Abstract
COVID-19 has severely devastated many lives across the globe. It has been speculated that stem cell-based therapy for COVID-19 treatment could be able to subsidize the effects. In preclinical and clinical studies, stem cell-based therapy has successfully eliminated inflammatory cytokines in ALI, ARDS, and COVID-19. Clinical trials have produced a variety of promising results for validating stem cell therapy in COVID-19 patients. For instance, exosome-based therapy (ExoFlow) showed an 87% survival status, and MSC-based therapy (Mesoblast) achieved an 83% survival rate in moderate to severe COVID-19 patients. This review debates the advantages of cell-free therapy, i.e., stem cell-derived exosome-based therapies, over stem cell-based therapy. This review aims to question whether the immunomodulatory effect of stem cells differs based on their origin and also tries to find possible answers for the best stem cells for treating SARS-CoV-2 infection.
Graphical abstract
The role of stem cells and their extracellular vesicles in the upregulation of regulatory immune cells, growth factors (EGF, FGF, VEGF), and anti-inflammatory cytokines (IL-6, INF-α, galectin-1, notch-1, PDL-1) that promote the tissue regeneration at the injured site. The right side of the image depicts the downregulation of inflammation-inducing immune cells, pro-inflammatory cytokines, and chemokines that could also enhance COVID-19 therapy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11010-022-04601-2.
Keywords: SARS-CoV2, COVID-19, Stem cell therapy, Immunomodulation, Stem cell-free therapy
Introduction
The novel Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) pandemic has spread across the globe and posed a great threat to human beings. The worst-hit countries based on death rates due to COVID-19 are the United States of America, Brazil, Mexico, India, Russia, and the United Kingdom [1]. The pandemic has hindered millions of people’s lives and affected economic conditions globally. Scientists and doctors are persistently taking measures to reduce and find a treatment for this pandemic disease. Research has succeeded in establishing various hypotheses and some clinical studies on new vaccines based on the current knowledge of coronavirus and the pathophysiology of the disease. Now, the re-emergence of the pandemic is worse. The most unfortunate situation is that the people from developing countries were also severely devastated economically. Many people lost their jobs and migrated from urban areas to rural areas due to the inability to manage their expenses. According to a survey, about 71% of household members lost their job, and about 61% of people shut down their small businesses [2]. Developing countries and the economic impact of COVID-19 did not spare developed countries. However, economic turbulence was lesser than in developing countries [3]. If this condition persists, then there would be a need to amend the strict lockdown, which would further create economic problems. Hence, there is a need to identify a treatment method that should not only cure coronavirus patients but also heal the detrimental effects that occurred during infection without providing any side effects.
Pathophysiology of COVID-19
The pathophysiology of the disease can be divided into three stages: the Asymptomatic stage (initial stage), the Symptomatic stage (mid-stage), and the Progressive to ARDS stage (terminal stage). The asymptomatic stage marks an entry point for the virus. The virus enters via the nasal route, and the spike protein on the virus interacts with human epithelial cells through an Angiotensin-converting enzyme (ACE-2) receptor [4]. S protein has an S1 subunit and an S2 subunit, which has a receptor-binding domain (RBD). It is observed that the ACE-2 receptor sequence from 331 to 524 residues binds to the S protein of the virus [5]. The S1 subunit forms a homotrimer and binds to the S2 portion [6]. The interaction between the S protein and the ACE-2 receptor is activated by multiple proteases like TMPRSS2 and cathepsin CTSL, which cleaves S1/S2 protein and triggers the viral membrane fusion process [7]. Furthermore, in silico analysis confirmed that the region surrounding K353 and N501 amino acids play a crucial role in the interaction. Interestingly, Villa et al. synthesized DNA aptamers sequences using SELEX technology that could bind to the ACE-2 receptor, thereby inhibiting the interaction between ACE-2 and S protein [8]. The two major variants of coronavirus, i.e., delta variant and omicron variant, show mutant sequences in the S protein. This mutation increases the virulence of the variants [9]. The Wuhan-Hu-1 (wild type) variant has 1273 amino acids, while the delta variant and omicron variant have 1271 and 1270 amino acids, respectively. Kumar et al. performed a computational comparative study by analyzing the docking score between spike proteins of these variants and human ACE-2 proteins. The docking score for the wild type was − 500.37, while for the omicron variant, it was − 539.81, and for the delta variant, it was − 529.62. These results revealed that the Omicron strains are more vulnerable to binding to ACE2 than the Delta strain. Thus, researchers predict that the virus has evolved gradually in immunocompromised patients, which might have played a crucial role in its virulence development [10].
This disease was previously believed to affect only the respiratory system, causing ARDS. However, some studies have shown that it could also affect the digestive, cardiovascular, urinary, and reproductive systems, apart from the respiratory system. One of the major speculations comes from the level of mRNA transcript of the ACE-2 receptor on the different cell surfaces of various tissues [11]. According to this study, the amount of ACE-2 receptors found on lung epithelial cells is comparatively less than those found in the duodenum, heart, gall bladder, small intestine, kidney, and testes [12]. However, it is unable to understand the pathophysiological condition of coronavirus and why it majorly affects the respiratory system rather than other systems. The probable reason might be that the respiratory system is the entry point for the virus and is more easily accessible than other parts of the body during the initial period. Later after the disease progresses, it may affect other body parts leading to multiple organ failures [12]. It is found that SARS-CoV-2 also affects various other cells like endothelial cells, pericytes, as well as astrocytes in the brain [6, 13, 14]. Scientists predict that SARS-CoV-2 could pass through olfactory mucosa and could reach brain cells [13, 15].
The symptomatic phase indicates the replication of the virus in the respiratory tract and the lungs. This leads to tissue damage and destroys the architecture of the lung tissue, which affects its functioning and leads to severe respiratory illnesses like shortness of breath, difficulties in breathing, and pneumonia-like symptoms. However, it is also seen in lesser numbers (1.0–3.8%) that coronavirus causes nausea and vomiting as early symptoms of COVID-19, thus affecting the digestive system [12]. At this stage, the immune system gets activated against the intruder, i.e., coronavirus. Innate and acquired immunity get elicited, which results in leukocytopenia [16]. The innate immune system produces various inflammatory cytokines and chemokines, which attract other immune cells to infiltrate the affected region. This creates immune-mediated lung injury and initiates ARDS.
During the progression phase of the disease, the cytokine-mediated injury shows adverse effects, leading to fatal conditions. Adverse effects were observed in the patients due to an increase in inflammatory monocytes and neutrophils, CD14+ CD16+ monocyte-derived macrophages, and pro-inflammatory cytokines [17, 18]. Bilateral diffuse alveolar damage, fibroblastic proliferation in the airways, and the circulation of hyperactivated CD4+ and CD8+ lymphocytes were also observed [16]. Some studies have also shown intra-alveolar hemorrhages and fibrin cluster formation, and abundant intra-alveolar neutrophilic infiltration. Apart from the lungs, the liver also showed mild sinusoidal dilatation [19]. This indicates that the tissues might be injured in this stage due to the viral outbreak from the cells and the inflammatory responses from immune cells. The Berlin definition classifies ARDS based on the PaO2/FiO2 ratio and chest scanned images and time period of respiratory distress [20]. Even though COVID-19 disease obeys the ARDS Berlin definition, there are distinct differences between COVID-19 pneumonia and ARDS [21]. The first pattern observed is multiple ground-glass lesions that evolve due to loss of aeration in the lung tissues. Furthermore, diffused alveolar damage, alveolar flooding in the presence of fibrin and hyaluran, and intense remodeling. These are some of the characteristic symptoms of COVID-19 [22]. This hampers respiratory function and causes organ damage. Inflammatory immunological reactions, i.e., cytokine storms, elevate the severity of the disease. Immune response against the virus is by secreting interferons, which also trigger other immune cells to infiltrate [23]. This is observed in some infectious and non-infectious diseases that lead to hyperinflammation. However, MSC-based stem cell therapies may neutralize this cytokine storm by activating suppressor cells as well as repairing and regenerating the injured sites. Researchers from many parts of the world are targeting and suppressing the inflammatory response.
Stem cell-based therapy
Stem cells are human-originated, undifferentiated cells that have the ability of self-renewal and the potential to differentiate into various tissue types. Stem cells can undergo immunomodulation, provide anti-inflammatory responses, and repair and regenerate injured tissues [24, 25]. Stem cells are generally of two major types based on their origin: embryonic stem cells (ESCs) [26] and adult stem cells. Human embryonic stem cells are pluripotent in origin, while adult stem cells are multipotent cells that have the potential to differentiate into particular cell lineages. There are different types of adult stem cells based on their location, i.e., bone marrow-mesenchymal stem cells (BM-MSCs) [27], dental pulp derived mesenchymal stem cells (DPSCs) [28], Warton Jelly mesenchymal stem cells (WJ-MSCs) [29], placental stem cells (P-MSCs) [30], amniotic fluid-derived stem cells [31], hair follicle stem cells [32], urinary stem cells [33], breast milk-derived stem cells [34], adipose tissue MSC [35], olfactory mucosa MSC [36], menstrual blood MSC [37], Umbilical cord Mesenchymal Stem Cells [38], induced pluripotent stem cells (iPSCs) [39]. ESCs are used in exceptional cases since the isolation of human embryonic cells is limited and cannot be extracted in large numbers because of ethical issues. The cytoplasmic contents of stem cells are different from differentiated cells; thus, it possesses regeneration and immunomodulatory characteristics [40]. The stemness of a cell is reduced in this transition from pluripotency to multipotency to unipotency (a differentiated cell). Some examples of cytoplasmic contents of stem cells are immunomodulatory factors, chemokines, secretomes, proteomes, growth factors, cytokines, and small molecules such as prostaglandin E2 (PGE-2), dibutryl cAMP, nitric oxide (NO), concavalin A, indoleamine 2,3-dioxygenase (IDO), and morphogenic factors [40, 41]. These secretomes have a paracrine effect on other cells, which might contribute to cell migration, stimulation, angiogenesis, or anti-apoptotic processes. MSCs play a critical role in regulating the inflammatory microenvironment and with immune cells like T-cells, neutrophils, B-cells, macrophages, natural killer cells, and dendritic cells [42]. Thus, changes occur due to the secretory by-products of stem cells that regulate the immunological aspects in the biological niche; this characteristic phenomenon of stem cells is known as Immunomodulation. Table 1 shows a list of secretion products of stem cells and their function on the effector cells. These secretion products provide an insight into the potential of stem cell therapy which might assist in treatment.
Table 1.
Comprehensive information on the stem cell cytokines and their effect on immune cells
| Stem cells | Immune cells | Secretion products | Function | References |
|---|---|---|---|---|
| MSCs | Monocyte/Macrophage | Interleukin-1 receptor antagonist (IL-1-RA) |
i. Conversion of monocytes/macrophages (type I) toward an anti-inflammatory/immune-regulatory (type 2) phenotype ii. Restricting the differentiation of cells into the type 1 phenotype |
[43] |
| IL-6 |
i. IL-6 induces the expression of IL-10 in anti-inflammatory monocytes ii. IL-6 represses the expression of inflammatory cytokines like IL-12p70, TNF-α, and IL-17 |
[44] | ||
| Dendritic cells | Galetin-1 | i. Inhibit CD83 expression, decrease the production of IL-12, and hampers DC maturation by disrupting the endocytosis process | [45] | |
| B-cells | IL-1-RA |
i. Reduction in plasmablast formation and inhibition of differentiation ii. Promote the induction of regulatory B-cells |
[43, 44] | |
| T-cells | Notch-1 |
i. Induction of T-regulatory cells ii. Induces anti-inflammatory proteins |
[46] | |
| DPSCs | Peripheral blood mononuclear cells (PBMCs) | Interferon-γ | i. Inhibits the proliferation of PBMCs | [47] |
| T-cells |
i. Inhibition of T-cell proliferation ii. Reduction in IL-17 production iii. Stimulation of regulatory T-cell (Treg) differentiation |
[48] | ||
| Natural killer cells (NK cells) | Hypoxia-inducible factor 1 | i. NK cell-mediated cell lysis is inhibited | [49] | |
| ESCs | T-cells | Fas-L | i. Induces apoptosis in activated T-cells | [50] |
| IL-10 | i. Reduces inflammation by regulatory T-cells | |||
| Human amnion-derived mesenchymal stem cells (HA-MSCs) | T-cells and B-cells | Programmed cell death ligand (PDL-1) | i. The binding of PDL-1 and PD-1 (Programmed cell Death-1) receptor suppresses the activated B-cells and T-cells | [51] |
| Human adipose-derived mesenchymal stem cells (AD-MSCs) | Neutrophils | Not mentioned |
i. The neutrophil influx was reduced, which decreased the inflammatory response of neutrophils in the injured site ii. Myeloperoxidase (MPO) is one of the most cytotoxic enzymes produced by neutrophils against bacteria, and its concentration was reduced |
[52] |
| i. The concentration of inflammatory cytokines like IL-6, TNF‐α, IL‐1β, and MIP‐2 was reduced |
Stem cell therapy in ARDS or ALI
Preclinical studies of stem cell therapy in ARDS or ALI
There are several successful cases of stem cell therapy treating lung-related diseases like influenza, Acute Respiratory Distress Syndrome (ARDS), Acute Lung Infection (ALI), and Chronic Obstructive Pulmonary Disease (COPD). ARDS symptoms are similar to the symptoms of COVID-19. Hence, some of the case studies are relatable. Table 2 shows the preclinical data of ARDS or ALI, which was treated with stem cell-based therapies.
Table 2.
