Abstract
A single intradermal administration of recombinant interleukin-7 (IL-7) has been shown to aggravate the course of murine schistosomiasis, to favor the development of Th2-associated antibodies specific for the parasite, and to alter migration kinetics and/or migratory route of the parasite within its vertebrate host. Here we show that after infection of IL-7-deficient mice with Schistosoma mansoni, the predominant parasite-specific humoral response follows a Th1 pattern, and the development of the parasite is greatly impaired. In IL-7-deficient mice, increased numbers of larvae reach the lungs and fewer larvae reach the liver, compared to control mice. In the absence of IL-7, female worms show an altered fecundity, leading to decreased numbers of eggs trapped in the tissues and to an amelioration of the pathology of the infected host. The most striking observation is the blockade of parasite growth in an IL-7-defective environment, leading to dwarf male and female worms. The results of this study have important implications for the role of IL-7 in the host-parasite relationship and show how parasites can disable or evade the host immune response.
The mammalian immune system is a complex network of interacting cell types and functions. Much of the regulation of cell growth and differentiation is accomplished by the elaboration of soluble factors, principally cytokines, that interact with specific receptors found on responsive cells. On the other hand, the precise adaptation of parasites in mammalian hosts implies that conditions required for their growth, differentiation, and maturation are present. Several reports describe the involvement of cytokines in the regulation of host-parasite relationships (reviewed in reference 8). During infection with Leishmania spp., it has been shown that interleukin-3 (IL-3) (12, 21), granulocyte-macrophage colony-stimulating factor (13, 29), and IL-2 (22) favored parasite growth. Tumor necrosis factor alpha (TNF-α) and gamma interferon have been reported to stimulate the growth of Trypanosoma spp. (3, 17). In the case of Schistosoma mansoni infection, there is little information concerning the effect(s) of host cytokine(s) on migration and/or maturation of the worm within its vertebrate host. This could be due to the complex life cycle of the parasite which involves different developmental stages and an arduous and intricate migration over a period of many days from the skin to the lungs and liver until the final residence in the systemic circulation system. There the worms mature sexually, male and female pairing occurs, and egg laying begins (11). It has been reported that the host’s cytokines do influence the egg laying (9), and it was recently shown that the parasitic worms require TNF-α to lay and excrete eggs (2, 15).
The stroma-derived cytokine IL-7 was originally described as a growth-promoting, and possibly differentiating, factor for B-lymphocyte precursors (25) and immature thymocytes (36). IL-7 is also involved in inflammatory reactions (33), in the induction of adhesion molecule expression (4), and in the activity of mature T cells and NK/LAK cells (1, 24). Despite the multiple and pleiotropic effects already described for IL-7 (reviewed in reference 23), disruption of the IL-7 gene in the murine germ line identifies IL-7 as a nonredundant cytokine (34).
Our previous reports showed that IL-7 is expressed in the skin of S. mansoni-infected mice from days 1 to 21 following parasite penetration (37) and that keratinocytes and dermal endothelial cells of infected human skin produced IL-7 (27). Furthermore, intradermal administration of recombinant IL-7 in mice prior to infection altered the migration of the parasite and led to an increased number of surviving adult parasites and to a more-severe liver pathology. At the same time, IL-7-treated mice had increased levels of Th2-associated specific antibodies (37). The above observations suggest that IL-7 influences both the host’s immune response and the development of the parasite.
To clarify issues regarding the contribution of IL-7 during S. mansoni infection, we performed a detailed examination of infection in mice with targeted inactivation of the IL-7 gene (IL-7−/− [34]). We examined migration of the larvae to the lungs, adult worm and egg burdens, hepatic pathology, and parasite-specific antibody responses. This study shows that S. mansoni development is strikingly impaired in an IL-7-deprived environment.
The data presented in this paper reinforce our previous observations (27, 37) and support the concept that IL-7 is one of the key host-derived cytokines involved in the complete and proper development of S. mansoni in the infected host. The role of IL-7 in the innate response of the S. mansoni-infected vertebrate host and its use for the benefit of the parasite are discussed.
MATERIALS AND METHODS
Collection of S. mansoni cercariae.
The life cycle of S. mansoni was maintained, using golden hamsters as the definitive hosts and Biomphalaria glabrata snails as the intermediate hosts. Cercariae of S. mansoni were obtained from infected B. glabrata snails by use of artificial light.
Infection of mice with S. mansoni.
Mice with a targeted deletion within the gene coding for IL-7 (IL-7−/−) were generated by von Freeden-Jeffry et al. (34). Animals were housed under microisolator caps in a quarantine room. Female littermate control mice (IL-7+/+) and female mutant mice were used at the age of 7 to 8 weeks. Animals were percutaneously infected as previously described (28), with 50 ± 5 cercariae of S. mansoni unless otherwise noted (namely, the experiments assessing migration to the lungs).
Quantification of adult worm and egg burdens.
Mice were sacrificed 42 and 80 days postinfection (p.i.) by intraperitoneal injection of 15 mg of pentobarbital (Sanofi Santé Animale, Gentilly, France) and 60 U of heparin (Sanofi Choay, Gentilly, France). Adult worms were harvested by perfusion of the portal venous system (10), and the numbers of female and male adult worms were determined with a light microscope. At the time of perfusion, livers were collected, weighed, and digested overnight with 4% potassium hydroxide, and eggs were counted in 500-μl samples under a microscope.
