Abstract
The use of iron supplementation for anemia in erythropoietic protoporphyria (EPP) is controversial with both benefit and deterioration reported in single case reports. There is no systematic study to evaluate the benefits or risks of iron supplementation in these patients. We assessed the potential efficacy of oral iron therapy in decreasing erythrocyte protoporphyrin (ePPIX) levels in patients with EPP or X-linked protoporphyria (XLP) and low ferritin in an open-label, single-arm, interventional study. Sixteen patients (≥18 years) with EPP or XLP confirmed by biochemical and/or genetic testing, and serum ferritin ≤30 ng/mL were enrolled. Baseline testing included iron studies, normal hepatic function, and elevated plasma porphyrins and ePPIX levels. Oral ferrous sulfate 325 mg twice daily was administered for 12 months. The primary efficacy outcome was the relative difference in total ePPIX level between baseline and 12 months after starting treatment with iron. Secondary measures included improvement in serum ferritin, plasma porphyrins, and clinical symptoms. Thirteen patients had EPP (8 females, 5 males) and 3 had XLP (all females) and the mean age of participants was 38.8 years (SD 14.5). Ten patients completed all study visits limiting interpretation of results. In EPP patients, a transient increase in ePPIX levels was observed at 3 months in 9 of 12 (75%) patients. Iron was discontinued in 2 of these patients after meeting the protocol stopping rule of a 35% increase in ePPIX. Seven patients withdrew before study end. Ferritin levels increased on iron replacement indicating an improvement in iron status. A decrease in ePPIX was seen in both XLP patients who completed the study (relative difference of 0.67 and 0.5 respectively). No substantial changes in ePPIX were seen in EPP patients at the end of the study (n = 8; median relative difference: -0.21 (IQR: −0.44, 0.05). The most common side effects of iron treatment were gastrointestinal symptoms. Hepatic function remained normal throughout the study. Our study showed that oral iron therapy repletes iron stores and transiently increases ePPIX in some EPP patients, perhaps due to a transient increase in erythropoiesis, and may decrease ePPIX in XLP patients. Further studies are needed to better define the role of iron repletion in EPP.
Trial registration: NCT02979249.
Keywords: Erythropoietic protoporphyria, X-linked protoporphyria, Photosensitivity, Clinical trial, Iron
Abbreviations: EPP, erythropoietic protoporphyria; XLP, X linked protoporphyria; ePPIX, erythrocyte protoporphyrin; FECH, ferrochelatase; ALAS2, 5-aminolevulinate synthase 2; IRE, iron-responsive element; IRP, IRE binding proteins; QoL, Quality of Life; DSMB, Data and Safety Monitoring Board
1. Introduction
Erythropoietic Protoporphyria (EPP) and X-Linked Protoporphyria (XLP) are rare disorders presenting clinically with phototoxicity usually starting in childhood [1]. In EPP, autosomal recessive loss-of-function mutations in the ferrochelatase (FECH) gene, reduce the enzyme activity to <35% of normal [1,2] resulting in the accumulation of predominantly metal-free protoporphyrin in marrow erythroid cells. XLP, a clinically similar disorder, results from gain of function mutations in erythroid-specific 5-aminolevulinate synthase 2 (ALAS2) leading to its increased enzymatic activity [3,4]. Cutaneous photosensitivity in both EPP and XLP is typically manifested by pain starting immediately or soon after exposure to sunlight, and with continued light exposure, redness and swelling of the sun exposed skin. The pain is severe and poorly responsive to analgesics [5]. Sun protection has been the primary mode of symptom prevention and medications such as beta-carotene, cysteine, and vitamin C have shown limited or no efficacy [6]. Afamelanotide (Scenesse™), an alpha-melanocyte stimulating hormone analogue, has been approved by the European Medicines Agency and the Food and Drug Administration for treatment of EPP. It darkens the skin and improves sunlight tolerance, but does not alter the underlying metabolic defect or lower levels of protoporphyrin [7].
