Abstract
Human peripheral blood monocytes became apoptotic following phagocytosis of Staphylococcus aureus. The consequences of heat stress for monocytes were studied with regard to the effect on S. aureus-induced apoptosis. Exposure of monocytes to 41.5°C for 1 h resulted in HSP72 expression and had no influence on phagocytosis of bacteria; moreover, phagocytosis of S. aureus immediately or shortly after heat shock had no effect on the S. aureus-induced monocyte apoptosis, as evidenced by DNA fragmentation assay. In contrast, cells which recovered from heat shock for 18 to 24 h, although active as phagocytes, were resistant to the S. aureus-induced apoptosis. The observed protective effect was related to the induction of HSP72, since blocking of HSP72 synthesis by an antisense oligomer abolished the protective effect of heat shock on bacterium-induced monocyte apoptosis.
Cells exposed to stressful conditions, including elevated temperatures, oxidative injury, heavy metals, and proinflammatory cytokines, increase synthesis of a multifunctional group of proteins referred to as stress proteins or heat shock proteins (HSPs). HSPs are classified into families according to apparent molecular mass and inducers. Because of the protective effect on vital cellular functions, all HSPs have been recently designated members of the larger family of proteins called molecular chaperons (15). Molecular chaperones recognize hydrophobic surfaces of nonnative forms of other proteins, preventing irreversible multimeric aggregation (8). This activity preserves native proteins and cellular integrity, particularly under stressful conditions. Several HSPs, called heat shock cognates (HSCs), are constitutively expressed. Moreover, the expression of some HSPs (HSP/HSC70 and HSP90) has been reported to be regulated under physiological (nonstressful) conditions such as cell cycle (27), differentiation (44), embryogenesis (6), and stimulation by growth factors (53). The HSP70 family includes both constitutive (HSC70) and stress-inducible (HSP70) proteins as well as the glucose-regulated protein GRP78. In addition to other functions, the ability of HSP70 to protect mammalian cells against stress-induced damages (1, 4, 52) seems particularly interesting. It has been shown that when HSP70 function is disrupted, exposure to stressing factors leads to the accumulation of cellular damage over a critical threshold and to cell apoptosis (10, 31, 43, 51). However, in some cell lines proapoptotic activity of overexpressed HSP has also been found (14, 30).
Recently, a role of cellular stress in functioning of the immune system has been recognized and attracted much interest. It was shown that mitogenic activation of peripheral blood mononuclear cells with phytohemagglutinin resulted in enhanced synthesis of predominantly HSC70 and HSP90 (17). Cellular stress reactions (or individual HSPs) were triggered when immune cells were activated by pathogens, their products, or inflammatory mediators. In monocytes, phagocytosis of erythrocytes induced the synthesis of many HSPs (65, 70, 90, and 110 kDa) (41), whereas phagocytosis of Staphylococcus aureus led to selective induction of HSP70 (26). The synthesis of HSP70 and HSP90 was also shown to be induced by the phorbol ester phorbol myristate acetate (through protein kinase C activation) (25). In addition, inflammatory cytokines were found to up-regulate cellular stress response in monocytes (5). On the other hand, heat shock (or chemical stress) may inhibit lipopolysaccharide-induced production of interleukin-1 (IL-1) and tumor necrosis factor alpha (TNF-α) in monocytes/macrophages (46, 47). Also, synthesis of reactive oxygen species was shown to be regulated by heat shock (41).
There is growing evidence that bacteria or their products can induce apoptosis in host cells, including monocytes/macrophages (19, 55), and it has been suggested that bacterium-induced apoptosis of monocytes/macrophages promotes an inflammatory response that causes tissue damage (56). Recently, we have demonstrated that phagocytosis of bacteria by peripheral blood monocytes leads to apoptosis of phagocytes within less than 24 h (2), indicating that the cellular stress reaction associated with phagocytosis of bacteria does not provide an effective protection against deleterious effect caused to the phagocyte by engulfed bacteria.
In this study, we investigated the reaction of stressed monocytes to the phagocytosis of bacteria and showed that prior heat shock may protect monocytes against subsequent contact with pathogens.
MATERIALS AND METHODS
Isolation and culture of cells.
Peripheral blood mononuclear cells were isolated by standard Ficoll-Paque (Pharmacia, Uppsala, Sweden) gradient centrifugation from heparin- or EDTA-treated blood from healthy donors. The cells were suspended in Hanks’ balanced salt solution supplemented with 1% autologous plasma and subjected to countercurrent centrifugal elutriation (Beckman JE-6B elutriation system equipped with a 5-ml Sanderson separation chamber) to obtain monocytes. Monocyte enrichment was confirmed by nonspecific esterase staining (85 to 95% positive) and/or expression of CD14 antigen (80 to 90% LeuM3 positive). Monocytes (5 × 106/ml) were washed once with cold RPMI 1640 and kept in an ice bath in RPMI 1640 culture medium supplemented with l-glutamine and 10% fetal calf serum, without antibiotics (all reagents were from GIBCO, Grand Island, N.Y.), until used. The medium used in these experiments allowed culture of monocytes for up to 48 h without the massive apoptosis (about 20% of annexin V binding and minimal DNA laddering) seen when some batches of fetal calf serum were used or when serum in medium was omitted.
Heat shock treatment.
Monocytes (2 × 106/ml), suspended in complete medium (with 50 μg of gentamicin [GIBCO] per ml) in siliconized glass tubes, were heat stressed at 41.5°C for 1 h. After the heat shock, cells were immediately transferred to standard culture conditions (37°C, 5% CO2, humidified atmosphere) at which experiments were carried out as indicated.
Antisense treatment.
Native (nonmodified) oligodeoxynucleotides (antisense and sense) were custom synthesized by GIBCO. HSP72 antisense oligomer (5′-CGCGGCTTTGGCCAT-3′) was complementary to the initiation codon and four downstream codons of human HSP72 mRNA (22). The corresponding sense oligomer (5′-ATGGCCAAAGCCGCG-3′) was used as a control. Freshly isolated monocytes suspended in complete culture medium were exposed to 2.5 or 5 μM HSP72 antisense or sense oligonucleotides for various lengths of time: 26 h for annexin V-propidium iodide (PI) staining, 42 h for the DNA-laddering experiment, and up to 48 h for HSP72 measurement by immunocytofluorimetry, depending on the time of culture termination. After 24 h of incubation, the culture medium was always replaced with fresh medium containing 5 μM HSP72 antisense or sense oligonucleotide.
Phagocytosis of bacteria.
