Abstract
Background and Objectives
Acute COVID-19 infection has been associated with neurological involvement. We report a case series of newly diagnosed patients with multiple sclerosis (MS) and neuromyelitis optica spectrum disorder (NMOSD) developed in a short period of time after acute COVID-19 infection.
Methods
New MS patients developing initial symptoms shortly after an acute COVID-19 infection were diagnosed based on the 2017 McDonald Criteria [Garcia-Ramos et al. in Cells, 2021]. The patients diagnosed with NMOSD met the 2015 International Panel criteria for the diagnosis of NMOSD (IPDN) [Thompson et al. in Lancet Neurol 17:162–173, 2018].
Results From the MS Patient Group
Ten patients were included who had developed initial MS symptoms after COVID-19 infection. Gender distribution was equal (50% male). The mean age was 28 (range 17–39) years. Average time to neurological presentation was between 2 and 6 weeks following acute COVID-19 infection. Partial transverse myelitis was the initial presentation in 40% of the cases, and 60% of patients had spinal cord lesions present at the moment of diagnosis. All patients showed enhancing lesions on brain magnetic resonance imaging (MRI). The presence of cerebrospinal fluid (CSF) oligoclonal bands was found in all six tested cases. The majority of patients (80%) were unvaccinated for COVID-19. The two vaccinated patients had received two doses of the monovalent COVID-19 messenger ribonucleic acid (mRNA) (Pfizer Biotech) vaccine and no booster, a year prior to contracting COVID-19.
Results From the NMOSD Group
Two patients with NMOSD were included. Positive aquoporin-4 protein antibody (AQP-4 Ab) was detected in serum in both cases [one Enzyme Linked immunosorbent assay (ELISA) and one cell based]. Both patients had mild COVID-19 infection prior to presentation, initial neurologic symptoms presented between 3 and 6 weeks after COVID-19 infection. Neither patients were vaccinated. Both responded partially to steroids, one developed a relapse 40 days after diagnosis.
Conclusion
COVID-19 infection has been linked to several neurological and immune-driven conditions. This study suggests that in susceptible individuals, acute COVID-19 infection may act as a trigger for developing MS and NMOSD.
Keywords: Multiple sclerosis, Neuromyelitis optica spectrum disorder, COVID-19
Key Summary Points
| There has been an increased number of autoimmune diseases associated with acute COVID-19 infection. |
| Previous Epstein–Barr infection has been associated with a higher risk of developing multiple sclerosis. |
| Acute COVID-19 infection has been associated with a hyperinflammatory immune state, which could mask or trigger autoimmunity. |
| In this case series we describe a group of patients who developed multiple sclerosis and neuromyelitis optica spectrum disorder after acute COVID-19 infection. |
Introduction
Neurological manifestations are common during and following COVID-19 infection [1]. The development of autoimmune diseases in this setting is less frequently reported; however, COVID-19 infection has been recognized as a trigger for multiple sclerosis (MS) exacerbation [1]. More than one-third of patients with COVID-19 develop neurological complications during the acute phase, and more than one-third develop neuropsychiatric complications as long as 6 months after infection [2]. There is increasing evidence that COVID-19 infection may lead to a dysregulation of the immune system, with the development of autoimmune disease. There several supposed mechanisms underlying the association between viruses and autoimmunity, including cross-reactive T-cell recognition, known as molecular mimicry, as well as bystander T-cell activation, ending in epitope dispersion, being the predominant mechanisms by which infection can lead to a T-cell-mediated autoimmune response. Another hypothesis is virus-induced decoy of the immune system, which ends up triggering autoimmunity [3]. We report a group of ten patients who were diagnosed with MS after an acute COVID-19 infection, as well as two cases of newly diagnosed neuro myelitis optica spectrum disorder (NMOSD) following the infection.
Methods
Patients who fulfilled the 2017 Mc Donald criteria for MS [4], presenting between weeks two and six after COVID-19 infection were included. Patients with previous MS diagnosis or report of neurologic symptoms suggestive of MS prior to COVID-19 infection were excluded. Demographic data as well as clinical and radiographic information were obtained. The recruitment period was from June 2021 to June 2022 in patients seen in the Texas Tech. University Health Sciences Center MS Clinic.
Findings from magnetic resonance imaging (MRI) were interpreted by a certified neuroradiologist. Following the reviewer’s observations, the studies and samples were subjected to additional evaluation by independent observers.