A comprehensive data on preclinical studies of stem cell therapy in ALI or ARDS
| Source of stem cells | Animal models | Type of injury model | Route of delivery and dosage | Key findings/outcome | References | |
|---|---|---|---|---|---|---|
| hBM-MSCs | C57BL/6 mice | H9N2 avian influenza virus (AIV) |
Intravenous 1 × 105 cells/100 μl |
∙ Post BM-MSC treatment, levels of inflammatory chemokines and cytokines reduced ∙ Increased expression of anti-inflammatory factors were observed |
[55] | |
| Female Balb/C mice | H5N1 Influenza A virus | 5 × 105 cells/100 μl |
∙ Survival and body weight of treated mice was greater than the control mice ∙ Wet-to-dry lung weight ratio of ALI induced and treated mice were less ∙ MSC treatment induced alveolar M2 phenotype macrophages, overexpression of CFTR (cystic fibrosis transmembrane conductance regulator) protein expression |
[56] | ||
| C57BL/6 mice | Bleomycin injected into tracheal lumen |
Intravenous 0.1 ml of 5 × 105 cells/ml BM-MSCs |
∙ Suppression of inflammatory cytokines and increased expression of anti-inflammatory cytokines (IFN-γ, IL-2, IL-4, and IL-1 α) were observed | [180] | ||
| C57BL/6 male mice | E. coli induction | 1 × 106 cells/30 µl | ∙ Neutrophile count and concentration of MIP-2 in BAL was reduced | [181] | ||
| BALB/C female mice | LPS induction |
Oral administration Two doses of 2.5 × 105/100 μl hBM-MSCs at 30 min interval |
∙ Neutrophile count and MPO (Myeloperoxidase) important marker for neutrophile infiltration was reduced ∙ Reduction in the expression of MCP-1, IL-1α, RANTES, IL-1β, IP-10, IL-17, IL-6, MIP-1α and were observed ∙ Upregulation of anti-inflammatory cytokine TNF-α-induced protein 6 (TSG-6), IL-1RN, and regenerating protein keratinocyte growth factor (KGF) |
[182] | ||
| C57BL/6 male mice | E. coli LPS induction |
Intratracheal 5 × 105/30 μl |
∙ Significantly increased the LXA4 (lipoxin A4) level while reduced the production of TNF-α and MIP-2 | [183] | ||
| Sprague–Dawley male rats | Ventilator-induction |
3 different routes of delivery ∙ Intratracheal ∙ Intraperitoneal ∙ Intravenous 4 different dosage ∙ 1 × 106 cells/kg ∙ 2 × 106 cells/kg ∙ 1 × 106 cells/kg ∙ 10 × 106 cells/kg |
∙ Better results were shown by intravenous and intratracheal route ∙ Lowest effective dose of hBM-MSCs was 2 × 106 cells/kg ∙ Enhanced lung repair, restoring oxygenation, improved lung compliance, reduced alveolar edema, and restored lung architecture |
[184] | ||
| Kunming mouse | Hypoxia induction |
Intraperitoneal 1 × 105 cells |
∙ Decrease in genes associated with fibrosis (TGFβ1 (transforming growth factor β1), collagen1α and TIMP-1 (tissue inhibitor of metalloproteinases1) ∙ Expression levels of IL-1, TNFα was reduced |
[185] | ||
| Sheep | Cotton smoke insufflation followed by instillation of live Pseudomonas aeruginosa |
Intravenous Lower dose—5 × 106 cells/kg Higher dose—10 × 106 cells/kg |
∙ Oxygenation was improved in treated sheep | [186] | ||
| hBM-MSC and mBM-MSC | Wistar male rats | Severe blunt chest trauma by single blast wave centered on the thorax |
Intravenous 5 × 106 cells/ml |
∙ Lower level of cytokines IL-1β, IL-6 and chemokines CXCL1, CCL2 ∙ Higher levels of TNF-α in the BAL |
[187] | |
| mBM-MSCs | C57BL/6 male mice | E. coli endotoxin induction |
– 7.5 × 105 cells/30 µl |
∙ Reduced concentration of MIP-2 and TNF-α while concentration of IL-1ra, IL-10, and IL-13 were increased | [188] | |
| C57Bl/6 female mice | Elastase induction |
Intravenous 1 × 106 cells |
∙ Reduction of peripheral lung tissue damage was observed in combination therapy of mBM-MSC and ATRA (All-trans retinoic acid) ∙ Reduction in MLIs (mean linear intercepts) and increases in S (alveolar surface area) values |
[189] | ||
| Sprague–Dawley male rats | Ventilator induction |
Intratracheal 4 × 106 cells/30 µl |
∙ Improvement in lung microvascular permeability, decreasing, lung wet: dry weights and reduction in BAL protein concentrations ∙ Lung neutrophil, macrophages and inflammatory cytokines (TNF-α, IL-6) accumulation was reduced ∙ Increase in KGF levels |
[190] | ||
| C57BL/6 male mice | E. coli K1 induced |
Intratracheal 7.5 × 105 cells/30 µl |
∙ Upregulation of lipocalin 2 inhibiting the bacteria ∙ mBM-MSCs reduced early pro-inflammatory responses in BALF |
[191] | ||
| C57BL/6 mice | E. coli endotoxin induced |
Intravenous 1 × 105 cells/0.05 ml |
∙ Decrease in IL-6, IL-10, MCP-1, MIP-1α, KC, IP-10, IL-1β, and IFN-γ ∙ Induction of M2 type of macrophage |
[192] | ||
| PDFGRa+ SCA1+ CD45− TER119− (PαS) expressed mBM-MSCs | C57BL/6 (C57BL/6NCrl) and B6.CgTg (TcraTcrb) 425Cbn/J mice | Klebsiella pneumoniae induced |
Intratracheal ∙ × 106 cells |
∙ Significantly reduced alveolitis and protein leakage, reduced alveolar TNF-α and IL-12p70 expression ∙ Decreased infiltration of respiratory DC cells |
[193] | |
| HGF (Hepatocyte growth factor) gene knockdown rBM-MSC and rBM-MSC | Sprague–Dawley male rats | LPS induction |
Intravenous 5 × 106 cells/100 µl |
∙ rBM-MSC decreased the lung wet weight: body weight ratio, inflammatory reactions while HGF knockdown rBM-MSC did not show these anti-inflammatory response | [194] | |
| VEGF gene knockout rBM-MSCs | Sprague–Dawley rats | LPS induction |
Intravenous 5 × 106 cells/100 µl |
∙ VEGF knockdown rBM-MSCs showed higher wet weight: body weight ratio and less pulmonary vascular permeability ∙ VEGF knockdown rBM-MSCs also showed less beneficial in treating inflammatory responses than rBM-MSCs |
[195] | |
| Overexpressed ACE-2 gene in mBM-MSCs | C57BL/6 male mice | LPS induction |
Intravenous 5 × 105 cells/100 µl |
∙ Lung injury, vascular permeability, neutrophil counts, histopathological index in BALF declined ∙ Reduction in the expression levels of IL-1β, Ang-2, and IL-6 was observed ∙ IL-10 protein levels increased |
[196] | |
| hBM-MSCs hUC-MSCs and CD362+ hUC-MSCs at (3,5,7,10 passage numbers) | Sprague–Dawley male rats | E. coli induction |
Intratracheal 1 × 107 cells |
∙ Passage number above 3 did not have much efficacy ∙ CD362+ hUC-MSCs were effective in reducing lung injuries ∙ Cryopreserved stem cells also showed efficacy along with antibiotic therapy |
[197] | |
| mAD-MSCs | C57BL/6 male mice | Pseudomonas aeruginosa induced |
Intratracheal Low dose 1 × 105 cells High dose 1 × 106 cells |
∙ Inhibition of alveolar neutrophil accumulation, decreased levels of MPO, MIP-2 and total proteins in BALF ∙ Inhibited the overproduction of prostaglandin E2, improved phagocytosis |
[198] | |
|
hCMSCs (human chorionic villi-derived MSCs) |
C57BL/6 male mice | LPS induction |
Intravenous 5 × 105 cells/200 µl |
∙ Reduction in Glucagon-like peptide-1 receptor (GLP-1R), specific surfactant protein C (SPC), Angiopoietin-1(Ang-1), and Fibroblast growth factor-10 (FGF-10) levels ∙ Combination of hCMSCs with liraglutide showed more therapeutic efficacy |
[199] | |
Loy H et al. provided an insight into the mechanism of human UC-MSCs cells and their regeneration properties in the lung injured site by conducting experiments on Influenza A(H5N1) virus-associated ALI [53]. UC-MSCs regulated ion transporter protein channels for clearance of the alveolar lumen. Immunomodulation properties were also exhibited by UC-MSCs, which decreased the inflammatory factors. Exosomes of UC-MSCs were also injected into the mice to evaluate whether exosomes also exhibited similar responses, and it was found that exosomes also showed convincing positive results [53]. The exosomes and their release contents might have contributed to treating ALI. Exploring the contents of exosomes and ensuring the contents contribute to treating ALI would provide a greater cause.
Kim et al. demonstrated the usefulness of human umbilical cord blood (UCB)-derived MSCs in treating the Escherichia coli-induced ALI mice model. The intratracheal injection of USB-MSCs downregulated the inflammatory responses (TNF-α, IL-1α, MIP-1α, IL-1β, MIP-1β, IL-6, RANTES, and IP-10) and by enhancing bacterial clearance [54]. The same group also studied the antibacterial effects of UCB-MSCs. After E. coli exposure, there was a significant upregulation in toll-like receptors TLR-4 and TLR-2 and β-defensin 2 (BD2) in MSCs, which was associated with bacterial clearance by regulating the TLR-4 signaling pathway [55]. The immunomodulatory function was also observed in hMSCs like USB-MSCs. hMSCs were used for treating pneumonia in E. coli-induced pneumonia rat models. The survival rate of these rat models was 100%, was visualized by an increase in arterial PO2, lung compliance, reduction in BAL protein, BAL neutrophils, alveolar E. coli counts, and reduced in histological injury, reduced alveolar thickening, and increased air-space volume [56]. However, optimization of the dose of hMSCs forms very essential criterion in using stem cell-based therapies. The use of hMSCs is more encouraged than UCB-MSCs since isolating UCB-MSCs from the umbilical cord is restricted due to availability. These provide evidence for using stem cells as therapeutics and the role of stem cells in downregulating inflammatory cytokines.
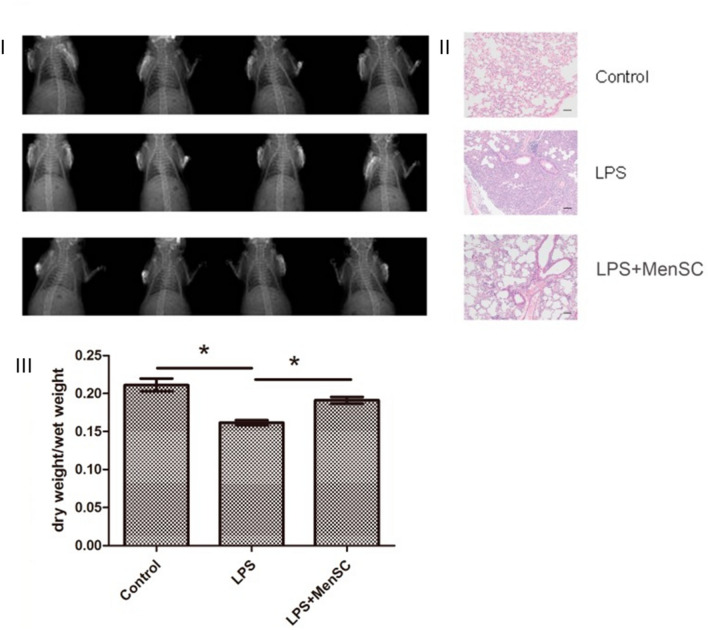
A combination of hUC-MSCs and FTY720 (a novel immunosuppressive agent) was investigated by Huang et al. in an LPS-induced lung injury mouse model. The clinical efficacy in alleviating lung injuries was higher with the combination treatment of hUC-MSCs and FTY720 than with using hUC-MSCs or FTY720 alone. Transcriptomic analysis by using a gene expression chip identified potential gene targets Nr1h4, Nol3, Cyp17a1, Prkg2, and Rps6ka6 related to ALI/ARDS [57]. Genetic expression analysis enabled the highlight of the possible genes involved in the aggravation of the infection. Thus, this study speculates that targeting these genes may provide alleviation to COVID-19 patients. Xiang et al. investigated the Menstrual Blood-Derived Mesenchymal Stem Cells (MenSCs) in treating LPS-induced ALI (Fig. 1). In the LPS-induced mice model, decreased inflammation and a severe reduction in the BALF were observed [58]. MenSCs may have reduced the inflammation which was observed in the histological tissues. Even though therapies might provide promisable results in pre-clinical studies, using menstrual blood for stem cell isolation is still dilemmatic. Due to sources of stem cell isolation, there is a requirement to use antibiotics to avoid secondary infections. Thus, there is uncertainty about the interference of these antibiotics in stem cell-based therapies. One of the major limitations of using MenSC is reproducibility, and each woman may have a different menstrual cycle and hormonal changes, which depend on the characteristics of the stem cells. Thus, the potential of the therapy may differ even between doses in the same patient.
Fig. 1.
Physiological changes occurred after stem cell injection. I X-rays images of saline-injected control group having no lesion in the lung tissue, LPS-induced rat depicting lung injury, hemorrhage, and inflammation, which were reduced after MenSC administration (LPS + MenSCs group). II H&E staining showing the pathological differences in the control group, LPS-induced group, and MenSC-treated group. III Lung dry/wet ratios of these models were evaluated. Scale bar: 50 µm Reproduced from [58] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
Silva and their team explored the best source of mesenchymal stem cells to treat pulmonary ARDS. Despite mesenchymal stem cell sources, MSC treatment reduced lung inflammation and lung fibrosis, thereby improving lung function and decreasing apoptosis in the lung, liver, and kidney. Their experiment showed that bone marrow BM-MSCs and adipose tissue AD-MSCs reduced TNF-α, IL-1β, KC, TGF-β, and VEGF levels but increased KGF. Lung-resident MSCs (L-MSCs) showed no significant differences in TNF-α, IL-1β, and KC, TGF-β, VEGF, and KGF levels. Hence, BM-MSCs and AD-MSCs showed greater potential than L-MSCs. However, further studies are required to confirm their definite mechanisms of action [59]. In another independent investigation, it was observed that L-MSCs had a crucial role in regulating Treg cells and Th17 cells in the LPS-induced ALI model. The administration of lung-resident L-MSC reduced lung damage and inflammation detected in the BALF. It was also observed that LRMSC could reduce inflammatory cytokines and increase the expression of KGF-2 surfactant protein C (SPC). This study found that the administration of LRMSC upregulated Tregs and downregulated Th17 cells by which LRMSCs improve lung injury [60]. Thus, the nature of MSCs changes based on the source, health conditions of the donor, and patients.
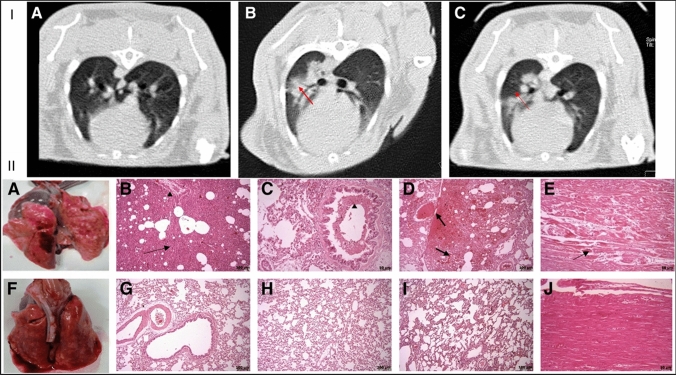
Mokhber Dezfouli et al. emphasized the beneficial effect of intrapulmonary autologous transplantation of bone marrow-derived mesenchymal stromal cells in treating LPS-induced ARDS in rabbits (Fig. 2). They confirmed that MSCs decrease the severity of clinical symptoms and inflammation. Lung CT images showed decreased inflammation and mucous accumulation, which was confirmed by cytokine profiling. (Fig. 2I). After MSC administration, inflammatory cytokines (TNF-α and IL-6) were found to be reduced in the blood and BAL. Necropsy and histopathological staining also further confirmed that no injury was observed in the treatment group (Fig. 2II) [61].
Fig. 2.
I CT scanned images of lung tissue of LPS-induced ARDS in the rabbit. (A) Transverse section of the lung. (B) Inflamed lungs were observed in the control group. (C) Decreased inflammation in the lungs after stem cell treatment. II Necropsy and histopathological findings in the LPS-induced ARDS in the rabbit. (A–E) Control group. (A) Lung tissue is showing hyperemia, hemorrhage, and edema. (B) Interstitial edema and pneumonia (arrow). (B, C) Inflammatory cell infiltration (arrowhead). (D) Hyperemia and severe hemorrhage in alveoli and parenchyma (arrows). (E) Myofibrils necrosis of heart (arrow). (F–K) Treatment group. (F) Lung tissue showing hyperemia and edema (lesser than in the control group). (G–I) Histopathological staining depicts lesser alveoli and parenchymal damage. (K) Heart without injured lesions. Reproduced from [61] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
Jackson and his group studied the crosstalk between MSCs and macrophages to understand its impact on the phagocytosis mechanism in the E. coli pneumonia mouse model of ARDS. They demonstrated that MSCs could transfer mitochondrial contents through nanotube (TNT)-like structures, which could enhance the phagocytic ability of macrophages and thus improve the function of macrophages against ARDS [62]. Li et al. demonstrated the beneficial effects of syngeneic MSCs treating H9N2 avian influenza virus (AIV)-induced acute lung injury in mice. After the infusion of MSCs through the caudal vein, a significant reduction of chemokine and proinflammatory cytokine levels was observed. Also, a reduction in the recruitment of inflammatory cells into the lungs was observed. The survival rate in the MSCs-treated group was 100%, whereas, in the control group, it was 80%. The histopathology studies showed a reduction in the alveolar space inflammation and improvement in the lung histopathology in the H9N2 AIV-induced lung injury model following MSC treatment. Arterial blood gas analysis was performed, which evaluates the partial pressure of arterial oxygen (PaO2), a saturation of arterial oxygen (SaO2), the partial pressure of arterial carbon dioxide (PaCO2), and pH values showed that MSCs infusion increased PaO2, SaO2, and pH values, decreased PaCO2, which indicate effective protection of the lungs. These results indicate the potential of MSCs for treating clinical avian influenza [63].
Chan et al. investigated influenza disease interventions in H5N1 Influenza A virus-induced Balb/C mice models [64]. Infection by the Influenza virus showed dysregulated alveolar fluid transport due to the inhibition of epithelial sodium channel activity. However, this condition was treated in BM-MSC-treated mice, and there was a significant increase in lung alveolar fluid permeability. The population of macrophages/monocyte in the bronchoalveolar lavage (BAL) fluid was preponderately alveolar macrophages (M2 phenotype) [64]. M2 phenotype macrophages are alternatively activated macrophages, unlike the classically activated macrophages (M1 phenotype). The polarization of M2-type cells might be due to the secretions of MSCs. The function of M2 phenotype macrophages is of the opposite nature to that of activated macrophages (M1 phenotype). They are regulatory in nature and have anti-inflammatory responses that are required to reduce the inflammation in injured lung tissues [65]. The concentrations of cytokine and chemokine proteins were evaluated, and significant improvement was observed in the MSC-treated group compared to the fibroblast-treated group. Overall, in vivo, MSC therapy reduced acute lung injury and increased the survival rate in aged H5N1-infected mice. These preclinical studies recommend that MSCs-based therapy might be highly beneficial to the patients [64]. Analysis of preclinical studies of ARDS enhances understanding of the potential of stem cells in lung lesion healing, immunomodulation for clearance of toxic substances, and lung tissue regeneration.