Staphylococcus aureus (ATCC 25923) was grown for 18 h on sugar broth, washed twice with a large volume of saline, and opsonized (30 min, 37°C) in the presence of 10% fresh human serum. After additional washing, the density of bacterial cells was measured spectrophotometrically (540 nm), and the cell number was calculated by using previously determined standard curves (based on CFU counts). Finally, the concentration of bacteria was adjusted to 109/ml of phosphate-buffered saline (PBS). To enable analysis of phagocytosis by flow cytometry, bacteria were incubated before opsonization for 2 h at 37°C in PBS containing 0.1% fluorescein isothiocyanate (FITC; BDH Chemicals Ltd., Poole, England). Monocytes (3 × 106) were incubated (37°C) in siliconized glass tubes with suspensions of opsonized bacteria in a total volume of 1 ml. The monocyte/bacterium ratio was 1:20. Monocytes without bacteria were also incubated in parallel. After 30 min of incubation, 1 ml of ice-cold complete medium with 50 μg of gentamicin (GIBCO) per ml was added; cells were centrifuged (110 × g, 8 min) to separate phagocytic cells from free bacteria and resuspended in complete medium. The monocytes (2 × 106/ml) were subsequently cultured at 37°C in 5% CO2, humidified atmosphere as indicated.
Confocal microscopy.
To enable analysis of phagocytosis by fluorescence microscopy, bacteria were incubated before opsonization for 2 h at 37°C in 50 mM Tris-Cl-buffered saline (pH 7.2) containing 10 μM SYTO17 (Molecular Probes Inc., Eugene, Oreg.). Prior to phagocytosis, monocytes were allowed to attach to glass coverslips submerged in culture medium in 35-mm-diameter cell culture dishes (Sarstedt Inc., Newton, N.C.) for 30 min at 37°C. Phagocytosis of SYTO17-stained bacteria was performed basically as described above for FITC-stained bacteria, but instead of centrifugation, culture dishes containing coverslips were gently rinsed with culture medium to remove the free bacteria. Monocytes were stained for 5 min with DiO (dioctadecyloxacarbocyanine; Molecular Probes) at a final concentration of 50 ng/ml in PBS. Monocytes attached to glass coverslips were placed in a microscope-stage microincubator (Life Science Research Cambridge, England) and kept at 37°C in culture medium during image collection. Images of monocytes and bacteria were collected by using a Bio-Rad MRC1024 confocal microscope equipped with an Ar-Kr laser (ALC). Excitation was 488 nm for DiO and 647 nm for SYTO17. A PlanApo 60× NA1.4 oil immersion lens was used.
Isolation of genomic DNA and gel electrophoresis.
DNA was isolated from monocytes pelleted from culture volume (1 ml) by centrifugation (280 × g). Lytic buffer (0.01 M Tris [pH 7.8], 0.005 M EDTA, 0.5% sodium dodecyl sulfate; 0.3 ml per cellular pellet) was added; and the sample was mixed vigorously and incubated at 65°C for 1 h to obtain viscous and clear cell lysates. The lysates were treated with RNase A (20 μg/ml; 37°C, 1 h) and proteinase K (20 μg/ml; 50°C, 1 h) and extracted twice with an equal volume of phenol-chloroform (1:1). DNA in the aqueous phase was precipitated at −20°C in 0.3 M sodium acetate–75% ethanol. Precipitates were pelleted by centrifugation (13,000 × g, 10 min, 4°C), washed with ice-cold 70% ethanol, and dried. For electrophoresis, DNA samples were dissolved in 50 μl of Tris-EDTA buffer. Gel loading buffer (25% Ficoll 400, 10 mM EDTA, 0.01% bromophenol blue, 0.01% xylenecyanol; 10 μl) was added, and the samples were heated at 65°C for 10 min. Aliquots corresponding to 106 cells were loaded per slot. Samples were subjected to electrophoresis in 2% agarose gel containing ethidium bromide (0.5 μg/ml in Tris-borate–1 mM EDTA buffer [pH 8.2]) at 5 V/cm for 90 min. DNA was visualized by UV light and photographed. The analyzed DNA fragments in the samples were compared with standard size fragments of the DNA marker φX174 HincII (Advanced Biotechnologies Ltd., Leatherhead, England). All other chemicals were purchased from Sigma Chemical Co., St. Louis, Mo.
Measurement of DNA concentration.
DNA samples (dissolved in Tris-EDTA buffer) were diluted 10-fold with 0.5 M HClO4. Freshly prepared diphenylamine reagent (200 μl) containing acetaldehyde (16 mg/ml) was added to a (100-μl) portion of each sample, and the tubes were incubated at 30°C for 18 h. Aliquots (150 μl) were transferred to flat-bottomed 96-well polystyrene microtiter plates, and the A600 was measured on an automated plate reader (Spectra Max 250; Molecular Devices Corp., Sunnyvale, Calif.). Chemicals were purchased from Sigma. DNA in the samples was determined from a standard curve prepared from salmon sperm DNA diluted in 0.5 M HClO4. Aliquots corresponding to 106 cells contained 100 μg of DNA.
Flow cytometry.
An early feature of apoptosis, the externalization of the anionic phospholipid phophatidylserine (13), was assessed by using an annexin V-FITC kit (Bender MedSystems, Vienna, Austria). Cell suspensions (200-μl aliquots) were washed with PBS and resuspended in binding buffer (10 mM HEPES-NaOH [pH 7.4], 140 mM NaCl, 2.5 mM CaCl2); 5 μl of FITC-labeled annexin V was added, and the mixture was incubated for 10 min in the dark at room temperature. After being washed, cells were resuspended in 0.2 ml of binding buffer and PI solution was added to a final concentration of 1 μg/ml of cell suspension. Ten thousand ungated cells were analyzed by fluorescence-activated cell sorting (FACS) in a FACScan flow cytometer (Becton Dickinson, San Jose, Calif.). In experiments with annexin V, bacteria were not labeled. To estimate phagocytosis of FITC-labeled bacteria, samples were analyzed with a FACScan flow cytometer (Becton Dickinson). During acquisition, the threshold was set to exclude free bacteria. Cells were acquired and analyzed ungated.
For the detection of HSP, 200-μl aliquots of monocytes were washed in PBS and then permeabilized with 0.5 ml of FACS permeabilizing solution (Becton Dickinson) for 15 min. After being washed, cells were resuspended in (PBS (pH 7.2) containing 1% bovine serum albumin and 0.1% sodium azide and then labeled with monoclonal antibodies (MAbs). To detect HSP72, anti-HSP72 MAb RPN1197 (Amersham International, Amersham, England) at a final dilution of 1:300 was used; HSP25/27 and HSP90 were detected with MAbs IAP-9 and AC-16 (Sigma). Isotype-specific controls (Becton Dickinson) were also included. After washing, FITC-conjugated goat anti-mouse immunoglobulins (DAKO A/S, Glostrup, Denmark) were added, and samples were incubated for 20 min in the dark. All procedures were performed at room temperature. After the final wash, cells were analyzed with a FACScan flow cytometer. Data were collected ungated. The analyses were performed by using the CellQuest program (Becton Dickinson).
