As exemplified by COVID-19 messenger RNA (mRNA) vaccines, RNA folds unprecedented opportunities for preventive and therapeutic approaches to various diseases. The increased usage of next generation sequencing, especially RNA sequencing (RNA-seq), has uncovered that most of the human genome are transcribed as RNA. Yet, only few percent of them correspond to exons of protein-coding genes, leaving behind a majority of transcribed RNAs as non-protein-coding RNAs (ncRNAs). These ncRNAs are currently categorized simply by their lengths as short and long non-coding RNAs (lncRNAs). Because the protein-centered research could not offer all the cures to various diseases, there is a high hope that these ncRNAs could be the keys to understand pathogeneses and potentially cure various diseases. However, the reality is that we are still far from understanding the mechanistic aspects of these ncRNAs, which hinders the therapeutic usages of ncRNAs. For example, microRNAs (miRNAs) are short (~ 22 nucleotides (nt)) ncRNAs that regulate the translation by binding to the 3′-untranslated regions (UTR) of mRNAs. Although their regulatory actions are robust, it is very difficult to pinpoint and finetune to one mRNA (thus, protein) as one miRNA is predicted to target hundreds of mRNAs. This is a problematic issue for using miRNAs as therapeutic targets as one miRNA might affect several important signaling pathways by binding to the 3′-UTR of several (but not all) components of each signaling pathway. As we know well, each signaling pathway is regulated by intertwined components (i.e., protein–protein interactions). Thus, it might be difficult to control the effects of miRNA for only intended target mRNA.
Other than miRNAs, there is a growing class of ncRNAs, which are called lncRNAs. LncRNAs include any ncRNAs longer than 200 nt. Because of their simple definition, many new lncRNAs are reported in recent years, which has proved to be difficult to understand and generalize the functions of ever-increasing number of lncRNAs. Compared to miRNAs and mRNAs, lncRNAs are generally expressed at low level because lncRNAs are expressed in a cell-type specific and time-dependent manner. Also, they are dysregulated in various diseases as most diseases result in alternations of cell types in a diseased tissue compared to the healthy one. By performing RNA-seq experiment, lncRNAs can be readily detected, which could be used as diagnostic biomarkers of a certain disease. However, their functions are not well defined for most lncRNAs. To consider lncRNAs as therapeutic targets, elucidating the mechanism of action of each dysregulated lncRNA in a certain disease is utmost importance. To this end, basic research in lncRNAs is urgently needed, which some are published in Cell Biology and Toxicology as below.
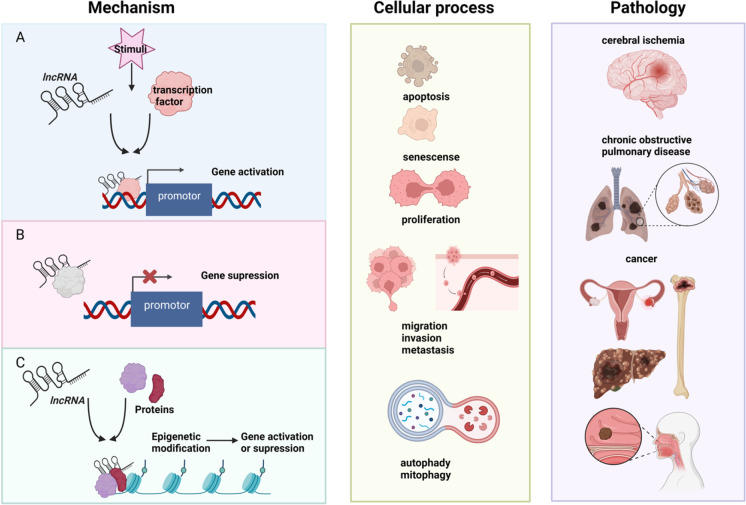
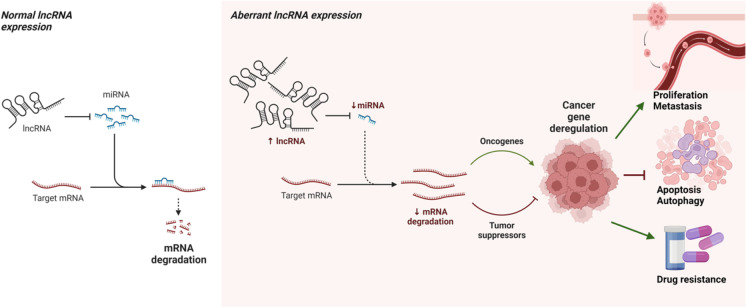
Probably the most well studied function of lncRNAs is epigenetic and transcriptional regulation by functioning as molecular scaffolds (Fig. 1). For example, the lncRNA CTSLP8 directly binds to PKM2 to promote its binding to the promoter region of c-Myc to regulate glycolysis in ovarian cancer cells, contributing to their chemotherapy resistance (Li et al. 2021). However, the careful investigations of the transcriptional repressor complex, polycomb repressive complex 2 (PRC2), clearly indicate that the component PRC2, EZH2, binds any RNAs, including lncRNAs, promiscuously (Yan et al. 2019), suggesting that the function of lncRNAs as molecular scaffolds for epigenetic and transcription factors need further detailed investigations. Instead, understanding the function of lncRNAs as miRNA sponges has become popular in recent years (Fig. 2). For example, the lncRNA OIP5-AS1 binds miRNA-129-5p, which targets IGF2BP2 to regulate resistance of glioblastoma cells to the chemotherapy drug temozolomide (Wang et al. 2021).
