Abstract
Neonatal bacterial meningitis remains a disease with unacceptable rates of morbidity and mortality despite the availability of effective antimicrobial therapy. Citrobacter spp. cause neonatal meningitis but are unique in their frequent association with brain abscess formation. The pathogenesis of Citrobacter spp. causing meningitis and brain abscess is not well characterized; however, as with other meningitis-causing bacteria (e.g., Escherichia coli K1 and group B streptococci), penetration of the blood-brain barrier must occur. In an effort to understand the pathogenesis of Citrobacter spp. causing meningitis, we have used the in vitro blood-brain barrier model of human brain microvascular endothelial cells (HBMEC) to study the interaction between C. freundii and HBMEC. In this study, we show that C. freundii is capable of invading and trancytosing HBMEC in vitro. Invasion of HBMEC by C. freundii was determined to be dependent on microfilaments, microtubules, endosome acidification, and de novo protein synthesis. Immunofluorescence microscopy studies revealed that microtubules aggregated after HBMEC came in contact with C. freundii; furthermore, the microtubule aggregation was time dependent and seen with C. freundii but not with noninvasive E. coli HB101 and meningitic E. coli K1. Also in contrast to other meningitis-causing bacteria, C. freundii is able to replicate within HBMEC. This is the first demonstration of a meningitis-causing bacterium capable of intracellular replication within BMEC. The important determinants of the pathogenesis of C. freundii causing meningitis and brain abscess may relate to invasion of and intracellular replication in HBMEC.
Citrobacter freundii is a member of the family Enterobacteriaceae and is often the cause of significant opportunistic infections. C. freundii has also been associated with neonatal meningitis and brain abscess (14). The mortality and morbidity rate of Citrobacter meningitis is unacceptably high. The fatality rate associated with neonatal meningitis is 25 to 50%; moreover, serious neurological sequelae result in 75% of survivors. Although the implication Citrobacter spp. in neonatal meningitis and brain abscess is clear, the mechanisms by which these organisms cause disease have been poorly investigated.
One of the least understood aspects of bacterial meningitis is the mechanisms by which bacteria traverse the blood-brain barrier. Escherichia coli K1 and group B streptococci (GBS), the two leading causes of bacterial meningitis in neonates, have offered excellent models for studying bacterial penetration of the blood-brain barrier. We have previously shown that E. coli K1 and GBS invade brain microvascular endothelial cells (BMEC) in vitro and are capable of penetrating the blood-brain barrier in the experimental newborn rat model of hematogenous meningitis (1, 15, 20). However, in contrast to Citrobacter spp., there is a much lower association of brain abscess formation with these organisms (8, 14). This observation suggests that Citrobacter spp. may utilize different pathogenic mechanisms for penetrating and/or replicating in the central nervous system. In an effort to understand the pathogenesis of Citrobacter spp. causing meningitis, we have used the in vitro blood-brain barrier model of human BMEC (HBMEC) to study the interaction between C. freundii and HBMEC. Here we report on the capacity of C. freundii to invade, replicate, and traverse HBMEC in vitro and present data on the eukaryotic mechanisms for the invasion process.
MATERIALS AND METHODS
Bacterial strains.
The C. freundii strain used in this study, 3009rif, is a spontaneous rifampicin-resistant mutant derived from urinary tract isolate 3009 (22) that retains wild-type morphology, growth characteristics, and invasive phenotype (data not shown). Bacteria were grown aerobically for 14 h at 37°C in brain heart infusion broth (Difco Laboratories, Detroit, Mich.) with rifampicin (100 μg/ml) selection. For confocal microscopy experiments, we generated a construct constitutively expressing gfp; this clone contains the promoter region of rpsM, which encodes the ribosomal protein S13. Oligonucleotides that hybridize 5′ and 3′ of the promoter region were generated based on the sequence of E. coli K12 rpsM. PCR was performed to amplify the rpsM promoter, using C. freundii chromosomal DNA as the template. The PCR product was then cloned immediately 5′ of the promoterless gfpmut3A construct in the promoter trap vector, pFPV25 (34). This clone was then electroporated into wild-type C. freundii and assessed for fluorescence. It was determined that the rpsM::gfpmut3A plasmid gave rise to highly fluorescent colonies (as visualized by fluorescence microscopy). This clone did not affect the growth rate, cell density, or ability of wild-type C. freundii to invade HBMEC.
HBMEC cultures.
HBMEC were isolated from a brain biopsy of an adult female with epilepsy by previously described methods (30). These cells were positive for factor VIII-Rag, carbonic anhydrase IV, and Ulex europaeus agglutinin I. They took up fluorescently labeled low-density lipoprotein and expressed gamma glutamyl transpeptidase, thus demonstrating their brain endothelial cell properties (30). HBMEC were subsequently immortalized by transfection with simian virus 40 large T antigen and maintained their morphological and functional characteristics for at least 30 passages (31, 32). The cells are polarized and exhibit a transendothelial electric resistance of at least 100 ohms/cm2 (20, 27). HBMEC were plated in 75-ml tissue cultures flasks previously treated with rat tail collagen-fibronectin and then cultured in RPMI 1640 supplemented with heat-inactivated 10% fetal calf serum (Gibco), 10% NuSerum IV (Becton Dickinson, Bedford, Mass.), 1% modified Eagle’s medium nonessential amino acids, heparin (5 U/ml), sodium pyruvate (1 mM), l-glutamine (2 mM), vitamins, and penicillin-streptomycin. Cultures were incubated at 37°C in a humid atmosphere of 5% CO2.
Invasion assays and inhibition studies.
Invasion assays were performed as previously described (1), using approximately 107 bacteria added to a well containing a confluent monolayer of HBMEC at a multiplicity of infection of 100. The number of intracellular bacteria was determined after the extracellular bacteria were eliminated by incubation of the monolayer with experimental medium containing gentamicin (100 μg/ml). The MIC of gentamicin for C. freundii was determined to be ≤1.0 μg/ml. Results are presented as percent invasion, determined as 100 × [(number of bacteria recovered)/(number of bacteria inoculated)]. Noninvasive E. coli HB101 was used as a negative control.
