schools (49 male students from Padad, 49 male students from Golestan and 25 male students from Kianpars). The most important inclusion criteria for children to be included in the study were: 1) No history of allergies, respiratory and other chronic illnesses; 2) No cold during one month to sampling; 3) No history of respiratory and other chronic illnesses in parents; 4) Lack of parental smoking; 5) A history of five years of living in the chosen sampling area; 6) School and home located inside the sampling region; 6) To belong to a same socioeconomic group and have a similar dietary intake [16].
Blood sampling and preparation procedure
On an appointed day, all of the participants were taken the blood samples. Firstly, written informed consent was obtained from the parents. 3 ml of venous blood obtained from every child by two skillful nurses in order to measure the level of IgE, IL-4 and IL-13. A total of 123 blood samples were taken by vacutainer needles. Previous studies have shown these needles do not add pollutions to the blood [17]. Blood sample tubes with a volume of 3 ml were centrifuged at room temperature for 10 min at 3000 RPM [18], aliquoted (200 μL serum supernatant was taken and discharged into microtube), and separated serum stored at −80 °C until the IgE, IL-4 and IL-13analyses [19].
Heavy metals analysis
The digested PM2.5-bound metal(loid)s were injected into the ICP-OES (Spectro Arcos) instrument to identify and quantify 6 elements of Arsenic (As), Cadmium (Cd), Chrome (Cr), Mercury (Hg), Nickel (Ni) and Lead (Pb). Limit of detection (LOD) and limit of quantitation (LOQ) were calculated based on the Center for Disease Control and prevention (CDC) guideline [20]. LOD, LOQ, blank samples (BS) and the deionized water (DW) specification for all elements are indicated in (Table 1).
Table 1.
The LOD, LOQ, DW and BS of ICP-OES for heavy metals analysis
| Element | LOD (ng mL−1) | LOQ (ng mL−1) | DW (ng mL−1) | BS (ng mL−1) |
|---|---|---|---|---|
| As | 0.358 | 1.074 | <0.358 | <0.358 |
| Cd | 0.49 | 1.47 | <0.49 | 1.4 |
| Cr | 0.96 | 2.88 | <0.96 | 3.78 |
| Hg | 0.351 | 1.053 | <0.351 | <0.351 |
| Ni | 0.286 | 0.858 | 0.356 | 2.93 |
| Pb | 2.2 | 6.6 | <2.2 | 4.3 |
LOD limit of detection, LOQ limit of quantitation, DW deionized water, BS blank sample
Cytokines (IL-4 and IL-13) and immunoglobulin E (IgE) measurements
IgE, IL-4 and IL-13 factors were measured by enzyme-linked immune sorbent assay (ELISA). Serum samples were taken from the freezer (−80 °C) and kept at room temperature for 30 min to become liquid. For uniformity of cell supernatants, micro tubes were shaken several times. Preparation of IgE,IL-4 and IL-13 were fulfilled using eBioscience sandwich ELISA Kits - Germany (product code base Kit: 15501647; IgE: 15521757; IL-4: 15511777; IL-13) according to the manufacturer’s protocol. Lastly, an ELISA Rader (BioTek; USA; ELx808) was used for sample determination at 450 nm as a Measurement and 620 nm as Reference [21].
Statistical analysis
The Excel and SPSS v.21 software were applied to data analysis. Firstly, the mean and standard deviation were calculated for all data. Shapiro Wilks test, Kruskal-Wallis (nonparametric test) and spearman correlation were used to evaluate the normality of the data and significance (P < 0.001) among sampling areas and IgE, IL-4 and IL-13 factors. The ANOVA test was used to study the relation between PM2.5-bound metal(loid)s and pre-inflammatory biomarkers (IgE, IL-4 and IL-13) (P < 0.001) in study areas.