Ferrochelatase, the enzyme deficient in EPP, catalyzes the insertion of ferrous iron into protoporphyrin IX (ePPIX) to form heme. Unexplained iron deficiency is common in EPP patients [8,9]. and iron availability may modulate ferrochelatase activity by regulating the amount of correctly spliced FECH mRNA [10]. Deficiency of ferrochelatase may also lead to a secondary increase in ALAS2 expression and further contribute to accumulation of ePPIX in EPP [10,11]. In XLP, over-expression of ALAS2 results in accumulation of ePPIX because FECH becomes rate limiting with marrow overproduction of ALA and other pathway intermediates [4].
ALAS2 is regulated by iron at the translational level. The 5′-untranslated region of ALAS2 mRNA contains an iron-responsive element (IRE) that acts as a binding site for the IRE-binding proteins (IRPs) 1 and 2. In an iron depleted state, the translation of ALAS2 mRNA is blocked by the binding of IRP1 or 2 to IRE, downregulating the active enzyme [10,11]. Thus, iron availability appears to play an important role in disease expression in both EPP and XLP.
Patients with EPP often have mild anemia, with evidence of iron deficiency, including microcytosis and low serum ferritin and iron levels [11]. In a Swedish cohort of 51 EPP patients, about 44% had low ferritin levels [8]. In a US report of 226 patients, 37.4% of EPP patients, 37.5% of XLP males and 44.4% of XLP females were anemic [9]. Other evidence of impaired erythropoiesis or abnormal iron metabolism is seldom described in these patients [12,13]. Studies demonstrate that the anemia in EPP results from an absolute iron deficiency [14]. A pilot study showed that patients with EPP and XLP absorb oral iron normally, with expected post dose increases in serum iron [15]. In addition, serum and urine hepcidin levels were not inappropriately increased in these subjects at baseline and did not increase with iron treatment [15].
Iron replacement in EPP is controversial, because some reports suggest clinical improvement [12,[16], [17], [18]] while others describe increased photosensitivity [[19], [20], [21]]. Therefore, some physicians treat iron deficient EPP patients with oral or intravenous iron [22] whereas others do not. Hemin therapy is combined with other treatments in protoporphyric hepatopathy, and may decrease erythrocyte and plasma protoporphyrin levels by repleting deficient iron stores as well as stabilizing deficient FECH [ 23]. Reports of iron supplementation in XLP are limited. Oral iron replacement in one patient with XLP with diminished iron stores decreased ePPIX levels [3,12]. In another XLP patient, iron treatment decreased ePPIX levels and improved liver damage and anemia. In XLP, iron repletion may increase the conversion of ePPIX to heme, as ferrochelatase levels are normal. Some investigators have recommended treatment of iron deficiency in XLP but not in EPP.
The purpose of this study was to provide prospective data on the treatment of iron deficient patients with EPP or XLP.
2. Materials and methods
2.1. Study design
This was a prospective, multi-center, single-arm, open label trial. The study (clinicaltrials.gov identifier NCT02979249) was funded by and performed from December 2016 to July 2019 at six sites of the Porphyrias Consortium of the National Institutes of Health Rare Diseases Clinical Research Network and conducted in accord with the Declaration of Helsinki. The institutional review board at each site reviewed and approved this study and an NIH-appointed data and safety monitoring board (DSMB) oversaw trial progress. Patients provided written informed consent.
2.2. Study population and intervention
Adults ≥18 years were included if they had a confirmed diagnosis of EPP or XLP and were enrolled in the longitudinal study of the Porphyrias Consortium. Inclusion criteria included marked elevation in erPPIX, with a predominance of metal-free protoporphyrin, molecular confirmation of either biallelic FECH variants (in EPP) or a gain of function ALAS2 mutation (in XLP), and a baseline serum ferritin of ≤30 ng/mL. Patients were excluded if they had previous intolerance to oral iron or a history of clinically significant liver dysfunction or liver or bone marrow transplant. Full list of inclusion and exclusion available at https://clinicaltrials.gov/ct2/show/NCT02979249.
Study drug (ferrous sulfate 325 mg BID containing 65 mg elemental iron) was sourced from two pharmacies (Par Pharmaceuticals and Major Pharmaceutical) and shipped to all study sites. Patients were prescribed 325 mg capsule for oral administration twice daily (total 650 mg daily) for 12 months.
