Abstract
Purpose:
To determine the effectiveness of laser vitreolysis in terms of contrast sensitivity function (CSF) and vision-related quality of life (VRQol) for symptomatic floaters due to posterior vitreous detachment (PVD).
Materials:
This is an interventional study that involved 57 eyes of 45 patients with symptomatic floaters for more than 3 months. Patients underwent one to three sessions of vitreolysis via Neodymiun-doped Yttrium Aluminum Garnet (Nd:YAG) laser. We examined the CSF using the computer programs Freiburg Acuity and Contrast Test (FrACT) and VRQoL survey using the National Eye Institute Visual Function Questionnaire-25 (NEI VFQ-25) before, and 1 month after, vitreolysis.
Results:
Twelve patients had both eyes lasered and 33 patients had one eye lasered. The mean CSF improved from 3.20 ± 0.85%W to 2.64 ± 0.63%W 1 month after vitreolysis. Each use of the laser showed a significant mean difference in CSF (%W) as analyzed by paired t-test before and after the first laser (0.29 ± 0.49%W [P ≤ 0.001]); after the first and second laser (0.35 ± 0.53%W [P = 0.01]); and after second and third laser (0.21 ± 0.31%W [P = 0.02]). There was improvement in the median of four subscales in NEI VFQ-25 scores post treatment: general vision (z = −3.30, P = 0.001), near activity (z = 3.396, P = 0.001, distance activity (z = −2.788, P = 0.005), and mental health (z = −2.219, P = 0.026). The mean scores increased to 79.55 ± 9.45 from the baseline 75.06 ± 9.69 (P ≤ 0.001). No adverse events were recorded 1 month after the laser treatments.
Conclusion:
Vitreolysis by Nd:YAG laser improved the CFS and VRQoL in symptomatic PVD patients.
Keywords: Contrast sensitivity function, floaters, laser vitreolysis, neodymiun-doped yttrium aluminum garnet laser, posterior vitreous detachment
Vitreous floaters in posterior vitreous detachment (PVD) cast shadows on the retina, resulting in reduced quality of vision and degradation of contrast sensitivity function (CSF).[1] PVD prevalence rate was 2.7% in a study in China.[2] Older age, female gender, and myopia are the risk factors for PVD.[2]
Symptomatic floaters were not treated in past decades. However, there is an underestimation of its adverse effects.[3,4] An electronic survey reported that 76% of smartphone users reported seeing floaters, and 33% of them had noticeable visual impairment.[3] On average, patients were willing to accept an 11% risk of death and 7% risk of blindness and were willing to trade off 1.1 years out of every 10 years of their remaining lives to be symptom-free.[4]
Visual acuity (VA) was not significantly affected in patients with symptomatic floaters.[5,6] However, CSF was diminished by 51.2% to 67% in PVD.[6,7,8,9,10] The National Eye Institute Visual Functioning Questionnaire-25 (NEI VFQ-25) was utilized as the subjective measurement of vision-related quality of life (VRQoL) in many studies. NEI VFQ-25 scores were 28.3% lower among patients with floaters (73.2 ± 15.6, N = 16) than in age-matched control subjects (93.9 ± 8.0, N = 12, P < 0.001).[8]
Treatments for symptomatic floaters include conservative observation, laser vitreolysis, and pars plana vitrectomy. Vitrectomy has been the standard treatment with a significant risk of complications, including endophthalmitis, retinal tear, retinal detachment, macular edema, cataract, or glaucoma.[1]
Neodymium-doped yttrium aluminium garnet (Nd:YAG) laser vitreolysis is an alternative treatment. It is non-invasive, painless, short duration, immediate effect, low-cost, and convenient, as it can be performed as a clinic-based procedure.[11] Nevertheless, clinical studies have demonstrated highly variable success rates from 0 to 100% in vitreolysis for the past 2 decades.[12]
Older techniques have a lower success rate and safety profile.[13,14] Inconsistency in energy delivery, lack of visualization of the vitreous in full depth, and suboptimal power usage in older techniques caused variable outcomes in vitreolysis.[15] For this study, we utilized a midvitreous contact lens and multi-modality Nd:YAG laser specifically designed for vitreolysis by its coaxial illumination technique, which allowed titration of red reflex by moving the slit-lamp beam to focus the beam on vitreous opacities.[15] for better visualization.