Patient’s who met the International Panel criteria for the diagnosis of NMOSD (IPDN) [5] after acute COVID-19 infection were included in the NMOSD patient group.
Criteria for COVID-19 severity was based on the National Institutes of Health (NIH) COVID-19 treatment guidelines [6].
All participants consented to participate in the study, and provided approval for their clinical information and images to be published. The study was approved by the institutional review board (IRB). COVID-19 infection was documented with a rapid antigen test in the hospital or outpatient clinic, and the results registered in the patient’s medical records. These patients were infected with COVID-19 at the peak of the Delta variant pandemic. The viral lineage was not identified.
Results from MS Patient Group
Ten patients were included who fulfilled the 2017 McDonald diagnostic criteria for MS. They had developed initial neurological symptoms between 2 and 6 weeks after an active COVID-19 infection. From this group, 80% of patients were not vaccinated for COVID-19. Two patients had received two doses of the monovalent COVID-19 messenger ribonucleic acid (mRNA) vaccine (Pfizer Biotec), with no booster between 10 and 12 months before the active COVID-19 infection. The sex ratio was 1:1. The mean age was 28 years (ranged 17–39 years) Demographic information is presented in Table 1. Most patients (90%) had mild COVID-19 symptoms including loss of taste, and mild flu-like symptoms including mild headache and myalgias. Only one patient had nausea, vomiting, and fever with no loss of smell or taste. None of the patients were hospitalized due to severe COVD-19 symptoms.
Table 1.
Demographic profile of patients with multiple sclerosis
| Demographic profile | Number of patients | Percentage |
|---|---|---|
| Age, years | ||
| ≤ 20 | 1 | 10 |
| 21–30 | 4 | 40 |
| 31–40 | 5 | 50 |
| Race | ||
| Hispanic | 3 | 30 |
| White | 4 | 40 |
| African American | 3 | 30 |
| Gender | ||
| Female | 5 | 50 |
| Male | 5 | 50 |
| COVID vaccination | ||
| Fully vaccinated | 2 | 20 |
| Non-vaccinated | 8 | 80 |
| Family history of multiple sclerosis | ||
| Positive | 4 | 40 |
| Negative | 6 | 60 |
Clinical manifestation of MS developed between 2 and 6 weeks of acute COVID-19 infection. Neurological presentation was variable (Table 2), with 40% of patients presenting with spinal cord involvement (partial transverse myelitis), 40% with optic neuritis, and two patients with brainstem and/or cerebellar involvement. Epstein–Barr virus Immunoglobulin G (Ig) was positive in all six patients that were tested. Cerebrospinal fluid (CSF) analysis was positive for oligoclonal bands in all tested cases. The majority (80%) of patients had good response to steroids [1 g of methylprednisolone intravenously (IV) for 5 days], with 90% improvement of neurological symptoms in 2 weeks.
Table 2.
Presenting symptoms at the moment of diagnosis in the group of patients with multiple sclerosis
| Presenting symptoms and laboratory findings | N (%) |
|---|---|
| Optic neuritis | 4 (40%) |
| Transverse myelitis | 4 (40%) |
| Brainstem, cerebellum | 2 (20%) |
| CSF oligoclonal bands* | 10 (100%) |
| CSF increased IgG index and synthesis rate > * | 8 (80%) |
*From total patients that had lumbar puncture done (N = 6)
Two male patients of African descent had incomplete recovery after IV solumedrol: both remained with gait disturbances. Nine out of the ten patients were started on disease-modifying therapy: seven on anti-B-cell therapy (ofatumumab), one on glatiramer acetate, and one on natalizumab. MRI images of three patients diagnosed with multiple sclerosis (Figs. 1, 2 and 3) are presented for illustration.
Fig. 1.