Isolation of RNA and reverse transcriptase-mediated PCR.
Total cellular RNA was isolated from cellular pellets containing 106 monocytes by using TRIZOL reagent (GIBCO) exactly as instructed by the manufacturer. Precipitated RNA was dissolved in 10 μl of sterile, RNase-free water and stored at −30°C when necessary. cDNA synthesis reactions were done in a total volume of 20 μl containing 10 μl of each RNA sample, 0.5 μg of oligo(dT)12–18 primer (GIBCO), and 200 U of SuperScript II RNase H− reverse transcriptase (GIBCO) according to the protocol provided with the enzyme. PCRs were set up in a total volume of 50 μl containing 5 μl of the cDNA, 0.2 mM deoxynucleoside triphosphates, 0.5 μM each oligonucleotide primer, 50 mM KCl, 1.5 mM MgCl2, and 2.5 U of Taq polymerase (GIBCO). PCRs were run in 0.5-ml tubes in an OmniGene thermal cycler (Hybaid, Teddington, England) equipped with a heated lid. Reactions were carried out at 94°C for 1 min, 60°C for 1 min, and 72°C for 1.5 min for 35 cycles (HSP72) or at 94°C for 1 min, 55°C for 1 min, and 72°C for 1.5 min for 35 cycles (β-actin), with final extension at 72°C for 10 min. The reaction products were then resolved on a nondenaturing 2% agarose (Sigma) gel and visualized by staining with ethidium bromide.
The following PCR primers were custom synthesized by GIBCO: for β-actin, 5′-AGCGGGAAATCGTGCGTG-3′ (sense) and 5′-GGGTACATGGTGGTGCCG-3′ (antisense); for HSP72, 5′-TTTGACAACAGGCTGGTGAACC-3′ (sense) and 5′-GTGAAGGATCTGCGTCTGCTTGG-3′ (antisense). The primers for β-actin were designed to match sequences in separate exons to avoid the contribution of genome-templated product in the signal analysis (48). The HSP72 primer pair was optimized for amplification by using the Gene Runner computer program (Hastings Software Inc.). The expected product lengths were 590 bp for HSP72 and 307 bp for β-actin.
RESULTS
Heat-shocked monocytes can effectively phagocytose bacteria.
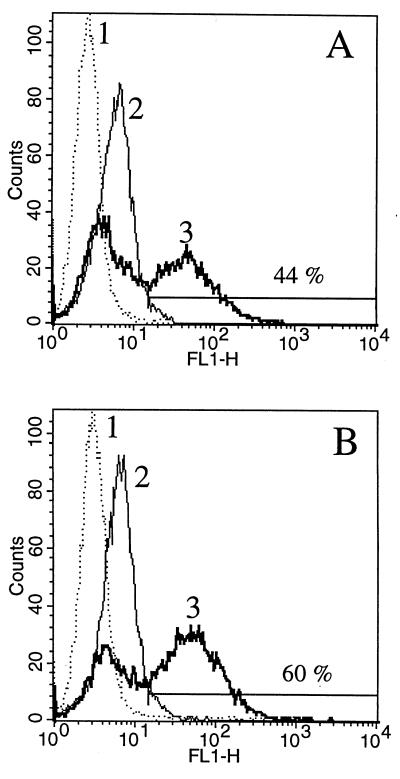
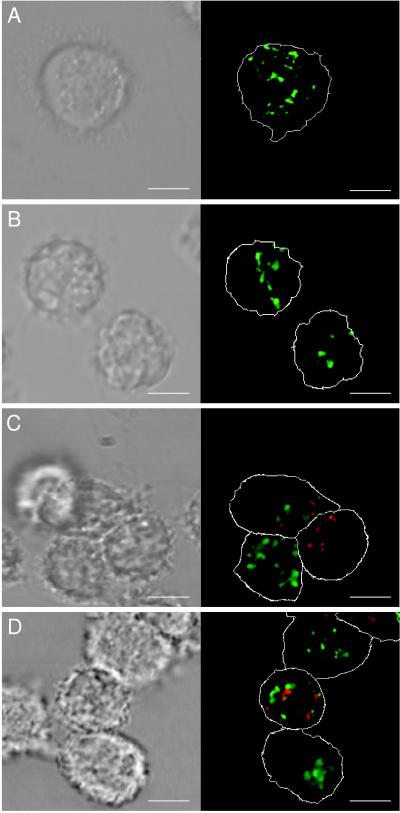
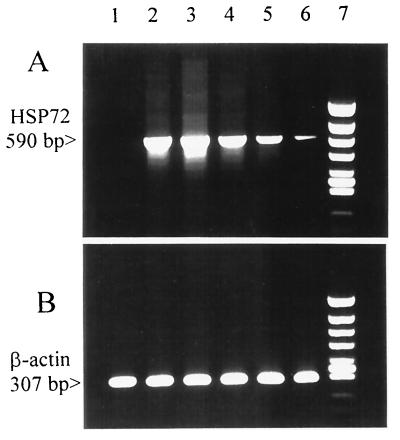
Since the phagocytosis of bacteria by monocytes was critical for this study, we wanted to show that monocytes exposed to heat stress were still effective as phagocytes. When heat-stressed cells were incubated with FITC-labeled S. aureus in a proportion 20 bacteria per cell, phagocytosis was observed in about 50% of monocytes (Fig. 1B), essentially the same as in nonstressed cells from the same donor (Fig. 1A). To confirm that the bacteria were indeed phagocytosed, confocal microscopy was used. Figure 2 demonstrates an optical section through the center of a monocyte following phagocytosis of bacteria stained with SYTO17. The image of mitochondria and cellular membranes stained with DiO clearly demonstrates that bacteria were endocytosed and could be detected inside the cells. The effectiveness of the applied heat shock was evaluated by measurement of HSP72 mRNA accumulation during first 5 h after the stress (Fig. 3). The control, untreated monocytes did not accumulate HSP72-specific transcript (lane 1). After exposure to elevated temperature, a strong HSP72-specific signal was observed in 1 h (lane 2), which after a peak at 2 h decreased to a nondetectable level. The observed reaction was in agreement with previous reports on this subject (17). The heat shock treatment that we used had no apparent influence on β-actin mRNA accumulation (Fig. 3).
FIG. 1.
Comparison by flow cytometry of phagocytic activities of resting (A) and heat-stressed (B) monocytes. Histograms of green fluorescence due to FITC-labeled bacteria engulfed by monocytes show data for control monocytes (tracing 1) and monocytes incubated with unlabelled and FITC-labeled (tracings 2 and 3, respectively) S. aureus. Percentages of bacterium-containing cells in the regions are shown. The left peak of tracing 3 corresponds to monocytes which do not ingested bacteria. Results of a typical experiment of three performed are presented.