Studies have examined symptomatic PVD patients treated with laser vitreolysis and assessed the efficacy of the treatment by looking into their CSF10 and VRQoL.[7,16,17] The majority of studies did not address the differences in CSF in various ethnicities. Interestingly, a study in Singapore demonstrated that the Chinese had lower CSF than other races (Malays, Indians, Eurasians, and others) among their adult population.[18]
Our study aims to determine the treatment efficacy of vitreolysis by evaluating the CSF and VRQoL in patients with symptomatic floaters due to PVD in a Malaysian population. The secondary objective was to investigate the safety of the vitreolysis procedure.
Methods
This was a prospective interventional study conducted from June 2017 until Oct 2020. The relevant Research and Ethics Committee approved the study FF-2017-336, which adhered to the tenets of the Declaration of Helsinki. Written informed consent was obtained from each subject after a full explanation of the procedure.
Study participants were recruited via referral from private ophthalmology centers, health clinics, and patient self-referral by emailing investigators. Flyers in hard and soft copy were distributed to promote this study to the public.
Subjects were eligible if they were 18 years and older; had symptomatic floaters for at least 3 months; and had been diagnosed with PVD with the presence of a Weiss ring or any form of vitreous opacity in the mid vitreous cavity. Subjects were excluded if there was high myopia (greater than − 6 diopters); asteroid hyalosis; floaters inaccessible with available lasers and lenses; floaters located near to the crystalline lens or the retina; the presence of corneal or lenticular opacities or any media opacity that may interfere with treatment; floaters deemed to require more than 5 treatment sessions (where surgical intervention is the best treatment course), elevated intraocular pressure (IOP) and on IOP lowering medication; found to have a retinal tear or retinal detachment (RD) on initial examination, history of RD repair in either eye; maculopathy of any kind; moderate or severe dry eye; ocular surgery of any kind in preceding 2 months; or psychiatric problems that may recur or worsen.
The sample size was determined by Cohen’s effect size.[19] The recommended sample size was 45 subjects, and a near moderate effect of 0.6 with a power of 80% was selected. Forty-five patients were recruited for laser vitreolysis.
The VA of recruited subjects was taken with ETDRS, followed by the Freiberg Acuity and Contrast Test (FrACT), which is available online.[20] Automated software with tumbling monochromatic Landolt C optotype was used. Patients were required to dark-adapt for 2 min, and the distance to the monitor was 2.9 m. This gave the mesopic reading. The Weber Index (WI) was automatically calculated by the program. A higher WI (%W) reflected a lower CSF. A brief history was taken from each patient who also drew the floaters they had seen subjectively.
The patient answered the NEI VFQ-25, which has been validated in two languages (Malay[21] and English[22] versions), with assistance from the investigator. Written permission was obtained from the author of the Malay version.
NEI VFQ-25 contains 25 targeted questions, representing 11 vision-related and general health subscales. Individual scores for all the sections were tabulated according to the described scoring algorithm published.[23]
Subsequently, the patients underwent slit-lamp examination, Goldman applanation tonometry, and then the eyes were dilated for vitreous and fundus examination using fundus biomicroscopy, and binocular indirect ophthalmoscopy (BIO), and Goldmann 3-mirror examination. Full pupil dilatation was achieved with tropicamide 1.0% (Mydriacyl, Alcon) and phenylephrine 2.5% (Myfrin, Alcon). Proparacaine 0.5% (Alcaine, Alcon) was applied to anesthetize the surface. Self-prepared povidone 5% eyedrop was also added to the conjunctival sac after the commencement of the COVID-19 pandemic in March 2020. The drawing of the floaters was correlated to the finding from the ophthalmoscopy. The distance from the floaters to the retina was assessed subjectively.
Next, optical coherence tomography (OCT) (Spectralis™, Heidelberg Engineering, Heidelberg, Germany) was used to scan the central macula thickness (CMT) before and 1 month after the treatment. Color photography of the fundus and vitreous was attempted by a fundus camera (TRC-50DX Type 1A, Topcon, Japan) to record the floaters. These two investigations were performed by different technicians with no less than 3 years experience.
Laser vitreolysis was performed by a single ophthalmologist who was trained in vitreoretinal diseases with over 10 years experience using Multi-Modality Nd:YAG laser (EllexUltraQ Reflex™, Ellex Medical, Australia) and contact lenses Ocular Karichkoff 21 mm OJKY-1 vitreous lens, Ocular Karichkoff off-Axis OJKPY-25 vitreous lens, or Ocular Peyman wide field OPY-25 (Ocular instrument, Washington, USA). YAG laser lens was used with polyacrylic coupling gel (Siccapos gel®, URSAPHARM).