For invasion inhibition studies, invasion assays were performed as described above except that the HBMEC were pretreated with experimental medium containing the indicated concentration of inhibitor. The inhibitors were maintained throughout the invasion period. For all inhibitors except microtubule-specific inhibitors, cells were pretreated for 30 min at 37°C. Pretreatment of microtubule inhibitors consisted of incubation with the compound for 1 h at 4°C and then 37°C for 30 min prior to assay. Control cells were treated with an equivalent amount of solvent lacking the active compound. Concentrations of solvents (dimethyl sulfoxide and ethanol) were always below 0.05%. None of the drugs used affected bacterial viability as estimated by bacterial growth during the assay. Potential inhibitors were tested at indicated concentrations for possible adverse effects on HBMEC, compared to cells without inhibitor, by examining cellular morphology, by examining confluency of the monolayer, and by trypan blue exclusion. Results were expressed as relative invasiveness, defined as percent invasion compared to bacteria in experimental medium alone, which was arbitrarily set at 100%.
Transcytosis experiments.
To examine the ability of C. freundii to transcytose polarized HBMEC monolayers, the double-chamber culture system of Transwell polycarbonate membrane filters was used as previously described (20, 27). Briefly, HBMEC were seeded onto the apical side of a 12-mm collagen-coated polycarbonate membrane with a pore size of 3 μm (Corning Costar Corp., Cambridge, Mass). The apical chamber of the Transwell contained 0.5 ml of HBMEC medium, while the basolateral chamber contained 1.5 ml of HBMEC medium.
Before transcytosis assays, the monolayers were washed, and fresh HBMEC medium was added without antibiotics. Then 108 bacteria were added to the apical chamber, and monolayers were incubated at 37°C in 5% CO2. Samples of 100 μl were collected from the basolateral chambers at incubation times of 0, 1, and 3 h, and plated for CFU, and an equivalent volume of medium was replaced. Simultaneously passive diffusion was measured via 3H-inulin. Noninvasive E. coli HB101 was used as a negative control.
TEM.
Transmission electron microscopy (TEM) was performed on HBMEC incubated with C. freundii for various times. At the designated time point, the HBMEC monolayer was washed four times with RPMI 1640 and gently scraped from the plastic surface. The cell slurry was then centrifuged for 10 min at 7,000 × g. The cell pellet was resuspended and fixed with 2.5% glutaraldehyde in 0.1 M phosphate-buffered saline (PBS). Cells were washed and postfixed with 2% OsO4 for 1 h, rinsed, dehydrated through graded ethanol solutions, and embedded in polypropylene oxide. Ultrathin sections were cut, mounted on colloidion one-hole grids, stained with uranyl acetate and lead citrate, and examined by TEM with a Philips CM transmission electron microscope.
Immunofluorescence.
For staining host cell elements, HBMEC were grown on eight-well chamber slides and bacteria were added as described above. At the designated time point, cells were washed three times with PBS and fixed with 3.7% formaldehyde–0.2% Triton X-100 in microtubule stabilization buffer (5) for 5 min at room temperature. Cells were then postfixed with 2% paraformaldehyde in PBS for 15 min at room temperature and subsequently permeabilized with 0.5% Triton X-100 in PBS for 20 min. The monolayers were first incubated with anti-α-tubulin monoclonal antibody (Sigma) in PBS containing 5% normal goat serum, washed three times with PBS, and then incubated with rhodamine labeled goat anti-mouse antibodies (Kierkegaard & Perry Laboratories) in PBS containing 5% normal goat serum. Cells were washed with PBS, chambers were removed, and slides were mounted. Stained cells were visualized with a Zeiss Axioscope confocal microscope.
RESULTS
Ability of C. freundii to invade HBMEC.
The ability of C. freundii to enter HBMEC was assessed via tissue culture invasion assays (gentamicin protection assay) as previously described (1). Experiments were performed to optimize and standardize the invasion assays for C. freundii. The frequency of C. freundii invasion was found to be optimal for an inoculum of approximately 107 CFU per well of HBMEC (an approximate multiplicity of infection of 100:1). The incubation time of the bacteria with the HBMEC that yielded the best and most reproducible results was found to be 1.5 h, with a subsequent 1-h incubation in medium containing gentamicin (100 μg/ml) to kill extracellular bacteria (data not shown). With standardized assay conditions, a representative experiment showed that approximately 0.38% ± 0.08% of the C. freundii inoculum was total cellular associated (representing number of bacteria attached and intracellular) and 0.12% ± 0.03% was invasive. Although the noninvasive bacteria control, E. coli HB101, showed similar levels of total cellular-associated inoculum (0.36% ± 0.17%), only 0.001% ± 0.00001% of the inocula invaded HBMEC.
Ability of C. freundii to survive and replicate within HBMEC.
To ascertain whether C. freundii survives and replicates within HBMEC, invasion assays were performed as described above except that the time between the 100-μg/ml gentamicin treatment and lysis of eukaryotic cells was lengthened. The extended incubation was done with medium containing a lower level of gentamicin (20 μg/ml, which was above the MIC). Time points examined were time zero (as in the standard invasion assay), and 2, 4, and 24 h. As shown in Table 1, C. freundii survived extended incubations with HBMEC and demonstrated an increase of intracellular CFU. An increase in recoverable bacteria was seen at 2 h (0.75% at time zero versus 3.3% at 2 h). Subsequent time points, however, revealed negligible or no increase in recoverable intracellular bacteria. These results suggest that C. freundii replicates within the HBMEC and can survive prolonged intracellular exposure. These data do not distinguish between exocytosis (or exit) of intracellular bacteria which are then subsequently killed by the gentamicin in the medium and the ability of C. freundii to replicate intracellularly long term.