Clinical study of stem cell therapy in ARDS or ALI
Stem cell therapy has been successful in treating ARDS and ALI. Although many preclinical studies support the idea of stem cell therapy, clinical trials would provide further insights into the dosage of stem cells required, possible hazardous effects, and side effects. Simonson et al. investigated the in vivo effect of non-HLA-matched allogeneic bone marrow-derived MSCs in two patients with severe refractory ARDS on a compassionate use basis. After an infusion of 2 × 106 cells per kilogram, both patients demonstrated improved lung function and were discharged from the ICU and hospital. No adverse changes were detected in both patients, including no changes in hemodynamic parameters or in oxygen saturation levels. Beneficial effects were associated with the reduction of inflammatory biomarkers such as interleukin-6 (IL-6), IL-8, and IFN-γ levels. Also, total K18 (epithelial cell death marker) and ccK18 (epithelial apoptosis marker) levels are decreased after the infusion of MSCs, suggesting lung epithelial cell recovery [66]. The findings clearly suggest the beneficial effect of MSCs in lung-protective roles in ARDS. However, analysis has been done only on a few patients, and there is a need for investigations on more patients to conclude the clinical efficacy of the MSCs.
Matthay et al. conducted clinical trials on 60 patients, of which 40 were treated with MSCs, and the rest were controls. The study showed that there was no adverse effect due to MSC treatment. However, in the treatment group, the oxygenation index was higher in the case of MSC-treated patients. The expression of proinflammatory cytokines and chemokines was severely decreased in the treatment group [67]. Another study by Zheng et al. was conducted using hAD-MSCs for treating ARDS. The results showed a decrease in the levels of SP-D proteins and the inflammatory cytokine IL-6. Patients were followed up for 28 days after the treatment in order to record any adverse effects of the hAD-MSC infusion [68]. Most of the clinical symptoms were ameliorated by combination therapy, and the MSC treatment regime has a death rate of 17. Four patients were followed for more than 5 years and had no adverse effects. A multi-centered study was conducted in UK and USA, which was two doses open-label. All the patients were monitored for any adverse effects or changes in PO2/FiO2 levels [69].
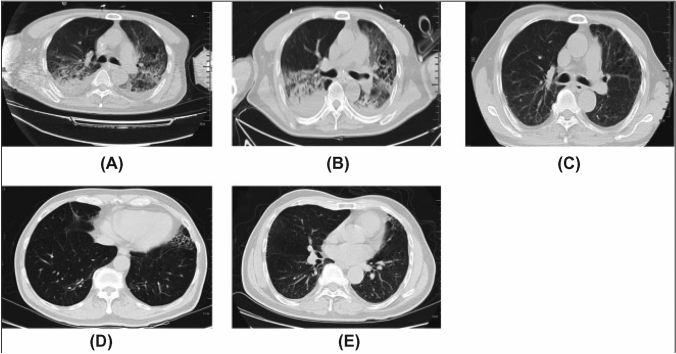
According to several clinical study reports, more than 80% of COVID patients had lymphopenia, and more than 50% of ICU patients had high levels of TNF-α and granulocyte colony-stimulating factors, two of the major causes of a cytokine storm [70]. Another study was conducted by Liu et al., where they investigated the clinical pathology and progression of SARS-CoV-2-infected ARDS patients. There were 109 COVID patients of the following categories: the elderly and those complicated with secondary diseases like diabetes, cerebrovascular disease, and kidney diseases. It was found that in each of these conditions, there are higher chances of acquiring SARS-CoV-2-induced ARDS [71]. Consequently, it is necessary to understand stem cell therapy for ARDS and ALI. A single-centered, open-labeled clinical trial was conducted for H7N9-induced ARDS in 17 patients. The mortality rate in the MSC-transplanted patients was significantly lowered in the experimental group. Before MSC treatment, the patients showed ground-glass opacities and fibrillations in the chest radiography images. After the treatment, radiographic images were followed up at different intervals, i.e., 1 week, 24 weeks, 1 year, and 5 years (Fig. 3). The patients started to improve after 1 week of treatment. Additionally, it was observed that MSC therapy did not have any adverse effects on the patients even after 5 years of follow-up (Fig. 3E) [72]. Hence, it can also be believed that stem cell-based therapies would provide beneficial results. The mode of action of stem cell therapies involves inhibiting the virus-induced cytokine storm by regulating macrophages (M1 phenotype), suppressing T-cells activation, increasing the concentration of Treg cells, suppressing dendritic cells, hampering NK cell-mediated cell lysis, affecting B-cell proliferation and antibody production, decreasing the levels of inflammatory factors, increasing anti-inflammatory factors, and regenerating injured tissues.
Fig. 3.
CT scanned images of four patients monitored for 5 years of MSC therapy—A Before MSC therapy, some fibrillation was observed. Radiologic changes included linear fibrosis, bronchiectasis, air bronchogram, ground-glass opacities isolated areas of pleural thickening, and hydrothorax post-MSC transplantation for B 1 week, C 24 weeks, D 1 year, and E 5 years. After MSC transplantation for 24 weeks and one year, all patients showed improvement on CT. Reproduced from [72] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
Averyanov et al. conducted the first-in-human high‐cumulative‐dose stem cell therapy for treating idiopathic pulmonary fibrosis (IPF). A high dose of BM-MSCs (1.6 × 109 cells/ml) was administrated to 20 patients [73]. The patients in the test group had increased lung function than the placebo group. The patients were monitored for 52 weeks to examine the adverse effects; however, no such adverse effects were observed [73]. Many in vivo and clinical studies exhibit that MSC-based therapies improve lung-related diseases like COPD, ARDS, and IPF. And the major symptoms of SARS-CoV2 is also inflammation of the lungs, pneumonia, and lung tissue damage. Since the pathophysiology of SARS-CoV2 is similar to ARDS and some other lung-related diseases, stem cell therapy for SARS-CoV2 might also provide similar results to ARDS. It also highlights that these therapies may be safe for COVID patients [74]. Table 3 also depicts evidence for using stem cell-based therapies against SARS-CoV2.
Table 3.
A detailed note on the clinical trials on stem cell therapy in ALI or ARDS
| Stem cell therapy | Route of delivery (dose) | Stage of the trial | No of patients | Results | Control | Country | Follow up period | References |
|---|---|---|---|---|---|---|---|---|
| AD-MSCs |
Intravenous (1 × 106 cells/kg) |
Phase I | 20 |
∙ Lower levels of IL-6 and SP-D (surfactant protein D) was observed ∙ No infusion toxicities or serious adverse events related to MSCs administration was observed |
Triple masking Placebo—Saline infusion |
China | 28 days | [139] |
|
Intravenous (1 × 106 cells/kg and 2 × 106 cells/kg) |
Phase I and II | 26 | – |
Quadruple masking Placebo—Saline infusion |
Spain | 1 year | [202] | |
| UC-MSCs |
Intravenous (1 × 106 cells/kg) |
– | 26 (Recruiting) | – | Placebo—Normal saline | China | 60 days | [203] |
|
Intravenous Low dose Medium dose High dose |
Phase I | 18 | – | Open label | Taiwan | 3 months | [204] | |
|
Intravenous (5 × 105 cells/kg) three doses per day |
Phase I and II | 20 | – | Open label | China | 14 days | [205] | |
|
Intravenous (5 × 107 cells/kg) and standard treatment |
Phase I and II | 40 | – | Quadruple masking Control—Standard treatment (hydroxychloroquine + Lopinavir/Ritonavir or Azithromycin and ventilation support) and placebo | Colombia | 6 months | [206] | |
| STem cells for ARDS Treatment (START) trials |
Phase I—Intravenous ∙ Low dose- 1 × 106 cells/kg ∙ Moderate dose- 5 × 106 cells/kg ∙ High dose-10 × 106 cells/kg Phase 2a—Intravenous ∙ High dose-10 × 106 cells/kg |
Phase I and Phase 2a completed |
Phase I—9 patients (3 in each category) Phase 2a—60 patients |
∙ All patients had no adverse effect on MSC infusion ∙ No significant changes were observed in mean serum creatinine, total bilirubin, ALT, arterial pressure ∙ 2 patients expired and one patient had a severe adverse effect (Not because of MSC transplantation) in Phase I and 1 patient died in Phase 2a for unrelated reasons ∙ Phase I showed a decline in lung injury score (LIS) was highest in the high dose patients ∙ Phase I showed reduced levels of IL-6, RAGE, and Ang-2 |
– | USA | 6 months | [59, 207, 208] |
| BM-MSCs | Intravenous (3 × 106 cells/kg) | Phase I completed | 20 | – | Open label | USA | 30 days | [209] |
| Intravenous | Phase II | 10 | – | Open label | South Korea | 28 days | [210] | |
| MB-MSCs | Intravenous (10 × 107 cells/kg) four times in 2 weeks | Phase I and II | 20 | – | Open label | China | 14 days | [28] |
| Cymera MSCs (iPSC and mesenchymoangioblast (MCA)-derived cells) | Intravenous (2 × 106 cells/kg) | Phase I and II | Recruiting (24) | – | Open label | Australia | 28 days | [98] |
Clinical studies of stem cell therapies in COVID-19
Clinical trials are essential for discovering any drug, therapy, or treatment and for considering the safety of the patients. Clinical trials reduce unwanted or inefficient medical treatment, which causes adverse reactions. The following are ongoing and completed stem cell-based therapies for COVID-19 listed on the U.S. National Library of Medicine (NLM) website, ClinicalTrials.gov. Clinical studies are ongoing worldwide, and researchers are trying to find solutions by discovering new drugs or therapies, or vaccines for COVID-19. In India, Stempeutics has successfully received approval from DCGI (Drugs Controller General of India) for phase 3 clinical trials for COVID treatment using BM-MSCs [75]. Ye et al. assessed the efficacy of human DP-MSCs in severely affected COVID-19 patients. Intravenous injection of DP-MSCs suspension (3.0 × 107 cells/dose) was provided thrice a week, while a placebo or saline was given to the control group. The assessment of the study was performed by counting immune cells, kidney and liver functionality tests, and immunological tests. These are some of the essential tests that provide information on whether stem cell therapies are effective and whether they cause any bystander effect on other surrounding microenvironments [76]. Safari et al. conducted a preclinical study by using a combination of BM-MSC along with nicorandil, which is an ATP-sensitive potassium channel opener. The combination of these two as therapeutic agents improved lung tissue damage and cell death [77]. Thus, this combinatorial approach might counteract COVID-19.
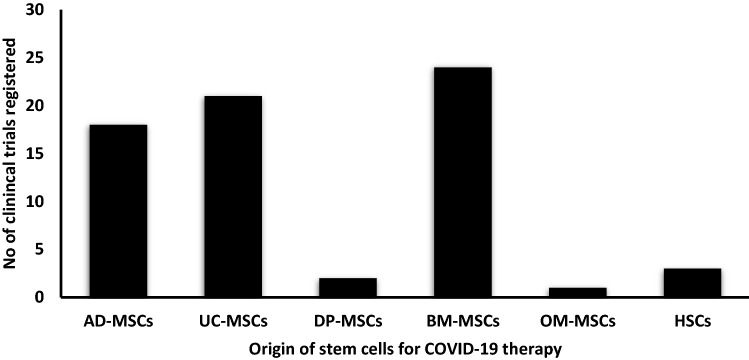
As of March 30th, 2022, there are 86 clinical trials registered on ClinicalTrials.gov. The table below is a comprehensive list of all the clinical studies (Supplementary Information Table S1) (Fig. 4) [78]. In our analysis, it is found that BM-MSCs (24 cases) and UC-MSCs (20 cases) are extensively used for stem cell-based therapy for COVID-19. However, now the question arises as to which stem cells could provide better results or promise better results and are suitable for the treatment. Or will there be any difference overall in treatment when stem cells are derived from different origins? Or which of these cells provides more concentration of anti-inflammatory by-products? Which cells are better obtained without many ethical issues? These are some of the questions that need to be answered when assessing both cells for treatments. Lee et al. compare the immunomodulatory effect of placenta-derived mesenchymal stem cells (P-MSCs) with BM-MSCs and AD-MSCs, and the study reveals that P-MSCs have much higher immunomodulatory capability than BM-MSCs and AD-MSCs [79]. However, the availability of P-MSCs is very scare due to a lack of awareness of placental stem cell banking is limited [80]. Furthermore, Atluri et al. also predict that hUC-MSCs could be the best source for COVID-19 treatments. UC-MSCs are better than BM-MSCs due to availability, scalability, faster doubling time, and non-invasive extraction [81]. Other than these two major cell types, few cases were also performed by cardiosphere derived cells (CDCs) [82], olfactory mucosal lining derived MSCs (OM-MSCs) [36], Haematopoietic stem cells (HSCs) [83], Stem Cell Educator-Treated Mononuclear Cells (SCE-MCs) [84], CAStem cells [Immunity- and matrix-regulatory cells (IMRCs) derived from ESCs] [85], P-MSCs [86], Extracorporeal derived MSC [87], DP-MSCs [88], AD-MSCs [89]. In the cases of CDCs, OM-MSCs, and HSCs, there is always a concern about whether these cells would differentiate into their respective lineages rather than perform their immunomodulation function. Furthermore, SCE-MCs function was quite different from the usual stem cell therapy. Stem Cell Educator (SCE) technology was patented by Tianhe Stem Cell Biotechnologies for treating autoimmune diseases like Type 1 diabetes, Alopecia Areata. SCE uses multipotent cord blood stem cells (CB-SCs). CB-SCs have proven their immunomodulatory properties in some autoimmune disorders. Because the properties of CB-SCs differ from those of other stem cells, researchers are attempting to incorporate these CB-SCs in order to combat COVID-19. However, this study is still in the preliminary stage [84]. Cell-free therapy has also undergone a clinical trial platform, and there were four studies that used exosomes or extracellular vesicles as their drug for intervention [90–93]. Out of these two studies was for the nasal route is their mode of drug delivery, which might be highly recommendable since coronavirus also targets the lung most [91, 92]. If the anti-inflammatory factors and growth factors from exosomes could target more of the lesions of the injured lung tissue, then this method may prove more advantageous than intravenous drug delivery.
Fig. 4.
Graph representing the clinical trials registered till 30th of March 2022 based on the origin of stem cells for COVID-19 therapy
Stem cell therapy has gained attention in the research community because of its self-renewal, pluripotency, and regenerative capacity. Moreover, there have been numerous studies on using stem cells for therapeutic usage for about two decades. Hence, many researchers have moved toward stem cell-based therapy for combating COVID-19. Many researchers have performed safety assessment clinical trials on the effect of stem cell secretions on COVID-infected tissues. Some studies have also shown evidence where pulmonary regeneration has happened similarly in vivo studies in humans. In vivo studies, the alveolar type II (AT2) has the capability to proliferate and differentiate into alveolar type I (AT1) cells mimicking the differentiation ability of stem cells. If stem cells get differentiated into AT2 cells, thereby enhancing pulmonary regeneration could be one of the beneficial improvements in achieving a cure for COVID [94]. Numerous researches have provided optimistic results [95].
A recent study conducted by Leng et al. demonstrated the potential clinical benefits of MSCs transplantation in 7 COVID-19 pneumonia patients (2 common, 4 severe, 1 critically severe) (Fig. 5). MSCs (1 × 106 cells/kg body weight) were transplanted by intravenous drip with saline, and patients were observed for 14-days. Remarkably, after 2–4 days of MSC transplantation, symptoms like pyrexia and dyspnea disappeared. Also, there was a decline in C-reactive protein level, aspartic aminotransferase, creatine kinase activity, and myoglobin, which suggested an immediate recovery of pulmonary function. Furthermore, mass cytometry (CyTOF) analysis showed a decrease in the number of active CXCR3+CD4+ T-cells, CXCR3+ NK cells, and CXCR3+CD8+ T-cells and an increase in CD14+CD11c+CD11bmid regulatory DC cell population. Similarly, TNF-α was also significantly decreased, while anti-inflammatory cytokine IL-10 level increased along with an increase of chemokines IP-10 and VEGF, which suggests the ability of MSCs in controlling cytokine release syndrome. Importantly, this is the first report that shows the negative gene expression of ACE2 and TMPRSS2 in MSCs, which suggests the safety of MSCs in COVID-19 treatment [96].