The laser settings were based on the EllexUltraQ reflex™ operator manual.[24] The laser was initiated with a lower power of 1.3 mJ and slowly escalated by observing the formation of gas bubbles from the floaters and observing the disruption and movement of the floaters. The number of shots per session was limited to approximately 600 if the floaters could not be eliminated with a lower number of shots. Laser power and the number of shots per session were recorded.
Thirty minutes after the laser, the patient was re-examined to check the IOP, retinal tear, and hemorrhage. Guttae dexamethasone sodium phosphate 0.1% (Maxidex®, Alcon) was prescribed every 6 h for 1 week. Patients were counseled regarding the symptoms of retinal tear or RD and advised to return should they experience floaters and flashes or curtain-like visual field loss.
Patients were reviewed 1 month later after the last treatment. Laser vitreolysis was completed if patients’ symptoms had resolved, floaters could not be found or were too close to the retina or lens. If patients were still symptomatic after three sessions, they could continue their treatment but were excluded from the study. Due to financial constraints, the study could not be extended to include patients more than 3 months from the initial treatment.
Statistical analysis was performed using the IBM Statistical Package for the Social Sciences software, version 22 (SPSS 22.0, Inc., Chicago, IL, USA). The mean change in CSF in WI at each session of the laser was compared with paired t-test. A subjective assessment of VRQoL by NEI VFQ-25 was analyzed by the Wilcoxon signed ranks test. The analysis of CSF was based on the number of eyes; however, the VRQoL analysis used the number of patients. Other continuous variables (e.g., VA, IOP, and CMT) were analyzed according to the number of eyes. The difference in CMT was compared using a paired-t test, whereas the IOP and CMT values were compared using the Wilcoxon signed ranks test. A P value of less than 0.05 was considered statistically significant.
Results
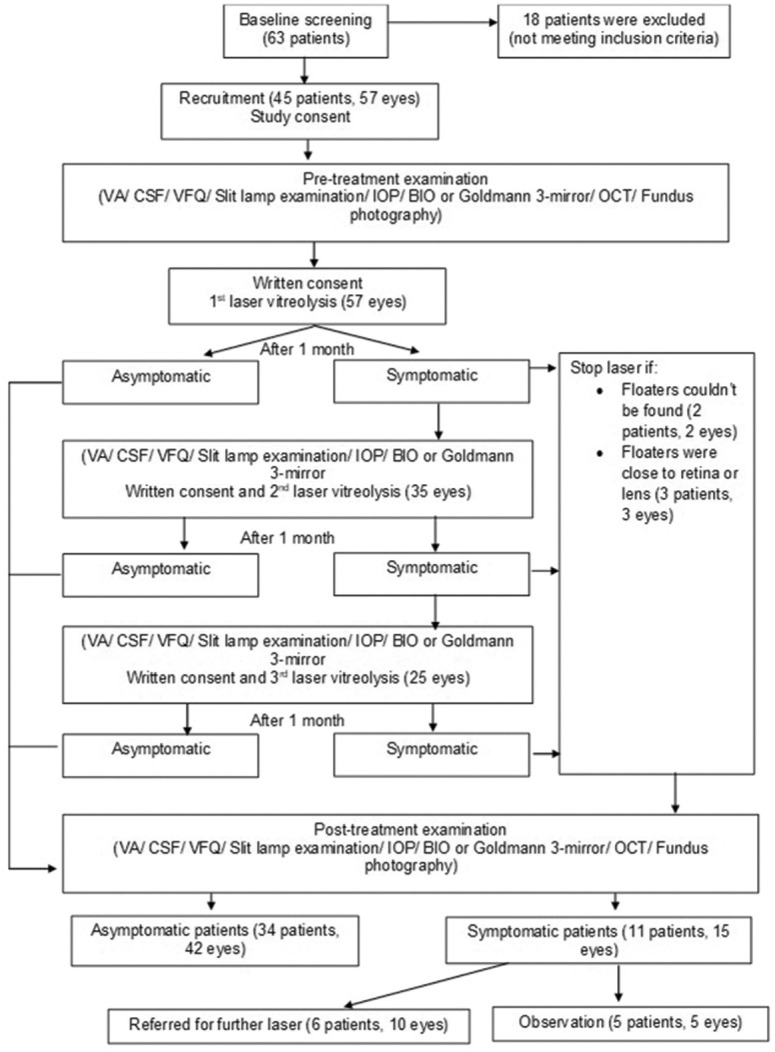
Fig. 1 shows the consort flow diagram of the study. Forty-five patients and 57 eyes were treated. Thirty-four (75.6%) patients became asymptomatic within three laser sessions. Six (13.3%) patients remained symptomatic and required additional laser treatment; all of them had mixed or amorphous types of floaters. The remaining five (11.1%) patients did not undergo laser due to the inability to visualize the floaters or the floaters were adjacent to the retina. Although not symptom-free, there were improvements compared to pre-treatment.