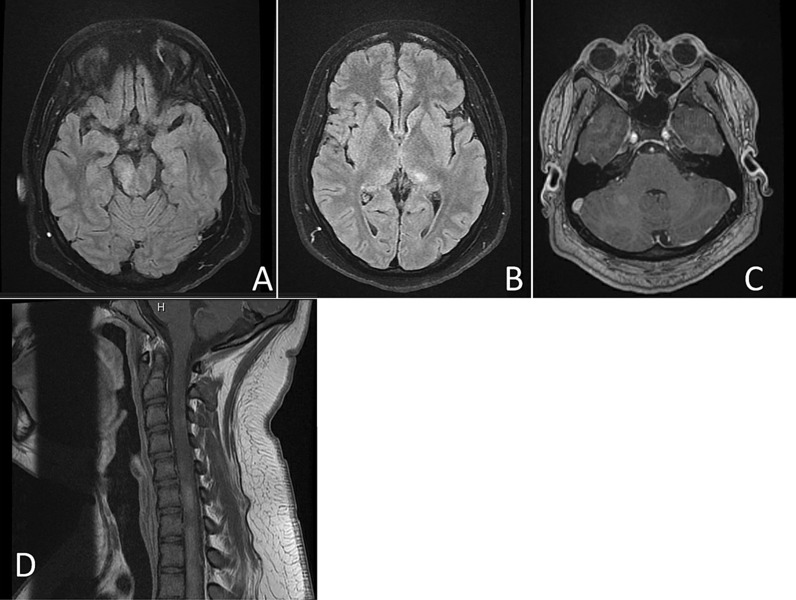
A 17-year-old Hispanic male who presented 3 weeks after acute COVID infection. Axial fluid attenuated inversion recovery (FLAIR) (A, B) images showing hyperintense lesions in the left hemisphere, pons, and right middle cerebellar peduncle, as well as enhancement in right cerebellar peduncle (c) and cervical cord lesion (d)
Fig. 2.
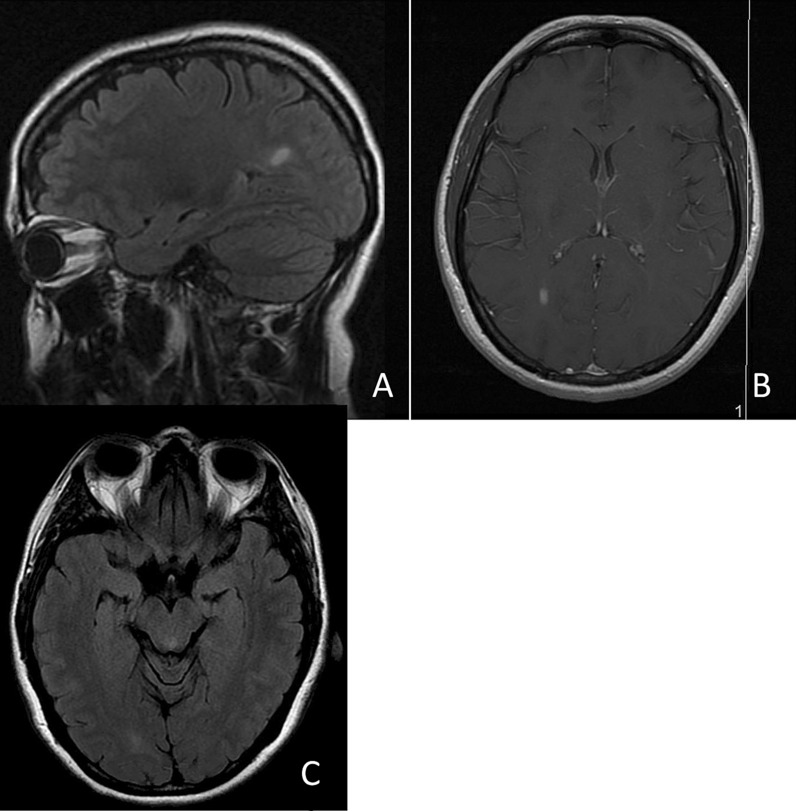
A 36-year-old Caucasian woman who developed right optic neuritis between three and four weeks after acute COVID infection: brain MRI showed multifocal white matter lesions. Sagittal FLAIR demonstrates hyperintense lesion in the left posterior corpus callosum (A), which was also enhancing (B). Axial Flair (C) displays a lesion in the left midbrain and right occipital hemisphere
Fig. 3.