Results and discussion
PM2.5 concentrations in study areas
Present study was conducted for the first time on evaluation and association among ambient air heavy metals and asthma and allergy pre-inflammatory factors (IgE, IL-4 and IL-13) in children in Ahvaz as a highly industrialized city in Iran. PM2.5 sampling was done in three sites. Table 2 shows that mean concentration of PM2.5 at each site exceeded the WHO daily (50 μg/m3) and annually (10 μg/m3) guideline reference values [22]. According to the Table 2 PM2.5 exposure levels from Padad region (68.56 μg/m3), were higher than those from Golestan (67.06 μg/m3) and Kianpars (55.31 μg/m3) regions, although no significant correlation (P = 0.031) was observed. High concentrations of PM2.5 in Ahvaz are related to vehicular traffics, vicinity to many industrial factories and Proximity to local and external dust sources [23]. In 2012 high level concentrations of PM have been reported in Ahvaz [14], which shows that Iran should develop a strategic plan to improve air quality in Ahvaz.
Table 2.
The significant relation of IgE and IL-4 levels of children and PM2.5-bound metal(loid)s from Padad, Golestan and Kianpars
| Parameter | Regions | P value | ||||||||
| Padad | Golestan | Kianpars | ||||||||
| N = 24 | N = 24 | N = 24 | ||||||||
| pollutants | CI5% | Mean | CI95% | CI5% | Mean | CI95% | CI5% | Mean | CI95% | |
| PM2.5 (μg/m3) | 61.56 | 68.56 | 75.57 | 59.27 | 67.06 | 74.84 | 45.94 | 55.31 | 64.67 | 0.031 |
| As (ng/m3) | 70.89 | 94.51 | 118.12 | 33.07 | 38.99 | 44.91 | 13.05 | 19.79 | 26.54 | <0.001 |
| Cd (ng/m3) | 7.52 | 8.06 | 8.59 | 6.64 | 7.24 | 7.84 | 4.23 | 4.44 | 4.67 | <0.001 |
| Cr (ng/m3) | 14.22 | 15.06 | 15.91 | 13.61 | 14.45 | 15.3 | 10.63 | 11.01 | 11.4 | <0.001 |
| Hg (ng/m3) | 6.51 | 7.11 | 7.72 | 6.24 | 6.42 | 6.59 | 4.15 | 4.21 | 4.28 | <0.001 |
| Ni (ng/m3) | 11.86 | 12.63 | 13.41 | 10.88 | 11.48 | 12.07 | 7.49 | 8.15 | 8.81 | <0.001 |
| Pb (ng/m3) | 200.68 | 298.38 | 396.1 | 385.88 | 443.77 | 501.66 | 165.81 | 209.3 | 252.7 | <0.001 |
|
Blood IgE and IL-4 |
N = 49 | N = 49 | N = 25 | p value | ||||||
|
IgE (pg/200landa) |
104.88 | 141.63 | 178.38 | 60.1 | 86.99 | 113.89 | 36.33 | 58.96 | 81.58 | 0.003 |
| IL-4 (pg/200landa) | 372.31 | 505.5 | 638.67 | 131.23 | 198.92 | 266.6 | 80.02 | 127.27 | 174.52 | <0.001 |
| IL-13 (pg/200landa) | 39.24 | 53.21 | 67.19 | 17.95 | 27.77 | 37.59 | 9.02 | 13.91 | 18.8 | <0.001 |
PM2.5-bound metal(loid)s in study areas
We found that the As, Cd, Cr, Ni and Hg levels in ambient air from Padad with significant difference (p < 0.001) were higher than those of Golestan and Kianpars areas. These might be due to the proximity of large number of industries (steel, carbon black, pipe production, oil refinery, reactor production, etc.) to this region. Pastuzka reported that As, Cd, Cr, Hg, Ni and Pb concentrations were higher in industrial sites [24]. Results of a study in Singapore showed that metallurgical industries, diesel vehicles and industrial sources with fossil fuel combustion emit Cd, Cr, Co and Ni into atmosphere [25]. In a study, road dust sample analyses in Ahvaz city showed that the heavy metal ranges across different sites were from 4.35–3577.06 mg kg − 1 for Pb, 32.40–1220.40 mg kg − 1 for Ni, 4.40–36.10 mg kg − 1 for Co, 22.40–312.40 mg kg − 1 for Cr, < 0.01–721.29 mg kg − 1 for Cu, 1.80–51.60 mg kg − 1 for As, 0.15–78 mg kg − 1 for Cd. The results of this study have shown that in industrial road dust of Ahvaz, the concentration of heavy metals were higher than those levels of the reference area [23]. In addition, the average levels of Hg, Cr, V, Sb, Co, Mn, Ni, Zn, Pb, Cu and Mo were higher than world soil values [26]. Keshavarzi et al. found that in Ahvaz, the coefficient of variation (CV) for As, Hg, Pb, Ni was >100 that reflects the exceptionally high variability of production resources, also for Cr and Cd were 100 > CV > 50 that shows the high variability of production resources [23]. This diversity in results can be due to the different heavy metal/metalloid resources in Ahvaz. The emitted heavy metals can float in the atmosphere or settle on the soil surface and enter children’s bodies through respiration and direct contact. However, the Pb concentration in Golestan was higher (p < 0.001) than Padad. It could be due to high traffic jam of vehicles in Golestan. A study has shown that Pb is emitted from gasoline and diesel fuels [27]. Pb is the only heavy metal with an annual average of 0.5 μg/m3 that is registered in Iran’s national atmosphere quality guideline. In the 1990s due to local regulations the use of leaded gasoline was banned in the developed countries and in 2002, the consumption of leaded gasoline in Iran was prohibited [22].