FIG. 2.
Demonstration of bacteria within heat-stressed monocytes. Transmitted light images (left column) and corresponding fluorescence confocal images (right column) of central planes of selected, representative monocytes are shown. Green, mitochondria (strong fluorescence) and cellular membranes (dim fluorescence). Red, bacteria stained with SYTO17. The positions of plasma membranes are marked with white dotted lines. Thickness of optical slices, approximately 700 nm; bar, 5 μm. Images represent control (A and C) and heat shock-treated (B and D) monocytes not exposed to S. aureus (A and B) and after phagocytosis of bacteria (C and D). Positions of plasma membranes, mitochondria, and bacteria in images C and D demonstrate that bacteria are present within the cell interior. The images are representative of one of three identical experiments performed.
FIG. 3.
Induction of stress-inducible HSP70 in monocytes after heat shock. (A) Accumulation of HSP72 mRNA in monocytes demonstrated by reverse transcriptase-mediated PCR. (B) Constitutive accumulation of β-actin mRNA in the same monocytes. Lanes: 1, control cells; 2 to 6, RNA isolated 1, 2, 3, 4, and 5 h after the heat shock; 7, DNA size marker (φX174 HincII). The data are from one representative experiment of five performed.
Heat-shocked monocytes preserve DNA integrity after phagocytosis of S. aureus.
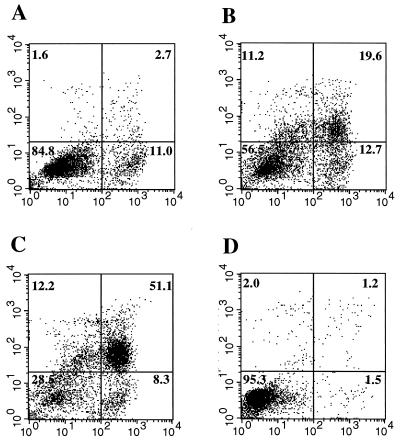
Immediately after phagocytosis of bacteria, monocytes showed remarkable redistribution of phosphatidylserine residues, which is considered an early marker of apoptosis (13, 50). As demonstrated by staining with annexin V (Fig. 4), 60 min after phagocytosis about 60% of monocytes exposed phosphatidylserine on the cell surface. Counterstaining with PI demonstrated that at this early stage the integrity of the plasma membrane was already compromised and permeable to the dye.
FIG. 4.
Redistribution of cell membrane phosphatidylserine and PI staining of monocytes after phagocytosis of S. aureus. Freshly isolated monocytes were incubated with S. aureus for 30 min to allow phagocytosis as described in Materials and Methods and exposed to annexin V-FITC and PI immediately (A) and 30 (B) and 60 (C) min later. (D) Control monocytes incubated for 90 min. In the dot plot analysis of ungated cells, the x axis represents annexin V staining and the y axis represents PI uptake. The percentages of cells in the quadrants are shown. The data are representative of one experiment of five performed.
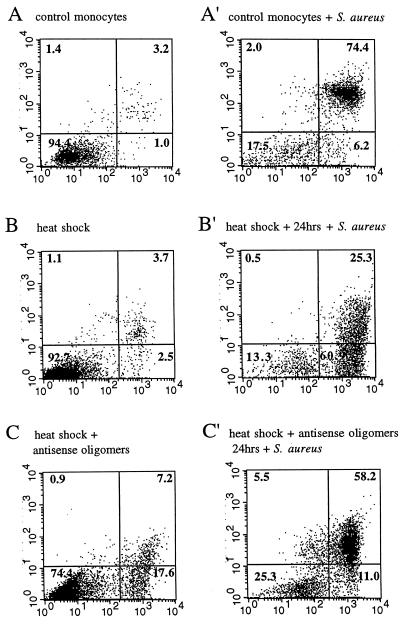
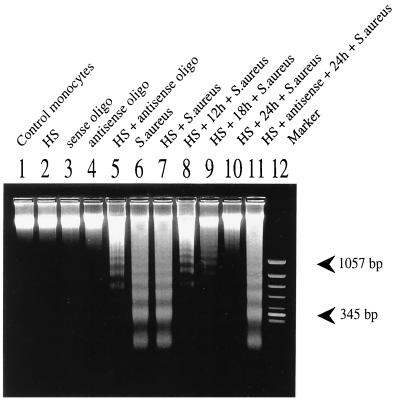
To study the effect of inducible HSP72 on monocyte apoptosis, it was necessary to perform experiments after culture time following heat shock to allow accumulation of the product. We also tested whether preculture time by itself would change the monocyte response to S. aureus phagocytosis. As shown in Fig. 5A and A′, early exposure of phosphatidylserine and PI staining were seen also in experiments in which monocytes were precultured for 24 h before phagocytosis of bacteria. Genomic DNA isolated from the same cultures 16 h later and resolved by gel electrophoresis revealed the characteristic ladder-like pattern formed by oligonucleosome-sized fragments (Fig. 6, lane 6), a typical feature of advanced apoptosis. In contrast, when monocytes were heat stressed prior to exposure to bacteria, under some conditions (see below) the DNA fragmentation was strongly inhibited. The time given to the monocytes to recover from heat shock was critical for the observed effect, demonstrating that some accumulating products of heat shock genes are involved. Depending on the cell donor, the resistance of heat-stressed monocytes to bacterium-induced DNA fragmentation was maximal when phagocytosis of bacteria occurred 18 to 24 h after the heat stress (Fig. 6, lanes 6 to 10). Prolonged (48 h from heat stress) incubation restored sensitivity of the heat-stressed monocytes to apoptosis (data not shown), suggesting that synthesis of a protecting factor is inducible and transient. Surprisingly, the preceding heat shock usually (in four of six experiments) had no effect on the proportion of annexin V-positive cells induced by phagocytosis, suggesting that the redistribution of phosphatidylserine residues in the cell membrane is not influenced by stress proteins (Fig. 5B and B′). In contrast, PI stainability and the fragmentation of DNA characteristic of apoptosis seemed to depend strongly on HSP. Although DNA laddering is not a quantitative method, the degree of DNA fragmentation appeared to parallel PI staining when both tests were performed on the same cultures.
FIG. 5.
Redistribution of cell membrane phosphatidylserine in resting and stressed monocytes. The cells were cultured in vitro for 26 h prior to annexin V-PI staining. (A) Control cells; (B) heat-shocked cells; (C) heat-shocked cells exposed to antisense oligomers (5 μM). The same populations after an additional 2-h incubation with S. aureus are shown in panels A′, B′, and C′. Other details of the dot plot analysis are as for Fig. 4.