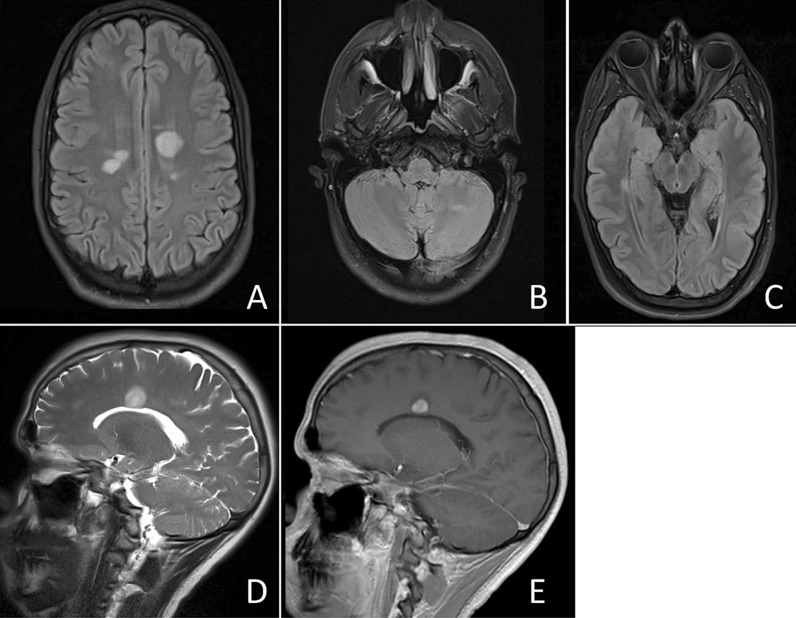
A 20-year-old Hispanic male presented 6 weeks after COVID infection. Brain MRI with multiple intracranial T2 hyperintensities at the bilateral parasagittal area (A) left cerebellum (B) and right medial temporal lobe (C). Left parasagittal lesion extending toward corpus collosum (D) and enhancing with contrast (E)
Results from NMOSD Patient Group
Patients with NMOSD in this study showed mild COVID-19 symptoms. Both patients in this group were young, female, and unvaccinated. One patient was Hispanic and the other was African American. Demographic information is presented in Table 3. Both patients were positive for aquaporin-4 antibodies Serum IgG (on ELISA and one cell based). One patient presented with longitudinally extended transverse myelitis (Fig. 4) and preexisting systemic lupus erythematosus, treated with mycophenolate mofetil and hydroxychloroquine. The patient developed progressive right leg weakness 3 weeks after acute COVID-19 infection. She denied any preexisting neurological symptoms. The other patient presented with opsoclonus consistent with brainstem involvement. High doses of intravenous steroids did not provide any improvement. The patient with transverse myelitis responded to an intravenous immunoglobulin (IVIG) 5-day course. The patient with opsoclonus responded to a 5-day course of plasmapheresis. Both patients were placed on an approved Food and Drug Administration (FDA) medication for NMOSD (inebilizumab and satralizumab). Since this was not a prospective study, baseline aquaporin-4 antibodies (AQ4 ab) were not available for these patients.
Table 3.
Demographic profile of NMOSD patients included in study; both were young female patients
| Demographic profile | Number of patients | Percentage |
|---|---|---|
| Age, years | ||
| 20–30 | 2 | 100 |
| Race | ||
| Hispanic | 1 | 50 |
| African American | 1 | 50 |
| Gender | ||
| Female | 2 | 100 |
| COVID vaccination | ||
| Non-vaccinated | 2 | 100 |
| Family history of autoimmune disorders | ||
| Negative | 2 | 100 |
Fig. 4.
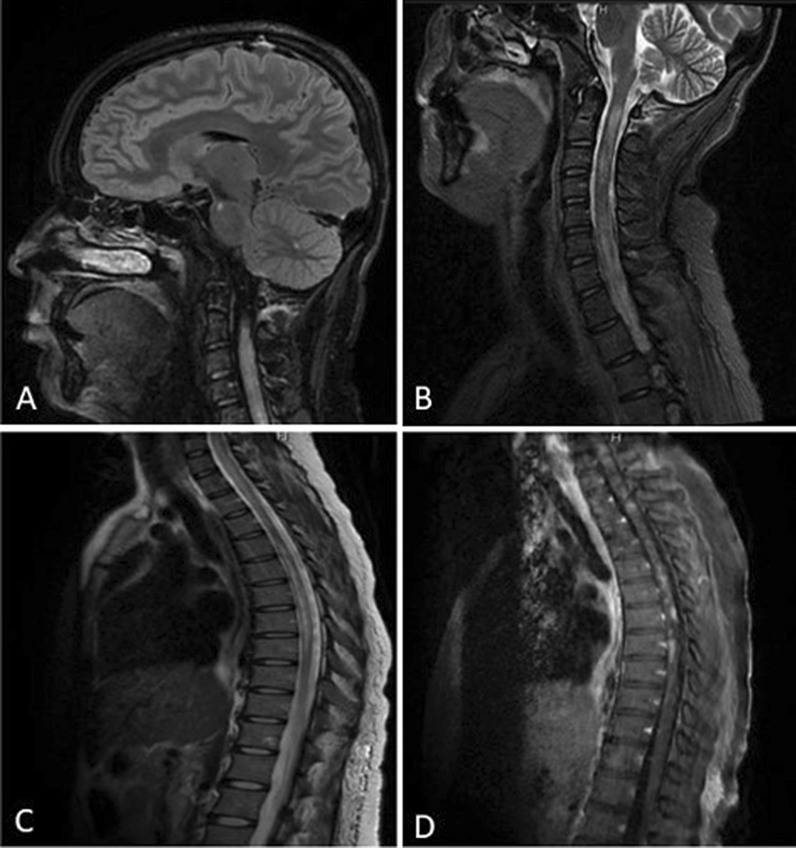
Brain and Spine MRI of NMOSD patient showing hyperintense lesion in the right pons (A), as well as hyperintense cervical and thoracic lesion (B, C), some of which enhance after gadolinium (D)
Discussion
Immune-driven conditions in the setting of acute COVID-19 infection have been previously reported, in particular autoantibodies for anti-cardiolipin, anti-β2-glycoprotein I, and antinuclear antibodies [7]. In the present study, we report 12 cases of central nervous system (CNS) immune-driven conditions after acute COVID-19 infection.