Figure 1.
Consort flow diagram
Demographical data are displayed in Table 1. The female-to-male ratio is 2:1. Malay and Chinese were almost equal in number. Most patients only complained of floaters in one eye. Most floaters were in a mixed pattern.
Table 1.
Baseline characteristics and demographic data of subjects
| Parameter | Values (percentage %) [range] |
|---|---|
| Total number of patients | 45 |
| Total number of eyes | 57 |
| Mean age (years) (range) | 60.4±11.14 [33-87] |
| Gender | |
| Female | 30 (66.7%) |
| Male | 15 (33.3%) |
| Ethnicity | |
| Malay | 24 (53.3%) |
| Chinese | 20 (44.5%) |
| Indian | 1 (2.2%) |
| Laterality | |
| Unilateral | 33 (73.3%) |
| Bilateral | 12 (26.7%) |
| Mean duration of floaters (months) | 18±3.59 [4-120] |
| Refractive error (eyes) | |
| Myopia | |
| Mild (0-1.5D) | 19 (33.3%) |
| Moderate (1.5-6.0D) | 15 (26.3%) |
| Emmetropia | 11 (19.3%) |
| Hyperopia | 12 (21.1%) |
| Lens status (eyes) | |
| Phakia | 36 (63.2%) |
| Pseudophakia | 21 (36.8%) |
| Pattern of floaters (eyes) | |
| Single Weiss ring | 8 (14.0%) |
| Clump/cloudy | 14 (24.6%) |
| Multiple mixed pattern (ring/streak/dot/amorphous/cloudy/clump) | 35 (61.4%) |
| Number of laser sessions (eyes) | |
| 1 | |
| 2 | 22 (38.6%) |
| 3 | 10 (17.5%) |
| Mean laser session | 25 (43.9%) |
| Laser setting | 2.05 times |
| Mean laser energy per shot (mJ) | 2.5±0.55 [1.3-4.0] |
| Mean number of laser shot per session | 348±138 [31-603] |
| Mean laser power per session (mJ) | 850.6±417.6 [74.0-1855.0] |
| Mean total laser power per eye (mJ) | 1730.8±936.5 [135.0-3732.0] |
The logMAR VA values were not significantly changed (median: 0.2 vs. 0.2, interquartile range [IQR]: 0.2–0.2 vs. 0.2–0.2, P = 1.00). The baseline CSF ranged from 1.49%W to 5.32%W with a mean of 3.20 ± 0.85%W. Table 2 shows the CSF value at baseline, first laser, second, and third laser sessions. When the WI was compared to the previous visit during each visit, there was a statistical improvement in the CSF.
Table 2.
Comparison of CSF by Weber Index (%W) before and after laser vitreolysis
| Mean Weber Index (%W)±SD | Paired differences | |||
|---|---|---|---|---|
|
| ||||
| Mean Weber Index (%W)±SD | 95% confidence interval of the difference | Significance (2-tailed) | ||
| Pair 1 | ||||
| Baseline CSF | 3.20±0.85 | 0.29±0.49 | 0.16-0.42 | <0.001 |
| CSF after first laser | 2.90±0.82 | |||
| Pair 2 | ||||
| CSF after first laser | 3.04±0.89 | 0.35±0.53 | 0.16-0.53 | 0.001 |
| CSF after second laser | 2.69±0.59 | |||
| Pair 3 | ||||
| CSF after second laser | 2.72±0.66 | 0.21±0.32 | 0.08-0.34 | 0.002 |
| CSF after third laser | 2.51±0.66 | |||
Analysis by paired t-test
Table 3 shows the comparison of VRQoL before and after the final treatment. There was a statistically significant improvement in four subscales: general vision, near activity, distance activity, and mental health. The mean score was 75.06 ± 9.69 at baseline and improved to 79.55 ± 9.44 after treatment (P = 0.00).
Table 3.