Iron was discontinued if patients met the safety endpoint of a sustained increase in erythrocyte total protoporphyrin of >35% over baseline that was sustained for two visits 2–4 weeks apart. This increase was considered clinically significant, since normal intraindividual variability in ePPIX levels are reported to be up to 25% [24]. Study drug could also be discontinued at the discretion of the site investigator if phototoxicity increased or other side effects became evident.
2.3. Outcome measures
The primary efficacy endpoint was the relative difference in total ePPIX levels between baseline and after 12 months after treatment. Secondary endpoints included ePPIX levels and EPP-specific quality of life (QoL) over time measured every 3 months from baseline to 12 months, and improvements in iron status, plasma porphyrin levels, and clinical symptoms. QoL was measured using a modified EPP-specific questionnaire. Participants returned to the study sites every three months for repeat lab tests and clinical assessments, including EPP symptoms. Data and laboratory elements captured at each time point included ePPIX and plasma porphyrin analyzed at a central lab, complete blood count, hepatic function tests and iron indices including ferritin. Adverse events were captured at each study visit and through monthly phone calls with the study coordinator. Compliance was assessed at each study visit by pill counts.
2.4. Statistical analysis
The trial was designed to detect an efficacy signal for iron using a Bayesian approach based on the posterior probability that the mean relative difference between baseline and 12-month ePPIX levels is >0. A posterior probably of 80% or higher was deemed reasonable assurance of an efficacy signal that would warrant additional study of iron repletion. Based on data from the longitudinal study [9], assuming the distribution of baseline ePPIXn is gamma (2.3, 861) and that baseline and 12 month values are correlated with r = 0.7, simulations indicated that a total sample size of 20 patients would allow us to detect an approximate quadrupling of the odds that iron is effective in lowering ePPIX if the true effect size is 10%.
Enrollment was halted due to low enrollment in March 2019, with agreement from the DSMB and National Institutes of Health, but follow-up visits continued. Total enrollment was 16 patients at 4 sites. Ten subjects had primary endpoint data at 12 months and were considered study completers (including those who discontinued study drug due to adverse events). Due to the smaller than planned sample, formal statistical analyses were not conducted. All analyses of the primary and secondary endpoints are descriptive using numbers and percentages for categorical measures and means, standard deviations, medians, interquartile ranges and ranges as appropriate for continuous measures. Analyses were conducted using SAS version 9.4 (SAS Institute Inc).
3. Results
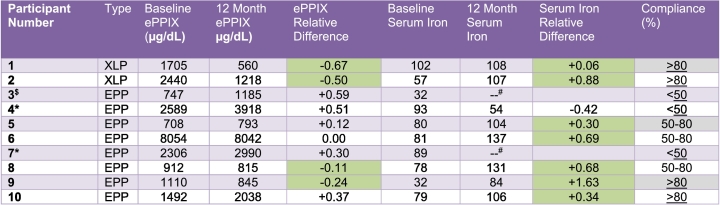
Seventeen patients were screened and 16 were enrolled with the mean age of 38.8 years (SD 14.5). One patient failed screening as serum ferritin was >30 ng/mL at baseline. Thirteen patients had EPP (8 female) and three had XLP (all female). All 16 patients were White and 69% were female. The disease-causing mutations of the 16 patients are shown in Supplementary Table 1. The median total ePPIX level at baseline was 1561 μg/dl (IQR: 1110–2589) for EPP patients and 1857 μg/dl (range: 1705–2440) for XLP patients. Among patients with follow-up through 12 months (n = 10), the median total ePPIX level at baseline was 1301 μg/dl (IQR: 829.5–2447.5) for EPP patients and 2072.5 μg/dl (range 1705–2440) for XLP patients. After 12 months of treatment, median total ePPIX levels were 1611.5 μg/dl (IQR: 830–3454) in EPP patients and 889 μg/dl (range: 560–1218) in XLP patients. The relative difference of 12-month vs. baseline ePPIX levels for study completers is shown in Table 1. Five of 8 (62.5%) EPP patients had increases in their ePPIX levels at 12 months vs. baseline, two had decreases, and one stayed approximately the same. The two XLP patients who completed 12 months of treatment both had a clinically significant decrease in ePPIX levels. 87.5% (7/8) of patients had an increase in serum iron levels from baseline to month 12. Among patients with complete data at baseline and 12 months, median ferritin levels increased from baseline to month 12 in both EPP (18.4 (IQR: 9.5, 23) to 36.5 (IQR: 22.0, 57.7) ng/mL) and XLP patients (22.5 (range: 18.4, 26.6) to 48.3 (range: 39.6–56.9) ng/mL). Transferrin saturation increased from baseline to month 12 in both EPP and XLP patients (Table 2). Hemoglobin levels increased from baseline in XLP patients. There were no clinically significant changes in liver enzymes throughout the study (Table 2). There was insufficient data to analyze and report on quality of life.