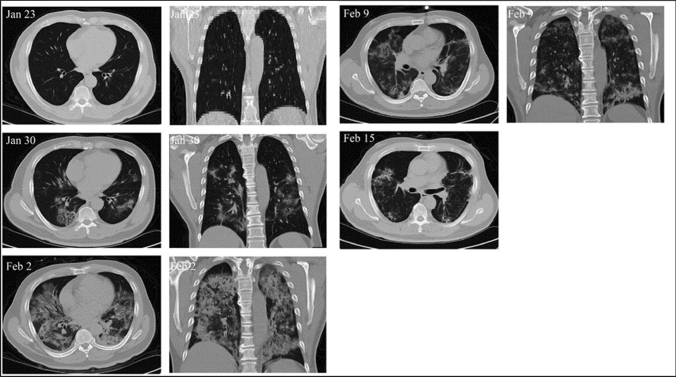
Fig. 5.
Examination of infected lungs of COVID-19 patient. On Jan 23, no signs of pneumonia were observed. On Jan 30, ground-glass opacity and pneumonia infiltration was observed on multiple lobes of the lungs. After 2 days of MSC treatment, on Feb 2, pneumonia invaded the whole lung, which had gradually reduced (Feb 9 and Feb 15). Reproduced from [96] and reprinted from Creative Commons Attribution License
Shu et al. conducted a clinical study on COVID patients using intravenous infusion of hUC-MSCs (Fig. 6) [97]. It was a single-center study where they found that the patients in the hUC-MSC treatment group recovered and improved, while three patients from the control group did not survive [97]. A similar study was also conducted by Meng et al., where they used UC-MSCs to treat 18 patients (9 in the treatment group and 9 in the control group). Both groups were provided with standard treatments. It was found that there was no adverse effect due to the administration of UC-MSCs. 2/9 of patients from the UC-MSC treatment group had shown symptoms of transient facial flushing and pyrexia. One of the patients suffered from transient hypoxia after 12 h of UC-MSCs administration [98].
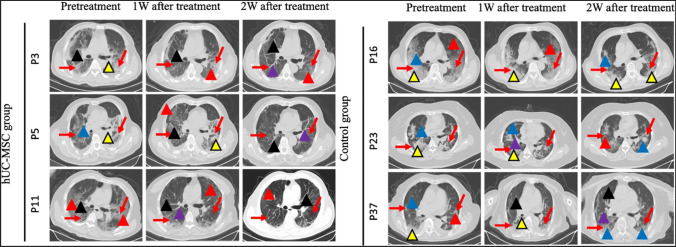
Fig. 6.
CT scanned images of hUC-MSC treated and control groups. CT imaging results for 6 patients at 3 time points (pretreatment, 1 week after treatment, and 2 weeks after treatment). The red arrows indicate inflammatory sites. The red triangles show the sites of the Crazy-paving pattern; the yellow triangles show the sites of consolidation; the blue triangles show the sites of GGO; the black triangles show the sites of interlobular septal thickening; the purple triangles show the sites of bronchial wall thickening. Reproduced from [97] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
Li et al. used menstrual blood from healthy females to extract mesenchymal stem cells for treating two critical COVID-infected patients [99]. These isolated cells were checked for genetic abnormalities by karyotyping. Menstrual blood-derived mesenchymal stem cells (MenSCs) were transplanted onto two patients, one 37-year-old female, and a 71-year-old male. Both these results showed promising results. This 37-year-old patient suffered from fever and dyspnea for almost 9 days and 4 days, respectively. Initially, drugs like oseltamivir and arbidol hydrochloride did not significantly improve the condition. Later, MB-MSCs infusion was administered, and symptoms gradually improved as levels of saturated O2, partial pressure of O2, and the fraction of inspired O2 improved. Along with these improvements, the lymphocyte counts increased, inflammation indicators decreased, initial chest CT scans showed large patchy high-density lesions, and after 6 and 10 days of treatment, absorption of the exudate lesions in the bilateral lungs was observed. MB-MSCs treatment was successful, and the patient was negative for COVID in the nucleic acid test. MB-MSC treatment also showed convincing results in 71-year-old male patients [99].
Another case in China, where COVID-19 pneumonia was treated by human umbilical cord Wharton’s jelly-derived MSCs. Before the treatment, the patient showed round-glass opacity and pneumonia filtration in both lungs. Along with this, a crazy-paving pattern was observed due to GGO with inter and intralobular septal thickening. Both these symptoms were reduced drastically after the intravenous infusion of WJ-MSCs. The patient improved significantly with a decrease in the symptoms, and gradual recovery of pulmonary function was observed post 1 week of discharge [100] (Fig. 7).
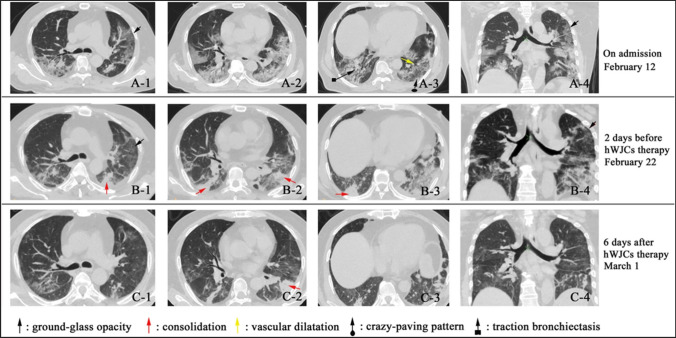
Fig. 7.
Chest CT images of the COVID-19 patients before and after stem cell treatment. I: CT images of the COVID-19 patient (A-1–A-4) before the treatment indicates ground-glass opacity (GGO), and pneumonia infiltration was observed throughout the lungs. B-1–B-4 images indicate the symptoms of the patient are slightly reduced, but the pneumonia was still significant. And after cell transplantation, images showed a decrease in pneumonia, lightening, and disappearance of ground-glass opacity. Reproduced from [100] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
In a study conducted by Singh et al., instead of the most widely used MSCs, they used Cardiosphere-derived cells (CDCs) [82]. CDCs are progenitor cells from heart tissue, and they have been used in clinical trials for myocardial infarction, heart failure, pulmonary arterial hypertension, Duchenne muscular dystrophy, and hypoplastic left heart syndrome. It is also proven that CDCs have immunomodulatory and anti-inflammatory functions, which are essential for treating the cytokine storm released by coronavirus-induced inflammation. Six patients were treated with CDC-based intravenous infusion. Four patients showed tremendous improvement. One patient had a slow recovery rate compared to the other four patients, and another 1/6 was the control. Levels of IL-1α and IL-1β did not vary much; however, levels of ferritin and C-reactive protein (CRP) decreased as expected. Even though the results are encouraging, however, there are certain concerns about using CDCs for COVID-19 treatment [82]. It has been shown that CDCs have limited ability to differentiate into non-cardiac lineage cells [101]. Hence, there are possibilities that it could differentiate into cardiac cells in other areas of the body, which is not desirable. Furthermore, it is also observed that CDCs have a higher potential for forming exosomes than MSCs, which concludes that CDCs could provide more beneficial results in cardiac-related stem cell therapies than COVID-19 [102].
Furthermore, various clinical cases demonstrated the success of stem cell therapy, and there was gradual commercialization via Pluristem Therapeutics, Inc. The company treated 7 patients suffering from ARDS and COVID-19-associated inflammations by administrating Pluristem’s PLX cells in 3 medical centers in Israel [103]. Another Australia-based company named Mesoblast also proves that MSC-based therapy has shown an 83% success rate in ventilator-driven COVID-19 patients [104]. Athersys, a commercial biotechnology-based company, has patented Multistem stem cell therapies for various other diseases like neurological, inflammatory, immune, and cardiovascular disease, along with treating COVID-19 [105, 106]. Similarly, there are some other companies that have been permitted by the FDA to conduct clinical trials, e.g., Cynata Therapeutics and Celltex Therapeutics [107, 108]. Cynanta is currently working on its Cymerus MSCs for Phase II trials, while Celltex is using AD-MSCs and is proceeding toward Phase II studies [109, 110].
Stem cell-derived extracellular vesicles (EV) or microvesicles (MV) or exosomes-based therapy for ARDS or COVID-19
Preclinical study of stem cell-derived EV or MV or exosomes-based therapy in ALI or ARDS
Another advancement of stem cell therapy is cell-free therapy using extracellular vesicles (EVs) of stem cells. Cell-free therapy is based on considering stem cells as a source for extracting therapeutic molecules such as EVs/MVs/exosomes instead of the whole stem cell as a therapeutic agent [111]. One of the advantages of cell-free therapies is these molecules can be formulated such that they can be administered as inhalants [112]. The International Society for Aerosols in Medicine (ISAM) recommends that in COVID-19, the primary route of infection is through the lungs, and inhalation as a route of delivery could lead to an effective treatment strategy [113]. Moreover, this route of delivery is being administered before the COVID-19 pandemic for other lung cancer diseases like asthma, COPD, cystic fibrosis, pneumonia, pulmonary hypertension, and ARDS [114]. Every cell ejects EVs in the form of exosomes (40–150 nm in diameter) or microvesicles (150–1000 nm in diameter) (MVs) [115]. The physiological function of EVs is communication, cell signaling, and defense [44]. MVs are products of exocytosis of the plasma membrane along with cellular components and cytoplasm, whereas exosomes originate from multivesicular bodies that are formed during endosomal maturation. Like MVs, exosomes also contain cellular components [116]. As mentioned earlier, stem cell-based therapy works on paracrine-based signaling. Similarly, EVs are regulated via the paracrine effect. However, one of the major advantages of cell-free therapies is that there is no risk of tumorigenicity and lower immunogenicity [115]. And there are various types of RNAs in the cytoplasm of a cell. Some of these RNAs are also likely to get trapped in the EVs. It was observed that EVs predominantly contain rRNAs, however, quantities of mRNAs, miRNAs, and tRNAs are relatively lesser [117]. The physiological and biological activities of exosomes are conserved and similar to those of their parent stem cells. EVs also possess anti-apoptotic, immunomodulatory, angiogenic, and tissue-regenerating functions [117]. Figure 8 depicts the immunopathology of COVID-19 and attempts to decipher the possible strategies of stem cells and their exosome-based therapy.
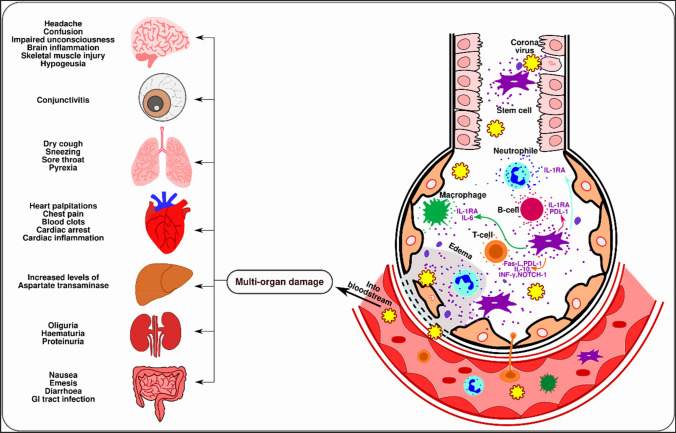
Fig. 8.
Immunopathogenesis of the coronavirus and the therapeutic potential of the MSC and their exosomes. Coronavirus infects ACE2-expressing cells and type II alveolar epithelial cells. The influx of T-cells, neutrophils, macrophages, and B-cells is induced by cytokines and chemokines secretion. The localization of the inflammatory cells at the injured site further leads to the production of pro-inflammatory cytokines, which is known as a cytokine storm, that acts as a major reason for ARDS in COVID-19. These inflammatory responses may cause lung fibrosis, apoptosis of the alveolar cells, edema, and organ failure. Immunomodulatory properties of stem cells are transferred to the injured site through exosomes that reduce inflammation-induced lung injury. The immunomodulatory properties of MSCs and their exosomes suppress inflammation and reduce inflammation-induced lung injury. Once the infected cells move into the bloodstream, the virus can move to many other organs causing various conditions. This figure was adapted from reference [118, 119]
For instance, Harting M et al. studied the cytokine profile of EVs, human PBMCs interaction with the exosomes of MSCs, and mouse splenocyte interaction with MSCs [120]. The cytokine profile of EVs showed 34 different inflammation-associated cytokines (sICAM-1, IL‐5, CCL5, IL‐6, IL‐10, CXCL12, IL‐13, RANTES, IP-10, Serpin E1). Furthermore, it was observed that the concentration of sICAM-1, CXCL12, and CCL5 were increased, and concentrations of IL‐10, IL‐6, IL‐5, and IL‐13 were reduced drastically. PBMCs interaction with EVs was performed to assess the uptake of EVs by granulocytes, lymphocytes, and monocytes. The order of uptake decreased in the following order: granulocytes, monocytes, and lymphocytes. Isolated splenocytes were activated using lipopolysaccharide (LPS) or concavalin A (ConA), which secreted IFN-γ and TNF-α when EVs interacted with activated splenocytes within 24 h, EVs reduced the concentration of IFN-γ and TNF-α [120]. The above-mentioned studies were in vitro-based studies; however, it should be considered that reciprocating the in vitro studies in vivo and then for clinical trials is the ultimate success for any experimental research. However, in vivo analysis of EVs might have provided intricate details, such as whether EVs induce tumorigenicity or other adverse effects.
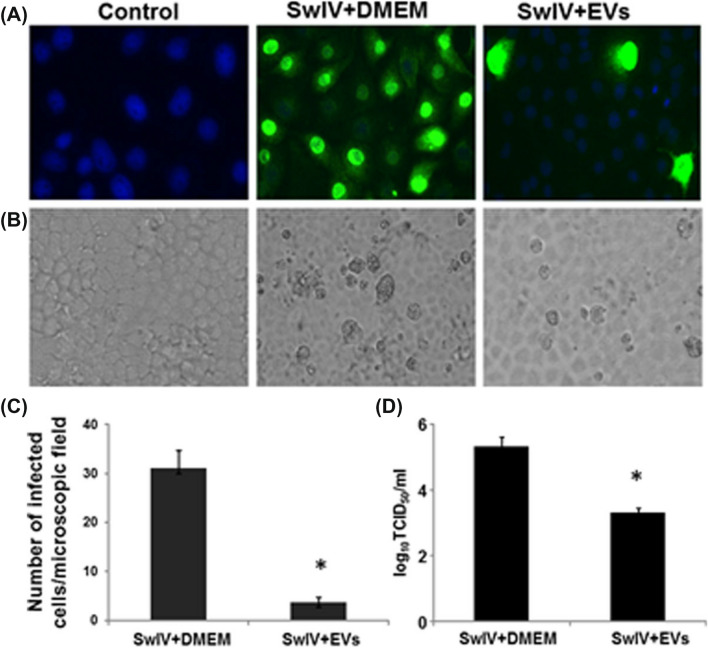
In a study conducted by Khatri et al., they studied the attenuation of the Influenza A-induced lung injury model in pigs by BM-MSCs-derived EVs [121] (Fig. 9). In vitro hemagglutination studies of different types of Influenza virus strains (swine/TX/98; H3N2, human/CA/09; H1N1, gull/MD/1995; H9N5, swine/MN/08; H1N1, chicken/NY/H7N2) were studied, and it was observed that EVs showed complete inhibition of hemagglutination activity within 1.25 and 5 μg/ml concentrations. MSC-EVs also drastically reduced the apoptosis of influenza-infected lung epithelial cells. In the SwIV-induced acute lung injury pig model, after 12 h of intratracheal administration of MSC-EVs (80 μg/kg body weight), there was a reduction of influenza virus infection by 100-folds. Anti-inflammatory cytokine IL-10 expression was increased while associated inflammatory cytokines (TNF-α and CXCL10) were reduced by BM-MSCs derived EVs [121]. EVs show improved results in treating respiratory-related diseases in in vitro and in vivo conditions. These studies show that there are possibilities for using cell-free therapies to treat ARDS for COVID-19, and Table 4 supports the use of cell-free therapies. In the current scenario, limited clinical trials are being performed using exosomes derived from MSCs.
Fig. 9.
SwIV influenza virus replication in lung epithelial cells. A, B Fluorescence and light microscopic images of Lung epithelial cells without influenza virus, Lung epithelial cells incubated with SwIV virus in DMEM media for 1 h, Lung epithelial cells incubated with SwIV virus subjected to 10 µg/ml MSC-EVs treatment for 1 h. C Graph representing the number of viral nucleoproteins expressed cells post 8 h of infection. D Virus titers of SwIV-infected cells and MSC-EV treated cells after 48 h of infection evaluated by titration performed using MDCK cells. Reproduced from [121] and reprinted from Creative Commons Attribution 4.0 licence (CC BY-4.0)
Table 4.