In vitro egg hatching assay.
Schistosome eggs were recovered from the livers of infected mice 80 days p.i. Briefly, livers were first treated with collagenase (Sigma) (1 mg per liver) for 1 h at 37°C and then homogenized on ice. Extracts were washed several times with ice-cold phosphate-buffered saline. About 200 eggs (approximately 50% mature eggs) were distributed into the wells of a 24-well plate (Nunc, Intermed S.A., Roskilde, Denmark) in calcium-free water, in a final volume of 1.5 ml. Egg hatching was performed by incubating the plates at 30°C for 90 min. Hatching was then stopped by the addition of 100 μl of 1% Lugol. Miracidia and living eggs (mature and immature) and nonliving eggs were counted under a phase-contrast microscope. Eggs were classified by using morphological criteria (32); immature eggs show a developing embryo, mature eggs have a fully developed miracidium, and dead eggs have a dark, retracted miracidium. Hatching assays were performed for each individual mouse, and data were expressed as follows: % Hatching = [number of eggshells/(number of mature eggs + number of shells)] × 100 and % Maturation = [(number of mature eggs + number of shells)/number of total eggs] × 100.
Histopathology and collagen measurement in the liver.
Livers were fixed in Bouin’s solution, embedded in paraffin, sectioned (6 μm), and stained for histopathological examination and collagen measurement. Sections were deparaffinized and incubated at room temperature for 2 h in the dark under continuous, mild agitation with a saturated solution of picric acid in distilled water containing 0.1% fast green FCF (Sigma, Saint Quentin Fallavier, France) which stained noncollagenous proteins and 0.1% Syrius red F3B (Gurr BDH Chemicals Ltd., Poole, England) which stained collagen. Half of the slides were then mounted for microscopical evaluation of the histopathology. The other half was destained for collagen determination as previously described (20). Data are reported as the numbers of micrograms of collagen per milligram of protein (mean ± standard error) for each individual mouse.
Evaluation of schistosomula migration to the lungs.
Mice infected with 500 cercariae were sacrificed at day 6, and their lungs were excised and chopped into the smallest possible fragments before transfer into a petri dish containing 0.2 ml of minimum essential medium (MEM). The moistened fragments were then transferred onto a 90-mm mesh (Hartmann-Larochette, Chatenois, France) which was placed on a 50-ml glass tube filled to the top with MEM. The young worms that migrated from the lungs into the MEM were collected after incubation at 37°C overnight and counted under a microscope.
Serum antibody determination by enzyme-linked immunosorbent assay (ELISA).
Sera were prepared from blood samples collected from infected IL-7+/+ and IL-7−/− animals at days 14, 21, 28, 35, and 42 p.i. Mice were individually tested for the presence of parasite-specific total immunoglobulin G (IgG), IgG1, and IgG2a.
For determination of specific anti-adult worm antigenic extract (anti-SWAP) or anti-egg antigenic extract (anti-SEA) antibody titers (total IgG, IgG1, and IgG2a), the wells in microtiter plates were coated overnight with 15 μg/ml of either parasitic extracts. After saturation with 3% bovine serum albumin in phosphate-buffered saline, plates were incubated with experimental sera (dilution 1:200) for 2 h at room temperature. Peroxidase-labelled goat anti-mouse IgG, IgG1, and IgG2a antibodies (Southern Biotechnology Associates, Inc., Birmingham, Ala.) were used for detection. ELISAs were performed with o-phenylenediamine (Sigma) and 0.001% hydrogen peroxide in phosphate-citrate buffer as substrate. Reaction was recorded with an automated plate reader at 492 nm (Multiskan MS; Labsystems, Helsinki, Finland).
Statistics.
Statistical significance was determined by using Student’s t test and P values of <0.05.
RESULTS
Parasite development and egg-induced pathology in IL-7-deficient mice.
Adult schistosomes dwell as pairs of male and female worms in the portal veins draining the large intestine of their vertebrate permissive host. The egg laying begins within 4 to 5 weeks p.i. (11).
(i) Adult worm and egg burdens in IL-7−/− mice.
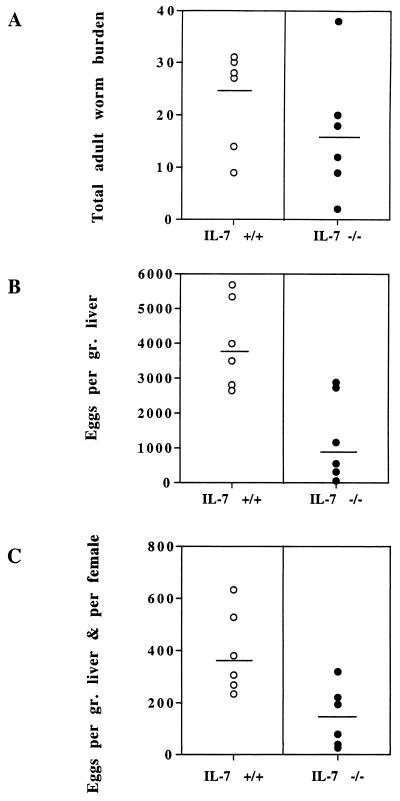
The number of male and female worms was assessed by total blood perfusion of IL-7+/+ and IL-7−/− S. mansoni-infected mice 42 days p.i. Livers were removed and weighed, and hepatic egg burdens were determined after alkali digestion of the organs (Fig. 1). IL-7−/− mice showed a reduced adult worm burden (28%) (Fig. 1A) with comparable ratios of males to females in the two groups of mice (not shown). In addition, the numbers of eggs expressed per gram of liver (Fig. 1B) and per female worm (Fig. 1C), were drastically decreased in IL-7−/− mice compared to the corresponding control animals (68 and 64%, respectively).