Table 1.
Relative difference in erythrocyte protoporphyrin and serum iron levels for study completers.
*Stopped study drug early due to primary safety endpoint being met (35% increase in ePPIX over baseline).
Green indicates an improvement in levels from baseline to end of study.
#Month 12 serum iron testing not done due to sample error.
$Discontinued iron due to increased EPP symptoms.
Table 2.
Lab results over time for all patients.
| Baseline | 3 Month | 6 Month | 9 Month | 12 Month | |
|---|---|---|---|---|---|
| Erythrocyte protoporphyrin (μg/dL) – Number of observations; Median (IQR); Range | |||||
| EPP | 13; 1561 (1110, 2589); 708–8054 | 12; 1823.5 (1201, 4323); 910–8695 | 9; 1357 (1114, 3919); 897–8885 | 8; 1191.5 (927.5, 3728); 792–8504 | 8; 1611.5 (830, 3454); 793–8042 |
| XLP | 3; 1857 (1705, 2440); 1705–2440 | 3; 1571 (1338, 1810); 1338–1810 | 2; 1102 (611, 1593); 611–1593 | 2; 1170.5 (942, 1399); 942–1399 | 2; 889 (560, 1218); 560–1218 |
| Iron (μg/dL) - Number of observations; Median (IQR); Range | |||||
| EPP | 12; 78.5 (53, 85); 32–107 | 11; 123 (86, 135); 36–223 | 8; 91.5 (71, 130.5); 41–162 | 8; 95.5 (60, 139.5); 27–182 | 6; 105 (84, 131); 54–137 |
| XLP | 3; 102 (57, 102); 57–102 | 3; 123 (104, 145); 104–145 | 2; 129 (120, 138); 120–138 | 2; 129 (101, 157); 101–157 | 2; 107.5 (107, 108); 107–108 |
| Ferritin (ng/mL) - Number of observations; Median (IQR); Range | |||||
| EPP | 13; 19.1 (13, 24); 4–29 | 11; 40 (29, 55); 4–75 | 8; 42.8 (23.7, 51); 6–59 | 8; 39.6 (33, 50.8); 4–67 | 8; 36.5 (22, 57.7); 5–77 |
| XLP | 3; 24 (18.4, 26.6); 18.4–26.6 | 3; 25.1 (23.2, 29); 23.2–29 | 2; 39.1 (37.7, 40.5); 37.7–40.5 | 2; 57.1 (50.4, 63.8); 50.4–63.8 | 2; 48.3 (39.6, 56.9); 39.6–56.9 |
| Transferrin Saturation (%)- Number of observations; Median (IQR); Range | |||||
| EPP | 11; 19.0 (17.0, 25.0); 6.0–31.0 | 11; 33.0 (25.0, 41.0); 7.0–56.0 | 8; 25.5 (16.5, 40.0); 13.0–50.0 | 8; 25.0 (17.5, 38.5); 6.0–60.0 | 6; 32.0 (25.0, 38.0); 17.0–41.0 |
| XLP | 3; 25.0 (18.0, 28.0); 18.0–28.0 | 2; 36.5 (31.0, 42.0); 31.0–42.0 | 2; 36.0 (35.0, 37.0); 35.0–37.0 | 2; 38.0 (28.0, 48.0); 28.0–48.0 | 2; 30.0 (29.0, 31.0); 29.0–31.0 |
| Plasma Porphyrins (μg/dL) - Number of observations; Median (IQR); Range | |||||
| EPP | 13; 4.1 (1.4, 5.6); 0.5–24 | 12; 5.4 (3.4, 9.5); 0.9–35.8 | 9; 5.1 (3.9, 8.2); 1.2–12.6 | 8; 4.1 (2.8, 7.6); 0.4–16.2 | 8; 4.2 (2.2, 8.9); 0.7–16.9 |
| XLP | 3; 1.4 (0.5, 1.7); 0.5–1.7 | 3; 2 (0.8, 2.3); 0.8–2.3 | 2; 1.6 (1.6, 1.6); 1.6–1.6 | 2; 1.8 (0.7, 2.9); 0.7–2.9 | 2; 1.2 (1, 1.3); 1–1.3 |
| Hemoglobin (g/dL) - Number of observations; Median (IQR); Range | |||||
| EPP | 13; 12.5 (12, 13.7); 8.6–14.2 | 12; 13.1 (12.2, 13.7); 11.6–14.9 | 8; 12.6 (12.1, 13.9); 11.5–14.8 | 8; 12.9 (12.2, 13.6); 11.5–15 | 8; 12.6 (11.7, 13.8); 11.3–15 |
| XLP | 3; 12.4 (11.8, 14.2); 11.8–14.2 | 3; 12.4 (12, 13.5); 12–13.5 | 2; 13.3 (12.9, 13.6); 12.9–13.6 | 2; 13.8 (13.5, 14.1); 13.5–14.1 | 2; 13.7 (13.5, 13.9); 13.5–13.9 |
| AST (U/L) - Number of observations; Median (IQR); Range | |||||
| EPP | 13; 21 (18, 28); 14–36 | 11; 29 (19, 38); 14–41 | 8; 25.5 (19.5, 28.5); 16–36 | 8; 19.5 (17, 24.5); 15–32 | 8; 22 (17.5, 24); 14–27 |