Comprehensive depiction of preclinical cases of using cell-free therapies in COVID-19
| Source of MSC-EV or MSC-MV or MSC-exosomes | Animal models | Type of injury model | Route of delivery and dosage | Key findings/outcome | References |
|---|---|---|---|---|---|
| hBM-MSCs-EVs | C57Bl/6 male mice | LPS induced | MVs released by 7.5 × 105 cells/ml |
∙ mtDNA transfer from MSC to alveolar macrophage diminished TNF-a and IL-8 production by 58 ± 8% and 30 ± 15% ∙ Levels of the M2 chemokines CCL18 (68 ± 21% reduction) and CCL22 (79 ± 12% reduction) |
[211] |
| C57Bl/6 male mice | E. coli endotoxin induced |
Intravenous 1 × 1010 EVs |
∙ EV increased phagocytic ability in immune cells ∙ EVs decreased MRP1 (multidrug resistant associated protein-1) expression and increased LTB4 (leukotriene B4) levels in BALF |
[212] | |
| hBM-MSCs-EVs from ARDS patients and healthy donors | CD1 male mice | LPS induced |
Three doses 0.8 × 103/ml 1.6 × 103/ml and 3.2 × 103/ml |
∙ EVsARDS had higher levels of (TβRI)/Alk5 and the Runx1 transcription factor | [213] |
| hBM-MSCs-EVs and mBM-MSCs-EVs | C57Bl/6 mice | Aspergillus hyphal induced allergic airway inflammation |
Intravenous EVs released by 3 × 107 cells/200 µl |
∙ Significantly ameliorated airway hyperreactivity, lung inflammation, and the antigen-specific CD4 T-cell Th2 and Th17 phenotype ∙ EVs from hBM-MSCs were more effective than hMSCs in reducing levels of IL-12 and chemokine keratinocyte chemoattractant (KC) in BALF ∙ Levels of IL-10 were increased by both EVs and stem cells |
[214] |
| hUC-MSC-EVs and hUC-MSC | Sprague–Dawley female rats | Hyperoxic induced |
Intratracheal Based on the severity of the rats—8 × 108 EVs/g 4.5 × 108 EVs/g 3 × 108 EVs/g |
∙ hUC-MSC-EVs and hUC-MSC reduced hypoxia induced damage ∙ EVs were better in terms of alveolarization and lung vascularization parameters |
[215] |
| hUC-MSC (naïve)-EVs and hUC-MSC (INF-γ activated)-EVs | Sprague–Dawley male rats | E. coli induced |
Intravenous 3–4 × 107 EVs |
∙ hUC-MSC (INF-γ activated)-EVs were more effective in attenuating than hUC-MSC (naive)-EVs ∙ Reduced alveolar protein leak, increased lung mononuclear phagocytes, enhanced endothelial nitric oxide synthase production |
[216] |
| hAD-MSC-EVs (young and aged donor) | C57Bl/6 mice | LPS induced |
Intravenous EVs—100 µg/200 µl hAD-MSC-EVs—1 × 106 cells/200 µl |
∙ Young hAD-MSC-EVs reduced the inflammatory cell accumulation and alveolar septal thickness ∙ Young hAD-MSC-EVs Significantly reduced protein, total cells, and neutrophils (by 37.4%, 43.2%, 42.8%, respectively) in the BALF ∙ Aged hAD-MSCs and aged hAD-MSC-EVs failed to show beneficial results |
[217] |
| hBM-MSCs-MVs | C57Bl/6 male mice | E. coli K1 induced |
Intravenous MVs released by 9 × 106 cells/90 µl |
∙ Enhanced monocyte phagocytosis of bacteria, reduced the influx of leukocytes by 40%, neutrophils by 53% and decreased the total protein concentration by 22% in the BALF ∙ Decreased inflammatory cytokine secretion and increased intracellular ATP levels in injured alveolar epithelial type 2 cells |
[218] |
| Ang-1 siRNA hBM-MSC MVs and hBM-MSC MVs | C57Bl/6 male mice | LPS induced |
Intratracheal MVs released by 1 × 106 cells/10 µl |
∙ Ang-1 mRNA deficient MVs increased the influx of neutrophils and MIP-2 levels in BALF ∙ bBM-MSC-MVs restored the pulmonary capillary permeability decreases levels of TNF-α and increased levels of IL-10 |
[219] |
| hMSCs-MVs | C57Bl/6 male mice | E. coli endotoxin induced |
Intratracheal MVs released by 7.5 × 105 cells/30 µl |
∙ MVs reduced extravascular lung water by 43% and reduced total protein levels in the BALF by 35% ∙ Reduced the influx of neutrophils and MIP-2 levels in the BALF by 73% and 49% respectively ∙ KGF siRNA pre-treated MSCs restricted the release of MVs thus states the importance of KGF |
[220] |
| hUC-MSC-MVs and downregulated miR100-hUC-MSC-MVs | Sprague–Dawley male rats | Bleomycin induced |
Intratracheal 1 × 106 MVs/10 µl |
∙ hUC-MSC-MVs elevated the miR-100 levels, whereas downregulated miR100-hUC-MSC-MVs inhibited the mi-R100 levels ∙ hUC-MSC-MVs reduced levels of LC3 II and Beclin-1 |
[221] |
| hUC-MSC derived exosome | BALB/C female mice | Avian influenza A (H5N1) induced |
Intravenous Exosomes released by 5 × 105 cells |
∙ hUC-MSCs are more efficient than hBM-MSCs in correcting impaired alveolar fluid clearance and alveolar protein permeability ∙ Reduced IL-6, IFN-β, IFN-γ1, MCP-1, ISG-15, IL-1β, IP-10, Mx-1, RANTES, and increased the levels of IL-4, IL-10, IL-11, IL-13, IL-1RA |
[44] |
| hAD-MSC derived exosomes | Sprague–Dawley rats | Hypoxia induced |
Intraperitoneal 3.4 × 109 exosomes/50 µl |
∙ Enhanced lung blood vessel density and reduce RV hypertrophy ∙ Exosome injection preserved alveolar growth in hyperoxia exposure rats |
[222] |
| mBM-MSCs derived exosomes and mBM-MSCs | Sprague–Dawley male rats | Intestinal ischemia–reperfusion induced |
Subcutaneous 5–10 μg/500 μl |
∙ Levels of pro-inflammatory cytokines like TNF-α, IL-6, and IL-1β were decreased ∙ Reduced expression of decreased TLR4 and NF-κB levels in rat lung tissue ∙ Both EVs and whole MSCs showed similar results |
[223] |
| mRNA-30b-3p-overexpressing mBM-MSCs derived exosomes | C57Bl/6 mice | LPS induction |
Intravenous 100 µg/200 µl |
∙ Alleviation was observed in edema and thickening of alveolar septum, alveolar hemorrhage, alveolar wall and inflammatory cell infiltration by mRNA-30b-3p overexpressing mBM-MSCs exosomes ∙ Reduction of SAA3, IL-1β, TNF-α, IL-6 and increase of IL-10 was greater in mRNA-30b-3p overexpressing mBM-MSCs exosomes than mBM-MSCs exosomes |
[224] |
| mAD-MSC derived exosomes | Sprague–Dawley male rats | Sepsis syndrome induction by cecal ligation and puncture |
Intravenous 100 µg purified from mAD-MSCs |
∙ Lower levels of CD11b/c/Ly6G/MIF (macrophage migration inhibitor factor) were found in blood and BALF | [225,226] |
Clinical studies of stem cell-derived EV or MV or exosome-based therapy in COVID-19
It is clear from the studies that exosome-based therapies can be more beneficial than stem cell-based therapies. Scientists have also tried many strategies to improve exosome contents for a better immunomodulatory effect. However, there are certain criteria to be considered when cell-free bodies are used for the treatment. It was found that extracellular vesicles have higher expression of tissue factor CD142 has been correlated to the severity of the infection [122]. CD142 acts as a procoagulant that initiates clotting leading to the systemic inflammatory response [123]. Thus, it is speculated that CD142 could be considered a biomarker for COVID-19 diagnosis. Furthermore, cell-free vesicles need to be thoroughly investigated for the absence of CD142 in the treatment regimens.
Researchers have hypothesized that using recombinant DNA technology increases the anti-inflammatory cytokines, increasing miRNA, which could further enhance the immunomodulatory effects. It has been estimated through MirTarget analysis that of 2565 miRNAs, miR-6864-5p, miR-4778-3p, and miR-5197-3p show high binding affinity and low Gibb’s free energy with the gRNA of MERS-CoV, SARS-CoV, and COVID-19 viruses [124]. Using recombinant DNA technology, if the expression of these miRNA is enhanced in stem cells and their exosomes, along with other anti-inflammatory factors, could possibly try to cure the disease. A similar study conducted with miRNA-377-3p showed a decrease in lung injury in the LPS-induced mice model. Although cell-free based therapies, some challenges need to be addressed, for instance, exosome homing in the specific injured regions. Sengulta et al. conducted a clinical trial by administrating ExoFlow (exosomes derived from BM-MSC). The results showed an 87% survival rate in the patients, and the rest of 16% of the patients expired due to reasons unrelated to the treatment. Efficacy of the exosomes was evaluated by the increase in the lymphocyte count by 46% and the reduction in the mean neutrophil count (32%), mean C-reactive protein (77%), ferritin (43%), and D-dimer (42%). These results provide positive hope for treating COVID-19 [90]. Table 5 shows the clinical cases of treating COVID-19 with cell-free therapies.
Table 5.
Clinical trials based on stem cell therapy EVs or exosomes for COVID-19
| Stem cell therapy | Route of delivery (dose) | Stage of the trial | No of patients | Results | Control | Country | Follow up period | References |
|---|---|---|---|---|---|---|---|---|
| Exosomes from MSCs | A single intravenous dosage of 15 ml | Completed | 24 |
i. 17 patients (71%) recovered ii. 3 patients (13%) remained critically ill and later stabilized iii. 4 patients (16%) died (Reasons unrelated to the treatment) iv. Significant improvements were observed in absolute neutrophil count, lymphocyte count, reduction in CRP, ferritin, and D-dimer |
[90] | |||
| Inhalation of aerosols five times per day (2 × 108 nanovesicles) | Completed | 24 | – | Open-label | China | 28 days | [91] | |
| Intravenous infusion of MSC-derived extracellular vesicles for 60 min | – | Not yet recruited | – | Intermediate-size population | – | – | [125] | |
|
Intravenous Group I—Escalating dose of MSC-exosomes delivered every other day: (2:4:8) (2 × 109, 4 × 109, 8 × 109/ml) Group II—Escalating dose of MSC-exosomes delivered every other day: (8:4:8) (8 × 109, 4 × 109, 8 × 109/ml) Group III—Escalating dose of MSC-exosomes delivered every other day: (8:8:8) (8 × 109, 8 × 109, 8 × 109/ml) Other Name: Ardoxso |
Phase I and II | Not yet recruited | – | Double masking | USA | 90 days | [126] | |
|
Inhalation Group I—Two times a day for 10 days, inhalation of 3 ml special solution containing 0.5–2 × 1010 of nanoparticles (exosomes) of the EXO I (first type) Group II—Two times a day for 10 days inhalation of 3 ml special solution containing 0.5–2 × 1010 of nanoparticles (exosomes) of the EXO II (second type) |
Phase I and II | Recruiting by invitation (90) | – | Group III—Placebo exosome-free solution inhalation | Russia | 30 days | [92] | |
| Extracellular vesicles from MSCs |
Intravenous Group II—10 ml ExoFlo and 90 ml saline Group III—15 ml ExoFlo and 85 ml saline |
Phase II | Recruiting (30) | – | Group I—100 ml saline | USA | 90 days | [127] |
|
Intravenous Group II—15 ml ExoFlo and 85 ml saline |
Phase I and II | Not yet recruited (60) | – | Group I—100 ml saline | USA | 61 days | [128] | |
| Exosome derived from human amniotic fluid (organicell flow) |
Intravenous Test group—2–5 × 1011 particles/ml injection volume 1 ml |
Phase I and II | Not yet recruited (20) | – | Double masking Placebo—1 ml saline | Russia | 60 days | [93] |
Precautions and criteria for using stem cells
The successful translation of stem cell-based therapies for COVID-19 depends on various factors related to the production, packaging, and delivery of stem cells. Isolation of stem cells, maintaining the stemness of cells, improving the stemness quality, characterization of stem cells, Good Manufacturing Product (GMP) handling, shelf life, the dosage of stem cells, and the half-life of stem cells. Stem cell therapies require a higher quantity and continuous supply for treatment. In this case, due to the compromised immune system of the patients, autologous stem cells are not the best option, hence, there is a requirement for pooled heterologous stem cells handled under the GMP facility [129]. As mentioned in the research, the average concentration of stem cells required for one dosage of intravenous injection of stem cells is one million cells or, in some cases, more than a million [35, 38, 130]. Multicentric clinical trials for stem cell therapy or cell-free therapies require billions and trillions of cells.
ESCs may not meet this condition since ESCs are derived from blastocytes’ inner cell mass, which is obtained after 5 days of culturing of the zygote [131]. It is unethical to extract an enormous number of stem cells for a particular trial or treatment. However, according to the pluripotency of ESCs, it might be the best candidate for stem cell therapies. Nevertheless, adult stem cells have produced convincing results. In the isolation of MSCs from bone marrow, it is found that repeated aspirations may decrease the yield of MSCs. Thus, initial aspiration is highly crucial for the culture and expansion of cells. MSCs yield also depends on an individual’s age and health status [131]. Properties of stem cells include the self-renewal ability to proliferate, differentiate, and regenerate. These properties depend on the stemness of the cell. It is recommended that the MSCs should have 3–5 passage numbers for stem cell therapies. This leads to risk in achieving the required quantity of cells by expansion affecting the stemness. On the other hand, there are studies that demonstrate that antioxidants, expression of the MRPS18-2 (mitochondrial ribosomal protein S18-2), expression of the retinoblastoma-associated protein (RB), ligand-receptor interaction between cell–cell adhesion through N-cadherin, and scrapie responsive gene 1 (SCRG1)/bone marrow stromal cell antigen-1 (BST1) could maintain the stemness of the cell [132–134]. These study results are obtained in small-scale studies, and the scale-up of these studies is cumbersome.
The development of clinical-grade stem cells for therapies should be conducted according to the GMPs. There are strict rules and regulations set by various organizations like the United States Food and Drug Administration (USFDA), the European Medicines Agency (EMA), the Japanese Pharmaceuticals and Medical Devices Agency (PMDA), and other regulatory agencies. GMPs ensure to scrutinize the potential risks involved and impose rules for manufacturing, testing, and application of the final product [129]. Stem cells must be evaluated for molecular cytogenetic disorders or aberrations to avoid oncogenic activities arising due to in vitro extensive expansion of cells. TF/CD142 expression must be significantly less in the stem cells as these proteins ameliorate the MSC’s hemocompatibility, thromboembolism, and massive intravascular thrombosis. Deficient TF/CD142 expression reduces anticoagulant activity in the in vivo system [135]. Some of the other essential tests include phenotypic pluripotency assays, histone modification, DNA methylation assays, and viral and bacterial contamination tests [136]. The shelf life of stem cells is very short since they cannot be passaged more than 3–5 times. However, stem cells can be cryopreserved and stored. Clinical-grade stem cells are a paramount requirement for these therapies since mishandling stem cells could cause adverse effects or even death in patients. Stem cell therapies are a boon with tons of restrictions. Even though many studies report the successful regeneration and recovery of patients worldwide, the fate of a stem cell inside the human body is unpredictable.
Conclusion
Worldwide prevailing studies on clinical trials are dominantly working on discovering new anti-viral drugs and vaccines, and very little research is being concentrated on stem cells or their exosomes as therapeutic candidates. This review article aims to focus light on these aspects for those considering these areas as potential candidates. Stem cell-based therapies have a paracrine effect on the nearby cell, which guides the damaged cells to regenerate and boosts the immune system by producing cytokines and chemokines. However, there is a certain hypothesis for stem cell-based researchers that is also encouraged by researchers nowadays. There are some similarities in the symptoms when compared to ARDS and COVID-19; moreover, stem cell-based therapies and cell-free (exosome) therapies have shown great recovery in these patients. Hence, researchers and clinicians have to invest in the idea of using these therapies for treating COVID-19. And several clinical studies mention that stem cells and their derivatives are safe and efficient.