Pro-inflammatory biomarkers (IgE, IL-4 and IL-13) levels in children
Pro-inflammatory biomarkers were measured in order to determine the allergic and inflammatory status in children. Table 2 indicates the average concentration of pro-inflammatory biomarkers including IgE, IL-4 and IL-13 in children blood serum of the ages 6 to 9 years olds in different sampling areas. The results showed IgE, IL-4 and IL-13 levels of children from padad region were higher (P = 0.003), (P < 0.001) and (P < 0.001) respectively than two other studied areas. Several studies have shown that the IL-6, IL-8 and IgE of the human population living in industrial areas were high [21, 27]. Few researches have shown the effect of chromium on levels of serum IL-2 and IL-6 in pigs [28] An in vitro experimental research showed that chromium could increase the level of IL-4 and IFN-γ [29]. In addition, Richard Hayse reported a significant association between chromium concentrations in ambient air and lung cancer in industrial workers [30].
Spatial distribution of children’s pre-inflammatory (IgE, IL-4 and IL-13) biomarkers
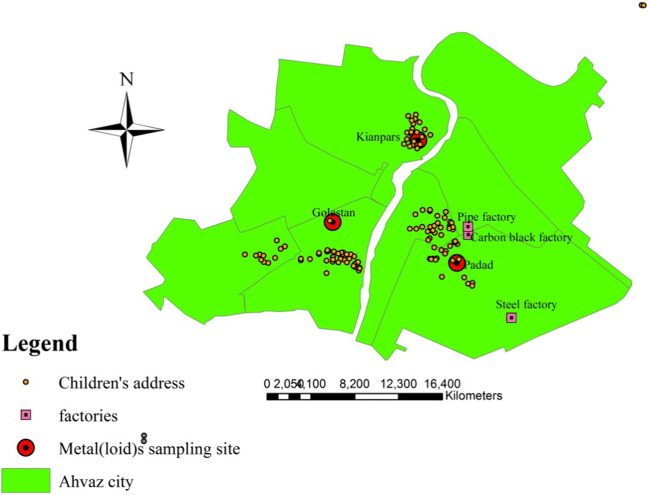
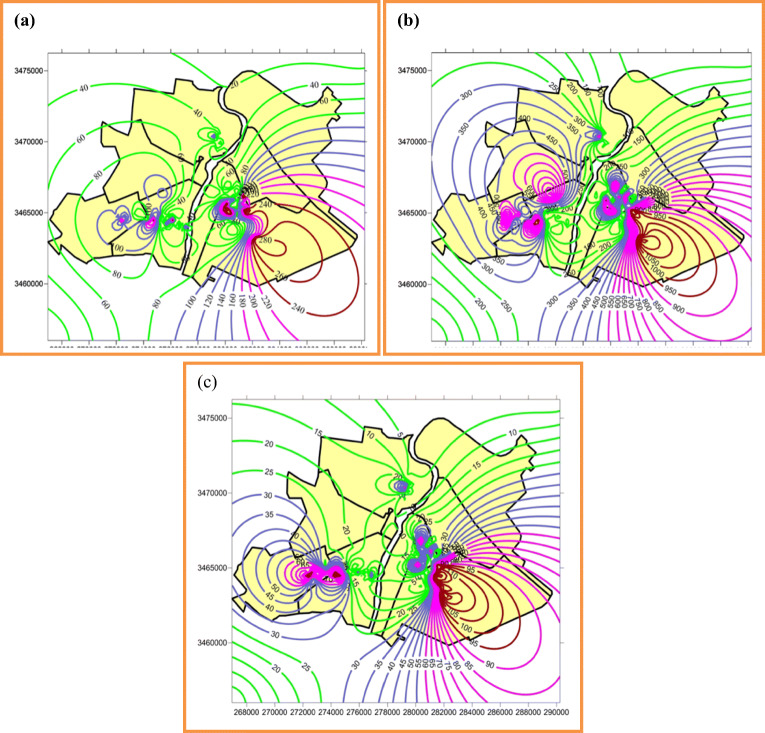
Children’s life patterns in Ahvaz city indicated this group spends most of their after-school hours in outdoor playing different hobbies [31]. Therefore, children are highly susceptible to inhalation and contact with these contaminants. Figure 2 shows the location of PM2.5-bound metal(loid)s sampling sites, industries and participants locations in different areas of Ahvaz. Blood samples were taken from children living in three areas: Padad (industrial), Golestan (vehicles traffic) and Kianpars (residential) and geographical information of each child was applied to compare the spatial distribution of pre-inflammatory (IgE, IL-4 and IL-13) biomarkers in the studied areas. Figures 3a to c show the spatial distribution of children’s IgE, IL-4 and IL-13 in the studied areas. Green curves in maps indicate desirable concentrations of IgE and IL-4, blue curves indicate acceptable concentrations, while pink lines indicate IgE, IL-4 and IL-13 concentrations over the reference, moreover, a warning red lines show the hazard zone of very high levels of blood toxic metals. Figure 3a and c show an abnormal increase level of 240 to 400 pg/200landa for IgE, 950 to 1400 pg/200landa for IL-4 and 90 to 128 pg/200landa in the Padad area. The increasing