The comparison of VRQoL by NEI VFQ-25 before and 1 month after laser vitreolysis
| Subscales | Median Of pre-treatment | Interquartile range (IQR) Pre-treatment | Median of post-treatment | Interquartile range (IQR) Post treatment | Z | Asymptomatic sig (2 tailed) P |
|---|---|---|---|---|---|---|
| General health | 50.00 | 50.00-75.00 | 50.00 | 50.00-75.00 | -0.164 | 0.870 |
| General vision | 60.00 | 60.00-80.00 | 80.00 | 60.00-80.00 | -3.300 | 0.001 |
| Ocular pain | 75.00 | 62.50-87.50 | 75.00 | 62.50-100.00 | -1.780 | 0.075 |
| Near activity | 66.67 | 58.33-83.33 | 83.33 | 58.33-83.33 | -3.396 | 0.001 |
| Distance activity | 75.99 | 58.33-91.67 | 83.33 | 75.00-100.00 | -2.788 | 0.005 |
| Social function | 100.00 | 75.00-100.00 | 100.00 | 75.00-100.00 | -1.209 | 0.227 |
| Mental health | 75.00 | 62.50-87.50 | 81.25 | 71.88-93.75 | -2.219 | 0.026 |
| Role difficulty | 62.50 | 50.00-87.50 | 75.00 | 50.00-100.00 | -1.534 | 0.125 |
| Dependency | 83.33 | 75.00-100.00 | 91.67 | 75.00-100.00 | -1.180 | 0.238 |
| Driving | 66.67 | 50.00-83.33 | 75.00 | 58.33-83.33 | -1.754 | 0.079 |
| Color vision | 100.00 | 75.00-100.00 | 100.00 | 75.00-100.00 | -0.228 | 0.820 |
| Peripheral vision | 75.00 | 75.00-100.00 | 100.00 | 75.00-100.00 | -1.159 | 0.247 |
Analysis by Wilcoxon signed ranks test
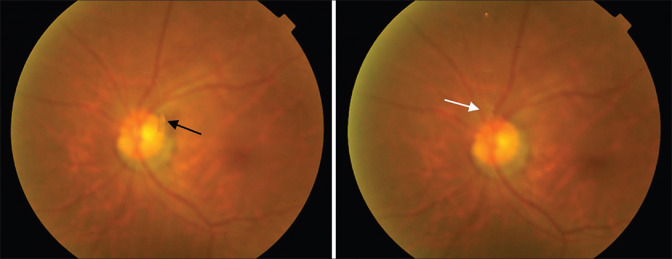
Patients were reviewed 1 month after vitreolysis. Fig. 2 gives an example of the posterior findings after successful vitreolysis in a subject from the study. No adverse events were found. OCT was used to measure the CMT revealed the mean CMT of 269.19 ± 24.71 mm became 268.77 ± 24.07 mm; however, the difference was not significant (P = 0.527) 1 month after laser treatment. The median IOP was 14 mmHg, which was equal before and 1 month after the treatment (IQR = 12–14 mmHg vs. 12–14 mmHg, P = 0.742).
Figure 2.
Fundus photography before and after laser vitreolysis. (Left) Black arrow shows a single small clump floater in front of the optic disc; (Right) White arrow shows minimal remnant of vitreous opacity left after vitreolysis. Patient was asymptomatic after one session of laser so no further treatment was given
Discussion
The study was able to answer the objective of the study. Vitreolysis improves the CSF after each session. It safely improves at least four of the subscales of the NEI VFQ-25.
Contrast sensitivity function (CSF)
The CSF in patients.[5] with PVD from other studies ranged from 1.19%W (normal) to 5.59%W (worse),[5,8] whereas, in our study ranged from 1.49%W to 5.32%W with a mean of 3.20 ± 0.85%W. There was an 18.56% improvement in our study after vitreolysis. Patients who required more than one session showed significant improvement at each subsequent treatment.
A study mentioned that amorphous cloud opacities required higher energy than solitary Weiss ring to remove by vitreolysis.[15] Better results and higher patient satisfaction scores were notably seen with solitary Weiss rings.[15] This is concordant with our study, all six (10.5%) patients who required more than three sessions presented with multiple amorphous vitreous opacities. Nineteen out of the 22 eyes that underwent a single session of laser were the eyes with Weiss ring or solitary opacities. Most eyes in our study had mixed patterns of floaters that needed more than one session, which meant higher total power.