TABLE 1.
C. freundii invasion and replication in HBMEC
| Organism | % Invasiona (mean ± SD)
|
|||
|---|---|---|---|---|
| 0 hb | 2 h | 4 h | 24 h | |
| C. freundii | 0.75 ± 0 | 3.3 ± 0.46 | 2.3 ± 0.07 | 3.7 ± 1.2 |
| E. coli HB101 | 0.005 ± 0.003 | 0.005 ± 0.003 | 0.005 ± 0.003 | 0.0003 ± 0 |
Defined as percentage of CFU that invaded HBMEC and survived gentamicin treatment.
Amount of time between addition of gentamicin and enumeration of intracellular bacteria.
TEM.
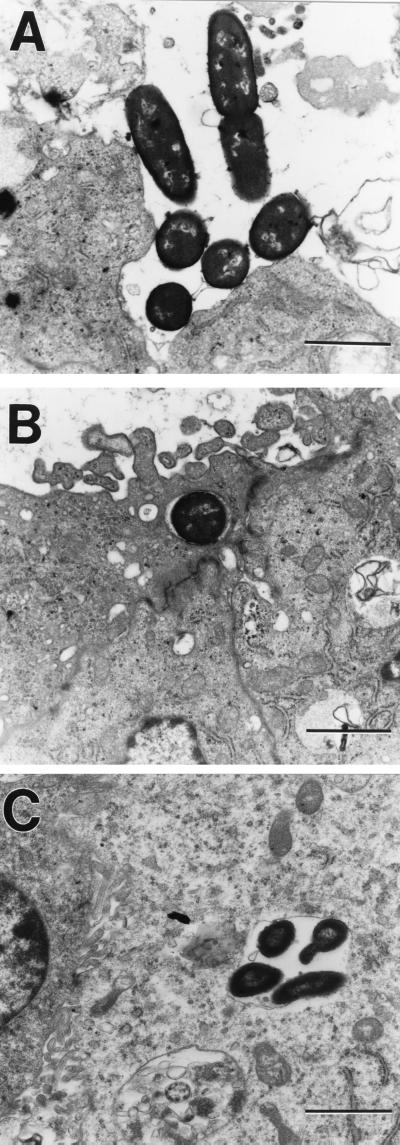
TEM was used to characterize the interaction between C. freundii and HBMEC. Infected monolayers were fixed at different times after the addition of bacteria and processed for TEM examination. Figure 1A shows entry of C. freundii after 45 min of incubation with HBMEC. Intimate interaction between the bacteria and the cell surface was observed, with some visible condensation of electron-dense particles accumulating within the vicinity of contact. After 45 min of incubation, C. freundii was occasionally found to be intracellular. After extended incubation for a total of 1.5 h, C. freundii was observed intracellularly in single membrane vacuole-like structures (Fig. 1B).
FIG. 1.
TEM demonstrating C. freundii invasion of HBMEC at a magnification of ×14,000. (A) Extracellular C. freundii attached to HBMEC; (B) intracellular C. freundii found within membrane-bound vacuole-like structures; (C) C. freundii replicating within vacuole-like structures.
To distinguish between the ability of C. freundii to survive and to replicate within HBMEC, the following experiment was performed. C. freundii was incubated with HBMEC initially as in the standard invasion assay, and then incubation was continued for 4 h in the presence of a low level of gentamicin. As shown in Fig. 1C, HBMEC vacuole-like structures contained multiple bacteria (four to six per vacuole), and in some instances there was evidence of actively dividing bacteria. Multiple bacteria in a vacuole may represent either intracellular replication or vacuole coalescence. However, since in nearly all cases the vacuoles appeared to contain actively dividing bacteria, the latter seems unlikely. These results corroborate the quantitative invasion assays presented above and demonstrate the ability of C. freundii to replicate within vacuole-like structures of HBMEC.
Effects of eukaryotic inhibitors on C. freundii invasion of HBMEC.
To identify the eukaryotic cellular components necessary for C. freundii invasion, we analyzed the effects of various eukaryotic inhibitors on C. freundii invasion of HBMEC.
(i) Role of microfilaments.
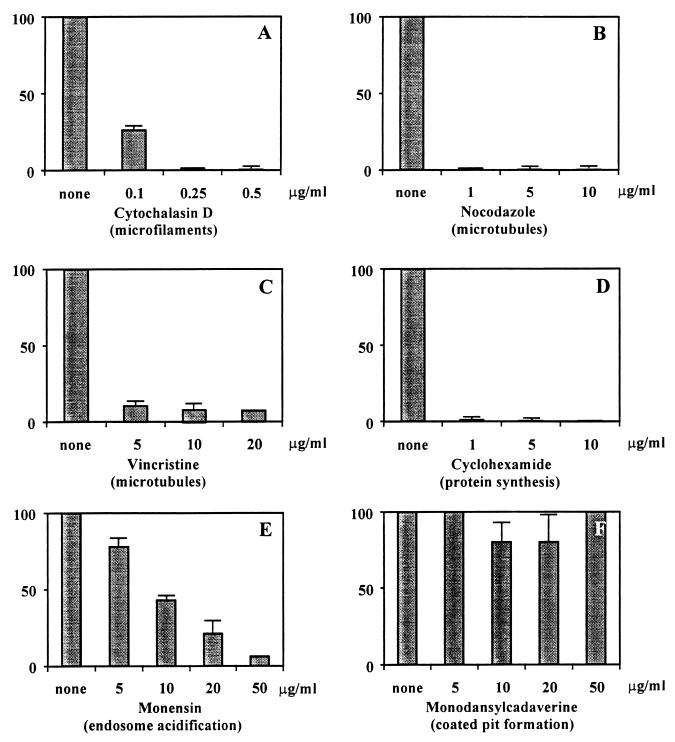
The role of actin-based cytoskeleton in C. freundii invasion was examined by using cytochalasin D, an agent that causes microfilament depolymerization in eukaryotic cells (33). We used various concentrations of cytochalasin D to pretreat HBMEC and compared the abilities of C. freundii to invade treated and untreated HBMEC. As shown in Fig. 2A, cytochalasin D had a profound effect on the ability of C. freundii to invade HBMEC. At a cytochalasin D concentration of 0.1 μg/ml, the frequency of invasion was reduced by 75%, while increasing the concentration of cytochalasin D to 0.25 μg/ml led to nearly 100% inhibition of C. freundii invasion. HBMEC treated with cytochalasin D at concentrations of 0.1 to 0.5 μg/ml did not show altered cell morphology.