| XLP | 3; 23 (19, 31); 19–31 | 3; 23 (20, 27); 20–27 | 2; 26.5 (24, 29); 24–29 | 2; 26.5 (24, 29); 24–29 | 2; 15 (14, 16); 14–16 |
| ALT (U/L) - Number of observations; Median (IQR); Range | |||||
| EPP | 13; 27 (19, 33); 10–78 | 11; 27 (16, 41); 12–93 | 8; 27.5 (22, 39.5); 17–55 | 8; 23 (17, 26); 15–33 | 8; 21.5 (16.5, 26.5); 16–42 |
| XLP | 3; 27 (16, 27); 16–27 | 3; 28 (20, 37); 20–37 | 2; 33 (28, 38); 28–38 | 2; 30.5 (26, 35); 26–35 | 2; 22.5 (17, 28); 17–28 |
Adverse events were mostly mild in intensity and are listed in Table 3. Those considered related or probably related to study drug were gastrointestinal, including constipation, abdominal pain, and dyspepsia. Two patients reported an increase in photosensitivity, which in one was assessed as possibly related to study drug. Both discontinued iron, and one continued to be followed for the course of the study (Subject #3, Table 1) and an increase in ePPIX was observed. The other participant was followed until month 6 and there was no significant increase in ePPIX. Three serious adverse events were all considered unrelated to study drug.
Table 3.
Adverse events during treatment with oral iron.
| Participant Number | Type | Serious | Adverse Event | Causality by Study Drug |
|---|---|---|---|---|
| 1 | XLP | No | Abdominal pain | Definitely not related |
| Yes | Diarrhea | Definitely not related | ||
| 4 | EPP | No | Dyspepsia | Definitely related |
| No | Elevated ePPIX 35% over baseline | Possibly related | ||
| No | Ovarian cyst rupture | Definitely not related | ||
| No | Productive cough | Definitely not related | ||
| No | Urinary tract pain | Definitely not related | ||
| 5 | EPP | No | Stomach pain | Possibly related |
| 7 | EPP | No | Elevated ePPIX 35% over baseline | Possibly related |
| 8 | EPP | No | Mucosal infection | Definitely not related |
| 11 | EPP | No | Increased Photosensitivity | Possibly related |
| 12 | EPP | No | Nausea | Definitely related |
| No | Constipation | Definitely related | ||
| 13 | EPP | No | Sinusitis | Definitely not related |
| Yes | Miscarriage | Definitely not related | ||
| 14 | XLP | Yes | Conjunctivitis | Definitely not related |
| 15 | EPP | No | Constipation | Probably related |
| No | Increased Photosensitivity | Probably not related |
Seven patients (43.8%) withdrew before study completion. Of these, three were lost to follow up (one withdrew due to mild gastrointestinal side effects). Two patients withdrew to participate in another clinical trial, one withdrew as she was planning a pregnancy (a study exclusion), and one withdrew as she noticed an increase in symptoms within the first three months of treatment. One of the two patients who withdrew to participate in another clinical trial was followed for ∼11 months and her lab values at the time of withdrawal were counted towards the 12-month visit. Treatment was stopped in two patients after they met the protocol-stopping rule of an increase in total erythrocyte protoporphyrin level of >35%, and then continued visits in the study for the full 12 months. Of the 10 patients who completed the study 4 were > 80% compliant with the study medication, 3 were 50–80%, and 3 were < 50% compliant; both XLP patients were > 80% compliant.