FIG. 6.
Expression of HSP72 correlates with maintenance of DNA integrity after phagocytosis of bacteria, determined by electrophoresis of DNA isolated from 42-h cultures of monocytes. At 18 h before culture termination, cells shown in lanes 6 to 11 were allowed to phagocytose S. aureus. Lanes: 1, control, untreated monocytes; 2, heat shock-treated (HS) cells; 3 and 4, monocytes treated with sense and antisense oligomers, (oligo), respectively; 5, heat shock-treated cells cultured in the presence of antisense oligomers; 6, untreated cells after phagocytosis of S. aureus; 7 to 10, cells exposed to heat shock immediately before and 12, 18, and 24 h before phagocytosis of S. aureus respectively; 11, as for lane 10 except that cells were cultured in the presence of antisense oligomers; 12, DNA size marker (φX174 Hinc II). Each slot was loaded with 100 μg of genomic DNA (corresponding to 106 monocytes used for preparation). Data from one of three experiments performed are shown.
Heat shock-induced resistance to apoptosis is HSP72 dependent.
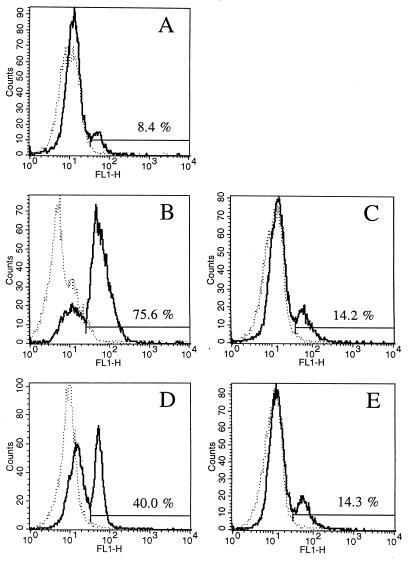
When the monocytes were treated with the HSP72 antisense oligomer, heat stress did not protect the cells against bacterium-induced apoptosis (Fig. 5C and C′; Fig. 6, lanes 10 and 11). The degree of DNA fragmentation and intensity of annexin V-PI staining resembled that obtained with resting monocytes exposed to bacteria. Moreover, without any contact with bacteria, a higher proportion of antisense-treated than control monocytes entered apoptosis after heat treatment (as detected by annexin-PI staining [Fig. 5C] and some DNA laddering [compare lanes 1, 4, and 5 in Fig. 6]). To confirm that in the antisense-treated monocytes the expression of HSP72 was indeed changed and that these changes were oligomer specific, cells cultured for 24 h with oligonucleotides were stained with MAbs to HSP72, HSP27, and HSP90. As shown by subsequent flow cytometry, the treatment with HSP72 antisense oligonucleotide efficiently blocked expression of inducible HSP72 form (Fig. 7). As expected (51), the expression of HSP27 and HSP90 was not influenced by the HSP72 antisense oligomer (data not shown).
FIG. 7.
Heat shock-induced, transient expression of HSP72 and its inhibition by an antisense oligomer. Monocytes were cultured for 24 h with no treatment (A), heat shocked and cultured for 24 (B) or 48 (C) h, or heat shocked and cultured for 24 h in the presence of 2.5 (D) or 5 (E) μM (E) antisense oligonucleotides. Intensity of fluorescence (x axis) indicates the expression of HSP72 in monocytes. The percentages of cells with HSP72 expression in the regions are shown. Dotted line, isotype control; solid line, anti-HSP72 MAb.
DISCUSSION
In this study we have shown that in monocytes, HSP72 provides efficient protection against apoptosis triggered by phagocytosis of S. aureus. To investigate the effect of heat stress on monocytes, we used two different methods for measurement of apoptosis: staining with annexin V-PI (to estimate cell viability and detect early apoptosis) and analysis of genomic DNA integrity (to show late apoptosis). We have shown previously that phagocytosis of extracellular bacteria by peripheral blood monocytes leads to apoptosis of phagocytic cells, demonstrated as proved by TUNEL (terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling) technique and DNA fragmentation (2). In monocytes, DNA fragmentation was observed 2 h after phagocytosis of S. aureus, but clear-cut DNA laddering was reproducibly observed some hours later (2); therefore, 18 h after phagocytosis was the time point chosen for analysis of monocyte DNA. To monitor monocyte apoptosis at the single-cell level, we tried staining with PI combined with FITC-conjugated annexin V, which binds to surface-exposed phosphatidylserine (13, 50). This method allowed us to monitor the proportion of apoptotic cells in control monocyte populations and in cells exposed to heat stress but was not suitable for evaluation of monocyte apoptosis after phagocytosis of bacteria. As early as 60 to 90 min after phagocytosis of S. aureus, more than a half of the monocytes were PI positive. Although PI-positive cells are generally considered necrotic or dead (50), this apparently was not the case in our study. First, DNA fragmentation is clearly seen only some hours later (2). Second, monocytes after phagocytosis of bacteria accumulate IL-1 mRNA and release over 20 h a large amount of biologically active (ICE-processed [21]) IL-1β (16a). A possible explanation for the observed early PI staining is membrane depolarization which without causing cell death allows the dye to penetrate the cell. Such a phenomenon was previously reported for monocytes and macrophages exposed to ATP (21, 39) and was associated with processing of the native form of IL-1β (21). Moreover, it has been shown that macrophage membrane depolarization can be induced by bacterial products (29). Despite the above-mentioned limitation, PI stainability correlated better with monocyte DNA laddering than with binding of annexin V to the cell surface, suggesting that the use of the annexin binding assay for evaluation of monocyte apoptosis needs more systematic studies.
Monocyte apoptosis as a consequence of phagocytosis occurs despite the fact that the uptake of S. aureus increases synthesis of HSP72 (26), a protein with well-established antiapoptotic activity in different cell types (10, 20, 23, 28, 45, 54). In our experiments the protective effect on monocytes was not seen when cells were exposed to bacteria shortly after heat shock. This however, did not exclude the possibility that accumulation of HSP prior phagocytosis of bacteria can be essential for phagocyte protection. So that the cells could accumulate the necessary amount of the inducible HSP72 protein, phagocytosis was performed 18 to 24 h after stress. Indeed, under this condition, stress rendered monocytes resistant to apoptotic DNA laddering and decreased the number of PI-positive cells in a population which phagocytosed S. aureus. Annexin V binding was, however, not reduced in most experiments, and therefore we speculate that the bacterium-induced changes in cell membrane integrity are regulated independently from mechanisms responsible for membrane permeabilization and leading to DNA fragmentation.