A retrospective comparative study by Nguyen et al.[10] found that there was 130% degradation of CSF in untreated vitreous floaters. However, there were no significant changes in CSF (P = 0.17) after Nd:YAG vitreolysis in 38 patients, differing from our study, possibly because there was a control group. Our study compared the changes to patients’ baseline. This may have resulted in some bias. Nevertheless, our study has a larger number of treated eyes. Both studies were conducted under mesopic lighting that introduces a possibility of inaccuracy because the impact of vitreous floaters varies depending on ambient and background lighting. Unlike the previous study, our study was performed with only a single experienced vitreoretinal surgeon to provide homogeneity in intervention and minimize variability in surgeons’ performance.
Visual-related quality of life
Subjective VRQoL in Nd:YAG treated patients is measured using NEI VFQ-25 in a few studies.[7,16,17] The general composite score in NEI VFQ-25 increased from 71.44 ± 12.77 to 88.54 ± 12.73 6 months after laser vitreolysis.[16] There was a significant improvement in subscale groups such as general vision,[7,16] peripheral vision,[7,16] role difficulties,[7,16] dependency,[7,16] and near vision.[17] We had similar findings in the improvement of general vision and near vision. Additionally, our study demonstrated significant improvement in distance vision and mental health as symptomatic floaters may be associated with depression and anxiety.[4] Our result is encouraging because laser vitreolysis improved the mental well-being of our patients.
Most of our patients’ near vision was affected by floaters, especially during reading and looking at the bright screen of electronic gadgets due to the white background that causes the floaters to be noticed obviously. Therefore, removal of the opacities will improve near vision.[17]
Other parameters that were studied
No adverse effects on the visual acuity, IOP, and macular thickness were noticed, providing reassurance to patients with glaucoma and patient prone to macular edema such as diabetes. However, a formal study is still required.
Discussion of laser setting
Katsanos et al.[25] proposed that laser vitreolysis was indicated if the floaters were more than 2 mm from the retina and more than 5 mm from the crystalline lens. We excluded patients with floaters that were too close to the retina to prevent complications. However, the nature of the PVD with liquefied vitreous causes high mobility in floaters. The impact of the laser shot can cause the further thrust of the floaters nearer to the retina. We abort the procedure when that happened.
A study using the B-scan ultrasound probe to view the behavior of the vitreous during laser procedure had found no movement of vitreous 1 mm or greater around the plasma creation and posterior hyaloid despite a high energy setting of 7 mJ.[15] From our experience, the surgeon should continuously gauge the distance of floaters to the retina during the procedure to avoid retinal hemorrhage from lasering the retina. In principle, if the retina was in focus behind the floater when the aiming beam was in focus on the floater, treatment was not performed.
Shah’s study that only recruited patients with symptomatic Weiss ring appearance revealed greater improvement in a symptom of floaters (54%) that were treated by laser vitreolysis than controls (9%)[7] is similar to our study where patients enjoyed a high rate of symptom improvement after one treatment session. However, Shah’s study did not include amorphous floaters unlike ours. They had a mean of 218 laser shots with a mean power of 1316 mJ in a single laser session. Our study, therefore, resulted in a higher mean power (1730.8 mJ) and more shots (348 shots) and sessions (mean: 2.05) overall.
The energy of the vitreolysis laser should start at the minimum level and be titrated up according to the optical breakdown ability in the vitreous cavity. The lower energy levels disrupt floaters without eliminating them.[25] Delaney’s study using relatively low energy (the total mean energy of 510 mJ) resulted in a 38.3% success rate.[14] Conversely, excessively high energy can cause the formation of cavitation bubbles that move within the vitreous cavity-causing microscopic retinal damage.[25] Besides, the tissue evaporation and the mechanical effect from the shock waves may cause retinal damage and even RD.[25] However, our study safely use a higher total mean energy level of over 1000 mJ.
Vitrectomy was stated as a definitive cure for symptomatic floaters. A study by Sebag et al.[8] treated their patients using 25 G vitrectomy. Sixteen patients’ CSF improved from 4.0 ± 2.3%W to 2.2 ± 1.5%W. In our study, CSF improved from 3.20 ± 0.85%W to 2.51 ± 0.66%W. The post vitrectomy CSF was greater because the CSF was worse at the start. Both procedures improved CSF levels. Unlike laser vitreolysis, which only removes culpable floaters, vitrectomy removes most if not all vitreous opacities. No retinal breaks, infection, or glaucoma were found after a mean of 17.5 months of follow-up in 60 patients after vitrectomy for floaters. However, 23.5% of patients had cataract removal in an average of 15 months after vitrectomy. Vitrectomy, the more-invasive technique, is also generally safe with low rate of adverse events. Hence, more extensive or dense floaters, and in older age patients with cataracts can be given the option of vitrectomy.