FIG. 2.
Effects of different eukaryotic cell function inhibitors on C. freundii invasion of HBMEC. The inhibitors at indicated concentrations were added before addition of the bacteria and were present until gentamicin treatment (see Materials and Methods). Results are presented as relative invasiveness (see Materials and Methods) and represent the mean ± standard deviation of at least three individual experiments performed in duplicate.
(ii) Role of microtubules.
To establish an involvement of microtubules in C. freundii invasion of HBMEC, invasion assays were performed with different microtubule inhibitors. Pretreatment of HBMEC with nocodazole, a microtubule-depolymerizing agent (10), led to a dramatic effect on C. freundii invasion of HBMEC. As shown in Fig. 2B, cells pretreated with nocodazole at 1.0 μg/ml demonstrated a 99% decrease in C. freundii invasion. Similar effects were seen when cells were treated with another microtubule-destabilizing agent, colchicine (data not shown). In addition, when the microtubule-stabilizing agent vincristine was used, C. freundii invasion was decreased by nearly 90% (Fig. 2C).
(iii) Role of HBMEC protein synthesis.
To examine whether de novo eukaryotic protein synthesis plays a role in C. freundii invasion, invasion assays were performed with cycloheximide-treated HBMEC. Cycloheximide concentrations as low as 1.0 μg/ml profoundly inhibited the invasion of C. freundii (i.e., 98% decrease in invasion as compared to untreated HBMEC) (Fig. 2D). [35S]methionine incorporation experiments determined that 0.1 μg of cycloheximide per ml inhibited protein synthesis in HBMEC (data not shown).
(iv) Endosome acidification but not coated pit formation is required for C. freundii invasion.
To examine the role of endosome acidification in the C. freundii invasion process, the inhibitor monensin was used in invasion assays. Monensin is a cationic ionophore that has been shown to increase the pH of intracellular vacuoles (18). Pretreatment of HBMEC with monensin at concentrations of 5 to 50 μg/ml was found to inhibit the susceptibility of HBMEC to C. freundii invasion or survival in a dose-dependent manner (Fig. 2E).
Clathrin-coated pit formation has been shown to be inhibited in eukaryotic cells by monodansylcadaverine (MDC) or oabain (3, 17). Preincubation of HBMEC with MDC at concentrations of 5 to 50 μg/ml showed no effect on the ability of C. freundii to invade HBMEC (Fig. 2F). Similar results were seen when oabain was used to inhibit coated pit formation in HBMEC (data not shown). In contrast, 20 μg of MDC per ml inhibited the ability of E. coli K1 to invade HBMEC by 50%, similar to results previously reported by Prasadarao et al. (24). MDC at concentrations higher than 50 μg/ml appeared to be toxic to the HBMEC and thus could not be assayed. However, control experiments revealed that HBMEC pretreated with 50 μg of MDC per ml significantly protected the eukaryotic cell from diphtheria toxin toxicity (data not shown).
Taken together, the above results suggest that C. freundii invasion of HBMEC is dependent on microfilaments, microtubules, de novo protein synthesis, and endosome acidification but not coated pit formation.
Microtubule aggregation is associated with C. freundii invasion of HBMEC.
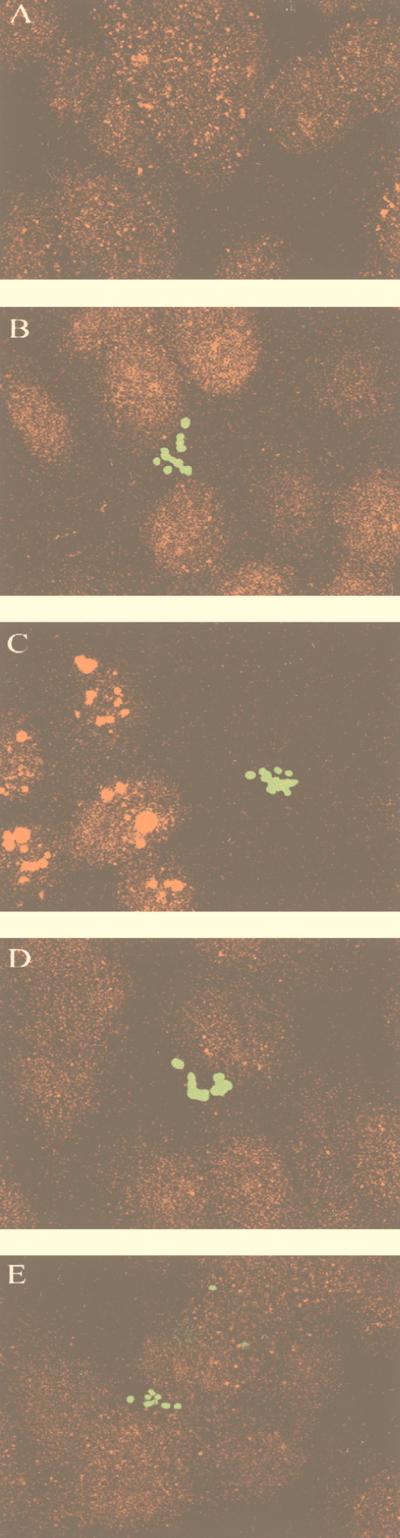
To further substantiate the apparent role of microtubules in C. freundii invasion of HBMEC, we examined by fluorescence microscopy whether there were changes in the microtubule network. Anti-α-tubulin staining of noninfected HBMEC showed uniform staining of microtubules (Fig. 3A). However, when C. freundii was incubated with HBMEC for 30 min, the microtubules appeared to be in aggregates (Fig. 3C). This observed aggregation was a time-dependent process; no effect on the microtubules was seen when bacteria and HBMEC were incubated for 5 min (data not shown), and only slight effects were seen when the incubation time was increased to 15 min (Fig. 3B). The microtubule aggregation-staining pattern did not appear to coexist with bacterial binding; other areas of HBMEC that did not have C. freundii bound also demonstrated pronounced microtubule clumping. Furthermore, this microtubule aggregation was not observed when C. freundii was incubated for 30 min with nocodazole (5 μg/ml)-pretreated HBMEC (Fig. 3D). Of interest, microtubule aggregation was not seen when bacteria were incubated for 30 min with cytochalasin D-pretreated HBMEC (Fig. 3E). Control experiments performed with noninvasive E. coli HB101 or invasive E. coli K1 did not show alterations in the HBMEC microtubule staining pattern (data not shown). These results indicate that a microtubule-depolymerizing agent can inhibit the formation of C. freundii-dependent microtubule aggregates and that a microfilament-depolymerizing agent (cytochalasin D) may have direct or indirect effects on the microtubule-dependent process of C. freundii invasion of HBMEC.