trend of children’s IgE, IL-4 and IL-13 observed in the northwest of steel factory and west of carbon black and piping factories, are affected by prevailing wind speed and direction, which is from southeast to northwest [32]. In the present study, children from Padad had higher IgE, IL-4 and IL-13 compared to volunteers from the two other areas, which may point out that children living in industrial area were more at risk of allergic, atopic, asthmatic and other chronic diseases. Several studies demonstrated that living in industrial area poses harmful long-term effects on the health of the present and future generations [33, 34]. In addition, some researches showed the role of IgE and IL-4 secretion on allergic patients and childhood asthma and other chronic diseases [13, 35].
Fig. 2.
Location of PM2.5-bound metal(loid)s, industries and children blood IgE, IL-4 and IL-13 in studied areas
Fig. 3.
Spatial distribution of children’s blood pro-inflammatory factors. a (IgE), b (IL-4) and c (IL-13)
Conclusions
This was the first study that simultaneously surveyed the increasing of children’s pre-inflammatory lung biomarkers (IgE, IL-4 and IL-13) in industrial, vehicle traffic and reference areas. Our findings suggested that in the industrial area exposure to As, Hg, Cr, Ni and Cd levels of PM2.5-bound metal(loid)s were higher compared with the vehicle traffic and reference areas and there were significant relationships with IgE, IL-4 and IL-13. Our results indicated the children residing at industrial areas are at very high risk of the allergic, asthmatic, atopic diseases, and early death. Due to the high quantity and diversity of pollutants in industrial areas it is recommended to evaluate other more potential confounders such as PAH markers and other blood heavy metals. The next step of the study could survey the levels of other pro-inflammatory biomarkers in connection with chronic and acute diseases.
Acknowledgments
This paper was a part of a funded Ph.D. thesis of Amir Zahedi, Ahvaz Jundishapur University of Medical Sciences (AJUMS), and the financial support of this study (ETRC-9705) and Ethics Code in Research (IR.AJUMS.REC.1397.324) was provided by AJUMS. The authors would like to express our sincere gratitude to the Khozestan Department of environment, Ahvaz West and East Health Centers and Environmental Technologies Research Center (ETRC), Ahvaz Jundishapur University of Medical Sciences.