Safety
Complications of laser vitreolysis include elevated IOP leading to IOP spikes; cataracts, including posterior capsule defects requiring cataract surgery; retinal tear; retinal detachment; retinal hemorrhages; scotomas; and an increased number of floaters.[13,15,26] Many studies show no adverse outcome, with most retrospective studies with follow-up of at least 6 months being relatively short to conclude the safety of this procedure.[7,10,14,16] Our study confirmed this; however, our follow-up was much shorter.
Strengths and limitations
The limitation of our study was the inclusion of floaters of various kinds. Another consideration is the treatment period of the study as limiting laser to three sessions does not indicate the real-life situation. Our study excluded those who needed more than three vitreolysis. The question arises regarding the efficacy of laser vitreolysis in this group of patients when vitrectomy is not an option. Furthermore, the post-laser monitoring of 1 month may be too short to observe late complications of Nd:YAG laser vitreolysis.
The strength of our study was that a single operator performed laser vitreolysis using a standardized technique. The data were collected for the first time in Southeast Asian patients in a prospective manner.
A better study design for future studies is to use a randomized controlled group with a larger sample size for the treatment efficacy and a longer observation.
Conclusion
Vitreolysis by Nd: YAG laser improved the CSF as measured by FrACT. Vitreolysis also improved VRQoL as measured by NEI VFQ-25 overall and in the subscales of general vision, near vision, distance vision, and mental health. No adverse events were seen in the short term.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Huang LC, Yee KMP, Wa CA, Nguyen JN, Sadun AA, Sebag J. V. B.8. Vitreous Floaters and Vision:Current Concepts and Management Paradigms. 2014:771–88. 10.1007/978-1-4939-1086-1_45. [Google Scholar]
- 2.Shen Z, Duan X, Wang F, Wang N, Peng Y, Liu DTL, et al. Prevalence and risk factors of posterior vitreous detachment in a Chinese adult population:The Handan eye study. BMC Ophthalmol. 2013;13:1–6. doi: 10.1186/1471-2415-13-33. doi:10.1186/1471-2415-13-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Webb BF, Webb JR, Mary CS, North CS. Prevalence of vitreous floaters in a community sample of smartphone users. Int J Ophthalmol. 2013;6:402–5. doi: 10.3980/j.issn.2222-3959.2013.03.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kim Y-K, Moon SY, Yim KM, Seong SJ, Hwang JY, Park SP. Psychological distress in patients with symptomatic vitreous floaters. J Ophthalmol. 2017:1–9. doi: 10.1155/2017/3191576. doi:10.1155/2017/3191576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mamou J, Wa CA, Yee KMP, Silverman RH, Ketterling JA, Sadun AA, et al. Ultrasound-based quantification of vitreous floaters correlates with contrast sensitivity and quality of life. Invest Ophthalmol Vis Sci. 2015;56:1611–7. doi: 10.1167/iovs.14-15414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Garcia GA, Khoshnevis M, Yee KMP, Nguyen-Cuu J, Nguyen JH, Sebag J. Degradation of contrast sensitivity function following posterior vitreous detachment. Am J Ophthalmol. 2016;172:7–12. doi: 10.1016/j.ajo.2016.09.005. [DOI] [PubMed] [Google Scholar]
- 7.Shah CP, Heier JS. YAG laser vitreolysis vs sham YAG vitreolysis for symptomatic vitreous floaters:A randomized clinical trial. JAMA Ophthalmol. 2017;135:918–23. doi: 10.1001/jamaophthalmol.2017.2388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sebag J, Yee KMP, Wa CA, Huang LC, Sadun AA. Vitrectomy for floaters:Prospective efficacy analyses and retrospective safety profile. Retina (Philadelphia, Pa) 2014;34:1062–8. doi: 10.1097/IAE.0000000000000065. [DOI] [PubMed] [Google Scholar]