FIG. 3.
Confocal immunofluorescence microscopy of gfp-expressing C. freundii and rhodamine-stained HBMEC microtubules. The superimposed images were generated by the LSM conversion program and subsequently labeled in Adobe Photoshop. (A) No bacteria added to HBMEC; (B to E) C. freundii incubated for 15 min with HBMEC (B), for 30 min with HBMEC (C), for 30 min with nocadazole (5 μg/ml)-pretreated HBMEC (D), and for 30 min with cytochalasin D (0.25 μg/ml)-pretreated HBMEC (E). HBMEC microtubules were stained by indirect immunofluorescence as described in Materials and Methods. All panels are of equal magnification (×4,000).
C. freundii transcytoses polarized HBMEC monolayers.
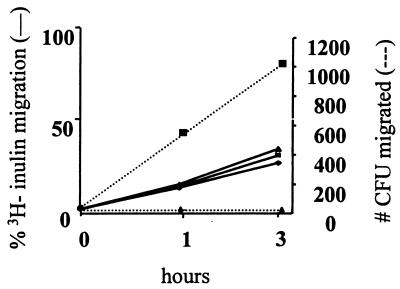
We and others have previously used Transwell experiments as a model system for studying bacterial transcytosis through an intact polarized HBMEC monolayer constituting the blood-brain barrier (20, 27). Briefly, bacteria are added to the apical chamber of polarized HBMEC in the Transwell. After a designated time, the bottom chamber (basolateral side of HBMEC) is sampled to ascertain bacterial penetration through the HBMEC. E. coli HB101 is used as a noninvasive bacteria control. 3H-inulin (4,000 Da) is simultaneously added in the apical chamber and collected with the bacteria from the basolateral chamber to assay for passive diffusion. As shown in Fig. 4, C. freundii could cross the polarized monolayer in a time-dependent process, whereas noninvasive HB101 demonstrated no HBMEC penetration. The levels of 3H-inulin migration were similar for all conditions (i.e., no bacteria versus invasive or noninvasive bacteria added), which indicates that the migration of C. freundii occurs principally through the HBMEC and is not passive and that the integrity of the cells has not been altered.
FIG. 4.
Transcytosis of C. freundii across polarized HBMEC monolayers. HBMEC were grown to confluence on Transwell filters as described in Materials and Methods. Bacteria were added to the apical side. Samples were collected from the basolateral chambers at indicated times and plated for CFU (right axis). Simultaneously, passive diffusion was measured via 3H-inulin (left axis). ⧫, no bacteria; ■, C. freundii; ▴, HB101. Results are presented as a representative assay of four independent experiments, each performed in triplicate and all yielding similar results.
DISCUSSION
Citrobacter is a significant cause of opportunistic infections; C. diversus is associated with approximately 40% of presenting cases, while C. freundii represents approximately 29% (11). Citrobacter spp. cause neonatal meningitis and have an unusual propensity for causing brain abscess (8, 14). The pathogenesis of Citrobacter spp. causing meningitis and brain abscess is not well characterized; however, as with other meningitis-causing bacteria, penetration of the blood-brain barrier must occur. The present study was undertaken to better understand the potential interactions of Citrobacter with the blood-brain barrier. C. freundii was chosen as a model bacterium for these studies because the bacterial genetics are better defined and a genomic library is available for eventual studies regarding the molecular basis of Citrobacter invasion and replication in HBMEC. Experiments performed with a cerebrospinal fluid isolate of C. diversus yielded similar results (data not shown), suggesting that the frequency and mechanism of HBMEC invasion for these two species may be alike.