Authors’ contributions
Amir Zahedi: Conceptualization, Methodology, Investigation, Resources, Writing - Original Draft. Mohammad Sadegh Hassanvand: Conceptualization, Methodology, Investigation, Writing - Original Draft. Nematollah Jaafarzadeh: Conceptualization, Methodology, Resources, Investigation, Supervision, acquisition, Project administration. Ata Ghadiri: Methodology, Investigation, Resources, Review & Editing. Mansour Shamsipour: Validation, Formal analysis. Mohamad Ghasemi Dehcheshmeh: Methodology, Resource.
Declarations
Competing interests
The authors declare they have no conflict of interest in preparation of the manuscript.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Ashrap P, Watkins DJ, Mukherjee B, Boss J, Richards MJ, Rosario Z, Vélez-Vega CM, Alshawabkeh A, Cordero JF, Meeker JD. Predictors of urinary and blood metal (loid) concentrations among pregnant women in northern Puerto Rico. Environ Res. 2020;183:109178. doi: 10.1016/j.envres.2020.109178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Irato P, Santovito G, Cassini A, Piccinni E, Albergoni V. Metal accumulation and binding protein induction in Mytilus galloprovincialis, Scapharca inaequivalvis, and Tapes philippinarum from the lagoon of Venice. Arch Environ Contam Toxicol. 2003;44(4):0476–0484. doi: 10.1007/s00244-002-1262-8. [DOI] [PubMed] [Google Scholar]
- 3.Kastury F, Smith E, Karna RR, Scheckel KG, Juhasz A. Methodological factors influencing inhalation bioaccessibility of metal (loid) s in PM2. 5 using simulated lung fluid. Environ Pollut. 2018;241:930–937. doi: 10.1016/j.envpol.2018.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan Y, Tian S, Li X, Sun Y, Li Y, Wentworth GR, Wang Y. Trace elements in particulate matter from metropolitan regions of northern China: sources, concentrations and size distributions. Sci Total Environ. 2015;537:9–22. doi: 10.1016/j.scitotenv.2015.07.060. [DOI] [PubMed] [Google Scholar]
- 5.Organization WH. WHO strategy for prevention and control of chronic respiratory diseases: World Health Organization 2002. https://apps.who.int/iris/handle/10665/67369.
- 6.Nhung NTT, Schindler C, Dien TM, Probst-Hensch N, Perez L, Künzli N. Acute effects of ambient air pollution on lower respiratory infections in Hanoi children: an eight-year time series study. Environ Int. 2018;110:139–148. doi: 10.1016/j.envint.2017.10.024. [DOI] [PubMed] [Google Scholar]
- 7.Holsapple MP, Paustenbach DJ, Charnley G, West LJ, Luster MI, Dietert RR, Burns-Naas LA. Symposium summary: children's health risk—what's so special about the developing immune system? Toxicol Appl Pharmacol. 2004;199(1):61–70. doi: 10.1016/j.taap.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 8.Chen Z, Cui L, Cui X, Li X, Yu K, Yue K, Dai Z, Zhou J, Jia G, Zhang J. The association between high ambient air pollution exposure and respiratory health of young children: a cross sectional study in Jinan, China. Sci Total Environ. 2019;656:740–749. doi: 10.1016/j.scitotenv.2018.11.368. [DOI] [PubMed] [Google Scholar]
- 9.Ygberg S, Nilsson A. The developing immune system–from foetus to toddler. Acta Paediatr. 2012;101(2):120–127. doi: 10.1111/j.1651-2227.2011.02494.x. [DOI] [PubMed] [Google Scholar]
- 10.Patel MM, Hoepner L, Garfinkel R, Chillrud S, Reyes A, Quinn JW, Perera F, Miller RL. Ambient metals, elemental carbon, and wheeze and cough in New York City children through 24 months of age. Am J Respir Crit Care Med. 2009;180(11):1107–1113. doi: 10.1164/rccm.200901-0122OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dunea D, Iordache S, Liu H-Y, Bøhler T, Pohoata A, Radulescu C. Quantifying the impact of PM 2.5 and associated heavy metals on respiratory health of children near metallurgical facilities. Environ Sci Pollut Res. 2016;23(15):15395–15406. doi: 10.1007/s11356-016-6734-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Oettgen HC, Geha RS. IgE regulation and roles in asthma pathogenesis. J Allergy Clin Immunol. 2001;107(3):429–441. doi: 10.1067/mai.2001.113759. [DOI] [PubMed] [Google Scholar]