FIG. 1.
Total adult worm burden (A), numbers of eggs expressed per gram of liver (B), and numbers of eggs expressed per gram of liver and per female worm (C) in IL-7+/+ and IL-7−/− S. mansoni-infected animals. Each datum point shows the value for one mouse (six mice per group), and the horizontal bar shows the mean for that group of mice. This experiment is representative of four independent experiments. Statistical analysis using the Student’s t test gave P values of 0.15 (A), 0.002 (B), and 0.05 (C).
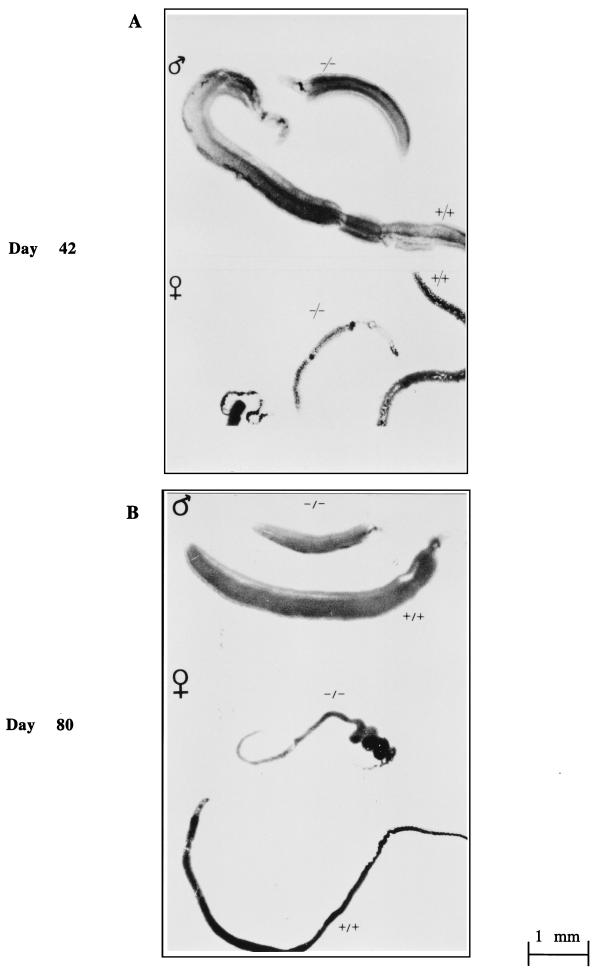
(ii) Morphology of worms in IL-7−/− mice.
An important difference was noticed in the parasite morphology 42 days after infection. Indeed, all male and female worms were strikingly smaller in IL-7−/− mice than in IL-7+/+ mice (Fig. 2A). To ascertain whether this size reduction corresponded to a delay or blockade of worm maturation, we performed another series of infection of IL-7+/+ and IL-7−/− mice and collected the parasites 80 days p.i. when they should have attained their full size and maturity. Again, as shown in Fig. 2B, worms were smaller in the group of IL-7−/− animals.
FIG. 2.
Morphology of worms (males and females) collected from IL-7+/+ infected animals (+/+) and IL-7−/− infected animals (−/−) at day 42 (A) and day 80 (B) following infection with S. mansoni cercariae.
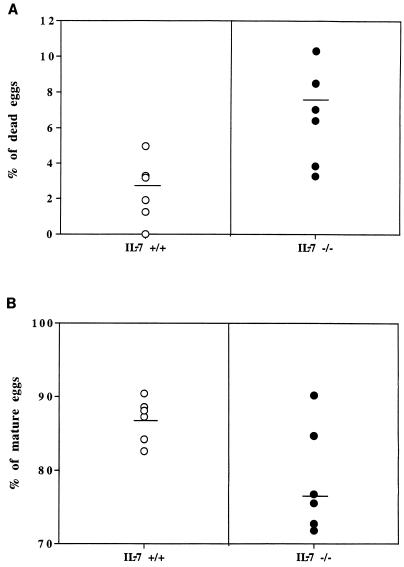
(iii) Fecundity of female worms in IL-7−/− mice.
Figure 1C shows the number of hepatic eggs laid per gram of liver and per female worm in both strains of mice. We further determined the percentages of viable (mature and immature) and dead eggs in the livers of IL-7+/+ and IL-7−/− S. mansoni-infected animals. This experiment showed that female worms developing in IL-7−/− mice laid significantly more dead eggs (63%) (Fig. 3A) and fewer mature eggs than the worms in control mice (10%) (Fig. 3B). Nevertheless, when the capability of mature eggs to hatch in vitro was determined, no statistical difference was observed between the experimental groups (data not shown).