The blood-brain barrier is a complex structure that consists of the choroid plexus epithelium and the brain capillary endothelium. The presence of tight junctions and low pinocytotic activity for the endothelial cells results in the restriction of macroelements passing through the blood-brain barrier. At this time, it is not known where in the blood-brain barrier C. freundii penetrates, but the choroid plexus was found to be rarely involved in the infant rat model of experimental hematogenous Citrobacter meningitis (16). In addition, endothelial microvascular cells cover the largest surface area of the blood-brain barrier, and other meningitis-causing bacteria have been shown to invade microvascular endothelial cells in vitro (13, 20, 25). We therefore selected HBMEC for our study. Tissue culture invasion assays and TEM studies provided evidence that C. freundii invades HBMEC. Results from invasion assays performed in the presence of various eukaryotic cellular inhibitors suggest that the invasion of C. freundii into HBMEC is a microfilament-, microtubule-, de novo protein synthesis-, and endosome acidification-dependent process. Extended invasion assays determined that C. freundii can survive and replicate intracellularly for prolonged periods in vitro. TEM analyses revealed the intracellular location of individual and multiple C. freundii cells to be within single membrane vacuole-like structures. Transwell experiments demonstrated that C. freundii could traverse a polarized monolayer of HBMEC, whereas noninvasive E. coli could not. Furthermore, our preliminary data shows that C. freundii penetrates the blood-brain barrier in the neonatal rat model of experimental hematogenous meningitis (21). Taken together, these findings suggest that C. freundii invades vacuoles, possibly replicates, transcytoses through the HBMEC, is released into the basolateral side, and thus penetrates the blood-brain barrier.
Invasion of eukaryotic cells by C. freundii has been reported (22, 35). However, this is the first report on the invasion of HBMEC by C. freundii. Curiously, the eukaryotic requirements for C. freundii invasion are as diverse as the cell types that C. freundii has been shown to invade. For example, the clathrin-coated pit inhibitor MDC has been shown to inhibit C. freundii invasion in all other cell types assayed (e.g., human vascular, intestinal, and bladder epithelial cells) except, as shown in this study, HBMEC. In addition, other meningitis-causing bacteria characterized thus far enter HBMEC in a route(s) that is dependent on microtubules and is MDC sensitive (20, 24, 27). Clathrin-coated pit inhibitors MDC and ouabain have not been shown to inhibit all receptors; thus, it may be that the receptor necessary for C. freundii invasion of HBMEC is not affected by the inhibitor MDC or ouabain. Although evidence collected so far suggests that C. freundii entry into HBMEC may not occur through an MDC- or oabain-sensitive receptor-mediated route, it does appear that endosome acidification and de novo protein synthesis are both required. The available data suggest two possible scenarios. Endosome acidification may be needed as an environmental trigger for intracellular bacterial survival. Similar requirements have been characterized for Salmonella epithelial invasion (26). Alternatively, endosome acidification and protein synthesis may be required for the separation of ligand-receptor complex, synthesis of receptor, and/or presentation of receptor to HBMEC surface in order for C. freundii invasion to occur. The latter scenario is reminiscent of other invasive pathogens, where contact of the viable organism is needed for modulation of eukaryotic cell adhesion molecules that are necessary for invasion (e.g., Streptococcus pneumoniae and platelet-activating factor receptor) (2). Experiments are in progress in our laboratory to distinguish between these proposed scenarios.
Invasion assays performed in the presence of microtubule inhibitors (both depolymerizing and stabilizing agents) significantly decreased the ability of HBMEC to take up C. freundii. Confocal microscopy experiments with anti-α-tubulin antibodies showed that microtubules aggregate after HBMEC come in contact with C. freundii. The microtubule aggregation was a time-dependent process; no aggregation was seen at 5 min, little seen in 15 min, and clear-cut aggregation was observed after 30 min of incubation of C. freundii with HBMEC. This microtubule aggregation was inhibited when cells were treated with either microtubule inhibitors or microfilament-inhibiting agents. Of interest, the microtubule aggregation staining pattern did not colocalize with bacterial binding and areas of HBMEC which did not show C. freundii binding also demonstrated pronounced microtubule clumping. This suggests that the contact of the bacteria with HBMEC may globally stimulate microtubule aggregation. Whether the microtubule aggregation is a result of a secreted bacterial factor or paracrine response to bacteria binding to HBMEC remains to be seen. Furthermore, the aggregation of microtubules in response to C. freundii binding may be related to the postulated receptor presentation via de novo protein synthesis and endosome acidification. It has been previously shown that the transport of many receptors to and from the cell surface is dependent on microtubules (10). Therefore, one explanation for the inhibitory effect of microtubule inhibitors on entry of C. freundii into HBMEC is that the agents may decrease the numbers of HBMEC receptors that mediate C. freundii invasion. Experiments are under way to discern between these possibilities.
Microtubules have previously shown to be required for invasion of many pathogens (e.g., Neiserria gonorrheae, Haemophilus influenzae, enteropathogenic and enterohemorrhagic E. coli, and Campylobacter jejuni (4, 9, 22, 23, 29). General thinking has been that although these pathogens may enter through microtubule-dependent pathways, they usually do not replicate intracellularly (6). The data acquired in this study from extended invasion assays and TEM analysis suggest that C. freundii may be an exception to that generalization. In contrast to what has been described for another intravacuole-replicating bacterium, Legionella pneumophila (12), there was no appearance of mitochondria or ribosomes in close proximity to the bacteria. This suggests that C. freundii may not use these organelles to directly obtain energy or that recruitment of specific host cell proteins may not be required for intracellular survival and proliferation (as in the case of L. pneumophila). Of particular relevance to central nervous system infections, other meningitis-causing bacteria such as E. coli K1, GBS, and S. pneumoniae have similarly been shown to invade (1, 13, 25) or invade and transcytose (20, 27) BMEC; however, the organisms have not been found to replicate within HBMEC. As described above, Citrobacter meningitis has been documented for its high frequency of brain abscess formation. Whether replication within HBMEC vacuoles is unique for Citrobacter and if there is a correlation with abscess formation remains to be determined.