- 13.Der Zee V, Groot D. The role of IL-13 in IgE synthesis by allergic asthma patients. Clin Exp Immunol. 1998;111(1):129–135. doi: 10.1046/j.1365-2249.1998.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shahsavani A, Naddafi K, Haghighifard NJ, Mesdaghinia A, Yunesian M, Nabizadeh R, et al. The evaluation of PM10, PM2. 5, and PM1 concentrations during the middle eastern dust (MED) events in Ahvaz, Iran, from April through September 2010. J Arid Environ. 2012;77:72–83. doi: 10.1016/j.jaridenv.2011.09.007. [DOI] [Google Scholar]
- 15.Atari L, Esmaeili S, Zahedi A, Mohammadi MJ, Zahedi A, Babaei AA. Removal of heavy metals by conventional water treatment plants using poly aluminum chloride. Toxin Rev. 2018;38(2):1–8. [Google Scholar]
- 16.Gao X, Colicino E, Shen J, Kioumourtzoglou M-A, Just AC, Nwanaji-Enwerem JC, Coull B, Lin X, Vokonas P, Zheng Y, Hou L, Schwartz J, Baccarelli AA. Impacts of air pollution, temperature, and relative humidity on leukocyte distribution: an epigenetic perspective. Environ Int. 2019;126:395–405. doi: 10.1016/j.envint.2019.02.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Olmedo P, Pla A, Hernández A, López-Guarnido O, Rodrigo L, Gil F. Validation of a method to quantify chromium, cadmium, manganese, nickel and lead in human whole blood, urine, saliva and hair samples by electrothermal atomic absorption spectrometry. Anal Chim Acta. 2010;659(1–2):60–67. doi: 10.1016/j.aca.2009.11.056. [DOI] [PubMed] [Google Scholar]
- 18.Dai Y, Huo X, Zhang Y, Yang T, Li M, Xu X. Elevated lead levels and changes in blood morphology and erythrocyte CR1 in preschool children from an e-waste area. Sci Total Environ. 2017;592:51–59. doi: 10.1016/j.scitotenv.2017.03.080. [DOI] [PubMed] [Google Scholar]
- 19.Cao S, Duan X, Zhao X, Ma J, Dong T, Huang N, Sun C, He B, Wei F. Health risks from the exposure of children to as, se, Pb and other heavy metals near the largest coking plant in China. Sci Total Environ. 2014;472:1001–1009. doi: 10.1016/j.scitotenv.2013.11.124. [DOI] [PubMed] [Google Scholar]
- 20.Kennedy ER. Guidelines for air sampling and analytical method development and evaluation. vol 95–117. US Department of Health and Human Services, Public Health Service, Centers …; 1995.
- 21.Longhin E, Holme JA, Gualtieri M, Camatini M, Øvrevik J. Milan winter fine particulate matter (wPM2. 5) induces IL-6 and IL-8 synthesis in human bronchial BEAS-2B cells, but specifically impairs IL-8 release. Toxicol in Vitro. 2018;52:365–373. doi: 10.1016/j.tiv.2018.07.016. [DOI] [PubMed] [Google Scholar]
- 22.Hassanvand MS, Naddafi K, Faridi S, Nabizadeh R, Sowlat MH, Momeniha F, et al. Characterization of PAHs and metals in indoor/outdoor PM10/PM2. 5/PM1 in a retirement home and a school dormitory. Sci Total Environ. 2015;527:100–110. doi: 10.1016/j.scitotenv.2015.05.001. [DOI] [PubMed] [Google Scholar]
- 23.Keshavarzi B, Najmeddin A, Moore F, Moghaddam PA. Risk-based assessment of soil pollution by potentially toxic elements in the industrialized urban and peri-urban areas of Ahvaz metropolis, southwest of Iran. Ecotoxicol Environ Saf. 2019;167:365–375. doi: 10.1016/j.ecoenv.2018.10.041. [DOI] [PubMed] [Google Scholar]
- 24.Pastuszka JS, Rogula-Kozłowska W, Zajusz-Zubek E. Characterization of PM10 and PM2. 5 and associated heavy metals at the crossroads and urban background site in Zabrze, upper Silesia, Poland, during the smog episodes. Environ Monit Assess. 2010;168(1):613–627. doi: 10.1007/s10661-009-1138-8. [DOI] [PubMed] [Google Scholar]
- 25.Balasubramanian R, Qian WB, Decesari S, Facchini M, Fuzzi S. Comprehensive characterization of PM2. 5 aerosols in Singapore. J Geophys Res: Atmos. 2003;108(D16).