FIG. 3.
Percentages of dead eggs (A) (P = 0.005) and mature eggs (B) (P = 0.014) laid by females in the livers of IL-7+/+ and IL-7−/− animals. Each datum point shows the value for one mouse, and the horizontal bar shows the mean for that group.
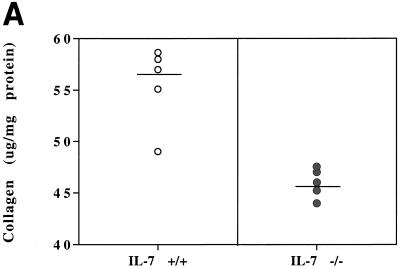
(iv) Liver pathology in IL-7−/− mice.
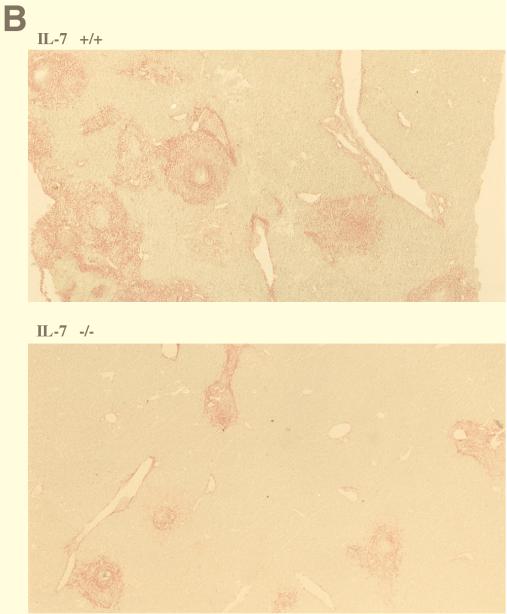
The key pathogenic event during S. mansoni infection is granuloma formation around eggs trapped in the liver. The immunopathology results from cellular infiltration and residual fibrosis surrounding the eggs (5). Measurement of the collagen deposits in the livers of mice after 80 days of infection showed a markedly reduced level of collagen in the livers of IL-7−/− infected mice (15%) (Fig. 4A). Corresponding liver sections observed before destaining visualized the reduced collagen deposition in the livers of IL-7−/− mice, yet inflammatory reactions developed around eggs trapped in the hepatic tissue of IL-7-deficient animals (Fig. 4B).
FIG. 4.
Pathology of livers of IL-7+/+ and IL-7−/− animals infected with S. mansoni for 80 days. (A) Collagen measurement (in micrograms per milligram of protein). Results are expressed for each individual mouse and are representative of three independent experiments. The horizontal bars show the means for the two groups of mice which were significantly different (P = 0.001). (B) Histological staining for collagen on liver sections of IL-7+/+ and IL-7−/− infected mice 80 days p.i. Syrius red F3B stained collagen in red. Magnification, ×100.
This series of data demonstrates the impairment of S. mansoni development in IL-7-deficient mice. IL-7−/− mice infected with S. mansoni larvae do not support normal parasite growth and development. Consequent to the reduction of number of eggs, liver pathology is reduced. Finally, male and female parasites which developed in IL-7−/− mice are stunted and could be considered “dwarf” parasites.
Migration of S. mansoni to the lungs in IL-7-deficient mice.
Development and maturation of schistosomes are intimately associated with their migration in the definitive vertebrate host (11). Therefore, we suspected that IL-7 deficiency could alter the capacity of the parasite to properly migrate from the skin to the lungs and then to the liver, as previously hypothetized (37). We thus investigated migration of the parasite to the lungs in IL-7+/+ and IL-7−/− mice 6 days after parasite penetration.
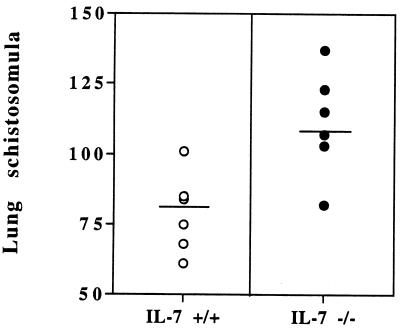
More schistosomula reached the lungs in IL-7−/− mice than in IL-7+/+ animals (28%) (Fig. 5). This observation is consistent with the injection of recombinant IL-7 prior to infection, which led to the opposite effect on parasite migration to the lungs (37). Therefore, the impairment of S. mansoni development and maturation in IL-7-deficient mice is associated with an alteration of the migratory route of the parasites to the lungs and to the liver.
FIG. 5.
Migration of S. mansoni schistosomula to the lungs in IL-7+/+ and IL-7−/− S. mansoni-infected mice day 6 p.i. Each datum point shows the value for one mouse. The horizontal bars show the mean values for the two experimental groups which were significantly different (P = 0.0035). This experiment was performed twice and gave similar results.
Specific humoral immune response of IL-7-deficient mice.
IL-7−/− mice have large reductions in lymphoid tissue cellularity in both the T- and B-cell lineages but have a normal ratio of CD4+ to CD8+ subsets (34). One important difference between the CD4+ T cells of Th1 and Th2 subsets is their ability to stimulate the production of certain Ig isotypes (7).