To see whether HSP72 is responsible for the protection of monocytes, the synthesis of this chaperone was abrogated by using an antisense oligomer. Since monocytes spontaneously uptake high amounts of native oligonucleotides from culture medium (18), we used nonmodified oligonucleotides without a carrier. The effectiveness of an antisense oligonucleotide with the same sequence as the one applied in this work has been shown by others (51) and confirmed in this report by the lack of HSP72 accumulation after heat shock, as determined by flow cytometry. In contrast, the treatment did not affect two other major stress proteins, HSP27 and HSP90, measured under the same conditions. The treatment with oligonucleotides neither compromised cell viability nor interfered with phagocytosis; moreover, β-actin mRNA accumulation did not decline (not shown). However, annexin-PI and DNA laddering indicated that the heat-shocked monocytes treated with HSP72 antisense oligonucleotide entered apoptosis without any contact with bacteria, indicating that thermal stress may be lethal for nonprotected monocytes.
The inhibition of apoptosis by prior heat shock was not seen in monocytes treated with the HSP72 antisense oligonucleotide. Therefore, it is conceivable that HSP72 was necessary for protection of monocyte DNA against apoptotic fragmentation triggered by phagocytosis of bacteria. Since the analysis of HSP72 provided a satisfactory explanation of the observed phenomena, involvement of the other HSPs in bacterium-induced apoptosis was not investigated. Although the protective role of HSP27 and HSP90 in S. aureus-induced monocyte apoptosis was largely excluded in experiments with the use of HSP72 antisense oligonucleotides, we are aware of the possible role of various HSPs in apoptosis induced by other factors. Obvious candidates to study in the context of apoptosis are the small HSPs (HSP25/27 and αB-crystallin). HSP27 and αB-crystallin were recently shown to be essential for cellular protection against TNF-α-induced tumor cell death (33, 34), and HSP25 was found to be necessary for protection against TNF-α-induced oxidative DNA damage (37). Unlike HSP72, the small HSPs are present in resting cells, sometimes in abundant amounts (7, 16, 49a). Thus, their concentrations are not limiting for the protective function.
In the case of S. aureus-induced apoptosis, we are far from understanding how the phagocytosed bacterium delivers the death signal to the phagocyte. The staphylococcal alpha-, beta-, and gamma-toxins as well as leukocidin are obvious candidates since they interact with cell membranes and are cytotoxic (49). To date, their involvement in monocyte apoptosis has been neither proved nor formally excluded. S. aureus mutants with inactivated alpha- or beta-toxin genes by insertional mutagenesis and allelic replacement (36) seem to offer a good model for such studies. Our preliminary data (42) suggest, however, that strains deficient in alpha- or beta-toxin and/or deficient in protein A induce monocyte apoptosis comparably to the parental, toxin-expressing strain. In addition, leukocidin, which kills phagocytic cells in vitro, is not responsible for the observed effect, since under our experimental conditions phagocytosis of S. aureus does not trigger apoptosis of polymorphonuclear leukocytes (2).
The two major human phagocytes, polymorphonuclear leukocytes and monocytes, respond differently to the phagocytosis of S. aureus (2, 41). On the other hand, monocytes differently react to the phagocytosis of different bacteria regarding both induction of apoptosis and stimulation of stress proteins synthesis (2, 3, 9, 11, 32). It has been suggested that the cellular stress machinery is involved in regulation of apoptosis, and stress-inducing and apoptotic signals may partly have the same molecular basis (38). HSP70 was found to inhibit stress-induced apoptosis by interfering with the stress-activated protein kinase/Jun N-terminal kinase signaling pathway and by inhibiting caspase 3 processing (35). This might explain the reduced DNA fragmentation observed in stressed cells since DNase activation is caspase 3 dependent (12). The negative correlation between DNA fragmentation and the level of HSP72 reported in this paper demonstrates that apoptosis may be inhibited at the terminal stage. This supports the recent finding that apoptosis is inhibited by HSP70-downstream pro-caspase 3 (24).
Taken together, our data clearly show that monocytes exposed to stress are more resistant to apoptotic signals provided during exposure to pathogen. Stressed phagocytes effectively kill bacteria (41), and macrophages which express HSP70 are more resistant to apoptosis induced by hypoxia (54). Collectively, these features improve the effectiveness of the defense mechanisms important during soft tissue infections caused by S. aureus and other pathogens and may represent more general mechanism of response to injury.
ACKNOWLEDGMENTS
We are grateful to B. Rajwa and T. Bernaś for analysis and processing of confocal images and to J. Potempa for critical reading of the manuscript.
This work was partially supported by grants 4 P05A 077 14 and 2224/4/91 from the Committee of Scientific Research and by grant 994/94 from the Foundation of Polish-German Cooperation in Warsaw.
REFERENCES
- 1.Angelidis C E, Lazaridis I, Pagoulatos G N. Constitutive expression of heat-shock protein 70 in mammalian cells confers thermoresistance. Eur J Biochem. 1991;199:35–39. doi: 10.1111/j.1432-1033.1991.tb16088.x. [DOI] [PubMed] [Google Scholar]
- 2.Baran J, Guzik K, Hryniewicz W, Ernst M, Flad H-D, Pryjma J. Apoptosis of monocytes and prolonged survival of granulocytes as a result of phagocytosis of bacteria. Infect Immun. 1996;10:4242–4248. doi: 10.1128/iai.64.10.4242-4248.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barazzone C, Kantengwa S, Suter S, Polla B S. Phagocytosis of Pseudomonas aeruginosa fails to elicit heat shock protein expression in human monocytes. Inflammation. 1996;20:243–262. doi: 10.1007/BF01488202. [DOI] [PubMed] [Google Scholar]
- 4.Bellmann K, Jaattela M, Wissing D, Burkart V, Kolb H. Heat shock protein hsp70 overexpression confers resistance against nitric oxide. FEBS Lett. 1996;391:185–188. doi: 10.1016/0014-5793(96)00730-2. [DOI] [PubMed] [Google Scholar]