Fig. 1.
The mechanisms of action of lncRNAs in the regulation of different cellular processes and human pathology. A LncRNAs can act as molecular signaling mediators to activate the gene expression by binding to transcription factors (Li et al. 2021, Zhou et al. 2021). B LncRNAs can suppress the gene expression by removing transcription factors from a specific genomic location (Ye et al. 2021). C LncRNAs can support the assembly of protein complexes that link the factors together to regulate the gene expression through epigenetic modulation (Lee et al. 2022, Xue et al. 2022). The figure created with BioRender.com, accessed on 2 November 2022
Fig. 2.
LncRNA-mediated gene expression by binding to miRNAs, which in turn blocks the translation of the target mRNAs. In cancer, the sponging of miRNAs by lncRNA affects the tumor growth in several mechanisms depending on the target mRNAs of bound miRNAs (Liu et al. 2021, Wang et al. 2021, Yang et al. 2021). The figure created with BioRender.com, accessed on 2 November 2022
As briefly summarized above, the mechanisms of action of lncRNA are diverse. It is also clear that most lncRNAs exert their actions by binding to other macromolecules (i.e., DNA, RNA, and/or proteins). Thus, it is safe to assume that lncRNAs finetune the signaling pathways. This is supported by the fact that most knockout (KO) mice of lncRNAs do not result in embryonic lethality. Furthermore, most of these KO mice develop normally and show some phenotypes only when challenged with disease models (e.g., transverse aortic constriction to induce cardiac hypertrophy) (Gao et al. 2020). Nevertheless, correctly placing lncRNAs in each signaling pathway is important task now as the number of lncRNAs is increasingly rapidly while that of protein-coding genes is decreasing due to better annotation of genome. Given that many lncRNAs are upregulated in a disease tissue, inhibiting these lncRNAs might affect the dysregulated signaling pathways that contribute to the pathogenesis of a certain disease. Furthermore, by overexpressing an lncRNA, it might be possible to finetune the signaling pathway that might be dysregulated in a diseased tissue. Such approaches are only possible if and only if the mechanism of action of each lncRNA is fully understood. What is not being reported nor investigated well to date is the identification of isoforms of each lncRNA. As we have learned from proteins and their isoforms, each isoform has its own expression pattern and functionality. Thus, it is not far-fetched to assume that each lncRNA isoform has its own distinctive function. This point should be investigated further if lncRNAs to be successfully used as therapeutic targets for various diseases.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Gao F, Cai Y, Kapranov P, Xu D. Reverse-genetics studies of lncRNAs-what we have learnt and paths forward. Genome Biol. 2020;21(1):93. doi: 10.1186/s13059-020-01994-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee KY, Ho SC, Sun WL, Feng PH, Lin CW, Chen KY, et al. Lnc-IL7R alleviates PM2.5-mediated cellular senescence and apoptosis through EZH2 recruitment in chronic obstructive pulmonary disease. Cell Biol Toxicol. 2022. 10.1007/s10565-022-09709-1. [DOI] [PubMed]
- Li X, Zhang Y, Wang X, Lin F, Cheng X, Wang Z, et al. Long non-coding RNA CTSLP8 mediates ovarian cancer progression and chemotherapy resistance by modulating cellular glycolysis and regulating c-Myc expression through PKM2. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09650-9. [DOI] [PMC free article] [PubMed]
- Liu Q, Ran R, Song M, Li X, Wu Z, Dai G, et al. LncRNA HCP5 acts as a miR-128–3p sponge to promote the progression of multiple myeloma through activating Wnt/β-catenin/cyclin D1 signaling via PLAGL2. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09628-7. [DOI] [PubMed]
- Wang X, Li X, Zhou Y, Huang X, Jiang X. Long non-coding RNA OIP5-AS1 inhibition upregulates microRNA-129–5p to repress resistance to temozolomide in glioblastoma cells via downregulating IGF2BP2. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09614-z. [DOI] [PubMed]
- Xue LX, Chen SF, Xue SX, Liu PD, Liu HB. LncRNA TUG1 compromised neuronal mitophagy in cerebral ischemia/reperfusion injury by targeting sirtuin 1. Cell Biol Toxicol. 2022. 10.1007/s10565-022-09700-w. [DOI] [PubMed]
- Yan J, Dutta B, Hee YT, Chng WJ. Towards understanding of PRC2 binding to RNA. RNA Biol. 2019;16(2):176–184. doi: 10.1080/15476286.2019.1565283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang R, Liu Z, Cao H, Shi Y. LINC01089, suppressed by YY1, inhibits lung cancer progression by targeting miR-301b-3p/HPDG axis. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09643-8. [DOI] [PubMed]
- Ye J, Fu Y, Wang Z, Yu J. Long non-coding RNA FOXP4-AS1 facilitates the biological functions of hepatocellular carcinoma cells via downregulating ZC3H12D by mediating H3K27me3 through recruitment of EZH2. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09642-9. [DOI] [PMC free article] [PubMed]
- Zhou Y, Jin Q, Chang J, Zhao Z, Sun C. Long non-coding RNA ZMIZ1-AS1 promotes osteosarcoma progression by stabilization of ZMIZ1. Cell Biol Toxicol. 2021. 10.1007/s10565-021-09641-w. [DOI] [PubMed]