In mice infected with schistosomes, Th2 responses have convincingly been involved in the development of granulomatous lesions around tissue-trapped parasite eggs (14). We previously demonstrated that a single intradermal injection of recombinant IL-7 prior to S. mansoni infection, led to an increase of the Th2 responses towards the egg antigenic extracts (37).
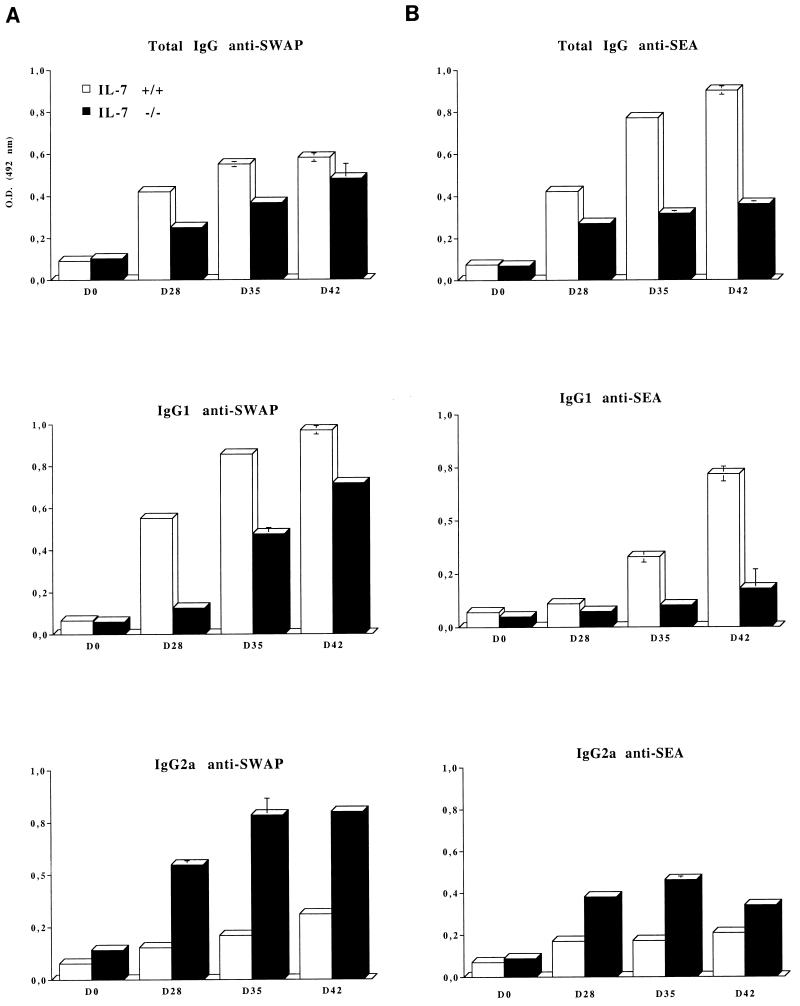
We therefore analyzed the isotype profiles of IgG specific for both SWAP and SEA during the course of infection in IL-7−/− and IL-7+/+ mice. The data represented in Fig. 6 show that for both SWAP (Fig. 6A) and SEA (Fig. 6B) antigens, the specific IgG1 antibodies are lower in IL-7−/− mice compared to the control mice, whereas specific IgG2a antibodies predominate in the IL-7−/− mice.
FIG. 6.
Specific antibody response in IL-7+/+ and IL-7−/− mice during the course of S. mansoni infection. Total IgG, IgG1, and IgG2a specific for SWAP (A) egg-specific IgG, IgG1, and IgG2a (SEA) (B) are shown. In this experiment, seven animals per group were infected and repeatedly bled on days 0, 28, 35 and 42 following infection. For each time point, sera from the 7 animals were pooled and tested by specific ELISA. Each test was done in triplicate. The y-axis label (optical density at 492 nm [O.D. (492 nm)] and symbol key shown in the top bar graph in panel A apply to all bar graphs in the figure.
DISCUSSION
With the advent of selective gene knockout technology, a number of studies have used mice with targeted disruption of cytokine genes to assess the contribution of these molecules to immune and inflammatory responses (16). However, there is still little information on the use of these models for studying the participation of cytokines in the innate host-parasite partnerships. For the parasite S. mansoni, it was demonstrated that adult worms induced the expression of hepatic TNF-α required for egg laying and excretion of eggs from the host (2, 19). In this study, we reported that the absence of the bioactive IL-7 molecule results in the impairment of the development of the parasite S. mansoni in its vertebrate host.
We found that IL-7-deficient mice (IL-7−/−) (34) infected with S. mansoni cercariae did not support normal parasite development. Compared with control immunocompetent mice, schistosome-infected IL-7−/− mice had fewer adult worm pairs. In addition, in vitro, the number of mature eggs produced by these worm pairs was reduced and they released more dead eggs. Consequent to the decreased egg numbers, liver pathology of IL-7−/− infected mice was improved and the humoral specific response during the course of infection was predominantly of the Th1 type. Of note are our previous observations of the injection of recombinant IL-7 leading to an increased number of worm pairs, a more severe hepatic pathology, and a dominant Th2-related humoral response (37). Since in schistosome-infected mice, eggs are the primary stimulus of the Th2 response (14), the effects of IL-7 on the Th1 and Th2 immune responses would instead be indirectly due to differential egg burdens in the IL-7-treated mice and IL-7−/− mice compared to that of control mice.