Cytochalasin D inhibits C. freundii invasion into HBMEC; however, using immunostaining, we found no detectable reorganization of microfilaments when C. freundii interacted with HBMEC (data not shown). In addition, cytochalsin D pretreatment of HBMEC inhibited the bacterium-dependent microtubule aggregation as visualized by confocal microscopy. There may be several explanations for these results. The cytochalsin D effect on bacterium-dependent microtubule aggregation may be due to indirect effects of the microfilament inhibitor on the microtubule network. For example, microtubules have been observed to act as anchoring structures for F-actin (28). Therefore, disruption of the microfilament network may affect the microtubule network and thus indirectly affect the microtubule-dependent C. freundii invasion of HBMEC. Alternatively, an actin-dependent invasion step may precede a microtubule-dependent step in the C. freundii invasion of HBMEC. This initial step may result in microfilament reorganization when bacteria are initially in contact with the HBMEC; however these events may be transient, and the experimental design utilizing immunofluorescence microscopy may not adequately detect their occurrence. A similar situation is noted for Yersinia invasin-mediated invasion (36). Therefore, if the initial stages of invasion are prevented by cytochalasin D, the subsequent stages of invasion which are microtubule dependent are not triggered. It has previously been shown that actin functions in the translocation of actin-binding protein factors to the plasma membrane as well as in cytosolic signaling (19). In addition, cytochalasin D inhibits Salmonella entry via disruption of the translocation of actin-binding proteins to the bacterial entry site (7). It is possible that in the case of C. freundii invasion of HBMEC, actin microfilaments are necessary for cytosolic signaling and/or bacterial penetration at the plasma membrane, and microtubules may be necessary for the transportation of membrane-bound bacteria from the plasma membrane toward the basolateral side (or just deeper into the cell). Thus, a disruption at either stage of invasion would result in a “traffic jam.”
In summary, the results presented here indicate that C. freundii can invade, multiply within, and transcytose HBMEC in vitro. Determining the genetic basis for these phenotypes will provide significant insight into the pathophysiology of Citrobacter meningitis and potentially aid in developing novel therapeutic and preventative strategies. Furthermore, an extensive molecular comparative analysis of Citrobacter with other meningitis-causing bacteria may shed light on Citrobacter’s unique property of brain abscess formation.
ACKNOWLEDGMENTS
We thank Carol A. Wass for excellent technical assistance, Hiroyuki Shimada for the TEM studies, Ernesto Barron at the USC School of Medicine Doheny Eye Institute for the confocal microscopy studies (EM Core grant EY03040), and Tobias Oelschlaeger and Joseph St. Geme III for providing Citrobacter strains.
This work was supported by Public Health Service grant NS 26310 to K.S.K. and CHLA Research Institute AIDS/Host Defense Program grant to J.L.B.
REFERENCES
- 1.Badger J L, Kim K S. Environmental growth conditions influence the ability of Escherichia coli K1 to invade brain microvascular endothelial cells and confer serum resistance. Infect Immun. 1998;66:5692–5697. doi: 10.1128/iai.66.12.5692-5697.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cundell D R, Gerard N P, Gerard C, Idanpaan-Heikkila I, Tuomanen E I. Streptococcus pneumoniae anchor to activated human cells by the receptor for platelet-activating factor. Nature. 1995;377:435–438. doi: 10.1038/377435a0. [DOI] [PubMed] [Google Scholar]
- 3.Davies P J, Davies D R, Levitzki A, Maxfield F R, Milhaud P, Willingham M C, Pastan I H. Transglutaminase is essential in receptor-mediated endocytosis of alpha 2-macroglobulin and polypeptide hormones. Nature. 1980;283:162–167. doi: 10.1038/283162a0. [DOI] [PubMed] [Google Scholar]
- 4.Donnenberg M S, Donohue-Rolfe A, Keusch G T. A comparison of HEp-2 cell invasion by enteropathogenic and enteroinvasive Escherichia coli. FEMS Microbiol Lett. 1990;57:83–86. doi: 10.1016/0378-1097(90)90417-o. [DOI] [PubMed] [Google Scholar]
- 5.Filler S G, Swerdloff J N, Hobbs C, Luckett P M. Penetration and damage of endothelial cells by Candida albicans. Infect Immun. 1995;63:976–983. doi: 10.1128/iai.63.3.976-983.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Finlay B B, Falkow S. Common themes in microbial pathogenicity revisited. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finlay B B, Ruschkowski S, Dedhar S. Cytoskeletal rearrangements accompanying salmonella entry into epithelial cells. J Cell Sci. 1991;99:283–296. doi: 10.1242/jcs.99.2.283. [DOI] [PubMed] [Google Scholar]
- 8.Graham D R, Band J D. Citrobacter diversus brain abscess and meningitis in neonates. JAMA. 1981;245:1923–1925. [PubMed] [Google Scholar]
- 9.Grassme H U, Ireland R M, van Putten J P. Gonococcal opacity protein promotes bacterial entry-associated rearrangements of the epithelial cell actin cytoskeleton. Infect Immun. 1996;64:1621–1630. doi: 10.1128/iai.64.5.1621-1630.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gruenberg J, Griffiths G, Howell K E. Characterization of the early endosome and putative endocytic carrier vesicles in vivo and with an assay of vesicle fusion in vitro. J Cell Biol. 1989;108:1301–1316. doi: 10.1083/jcb.108.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hodges G R, Degener C E, Barnes W G. Clinical significance of Citrobacter isolates. Am J Clin Pathol. 1978;70:37–40. doi: 10.1093/ajcp/70.1.37. [DOI] [PubMed] [Google Scholar]