4. Discussion
This pilot study systematically evaluated the role of iron supplementation in iron deficient patients with EPP or XLP. This is clinically important because >40% of patients with these disorders are mildly anemic, likely due to iron deficiency. Previous experience with iron replacement in protoporphyrias has been based on single or small group reports without adequate clinical or standardized laboratory follow up and limited and conflicting results.
We captured data over 12 months and noted a transient increase in total ePPIX levels around Month 3 in some EPP patients but not in a smaller number of XLP patients. Total ePPIX levels increased 81% from baseline within the first month in one EPP patient, accompanied by increases in ferritin and an increase in hemoglobin from 7.9 g/dl to 9.1 g/dl. The increase in ePPIX likely corresponded to an expected increase in erythropoiesis with iron replenishment. This corresponded with an increase in photosensitivity in at least one patient. Treatment was discontinued in two patients based on an increased ePPIX level > 35% over baseline (a stopping rule) despite absence of other safety concerns.
Iron replacement led to an increase in serum iron, ferritin, and transferrin saturation in EPP and XLP patients over the 12-month study period. This suggests that the iron deficiency seen in these patients is not due an impaired iron absorption, and supports the study of Bossi et al [15] also indicating that iron absorption is normal in EPP and XLP patients. Hemoglobin levels increased from baseline in XLP patients but there were no changes from baseline in EPP patients.
A decline in ePPIX occurred in all 3 XLP patients by month 3 and persisted in study completers. A significant and consistent decline in ePPIX levels did not occur over 12 months in the EPP patients, although an improvement was seen in two patients. Both patients had a compliance of >50%, however given the limited sample size no conclusions could be drawn Compliance with treatment was variable which could have affected results. Baseline ePPIX levels ranged from 708 to 8054 μg/dL, and these levels did not appear to correlate to the amount of change in ePPIX at 12 months. However, interpretation of these results was complicated by the small number of patients who completed all the study time points. The most frequent additional side effects were gastrointestinal symptoms which were mostly mild and self-limiting. Unlike previous reports of iron treatment in EPP [13], we did not see significant or persistent elevations in hepatic function tests.
Our study was limited by the small number of participants who completed all study visits (10 of 16, 62.5%) and lack of formal statistical analysis. Reasons for early withdrawal included patients lost to follow up, competing therapeutic clinical trials, increases in photosensitivity, and the predetermined treatment stopping rule. Nevertheless, our experience and other reports indicate that iron replacement is beneficial in XLP by reducing ePPIX levels and likely decreases symptoms. Results from this study suggest that iron (ferrous sulfate 325 mg or equivalent every other day) [25] can be supplemented in EPP patients with close monitoring of ePPIX levels and hepatic function tests, and may be desirable in those with symptoms of iron deficiency and a ferritin of <30 ng/mL. However, additional studies are needed to make recommendations that are more definitive in EPP.
5. Conclusion
In this pilot clinical trial, oral iron therapy led to reduction in protoporphyrin levels in XLP patients. No clinically significant changes were seen in EPP patients and no safety concerns were identified. Our findings support the existing literature that iron supplementation should be recommended in iron deficient XLP patients. Iron therapy can be attempted in EPP patients with close monitoring, particularly if iron deficiency is symptomatic.