It has been reported recently that immune cells infected by COVID-19 can trigger massive inflammatory response by monocytes and macrophages [8], as well as convincing evidence that the virus can infect and replicate in cells and activate inflammasomes, which are large molecules that trigger a cascade of inflammatory responses that end in cell death [8]. Studies show that the COVID-19 virus can infect and replicate in macrophages in human lungs, as well in a mouse model of the human immune system [9].
In MS patients, there seems to be a strong association with previous Epstein–Barr virus infection and the development of MS [10]. There are a few case reports in the literature of newly diagnosed MS with concurrent COVID-19 infection [10]. An association with Epstein–Barr has not been clearly demonstrated for NMOSD [11].
In this study, the two patients in the NMOSD group showed mild COVID-19 symptoms. Both patients were young, female, and unvaccinated. One patient was Hispanic, the other was African American. Both also had unremarkable CSF studies and poor response to steroids. Atypical features were also noted in one, including opsoclonus at presentation, with nonsignificant radiographic finding on magnetic resonance imaging (MRI). Cases of NMOSD with minimal hyperintense brainstem lesions have been reported [12]. Concomitant autoimmune disorders are not uncommon with NMOSD, in particular in AQP4-IgG antibody positive patients: most common autoimmune disorders target nuclear and cytoplasmic antigens detected in diseases such as systemic lupus erythematosus, Sjogren, and anti-phospholipid antibody syndromes [13]. Lupus erythematosus was previously diagnosed in one of our patients.
The correlation of NMOSD after acute COVID-19 infection is not fully understood. A recent article reported seven NMOSD cases, six of which were aquaporin positive after COVID-19 infection [14]. Demyelinating changes may occur owing to a hyperinflammatory state with the release of cytokines caused by infection leading to glial activation, or it may occur as part of a delayed immune response [15]. Molecular mimicry of SARS-CoV2 antigens and neurological self-antigens could be other theoretically proposed potential mechanisms. Our case series reports adds to the evidence for autoimmune disorders following acute COVD-19 infections; larger studies are needed to evaluate the implication for future therapies.
COVID-19 infections were prevalent in our geographic area of West Texas (1033 new cases) around the time these patients had the infection; therefore epidemiologically, patients attending the MS center were environmentally predisposed.
This is a case series report, larger studies are needed to make any formal conclusions about the direct association of COVID-19 acute infection and the development of CNS autoimmune disorders.
Conclusions
The association between COVID-19 infection and the diagnosis of multiple sclerosis and NMOSD remains unclear, but theoretically it may unmask or trigger the abnormal immune system contributing to the development of such CNS auto-driven neurological conditions. We cannot conclude that COVID-19 acute infection was the only factor contributing to the development of MS or NMOSD: larger studies would be needed to make such a conclusion.
Acknowledgements
We thank the participants of the study.
Funding
No funding or sponsorship was received for this study or publication of this article.
Author Contributions
Mirla Avila MD (design, statistics, reporting, editing) Yuanyuan Tan MD (design, statistics, reporting, editing), Roberto Hernandez MD (reporting, table design), Hafsa Zuberi (design), Victor M. Rivera MD (editing).