- 9.Garcia GA, Khoshnevis M, Yee KMP, Nguyen JH, Nguyen-Cuu J, Sadun AA, et al. The effects of aging vitreous on contrast sensitivity function. Graefes Arch Clin Exp Ophthalmol. 2018;256:919–25. doi: 10.1007/s00417-018-3957-1. [DOI] [PubMed] [Google Scholar]
- 10.Nguyen JH, Nguyen-Cuu J, Yu F, Yee KM, Mamou J, Silverman RH, et al. Assessment of vitreous structure and visual function after neodymium:Yttrium-aluminum-garnet laser vitreolysis. Ophthalmology. 2019;126:1517–26. doi: 10.1016/j.ophtha.2019.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Luo J, An X, Kuang Y. Efficacy and safety of yttrium-aluminium garnet (YAG) laser vitreolysis for vitreous floaters. J Int Med Res. 2018;46:4465–71. doi: 10.1177/0300060518794245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Milston R, Madigan MC, Sebag J. Vitreous floaters:Etiology, diagnostics, and management. Surv Ophthalmol. 2016;61:211–27. doi: 10.1016/j.survophthal.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 13.Little HL, Jack RL. Q-switched neodymium:YAG laser surgery of the vitreous. Graefe's Archr Clin Exp Ophthalmol. 1986;224:240–6. doi: 10.1007/BF02143063. [DOI] [PubMed] [Google Scholar]
- 14.Delaney YM, Oyinloye A, Benjamin L. Nd:YAG vitreolysis and pars plana vitrectomy:Surgical treatment for vitreous floaters. Eye (London, England) 2002;16:21–6. doi: 10.1038/sj.eye.6700026. [DOI] [PubMed] [Google Scholar]
- 15.Singh IP. Modern vitreolysis-YAG laser treatment now a real solution for the treatment of symptomatic floaters. Surv Ophthalmol. 2020;65:581–8. doi: 10.1016/j.survophthal.2020.02.006. [DOI] [PubMed] [Google Scholar]
- 16.Sun X, Tian J, Wang J, Zhang J, Wang Y, Yuan G. Nd:YAG laser vitreolysis for symptomatic vitreous floaters:Application of infrared fundus photography in assessing the treatment efficacy. J Ophthalmol. 2019;2019 doi: 10.1155/2019/8956952. doi:10.1155/2019/8956952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Souza CE, Lima LH, Nascimento H, Zett C, Belfort R., Jr Objective assessment of YAG laser vitreolysis in patients with symptomatic vitreous floaters. Int J Retina Vitreous. 2020;6:1–5. doi: 10.1186/s40942-019-0205-8. doi:10.1186/s40942-019-0205-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Oen FT, Lim TH, Chung MP. Contrast sensitivity in a large adult population. Ann Acad Med Singap. 1994;23:322–6. [PubMed] [Google Scholar]
- 19.Cohen J. Statistical power analysis for the behavioral sciences. Academic Press Inc; New York: 2013. [Google Scholar]
- 20.Freiburg Visual Acuity &Contrast Test –Homepage. [Last accessed on 2021 Jul 07]. Available from: https://michaelbach.de/fract/
- 21.Azreen RA, Adlina AR, Mohtar I, Ahmad MS, Shatriah I. The study of correlation of quality of life assessment with visual fuction among kelantan glaucomatous patients. Med J Malaysia. 2010. [Last accessed on 2021 Jul 07]. Available from: http://www.e-mjm.org/2010/CRC_2010_supA.pdf .
- 22.Visual Function Questionnaire (VFQ-25) |RAND. [Last accessed on 2021 Jul 07]. https: //www.rand.org/health-care/surveys_tools/vfq.html .
- 23.The National Eye Institute 25-Item Visual Function Questionnaire (VFQ-25) [Google Scholar]
- 24.PROCEDURE GUIDE:VITREOUS OPACITIES. [Last accessed on 2021 Jul 07]. Available from: https://www.ellex.com/uploads/Ellex-Laser-Procedure-Guide-Vitreolysis-Opacities-VB0002H-A4-ELECTRONIC.pdf .
- 25.Katsanos A, Tsaldari N, Gorgoli K, Lalos Fotios, Stefaniotou M, Asproudis I. Safety and efficacy of YAG laser vitreolysis for the treatment of vitreous floaters:An overview. Adv Ther. 2020;37:1319–27. doi: 10.1007/s12325-020-01261-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hahn P, Schneider EW, Tabandeh H, Wong RW, Emerson GG. Reported complications following laser vitreolysis. JAMA Ophthalmol. 2017;135:973–6. doi: 10.1001/jamaophthalmol.2017.2477. [DOI] [PMC free article] [PubMed] [Google Scholar]