- 5.Belka C, Ahlers A, Scott C, Gaestel M, Herrmann F, Brach M A. Interleukin (IL)-6 signaling leads to phosphorylation of the small heat shock protein (Hsp)27 through activation of the MAP kinase and MAPKAP kinase 2 pathway in monocytes and monocytic leukemia cells. Leukemia. 1995;9:288–294. [PubMed] [Google Scholar]
- 6.Bensaude O, Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2:173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beresford P J, Jaju M, Friedman R S, Yoon M J, Lieberman J. A role for heat shock protein 27 in CTL-mediated cell death. J Immunol. 1998;161:161–167. [PubMed] [Google Scholar]
- 8.Bukau B, Horwich A L. The Hsp70 and Hsp60 chaperone machines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 9.Chen Y, Smith M R, Thirumalai K, Zychlinski A. A bacterial invasin induces macrophage apoptosis by binding directly to ICE. EMBO J. 1996;15:3853–3860. [PMC free article] [PubMed] [Google Scholar]
- 10.Dix D J, Allen J W, Collins B W, Mori C, Nakamura N, Poorman-Allen P, Goulding E H, Eddy E M. Targeted gene disruption of Hsp70-2 results in failed meiosis, germ cell apoptosis, and male infertility. Proc Natl Acad Sci USA. 1996;93:3264–3268. doi: 10.1073/pnas.93.8.3264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Durrbaum-Landmann I, Grecken J, Flad H D, Ernst M. Effect of in vitro infection of human monocytes with low numbers of Mycobacterium tuberculosis bacteria on monocyte apoptosis. Infect Immun. 1996;64:5384–5389. doi: 10.1128/iai.64.12.5384-5389.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A, Nagata S. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature. 1998;391:43–50. doi: 10.1038/34112. [DOI] [PubMed] [Google Scholar]
- 13.Fadok V A, Voelker D R, Campbell P A, Cohen J J, Bradton D L, Henson P M. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J Immunol. 1992;148:2207–2216. [PubMed] [Google Scholar]
- 14.Galea-Lauri J, Richardson A J, Latchman D S, Katz D R. Increased heat shock protein 90 (hsp90) expression leads to increased apoptosis in the monoblastoid cell line U937 following induction with TNF-a and cycloheximide. J Immunol. 1996;157:4109–4118. [PubMed] [Google Scholar]
- 15.Georgopoulos C, Welch W J. Role of the major heat shock proteins as molecular chaperones. Annu Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125. [DOI] [PubMed] [Google Scholar]
- 16.Golenhofen N, Ness W, Koob R, Htun P, Schaper W, Drenckhahn D. Ischemia-induced phosphorylation and translocation of stress protein alphaB-crystallin to Z lines of myocardium. Am J Physiol. 1998;274:H1457–H1464. doi: 10.1152/ajpheart.1998.274.5.H1457. [DOI] [PubMed] [Google Scholar]
- 16a.Guzik, K., and J. Pryjma. Unpublished data.
- 17.Hansen L K, Houchins J P, O’Leary J J. Differential regulation of HSC70, HSP70, HSP90α, and HSP90β mRNA expression by mitogen activation and heat shock in human lymphocytes. Exp Cell Res. 1991;192:587–596. doi: 10.1016/0014-4827(91)90080-e. [DOI] [PubMed] [Google Scholar]
- 18.Hartmann G, Krug A, Bidlingmaier M, Hacker U, Eigler A, Albrecht R, Strasburger C J, Endres S. Spontaneous and cationic lipid-mediated uptake of antisense oligonucleotides in human monocytes and lymphocytes. J Pharmacol Exp Ther. 1998;285:920–928. [PubMed] [Google Scholar]
- 19.Hayashi T, Catanzaro A, Rao S P. Apoptosis of human monocytes and macrophages by Mycobacterium avium sonicate. Infect Immun. 1997;65:5262–5271. doi: 10.1128/iai.65.12.5262-5271.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He L, Fox M H. Variation of heat shock protein 70 through the cell cycle in HL-60 cells and its relationship to apoptosis. Exp Cell Res. 1997;232:64–71. doi: 10.1006/excr.1997.3494. [DOI] [PubMed] [Google Scholar]
- 21.Hogquist K A, Nett M A, Unanue E R, Chaplin D D. Interleukin 1 is processed and released during apoptosis. Proc Natl Acad Sci USA. 1991;88:8485–8489. doi: 10.1073/pnas.88.19.8485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hunt C, Morimoto R I. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci USA. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jaattela M, Wissing D, Bauer P A, Li G C. Major heat shock protein hsp70 protects tumor cells from tumor necrosis factor cytotoxicity. EMBO J. 1992;11:3507–3512. doi: 10.1002/j.1460-2075.1992.tb05433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jaattela M, Wissing D, Kokholm K, Kallunki T, Egeblad M. Hsp70 exerts its anti-apoptotic function downstream of caspase-3-like proteases. EMBO J. 1998;17:6124–6134. doi: 10.1093/emboj/17.21.6124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jacquier-Sarlin M R, Jornot L, Polla B S. Differential expression and regulation of hsp70 and hsp90 by phorbol esters and heat shock. J Biol Chem. 1995;270:14094–14099. doi: 10.1074/jbc.270.23.14094. [DOI] [PubMed] [Google Scholar]
- 26.Kantengwa S, Polla B S. Phagocytosis of Staphylococcus aureus induces a selective stress response in human monocytes-macrophages (Mφ): modulation by Mφ differentiation and by iron. Infect Immun. 1993;61:1281–1287. doi: 10.1128/iai.61.4.1281-1287.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kao H T, Capasso O, Heintz N, Nevins J R. Cell cycle control of the human HSP70 gene: implications for the role of a cellular E1A-like function. Mol Cell Biol. 1985;5:628–633. doi: 10.1128/mcb.5.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim Y M, de Vera M E, Watkins S C, Billiar T R. Nitric oxide protects cultured rat hepatocytes from tumor necrosis factor-alpha-induced apoptosis by inducing heat shock protein 70 expression. J Biol Chem. 1997;272:1402–1411. doi: 10.1074/jbc.272.2.1402. [DOI] [PubMed] [Google Scholar]
- 29.Kluftinger J L, Lutz F, Hancock R E. Pseudomonas aeruginosa cytotoxin: periplasmic localization and inhibition of macrophages. Infect Immun. 1989;57:882–886. doi: 10.1128/iai.57.3.882-886.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liossis S N, Ding X Z, Kiang J G, Tsokos G C. Overexpression of the heat shock protein 70 enhances the TCR/CD3- and Fas/Apo-1/CD95-mediated apoptotic cell death in Jurkat T cells. J Immunol. 1997;158:5668–5675. [PubMed] [Google Scholar]