- 12.Horwitz M A. Formation of a novel phagosome by the Legionnaires’ disease bacterium (Legionella pneumophila) in human monocytes. J Exp Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Huang S H, Wass C, Fu Q, Prasadarao N V, Stins M, Kim K S. Escherichia coli invasion of brain microvascular endothelial cells in vitro and in vivo: molecular cloning and characterization of invasion gene ibe10. Infect Immun. 1995;63:4470–4475. doi: 10.1128/iai.63.11.4470-4475.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Joaquin A, Khan S, Russel N, al Fayez N. Neonatal meningitis and bilateral cerebellar abscesses due to Citrobacter freundii. Pediatr Neurosurg. 1991;17:23–24. doi: 10.1159/000120561. [DOI] [PubMed] [Google Scholar]
- 15.Kim K S, Itabashi H, Gemski P, Sadoff J, Warren R L, Cross A S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J Clin Investig. 1992;90:897–905. doi: 10.1172/JCI115965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline M W, Kaplan S L, Hawkins E P, Mason E O., Jr Pathogenesis of brain abscess formation in an infant rat model of Citrobacter diversus bacteremia and meningitis. J Infect Dis. 1988;157:106–112. doi: 10.1093/infdis/157.1.106. [DOI] [PubMed] [Google Scholar]
- 17.Larkin J M, Brown M S, Goldstein J L, Anderson R G. Depletion of intracellular potassium arrests coated pit formation and receptor-mediated endocytosis in fibroblasts. Cell. 1983;33:273–285. doi: 10.1016/0092-8674(83)90356-2. [DOI] [PubMed] [Google Scholar]
- 18.Mellman I, Fuchs R, Helenius A. Acidification of the endocytic and exocytic pathways. Annu Rev Biochem. 1986;55:663–700. doi: 10.1146/annurev.bi.55.070186.003311. [DOI] [PubMed] [Google Scholar]
- 19.Miyamoto S, Teramoto H, Coso O A, Gutkind J S, Burbelo P D, Akiyama S K, Yamada K M. Integrin function: molecular hierarchies of cytoskeletal and signaling molecules. J Cell Biol. 1995;131:791–805. doi: 10.1083/jcb.131.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nizet V, Kim K S, Stins M, Jonas M, Chi E Y, Nguyen D, Rubens C E. Invasion of brain microvascular endothelial cells by group B streptococci. Infect Immun. 1997;65:5074–5081. doi: 10.1128/iai.65.12.5074-5081.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Oelschlaeger, T. A., J. L. Badger, and K. S. Kim. 1999. Unpublished results.
- 22.Oelschlaeger T A, Guerry P, Kopecko D J. Unusual microtubule-dependent endocytosis mechanisms triggered by Campylobacter jejuni and Citrobacter freundii. Proc Natl Acad Sci USA. 1993;90:6884–6888. doi: 10.1073/pnas.90.14.6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oelschlaeger T A, Tall B D. Invasion of cultured human epithelial cells by Klebsiella pneumoniae isolated from the urinary tract. Infect Immun. 1997;65:2950–2958. doi: 10.1128/iai.65.7.2950-2958.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Prasadarao, N. V., M. F. Stins, C. A. Wass, H. Shimada, and K. S. Kim. Outer membrane protein A promoted cytoskeletal rearrangement of brain microvascular endothelial cells is required for Escherichia coli invasion. Submitted for publication. [DOI] [PMC free article] [PubMed]
- 25.Prasadarao N V, Wass C A, Weiser J N, Stins M F, Huang S H, Kim K S. Outer membrane protein A of Escherichia coli contributes to invasion of brain microvascular endothelial cells. Infect Immun. 1996;64:146–153. doi: 10.1128/iai.64.1.146-153.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rathman M, Sjaastad M D, Falkow S. Acidification of phagosomes containing Salmonella typhimurium in murine macrophages. Infect Immun. 1996;64:2765–2773. doi: 10.1128/iai.64.7.2765-2773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ring A, Weiser J N, Tuomanen E I. Pneumococcal trafficking across the blood-brain barrier. Molecular analysis of a novel bidirectional pathway. J Clin Investig. 1998;102:347–360. doi: 10.1172/JCI2406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rubino S, Fighetti M, Unger E, Cappuccinelli P. Location of actin, myosin, and microtubular structures during directed locomotion of Dictyostelium amebae. J Cell Biol. 1984;98:382–390. doi: 10.1083/jcb.98.2.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St. Geme J W, III, Falkow S. Haemophilus influenzae adheres to and enters cultured human epithelial cells. Infect Immun. 1990;58:4036–4044. doi: 10.1128/iai.58.12.4036-4044.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stins M F, Gilles F, Kim K S. Selective expression of adhesion molecules on human brain microvascular endothelial cells. J Neuroimmunol. 1997;76:81–89. doi: 10.1016/s0165-5728(97)00036-2. [DOI] [PubMed] [Google Scholar]
- 31.Stins M F, Prasadarao N V, Zhou J, Arditi M, Kim K S. Bovine brain microvascular endothelial cells transfected with SV40- large T antigen: development of an immortalized cell line to study pathophysiology of CNS disease. In Vitro Cell Dev Biol Anim. 1997;33:243–247. doi: 10.1007/s11626-997-0042-1. [DOI] [PubMed] [Google Scholar]
- 32.Stins, M. F., N. V. Prasadrao, J. L. Badger, F. Gilles, and K. S. Kim. 1999. Unpublished results.
- 33.Tanenbaum S W. Cytochalasins: biochemical and cell biological aspects. New York, N.Y: North-Holland; 1978. [Google Scholar]
- 34.Valdivia R H, Falkow S. Bacterial genetics by flow cytometry: rapid isolation of Salmonella typhimurium acid-inducible promoters by differential fluorescence induction. Mol Microbiol. 1996;22:367–378. doi: 10.1046/j.1365-2958.1996.00120.x. [DOI] [PubMed] [Google Scholar]
- 35.Woods C R, Jr, Mason E O, Jr, Kaplan S L. Interaction of Citrobacter diversus strains with HEp-2 epithelial and human umbilical vein endothelial cells. J Infect Dis. 1992;166:1035–1044. doi: 10.1093/infdis/166.5.1035. [DOI] [PubMed] [Google Scholar]
- 36.Young V B, Falkow S, Schoolnik G K. The invasin protein of Yersinia enterocolitica: internalization of invasin-bearing bacteria by eukaryotic cells is associated with reorganization of the cytoskeleton. J Cell Biol. 1992;116:197–207. doi: 10.1083/jcb.116.1.197. [DOI] [PMC free article] [PubMed] [Google Scholar]