Supplementary Information
Below is the link to the electronic supplementary material.
Abbreviations
- ACE-2
Angiotensin-converting enzyme-2
- AIV
Avian influenza virus
- ALI
Acute lung injury
- ALT
Alanine aminotransferase
- Ang-1
Angiopoietin-1
- Ang-2
Angiopoietin-2
- ARDS
Acute respiratory distress syndrome
- AT1
Alveolar epithelial cells type I
- AT2
Alveolar epithelial cells type II
- ATP
Adenosine triphosphate
- ATRA
All-trans retinoic acid
- BAL
Bronchoalveolar lavage
- BALF
Bronchoalveolar lavage fluid
- BD2
β-Defensin 2
- BM-MSCs
Bone marrow-mesenchymal stem cells
- BST1
Bone marrow stromal cell antigen-1
- cAMP
Cyclic adenosine monophosphate
- CAStem cells
Immunity- and matrix-regulatory cells (IMRCs) derived from embryonic stem cells
- CB-SCs
Cord blood stem cells
- ccK18
Epithelial apoptosis marker
- CCL18
Chemokine (C–C motif) ligand 18
- CCL2
Chemokine (C–C motif) ligand 2
- CCL22
Chemokine (C–C motif) ligand 22
- CD14+
Cluster of differentiation 14
- CD362+
Cluster of differentiation 362
- CD4+
Cluster of differentiation 4
- CD8+
Cluster of differentiation 8
- CDCs
Cardiosphere derived cells
- CINC-1
Cytokine-induced neutrophil chemoattractant-1
- COVID-19
Coronavirus Disease 2019
- CRP
C-reactive protein
- CXCL1
Chemokine (C-X-C motif) ligand 1
- Cyp17a1
Cytochrome P450 17A1 gene
- DPSCs
Dental pulp-derived mesenchymal stem cells
- EGF
Epidermal growth factor
- EMA
European Medicines Agency
- ERK
Extracellular-signal-regulated kinase
- ESCs
Embryonic stem cells
- EVs
Extracellular vesicles
- FGF
Fibroblast growth factor
- FGF-10
Fibroblast growth factor-10
- FN
Fibronectin
- GLP-1R
Glucagon-like peptide-1 receptor
- GM-CSF
Granulocyte–macrophage colony-stimulating factor
- GMP
Good Manufacturing Product
- HA-MSCs
Human amnion-derived mesenchymal stem cells
- hCMSCs
Human chorionic villi-derived mesenchymal stem cells
- HGF
Hepatocyte growth factor
- HIF-1
Hypoxia-inducible factor 1
- HSCs
Haematopoietic stem cells
- IDO
Indoleamine 2,3-dioxygenase
- IL-1
Interleukin-1
- IL-10
Interleukin-10
- IL-12p70
Interleukin-12p70
- IL-17
Interleukin-17
- IL-1RN
Interleukin-1 receptor antagonist gene
- IL-1α
Interleukin-1α
- IL-1β
Interleukin-1β
- IL-4
Interleukin-4
- IL-6
Interleukin-6
- INF-α
Interferon α
- IP10/CXCL-10
Interferon gamma-induced protein 10
- IPF
Idiopathic pulmonary fibrosis
- iPSCs
Induced pluripotent stem cells
- JNK
C-Jun N-terminal kinase
- K18
Epithelial cell death marker
- KC
Neutrophil chemokines KC
- KGF
Regenerating protein keratinocyte growth factor
- LPS
Lipid polysaccharide
- LRMSC
Lung-resident mesenchymal stem cells
- LTB4
Leukotriene B4
- LXA4
Lipoxin A4
- MB-MSC
Menstrual blood mesenchymal stem cells
- MCP-1
Monocyte chemoattractant protein-1
- MERS-CoV
Middle east respiratory syndrome coronavirus
- MIG
Monokine induced by IFN-γ
- MIP-1α
Macrophage Inflammatory Protein-1α
- MIP-2
Macrophage Inflammatory PROTEIN-2
- MLIs
Mean linear intercepts
- MPO
Myeloperoxidase
- mRNA
Messenger RNA
- MRP1
Multidrug-resistant associated protein-1
- MRPS18-2
Mitochondrial ribosomal protein S18-2
- mtDNA
Mitochondrial DNA
- MVs
Microvesicles
- NLM
National Library of Medicine
- NO
Nitric oxide
- Nol3
Nucleolar protein 3 gene
- Notch-1
Notch homolog 1, translocation-associated (Drosophila)
- Nr1h4
Nuclear receptor subfamily 1, group H, member 4 gene
- OF-MSC
Olfactory mucosa mesenchymal stem cells
- OM-MSCs
Olfactory mucosal lining derived MSCs
- PaCO2
Partial pressure of arterial carbon dioxide
- PaO2
Partial pressure of arterial oxygen
- PDL-1
Programmed death ligand-1
- PGE-2
Prostaglandin E2
- PMDA
Pharmaceuticals and Medical Devices Agency
- Prkg2
Protein kinase cGMP-dependent 2 gene
- RANTES/CCL5
Regulated on Activation, Normal T Expressed and Secreted
- RB
Retinoblastoma-associated protein
- RBD
Receptor-binding domain
- Rps6ka6
Ribosomal protein S6 kinase gene
- Runx1
Transcription factor
- SaO2
Saturation of arterial oxygen
- SARS-CoV2
Severe Acute Respiratory Syndrome Coronavirus-2
- SCE
Stem Cell Educator
- SCE-MCs
Stem Cell Educator-Treated Mononuclear Cells
- SCRG1
Scrapie responsive gene 1
- siRNA
Small interference RNA
- SPC
Specific surfactant protein C
- SP-D
Surfactant protein D
- TGFβ1
Transforming growth factor β1
- Th17
T helper 17 cells
- TIMP-1
Tissue inhibitor of metalloproteinases-1
- TLR-2
Toll-like receptors-2
- TLR4
Toll-like receptor 4
- TMPRSS2
Transmembrane serine protease 2
- TNF-α
Tumor necrotic factor α
- TNT
Tunneling nanotubes
- Treg
Regulatory T-cells
- TSG-6
Tumor necrosis factor-α-induced protein 6
- UCB-MSCs
Human Umbilical Cord Blood Mesenchymal Stem Cells
- UC-MSCs
Umbilical cord-derived stem cells
- USFDA
United States Food and Drug Administration
- VEGF
Vascular endothelial growth factor
- WJ-MSCs
Warton Jelly mesenchymal stem cells
Author contributions
DM drafted the manuscript, and RL revised and approved the manuscript.
Funding
Financial support from the Department of Biotechnology, New Delhi, for the DBT Ramalingaswami Re-entry Fellowship project (BT/RLF/Re-entry/44/2018) and Science and Engineering Research Board (SERB), New Delhi, for a Core Research Grant (CRG) (CRG/2020/001213), Board of Research in Nuclear Sciences (BRNS) 54/14/03/2022-BRNS/10207, and Vellore Institute of Technology (VIT) SEED GRANT 2020-21, 2021-2022 are kindly acknowledged.
Data availability
Not applicable.
Declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
Not applicable.
Consent to participate
Not applicable.
Consent to publication
Not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard | WHO coronavirus (COVID-19) dashboard with vaccination data. (2021). https://covid19.who.int/. Accessed 27 Apr 2021
- 2.Bottan NL, Vera-Cossio DA, Hoffmann B. The unequal impact of the coronavirus pandemic: evidence from seventeen developing countries. PLoS ONE. 2020 doi: 10.18235/0002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.U.C. Bureau (2021) Initial impact of COVID-19 on U.S. economy more widespread than on mortality
- 4.Astuti I. Ysrafil, severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2): an overview of viral structure and host response. Diabetes Metab Syndr. 2020 doi: 10.1016/j.dsx.2020.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tai W, He L, Zhang X, Pu J, Voronin D, Jiang S, Zhou Y, Du L. Characterization of the receptor-binding domain (RBD) of 2019 novel coronavirus: implication for development of RBD protein as a viral attachment inhibitor and vaccine. Cell Mol Immunol. 2020 doi: 10.1038/s41423-020-0400-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wu Y, Zhao S. Furin cleavage sites naturally occur in coronaviruses. Stem Cell Res. 2020 doi: 10.1016/j.scr.2020.102115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Simmons G, Zmora P, Gierer S, Heurich A, Pöhlmann S. Proteolytic activation of the SARS-coronavirus spike protein: cutting enzymes at the cutting edge of antiviral research. Antivir Res. 2013;100:605–614. doi: 10.1016/j.antiviral.2013.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villa A, Brunialti E, Dellavedova J, Meda C, Rebecchi M, Conti M, Donnici L, De Francesco R, Reggiani A, Lionetti V, Ciana P. DNA aptamers masking angiotensin converting enzyme 2 as an innovative way to treat SARS-CoV-2 pandemic. Pharmacol Res. 2022;175:105982. doi: 10.1016/j.phrs.2021.105982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Computational comparison of SARS-CoV-2 Delta and Omicron variant binding affinity with ACE2. (2021). https://www.news-medical.net/news/20211208/Computational-comparison-of-SARS-CoV-2-Delta-and-Omicron-variant-binding-affinity-with-ACE2.aspx. Accessed 24 Jan 2022
- 10.Kumar S, Thambiraja TS, Karuppanan K, Subramaniam G. Omicron and Delta variant of SARS-CoV-2: a comparative computational study of spike protein. J Med Virol. 2021 doi: 10.1002/JMV.27526. [DOI] [PubMed] [Google Scholar]
- 11.ACE2 angiotensin I converting enzyme 2 [Homo sapiens (human)]—Gene—NCBI. (2018). https://www.ncbi.nlm.nih.gov/gene/59272#gene-expression. Accessed 10 Sept 2020
- 12.Ng SC, Tilg H. COVID-19 and the gastrointestinal tract: more than meets the eye. Gut. 2020 doi: 10.1136/gutjnl-2020-321195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Francistiová L, Klepe A, Curley G, Gulya K, Dinnyés A, Filkor K. Cellular and molecular effects of SARS-CoV-2 linking lung infection to the brain. Front Immunol. 2021 doi: 10.3389/fimmu.2021.730088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu F, Han K, Blair R, Kenst K, Qin Z, Upcin B, Wörsdörfer P, Midkiff CC, Mudd J, Belyaeva E, Milligan NS, Rorison TD, Wagner N, Bodem J, Dölken L, Aktas BH, Vander Heide RS, Yin X-M, Kolls JK, Roy CJ, Rappaport J, Ergün S, Qin X. SARS-CoV-2 infects endothelial cells in vivo and in vitro. Front Cell Infect Microbiol. 2021 doi: 10.3389/fcimb.2021.701278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marshall M. COVID and the brain: researchers zero in on how damage occurs. Nature. 2021;595:484–485. doi: 10.1038/d41586-021-01693-6. [DOI] [PubMed] [Google Scholar]
- 16.Zhong J, Tang J, Ye C, Dong L. The immunology of COVID-19: is immune modulation an option for treatment? Lancet Rheumatol. 2020;2:e428–e436. doi: 10.1016/S2665-9913(20)30120-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeyanathan M, Afkhami S, Smaill F, Miller MS, Lichty BD, Xing Z. Immunological considerations for COVID-19 vaccine strategies. Nat Rev Immunol. 2020 doi: 10.1038/s41577-020-00434-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen IY, Moriyama M, Chang MF, Ichinohe T. Severe acute respiratory syndrome coronavirus viroporin 3a activates the NLRP3 inflammasome. Front Microbiol. 2019;10:50. doi: 10.3389/fmicb.2019.00050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tian S, Xiong Y, Liu H, Niu L, Guo J, Liao M, Xiao SY. Pathological study of the 2019 novel coronavirus disease (COVID-19) through postmortem core biopsies. Mod Pathol. 2020;33:1007–1014. doi: 10.1038/s41379-020-0536-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Fanelli V, Vlachou A, Ghannadian S, Simonetti U, Slutsky AS, Zhang H. Acute respiratory distress syndrome: new definition, current and future therapeutic options. J Thorac Dis. 2013;5:326–334. doi: 10.3978/j.issn.2072-1439.2013.04.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matthay MA, Thompson BT, Ware LB. The Berlin definition of acute respiratory distress syndrome: should patients receiving high-flow nasal oxygen be included? Lancet Respir Med. 2021;9:933–936. doi: 10.1016/S2213-2600(21)00105-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ball L, Silva PL, Giacobbe DR, Bassetti M, Zubieta-Calleja GR, Rocco PRM, Pelosi P. Understanding the pathophysiology of typical acute respiratory distress syndrome and severe COVID-19. Expert Rev Respir Med. 2022;16:437–446. doi: 10.1080/17476348.2022.2057300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mahmudpour M, Roozbeh J, Keshavarz M, Farrokhi S, Nabipour I. COVID-19 cytokine storm: the anger of inflammation. Cytokine. 2020;133:155151. doi: 10.1016/j.cyto.2020.155151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhao Q, Ren H, Han Z. Mesenchymal stem cells: immunomodulatory capability and clinical potential in immune diseases. J Cell Immunother. 2016;2:3–20. doi: 10.1016/j.jocit.2014.12.001. [DOI] [Google Scholar]
- 25.Wong VW, Sorkin M, Gurtner GC. Enabling stem cell therapies for tissue repair: current and future challenges. Biotechnol Adv. 2013;31:744–751. doi: 10.1016/j.biotechadv.2012.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laowtammathron C, Chingsuwanrote P, Choavaratana R, Phornwilardsiri S, Sitthirit K, Kaewjunun C, Makemaharn O, Terbto P, Waeteekul S, Lorthongpanich C, U-Pratya Y, Srisook P, Kheolamai P, Issaragrisil S. High-efficiency derivation of human embryonic stem cell lines using a culture system with minimized trophoblast cell proliferation. Stem Cell Res Ther. 2018;9:138. doi: 10.1186/s13287-018-0866-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Baghaei K, Hashemi SM, Tokhanbigli S, Rad AA, Assadzadeh-Aghdaei H, Sharifian A, Zali MR. Isolation, differentiation, and characterization of mesenchymal stem cells from human bone marrow. Gastroenterol Hepatol Bed Bench. 2017;10:208–213. doi: 10.22037/ghfbb.v0i0.1089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pisciotta A, Carnevale G, Meloni S, Riccio M, De Biasi S, Gibellini L, Ferrari A, Bruzzesi G, De Pol A. Human dental pulp stem cells (hDPSCs): isolation, enrichment and comparative differentiation of two sub-populations Integrative control of development. BMC Dev Biol. 2015;15:14. doi: 10.1186/s12861-015-0065-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mennan C, Wright K, Bhattacharjee A, Balain B, Richardson J, Roberts S. Isolation and characterisation of mesenchymal stem cells from different regions of the human umbilical cord. BioMed Res Int. 2013 doi: 10.1155/2013/916136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Pelekanos RA, Sardesai VS, Futrega K, Lott WB, Kuhn M, Doran MR. Isolation and expansion of mesenchymal stem/stromal cells derived from human placenta tissue. J Vis Exp. 2016;2016:54204. doi: 10.3791/54204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Spitzhorn LS, Rahman MS, Schwindt L, Ho HT, Wruck W, Bohndorf M, Wehrmeyer S, Ncube A, Beyer I, Hagenbeck C, Balan P. Isolation and molecular characterization of amniotic fluid-derived mesenchymal stem cells obtained from caesarean sections. Stem Cells Int. 2017 doi: 10.1155/2017/5932706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Call M, Meyer E, Kao W, Kruse F, Schloetzer-Schredhardt U. Hair follicle stem cell isolation and expansion. Bio-Protocol. 2018 doi: 10.21769/bioprotoc.2848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pavathuparambil Abdul Manaph N, Al-Hawaas M, Bobrovskaya L, Coates PT, Zhou XF. Urine-derived cells for human cell therapy. Stem Cell Res Ther. 2018;9:189. doi: 10.1186/s13287-018-0932-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hassiotou F, Beltran A, Chetwynd E, Stuebe AM, Twigger AJ, Metzger P, Trengove N, Lai CL, Filgueira L, Blancafort P, Hartmann PE. Breastmilk is a novel source of stem cells with multilineage differentiation potential. Stem Cells. 2012;30:2164–2174. doi: 10.1002/stem.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sánchez-Guijo F, García-Arranz M, López-Parra M, Monedero P, Mata-Martínez C, Santos A, Sagredo V, Álvarez-Avello J-M, Guerrero JE, Pérez-Calvo C, Sánchez-Hernández M-V, Del-Pozo JL, Andreu EJ, Fernández-Santos M-E, Soria-Juan B, Hernández-Blasco LM, Andreu E, Sempere JM, Zapata AG, Moraleda JM, Soria B, Fernández-Avilés F, García-Olmo D, Prósper F. Adipose-derived mesenchymal stromal cells for the treatment of patients with severe SARS-CoV-2 pneumonia requiring mechanical ventilation. A proof of concept study. EClinicalMedicine. 2020 doi: 10.1016/j.eclinm.2020.100454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Treatment of Covid-19 associated pneumonia with allogenic pooled olfactory mucosa-derived mesenchymal stem cells—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04382547?term=stem+cell&cond=sars-cov-2&draw=2&rank=13. Accessed 23 Aug 2020