Similarly, the influence of IL-7 on parasite migration from the skin to the lungs was different in recombinant IL-7-injected mice and in IL-7−/− mice. Fewer larvae reached the lungs of IL-7-treated mice, whereas more reached the lungs of IL-7−/− animals. One could propose that IL-7 produced in the skin (27, 37) drove the proper migration of the parasite from the skin to the lungs. In addition, we showed that the parasite directly triggered IL-7 release from endothelial cells of dermal vessels (27), further support for the notion that at some point between the penetration of this endovascular parasite in the skin to its migration to the lungs, S. mansoni encountered IL-7. Interactions between S. mansoni and its vertebrate host might be relevant for several features of the life cycle of the parasite, particularly for its migration and homing. Those interactions require direct contact between the parasite and the mammalian cells, which involve host cell-parasite adhesion (6).
Among parasite adhesive motifs that may have implications for the host-parasite relationship is the Lewisx (Lex) determinant (30). Recently, Lex was shown to be involved in the mechanisms of cytotoxicity against schistosomula (31), but the precise role of this determinant in parasite survival in infected animals is unknown. We have preliminary data showing that stunted parasites of IL-7−/− infected mice expressed higher levels of Lex on their surface (38). We are presently investigating the expression of adhesion molecules, particularly ligands of Lex, on the host tissues. Differential expression of adhesion molecules on either the worm or the host’s tissues could be a partial explanation for the mechanisms by which IL-7 acts on parasite migration.
Unexpectedly, schistosomes which developed in IL-7−/− mice were smaller than those in control mice. Both male and female worms were smaller, and this phenomenon was definitely a blockade, and not a delay, since it occurred at a time when the worms were fully developed (i.e., day 80 p.i.). Interestingly, parasites developing in IL-7−/− mice were sexually differentiated, and they mated and laid eggs, even if in reduced number. These worms could thus be called “dwarf” parasites.
IL-7 knockout mice are deficient in several aspects of the immune system (34) due to the lack of IL-7. However, none of the numerous immunodeficient mice so far infected with S. mansoni allowed the development of such dwarf parasites, which means that the effects we report for IL-7 on the development of S. mansoni are not due to the immunodeficiency of the animals but are due (directly or indirectly) to IL-7. To our knowledge, the unique report in the literature of reduced-size S. mansoni worms corresponded to hypothyroid mice (35) in which the parasite phenotype was similar to the phenotype of IL-7−/− infected mice. This raises questions about the relationship between IL-7 and thyroid hormones. Experiments are presently under way to investigate this matter. Various hormones of vertebrate hosts have been implicated in the stimulation or induction of growth or sexual reproduction of their parasites (18). Perhaps IL-7 deficiency creates a hormonal environment in the host to which schistosomes adapt by developing ad minima as male and female dwarf worms, laying eggs to ensure its transmission to new hosts.
In conclusion, our study reveals an unexpected and central role for IL-7 in the development of the skin-penetrating parasite S. mansoni in its definitive vertebrate host. This contention is supported both by the results of our previous sets of experiments (27, 37) and by the present results. The signals delivered through IL-7 to the parasite in the skin and throughout its migration in the host allow proper migration, maturation, and development of the worms. Further studies to determine the mechanism of action of IL-7 in the modulation of host’s immune response and how it affects parasite development are needed. Ligand binding studies with radiolabelled IL-7 so far suggest that this cytokine does not directly bind on the parasite surface (our unpublished observation). Therefore, the observed effects of IL-7 (27, 37; this report) can be attributed to alterations of the host’s immune and/or endocrine responses. For this particular aspect, infection of IL-7 receptor-deficient mice (26) could help in dissociating the effects of IL-7 on the host’s responses from those acting directly on the parasite. The similar phenotype of the parasites (namely, male and female worms of reduced size) developing in IL-7−/− and hypothyroid animals (35) could mean that IL-7 exerts effects on parasites, possibly by increasing their responsiveness to host endocrine factors. Understanding the level of interaction(s) between IL-7, the endocrine system, and S. mansoni is obviously critical to the host-parasite interactions.
Clearly, IL-7 is a key cytokine in the host-parasite relationship which increases the permissiveness of the definitive host to infection, far beyond the development of the host’s immune response.
ACKNOWLEDGMENTS
This work was supported in part by the Centre National de la Recherche Scientifique, the Institut Pasteur de Lille, and Lille II University. O. Roye and S. Nutten were supported by grants from the Région Nord-Pas de Calais and from the Institut Pasteur de Lille.
We thank J. Fontaine for many helpful discussions on the parasite. The critical views on our work of D. D. Dombrovicz, C. Dissous, and R. Pierce were of great value. The assistance of S. Vanwigene in preparation of the parasite, E. Fleurbaix in breeding mice, and J.-M. Merchez in photography is greatly appreciated.