- 31.McCormick T S, McColl K S, Distelhorst C W. Mouse lymphoma cells destined to undergo apoptosis in response to thapsigargin treatment fail to generate a calcium-mediated grp78/grp94 stress response. J Biol Chem. 1997;272:6087–6092. doi: 10.1074/jbc.272.9.6087. [DOI] [PubMed] [Google Scholar]
- 32.McNeil G, Virji M, Moxon E R. Interactions of Neisseria meningitidis with human monocytes. Microb Pathog. 1994;16:153–163. doi: 10.1006/mpat.1994.1016. [DOI] [PubMed] [Google Scholar]
- 33.Mehlen P, Schulze-Osthoff K, Arrigo A P. Small stress proteins as novel regulators of apoptosis. Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J Biol Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 34.Mehlen P, Kretz-Remy C, Preville X, Arrigo A P. Human hsp27, Drosophila hsp27 and human alphaB-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNF-alpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- 35.Mosser D D, Caron A W, Bourget L, Denis-Larose C, Massie B. Role of the human heat shock protein hsp70 in protection against stress-induced apoptosis. Mol Cell Biol. 1997;17:5317–5327. doi: 10.1128/mcb.17.9.5317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Reilly M, Azavedo J C S, Kennedy S, Foster T J. Inactivation of the alpha-haemolysin gene of Staphylococcus aureus 8325-4 by side-directed mutagenesis and studies on the expression of its hemolysins. Microb Pathog. 1986;1:125–138. doi: 10.1016/0882-4010(86)90015-x. [DOI] [PubMed] [Google Scholar]
- 37.Park Y M, Han M Y, Blackburn R V, Lee Y J. Overexpression of HSP25 reduces the level of TNF alpha-induced oxidative DNA damage biomarker, 8-hydroxy-2′-deoxyguanosine, in L929 cells. J Cell Physiol. 1998;174:27–34. doi: 10.1002/(SICI)1097-4652(199801)174:1<27::AID-JCP4>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 38.Pena L A, Fuks Z, Kolesnick R. Stress-induced apoptosis and the sphingomyelin pathway. Biochem Pharmacol. 1997;53:615–621. doi: 10.1016/s0006-2952(96)00834-9. [DOI] [PubMed] [Google Scholar]
- 39.Picello E, Pizzo P, Di Virgillo F. Chelation of cytoplasmic Ca2+ increases plasma membrane permeability in murine macrophages. J Biol Chem. 1990;265:5635–5639. [PubMed] [Google Scholar]
- 40.Polla B S, Kantengwa S. Heat shock proteins and inflammation. Curr Top Microbiol Immunol. 1991;167:93–105. doi: 10.1007/978-3-642-75875-1_6. [DOI] [PubMed] [Google Scholar]
- 41.Polla B S, Stubbe H, Kantengwa S, Maridonneau-Parini I, Jacquier-Sarlin M R. Differential induction of stress proteins and functional effects of heat shock in human phagocytes. Inflammation. 1995;19:363–378. doi: 10.1007/BF01534393. [DOI] [PubMed] [Google Scholar]
- 42.Pryjma, J., et al. Unpublished data.
- 43.Riabowol K T, Mizzen L A, Welch W J. Heat shock is lethal to fibroblasts microinjected with antibodies against HSP70. Science. 1988;242:433–436. doi: 10.1126/science.3175665. [DOI] [PubMed] [Google Scholar]
- 44.Richards F M, Watson A, Hickman J A. Investigation of the effects of heat shock and agents which induce a heat shock response on the induction of differentiation of HL-60 cells. Cancer Res. 1988;48:6715–6720. [PubMed] [Google Scholar]
- 45.Samali A, Cotter T G. Heat shock proteins increase resistance to apoptosis. Exp Cell Res. 1996;223:163–170. doi: 10.1006/excr.1996.0070. [DOI] [PubMed] [Google Scholar]
- 46.Schmidt J A, Abdulla E. Down-regulation of IL-1β biosynthesis by inducers of heat shock response. J Immunol. 1988;141:2027–2034. [PubMed] [Google Scholar]
- 47.Snyder Y M, Guthrie L, Evans G F, Zuckerman S H. Transcriptional inhibition of endotoxin-induced monokine synthesis following heat shock in murine peritoneal macrophages. J Leukoc Biol. 1992;51:181–187. doi: 10.1002/jlb.51.2.181. [DOI] [PubMed] [Google Scholar]
- 48.Toellner K M, Scheel-Toellner D, Sprenger R, Duchrow M, Trumper L H, Ernst M, Flad H-D, Gerdes J. The human germinal centre cells, follicular dendritic cells and germinal centre T cells produce B cell-stimulating cytokines. Cytokine. 1995;7:344–354. doi: 10.1006/cyto.1995.0044. [DOI] [PubMed] [Google Scholar]
- 49.Tomita T, Kamio Y. Molecular biology of the pore-forming cytolysins from Staphylococcus aureus, alpha- and gamma-hemolysins and leukocidin. Biosci Biotechnol Biochem. 1997;61:565–572. doi: 10.1271/bbb.61.565. [DOI] [PubMed] [Google Scholar]
- 49a.van de Klundert F A, Gijsen M L, van den Ijssel P R, Snoeckx L H, Jong W W. alpha B-crystallin and hsp25 in neonatal cardiac cells—differences in cellular localization under stress conditions. Eur J Cell Biol. 1998;75:38–45. doi: 10.1016/s0171-9335(98)80044-7. [DOI] [PubMed] [Google Scholar]
- 50.Vermes I, Haanen C, Steffens-Nakken H, Reutelingsperger C. A novel assay for apoptosis. Flow cytometric detection of phosphatidylserine expression on early apoptotic cells using fluorescein labelled Annexin V. J Immunol Methods. 1995;184:39–51. doi: 10.1016/0022-1759(95)00072-i. [DOI] [PubMed] [Google Scholar]
- 51.Wei Y Q, Zhao X, Kariya Y, Teshigawara K, Uchida A. Inhibition of proliferation and induction of apoptosis by abrogation of heat-shock protein (HSP) 70 expression in tumor cells. Cancer Immunol Immunother. 1995;40:73–78. doi: 10.1007/BF01520287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wissing D, Jaattela M. HSP27 and HSP70 increase the survival of WEHI-S cells exposed to hyperthermia. Int J Hyperthermia. 1996;12:125–138. doi: 10.3109/02656739609023695. [DOI] [PubMed] [Google Scholar]
- 53.Wu B J, Morimoto R. Transcription of the human hsp70 gene is induced by serum stimulation. Proc Natl Acad Sci USA. 1985;82:6070–6074. doi: 10.1073/pnas.82.18.6070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yun J K, McCormick T S, Villabona C, Judware R R, Espinosa M B, Lapetina E G. Inflammatory mediators are perpetuated in macrophages resistant to apoptosis induced by hypoxia. Proc Natl Acad Sci USA. 1997;94:13903–13908. doi: 10.1073/pnas.94.25.13903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Zychlinsky A, Prevost M C, Sansonetti P J. Shigella flexneri induces apoptosis in infected macrophages. Nature. 1992;358:167–169. doi: 10.1038/358167a0. [DOI] [PubMed] [Google Scholar]
- 56.Zychlinsky A, Sansonetti P J. Apoptosis as a proinflammatory event: what can we learn from bacteria-induced cell death? Trends Microbiol. 1997;5:201–204. doi: 10.1016/S0966-842X(97)01044-5. [DOI] [PubMed] [Google Scholar]