- 37.Using human menstrual blood cells to treat acute lung injury caused by H7N9 bird flu virus infection—full text view—ClinicalTrials.gov. (2014). https://clinicaltrials.gov/ct2/show/NCT02095444?term=NCT02095444&draw=2&rank=1. Accessed 20 Sept 2020
- 38.Peng H, Gong T, Huang X, Sun X, Luo H, Wang W, Luo J, Luo B, Chen Y, Wang X, Long H, Mei H, Li C, Dai Y, Li H. A synergistic role of convalescent plasma and mesenchymal stem cells in the treatment of severely ill COVID-19 patients: a clinical case report. Stem Cell Res Ther. 2020;11:291. doi: 10.1186/s13287-020-01802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki T. Methods of induced pluripotent stem cells for clinical application. World J Stem Cells. 2015;7:116. doi: 10.4252/wjsc.v7.i1.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hwang NS, Varghese S, Elisseeff J. Controlled differentiation of stem cells. Adv Drug Deliv Rev. 2008;60:199–214. doi: 10.1016/j.addr.2007.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wang M, Yuan Q, Xie L. Mesenchymal stem cell-based immunomodulation: properties and clinical application. Stem Cells Int. 2018 doi: 10.1155/2018/3057624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhou Y, Yamamoto Y, Xiao Z, Ochiya T. The immunomodulatory functions of mesenchymal stromal/stem cells mediated via paracrine activity. J Clin Med. 2019;8:1025. doi: 10.3390/jcm8071025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Luz-Crawford P, Djouad F, Toupet K, Bony C, Franquesa M, Hoogduijn MJ, Jorgensen C, Noël D. Mesenchymal stem cell-derived interleukin 1 receptor antagonist promotes macrophage polarization and inhibits B cell differentiation. Stem Cells. 2016;34:483–492. doi: 10.1002/stem.2254. [DOI] [PubMed] [Google Scholar]
- 44.Weiss ARR, Dahlke MH. Immunomodulation by mesenchymal stem cells (MSCs): mechanisms of action of living, apoptotic, and dead MSCs. Front Immunol. 2019 doi: 10.3389/fimmu.2019.01191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang Y, Ge XH, Guo XJ, Bin Guan S, Li XM, Gu W, Xu WG. Bone marrow mesenchymal stem cells inhibit the function of dendritic cells by secreting galectin-1. BioMed Res Int. 2017 doi: 10.1155/2017/3248605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Negi N, Griffin MD. Effects of mesenchymal stromal cells on regulatory T cells: current understanding and clinical relevance. Stem Cells. 2020;38:596–605. doi: 10.1002/stem.3151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Andrukhov O, Behm C, Blufstein A, Rausch-Fan X. Immunomodulatory properties of dental tissue-derived mesenchymal stem cells: implication in disease and tissue regeneration. World J Stem Cells. 2019;11:604–617. doi: 10.4252/wjsc.v11.i9.604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Özdemir AT, Özgül Özdemir RB, Kırmaz C, Sarıboyacı AE, Ünal Halbutoğlları ZS, Özel C, Karaöz E. The paracrine immunomodulatory interactions between the human dental pulp derived mesenchymal stem cells and CD4 T cell subsets. Cell Immunol. 2016;310:108–115. doi: 10.1016/j.cellimm.2016.08.008. [DOI] [PubMed] [Google Scholar]
- 49.Martinez VG, Ontoria-Oviedo I, Ricardo CP, Harding SE, Sacedon R, Varas A, Zapata A, Sepulveda P, Vicente A. Overexpression of hypoxia-inducible factor 1 alpha improves immunomodulation by dental mesenchymal stem cells. Stem Cell Res Ther. 2017;8:208. doi: 10.1186/s13287-017-0659-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Grinnemo KH, Kumagai-Braesch M, Månsson-Broberg A, Skottman H, Hao X, Siddiqui A, Andersson A, Strömberg AM, Lahesmaa R, Hovatta O, Sylven C, Corbascio M, Dellgren G. Human embryonic stem cells are immunogenic in allogeneic and xenogeneic settings. Reprod Biomed Online. 2006 doi: 10.1016/S1472-6483(10)60663-3. [DOI] [PubMed] [Google Scholar]
- 51.Bulati M, Miceli V, Gallo A, Amico G, Carcione C, Pampalone M, Conaldi PG. The immunomodulatory properties of the human amnion-derived mesenchymal stromal/stem cells are induced by INF-γ produced by activated lymphomonocytes and are mediated by cell-to-cell contact and soluble factors. Front Immunol. 2020;11:54. doi: 10.3389/fimmu.2020.00054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Perlee D, Vos AF, Scicluna BP, Mancheño P, Rosa O, Dalemans W, Nürnberg P, Lombardo E, Poll T. Human adipose-derived mesenchymal stem cells modify lung immunity and improve antibacterial defense in pneumosepsis caused by Klebsiella pneumoniae. Stem Cells Transl Med. 2019;8:sctm.18-0260. doi: 10.1002/sctm.18-0260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Loy H, Kuok DIT, Hui KPY, Choi MHL, Yuen W, Nicholls JM, Peiris JSM, Chan MCW. Therapeutic implications of human umbilical cord mesenchymal stromal cells in attenuating influenza A(H5N1) virus-associated acute lung injury. J Infect Dis. 2019;219:186–196. doi: 10.1093/infdis/jiy478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chang YS, Choi SJ, Sung DK, Kim SY, Oh W, Yang YS, Park WS. Intratracheal transplantation of human umbilical cordblood-derived mesenchymal stem cells dose-dependently attenuates hyperoxia-induced lung injury in neonatal rats. Cell Transplant. 2011;20:1843–1854. doi: 10.3727/096368911x565038a. [DOI] [PubMed] [Google Scholar]
- 55.Sung DK, Chang YS, Sung SI, Yoo HS, Ahn SY, Park WS. Antibacterial effect of mesenchymal stem cells against Escherichia coli is mediated by secretion of beta-defensin-2 via toll-like receptor 4 signalling. Cell Microbiol. 2016 doi: 10.1111/cmi.12522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Devaney J, Horie S, Masterson C, Elliman S, Barry F, O’Brien T, Curley GF, O’Toole D, Laffey JG. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax. 2015;70:625–635. doi: 10.1136/thoraxjnl-2015-206813. [DOI] [PubMed] [Google Scholar]
- 57.Huang Z, Liu H, Zhang X, Wen G, Zhu C, Zhao Y, Niu W, Qin Y, Chen H, Bai C, Liu G. Transcriptomic analysis of lung tissues after hUC-MSCs and FTY720 treatment of lipopolysaccharide-induced acute lung injury in mouse models. Int Immunopharmacol. 2018;63:26–34. doi: 10.1016/j.intimp.2018.06.036. [DOI] [PubMed] [Google Scholar]
- 58.Xiang B, Chen L, Wang X, Zhao Y, Wang Y, Xiang C. Transplantation of menstrual blood-derived mesenchymal stem cells promotes the repair of lps-induced acute lung injury. Int J Mol Sci. 2017;18:1–16. doi: 10.3390/ijms18040689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Silva LHA, Antunes MA, Dos Santos CC, Weiss DJ, Cruz FF, Rocco PRM. Strategies to improve the therapeutic effects of mesenchymal stromal cells in respiratory diseases. Stem Cell Res Ther. 2018;9:1–9. doi: 10.1186/s13287-018-0802-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang L, Shi M, Tong L, Wang J, Ji S, Bi J, Chen C, Jiang J, Bai C, Zhou J, Song Y. Lung-resident mesenchymal stem cells promote repair of LPS-induced acute lung injury via regulating the balance of regulatory T cells and Th17 cells. Inflammation. 2019;42:199–210. doi: 10.1007/s10753-018-0884-6. [DOI] [PubMed] [Google Scholar]
- 61.Mokhber Dezfouli MR, Jabbari Fakhr M, Sadeghian Chaleshtori S, Dehghan MM, Vajhi A, Mokhtari R. Intrapulmonary autologous transplant of bone marrow-derived mesenchymal stromal cells improves lipopolysaccharide-induced acute respiratory distress syndrome in rabbit. Crit Care. 2018;22:1–13. doi: 10.1186/s13054-018-2272-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jackson MV, Morrison TJ, Doherty DF, McAuley DF, Matthay MA, Kissenpfennig A, O’Kane CM, Krasnodembskaya AD. Mitochondrial transfer via tunneling nanotubes is an important mechanism by which mesenchymal stem cells enhance macrophage phagocytosis in the in vitro and in vivo models of ARDS. Stem Cells. 2016;34:2210–2223. doi: 10.1002/stem.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Y, Xu J, Shi W, Chen C, Shao Y, Zhu L, Lu W, Han XD. Mesenchymal stromal cell treatment prevents H9N2 avian influenza virus-induced acute lung injury in mice. Stem Cell Res Ther. 2016;7:1–11. doi: 10.1186/s13287-016-0395-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chan MCW, Kuok DIT, Leung CYH, Hui KPY, Valkenburg SA, Lau EHY, Nicholls JM, Fang X, Guan Y, Lee JW, Chan RWY, Webster RG, Matthay MA, Peiris JSM. Human mesenchymal stromal cells reduce influenza A H5N1-associated acute lung injury in vitro and in vivo. Proc Natl Acad Sci USA. 2016;113:3621–3626. doi: 10.1073/pnas.1601911113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Orecchioni M, Ghosheh Y, Pramod AB, Ley K. Macrophage polarization: different gene signatures in M1(Lps+) vs. classically and M2(LPS−) vs. alternatively activated macrophages. Front Immunol. 2019;10:1084. doi: 10.3389/fimmu.2019.01084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Surgery C, Institutet K, Cell S, Mass P, Surgery C (2015) Tissue engineering and regenerative medicine in vivo effects of mesenchymal stromal cells in two patients with severe acute respiratory distress syndrome. 1199–1213 [DOI] [PMC free article] [PubMed]
- 67.Matthay MA, Calfee CS, Zhuo H, Thompson BT, Wilson JG, Levitt JE, Rogers AJ, Gotts JE, Wiener-Kronish JP, Bajwa EK, Donahoe MP, McVerry BJ, Ortiz LA, Exline M, Christman JW, Abbott J, Delucchi KL, Caballero L, McMillan M, McKenna DH, Liu KD. Treatment with allogeneic mesenchymal stromal cells for moderate to severe acute respiratory distress syndrome (START study): a randomised phase 2a safety trial. Lancet Respir Med. 2019;7:154–162. doi: 10.1016/S2213-2600(18)30418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zheng G, Huang L, Tong H, Shu Q, Hu Y, Ge M, Deng K, Zhang L, Zou B, Cheng B, Xu J. Treatment of acute respiratory distress syndrome with allogeneic adipose-derived mesenchymal stem cells: a randomized, placebo-controlled pilot study. Respir Res. 2014;15:1–10. doi: 10.1186/1465-9921-15-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bellingan G, Jacono F, Bannard-Smith J, Brealey D, Meyer N, Thickett D, Young D, Bentley A, McVerry B, Wunderink RG, Doerschug KC, Summers C, Rojas M, Jenkins ED, Ting A (2019) Primary analysis of a phase 1/2 study to assess MultiStem® cell therapy, a regenerative advanced therapy medicinal product (ATMP), in acute respiratory distress syndrome (MUST-ARDS), vol 174, p A7353. 10.1164/ajrccm-conference.2019.199.1_meetingabstracts.a7353
- 70.Zhao RC. Stem cell-based therapy for coronavirus disease 2019. Stem Cells Dev. 2020;29:679–681. doi: 10.1089/scd.2020.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Liu Y, Sun W, Li J, Chen L, Wang Y, Zhang L, Yu L. Clinical features and progression of acute respiratory distress syndrome in coronavirus disease 2019. medRxiv. 2020 doi: 10.1101/2020.02.17.20024166. [DOI] [Google Scholar]
- 72.Chen J, Hu C, Chen L, Tang L, Zhu Y, Xu X, Chen L, Gao H, Lu X, Yu L, Dai X, Xiang C, Li L. Clinical study of mesenchymal stem cell treatment for acute respiratory distress syndrome induced by epidemic influenza A (H7N9) infection: a hint for COVID-19 treatment. Engineering. 2020 doi: 10.1016/j.eng.2020.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Averyanov A, Koroleva I, Konoplyannikov M, Revkova V, Lesnyak V, Kalsin V, Danilevskaya O, Nikitin A, Sotnikova A, Kotova S, Baklaushev V. First-in-human high-cumulative-dose stem cell therapy in idiopathic pulmonary fibrosis with rapid lung function decline. Stem Cells Transl Med. 2020;9:6–16. doi: 10.1002/sctm.19-0037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Geiger S, Hirsch D, Hermann FG. Cell therapy for lung disease. Eur Respir Rev. 2017 doi: 10.1183/16000617.0044-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Bian L, Gao Q, Gao F, Wang Q, He Q, Wu X, Mao Q, Xu M, Liang Z. Impact of the Delta variant on vaccine efficacy and response strategies. Expert Rev Vaccines. 2021;20:1201–1209. doi: 10.1080/14760584.2021.1976153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ye Q, Wang H, Xia X, Zhou C, Liu Z, Xia ZE, Zhang Z, Zhao Y, Yehenala J, Wang S, Zhou G, Hu K, Wu B, Wu CT, Wang S, He Y. Safety and efficacy assessment of allogeneic human dental pulp stem cells to treat patients with severe COVID-19: structured summary of a study protocol for a randomized controlled trial (Phase I/II) Trials. 2020;21:520. doi: 10.1186/s13063-020-04380-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Safari A, Lionetti V, Razeghian-Jahromi I. Combination of mesenchymal stem cells and nicorandil: an emerging therapeutic challenge against COVID-19 infection-induced multiple organ dysfunction. Stem Cell Res Ther. 2021;12:404. doi: 10.1186/s13287-021-02482-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Search of: stem cells and COVID—list results—ClinicalTrials.gov. (2022). https://clinicaltrials.gov/ct2/results?cond=stem+cells+AND+COVID&term=&cntry=&state=&city=&dist=. Accessed 24 Jan 2022
- 79.Lee JM, Jung J, Lee H-J, Jeong SJ, Cho KJ, Hwang S-G, Kim GJ. Comparison of immunomodulatory effects of placenta mesenchymal stem cells with bone marrow and adipose mesenchymal stem cells. Int Immunopharmacol. 2012;13:219–224. doi: 10.1016/j.intimp.2012.03.024. [DOI] [PubMed] [Google Scholar]
- 80.Pisula A, Sienicka A, Stachyra K, Kacperczyk-Bartnik J, Bartnik P, Dobrowolska-Redo A, Romejko-Wolniewicz E. Women’s attitude towards umbilical cord blood banking in Poland. Cell Tissue Bank. 2021;22:587–596. doi: 10.1007/s10561-021-09914-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Atluri S, Manchikanti L, Hirsch JA. Expanded umbilical cord mesenchymal stem cells (UC-MSCs) as a therapeutic strategy in managing critically ILL COVID-19 patients: the case for compassionate use. Pain Physician. 2020;23:E71–E84. [PubMed] [Google Scholar]
- 82.Singh S, Chakravarty T, Chen P, Akhmerov A, Falk J, Friedman O, Zaman T, Ebinger JE, Gheorghiu M, Marbán L, Marbán E, Makkar RR. Allogeneic cardiosphere-derived cells (CAP-1002) in critically ill COVID-19 patients: compassionate-use case series. Basic Res Cardiol. 2020;115:36. doi: 10.1007/s00395-020-0795-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Role of immune and inflammatory response in recipients of allogeneic haematopoietic stem cell transplantation (SCT) affected by severe COVID19—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04349540?term=stem+cell&cond=sars-cov-2&draw=2&rank=22. Accessed 23 Aug 2020
- 84.Stem cell educator therapy treat the viral inflammation in COVID-19—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/study/NCT04299152?term=stem+cell&cond=sars-cov-2&draw=2&rank=26. Accessed 23 Aug 2020
- 85.Safety and efficacy of CAStem for severe COVID-19 associated with/without ARDS—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04331613?term=stem+cell&cond=sars-cov-2&draw=2&rank=49. Accessed 23 Aug 2020