REFERENCES
- 1.Alderson M R, Sassenfeld H M, Widmer M B. Interleukin 7 enhances cytolytic T lymphocyte generation and induces lymphokine-activated killer cells from human peripheral blood. J Exp Med. 1990;172:577–587. doi: 10.1084/jem.172.2.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amiri P, Locksley R M, Parslow T G, Sadick M, Rector E, Ritter D, McKerrow J H. Tumor necrosis factor-alpha restores granuloma and induces parasite egg-laying in schistosome-infected SCID mice. Nature. 1992;356:604–607. doi: 10.1038/356604a0. [DOI] [PubMed] [Google Scholar]
- 3.Bakhiet M, Olsson T, Eldund C, Höjeberg B, Holmberg K, Lorentzen J, Kristensson K. A Trypanosoma brucei-derived factor that triggers CD8+ lymphocytes to interferon-γ secretion: purification, characterization and protective effects in vivo by treatment with a monoclonal antibody against the factor. Scand J Immunol. 1993;37:165–178. doi: 10.1111/j.1365-3083.1993.tb01753.x. [DOI] [PubMed] [Google Scholar]
- 4.Barth C, O’Connell P, Zanker B, Strom T B. Interleukin-7 (IL-7) and its effect on the expression of the intercellular adhesion molecule ICAM-1 in T cells. Transplant Proc. 1991;23:824–825. [PubMed] [Google Scholar]
- 5.Boros D L, Warren K S. Delayed hypersensitivity-type granuloma formation and dermal reaction induced and elicited by a soluble factor isolated from Schistosoma mansoni eggs. J Exp Med. 1970;132:488–507. doi: 10.1084/jem.132.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Butterworth A E, Vadas M A, Wassom D L, Dessein A, Hogan M, Sherry B, Gleich G J, David J R. Interactions between human eosinophils and schistosomula of Schistosoma mansoni. II. The mechanism of irreversible adherence. J Exp Med. 1979;150:1456–1471. doi: 10.1084/jem.150.6.1456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coffman R L, Seymour B W, Lebman D A, Hiraki D D, Christiansen J A, Shrader B, Chewinski H M, Savelkoul H F J, Finkelman F D, Bond M W, Mosmann T R. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 8.Denis M, Campbell D, Gregg E O. Cytokine stimulation of parasitic and microbial growth. Res Microbiol. 1991;142:979–983. doi: 10.1016/0923-2508(91)90008-x. [DOI] [PubMed] [Google Scholar]
- 9.Doenhoff M J, Musallam Bain J, McGregor A. Studies of the host-parasite relationship in Schistosoma mansoni-infected mice: the immunological dependence of parasite egg secretion. Immunology. 1978;35:771–778. [PMC free article] [PubMed] [Google Scholar]
- 10.Duval R H, DeWitt W B. An improved perfusion technique for recovering adult schistosomes from laboratory animals. Am J Trop Med Hyg. 1967;16:483–486. doi: 10.4269/ajtmh.1967.16.483. [DOI] [PubMed] [Google Scholar]
- 11.Faust E C, Jones C A, Hoffman W A. Studies on schistosomiasis mansoni in Puerto Rico. III. Biological studies. 2. The mammalian phase of the life cycle. Puerto Rico J Public Health Trop Med. 1934;10:133–196. [Google Scholar]
- 12.Feng Z Y, Louis J, Kindler V, Pedrazzini T, Eliason J F, Behin R, Vassalli P. Aggravation of experimental cutaneous leishmaniasis in mice by administration of interleukin-3. Eur J Immunol. 1988;18:1245–1251. doi: 10.1002/eji.1830180815. [DOI] [PubMed] [Google Scholar]
- 13.Greil J, Bodendorfer B, Röllinghoff M, Solbach W. Application of recombinant granulocyte-macrophage colony-stimulating factor has a detrimental effect in experimental murine leishmaniasis. Eur J Immunol. 1988;18:1527–1533. doi: 10.1002/eji.1830181009. [DOI] [PubMed] [Google Scholar]
- 14.Grzych J M, Pearce E J, Cheever A, Claulada Z A, Caspar P, Hieny S, Lewis F A, Sher A. Egg deposition is the major stimulus for the production of Th2 cytokines in murine schistosomiasis mansoni. J Immunol. 1991;146:1322–1327. [PubMed] [Google Scholar]
- 15.Joseph A L, Boros D L. Tumor necrosis factor plays a role in Schistosoma mansoni egg-induced granulomatous inflammation. J Immunol. 1993;151:5461–5471. [PubMed] [Google Scholar]
- 16.Koller B H, Smithies O. Altering genes in animals by gene targeting. Annu Rev Immunol. 1992;10:705–730. doi: 10.1146/annurev.iy.10.040192.003421. [DOI] [PubMed] [Google Scholar]
- 17.Kongshavn P A L, Ghadirian E. Enhancing and suppressive effects of tumour necrosis factor/cachectin on Trypanosoma musculi growth. Parasite Immunol. 1989;10:581–588. doi: 10.1111/j.1365-3024.1988.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 18.Lawrence P O. Hormonal effects on insects and other endoparasites in vitro. In Vitro Cell Dev Biol. 1991;27:487–496. doi: 10.1007/BF02631150. [DOI] [PubMed] [Google Scholar]
- 19.Leptak C L, McKerrow J H. Schistosome egg granulomas and hepatic expression of TNF-alpha are dependent on immune priming during parasite maturation. J Immunol. 1997;158:301–307. [PubMed] [Google Scholar]