Disclosures
There was no funding for this study, no commercial associations that might pose a competing interest in connection with the submitted material. Mirla Avila has served as an advisor for Biogen, EMD Serono, BMS, and has no competing interests with the submitted material. Authors Yuanyuan Tan, and Roberto Hernandez, Hafsa Zuberi and Victor M Rivera have nothing to disclose.
Compliance with Ethics Guidelines
All participants provided informed consent for publication. IRB approval was obtained. FWA #0,006,767. Lubbock IRB#00,000,096 IRB#L23-019. IRB approval study title name: Multiple Sclerosis and Neuromyelitis Optica Spectrum Disorder: onset following acute COVID-19 infection IRB #: L23-019 SUBMISSION REFERENCE #091,151.
Data Availability
All data generated or analyzed during this study are included in this published article.
Footnotes
The original online version of this article was revised to correct the author name Yuanyuan Tan.
Change history
2/23/2023
A Correction to this paper has been published: 10.1007/s40120-023-00447-y
References
- 1.Garjani A, Middleton RM, Hunter R, Tuite-Dalton KA, Coles A, Dobson R, et al. COVID-19 is associated with new symptoms of multiple sclerosis that are prevented by disease modifying therapies. Mult Scler Relat Disord. 2021;52:102939. doi: 10.1016/j.msard.2021.102939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Moore L, Ghannam M, Manousakis G. A first presentation of multiple sclerosis with concurrent COVID-19 infection. eneurologicalSci. 2021;22:100299. doi: 10.1016/j.ensci.2020.100299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garcia-Ramos AE, Martin-Nares E, Herandez-Molina G. New onset of autoimmune diseases following COVID-19 diagnosis. Cells. 2021 doi: 10.3390/cells10123592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson AJ, Banwell BL, Barkhof F, Carroll WM, et al. Diagnosis of multiple sclerosis:2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- 5.Wingerchurk DM, Banwell BL, Bennett JL, Cabre P, Carroll W, et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology. 2015;85(2):177–189. doi: 10.1212/WNL.0000000000001729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.https://www.covid19treatmentguidelines.nhi.gov
- 7.Steelman AJ. Infection as an environmental trigger of multiple sclerosis disease exacerbation. Front Immunol. 2015;6:520. doi: 10.3389/fimmu.2015.00520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miriam M, Jerome M. Pathological inflammation in patients with COVID-19: a key role for monocytes and macrophages. Nat Rev Immunol. 2020;20(6):355–362. doi: 10.1038/s41577-020-0331-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derksen V, Kissel T, Lamers-Karnebeek FBG, van der Bijl AE, Venhuizen AC, Huizinga TWJ, et al. Onset of rheumatoid arthritis after COVID-19: coincidence or connected? Ann Rheum Dis. 2021 doi: 10.1136/annrheumdis-2021-219859. [DOI] [PubMed] [Google Scholar]
- 10.Aloisi F, Salvetti M. Epstein-Barr virus and multiple sclerosis: supporting causality. Lancet Neurol. 2022;21(4):300–301. doi: 10.1016/S1474-4422(22)00086-2. [DOI] [PubMed] [Google Scholar]
- 11.Tselis AC. Epstein-Barr virus infections of the nervous system. Handb Clin Neurol. 2014;123:285–305. doi: 10.1016/B978-0-444-53488-0.00013-4. [DOI] [PubMed] [Google Scholar]
- 12.Kim W, Kim S-H, Huh S-Y, Kim HJ. Brain abnormalities in neuromyelitis optica spectrum disorder. Mult Scler Int. 2012;2012:735486. doi: 10.1155/2012/735486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rivera VM, Hamuy F, Rivas V, Garcia F, et al. status of the neuromyelitis Optica spectrum disorder and Latin America. Mult Scler Related Disorders. 2021;53:103083. doi: 10.1016/j.msard.2021.103083. [DOI] [PubMed] [Google Scholar]
- 14.Feizi P, Sharma K, Pasham SR, Nirwan L, et al. Central nervous sytem (CNS) inflammatory demyelination disease associated with COVID-19: a case series and review. J Neuroimmunol. 2022;371:577939. doi: 10.1016/j.jneuroim.2022.577939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rafique S, Wasim A, Sultan T, Ahmad A. Post-COVID neuromyelitis optica spectrum disorder. J Coll Physicians Surg Pak. 2021;31(7):138–140. doi: 10.29271/jcpsp.2021.Supp2.S138. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data generated or analyzed during this study are included in this published article.