- 86.Treatment of coronavirus COVID-19 pneumonia (pathogen SARS-CoV-2) with cryopreserved allogeneic P_MMSCs and UC-MMSCs—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/study/NCT04461925?term=stem+cell&cond=sars-cov-2&draw=2&rank=31. Accessed 23 Aug 2020
- 87.A study of cell therapy in COVID-19 subjects with acute kidney injury who are receiving renal replacement therapy—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04445220?term=stem+cell&cond=sars-cov-2&draw=2&rank=63. Accessed 23 Aug 2020
- 88.Novel coronavirus induced severe pneumonia treated by dental pulp mesenchymal stem cells—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/study/NCT04302519?term=stem+cell&cond=sars-cov-2&draw=2&rank=27. Accessed 23 Aug 2020
- 89.Clinical trial to assess the safety and efficacy of intravenous administration of allogeneic adult mesenchymal stem cells of expanded adipose tissue in patients with severe pneumonia due to COVID-19—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/NCT04366323?term=stem+cell&cond=sars-cov-2&draw=2&rank=1. Accessed 21 Aug 2020
- 90.Sengupta V, Sengupta S, Lazo A, Woods P, Nolan A, Bremer N. Exosomes derived from bone marrow mesenchymal stem cells as treatment for severe COVID-19. Stem Cells Dev. 2020;29:747–754. doi: 10.1089/scd.2020.0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.A pilot clinical study on inhalation of mesenchymal stem cells exosomes treating severe novel coronavirus pneumonia—no study results posted—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/results/NCT04276987. Accessed 4 Aug 2020
- 92.Evaluation of safety and efficiency of method of exosome inhalation in SARS-CoV-2 associated pneumonia—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04491240?term=stem+cell&cond=sars-cov-2&draw=2&rank=57. Accessed 23 Aug 2020
- 93.Organicell flow for patients with COVID-19—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/NCT04384445?term=NCT04384445&draw=2&rank=1. Accessed 23 Aug 2020
- 94.Chen J, Wu H, Yu Y, Tang N. Pulmonary alveolar regeneration in adult COVID-19 patients. Cell Res. 2020;30:708–710. doi: 10.1038/s41422-020-0369-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Shetty AK. Mesenchymal stem cell infusion shows promise for combating coronavirus (COVID-19)-induced pneumonia. Aging Dis. 2020;11:462–464. doi: 10.14336/AD.2020.0301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Leng Z, Zhu R, Hou W, Feng Y, Yang Y, Han Q, Shan G, Meng F, Du D, Wang S, Fan J, Wang W, Deng L, Shi H, Li H, Hu Z, Zhang F, Gao J, Liu H, Li X, Zhao Y, Yin K, He X, Gao Z, Wang Y, Yang B, Jin R, Stambler I, Lim LW, Su H, Moskalev A, Cano A, Chakrabarti S, Min KJ, Ellison-Hughes G, Caruso C, Jin K, Zhao RC. Transplantation of ACE2- mesenchymal stem cells improves the outcome of patients with covid-19 pneumonia. Aging Dis. 2020;11:216–228. doi: 10.14336/AD.2020.0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Shu L, Niu C, Li R, Huang T, Wang Y, Huang M, Ji N, Zheng Y, Chen X, Shi L, Wu M, Deng K, Wei J, Wang X, Cao Y, Yan J, Feng G. Treatment of severe COVID-19 with human umbilical cord mesenchymal stem cells. Stem Cell Res Ther. 2020;11:1–11. doi: 10.1186/s13287-020-01875-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Meng F, Xu R, Wang S, Xu Z, Zhang C, Li Y, Yang T, Shi L, Fu J, Jiang T, Huang L, Zhao P, Yuan X, Fan X, Zhang JY, Song J, Zhang D, Jiao Y, Liu L, Zhou C, Maeurer M, Zumla A, Shi M, Wang FS. Human umbilical cord-derived mesenchymal stem cell therapy in patients with COVID-19: a phase 1 clinical trial. Signal Transduct Target Ther. 2020 doi: 10.1038/s41392-020-00286-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tang L, Jiang Y, Zhu M, Chen L, Zhou X, Zhou C, Ye P, Chen X, Wang B, Xu Z, Zhang Q, Xu X, Gao H, Wu X, Li D, Jiang W, Qu J, Xiang C, Li L. Clinical study using mesenchymal stem cells for the treatment of patients with severe COVID-19. Front Med. 2020 doi: 10.1007/s11684-020-0810-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhang Y, Ding J, Ren S, Wang W, Yang Y, Li S, Meng M, Wu T, Liu D, Tian S, Tian H, Chen S, Zhou C. Intravenous infusion of human umbilical cord Wharton’s jelly-derived mesenchymal stem cells as a potential treatment for patients with COVID-19 pneumonia. Stem Cell Res Ther. 2020;11:207. doi: 10.1186/s13287-020-01725-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Oldershaw R, Owens WA, Sutherland R, Linney M, Liddle R, Magana L, Lash GE, Gill JH, Richardson G, Meeson A. Human cardiac-mesenchymal stem cell-like cells, a novel cell population with therapeutic potential. Stem Cells Dev. 2019;28:593–607. doi: 10.1089/scd.2018.0170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Li TS, Cheng K, Malliaras K, Smith RR, Zhang Y, Sun B, Matsushita N, Blusztajn A, Terrovitis J, Kusuoka H, Marbán L, Marbán E. Direct comparison of different stem cell types and subpopulations reveals superior paracrine potency and myocardial repair efficacy with cardiosphere-derived cells. J Am Coll Cardiol. 2012;59:942–953. doi: 10.1016/j.jacc.2011.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pluristem reports preliminary data from its COVID-19 compassionate use program, treating seven patients with acute respiratory failure Nasdaq:PSTI. (2020). https://www.globenewswire.com/news-release/2020/04/07/2012754/0/en/Pluristem-Reports-Preliminary-Data-from-its-COVID-19-Compassionate-Use-Program-Treating-Seven-Patients-with-Acute-Respiratory-Failure.html. Accessed 22 Aug 2020
- 104.Mesoblast reports 83% survival in ventilator-dependent COVID-19 patients following stem cell therapy | 2020-04-24 | BioWorld. (2020). https://www.bioworld.com/articles/434640-mesoblast-reports-83-survival-in-ventilator-dependent-covid-19-patients-following-stem-cell-therapy. Accessed 23 Aug 2020
- 105.MultiStem administration for COVID-19 induced ARDS (MACoVIA)—tabular view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/record/NCT04367077?term=stem+cell&cond=sars-cov-2&draw=2&rank=62. Accessed 23 Aug 2020
- 106.Athersys, Inc.—home. (2020). https://www.athersys.com/home/default.aspx. Accessed 23 Sept 2020
- 107.Cynata therapeutics kicks off COVID-19 trial enrolment. (2020). https://www.outsourcing-pharma.com/Article/2020/09/09/Cynata-Therapeutics-kicks-off-COVID-19-trial-enrollment. Accessed 23 Sept 2020
- 108.Celltex therapeutics gets FDA nod for Phase II Covid-19 trial. (2020). https://www.clinicaltrialsarena.com/news/celltext-fda-nod-phaseii-trial/. Accessed 23 Sept 2020
- 109.The MEseNchymal coviD-19 trial: a pilot study to investigate early efficacy of MSCs in adults with COVID-19—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/NCT04537351?term=NCT04537351&draw=2&rank=1. Accessed 20 Sept 2020
- 110.Autologous adipose-derived stem cells (AdMSCs) for COVID-19—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/study/NCT04428801?term=stem+cell&cond=sars-cov-2&draw=2&rank=4. Accessed 21 Aug 2020
- 111.Evangelista A, Soares MBP, Villarreal CF. Cell-free therapy: a neuroregenerative approach to sensory neuropathy? Neural Regen Res. 2019;14:1383–1384. doi: 10.4103/1673-5374.253522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Popowski KD, Dinh P-UC, George A, Lutz H, Cheng K. Exosome therapeutics for COVID-19 and respiratory viruses. VIEW. 2021;2:20200186. doi: 10.1002/VIW.20200186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mitchell JP, Berlinski A, Canisius S, Cipolla D, Dolovich MB, Gonda I, Hochhaus G, Kadrichu N, Lyapustina S, Mansour HM, Darquenne C, Clark AR, Newhouse M, Ehrmann S, Humphries R, Boushey H. Urgent appeal from international society for aerosols in medicine (ISAM) during COVID-19: clinical decision makers and governmental agencies should consider the inhaled route of administration: a statement from the ISAM Regulatory and Standardization Issues Networking Group. J Aerosol Med Pulm Drug Deliv. 2020;33:235–238. doi: 10.1089/jamp.2020.1622. [DOI] [PubMed] [Google Scholar]
- 114.Eedara BB, Alabsi W, Encinas-Basurto D, Polt R, Ledford JG, Mansour HM. Inhalation delivery for the treatment and prevention of COVID-19 infection. Pharmaceutics. 2021;13:1077. doi: 10.3390/pharmaceutics13071077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Yin K, Wang S, Zhao RC. Exosomes from mesenchymal stem/stromal cells: a new therapeutic paradigm. Biomark Res. 2019;7:8. doi: 10.1186/s40364-019-0159-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Stahl PD, Raposo G. Extracellular vesicles: exosomes and microvesicles, integrators of homeostasis. Physiology (Bethesda, Md.) 2019;34:169–177. doi: 10.1152/physiol.00045.2018. [DOI] [PubMed] [Google Scholar]
- 117.Gomzikova MO, James V, Rizvanov AA. Therapeutic application of mesenchymal stem cells derived extracellular vesicles for immunomodulation. Front Immunol. 2019 doi: 10.3389/fimmu.2019.02663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.MahsaTaghavi-Farahabadia MH, Mahmoudib M, Soudic S, Hashemi SM. Hypothesis for the management and treatment of the COVID-19-induced acute respiratory distress syndrome and lung injury using mesenchymal stem cell-derived exosomes. Med Hypotheses. 2020;144:109865. doi: 10.1016/j.mehy.2020.109865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bhaskar S, Sinha A, Banach M, Mittoo S, Weissert R, Kass JS, Rajagopal S, Pai AR, Kutty S. Cytokine storm in COVID-19—immunopathological mechanisms, clinical considerations, and therapeutic approaches: the REPROGRAM Consortium Position Paper. Front Immunol. 2020 doi: 10.3389/fimmu.2020.01648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Harting MT, Srivastava AK, Zhaorigetu S, Bair H, Prabhakara KS, Toledano Furman NE, Vykoukal JV, Ruppert KA, Cox CS, Olson SD. Inflammation-stimulated mesenchymal stromal cell-derived extracellular vesicles attenuate inflammation. Stem Cells. 2018;36:79–90. doi: 10.1002/stem.2730. [DOI] [PubMed] [Google Scholar]
- 121.Khatri M, Richardson LA, Meulia T. Mesenchymal stem cell-derived extracellular vesicles attenuate influenza virus-induced acute lung injury in a pig model. Stem Cell Res Ther. 2018 doi: 10.1186/s13287-018-0774-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Burrello J, Caporali E, Gauthier LG, Pianezzi E, Balbi C, Rigamonti E, Bolis S, Lazzarini E, Biemmi V, Burrello A, Frigerio R, Martinetti G, Fusi-Schmidhauser T, Vassalli G, Ferrari E, Moccetti T, Gori A, Cretich M, Melli G, Monticone S, Barile L. Risk stratification of patients with SARS-CoV-2 by tissue factor expression in circulating extracellular vesicles. Vascul Pharmacol. 2022;145:106999. doi: 10.1016/j.vph.2022.106999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Balbi C, Burrello J, Bolis S, Lazzarini E, Biemmi V, Pianezzi E, Burrello A, Caporali E, Grazioli LG, Martinetti G, Fusi-Schmidhauser T, Vassalli G, Melli G, Barile L. Circulating extracellular vesicles are endowed with enhanced procoagulant activity in SARS-CoV-2 infection. EBioMedicine. 2021;67:103369. doi: 10.1016/j.ebiom.2021.103369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Ivashchenko ARakhmetullina A, Aisina D (2020) How miRNAs can protect humans from coronaviruses COVID-19, SARS-CoV, and MERS-CoV. 10.21203/rs.3.rs-16264/v1
- 125.Expanded access protocol on bone marrow mesenchymal stem cell derived extracellular vesicle infusion treatment for patients with COVID-19 associated ARDS—full text view—ClinicalTrials.gov. (2020). https://clinicaltrials.gov/ct2/show/NCT04657458?term=NCT04657458&draw=2&rank=1. Accessed 23 Apr 2021
- 126.The use of exosomes for the treatment of acute respiratory distress syndrome or novel coronavirus pneumonia caused by COVID-19—tabular view—ClinicalTrials.gov. (2021). https://clinicaltrials.gov/ct2/show/record/NCT04798716?term=NCT04798716&draw=2&rank=1. Accessed 23 Apr 2021
- 127.NCT05125562, bone marrow mesenchymal stem cell derived extracellular vesicles infusion treatment for mild-to-moderate COVID-19: a Phase II clinical trial—full text view—ClinicalTrials.gov. (2021). https://clinicaltrials.gov/ct2/show/NCT05125562?term=NCT05125562&draw=2&rank=1. Accessed 28 Dec 2021
- 128.NCT05116761, ExoFloTM infusion for post-acute COVID-19 and chronic post-COVID-19 syndrome—full text view—ClinicalTrials.gov. (2021). https://clinicaltrials.gov/ct2/show/NCT05116761?term=NCT05116761&draw=2&rank=1. Accessed 28 Dec 2021
- 129.Rohani L, Johnson AA, Naghsh P, Rancourt DE, Ulrich H, Holland H. Concise review: molecular cytogenetics and quality control: clinical guardians for pluripotent stem cells. Stem Cells Transl Med. 2018;7:867–875. doi: 10.1002/sctm.18-0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Wilson JG, Liu KD, Zhuo H, Caballero L, McMillan M, Fang X, Cosgrove K, Vojnik R, Calfee CS, Lee JW, Rogers AJ, Levitt J, Wiener-Kronish J, Bajwa EK, Leavitt A, McKenna D, Thompson BT, Matthay MA. Mesenchymal stem (stromal) cells for treatment of ARDS: a phase 1 clinical trial. Lancet Respir Med. 2015;3:24–32. doi: 10.1016/S2213-2600(14)70291-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.de Wert G, Mummery C. Human embryonic stem cells: research, ethics and policy. Hum Reprod. 2003;18:672–682. doi: 10.1093/humrep/DEG143. [DOI] [PubMed] [Google Scholar]
- 132.Liao N, Shi Y, Zhang C, Zheng Y, Wang Y, Zhao B, Zeng Y, Liu X, Liu J. Antioxidants inhibit cell senescence and preserve stemness of adipose tissue-derived stem cells by reducing ROS generation during long-term in vitro expansion. Stem Cell Res Ther. 2019;10:306. doi: 10.1186/s13287-019-1404-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Mushtaq M, Kovalevska L, Darekar S, Abramsson A, Zetterberg H, Kashuba V, Klein G, Arsenian-Henriksson M, Kashuba E. Cell stemness is maintained upon concurrent expression of RB and the mitochondrial ribosomal protein S18–2. Proc Natl Acad Sci USA. 2020;117:15673–15683. doi: 10.1073/pnas.1922535117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Chosa N, Ishisaki A. Two novel mechanisms for maintenance of stemness in mesenchymal stem cells: SCRG1/BST1 axis and cell–cell adhesion through N-cadherin. Jpn Dent Sci Rev. 2018;54:37–44. doi: 10.1016/j.jdsr.2017.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Oeller M, Laner-Plamberger S, Hochmann S, Ketterl N, Feichtner M, Brachtl G, Hochreiter A, Scharler C, Bieler L, Romanelli P, Couillard-Despres S, Russe E, Schallmoser K, Strunk D. Selection of tissue factor-deficient cell transplants as a novel strategy for improving hemocompatibility of human bone marrow stromal cells. Theranostics. 2018;8:1421–1434. doi: 10.7150/thno.21906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zakrzewski W, Dobrzyński M, Szymonowicz M, Rybak Z. Stem cells: past, present, and future. Stem Cell Res Ther. 2019;10:68. doi: 10.1186/s13287-019-1165-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Not applicable.