- 26.Najmeddin A, Keshavarzi B, Moore F, Lahijanzadeh A. Source apportionment and health risk assessment of potentially toxic elements in road dust from urban industrial areas of Ahvaz megacity, Iran. Environ Geochem Health. 2018;40(4):1187–1208. doi: 10.1007/s10653-017-0035-2. [DOI] [PubMed] [Google Scholar]
- 27.Rodríguez-Cotto RI, Ortiz-Martínez MG, Rivera-Ramírez E, Mateus VL, Amaral BS, Jiménez-Vélez BD, Gioda A. Particle pollution in Rio de Janeiro, Brazil: increase and decrease of pro-inflammatory cytokines IL-6 and IL-8 in human lung cells. Environ Pollut. 2014;194:112–120. doi: 10.1016/j.envpol.2014.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wu B. Relationship between the chromium or chromium compounds on immune functions in animals. J Vet Med Health. 2017;1:101. [Google Scholar]
- 29.Shrivastava R, Upreti R, Seth P, Chaturvedi U. Effects of chromium on the immune system. FEMS Immunol Med Microbiol. 2002;34(1):1–7. doi: 10.1111/j.1574-695X.2002.tb00596.x. [DOI] [PubMed] [Google Scholar]
- 30.Hayes RB. Review of occupational epidemiology of chromium chemicals and respiratory cancer. Sci Total Environ. 1988;71(3):331–339. doi: 10.1016/0048-9697(88)90205-7. [DOI] [PubMed] [Google Scholar]
- 31.Soori H. Children's indoor and outdoor play patterns in Ahwaz City: implications for injury prevention. EMHJ-East Mediterr Health J. 2006;12(3–4):372–381. [PubMed] [Google Scholar]
- 32.Kim KH, Lee S-B, Woo D, Bae G-N. Influence of wind direction and speed on the transport of particle-bound PAHs in a roadway environment. Atmos Pollut Res. 2015;6(6):1024–1034. doi: 10.1016/j.apr.2015.05.007. [DOI] [Google Scholar]
- 33.Chimonas M-AR, Gessner BD. Airborne particulate matter from primarily geologic, non-industrial sources at levels below National Ambient air Quality Standards is associated with outpatient visits for asthma and quick-relief medication prescriptions among children less than 20 years old enrolled in Medicaid in Anchorage, Alaska. Environ Res. 2007;103(3):397–404. doi: 10.1016/j.envres.2006.08.013. [DOI] [PubMed] [Google Scholar]
- 34.Wichmann FA, Müller A, Busi LE, Cianni N, Massolo L, Schlink U, Porta A, Sly PD. Increased asthma and respiratory symptoms in children exposed to petrochemical pollution. J Allergy Clin Immunol. 2009;123(3):632–638. doi: 10.1016/j.jaci.2008.09.052. [DOI] [PubMed] [Google Scholar]
- 35.Kabesch M, Schedel M, Carr D, Woitsch B, Fritzsch C, Weiland SK, von Mutius E. IL-4/IL-13 pathway genetics strongly influence serum IgE levels and childhood asthma. J Allergy Clin Immunol. 2006;117(2):269–274. doi: 10.1016/j.jaci.2005.10.024. [DOI] [PubMed] [Google Scholar]