- 20.Lopez De Leon A, Rodjkind M. A simple micromethod for collagen and total protein determination in formalin-fixed-embedded sections. J Histochem Cytochem. 1985;33:737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- 21.Louis J A, Pedrazzini T, Titus R G, Müller I, Farrell J P, Kindler V, Vassalli P, Marchal G, Milon G. Subsets of specific T cells and experimental cutaneous leishmaniasis. Ann Inst Pasteur/Immunol. 1987;138:755–758. doi: 10.1016/s0769-2625(87)80032-6. [DOI] [PubMed] [Google Scholar]
- 22.Mazingue C, Cottrez-Detoeuf F, Louis J, Kweider M, Auriault C, Capron A. In vitro and in vivo effects of interleukin-2 on the protozoan parasite Leishmania. Eur J Immunol. 1989;19:487–491. doi: 10.1002/eji.1830190312. [DOI] [PubMed] [Google Scholar]
- 23.Mertsching E, Meyer V, Linares J, Lombard-Platet S, Ceredig R. Interleukin-7, a non-redundant potent cytokine whose over-expression massively perturbs B-lymphopoiesis. Int Rev Immunol. 1998;16:285–308. doi: 10.3109/08830189809042998. [DOI] [PubMed] [Google Scholar]
- 24.Morrissey P J, Goodwin R G, Nordan R P, Anderson D, Grabstein K H, Cosman D, Sims J, Lupton S, Acres B, Reed S G. Recombinant interleukin 7, pre-B cell growth factor, has costimulatory activity on purified mature T cells. J Exp Med. 1989;169:707–716. doi: 10.1084/jem.169.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Namen A E, Schmierer A E, March C J, Overell R W, Park L S, Urdal D L, Mochizuki D Y. B cell precursor growth-promoting activity. Purification and characterization of a growth factor active on lymphocyte precursors. J Exp Med. 1988;167:988–1002. doi: 10.1084/jem.167.3.988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Peschon J J, Morrissey P J, Grabstein K H, Ramsdell F J, Marakovsky E, Gliniak B C, Park L S, Ziegler S F, Williams D E, Ware C B, et al. Early lymphocyte expansion is severely impaired in IL-7 receptor-deficient mice. J Exp Med. 1994;180:1955–1960. doi: 10.1084/jem.180.5.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Roye O, Delhem N, Trottein F, Remoue F, Nutten S, Decavel J-P, Delacre M, Martinot V, Cesbron J-Y, Auriault C, Wolowczuk I. Dermal endothelial cells and keratinocytes produce interleukin-7 in vivo after human Schistosoma mansoni percutaneous infection. J Immunol. 1998;161:4161–4168. [PubMed] [Google Scholar]
- 28.Smithers S R, Terry R J. Immunity in schistosomiasis. Ann N Y Acad Sci. 1969;160:826–840. doi: 10.1111/j.1749-6632.1969.tb15904.x. [DOI] [PubMed] [Google Scholar]
- 29.Solbach W, Greil J, Röllinghoff M. Anti-infectious responses in Leishmania major-infected Balb/c mice injected with recombinant granulocyte-macrophage colony-stimulating factor. Ann Inst Pasteur/Immunol. 1987;138:759–762. doi: 10.1016/s0769-2625(87)80033-8. [DOI] [PubMed] [Google Scholar]
- 30.Srivatsan J, Smith D F, Cummings R D. The human blood fluke Schistosoma mansoni synthesizes glycoproteins containing the Lewisx antigen. J Biol Chem. 1992;267:20196–20203. [PubMed] [Google Scholar]
- 31.Trottein F, Nutten S, Papin J-P, Leportier C, Poulain-Godefroy O, Capron A, Capron M. Role of adhesion molecules of the selectin-carbohydrate families in antibody-dependent cell-mediated cytotoxicity to schistosome targets. J Immunol. 1997;159:804–811. [PubMed] [Google Scholar]
- 32.Vercruysse J, Southgate V R, Rollinson D, Hilderson H M. Observations on the infectivity and fecundity of Schistosoma curassoni from Senegal in albino mice. J Helminthol. 1988;62:103–109. doi: 10.1017/s0022149x00011329. [DOI] [PubMed] [Google Scholar]
- 33.Viney J L. Altering cytokine soups: a recipe for inflammatory bowel disease? Gut. 1998;42:607–608. doi: 10.1136/gut.42.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Freeden-Jeffry U, Viera P, Lucian L A, MacNeil T, Burbach S E G, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wahab A M F, Warren K S, Levy R P. Function of the thyroid and the host-parasite relation in murine schistosomiasis. J Infect Dis. 1971;124:161–171. doi: 10.1093/infdis/124.2.161. [DOI] [PubMed] [Google Scholar]
- 36.Watson J D, Morrissey P J, Namen A E, Conlon P J, Widmer M B. Effect of IL7 on the growth of fetal thymocytes in culture. J Immunol. 1989;143:1215–1222. [PubMed] [Google Scholar]
- 37.Wolowczuk I, Delacre M, Roye O, Giannini S L, Auriault C. Interleukin-7 in the skin of Schistosoma mansoni-infected mice is associated with a decrease in interferon-γ production and leads to an aggravation of the disease. Immunology. 1997;91:35–44. doi: 10.1046/j.1365-2567.1997.00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolowczuk, I. Unpublished observations.