Funding
The Porphyrias Consortium (U54DK083909) is part of Rare Diseases Clinical Research Network (RDCRN), an initiative of the Office of Rare Diseases Research (ORDR), NCATS. This consortium is funded through collaboration between NCATS and NIDDK.
Declaration of Competing Interest
The authors report no related conflicts of interest.
Acknowledgments
We thank the patients for their participation in this study. We would also like to thank the research coordinators at the study sites.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ymgmr.2022.100939.
Appendix A. Supplementary data
FECH and ALAS2 mutations of study participants.
CONSORT flow diagram.
Data availability
Data will be made available on request.
References
- 1.Anderson K.E., Sassa S., Bishop D.F., Desnick R.J. In: The Metabolic and Molecular Basis of Inherited Disease. 8th edn. Scriver C.R., Beaudet A.L., Sly W.S., Valle D., Childs B., Vogelstein B., editors. McGraw-Hill; New York: 2022. Disorders of heme biosynthesis: X-linked sideroblastic anemias and the porphyrias; pp. 2991–3062. [Google Scholar]
- 2.Whatley S.D., Mason N.G., Holme S.A., Anstey A.V., Elder G.H., Badminton M.N. Molecular epidemiology of erythropoietic protoporphyria in the U.K. Br. J. Dermatol. 2010;162(3):642–646. doi: 10.1111/j.1365-2133.2010.09631.x. [DOI] [PubMed] [Google Scholar]
- 3.Whatley S.D., Ducamp S., Gouya L., Grandchamp B., et al. C-terminal deletions in the alas2 gene lead to gain of function and cause x-linked dominant protoporphyria without anemia or iron overload. Am. J. Hum. Genet. 2008;83:408–414. doi: 10.1016/j.ajhg.2008.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Balwani M., Doheny D., Bishop D.F., et al. Loss-of-function ferrochelatase and gain-of-function erythroid 5-aminolevulinate synthase mutations causing erythropoietic protoporphyria and X-linked protoporphyria in north American patients reveal novel mutations and a high prevalence of X-linked protoporphyria. Mol. Med. 2013;19(1):26–35. doi: 10.2119/molmed.2012.00340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balwani M. Erythropoietic protoporphyria and X-linked protoporphyria: pathophysiology, genetics, clinical manifestations, and management. Mol. Genet. Metab. 2019;128(3):298–303. doi: 10.1016/j.ymgme.2019.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Minder E.I., Schneider-Yin X., Steurer J., Bachmann L.M. A systematic review of treatment options for dermal photosensitivity in erythropoietic protoporphyria. Cell. Mol. Biol. (Noisy-le-grand) 2009;55(1):84–97. [PubMed] [Google Scholar]
- 7.Harms J.H., Lautenschlager S., Minder C.E., Minder E.I. Mitigating photosensitivity of erythropoietic protoporphyria patients by an agonistic analog of alpha-melanocyte stimulating hormone. Photochem. Photobiol. 2009;85(6):1434–1439. doi: 10.1111/j.1751-1097.2009.00595.x. [DOI] [PubMed] [Google Scholar]
- 8.Wahlin S., Floderus Y., Stål P., Harper P. Erythropoietic protoporphyria in Sweden: demographic, clinical, biochemical and genetic characteristics. J. Intern. Med. 2011;269(3):278–288. doi: 10.1111/j.1365-2796.2010.02236.x. [DOI] [PubMed] [Google Scholar]
- 9.Balwani M., Naik H., Anderson K.E., et al. Clinical, biochemical, and genetic characterization of north American patients with erythropoietic protoporphyria and X-linked protoporphyria. JAMA Dermatol. 2017;153(8):789–796. doi: 10.1001/jamadermatol.2017.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barman-Aksözen J., Béguin C., Dogar A.M., et al. Iron availability modulates aberrant splicing of ferrochelatase through the iron- and 2-oxoglutarate dependent dioxygenase Jmjd6 and U2AF(65.) Blood Cells Mol. Dis. 2013;51(3):151–161. doi: 10.1016/j.bcmd.2013.05.008. [DOI] [PubMed] [Google Scholar]
- 11.Barman-Aksözen J., Minder E.I., Schubiger C., et al. In ferrochelatase-deficient protoporphyria patients, ALAS2 expression is enhanced and erythrocytic protoporphyrin concentration correlates with iron availability. Blood Cells Mol. Dis. 2015;54(1):71–77. doi: 10.1016/j.bcmd.2014.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Holme S.A., Worwood M., Anstey A.V., et al. Erythropoiesis and iron metabolism in dominant erythropoietic protoporphyria. Blood. 2007;110(12):4108–4110. doi: 10.1182/blood-2007-04-088120. [DOI] [PubMed] [Google Scholar]
- 13.Landefeld C., Kentouche K., Gruhn B., et al. X-linked protoporphyria:Iron supplementation improves protoporphyrin overload, liver damage and anaemia. Br. J. Haematol. 2016;173(3):482–484. doi: 10.1111/bjh.13612. [DOI] [PubMed] [Google Scholar]
- 14.Barman-Aksoezen J., Girelli D., Aurizi C., et al. Disturbed iron metabolism in erythropoietic protoporphyria and association of GDF15 and gender with disease severity. J. Inherit. Metab. Dis. 2017;40(3):433–441. doi: 10.1007/s10545-017-0017-7. [DOI] [PubMed] [Google Scholar]
- 15.Bossi K., Lee J., Schmeltzer P., et al. Homeostasis of iron and hepcidin in erythropoietic protoporphyria. Eur. J. Clin. Investig. 2015;45(10):1032–1041. doi: 10.1111/eci.12503. [DOI] [PubMed] [Google Scholar]
- 16.Gordeuk V.R., Brittenham G.M., Hawkins C.W., et al. Iron therapy for hepatic dysfunction in erythropoietic protoporphyria. Ann. Intern. Med. 1968;105:27–31. doi: 10.7326/0003-4819-105-1-27. [DOI] [PubMed] [Google Scholar]
- 17.Holme S.A., Thomas C.L., Whatley S.D., et al. Symptomatic response of erythropoietic protoporphyria to iron supplementation. J. Am. Acad. Dermatol. 2007;56(6):1070–1072. doi: 10.1016/j.jaad.2006.11.030. [DOI] [PubMed] [Google Scholar]
- 18.Kniffen J. Protoporphyrin removal in intrahepatic porphyrastasis. Gastroenterology. 1970;58:1027. [Google Scholar]
- 19.Milligan A., Graham-Brown R.A., Sarkany I., Baker H. Erythropoietic protoporphyria exacerbated by oral iron therapy. Br. J. Dermatol. 1988;119:63–66. doi: 10.1111/j.1365-2133.1988.tb07102.x. [DOI] [PubMed] [Google Scholar]
- 20.Baker H. Erythropoietic protoporphyria provoked by iron therapy. Proc. R. Soc. Med. 1971;64(6):610–611. doi: 10.1177/003591577106400609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barman-Aksoezen J., Schneider-Yin X., Minder E.I. Iron in erythropoietic protoporphyrias: Dr. Jekyll or Mr. Hyde? J. Rare Dis. Res. Treat. 2017;2:1–5. [Google Scholar]
- 22.Bentley D.P., Meek E.M. Clinical and biochemical improvement following low-dose intravenous iron therapy in a patient with erythropoietic protoporphyria. Br. J. Haematol. 2013;163(2):289–291. doi: 10.1111/bjh.12485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.McGuire B.M., Bonkovsky H.L., Carithers R.L., Jr., et al. Liver transplantation for erythropoietic protoporphyria liver disease. Liver Transpl. 2005;11(12):1590–1596. doi: 10.1002/lt.20620. [DOI] [PubMed] [Google Scholar]
- 24.Gou E., Weng C., Greene T., et al. Longitudinal analysis of erythrocyte and plasma protoporphyrin levels in patients with protoporphyria. J. Appl. Lab. Med. 2018;3(2):213–221. doi: 10.1373/jalm.2017.025874. [DOI] [PubMed] [Google Scholar]
- 25.Auerbach M. In: UpToDate, Waltham, MA. UpToDate, Means R.T. Jr., editors. 2022. Treatment of iron deficiency anemia in adults. (Accessed on November 7, 2022) [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
FECH and ALAS2 mutations of study participants.
CONSORT flow diagram.
Data Availability Statement
Data will be made available on request.